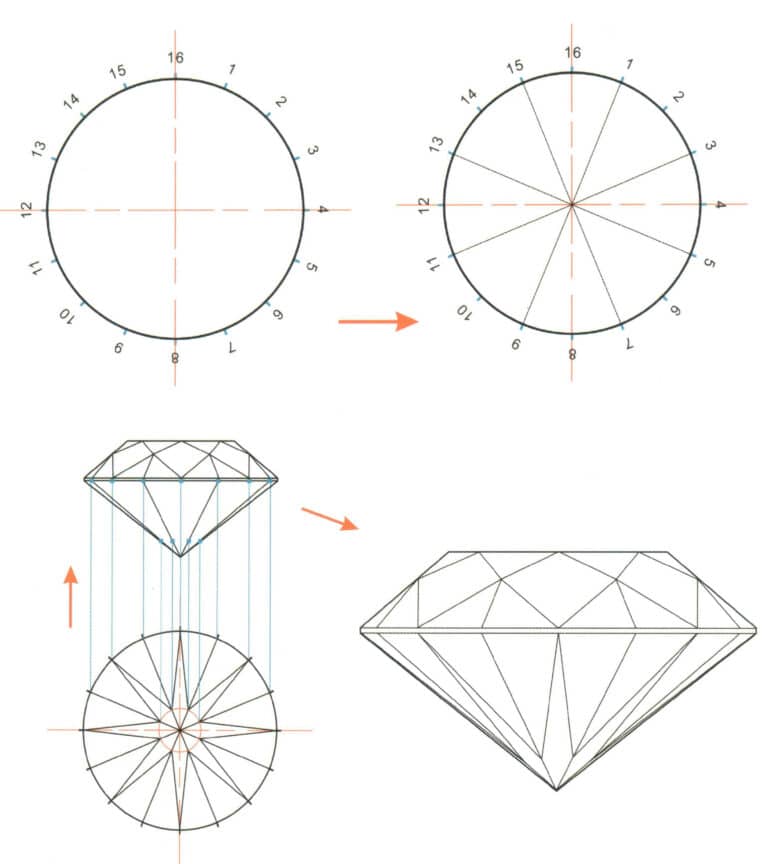
How to Elevate Your Jewelry with Surface Treatment Techniques
Mastering Surface Treatments for Jewelry: From Polishing to Nano-Spraying
The surface treatment process of jewelry is a technical treatment that uses various methods, such as physical, chemical, electrochemical, and mechanical, to change the texture, color, and feel of the accessory’s surface, prevent corrosion, beautify decoration, and extend its service life. It greatly enriches the decorative effect of accessory products, broadens the available means of accessory design, presents a more vibrant and diverse style for jewelry, and provides consumers with more personalized choices. It significantly improves the surface effect, service life, and economic added value of accessory products.
There are many types of surface treatment processes for modern popular jewelry, with commonly used methods mainly including polishing, electroplating, chemical plating, chemical electrochemical conversion films, physical vapor deposition, enamel, epoxy resin, and surface nano-spraying.

Swimming Plating color options
Table of Contents
Section I Polishing Technology
1. Mechanical Polishing
Mechanical polishing is treating the surface of jewelry using polishing machinery and abrasive media. Common methods of mechanical polishing include the following categories.
(1) Cloth Wheel Polishing
Cloth wheel polishing is completed using a polishing wheel mounted on a machine. Polishing paste is periodically applied to the working surface of the polishing wheel. In contrast, the surface of the workpiece being processed is pressed forcefully against the working surface of the polishing wheel, which is in a high-speed rotation state. With the help of the fibers of the polishing wheel and the polishing paste, the surface of the workpiece being processed achieves a mirror-like appearance (Figure 11-1). It is currently generally believed that the principle of cloth wheel polishing machines is the high temperature generated by the friction between the high-speed rotating polishing wheel and the surface of the workpiece being processed can cause plastic deformation of the workpiece surface, filling in the microscopic recesses on the surface of the workpiece being processed; at the same time, the high temperature generated during polishing can also quickly form a very thin layer of oxide film on the surface of the workpiece being processed. The exposed substrate surface is again oxidized when removing this oxide film layer. The process continues in this cycle until polishing is complete, resulting in a flat and smooth surface.
(2) Vibration Polishing
It is installed with a vibrating motor in the vibrating disc of the vibrating polishing machine, and the vibrating disc is connected to the base through vibrating springs. When the vibrating grinder is started, the vibrating motor generates a strong excitation force, driving the grinding media in the vibrating disc to produce motion in three directions: vertical vibration, inward-to-outward flipping, and spiral clockwise rotation, thereby creating a grinding effect on the surface of the jewelry to achieve a polished finish (Figure 11-2 ).

Figure 11-1 Cloth Wheel Polishing

Figure 11-2 Vibration Polishing
(3) Roller Polishing
Its working principle is as follows: Four hexagonal rollers are evenly mounted on the circumference of the rotating body. The rollers rotate with the rotating body while also revolving around their axis under the action of the sprocket system (in the opposite direction). The planetary motion of the rollers causes the material inside the rollers to always remain on one side of the outer wall of the rollers due to centrifugal force, creating a flow layer on the surface. Within this flow layer, the grinding stones and workpieces generate relative motion, performing fine cutting and pressing on the surface of the workpiece, thereby achieving a polished surface on the workpiece (Figure 11-3).
(4) Vortex polishing
Its working principle is: utilizing the centrifugal force generated by the high-speed rotation of the bottom turntable, strong friction is produced between the workpiece and the abrasive under the action of a fixed groove, forming a spiral vortex operation, causing high-speed rotational friction and spiral flipping of the workpiece and abrasive. This allows the polished jewelry to have burrs removed and polished evenly in a very short time, achieving an ideal polishing effect. The base of the polishing machine is a rotating disc within a container, with the top of the container open and the walls of the container not rotating. The gap between the container and the disc can be less than 0.05 mm, allowing the use of the finest walnut shell particles (Figure 11-4).

Figure 11-3 Roller Polishing

Figure 11-4 Vortex Polishing
(5) Dragging Polishing
When working, the workpiece is dragged over the polishing medium while it does not move. Each workpiece has its own support position, the surfaces between the workpieces will not come into contact, thus preventing surface damage. Compared to traditional polishing methods, it creates greater relative motion and stronger processing force, significantly reducing processing time. It has great advantages for heavy workpieces. The drag polishing method is particularly suitable for heavy rings, clasps, and watch cases and is also suitable for many other workpieces that can be suspended on fixed brackets (Figure 11-5).

Table 11-1 Characteristics of Different Polishing Processes
| Polishing methods | Polishing medium | Grinding Medium | Advantages | Disadvantages | Suitable workpiece |
|---|---|---|---|---|---|
| Vibration polishing | Wood chips, porcelain pieces, walnut shell particles, corn flour, steel balls | Ceramics, plastic | Cheap, large items, stamped parts | With long processing time, low pressure, indentations, and poor smoothness effect, achieving ideal results during dry processing is impossible. | Small chain, mechanics chain |
| Barrel polishing | Wooden cube, wooden needle, walnut shell particles, corn flour, steel ball | Ceramics, plastic | Cheap | Long processing time, inconvenient processing, the surface has dust, the surface is pressed | Various jewelry pieces |
| Vortex polishing | Walnut shell particles, porcelain pieces, plastic | Ceramics, plastic | With high efficiency and short processing time, the machine completes the 70% workload, fewer processes, clean jewelry, easy handling, high surface quality | It can only handle lightweight workpieces (maximum 20 g) and cannot process small chain gemstone settings. | Most jewelry, industrial products, and watch cases |
| Drag polishing | Walnut shell particles | Walnut shell Granule | It can polish large, heavy workpieces without impact or collision, with short processing time, easy handling, and high surface quality | No wet grinding | Various jewelry pieces that can be fixed on a shelf |
2. Chemical Polishing
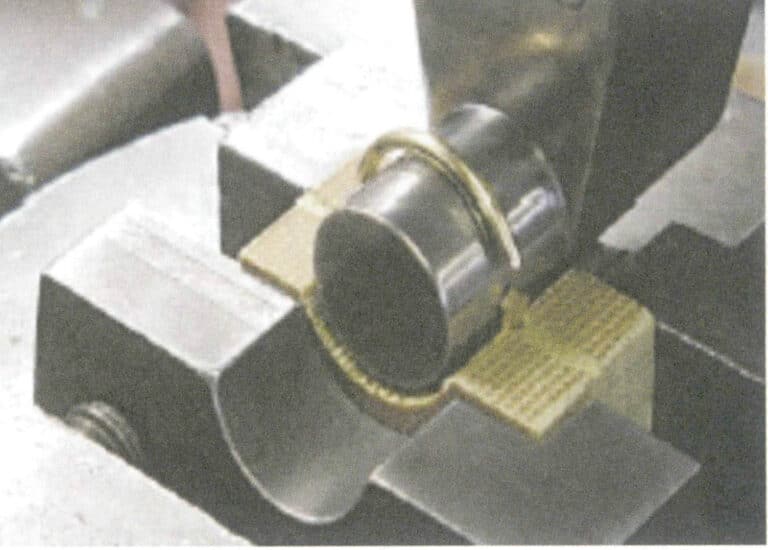
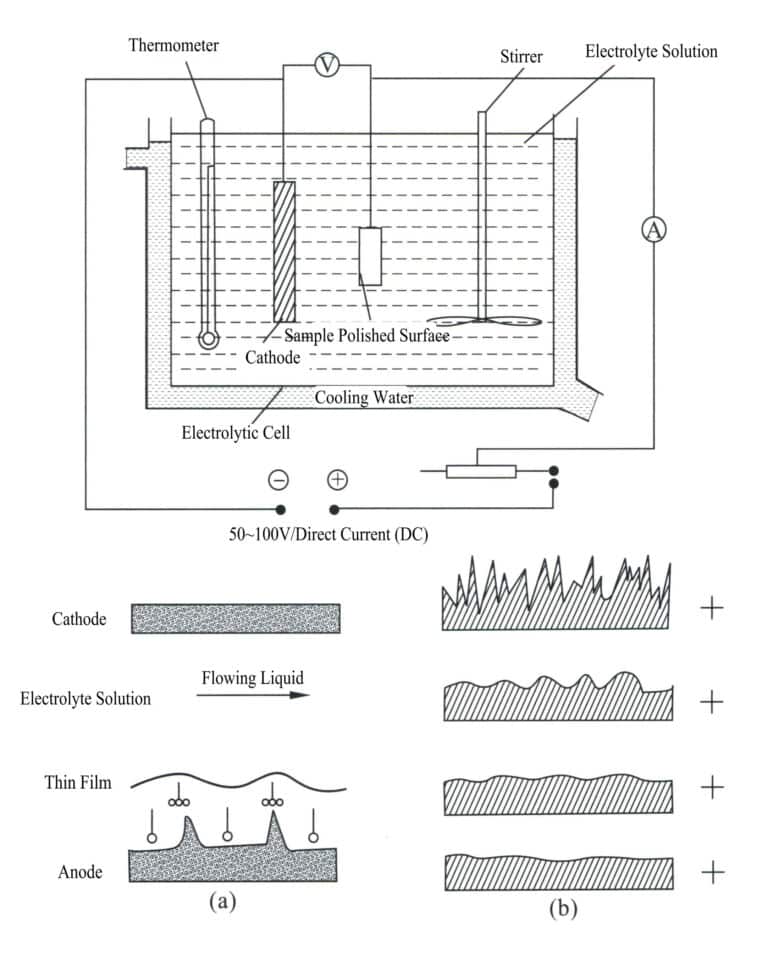
3. Electrochemical Polishing
Section II Electroplating Process
1. Basic Knowledge of Electroplating for Jewelry
(1) Types of Electroplating for Jewelry
According to the purpose of the coating, electroplating for jewelry can be divided into protective coatings and decorative coatings.
- Protective coatings. The main purpose is to prevent metal corrosion. Commonly used coatings such as galvanized and tin layers belong to this category. Black metals are usually protected by galvanized layers under general atmospheric conditions, while tin layers are generally used to protect black metals that come into contact with organic acids.
- Decorative coatings. Primarily for decorative purposes, but also with a certain level of protection. Composite coatings mostly form multiple layers, as it is difficult to find a single coating that meets the requirements for decorative coatings. Typically, a base layer is first plated onto the substrate, followed by a surface layer, and sometimes an intermediate layer is also plated. For example, electroplated precious metals and imitation gold electroplating are widely used, especially in some valuable jewelry and small hardware jewelry, with a relatively high usage and production volume. Mainly involves electroplated precious metals and various alloys.
According to the electrochemical relationship between the coating and the substrate metal during corrosion, decorative electroplating can be divided into anodic and cathodic coatings.
- Anodic coating. Refers to the coating that acts as the anode and dissolves first when a corrosion micro-battery is formed with the substrate metal, such as zinc plating on iron. This type of coating not only provides mechanical protection to the substrate but also offers chemical protection.
- Cathodic coating. Refers to the coating acting as a cathode when it forms a corrosion micro-battery with the substrate metal. For example, tin plating on iron. This type of coating can only provide mechanical protection to the substrate; once the coating is damaged, it fails to protect the substrate and accelerates the substrate’s corrosion rate.
(2) The Basic Process of Metal Electroplating
Electrodeposition is an electrochemical and redox process. During electrodeposition, the metal component acts as the cathode, the metal or alloy to be plated acts as the soluble anode or a titanium mesh is used as the insoluble anode, connected to the negative and positive terminals of the power supply, and immersed in an electrolyte containing the plating components. Under the action of the current, a deposition layer can be obtained on the surface of the ornament (Figure 11-7).
