Jelajahi Bahan Perak dan Paduan Perak murni yang digunakan untuk Perhiasan
A Comprehensive Guide To Pure Silver and Silver Alloy Materials' Properties And Features
Silver has an alluring white luster, high chemical stability, and collectible aesthetic value, making it highly favored by people (especially women), thus earning the title of “the metal of women.” It is widely used for jewelry, decorative items, silverware, tableware, congratulatory gifts, medals, and commemorative coins. Silver jewelry has a broad market in developing countries, and silver tableware is popular among families. Silver commemorative coins are exquisitely designed, issued in limited quantities, and have the function of preserving and increasing value, making them highly sought after by coin collectors and investors.

Daftar Isi
Section Ⅰ Basic Properties of Silver
1. Physical Properties of Silver
Silver is an element in group IB of the 5th period of the periodic table, with the element symbol Ag, atomic number 47, and relative atomic mass 107.870. Silver has a very high reflectivity for visible light, reaching 92%-96% in the wavelength range of 380-780 nm, the highest among all metallic elements, significantly higher than other precious metal elements (Figure 4-1). Therefore, silver appears bright
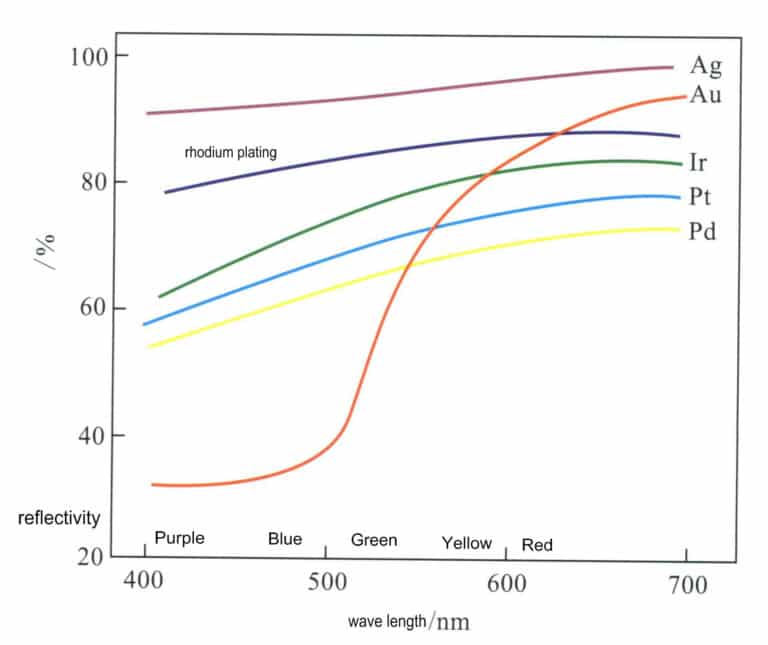
The main physical properties of silver are shown in Table 4-1. At room temperature, the density of silver is 10.49 g/cm3, and as the temperature increases, the density of silver decreases, dropping to 9.35 g/cm3 just before it melts. Silver is the best conductor of electricity and heat among all metals, which increases the difficulty for 3D printing and laser welding, as the heat applied locally quickly conducts to the surroundings, making it hard to concentrate heat.
Table 4-1 Main Physical Properties and Index Values of Silver
| Sifat Fisik | Nilai Indeks | Sifat Fisik | Nilai Indeks |
|---|---|---|---|
| Color Coordinates | L* = 95.8, a* =-0.7, b* = 5.3 | Linear expansion coefficient (0-100℃) | 19.2 x 10-6/℃ |
| Density (20℃) | 10.49 g/cm3 | Resistivitas (25 ℃) | 1.59 x 10-6Ω⸳cm |
| Titik leleh | 961.78 | Kapasitas panas spesifik (25 ℃) | 25.41 J/(mol⸳K) |
| Titik didih | 2177 | Panas fusi | 11.30 kJ/mol |
| Vapor Pressure ( Melting ) | 0.38 Pa | Panas penguapan | 284.6 kJ/mol |
| Konduktivitas termal (25 ℃) | 433 W/(m⸳K) | Debye temperature ϴd | 215 K |
| Difusivitas termal (0 ℃) | 1.75 m2/s | Kerentanan magnetik | -0.15 x 10-6 cm3/g |
2. Chemical Properties of Silver
The chemical properties of silver are not reactive, and its chemical stability is better than that of metals like iron and copper. It does not react with oxygen, hydrogen, inert gases, and organic gases at room temperature, and even at high temperatures, it does not react with hydrogen or inert gases, making it prone to corrosion and discoloration.
Silver has a strong affinity for sulfur, and in atmospheres containing harmful substances such as H2S, SO2, COS (carbonyl sulfide), and in aqueous solutions containing sulfides, it is prone to corrosion, forming insoluble black Ag2S compounds on its surface, and the corrosion behavior mostly exhibits electrochemical characteristics. When silver is left in the air, its surface gradually forms black Ag2S, causing jewelry to become dull and discolored. This property of silver severely affects its value as a precious metal. Ag2S can decompose into metallic silver and SO2 when heated in the air.
At room temperature, silver dissolves in nitric acid and concentrated sulfuric acid but is insoluble in hydrochloric acid and dilute sulfuric acid. It dissolves in hydrochloric acid, sulfuric acid, nitric acid, and aqua regia when heated. Like gold, silver readily reacts with aqua regia and saturated chlorinated acids; silver forms an AgCl precipitate, which can be used to separate gold and silver.
Like gold, silver has good corrosion resistance in alkaline solutions and molten alkali metals, making it a common crucible material for molten NaOH and KOH.
Silver can slowly combine with halogens at room temperature, but under heating conditions, silver can react very quickly with halogens to form silver halides. Silver dissolves in certain complexing agents saturated with air (such as cyanides of alkali metals from group ⅠA and alkaline earth metals from group ⅡA, oxygen-containing cyanide solutions, and acidic thiourea solutions containing Fe3+ ), forming stable complexes (Table 4-2).
Table 4-2 Behavior of silver in various corrosive media
| Media korosif | Keadaan sedang | Suhu | The degree of corrosion of silver | |||
|---|---|---|---|---|---|---|
| Media korosif | Keadaan sedang | Suhu | Almost No Corrosion | Slight Corrosion | Korosi sedang | Korosi parah |
| Asam sulfat | 98% | 18℃ | Ya. | |||
| Asam sulfat | 98% | 100℃ | Ya. | |||
| Asam nitrat | 0.1 mol/L | Suhu ruangan | Ya. | |||
| Asam nitrat | 70% | Suhu ruangan | Ya. | |||
| Asam nitrat | Smoke (>90%) | Suhu ruangan | Ya. | |||
| Asam klorida | 36% | 18℃ | Ya. | |||
| Asam klorida | 36% | 100℃ | Ya. | |||
| Hydrofluoric acid | 40% | Suhu ruangan | Ya. | |||
| Aqua regia | 75%HCl + 25%HNO3 | Suhu ruangan | Ya. | |||
| Hydrogen sulfide | Kelembaban | Suhu ruangan | Ya. | |||
| Asam fosfat | > 90% | Room temperature-100℃ | Ya. | |||
| Klorin | Klorin kering | Suhu ruangan | Ya. | |||
| Klorin | Klorin basah | Suhu ruangan | Ya. | |||
| Asam sitrat | Room temperature-100℃ | Ya. | ||||
| Merkuri | Suhu ruangan | Ya. | ||||
| Iron(I II) chloride solution | Suhu ruangan | Ya. | ||||
| Sod ium hydroxide solution | Suhu ruangan | Ya. | ||||
| Larutan amonia | Suhu ruangan | Ya. | ||||
| Larutan kalium sianida | Suhu kamar ~ 100 ℃ | Ya. | ||||
| Natrium hidroksida cair | 350℃ | Ya. | ||||
| Natrium peroksida cair | 350℃ | Ya. | ||||
| Molten sodium sulfate | 350℃ | Ya. | ||||
Silver can form compounds with various substances and exists in the form of monovalent ions in these compounds, such as AgNO3, Ag2O, AgCl, AgBr, AgCN, Ag2SO4, etc. AgNO3 is commonly used as the main salt for cyanide-free silver plating and is a source of silver ions. Silver nitrate solution contains a large number of silver ions, making it highly oxidative, easily decomposing in light, can cause protein coagulation, and has certain corrosive effects on the skin, so it should be stored in brown bottles. Ag2O is a black-brown powder with poor thermal stability, decomposing into silver and oxygen when heated. AgCl is insoluble in water but easily soluble in KCN, NaCN, and other substances. AgCl suspended in dilute sulfuric acid can be easily reduced to silver by negatively charged metals such as zinc, iron, etc., and this simple method is widely used for refining silver.
The properties of AgBr are similar to those of AgCl, dissolving in ammonium salts, thiosulfates, sulfites, and cyanide solutions, and can be easily reduced to metallic silver. The photosensitive properties of silver halides are the most important characteristics; under the influence of light, they decompose into silver and free halogens. This property of silver halides is utilized to produce photographic film, photo paper, and sensitized membranes.
3. Mechanical Properties
The main mechanical properties of pure silver are shown in Table 4-3. Pure silver is very soft, with good ductility and malleability, second only to gold in ductility, capable of being pressed into thin sheets and drawn into fine wires; 1 gram of silver can be drawn into a wire 1800m long and rolled into foil with a thickness of 10μm. However, when silver contains small amounts of impurities such as, Sb, Bi, and Pb, it becomes brittle, and ductility significantly decreases, with the effect of Pb being the most pronounced.
Table 4-3 shows the main mechanical properties of annealed pure silver.
| Mechanical properties | Nilai Indeks | Mechanical properties | Nilai Indeks |
|---|---|---|---|
| Brinell hardness HB/N/mm2 | 25 | Cross-sectional shrinkage rate /% | 80 ~ 95 |
| Kekuatan tarik / MPa | 140 ~ 160 | Elastic modulus E/GPa | 82 |
| Kekuatan luluh / MPa | 20 ~ 25 | Shear modulus G/GPa | 28 |
| Tingkat perpanjangan /% Tingkat perpanjangan | 40 ~ 50 | Compressive modulus B/GPa | 101.8 |
Pure silver can be strengthened through cold working Figure 4-2. The processing rate affects the mechanical properties of silver. The first processing rate of annealed pure silver can reach 99%. As the processing rate increases, the hardness, tensile strength, and yield strength of silver all rise, while the elongation rate rapidly decreases, and the rate of work hardening shows a pattern of initially fast and then slow. However, due to pure silver’s low stacking fault energy, its work-hardening effect is not significant, and the strength and hardness after processing remain very low, making it difficult to meet the strength requirements for setting jewelry.
