How to do Maintenance and Care of Precious Metal Jewelry?
Ultimate Guide to Precious Metal Jewelry Care: Maintenance, Repair, and Restoration
Introduction:
Precious metal jewelry uses precious metals as the base material, carrying functions such as value preservation and appreciation, decoration, wearability, and symbolic commemoration. In daily wear, jewelry inevitably experiences deformation, breakage, wear, corrosion, and discoloration, which affect its usability and decorative effect and must be restored through maintenance and repair.

Table of Contents
Section Ⅰ Deformation and Breakage of Precious Metal Jewelry
1. Deformation
While wearing jewelry, it is inevitably subjected to external forces. When the external stress exceeds the yield strength of the material itself, it will cause permanent plastic deformation, leading to a change in shape.
1.1 Influencing Factors and Improvement Measures
(1) Yield strength of materials.
The lower the yield strength of a material, the poorer its ability to resist deformation, making it more prone to deformation. Among common jewelry materials such as gold, silver, and platinum, high-purity gold, silver, and platinum jewelry generally have low strength, especially when annealed, making them highly susceptible to deformation, as shown in Figure 7-1. By adding alloying elements for alloying treatment and utilizing solid solution strengthening fine grain strengthening, and dispersion strengthening, the strength of the materials can be effectively improved, thereby enhancing the resistance of precious metal jewelry to deformation.

(2) The wall thickness of jewelry.
Jewelry wall thickness is an important factor affecting the deformation, under the same external force, the smaller the wall thickness or diameter of the jewelry, the higher the external force (stress) per unit area, the more likely to cause deformation of the jewelry, especially with the openwork decoration of high-purity jewelry. For example, traditional filigree silver jewelry is made from fine silver wires to create hollow decorations. During the manufacturing process, a lot of welding is used, resulting in poor resistance to deformation. A slight oversight can lead to deformation, as Figure 7-2 shows.

For set jewelry, to ensure the stability of the gemstones, the prongs, pins, or borders used to secure the gemstones must have a specific thickness or diameter to prevent them from deforming or even breaking, which could cause the gemstones to fall out and be lost. Taking a four-prong setting for a round gemstone as an example, the light industry standard “Quality Evaluation Specification for Precious Metal Jewelry Craft” (QB/T 4189-2011) specifies the relationship between the prong diameter, prong groove depth ratio, prong height ratio, and gemstone diameter, as shown in Table 7-1.
Table 7-1 Requirements for the Firmness of Gemstone Settings with Four Claws
| Gemstone Diameter /mm | Claw Diameter /mm | Claw Slot Depth Ratio (H1) | Claw Retention Height Ratio (H2) |
|---|---|---|---|
| 2.5 - 2.8 | ≥0.40 | ≤ 1/2 | ≥55% |
| 2.9 - 4.1 | ≥0.50 | ≤ 1/2 | ≥55% |
| 4.2 - 5.2 | ≥0.65 | ≤ 3/4 | ≥55% |
| 5.3 - 6.8 | ≥0.75 | ≤ 3/4 | ≥55% |
| 6.9 - 15.0 | ≥0.8 | ≤ 3/4 | ≥55% |
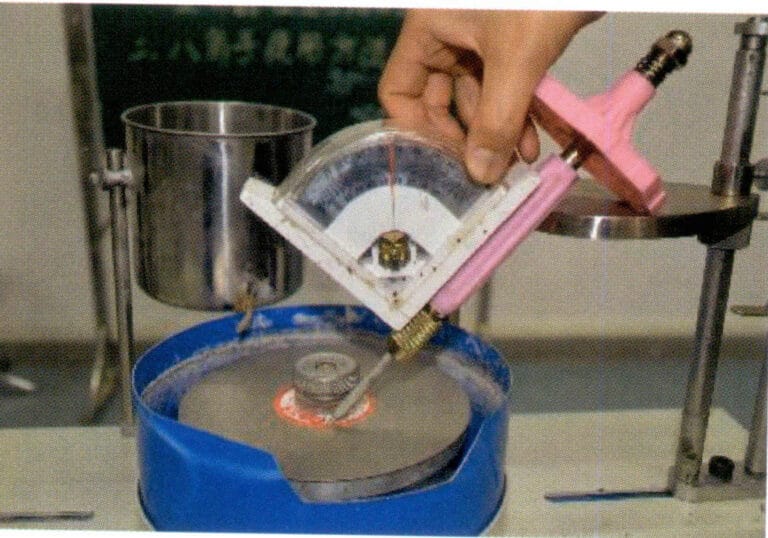
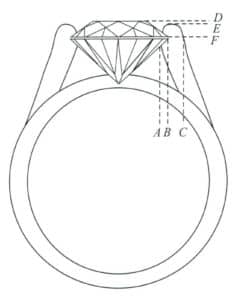
Among them, the prong groove depth ratio refers to the opening on the prong, the “V” shaped groove that easily appears in silver filigree jewelry, when viewed from the side in the direction of the gemstone’s waist edge, measures the percentage of the groove depth to the prong diameter, represented by H1, as shown in Figure 7-3, H1 = AB/AC; the prong height ratio is the percentage of the height from the gemstone’s waist edge to the prong tip compared to the height from the waist edge to the gemstone’s table surface, represented by percent, H2 = FE/FD.
Traditional solid gold jewelry is sometimes set with gemstones. Due to the low strength of solid gold materials, they are prone to deformation, which can lead to the loss of gemstones. Therefore, the industry standard “Durability of Gemstone Setting in 24K Gold Jewelry” (QB/T 4114 – 2010) specifies product categories, setting methods, quality, and corresponding durability requirements for settings, as shown in Table 7-2. Among them, the durability of the setting refers to the firmness with which gemstones are set in precious metal jewelry in different ways, represented by the vertical force applied to the bottom of the gemstone required to detach it from the setting. To meet the durability requirements for 24K gold jewelry settings, the most basic requirement is to control the thickness of the prongs (edges).
Table 7-2 specifies the durability values for gemstone settings in 24K gold jewelry
| Product Category | Inlay Method | Inlay Firmness/N |
|---|---|---|
| Men's Ring | Prong Setting | 60 |
| Men's Ring | Bezel setting | 80 |
| Women's ring | Prong Setting | 20 |
| Women's ring | Bezel setting | 40 |
| Ear studs (earplugs) | Prong Setting | 20 |
| Ear studs (earplugs) | Bezel setting | 30 |
| Bracelet | Prong Setting | 20 |
| Bracelet | Bezel setting | 30 |
Although increasing wall thickness is an effective way to improve the deformation resistance of jewelry, however, increasing wall thickness can sometimes lead to issues. Taking electroformed hard 24K gold hollow jewelry as an example, the overall requirements are sufficient to color, high strength, and lightweight. However, as wall thickness increases, it will lead to an increase in gold weight, raising the product price and reducing market attractiveness; secondly, the clarity of the surface of the electroformed piece decreases, especially in some fine decorative areas; thirdly, the internal stress of the electroformed piece increases, which will increase its brittleness.
(3) Jewelry structure.
Different structures have varying abilities to withstand external forces. For diamond-set jewelry, a hollow structure is generally used at the bottom of the setting to reduce the weight of the jewelry base and enhance the brightness of the diamond. However, this compromises the strength of the setting, especially for wax-set cast diamond jewelry, which can easily lead to deformation of the setting and cause stones to fall out, as shown in Figure 7-4. Therefore, it is necessary to add a certain number of supports at the bottom of the setting, as shown in Figure 7-5, to ensure that the setting has sufficient strength without significantly affecting the brightness of the diamond.
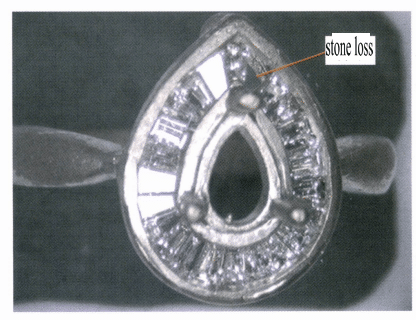
Figure 7-4 Insufficient setting strength can easily lead to stone loss.
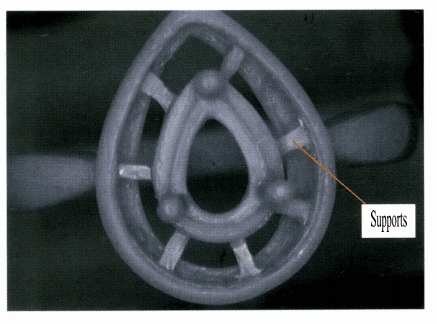
Figure 7-5 Supports added at the bottom of the setting
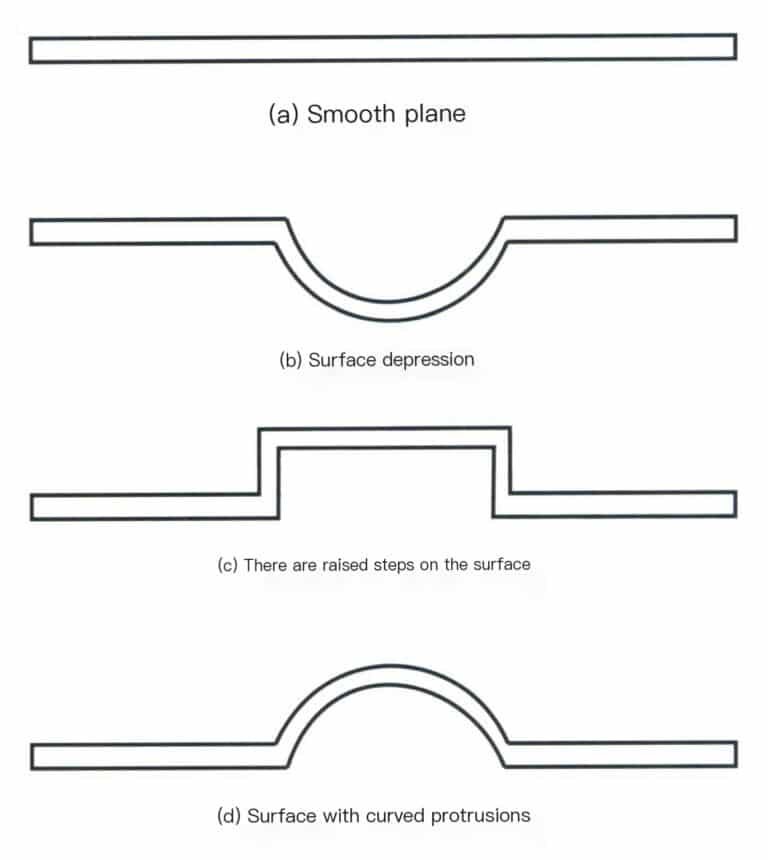
In electroformed hard gold jewelry, if the surface of the jewelry product is a smooth plane without curvature, as shown in Figure 7-6(a) when its area exceeds 1cm2, its ability to withstand external forces significantly decreases, and a slight pressure in the middle may cause a dent.