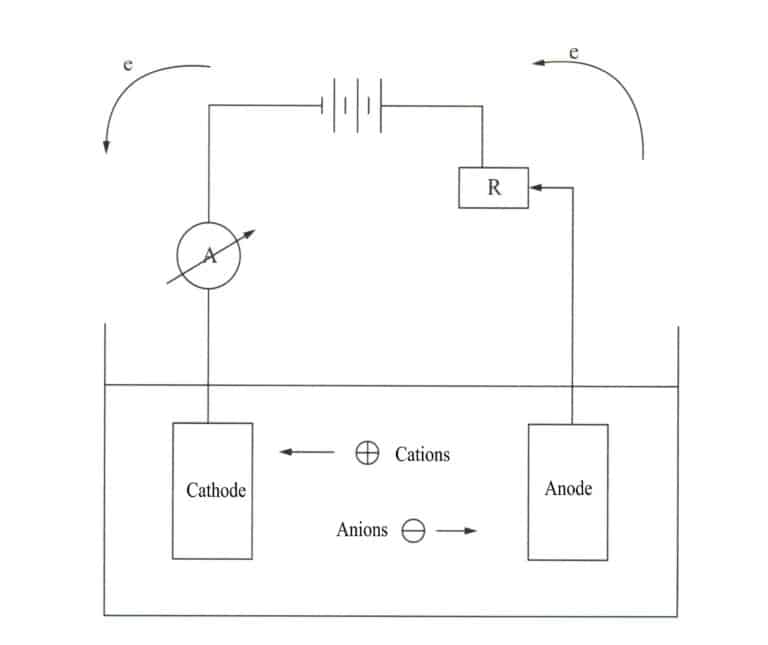
The metal electroplating process reduces metal or its complex ions to metal at the cathode. Since the plated metal has a crystalline structure like ordinary metals, the electroplating process is also known as the electro crystallization process. It includes the following three steps.
- Mass transfer process. Metal ions or metal complex ions are continuously transported to the electrode surface from the electrolyte through steps such as diffusion, convection, and electro-migration.
- Electrochemical process. Metal ions or metal complex ions dehydrate and adsorb on the surface of the cathode to release electricity and reduce to metallic atoms.
- Crystallization process. Metal atoms arrange themselves on the cathode to form metal crystals of a certain shape, and crystallization usually occurs in nucleation and growth.
The fineness of the crystals is determined by the rate of nucleation and the rate of growth. If the rate of nucleation is faster than the rate of growth, more crystals will be produced, resulting in finer and denser grains; conversely, the grains will be coarser.
2. Electroplated Copper and Copper Alloys
The copper plating is pink, uniform, and delicate, with different shades depending on the process. In electroplating, copper plating is widely used, mainly as a base layer and intermediate layer, and can also be used as a surface layer, such as imitation gold plating.
The currently used copper plating processes mainly include cyanide, acidic sulfate, and pyrophosphate copper plating. Among them, cyanide copper plating solution is highly toxic, severely pollutes the environment, harms human health, and has been listed as a process to be eliminated. In addition, amino sulfonate copper plating, organic amine copper plating, citrate-tartrate copper plating, and HEDP copper plating have also seen development and application in recent years.
In the 1970s, research focused on replacing cyanide electroplating with non-cyanide electroplating, which led to pyrophosphate and sulfate copper plating. Still, they could not be directly plated onto steel substrates as a base layer.
2.1 Copper Plating with Sulfate
Copper plating with sulfate is widely used in protective decorative electroplating, plastic electroplating, and thick plating copper for electroforming printed circuit boards. It can be divided into two types: one is a high copper low acid plating solution used for parts electroplating, which has a smooth and bright coating with good toughness; the other is a high acid low copper plating solution used for printed circuit board electroplating, which has excellent dispersion and coverage capabilities, making it very suitable for hole plating, with a uniform and delicate coating.
(1) Main Components of the Plating Solution
① Copper sulfate. It is the main salt that provides the necessary Cu2+ for electrodeposition. When the concentration is too low, it will reduce the upper limit of current density, decrease deposition speed, and affect the brightness of the coating. When the concentration is too high, it will reduce the dispersion ability of the plating solution, and due to the solubility limit of copper sulfate, copper sulfate crystals will precipitate, with being 180~220g/L appropriate.
② Sulfuric acid. Its main function is to increase the conductivity of the solution. When the concentration is too low, it leads to incomplete oxidation of the anode copper, producing Cu2O, resulting in “copper powder” or burrs in the coating. At the same time, the dispersion ability of the plating solution decreases. When the sulfuric acid concentration is appropriate, the current density range of the coating is wide, the coating is bright, and the leveling ability reaches optimal results. If the sulfuric acid concentration is too high, it affects the brightness and leveling of the coating.
③ Chloride ions. They are anode activators that can help the anode dissolve normally, inhibit the production of Cu+, improve the brightness and leveling ability of the coating, and reduce internal stress in the coating. If the chloride ion concentration is too low, it leads to the formation of dendritic coatings, with the high current area prone to burning, and the coating is likely to develop pits or pinholes. If the chloride ion concentration is too high, a white gel-like film layer appears on the anode surface, and no matter how much brightener is added, the coating will not be bright. The appropriate chloride ion concentration is 40~100ml/L.
④ Additives. An excellent combination of additives can produce stable plating solutions, high product qualification rates, and high work efficiency. Currently, many additives and their materials have been commercialized. The requirements for additives vary depending on the type of plating. For example, decorative coatings place more emphasis on the brightness, speed, and leveling of the coating; protective decorative coatings focus more on the leveling and flexibility of the coating; circuit board coatings require excellent low current zone performance, uniform coating distribution, and coating ductility, among others. Copper plating additives mainly consist of four parts: carriers, brighteners, leveling agents, and wetting agents.
- Carrier: A good carrier can maximize the effectiveness of brightening and leveling agents. Carriers are mostly formulated from surfactants, and it is impossible to achieve the best effect with a single material, such as polyether compounds, tetraether anionic compounds of ethylenediamine, etc.
- Brightening agents and leveling agents: Organic polysulfide compounds, organic polysulfides, polysulfide organic sulfonates, organic dyes, etc., have brightening and leveling effects in combination with carriers. Both effects may appear in the same material, with dyes focusing more on leveling ability.
- Wetting agents: can improve the wetting action of the plating solution. Commonly used are non-ionic or anionic surfactants, such as polyethylene glycol, OP emulsifiers, etc. Bright acid copper uses air stirring and can only choose low-foam wetting agents.
(2) Electrode reaction of copper plating with sulfuric acid
Cathode: Cu2+ + 2e=Cu φ0Cu2+/Cu = +0.34V
Cu2+ + e=Cu+ φ0Cu2+/Cu+ = +0.17V
Cu+ + e=Cu φ0Cu+/Cu = +0.51V
Due to the standard electrode potential of Cu2+ being much more positive than hydrogen, hydrogen gas will not be released at the cathode, but when it is not sufficiently reduced, it will appear Cu+. From the perspective of standard electrode potential, the reaction of reducing to Cu is more likely to occur, and the reduction of Cu will lead to a rough coating, which should be avoided.
Anode: Copper anode in sulfuric acid solution undergoes anode dissolution, providing the copper ions needed in the plating solution, namely: Cu-2e=Cu2+.
At the same time as Cu2+ is generated, it is inevitably generated Cu+, namely: Cu-e=Cu+. When Cu+ Appears and enters the solution; if there is enough sulfuric acid and air in the solution, Cu+ can be oxidized, namely:4Cu++O2+4H+=4Cu2++2H2O, When the concentration of sulfuric acid in the solution is insufficient, Cu+ will hydrolyze, namely:2Cu++2H2O=2CuOH+2H+=Cu2O+H2O. At this time, Cu2O will deposit on the cathode by electrophoresis, producing burrs. Due to the instability of Cu+, disproportionation reactions can also occur, namely:2Cu+=Cu2++Cu, and the generated Cu will also deposit on the coating by electrophoresis, producing copper powder, burrs, and roughness. Therefore, during the electroplating process, the appearance of Cu+ should be avoided as much as possible; using phosphorus-containing copper anodes and stirring the plating solution with air can solve the problem.
2.2 Pyrophosphate Copper Plating
Copper plating with pyrophosphate cannot be directly plated on iron and zinc substrates. It is mostly used on zinc alloy substrates before acid sulfate copper plating to protect the substrate from strong acid corrosion and ensure the quality of the coating combination. It is also used in plastic metallization electroplating processes, but its application in hardware electroplating is limited.
The bright phosphoric acid copper plating crystals have good dispersion and coverage capabilities. The cathodic current efficiency is high, but long-term use can lead to phosphate accumulation, which reduces the deposition rate.
(1) Main Components of the Plating Solution
- Copper pyrophosphate. It is the main salt of the plating solution, providing copper ions. If the copper content is too low, it decreases the current density, resulting in poor gloss and leveling of the coating; if the copper content is too high, it will reduce cathodic polarization, leading to a rough coating. The copper content in the plating solution must be maintained at a certain ratio with potassium pyrophosphate.
- Potassium pyrophosphate. It is the main complexing agent, and when the pH value is 8, the main form of the complex is [Cu(P2O7)2]6-, maintaining [P2O74-]:[Cu2+]=7~8 in the plating solution is relatively appropriate; if the ratio is too large, it leads to reduced current efficiency, pinholes in the coating, and the plating solution being prone to turbidity.
- Citric acid amine. It is an auxiliary complexing agent and anode depolarizer. It can improve anode dissolution, enhance the dispersion ability of the plating solution, and increase the brightness of the coating. If the content is too low, anode dissolution will be poor, the dispersion ability of the plating solution will decrease, and “copper powder” will be produced. Generally, the appropriate content is around 10~30g/L.
(2) Electrode reaction of copper plating with pyrophosphate
Cathodic reaction:[Cu(P2O7)2]6- + 2e6- = Cu + 2P2O74-
2H2O + e = H2 + 2OH–
Anodic reaction:Cu + 2P2O74- -2e = [Cu(P2O7)2]6-
When the anode is passivated, oxygen is released:4OH– – 4e = O2 + 2H2O
When anodic oxidation is incomplete, Cu + occurs: Cu – e = Cu+
The last two reactions need to be carefully monitored to prevent occurrence.
2.3 Imitation Gold Electroplating
In recent years, due to the development of decorative electroplating in construction, hardware, lighting, and ornaments, imitation gold plating has been widely used.
(1) Main Categories of Imitation Gold Electroplating
The imitation gold plating can be made of copper-zinc, copper-tin, or copper-tin-zinc alloys, or post-processing copper-zinc alloys can produce it to create a realistic gold effect. The imitation gold effect can achieve colors such as 18K, 4K and rose gold. Copper-tin alloys (bronze) can be divided into three categories based on tin content: low-tin bronze with a tin content of 5%~15%, which appears pink to golden yellow; medium-tin bronze with a tin content of 15%~40%, which appears yellow; and high-tin bronze with a tin content of 40%~50%, which appears silver-white.
The gold-like plating has a very short electroplating time, and its luster mainly relies on the underlying layer for support, usually plated on a bright nickel layer or other white and shiny plating. Brass plating can also serve as a decorative thin gold layer’s base and a protective and lubricating layer. Brass is prone to discoloration in the air, so it must undergo anti-discoloration treatment when used as a surface layer or thin gold layer’s base, such as being sprayed with an organic coating or coated with cathodic electrophoretic paint. In recent years, to prevent skin allergies to nickel in jewelry plating, white copper-zinc alloy can be used as a low-grade substitute for nickel plating, and it can also serve as a base for chrome and for white and required white coatings in toy metal decorations.
The key to obtaining an alloy through the simultaneous co-deposition of two metals is that their deposition potentials must be close, and the cathodic polarization must ensure that the two metals deposit in the desired ratio. The complex ions present in the cyanide plating solution are mainly Cu(CN)32- and Zn(CN)42-. The stability of copper cyanide ions is much higher than that of zinc cyanide ions, and the cathodic polarization of copper is far greater than that of zinc. Therefore, to achieve a coating that meets the requirements, it is necessary to strictly control the total cyanide, free cyanide, copper-zinc ratio, pH value, and factors such as temperature, current density, and stirring.
(2) Imitation Gold Electroplating Process Flow
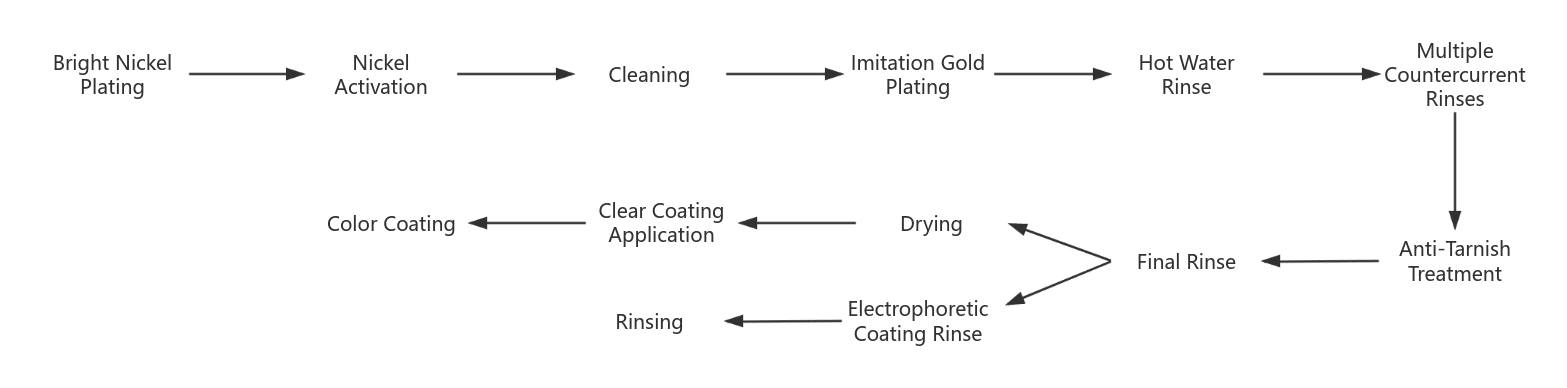
There are two major challenges in copper alloy imitation gold plating: one is how to maintain the luster of the plating and prevent discoloration of the copper alloy plating; the second is how to achieve a realistic effect. Therefore, a reasonable electroplating process and post-treatment become crucial. The commonly used imitation gold electroplating process is as follows:
- Before the workpiece is plated with bright nickel, it must undergo pretreatment. The bright nickel plating ultimately has a whitish tone, which can enhance the brilliance of the imitation gold layer.
