The Ultimate Guide to 16 kinds of Synthetic Gemstones
Characteristics, Synthetic making methods and comparison
Introduction:
Due to the development of contemporary synthetic technology, almost all natural gemstones can be synthesized in the laboratory, and their characteristics are increasingly similar to those of natural ones, even reaching a level that is difficult to distinguish.

Table of Contents
Section I Synthetic Diamond
Gem-quality synthetic diamonds are mainly produced using the High-Temperature High Pressure (HTHP) BARS press, with the main producing countries for jewelry-grade synthetic diamonds being Russia, Ukraine, and the United States. The main physical and chemical properties of HTHP synthetic diamonds are similar to those of natural diamonds.
1. Characteristics of diamond synthesis by the crystal seed catalyst method
(1) External characteristics of crystals
The crystal shape is generally cubic {100} and octahedral {111} in aggregation. Diamonds synthesized by the “BARS” method may exhibit slightly distorted branching patterns, wavy growth features, and residual crystal flakes. At low temperatures, the edges of the crystal faces often protrude while the center is concave; at high temperatures, the entire crystal becomes rounded. Under a microscope, growth textures and color differences in different growth areas can be observed.
(2) Color
Synthetic diamond crystals are generally light yellow, orange-yellow, or brown. Those grown at low temperatures are lighter in color, while those grown at high temperatures are darker. The color significantly depends on the catalyst alloy used. If the catalyst is Fe-Al, the resulting crystal is colorless; if it contains B (boron), it appears blue, and if it contains Ni (nickel), it appears brownish-yellow. The color distribution is uneven, with color bands visible along the edges of the octahedral crystals.
(3) Characteristics of inclusions
The main inclusions are catalytic metals that appear isolated or clustered on the crystal surface or oriented along the boundaries of internal growth zones, presenting rounded, elongated, point-like, or needle-like shapes. The purity levels are mainly in the P and SI ranges. The growth patterns of HTHP synthetic diamonds develop differently depending on the growth zone. The growth patterns in the octahedral growth zone are straight and may have reddish-brown needle-like inclusions (visible only under cathodic luminescence); the cubic growth zone has no growth patterns but may have black cross inclusions; the edges of the square octahedral growth zone develop straight growth patterns.
(4) Optical characteristics
There is often very weak anomalous birefringence. The color change of interference colors is not significant, less pronounced than that of natural diamonds.
(5) Luminescence
Under ultraviolet light, X-rays, and cathode rays, it exhibits regular zoned luminescence, with different growth zones emitting different light colors, forming regular geometric patterns.
(6) Absorption Spectrum
Type I b generally shows no obvious absorption; sometimes, due to cooling effects during the growth process, it may cause absorption at 658 nm. Type I b + I a shows several clear absorption lines at 600- 700 nm, while natural diamonds have an absorption line at 415 nm (see Table 2-5).
Table 2-5 Identification Characteristics of Synthetic Diamonds and Natural Diamonds
| Item | Natural Diamond | Synthetic diamond |
|---|---|---|
| Color | Mostly colorless, light yellow, light brown, brown, and also green, golden yellow, blue, and pink | Mostly light yellow, light brownish yellow, also colorless, green, and blue, with uneven colors, visible color bands arranged parallel to the octahedral crystal edges |
| Type | Mostly type I a, also type I b, II a, II b, and their mixed types | Mostly type I b, but also type II a, la + I b and II a + II b (mixed types) |
| Crystal form | Often presents as octahedral, rhombic dodecahedral and their aggregates, with triangular growth hills resembling disintegration on the crystal faces | Often presents as cubic, octahedral, rhombic dodecahedral and cubic octahedral, with unusual branching patterns, wavy growths, and residual crystal flakes on the crystal faces |
| Inclusion | Visible mineral inclusions such as diamonds, peridot, garnet, spinel, and pyroxene; type I b diamonds often contain dark needle-like or plate-like inclusions | Common crystal catalyst inclusions appear shiny under reflected light and black opaque under transmitted light, approximately 1 mm long, generally rounded or elongated, appearing isolated or in groups, often parallel to the crystal surface or distributed along the boundaries of internal growth zones; some inclusions are pointed or needle-like. |
| Luminescence | Irregular zoned luminescence phenomenon | Regular zoned luminescence phenomenon under ultraviolet light, X- rays, and cathode rays |
| Absorption spectrum | Type I a "Cape" color has 1 or several clear absorption lines, such as at 415 nm, 453 nm, 478 nm | Type I b generally has no obvious absorption, sometimes due to the cooling effect of synthetic diamonds causing absorption at 658 nm; Type I b + I a at 600- 700 nm |
| Magnetic | Non-magnetic | Magnetic due to the presence of iron inclusions |
2. Chemical vapor deposition method for synthesizing diamond films (CVD synthesized diamond)
(1) Physical properties
The physical properties such as hardness, thermal conductivity, density, elasticity, and translucency are close to or reach those of natural diamonds. CVD-synthesized diamonds are plate-like, with {111} and {110} faces not developed; colors are mostly brown and light brown or colorless and blue. They exhibit strong anomalous extinction under orthogonally polarized light, varying in different directions.
