What Makes Tungsten Steel Jewelry Stand Out: Materials, Features, and Production Techniques
Discover Tungsten Steel Jewelry: High-Quality, Durable, and Stylish Choices for Your Collection

Plain tungsten steel ring
Table of Contents
Section I Introduction to Tungsten Steel Materials
1. Metal Tungsten
1.1 The Discovery of Tungsten
The Latin meaning of tungsten is “white foam in the wolf’s mouth.” How could tungsten be associated with carnivorous animals? It turns out that a long time ago when people were refining tin from ore, they discovered that whenever the ore contained a certain type of heavy brownstone, the tin yield would drop sharply. This heavy stone would consume tin just like a wolf devours a sheep. Therefore, tungsten was named “white foam in the wolf’s mouth.”
Gallium accounts for about one hundred thousandth of the Earth’s crust, belongs to rare metals, and is an important strategic material. In nature, there are tungsten-manganese-iron ores (also called black tungsten ores) and yellow-gray calcium tungstate ores (also called white tungsten ores). China has the largest reserves of tungsten ore in the world. The Nanling region in China is the richest area of gallium ore in the world, especially southern Jiangxi, known as the “metallic hometown.” Dayu in Jiangxi and Shizhu Garden in Hunan have the world’s largest gallium mines.
As early as the 18th century, humans discovered tungsten, but it was not until 1850 that pure metallic tungsten was produced by Wöhler, after which tungsten was widely used.
1.2 Properties of Tungsten
(1) Physical Properties
Tungsten is a rare high-melting-point metal belonging to group VIB of the sixth period (the second longest period) in the periodic table. The element symbol is W, the atomic number is 74, and the relative atomic mass is 183.85. The main physical properties of tungsten are as follows.
① Color. Pure tungsten is a silvery-white metal resembling steel; only powdered or fine-wire tungsten is gray or black. Light bulbs turn black after prolonged use because a layer of tungsten powder is on the inner wall of the bulb.
② Melting point. Tungsten has a high melting point, very low vapor pressure, and a relatively low evaporation rate. Among all metals, tungsten is the hardest to melt and the least likely to vaporize, so it is called a “high melting point metal.” Its melting point reaches 3410℃, and its boiling point is 5927℃. When the light bulb is lit, the temperature of the filament exceeds 3000℃. At such a high temperature, only tungsten can withstand it, while most other metals will melt into liquid or even turn into vapor.
③ Density. The density of tungsten is very high, reaching 19.35g/cm3, similar to that of gold; hence, its original Swedish meaning is “heavy.”
④ Hardness. Tungsten is very hard, and using the hardest diamond as a drawing die, a 1 mm diameter tungsten wire is drawn through more than 20 gradually smaller diamond holes, reducing it to a filament with a diameter of only a few hundredths of a millimeter. 1 kg of tungsten ingot can be drawn into fine wire up to 400 km long. Incandescent lamps, vacuum tubes, and even the new “iodine tungsten lamps” developed in our country in recent years all use tungsten as the filament.
(2) Chemical Properties
The chemical properties of tungsten are very stable; even when heated, it does not react with hydrochloric acid or sulfuric acid, and it does not dissolve in aqua regia. In aqua regia, tungsten only undergoes slow surface oxidation. Only a highly corrosive mixture of hydrofluoric and nitric acid can dissolve tungsten.
1.3 Uses of Tungsten
Tungsten is widely used in modern technology in both pure metal and alloy states. The most important in the alloy state are alloy steel, tungsten carbide-based hard alloys, wear-resistant alloys, and high-temperature alloys. Tungsten is mainly applied in the following industrial fields.
(1) Steel Industry
Tungsten is mostly used to produce special steel. Widely used high-speed steel contains 9%~24% tungsten, 3.8%~4.6% chromium, 1%~5% vanadium, 4%~7% cobalt, and 0.7%~1.5% carbon. The characteristic of high-speed steel is that it can be self-quenched at a high tempering temperature in air (700~800℃ ), thus maintaining high hardness and wear resistance under 600~650℃. The alloy tool steel containing tungsten contains 0.8%~1.2% tungsten; chromium tungsten silicon steel contains 2%~2.7% tungsten; chromium tungsten steel contains 2%~9% tungsten; chromium tungsten manganese steel contains 0.5%~1.6% tungsten. Tungsten-containing steel is used to manufacture various tools, such as drill burs, milling cutters, wire drawing dies, female and male molds, pneumatic tool parts, etc. Tungsten magnet steel is a permanent magnet containing 5.2%~6.2% tungsten, 0.68%~0.78% carbon, and 0.3%~0.5% tungsten. Tungsten cobalt magnet steel contains 11.5%~14.5% tungsten, 5.5%~6.5% molybdenum, and 11.5% ~12.5% cobalt as hard magnetic materials. They have high magnetic strength and coercivity.
(2) Tungsten Carbide-Based Cemented Carbide
Tungsten carbide has high hardness, wear resistance, and refractory properties. These alloys contain 85%~95% tungsten carbide and 5%~14% cobalt, with cobalt serving as a binder metal, providing the necessary strength to the alloy. They are mainly used in certain alloys for processing steel, containing titanium, tantalum, and niobium carbides. All these alloys are made with powder metallurgy. When heated to 1000~1100℃, they still have high hardness and wear resistance. The cutting speed of carbide tools far exceeds that of the best steel tools. Carbide is mainly used for cutting tools, mining tools, and drawing dies.
(3) Heat-Resistant and Wear-Resistant Alloys
As the most difficult metal to melt, tungsten is a component of many heat-resistant alloys, such as 3%~15% tungsten, 25%~35% chromium, 45%~65% cobalt, and 0.5%~2.75% carbon, mainly used for parts that require high wear resistance. For example, valve components in aircraft engines, working parts of hot cutting tools for molds, impellers of bath wheel machines, excavation equipment, and surface coatings of plowshares. In aviation and rocket technology, as well as in other sectors that require high thermal strength for machine parts, engines, and some instruments, tungsten and other molten metal alloys (tantalum, niobium, molybdenum, rhenium) are used as heat-resistant materials.
(4) Contact Materials and High-Density Alloys
The aluminum-copper ( 10%~40% copper) and tungsten-silver alloys manufactured using powder metallurgy methods have good electrical conductivity, copper and silver thermal conductivity, and tungsten’s wear resistance. Therefore, they become very effective contact materials for manufacturing working components such as knife switches, circuit breakers, and spot welding electrodes. High-density alloys composed of 90%~95% tungsten, 1%~6% nickel, and 1%~4% copper, as well as alloys using iron instead of copper ( ~5%), are used to manufacture gyroscope rotors, aircraft, balance weights for control surfaces, radiation shields for radioactive isotopes, material baskets, etc.
(5) Electric Vacuum Lighting Materials
Tungsten produces electronic tubes, radio electronics, and X-ray technology through tungsten wire, strips, and various forged components. Tungsten is the best material for incandescent lamp filaments and spiral wires. High operating temperatures (2200-2500 °C) ensure high luminous efficiency, while low evaporation rates guarantee a long lifespan for the filaments. Tungsten wire manufactures directly heated cathodes and grids for electronic oscillators, high-voltage rectifiers, and side-heated cathode heaters in various electronic instruments. Tungsten is used for the anodes and cathodes of X-ray tubes and gas discharge tubes, as well as contacts for radio equipment and electrodes for atomic hydrogen welding guns. Beryllium wire and beryllium rods are heaters for high-temperature furnaces (up to 3000 °C). Tungsten heaters operate in hydrogen atmospheres, inert atmospheres, or vacuum.
(6) Compounds of Tungsten
Sodium tungstate is used in the production of certain types of paints and pigments, in the textile industry for fabric weighting, and mixed with ammonium sulfate and ammonium phosphate to manufacture fireproof and waterproof fabrics; it is also used in the production of metal tungsten, tungsten sulfate, and tungsten salts, as well as in dyes, pigments, inks, electro-plating, and more; it is also used as a catalyst, among other things. Tungstic acid is a mordant and dye in the textile industry and is used as a catalyst for producing high-octane gasoline in the chemical industry. Tungsten disulfide is used as a solid lubricant and catalyst in organic synthesis, such as in the production of synthetic gasoline.
2. Tungsten Carbide Cemented Carbide
2.1 Tungsten Carbide
(1) Physical Properties of Tungsten Carbide
The main compound of carbon and tungsten is tungsten carbide, with the chemical formula WC. It is a black hexagonal crystal with a metallic luster hardness similar to a diamond and is a good conductor of electricity and heat. Melting point is 2870℃, boiling point is 6000℃, hardness is HV 2200, and relative density is 15.63g/cm3. Pure tungsten carbide is brittle, but adding a small amount of metals such as titanium and cobalt can reduce brittleness. Another compound of tungsten and carbon is tungsten dicarbide, with the chemical formula W2C, melting point of 2860℃, boiling point of 6000℃, hardness of HV 3000, and relative density of 17.15g/cm3. Its properties, manufacturing methods, and uses are the same as those of tungsten carbide.
In carbonized tungsten, carbon atoms are embedded in the gaps of the tungsten metal lattice without destroying the original metal lattice, forming a gap-solid solution. Therefore, these compounds are also called interstitial compounds.