- Nickel activation aims to remove the passivation layer on the bright nickel surface to improve the bonding strength with the surface layer. The method involves cathodic treatment in the electro-degreasing solution for 3~5 minutes, followed by activation with 5% sulfuric acid after rinsing with water and thoroughly rinsing before entering the imitation gold plating tank.
- After the electroplated imitation gold coating, the workpiece surface is cleaned with hot water and rinsed with countercurrent washing.
- Anti-discoloration treatment aimed at preventing the coating from discoloring after washing. Common passivation processes include potassium dichromate or benzotriazole.
- The cathodic electrophoresis is treated with acrylic-type cathodic electrophoresis paint or with transparent coatings such as acrylic-type varnish or sprayed or dipped with organic silicon transparent coatings.
- Varnish or paint coloring. To create a realistic imitation gold effect in appearance and to compensate for the shortcomings of the tone of the imitation gold plating, a transparent coating can be applied in gold color.
3. Electroplated Nickel
3.1 Bright Nickel
Bright nickel is one of the most widely used coatings today. It is based on watt nickel, with additives added to achieve a bright and smooth nickel plating.
(1) Main Components of the Plating Solution
① Nickel. The sources of nickel ions can be nickel sulfate, nickel chloride, nickel aminosulfonate, etc. Nickel ions are the main component of the plating solution, generally with a content of 52~70g/L. A high concentration of nickel ions allows for an increased current density. It improves the deposition rate, but if the concentration is too high, the dispersion ability of the plating solution decreases, which can lead to no plating in low current areas. If the concentration of nickel ions is too low, the deposition rate decreases, and in severe cases, the high current area can become burnt.
② Buffer. Boric acid is the best buffer in the nickel plating solution, and its minimum effective concentration is not less than 30g/L; generally, taking in the plating solution is appropriately 40~50g/L. Boric acid can also enhance cathodic polarization, improve solution conductivity, and improve the mechanical properties of the coating.
③ Wetting agents. During the electroplating process, hydrogen is released at the cathode. Wetting agents can reduce the surface tension of the plating solution, increasing the wetting effect of the solution on the surface of the workpiece, making it difficult for hydrogen bubbles generated during electroplating to remain on the cathode surface, thus preventing the formation of pinholes and blemishes. Wetting agents are composed of surfactants, which can be classified into high-foam wetting agents and low-foam wetting agents. High-foam wetting agents include sodium dodecyl sulfate, while low-foam wetting agents include sodium diethylhexyl sulfate.
④ Brightening agents. Including primary brightening agents, secondary brightening agents, and auxiliary brightening agents.
- Primary brightener: Its main function is to refine the grain size and reduce the sensitivity of the plating solution to metal impurities, with a general dosage of approximately 1~10g/L, a nickel plating layer containing approximately 0.03%S. Typical primary brighteners include saccharin, diphenyl sulfonium ammonium (BB1), toluene sulfonium ammonium, benzenesulfonic acid, 1,3,6 naphthalene sulfonic acid, benzene sulfonic acid, and benzene sulfonic acid sodium (BSS), etc.
- Secondary brighteners create a noticeable gloss on the coating but also introduce stress and brittleness to the coating and sensitivity to impurities. The dosage must be strictly controlled, and when used in conjunction with primary brighteners, they can produce a fully bright coating. Typical secondary brighteners include 1,4-butanediol, propargyl alcohol, hexanol, pyridine, thiourea, etc.
- Auxiliary brighteners: They assist in brightening the coating, improve the coverage ability of the coating, and reduce the sensitivity of the plating solution to metal impurities. Typical auxiliary brighteners include sodium allyl sulfonate, sodium vinyl sulfonate, sodium propargyl sulfonate, etc.
⑤ Nickel-plated commodity additives. Various intermediates are combined based on their respective performance characteristics, resulting in several types.
- Bath starter (softening agent): mainly composed of primary and auxiliary brightening agents.
- Brightening agent (main brightening agent): Composed of one or more compounds, the main component being a secondary brightening agent, supplemented by other components.
- Surfactants: There are two types: low foam and high foam.
- Purging agents: such as iron removers, copper removers, zinc removers, low zone positioning agents, etc.
(2) Electrode Reaction
Cathode: Ni2+ + 2e = Ni 2H+ + 2e = H2
Anode: Ni = Ni2+ + 2e 4OH– = 2H2O + O2 + 4e
3.2 Electroplated Black Nickel and Gunmetal Satin
Black and gunmetal nickel (black pearl) plating is mainly used for optical coatings and decorative antique coatings, generally plated on bright nickel, copper, bronze, and zinc coatings, with a thickness not exceeding 2μm. This type of coating is hard and brittle, with poor corrosion resistance, and the surface of the coating needs to be protected with varnish.
The appearance of black and gunmetal colors is due to the different blackening materials in the plating solution. The black nickel plating layer contains a higher amount of non-metallic phases, such as zinc-containing black nickel plating, which generally has a nickel mass fraction of 40%~60%, zinc of 20%~30%, sulfur of 10%~15%, and organic matter of around 10%, making it a mixture of nickel, zinc, nickel sulfide, zinc sulfide, and organic matter.
The working temperature of the plating solution, pH value, and current density all affect the darkness of the coating. If the coating is not dark or has colors or appears yellow, first check the conductivity, then check if the current is too high or too low; if the temperature is too high, and then check if the concentrations of thiocyanate, zinc sulfate, ammonium molybdate, etc., in the plating solution are insufficient.
3.3 Electroplated Pearl Nickel
Pearl nickel (satin nickel) has fine crystallization, low porosity, low internal stress, good corrosion resistance, and a soft tone. It does not leave marks when touched by hand, is valued and loved in decorative electroplating, is widely used as a chrome, silver, and gold base layer, and can be used directly for surface layers, especially in applications such as watches and jewelry.
The main process of satin nickel electroplating is to add certain organic substances, such as anions and amphoteric substances, to the plating solution, which, under electrolytic conditions, form precipitates with a diameter similar to that of colloidal particles. These precipitates co-deposit with nickel on the cathode, producing a satin nickel coating with a pearlescent luster. By selecting the types and concentrations of additives, the diameter of the precipitates can be controlled. The pearl nickel process is almost entirely composed of patented commercial additives.
The defect commonly occurring in pearl nickel is “bright spots,” which can be resolved by removing contaminants and thoroughly stirring the plating solution before use.
4. Electroplated Silver and Silver Alloys
The element symbol for silver is Ag, the relative atomic mass is 107.9; the standard electrode potential is 0.799 V, and the electrochemical equivalent is 4.025/(A.h)
Silver and silver alloy coatings have excellent conductivity, low contact resistance, solderability, and strong reflective and decorative properties. They are widely used in tableware, musical instruments, jewelry, and more as decorative coatings.
The silver-plated layer reacts with sulfur in the air, forming silver oxide and black silver sulfide. It can also easily turn black when in contact with sulfur-containing materials such as plastics and rubber, and the oxygen in the air contributes to the darkening. The discoloration of the silver-plated layer severely affects the components’ appearance and impacts the coating’s solderability and electrical performance.
Silver plating solutions are still mainly based on cyanide silver plating, which produces a fine, pure white layer with good dispersion and coverage capabilities, and the process is relatively stable. However, it has a high cyanide content and is highly toxic. Both domestic and international research has been conducted on non-cyanide silver plating processes, and there are now commercial supplies available, such as NS silver plating, niacin silver plating, thiosulfate silver plating, dibutyryl imide silver plating, and imidazole-sulfonic acid silver plating, among others. Non-cyanide silver plating has progressed slowly due to its less delicate appearance than cyanide plating, less convenient maintenance, and inconsistent raw material supply.
4.1 Cyanide Silver Plating
From the first silver plating patent in 1840 to now, cyanide silver plating has more than 160 years of history. Cyanide silver plating has always been dominant in the generation of silver plating. The development of cyanide silver plating in the 1970s introduced brighteners that directly plated a bright silver layer from the plating solution, eliminating the polishing process, improving efficiency, and saving a large amount of silver. Bright silver plating has become the mainstream of cyanide silver plating.
(1) Main Components of Cyanide Silver Plating Solution
① Silver. It is the main salt in the plating solution, existing as silver cyanide complex ions. The source of silver may be AgNO3, AgCl, AgCN, KAg(CN)2, but AgNO3 and AgCl is best converted to AgCN or KAg(CN)2, then added to the plating solution. The Ag in the plating solution is maintained at 20~40g/L. If the silver concentration is too high, the coating crystallizes coarsely and appears yellow; if the silver concentration is too low, the current density range is too narrow, and the deposition rate decreases.
② Potassium cyanide. It is a complexing agent; in addition to complexing with Ag, a certain amount of free potassium cyanide is beneficial for the stability of the plating solution, normal dissolution of the anode, and the dispersion ability of the plating solution. The data, in general, mostly refers to free KCN. If its concentration is too high, the deposition rate of the plating solution is slow; if the concentration is too low, the plating layer is prone to yellowing, and the silver anode is easily passivated, resulting in a slow deposition rate.
③ Potassium hydroxide, potassium carbonate. It can improve the conductivity of the plating solution, help with the dispersion ability of the plating solution, and enhance the brightness of the coating.
④ Sodium tartrate. It can reduce anode polarization, prevent passivation, and promote silver anode dissolution.
⑤ Brightening agents. Adding brightening agents can achieve a fully bright coating and expand the current density range, but suitable brightening agents need to be selected for silver coatings used for different purposes. For decorative coatings, the thickness requirement is not high, but the requirements for the coating’s color (whiteness and brightness) are particularly high, making it unsuitable to use additives containing metals. For functional coatings used in electrical and electronic applications, the requirements for coating thickness and electrical performance are higher, and some may consider the hardness requirements of the coating, allowing for the addition of metal salts such as potassium antimonate.
Non-metallic brighteners often contain sulfur, which can produce a bright white silver plating, but their lifespan is not long enough. It will decompose if not used in the plating solution promptly. Metallic brighteners, such as antimony, selenium, tellurium, cobalt, and nickel, can improve the brightness of the plating and increase hardness, making them more suitable for hard silver plating.
(2) Electrode Reaction of Cyanide Silver Plating
Cathode:
The silver cyanide complex ion is directly reduced at the cathode: Ag(CN)2- + e = Ag + 2CN–
Adverse reaction: 2H2O + 2e = H2 + 2OH–
Anode:
Using soluble silver anode: Ag + 2CN– = Ag(CN)2- + e
When using insoluble anodes:4OH– = 2H2O + O2 + 4e
The current efficiency of cyanide silver plating is high, with both cathode and anode current efficiencies close to 100%.
4.2 Discoloration of the Silver Plating
Silver-plated jewelry, when placed in the air or used for some time, comes into contact with harmful gases or substances containing sulfur in the air, causing the plating to corrode and discolor, seriously affecting the appearance of the jewelry. The main reasons for this are as follows.
- The silver-plated layer itself can easily react and turn yellow in a humid atmosphere containing sulfides, and in severe cases, it can turn black.
- Improper operation of the silver plating process. Inadequate cleaning after plating leaves traces of silver salts on the surface, and this ionized silver is prone to discoloration. The plating solution is contaminated or not pure enough, with metal ions such as copper, iron, and zinc, resulting in low purity of the plating layer. Improper operation leads to a rough plating layer with high porosity. Surfaces with high porosity are prone to accumulate moisture and corrosive media.
- Improper packaging and storage after silver plating. The reasons for discoloration mainly include: first, silver-plated jewelry is directly exposed to light, causing silver atoms to be affected by ultraviolet rays, transforming into silver ions, which accelerates the discoloration speed; second, storage in a humid and high-temperature environment is prone to discoloration; third, poor sealing of the packaging can cause the packaging materials to react with the silver plating.
4.3 Silver-Plated Layer Anti-Discoloration Treatment Process
To prevent the silver layer from discoloring, silver layer passivation processes are often used in production, which typically include the following methods: chemical passivation, electrochemical passivation, immersion in anti-discoloration agents, electroplating with precious metals, and immersion in organic protective films.
(1) Chemical Passivation
After thorough water washing, the plated parts that have been non-bright silver plated should be immediately treated with chromic acid.
- Chromic acid treatment. Chromium anhydride:80~85g/L; sodium chloride:15~20g/L; temperature: room temperature; time: 5~15 seconds. After chromic acid treatment, a relatively loose yellow film is formed on the surface of the silver plating.
- Stripping process. Ammonia water: 300~500ml/L; room temperature; time: 20~30 seconds.
- Idemitsu. Nitric acid or hydrochloric acid mass fraction of 5%~10%; room temperature; time: 5~20 seconds. After the silver plating layer undergoes the above processes, it is subjected to chemical pinning to achieve better results. The chemical passivation film layer is very thin, which has little effect on contact resistance. Still, the passivation film structure is not dense enough, and its ability to prevent discoloration is not strong, so electrochemical passivation can be carried out next.
(2) Electrochemical Passivation
It can be done after chemical passivation or directly after bright silver plating. Use the silver plating layer as the cathode and stainless steel as the anode. Through electrolysis, a relatively dense needle-like film is formed on the surface of the silver layer, which has a higher resistance to discoloration than the chemical passivation film. If chemical passivation is combined with electrolysis, the effect is even better.
