How to Produce Synthetic Gemstones --- 8 kinds of Synthesid methods
Learn about the history, principles, and 8 kinds of Synthesid methods & processes of synthetic gemstones
Introducere:
The field of synthetic gemstones has seen remarkable advancements, bridging the gap between natural rarity and artificial replication. From the historical fusion of rubies by E. D. Clarke to the modern high-pressure, high-temperature synthesis of diamonds, the journey has been transformative. The principles of gem synthesis, rooted in understanding natural gem formation through endogenic, exogenic, and metamorphic processes, have paved the way for advanced laboratory techniques. Methods such as flame fusion, hydrothermal growth, and flux melting have been instrumental in creating gemstones like corundum and emeralds. The economic assessment of these synthetic methods ensures their profitability, while maintaining the exquisite quality and appearance of natural gems. The future of gem synthesis lies in refining these techniques, ensuring the stability and beauty of synthetic gemstones, and expanding their applications in the jewelry industry and beyond. As the demand for gemstones grows, synthetic gemstones offer a sustainable and ethical alternative, promising a brilliant future for this dynamic field.
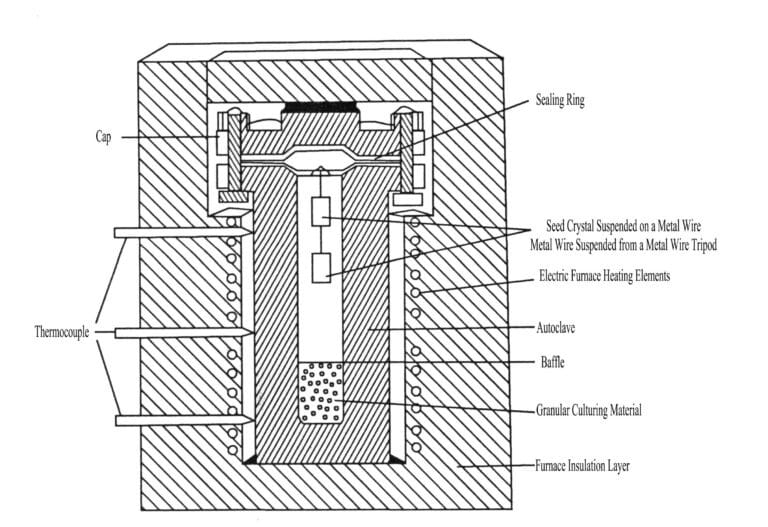
Tabla de conținut
Section I History of Gem Synthesis
The history of synthetic gemstones began in 1819 when E. D. Clarke fused two rubies using a hydrogen-oxygen blowpipe flame. Over 200 years, the development process has evolved from simple to complex, from low-level to high-level. Although the research and development of synthetic gemstones in our country started relatively late (in the 1950s), it has quickly progressed, and various synthetic gemstones can now be produced to meet market demands.
To help readers understand the development history of synthetic gemstones, a brief history of synthetic gemstones has been compiled (Table 2-1) for reference.
Table 2-1 Brief History of Synthetic Gemstones
| Year | Inventors and Improvers | Metoda | Synthetic Gemstone Varieties |
|---|---|---|---|
| 1902 | A. Werner Leaf (France) | Flame Fusion Method | Synthetic Ruby |
| 1908 | G. Spezia (Italy) | Metoda hidrotermală | Synthetic crystal |
| 1910 | A. Vernay (France) | Flame fusion method | Synthetic Blue Spinel |
| 1928 | Richard Nacken (Germany) | Flux Method | Synthetic Emerald (1ct) |
| 1934 | H. Espik (Germany) | Metoda fluxului | Synthetic emerald |
| 1940 | C. Chatham (USA) | Metoda fluxului | Synthetic emerald |
| 1947 | Lind, Inc. USA | Flame fusion method | Synthetic star ruby, sapphire |
| 1948 | National Lead Company, USA | Flame fusion method | Synthetic Rutile |
| 1955 | Riley Company (USA) | Vapor Phase Precipitation Method | Moissanit sintetic |
| 1958 | Laodis and Bauman | Hydrothermal Method | Synthetic ruby and green, colorless sapphire |
| 1959 | Shchepanov (Soviet Union) | Melt guide mold method | White sapphire |
| 1960 | United States, former Soviet Union | Vapor Phase Precipitation Method | Synthetic diamond polycrystalline film of white sapphire |
| 1960 | Schepanov (Soviet Union) | Melt-guided mold method | Synthesis of ruby, sapphire, and cat's eye, Etc. |
| 1964 | Mei and J.C. Shaa | Metoda hidrotermală | White sapphire |
| 1965 | Linde Group, USA | Metoda hidrotermală | Synthetic Emerald (Commercial Production) |
| 1966 | D.L. Wood and A Bauman | Hydrothermal Method | Cristal albastru |
| 1970 | General Electric Company | High-temperature and high- pressure method | Synthetic diamond (gem-quality diamond) |
| 1971 | Label (USA) | Mold guiding method | White sapphire |
| 1972 | P. Gilson (France) | Chemical precipitation method | Synthetic opal, synthetic turquoise |
| 1987 | Wang Chonglu (China) | Melt guide mold method | Synthetic Ruby Cat's Eye |
| 1990 | A.S. Kliber (Soviet Union) | Hydrothermal Method | Acvamarin sintetic |
| 1990 | De Beers Laboratory South Africa | High temperature and ultra-high pressure | 14.2ct synthetic diamond |
| 1993 | Guangxi Gem Research Institute, China | Metoda hidrotermală | Rubin sintetic |
| 1995 | China | Vapor Phase Precipitation Method | Black polycrystalline synthetic diamond |
| 2001 | Gemstone Research Institute of Guangxi, China | Metoda hidrotermală | Synthetic emerald (close to natural) |
Section II Principles of Gem Synthesis
Before synthesizing artificial gemstone, it is essential to understand how natural gems are formed in nature.
Gems are beautiful minerals. Minerals are naturally occurring crystalline substances with a specific chemical composition and internal structure, formed by geological or cosmic processes and relatively stable under certain physical and chemical conditions. They are the basic building blocks of rocks (such as jade). Minerals (gems) have specific chemical compositions, internal structures, and certain forms and physical and chemical properties, allowing us to identify different types of minerals (gems). However, due to the complexity of the formation environment, the composition, structure, form, and properties of minerals (gems) can vary within a certain range.
When the external conditions change or exceed the stable range of the minerals (gems), they may transform into other stable minerals (gems) under the new conditions.
Therefore, before synthesizing gems, one should thoroughly study the composition, structure, form, properties, genesis, occurrence, uses, and intrinsic relationships among the corresponding natural gems (minerals), as well as the temporal and spatial distribution patterns of natural gems and their formation and change processes.
The chemical composition of gemstones is the material basis for their formation and is one of the most essential factors determining various properties of gemstones. It is very sensitive to slight changes in the conditions of gemstone formation, especially the coloring elements. The form in which coloring elements exist in gemstones depends on the chemical behavior of the elements with atoms or ions and the geological environment and physicochemical conditions in which they are found. Therefore, before developing synthetic gemstones, it is necessary to understand the reasons and processes for forming natural gemstones.
1. The Formation of Natural Gemstones
The formation of gemstones is usually classified according to the geological processes of mineralization. According to the nature and energy source of the process, the geological process of gemstone formation can be divided into three types: endogenous process, exogenous process and metamorphism.
(1) Endogenic Processes
Endogenic processes refer to various geological processes that lead to the formation of gemstones due to the Earth’s internal heat. This includes a variety of complex mineralization processes such as magmatic processes, volcanic processes, Pegmatitic action and hydrothermal processes.
(a) Magmatic action:
Refers to the process of gem (mineral) formation from magma melts rich in volatile components under high temperature (700-1300) and high pressure (5 x 108 –20 x 108 Pa), which cool and crystallize under geological stress. Examples include Peridot, pyroxene, hornblende, feldspar, quartz, pure diamond, platinum group natural elements, etc., all formed during magmatic action.
(b) Volcanic action:
Refers to the entire rock formation and mineralization process when magma from deep underground intrudes along weak zones in the crust to the surface or erupts directly, rapidly cooling. Gemstones related to volcanic action include zeolite, opal, agate, calcite, realgar, orpiment, Peridot, ruby, and sapphire found in deep-source inclusions.
(c) Pegmatitic action:
Refers to the process of rock formation and mineralization occurring under high temperatures (400- 700) and high pressure (1 x 108 – 3 x 108 Pa) conditions at greater depths underground (3- 8 km). The gemstones formed by
Pegmatitic action has large crystals, rich in Si, K, Na and volatiles (F, Cl, B, OH), such as quartz, feldspar, amethyst, topaz, tourmaline, beryl, spodumene, and amazonite.
(d) Hydrothermal action:
Refers to the process of gem formation from gas-water solutions to hot-water solutions, classified into three
Types based on temperature: high temperature (500-300), medium temperature (300-200), and low temperature (200-50). Gemstones related to hydrothermal action include beryl, topaz, tourmaline, quartz, fluorite, barite, calcite, cinnabar, as well as cassiterite, bismuthinite, natural gold, argentite, etc. The hydrothermal method in synthetic processes mimics hydrothermal mineralization.