(2) Chemical Properties of Tungsten Carbide
The chemical properties of tungsten carbide are stable, insoluble in water, hydrochloric acid, and sulfuric acid, but easily soluble in a mixed acid of nitric acid and hydrofluoric acid.
There are two stable tungsten oxides, WO2 and WO3. Among them, WO3 is the most thermodynamically stable under low temperature and atmospheric pressure conditions. Therefore, the direct oxidation of tungsten often leads to its formation. The oxidation rate of W is closely related to temperature and is also influenced by the atmosphere; in a humid atmosphere, the oxidation rate increases significantly above 300℃.
The oxidation of WC under dry gas is very slow, forming WO3. In a humid atmosphere, the oxidation behavior of WC is similar to that of W, but compared to W, WC has stronger antioxidant properties. When WC is exposed to air with relative 95% humidity, the formed oxide layer is significantly thinner than the oxide layer formed on W under the same conditions. The reason for the passivation of the WC surface is not yet fully understood. Still, it can be assumed that the crystalline diamond structure of WC is disturbed in the surface region, resulting in unsaturated W atoms. These W atoms will quickly oxidize to easily form WO3 and dissolve in water. When all the unsaturated W atoms are oxidized and dissolved in this way, the outermost layer of the crystal will contain only carbon atoms. One possibility is that these carbon atoms will form covalent bonds with the carbon atoms in the second layer, resulting in a very stable surface structure, which gives tungsten steel decorative materials, primarily composed of carbide, good antioxidant properties.
(3) Composition Indicators of Tungsten Carbide Powder
Tungsten steel material is produced using powder metallurgy, and tungsten carbide powder is the basic material for powder metallurgy, which has specific quality requirements. Table 6-1 shows the quality specifications for tungsten carbide powder, and Table 6-2 shows the chemical composition indicators for tungsten carbide powder.
Table 6-1 Tungsten Carbide Powder Quality Specifications
| Category | Fisher average particle size /μm) | Total carbon amount /% | Free carbon/% |
|---|---|---|---|
| WC-1 | ≤1.0 | 6.08~6.18 | ≤0.08 |
| WC-2 | 1~1. 99 | 6.08~6. 18 | ≤0.08 |
| WC-3 | 2~3. 99 | 6.08~6.18 | ≤0.08 |
| WC-4 | 4~5. 99 | 6.08~6.18 | ≤0.08 |
| WC-5 | 6~7. 99 | 6.08~6.18 | ≤0.08 |
| WC-6 | 8~11. 99 | 6.08~6. 18 | ≤0.08 |
| WC-7 | 12~15.99 | 6.08~6. 18 | ≤0.08 |
| WC-8 | ≥16 | 6.08~7. 18 | ≤0.08 |
| (State Administration for Technical Supervision, 1990) | |||
Table 6-2 Chemical Composition Indicators of Tungsten Carbide Powder
| WC | Fe | Mo | Al | Si | Ca | Mn | Mg | Ni | Na |
|---|---|---|---|---|---|---|---|---|---|
| ≥99.8 | ≤0.04 | ≤0.010 | ≤0.001 | ≤0.01 | ≤0. 005 | ≤0.002 | ≤0.002 | ≤0.005 | ≤0.003 |
| ≥99. 7 | ≤0. 06 | ≤0. 015 | ≤0.002 | ≤0. 01 | ≤0.008 | ≤0.002 | ≤0.004 | ≤0.008 | ≤0.005 |
| (State Administration for Technical Supervision, 1990) | |||||||||
(4) Tungsten Carbide Powder Particle Size
The particle size of tungsten carbide powder significantly affects the material’s performance. Refinement of WC grains can noticeably improve the alloy’s performance. Ultra-fine grain tungsten steel has high hardness, good wear resistance, and very high strength and toughness.
2.2 Binder
In tungsten steel powder metallurgy, a binder bonds the powder together. Depending on the different stages and functions of production, binders are divided into organic and metal.
(1) Organic Binder
In powder metallurgy injection molding, organic binders are often used to bond metal powder particles, allowing the mixture to have rheological and lubricating properties after heating in the injection machine’s barrel. That is, the binder acts as a carrier that drives the flow of the powder. Therefore, the binder selection is key to the entire powder injection molding process. The requirements for organic binders are: ① low dosage, able to achieve better rheological properties with less binder; ② non-reactive, during the process of removing the binder, there is no chemical reaction with the metal powder; ③ easy to remove, leaving no carbon residue in the product.
The organic binder is removed after sintering and does not constitute the final composition of the material.
(2) Metal Binder
In general, powder metallurgy uses metal binders to bond the powder together. The carbides and the binding metal determine the properties of tungsten steel. They vary significantly due to WC content, WC grain size, and alloy additives. The impact of carbides on performance in composite materials is reflected in hardness and wear resistance, while the metal or alloy binder is reflected in strength and toughness. Metals commonly used as tungsten steel binders include Co, Ni, Fe, Fe-Ni, Ni-Co, Ni-Cr3C2-P, Fe-Ni-Co and so on.
① Cobalt. Cobalt is an excellent binder for WC and WC-TiC-based cemented carbide. Since the invention of WC-Co hard alloys in 1926, cobalt-bonded alloys of this type have dominated the market due to the unique properties of the Co and Co-W-C ternary systems. It is well known that the solubility of WC and Co is very high and varies greatly with temperature. The excellent wettability of WC and liquid Co, as well as the good performance of Co-W-C metal binders, have made the use of Co-dominant in hard alloys.
In the WC-Co hard alloy, the vertical cross-section of the W-C-Co ternary phase diagram along the Co-WC line is shown in Figure 6-1. Taking the WC-Co alloy with a WC content of 60% as an example, before the appearance of the liquid phase, the solubility of WC in Co increases with the temperature rise, and at the eutectic temperature (approximately 1340℃), the liquid phase of the eutectic composition begins to appear in the sintered body. At the sintering temperature (1400℃ ) and when held at that temperature, the sintered body consists of the liquid phase and the remaining solid WC phase. During cooling, WC first precipitates from the liquid phase, and when the temperature falls below the eutectic temperature, an alloy with a WC+γ two-phase structure is formed.

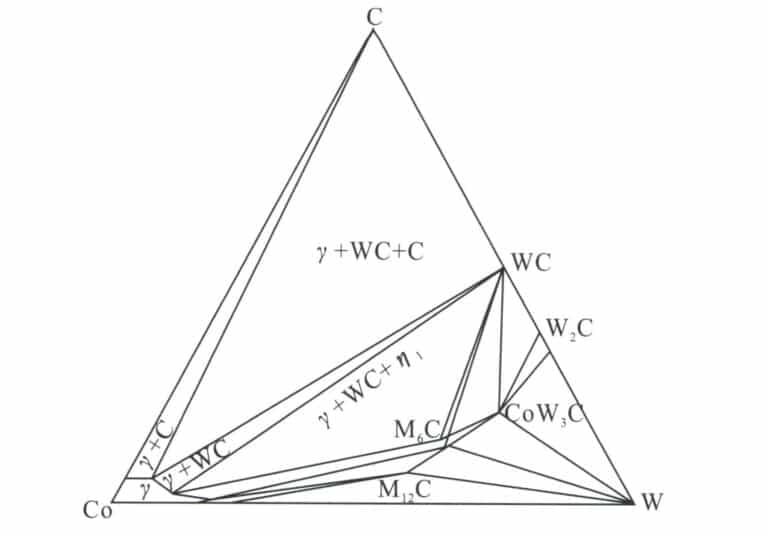
The phase composition of the alloy is related to the alloy composition, sintering process, etc. In actual production, it is relatively easy to control the phase composition of the alloy to avoid the generation of phases that may lead to the deterioration of alloy performance. Doping some other components into WC-Co alloys can change the width of the γ+WC two-phase region; for example, adding a small amount of TaC(0.5%~3%) to the WC-10%Co alloy increases the 6.03%~6.22% width of the phase region, and the width of the phase region increases with the amount of TaC added, with TiC and NbC having similar effects. Additionally, Ni can rapidly expand the phase region of low carbon content, reducing the sensitivity of the alloy phase composition to carbon content.
The performance of WC-Co type tungsten steel is directly related to the morphology of the bonding phase Co layer. When Co transitions from fcc to hcp structure, it reduces the ability for plastic deformation and suppresses crack formation. The addition of rare earth elements significantly impacts the phase structure, composition, and phase transformation of WC-Co alloys, mainly because rare earth elements can inhibit the transition of the Co bonding phase layer to the hcp structure.
Cobalt is an expensive and scarce metal with extremely limited reserves. As a result, it is facing a serious problem of resource shortage, and prices are continuously rising, so there is a need to find alternative materials for cobalt.