(2) Structural defects
There are many (111) twins, (111) stacking faults, or dislocations. Under magnification, irregular dark inclusions and point-like inclusions can be seen, with parallel growth color bands.
(3) Electrical conductivity
Blue synthetic diamond thin layers are conductive and evenly distributed over the entire surface of the faceted diamond.
(4) Infrared spectrum
Diamond films are polycrystalline, with a granular structure on the surface and characteristic peaks near 1332 cm-1, full width at half, maximum (FWHM) and even a broad peak appear near 1500 cm-1. Under ultraviolet irradiation, weak orange-yellow fluorescence usually occurs.
Section II Synthetic Moissanite (synthetic calcarenite)
Synthetic Moissanite is mainly produced by the Lely method and was first launched in cities like Atlanta, USA, in June 1998. Its gemological characteristics are as follows:
(1) Color
Colorless to pale yellow, light gray, light green, light brown, light blue, green, and gray, influenced by trace amounts of nitrogen and aluminum impurities. For example, yellow (containing nitrogen 0.01%), green (containing nitrogen 0.1%), blue-green (containing Nitrogen 10%), blue (containing high amounts of aluminum). Colorless crystals do not contain nitrogen or reduce the influence of nitrogen by adding trace elements of aluminum.
(2) Luster
Transparent, sub-adamantine luster.
(3) Crystal system and optical properties
Hexagonal crystal system, sphalerite-type structure. It often occurs in massive form with a uniaxial positive optical property.
(4) Refractive Index and Dispersion
Refractive index 2.648-2.691, birefringence 0.043, focusing on the bottom tip allows seeing the tabletop and the facet reflections of the crown. Reflectivity is about 21.0%, dispersion 0.104.
(5) Density and Hardness
Density 3.20-3.24 g/cm3, Mohs hardness around 9.25. The crystal’s toughness is excellent.
(6) Inclusions
Long, slender white tubular objects, irregular cavities, small SiC crystals, negative crystals, and dark metallic luster spherical objects can be arranged linearly with three or more particles, and there are also some cloud-like, dispersed, pinpoint-like inclusions, possibly containing bubbles.
(7) Absorption spectrum
No characteristic absorption spectrum was observed. Nearly colorless Synthetic Moissanite has a weak absorption below 425 nm.
(8) Luminescence
Exhibits luminescence, with a few showing medium to weak orange fluorescence under longwave light, very few showing weak orange fluorescence under shortwave light; a very small number exhibit medium to weak yellow fluorescence under X-rays.
(9) Thermal Conductivity
The thermal conductivity is 230- 490w/(m·k), 1w/(m·k) = 1.163kcal/(m·h·k).
(10) Electrical Conductivity
Absorption below 1800 cm-1, there are several strong and sharp absorption peaks in the 2000-2600 cm-1 region, and only a few absorption peaks can be seen in the 3000-3200 cm-1 region.
(11) Infrared Spectrum
The following absorption shows that there are several strong and sharp absorption peaks in the area, and a few absorption peaks can barely be seen in the range.
(12) Simple methods for distinguishing diamonds
① Illumination method
Mix diamonds with Synthetic Moissanite and pour the mixture into a plastic tray, submerging the gems in water. Place a sheet of white paper 25 mm below the plastic tray and illuminate from 15 cm above the gems using a fiber optic lamp or flashlight. It is better to cover the light source with a plate that has a slit and to perform the test in a dark room. Under illumination, move the plastic tray from one side to the other; Synthetic Moissanite will show vibrant colors, while diamonds will only emit white light.