Figures 7-6(b) to 7-6(d) show the modified structures. Figure 7-6(b) shows a depression formed in the plane’s center. The indentation slightly increases the ability to withstand external forces to a certain extent, but when the depth-to-width ratio of the indentation is slightly larger, there may be insufficient wall thickness at the indentation area, leading to cracking and falling off; Figure 7-6(c) shows a step-like protrusion formed in the center of the plane, which provides almost no benefit for withstanding external forces and may even be more prone to deformation under compression; Figure 7-6(d) shows a curved protrusion formed in the center of the plane, which greatly improves the ability of the casting to withstand external forces. The wall thickness is also basically uniform. Therefore, when designing jewelry structures, it is essential to consider the production process’s feasibility and the casting’s deformation resistance, as not all jewelry that meets aesthetic viewpoints can be produced.
Generally, curved structures are preferred, and the deformation resistance varies with different shapes of protrusions. Figure 7-7 shows three surface protrusions of the same area but different shapes, among which the deformation resistance of Figure 7-7(a) is lower than that of Figures 7-7(b) and 7-7(c).
![Figure 7-7 Three surface protrusions of the same area but different shapes [(a) - (c), enhanced deformation resistance]](https://sobling.jewelry/wp-content/uploads/2024/09/6.png)
Many electroformed hard gold jewelry pieces have flat bottoms with a large area. To increase their ability to withstand external forces, small holes can be densely punched into the flat bottom of the original piece, allowing the electroformed gold also to have such holes on the bottom surface, as shown in Figure 7-8. This structure can significantly improve the deformation resistance of the flat surface. Multiple hollowing methods can be employed when the jewelry has a larger shape, as shown in Figure 7-9. This structure creates a special decorative effect and benefits deformation resistance.
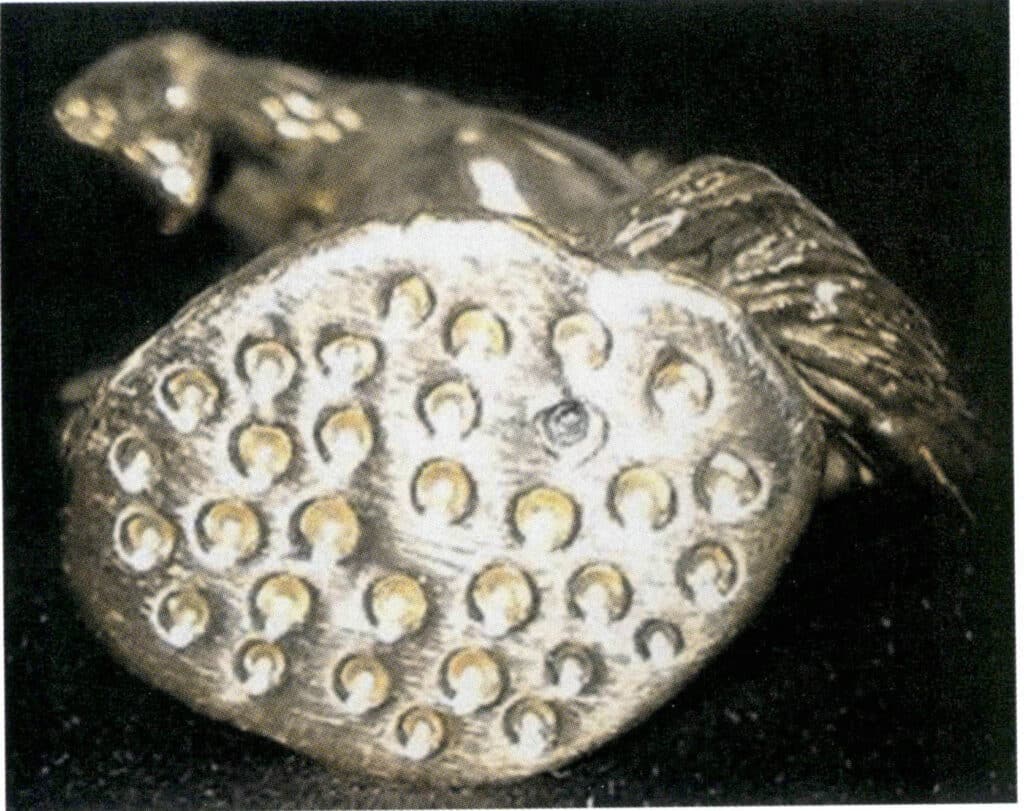
Figure 7-8 Punching holes in the bottom flat surface of jewelry to prevent deformation.

Figure 7-9 Multiple hollowing on the surface of jewelry to prevent deformation
(4) Jewelry processing technology.
Different processing techniques for jewelry result in significant differences in the strength of the final products. As an example, 24K gold jewelry’s cast hardness is only around HV30, and the annealed state is even lower, making it prone to deformation. However, its strength can be significantly increased if cold working is used for shaping. In recent years, hardened 24K gold has appeared on the market, and besides the strengthening effect of trace alloying elements, a crucial part of the strengthening comes from cold working. These products, when worn, can quickly lose strength and hardness if exposed to high temperatures, making them susceptible to deformation.
(5) Wearing and usage methods.
Jewelry styles are diverse, and deformation can be corrected for jewelry with simple structures through reshaping repairs. However, not all deformed jewelry can be restored; some complex structured pieces or closed hollow jewelry are difficult to repair after being compressed and deformed. For example, the closed hollow silver bracelet shown in Figure 7-10 is nearly impossible to restore without damage after its surface is dented. More and more personalized jewelry pieces are made through 3D printing, many of which have very delicate and complex structures, leading to a high probability of deformation. For instance, the pendant shown in Figure 7-11 has multiple layers of hollow structure, and when the inner structure deforms, the repair difficulty is very high.

Figure 7-10 Closed Hollow Silver Bracelet

Figure 7-11 3D Printing Multi-layer Hollow Pendant Repair Difficulty After Deformation
Therefore, for jewelry with delicate and complex structures, reducing deformation largely depends on the method of wearing and daily maintenance. Care should be taken during the wearing process to avoid collisions or pressure. Rings should be removed during physical labor or intense exercise, not only to protect the ring but also to protect one’s fingers. When wearing solid gold necklaces and bracelets, care should be taken not to pull too hard to avoid deforming the jewelry.
1.2 Repairing Deformed Jewelry
(1) Deformed Rings.
For solid gold and silver rings, the ring should not be deformed by hand. When the degree of deformation is relatively minor, you can find a cylindrical object and the inner diameter of the ring is almost the same, and then put the ring on the top, on a flat table or glass plate, rolling a few times, so that the ring can be restored to the shape of the circle. If it is a K gold or silver alloy ring, you can use a ring bar and a rubber mallet to solve the problem. Press the deformed part of the ring onto the ring bar, and use the rubber mallet to slowly beat the surface of the ring, while beating and rotating, until the ring becomes round, as shown in Figure 7-12. Keep in mind the desired ring size, and repeat the measurement with the ring mallet to prevent the ring from becoming larger due to hammering. If there is no ring rod and rubber hammer, you can find a slightly smaller diameter round metal sleeve or cylinder, wrapped in a soft cloth outside the hammer, the deformed part of the ring towards yourself, gently hit the hammer a few times, be careful not to rush, always check the curvature of the ring, rather than more than a few times, otherwise it is likely to hit too much, will be a section of the ring to knock the flat or the ring inch bigger. If the ring is seriously deformed, it should be sent to a jewelry store for after-sale treatment, where it will be restored and repaired by a professional technician using professional tools.

(2) Bracelet deformation.
Unlike rings, bracelets are larger and can be more difficult to restore to their original shape after deformation. The repair method should be determined based on the product’s material, structure, and degree of deformation. For silver bracelets with smaller widths and wall thicknesses, you can adjust the shape by hand or with certain tools, carefully applying gentle force to avoid breaking them. When using tools to restore the shape, it is best to place a cloth under the bracelet and then shape it with the tool to avoid scratching the surface. You can wrap a conical tool with a silk cloth and rotate it slowly. For solid gold bracelets, you can refer to the method for silver bracelets. A K gold bracelet can be gently tapped on a conical mold if it is deformed. Severely deformed bracelets should be taken to a jewelry store for professional repair.
(3) Earring deformation.
If the deformation is slight, you can correct it yourself. Be careful not to apply too much force when correcting the earring; use gentle pressure to gradually restore the deformed earring to its original shape. Before shaping, finding a reference object, such as a ruler, is best to accurately straighten the deformed earring. For more severe deformations, it should be handed over to a professional repair technician at a jewelry repair shop for restoration.
(4) Necklace deformation.
If the deformation is slight, first check the deformed area at the knot, then use tweezers to straighten the deformed part until it is no longer knotted. For more severe deformations, it should be handed over to a professional repair technician at a jewelry repair shop for restoration.
(5) Choker deformation.
For slight deformations, you can place a slightly larger round enamel bowl upside down on the table, cover it with silk cloth, and place the choker on the inverted bowl. Gently tap the deformed area while continuously rotating and changing positions. Be careful not to re-tap at the joints to avoid deforming the hollow joints, making it difficult to fasten. For more severe deformations, it should be handed over to a professional repair technician at a jewelry repair shop for restoration.
2. Fracture
The fracture of precious metal jewelry refers to a serious failure where cracks occur under external forces or internal stresses, leading to complete disconnection and inability to wear normally.
2.1 Causes of Fracture
(1) Chain Fracture.