② Nickel. As a relatively inexpensive and abundant metallic element, nickel is quite rich in resources in our country. If nickel can replace cobalt as a binder for hard alloys, it will greatly reduce the production cost of hard alloys. Nickel and cobalt belong to the iron group of elements; nickel has a structure and properties similar to cobalt, but there are still some differences. It has long been attempted to use pure nickel instead of cobalt as a binder for hard alloys, but the performance of the resulting hard alloys is poor. Nickel’s wettability on tungsten carbide grains is not as good as that of cobalt, leading to nickel aggregation, abnormal growth of tungsten carbide grains, and voids in the products. Therefore, replacing cobalt with pure nickel to produce hard alloys cannot ensure good alloy performance; adding an appropriate amount of other metal elements to the binder is necessary to improve and enhance the alloy performance. The choice of additives is key to the success of nickel replacing cobalt; the additives should address the issues of nickel aggregation and abnormal growth of tungsten carbide grains in nickel-cobalt alloys and should also enhance the bonding phase and improve nickel’s wettability on tungsten carbide grains, ensuring a good combination of hard and brittle tungsten carbide with soft and ductile metallic nickel.
③ Iron. Steel-bonded hard alloys have a wide range of process characteristics, good physical and mechanical comprehensive properties, and excellent chemical stability. Iron is the main element of the bonding phase in steel-bonded hard alloys and can improve the strength and plasticity of the alloy. When iron is used solely as a binder, it has high surface tension, poor wettability, coarse grains, and many pores. Adding some other elements to the steel-bonded hard alloys, such as C, Cr, Mo, W, Mn, B, etc, is essential to achieve the necessary structure and properties.
④ Ni-Cr3C2-P. Since the strength of WC-pure Ni alloy is lower than that of WC-Co alloy, it is necessary to alloy Ni. Cr3C2 is a commonly used additive that can increase the strength of the alloy, improve its oxidation and corrosion resistance, and limit the growth of WC grains to obtain a fine structure. However, when the content of Cr3C2 is too high, the pore size will increase accordingly.
Due to the higher sintering temperature of the WC-Ni alloy, the solubility of tungsten carbide in nickel is higher, so WC-Ni alloys often have higher porosity, and tungsten carbide grains are also prone to coarsening. In the Ni-P alloy, a small amount of phosphorus is added in the form of intermediate Ni-P alloy; the low melting point has high liquid flowability and strong adhesion to metals and refractory compounds; phosphorus can make the WC-Ni bonding phase changeable in the alloy, activate the sintering process, and lower the sintering temperature, thereby avoiding the growth of carbide grains and producing materials with low porosity and high strength.
⑤ Fe-Ni-Co. Due to certain unique properties of cobalt, it still dominates the market as a binder. However, its hexagonal close-packed (hcp) crystal structure affects the plastic deformation properties of the alloy. The newly developed Fe-Ni-Co binder can improve the fatigue strength and toughness of the alloy by selecting the appropriate Fe:Ni:Co ratio. The alloy has a mixed crystal structure and excellent physical properties, making it a potential alternative to hard alloy binders.
2.3 No Binder
As mentioned, the tungsten carbide cemented carbide material is formed by adding a binder to the carbide yttrium powder. Due to the high melting point of WC, it is almost impossible to sinter pure WC alone using conventional sintering methods (which require some liquid phase) without a low melting point binder like Co. Adding the binder reduces the hardness, corrosion resistance, and oxidation resistance of the material and complicates the production process. It is also prone to thermal stress due to the difference in thermal expansion coefficients with WC. Moreover, conventional sintering methods cannot effectively suppress grain growth during the sintering process, making it difficult to obtain ultrafine hard materials.
In recent years, the discharge plasma sintering technology has emerged, which applies direct current pulsed voltage generated by a special power control device to the pressed powder sample. The spark discharge between the powders allows for the concentration of high-energy pulses (high-temperature plasma) in the bonding areas between particles, causing the surface of the tungsten carbide to melt and bond together. This technology features surface purification and high-speed sintering and effectively suppresses grain growth during the sintering process, becoming a new direction in powder metallurgy processes.
3. Decorative Tungsten Steel Material
3.1 Requirements for Decorative Tungsten Steel Material
In the jewelry industry, tungsten steel is often referred to as tungsten gold, not only because tungsten is a rare metal with low abundance on Earth but also due to its physical and chemical properties related to tungsten steel. The tungsten steel used for jewelry is not the traditional tungsten alloy steel but a hard alloy produced using tungsten carbide as the main raw material through powder metallurgy methods. Compared to general hard alloys, it has the following requirements.
(1) Requirements for Tungsten Carbide Content
WC is a new functional material with high hardness, thermal stability, and wear resistance. The surface effect of tungsten steel jewelry is closely related to its composition, requiring the tungsten carbide content in tungsten steel materials to reach a certain amount, usually requiring the tungsten carbide component in the material to be above 80% to be called tungsten gold. A laboratory at a certain university in the United States found through research and analysis that when the tungsten carbide content in tungsten steel materials reaches 85.7%, the polishing brightness of the jewelry is highest, and the effect is best. This number is also the international standard in the industry, and its accuracy directly determines the quality of tungsten steel jewelry. Of course, reaching this standard is also very difficult, presenting a technical bottleneck for most manufacturers, making it hard to produce high-quality tungsten steel jewelry. Only a few countries, such as China, South Korea, and Japan, can meet this standard.
(2) Binder Requirements
Jewelry materials generally require no harmful effects on the human body, no magnetic properties, and good corrosion and oxidation resistance. Therefore, cobalt is rarely used as a binder in tungsten steel for jewelry, while nickel-based alloys are widely used as binders. Hard alloys WC-Ni-Cr3C2-P are ideal materials for making jewelry.
(3) Factors Affecting the Performance of Tungsten Steel Materials used in Accessories
The performance of tungsten steel materials is not only related to the grain size of WC but also largely depends on the phase composition, microstructure, and its form in the alloy. In actual production, due to the influence of raw materials and sintering processes, the alloy usually contains a more complex organizational structure. Therefore, during production, it is necessary to strictly control the quality of raw materials and formulate and implement production processes such as mixing, ball milling, and sintering.
3.2 Common Issues with Tungsten Steel Materials for Jewelry
(1) Sand Eye (hole)
Clear boundary circular or flaky black holes appear on the product’s surface, and the amount of pores is represented by porosity, which is generally assessed by comparing it with standard images. The main reason for the formation of pores is insufficient sintering temperature or holding time, leading to under-sintering. The causes of sand holes may include the following.
① High impurity content. The impurities in WC-Ni hard alloys are mainly brought in by tungsten trioxide and nickel oxide, among which, K2O, Na2O, MgO, CaO, SiO2, Al2O3 at the sintering temperature, do not melt themselves and cannot be wetted by the liquid phase but instead worsen the wettability of the liquid phase to carbides, so when their content is slightly high, the B-type porosity (10~25㎛) of the alloy significantly increases.
② The component allocation ratio is inappropriate. First, when the content of Cr3C2 is too high, and the content of the WC-Ni hard alloy is excessive, the pore size will increase. Second, when the content of Ni-P is low, the low melting point Ni-P has very high liquid flowability and strong adhesion to metals and difficult-to-wet compounds; phosphorus in WC-Ni alloys can make the bonding phase changeable, activate the sintering process and lower the sintering temperature, thus avoiding the growth of carbide grains and producing materials with fewer pores and higher strength. If too little Ni-P is added to the WC-Ni alloy, it will not serve the purpose of an additive and will not achieve the desired effect.
③ The impact of technology and operations is reflected in the following six aspects.
One is improper wet grinding. Due to inaccurate addition of anhydrous ethanol, insufficient ball quantity or small ball diameter, slack belts reducing grinding cylinder speed, or even occasional mid-process or later shutdowns, the grinding efficiency is reduced, leading to uneven component mixing. As a result, certain carbides lack a liquid phase, making it difficult to fully shrink during the sintering process, leaving residual pores in the alloy.
The second is nickel aggregation. Even when using very fine nickel powder as the raw material, during wet grinding, the nickel powder will coarsen into large nickel aggregates (containing a small amount of fine WC), which can form large pore defects during the sintering of the pressed mixture.
Third, the mixture’s oxygen content is relatively high. This can lead to carbon deficiency, oxidation, and increased dirtiness in the alloy.
The fourth issue is uneven wax mixing. Due to the low solubility of paraffin in gasoline at room temperature, and the amount of paraffin used is usually more than double that of synthetic rubber, the volume of the paraffin-gasoline solution required for a certain amount of mixture increases accordingly. This not only makes mechanical mixing difficult but also leads to a significant amount of the solution floating on the mixture during manual mixing; if the drying process is not timely, a considerable amount of paraffin often floats on the surface of the material, resulting in uneven mixing, which is expelled during the low-temperature stage of sintering, leaving larger pores afterward.
The fifth is hard nickel particles. Due to the high reduction temperature or prolonged holding time when reducing nickel oxide, the resulting nickel powder contains hard particles, and excessively hard nickel particles cannot be crushed by pressure during compaction. Because individual nickel particles are relatively dense, larger voids must be when the crushed relative density is the same.
Six is vacuum sintering. For the pressed parts that have undergone dewaxing and pre-sintering, during the mid-stage of the vacuum sintering process, the vacuum degree in the furnace decreases due to the intense carbon-oxygen reaction and the release of a large amount of gas. At this time, the heating rate should be slowed down to allow the gas to be discharged outside the furnace. To make the carbon-oxygen reaction as complete as possible, in addition to increasing the vacuum inside the furnace, it should also be kept at 1200~1250℃ for insulation, which effectively reduces the alloy’s porosity. Otherwise, a rapid heating rate and insufficient insulation time will increase the alloy’s porosity.
