What Makes Ceramic Jewelry Unique: Materials, Design, and Craftsmanship
Discover the Charm of Ceramic Jewelry: Innovative Design & Techniques
Ceramic ornaments refer to decorative items made from various ceramic materials or combined with metals and other materials that adorn the human body and its related environment (including jewelry, ornaments, etc.).
Ceramics, as a unique artistic medium, have their material characteristics and historical cultural connotations. From a material perspective, ceramics possess a gentle texture, variable glazes, rich textures, and the element of chance in their production. This endows ceramic art with a charm that cannot be achieved with other materials. At the same time, ceramic materials have superior properties such as high hardness, wear resistance, acid resistance, alkali resistance, cold resistance, and heat resistance, making them unmatched in modern decoration. Through modern materials science and technology, nanoceramic technology can change the fatal weakness of ceramics, which is being brittle, transforming them into a new material for jewelry high in strength and toughness, providing more possibilities for jewelry design.
Ceramic jewelry are a new type of accessory with a novel and unique style. They may stand out with unusual shapes, excel in glaze colors, or showcase new forms in decoration, creating an artistic image rich in meaning and elegance.

Chanel 18K diamond-set precision ceramic ring
Table of Contents
Section I Introduction to Ceramic Materials
1. The Concept of Ceramics
Ceramics is a general term for a type of material and its indispensable products in human life and production. It has undergone thousands of years of development in human history. Traditionally, ceramics refers to various products made from clay as the main raw material mixed with other natural mineral raw materials through crushing and mixing→shaping→ sintering. Common daily-use ceramic products, architectural ceramics, and electrical porcelain belong to traditional ceramics. Since its main raw materials are silicate minerals (such as clay, feldspar, quartz, etc.) sourced from nature, it can be classified as silicate materials and products. The traditional ceramic industry can be categorized alongside glass, cement, enamel, and refractory materials under the “silicate industry.”
With the development of modern science and technology, it is necessary to fully use ceramic materials’ physical and chemical properties. In the past century, many new types of ceramics have emerged, such as oxide, piezoelectric, and various high-temperature and functional ceramics. Although their production process is still fundamentally based on the traditional ceramic production methods of raw material processing→forming→sintering, the raw materials used are no longer traditional ceramic materials like clay or are used very little. It has expanded to chemical raw materials and synthetic minerals, even non-silicate and non-oxide raw materials, and the composition range has also extended to the scope of inorganic non-metallic materials, with many new processes emerging. Therefore, the broad concept of ceramics is a general term for inorganic non-metallic solid materials and products manufactured using ceramic production methods, and the term ceramics used internationally does not have a unified boundary in various countries.
2. Classification of Ceramics
There are many types of ceramics and various classification methods. Based on their concept and use, ceramics can be divided into two main categories: ordinary ceramics and special ceramics.
Ordinary ceramics refer to traditional ceramics within the concept of ceramics. This category of ceramic products is the most common and widely used in people’s daily lives and production. Depending on their application areas, they can be divided into daily-use ceramics (including artistic display ceramics), architectural sanitary ceramics, chemical ceramics, porcelain, electrical ceramics, and other industrial ceramics. Daily-use ceramics are the oldest and most commonly used traditional ceramics among various ceramic products. These ceramic products have the broadest practicality and aesthetic appeal and are also the result of the organic combination of ceramic science and technology with arts and crafts. Decorative ceramics also belong to this category of products. Decorative ceramics can be defined as products made from aluminum silicate minerals or certain oxides as the main raw materials, designed according to specific styles and produced through particular chemical processes at high temperatures and under certain atmospheres (oxidation, carbonization, nitridation, etc.) to achieve the desired form, with a surface coated with various aesthetically pleasing glazes or specific decorations. Some porcelain also exhibits varying degrees of translucency. The body comprises one or more crystals, amorphous binders, pores, or microstructures relative to the clinker encapsulation.
Special ceramics refer to ceramic materials and products involved in the broad concept of ceramics beyond ordinary ceramics. Special ceramics are ceramic products required for various modern industries and cutting–edge scientific technologies and the raw materials and production processes are significantly different and more advanced than ordinary ceramics. In terms of performance, special ceramics possess different special properties and functions, such as high strength, high hardness, corrosion resistance, electrical conductivity, insulation, and special functions in magnetism, electricity, optics, acoustics, and bioengineering, which allows for their extensive application in high temperature, mechanical, electronic, aerospace, and medical engineering fields. In terms of composition, traditional ceramics are determined by the components of clay, so ceramics from different regions and kilns have different textures. Since the raw materials for special ceramics are pure compounds, their composition is determined by artificial ratios, and the quality of their properties is determined by the purity of the raw materials and the processes rather than by the place of origin. In terms of preparation processes, the limitations of traditional ceramics, which primarily rely on kilns for production, have been broken through with the widespread use of methods such as vacuum sintering, protective atmosphere sintering, hot pressing, and hot isostatic pressing. In terms of raw materials, the limitations of traditional ceramics, which primarily use clay as the main raw material, have been surpassed; special ceramics generally use oxides, nitrides, silicides, borides, carbides, and other materials as the main raw materials.
3. Composition of Ceramic Materials
Ceramic materials belong to inorganic non-metallic materials, mostly oxides containing silicon and other elements. Their raw material composition mainly consists of four parts: raw materials for the ceramic body, raw materials for the glaze, coloring materials for decoration, and raw material additives.
(1) Raw Materials for the Ceramic Body
Generally, they are natural mineral raw materials, which can be divided into clay-based materials, siliceous materials, calcium-magnesium mineral raw materials, and other porcelain sand materials based on their physical and chemical properties.