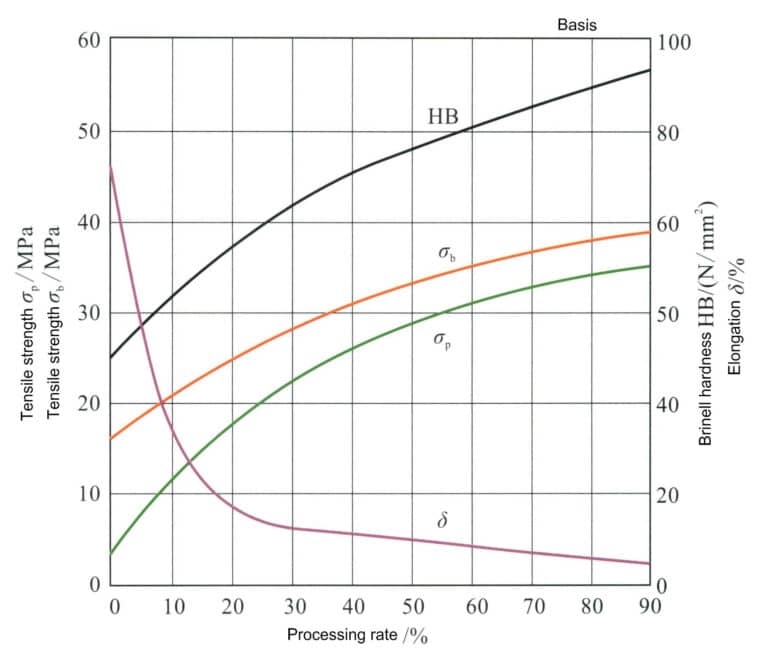
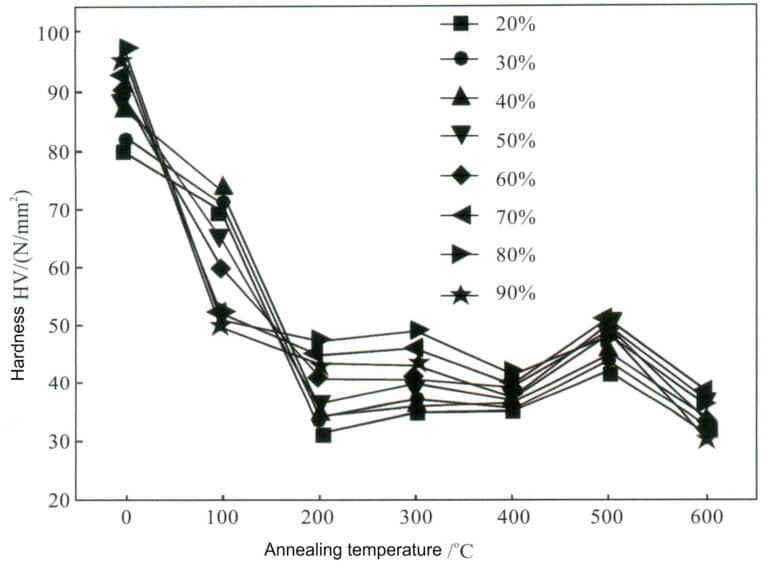
The low stacking fault energy of pure silver means that its work-hardening effect is not significant, and the strength and hardness after processing remain very low, making it difficult to meet the strength requirements for setting jewelry. The mechanical properties of silver in the processed hardened state change rapidly after annealing treatment. As the annealing temperature increases, the hardness of pure silver gradually decreases at different processing rates, but the rate of decrease needs to be more consistent. When the processing rate is below 50%, the hardness decreases the fastest at an annealing temperature of 200℃; when the processing rate is above 70%, the hardness decreases the fastest at an annealing temperature of 100℃ (Figure 4-3).
Another characteristic of pure silver in the processed state is that it is prone to “natural aging softening,” meaning that the strength and hardness of the processed profile or product gradually decrease during natural placement, which is unfavorable for wearing jewelry. The softening of pure silver during natural aging is actually caused by recovery or even the formation of recrystallized structures. Research shows that the change in strength of pure silver after cold deformation is related to material purity, amount of deformation, aging temperature, and placement time. Polycrystalline pure silver can even experience natural aging softening at temperatures below 20℃; the softening rate depends on the deformation and the impurity content in pure silver. The magnitude of the processing deformation also greatly affects aging softening. Silver with a purity of 99.999% begins to soften after being deformed by 99% and held for 10 hours at 20℃, while after 50% deformation, it can be maintained for 100 hours at 20℃ before starting to soften.
4. Process Performance
Silver has a relatively low melting point and can be melted using flame heating, induction heating, and resistance heating methods. However, during the melting of silver, a phenomenon known as “silver rain,” characterized by metal splashing, often occurs, leading to significant losses. When melting silver in an environment with poor atmospheric or vacuum conditions, the volatility of silver is relatively high, and it is even higher in an oxidizing atmosphere than in a reducing atmosphere.
Silver is prone to the formation of porosity defects during casting, and the principle of their formation is closely related to the properties of silver. According to casting formation theory, the main reason for the generation of porosity is that during the solidification process, the solubility of gas in the molten metal decreases with the drop in temperature, leading to gas supersaturation, precipitation, and bubble growth, which are not expelled in time, resulting in pores. The pores in silver castings are related to the oxygen absorbed by the molten metal. From the binary phase diagram Ag-O (Figure 4-4), it can be seen that when the saturated oxygen silver melt solidifies, it begins to solidify at about 951℃ below the silver melting point (961.78℃), and solidification is completed at about 931℃.
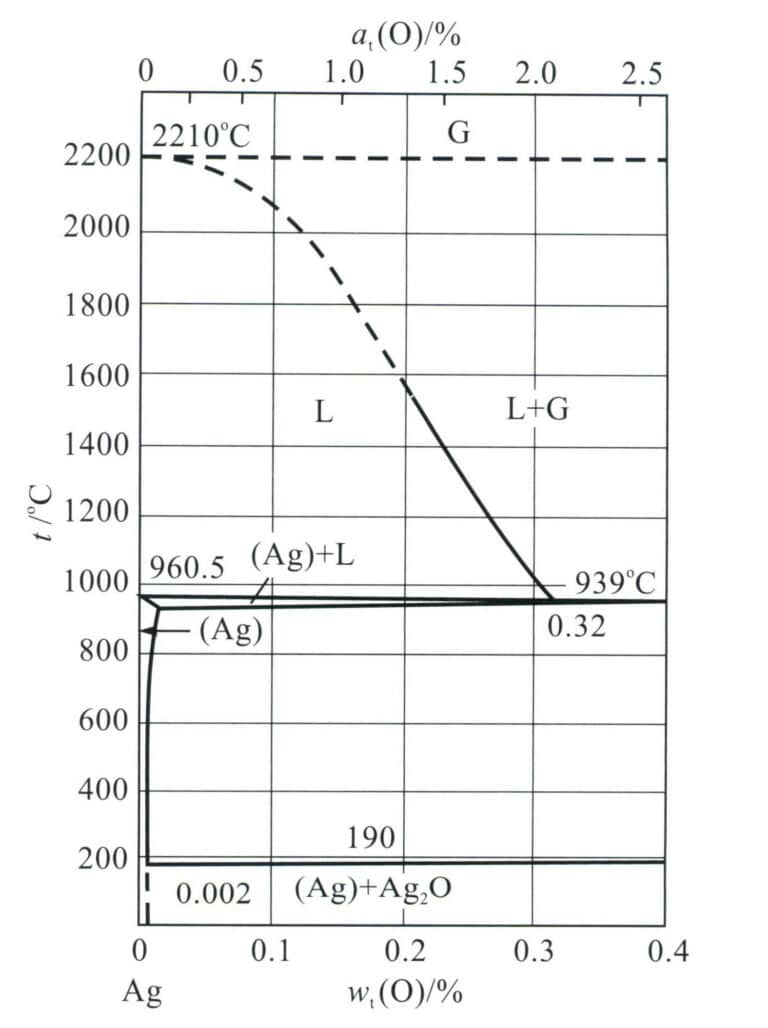
Note: G represents the gas phase; (Ag) +L represents the solid-liquid two-phase region,
where (Ag) represents the silver based solid solution, and L represents the liquid phase.
Table 4-4 shows the solubility of oxygen dissolved as atoms in silver in an oxygen atmosphere at 1 atm. The solubility of oxygen in molten silver just above the melting point is the highest, approximately 3200 x 10-6, reaching 21 times its own volume. As the temperature increases, the silver liquid’s superheating degree increases and oxygen’s solubility decreases.
When the silver liquid solidifies, the solubility of oxygen in solid silver significantly decreases. The solubility of oxygen in solid silver at 931℃ reaches its maximum, approximately 60 x10-6. As the temperature drops, the solubility of oxygen in solid silver rapidly decreases, and at room temperature, silver can hardly absorb oxygen. The solubility of oxygen is not only related to temperature but also to the partial pressure of oxygen. As the partial pressure of oxygen increases, the solubility increases, and the reaction between silver and oxygen also changes.
Table 4-4 shows the solubility of oxygen in silver in an oxygen atmosphere at 1 atm.
| Suhu / ℃ | Suhu / ℃ | 200 | 400 | 600 | 800 | 973 | 1024 | 1075 | 1125 |
|---|---|---|---|---|---|---|---|---|---|
| Oxygen content | x10-6 | 0.03 | 1.4 | 10.6 | 38.1 | 3050 | 2950 | 2770 | 2640 |
| Oxygen content | mm3/g | - | - | - | - | 2135 | 2056 | 1939 | 1849 |
During solidification, substances dissolved in the silver liquid, such as O, N, and H, are expelled to the solid-liquid interface. Once their saturation solubility is exceeded, they will be released. The formation of gas pores consists of two stages: nucleation and growth. Nucleation must overcome the combined effects of atmospheric pressure, metal static pressure, and additional pressures from surface tension. Only when the pressure of the gas being precipitated exceeds the total value of the external pressures can a bubble core be formed. The bubble further grows, and when it becomes large enough, the buoyancy acting on it increases, causing it to rise and detach. When the wetting angle of the bubble with the solid phase surface is ϴ> 90, it is easy to detach; when ϴ< 90, it is not easy to detach. If the dendrite growth rate during solidification is greater than the rising speed, the growing dendrites will completely enclose the bubbles, forming gas pores.
Silver is soft in texture, making it suitable for handmade jewelry. In traditional silver jewelry making, pure silver is extensively used to create filigree and woven jewelry, often employing techniques such as hammering and engraving to form decorative patterns on the surface of the jewelry. In modern jewelry production, cold processing techniques such as rolling, drawing, stamping, and hydraulic pressing are also widely used to process silver jewelry, taking advantage of the excellent ductility of pure silver. Techniques like spinning, deep drawing, and deep stamping are frequently used to create silver bowls, silver cups, and other crafts.
Section II The Purity and Classification of Silver Jewelry
1. Purity Marking of Silver Jewelry
For silver jewelry, the purity is indicated by a combination of the thousandth purity and silver, Ag, or S (S is the abbreviation for silver in English). For example, silver jewelry containing 92.5% can be marked with a purity label such as 925 silver, 925Ag, 925 S, or 925. For silver jewelry with a purity not lower than 99%, the purity label is marked as fine silver, 990 silver, 990 Ag, or S 990. The commonly referred thousandth silver in the market (with a silver content not lower than 99.9% ) is uniformly labeled as pure silver.
2. Classification of Silver Jewelry Purity
Silver is widely used in the production of jewelry, crafts, and other decorative items. Based on the purity of silver, it can be divided into high-purity jewelry silver and ordinary-purity jewelry silver.
2.1 High-Purity Jewelry Silver
As the name suggests, high-purity jewelry silver refers to silver with a high purity level, which can be further subdivided into:
(1) Pure Silver.
Theoretically, the silver content should be 100%. However, just as “gold is not completely pure,” silver is also not. Even with today’s scientific and technological levels, it is very difficult to smelt silver with a purity of 100%, and one can only get close to this purity value. Pure silver is also known as “fine silver,” and it is named for the unique patterns that form on the surface during the melting, refining, and condensing processes. In terms of jewelry materials, excessively pursuing the purity of silver is neither necessary nor practical. Therefore, silver with a purity not lower than 99.6% in the industry is generally classified as fine silver. Silver with a content not lower than 99.9% is referred to as pure silver.
(2) 990 fine silver.
The silver content must be at least 990‰. 990 fine silver was commonly used in the past as standard silver for circulation and trading. It can be used as collateral for property, as the silver backing of corporate consortiums, and as a medium for trade exchanges.
Pure silver and 990 silver have a higher grade, making them softer in texture. They are generally only used for unembellished silver jewelry, with traditional-style silver ornaments being the most common.
2.2 Ordinary-purity jewelry silver
Ordinary-purity jewelry silver by adding a small amount of other metals to pure silver or sterling silver, resulting in a harder texture of silver. These kinds of silver are generally based on Ag-Cu alloys, as copper’s physical and chemical properties are similar to those of silver, which can give colored silver toughness and maintain good ductility. Additionally, some alloying elements can suppress the dulling effect of air on silver jewelry to a certain extent. Therefore, the surface luster of many colored silver ornaments is less prone to change compared to pure silver and sterling silver. Ordinary-purity jewelry silver mainly includes the following categories:
(1) 980 silver.
Indicates a silver content of 98%, with a purity mark of 980 S. This colored silver is slightly harder than pure silver and sterling silver and is mostly used for making value-preserving jewelry.
(2) 958 silver.
It has a silver content of 95.8%, the second standard silver jewelry alloy in 12th century England, known as Britannia silver. It has lower hardness and is not suitable for setting gemstones.
(3) 925 silver.
Indicates a silver content of 92.5%, known as “sterling silver,” when only Cu is used as the alloying element. This is the first standard jewelry silver alloy in 12th century England, still in use today, with a history of over 800 years, and is widely accepted and used around the world. This silver has a certain hardness and toughness, making it suitable for making rings, necklaces, brooches, hairpins, and other jewelry, and is conducive to setting gemstones.