(2) Exogenic action
Exogenic processes refer to various geological processes, including weathering and sedimentation ,that form gemstones under low temperature and pressure on the surface or near the surface due to the participation of solar energy, water, atmosphere and biological factors.
(a) Weathering:
Under external forces, the original rock (raw ore) undergoes mechanical fragmentation and chemical decomposition. Weather-resistant gemstones are disintegrated into sand deposits, such as diamonds, rubies, sapphires, opals, and zircon, while easily weathered minerals form surface gemstones like chalcedony, opal, malachite, and azurite at the surface.
(b) Sedimentation:
Mainly occurs in rivers, lakes, and oceans, referring to the process where weathered products from the surface are transported to suitable environments and deposited to form new minerals (gemstones) or mineral combinations. For example, mechanical sediments include natural gold, platinum, diamonds, cassiterite, and zircon; biochemical sediments include calcite, apatite, jet, amber, and coral.
(3) Metamorphic processes
Metamorphism refers to the process in which rocks that have already formed at greater depths below the surface change their geological and physicochemical conditions due to tectonic movements, magmatic activity, and changes in geothermal flow, resulting in changes in composition and structure while largely maintaining a solid state, leading to the formation of a series of metamorphic minerals (gems) that form rocks (jade).
Based on the different causes and physicochemical conditions, metamorphism can be divided into contact metamorphism and regional metamorphism.
(a) Contact metamorphism:
It refers to a metamorphism caused by magmatic activity that occurs in the contact zone between the magmatic intrusion and the surrounding rock at a shallow depth (2- 3 km) underground. According to the different metamorphic factors and characteristics, it can be divided into thermal metamorphism and contact metamorphism.
- Thermal metamorphism: This refers to the process where the intrusion of magma into the surrounding rock causes the minerals in the surrounding rock to undergo recrystallization due to the heat and volatiles from the intruding magma, resulting in larger grains or metamorphic crystallization, and the recombination of components to form new minerals and mineral assemblages. Common gems include ruby, cordierite, Wollastonite, sanidine.
- Contact metasomatism: This occurs when the volatiles and hydrothermal fluids that are released during the late crystallization of the magma at the contact with the surrounding rock cause significant metasomatic changes in the surrounding rock and the intrusion, forming new rocks (jade). Contact metasomatism is most likely near the contact zone between intermediate-acidic intrusions and carbonate rocks. Due to double metasomatism, the result is that the rocks near the contact zone change composition, structure, and texture, forming a series of gems or jade, with the most common being diopside, augite, Andradite, and Grossularite, as well as later occurrences of tremolite, actinolite, epidote, plagioclase, and hornblende. New mineral assemblages can form jade types such as pyroxene, hornblende, serpentine, and carbonate jade.
(b) Regional metamorphism:
Refers to the metamorphic processes that occur over large areas due to regional tectonic movements. The mineral composition and structural characteristics of the original rock change as a result of the combined effects of major physical and chemical factors such as temperature (200- 800), pressure (4 x 108– 12 x 108 Pa ), stress, and chemically active fluids primarily composed of H2O CO2.
The metamorphic minerals (gems) and their combinations formed by regional metamorphism mainly depend on the composition and Degree of metamorphism of the original rock. If the main components of the original rock are SiO, CaO, MgO, FeO, it is easy to form tremolite, actinolite, tremolite and calcium-iron pyroxene after metamorphosis. If the original rock mainly consists of clay minerals composed of SiO2 IA2O3, its metamorphic products will include quartz or corundum and the mineral symbiosis of one of Al2SiO5 homogenous three-phase variants. Low-temperature and high-pressure environments favor the formation of kyanite, while the temperature and pressure for the formation of andalusite are relatively low.
It should be mentioned that the geological processes that form gems are a comprehensive manifestation of various factors. Those above endogenic, exogenic, and metamorphic processes are not isolated or completely separate from each other. In other words, the formation, stability, and evolution of gems depend on the geological environment and the physical and chemical conditions they are in, which means they depend on geological processes and factors such as temperature, pressure, the concentration of components, acidity, and alkalinity (PH) of the medium, redox potential, chemical potential(µi), fugacity (fi), activity (ai), and time. Gems are the products of the combined effects of various physical and chemical factors in specific geological processes, and the physical and chemical conditions can vary significantly in different geological processes and stages of the same geological process. It should be noted that the relationship between the formation of gems and some of their properties and free energy. The formation and enrichment of gems are constrained by the activity of chemical components in the system, and the stability of gems depends on the degree of openness and closure of the geological system. When analyzing the genesis of gems, comprehensive consideration should be made to draw reasonable inferences and lay a theoretical foundation for the artificial synthesis of natural gems.
2. Designing experimental schemes for gem synthesis
Based on the formation environment and conditions of corresponding natural gemstones, crystal materials are synthesized in the laboratory by simulating similar mineralization processes. For example, mineralogists recognized in 1797 that diamonds are pure crystals composed of carbon atoms with a cubic crystal structure formed under high temperature and high-pressure conditions deep underground. People then created high-temperature and high-pressure environments in the laboratory to crystallize carbon into diamond crystals. In 1953, the Swiss ASEA laboratory finally synthesized industrial-grade diamonds using high-temperature and high-pressure methods. By 1970, General Electric in the United States had synthesized gem-grade diamonds. By the end of 1995, black diamond polycrystalline film products synthesized using the CVD method entered the jewelry market in our country.
Therefore, the synthesis of gemstones must be based on the formation mechanisms of natural gemstones, designing various synthesis methods. In synthesizing gemstones in the laboratory, a reasonable process plan is gradually established by selecting the best options.
3. Process technology and economic benefit assessment
Through various experimental trials, effective synthesis methods are established, and the economic benefits of the selected methods are assessed. In other words, while synthesizing ideal synthetic gemstones using reasonable methods, evaluating the economic value of the gemstones synthesized by these methods is necessary to determine if they are profitable. If the synthesized gemstones are priced higher than their corresponding natural ones, it is unsuitable for large-scale production; such methods only have scientific significance and no commercial value.
4. Select crystal growth processes and test crystal qualification rates.
Currently, gemologists have developed many methods for artificially growing crystals. Although these methods can adapt to the production of various synthetic gemstones, a comprehensive and detailed study of the selected synthetic method should be conducted during the production process. This includes precisely determining various crystal growth parameters to ensure the size and specifications of the crystals, and eliminating various defects that occur during crystal growth in order to achieve the exquisite quality of high-quality natural gemstones with no obvious differences from natural gemstones.
Section III Gemstone Synthesis Process
Synthetic gemstones (crystallites) are crystalline solids with a lattice structure, and their synthesis is actually a process of arranging the points (atoms, ions, or molecules) that make up the crystal according to the lattice structure law under certain artificially controlled conditions. Although the synthesis of gemstones in many ways, but from the transformation of the physical phase, the crystal growth process can be divided into: gas phase a crystallization of the solid phase e → the liquid phase a crystallization of the solid phase → amorphous solid phase a crystallization of the solid phase → a crystallization of the solid phase → another crystallization of the solid phase and so on four kinds of types.
The liquid phase can be either a solution or a melt. The thermodynamic conditions leading to the first two phase transitions are oversaturation (concentration greater than solubility), which leads to the third phase transition, spontaneous nucleation and growth, and the fourth phase transition, which is due to changes in the external temperature and pressure conditions that make the original crystalline solid phase unstable and form another type of crystal. Based on this, at present, the main production processes used for synthesizing gemstones are flame melting method, hydrothermal method, flux method, melt method, high temperature and ultra-high pressure method, chemical precipitation method and so on.