Clay-based raw materials in ceramic production formulas stem from their plasticity. They combine with porcelain sand-based raw materials to strengthen the products, ensuring they remain undamaged during transportation and decoration on the production line. They account for nearly 10%~40% of all the composition. Porcelain sand-based raw materials mainly come from mines and are the primary component of ceramic raw materials, generally accounting for nearly 50%~90%. The types and typical minerals are as follows: when clay and porcelain sand are combined, ground to a certain fineness, and fired at an appropriate temperature, they form ceramic bodies with various water absorption rates, shrinkage rates, and different physical and chemical properties.
(2) Raw Materials for the Glaze
Most of them are standardized raw materials formed by deep processing and full synthesis of some natural minerals and some chemical raw materials, such as quartz, kaolin, alumina, manganese dioxide, and ferric oxide. Synthetic frit materials have also appeared with the emergence of low-temperature fast-firing technology in modern ceramics. Their different combinations can create glazes with varying textures and extremely rich effects, using them to cover the ceramic body’s surface, resulting in many artistic decorative effects.
(3) Pigments
Pigment is the coloring agent applied to body glaze, which is generally added directly to the body material and glaze during use. Common coloring agents in ceramics include ferric oxide, copper oxide, cobalt oxide, manganese oxide, and titanium dioxide, which present red, green, blue, purple, and yellow.
(4) Additive
Some additives used in ceramic production can be called the “salt and monosodium glutamate” of the ceramic industry, as they can significantly improve many properties in producing ceramic body glaze materials. For example, using a small amount of sodium tripolyphosphate under low moisture conditions can help the slurry achieve good dilution. Additives can be systematically classified according to their functions into deflocculants, wetting agents, preservatives, etc.
4. The Properties of Ceramic Materials
The properties of ceramic materials include physical properties, chemical properties, mechanical properties, thermal properties, electrical properties, magnetic properties, and optical properties, among others. This section focuses on analyzing and explaining the general performance characteristics of ceramic materials.
(1) Physical Properties
① Thermal properties. The thermal properties of ceramic materials refer to aspects such as their melting point, heat capacity, thermal expansion, and thermal conductivity.
The melting point of ceramic materials is generally higher than that of metals, with some reaching above 3000℃. They have superior high-temperature strength compared to metals, making them commonly used high-temperature resistant materials in engineering.
Ceramics’ coefficient of linear expansion is relatively small, much lower than that of metals; their thermal conductivity mainly relies on the thermal vibrations of atoms. The thermal conductivity of different ceramic materials varies; some are good insulating materials, while others are good thermal conductive materials, such as boron nitride and silicon carbide ceramics.
Thermal stability refers to a material’s ability to resist damage when subjected to rapid temperature changes. Materials with a large thermal expansion coefficient, poor thermal conductivity, and low toughness have low thermal stability. Most ceramics have poor thermal conductivity and low toughness, thus exhibiting poor thermal stability. However, some ceramics, such as silicon carbide, have high thermal stability.
② Conductivity. Most ceramics have good insulating properties, but some, such as piezoelectric and superconducting, have certain conductivity.
③ Optical properties. Ceramics are generally opaque, but with the development of technology, new types of ceramics have been developed, such as materials for solid laser devices, optical fiber materials, and optical storage materials.
(2) Chemical Properties
The structure of ceramics is very stable, and under normal circumstances, it is unlikely to react with oxygen in the medium. It does not oxidize at room temperature; even above 1000℃, it will not oxidize. It also has strong resistance to corrosion from acids, bases, and salts and can resist the erosion of molten metals (such as aluminum, copper, etc.).
(3) Mechanical Properties
The elastic modulus of ceramics is generally high, making them very difficult to deform. Some advanced ceramics have good elasticity and can be made into ceramic springs. Ceramics have a very high hardness, with the hardness of most ceramics far exceeding that of metals. Ceramics have good wear resistance, making them a good material for manufacturing various easily damaged components with special requirements. The tensile strength of ceramics is low, but the flexural strength is relatively high, and the compressive strength is even higher, generally an order of magnitude greater than the tensile strength.
Ceramic materials have high hardness and high elastic modulus because of the structure of their internal ionic crystals. Ceramic materials are mostly ionic crystals formed by ionic bonds; covalent bonds also form covalent crystals. In these crystal structures, the bond energy is high, and the combination of positive and negative ions is strong, resisting elastic deformation under external forces and exhibiting strong capabilities for scratching and indentation, thus showing characteristics of high elastic modulus and hardness. In addition, this type of crystal structure has obvious directionality, so the slip systems in polycrystalline ceramics are very few, and they hardly produce plastic deformation under external forces, often resulting in brittle fracture, which is the fatal drawback of ceramics as engineering materials. Due to the brittleness of ceramics, their impact resistance is very low, and their fatigue resistance is also poor.
With the advancement of materials science and technology, precision ceramic materials with superplasticity have been studied in recent years, which can achieve a strain of about 300% before fracture. As shown in Figure 7-1, the ceramic plate is 3 m long, 1 m wide, and only a thickness of 3 mm, can be bent along the length direction. Common precision ceramic materials include alumina and zirconia; their properties are shown in Table 7-1.