② Heating method
- Heat these gems using an oven, electric furnace, or a 250 W incandescent lamp; at this time, Synthetic Moissanite turns bright yellow, while diamonds do not change color.
- Place the outer flame of a match or lighter directly beneath the gem; diamonds do not change color, while Synthetic Moissanite turns yellow but returns to its original state after annealing.
③ Dispersion method
Place the diamond face down in a shallow, clean glass dish, fully submerged in tap water, and illuminate it vertically with a penlight; Synthetic Moissanite exhibits bright spectral color flashes, while diamonds have less bright colored flashes.
④ Specific Gravity Method
Place the gemstone in diiodomethane heavy liquid; Synthetic Moissanite floats up, and the diamond sinks.
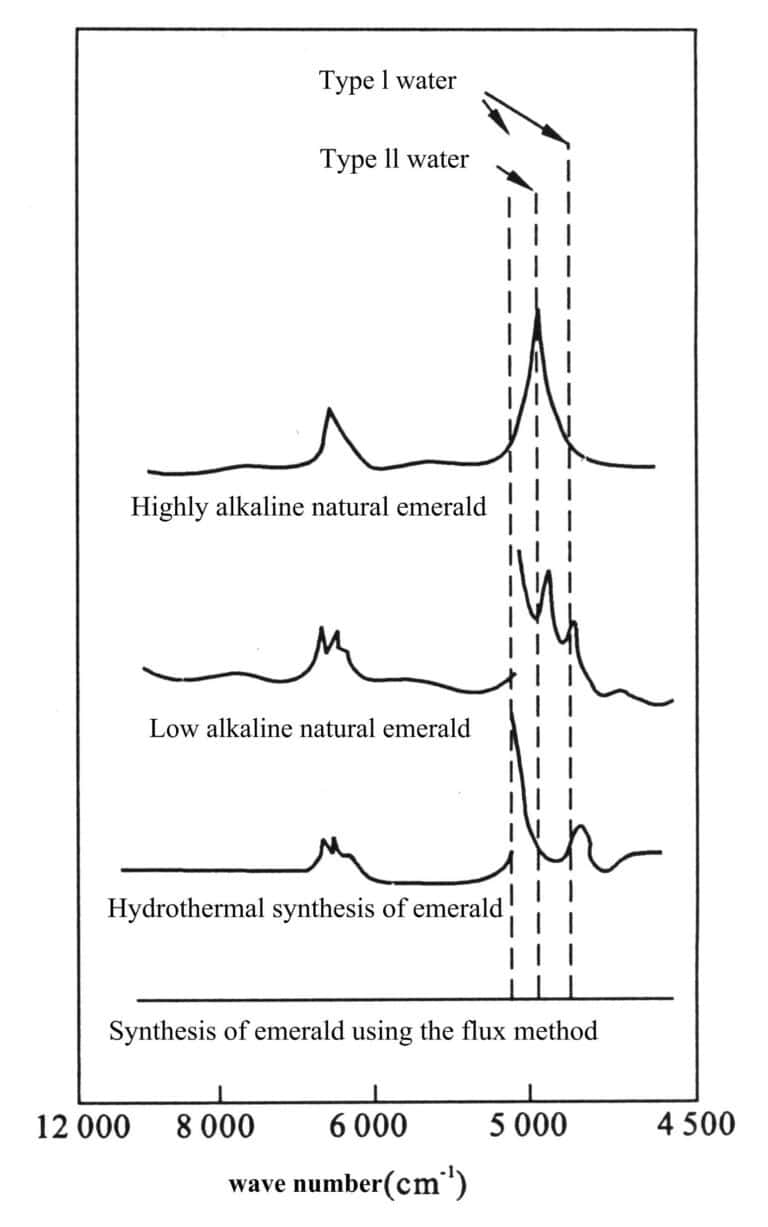
Section III Synthetic Emerald
The methods for synthesizing emeralds mainly include a hydrothermal method and a flux method. The physical characteristics, such as the refractive index and density of the synthesized product, are very close to those of natural emeralds, with the main difference being in internal features and infrared spectral characteristics. Different production processes also have variations.
1. Hydrothermal Method for Synthesizing Emerald
The hydrothermal Synthesis of emerald includes Russian synthetic emerald, Linde method synthetic emerald, Biron method synthetic emerald, Lechleitner method synthetic emerald, and the hydrothermal Synthesis of emerald in Guilin, China. The characteristics of different hydrothermal methods for synthesizing emeralds are shown in Table 2-6.