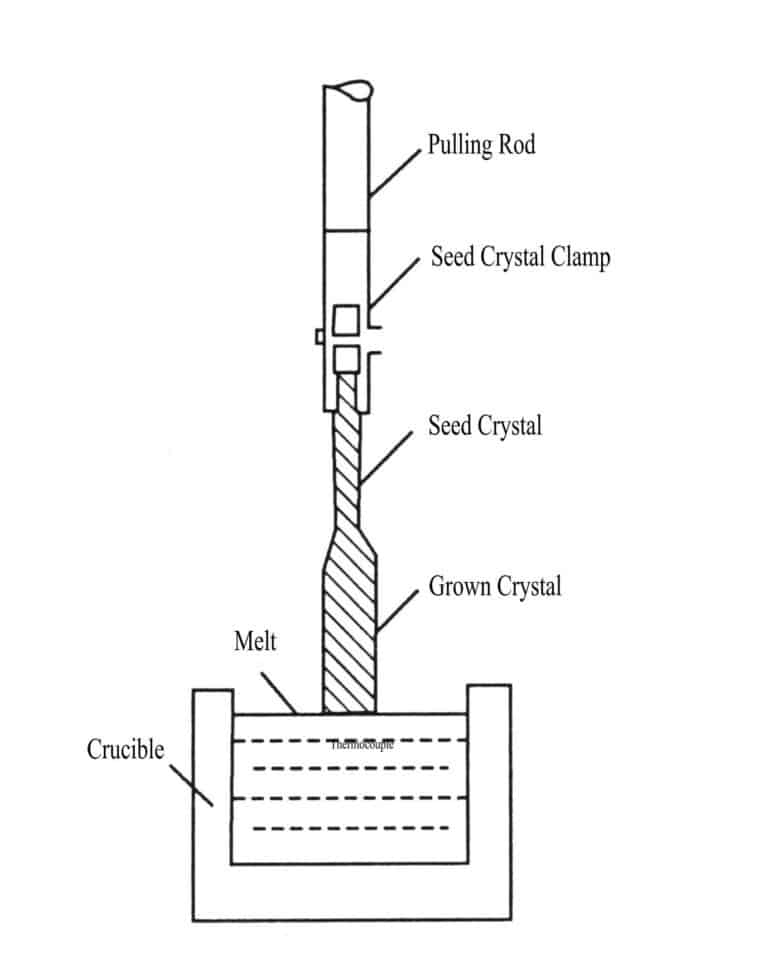
1. Flame Fusion Method
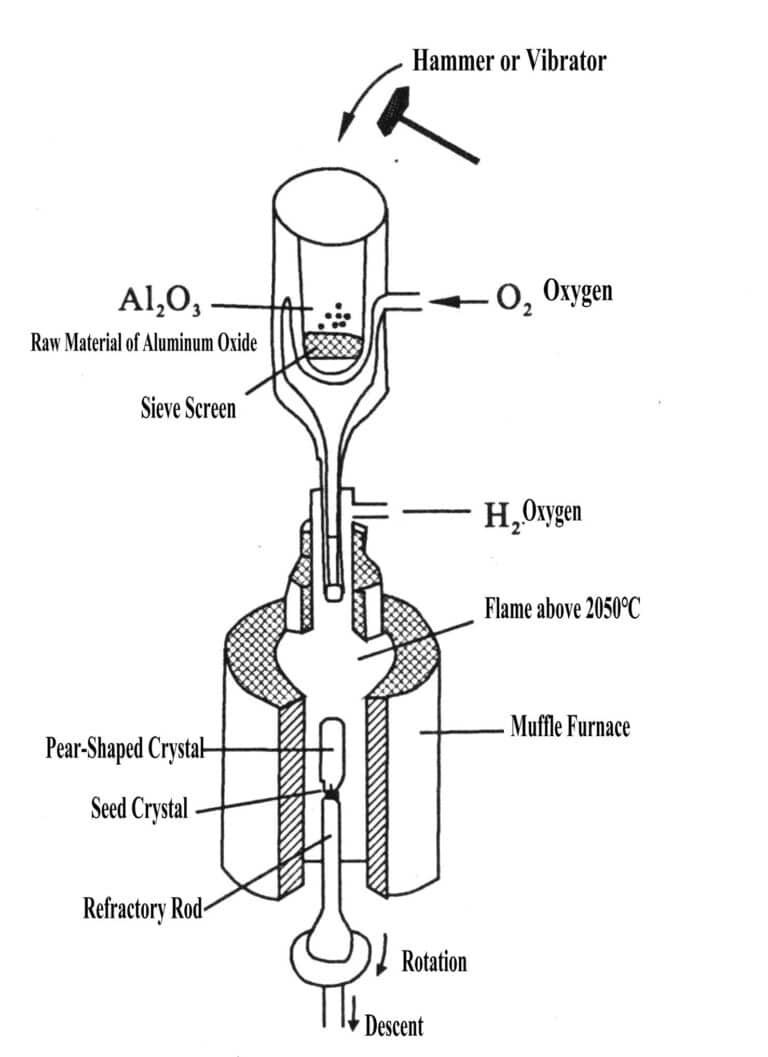
Using the high temperature generated by a hydrogen-oxygen flame, the raw material powder for synthesizing gemstones is heated and melted during its descent in a vibrating feed tube. The molten melt falls onto the seed crystal at the top of the crystal rod on the support, and as it slowly descends due to heat dissipation, it condenses and crystallizes into pear-shaped crystals (Figure 2-1). The process of growing crystals using this method simulates the transition from the liquid phase (melt) to the crystalline phase in the magmatic mineralization process.
1.1 Process Flow
The process of growing gem crystals by flame fusion mainly includes four steps: raw material purification, powder preparation, crystal growth, and annealing treatment.
(1) Raw Material Purification
The raw materials should be abundant in source and low in cost, and the purification method should be simple and effective.
(2) Powder Preparation
The powder material requires high purity, complete chemical reaction, and small volume capacity, and the crystal structure should be conducive to crystal growth.
(3) Crystal Growth
The process of crystal growth can be divided into three stages: seed crystal, expansion, and isometric growth.
Throughout the crystal growth, the feeding system must provide a uniform material supply to ensure that all the powder is melted into tiny liquid beads; The temperature of the gas burner reaches 2900℃ , and constitutes the shape of the three-layer flame and the orderly change of temperature; The crystallization furnace is required to create good heat preservation conditions for the growing crystals, and facilitate gas flow and no accumulation of powder; The lowering mechanism is required to ensure that the starting position can make the top temperature of the crystal higher than the melting point of the crystal but lower than the boiling point of the crystal, and ensure that there is a melting layer of 2~ 3 mm thick.
(4) Annealing treatment
After placing the synthetic crystal in a high-temperature furnace, slowly raise the temperature to the predetermined level, then maintain a constant temperature for a long time and slowly anneal to release the thermal stress of the synthetic gemstone crystal, preventing the crystal from cracking due to heat.
1.2 Production equipment
(1) Feeding system
The powder material should fall smoothly and evenly, melting into tiny droplets when passing through the burner.
(2) Hydrogen-oxygen burner
The gas structure should be good, with an appropriate hydrogen-oxygen supply ratio, a flame that is three-layered, and a stable temperature at 2900℃ while minimizing powder loss as much as possible.
(3) Crystallization furnace
The furnace body should maintain stable insulation, the furnace chamber should be streamlined, without powder accumulation, and should not cause gas turbulence, with a small temperature gradient.
(4) Descent mechanism
It should be adapted to the crystal growth temperature to ensure that the solid-liquid interface of the crystals is stable and the descent is uniform and smooth, the same as the crystallization rate. And ensure that the top of the seed crystal has 2- 3 mm molten layer.
1.3 Specific example: Flame fusion method for synthesizing corundum gemstones
(1) Selection of raw materials
Currently, both domestically and internationally, the flame fusion method for synthesizing corundum gemstones uses ammonium aluminum sulfate (also known as Ammonium alum) is the preferred raw material for preparing γ-AI2O3 powder, with the following advantages:
① Ammonium aluminum sulfate has abundant raw materials, low prices, and simple and effective purification methods;
② The roasted product of ammonium aluminum sulfate is loose and has good fluidity;
③ Ammonium aluminum sulfate has high solubility and can be purified using a simple crystallization method. Moreover, during the recrystallization process and its impurity removal effect are very good. It only requires 3 – 4 times of recrystallization for the purity of ammonium aluminum sulfate to reach 99.9% – 99.99%.
(2) Preparation and purification of raw materials
① Preparation of ammonium aluminum sulfate. Mix aluminum sulfate and ammonium sulfate in a ratio of =2.5 :1 and mix evenly, then prepare with a material-to-water ratio of 1 : 1.5 heat to boiling, completely dissolve, and slowly cool to crystallize to obtain ammonium aluminum sulfate.
② Purification of ammonium aluminum sulfate. Dissolve the synthesized ammonium aluminum sulfate in distilled water or deionized, water is then recrystallized repeatedly 3- 5 times to obtain a raw material with a higher purity of 99.9% or higher.
(3) Preparation of colored synthetic corundum gemstone powder.
The composition of colored synthetic corundum gemstone powder is γ-AI2O3 and a small amount of coloring agent. The coloring agents are mostly oxides of transition elements or rare earth elements, which introduce chromophore ions into the lattice, causing the crystal to selectively absorb visible light, thereby coloring the crystal.
Colored synthetic corundum gemstone powder is obtained by adding coloring agents to the raw material ammonium aluminum sulfate, dehydration, and calcination. The specific method is to prepare the coloring agent into a solution of a certain concentration and add it to the ammonium aluminum sulfate as required. After heating, the ammonium aluminum sulfate dissolves, and the coloring agent is evenly distributed in the ammonium aluminum sulfate solution. The mixture of ammonium aluminum sulfate and coloring agent is then placed in a dehydration furnace for dehydration and in a calcination furnace for calcination, thus ensuring that the coloring agent is evenly distributed in the powder.
In synthetic corundum gemstones, the types and amounts of coloring agents added vary, resulting in different colors of the gemstones.
(4) Growth of synthetic corundum gemstones
The process conditions and operational steps for all corundum gemstones’ flame fusion growth are similar.