Table 7-1 Performance of Precision Ceramics
| Physical properties | Alumina ceramic | Zirconia ceramic |
|---|---|---|
| Quality fraction/% | Aluminum oxide>99. 8% | Zirconia>97% |
| Density /(g · cm-3) | 3.93 | 6.05 |
| Hardness HV | 2300 | 1300 |
| Compressive strength /MPa | 4500 | 2000 |
| Bending strength /MPa | 595 | 1000 |
| Young's modulus/GPa | 400 | 150 |
| Fracture toughness K/(MPa · m½) | 5〜6 | 15 |
Section II Ceramic Jewelry
1. Overview of the Development of Ceramic Jewelry
The famous French porcelain artist Bernardaud proposed the concept of “ceramic jewelry.” Faced with difficulties in his ceramic shop and a decline in porcelain sales, he suggested expanding the variety of porcelain products by creating ceramic jewelry. The initial ceramic jewelry was ceramic rings, designed simply and elegantly. These caused a great sensation upon their launch in France and were favored by customers.
Professor Klaus Dembrowski from Germany is the world’s first ceramic jewelry designer. Since 1972, he has been engaged in the research and design of ceramic jewelry at the institution where he teaches, and his works have won multiple national and international awards. Other famous ceramic jewelry designers from Germany include Pierre Cardin and Barbara Gotthoff.
Ceramic jewelry have a history of several decades since they were first introduced. During this time, European countries have seen a significant emergence of ceramic jewelry, with varying degrees of development in countries like France and Germany; Asian countries such as South Korea and Japan have also introduced many new styles of ceramic jewelry, which are elegant and cute and are very popular among consumers, making them a great choice for gifts.
With the emergence of high-toughness zirconia precision ceramic materials, using ceramic materials in jewelry design has become one of the hottest trends in recent years. Many jewelry brands have launched ceramic jewelry, with the most representative being the black and white ceramic wedding rings in Chanel’s Ultra collection; Italy’s Damiani has also combined white and black ceramics with gold and diamonds to create a new fashion trend; additionally, Cartier’s black and white diamond ceramic bracelets and rings, as well as Bulgari’s rose gold three-ring black and white ceramic ring, are also leading the new trend in ceramic jewelry. Major brands are creating high-end jewelry by mixing uniquely creative high-precision ceramics with other metal powders, which possess a quality as precious as that of precious metals, and their distinctive designs can bring a more modern and avant-garde atmosphere to traditional jewelry.

Figure 7-2 Chanel 18K diamond-set precision ceramic ring

Figure 7-3 Damiani precision ceramics
2. Characteristics of Ceramic Jewelry
The materials used in ceramic jewelry are sourced from the earth and stones of nature, which possess many natural characteristics. Due to the close relationship between humans and nature, the earth and stones from nature hold a special significance for people. Ceramic materials have excellent properties such as high hardness, wear resistance, acid resistance, alkali resistance, cold resistance, and heat resistance, and they are low in harm, environmentally friendly, energy-saving, and healthy. The trace elements contained in the raw materials are beneficial to human health. Research has confirmed that ceramics have health benefits, such as improving metabolism and promoting blood circulation. Ceramics can emit beneficial infrared rays on humans at room temperature, and the infrared rays it emits match the wavelength of the infrared rays emitted by the human body. Thus, a resonance phenomenon can occur when ceramics are close to the human body. In addition, due to changes in people’s aesthetic concepts, the traditional value of preservation of jewelry has been abandoned, and ceramic jewelry have placed more emphasis on decoration, becoming a new type of “green jewelry.”
Ceramic jewelry are flowing and dynamic, with dazzling and vibrant colors, rich hues, unique shapes, and wonderful artistic concepts. Worn on fingers, ears, wrists, or necks, they possess a cold and elegant beauty akin to gemstones and jade, surpassing the artistic effects of amber and agate. The colorful and vivid glazes, with their jade-like warmth, icy textures, and sparkling crystalline luster, evoke a sense of the enchanting beauty of the glazes, fully showcasing a charm akin to ice and fire. This is irreplaceable by jewelry made from other materials, as they expand the aesthetic vision of jewelry design and meet the diverse aesthetic needs of individuals with different personalities for modern accessories.
The production process of ceramic jewelry is simple and low-cost, making them truly affordable and of good quality. This is beneficial for the popularization of jewelry.
3. Categories of Ceramic Jewelry
The categories of ceramic jewelry are rich and diverse; common jewelry include:
(1) Ceramic rings. There are many types, including plain ceramic rings with ceramic as the band and rings with metal bands inlaid with ceramic.
(2) Ceramic bracelets. A typical example is the blue and white porcelain bracelet, which is decorated with natural cobalt materials painted on white clay, then covered with a transparent glaze, and fired at high temperatures in one go, allowing the color to fully penetrate the glaze, presenting a vibrant blue pattern that appears elegant and pure. Another type is the ice crack glaze ceramic bracelet. In ceramics, if the ceramic body and glaze are not properly designed in formulation and firing, a significant difference in their expansion coefficients can often cause cracks in the glaze surface. However, intentionally creating cracks in the glaze surface has charm, known in ceramics as crackle glaze. “Ice crack glaze” differs from crackle glaze in that the former has a multi-layered three-dimensional structure of cracks, resembling the layers of rose petals, combined with variations in glaze color, resulting in a very good artistic effect. In contrast, the latter has a single-layer crack.
(3) Ceramic necklace.
(4) Ceramic pendant.
(5) Ceramic earrings.
(6) Ceramic watch.
(7) Ceramic hairpin.
The typical examples of the above ceramic jewelry are as follows.