(4) 900 silver.
The silver content is 90%, with good strength and hardness. Originally designed mainly for making silver coins, it is also known as coin silver and later used for making jewelry.
(5) 800 silver.
Indicates a silver content of 80%; this silver has high hardness and good elasticity, making it suitable for making handbells, collar clips, and other jewelry.
There are lower purity types of silver, such as 700 silver, 600 silver, and 500 silver. It should be noted that silver’s chemical properties are not as stable as gold’s, especially when exposed to air, which can cause it to tarnish and lose its luster. Therefore, its status in precious metal jewelry has always been low, classified as low-grade precious metal jewelry with a value lower than platinum and gold.
Section III The Alloying of Pure Silver and Silver
1. Decorative Pure Silver
The culture of traditional handmade silver jewelry has a history of thousands of years. Traditional silver jewelry mainly uses handcraft techniques such as hammering, molding, filigree, and engraving, requiring soft and easy-to-shape materials. Therefore, the material is primarily pure silver, and the shapes and patterns mainly feature vines, flowers, auspicious beasts, and auspicious characters. This traditional craft culture has been passed down to this day and still has a certain market (Figure 4-5).

Jewelry companies generally purchase pure silver grains or pure silver ingots as raw materials when producing silver jewelry (Figure 4-6. Figure 4-7).


To ensure the quality of pure silver products, starting from the source of raw material grades is necessary. Pure silver is divided into three grades based on chemical composition: IC-Ag99.99, IC-Ag99.95, and IC-Ag99.90. The industry standard “Silver Granules” (YS/T 856-2012) specifies the requirements for the specifications of silver granules, with a particle size of 1-15 mm, and the chemical composition must meet the international standard requirements for silver ingots. The international standard “Silver Ingots” clearly specifies the chemical composition and impurity content of these three grades of pure silver ingots, as shown in Table 4-5.
Table 4-5 Requirements for the Chemical Composition of Pure Silver Ingots
| Kelas | wt (Ag) (≥)% | Impurity content (wt≤)/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kelas | wt (Ag) (≥)% | Cu | Pb | Fe | Sb | Se | Te | Bi | Pd | Total kotoran |
| IC-Ag99.99 | 99.99 | 0.0025 | 0.001 | 0.001 | 0.001 | 0.0005 | 0.0008 | 0.0008 | 0.001 | 0.01 |
| IC-Ag99.95 | 99.95 | 0.0250 | 0.015 | 0.002 | 0.002 | - | - | 0.001 | - | 0.05 |
| IC-Ag99. 90 | 99.90 | 0.0500 | 0.025 | 0.002 | - | - | - | 0.002 | - | 0.10 |
As mentioned earlier, the strength and hardness of traditional pure silver jewelry are very low. Even with cold processing, the level of work hardening could be higher due to silver being a low-stacking fault energy metal. Moreover, pure silver in a work-hardened state is prone to natural aging softening, making it easy to deform and wear during daily wear. Due to its low strength, it is also unsuitable for setting gemstones, making it difficult to create designs with a three dimensional effect. Additionally, pure silver is prone to dullness and discoloration in the air.
To improve the shortcomings of pure silver material, it is necessary to modify it using alloying or special processing techniques so that the modified material meets the corresponding jewelry color standards while having good comprehensive performance in physical, chemical, mechanical, and processing aspects.
2. Micro-alloyed Silver
The industry has developed high-purity silver that resists natural aging, softening, and dullness through micro-alloying methods or created high-hardness, high-purity silver jewelry using special processing techniques.
2.1 Micro-alloyed Silver
Research has found that adding trace alloying elements to pure silver can improve its strength and hardening rate, suppress the recovery process to some extent, raise the recrystallization temperature, and enhance the alloy’s hardening characteristics and resistance to natural aging softening. For example, adding trace rare earth elements (Y, La, Ce) to pure silver with a purity of 99.96% in amounts less than 0.11% results in a silver brazing solid solution alloy that has better corrosion resistance and higher resistance to aging softening compared to pure silver, making it suitable for use as a material for silver jewelry (Figure 4-8).
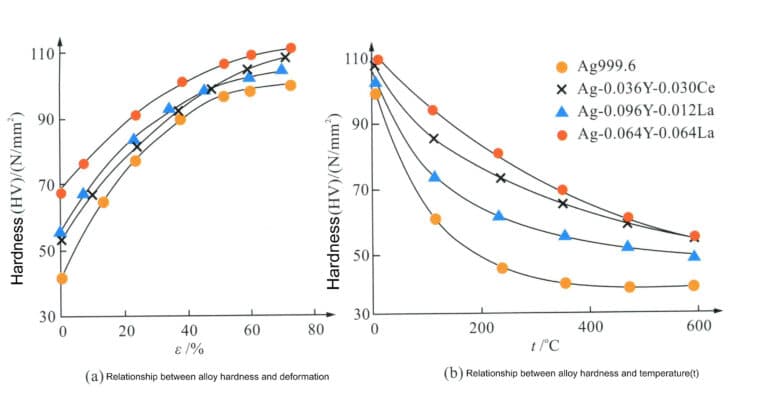
Similarly, adding 0.01% of Mn to ordinary pure silver, with a processing rate of 97%, results in a tensile strength of 340 MPa and a hardness of HV103 for Mn micro-alloyed pure silver, which can remain stable for 365 days at 25℃, while ordinary pure silver reverts to its pre-deformation strength and hardness levels in less than 30 days, its strength and hardness are basically restored to the level before deformation (Figure 4-9). Adding Mn can effectively refine the grain of silver, increase the number of grain boundaries, enhance deformation resistance, and play a role in strengthening and stabilizing mechanical properties.
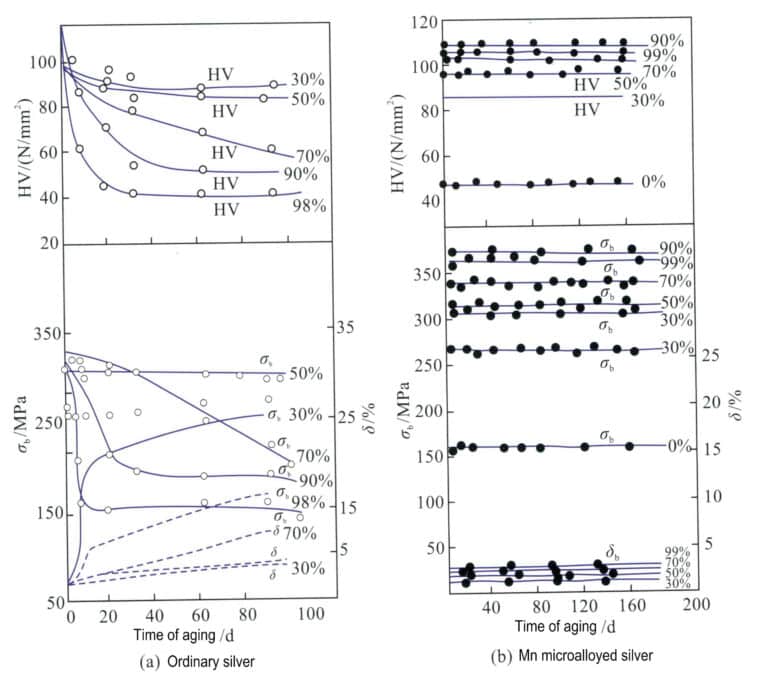
σb. Tensile strength; δ. Elongation; HV. Micro Vickers hardness; Ordinary pure silver; Mn micro-alloyed pure silver
2.2 Electroformed hard sterling silver
The electroformed hard 999 silver process is a jewelry-forming technique based on the principle of electrochemical deposition. By improving the formulation of the electroforming solution and parameters such as pH value, working temperature, organic brightener content, and stirring speed, the internal structure of the silver is enhanced, achieving a dense structure close to nanocrystals, significantly increasing the strength and hardness of the silver. It represents a breakthrough and innovation in traditional sterling silver jewelry.
The silver content of electroformed hard 999 silver is not less than 99.9%, meeting the quality standard for 999 silver, but its hardness is more than three times that of ordinary 999 silver jewelry. It has a hardness comparable to 925 silver, significantly improving pure silver jewelry’s deformation and wear resistance and meeting the requirements for gemstone inlays. Moreover, due to the hollow interior of the jewelry, its volume is four times that of ordinary pure silver jewelry at the same weight, allowing for the creation of three-dimensional and vivid shapes. The product has a good three-dimensional effect and features a combination of pure silver quality, 925 silver hardness, and traditional silver jewelry 1/3 weight (Figure 4-10).

3. Silver Alloy Systems for Jewelry
The strength performance of micro-alloyed silver largely relies on cold deformation hardening. However, once jewelry is subjected to heat during processes such as welding and polishing, its hardness quickly decreases, making it difficult to meet production and usage requirements. Therefore, appropriately reducing the fineness of silver through alloying to obtain a silver alloy with good overall performance is the main approach in the silver jewelry market, with silver alloys containing 92.5% being the most widely used. Theoretically, all elements that can dissolve in silver can produce a strengthening effect, but the degree of strengthening varies among different alloying elements. Moreover, many elements have a serious tendency to segregate at grain boundaries in silver. When micro-alloying to strengthen silver, the amount added is very small and can serve as beneficial alloying elements. However, once their content exceeds the solubility limit, it can lead to the embrittlement of silver. The commonly used alloying elements for silver alloys mainly include Cu, Zn, Pd, Pt, Sn, In, Si, Ge, and others.
3.1 Ag-Cu Alloy
The binary alloy phase diagram of Ag-Cu is shown in Figure 4-11. The Ag-Cu alloy is a eutectic alloy with a copper content at a eutectic point of 28.1%, occurring at 779℃. The maximum solubility of copper in silver is 8.8%. Within this range, as the copper content increases, the alloy’s melting point decreases until it reaches the eutectic temperature of the alloy. Therefore, adding copper to silver improves its casting performance. After solidification, the Ag-Cu alloy forms immiscible silver-rich and copper-rich solid solutions, significantly increasing the strength of the alloy. Aging treatment of the solid solution alloy at low temperatures can further produce precipitation strengthening. Thus, copper can produce a noticeable strengthening effect in silver and increase its recrystallization temperature.
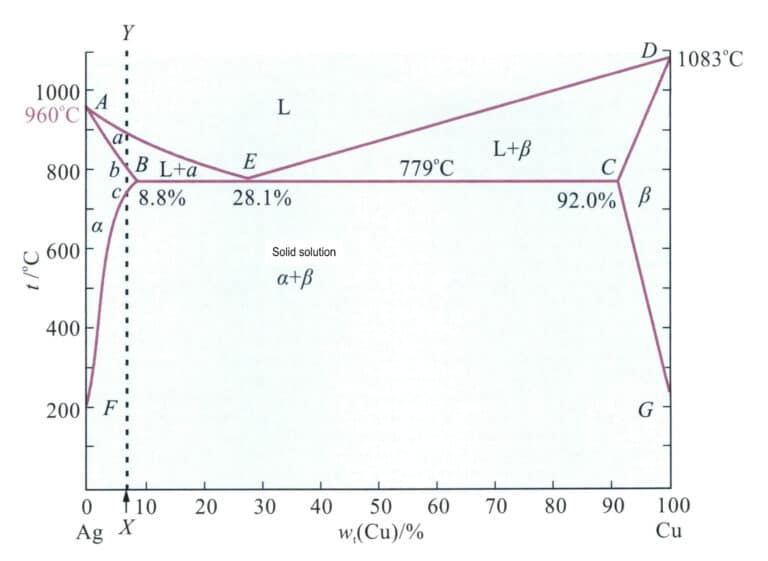
Note: 960℃ represents the melting point of pure silver; 1083℃ represents the melting point of pure copper; 8.8% is the maximum solubility of copper in silver; 28.1% is the copper content at the eutectic point; 779℃ is the eutectic temperature; 92.0% indicates that the maximum solubility of silver in copper is 100%-92.0% = 8.0%; point A point E represents the liquidus line; a is the silver-based solid solution; 0 is the copper-based solid solution; G represents the temperature at which the solubility of silver in copper decreases to 0 under equilibrium conditions.