(2) Delamination
Typically located at the edges, it appears similar to dirt under low magnification (100X) but is straighter and longer than dirt. Its total length is measured using an eyepiece micrometer during determination. The main reasons for the delamination in jewelry alloys are high pressing pressure, fine material particles, uneven wax mixing, overly wet or dry mixtures, poor mold smoothness, etc.
(3) Nickel Accumulation
Under low magnification, the alloy surface presents snowflake-like spots resembling plum blossoms and bamboo leaves. The reasons for the accumulation of nickel may be as follows.
- Wet grinding. Nickel powder coarsens into large nickel aggregates during wet grinding (containing a small amount of fine WC).
- The inappropriate component ratio, wet grinding, and vacuum sintering are poor. The particles are fine and highly active. Phosphorus also activates the sintering process, lowering the sintering temperature of the WC-Ni alloy. When the sintering temperature is high, the vacuum degree is high, the nickel content is high, and the sintering time is long, the problem of nickel aggregation is more prevalent, leading to significant evaporation or loss of nickel phase. The resulting nickel aggregates or “nickel pools” are internal causes, while the inappropriate component ratio, wet grinding, and poor vacuum sintering are merely external causes; that is, the aggregation of nickel phase → evaporation (volatilization) →loss, leaving behind fine WC.
(4) Carburization (graphite inclusion)
If small pores in nest-like aggregates or flaky shapes are observed under low magnification on uncorroded grinding discs, they are considered graphite inclusions. The degree of carburization can be checked against standard images and reported results. The main reasons for the carburization phenomenon in alloys are high total carbon and free acid content, insufficient dewaxing, and low O2 content in nickel powder.
Section II Characteristics of Tungsten Steel Jewelry
1. Advantages of Tungsten Steel Jewelry
Tungsten steel, also known as tungsten gold in the West, has qualities that are difficult to compare with other jewelry materials, as reflected in the following aspects.
(1) High hardness: Tungsten steel can reach a Mohs hardness 8.9~9.1, equivalent to natural sapphire. This high hardness makes tungsten steel very wear-resistant and not easily prone to scratches, deformation, and other issues.
(2) High brightness: After being highly polished, tungsten steel fully radiates a gem-like color and luster, with a brightness like that of a mirror.
(3) Tungsten steel is corrosion resistant; in artificial sweat testing, it does not corrode, fade, change color, allergy and rust, and the luster can be maintained for a long time, which is something other metals cannot achieve.
(4) Tungsten steel has a high density and strong texture, making it a noble choice for fashionable men.
2. The Disadvantages of Tungsten Steel Jewelry
Tungsten steel is very brittle and prone to breaking when subjected to impact during production and use; therefore, it cannot be inlaid with gemstones.
Tungsten steel is very hard to process, requiring diamond polishing tools for machining.
3. Identification of Tungsten Steel Jewelry
Tungsten steel is a popular material in the current fashion jewelry market, with good market response and product profits. However, some merchants pursue profits by passing off inferior goods as high quality, making it difficult for ordinary consumers to distinguish.
(1) The Difference between Tungsten Steel, Stainless Steel, and Titanium Alloy
The stainless steel and titanium alloy have been introduced earlier, and the three materials are fundamentally different.
Stainless steel is a high-alloy steel that can resist corrosion in air or chemical corrosive media because it contains chromium, which forms a very thin chromium film on the surface, isolating the steel from the oxygen that invades it and providing corrosion resistance. To maintain the inherent corrosion resistance of stainless steel, the steel must contain at least 12% chromium. The specific gravity of stainless steel is about 8g/cm3, its color, which is slightly white, and its hardness is only 1/7 about that of aluminum steel.
The titanium alloy used for accessories is generally industrial pure titanium, with a smaller specific gravity of only 4.51g/cm3 about of that of 1/3 tungsten steel. It is gray-white in color and hardness similar to stainless steel.
(2) Identification of Tungsten Steel Quality
Since the introduction of tungsten steel jewelry, it has been loved and sought after by fashion enthusiasts from all walks of life, especially in Europe and America, where people take pride in being able to wear tungsten steel jewelry. However, due to the hardness and rarity of tungsten steel materials, the manufacturing and processing techniques are extremely difficult, leading to many inferior tungsten steel products in the market, some of which can even harm people’s bodies. These so-called tungsten steel jewelry are prohibited from selling in Europe and America. The quality of tungsten steel jewelry can be identified mainly from the following aspects.
① Material composition. Tungsten is extremely rare on Earth, and the tungsten content in tungsten steel jewelry must reach 80% or above to be called tungsten steel. When the tungsten content in tungsten steel reaches 85.7%, the brightness is the highest, and the effect is optimal. Currently, many tungsten steel jewelry items on the market generally do not reach this content and may even be below 60%, so such tungsten steel jewelry is, of course, not very valuable.
② Appearance. Due to its hardness, tungsten steel jewelry is difficult to process at the edges and corners. If not handled well, it can have sharp edges that may cause injury to the body, and if over-processed, it fails to showcase the unique style of tungsten steel jewelry. Tungsten steel jewelry uses gemstone cutting and polishing techniques, and after fine polishing, it can achieve a gemstone-like luster and brilliance. Poor cutting and polishing techniques can greatly affect the surface appearance.
③ Size. The polishing of tungsten steel jewelry is almost entirely a manual process, making size control very difficult. When the control is improper, it is easy to have issues such as dimensional deviations and asymmetrical shapes.
④ Environmental protection and safety. This is currently the most concerning issue, both internationally and domestically. In terms of meaning, tungsten steel jewelry is also an alloy, and since it is an alloy, it contains other metal components. It is necessary to determine whether the metal elements contained are harmful to the human body, such as cobalt.
Section III Categories of Tungsten Steel Products
1. Plain Tungsten Steel Jewelry

Plain tungsten steel ring

Plain tungsten steel bracelets

Plain tungsten pendants

Plain tungsten belt buckles

Plain tungsten watch
Plain tungsten cufflinks

K gold-inlaid tungsten ring

Diamond-inlaid tungsten ring
2. Tungsten Steel Inlaid Jewelry
Copywrite @ Sobling.Jewelry — Custom jewelry manufacturer, OEM and ODM jewelry factory
Section IV Production Process of Tungsten Steel Accessories
1. Introduction to Powder Metallurgy Technology
(1) The History of Powder Metallurgy Development
Modern powder metallurgy technology, an industrial technology recognized worldwide, has three important milestones in its development.
- Overcame the difficulties arising from the casting process of refractory metals. In 1909, the production of electric lamp tungsten wires promoted the development of powder metallurgy; the emergence of powder metallurgy hard alloys in 1923 was hailed as a revolution in machining.
- In the 1930s, porous oil-containing bearings were successfully produced. Subsequently, the development of powder metallurgy iron-based mechanical parts fully utilized the advantages of powder metallurgy with little or no cutting.
- Develop new materials and new processes at a higher level. Following the emergence of metal ceramics and dispersion-strengthened materials in the 1940s, powder high-speed steel and powder high-temperature alloys appeared successively from the late 1960s to the early 1970s; high-strength parts can now be manufactured using powder metallurgy forging and hot isostatic pressing.
However, powder metallurgy technology has developed in recent years mainly because the automotive industry needs to produce large quantities of final or near-final products.
(2) Categories of Powder Metallurgy
① From the perspective of product forming methods, there are generally two types of powder metallurgy product forming: pressing and injection molding.
Press molding is the process of filling dry powder into a mold relying on gravity and forming it through external pressure. There are many types, and in actual industrial applications, press forming is widely used. Warm pressing, cold closed steel mold pressing, cold isostatic pressing, and hot isostatic pressing all belong to press molding.
Injection molding is the process of injecting a fine powder mixed with a large amount of thermoplastic binder into a mold.
② From the perspective of matrix materials, powder metallurgy is roughly divided into iron-based, copper-based, aluminum-based, stainless steel, magnetic materials, friction materials, magnetic steel, hard alloys, etc. However, this distinction is relatively coarse, as adding different metals, non-metals, and other additives to the matrix materials can achieve different effects, which need to be determined based on different performance requirements.
(3) Advantages of the Powder Metallurgy Process
- It is possible to produce workpieces with colors that change continuously or to combine two or more materials that are difficult to dissolve, which cannot be achieved with conventional production methods.
- The pressed blank that can be compressed to the final size has a high surface finish, requiring very little subsequent processing and adjustment, which can greatly save metal and cutting tools, reducing product costs.
- During the production process, the materials do not melt, so there is no fear of impurities brought in by crucibles and reducing agents, and sintering is generally carried out in a vacuum and reducing atmosphere, which is not afraid of oxidation and will not contaminate the materials, allowing for the production of high-purity materials.
- Can ensure the correctness and uniformity of the material composition ratio.
- Powder metallurgy is suitable for producing a large quantity of products with the same shape, significantly improving production efficiency, shortening production cycles, and greatly reducing production costs.