First, place the seed crystal at the top of the refractory clay rod to control the crystallization orientation, with the preferred orientation being 60°.
After the furnace is opened, the feeding system, burner, and descending mechanism begin to operate. The melting point of corundum is 2050℃ and the working temperature of the hydrogen-oxygen flame is 2900℃; the growth of synthetic ruby is H2 : O2 = (2.0 – 2.5); the growth of synthetic sapphire is H2 : O 2 = (2.8-3.0) 1 ;the growth of synthetic sapphire is H2 : O2 = (3.6-4). Adjust the position of the crystal rod so that the temperature at the top of the crystal is above the melting point 2050 and below the boiling point 2150℃, ensuring there is a molten layer of 2-3 mm . After the seed crystal expands, continue to grow to the desired size with constant diameter. Finally, the crystal should be left in the furnace to cool in its original state. The cooling conditions at this time also have a significant impact on crystal quality; if rapid cooling is used, a large temperature difference inside and outside the crystal will increase internal stress, making the crystal surface more brittle and prone to cracking.
During the growth of colored synthetic corundum crystals, the addition of coloring agents lowers the melting point of the powder, which also reduces the crystal growth temperature. Additionally, certain coloring ions have a distribution coefficient of less than 1 in corundum, leading to defects such as uneven color or brittleness in the crystals grown from these ions.
The crystal quality of corundum-type gemstones varies, usually being pear-shaped crystals of size 150- 750ct, with a diameter of up to 17 – 19 mm. Currently, the largest crystals produced can have a diameter of up to 32 mm.
(5) Annealing treatment of synthetic corundum-type gemstones
The main conditions for annealing treatment are temperature and time. Corundum-type gemstone crystals grown by flame fusion have significant internal stress due to a large temperature gradient, necessitating annealing treatment. Typically, a 50 mm pear-shaped crystal has a melting layer temperature of 2050℃ at the top, while the bottom may only be 100℃, resulting in internal stress in the crystal during the crystallization process that can reach 80-lOOMpa. If the internal stress is not relieved through annealing, the crystals are very prone to breaking during processing and use. Flame-fused synthetic corundum gemstone crystals used in jewelry are generally not annealed, but they all crack along the growth axis where the internal stress is greatest, and the cracked surface is used as the working surface for cutting and grinding.
Specific example: colorless synthetic sapphire
High-purityγ-AI2O3 powder obtained from calcined ammonium aluminum sulfate is uniformly fed through the combustion furnace [H2: O 2 = (2.0-2.5): 1], melting at high temperatures of 2900℃, and dripping onto high-quality seed crystals with a molten layer.
At the top, the descending mechanism descends, expanding the seed crystal shoulder, condensing, and crystallizing. When it grows to the predetermined size, the furnace is closed, allowing the crystal to cool inside the furnace.
To eliminate internal stress in the crystal, annealing treatment is still required, with an annealing temperature around 1800 ℃and a time of about 2 hours. Generally, sapphires used in jewelry do not undergo annealing treatment, but the table’s surface should be cut from the growth axis direction with the maximum internal stress.
1.4 Advantages and Disadvantages of Flame Fusion Method
Compared to other methods, the flame fusion method for growing crystals has the following characteristics.
(1) No crucible is needed, which can avoid contamination from the crucible;
(2) High temperature can be used to produce gemstones with higher melting points;
(3) Fast crystal growth rate, large output;
(4) Simple equipment, high labor productivity;
(5) Large flame temperature gradient, poor crystal quality;
(6) Temperature is difficult to control, and crystals are prone to large internal stresses, so annealing treatment is required;
(7) Strict requirements for the purity and particle size of the powder, high gloss, and high cost of raw materials;
(8) For volatile and easily oxidized materials, this method is usually not applicable for synthesizing gemstones.
2. Hydrothermal Method
Simulating the process of hydrothermal mineralization in nature, the hydrothermal method for growing crystal gemstones is conducted by transitioning from the liquid phase (solution) to the crystal phase in a water-containing system. Natural hydrothermal mineralization occurs under certain temperature and pressure conditions, and the mineralization solution has specific concentrations and PH values (the properties of the mineralizing solution vary depending on the type of gemstone crystal being grown). Experiments have shown that only in a high-pressure vessel can the conditions for simulating the natural growth of gemstone crystals be met. Therefore, the hydrothermal method is distinct from other systems for growing gemstone crystals. This method suits materials with low solubility at normal temperature and pressure but high solubility at high temperature and pressure.
2.1 Production Process
According to the transportation method of crystal growth, it can be divided into three production processes.
(1) Isothermal method
The isothermal method mainly utilizes the difference in solubility to grow crystals, with the raw materials being metastable substances and the seed crystals being stable. There is no temperature difference inside the high-pressure kettle, which is a characteristic of this method.
The disadvantage of the isothermal method is that it cannot grow large crystals with complete crystal forms.
(2) Oscillation method
The oscillation device consists of two cylinders at different temperatures. One cylinder contains the culture solution, while the other holds the seed crystal. The two cylinders are oscillated at set intervals to accelerate convection between them. Crystals are grown in a high-pressure environment using the temperature difference between the two cylinders.
(3) Temperature difference method
Temperature difference method is a method of growing crystals in a vertical autoclave, which is mostly used for synthesizing crystals, rubies, emeralds, aquamarines and so on. The crystal growth conditions are as follows:
① The minerals should have a certain solubility in the mineralizer solution and be able to form the desired single stable crystal phase;
② Minerals can reach supersaturation at appropriate temperature differences without spontaneous nucleation;
③ Crystal growth requires seed crystals of certain shapes and specifications, and the ratio of the total surface area of the raw materials to the total surface area of the seed crystals must be sufficiently large;
④ The temperature coefficient of the solution density must be sufficiently large to facilitate the convection of the crystal growth solution and the transport of solutes;
⑤ The high-pressure vessel must have high-temperature resistance and corrosion resistance.
2.2 Basic Equipment
The basic apparatus for the hydrothermal method mainly includes a high-pressure reactor, heater, temperature controller, and temperature recorder (Figure 22).

2.3 Specific example: Hydrothermal synthesis of crystals
(1) Principle of hydrothermal synthesis of crystals
The basic principle is to grow crystals in a supersaturated solution, where the temperature at the bottom of the high-pressure reactor is
Higher and gradually dissolves into the solution, while the temperature at the top is lower, SiO2 and slowly precipitates, growing on the placed seed crystal. During the synthesis of crystals, a certain amount of mineralizer must be added to change the original composition and properties of the solvent in order to increase the solubility of SiO2.
(2) The hydrothermal method for synthesizing crystals.
The process flow of synthesizing crystals using the hydrothermal method can be divided into four stages.
① Preparation stage. This includes the preparation of the solution, cutting and cleaning of seed crystals, calculating the volume of the culture material (melted quartz), seed crystals, seed crystal support plates, tying seed crystal metal wires, and the free space volume of the high-pressure vessel, filling degree calculations, as well as checking the dimensions of the sealing ring pressure ring, heating, and temperature measurement systems.
② Loading stage. Place the melted quartz into the high-pressure vessel, position the seed crystal support, pour in the alkaline solution (mineralizer solution), measure the liquid level height, install the sealing ring, seal the high-pressure vessel, then place the high-pressure vessel into the furnace, insert the thermocouple, and cover with an insulation cover, etc.
③ Growth stage. Power on the heating furnace to heat, raise the temperature of the high-pressure vessel, and adjust the temperature, regulating it to the desired temperature and controlling the temperature difference. During the production process, it is necessary to maintain a stable temperature (generally requiring temperature fluctuations within 5℃). After growth, stop the furnace and open the insulation cover, allowing the upper heat to dissipate faster than the lower part. After cooling, the high-pressure vessel can be removed from the furnace.
④ Opening the autoclave stage. When the temperature inside the autoclave drops to room temperature, the autoclave can be opened to take out the crystals. Then, pour out the residual solution and remaining fused quartz, and clean and inspect the grown crystals and the high-pressure autoclave.
2.4 Characteristics of the hydrothermal method
The typical conditions for crystal growth using the hydrothermal method are temperature 300-700℃, pressure 5.0 x 107– 3.0x 108 Pa.
(1) Able to grow materials that undergo phase transitions (such as α -quartz, etc.) and materials with high vapor pressure near their melting point (such as ZnO) or materials to be decomposed (VO2 ).
(2) Able to grow large and clean high-quality crystals.
(3) The crystals grown are closest to natural gemstone crystals.
(4) The equipment is expensive and is unsafe.
(5) High-quality seed crystals of appropriate size and suitable facets are needed.
(6) Due to the sealing of the high-pressure vessel, the entire growth process cannot be directly observed.
(7) The size of the high-pressure vessel controls the size of the crystals.
3. Flux Method
The flux method, as the name suggests, is a method in which minerals melt at a lower temperature with the help of a flux at high temperatures, allowing gem crystals to grow from the molten body.