(3) Immersion Electrical Contact Protective Agent
Dissolve the protective agent in an organic solvent and soak it for 1~2 minutes at a certain temperature, which has a protective effect on the surface.
(4) Electroplating Precious Metals
Electroplated gold, rhodium, palladium, palladium-nickel alloy (80% ), thickness of 0.1~0.2μm.
(5) Organic Protective Film
The thickness is generally above 5μm, and the protective effect is better. Acrylic or silicone-based transparent protective coatings can be immersed (sprayed) or cathodic electrophoretic acrylic electrophoretic paint. The requirements for the organic protective film mainly include good coating density, high transparency, coating hardness not less than HV4, and good adhesion to the substrate.
5. Electroplated Gold and Gold Alloys
The relative atomic mass of gold is 197, the standard electrode potential of monovalent gold is +1.68 V, the electrochemical equivalent of trivalent gold is +1.5 V, and the electrochemical equivalent of Au+ is 7.357g/(A.h) and the electrochemical equivalent of Au3+ is 2.44977g/(A.h).
Gold has extremely high chemical stability and is not corroded by hydrochloric acid, sulfuric acid, nitric acid, hydrofluoric acid, or alkalis. Gold’s electrical conductivity is second only to silver and copper. The thermal conductivity of gold is 70% of that of silver, and gold has excellent ductility. Due to its chemical stability, conductivity, and good solderability, gold is widely used in the decoration industry.
A decorative gold plating is generally used for craft jewelry, requiring the plating to have good color, luster, wear resistance, and no discoloration. The purity of the plating can be divided into pure gold and K gold, with pure gold having a gold content of above 99.9% and commonly used K gold types, including 22K, 18K and 14K. The thickness of the plating can be divided into thin gold and thick gold, with thin gold being able to be directly plated on substrates such as nickel, copper, and bronze, while thick gold requires a base layer first.
Gold plating began in the early 19th century, with the appearance of patents for gold plating applications in the late 1840s based on cyanide-based alkaline plating solutions. Due to the extreme toxicity of cyanide, both domestically and internationally, there has been ongoing research to develop cyanide-free and low-cyanide gold plating solutions, leading to the emergence of acidic pure gold plating, acidic hard gold plating, neutral gold plating, and non-cyanide gold plating. Gold plating solutions can be divided into four types: alkaline cyanide, acidic low-cyanide, neutral low-cyanide, and non-cyanide. Overall, low-cyanide and cyanide-free gold plating solutions still have certain gaps in stability and plating effects compared to alkaline cyanide gold plating solutions.
5.1 Cyanide Gold Plating
(1) Alkaline Cyanide Gold Plating
① The main components of alkaline cyanide gold plating solution. Alkaline cyanide gold plating solution has good dispersion ability, is a stable plating solution, is easy to operate and maintain, and can easily incorporate different alloy elements, such as Cu, Ni, Co, Ag, Cd, etc. to produce gold alloys of different hues. For example, adding nickel can yield a slightly white gold color, adding Cu and Cd producing rose gold; adding Ag can result in a light green gold plating. By controlling the concentration of alloy elements in the plating solution and the working conditions, almost any desired hue of gold plating can be achieved. The porosity of cyanide gold plating is relatively high, and its wear and corrosion resistance are poor. Due to the high cyanide content in gold plating, its usage has significantly decreased in recent years; however, in the jewelry industry, cyanide gold plating remains the most common type. The main components of alkaline cyanide gold plating solution are as follows.
- Potassium gold cyanide (containing 68.3% gold ). It is the main salt in the plating solution and the source of gold in the plating layer. The plating layer will appear red and rough if the Au content is too low. The quality of potassium gold cyanide is very important, and care should be taken during the selection and use of it. Potassium gold cyanide should be dissolved in deionized water before being added to the plating solution.
- Potassium cyanide (sodium cyanide). It is a complexing agent that can stabilize the plating solution and allow the electrode process to proceed normally. If the content is too low, the plating solution becomes unstable, resulting in a rough coating with poor color.
- Phosphate. It is a buffer that stabilizes the plating solution and improves the gloss of the coating.
- Carbonate. It is a conductive salt that can improve the conductivity of the plating solution and enhance its dispersion ability. However, if carbonate is not added when opening the tank and the solution is alkaline, over time, CO2 from the air will accumulate in the plating solution. When the accumulation of carbonates is excessive, it can cause the coating to become rough and produce spots.
- Alloy composition Cu, Ni, Co, Ag and Cd is mostly added with cyanide salts, and there are also those added with EDTA salts. Their concentrations must be properly controlled to obtain 14K, 16K, 18K and 23K alloy coatings of different proportions. Moreover, 16K gold-silver coatings and 18K gold-copper-cadmium coatings can be used as intermediate layers in thick gold plating combinations and plated to the required thickness.
② Electrode reaction of alkaline cyanide gold plating. The main salt in the cyanide gold plating solution is potassium gold cyanide KAu(CN)2. The cyanide complex ion Au(CN)2– is in the solution and discharged at the cathode, generating a gold plating layer.
Cathode:[Au(CN)2]– + e = Au + 2CN–
Adverse reaction:2H+ + 2e = H2
Anode:
Using soluble silver anode: Au + 2CN– – e = [Au(CN)2]–
When using insoluble anodes:2H2O – 4e = 4H+ + O2
A portion of CN– remaining in the solution is oxidized by the oxygen in the initial ecosystem, and possible products include CNO–, COO–, CO32-, NH3, (CN)2, etc., which accumulate in the solution and become pollutants.
(2) Acidic Cyanide Gold Plating Process
The basis for an acidic micro-cyanide gold plating solution is that gold cyanide complex ions do not decompose at a pH of 3.1. The pH of the acidic gold plating solution is 3.5~5.5. The pure gold plating is bright, uniform, delicate, and has a yellowish-red hue. Adding alloying elements Co, Ni, Sb, Cu, Cd and others to the plating solution produce gold alloys to meet the decorative industry’s requirements for different hues, such as the 22.5~23.5 gold plating layers of 1N14, 2N18, 3N.
In production, acidic gold plating is mainly divided into two types: thin gold and thick gold. Thin gold plating includes pre-gold and decorative gold. Pre-gold requires excellent adhesion to the substrate and the gold layer, while the pre-gold plating solution prevents contamination of the thick gold plating solution. Decorative gold can be pure gold or gold alloy, depending on the appearance requirements. Thick gold plating solutions include ordinary and high-speed gold plating solutions. The plating solution can be plated to the required thickness as needed. The main components of acidic cyanide gold plating solution are as follows:
- Potassium cyanide. With sufficient content, the main salt can produce a bright, finely crystalline gold plating. Insufficient content results in a narrow current density range, with the plating appearing red, rough, and having high porosity.
- Citrate. It has complexing, associating, and buffering effects. If the concentration is too high, the current efficiency decreases, and the solution is prone to aging; if the concentration is too low, the dispersion ability of the plating solution is poor.
- Phosphate. A buffering agent that can stabilize the plating solution and improve the gloss of the coating.
- Cobalt, nickel, antimony, copper, cadmium, silver, etc., are alloying elements that can improve the hardness and appearance of the coating, and their concentration should be strictly controlled.
5.2 Non-Cyanide Gold Plating
In the 1960s, cyanide-free gold plating was used in production, with plating solutions containing sulfites, thiosulfates, halides, the-succinic acid, etc., but the most widely used was the sulfite plating solution with [Au(SO3)2]3- as the complex anion.
The characteristics of sulfite plating solution are: the plating solution has good dispersion and covering ability, the plating layer has good leveling and ductility (the elongation can reach 70%~90%), can achieve mirror gloss, and the purity of the plating layer is high, with good weldability. Fast sedimentation rate, few pores. The coating has good adhesion to nickel, copper, and silver metals.
The disadvantage of the sulfite plating solution is that its stability is not as good as that of the cyanide plating solution, and the wear resistance of hard gold is poor. Currently, this process has a small market share but has potential.
6. Electroplating Rhodium
Rhodium plating is silvery-white, has a strong surface gloss, is unaffected by corrosive gases such as carbon dioxide and sulfides in the atmosphere, and has high stability against acids and bases, exhibiting strong corrosion resistance. The hardness of rhodium plating is ten times that of silver plating, with good wear resistance. As a decorative rhodium layer, it has a slightly bluish tint in white, a bright luster, wear-resistant, and high hardness, making it the highest-grade decorative coating. Due to the high hardness and brittleness of rhodium, if the coating is too thick, it can easily peel off. Therefore, for general fashion jewelry, it is common to first plate silver, palladium, or nickel as a base layer before rhodium plating.
Rhodium plating solution contains sulfates, phosphates, or aminosulfonates, the most commonly used sulfates. Its plating solution is easy to maintain, has high current efficiency, and fast deposition speed, making it suitable for jewelry processing.
(1) Main Components of Rhodium Sulfate Plating Solution
- Rhodium sulfate. It is the main salt of the plating solution, and when the rhodium content is appropriate, it can produce a finely crystalline bright coating. If the content is too high, the coating will not be white and rough; if the content is too low, the coating will turn yellow and have poor brightness. Generally, the rhodium content is controlled between 1.6~2.2.g/L.
- Sulfuric acid. Its main function is to maintain the stability of the plating solution and increase conductivity, and low sulfuric acid content will affect the brightness of the plating layer.
(2) Electrode Reaction of Rhodium Plating
Anodic reaction:4OH – 4e = 2H2O + O2 ↑
Cathodic reaction: Rh2+ + 2e = Rh
Cathodic side reaction:2H+ + 2e = H2 ↑
Section III Chemical Plating Process
1. Characteristics of Chemical Plating
Compared with electroplating, chemical plating has the following advantages.
(1) Electroless plating suits various substrate materials, including metals, semiconductors, and non-metallic materials.
(2) The thickness of the chemical plating is uniform and consistent, regardless of the shape or complexity of the workpiece; as long as appropriate technical measures are taken, a uniform coating can be obtained on the workpiece.
(3) For autocatalytic chemical plating, coatings of any thickness can be obtained, and even electroforming is possible. The coatings obtained by chemical plating have excellent chemical, mechanical, and magnetic properties (such as dense coatings and high hardness).
However, chemical plating also has some disadvantages: first, the lifespan of the chemical plating solution is relatively short; second, the plating speed is slow, and only below the critical plating speed can the coating quality be guaranteed.
2. Principle of Chemical Plating
Chemical plating is a metal deposition process in which metal ions are reduced on the metal surface through self-catalysis by appropriate reducing agents in the solution. It is a chemical redox reaction involving electron transfer and a chemical deposition process without an external power source. This type of chemical deposition can be divided into three categories.
(1) Replacement Plating
Place a metal with strong reducing properties (the substrate, the workpiece to be plated) into a solution of another metal salt with strong oxidizing properties. The substrate metal acts as a strong reducing agent, and the electrons it donates are accepted by the metal ions in the solution, depositing on the substrate surface to form a coating. This process is also called immersion plating. For example, copper displacing silver, where the copper workpiece as the substrate displaces silver from the solution, and the deposited silver layer covers the copper surface. The reduction reaction stops immediately when fully covered, resulting in a very thin coating. Because the reaction is based on the substrate metal’s corrosion, the coating’s bonding strength to the substrate is relatively poor. There are not many systems suitable for the immersion plating process in terms of substrate and plating solution, so the application of this process is limited.
(2) Contact Plating
After the metal to be gold-plated comes into contact with another auxiliary metal, it is immersed in a solution of metal salts, and the potential of the auxiliary metal should be lower than that of the deposited metal. After the metal workpiece and the auxiliary metal are immersed in the solution, they form a primary battery, with the auxiliary metal being the anode due to its strong activity, dissolving and releasing electrons. In contrast, the metal workpiece is the cathode, where the metal ions in the solution are reduced and deposited as a metal layer on the workpiece. This method lacks significance in practical application but can be used to initiate chemical plating on non-catalytic active substrates.
(3) Reduction Method
First, the chemical plating solution contains metal ions for the coating, and then electrons provided by an appropriate reducing agent is added to reduce metal ions to deposit a metal coating. This chemical plating reaction must be well-controlled in terms of speed; otherwise, deposition throughout the solution becomes meaningless. The reduction method involves depositing a metal coating on an active surface with catalytic ability. Due to the self-catalytic ability of the deposited layer during the plating process, this technique can continuously deposit to form a coating of a certain thickness that has practical value, which is the true meaning of the “chemical plating” process. The method of achieving metal deposition using a reducing agent on a self-catalytic active surface is the only wet deposition process that can replace electroplating.
3. Chemical Gold Plating
Chemical gold plating can be divided into reducing type and displacement type, depending on whether a reducing agent is used in the plating solution. The reducing type chemical gold plating solution includes gold salt, complexing agent, reducing agent, pH buffer, and other additives. Its reaction utilizes the reducing agent to reduce gold, which is then uniformly deposited on the substrate to achieve the desired thickness. Generally, a thicker gold layer can be deposited, with a thickness of around 1μm. Displacement-type chemical gold plating is carried out without an external reducing agent. Due to the potential difference between metals, a more active metal can displace a less active metal from the solution through a displacement reaction. For example, in displacement gold plating on a nickel substrate, the potential difference between gold and nickel allows nickel to displace gold from the plating solution to the surface of the nickel layer. The standard potential of gold is 1.68 V, while the standard potential of nickel is only -0.25 V, indicating a significant potential difference. When the nickel substrate is immersed in the displacement gold plating solution, a displacement reaction occurs, and a layer of gold quickly replaces the nickel surface. However, gold atoms have a larger volume, resulting in a relatively loose arrangement on the nickel surface with many pores. Therefore, in the subsequent gold immersion process, as time extends, the gold ions in the plating solution continue to undergo displacement reactions with nickel atoms through the pores on the surface of the gold layer.