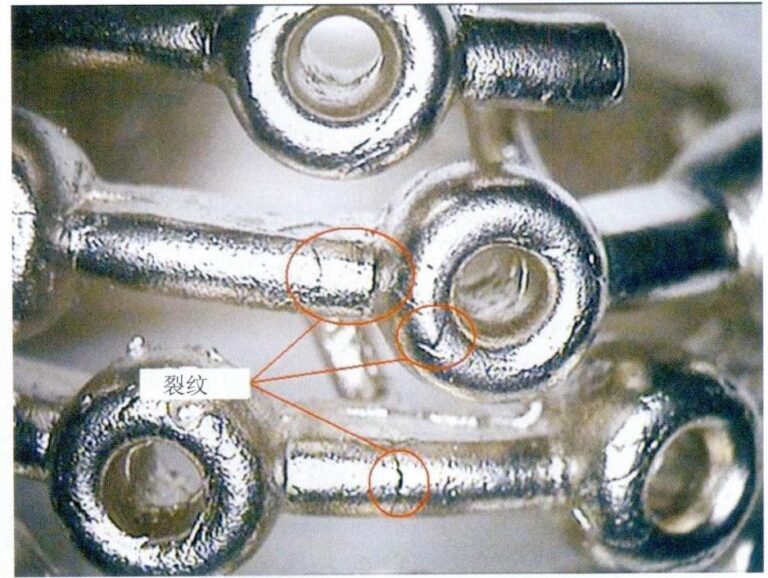
Chains are flexible components connected by links, and the strength of the link connection is crucial for the safe use of the chain. If the external force on the chain exceeds the connection strength, it may cause the chain to break, as shown in Figure 7-13. Chain fractures are influenced by both the quality of the chain itself and external factors.
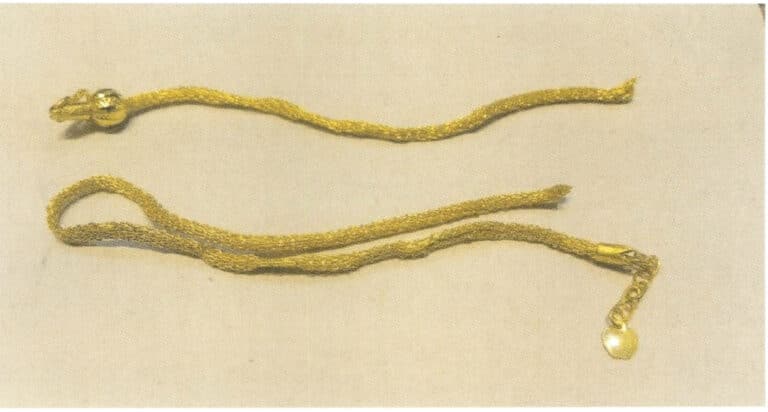
Chains are usually relatively thin, and the strength of precious metals is not high compared to other metal materials, with a limited force that can be sustained per unit area. Excessive force may lead to fracture when the chain is subjected to external pulling forces during wear.
Processing chains must go through processes such as smelting and casting, linking, welding, molding, polishing, or electroplating. The manufacturing quality of these processes may pose hidden dangers for chain breakage. For example, if the metallurgical quality of the molten metal is poor during smelting, and if there are inclusions or sand holes in the chain links, it will reduce the chain links’ effective cross-sectional area and lower the chain’s mechanical strength. If the chain links are bent back and forth repeatedly during linking, the shaping of the chain links will be reduced. If there are defects such as false welding or inclusions during welding, the strength of the welded area will be reduced. If the chain is excessively thinned during molding and polishing, it can easily lead to chain breakage. The “Quality Evaluation Standards for Precious Metal Jewelry” (QB/T 4189-2011) specifies requirements for the firmness of chain products, as shown in Table 7-3. Based on the quality of the chain, weights are selected for testing. The weights are hung on the jewelry hook, and then one side of the jewelry is hung on a support. If the chain does not break or come apart after being left still for 1 minute, it is considered to have passed the test.
Table 7-3 Requirements for the firmness of chain products
| Chain quality G/g | Select the weight /g |
|---|---|
| ≥2 | 300 |
| < 2 | 200 |
(2) The breakage of rose gold jewelry.
Rose gold is a red gold alloy primarily composed of Cu as the main alloying element. During the cooling process from high temperatures, an ordering transformation may occur, and the resulting ordered phase will reduce the material’s plasticity. Especially for 18K rose gold with a high Cu content, if it cools slowly in the sensitive temperature range where the transformation occurs, it is prone to ordering transformation, and the alloy will exhibit significant brittleness. A slight external force or impact may cause the jewelry to crack or completely break, as shown in Figure 7-14. This transformation can occur during the casting cooling stage and during annealing or welding processes. If cooling is slow, a certain degree of ordering transformation may also occur. Therefore, the main reason for the breakage of rose gold jewelry is its material properties. In addition to selecting appropriate repair methods, when thermally processing jewelry, one should adopt a slow cooling method to reduce thermal stress and minimize the total and organizational stress from the ordering transformation. During wear, avoiding exposing the jewelry to significant impacts, pulls, bends, or other external forces is also important.

(3) Fracture of white K gold with claws.
Jewelry may retain residual stress during processing, and while wearing the jewelry, the combined effects of residual stress and corrosive environments may lead to stress corrosion. Different jewelry materials have varying sensitivities to stress corrosion, with K platinum, which primarily contains Ni as a bleaching element, being more sensitive to stress corrosion compared to other materials; different grades of K gold also exhibit different tendencies towards stress corrosion, generally speaking, lower-grade K gold materials have a greater tendency for stress corrosion. Taking 9K gold as an example, when a round wire sample is immersed in a 10% ferric chloride solution, and a certain stress is applied to the sample after a certain period due to the preferential electrochemical dissolution of alloying elements such as Cu, Zn at dislocations, stacking faults, and grain boundaries, cracks may initiate. The stress causes fresh metal to be continuously exposed to the corrosive medium, leading to the gradual expansion of cracks, ultimately resulting in intergranular fracture of the sample, with the fracture morphology exhibiting typical brittle fracture, as shown in Figure 7-15.
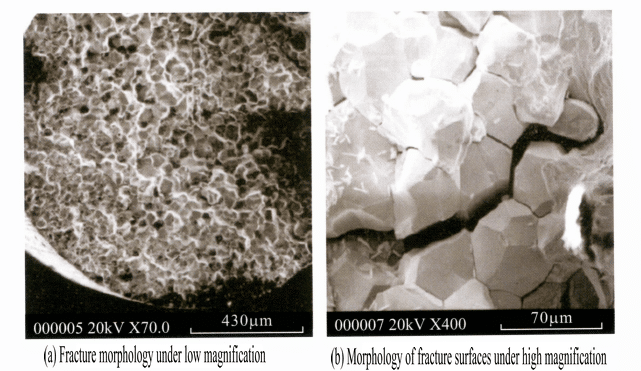
As mentioned earlier, the residual stress reduces the electrode potential of the alloy, so that the corrosion resistance of the material decreases, and it interacts with the corrosive environment to cause visible or potential cracks. The higher the residual stress, the more corrosive the corrosive medium, the more likely to exacerbate the role of stress corrosion cracking. Therefore, to effectively prevent the occurrence of stress corrosion cracks in jewelry, first of all, should choose stress corrosion tendency of small materials, in the production process to try to eliminate the residual stress and microcracks in the material, in the process of wear and use should pay attention to the daily maintenance, in the corrosive environment of the jewelry, pay attention to the usual cleaning and care, to avoid corrosive media for a long time in the local build-up!
2.2 Prevention and Repair of Jewelry Fracture
When purchasing jewelry, carefully examine the craftsmanship quality, especially for chain products like necklaces. Observe the thickness and uniformity of the chain, determine if any sections are overly thin, check if all welding points are in place, and look for any sand holes or cracks.
Since jewelry is usually delicate and fragile, proper wearing, usage, and daily maintenance are important to prevent jewelry from breaking. When wearing jewelry, avoid subjecting it to strong external forces, and do not pull on necklaces forcefully with your hands to prevent breakage. Jewelry should be removed when engaging in physical labor, bathing, or contacting corrosive substances.
When jewelry breaks, specialized welding tools, equipment, materials, and professional welding operations are required for repair. Collect all broken parts and send them to the jewelry after-sales service for professional maintenance.
Section II Wear and Fatigue of Precious Metal Jewelry
1. Wear of Precious Metal Jewelry
The wear of precious metal jewelry refers to the phenomenon where, during wearing, the shape and size decrease and the surface roughness increases due to friction, collision, and other actions.
The hardness value is an important indicator for measuring materials’ wear resistance. Different jewelry materials have different hardness levels, resulting in significant differences in wear resistance. The higher the material’s hardness, the better its ability to resist static or dynamic friction wear. Precious metals such as gold, silver, and platinum, which have high purity, usually have lower hardness and are prone to scratches during use. Moreover, pure precious metal jewelry often has decorative patterns carved on the surface to avoid monotony. Long-term wear can lead to wear and tear, causing the patterns to be worn down or even disappear, losing their original appearance. The hardness of precious metal alloys like K gold and silver alloys is significantly higher than that of pure precious metals, improving their resistance to friction wear, which helps maintain the surface brightness of the jewelry. However, surface wear marks may inevitably appear after a period of wear, as shown in Figure 7-16. In particular, most K white gold jewelry is plated with rhodium. When the surface plating wears off in certain areas, it creates a noticeable color contrast with the base, deteriorating the decorative effect of the jewelry.
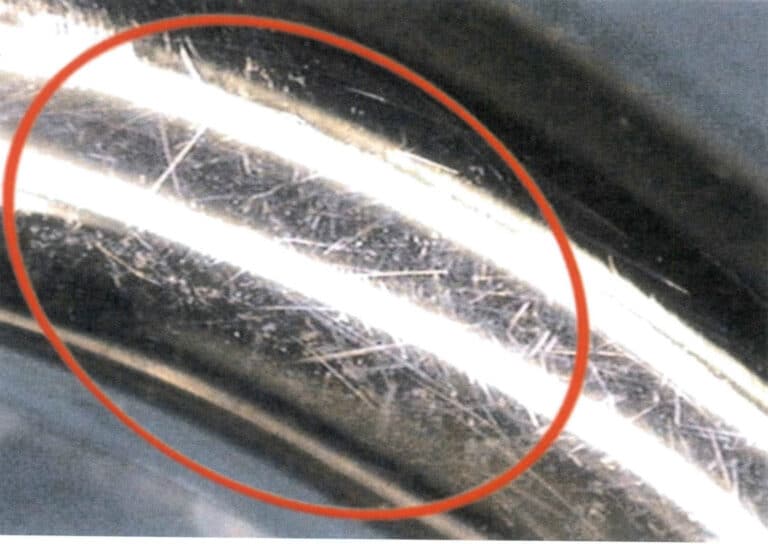
Therefore, when wearing and storing precious metal jewelry daily, the following points should be noted:
(1) Develop a habit of frequently putting on and taking off jewelry. When engaging in high-intensity sports or heavy physical work, try not to wear jewelry, especially low-hardness pure precious metal, as it can easily get damaged due to impact and friction. Jewelry should also be removed before entering the kitchen or going to bed to better maintain its surface brightness.