The main mechanical properties of the alloy are shown in Table 4-6. As the Cu content increases, the strength and hardness of the Ag-Cu alloy improve while the elongation correspondingly decreases.
Table 4-6 shows the main mechanical properties of the Ag-Cu alloy.
| Alloy grade | Hardness HB/(N/mm2) | Kekuatan tarik/MPa | Elongation/% | |||
|---|---|---|---|---|---|---|
| Alloy grade | Annea led state | Processed state | Annealed state | Processed state | Annea led state | Processed state |
| 95%Ag-5%Cu | 50 | 119 | 240 | 450 | 43 | 5 |
| 92.5%Ag-7.5%Cu | 57 | 118 | 260 | 470 | 41 | 4 |
| 90%Ag-10%Cu | 64 | 125 | 270 | 450 | 35 | 4 |
| 87.5%Ag-12.5%Cu | 70 | 127 | 260 | - | 38 | 4 |
| 80%Ag-20%Cu | 79 | 134 | 310 | 500 | 35 | 4 |
| 75%Ag-25%Cu | 82 | 135 | 320 | 540 | 33 | 4 |
After adding Cu to silver, its color has a certain impact. As the Cu content increases, the reflectivity of the Ag-Cu alloy to visible light gradually decreases (Figure 4-12), and the color of the alloy gradually changes from silver-white to light pink, pink, and even red.
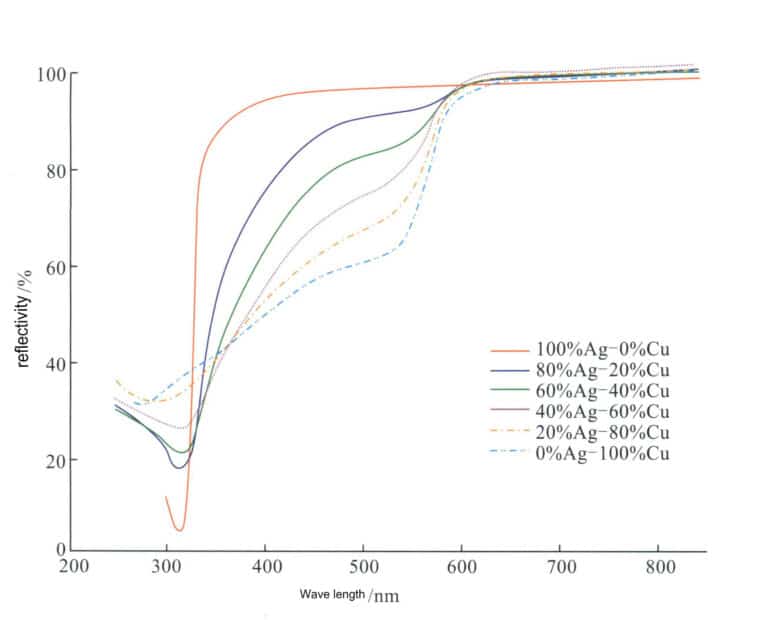
Cu is the most commonly used alloying element in Ag, and the traditional silver color is a binary alloy with Cu as the alloying element, with main grades including 980 silver, 925 silver, 900 silver, and 800 silver. The silver alloys in the current market are also basically is based on the Ag-Cu alloy as the base alloy. Although copper can improve silver’s strength, hardness, and casting performance, it does not improve its resistance to darkening and discoloration. Moreover, due to the two-phase structure of the alloy, there is a corrosion micro-battery effect in corrosive environments, making its corrosion resistance worse than that of a single-phase silver solid solution.
3.2 Ag-Pd Alloy
Research shows that adding a certain amount of precious metals to silver effectively improves its resistance to darkening and discoloration. Pd is silver’s preferred precious metal element, and Figure 4-13 shows the Ag-Pd binary alloy phase diagram.
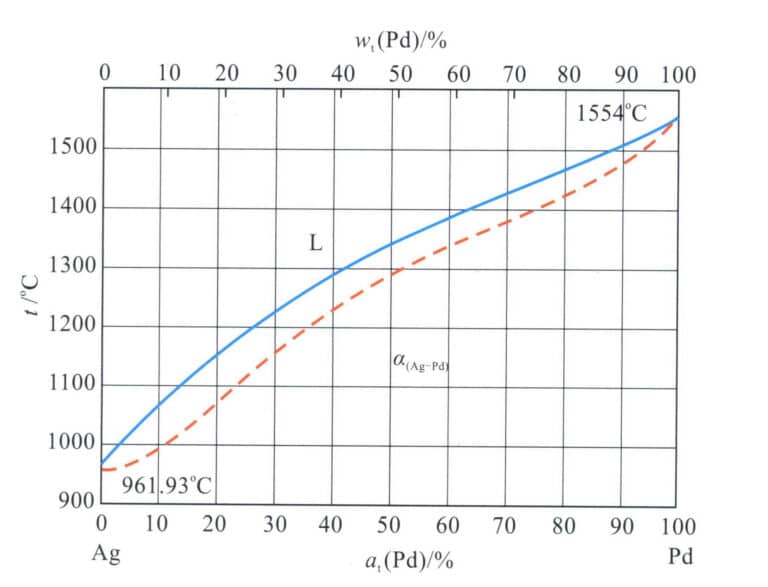
The alloy is infinitely soluble in liquid and solid phases, forming a continuous solid solution. Therefore, the strengthening effect of palladium on silver is generally modest, as shown in Table 4-7. Cold deformation can improve the strength and hardness of the alloy to a certain extent. However, it still cannot fully meet the strength requirements for embedded jewelry, necessitating the addition of other alloying elements for further reinforcement.
Table 4-7 Main properties of the annealed alloy
| Alloy grade | Melting temperature /℃ | Kepadatan / (g/cm)3) | Hardness HV/(N/mm2) | Kekuatan tarik / MPa | Thermal conductivity /[W/(cm⸳K)] |
|---|---|---|---|---|---|
| 95%Ag - 5%Pd | 980 ~ 1020 | 10.5 | 28 | 170 | 2.20 |
| 90%Ag - 10%Pd | 1000 ~ 1060 | 10.6 | 35 | 210 | 1.42 |
| 80%Ag - 20%Pd | 1070 ~ 1150 | 10.7 | 45 | 260 | 0.92 |
Palladium effectively improves the silver’s resistance to sulfide tarnishing. As the palladium content increases, the tendency for silver to tarnish and discolor due to sulfide significantly decreases. However, the melting point of the alloy increases, the crystallization interval also widens, and palladium is prone to gas absorption during melting, which increases the difficulty of smelting and casting, requiring melting under vacuum or inert gas protection.
Due to the continuous rise in the price of palladium, adding palladium significantly increases the cost of silver alloys. Therefore, in recent years, the application of palladium in silver has greatly decreased, and existing uses are mainly based on small additions.
Copywrite @ Sobling.Jewelry - Produsen perhiasan khusus, pabrik perhiasan OEM dan ODM
Section IV Sterling Silver and Its Modifications
Sterling originated from the name of a German coin maker, the Easterlings, in the 12th century. He brought advanced silver coin and silver alloy preparation techniques to England during the reign of Henry II, creating a silver alloy composed of 92.5%Ag and 7.5%Cu. This alloy was widely used and became the first brand of silver alloy in 12th-century England. In honor of this coin maker, the alloy was named sterling silver. Initially, sterling silver specifically referred to the 92.5%Ag-7.5%Cu alloy, but later, the range of alloys expanded, becoming a general term for all 925 silver. Since the 12th century, sterling silver has been widely used in silverware and silver jewelry, consistently serving as a standard-grade alloy, and is the oldest decorative silver alloy in history.
1. Characteristics of Sterling Silver
1.1 Mechanical Properties
According to Figure 4-11, the composition of the sterling silver alloy corresponds to the dashed line XY, and the intersection points with the phase boundary are A, B, C. The range from point B to point C is a single solid solution, and after slowly cooling below point C, a copper-rich solid solution phase will precipitate from the solid solution. Heating sterling silver to 800℃ for solid solution treatment yields a single solid solution, which can give the alloy excellent ductility and workability. Table 4-6 shows that the strength and hardness of solid solution in sterling silver are significantly higher than those of pure silver. Cold working of the solid solution sterling silver can achieve a good work hardening effect (Figure 4-14).
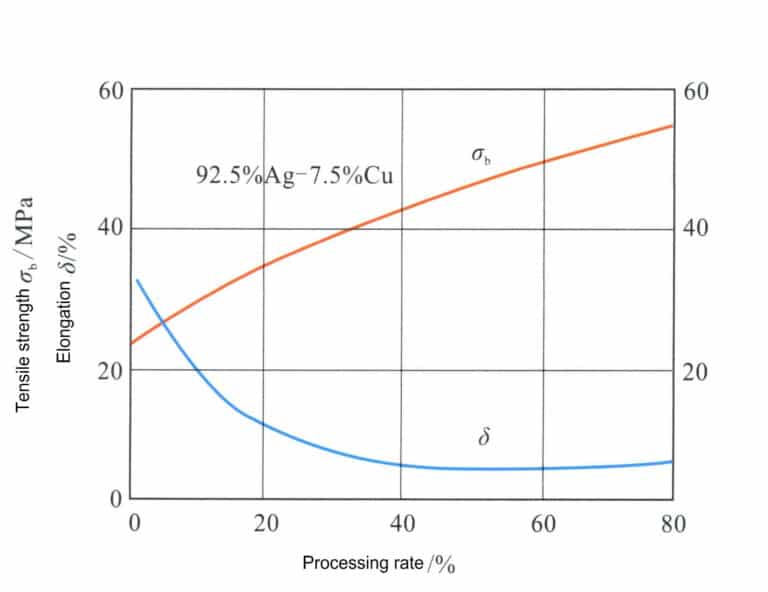
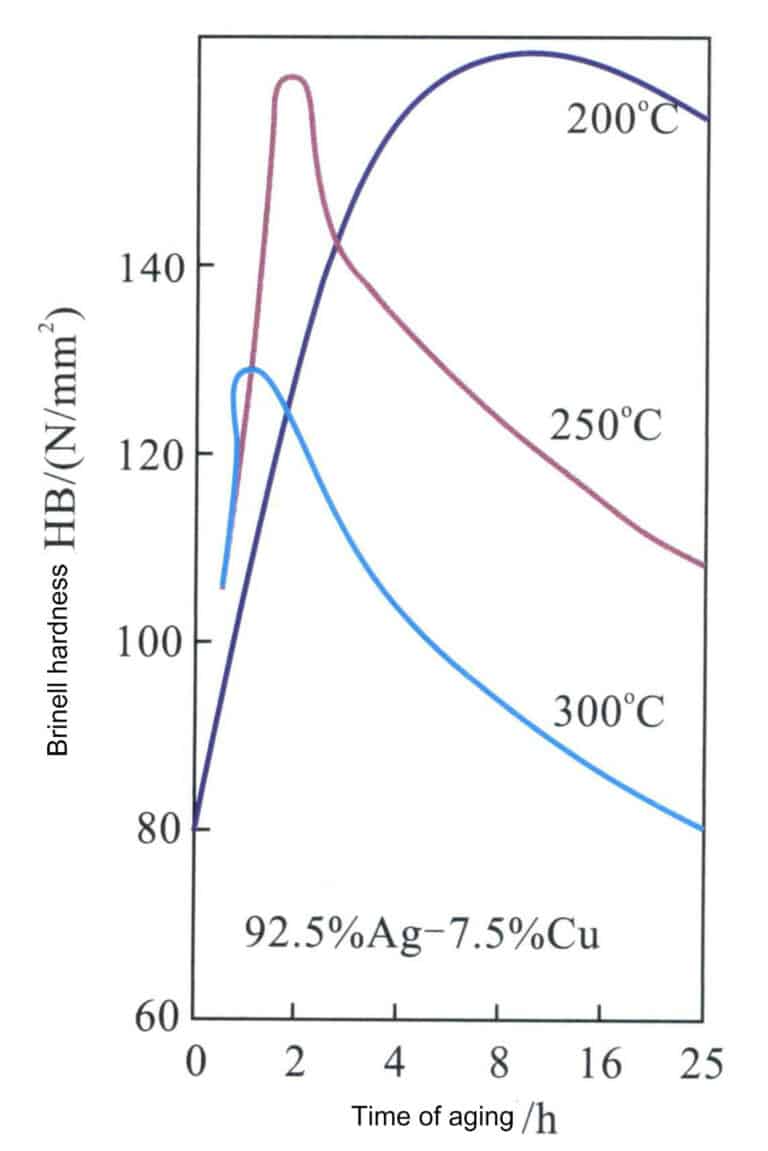
A prominent feature of sterling silver alloy is its excellent age-hardening characteristics, which can be altered through aging treatment (Figure 4-15). The solid solution of sterling silver undergoes aging treatment at 200-300℃, and when the aging temperature is 200℃, the highest hardness obtained is close to HV160, comparable to that of 18K gold alloy; however, a longer aging time is required to reach this peak value. As the aging temperature increases, the time to achieve peak hardness is greatly shortened, but the peak hardness also decreases accordingly. When the aging temperature reaches 300℃, the effect of age-hardening has significantly diminished.