2. The Process of Producing Tungsten Steel Jewelry Using Powder Metallurgy Technology
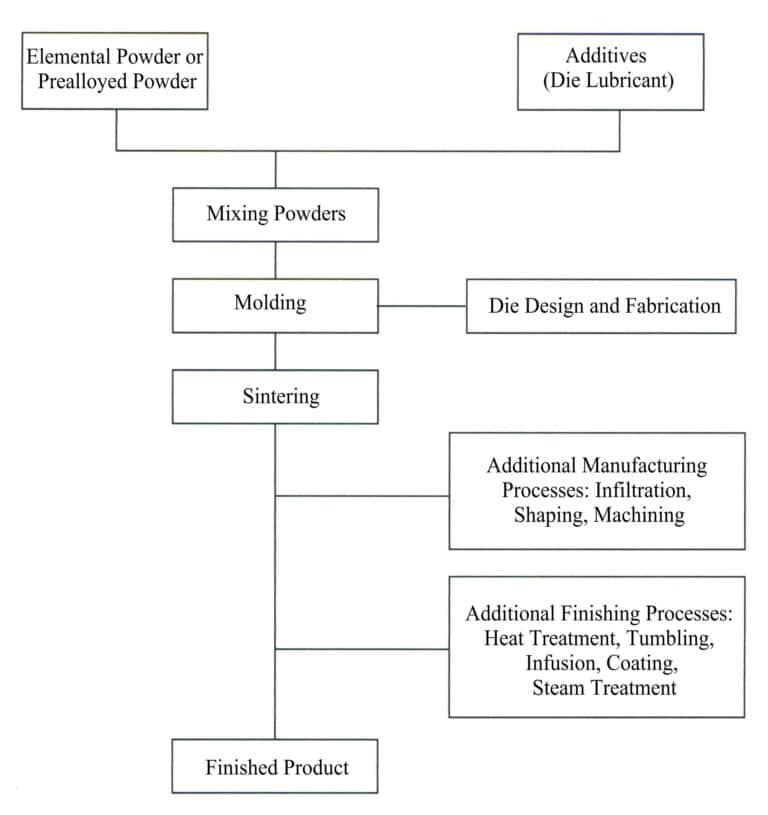
2.1 Preparation of Raw Material Powder
(1) Requirements for the Powder
In the powder metallurgy production process, the pressed products must have sufficient mechanical strength so that cracks do not occur during spraying, pressing treatment, and transfer to the sintering furnace. Mechanical strength results from cold welding between individual powder particles but is mainly due to the mutual mechanical bonding between the particles. Therefore, there are certain requirements for the size and shape of the powder; when the powder is too coarse, it adversely affects the green strength of the blank, making it easier to produce cracks when removing the blanks from the die. Fine powders have more contact points and are more ideal than coarse particle powders, while irregularly shaped powders bond less well, so spherical particles should be prioritized.
(2) Powder Preparation Method
The existing milling methods can be broadly divided into two categories: mechanical methods and physicochemical methods. Mechanical methods can be further divided into mechanical crushing methods and atomization methods; physicochemical methods are divided into electrochemical corrosion methods, reduction methods, chemical methods, reduction-chemical methods, gas phase deposition methods, liquid phase deposition methods, and electrolysis methods.
The atomization method is the most widely used, particularly suitable for producing alloy powders. Its basic method involves high-pressure gas or water flow to strike fine metal liquid streams into very fine droplets, solidifying solid particles in the atomization chamber. Atomization can be divided into gas atomization and water atomization. Gas atomization has a relatively slow solidification rate, and the surface of the droplets tends to form spherical particles due to surface tension. The solidification rate of the water atomization method is much faster than that of the gas atomization method, and the time is also much shorter, so the effect of surface tension is not exerted, making it easier to form irregular particles. The size of atomized powders is uneven, so the powders need to be sieved to achieve uniform and fine sizes.
In recent years, research has shown that the refinement of WC grains can significantly improve the performance of alloys. Ultra-fine grain tungsten steel has high hardness, good wear resistance, and very high strength and toughness. Currently, the main preparation methods for ultra-fine WC powder include the fixed reaction method, in-situ carburization reduction method, mechanical alloying method, and spray drying—fluidized bed method, among others.
(3) Powder Preparation Process Parameters
The higher the melting temperature, the greater the pressure of the spray atomization and the finer the powder. The average particle size of the powder obtained after atomization is 45㎛, 50% of the powder, smaller than the average size.
(4) Storage of Powder
Powder exposed to air for some time will absorb moisture or gas, which may result in cracks in the powder metallurgy blanks during rolling. Therefore, the powder should undergo vacuum heat treatment, and the process parameters can refer to the temperature of 180℃, with the vacuum degree being one millibar (1bar=105Pa). The powder is treated under vacuum and then vibrated to ensure uniform distribution, and then it is bagged and sealed according to the powder’s color category.
2.2 Mixing Powder
Powders in powder metallurgy can be divided into elemental or pre-alloyed metal powders. Elemental powders consist of single metal elements and can be used alone or mixed with other elemental powders to form an alloy. Pre-alloyed metal powders are alloyed during powder manufacturing, so each powder particle contains the same nominal composition. Tungsten carbide materials are generally produced using pre-alloyed metal powders.
The mixed powder is a uniform mixture of the main components, such as tungsten carbide powder, chromium carbide powder, graphite powder, nickel powder, and additives. Mold lubricant is a typical additive that can reduce the force required to eject defective finished products from the mold. Adding graphite powder provides carbon for the reduction of oxides, achieving the final carbon content of the sintered product.
After mixing the powder, place it in a dedicated mold for pressing. The design of the mold (and the pressed parts) should take into account both the powder’s flow characteristics and the mold’s pressing effect on the powder.
Although the metal powder is spherical, it does not flow according to fluid mechanics principles. This is because there is friction between the powder particles and the mold. Therefore, the design of the components should ensure that the powder can be properly distributed in the mold cavity. In addition, the lateral flow of the metal powder is also limited, which restricts the structural shapes that can be produced.
2.3 Molding and Pressing
Forming aims to produce a compact with a specific shape and size, ensuring a certain density and strength.
The molding methods are basically divided into pressure molding and non-pressure molding. Pressure molding is more common, and the most widely used method in pressure molding is compression molding.
(1) Mold
Molding first requires creating a corresponding mold based on the shape and size of the workpiece. Since very high pressure is used in powder pressing molding, there is significant friction between the metal powder and the mold wall. It is essential to ensure the quality and performance of the mold, meeting the requirements for precision, surface smoothness, and wear resistance. The mold structure design should facilitate the easy and smooth removal of blanks from the mold.
(2) Molding Process and Key Operation Points
The tungsten carbide powder’s operating parameters should be followed during pressing. Based on the characteristics of the jewelry piece, such as size and weight, preparatory work should be done before pressing, adjusting the position of the lower plunger to ensure the weight of the blank meets the requirements and adjusting the pressing pressure to ensure the height and density of the blank meet the requirements. After adjustments are completed, the mold should be fixed to the press plunger, and the powder is fed from the feed pipe into the vibrator and then sent into the mold cavity.
After the preparation work is completed, the first step of the pressing process is to place the control quantity of powder into a precisely sized die, with a die volume approximately 2.5 times that of the finished product. The powder is pressed by punches moving simultaneously up and down with pressure of 345~620MPa, and the pressed components are called “raw blanks.” The raw blanks are removed from the mold, and the process of refilling and compacting the powder is repeated. The entire cycle of forming and pressing takes about 6~10s. Therefore, the production speed can reach 600Pcs/h, and the efficiency is very high.
(3) Considerations for Molding and Pressing
When the equipment and operating process parameters are stable, the quality of the pressed blank is very stable, and the weight and dimensional consistency of batch products are good. However, if the process parameters are inappropriate, if there are inappropriate parts, and if there are quality issues with pressing, they are easy to have. Therefore, attention should be paid to the following matters during operation.
- The volume of the mold cavity determines the amount of powder loaded, which directly affects the weight of the blanks.
- The density of the blank is closely related to the pressing pressure. As the pressing pressure increases, the density increases, which is beneficial for obtaining dense workpieces with fewer pores. However, the pressing pressure is too high. In that case, the friction between the powder particles and the mold wall will gradually damage the mold, affecting the precision and quality of the blank and having a certain impact on the lifespan of the mold and punch.
- The friction between the powder and the mold wall also affects the surface finish of the pressed part, increases the difficulty of removing blanks from the mold, and makes the blank prone to cracking. After removing the blank, residual internal stress may also lead to cracking (Figure 6-4).
2.4 Blanks Sintering
The pressed material, after forming, achieves the required final physical and mechanical properties through sintering, a key process in powder metallurgy. During the sintering process, atoms move across the surface of the powder particles to the contact points formed during the pressing process. As the sintering time increases, the contact points grow, and the powder particles bond into a solid mass containing various sizes and shapes of pores. Sintering transforms the mechanical bonding between the powder particles into metallurgical bonding. Therefore, the mechanical properties of the final product can rival those of cast or forged products with the same chemical composition.