Ceramic Plain Ring

Metal and Ceramic Ring

Ceramic Bracelet

Crackle Glaze Ceramic Bracelet

Ceramic Necklace

18K Diamond Necklace with Ceramic
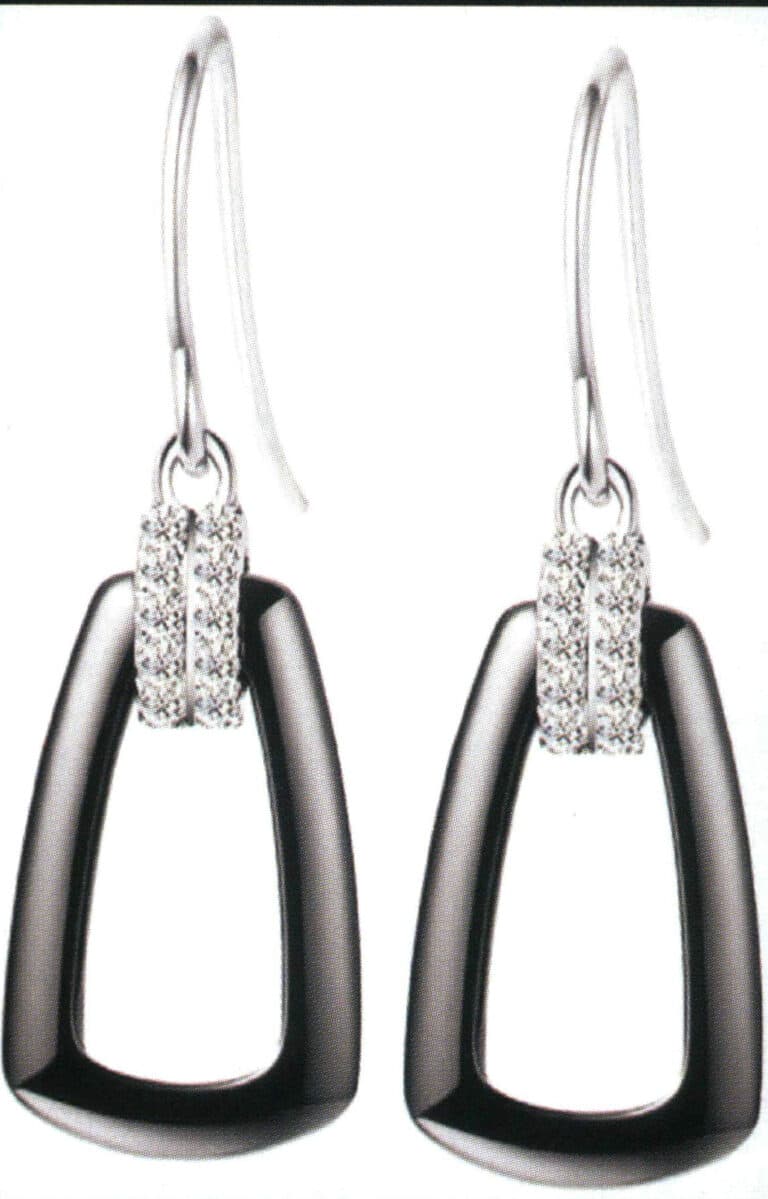
Ceramic Earrings

Ceramic Pendant

Ceramic Watch

Ceramic Hairpin
Copywrite @ Sobling.Jewelry — Custom jewelry manufacturer, OEM and ODM jewelry factory
Section III Production Process of Ceramic Jewelry
The main components of ceramic raw materials are silicon and aluminum. The composition of ceramics is not fundamentally different from that of rocks; the only difference is between natural and artificial. Ceramic jewelry are mostly sintered ceramics because they cannot flow molten liquid into molds like metals and plastics. They are made using the hot pressing method due to their inherent plastic deformation properties, so they are produced by powder forming followed by sintering. The production of ceramic jewelry can be divided into four main processes: raw material processing, clay body shaping, glazing, and sintering, which are clay preparation, shaping, glazing, and sintering, as shown in Figure 7-4.

1. Clay Preparation
There is a saying in the ceramics industry: “Raw materials are the foundation; firing is the key. ” This saying reflects the importance of processing raw and ceramic body materials in ceramic production. To achieve stable quality in ceramic jewelry, raw mineral materials with stable and reliable components and performance in powder preparation are necessary. After extracting porcelain stone and kaolin, they are processed through crushing, washing, and other procedures to remove coarse impurities from the raw materials, forming block materials, which are then refined, processed, and formulated into suitable body and glaze materials for various porcelain uses.
The purpose of clay preparation is, on the one hand, to remove impurities and, on the other hand, to combine clays from different sources with varying forming and firing properties into a mature clay that meets the needs of the maker, with a certain range of firing temperatures that can correspond with the glaze and firing temperature. Sometimes, sand is appropriately mixed in to enhance the support strength of the clay under high-temperature firing conditions and prevent the body from collapsing. Sometimes, in pursuit of the color of the fired clay, some coloring materials are added to create a “colored body.” The chemical composition of earthenware clay and porcelain clay is the same. Still, due to weathering and re-weathering, their physical properties have changed, resulting in earthenware clay having greater viscosity and plasticity. In contrast, porcelain clay is characterized by brittleness and a higher degree of vitrification at high temperatures.
2. Shaping
After the ceramic raw materials are prepared, the forming stage begins. Forming is adding plasticizers and other materials to the ceramic powder to create a paste, which is then further processed into semi-finished products of specific shapes and sizes. The purpose of shaping is to achieve a uniform and high-density body, and improving forming techniques is a key step in enhancing the reliability of ceramic products. There are various forming methods for ceramic jewelry, which need to be selected according to the characteristics of the product.