1.2 Casting Characteristics
The liquidus temperature of sterling silver is 898℃, and the alloy has a low melting point, making it suitable for melting in a graphite crucible.
However, sterling silver absorbs a large amount of oxygen in the molten state, which poses problems for smelting and casting. This property makes the alloy prone to volatilization at high temperatures or causes significant loss during the high-temperature cooling process due to splashing. For sterling silver, without sufficient deoxidizers, if protection is not applied during smelting, oxygen can easily accumulate, leading to copper oxidation, and jewelry castings are prone to defects such as porosity and oxidized inclusions. The oxidized copper in the castings can cause two types of problems: (1) the entire casting may have oxidized copper inclusions, which form hard spots when the inclusions are near the surface, protruding on the polished surface; (2) oxidized copper inclusions near shrinkage cavities appear as mottled cloudy spots on the polished surface, which are deep and difficult to clean off. Suppose the sterling silver melt is severely overheated or not protected for a long time. In that case, copper will oxidize severely, forming a viscous liquid surface, reducing the fluidity of the molten metal, leading to incomplete filling in some small areas of the casting, and often resulting in underfilling. The surface near the casting area appears red. In addition, the crystallization interval of sterling silver is relatively large, reaching 90℃, with significant differences between the liquid and solid phase components, tending towards paste-like solidification, with severe dendritic growth, while having lower fluidity, leading to a greater tendency for shrinkage porosity.
To prevent the accumulation of oxygen in the sterling silver melt, it is crucial to avoid contact between the molten metal and the atmosphere as much as possible during the smelting or casting process. Therefore, the following points should be noted.
(1) During electric furnace smelting, vacuum protection is used, or inert gases such as argon or nitrogen are employed for protection, which can eliminate oxygen in the smelting chamber and reduce the oxidation of the molten metal.
(2) When using a fire gun for smelting, the flame should be adjusted to a reducing yellow flame, covering the entire liquid surface to prevent the molten metal from absorbing oxygen. During electric furnace smelting, a reducing flame can sometimes be added at the mouth of the crucible to cover the molten metal.
(3) Sprinkle charcoal or anhydrous boric acid on the surface of the molten metal; they float on the surface of the silver liquid and can protect the silver liquid in two ways: a. forming a barrier between the molten metal and the air; b. reducing copper oxide. This method is unsuitable for centrifugal casting machines but works very well on manually operated vacuum casting machines.
(4) In the above method, it is also important to strengthen the protection of the molten metal during the pouring process. Especially when using a vacuum casting machine for pouring, since it is a manual pouring under vacuum conditions, it is necessary to protect the flow of molten metal. Typically, a reducing flame is used; when the gypsum mold is placed, the flame is ignited, and the flame must cover the pouring gate of the mold, which can eliminate the air inside the mold.
1.3 Polishing "red spot" phenomenon
Sterling silver often forms dark red spots on the surface during polishing, which severely affects the brightness and aesthetics of the polished surface, as well as the adhesion of the electroplated layer. This phenomenon is more pronounced on the surfaces of products that have undergone thermal processing, such as annealing and welding.
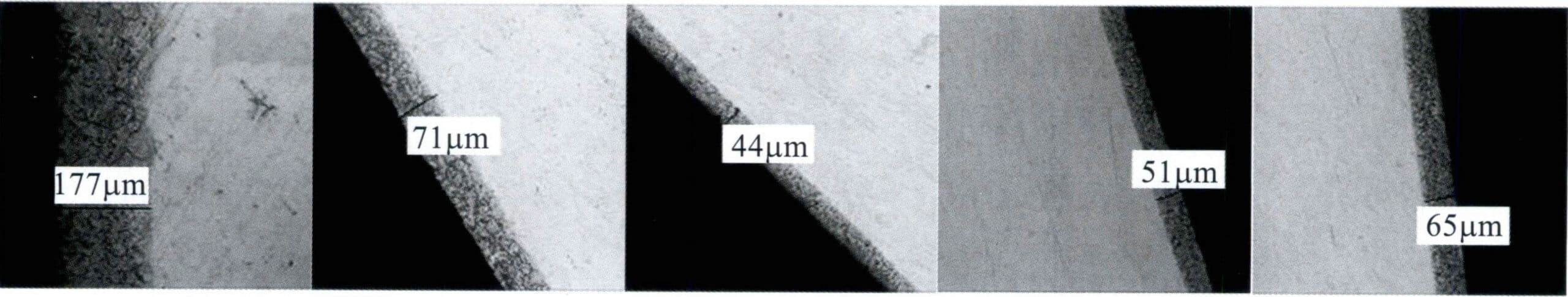
After heating the sterling silver block at 700℃ for 1.5 hours, the oxidation condition was observed under a microscope, revealing that the alloy not only formed an oxide layer on the surface but also developed an internal oxidation zone beneath the surface (Figure 4-16).

Sterling silver belongs to the Ag-Cu alloy. When the alloy comes into contact with oxygen in the air at high temperatures, selective oxidation of Cu occurs only after the temperature exceeds 400K. When the sample is immersed in dilute sulfuric acid, the surface layer of copper oxide can be removed. Therefore, after the sample is lightly polished, it can present a silver-white color. However, after further polishing, dark red spots appeared on the polished surface, damaging the reflective properties of the polished silver surface (Figure 4-17), indicating that oxidation products of Cu still exist in that area.

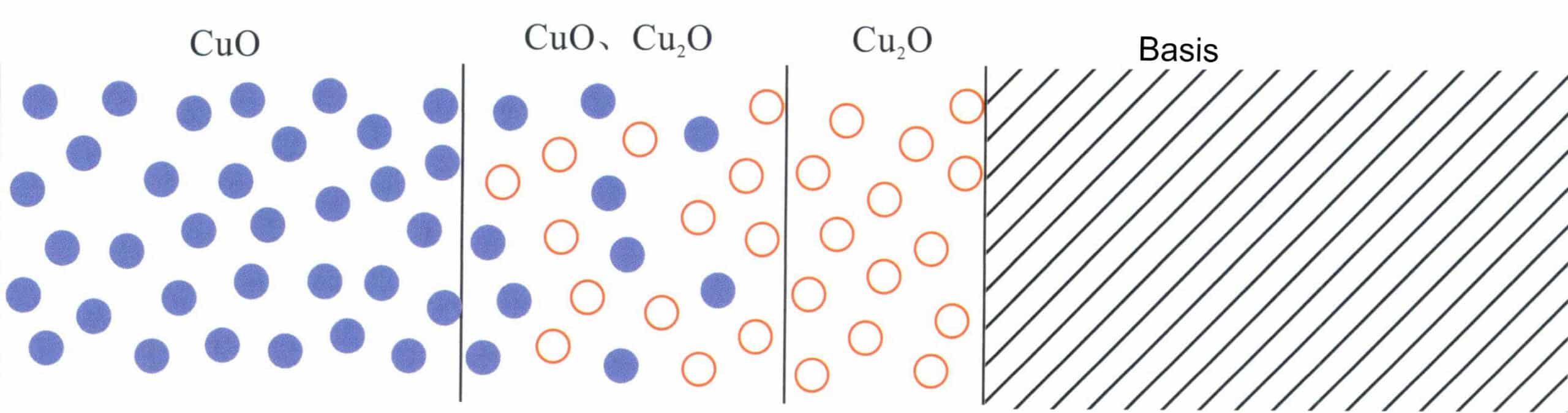
When copper comes into contact with oxygen in the air at high temperatures, such as during hot rolling, annealing, or welding, the surface of the copper workpiece first oxidizes to form red Cu2O and then further oxidizes to become black CuO. The oxidation of copper is not limited to the surface of silver alloys but may penetrate to a certain depth (Figure 4-18). According to the theory of high-temperature oxidation kinetics of alloys, when two diffuses simultaneously with Cu, there must be an accumulation of the Cu2O precipitate phase in the internal oxidation zone, and the alloy that has not undergone internal oxidation at the internal oxidation front will show depletion of Cu. Ag has a strong ability to absorb oxygen and transport oxygen to the interior of the metal at high temperatures. Hence, oxygen diffusion is dominant, and its permeability is much higher than that of Cu. Therefore, oxygen can penetrate the alloy surface’s sublayer, generating internal oxide precipitates. A direct pathway from the oxide to the alloy surface must exist to remove the sublayer’s copper oxide through acid etching. The copper content in sterling silver is only 7.5%, and its structure is a dual-phase solid solution without forming an oxide network, which means there is no direct pathway into the interior during acid immersion, allowing the internal Cu2O to remain intact. As a result, the surface of the oxidized sterling silver sample still exhibits black and irregular patches, known as “red spots.”
Research has found that the severity of the red spots on the surface of sterling silver is closely related to the heating temperature and heating time (Figure 4-19). The higher the heating temperature and the longer the heating time, the thicker the surface oxide film becomes, and the deeper the internal oxide layer penetrates into the substrate, making it difficult to remove through conventional polishing methods.

1.4 Darkening Color Change Phenomenon
Silver itself is prone to darkening and discoloration. The addition of Cu elements in sterling silver does not improve the alloy’s tendency to discolor. Moreover, the sterling silver alloy has a two-phase structure in both the cast and aged states, consisting of a silver-rich solid solution and a copper-rich solid solution, which are two incompatible phases. The potential difference between these two phases forms localized micro-battery reactions, increasing the electrochemical corrosion performance of the sterling silver alloy and reducing its corrosion resistance. Therefore, sterling silver jewelry is prone to corrosion and discoloration, severely affecting the appearance quality of silver ornaments.
2. The Influence of Alloying Elements on Sterling Silver
Due to the tendency of sterling silver to develop polishing red spots and dull discoloration issues, as well as the susceptibility to metallurgical and casting defects during melting, modification treatment is required. This involves alloying to enhance its overall performance while maintaining its good mechanical properties. Common alloying elements include Si, Zn, Sn, Ce, Ge, and others.
2.1 The impact of Silicon(Si) on sterling silver
Traditional sterling silver alloys tend to absorb gas during melting and pouring, making them prone to oxidation and resulting in larger gas pores in the castings. At the top are gas escape channels due to solidification gas absorption, accompanied by oxidized inclusions, and inclusions also exist within the castings. Adding a small amount of silicon to sterling silver can effectively improve its gas absorption tendency and oxidation resistance, resulting in better casting quality, reduced defects of gas pores and inclusions, and improved surface quality after polishing. From a thermodynamic perspective, the Gibbs free energy value for silicon-forming oxides is higher than that for copper oxides. Therefore, after adding an appropriate amount of silicon, the silicon in the molten metal preferentially reacts with oxygen, reducing gas pore defects. Due to the low density and high viscosity of silica, it can be removed from the surface of the molten metal using flux once it floats to the surface. The silicon added to sterling silver also helps improve the alloy’s resistance to oxidation and sulfide discoloration.
As the Si content increases, the grain structure will gradually become coarser, worsening the surface polishing effect and leading to the appearance of an “orange peel” effect on the surface. When the silicon content exceeds a certain value, the brittleness of the alloy significantly increases, making it prone to cracking during processing.
2.2 The Influence of Zinc(Zn) on Sterling Silver
Zn can lower the melting point of sterling silver, increase the fluidity of the melt, reduce shrinkage defects, and make the cast structure denser, but it has no significant effect on grain size. As an oxygen-active element, Zn, when added to sterling silver, preferentially reacts with oxygen in the molten metal, which can help reduce the oxidation of the molten metal due to absorbed oxygen. However, when the zinc content is too high, it can easily increase the amount of oxidized inclusions in the molten metal.