(2) When wearing pure gold jewelry, you should put on your clothes and only then wear the jewelry; when removing makeup, you should take off the jewelry first.
(3) When storing jewelry, it should be kept separately according to the properties of different materials. Refrain from mixing jewelry casually, as the materials have different hardness levels, and placing them together can easily cause surface wear due to collision and friction. For pure gold jewelry, it is recommended to wrap it in a soft cloth to avoid bumps.
(4) Develop the habit of regularly checking your jewelry, paying attention to whether there are any loose or worn areas. If such conditions are found, maintenance should be carried out promptly. Jewelry that is not suitable for repair can be exchanged for new items.
When the surface of precious metal jewelry is worn, it must be polished again to restore its original brightness. Jewelry made of low-hardness pure gold or pure silver is usually polished using agate knives or steel presses. In contrast, higher hardness K gold jewelry requires polishing with polishing cloth wheels. After polishing precious metal jewelry made of silver alloys or white K gold, additional processes are needed. Rhodium or gold plating is applied to further enhance the surface brightness of the jewelry, achieving a refreshed decorative effect.
Copywrite @ Sobling.Jewelry — Custom jewelry manufacturer, OEM and ODM jewelry factory
2. Fatigue of Precious Metal Jewelry Fasteners
2.1 Common Types of Jewelry Fasteners
Fastening and locking components are essential parts of the structures of precious metal jewelry, such as bracelets, necklaces, bangles, and earrings. Common types of fasteners include the following.
2.1.1 Fasteners used for chain-type items like bracelets or necklaces
(1) Lobster clasp. Figure 7-17 shows that its basic structure includes the clasp body, opening and closing button, and spring mechanism. The clasp body consists of a hook-shaped body, a connecting part integrated with a barrel-shaped body, and a hanging hole integrated with the connecting part. The opening and closing buttons are equipped with a mounting groove for installing the spring mechanism, and the mounting groove has a connecting pin integrated with the opening and closing buttons. The spring mechanism is fitted onto the connecting pin. The lobster clasp keeps the arm closed through the pressure of the built-in spring, making it convenient to wear.
(2) Spring loop clasp. As shown in Figure 7-18, when the arm of the clasp is pulled back, the spring loop opens, and when released, the pressure of the spring keeps the clasp arm tightly closed.
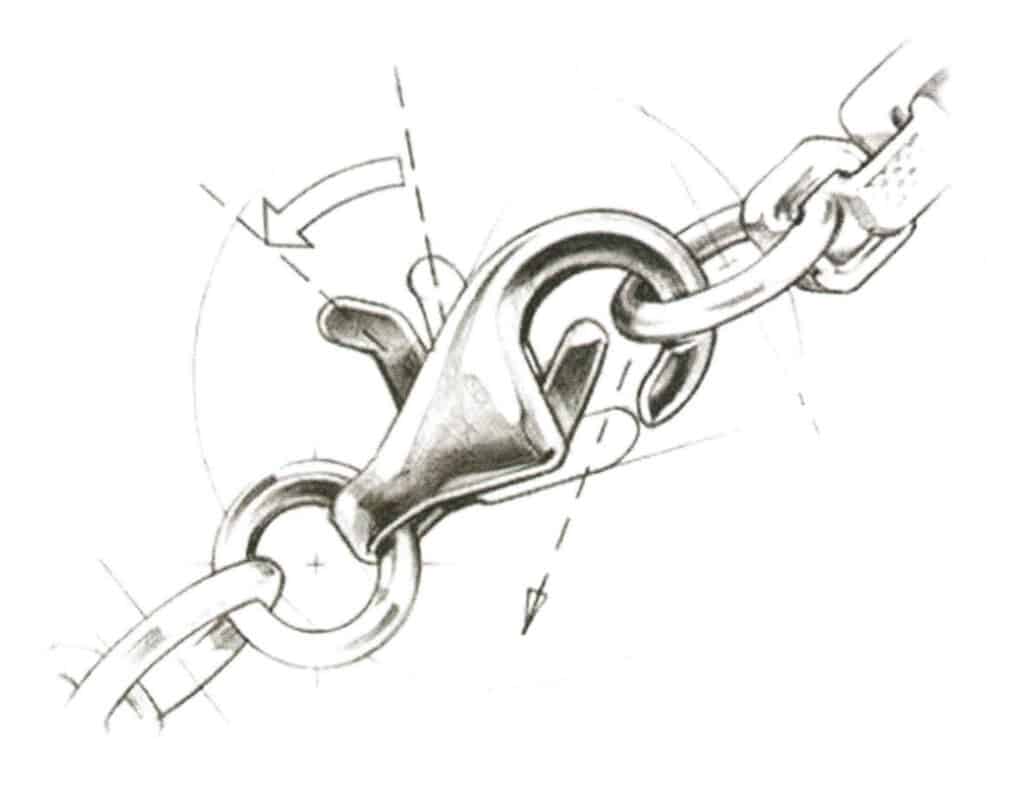
Figure 7-17 Schematic diagram of lobster clasp (1)
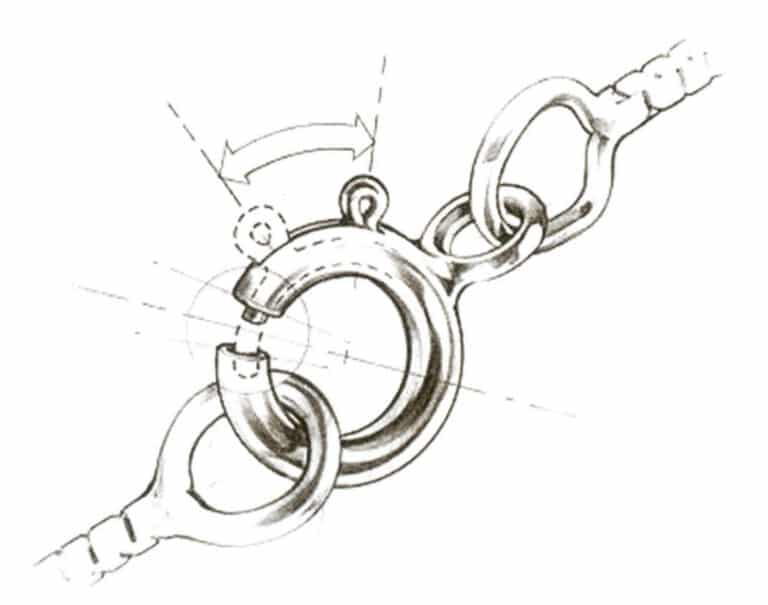
Figure 7-18 Schematic diagram of spring ring buckle
(3) Safety Plug clasp. As shown in Figure 7-19, open the hook of the squeeze hook, then turn it to the side to remove it from the lock. This structure helps prevent slipping off.
(4) Arc lock clasp. As shown in Figure 7-20, press the spring pin, insert the chain clasp into the socket, and after releasing the pin, it will lock the chain clasp.
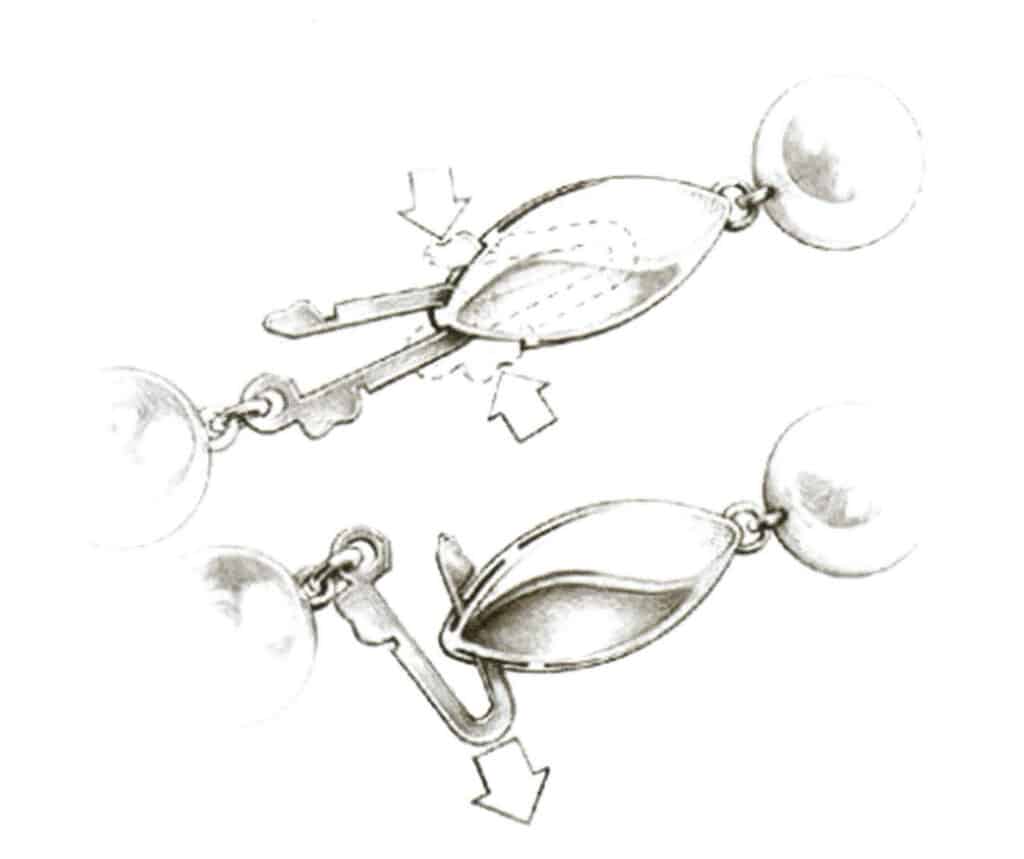
Figure 7-19 Schematic diagram of safety ring buckle
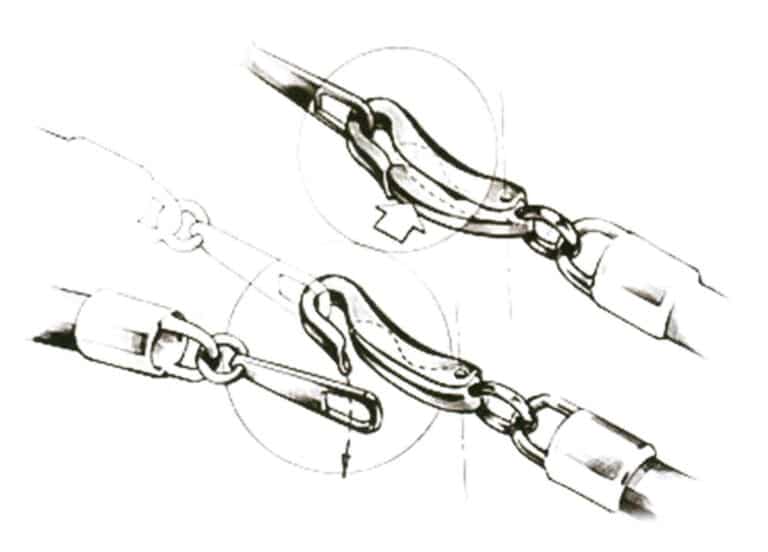
Figure 7-20 Schematic diagram of the arc lock
2.1.2 Earring-type fasteners
(1) Butterfly ear clip. As shown in Figure 7-21, it prevents the earring from slipping off through the notch on the ear pin and fits with the butterfly clip behind the ear clip.