A single personalized piece of jewelry can be directly shaped by hand. First, both hands knead the clay repeatedly, eliminating the air bubbles inside and making the clay more “mature.” Use the hand-sculpting method to shape the jewelry to the required dimensions. A wheel-throwing method can also be used for larger ceramic jewelry or figurines. After the ceramic body is shaped, it must be refined, smoothed with wet hands, and stamped. The purpose is to prevent the surface from cracking too early due to drying, to make the surface smooth, and to fill in and level out any uneven areas of the clay body.
Currently, most ceramic jewelry are produced in batches, generally requiring forming equipment and molds to improve production efficiency and achieve stable and consistent product quality.
(1) Compression Shaping
It is a method of adding organic binders to the powder material, filling the mixed plastic into a metal mold, and forming a shaped body with a certain strength after applying pressure. Its advantages are low cost and small dimensional errors of the shaped body. The pressure is within the range of 200~2000kgf/cm2(1kgf/cm2=98.0665kPam).
(2) Isostatic Pressing
It is a method for forming uniform powder shapes. Because it uses a rubber bag (mold), it is also called the rubber bag forming method. This method involves placing the powder into a rubber bag and then placing the rubber bag filled with powder into a hydrostatic chamber for shaping. The pressure in the hydrostatic chamber is evenly applied to the powder, thus achieving a well-formed body.
(3) Extrusion Shaping
It is a method of extruding the mixed plastic raw material from the mold hole, with the forming ceramic body material enters the cap from the supply hole in the cap, expands into a thin wall after subdivision, and then combines, thus obtaining a quality with good extensibility and cohesiveness. In extrusion forming, the choice of binder should optimize both the flowability and self-adhesion of the ceramic body material.
(4) Grouting Shaping
It is made from a slurry with fluidity created using water and other materials, which is injected into a porous gypsum mold. Water seeps into the gypsum mold through the contact surface, forming a hard layer on the surface. This forming method produces a shape on the inner surface of the gypsum mold that is the same as the shape of the formed body. It is further divided into double-sided slurry feeding method (solid injection method) and single-sided slurry feeding method (hollow injection method). The key tool for slurry forming is a special gypsum mold or a porous model made of other materials. When using a gypsum mold, in addition to reinforcing the mold wall with steel bars to withstand the pressure of the mold head, it is also necessary to appropriately distribute smaller-diameter porous hoses within the mold wall. These small tubes can quickly and evenly drain water during pressurized forming and blow air in to assist with demolding. When using a metal mold head, lubricants or heating methods can be employed to prevent sticking. When using a gypsum mold head, air is blown into the mold during demolding, causing the ceramic body to adhere to the mold head and separate from the mold. Finally, air is blown into the mold head again to detach the body from the mold head. The ceramic body from slurry forming does not need to dry with the mold, resulting in higher production efficiency compared to the production of plastic-molded irregular products, and the quality of the ceramic body is good, making it a promising new process.
(5) Hot Press Shaping
It is a method of forming by adding plastic to the powder and using the same method as resin molding. Although this method is suitable for forming complex parts, if the amount of adhesive exceeds 15%~25%, debinding becomes difficult. Currently, this method is not suitable for large, thick-walled products.
3. Drying
The drying of ceramics is one of the very important processes in ceramics production technology, and improper drying causes a large part of the quality defects in ceramic products. Drying is a relatively simple technical process, but it is widely applied; it not only affects the product quality and yield of ceramics but also affects the overall energy consumption of ceramic enterprises. The basic requirements for drying technology include fast drying speed, energy saving, high quality, and no pollution.
(1) Mechanism of the Ceramic Drying Process
The moisture content of ceramic bodies generally ranges between 5%~25%. The forms of binding between the ceramic body and moisture, the changes in the material during the drying process, and the factors affecting the drying rate are the theoretical basis for analyzing and improving dryers. When the ceramic body comes into contact with still air at a certain temperature and humidity, it will inevitably release or absorb moisture, causing the moisture content of the ceramic body to reach a certain equilibrium value. As long as the state of the air remains unchanged, the moisture content achieved in the ceramic body will no longer change with an increase in contact time; this value is the equilibrium moisture content of the ceramic body under that air state. The moisture lost by the wet ceramic body upon reaching equilibrium is called free moisture. In other words, the ceramic body’s moisture content consists of equilibrium and free moisture. Under certain air conditions, the limit of drying is to bring the ceramic body to its equilibrium moisture content.
The moisture contained in the body can be divided into physical and chemical water. The drying process only involves physical water, further divided into bound and unbound water. Unbound water exists in the large capillaries of the body and is loosely combined with the body. The evaporation of unbound water in the body is similar to that of water on a free liquid surface, where the partial pressure of water vapor on the body’s surface equals the saturated vapor pressure at its surface temperature. When unbound water is expelled from the body, the particles of the material come closer together, resulting in volume shrinkage; therefore, unbound water is also referred to as shrinkage water. Bound water is the water present in the micro-capillaries of the body (with a diameter less than 0.1㎛) and on the surface of colloidal particles, which is more firmly combined with the body (due to physicochemical interactions). Therefore, when bound water is expelled, the partial pressure of water vapor on the body’s surface will be less than the saturated vapor pressure at the body’s surface temperature. During the drying process, when the partial pressure of water vapor on the body’s surface equals the partial pressure of water vapor in the surrounding drying medium, the drying process stops, and moisture cannot continue to be expelled. At this point, the moisture contained in the body is called equilibrium water, which is a part of bound water, and its amount depends on the temperature and relative humidity of the drying medium. When bound water is expelled, the volume of the body does not shrink, making it relatively safe.