(1) Types of Sintering
According to the reactions involved in the sintering process, sintering is divided into single-component sintering and multi-component sintering. According to the state of the bonding phase during sintering, it is further divided into solid-phase sintering and liquid-phase sintering. In addition to ordinary sintering, there are special sintering processes such as loose sintering, infiltration, and hot pressing.
The sintering temperature is lower than the melting point of the metals and alloys used for the solid-phase sintering of single-component and multi-component systems. It is achieved through high-temperature heat treatment, which causes the powder particles to bond and densify the blank, resulting from solid-state diffusion without melting. The energy for diffusion is provided by thermal energy; therefore, a higher sintering temperature can lead to stronger bonding and higher density. For the liquid-phase sintering of multi-component systems, the sintering temperature is generally lower than the melting point of the refractory components and higher than the melting point of the easily melted components.
Due to the presence of some easily oxidizable elements in the composition of tungsten steel, sintering needs to be carried out under a controlled atmosphere, and a reducing atmosphere composed of 95%N2+5%H2 can be used.
(2) Requirements for the Sintering Furnace
Certain requirements for the sintering furnace are specified, such as a certain output, the ability to continuously sinter for more than 24 hours, the ability to stably reach the required sintering temperature, the allowance for the use of a reducing atmosphere, and the presence of devices for conveniently quenching workpieces.
These requirements can be met when using a rotary furnace. The furnace is divided into sections, and each section can hold a certain number of workpieces in refractory containers. The furnace rotates at regular intervals, which allows for periodic loading and unloading of blanks and ensures good temperature uniformity.
When the required sintering time is reached, the sintering process ends, and post-processing can be carried out after the blanks cool down.
2.5 Common Defects of Tungsten Steel Blanks
High-quality pressed blanks are the foundation for ensuring the quality of tungsten alloy jewelry. Due to the particularity of the production process, quality issues inevitably arise during pressing production. The following lists some typical causes of defects in blanks and improvement measures.
(1) Local Density Deviation
- The intermediate density is too low. The causes include excessive side area, rough mold wall, poor lubrication of the mold wall, and poor powder compressibility. Improvement measures include switching to bidirectional friction pressing, reducing the roughness of the mold wall, and adding lubricants to the mold wall or the powder.
- One end has a too-low density. The causes include a large length-to-diameter or length-to-thickness ratio, a rough mold wall, poor lubrication of the mold wall, and poor compressibility of the powder material. Improvement measures include switching to bidirectional pressing, reducing the roughness of the mold wall, and adding lubricants to the mold wall or the powder material.
- High or low density. The causes include improper compensation for the powder. Improvement measures include adjusting the amount of compensation powder.
- The density is low in thin-walled areas. The reasons for this include the local wall thickness ratio being too large and unidirectional pressing not being suitable. Improvement measures include adopting bidirectional pressing, reducing mold wall roughness, and adding additives to local areas of the mold wall.
(2) Crack
- Cracks at the corner. The causes include improper powder filling compensation, poor powder compressibility, and incorrect demolding method. Improvement measures include adjusting the compensation of powder filling, improving the compressibility of the powder, and using the correct demolding method; for external products, a pressure sleeve should be used, and the flange should be de-molded first with the pressure sleeve.
- Side cracking. The causes include the inner hole of the female mold decreasing in size along the demolding direction. For example, in processing, the forming part has been severely worn, and there are burrs at the outlet; the graphite powder in the raw material is segregated and layered; the upper and lower surfaces of the press are uneven, or the verticality and parallelism of the mold exceed the standard; poor powder compressibility. Improvement measures include machining a demolding taper along the demolding direction of the female mold, adding some lubricant to the raw material to avoid graphite segregation, improving the flatness of the press and mold, and improving the compressibility of the raw material.
- Diagonal cracks. The causes include poor mold rigidity, excessive pressing pressure, and poor powder pressing performance. Improvement measures include increasing the wall thickness of the female mold, switching to a circular mold sleeve, improving the powder pressing performance, and reducing the pressing pressure (to achieve the same density).
(3) Wrinkling
- Wrinkling at the inner corner of the platform. The causes include the large hole core rod being pressed down too early, the end platform already being formed, and when the thin-walled sleeve continues to be pressed, the powder flow breaks through the already formed area and reshapes it. Repeated cycles can lead to wrinkling. Improvement measures include increasing the final pressing amount of the large hole core rod, appropriately reducing the density of the thin-walled area, and appropriately reducing the radius at the corners.
- Outer spherical wrinkling. The causes include the already formed spherical surface during the pressing process being continuously broken by the flowing powder and constantly reformed as a result. Improvement measures include appropriately reducing the pressing density, using powders with a larger loose bulk density, final rolling to eliminate, and switching to elastic molding.
- Overpressure wrinkling. The causes include excessive local unit pressure, crushing the surface of the formed part, losing plasticity, and being unable to reshape during further pressing. Improvement measures include reasonably compensating for powder filling to avoid local overpressure and improving the powder pressing performance.
- Sharp edges removing. The causes include uneven density, low local density, improper demolding, such as not being straight during demolding, unreasonable mold structure, or bouncing during demolding, and storage and handling causing damage. Improvement measures include improving the pressing method to avoid low local density, improving demolding conditions, and being careful during operation.
- Localized peeling on the side. The causes include gaps at the seams of the assembled mold and steps at the seams of the assembled mold, which inevitably lead to localized peeling during demolding (i.e., the diameter of the sphere is greater than that of the column or the sphere and column are not concentric). Improvement measures include: the assembly of the mold should be seamless; there should only be steps at the seams that do not affect demolding (i.e., the diameter of the spherical part in the figure can be slightly smaller but not larger, and the sphere and column must be concentric).
(4) Surface Scratches
The causes are high roughness of the mold cavity surface or low hardness, mold wall forming nodules, and local areas of the mold cavity surface being gnawed or scratched. Improvement measures include increasing the hardness of the mold wall, reducing roughness, eliminating nodules, and enhancing lubrication.
(5) Size Deviation
Excessive mold wear and unreasonable process parameter selection are the reasons for this occurrence. Improvement measures include using hard alloy molds and adjusting process parameters.
(6) Excessive Eccentricity
The reasons for the occurrence are poor alignment of the mold installation, uneven powder filling, excessive mold gap, and short guiding section of the mold punch. Improvement measures include ensuring good mold alignment, using vibration or suction-type powder filling, reasonably selecting the gap, and increasing the guiding part of the mold punch.
2.6 Grinding and Polishing of Tungsten Steel Jewelry
Tungsten steel materials have high hardness, great brittleness, and low thermal conductivity, which makes grinding jewelry very difficult, especially for tungsten steel jewelry with large grinding allowances. High hardness requires a large grinding pressure, while a low thermal conductivity does not allow for excessive grinding heat, and high brittleness leads to a greater tendency for grinding cracks. Therefore, when sharpening tungsten steel jewelry, the grinding wheel must have good self-sharpening properties, a reasonable grinding process, and good cooling to ensure better heat dissipation conditions and reduce the occurrence of grinding cracks. Generally, when grinding tungsten steel jewelry, if the temperature exceeds 600℃, the surface layer of the jewelry will undergo oxidation discoloration, resulting in varying degrees of grinding burns. It can easily cause cracks in the tungsten steel jewelry in severe cases. These cracks are usually very small, and the grinding surface near the cracks often shows colors of different oxidation indices, such as blue, purple, brown, and yellow. When the crack is broken along the crack, there are often severe burn marks at the fracture of the crack, and the entire crack cross-section is often distinctly defined from the fresh fracture due to the infiltration of grinding oil.
The surface grinding and polishing methods for tungsten steel jewelry mainly include mechanical and electrolytic grinding and polishing.
(1) Mechanical Grinding and Polishing
① Polishing and grinding machinery. The polishing and grinding of aluminum steel is very similar to gemstone processing, and the commonly used equipment includes the following four types.
Molding machine: This grid has circular and contoured shapes, featuring uniform dimensions and high precision.
Grinding equipment: There are several types of grinding tungsten steel to shape it, including wheel grinders, disc grinders, belt grinders, and roll grinders, depending on the grinding method and tools used. Among them, wheel grinders are mainly used for chamfering and shaping tungsten steel blank material; disc grinders are mainly used for flat grinding of blank material; belt grinders are mainly used for curved surface grinding; roll grinders are mainly used for grinding away the edges of blank materials to make them smooth.
Polishing equipment: Common polishing equipment includes drums, vibrating barrels, etc.
Drilling equipment: The commonly used drilling equipment includes ultrasonic and laser drilling machines.
② Grinding and polishing abrasives and tools. Tools are the most important cutting, grinding, and polishing instruments in tungsten processing. Depending on their role in processing, they can be divided into three main categories: cutting tools, grinding tools, and polishing tools. If classified according to the attachment relationship between the tools and abrasives, there are also free and bonded abrasive tools.
Due to the variety of types, models, and specifications of abrasives and tools, it is necessary to select the appropriate characteristic parameters for different tungsten steel accessories in order to achieve satisfactory results.
a. Abrasives for grinding tools. Many types of abrasives are available, and their selection is often directly related to the material properties of the workpiece being processed. Due to the high hardness of the material itself, superhard abrasives are generally selected for tungsten steel jewelry.