(1) Composition and Process Conditions of Chemical Gold Plating Solution
The chemical gold plating solution contains gold ion compounds (i.e., gold salts), complexing agents, pH buffers, reducing agents, stabilizers, and other main components.
① Gold salts and complexing agents. Suitable gold ion compounds include cyanide gold salts KAu(CN)2, KAu(CN)4, water-soluble gold compounds HAuCl4, KAuCl4, NH4AuCl4, Na3Au(SO3)2, Na3Au(S2O3)2, and gold compounds Au(OH)3 with lower solubility. They can be used alone or in combination. The gold ion concentration is generally 0.001~0.1mol/L. If the gold ion concentration is below 0.001mol/L, practical gold deposition rates cannot be achieved; if the gold ion concentration is above 0.1mol/L, gold precipitates are likely to form, and gold compounds cannot fully exert their role in chemical plating, leading to the waste of gold, which is economically unfavorable. The addition of complexing agents to the plating solution aims to form complexes with the metal components in the solution while acting as a buffer to suppress changes in the pH of the plating solution. Available complexing agents include EDTA·2Na, K2SO3, Na2SO3, K2S2O3 and others.
② Reducing agents and additives. The main reducing agents currently used in research include dimethylamine borane (DMAB), sodium hypophosphite, hydrazine, borohydrides, hydrazine borane, thiourea, sodium ascorbate, and titanium trichloride, among others.
③ Process conditions. The pH value of the plating solution is generally 5~9, with an optimal range of 6~8. A lower pH value improves the adhesion of the gold plating layer, but excessively low pH values can easily produce harmful gases and corrosion. A high pH value makes the plating solution strongly alkaline, which can dissolve the coating on the surface of the plated item. Depending on the type and concentration of gold ion compounds and complexing agents, it is appropriate to select alkaline solutions NaOH, KOH, NH4OH or inorganic acid solutions H2SO4, H3PO4, H3BO3 to adjust the pH value of the plating solution. The plating temperature is generally 50~90℃, the best being 60~85℃. A lower operating temperature is particularly suitable for items that are not heat-resistant, and it can also save energy and ensure safe operation.
(2) Cyanide-Free Chemical Gold Plating
Developing cyanide-free chemical gold plating, which replaces CN– in the gold plating solution with non-cyanide alternatives, is an important direction in chemical gold plating. It represents a significant advancement based on cyanide gold plating and has seen considerable domestic and international development in recent years. The current cyanide-free gold plating solutions mainly include sulfite gold plating, thiosulfate gold plating, halide gold plating, and thiocyanate gold plating, among which sulfite gold plating has better practical value. The following mainly introduces the sulfite gold plating system.
Sulfite system: In 1842, sulfite gold salt was first used as a gold source for cyanide-free electroplating of gold; it was later applied in chemical gold plating solutions, referred to as sulfite gold plating solutions. The reducing agents used in this gold plating solution include sodium hypophosphite, formaldehyde, hydrazine, borohydrides, DMAB, sodium ascorbate, thiourea and its derivatives, and phenyl compounds, among others. To obtain a practical cyanide-free gold plating solution, a small amount of stabilizers needs to be added to the plating solution, such as EDTA, triethanolamine, NTA, benzotriazole, 2-mercaptobenzothiazole, etc. These additives can form complex chelating agents with the monovalent gold ions in sulfite gold salt, thereby improving the stability of the plating solution.
4. Chemical Nickel Plating
The chemical nickel plating layer is independent of the coating thickness and the shape of the part and has high hardness, good wear resistance, natural lubricity, and excellent corrosion resistance, which is why it is known as the “designer’s coating.” Designers can find suitable options in the coating system based on the properties required for the parts.
Composition and Process Conditions of Chemical Nickel Plating Solution
The chemical nickel plating solution is crucial for the chemical plating process’s stability and the coating’s quality. Since the development of chemical plating, many types of plating solutions have been developed. Among them, the most commonly used solution combines nickel sulfate as the main salt and sodium hypophosphite as the reducing agent, along with appropriate additional components to adjust stability, complexation, and other properties.
(1) Nickel salts. There are mainly two types: nickel sulfate and nickel chloride. During the plating process, if the concentration of nickel salts is too low, the reaction rate is slow, making it difficult to form a plating layer. If the concentration is too high, it leads to some nickel ions being free in the plating solution, reducing the stability of the solution, which can easily result in a rough plating layer and even trigger the decomposition of the plating solution. Therefore, it is essential to maintain an appropriate content of nickel salts in the plating solution and to accurately analyze and appropriately supplement the nickel salt content during the process.
(2) Reducing agent. The common reducing agent is sodium hypophosphite. The role of the reducing agent is to catalyze dehydrogenation to provide lively primary hydrogen atoms to reduce the nickel ions to metallic nickel. The reducing agent’s content significantly impacts the deposition rate; increasing the concentration of the reducing agent can accelerate the deposition rate, but the concentration of the reducing agent should not be too high. Otherwise, the plating solution is prone to self-decomposition, which destroys the stability of the plating solution, and the deposition rate will also reach a limit value.
(3) Complexing agents. Commonly used complexing agents include glycolic acid, malic acid, tartaric acid, citric acid, and lactic acid. Adding complexing agents controls the deposition rate of nickel ions. The addition of complexing agents must consider the ability to complex all nickel ions and the deposition rate of the plating solution to maintain an appropriate ratio of the components. Complexing agents can reduce the concentration of free ions and the equilibrium potential in the solution while also adsorbing on the plated parts’ surface, enhancing the plated parts’ surface activity and accelerating the release of hydrogen ions from hypophosphite. Using composite complexing agents can effectively improve the stability of the plating solution and the deposition rate and make the surface of the coating bright and dense.
(4) pH value adjusters and buffers. The pH value of the plating solution has a significant impact on the deposition rate, reducing agent utilization and coating performance. Since H+ is a byproduct of the reduction reaction, the pH value of the plating solution will decrease as the reaction proceeds. Therefore, adjusting and controlling the pH value during chemical plating is crucial. pH value adjusters are usually NaOH, KOH or alkaline compounds such as carbonates, ammonia, etc. If it is necessary to lower the pH value, inorganic or organic acids must be added. Adding buffers prevents instability in the deposition rate caused by drastic changes in pH value during the deposition reaction. The anions of the buffer combine to form weak acid molecules with very low ionization, thus controlling the drastic changes in the pH value of the plating solution.
(5) Stabilizers. During the plating process, active crystalline cores inevitably form in the plating solution for various reasons, causing the solution to decompose and fail. By adding stabilizers, these active crystalline cores can be poisoned, losing their self-catalytic effect, thus preventing the decomposition of the plating solution. Stabilizers have become a technical secret in the chemical nickel plating process. Common stabilizers include lead ions, tin sulfides, etc.
(6) Promoters. The addition of complexing agents to the chemical nickel plating solution generally leads to a decrease in deposition rate. If added in excess, it can cause the deposition rate to become very slow or even unusable. Small amounts of organic acids are often added to the plating solution to increase the deposition rate, and these organic acids are referred to as promoters.
(7) Temperature. Temperature is the most important parameter affecting the deposition rate of chemical nickel plating. The catalytic reaction of chemical nickel plating generally can only be achieved under heating conditions, and many individual reaction steps of chemical nickel plating only show a significant deposition rate above 50℃. The operating temperature of the acidic plating solution for chemical nickel-phosphorus alloy is generally around 85~95℃, while general alkaline chemical plating solutions can deposit within a moderate temperature range. As the temperature increases, the deposition rate accelerates. However, raising the plating solution temperature will accelerate the increase of hypophosphite, making the plating solution unstable. During operation, the plating solution should be stirred evenly, and care should be taken to prevent local overheating of the plating solution, maintaining a stable working temperature to avoid severe self-decomposition of the plating solution and adverse consequences such as delamination of the coating.
(8) pH value. With the increase of the pH value of the plating solution, the deposition rate accelerates, and the solubility of phosphite decreases, which can easily lead to the self-decomposition of the plating solution. Suppose the pH value of the plating solution is too high. In that case, the reaction of hypophosphite oxidizing to phosphite accelerates, and the catalytic reaction turns into a spontaneous reaction, causing the plating solution to fail quickly. As the pH value increases, the phosphorus content in the coating decreases. When the pH value is too low, the reaction cannot proceed, such as in acidic plating solutions, when the pH value<3, it isn’t easy to deposit a nickel-phosphorus alloy coating.
(9) The effect of stirring. The diffusion process influences the chemical nickel plating process, and stirring the chemical plating solution helps to increase the transfer rate of reactants to the workpiece surface while also facilitating the removal of reaction products. Essentially, stirring alters the chemical composition and pH value within the diffusion layer at the workpiece/solution interface. Stirring methods include mechanical stirring, magnetic stirring, ultrasonic dispersion, and chemical dispersion methods. Additionally, when chemical nickel plating is conducted under heating conditions, the large amount of hydrogen gas released can create a “self-stirring” effect. Among these, mechanical stirring is simple and easy to implement, generally using external shear and impact forces to fully disperse particles in the medium; however, it does not effectively address the overall stirring of the plating solution, especially at the bottom of the beaker. Magnetic stirring utilizes a magnetic rotor to generate stirring through rotation in the plating solution. It is very effective for stirring the solution at the bottom, making it beneficial for composite plating solutions containing settling particles. However, magnetic stirrers typically only heat the bottom of the plating tank, and this heating method can easily cause local overheating of the bottom solution, which may adversely affect the plating solution’s stability and the coating’s performance. Ultrasonic dispersion has been widely recognized and effective in recent years, utilizing ultrasound’s high energy and cavitation effect to crush aggregated microparticles for dispersion. However, due to the immense energy of ultrasound, this dispersion should be applied intermittently during plating. Better results can be achieved if supplemented with a certain intensity of mechanical stirring.
5. Chemical Copper Plating
Chemical copper plating technology is mainly applied to non-metal surfaces such as plastics and wood in decorative items. Whether for decorative or functional plastic electroplating, most require chemical copper plating to ensure a good conductive base layer and achieve a good coating. Compared to other methods of metalizing plastic surfaces, chemical copper plating is the most economical and simplest method.
(1) Common Methods of Chemical Copper Plating
The chemical copper plating solution mainly consists of copper salts, reducing agents, complexing agents, stabilizers, adjusting agents, and other components. Currently, the widely used chemical copper plating solution uses copper sulfate as the main salt and formaldehyde as the reducing agent, mainly composed of two parts: one is a solution containing copper sulfate, potassium sodium tartrate, sodium hydroxide, sodium carbonate, and nickel chloride; the other is a solution containing the reducing agent formaldehyde. These two solutions must be prepared separately in advance and then mixed for use. In alkaline solutions, formaldehyde mainly exists as methylene glycol and its anions. During the chemical copper plating process, formaldehyde rapidly undergoes a disproportionation reaction, producing its redox products and leading to premature aging of the plating solution. Since no coordinating agents exist in the plating solution, only a small amount of these oxides can be dissolved while most continue accumulating. A typical plating solution formula and working conditions are 5g/L copper sulfate, 25g/L potassium sodium tartrate, 7g/L sodium hydroxide, 10ml/L formaldehyde, and time 20~30 seconds.
(2) Chemical Copper Plating on Non-Metallic Surfaces
With the expansion of the application field of chemical copper plating, the technology for chemical copper plating on non-metal surfaces has also gradually matured. For example, maskless producing the copper interconnection wires on ceramic substrates has been achieved by combining laser micro-etching technology with chemical plating. On this basis, a chemical copper plating method has been further established and implemented on the surfaces of non-metallic materials such as ceramics without the need for catalytic activation. This method simplifies the chemical copper plating process, has good coating performance, stable plating solution, and fast plating speed; it also saves precious metals and reduces production costs. Chemical metal plating is one of the main methods for metalizing plastic surfaces.
After the plastic is metalized, it can be further processed through chemical plating or electroplating to obtain plastic products with wear resistance, heat resistance, thermal stability, and special functions.
Chemical copper plating is also applied to the surface treatment of wooden materials, and the treated materials have better decorative and corrosion-resistant properties, which can enhance the added value of the products. Japan has made certain progress in researching wood copper plating and gold plating processes in recent years. The treatment objects are mainly wood from commonly used tree species such as Japanese cedar, and the test materials include wood chips and small square timber. The treatment process first involves ultrasonic treatment of the test materials in aqueous and organic solvent immersion solutions, degreasing and removing components that hinder the plating film, then sealing the resin channels with polyethylene glycol toluene solution, attaching the catalyst, and finally performing chemical plating, which requires several drying steps.
Despite the significant development of the chemical copper plating process, the following aspects still need further improvement: the relationship between the stability of chemical copper plating and plating speed; the kinetic study of chemical copper plating in multi-complex systems; the impact of additives on coating performance; the relationship between the microstructure of the coating and the surface morphology of the substrate; alternatives to formaldehyde, etc.
Copywrite @ Sobling.Jewelry — Custom jewelry manufacturer, OEM and ODM jewelry factory
6. Chemical Plating Example: Electroplating of Leaf Veins
Leaf vein electroplating, also known as leaf decoration electroplating, first selects artistic, hard, and dense-veined leaves. After removing chlorophyll to expose the leaf veins undergo surface metallization and electroplating processing.
These leaves have been shaped and processed to maintain their original, realistic appearance and reflect the elegance and luxury after electroplating (Figure 11-8).

The main processes of leaf vein decorative electroplating are divided into three parts: leaf vein treatment, surface metallization (chemical plating), and decorative electroplating.
(1) Leaf Vein Treatment
Place the freshly picked leaves in an alkaline aqueous solution for soaking to remove chlorophyll, allowing the surface to display a relatively intact natural leaf vein pattern. The soaking solution is prepared with sodium hydroxide. After soaking for several days, chlorophyll can be removed, but this method takes a long time, and the degree of corrosion is difficult to control. Adding some sodium carbonate to the sodium hydroxide solution and heating it to a boil can quickly remove chlorophyll, with the leaves changing from green to yellow-green ideal. After washing the boiled leaves, if there are still traces of chlorophyll remaining on the veins, a soft brush must gently scrub along the veins, ensuring that the veins remain intact as a standard.