(2) The Drying Process of the Ceramic Body
Taking the convective drying process as an example, the drying process of the ceramic body can be divided into three simultaneous and interrelated processes: heat transfer, external diffusion, and internal diffusion.
Heat transfer process: The heat of the drying medium is transferred to the surface of the workpiece by convection and then conducted from the surface to the interior of the workpiece. The moisture on the surface of the workpiece receives heat and vaporizes, changing from liquid to gas.
External diffusion process: The water vapor generated on the surface of the ceramic body moves from the surface to the drying medium by diffusion through the laminar flow layer under the influence of a concentration difference.
Internal diffusion process: Due to the evaporation of moisture from the surface of the wet body, a humidity gradient is created inside, promoting the diffusion of moisture from the inner layer with a higher concentration to the outer layer with a lower concentration, referred to as moisture conduction or moisture diffusion.
Under stable drying conditions, the surface temperature of the ceramic body, moisture content, drying rate, and time have a certain relationship. Based on the changing characteristics of their relationships, the drying process can be divided into three stages: heating stage, constant rate drying stage, and falling rate drying stage.
During the heating stage, since the heat transferred from the drying medium to the surface of the body in a unit of time is greater than the heat consumed by the evaporation of surface moisture, the temperature of the heated surface gradually rises until it equals the wet bulb temperature of the drying medium. At this point, the heat gained by the surface and the heat consumed by evaporation reach a dynamic equilibrium, and the temperature remains constant. In this stage, the body’s moisture content decreases, and the drying rate increases.
The constant rate drying stage continues to expel non-combined water. Since the moisture content of the body is relatively high, the amount of water evaporated from the surface can be replenished from the inside, meaning that the internal moisture movement speed (internal diffusion rate) equals the surface moisture evaporation rate, which also equals the external diffusion rate, thus keeping the surface in a moist state. Additionally, the heat transferred from the medium to the body’s surface equals the heat required for the vaporization of the moisture. Hence, the body’s surface temperature remains constant, equal to the wet bulb temperature of the medium. The water vapor partial pressure at the body’s surface equals the saturated water vapor partial pressure at the surface temperature, and the drying rate is stable. Hence, it is called the constant rate drying stage. This stage is focused on expelling non-combined water, so the body will experience volume shrinkage, with the amount of shrinkage being linearly related to the reduction in moisture content. If not operated properly, drying too quickly can easily deform and crack the body, resulting in dry waste. At the end of the constant drying phase, the moisture content of the material drops to a critical value. At this point, although the internal moisture of the material is still free water, bound water begins to appear in the surface layer.
In the falling rate drying stage, the body’s moisture content decreases, and the internal diffusion rate cannot keep up with the evaporation rate of surface moisture and the external diffusion rate. The surface is no longer moist, and the drying rate gradually decreases. As the heat required for surface moisture evaporation decreases, the temperature of the material begins to rise gradually. The vapor pressure of water vapor on the material’s surface is less than the saturated vapor pressure of water vapor at the surface temperature. This stage expels bound water; the body does not undergo volume shrinkage and will not produce drying waste. When the moisture expelled from the material equals the equilibrium moisture, the drying rate becomes zero, and the drying process terminates. Even if the drying time is extended, the moisture of the material will no longer change. At this time, the material’s surface temperature equals the medium’s dry bulb temperature, and the surface vapor pressure equals the vapor pressure of the medium. The drying speed in the slow drying stage depends on the internal diffusion rate; hence, it is also called the internal diffusion control stage. At this time, factors such as the material’s structure, shape, and size affect the drying rate.
(3) Factors Affecting Drying Rate
Factors affecting the drying rate include heat transfer and external and internal diffusion rates.
① Accelerate the heat transfer rate. To accelerate the heat transfer rate, the following three points should be achieved: first, increase the temperature of the drying medium, such as raising the temperature of the hot gas in the drying kiln, increasing the hot air furnace, etc., but the surface temperature of the ceramic body should not rise too quickly to avoid cracking; second, increase the heat transfer area: for example, change from single-sided drying to double-sided drying, stack the ceramic bodies in layers or reduce the number of layers, increasing the contact area with the hot gas; third, improve the convective heat transfer coefficient.
② Increase the external diffusion rate. When drying is in the constant rate drying stage, the external diffusion resistance becomes the main contradiction affecting the overall drying rate. Therefore, reducing external diffusion resistance and increasing external diffusion rate have the greatest impact on shortening the entire drying cycle. External diffusion resistance mainly occurs in the boundary layer, so the following three points should be addressed: first, increase the medium flow rate to reduce the boundary layer thickness, thereby improving the convective heat transfer coefficient; the convective mass transfer coefficient can also be increased to facilitate an increase in drying speed; second, reduce the water vapor concentration of the medium and increase the mass transfer area, which can also improve drying speed; third, increase the internal diffusion rate of moisture.
The internal diffusion rate of moisture is jointly influenced by moisture diffusion and thermal diffusion. Moisture diffusion is the movement of water caused by a humidity gradient within the material. In contrast, thermal diffusion is the movement caused by a temperature gradient within the material. To increase the internal diffusion rate, the following five points should be addressed: first, align the directions of thermal diffusion and moisture diffusion, that is, try to make the temperature at the center of the material higher than that at the surface, such as through far-infrared heating or microwave heating; second, when the directions of thermal diffusion and moisture diffusion are aligned, enhance heat transfer to increase the temperature gradient within the material; when the two are opposite, strengthening the temperature gradient, although it increases the resistance to thermal diffusion, can enhance heat transfer, raise the material temperature, and increase moisture diffusion, thus accelerating drying; third, reduce the thickness of the body, changing from single-sided drying to double-sided drying; fourth, lower the total pressure of the medium, which is beneficial for increasing the moisture diffusion coefficient, thereby increasing the moisture diffusion rate; fifth, consider other factors related to the properties and shape of the ceramic body.