The crystal growth process using the flux method is similar to the formation of minerals during the crystallization differentiation process of magma. It is akin to the hydrothermal crystal growth method, except that the flux replaces the aqueous solvent. Therefore, the flux method can also be referred to as the high-temperature melt solution, flux method, or molten salt method. This method plays an important role in crystal synthesis; as early as the mid-19th century, someone used this method to synthesize rutile, but it was overlooked due to the rise of flame fusion methods, and only in recent years has it been widely applied.
3.1 Classification of the flux method
The flux method can be divided into two main categories based on the nucleation and growth methods of crystals.
(1) Spontaneous nucleation method
The first step in the crystal growth process is the formation of crystal nuclei. Nucleation is a phase transition process, that is, the formation of small solid crystal buds in the mother liquid phase.
The change in the system’s free energy during this phase transition process is: △G = △G µ + △Gs .
In the formula: △G µ, is the change in the system’s free energy when the new phase is formed, and △G µ< 0; △Gs is the surface energy at the interface between the new phase and the old phase, and △Gs > 0. This means that the formation of crystal nuclei causes a decrease in the system’s free energy as the system transitions from the liquid phase to the crystal phase with lower internal energy, while also increasing the system’s free energy due to the added liquid-solid interface. Experiments show that the main external factors affecting nucleation are super-cooling and supersaturation. There is a lag phenomenon in the phase transition of nucleation, meaning that when the temperature drops to the phase transition point, or when the concentration just reaches saturation, nucleation cannot be observed. Nucleation always requires a certain degree of super-cooling or supersaturation.
Additionally, nucleation can be divided into homogeneous nucleation and heterogeneous nucleation. Homogeneous nucleation occurs at an equal rate at any point in the system, while heterogeneous nucleation occurs at certain points in the system where the nucleation rate is higher than at other points.
Homogeneous nucleation can only occur under ideal conditions; in reality, the nucleation process is always heterogeneous, meaning there are always impurities, uneven heat flow, and uneven container walls in the system. These homogeneities effectively lower the energy barrier for nucleation, allowing nuclei to preferentially form at these locations. Therefore, the artificial synthesis of gemstones always deliberately creates homogeneities to facilitate nucleation, such as by adding seed crystals or nucleating agents.
This method can be divided into three types based on the different ways of obtaining supersaturated solutions: slow cooling method, reaction method, and evaporation method, among which the slow cooling method is widely used due to its simple equipment (Figure 2-3).
① The slow cooling method involves melting all crystal materials in a flux and then slowly cooling them in a high-temperature furnace, allowing the crystals to spontaneously nucleate and gradually grow. This method can be used to produce synthetic corundum and synthetic Yttrium Aluminum Garnet.
② The reaction method involves melting the flux with the raw materials of the crystal to be grown and causing a chemical reaction. Under certain supersaturation conditions, the crystals nucleate and then grow.
③ The evaporation method involves evaporating the solvent under constant temperature conditions, causing the melt to reach a supersaturated state, thereby allowing crystals to precipitate and grow from the melt. For example, crystal growth like CeO2, YbCrO3
Copywrite @ Sobling.Jewelry - Producător de bijuterii personalizate, fabrică de bijuterii OEM și ODM
(2) Seed crystal growth method
This method is a crystal growth technique that involves adding seed crystals to the melt. Its characteristic is that it only allows crystals to crystallize and grow on the seed crystals, overcoming the disadvantage of excessive grain formation during spontaneous nucleation. It can be divided into several methods based on different crystal growth processes.
① Seed crystal rotation method. The seed crystal’s rotation helps stir the molten flux, allowing it to diffuse towards the crystal, accelerating crystal growth and reducing inclusions [Figure 2-3(b)].
② Top seed crystal rotation and lifting method. This method combines the seed crystal rotation and lifting method with the melt lifting method. It allows the raw materials to melt in the flux at the high-temperature zone at the bottom of the crucible, forming a saturated molten liquid; under the action of rotation and stirring, it diffuses and convicts to the relatively low-temperature zone at the top, forming a supersaturated molten liquid, which crystallizes and grows on the seed crystal. As the seed crystal continues to rotate and lift, the crystal gradually grows on the seed crystal. The advantage of this method is that it can avoid thermal stress on the crystals, and the remaining melt can be reused with crystal materials and flux.
③ Bottom seed crystal water cooling method. When the flux is highly volatile, this method can obtain good crystals. Water cooling ensures the seed crystal’s growth and suppresses nucleation on the surface of the melt and other parts of the crucible, thus ensuring that the crystals only grow on the seed crystal.
3.2 Selection of Flux
The growth of crystals using flux methods requires the presence of a flux. As a flux, it must have the property of dissolving the material to be crystallized when melted while being resistant to decomposition and volatilization. Therefore, how to select a flux becomes a key factor in crystal growth, as it will affect the quality of crystal growth and the growth process (Table 2-2).
Table 2-2 Luster levels of freshwater pearl
| Flux | Punct de topire/ ℃ | Boiling point/ ℃ | Density (g/cm3 ) | Solvent (Melting fluxes) | Example of crystal growth |
|---|---|---|---|---|---|
| B2O3 | 450 | 1250 | 1.8 | Hot water | Li0.5Fe2.5O4, FeBO3 |
| BaCl2 | 962 | 1189 | 3.9 | Water | BaTiO3, BaFe12O19 |
| BaO - 0.62 B2O3 | 915 | - | About 4.6 | Hydrochloric acid, Nitric acid | YIG, YAG, NiFe2O4 |
| BaO - Ba F2 -B2O3 | 800± | - | About 4.7 | Hydrochloric acid, Nitric acid | YIG, RFeO3 |
| BiF3 | 727 | 1027 | 5.3 | Hydrochloric acid, Nitric acid | HfO2 |
| Bi2O3 | 817 | 1890 decomposition | 8.5 | Alkali, Nitric Acid | Fe2O3 , Bi2Fe4O9 |
| CaCO3 | 782 | 1627 | 2.2 | Water | CaFeO4 |
| CdCO3 | 568 | 960 | 4.05 | Water | CdCrO4 |
| KCl | 772 | 1407 | 1.9 | Water | KNbO3 |
| KF | 856 | 1502 | 2.5 | Water | BaTiO3, CeO2 |
| LiCl | 610 | 1382 | 2.1 | Water | CaCrO4 |
| MoO3 | 795 | 1155 | 4.7 | Acid nitric | Bi2M02O9 |
| Na2B4O7 | 724 | 1575 | 2.4 | Water, acid | TiO2, Fe2O3 |
| NaCl | 808 | 1465 | 2.2 | Water | SrSO4, BaSO4 |
| Na | 995 | 1704 | 2.2 | Water | BaTiO3 |
| PbCl2 | 498 | 954 | 5.8 | Water | PbTiO3 |
| PbF2 | 822 | 1290 | 8.2 | Acid nitric | IA2O3, MgAl2O4 |
| PbO | 886 | 1472 | 9.5 | Acid nitric | YIG, YFeO3 |
| PbO - 0.2 B2O3 | 500 | - | About 5.6 | Acid nitric | YIG, YAG |
| PbO - 0.85 | 500± | - | About 9 | Acid nitric | YIG, YAG, RFeO3 |
| PbF2 | 580± | - | About 9 | Acid nitric | ( Bi, Ca)3 (Fe, V)5O12 |
| PbO - B2O3 | 720 | - | About 6 | Hydrochloric acid, Nitric acid | YAG, YIG |
| 2PbO · V2O5 | 670 | 2052 | 3.4 | Acid clorhidric | RVO4, TiO2, Fe2O3 |
| V2O5 | 705 | - | 2.66 | Hot alkali, Acid | RVO4 |
| Li2NoO4 | 698 | - | 4.18 | apă | BaMoO4 |
| Na2WO4 | Fe2O3, AI2O3 |
The basic principles for selecting a flux are:
(1) High solubility that varies with temperature, facilitating crystal growth.
(2) As low a melting point and viscosity as possible and as high a boiling point as possible to allow for rapid crystal growth over a wide temperature range.
(3) Volatility should be low, toxicity and corrosiveness should be minimal, and it should be easy to remove for environmental protection and safe production.
(4) Should not form intermediate compounds with crystal components, allowing the growth of crystals to be the only stable phase.
3.3 Characteristics of the Flux Method
The flux method has the following characteristics compared to other methods:
(1) Strong applicability, capable of producing various gemstone materials.
(2) Low growth temperature, which not only saves energy consumption but also conserves high-temperature materials.
(3) Can produce gemstone crystals with volatile components that decompose near the melting point.
(4) The flux method can grow crystals below its phase transition temperature, avoiding destructive phase changes.
(5) The grown crystals have good quality, and the equipment is simple and easy to operate.
(6) The crystal growth rate is slow, the growth cycle is long, and the crystals are small and prone to contain cations from the flux.
(7) Many fluxes have varying degrees of toxicity, and their volatiles often corrode or contaminate the furnace body.