Table 2-6 Characteristics of different hydrothermal methods for synthesizing emerald.
| Variety | Refractive index | Birefringence | Density (g/cm3) | Ultraviolet fluorescence | Inclusion | Other Characteristics | Angle between growth lines and the Z-axis |
|---|---|---|---|---|---|---|---|
| Lechleitner (Australia) | 1.570 ~ 1.605; 1.559 ~ 1.566 | 0.005 ~ 0.010; 0.003 ~ 0.004 | 2.65 ~ 2.73 | Red | Seed crystal, cross-fractures | Layering visible in oil immersion, orthogonal polarized light wavy disappearance | 30 ° |
| Linde (USA) | 1.567 ~ 1.572 | 0.005 | 2.67 ± | Strong red | Gas and feather-like two-phase gas¬-liquid inclusions, parallel nail-like or needle-like inclusions, silicate beryllium | There is absorption of H2O in the infrared spectrum, containing type I water | 36 ~ 38 ° |
| Refined pool method (Australia) | 1.570 ~ 1.575 | 0.005 | 2.694 | Weak and none | Cloud -like window screen inclusions | There is absorption of H2O in the infrared spectrum, including Cl | 22 ~ 23 ° |
| China (Guilin) | 1.570 ~ 1.578 | 0.006 | 2.67 ~ 2.69 | Bright red | Three-phase fishing-shaped inclusions, sometimes appearing individually, resembling wheat seedlings when appearing in groups, silicate beryllium | Contains I and II type water | |
| Biron (Australia) | 1.570 ~ 1.578 | 0.007 ~ 0.008 | 2.68 ~ 2.70 | Strong red | Biphasic nail¬-shaped inclusions, silicate beryllium crystals, white comet-shaped and bead-like particles, flux feather-like inclusions, and dark metallic inclusions | Contains Type I and II water, Cl | 32 ~ 40 ° |
| Russia (Old) (New) | 1.572~ 1.578; 1.579 ~ 1.584 | 0.006 ~ 0.007 | 2.68 ~ 2.70 | Weak Red | A thousand tiny brown particles, a cloud form | Contains Type I and II water | 32 ~ 32 ° ; 43 ~ 47 ° |
(1) Color
Vivid green.
(2) Structure of the water content
Type I water is predominant, with some Type II water.
(3) Infrared Spectroscopy
Although the hydrothermal Synthesis of emerald contains both Type I and Type II water, it has different peak positions and intensities for the stretching and bending vibrations of water molecules. The hydrothermal Synthesis of emeralds shows absorption in the mid-.
Infrared at 4357 cm-1, 4052 cm-1 and 3490 cm-1, 2995 cm-1, 2830 cm-1, 2745 cm-1 , which can distinguish it from natural emerald (see Figure 2-9).
(4) Inclusions
There are often two-phase inclusions, needle-like or nail-like beryl, and voids, with solid-liquid inclusions distributed on individual planes and arranged parallel to each other on the same plane. In some cases, there are birefringent crystals, cavities filled with multiple phases, and planar shapes of seed crystals with twisted white feather-like, fibrous, and cotton-like inclusions. Slag-like inclusions are distributed planarly, and the crystal surface exhibits unique growth ripples. The undulating or serrated growth lines and color bands inside the crystal are mostly parallel to the seed crystal plate, with an angle of intersection with the Z axis between 22 ° – 40 ° , and exhibit irregular sub-grain boundaries that are nearly vertical to the color bands, forming angular patterns.
The grain boundaries are nearly vertical to the color bands, forming angular patterns.
The synthetic emerald produced by the hydrothermal method in Guilin, China, belongs to the chlorine-containing alkali-free series and only has Type I water peaks. The hook-shaped inclusions parallel to the C axis are often Chrysoberyl and sometimes beryl. The distribution of solid phase inclusions is related to the seed crystal boundaries, and the arrangement direction of the needle-like inclusions is perpendicular to the seed crystal and the main growth surface.
(5) Special Optical Effects
Under black background conditions, red will appear at certain angles when illuminated with a strong light source.
(6) Fluorescence
Strong red fluorescence.
(7) Color Filter Observation
Bright red color.
2. Synthesis of emerald using flux method
Manufacturers producing synthetic emeralds using the flux method include Chatham, Gilson, and Lennox. The characteristics of synthetic emeralds from different manufacturers vary slightly (see Table 2-7).