(2) Omega ring buckle. Figure 7-22 shows that it secures the earring by closely fitting the ” Ω ” ring buckle against the earlobe, applying less pressure to the earlobe.
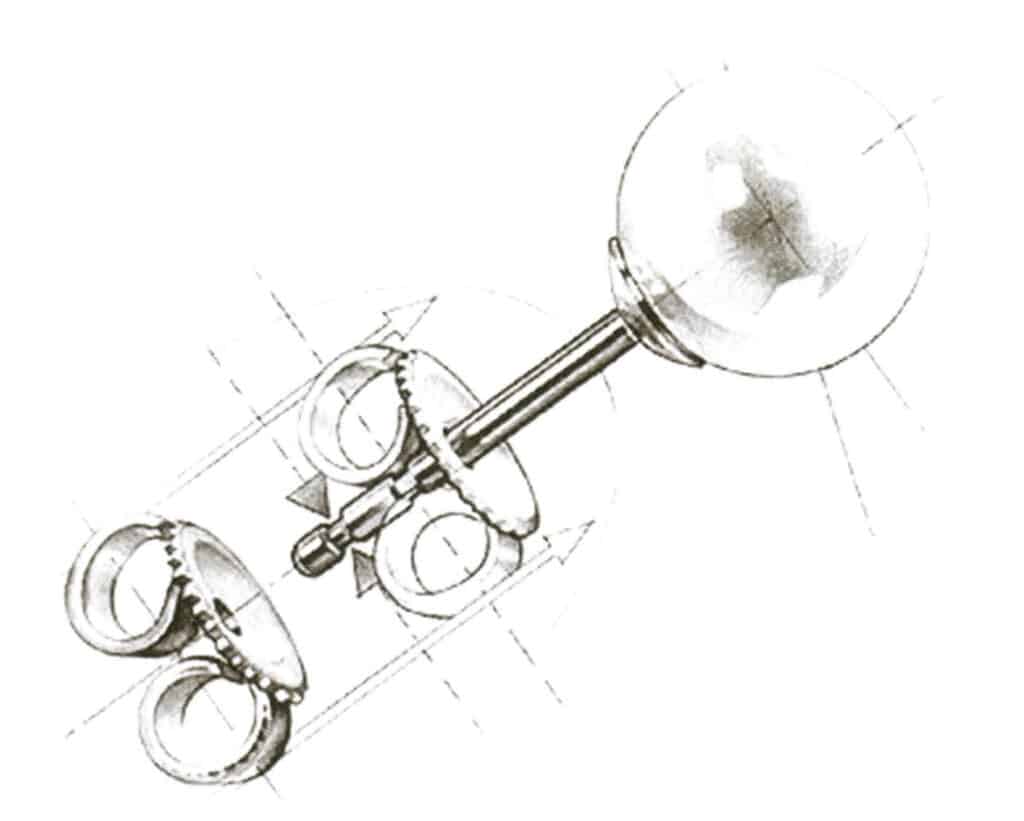
Figure 7-21 Schematic diagram of the butterfly ear pressure
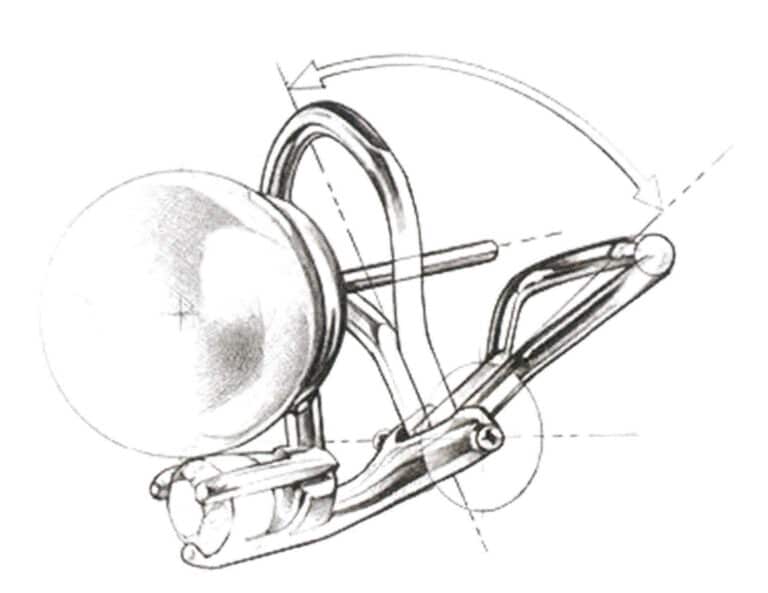
Figure 7-22 Schematic diagram of the Omega ring buckle
(3) Hinge buckle. As shown in Figure 7-23, closing the earring and applying slight pressure can lock the earring, and the back of the ring buckle is engaged together, secured with a small notch.
(4) Screw the earplugs. As shown in Figure 7-24, the earring is fixed through the back of the screw post, which helps ensure the safety of inlaid precious gemstone earrings.
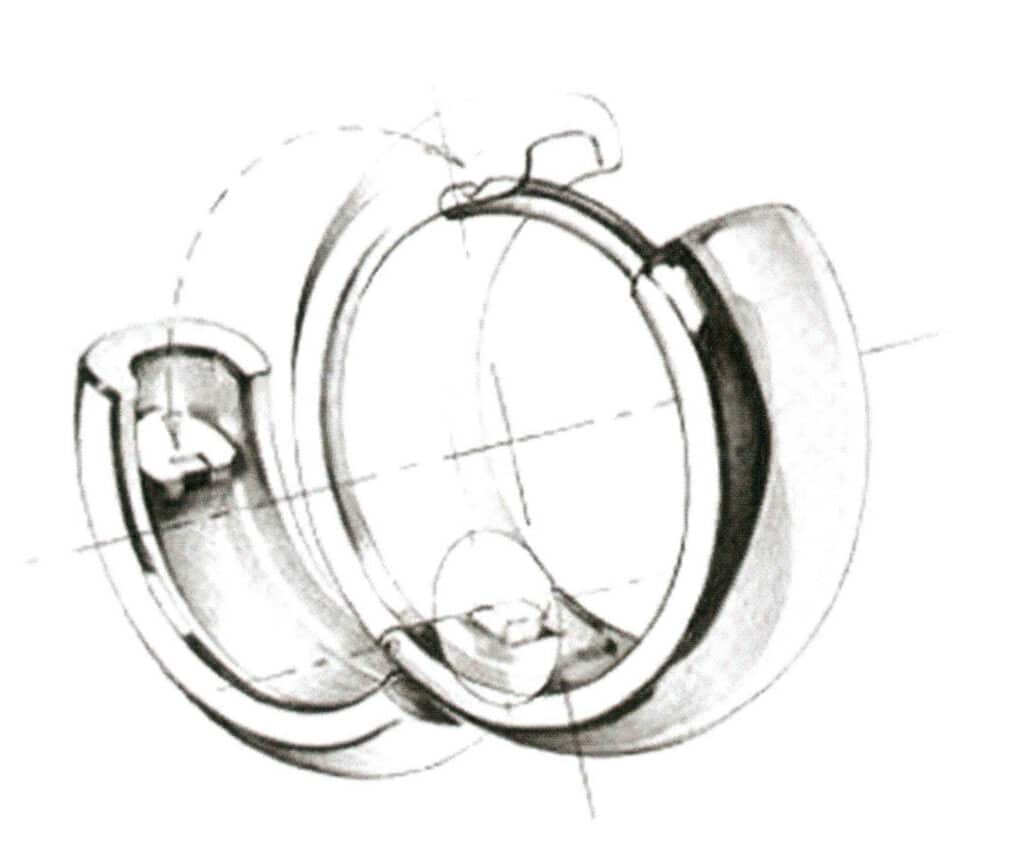
Figure 7-23 Hinge Buckle Schematic
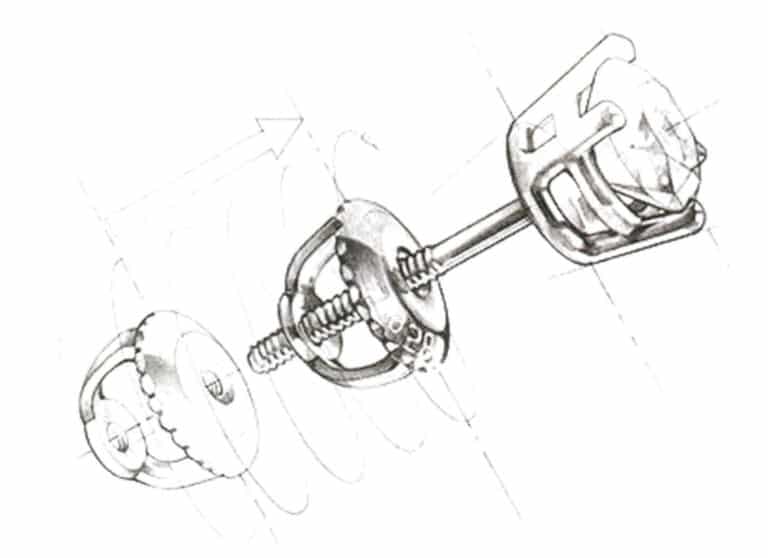
Figure 7-24 Spiral Ear Plug Schematic
(5) Hinge Ear clap. Figure 7-25 shows that the hinge allows the earrings to open and close, and the spring clip ear clap fits with the circular notch on the earpin to secure the earrings.