4. Melting method
The method of producing crystals using a crucible is commonly referred to as the melt method. The production processes for gemstones mainly include the crystal pulling method, melt casting method, melt bottom cooling method, crucible descent method, bubble growth method, and arc melting method, among others. The crystal pulling and casting methods are currently the most commonly used among these. The melt method for growing crystals belongs to the non-uniform nucleation type of synthesis method.
4.1 Crystal pulling method
This production process utilizes seed crystals to pull crystals from the melt. This method can grow large, high-quality single crystals without dislocations. It has successfully grown many gemstone materials of practical value. For example, the Zhejiang Juhua Gem Factory successfully grew internationally advanced colorless sapphire LED crystals for lighting using the bubble growth pulling method in 1999; it also grew colorless sapphire crystals with a diameter of 250 mm and weighing about 20 kg for optical-grade window materials used in missiles and drones using the melt pulling method; in 2001, rare-earth doped alumina garnet crystals for laser were grown by this method.
(1) Process principles and procedures
Place the raw materials into the crucible, heat and melt them, and adjust the temperature inside the furnace so that the temperature of the upper melt is slightly higher than the melting point. Allow the seed crystal on the seed crystal rod to contact the melt surface, and after the surface of the seed crystal melts slightly, lower the temperature to the melting point, pull and rotate the seed crystal rod, causing the top of the melt to be in a super-cooled state and crystallize on the seed crystal. In this way, cylindrical crystals are grown during the continuous pulling and rotating of the seed crystal rod (see Figure 2-4). When the growing crystal reaches a certain size and leaves the melt surface, it should be gradually cooled in the post-heater to prevent the crystal from cracking due to internal stress caused by a rapid temperature drop.
(2) Quality control factors
① Quality of the seed crystal: requires no dislocations or low dislocation density with a surface free of damaged layers, capable of fully wetting the melt with the seed crystal.
② Temperature control: requires that the temperature distribution in the melt at the solid-liquid interface is exactly the melting point, ensuring that the melt around the seed crystal has a certain degree of super-cooling, while the temperature in other areas is above the melting point.
③ Pulling speed and rotation speed depend on the diameter of the crystal to be grown, melt temperature, dislocations, inclusions, and component super-cooling. In addition, the shape of the solid-liquid interface (plane) is also an important parameter that determines crystal quality.
④ Impurities: The types and quantities of impurities have different effects on the quality of the crystal.
(3) Characteristics of crystals grown by the pulling method
① The entire process of crystal growth can be directly observed.
② The growing crystal does not contact the crucible, avoiding nucleation on the crucible wall and the compressive stress from the crucible wall on the crystal.
③ There are few crystal defects and high-quality oriented crystals can be obtained relatively quickly.
④ Crucibles and other materials easily contaminate crystals.
⑤ The vibration of mechanical transmission devices, fluctuations in temperature, and the complex liquid flow in the melt can all affect crystal quality.
4.2 Melt-guided method
(1) Process principles and procedures
In the 1960s, the melt-guided method developed from the pulling method is a growth technology that can directly pull crystals with various cross-sectional shapes from the melt, essentially a variant of the pulling method. Its name should be edge-limited thin film feeding pulling growth technology (EPG method).
This method involves heating and melting the material for growing crystals in a high-temperature crucible, placing a mold with capillaries into the melt, and then rising along the capillaries to the top of the mold with a certain cross-sectional shape. The seed crystal is immersed in the melt at the top of the mold, and after the surface of the seed crystal remits, it is gradually pulled up. This continues until the melt expands to the edge of the cross-section at the top of the mold, at which point pulling is resumed, allowing the crystal to enter the equated growth stage, where the crystal grows continuously according to the size and shape of the cross-section at the top of the mold (Figure 2-5).
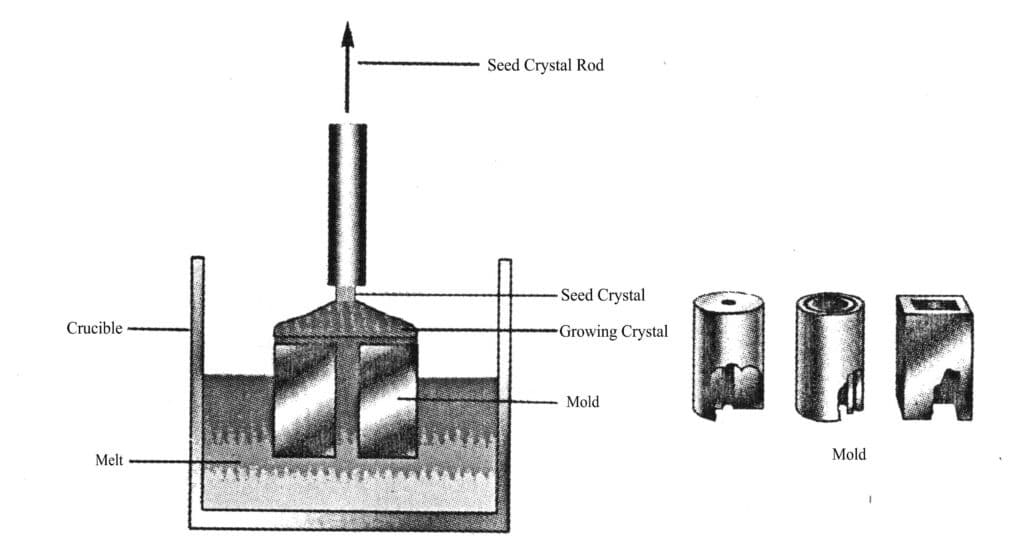
The key to growing crystals using the melt mold method is mold design and the temperature field within the furnace. The mold design must consider whether the melt has wetting properties with the mold material and whether there are chemical reactions, and the melting point of the mold material must be higher than that of the crystal; the temperature field design must ensure that the temperature at the mold opening is appropriate.
There are two different types of mold methods:
① Scepanov Method: Schepanov proposed this method from the Soviet Union in the 1960s. It involves placing a mold with a narrow slit in the melt, allowing the melt to rise to the top of the mold through capillary action, and upon contact with the seed crystal, the crystal is continuously pulled into the shape defined by the mold’s narrow slit as the seed crystal is lifted. The advantage of this method is that it does not require the mold material to be wetted by the melt.
② EPG method: It is a molding method successfully researched by Dr. H.E. Rapeal of the TYCO laboratory in the United States in the early 1970s, also known as edge-limited thin film feed growth technology. The primary condition for this method is that the melt must wet the mold material, and there must be no chemical reaction between them. Under the conditions that the wetting angle ɵ ,o < ɵ < 90° , the melt rises to the top of the mold due to capillary action, and the shape and size of the crystal cross-section are strictly determined by the shape and size of the top edge of the mold, rather than by the capillary slit.
This method’s specially shaped crystal materials can eliminate the heavy cutting, forming, and other mechanical processing procedures required for gemstone crystal processing, reduce material processing losses, save processing time, and thus significantly lower product costs.
(2) Characteristic of the melt molding method
① It can directly pull out specified shapes such as wires, tubes, rods, sheets, plates, and other special crystals from the melt.
② It can obtain uniformly composed doped crystals.
③ Easy to grow eutectic compound crystals with constant composition and good optical uniformity without growth patterns.
④ The crystals may contain conductive metal and seed crystal traces and defects.
⑤ Crystals often contain gaseous inclusions.
5. Cold crucible melting shell method
The cold crucible melting shell method for growing crystals does not require a special high-temperature material crucible. Still, it uses the crystal material to be grown as the “mold,” melting it internally through a high-frequency oscillator, serving as a conductive “crucible” melt. A cooling device is set up externally to keep the surface un-melted, forming an un-melted shell that acts as a crucible. The already melted crystal material crystallizes and grows based on the principle of crystal growth by the descending crucible method (Figure 2-6). This method grows crystals by transforming an amorphous solid phase into another form close to a solid phase through a liquid phase (melt).
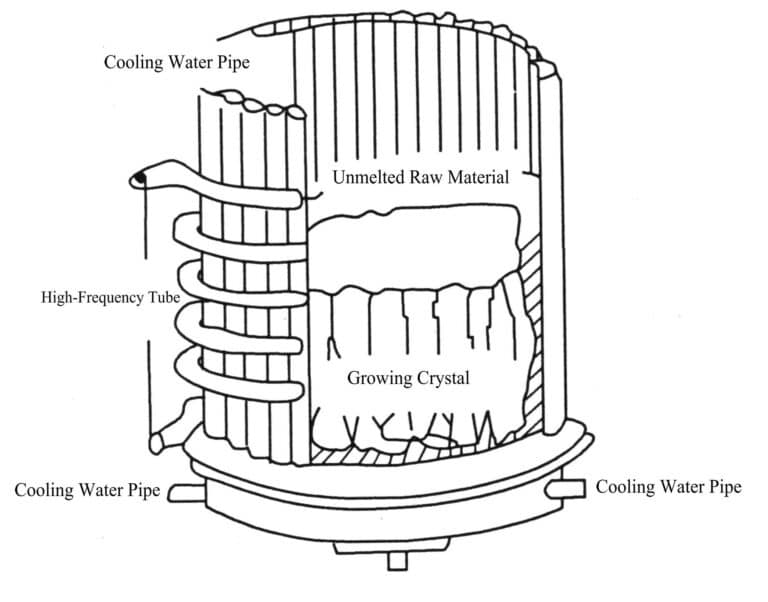
This method is mainly used to produce cubic lead oxide crystal materials. Since China began producing artificial cubic lead oxide in 1983, significant improvements have been made in equipment. Initially, each high-frequency furnace could only produce 5 kg per batch, but now it can produce 400 kg of artificial cubic zirconia, greatly increasing output and reducing costs; at the same time, the crystals produced previously were relatively small, weighing only a few dozen grams, but now can reach over 1980g per unit, and the colors are also more diverse.