(4) Classification of Drying Technologies
Drying can be divided into natural and artificial, depending on whether a drying system controls it. Since artificial drying is people controlling the drying process, it is also called forced drying.
It can be classified into four types according to different drying methods.
- Convective drying. Its characteristic is using gas as the drying medium, blowing at a certain speed over the surface of the workpiece to facilitate drying.
- Radiation drying. This method uses infrared, microwave, and other electromagnetic radiation energy to irradiate the dried body, allowing it to dry.
- Vacuum drying. This method involves drying the ceramic body under vacuum (negative pressure). The green body does not need to be heated, but pumping equipment is required to create a certain level of negative pressure, so the system needs to be sealed, making continuous production difficult.
- Combined drying. Its characteristic is the comprehensive use of two or more drying methods to leverage their respective strengths, complementing each other, which often results in an ideal drying effect.
Some drying methods can also be divided into batch dryers and continuous dryers based on whether the drying process is continuous. Continuous dryers can further be classified into co-current, counter-current, and mixed flow based on the direction of movement of the drying medium and the workpiece. They can also be categorized into chamber dryers, tunnel dryers, etc., based on their shape.
4. Sintering
After the ceramic jewelry are shaped and refined, they can be fired. The sintering temperature and material selection determine the ceramics’ characteristics.
(1) Sintering Mechanism
Sintering is the process of placing granular ceramic green bodies in a high-temperature furnace to densify them into a strong solid material. Sintering begins with eliminating voids between body material particles, allowing the corresponding adjacent particles to bond into dense bodies. However, the sintering process must meet two basic conditions: ① there should be a mechanism for mass transport; ② there must be an energy (thermal energy) to promote and sustain mass transport.
Currently, the sintering mechanisms of fine ceramics have four sintering modes: gas phase sintering, solid phase sintering, liquid phase sintering, and reactive liquid sintering. Their material structural mechanisms and sintering driving forces are different. The main sintering mechanisms are liquid phase and solid phase sintering, especially for traditional ceramics and most electronic ceramics, which rely on liquid phase formation, viscous flow, and dissolution-precipitation processes. In contrast, the sintering of high-purity, high-strength structural ceramics mainly relies on solid phase sintering, which achieves material migration through grain boundary diffusion or lattice diffusion.
(2) Kilns Used for Ceramic Sintering
Ceramic materials and products can be fired in various kilns, either intermittent or continuous kilns. The former is periodic and suitable for small batches or special firing methods. The latter is used for large-scale production and relatively low firing conditions. The most widely used kiln for ceramic jewelry is the electric furnace. The firing temperature and the required atmosphere determine the type of kiln to be selected. According to the classification of traditional ceramic firing temperatures, temperatures below 1100℃ are low-temperature sintering, between 1100~1250℃ are medium-temperature sintering, between 1250~1450℃ are high-temperature sintering, and above 1450℃ are ultra-high-temperature sintering.
(3) Main Sintering Techniques for Ceramics
There are several technical methods for ceramic sintering.
① Normal pressure sintering (also known as pressureless sintering). It refers to the free sintering of the ceramic body under atmospheric pressure conditions. Sintering begins without external forces when the temperature generally reaches the material’s melting point between 0.5~0.8. At this temperature, solid-phase sintering can cause sufficient atomic diffusion. In contrast, liquid-phase sintering can promote the formation of a liquid phase or generate a liquid phase through chemical reactions to facilitate diffusion and viscous flow. Accurately formulating the firing curve is crucial in normal pressure sintering. An appropriate heating regime can ensure that the products reduce cracking and structural defects, thereby improving the yield.
② Hot pressing sintering and hot isostatic pressing sintering. Hot pressing sintering refers to applying a certain pressure (10~40Mpa) during the sintering process, which promotes the material’s accelerated flow, rearrangement, and densification. The temperature used in hot pressing sintering is generally 100℃ lower than that of conventional pressure sintering, mainly depending on the different products and whether a liquid phase is generated. The hot pressing sintering method uses pre-formed shapes or directly fills the powder into the mold, making the process relatively simple. Products made using this sintering method have high density, theoretical density reaching 99%, and excellent performance. However, this sintering method is unsuitable for producing complex-shaped products, has a smaller production scale, and higher costs.
Continuous hot pressing sintering has high production efficiency, but the costs of equipment and molds are relatively high, and it is not conducive to the firing of overly thick products. Hot isostatic pressing sintering can overcome the above shortcomings and is suitable for the production of complex-shaped products. Currently, some high-tech products, such as ceramic bearings, mirrors, nuclear fuel needed for military applications, and gun barrels, can also use this sintering process.
③ Reactive sintering. This method of sintering materials results from the interaction between the gas or liquid phase and the matrix material. The most typical representative products are reaction-sintered silicon carbide and reaction-sintered silicon nitride. The advantages of this sintering method are its simplicity, the ability to slightly process or not process the products, and the capability to prepare complex-shaped products. The disadvantages are residual unreacted products in the final products, the structure is difficult to control, and achieving complete reactive sintering for thicker products is challenging.