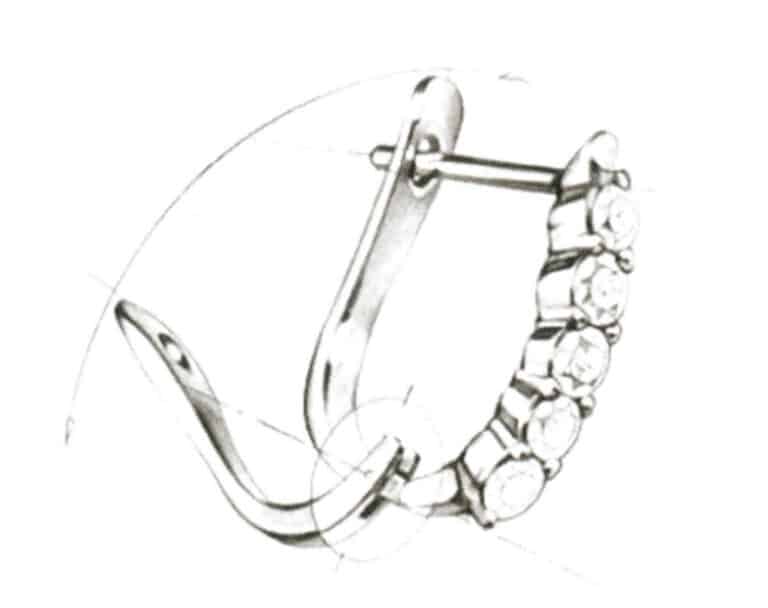
Figure 7-25 Hinge Ear Pressure Diagram
2.1.3 Fasteners for Bracelets
(1) Hidden Buckle. As shown in Figure 7-26, commonly known as “tongue clasp,” the plug tongue made of spring clips connects with the clasp box, and sometimes a number 8-shaped safe Latch is set on the box side to enhance the fastening security. When in use, press the tongue to insert it, after loosening, the box blocks the spring piece, and the safety buckle is tightened inside the box.
(2) Box Buckle. As shown in Figure 7-27, press the button to release the snap ring from the locked position, then slide the buckle out of the box.
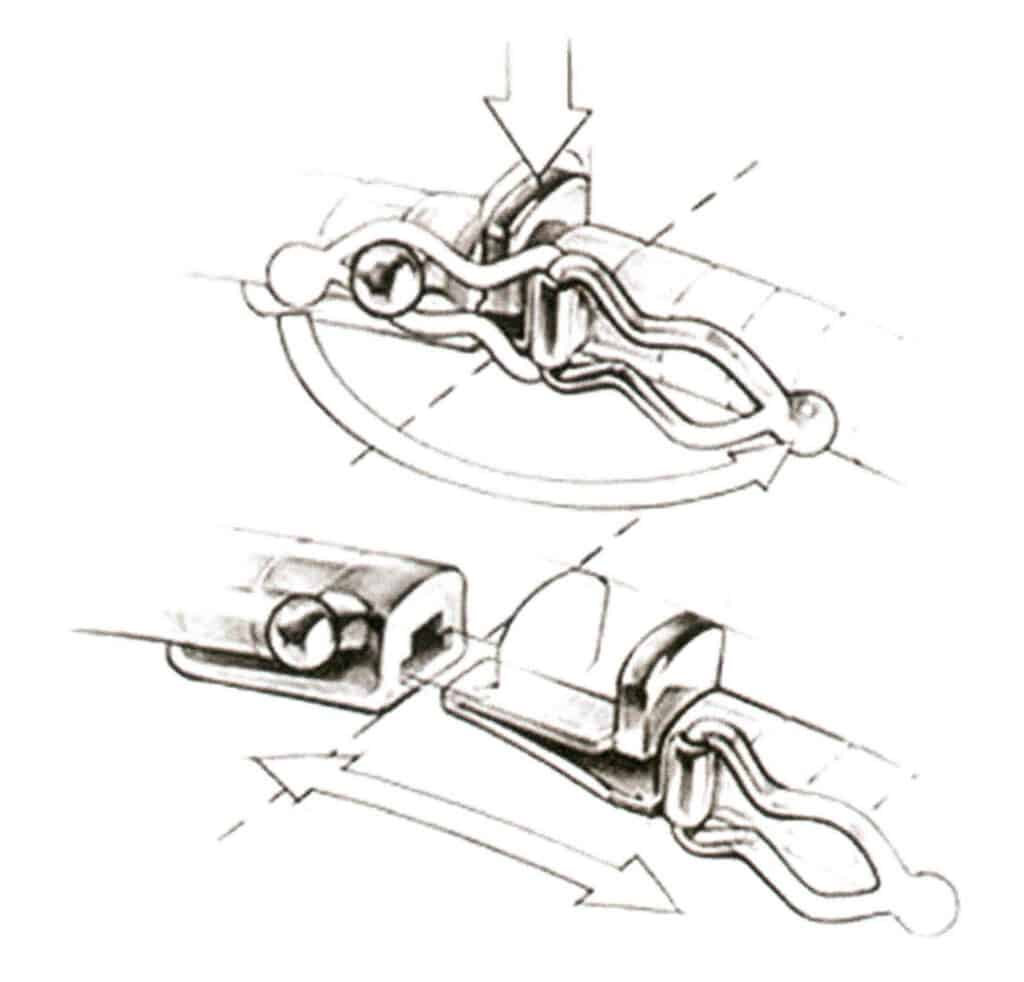
Figure 7-26 Hidden Buckle Diagram

Figure 7-27 Box Buckle Diagram
2.2 Fatigue Failure of Fasteners
Jewelry fasteners utilize the principles of compression and rebound to achieve locking and unlocking. As repeated openings and closings increase, their elasticity gradually deteriorates, and may even fail. This phenomenon is because the metal components experience fatigue, which refers to the failure of jewelry parts under cyclic loading. Even if the stress on the material is far below its static strength, this type of structural damage can still occur.
The lifespan of fasteners is related not only to the material and manufacturing process but also to the method of use. Excessive force in pressing or pulling the fasteners can easily cause damage; repeatedly pressing and pulling the fasteners reduces the fatigue life of the springs, leading to premature failure. Therefore, when wearing them, one should be careful and gentle with force and regularly check the jewelry. If there is insufficient elasticity or poor fitting of the fasteners, they should be promptly sent to a professional repair point for maintenance. If repair is not suitable, consider exchanging it for a new one.
Section III The Discoloration and Maintenance of Metal Jewelry
Precious metal jewelry in the wearing process, often appear dirty, discoloration phenomenon, affecting the decorative effect. Therefore, it is necessary to explore the causes of dirty jewelry discoloration, maintenance and cleaning methods.
1. Dirt and Discoloration on the Surface of Precious Metal Jewelry
1.1 Surface dirt on precious metal jewelry
Newly purchased precious metal jewelry has no dirt accumulation and a strong luster. During wear, dust in the air can settle on the surface of the jewelry, and the oils secreted by the skin can also adhere to the surface and in the crevices of the jewelry, especially in intricately carved designs where dust is more likely to accumulate. Allowing dust and oil to adhere for a long time not only causes the jewelry to lose its luster but also makes it easier for bacteria to breed, especially in summer when temperatures are high, and sweat on the skin increases, making it easier for the dust to adhere to the surface of the jewelry, becoming a breeding ground for bacteria, as shown in Figure 7-28.

1.2 Discoloration of the surface of precious metal jewelry
1.2.1 Discoloration of the surface of gold jewelry
(1) The surface of the gold jewelry turns white. Gold has stable chemical properties, and generally, the surface of gold jewelry does not change when worn or used. However, in certain special environments, the surface of gold jewelry may appear whitish, as shown in Figure 7-29.

The chemical properties of gold are very stable, and a noticeable color that is not characteristic of gold itself can easily be mistaken for a problem with the gold’s quality. However, using XRF detection, the color of the non-discolored areas is qualified, while significant amounts of Hg can be detected in the discolored areas. This is because gold jewelry has come into contact with Hg (commonly known as mercury), causing a chemical reaction between Au and Hg, forming white gold-mercury compounds (amalgam). The whitening of gold jewelry is closely related to the concentration of Hg in the environment and can serve as an indicator of environmental quality. Additionally, commonly used thermometers, barometers, and vapor lamps contain mercury, as do many cosmetics (which contain whitening mercury), medications, and disinfectants most women use may also contain Hg. If one is not careful and the skin, which has cosmetics on it, comes into contact with gold jewelry, the jewelry absorbs trace amounts of Hg, and over time, this can form a gold-mercury amalgam, causing the surface color of the gold jewelry to turn white and become brittle. During detection, Hg can be detected using a spectrometer.
Due to the low hardness of gold, another reason for the whitening of gold jewelry is that when gold jewelry is worn together with white jewelry such as platinum, K white gold, or silver, the friction between the jewelry may transfer the metal from the white jewelry onto the gold jewelry. This color change only occurs in the areas that can be rubbed and is only on the surface, appearing as scratch marks.
(2) The surface of the gold jewelry shows red rust spots. Although gold has very stable chemical properties and generally does not oxidize or change color in the atmosphere, sometimes brownish-red “rust spots” can appear in some products, such as the electroplated 24K gold ornament shown in Figure 7-30. According to the standard “Methods” (GB 11887-2012), the minimum gold content for 24K gold jewelry is 999%. If the jewelry does not meet the standard, the more impurities it contains, the more susceptible it is to environmental influences during wearing and storage, leading to red rust spots.