The atomic size difference between Zn and Ag is 7.76%, Cu, and the atomic size difference with Ag is 11.50%, Zn. The strengthening effect of Ag is less strong than that of Cu. When Zn partially replaces Cu in sterling silver, the casting and annealed hardness are reduced. When the Zn content exceeds 3.36%, the casting hardness of sterling silver is only around HV50, making it difficult to meet the jewelry inlay requirements and wear resistance requirements. Zn has an adverse effect on the processing performance of the alloy, reducing its plasticity. Excessive Zn content will cause issues such as delamination, scaling, and cracking during processing.
The electrode potential of Zn is lower than Ag and Cu, and a passivation film will spontaneously form on the Sterling silver surface, slowing down electrochemical corrosion and improving the alloy’s resistance to sulfide discoloration and oxidation. As the Zn content increases, Sterling Silver. The thickness of the oxide film gradually decreases. Still, when the Zn content exceeds 3.5%, Sterling silver is more prone to oxidation inclusions, which is detrimental to its electrochemical corrosion resistance and anti-sulfide discoloration performance.
2.3 The effect of Stannum(Sn) on Sterling silver
Adding Sn to sterling silver can lower the alloy’s melting point, increase the fluidity of the melt, and reduce the alloy’s shrinkage, resulting in a denser cast structure. A small amount of Sn can also refine the alloy’s structure; however, when the Sn content exceeds 2%, the alloy’s structure shows a noticeable coarsening, forming relatively large dendrites and shrinkage and segregation.
In sterling silver alloys, the partial replacement of Cu with Sn generally improves the initial hardness of the alloy in the cast state. As the Sn content increases, the hardness of the alloy first rises and then decreases. When the Sn content approaches 1%, the hardness reaches its maximum value; however, the ductility of the alloy is significantly affected, which may lead to cracking during cold working.
Sn is also an oxygen-active element. A certain amount of Sn can form a dense oxide film on the alloy’s surface, protecting the substrate. Sn can improve the electrochemical corrosion resistance of sterling silver. As the Sn content increases, the alloy’s electrochemical corrosion resistance improves. When the Sn content exceeds 2%, the alloy’s sulfide discoloration and oxidation resistance are significantly enhanced.
2.4 The impact of Cerium (Ce) on sterling silver
The addition of rare earth element Ce to sterling silver significantly impacts its structure. Ce can effectively purify the metal liquid and reduce gas content. When the Ce content is below 0.05%, it can refine the grain structure, mainly because Ce acts as a grain refiner during the solidification process of the metal liquid, reducing the degree of shrinkage porosity and improving density during annealing; Ce can also hinder grain boundary migration, thereby maintaining a fine grain structure.
Trace amounts of Ce can improve the mechanical properties of sterling silver, increasing its strength and hardness, enhancing ductility, and improving the alloy’s work-hardening effect. When the Ce content is further increased, it is prone to segregation at the grain boundaries, worsening the alloy’s processing performance and making it more susceptible to oxidation inclusions.
After adding trace amounts of Ce, the electrochemical corrosion resistance, oxidation spot resistance, and sulfide discoloration resistance of sterling silver can be greatly improved. When the Ce content exceeds 0.075%, the corrosion resistance of sterling silver will decline.
2.5 The impact of Ge on sterling silver
When Ge is added to sterling silver, and its content is between 0.2% and 0.8%, the cast hardness of sterling silver is higher, and its work hardening performance, electrochemical corrosion resistance, and discoloration resistance are all improved compared to sterling silver, reflecting better overall performance. When the Ge content is too low, the performance improvement of sterling silver is not significant; however, when the content is too high, it can easily cause grain coarsening of the alloy, leading to a decline in the overall performance of the alloy.
Section V The Discoloration and Protection of Silver
Silver and its alloy materials are widely used in the jewelry industry, and they have a significant characteristic: they easily undergo tarnishing and discoloration. After silver alloys discolor, the surface gloss greatly decreases, severely affecting the appearance quality of the jewelry and increasing the difficulty of the alloy processing.
1. The reason for silver tarnishing
The chemical potential of pure silver is +0.799 V, which is relatively high compared to the standard potential of hydrogen, classifying it as an inert metal. Under normal conditions, it does not react chemically with acids or bases, only reacting with strong oxidizing concentrated acids (such as concentrated nitric acid and hot concentrated sulfuric acid). However, silver jewelry will gradually tarnish after being worn for some time or even when placed in a storage box for a certain duration, and traditional sterling silver is particularly prone to tarnishing. The reasons for silver tarnishing can be summarized as follows.
1.1 Silver sulfide discoloration
Silver and its alloys are prone to corrosion and discoloration in environments containing H2S, SO2, and COS. Silver is very sensitive to H2S gas, and when the concentration of H2S in the atmosphere reaches 0.2 x 10-9 (volume fraction), it is sufficient to corrode silver, resulting in the formation of black Ag2S, namely:
4Ag + 2 H2S + O2 = 2Ag2S + 2H2O
The rate of silver sulfide discoloration in the atmosphere of H2S follows the Wagner diffusion kinetic mechanism, and when the content of H2S increases or when H2S coexists with other gases such as NO2, O2, the discoloration rate of silver sulfide intensifies. The SO2 in the air can also be converted into S2-to form Ag2S, causing silver discoloration. The sensitivity of SO2 is not as high as that of H2S, but when SO2 coexists with other gases such as NO2 and O2, the rate of sulfide discoloration will also intensify.
Silver is very sensitive to oxygen-containing sulfide solutions. The discoloration occurs slowly when silver is immersed in an oxygen-free Na2S solution. However, if the sample is taken out and the sodium sulfide solution attached to the silver surface comes into contact with oxygen, the silver sample will quickly show obvious discoloration. The longer it is exposed to air, the more severe the discoloration becomes. The sequence of color changes is silver, white → yellow → brown → blue. This is because the standard electrode potential of Ag (0.779 V) is lower than that of O at 1.229 V. Ag is thermodynamically unstable when oxygen is present, first by O2 being oxidized to Ag+ and then combining with Ag+ and S2-to form an insoluble compound Ag2S. The higher the concentration of sulfides, the more severe the discoloration. The chemical reaction of silver in oxygen-containing Na2S aqueous solution is:
4Ag + 2H2O + O2 + 2S2-= 2Ag2S↓+4OH–
Ag-Cu Alloys are more prone to sulfide formation and produce black Cu2 S because Cu is more easily sulfide than Ag; thus, they discolor more easily than pure silver.
1.2 Electrochemical corrosion in humid environments
In humid environments, the unevenness of the silver surface state (uneven alloy composition or physical states such as internal stress, surface smoothness, etc.) can cause different potentials in different areas of the metal surface beneath the water film, creating a potential difference between these areas. Two adjacent areas with different potentials are connected, with the water film acting as an electrolyte to transport ions and the metal serving as a conductor for electron transport, forming an electric cycle. This creates the effect of a short-circuit battery, forming many corrosion micro-batteries on the metal surface. The cast structure of sterling silver generally consists of a two-phase structure made up of silver-rich solid solution and copper-rich solid solution. In humid environments, the copper-rich solid solution phase becomes the anode of the corrosion micro-battery, making the alloy more susceptible to corrosion and discoloration. Higher-quality silver can also undergo electrochemical corrosion due to impurities, and in humid environments containing salt, the silver surface often transforms into silver chloride, a gray-brown adhesive substance resembling soil.
1.3 The effect of ultraviolet light on silver discoloration
Light, as an external energy source, can promote the ionization of metal ions, thereby accelerating the reaction between silver and the corrosive medium, that is accelerating the discoloration reaction of silver. When the surface of the silver-plated layer is irradiated with light of different wavelengths, the results are shown in Table 4-8. It can be seen that the silver-plated layer is prone to discoloration after absorbing ultraviolet light, and the ability of the irradiated light wavelength to cause discoloration increases as the wavelength decreases.
Table 4-8 The effect of irradiated light wavelength and irradiation time on the discoloration of the silver-plated layer
| Irradiated light wavelength/nm | Irradiation time /hours | ||||
|---|---|---|---|---|---|
| Irradiated light wavelength/nm | 6 | 12 | 18 | 24 | 48 |
| 253.7 | Unchanged | Focal Macula | Yellow Brown | Brown-Black | All Black |
| 365.0 | Unchanged | Unchanged | Unchanged | Kuning | - |
| Sunlight | Unchanged | Unchanged | Unchanged | Localized macular | - |
According to the results of X-ray photoelectron spectroscopy and Auger spectroscopy analysis, the color change of the silver-plated layer under ultraviolet light is mainly composed of Ag2O, AgO, AgCl, and corresponding silver compounds.
2. Ways to prevent silver discoloration
Regarding the discoloration of silver and its alloys, considerable research has been conducted both domestically and internationally on how to improve the discoloration resistance of silver. From the perspective of promoting discoloration resistance, it can be summarized into two main categories: surface modification treatment of silver alloys and the development of discoloration-resistant silver alloys.
2.1 Surface modification technology to prevent silver discoloration
Surface modification involves using chemical or physical methods to form an inert film on the surface of silver jewelry, isolating the silver substrate from corrosive media in the environment, blocking the reactions between light, oxidants, corrosive media, and silver, and preventing the occurrence of discoloration. Depending on the type of film formed, surface modification can be categorized into several major types: electroplating, immersion, chemical passivation, electrochemical passivation, organic adsorption passivation, resin coating, and self-assembled films.
Rhodium plating is the most widely used surface modification method for sterling silver jewelry. By coating a thin film of rhodium on the surface of the jewelry, a bright, mirror-like appearance can be achieved, and the rhodium layer has high hardness and good chemical stability, which can enhance the wear resistance and discoloration resistance of silver jewelry. However, due to production costs and the surface brightness effect, the rhodium layer on jewelry is usually very thin and can easily wear off during use, losing its protective effect.
Chemical passivation or electrochemical passivation methods can form an inorganic passivation film on the surface of silver. Chromate passivation is a commonly used chemical passivation method for silver craft jewelry, which generates a layer of silver oxide and silver chromate in an acidic or alkaline solution containing hexavalent chromium compounds. Electrochemical passivation utilizes the principle of cathodic reduction to generate a film layer composed of silver chromate, chromic acid, basic silver chromate, and basic chromic acid on the surface of silver. These film layers have good passivation effects, can reduce the free energy of the alloy surface, and serve to prevent discoloration while having no significant impact on the appearance of silver crafts. However, issues include the film layer needing to be more non-dense, poor mechanical stability, complex structure, difficulty covering edges, and environmental impact.
The application of methods such as immersion, spraying, and coating to form an organic protective film on the surface of silver can improve its anti-tarnish performance, and considerable research has been conducted in this area both domestically and internationally. Benzotriazole, tetrazolium, and various sulfur-containing compounds can form complexes on silver. Composite films can be formed, and some water-soluble polymers are added to create film agents, but the resulting film layer needs to be more dense, and the anti-tarnish effect could be better. Some protective agents are oil-soluble anti-tarnish agents based on paraffin and long-chain quaternary ammonium salts, which can form a solid lubricating layer on the surface of silver, providing a good anti-tarnish effect. However, their resistance to solution corrosion is poor, and using hot gasoline as a solvent poses significant risks. Additionally, after coating a layer of wax on the surface, the brightness and reflectivity of the alloy will be greatly reduced. Spraying acrylic varnish, polyurethane varnish, and organic silicone transparent varnish on the surface of silver alloys can enhance their anti-tarnish ability. Still, the coating must also have sufficient thickness to provide a certain anti-tarnish effect, which can also affect the appearance of silver craft jewelry.
Traditional protective agents do not perform well in protecting the gaps of silver craft jewelry, while molecular self-assembly systems such as alkyl thiols, organ silanes, and Schiff bases can form protective films on the surface of silver jewelry. These films have characteristics such as dense and uniform structure, unaffected by the shape of the substrate surface, free of metallic impurities, and do not affect the appearance of the substrate, demonstrating excellent anti-tarnish ability for silver, making them one of the promising processes for surface treatment of tarnished silver craft jewelry.
Overall, surface modification processes have characteristics such as low cost, simple and practical processes, and certain anti-tarnish performance. However, due to the thinness of the generated film, the exposed silver substrate will still come into contact with corrosive media and tarnish once it is scratched.