(2) Surface Metallization
Surface metallization is a treatment method that makes the surface of general non-metal materials conductive, preparing for the next electroplating step, which can be achieved through sensitization, activation, reduction, and chemical plating. Chemical nickel plating makes the surface of the leaf veins conductive, with the basic formula and process conditions being 26~28g/L nickel sulfate, 35g/L sodium hypophosphite, 20g/L citric acid, and other appropriate amounts. The operating process conditions are pH value being 4.6~4.8 and temperature at 90℃.
(3) Decorative Electroplating
After bright copper plating, mid-term production can be carried out: use spot welding to configure hanging parts, such as positioning pins, hooks, and other hangers. The material for the hanging parts is generally fine purple copper wire. Before spot welding, soak the fine copper wire in an acid solution for a short time (less than 30 seconds), and then cover it with solder for spot welding.
(4) Bright Electroplated Nickel
The nickel plating process, which is mainly used to prevent the penetration of copper and gold plating, refers to the previous one.
(5) Electroplated Gold
Finally, a thick gold plating is applied to the surface.
Section IV Chemical and Electrochemical Conversion Film Process for Popular Jewelry
Chemical and electrochemical conversion film technology is a technique that uses chemical or electrochemical means to bring metal into contact with a specific chemical treatment solution, thereby forming a layer on the metal surface that has good adhesion and can protect the base metal from the effects of water and other corrosive media, can improve the adhesion and aging resistance of organic coatings, or can impart decorative properties to the surface.
In the jewelry industry, chemical and electrochemical conversion film technology has been widely applied, forming colored films or interference films through surface conversion, creating various decorative colors and surface coloring effects, improving the appearance of materials, and enhancing corrosion resistance. This includes surface coloring treatments for copper jewelry, stainless steel jewelry, titanium jewelry, aluminum jewelry, and silver jewelry.
1. Chemical Coloring Process of Copper and Copper Alloy Ornaments
Copper alloy coloring is mainly applied to craft jewelry. Most copper compounds have intense colors, and the decorative colors on the surface of copper and its alloys almost cover the entire color spectrum through chemical coloring. Currently, the ones that are accepted by the market and can be produced on an industrial scale are primarily green (copper carbonate), black (copper sulfide), blue (alkaline copper ammonia complex), black (copper oxide), and red (cuprous oxide).
(1) The Chemical Reaction Mechanism of Surface Coloring
The surface coloring of copper and its alloys is the interaction of metallic copper with the coloring solution, forming an oxide layer, sulfide layer, and other compound films on the metal surface. Different coloring effects can be achieved by selecting different coloring formulas and conditions. For example, sulfur-based solutions that can be utilized include sulfides (such as potassium sulfide, ammonium sulfide, etc.), sodium thiosulfate, polysulfides (such as potassium persulfate), etc. The coloring principle is based on the characteristic reaction of sulfur with copper to produce copper sulfide, and under different reaction conditions and the participation of other components in the formula, colors such as black, brown, dark antique copper, blue, and purple can be formed. The interaction of copper with ammonia and chromium, along with the participation of other ions in the formula, can also produce various coloring effects under different reaction conditions. The addition of oxidants in the coloring formula can promote the reaction, but excessive oxidants can affect the quality of the oxide film.
(2) Chemical Coloring Process of Copper
- The color is antique copper. Immerse pure copper or copper-plated ornaments (the thickness of the copper plating must be greater than 5μm) in the coloring solution below and shake continuously. It will quickly turn brown and deepen over time. When it reaches a certain thickness, oxygen will start to precipitate; at this point, it should be removed, cleaned, and dried for polishing, or the colored piece can be rubbed with leather scraps in a drum. The surface layer of the convex part is ground away to reveal some of the original copper color. The parts will exhibit a gradient of color from light to dark from the convex to the concave surfaces, creating an elegant antique style. This antique color tone is favored in craft ornaments on the international market. The typical process specification for achieving antique copper color is 40~120g/L basic copper carbonate, 200ml/L ammonia, reacting at room temperature for 5~15 minutes.
- Copper coloring blue. Optional copper coloring blue process scheme: 130g/L copper sulfate, 13g/L ammonium chloride, 30ml/L ammonia, and 10ml/L acetic acid react for several minutes at room temperature.
- Copper coloring green. Optional copper coloring green process schemes: 32g/L calcium chloride, 32g/L copper nitrate, 32g/L ammonium chloride, react for a few minutes under 100℃.
- Copper coloring ancient green. Optional copper coloring ancient green process specifications: 5~10g/L sulfuric acid brocade, 10~15g/L ammonium sulfate, 25~30g/L sodium thiosulfate, water 200 ml, react for several minutes under 30~50℃.
- Copper coloring brown. Optional copper coloring brown process specifications: 6g/L copper sulfate, 4g/L copper acetate, 1g/L alum, react for 10 minutes under 95~100℃.
- Copper coloring golden yellow. Optional copper coloring golden yellow process schemes: 0.8g/L potassium sulfide, 1g/L ammonium sulfide, 0.3g/L barium sulfide, 4g/L sodium sulfide, 0.13g/L potassium permanganate, and 0.7g/L hydrogen peroxide react for several minutes at room temperature.
- Copper coloring red. Optional copper red process scheme: 25g/L copper sulfate, 200g/L sodium chloride, react for 5~10 minutes under 50℃.
- Copper coloring black. Optional copper blackening process schemes: 5~12.5g/L potassium sulfide and 20~200g/L ammonium chloride, which react for several minutes at room temperature.
(3) Chemical Coloring Process of Copper Alloys
Coloring with brass is relatively simple, with copper alloys followed by bronze, aluminum, and band bronze. The chemical coloring of copper alloys is widely used in craft jewelry.
- Copper alloy coloring red. Optional copper alloy red process schemes: 2g/L iron nitrate, 2g/L sodium sulfite, react for a few minutes under 75℃.
- Copper alloy coloring orange. Optional copper alloy orange process schemes: 25g/L sodium hydroxide and 50g/L copper carbonate, which react for a few minutes under 60~75℃.
- Copper alloy coloring brown. Optional process scheme for brown copper alloy: 12.5g/L barium sulfide, reacting for several minutes under 50℃.
- Copper alloy coloring chocolate color. Optional processes for copper alloy with chocolate color: 25g/L copper sulfate, 25g/L ammonium nickel sulfate, 25g/L potassium chlorate, react for a few minutes under 100℃.
- Copper alloy coloring ancient green color. Optional processes for achieving the ancient green color of copper alloy: 350g/L ammonium chloride, 200g/L copper acetate, react for a few minutes under 100℃.
- Copper alloys coloring blue. Optional processes for blue copper alloys: 2g/L sodium sulfite, 1g/L lead acetate, react for a few minutes under 100℃.
- Copper alloy coloring black. Optional process scheme for blackening copper alloy: 25g/L copper sulfate, a small amount of ammonia, reacts for a few minutes under 80~90℃.
(4) Factors Affecting Coloring Effects
The chemical coloring of copper and copper alloy jewelry is sometimes unstable, and the main factors affecting the coloring effect are the following three.
① The influence of substrate composition. Generally speaking, substrates with a high copper content are more likely to achieve a pure and attractive appearance. As the copper content decreases, the presence of other alloying elements, which are more reactive than copper, will affect the purity of the corrosion products and the color of the copper coating. For example, the corrosion products of zinc, tin, aluminum, etc., will mix white hydroxides into the film layer, causing the film layer to appear white. In this case, the content of stabilizers can be appropriately increased. When a β phase is present in the material’s metallographic structure, due to its poor corrosion resistance, severe pitting will occur, resulting in a large accumulation of zinc hydroxide at the pit openings, leading to obvious white spots and rendering the material unusable. At this time, reducing the stabilizer content can achieve good results.
② The impact of the workpiece surface condition. For deep-drawn or deep-stretched parts, in areas of severe deformation, 80% mechanical energy is stored in the form of crystal defects such as dislocations and vacancies, which have high chemical reactivity, leading to localized color differences. Stress relief annealing can be used to eliminate these issues. Additionally, the consistency of the surface roughness of the parts also has a certain impact on color consistency.
③ The impact of special climatic conditions. The coloring of copper alloy jewelry after applying the coloring solution may experience instability due to changes in climate during the color development reaction in the air. There may be insufficient or excessive reaction time for special climates, which requires appropriate control of the coloring time to resolve.
2. The Coloring Process of Silver and its Alloy Jewelry
The coloring treatment of silver and its alloys is mainly used for decorative craft jewelry, forming sulfides on its surface, which can create shades of black, blue-black, light gray, dark gray-green, gray-green, ancient silver, yellow-brown, green-blue, and so on.
(1) Silver Coloring Ancient Silver Color
Selectable silver alloy antique silver color process scheme: 25g/L potassium sulfide, 38g/L ammonium chloride, and 2g/L barium sulfide react at room temperature until the desired tone is reached.
(2) Silver Coloring Black
Selectable silver alloy with black process scheme: 15g/L potassium sulfide, 40g/L ammonium chloride, and a small amount of ammonia water react under 80℃ until the desired tone is reached.
(3) Silver Coloring Blue-Black
Selectable silver alloy with blue-black color process scheme: 2g/L potassium sulfide, 6g/L ammonium chloride, reacting under 60~80℃ until the desired hue is reached.
(4) Silver Coloring Blue and Yellow
Selectable silver alloy with blue-yellow process scheme: 1.5g/L potassium sulfide, reacting under 80℃ until the desired hue is reached.
(5) Silver Coloring Green
Selectable silver alloy with blue-green process scheme: 300g/L hydrochloric acid and 100g/L iodine react at room temperature until the desired hue is reached.
(6) Silver Coloring Yellow-Brown
Selectable silver alloy yellow-brown process scheme: 5g/L barium sulfide reacts at room temperature until the desired hue is reached.
3. Coloring Process of Zinc and its Alloy Jewelry
The surface conversion film formed by chromate passivation treatment of zinc and its alloys also has coloring functions, generally resulting in various colors such as rainbow, grass green, brownish yellow, green, military green, and black.
(1) Zinc Coloring Black
Selectable zinc blackening process: For 10~15 minutes, 40~50g/L copper sulfate and 30~40g/L potassium chloride react at room temperature.
(2) Zinc turns red
Selectable zinc red process scheme: copper sulfate, tartaric acid, and ammonia react at room temperature for minutes.
(3) Zinc Coloring Red
Selectable zinc coloring red craft scheme: 60g/L copper sulfate, 80g/L sodium carbonate, 60ml/L potassium bitartrate, react at room temperature for 3~5 minutes.
(4) Zinc Alloy Coloring Gray
Selectable zinc alloy gray coating process scheme: 20~25g/L copper sulfate, 50ml/L ammonia, 30g/L ammonium chloride, react for 3~5 minutes under 20~25℃.
(5) Zinc Alloy Coloring Green
Selectable zinc alloy green process scheme: 80~100g/L potassium dichromate, 25~30g/L sulfuric acid, 150g/L hydrochloric acid, react for 0.5~1 minutes under 30~40℃.
(6) Zinc Alloy Coloring Black
Selectable zinc alloy blackening process: 140~160g/L copper sulfate and 80~90g/L potassium chlorate react for 3~5 minutes under 20~25℃.
4. The Oxidation Coloring Process of Stainless Steel Jewelry
Stainless steel anodizing refers to forming an anodized color layer on the surface of stainless steel. It utilizes the colorless and transparent oxide film to create color through light interference, resulting in a durable hue formed by this film layer.
Typical methods for coloring stainless steel through oxidation include chemical and oxidation coloring methods. Chemical coloring methods include the Inco method and the Acheson method. The Inco method involves immersing the steel in a high-temperature sulfuric acid solution for coloring. In contrast, the Acheson method involves electrolysis in a medium-temperature solution to form a thin oxide film on the stainless steel surface, with color controlled by changing the thickness of the oxide film. The oxidation coloring method involves heating in the atmosphere or a controlled atmosphere to form an oxide film; this method is simple, but it is difficult to control the color, so it is limited to small stainless steel ornaments.
(1) Inco Method
In 1972, Inco established the color management and color-enhancing film technology for the acidic solution oxidation method, and its processing steps are as follows:
Pre-treatment → Water washing→Color development treatment→Water washing→Hard film treatment→Water washing→Drying
After pretreatment, the stainless steel plate is immersed in a 80~85℃ mixed solution of chromic acid and sulfuric acid. This treatment can form a chromium-based oxide film on the surface of the stainless steel, which can vary in thickness from hundreds to thousands depending on the treatment time. Due to light interference, colors can appear in the order of tea color: blue, gold, purple, green, etc. The growth of the oxide film, that is, the color change, can alter the immersion potential of the stainless steel, mastering this potential change for color management is the first feature of the Inco method (color development process). However, the color development film is porous at this stage and has poor wear resistance. Therefore, cathodic electrolysis should continue in a mixed aqueous chromic acid and phosphoric acid solution to strengthen the color development film. This treatment can seal the pores of the porous color development film, significantly improving wear and corrosion resistance. This is the second characteristic of the Inco process (hard film treatment).
Due to some issues with the process, the color in this technology is difficult to control, and the management requirements for raw materials are strict.
(2) Acheson Method
In 1985, Kawasaki Steel successfully developed the alternating current electrolysis method, also known as the Acheson method. This method involves electrolyzing stainless steel in a mixed solution of chromic acid and sulfuric acid below 60℃, forming an oxide film on the surface. Due to the repeated alternating anode electrolysis for a specified number of times, coloration and film hardening can occur simultaneously. Color control can be achieved by controlling the total electric charge. Stainless steel colored using the Acheson method has bright colors and can display unique colors such as bronze and yellow.