However, when testing the gold content of jewelry with red rust spots on the market, the overall gold content generally meets the standard requirements. The “rust spot” issue is usually due to inadequate control during the production process. For example, in the electroforming process, unreasonable setting of process parameters can lead to localized pinholes; in stamping production, a dirty and dusty environment or using the same equipment to press gold and other materials can cause foreign impurities to be pressed into the surface of pure gold due to its softness; during the casting of 24K gold jewelry, defects such as shrinkage cavities and sand holes may occur. These pore defects and impurity spots are not thoroughly treated during acid immersion and cleaning, causing the jewelry or ornament to erode due to environmental influences during wearing and display, resulting in corrosion spots.
(3) The surface of the gold jewelry turns black. Long-term use of gold jewelry can lead to darkening of color, which is a common phenomenon. This is mainly due to the trace elements such as Ag and Cu in the gold jewelry reacting with sweat and other substances, resulting in oxidation and darkening of the surface. However, some jewelry may show significant blackening shortly after the sale, particularly at the joints of different parts, and this blackening phenomenon can occur within a few weeks after the sale, making it easy for consumers to doubt the quality of the jewelry.
Based on micro-area composition analysis of the blackened areas of the jewelry, it can be found that the composition at the joints is significantly lower than the purity of the main precious metal of the jewelry, especially with a noticeably higher content of Ag, which can react with O or S to form black Ag2O or Ag2S. Therefore, the high silver content mainly causes the blackening at the joints of the jewelry. Necklaces, bracelets, and other jewelry sometimes require solder for the sake of craftsmanship, which connects the various parts of the jewelry. Since national standards only specify the overall precious metal purity of the jewelry without corresponding requirements for solder, different manufacturers use different solder compositions for various reasons. To reduce costs and simplify the process, some merchants use solder with a purity significantly lower than that of the main precious metal, and high silver content solder can lead to blackening at the joints of the jewelry.
1.2.2 The discoloration phenomenon of K gold jewelry
K gold jewelry, after prolonged wear, may lose its luster on some parts of the surface, resulting in discoloration phenomena such as black spots, red spots, white haze, or varying shades of color. The main reasons for the discoloration of K gold jewelry are as follows:
(1) chemical reaction with external substances lead to corrosion and discoloration of jewelry. k gold is gold and other alloying elements of gold alloy materials, the most commonly used alloying elements for Cu, Ag, Zn, white k gold also often contains bleaching element Ni, the chemical stability of these alloying elements is worse than gold, jewelry is easy to produce a reaction with chemicals, such as sweat, cosmetics (perfume, sunscreen, etc.), Chemicals, etc., in long-term use, it is also easy to react with trace acids, alkalis, sulfides, halides in the air, thus making K gold jewelry yellow or black, causing jewelry discoloration. Among them, to Cu as the main alloying element of rose gold jewelry, its surface is because of the high content of Cu, easy to oxidation corrosion and become dull, as shown in Figure 7-31.

Human sweat is the main environmental factors that cause corrosion and discoloration of jewelry, sweat contains some chloride, lactic acid, urea and other components, they can react with Cu, Ag and other alloying elements, resulting in deep black chemicals, thus making the jewelry black, but also fall on the skin to leave a very obvious stains. Different people have different physical qualities, and the corrosiveness of sweat is also somewhat different. Therefore, when wearing the same jewelry, the time and degree of discoloration will often be somewhat different. Some cheap cosmetics and hair dyes often contain Pb, when gold jewelry encountered with such a chemical substance is easy to blacken. For health care workers and chemical laboratory staff, if they wear karat gold jewelry in their daily work environment, it is easy for discoloration to occur because of the chemicals in the surrounding environment.
Soldering is often required to assemble components and repair defects during the production of K gold jewelry. To facilitate proper soldering, a solder with a melting point lower than the base material, good wettability, and good fluidity needs to be prepared with a higher Ag content. The difference in composition between the solder and the base material results in inconsistent chemical and electrochemical properties, causing the soldered area to corrode preferentially and lead to discoloration, as shown in Figure 7-32.
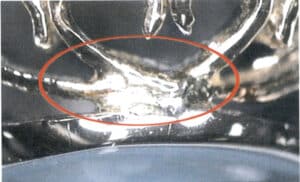
(2) Wear of the plating causes a color contrast on the surface. The surface of K gold jewelry is often treated with electroplating, especially for the commonly seen white K gold jewelry in the market, an alloy obtained by melting gold with metals like silver, zinc, and nickel in certain proportions. Although its color is similar to white, it will more or less have some yellowish tones. Therefore, a layer of rhodium or other metals is usually plated on its surface during jewelry-making. If worn for a long time without proper maintenance, the plating can wear off in certain areas, revealing its original color and causing a color contrast on the jewelry’s surface.
1.2.3 Discoloration phenomenon of silver jewelry
(1) The surface of silver jewelry turns black. Due to the lower chemical stability of silver compared to gold and platinum, silver reacts with many chemicals, substances will react and cause the surface to turn black, including sulfides (sulfides and sulfites) and halides (iodides, bromides, chlorides), especially sensitive to sulfides. These substances commonly appear around us in daily life, such as in the air, in the kitchen H2S, in foods like century eggs, fermented tofu, and the sulfites in pickled vegetables, in cosmetics with sulfide additives, in sulfur-scented detergents, hot springs, and perfumes. When they come into contact with silver jewelry, they will cause the surface of the silver jewelry to generate black sulfides.
In addition, silver jewelry is prone to chemical reactions with ozone, and the direct action of ozone on silver can produce gray-black silver oxide. Therefore, silver jewelry should not be placed near air-negative ion generators or disinfection cabinets (ozone sterilization).
(2) Silver jewelry first turns white and then black. The sweat excreted by the human body contains chlorides and the bleaching powder commonly used in tap water purification (mainly composed of hypochlorous acid) and chlorine, as well as the bleaching agents (mainly chlorine-containing) in laundry detergent. Under certain conditions, Cl can react with Ag to form white AgCl, which can easily oxidize to black silver chloride in the air, causing discoloration and corrosion of the jewelry surface.
1.2.4 The phenomenon of discoloration of platinum jewelry
The chemical properties of platinum are very stable, and under normal circumstances, it is not prone to discoloration. The national standard “Regulations and Naming Methods for Precious Metal Purity in Jewelry” (GB 11887-2012) specifies the minimum platinum content for different grades of platinum alloys and sets clear requirements for elements harmful to human health, while no requirements are made for other metal elements.
In addition to quality issues during the production and manufacturing process of jewelry, the reasons for the discoloration of platinum jewelry may include the following situations:
(1) Other elements mixed into platinum jewelry may cause red, white, purple, and black spotted color areas to appear on their surface, and the area of this color change is usually very small.
(2) Wearing platinum jewelry together with gold jewelry or bumping it against other metal products in daily life can cause the surface of the platinum jewelry to turn yellow. Due to the different materials of the two types of jewelry, their hardness differs, there is a difference in hardness, and during use, the friction between the two will cause the gold jewelry to rub against the surface of the platinum jewelry. Upon careful observation of the jewelry’s surface, this color change only occurs at the points of friction. It appears as scratches on the surface, which can be restored by re-polishing.
(3) Platinum itself does not exhibit the “amalgam” phenomenon, but if other elements are mixed into platinum jewelry, the “amalgam” phenomenon can occur. Mercury can form amalgams with all metals with a lower atomic number except iron. If care is not taken to avoid contact with platinum jewelry, gray-white spots may form due to amalgamation.
(4) Platinum jewelry contains other elements. If placed in an environment containing Sulphur for a long time, Sulphur may react with the internal impurity elements and cause discoloration spots.
1.3 Wearing and Maintenance of Precious Metal Jewelry
1.3.1 Precautions for Wearing Precious Metal Jewelry
People enjoy wearing precious metal jewelry. However, due to a lack of understanding or attention to the wearing requirements during use, issues such as discoloration, scratches, and breakage can occur, severely affecting the aesthetics and usability of the jewelry. Therefore, one should pay attention to proper use and maintenance when wearing precious metal jewelry.
(1) When wearing gold jewelry, avoid colliding or rubbing against other hard objects. Gold jewelry has a lower hardness and is prone to wear. Suppose it comes into contact with hard objects. In that case, it can not only deform beautifully crafted jewelry but also damage the surface, causing scratches that weaken the intensity of light reflection on the jewelry’s surface, reducing the metallic luster of the gold jewelry. This not only diminishes the beauty of the gold jewelry but also greatly reduces its value. In northern regions, during seasons with heavy sandstorms, wearing gold jewelry can easily cause the surface to lose its luster due to friction with dust particles (mainly composed of SiO2 ) suspended in the air.
(2) Gold and platinum rings should not be worn together, as friction between the two can cause the surface of the gold ring to become partially coated with platinum, resulting in a whitish appearance.
(3) Try to wear jewelry as little as possible in chemically polluted areas; be careful not to let jewelry be exposed to humid environments or direct sunlight for long periods, as this can easily cause the surface of the jewelry to oxidize and discolor. Silver jewelry, especially, should be kept in a dry and cool environment. Jewelry should be kept to a minimum contact with cosmetics such as perfume, hair spray, and floral water; makeup should be done before wearing jewelry to reduce contact with such liquids.
(4) Do not wear jewelry when soaking in hot springs or swimming at the beach, especially silver jewelry and low-karat gold jewelry, as they can undergo varying degrees of chemical reactions when exposed to seawater or hot spring water. Jewelry should also be removed when bathing at home, as the bleach in tap water can corrode jewelry materials, and prolonged exposure can still cause discoloration of the jewelry surface.
1.3.2 Daily Maintenance of Precious Metal Jewelry
People are cautious when purchasing jewelry but often need to pay more attention to the maintenance aspect while wearing it. Poor maintenance of a beautiful and high-quality piece of jewelry fails to achieve the decorative effect and damages its true value, thus affecting both decoration and value retention. Therefore, users of precious metal jewelry should understand the general characteristics of precious metal materials and gemstones and pay attention to the daily maintenance of jewelry, which mainly includes the following aspects:
(1) Regularly dusting. During use, jewelry often accumulates dust and oil secreted by the body in the gaps of the jewelry due to airborne particles. Therefore, jewelry needs to be regularly dusted to maintain the cleanliness and proper appearance of the jewelry surface Luster.