The melting shell method for producing cubic zirconia crystals usually requires the purity of ZrO2 powder and stabilizer to be Y2O3 stabilizer to be 99%-99.9%. The impurity content should be less than0.005%-0.01% (NiO, TiO2, Fe2O3, etc.), to produce colored cubic Zirconia, it is only necessary to add coloring agents to the mixture to produce various colors crystals, especially the blue and green crystals, can imitate sapphires and emeralds (Table 2-3).
Table 2-3 Coloring agents and their corresponding body colors in synthetic CZ
| Coloring agent | Mass percentage content | Crystal color |
|---|---|---|
| Ce2O3 | 0.15 | Roșu |
| Pr2O3 | 0.1 | Galben |
| Nd2O3 | 2.0 | Violet |
| Ho2O3 | 0.13 | Galben deschis |
| Er2O3 | 0.1 | Roz |
| V2O5 | 0.1 | Galben-verde |
| Cr2O3 | 30.3 | Olive Green |
| Co2O3 | 0.3 | Deep Purple |
| CuO | 0.15 | Light Green |
| Nd2O3 + Ce2O3 | 0.09 + 0.15 | Rose Red |
| Nd2O3 + CuO | 1.1 + 1.1 | Light Blue |
| CO2O3 + CuO | 0.15 + 1.0 | Violet Albastru |
| CO2O3 + V2O5 | 0.08 + 0.08 | Maro |
6. Zone Melting Method
6.1 Principle
According to research by scientists such as Pu Fan, during the process of regional melting growth of crystals, the driving force for material transport comes from the density difference between a solid phase and a liquid phase of a substance. If the liquid phase density is greater than the solid phase density (volume contraction during melting), the material is transported towards the melting zone; otherwise, the material is transported in the opposite direction. Therefore, regional melting technology can control or redistribute the fusible impurities in the raw materials. By using one or several melting zones to repeatedly pass through the raw materials in the same direction to remove harmful impurities, the regional homogenization process (where the melting zone passes back and forth in both directions) can also effectively eliminate the segregation effect, uniformly incorporating the desired impurities into the crystal, and can to some extent control and eliminate structural defects such as dislocations and inclusions.
6.2 Process
The regional melting method is divided into containerized regional melting (Figure 2-7) and non-containerized regional melting. The growth of gemstone crystals often uses the non-crucible regional melting method, also known as the floating zone method (FZM).
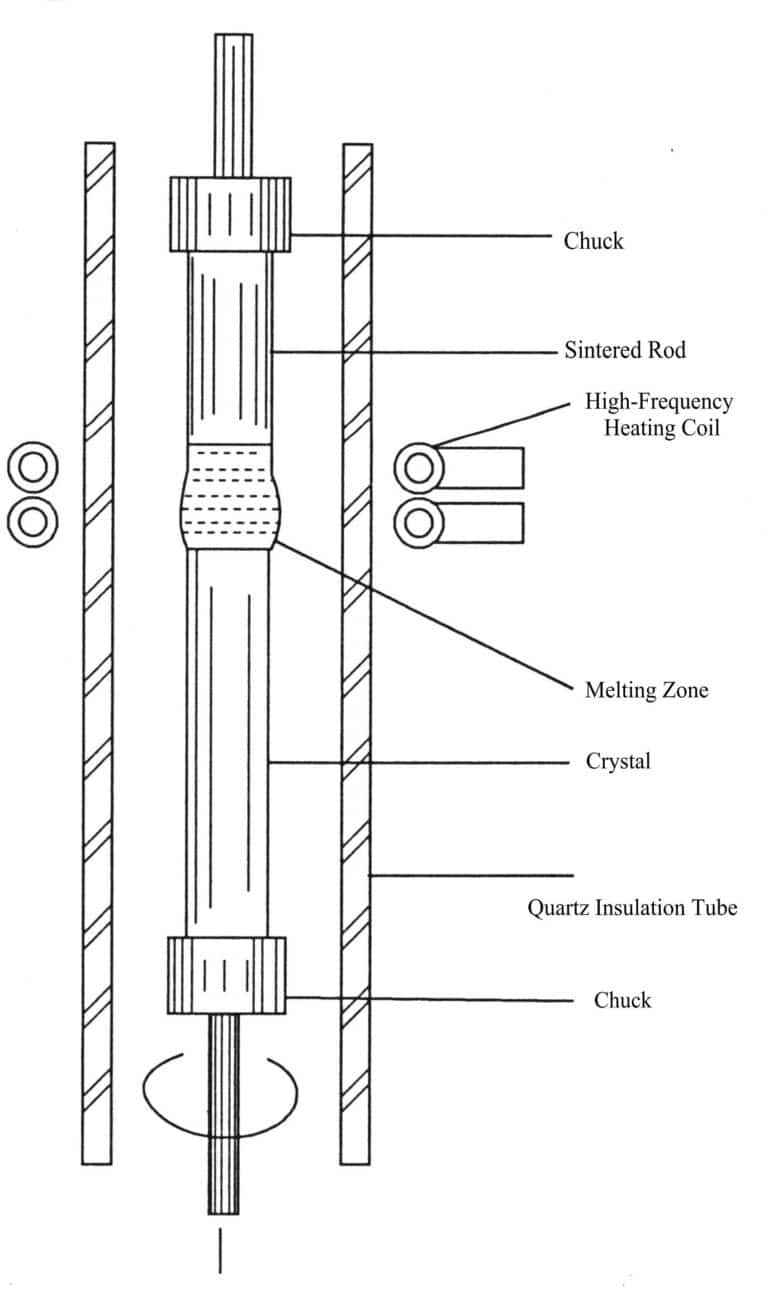
The process of the floating zone method is as follows: first, the crystal material is sintered or pressed into a rod shape and then fixed with two chucks; the sintered rod is vertically placed into the insulation tube, rotated and lowered (or moved by an accelerator) to melt the rod material; the molten zone is in a floating state, supported only by surface tension without allowing the liquid to fall, thus obtaining a purified or recrystallized single crystal.
Induction heating is the most widely used method in the floating zone synthesis of gemstone crystals, which can be applied in a vacuum or in any inert oxidizing or reducing atmosphere.
The movement of the molten zone can be achieved in two ways: one is that the raw material sintered rod remains stationary while the heater moves; the other is that the heater remains stationary while the raw material sintered rod moves.
The actual temperature distribution in the molten zone often depends on the characteristics of the power and heat source, the cooling device, the sintered rod’s thermal conductivity, and the solute concentration in the liquid phase, among other factors. The general requirement is that the temperature within the molten zone should be greater than the melting temperature of the raw material, while the temperature outside the molten zone should be less than the melting temperature of the raw material.
6.3 Characteristics of the zone melting method
(1) No crucible impurity contamination in the crystal.
(2) Good crystal quality, with very few inclusions and growth lines.
(3) High purity, very clean internally.
(4) A sudden change in process conditions during crystal growth can cause chaotic growth lines and uneven color in the crystal.
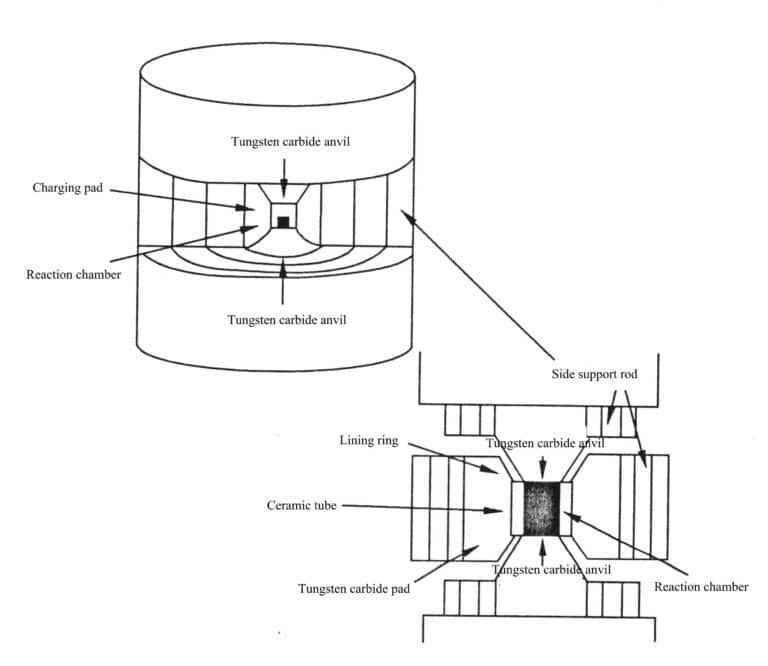
7. High-Temperature and Ultra-High-Pressure Method
The high-temperature and ultra-high-pressure method for synthesizing gemstone crystal materials refers to the use of high-temperature(above 500℃) and ultra-high pressure (above 1.0x 109 Pa) equipment to cause the synthetic gemstone raw materials (powder samples) to undergo phase changes or melting and subsequently crystallize under high-temperature and ultra-high-pressure conditions, similar to metamorphic processes. This method is mainly used to produce diamonds, jade, and others.