In addition to the reaction sintering of silicon carbide and silicon nitride, a new method for reaction sintering of aluminum oxide has recently emerged. This method can utilize the oxidation reaction of Al powder to prepare Al2O3 and Al2O3-Al composites with good material properties.
④ Liquid phase sintering. Many oxide ceramics use low melting point additives to promote material sintering. The addition of additives generally does not affect the material’s performance or may even positively affect certain functions. As additives are used for high-temperature structures, it is important to note that glass at the grain boundaries is a major factor causing a decline in high-temperature mechanical properties. If a liquid phase with a high melting point or viscosity is selected, or if a suitable liquid phase composition is chosen, followed by high-temperature heat treatment to precipitate certain crystal phases at the grain boundaries, it can improve the material’s creep resistance.
⑤ Microwave sintering method. It is a method that uses microwave energy for direct heating and sintering. Currently, there are microwave sintering furnaces with a volume of 1m3 and a firing temperature of up to 1650℃. The temperature can exceed 2000℃ if a controlled atmosphere graphite-assisted heating furnace is used. A microwave continuous heating tunnel furnace device with a length of 15 m has also appeared. Using microwave ovens to sinter ceramics is superior to other kilns regarding product quality and energy consumption reduction.
⑥ Arc plasma sintering method. Its heating method differs from hot pressing; it applies a pulsed power supply to the product while applying stress, toughening, and densifying the material simultaneously. Experiments have shown that this method sinters fast, enabling materials to form fine-grained high-density structures, which is expected to be more suitable for sintering nanoscale materials. However, it is still in the research and development stage, and many issues must be explored in depth.
⑦ Self-manufactured sintering method. It is a method for producing precision ceramic products through the rapid exothermic chemical reaction of the material itself, saving energy and reducing costs.
⑧ Gas phase deposition method. It is divided into two categories: the physical gas phase method and the chemical gas phase method. The main types in the physical method are sputtering and evaporation deposition. Sputtering involves bombarding a flat target material with electrons in a vacuum, exciting the target material’s atoms, which then coat the sample substrate. Although the coating speed is slow and only used for thin coatings, it allows for control of purity and does not require substrate heating. The chemical vapor deposition method involves heating the substrate while introducing reactive gases or gas mixtures, which decompose or react at high temperatures to deposit products on the substrate, forming dense materials. The advantage of this method is that it can produce high-density fine crystalline structures, and the materials’ optical transparency and mechanical properties are better than those obtained from other sintering processes.
5. Glazing
The ceramic body is composed of crystalline phases generated after high-temperature firing, glass phases, unreacted quartz in the raw materials, and pores. The crystalline phase material can improve ceramic products’ physical and chemical properties, such as increasing mechanical strength, wear resistance, and thermal stability. Still, it has poor light transmittance and a rough cross-section. The glass phase material fills around the crystalline phase material to make it a coherent whole, enhancing the overall performance of the ceramic. Still, the glass phase is brittle, with poor thermal stability and wear resistance, so the glass phase must be controlled within a certain range. The glass phase can improve the light transmittance of ceramics, making the cross-section finer.
Ceramics can be divided into glazed and unglazed, but the vast majority need to be glazed for ornaments. If there is no glaze on ceramic ornaments, no matter how beautiful the shape or how new the style is, they will lose their charm. Ceramics are the art of fire, and various changes occur due to the action of fire, but mainly, it is the glaze that changes in the fire. The glaze on the surface of glazed ceramics is very similar to glass, giving ceramic utensils a smooth and shiny surface. It serves a decorative purpose, making ceramics visually appealing, and enhances the mechanical strength, surface hardness, and resistance to chemical corrosion of ceramics. Additionally, because the glaze is a smooth glass-like substance with very few pores, it is easy to clean dirt, providing convenience for the user.
Glaze, like the ceramic body, is produced from rocks or soil, but it differs from the body in that it melts more easily in fire. When the intense heat in the kiln causes the material to reach a semi-melted state, the raw materials for the glaze must be completely melted into a liquid state. After cooling, this liquid solidifies to become a glaze. Glaze is a glassy layer on the surface of non-absorbent porcelain; the sintered glaze is silicate, and the silicate source is plant ash and feldspar.
Ceramics fired with various colored metal oxides were added to the glaze to display rich colors. The glazes used for ceramic ornaments are very diverse, mainly including red glaze, cyan glaze, green glaze, yellow glaze, blue glaze, white glaze, black glaze, purple glaze, Ru kiln glaze, tea powder glaze, and many others. In addition to colored glazes, there are many types, such as crystalline glaze, crackle glaze, and matte glaze. Colored glazes are further divided into high-temperature and low-temperature glazes, with more than 60 types of high-temperature colored glazes and more than 30 types of low-temperature colored glazes, and the variety of raw materials is numerous. The porcelain clay used for ceramic ornaments is relatively refined in material selection, and most are coated with high-temperature glaze. The glaze makes the surface of the objects waterproof, gives them a luster, provides a clean and bright feeling, and increases their strength, making them easy to clean. Through these glaze colors, ceramic ornaments can present a rich artistic effect.
Several methods for glazing include dipping, pouring, brushing, and spraying. Dipping involves immersing the entire piece in a suitably thick glaze, allowing it to naturally absorb to a certain thickness. Brushing involves using a brush dipped in glaze to apply it to the piece, and using the side of the brush can create special effects. Spraying involves using a sprayer to apply glaze to the body. The glazing method can be chosen according to the design of the ceramic ornament, followed by low-temperature sintering, and finally, hanging them on specially designed stands to dry, resulting in exquisite ceramic ornaments.