Table 2-7 Characteristics of emerald synthesized by different flux methods
| Variety | Refractive index | Birefringence | Density (g/cm3) | Ultraviolet fluorescence | Inclusion | Other Characteristics | Growth rings |
|---|---|---|---|---|---|---|---|
| Chatham (USA) | 1.560 ~ 1.563 | 0.007 | 2.65± | Strong Red | Feather-like, veil-like envelope and silicon tantalum crystal | No H2 O in the infrared spectrum | C(0001); m(1010); u(1120) |
| Gilson I type (French) | 1.559 ~ 1.569 | 0.005 | 2.65 ± 0.01 | Orange -red | Feather-like inclusions, rectangular beryllium silicate crystals | No H2 O in the infrared spectrum | |
| Gilson II type (French) | 1.562 ~ 1.567 | 0.003 ~ 0.005 | 2.65 ± 0.01 | Red | Same as above | Same as above, the product is very rare | |
| Gilson N type (French) | 1.571 ~ 1.579 | 0.006 ~ 0.008 | 2.68 ~ 2.69 | None | Fibrous, bundle-like solid flux package, platinum and beryllium silicate | As above, there is characteristic absorption at 427 nm | |
| Lennix (French) | 1.556 ~ 1.566 | 0.003 | 2.65 ~ 2.66 | Red | Opaque tubular package, silicon beryllium stone and emerald-like crystals, filled with flux in the cracks | Opaque tubular package, silicon beryllium stone and emerald-like crystals, filled with flux in the cracks |
Quoted from “System Gemology” (2006)
(1) Infrared spectrum
Water-free, therefore, there is no absorption of any water (see Figure 2-9). If Fe is added (Gilson N-type), there is an absorption band at 427 nm in the violet region, which is absent in natural emeralds.
(2) Inclusions
Solid melt inclusions that have not melted often fill along cracks and cavities, appearing feather-like, fibrous, or bundled, like fluttering window curtains; step-like coarse-grained flux inclusions; some parallel band-like or line-like features, either consistently extending towards the six-faced prism surface or forming a certain angle with the prism surface, some appearing along the crystal axis direction, making the six-faced external contour looks like it has a cavity; sometimes there are solid inclusions of crucible material (platinum) and silicate beryllium ; sometimes traces of natural seed crystals can be seen (darker in color), with the dark emerald part surrounding the seed crystal showing the same inclusion characteristics. These inclusions can be divided into five types:
- Curved feather-like inclusions resembling veils or straw;
- Laughing-shaped hook-like inclusions;
- Gas-liquid two-phase inclusion type;
- Small pile-like crystal type;
- Rare dark-colored conical wrapping body type.
(3) Component analysis
Contains metal cations with fluxing agents such as Mo and V, while natural emerald does not.
(4) Luminescence
Red fluorescence. The transmittance of Chatham synthetic emerald under shortwave (below 230 nm) is much stronger than that of natural emerald (which does not transmit below 295 nm).
The emeralds synthesized by the flux mentioned above or the hydrothermal method are very similar to natural emeralds and are generally difficult to distinguish. The main basis for identification is the analysis of their internal features and infrared spectral characteristics using a microscope and infrared spectrometer (Table 2-8).
Table 2-8 Differences between natural emerald and emerald synthesized by flux method and hydrothermal method
| Types | Synthesis of emerald by flux method | Synthesis of emerald by hydrothermal method | Natural emerald | |
|---|---|---|---|---|
| Density (g/cm3) | 2.65 ~ 2.67 | 2.67 ~ 2.69 | 2.69 ~ 2.74 | |
| Ne | 1.560 ~ 1.563 | 1.566 ~ 1.576 | 1.565 ~ 1.586 | |
| No | 1.563 ~ 1.566 | 1.571 ~ 1.578 | 1.570 ~ 1.593 | |
| Birefringence | 0.003 ~ 0.005 | 0.005 ~ 0.006 | 0.005 ~ 0.009 | |
| Internal characteristics | Silicon beryllium stone, platinum sheet, curved vein-like cracks, two-phase inclusions | Silicon beryllium stone, small two-phase inclusions | Mica, tremolite, actinolite, pyrite, calcite, three-phase inclusions | |
| Water | None | Contains Type I water and Type II water | Contains Type I water and Type II water | |
| Potassium | Variable | None | Variable | |
| Infrared spectrum | No water absorption peak |
(According to Kurt Nassan, 1979)
Section IV Synthesis of corundum gemstones
1. Flame fusion method for synthesizing corundum gemstones
(1) Synthesis of rubies
① Internally relatively clean, with no bubbles or occasionally seen bubbles. The bubbles are small and few, mostly spherical, and rarely tadpole-shaped. If the production process is unstable, a large number of point-like bubbles may form clusters, distributed in bands or cloud-like patterns. Occasionally, un-melted aluminum oxide powder and red chromium oxide powder appear crumb-like.