2.2 Anti-tarnish silver alloys through overall alloying
As early as 1927, the National Bureau of Standards in the United States proposed after research that there is no other way to completely prevent the sulfidation reaction of silver unless alloyed with other precious metal elements. To suppress the formation of silver sulfides, it is necessary to form alloys with 40% palladium, 70% gold, or 60% platinum. However, it is undeniable that improving the tarnish resistance of silver alloys through alloying is still a necessary and effective method. Many countries worldwide are still striving to develop new types of tarnish-resistant silver alloys, achieving some research results. The main alloying elements that form tarnish-resistant silver alloys can be classified into three categories.
(1) Alloying with precious metals.
Among all precious metal elements, silver has relatively active chemical properties. Adding precious metal elements with higher chemical potentials, such as Au, Pd, and Pt, can improve the electrode potential of silver alloys and enhance their tarnish resistance. For example, adding 5% Pd to sterling silver significantly improved the tarnish resistance of the silver alloy, which showed no significant discoloration or corrosion after ten days in a chlorine or ammonia atmosphere. The elongation of the alloy is between 15%-26%, and it can be produced using conventional casting and mechanical forming methods. Similarly, in the series of tarnish-resistant silver alloys containing platinum, when the Pt content is 1%, the tarnish resistance is more than three times that of sterling silver; when containing Pt 3.5%, the tarnish resistance is more than six times that of sterling silver; when containing Pt 5%, the tarnish resistance is more than eight times that of sterling silver. Silver alloys containing platinum can significantly refine the grain size while increasing hardness; the alloy also has excellent plasticity; it enhances the brightness of the alloy, approaching the color of platinum, and prevents the appearance of red spots. Alloying with precious metals significantly increases the material cost of tarnish-resistant silver, and its market application is relatively limited.
(2) Alloying with rare earth metals.
Many studies have shown that adding trace amounts of rare earth elements to silver or silver alloys helps improve the alloy’s resistance to sulfidation discoloration. The most widely used rare earth elements include Yttrium(Y), cerium(Ce), lanthanum(La), etc. For example, when rare earth elements are added to pure silver if the rare earth content is below 0.11%, it can exhibit better sulfidation discoloration resistance than pure silver. The addition of rare earth elements refines the grain size of the cold-deformed recrystallization structure, and the dispersed silver-rare earth compound second phase formed through fragmentation and re-aggregation effectively strengthens the silver alloy and improves the thermal stability of the alloy, demonstrating a high resistance to aging softening. Most tarnish-resistant silver developed domestically has chosen rare earth elements as alloying elements.
(3) Alloying with other oxygen-active elements.
Adding oxygen-active elements such as Zn, Si, Sn, In, and Ge to the Ag-Cu alloy can improve the sulfidation and oxidation discoloration resistance of silver alloys. This is currently the most common type of tarnish-resistant silver alloy on the market. Italy, the United States, Germany, and others have developed various tarnish-resistant silver alloy fillers, achieving sulfidation discoloration effects that reach more than five times that of sterling silver. The principle of its tarnish resistance: these elements belong to oxygen-active elements, and their oxides have lower free energy than copper oxides, with a stronger affinity for oxygen, allowing for the formation of more stable oxides. Before forming Ag2S, these oxides form a dense protective film layer, acting as a barrier to protect the silver matrix.
Section VI Performance Evaluation and Common Issues of Tarnish-Resistant Silver
1. Performance Evaluation of Anti-tarnish Silver
Various anti-discoloration silver filling materials have appeared on the market, with performance varying widely. Adopting appropriate methods to evaluate their performance is necessary, providing a basis for selecting suitable filling materials.
1.1 Evaluation Methods for Anti-Discoloration Performance
Anti-discoloration performance is one of silver alloys’ most important performance indicators, mainly including resistance to sulfide discoloration and oxidative red spots, which must be tested through experiments.
1.1.1 Evaluation Method for Resistance to Sulfide Discoloration
According to the conditions and locations used in the experiment, it is divided into outdoor and laboratory testing methods.
(1) Outdoor testing method
The outdoor testing method involves placing silver alloy samples in a real environment to observe the time the samples remain unchanged in color and the specific phenomena of color change that occur to evaluate the alloy’s resistance to color change. This method can more accurately reflect the alloy’s resistance to color change, but it has its own drawbacks: (1) The time to obtain test results is relatively long; for example, in certain environments, alloys with good corrosion resistance may take years to yield results; (2) The reproducibility of the results is low. Due to differences in regions and times, the natural environment will vary to some extent. Therefore, the results of the same alloy tested in different regions will differ significantly; even in the same region at different times, the test results will also vary.
Due to the long testing time in natural environments, accelerated corrosion methods are sometimes used to obtain results more quickly. For example, they are placing the alloy in harsh environments such as near electroplating workshops or boiler flue gas or exposing it to ultraviolet radiation in the atmosphere for testing. However, these methods are easily affected by environmental pollution and other factors, leading to significant differences in the degree of similarity, authenticity, and reproducibility of the test results compared to natural corrosion color change. Therefore, they are not advisable.
(2) Laboratory testing method
According to the corrosive media used in laboratory tests, it can be divided into two methods: liquid phase tests and gas phase tests.
(2.1) Liquid phase test method.
The more commonly used methods are the sulfide solution and artificial sweat immersion methods. The former involves immersing the sample in a solution of sodium sulfide or ammonium sulfide at a certain concentration, using the Tuccillo-Nielsen method, which is quite general. The sample is fixed on a rotating wheel and is periodically immersed in a solution of concentration 0.5% or 2% Na2S solution at a speed of 1r/min. It can effectively detect the discoloration resistance of silver alloys under the action of oxygenated sodium sulfide solution. The latter involves preparing artificial sweat according to relevant standards, immersing the sample in sweat with a certain pH value and temperature, usually around pH 6.5, at a temperature of 30℃ or 37℃. During the immersion test, it is necessary to maintain the stability of the solution temperature, and the sample and the comparison sample should be tested under the same conditions. The color changes of the comparison sample after different immersion times can be accurately measured using a colorimeter to determine the degree of discoloration of the sample.
(2.2) Gas phase test method.
Using gas phase test methods to examine the discoloration resistance of silver alloys and silver coatings has become quite common, forming international and domestic standards. Gas phase tests can be conducted in static or flowing gas. The gas contains substances that can cause discoloration of silver alloy materials, such as H2S, SO2, Cl2, and NO2, which can be a single gas or a mixture of two or more gases; the gas can be introduced or generated through chemical reactions. Common gas phase test methods mainly include:
A. H2S test method. This method uses H2S to conduct accelerated corrosion tests, widely used in the electronics industry for evaluating the discoloration resistance of electronic components and electrical contact materials, with multiple international and national standards. These standards include those using high-concentration H2S atmospheres as well as low-concentration ones. However, there is no specific test standard for discoloration resistance in the jewelry industry, leading to various practices, some referencing electronic industry standards for testing, while others choose their testing conditions. A typical testing method is the Thioacetamide (TAA) method, which is a strict standard for measuring silver jewelry, corresponding to the international standard Metallic Coatings-Thioacetamide corrosion test (TAA test) (BS EN ISO 4538-1995). Due to the high concentration of H2S atmosphere, some silver alloy surfaces are prone to discoloration, and the corrosion film layer may become loose and peel off, which can affect the accuracy and reproducibility of the results to some extent. The discoloration conditions of different types of silver alloys after H2S corrosion for 3 hours are compared as shown in Figure 4-20, where the concentration of H2S is 13 x 10-6, relative humidity is 75%, and temperature is 30℃.

B. SO2 Test method. SO2 Can accelerate the corrosion of silver alloys, typical methods include the “Corrosion Test of Metals and Other Inorganic Coatings under Condensation Conditions of Sulfur Dioxide”, which uses a certain volume, heated closed acrylic test chamber, introducing a certain concentration of SO2 gas, tested over three cycles (non-continuous exposure). Using a single SO2 gas for corrosion testing has a longer test cycle, and assessing the corrosion results between samples is somewhat difficult.
C. Mixed gas test method. The corrosion products of this method are relatively close to actual conditions, and the test results are relatively stable. This method is conducted in a specially designed test environment, with humidity at 75%, the temperature at 25℃, H2S, and concentrations of 0.8mg/L, SO2, and 3mg/L, updated three times per hour. Japan invented a mixed gas accelerated corrosion method for testing the corrosion status of silver alloys in electronic devices, composed of air, H2S, and NO2, where H2S is the main factor causing discoloration, and NO2 acts as a catalyst to accelerate the reaction between silver and H2S, allowing the formation of corrosion products in a shorter time.
1.1.2 Evaluation method for anti-oxidation red spot performance
There are generally two methods to evaluate silver alloys’ anti-oxidation red spot performance. (1) Place the sample in an electric furnace for heating, controlling the atmosphere, heating temperature, and insulation time, then take a cross-section of the sample to observe the oxidation film under a microscope (Figure 4-21). Polish the sample and observe the red spot condition on the polished surface. This method can stably control test conditions, and the test accuracy is relatively good. (2) Heat the sample with a torch to a certain temperature, then stop heating and let the sample cool naturally to room temperature, repeating the above operation several times, taking a cross-section of the sample to observe the oxidation film condition, and polishing to observe the red spot condition on the sample surface; this method has a larger human factor.
1.2 Evaluation of Process Performance
Silver alloys used for making jewelry need to have good resistance to sulfide discoloration and oxidation spots and require good mechanical and process performance, which often presents contradictions in joint development. Some alloying elements are beneficial for discoloration resistance. Still, when their content reaches a certain level, they may negatively impact the alloy’s casting and processing performance, leading to a decline in the overall performance of the alloy; In contrast, some alloying elements can improve the mechanical properties of silver, they may not be favorable for its discoloration resistance. Therefore, when selecting discoloration-resistant silver alloys, it is necessary to evaluate their discoloration resistance while fully considering the performance requirements of the alloy for different processing techniques. For example, the melting method can affect the oxidation resistance of the alloy; the same alloy can yield different results when melted using a torch, induction heating in the atmosphere, or melted in a protective atmosphere or vacuum; similarly, jewelry production may use casting methods, stamping methods, or welding methods, each of which has a different emphasis on the process performance requirements of the alloy, requiring separate evaluations from the perspectives of castability ability, cold working performance, welding performance, etc., and fully consider the process operability of the alloy to avoid operational issues that may arise from a too-narrow process range.
1.3 Evaluation of Safety and Cost-Effectiveness
The silver alloy used for jewelry must meet safety requirements, and the content of toxic and harmful impurity elements must not exceed international standards. Additionally, the silver alloy’s comprehensive performance and material cost should be evaluated in terms of cost-effectiveness.
2. Common issues with anti-tarnish silver for jewelry
The issues with anti-tarnish silver for jewelry in the market mainly include the following aspects.
2.1 Insufficient anti-tarnish performance
When jewelry companies discuss the silver jewelry business, the most direct question from customers is how long the silver jewelry can remain untarnished. Many customers require it to remain untarnished for at least one year, but companies need help guaranteeing this. Besides the influence of usage environment and storage methods, a significant reason is that the anti-tarnish performance of the alloy itself is not outstanding. Silver alloys containing precious metal elements like Pd and Pt have better anti-tarnish performance, but their relatively high prices deter many companies, as customers often need to specify the use of such alloys or pay extra for them. The market is predominantly occupied by anti-tarnish silver alloys that use oxygen-active element alloying. Theoretically, the dense oxide film formed by these elements should prevent the internal metal from further sulfiding and oxidizing, thereby improving the silver alloy’s resistance to sulfide and oxidative discoloration. However, it should be noted that the microstructure of the base alloy, the distribution of alloying elements in the base, and the structure and mechanical properties of the surface oxide film can significantly affect the structure of the oxide film. If the formed alloy oxide film is unevenly distributed, loose and coarse, or has micro-cracks, it will not provide effective protection. In other words, different alloys with different component ratios will yield different anti-tarnish results. Even if the same alloy manufacturer uses the same alloy formula, if the company does not strictly adhere to the specified melting and casting process standards during production, the results may also vary.