Methods to achieve high temperature and ultra-high pressure include static pressure methods and explosive methods (explosives, nuclear explosions).
7.1 Methods for synthesizing diamonds
There are dozens of methods for artificially manufacturing diamonds and successful methods can be divided into three main categories:
(1) Static Pressure Method
① Static Pressure Catalyst Method
② Static Pressure Direct Conversion Method
③ Seed Catalyst Method
(2) Explosive Method (Dynamic Method)
① Explosion Method
② Liquid Discharge Method
③ Direct Transformation of Hexagonal Diamond Method
(3) Growth Method in the Metastable Region
① Vapor phase method
② Liquid phase epitaxy method
③ Gas-liquid-solid phase epitaxy method
④ Atmospheric pressure high-temperature synthesis method
The method commonly used for synthesizing diamonds is the seed catalyst method (Figure 2-8). In 1963, China produced industrial-grade synthetic diamonds using high-temperature and ultra-high-pressure methods, where each Synthesis could only yield small particles of synthetic diamonds. Each Synthesis can produce 60ct of synthetic diamonds with significantly larger particles.
7.2 Methods for synthesizing jadeite
(1) Weigh the chemical reagents (sodium silicate and aluminum silicate), mix, heat, and melt to form jade glass material (NaAlSi2O5).
(2) Crush the jade glass material into powder, mix with coloring agents, and load into a high-purity graphite crucible in 140℃ bake for more than 24 hours , then perform high-temperature ultra-high pressure (1100℃treatment for 5.9 x 107 Pa (4 hours), cuts off power to cool down, and condenses crystallize into a hard jade aggregate.
Laboratory observation: Under the color filter, some synthetic products appear red, while others appear green, indicating that some chromium ions have entered the lattice while others have not yet entered the lattice.
The key to synthesizing jadeite to meet gemstone-grade requirements is to make it reach clarity and allow Cr3+ to enter the lattice.
Types of coloring agents that can color jadeite are shown in Table 2-4.
Table 2-4 The effect of different colorants at different concentrations on the color of jadeite
| Colorant | The color change of jade glass material as the content varies from 0.01%-10% from small to large |
|---|---|
| Chromium oxide | Lemon yellow →Yellow-green→Green-yellow→Dark green→Olive green→Light blue |
| Cobalt oxide | Azure blue→Dark cobalt blue |
| Nickel oxide | Light lotus color→Lotus color → Purple→Blue-purple →Dark blue |
| Copper oxide | Light blue→Sky blue→Sea blue→Deep ink blue |
| Manganese oxide | Light lilac →Lilac → Deep lilac→ Purple |
| Iron oxide | White→Light yellow-green→Light yellow-brown |
| Titanium oxide | Gray→ Light gray →White |
| Neodymium oxide | Purplish-red under fluorescent light →Bluish-purple under sunlight ( color change effect ) |
| Lutetium oxide | With a fresh green hue |
| Vanadium pentoxide | White with a blue hue →White with a red hue |
| Cerium oxide | White→ with a slight reddish tint |
| Tin dioxide | White with a greenish tint → white with a slight reddish tint |
| Ferric oxide | White with a slight yellowish tint |
| Selenite | A white color with a pinkish hue |
8. Chemical precipitation method
The chemical precipitation method mainly includes chemical vapor deposition and chemical liquid phase deposition. Crystal growth occurs through the transformation from liquid or gas to crystal phase. For example, using the chemical liquid phase deposition method to synthesize polycrystalline gemstone materials such as opal, turquoise, lapis lazuli, and malachite, as well as using the chemical vapor deposition method to synthesize polycrystalline diamond films, large-grain diamonds, and silicon carbide single crystal materials.
8.1 Vapor phase synthesis of diamond films
The gas produced from low molecular weight hydrocarbons mixed with hydrogen is dissociated under certain temperature and pressure conditions, generating carbon ions in a plasma state. Then, guided by an electric field, the carbon ions grow into polycrystalline diamond film layers on a diamond or non-diamond (Si, SiO2, Al2O3, SiC, Cu . etc.) substrate.
There are various CVD methods: hot wire CVD, microwave plasma CVD, DC plasma CVD, laser plasma CVD, plasma-enhanced PECVD, and flame methods. Based on the principle of plasma generation, all CVD methods can be divided into four categories: pyrolytic CVD, DC plasma CVD, radio frequency plasma, and microwave plasma CVD.
8.2 Vapor Phase Precipitation Method for synthesizing silicon carbide
The structure of silicon carbide SiC has more than 150 configurations. Currently, only the 4H and 6H configurations of α- SiC can grow into large crystals belonging to the hexagonal phase.
(1) Ajfa Method: Mix carbon (petroleum coke or anthracite C) with sand (SiO2) and a small amount of sawdust and salt, place it into a graphite rod wrapped with the mixture, apply electricity and heat to 2700℃ to produce SiC(SiO2 + 3C→SiC + 2CO) .
(2) Lely Method: The raw powder for producing silicon carbide single crystals is heated and sublimated into gas after passing through a porous graphite tube, crystallizing directly on the seed without going through the liquid phase, resulting in pear-shaped SiC single crystals.
8.3 Example: Synthesis of Opal by Chemical Precipitation
(1) Principle of Opal Synthesis
From a chemical composition perspective, the components of opal consist of water-containing 3%-10%silica, where the spheres in its structure are made up of amorphous silica or quartz and water, The ratio of silica to water varies slightly, usually containing more silica, provide sufficient refractive index differences for diffraction. For the above reasons, opal has a special play-of-color effect. The colors of the play-of-color are related to the size of the silica spheres: when the diameter of the spheres is less than 138 nm, only ultraviolet light is diffracted, and the play-of-color effect is not observed; when the diameter is 138 nm, purple play-of-color predominates; at a diameter of 241 nm, various colors from first-order red to first-order purple appear, which is also the best quality and most richly colored opal; when the diameter exceeds 333 nm, diffraction is limited to infrared light, and opal will not exhibit the play-of-color effect. Opal is usually composed of aggregates of different particles, each particle arranged in layers of uniformly sized spheres, forming a three-dimensional grating. Therefore, on an Opal polishing surface, you can see some color maps composed of small pieces of color, the size of the color area is between 1 – 10 mm, which is determined by the size of the SiO2 sphere particles.
The revelation of the mysteries within opal provides a theoretical basis for the Synthesis and imitation of opal. Although the principle is simple, it was not until 1972 that P. Gilson successfully synthesized synthetic opal for the first time. Practical synthetic opal began to be marketed in 1974.
(2) The process of artificially synthesizing opal.
Although the synthesis method of opal is a strictly confidential trade secret, it is generally believed that the production process for synthetic opal can be divided into three steps:
① Formation of silica spheres. This is generally achieved using certain high-purity organosilicon compounds, such as tetraethyl orthosilicate, which generate monodisperse silica spheres through controlled hydrolysis. Typically, tetraethyl orthosilicate is usually dispersed in the form of small drops in the aqueous solution of ethanol, adding ammonia and other weak bases and stirring, so that it is converted into water-containing silica spheres.
During the reaction process, care must be taken to control the speed and concentration of the reactants, so that the prepared silica spheres have the same size. Depending on the type of opal required, the diameter of the resulting spheres may vary. (The sphere diameter is 200 nm, 300 nm etc.)
② Precipitation of silica spheres. The dispersed silica spheres are precipitated in a solution with controlled acidity and alkalinity. This step may take more than a year. Once precipitated, these spheres will automatically present the most closely packed arrangement.
③ Compaction of spheres and generation of synthetic opal. This step is crucial for achieving gem-quality requirements and is the most difficult. The product from the second step is similar to barium feldspar, which is very brittle and will quickly dry out and lose its color, so the spheres must be compacted. The method for compacting the spheres is to apply hydrostatic pressure to them. They are placed in a steel piston during pressurization, and a pressure-transmitting liquid is added. As the amount added increases, hydrostatic pressure is applied in all directions to the precipitated spheres without causing deformation.
There are several synthetic opal varieties, including white opal, black opal, and fire opal. The main producing countries are France and Japan.