② Bright colors, overly pure, can have deep red, orange-red, purple-red, and many other colors, often giving a feeling of “fake”.
③ It has a wider arc-shaped growth pattern runs through the entire sample. Due to technological improvements, the curvature of the growth pattern has relatively decreased, appearing relatively straight over a smaller range. During the processing and polishing process, feather-like cracks may occur, and cracks may also form during subsequent heat treatment. If filled with resin, a false fingerprint-like inclusion may form inside the cracks.
④ Since the surface is parallel or nearly parallel to the Z-axis orientation, there is a noticeable dichroism in the surface direction.
⑤ Under ultraviolet light, it exhibits a medium to strong red fluorescence.
⑥ There may be a red phosphorescence phenomenon after X-ray irradiation.
(2) Synthesis of sapphire
① Various colors: blue sapphires appear blue from the top view and purple-blue from the side view.
② Gas inclusions, solid inclusions, growth lines, and pleochroism are similar to synthetic rubies, as seen in fluorescence and absorption spectra in Table 2-9. Sometimes, blue substances may accumulate around bubbles, making them easy to detect.
③ The iron absorption line at 450 nm in natural sapphires may disappear or be very weak and blurred.
Table 2-9 Comparison of Characteristics of Flame-Fusion Synthetic Corundum Gemstones
| Gem varieties | Growth Structure | Inclusions | Spectrum | Ultraviolet fluorescence | Other Characteristics |
|---|---|---|---|---|---|
| Ruby | Hexagonal ribbon | Rutile, healing fissures | Cr spectrum | Strong one middle | Vertical C-axis |
| Synthetic Ruby | Curved Growth Lines | Bubbles, Powder | Cr Spectrum | Very strong | No orientation |
| Sapphire | Hexagonal color band | Rutile, healed cracks ,crystal inclusions | 450 nm narrowband | Weak, orange-red (long wave) | Straight crackling |
| Synthetic sapphire | Curved growth lines | Bubbles, small bubble clusters, powder | Missing | Weak, blue-white (shortwave) | Curved cracks |
| Yellow sapphire| stone | Hexagonal color band | Rutile, healed cracks, crystal inclusions | 450 nm narrow band, or none | No middle, non-fluorescent with absorption bands, conversely yellow fluorescence | Fe3+ or Mg2+ is the color agent, and does not contain Ni |
| Synthetic Yellow Sapphire | Curved Color Band (Blue Glass Filter) | Bubbles, Small Bubble Clusters, Powder | Absence | Weak and none | Containing Ni, Ni2+ is a coloring agent |
| Green Sapphire | Hexagonal Color Band | Rutile, Healing Fractures, Crystal Inclusions | 450nm Narrow Band | No Fluorescence | Fe3+ Fe/ Ti is a coloring agent |
| Synthetic Green Sapphire | Curve d Growth Lines | Bubbles, Small Bubble Clusters, Powder | Missing | Middle school weak, orange | Ni, Co, Ni2+ Co as a coloring agent |
| Color¬ changing sapphire | Hexagonal color band | Rutile, healed cracks. Crystal inclusions | Cr Spectrum | Weak, red | Fe3+ Fe/ Ti are chromogenic agents and contain almost no V |
| Synthetic color-changing sapphire | Curved growth lines | Bubbles, small bubble clusters, powder | 470 nm fine line | Weak, bluish-white (short wave) | Containing V, V3+ is the chromogenic agent |
| Colorless sapphire | Weak hexagonal color band | Rutile, healed fractures, crystal inclusions | None | Medium weak, yellow fluorescence | No Platt effect |
| Synthetic colorless sapphire | None | Bubbles, small bubble clusters, powder | None | Medium weak, blue-white fluorescence | Platt effect |
(3) Synthetic Star Ruby (Blue) Sapphire
① Color, Transparency: Synthetic Star Light Red Sapphire is pink to red, semi-transparent to transparent; Synthetic Star Light Blue Sapphire has milky blue to blue, white to gray, purple, green, yellow, brown, black, and semi-transparent.