Traditional silicon carbide grinding wheels for grinding tungsten steel have gradually been eliminated due to their low grinding efficiency, high grinding force, poor self-sharpening, and high local surface temperatures in the grinding contact area (up to around 1100℃), which result in poor tool edge quality, rough surface finish, and high scrap rates. In contrast, synthetic diamond grinding wheels are widely used in the grinding of tungsten steel tools due to their high grinding efficiency, lower grinding force, good self-sharpening, sharp diamond edges that are not prone to pinning, and lower local surface temperatures in the grinding contact area (generally around 400℃). The varieties, codes, and application ranges of synthetic diamonds are shown in Table 6-3.
Table 6-3 Types of Synthetic Diamond, Codes and Application Scope (GB/T 23536-2009)
| Types and codes of synthetic diamonds | Scope of use | ||
|---|---|---|---|
| Variety | Code | Granularity | Recommended use |
| Narrow range | |||
| Abrasive grade | RVD | 35/40〜325/400 | Ceramic, resin bonded grinding tools; grinding tools, etc |
| MBD | Metal bond grinding tools, electro-plated products, etc | ||
| Cutting grade | SMD | 16/18〜70/80 | Saws, drilling tools, electro-plated products, etc |
| Adjustment grade | DMD | 30/35 | Trimming tools: single or multi-grain trimmers, etc |
| Micro powder | MPD | M0/0. 5〜M36/54 | Precision grinding, polishing tools, polycrystalline composite materials, etc |
In recent years, with the application of new materials, CBN (cubic boron nitride) grinding wheels have shown very good processing effects, and the finishing on CNC forming grinding machines, coordinate grinding machines, and CNC internal and external cylindrical grinding machines is better than that of other types of grinding wheels.
In grinding processing, it is important to dress the grinding wheel in a timely manner to maintain its sharpness. When the grinding wheel becomes dull, it will slide and squeeze on the workpiece surface, causing burns and reducing its strength.
b. Bonding agents for abrasives. Bonding agents are materials that bind many small abrasive particles together to form abrasives. Common bonding agents include two main categories: resin and metal. Different bonding agents have different characteristics and applications (Table 6-4).
Table 6-4 Types, Characteristics, and Application Scope of Binders
| Binder name | Code | Characteristics | Scope of application |
|---|---|---|---|
| Resin binder | B | The grinding tool has good self-sharpening, is not easily clogged, generates little heat, is easy to dress, has good polishing properties, is wear-resistant, but has poor heat resistance and is not suitable for heavy load grinding. | Diamond grinding tools are used for the semi-finishing and finishing hard alloys, tools, and non-metals; cubic boron nitride tools are used for the semi-finishing and finishing high-speed steel, tool steel, stainless steel, and heat-resistant steel. |
| Metal binder (electro-plated nickel) | Me | Strong bonding force, sharp cutting edge, high processing efficiency, but limited by the coating, thin working layer, short service life | It is mainly used for glass processing and processing of ferrite magnetic materials. It has good precision and is used for semi-fine grinding, fine grinding, and shaping grinding. It can also be used to manufacture ultra-thin and special-shaped opening grinding tools and shaping grinding wheels. |
| Bronze binder | M | It has a strong bonding force, good wear resistance, low tool consumption, and can withstand larger loads. However, it has poor self-sharpening properties and can heat up and clog if used improperly. | Diamond tools are used for cutting, rough grinding, fine grinding, and shaping glass, ceramics, and gemstones; cubic boron nitride tools are used for grinding materials such as alloy steel. |
c. Grit of the abrasive. The grit of the abrasive is closely related to grinding efficiency, precision, etc. The principle for selecting grit is to choose coarser grit as much as possible while meeting the surface roughness requirements of the workpiece to improve grinding efficiency. Generally, the corresponding relationship between abrasive grit and workpiece surface roughness is shown in Table 6-5.
Table 6-5 Correspondence between Tool Grit Size and Workpiece Surface Roughness
| Abrasive particle size code | 70/80〜 100/120 | 100/120〜 140/170 | 140/170〜 230/270 | 270/325〜 10/20 | 8/12 〜 2.5/5 | 2.5/5〜 0/2 |
|---|---|---|---|---|---|---|
| Workpiece surface roughness Ra/㎛ | 3. 2 〜 0. 8 | 0.8 〜0.4 | 0.4 〜0.2 | 0. 2 ~ 0. 1 | 0. 1 ~ 0.05 | 0.05 〜 0.025 |
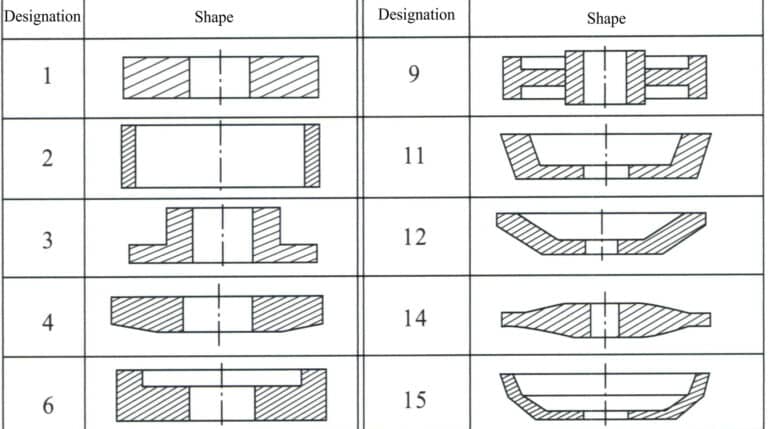
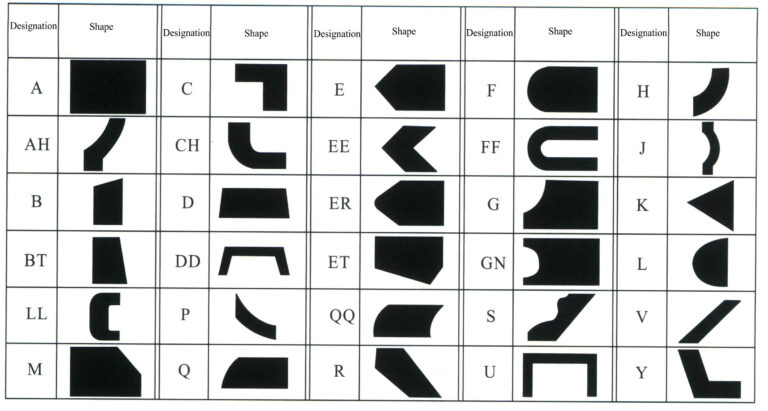
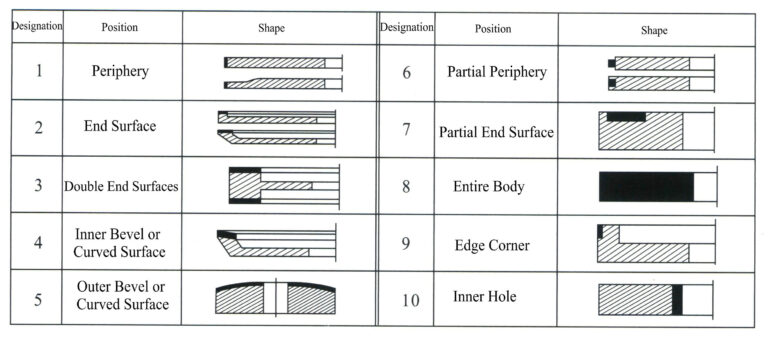
d. Tool shape. The tool shape mainly involves the substrate’s basic shape, the abrasive layer’s cross-sectional shape, and the abrasive layer’s position on the substrate. The national standard GB/T6409.1-94 (referencing ISO 6104-79) specifies the tool marking method to facilitate standardization. The tool marking consists of shape code + basic dimensions + abrasive code + abrasive particle size code + binder code + concentration code. Among these, the shape code indicates the basic shape of the substrate and the cross-sectional shape of the grinding layer, as well as the positional relationship between the two (Figures 6-5 to 6-7); the basic dimensions represent the basic dimensions of the substrate and the grinding layer; the abrasive code indicates the variety code for synthetic diamond or cubic boron nitride; the particle size code indicates the code for the coarseness of the abrasive; the binder code indicates the category code of the binder, with resin—B, metal—M, ceramic—V; the concentration code indicates the code for the ratio of abrasive in the abrasive layer. For example, tool marking: 1A14 100×25×127×10 CBN 100/120 B 100.
③ Auxiliary materials. In the processing of tungsten steel, in addition to various abrasives and grinding tools, various auxiliary materials are also needed, including grinding fluids, cooling fluids, bonding materials, cleaning materials, etc.
- Tungsten steel grinding fluid. Tungsten steel is a hard and brittle material. To reduce the wear of the abrasive medium during grinding and polishing and to prevent cracking of the workpiece, a series of efficient grinding fluids have been developed in the industry. They are particularly suitable for tungsten steel and other cobalt-containing processing materials. During the processing, they ensure that the cobalt in the workpiece material does not dissolve into the grinding fluid. The processed workpiece can maintain its original bending strength and fracture toughness to the maximum extent without changing its hardness. They can be used with various grinding wheels or grinding particles on the workpiece surface, and they are suitable for centerless grinding, external cylindrical grinding, grinding discs, and other processes. They feature fast chip deposition and no foaming and will not produce residues on machinery and parts.