The color of the stainless steel surface mainly depends on factors such as the chemical composition of the surface film, its microstructure, surface smoothness, film thickness, and the angle of incident light. Generally, thin oxide films display blue or brown, medium thickness films show golden yellow or red, thick films appear green, and the thickest ones appear black. Overall, the coloring process of stainless steel is relatively difficult, with high process requirements, and its color uniformity is not easy to control.
5. Anodizing Treatment of Aluminum Alloy Jewelry
Aluminum alloy jewelry are widely colored using the anodizing method. Aluminum is one of the easiest metals to color. Through anodizing treatment, a thick and dense oxide film layer is formed on the aluminum surface, significantly changing the corrosion resistance of the aluminum alloy and improving hardness, wear resistance, and decorative performance. The methods for coloring aluminum oxide films mainly include electrolytic coloring, chemical dyeing, and electrolytic dyeing.
(1) Electrolytic Coloring Method
Electrolytic coloring is the process of anodizing and coloring completed in the same solution, directly forming a colored oxide film on aluminum alloys. Therefore, the electrolytic coloring method is also known as the one-step electrode coloring method. Electrolytic coloring is caused by the selective absorption of certain wavelengths of light by the film layer while the remaining wavelengths are reflected. The selective absorption is related to the oxidation state of the alloying elements in the film layer or the state of the electrolyte composition combined with the substances in the oxide film.
(2) Chemical Coloring Method
The anodic oxide film of aluminum jewelry can be impregnated and colored with inorganic or organic dyes. The coloring agents are deposited in the upper part of the pores of the oxide film. The most suitable oxide film for coloring is obtained through sulfuric acid anodization.
(3) Electrolytic Coloring Method (Two-Step Electrolytic Coloring Method)
After anodizing aluminum or aluminum alloys, they are immersed in a solution of more expensive metal salts, and coloring is achieved by using alternating current for polarization or direct current for cathodic polarization. Under the action of the electric field, metal ions are reduced and deposited at the bottom of the pores in the oxide film, resulting in the coloring of aluminum and its alloys.
Section V Physical Vapor Deposition Process
Physical Vapor Deposition is abbreviated as PVD. Physical Vapor Deposition is a physical vapor reaction growth method, and the deposition process occurs under vacuum or low-pressure gas discharge conditions. The source of the deposited material is a solid material, which, after “evaporation or sputtering,” generates a new solid material deposition layer on the surface of the part that is completely different in performance from the substrate.
Physical vapor deposition technology is increasingly applied to jewelry, with surface decorative coatings for crafts. The colors of the coatings are diverse, including deep gold, light gold, coffee, antique bronze, gray, black, gray-black, and multicolored, which can significantly improve the appearance color of craft jewelry and enhance characteristics such as wear resistance and corrosion resistance.
1. Classification of Physical Vapor Deposition Processes
Physical vapor deposition processes are divided into several types, including vacuum evaporation coating, ion plating, and magnetron sputtering coating, with the latter two belonging to the category of plasma vapor deposition. The deposition of the film layer occurs under low-pressure plasma gas discharge conditions, where the film layer particles gain higher energy in the electric field, resulting in significant improvements in the organization, structure, and adhesion of the film layer compared to vacuum evaporation coating. With the development of high technology, various new technologies for ion plating and magnetron sputtering coating have gradually matured and are continuously promoted and applied in various fields.
(1) Vacuum Evaporation Coating
Vacuum evaporation coating (evaporation) is vaporizing and evaporating the coating material through high-temperature heating under vacuum conditions and depositing it on the substrate surface to form a solid thin film. Evaporation technology is one of the earlier developed technologies in vacuum coating technology. Compared to sputtering and ion plating, the evaporation method is relatively simple, but under appropriate process conditions, it can produce very pure, thin film coatings with specific structures and properties. Therefore, evaporation technology still plays an important role in optics, semiconductor devices, and plastic metallization fields.
The vacuum evaporation coating has the following technical characteristics:
- The vacuum evaporation coating process includes the evaporation of the coating material, the transport of vapor atoms, and the nucleation and growth of vapor atoms on the substrate surface, which consists of evaporation, transport, and deposition.
- The deposition vacuum of vacuum evaporation coating is high, generally at 10-3~10-5Pa. The film layer particles hardly collide with gas molecules or other metal vapor atoms, reaching the workpiece directly.
- The energy of the film layer particles reaching the workpiece is the thermal energy carried during evaporation. Since the workpiece is not biased in vacuum evaporation coating, the metal atoms rely solely on the vaporization heat during evaporation, which is approximately 0.1~0.2eV. Therefore, the energy of the film layer particles is low, the bonding strength between the film layer and the substrate is small, and it isn’t easy to form compound coatings.
- The vacuum evaporation coating layer is formed under a high vacuum, and the layer particles in the vapor are atoms state, forming small cores on the surface of the workpiece, growing into a dense structure.
- The vacuum evaporation coating layer is obtained under high vacuum conditions. Generally, the coating can only be deposited on the side of the workpiece facing the evaporation source. In contrast, the side and back of the workpiece hardly receive any coating, resulting in poor coating quality.
(2) Magnetron Sputtering Coating
Under certain vacuum conditions, bombarding the surface of a material with charged particles of tens of electron volts or higher kinetic energy allows the atoms of the bombarded material to gain enough energy to escape the binding of the original material lattice and enter the gas phase. This technology is called sputtering technology. The sputtered gas phase elements are used for deposition to form a film, and this deposition process is referred to as sputter coating, as shown in Figure 11-9. When depositing hard coatings, the workpiece needs to be connected to a negative bias power supply called magnetron sputtering ion plating or bias sputtering.
Sputter coating is currently the most widely used coating technology in PVD technology, with the following advantages:
- The quality of the sputtered coating is good, and the adhesion to the substrate is very strong.
- The application range of sputter coating is wide and suitable for various materials, and high melting point materials can also be easily sputtered.
- The thickness distribution of the sputter-coated film is relatively uniform.
- The sputtering coating process has good repeatability, is easy to achieve process control automation, and can be produced continuously on a large scale.
(3) Ion Plating
Ion plating is a process that takes place under vacuum conditions, using gas discharge to partially ionize gas or evaporated materials. While the gas ions or ions of the evaporated materials bombard the surface, the evaporated material or its reaction products are deposited onto the substrate.
In the following aspects, Ion plating combines the fast deposition rate of evaporation plating with the surface cleaning characteristics of ion bombardment in sputtering plating.
- The film layer has good adhesion to the substrate and dense film structure.
- It can improve the film’s ability to cover complex shaped surfaces, also known as the diffraction capability during the film deposition process.
- The controllable parameters of the film layer organization are numerous, the overall energy of the film layer particles is high, and it is easy to perform reactive deposition, allowing for the acquisition of compound coatings at lower temperatures.
2. The Application of PVD Coating Technology in the Jewelry Industry
2.1 Zirconium Nitride Decorative Coating
(1) Pre-Treatment for Coating
The vacuum coating process requires a high level of cleanliness for the sample surface and the vacuum furnace chamber, which is necessary for obtaining high-quality film layers. Therefore, the samples must be cleaned, and the vacuum furnace must be cleaned before coating.
Cleaning the sample surface is key to film formation. The sample surface must be clean and free of rust spots and oil stains, so the sample should be strictly cleaned before entering the furnace to remove oil, dirt, rust, and moisture. The cleaned sample should not be touched by hand to prevent secondary contamination.
The pre-treatment process flow for workpiece coating is as follows:
- Ultrasonic cleaning (1% metal cleaning agent), temperature: 50℃, time: 3 minutes.
- Ultrasonic cleaning (5% metal cleaning agent), temperature: 50℃, time: 2 minutes.
- Hot water cleaning, temperature: 50℃, time: 30 seconds.
- Rinse with cold water at room temperature for 30 seconds.
- Rinse with deionized water at room temperature for 20 seconds.
- Alcohol dehydration.
- Drying or air drying.
- Cleaning of the vacuum furnace. Wipe the dirt, attachments, and deposits inside the vacuum furnace with a dry cloth, and use a scraper to remove attachments from the corners.
(2) Coating Process Flow
① Pre-evacuation. After the sample is placed in the vacuum chamber, close the furnace door, turn on the mechanical pump for rough pumping, and when the vacuum reaches 0.1 Pa, turn on the oil diffusion pump for fine pumping. The vacuum process ends when the vacuum in the chamber reaches 6.5×10-3Pa.
② Glow discharge cleaning. The function of glow discharge cleaning involves many electrons moving toward the anode during the cleaning process. The high-speed movement of electrons ionizes the argon gas in the vacuum chamber, creating a glow phenomenon and generating softer argon ion movement. The moving argon ions, under the influence of the biased electric field, bombard the surface of the workpiece, colliding and removing the adsorbed gases and impurity atoms from the surface, exposing a clean surface of the workpiece material. At the same time, it increases the micro-roughness of the workpiece surface, which is beneficial for enhancing the bonding strength between the film layer and the workpiece. After introducing a certain argon gas flow to raise the pressure to 4×100Pa in the furnace, the bias power supply applied to the workpiece is turned on (the voltage and pulse duty cycle are adjustable). Here, the bias value is positive, and argon ions are used to clean the furnace wall, target surface, workpiece, and turntable. The cleaning time is determined based on the material and cleanliness of the workpiece.
③ Main bombardment. The role of the main bombardment is to apply a negative bias to the workpiece, which accelerates a large number of target material ions that are sputtered out. Positively charged target material ions move towards the workpiece under the influence of the negative bias, forming high-speed ion clusters with considerable energy. These ion clusters perform a certain degree of cleaning, activation, and implantation on the workpiece. The effect of the main bombardment: a large number of metal ions are implanted into the surface of the workpiece, and the implanted ions cause lattice restructuring with the substrate. Some metal ions with slightly lower energy levels produce a diffusion layer due to smaller implantation forces. Ions moving toward the workpiece may collide with it, and some will sputter and eject due to lower energy levels or differing incident angles. These ions will then collide with the next moving ions, forming secondary energy-level ions that ultimately deposit on the surface of the workpiece.
After cleaning, the vacuum chamber continues to evacuate to achieve 6.5×10-3Pa. Then, a certain flow rate of argon gas is introduced to achieve a total pressure of 0.3 Pa in the furnace; the values of pulse bias and duty cycle are adjusted (specific values depend on the requirements of the film layer); the power (voltage, current) of the cathode target’s intermediate frequency power supply is set to a certain value; the sample is subjected to main bombardment, with the bombardment time determined by process requirements.
④ Adding nitrogen gas for deposition coloring. At the end of the main bombardment process, an appropriate flow of reaction gas N2 will be introduced (at this time, the vacuum degree will slightly decrease). Manually adjust the nitrogen flow meter’s potentiometer to control the nitrogen flow based on the partial pressure of nitrogen (percentage) displayed by the mass spectrometer. At this time, the pulse bias, duty cycle, and the power (voltage, current) values of the cathode target’s intermediate frequency power supply can be adjusted according to process requirements; the sample is subjected to deposition coloring, and the deposition time is determined based on process requirements. In this way, metal ions will move towards the workpiece under appropriate bias, and during the movement, they will collide with the reaction gas to form a metallic compound film. At the same time, the movement speed will also decrease and deposit on the surface of the workpiece.
⑤ Passivation. The role of passivation is that the freshly coated film layer is a relatively active structure with a certain temperature. If it comes into contact with the atmosphere immediately, it will react again, causing a color change. Therefore, after the coating is completed, it should be cooled down, and a suitable amount of argon gas should be introduced, which protects the surface film layer of the workpiece with inert gas, allowing the film layer to be more stable with the temperature decreasing; on the other hand, it allows the target surface to absorb a large amount of argon gas and become saturated, thereby maintaining a fresh target surface and preventing the target surface from absorbing other gases after being filled with atmosphere, thus protecting the sputtering target surface from contamination. The maximum argon gas flow during the filling process is about 2 minutes.
⑥ Inflatable cooling. When the temperature of the vacuum chamber drops to 120℃, it introduces air to atmospheric pressure.
⑦ Opening the furnace and take out the sample. Open the furnace door, put on clean gloves to remove the sample, and seal the sample for color measurement, film thickness measurement, and Auger semi-quantitative analysis.
(3) Color Control of Zirconium Nitride Film
As a decorative ZrN gold-like film, the color of the film layer is particularly crucial. Due to the numerous process parameters of the medium-frequency reactive magnetron sputtering technology, many factors affect the film’s color. Under certain other process parameters, the nitrogen partial pressure and sputtering power are vital in the film’s color. As the nitrogen partial pressure increases, the color of the ZrN film changes from silver-white→light yellow→gold yellow→reddish gold→deep red. As the sputtering power increases, the color of the ZrN film changes from deep yellow→gold yellow →light yellow. By comparing the colors of ZrN films with those of gold and gold alloys, the desired gold yellow can be obtained by adjusting the nitrogen partial pressure during deposition.
2.2 Titanium Nitride Decorative Coating
(1) Magnetron Sputtering Technology for the Deposition of Titanium Nitride Process
① Install the workpiece. After installing the workpiece on the workpiece fixture, close the coating chamber.
② Vacuum extraction. Start the vacuum pump, and when the vacuum degree reaches 6 Pa, open the diffusion high spring and extract the vacuum to achieve 6×10-3Pa.
③ Baking heating. Turn on the baking heating power supply to heat the workpiece to the predetermined temperature.
④ Bombardment purification. Introduce working gas into the coating chamber, maintain a vacuum degree of 1~3Pa, bombardment voltage of 1000~3000 V, the workpiece generates glow discharge, argon ions bombard and purify the workpiece, bombardment for 10~20 minutes.