② Arc-shaped growth lines are generally parallel to the base, and bubbles are often distributed along the arc-shaped growth layers. Tiny rutile inclusions are densely arranged in three directions, appearing misty.
③ The star lines are fine and narrow, complete, clear, and distributed on the sample’s surface without asterism.
The distinguishing features of synthetic star ruby (blue) and natural stones are shown in Table 2-10.
Table 2-10 Characteristics of flame-fusion synthetic star ruby (blue)
| Item | Synthetic | Natural | |
|---|---|---|---|
| Surface features | Star light | Starlight floats on the surface, exceptionally bright, not soft | Starlight emanates from within the crystal, soft |
| Star lines | Star lines are continuous, fine, straight, and uniform; the intersections of the star lines are clear, and there is no widening or brightening phenomenon at the junctions floating on the surface (no gem-like luster) | The star lines vary in width, extending forward in a wavy pattern, with the intersections of the star lines becoming wider and brighter (glory) | |
| Internal features | Curved growth lines can be observed (especially clear on the convex back of the gemstone) along with extremely fine white powder and scattered rutile inclusions | Angular inclusions are visible, and there is a color banding phenomenon | |
| Ultraviolet fluorescence | Long wave | Synthetic star ruby exhibits a very strong bright red color | Natural star ruby exhibits a weak red color |
| Short wave | Synthetic star ruby exhibits a very strong bright red color, synthetic blue sapphire exhibits a blue-white color | Natural star ruby exhibits a weak red color, natural star blue sapphire exhibits a sensual quality | |
2. Hydrothermal Synthesis of sapphire gemstones
(1) External Characteristics of Crystals
① The shape of crystals is mostly thick plate-like or plate-like, with common forms being hexagonal bipyramids {2241} and {2243},followed by rhombohedra{0111}, and occasionally negative trigonal bipyramids{3581} and parallel double faces{0001}.
② Various growth patterns are commonly developed on the hexagonal bipyramid crystal faces. Common patterns include tongue-shaped or droplet-shaped growth hills, step-like growth terraces, grid-like growth textures, and irregular growth striations, with occasional radial fibrous streaks. These growth patterns are closely related to the temperature, pressure, mineralizers, solvent flow direction, and temperature gradient during the crystal growth process. They represent a form of the crystal’s internal embedded structure and growth dislocations.
③ Cracking phenomena can occur in crystals. There are two situations for cracking in synthetic rubies: one is cracking along the seed crystal face (mainly due to the large stress between the crystal and the seed crystal); the other is regular network cracking on the {2243}crystal face (determined by the structure and growth conditions of the crystal). There are three types of cracking in synthetic yellow sapphire crystals: one is two groups of cracking along the direction of the crystal rhombohedron; one is cracking along the center of the seed crystal plate; and the other is cracking along the interface between the seed crystal and the crystal. T The reason for the cracking of the latter is more complicated, and it may be related to the lattice mismatch or crystal distortion between the fine crystal and the crystal. However, some soluble impurities or gelatinous mechanical mixing in the crystals, as well as the thermal fluctuations caused by the uneven heat flow impact during the growth process, may be the main reasons for the cracking of synthetic yellow sapphire crystals.
(2) Internal Characteristics
① Gas-liquid two-phase inclusions. They may be distributed separately or in a fingerprint-like pattern on the healed fracture surface, resembling a net structure. They have a stronger three-dimensional sense and more regularity than the fingerprint-like inclusions in natural sapphire. Characteristic nail-shaped fluid inclusions are often densely oriented.
The edges of the single inclusions in synthetic rubies are smooth and relatively regular, with a gas-liquid volume ratio of 20%. The individual or bead-like distributed gas-liquid two-phase inclusions in synthetic yellow gemstone crystals are approximately 0.02 -0.05 mm in size, oval or irregular in shape, with a gas-liquid ratio of 15%-25%, generally isolated and distributed far from the seed crystals, and their morphological characteristics are very similar to the fluid inclusions in natural yellow sapphires. The two are difficult to distinguish under a microscope.