Dust removal methods include gently brushing with a soft-bristle brush, wiping with a soft cloth, and blowing with a rubber air blower.
(2) Regular cleaning. Due to cosmetics, oils, detergents, pharmaceuticals, trace acids, alkalis, sulfur, and other chemical pollutants in the atmosphere and environment, precious metal jewelry is prone to surface corrosion, leading to spots and discoloration. Therefore, it is best to clean the jewelry regularly.
Cleaning method: First, pour a small amount of detergent into warm water, place the precious metal jewelry in it, gently brush with a soft brush, and then rinse thoroughly with clean water; afterward, use a soft cloth to absorb and wipe dry. If there is still a damp feeling, it can be placed under a desk lamp to dry, restoring its shine.
(3) Regularly oiling. Precious metal jewelry’s spring or small switch mechanism should be kept lubricated to reduce daily wear. Usually, one or two drops of sewing machine oil can be added after cleaning. Sewing machine oil evaporates easily, is easy to clean, and does not create grease stains. However, wiping off any excess oil sticking to the precious metal jewelry with a soft cloth after each oiling is important to avoid attracting dust.
(4) Regularly check. Frequently check precious metal jewelry for any abnormalities to detect and address issues promptly.
(5) Pay attention to storage conditions. If precious metal jewelry is not in use, it should be stored away before storing; first, clean all parts of the jewelry to keep it clean. After removing dirt, be sure to dry and wipe it clean. Additionally, prepare a well-sealed box (preferably a jewelry-specific box) for storage; the lining can be cloth or plastic, but it should be appropriately sized, generally slightly larger than the jewelry volume. Line the box with some cotton or sponge, and prepare a small packet of desiccant wrapped in gauze. Place the clean jewelry in the box along with the desiccant, seal the lid tightly, keep the box away from substances like mothballs, and try to store it in a dry environment. When storing jewelry made of different materials, wrap it in cloth before placing it in the jewelry box to avoid collisions and friction.
During storage, pay attention to whether the desiccant inside the box has become damp. If it has, remove the desiccant and dry it in the sunlight before reusing it. At the same time, you can wipe the stored jewelry and check for any changes in luster; if there are changes, address them promptly. For larger, finely crafted, artistic jewelry, a transparent acrylic box can be used as a storage tool if there are no suitable boxes. This protects the jewelry while allowing it to be admired, but care should be taken to avoid direct sunlight and ensure safe placement to prevent impacts.
Section IV Cleaning and Renovation of Precious Metal Jewelry
1. Cleaning of Precious Metal Jewelry
Precious metal jewelry, during wearing and use, can experience a decrease in luster due to corrosion, discoloration, and the adhesion of dirt, which affects the aesthetic and appearance of the jewelry to varying degrees. Therefore, cleaning jewelry has become an important topic in the jewelry industry. Cleaning refers to using chemical methods to clean the surface of the jewelry, removing dirt from the surface and crevices, making it cleaner, and presenting the true state of the jewelry’s surface.
Based on whether cleaning and refurbishment cause loss of precious metals, it can be divided into two main categories: damaging cleaning and non-damaging cleaning.
Damaging cleaning refers to removing foreign impurities from jewelry through chemical reactions. This method can result in a loss of quality for the jewelry. Damaging cleaning most commonly uses aqua regia, which is a strong oxidizing acidic substance (1:3 composed of nitric acid and hydrochloric acid) that can react not only with dust and oil stains but also with almost all metal elements and their compounds in precious metal jewelry, causing gold, silver, platinum, and other precious metals to dissolve into the solution, resulting in a loss of jewelry quality. This is what is commonly referred to as the “gold washing” phenomenon. Generally speaking, the longer the cleaning time, the greater the loss of jewelry quality. Of course, besides aqua regia, other acidic solutions can also be used to treat certain jewelry that has experienced discoloration. For example, the Cu2O on the surface of K rose gold can be H2SO4 cleaned, which can restore the dull and discolored surface to brightness. Still, sometimes, it may introduce new impurities and cause secondary discoloration.
Non-damaging cleaning mainly deals with contaminating precious metal jewelry with organic substances such as dust and oil stains. According to the principle of similar solubility, organic reagents can dissolve and remove organic dirt from the surface of the jewelry, restoring its original color. Available cleaning agents include acetone, ether, ethanol, isopropanol, and butyl acetate. The cleaning method is also very simple: place the jewelry in the solution and stir for a moment, with the solution concentration determined based on the actual situation. Jewelry that has been worn for a long time and is heavily soiled can be boiled in hot water for about 10 minutes to speed up the cleaning process. If conditions permit, it can also be directly burned over an alcohol lamp. The most commonly used cleaning agent is ethanol solution.
1.1 Cleaning of Gold Jewelry
(1) After the surface of gold jewelry gets dirty, it can be rinsed with a developing solution from photo-developing powder mixed with 30-40℃ warm water, diluted with 1x clean water. Soak the gold jewelry for a few minutes, then use a soft brush to scrub off the dirt, and rinse several times with clean water to restore the jewelry’s luster. If, after cleaning, a thin layer of transparent colorless nail polish is applied with a soft cloth, the jewelry can also become brilliantly shiny.
Additionally, organic solvents can be used to dissolve and remove dirt from the surface of the jewelry, with the concentration of the solution determined based on the actual situation.
(2) When the surface of gold jewelry fades or slightly darkens, applying some toothpaste and rubbing it repeatedly with a soft cloth can restore the color.
(3) If the surface of gold jewelry is severely discolored, it can be treated with an ultrasonic cleaning machine or specialized cleaning solution. Ultrasonic cleaning machines typically use ultrasonic cleaning liquid, which vibrates continuously under ultrasonic action to remove dirt and discoloration from the surface of the gold jewelry. The standard formula for ultrasonic cleaning liquid is 1000mL of 40℃ warm water, 100g of chromic anhydride, and 30mL of sulfuric acid. The specialized cleaning solution is a specifically formulated dilute sulfuric acid solution used for cleaning the surface of gold jewelry using the “sulfuric acid stain removal method.”
1.2 Cleaning Silver Jewelry
(1) For silver jewelry with slight discoloration, you can polish it with toothpaste or soak it in a baking soda (NaHCO3) solution, then clean it with a soft brush; you can also soak it in an oxalic acid solution with a concentration below 50%. All three methods can remove the slight discoloration on the surface of silver jewelry, making it shine like new.
(2) For silver jewelry that has developed spots due to moisture, use warm edible vinegar, wipe it off with a soft cloth, and then rinse it with clean water. Of course, you can also polish it with toothpaste.
(3) The following methods can be used for cleaning for severely tarnished silver jewelry.
- Soak in a baking soda ( NaHCO3 ) solution, then add a few pieces of aluminum foil under the silver jewelry and heat them together to remove the black spots and restore the original color. The principle is that aluminum reacts with NaHCO3 to release hydrogen gas, quickly reducing the sulfide black spots to silver and releasing sulfur.
- Wash with a hot, soapy water solution of 1%, then moisten the surface with sodium thiosulfate solution and wipe with a cloth to clean the surface of the silver jewelry.
- Ultrasonic or home cleaning methods in phosphoric acid cleaning solution are effective. The phosphoric acid cleaning solution formula is 1000mL of 50℃ Warm water, 200mL of phosphoric acid, and 30g of concentrated laundry detergent. The working principle of this formula is that the laundry detergent acts as a catalyst, enhancing the liquid’s wetting ability; phosphoric acid acts as a reactant, reacting with silver sulfide (Ag2S) to form yellow phosphoric silver precipitate; warm water acts as a diluent, increasing the descaling effect.
- Ammonia solution is used as a cleaning liquid; this method is widely used in the traditional jewelry industry. However, its effect could be better than that of a phosphoric acid cleaning solution.
- Soak in a saturated sodium thiosulfate solution at room temperature while continuously agitating the workpiece until the discoloration products on the surface of the silver jewelry are completely removed.
- Soak in a solution containing thiourea 8%, concentrated hydrochloric acid 5.1%, water-soluble fragrance 0.3%, wetting agent 0.5%, and water 86.1% at room temperature until the discoloration products on the surface of the silver jewelry are completely removed.
The above method can not only clean the black spots, dust, and grease on the surface of silver jewelry but also restore the original luster of the silver jewelry to a certain extent.
2. Renovation of Precious Metal Jewelry
During the wearing and use of jewelry, it is inevitable to encounter bumps and friction, resulting in pits and scratches on the surface, decreasing luster. Renovation is needed to restore its decorative effect. Renovation involves relatively specialized tools, equipment, and techniques; sending it to a professional jewelry repair and maintenance point is generally advisable for processing.
2.1 Renovation of Gold Turning White
(1) Pure gold jewelry will undergo a chemical change when it encounters mercury, appearing mottled white. Using a torch to heat it until red hot, the mercury will evaporate at high temperatures, restoring its original color. Then, use an agate knife, steel press, etc., to polish the surface of the jewelry.
(2) When the surface of gold jewelry is scratched with platinum and turns white, it cannot be treated with fire, as the melting point of platinum is higher than that of gold. This layer of gold needs to be gently pressed and polished with an agate knife to restore the original luster of the gold.
2.2 Removal of the discoloration layer on the surface of jewelry
Chemical films such as oxides and sulfides that cause discoloration on the surface of jewelry are generally removed by chemical soaking methods. If they are difficult to soak or prone to secondary discoloration, mechanical polishing, and other methods will be used to remove them.
2.3 Restoration of the brightness of the jewelry surface
Pits and scratches caused by bumps and friction on the surface of jewelry must be removed through sanding, grinding, and polishing with cloth wheels, making the surface smooth and shiny. For precious metal jewelry made of silver alloys, white K gold, and other materials, rhodium plating or gold plating should be done after polishing to further enhance the brightness of the jewelry surface, achieving a refreshed decorative effect.
2.4 Restoration of the texture on jewelry surfaces
During the wearing process of precious metal jewelry, pieces with textured surfaces, such as sandblasted, brushed, or engraved finishes, will gradually wear down and lose their texture. Using equipment or tools like sandblasting, brushing, or engraving is necessary to reprocess the jewelry surface and restore the desired texture effect.