⑤ Titanium nitride deposition. First, deposit the base layer, reduce the workpiece bias to about 500 V, introduce argon gas, and adjust the vacuum to 0.3~0.5Pa. Turn on the magnetron sputtering target power supply. A glow discharge occurs on the target surface, and a high-density flow of argon ions ejects titanium atoms from the target surface. Next, deposit the titanium nitride coating, requiring the introduction of nitrogen gas, with the vacuum maintained within the range of 0.5~0.7Pa. Due to the balanced metal ionization rate of the magnetron sputtering being only 5%~10%, the reactivity of the metal is relatively low, resulting in a narrow process range (nitrogen content) for obtaining nitrogenized needle-like compounds coating. Compared to glow discharge, to achieve high-quality titanium nitride coatings, it is essential to strictly control the amount of titanium sputtering and the argon-nitrogen ratio. Otherwise, the deposition rate will be very low, and the color will be difficult to control.
⑥ Remove the workpiece. When the film thickness reaches the specified requirements, inflate the coating chamber, open the coating chamber, and remove the workpiece.
(2) The Effect of Process Parameters on the Properties of Titanium Nitride Films
① The effect of deposition temperature on the properties of nitride thin films. The deposition temperature affects the hardness and structure of TiN coatings; the hardness and adhesion of the TiN film deposited near 500℃ are the highest. Excessive and high temperatures are detrimental to the surface hardness of the TiN film. If the deposition temperature is too low, it reflects a short pre-sputtering time or low sputtering energy, affecting the film’s density, leading to insufficient nucleation of the TiN film and coarsening of the structure, resulting in a decrease in hardness. Conversely, if the deposition temperature is too high, it can cause overheating on the sample surface. Suppose the deposition temperature exceeds the tempering temperature of the substrate material. In that case, it will reduce substrate hardness, preventing a good bond with the film and causing a decrease in film hardness. Therefore, increasing the deposition temperature is beneficial for improving the hardness of the TiN film, provided that the sample surface does not overheat.
② The effect of deposition voltage on the properties of titanium nitride films. As the deposition voltage increases, the fracture morphology of the prepared TiN films becomes finer, and the microhardness and the deposition rate of the films increases. Increasing the discharge voltage under the same deposition conditions also means enhancing the plasma intensity, which promotes the chemical reactions in a direction favorable for product formation. Therefore, increasing the deposition voltage can increase the deposition rate of TiN films.
③ The effect of the workpiece on the performance of titanium nitride films. The quality of the film’s performance on the workpiece is not only related to the properties of the film itself but also to the properties of the workpiece material, especially the hardness of the workpiece material. Hard films exhibit their superior wear resistance only on relatively hard substrate materials. If the hardness of the substrate material varies, the bonding strength between the TiN film and the substrate will also differ. The greater the substrate hardness, the better the bonding between the TiN film and the substrate. In practical applications, efforts should be made to maintain a high hardness of the substrate material under certain deposition temperature conditions to improve the quality of the film. The smaller the surface roughness of the substrate, the higher the bonding strength between the titanium nitride film and the substrate, with polishing being the best for substrate surface roughness.
Section VI Enamel Craftsmanship
Enamel is a beautiful and colorful craft art made by attaching various colored glazes to a metal base (using materials such as gold, silver, copper, titanium, etc.) and firing it at high temperatures. Enamel colors are vibrant, have a gem-like luster and texture, resist corrosion, wear, and high temperatures, are waterproof and moisture-proof, are hard and solid, and do not age or deteriorate. Enamel has a long history of application in craft jewelry; in ancient centuries, it was used in the design of various decorative items for jewelry boxes, and by the 15th century, watchmakers incorporated it into watchmaking, adding bright colors to watches. The meticulous firing methods of enamel are intriguing, and its rich color expressiveness has pushed it to the forefront of fashion trends. Enamel jewelry wins with color and often attracts the attention of jewelry designers, especially when enamel is used for surface decoration on metal jewelry, greatly enhancing the artistic expressiveness of the pieces.
Enamel is commonly known as “Shaoqing” in southern China, “Shaolan” in northern China, and “shippō-yaki” in Japan.
The processing methods of jewelry enamel are divided into three types: painted enamel, champlevé enamel, and cloisonné enamel.
(1) Painted Enamel
The enamel painting craft originated in Western Europe and France and was brought to Guangdong by European merchants and missionaries during the Kangxi period of the Qing Dynasty. The art of painted enamel was first manufactured in Guangdong, where it is commonly referred to as “Shaoqing,” “Guang enamel,” or “Foreign enamel.” The emperor and ministers quickly appreciated and valued this exceptionally exquisite craft upon its arrival in China. During the Qing Dynasty, Emperors Kangxi, Yongzheng, and Qianlong established enamel workshops in both the Beijing Imperial Palace and Guangdong, and they frequently selected outstanding enamel painters from Guangdong to serve in the capital, producing large quantities of enamelware and enamel jewelry for royal use.
Painted enamel is the most difficult of the three main types of enamel techniques. The biggest advantage of painting directly on metal is that the patterns and lines can be richer, allowing for more detailed and complex designs that are unrestrained. However, omitting the step of using metal wire to outline will greatly increase the difficulty of firing the enamel. First, there is the issue of color mixing between different colors; without metal wire to separate the different colors, if the enamel materials are not mixed properly, it can lead to a chaotic situation of color bleeding.
Making painted enamel involves applying several layers of white glaze, or porcelain white, onto a metal base. Porcelain white is a white solid that must be ground into powder and mixed with appropriate water. A small brush can be applied evenly on the metal base. The firing temperature for porcelain is generally between 720~820℃. After sintering, a white base plate is obtained, and various colors of enamel are painted on this base plate, intricately depicting patterns like in oil painting. The biggest issue is color mixing; if the colors are mixed too much, the patterns will become blurred after sintering, ruining the artwork. Therefore, after depicting a section, it must be sintered first. The next section can be painted, followed by another sintering, which must be repeated until the work is completed. Sometimes, it requires dozens of repetitions of sintering, and if it gets damaged at any point, the entire piece will be ruined, making the craft of painted enamel highly valuable.

(2) Cloisonné Enamel
The cloisonné enamel technique is relatively complex, yet it is the most familiar and proudest enamel craft for the Chinese, with the famous “Jingtai Blue” being a type of cloisonné enamel. Its characteristic is that metal wires are first used on a metal base to outline the pattern, fixed with a natural adhesive, and then welded onto the metal base. Different colors of enamel glaze are then filled into the outlined contours made from finely ground minerals and metal oxides. The metal pieces are placed in a special furnace at a temperature of 800℃ for firing, causing the glaze to change color due to the high temperature. Since different metal oxides require different temperatures to change color, the enamel pieces may undergo multiple firings. Finally, the metal wires are smoothed, and a colorless transparent protective agent is applied for protection.

(3) Champlevé Enamel
The technique is similar to cloisonné enamel. The surface patterns of metal ornaments are formed using techniques such as engraving, stamping, or etching. First, patterns are carved on the metal base, leaving grooves within the carved outlines. To create vivid patterns with lines of varying thickness, the enamel painter grinds the enamel paint into a powder, mixes it with a small amount of water, and then fills the grooves with enamel, brushing a thin layer of painted patterns. Once completed, it is left to air dry and then placed in a high-temperature kiln above for firing, with each layer of paint needing to be fired for 40~60 seconds in the high-temperature kiln of 800℃. After multiple repetitions of filling with enamel and firing and polishing, a smooth and lustrous work is ultimately formed.

Section VII Resin Casting Process
The resin casting process is widely used in popular jewelry. According to the nature of the resin, it is divided into two main categories: soft resin and hard resin. Soft resin has a lower hardness and is not suitable for grinding and polishing but can be used for surface coating of craft jewelry; hard resin has a high hardness and can be processed by grinding and polishing to achieve a smooth and shiny effect.
Generally, epoxy resin crystal drop glue is used, which is composed of high-purity epoxy resin, curing agents, and other substances. Its cured product has the characteristics of being water-resistant, chemically resistant, and crystal clear. In addition to providing good protection for the surface of craft ornaments, this crystal drop glue can also enhance the surface gloss and brightness. Adding various pigments to the glue can form a rich color series, further enhancing the surface decorative effect, making it suitable for surface decoration and protection of crafts made from materials such as metal, ceramics, glass, and acrylic.
Epoxy resin has a surface effect similar to enamel and is often used as a faux enamel product, sometimes referred to as “soft enamel.” However, there are significant differences between the two. Enamel is made from enamel pigments fired at high temperatures; it is very hard and stable, has good durability, and does not age or deteriorate, but is prone to metal oxidation and softening, making it unsuitable to use for ornaments inlaid with gemstones; resin belongs to the category of organic compounds made from plastic. Products of this type of plastic resin do not need to be sintered in a high-temperature furnace to achieve color. It is sufficient to apply liquid color resin on the metal and let it air dry naturally or dry it in a baking box, making the production simple and not harmful to the metal, suitable for the final treatment of inlaid gemstone jewelry. However, due to the material properties of the resin, it is not durable, not heat-resistant, and is prone to corrosion and wear. After a certain period, it is likely to show aging, fading, and loss of luster. It is of lower grade and does not retain value. The basic process of resin is as follows.
- First, prepare the necessary tools and equipment, such as weighing instruments, glue adjusting tools, workpiece carriers, drying equipment, and the workpieces for the dripping glue operation.
- Place or adjust the balance scale (electronic scale), oven, workpiece carrier, and workbench level.
- Weigh A glue using a dry and clean wide-mouth flat container while proportionally weighing B glue (generally at a weight ratio of 3:1)
- Use a round glass rod (or round wooden stick) to stir the AB mixture to the left, right, or in a clockwise or counterclockwise direction. At the same time, the container (vessel) is preferably tilted at an angle of 45° and continuously rotated, stirring for about 1~2 minutes.
- Pour the mixed AB glue into a soft plastic bottle with a pointed nozzle for dripping, and drop the glue onto the desired accessory position.
- When the area of the resin drop is slightly larger, or the amount of resin is relatively more, to accelerate the elimination of bubbles in the glue, a gas torch fueled by liquefied gas can be used to promote defoaming. During defoaming, the torch’s flame should be adjusted to a complete combustion state, and the flame should ideally be kept about 25 cm away from the surface of the workpiece. The movement speed of the torch should not be too fast or too slow; a moderate speed should be maintained.
- After the bubbles are eliminated, the workpiece can be placed horizontally in the oven for heating and curing. The temperature should first be adjusted to 40℃ around 30 minutes and then raised to 60~70℃ until the glue is fully cured.

Section VIII Etching Technology
Etching is the process of removing unwanted metal to a certain depth using chemical methods. Its principle is similar to the production of circuit boards, where the metal parts to be retained are covered with an acid-resistant coating, and then an acidic solution is used to etch away the unwanted metal parts; the processing of negative patterns is exactly the opposite of that of positive patterns.
The etching process is suitable for the processing and producing antique-style crafts and jewelry with special surface style requirements. In recent years, the export volume of metal paintings, crafts, and hollow art pieces processed by etching methods in our country has greatly increased, becoming a new branch of craft jewelry.
Etching processes can be divided into chemical etching, electrolytic etching, laser etching, and ultrasonic etching.
1. Chemical Etching Process
Cover the areas that need not be etched with a corrosion-resistant coating, then immerse the workpiece in a special etching solution. The formula for the solution is shown in the table 11-2. Ensure that the areas to be etched are in full contact with the solution, and if necessary, heating and stirring can be applied. Control the etching time based on the required etching depth and the etching rate obtained from experiments to achieve the desired etching result.
Table 11-2 Partial Metal Etching Solution Formulas
| Ingredients and process conditions | Etching stainless steel | Etching copper alloy | Etching aluminum |
|---|---|---|---|
| Ferric chloride | 600 ~ 800 | 600 ~ 650 | 450 ~ 550 |
| Hydrochloric acid/(g・L -1) | 80 ~ 120 | ||
| Phosphoric acid/(g・L -1) | 20 ~ 30 | ||
| Copper sulfate/(g・L -1) | 200 ~ 300 | ||
| Sulfuric acid/(g・L -1) | 90 ~ 100 | 10 ~ 20 | |
| Nitric acid/(g・L -1) | 8 ~ 12 | ||
| Etching accelerator/(g・L -1) | 80 ~ 100 | ||
| Temperature/℃ | 10 ~ 45 | 15 ~ 50 | 20 ~ 40 |
| Time/min | 15 ~ 20 | 10 ~ 15 | 10 ~ 20 |
2. Electrolytic Etching
Section IX Nano ~ Spraying Plating Technology

1. The Principle of Nano ~ Spraying and Plating
2. Characteristics of Nano ~ Spraying Plating
(1) Green and environmentally friendly. No three waste emissions, non ~ toxic and harmless, no harmful heavy metals.
(2) Low investment and low cost. Compared to electroplating, the equipment investment is small, and there is no need to equip a wastewater treatment system.
(3) The operation is both safe and simple, with high efficiency. There will be no harm if the operator accidentally sprays it on their hands or other body parts. There is no need for water electroplating treatment and complex preliminary conductive layer treatment.
(4) It can be positioned for spray plating and can spray plate in different colors on the same product. Various colors at will.
(5) The processed parts are not limited by size and shape, are not restricted by various materials, can be recycled, and save resources.
(6) The application range of color diversity is extensive. The sprayed products can be coated with gold, silver, chrome, nickel, or sand ~ colored mirror effects. They can also be coated with various other colors, such as golden yellow, brass color, antique gold yellow, cannon copper color (red, yellow, purple, green, blue), and other high ~ gloss effects. It can be sprayed on various materials such as metal, resin, plastic, glass, ceramics, acrylic, and wood, and can be widely used in various surface decoration industries such as crafts and jewelry.
(7) Products made with nano ~ spraying have excellent adhesion, impact resistance, corrosion resistance, weather resistance, wear resistance, scratch resistance, and good rust prevention capabilities.