- Coolant. Commonly used coolants include water, quinone oil, and emulsions. Choosing the right coolant is crucial. Proper use of cooling and lubricating fluids plays three major roles: cooling, washing, and lubrication while keeping the cooling lubricant clean, thus controlling grinding heat within an allowable range to prevent thermal deformation of the workpiece. Improving cooling conditions during grinding, such as using oil-immersed grinding wheels or internal cooling grinding wheels, is important. Introducing the cutting fluid into the center of the grinding wheel allows the cutting fluid to directly enter the grinding area, providing effective cooling and preventing burns on the workpiece surface. Therefore, the proper use and maintenance of grinding fluids are essential during the grinding process.
- Bonding material. Mainly used to bond tungsten steel to the operating rod for processing.
- Cleaning materials. These are mainly used to clean oil stains, dust, and other contaminants from adhesive and tungsten steel surfaces.
④ Grinding operation process. When tungsten steel jewelry is being ground, improper operation or inappropriate selection of grinding wheels can easily lead to excessive grinding temperatures, causing the alloy surface to overheat or reducing its toughness and increasing brittleness, affecting the quality of tungsten steel products. Establishing a reasonable grinding process is a prerequisite, as it is the foundation for ensuring the grinding processing of tungsten steel products. It is important to reasonably select the grinding amount, using a fine grinding method with a smaller radial feed rate or even precision grinding. For example, appropriately reducing the radial feed rate and wheel speed while increasing the axial feed rate can reduce the contact area between the wheel and the workpiece, improving heat dissipation conditions and thus effectively controlling the increase in surface temperature.
(2) Electrolytic Grinding Processing
In the past, the mechanical grinding and polishing of tungsten steel almost remained at the level of mechanical processing methods. This method involves complex equipment and requires processes such as grinding with diamond wheels→manual grinding with emery paper→ manual grinding with fine cotton sand, which are complicated and time-consuming. Not only is the efficiency low and the cost high, but a greater drawback is that repeated mechanical grinding can easily generate stress and cracks on the surface and inside the alloy, reducing its lifespan and even causing the alloy to become brittle and damaged. Electrolytic grinding processing utilizes the combined effects of electrolytic processing and mechanical grinding to process hard alloys, with electrolytic processing playing a major role, accounting for about 80%~90%, while mechanical grinding only accounts for 10%~20%. The production efficiency is 4~8 times higher than that of general mechanical grinding. At the same time, it is easy to change the electrical parameters, merging the rough and fine processes into one, shortening the production cycle and reducing processing costs, making it a promising method for processing tungsten steel.
① Structure and principle. Electrolytic grinding mainly consists of three parts: a DC power supply, a machine tool, and a hydraulic system, as shown in Figures 6-8.
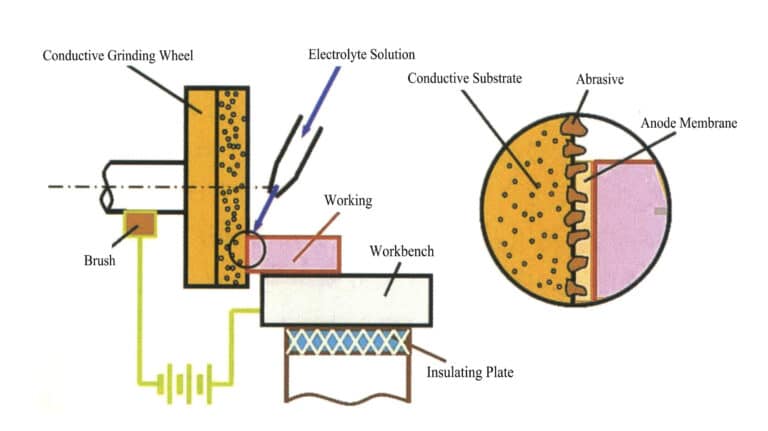
During electrolysis grinding, the tungsten steel workpiece is connected to the positive terminal of a DC power supply, while the diamond conductive grinding wheel is connected to the negative terminal. Both maintain a certain contact pressure, keeping a specific electrolytic gap with the protruding abrasives (diamonds) on the wheel surface, and an electrolyte is supplied to the gap. When the power is turned on, an electrochemical reaction occurs on the workpiece surface. The hard alloy is electrolyzed, and a very thin oxide film (electrolytic film) is formed on its surface, which has a hardness far lower than the hard alloy itself. This oxide film is continuously scraped off by the high-speed rotating diamond grinding wheel and carried away with the electrolyte. This exposes a new workpiece surface, continuing the electrolysis reaction. The electrolysis and the removal of the oxide film alternate, allowing the tungsten steel to be continuously processed to form a smooth surface and achieve a certain dimensional accuracy.
When selecting electrochemical equipment, the grinding machine structure must be sufficiently rigid to maintain accuracy between the grinding wheel and the workpiece even under high bending stress. The machine tool requires some corrosion-resistant auxiliary equipment for pressurizing and filtering the electrolyte. Control devices, fixtures, and mechanical and electrical systems should be made of suitable materials or coated to operate in a salt spray environment. Electrolytic grinding wheels with diamond abrasive conductive wheels can conduct electricity. At the same time, non-conductive abrasive wheels can also be used, but their performance is not as good as that of diamonds. The material for the electrolyte nozzle is generally made of heat-resistant acrylic or other equivalent insulating materials. Workpiece fixtures are made of copper or copper alloy materials. The design should ensure that the parts with cathode and anode polarity are insulated from each other during electrolytic grinding to ensure the machine tool is operating normally.
② Grinding electrolyte and electrolytic grinding wheel. Electrolytic grinding is based on electrochemical dissolution. The choice of electrolyte significantly impacts the productivity, processing accuracy, and surface quality of electrolytic grinding. The chemical substances used to prepare the electrolyte include sodium nitrite, sodium nitrate, sodium dihydrogen phosphate, sodium chloride, sodium borate, potassium chromate, etc. For example, 6.3% sodium nitrite, 0.3% sodium nitrate, 2% sodium dihydrogen phosphate, and 1.4% sodium borate, with pH value controlled at 8~9.
Electrolytic grinding of hard alloys generally uses diamond conductive grinding wheels due to the regular shape of diamond abrasives, high hardness, ability to maintain a uniform electrolytic gap for a long time, and high productivity, allowing for separate mechanical grinding during fine grinding. Diamond electrolytic grinding wheels can be divided into metal-bonded and electro-plated diamond grinding wheels. The former is used for electrolytic grinding of tungsten steel’s flat surfaces and inner and outer circles; the latter is used for electrolytic shaping grinding of large quantities of workpieces with a single shape and inner circle grinding of small holes.
③ Grinding process parameters. In the electrochemical grinding process, current density is the main factor determining productivity, which increases with electrochemical density. However, if the current density is too high or too low, it will reduce processing accuracy and surface quality. In actual production, it is not advisable to increase the voltage without limit, as excessive voltage can cause spark discharge, affecting the surface quality of the workpiece.
When electrochemical grinding tungsten steel, the productivity is highest at a current density of 110A/cm2, the actual current density used is 15~60A/cm2, and the voltage is 7~10V. The current during rough grinding is 20~30A/cm2, and during fine grinding, it is 5~6A/cm2.
At a certain voltage, a small processing gap can achieve a higher current density, improve productivity, and achieve a smooth and precise processing surface. However, if the gap is too small, the electrolyte is difficult to introduce or distribute evenly, which can easily lead to spark discharge and accelerate the wear of the grinding wheel. The generally used processing gap is 0.025~0.05mm.
As the grinding pressure increases, the productivity also increases. With the continuous increase in pressure, the electrolytic gap decreases, making it easy to produce spark discharge. Conversely, if the grinding pressure is too low, the oxide film removal is insufficient, decreasing processing efficiency and surface quality. Therefore, the grinding pressure should be based on the principle of not producing spark discharge and being able to fully scrape off the oxide film. Generally, a grinding pressure of 0.2~0.5MPa is recommended.
The contact area increases, allowing the DC power supply to automatically input a larger current, thereby improving productivity while the surface quality remains good. Therefore, during electrolytic grinding, efforts should be made to maintain the maximum contact area between the grinding wheel and the workpiece.
Increasing the grinding wheel speed can ensure a sufficient supply of electrolytes in the electrolytic gap, alternating quickly while enhancing the mechanical grinding effect, thus improving productivity. However, it should not be too high. The general grinding wheel linear speed is 1200~2100 m/min.
The electrolyte flow should ensure it enters the electrolysis gap sufficiently and evenly. Generally, the electrolyte flow for vertical electrolysis flat grinding machines is 5~15L/min, and that for internal and external circular electrolysis grinding machines is 1~6L/min. Installing the electrolyte nozzle is also important, as it helps limit the electrolysis action to the processing gap between the grinding wheel and the workpiece. The nozzle must be securely installed close to the outer surface of the grinding wheel and equipped with an air scraper so that the nozzle will break the air layer at the outer edge of the rotating grinding wheel.