2.2 The problem of insufficient hardness
Many companies have reported that the hardness of tarnish-resistant silver alloys is much lower than that of sterling silver, making them relatively easy to deform and unable to meet the requirements for mold making, elastic parts, etc. This is indeed the case. For silver alloys that improve tarnish resistance with Pd, Pt precious metal elements, due to their similar crystal structure to silver and high solubility, the strengthening effect is poor, and the initial hardness is generally low. Zn is commonly used as the main alloying element for tarnish-resistant silver alloys alloyed with other elements, and the strengthening effect could be better. Therefore, most tarnish-resistant silver alloys have low as-cast hardness, usually below HV60, which is insufficient for jewelry products with certain strength requirements. Although alloys can increase hardness through deformation processing, only casting processes can be used for the production of most set jewelry, making deformation methods unsuitable. Of course, some alloys can improve hardness through aging treatment, but they are often not used or misused in actual production because jewelry-making involves a multi-process craft. During the mold-making stage, gemstone setting stage, and even polishing stage, the workpiece may undergo welding or heating, usually done by operators using flame heating, with heating temperature, heating time, and cooling speed being quite arbitrary, making it generally difficult to achieve the effects expected by alloy suppliers. Additionally, once the jewelry has been set with gemstones, it is no longer suitable to use aging treatment methods to increase hardness, as high-temperature quenching can easily damage the gemstones.
Therefore, from a practical application perspective, it is necessary to improve the alloys’ as-cast and annealed hardness. From China’s resource advantages, rare earth elements should be worth considering.
2.3 Issues related to casting
The vast majority of jewelry is formed through casting, and several jewelry companies have established their casting departments. Companies generally only purchase filler from alloy suppliers and then buy pure silver to prepare the required silver alloy. During the casting production process, many companies are often troubled by various casting issues such as porosity, sand holes, shrinkage, inclusions, and cracks, which affect the normal order of production and increase production costs.
Sterling silver, with Cu as the main alloying element, is prone to defects such as porosity and oxidized inclusions if not protected during melting, increasing the molten metal’s viscosity. The oxidized film formed at the front of the molten metal increases surface tension, leading to increased filling resistance and affecting forming performance. The resulting defects, such as porosity and inclusions, significantly increase the difficulty of subsequent polishing.
Silver alloys primarily composed of Pd, Pt, and other precious metal elements have a higher melting point, increasing the likelihood of porosity. This is because jewelry mainly uses gypsum molds, and gypsum has poor thermal stability; the higher the alloy’s melting point, the greater the possibility of thermal decomposition of gypsum, leading to porosity in the castings.
Different anti-tarnish silver alloys contain different types and amounts of oxygen-active elements, exhibiting varying casting performances. Si oxide has a low density and high viscosity, making it easy to float to the surface of the molten metal and be removed with the help of flux, which is beneficial for fluidity and filling performance. However, excessive Si can lead to thermal cracking and polishing issues; removing Zn and Sn oxides is more difficult. For silver alloys containing a large amount of low-melting-point oxygen-active elements (such as Zn, Sn, In ), using a torch for melting can easily produce volatiles and oxidized inclusions, and using induction heating can also lead to similar problems due to overheating. A small amount of rare earth added can improve filling properties. Still, the amount of rare earth increases to a certain extent. In that case, the formed rare earth oxides will increase the viscosity of the molten metal, counteracting the purifying effect of the rare earth and negatively affecting casting performance.
Among the issues above, porosity is one of the most prominent problems. The porosity generation is closely related to the properties of the silver alloy itself. As mentioned earlier, silver has a typical characteristic of absorbing a large amount of oxygen in the molten state, causing the molten metal to splash at high temperatures, resulting in significant losses easily. During the solidification process of casting, the solubility of gas in the molten metal decreases with the drop in temperature, leading to gas supersaturation, precipitation, and enlargement, forming bubbles that fail to be discharged in time, resulting in porosity (Figure 4-22).

From the mechanism of pore formation, it can be seen that there are mainly two approaches to reduce or avoid pore defects: (1) minimize the amount of gas entering the molten metal; (2) take measures to release the gas in the molten metal before pouring.
(1) Reduce the gas entering the molten metal.
First, the quality of the raw materials must be controlled. The raw materials should be dry and clean, not damp or oily. The purchased pure silver materials come in forms such as granules, bars, and plates, with granules being the most common. Since granules are formed by metal droplets rapidly cooling in water, they can sometimes be hollow and even contain water inside, which can introduce a large amount of gas during melting. They must be thoroughly dried or pre-melted into ingots before use. If the surface of the raw materials has oil or other organic impurities, it will also introduce gas, especially when reusing scrap from stamped jewelry, which often adheres to oil. It should be degreased and cleaned thoroughly before use. The ratio of new and reused materials should be reasonably controlled when mixing materials. Each time the raw materials are melted and cast, they will be contaminated, including gas absorption, reaction with gypsum molds, and residual inclusions. Therefore, the amount of reused materials should generally be controlled within 50%.
Secondly, effective protective measures should be taken during melting. Different enterprises have different production conditions, and the melting methods used also vary. Common melting methods include gas-oxygen flame, acetylene oxygen flame, high-frequency induction furnace, and medium-frequency induction furnace. When melting in the open atmosphere, the molten metal will inevitably absorb gas; the larger the liquid surface and the longer the contact time, the greater the tendency to absorb gas, mainly oxygen from the air. When using flame melting, it also includes oxygen brought in by the oxidizing flame. Therefore, protective measures should be taken during the melting process to reduce the amount of gas absorption if silver is melting in the atmosphere; crushed charcoal, graphite sheets, or dehydrated borax can be used as a cover. If flame melting is used, it should be adjusted to an orange-yellow reducing flame, and the melting time should be a manageable length. If conditions permit, priority should be given to vacuum induction melting, which can be done under low vacuum with protective gas. This involves evacuating the melting chamber first, then backfilling it with nitrogen or argon as protective gas, followed by heating and melting. Nitrogen is cheaper, but it has a certain solubility in silver, which poses a risk of causing pores; argon is more expensive but is more stable in silver and should be prioritized.
(2) Promote gas release in the molten metal before pouring.
Under atmospheric conditions, molten silver inevitably absorbs gas. To avoid the formation of gas pores, the molten metal should undergo degassing treatment before pouring, allowing as much gas dissolved in the silver liquid as possible to be released. Two methods can be adopted:
A. Using floating gas bubbles for degassing. By blowing fine, dense argon bubbles into the bottom of the molten metal using a breathable plug, it does not react with the molten metal and becomes floating gas bubbles. The bubbles serve as a vacuum space for the gases dissolved in the molten metal, allowing the dissolved gases to diffuse into the floating bubbles, becoming gaseous molecules that rise with the bubbles. When the floating bubbles rise to the surface of the molten metal, the gas inside the bubbles escapes into the atmosphere, achieving the goal of reducing the gas content in the molten metal.
B. Using condensation method for degassing. The silver liquid is slowly cooled to the solidification temperature, causing most of the dissolved oxygen and other gases to precipitate due to the decrease in solubility with the drop in temperature, thus achieving the purpose of degassing. It is then rapidly heated back to the pouring temperature for casting. For silver liquids with poor raw material quality and high gas content, multiple cycles of condensation and remelting can be performed to improve their quality.
2.4 Issues in cold processing
Issues in cold processing in the production process of silver jewelry, common cold deformation processing techniques include cold volume forging such as cold heading, cold extrusion, stamping, etc., and sheet metal stamping such as stretching, blanking, trimming, punching, etc. Material rolling, such as cold rolling, roll forming, etc., and the following issues are often encountered during processing.
(1) Surface sand holes of profiles.
The surface quality of profiles depends not only on the surface quality of the ingot but is also closely related to the surface quality of the rollers. When the roller surface has scratches or local damage, it will be replicated on the profile surface; when dust or other debris accumulates on the roller surface, it will be pressed into the profile surface, worsening the surface quality of the profile (Figure 4-23). Therefore, during production, the roller surface should be frequently wiped to prevent dust and other impurities from accumulating and scratching the rollers or being pressed into the strip surface. The rollers should be covered when not in use to protect the surface. The diameter of the finished product rollers should be small and highly polished or electroplated to achieve a mirror effect.

(2) Annealing defects.
This includes blistering, abnormal grain growth, and incomplete annealing.
A. Blistering. Blistering on the surface of sheets and strips is caused by gas holes within the ingot or reactions between the ingot and the atmosphere during annealing. The pressure increases when the bubbles are heated, causing the metal surrounding the bubble to expand and form blisters (Figure 4-24). This issue can generally be avoided by controlling the casting or annealing conditions. For example, it enhances deoxidation during the melting process, reduces the dissolved oxygen content and oxidation in the molten metal, controls the annealing temperature, and avoids using hydrogen-rich annealing atmospheres.
B. Abnormal grain growth. Silver will exhibit significant grain growth when the annealing temperature is too high or the annealing time at high temperature is too long (Figure 4-25). Excessively coarse grains affect mechanical properties and severely impact the surface quality of processed jewelry. Therefore, the annealing process should be reasonably formulated based on the size and quality of the profile.
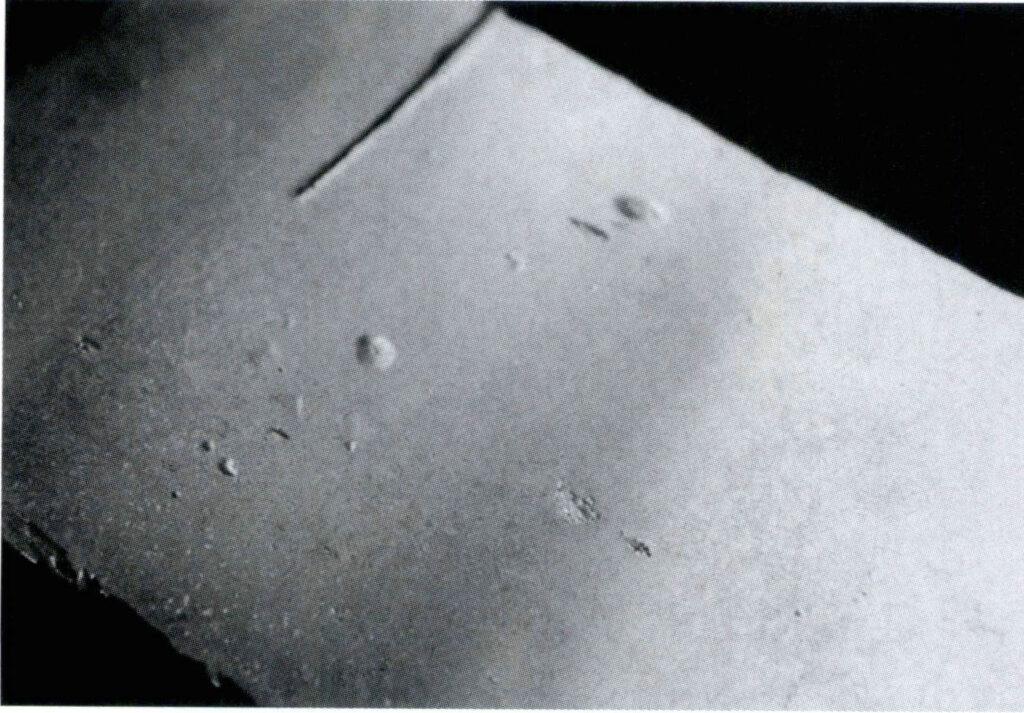
Figure 4-24 Bubbles appearing on the surface of silver profiles after annealing

Figure 4-25 Abnormal grain growth caused by improper annealing of silver
C. Incomplete annealing. Different silver alloy materials have different recrystallization temperatures. If the annealing temperature is too low, the amount loaded into the furnace is too large, or uneven heating is applied with a torch, incomplete annealing may occur. In this case, residual stress will remain within the profile, affecting subsequent processing and leading to defects such as cracks.
2.5 Issues related to polishing
Jewelry has high requirements for surface quality, and most jewelry must undergo polishing to achieve a mirror-like finish. Polishing of anti-tarnish silver alloys often encounters several typical problems, such as orange peel surface, dents, scratches, and comet tails. When the alloy’s grain is coarse, it is prone to forming an orange peel surface; when there are shrinkage or porosity defects in the alloy, it is likely to form polishing dents; when high hardness segregated phases or inclusions appear within the alloy’s grain structure, it is easy to form polishing scratches and comet tails.
To achieve a good surface polishing effect, in addition to correctly executing the polishing operation process, the properties of the alloy itself also have an important impact. Grain refinement and improvement of casting performance are the main ways to enhance polishing performance.