② Bubbles appear in clusters. In early synthetic rubies, many clusters of bubbles are often densely distributed as tiny bubbles of 0.01 mm on the seed crystal chips, seed crystal covers, or hanging gold wires. It is generally difficult to see such inclusions in synthetic corundum gemstones.
③ Presence of seed crystal chips. If the gemstone crystal is placed in naphthalene bromide immersion oil, it can be identified by the irregular wavy growth boundary between the seed crystal chips and the growth layers.
④ Solid metal inclusions. Gold microcrystal aggregates are distributed dot-like or clumpy, originating from the gold lining or hanging wires of high-pressure vessels.
A grayish-white Al(OH)3 powder can also be seen in synthetic ruby crystals, resembling bread crumbs, and is opaque. It is mostly distributed in a dotted and planar manner near the seed crystal.
In synthetic yellow sapphire crystals, fusible impurity inclusions can also be found, mostly in irregular dendritic, radial, or irregular granular forms, colorless and transparent, with medium protrusions. Under orthogonal polarized light, the interference color sequence is relatively high (related to thickness), and it is often unevenly distributed at the interface between the crystal and the seed crystal; a gel-like mechanical mixture with a regular or irregular network shape can also be observed, which is colorless or light yellow-green, transparent, with medium to high protrusions, existing only at the cracks between the crystal and the seed crystal, and often associated with fusible impurity inclusions or fluid inclusions.
⑤ Growth textures and color bands. Synthetic ruby crystals exhibit dark red and orange-red growth bands, distributed in straight banded patterns, resembling “Polymer twin”; some synthetic yellow sapphire crystals have more developed microwave-patterned growth textures, which are mostly directional and extend along the direction of the seed crystal.
⑥ Smoky-cracked. Due to cracking phenomena, smoky fissures can be seen in early synthetic rubies, and they are relatively developed. Currently, most hydrothermal synthetic ruby crystals are relatively clean inside.
(3) Spectral and ultraviolet fluorescence characteristics
① Ultraviolet to visible light spectral characteristics: Ruby synthesized by the hydrothermal method in Guilin. The spectral band at 241 nm in the ultraviolet region is important evidence to distinguish natural rubies.
② Infrared spectral characteristics: Rubies synthesized by the hydrothermal method in Guilin generally exhibit the stretching vibration spectral bands of 3307 cm-1, 3231 cm-1, 3184 cm-1, 3013 cm-1, and a series of infrared absorption spectra of OH or crystalline water vibrations within the range of Al – OH and 2364 cm -1 2348 cm-1.
③ Ultraviolet fluorescence characteristics: Rubies synthesized by the hydrothermal method exhibit stronger and brighter red fluorescence than natural rubies. Synthetic yellow sapphires are inert under long waves, while most synthetic crystals show banded fluorescence under short waves; seed crystals exhibit medium to weak blue-white fluorescence, with a few also being inert under short waves.
3. Characteristics of corundum-type gemstones synthesized by the flux method
(1) Ruby is synthesized by the flux method.
① The bubble monomers appear broken yet unbroken, connected yet unconnected, with a significant contrast to the surroundings.
② Yellow to pink block-like flux agent inclusions are visible, appearing mostly opaque under transmitted light and light yellow to orange-red with a metallic luster under reflected light. They come in various forms: branched, fence-like, net-like, twisted cloud-like, tubular, droplet-like, comet-like, etc.
③ Platinum is a common type of inclusion with a metallic luster in triangular, hexagonal, or other shapes.
④ Unique cloud-like bubble aggregates or broom-like inclusions can be seen around the seed crystals, with occasional coarse flux agent inclusions and seed crystals with blue edges.
⑤ Synthetic rubies may contain Pb, B and other flux cation species.
⑥ Under shortwave ultraviolet light, it exhibits a strong red fluorescence, which is different from natural rubies (which show weak to moderate red fluorescence). Some varieties have special fluorescence due to rare earth elements that can be used for identification.
➆ The color is quite rich, displaying various shades of red. There may be a swirling color unevenness phenomenon (in Lamra synthetic products), blue triangular growth bands (in Russian synthetic products), straight growth rings, and uneven color blocks.
(2) Flux method for synthesizing sapphire
① Internal characteristics: Residual flux, color bands, platinum flakes, etc., are the same as those in flux method synthesized rubies.
② Fluorescence: Under ultraviolet light, residual flux can exhibit various strong fluorescence colors such as pink, yellow-green, and brown-green.
③ Absorption spectrum: Absorption lines may be missing 460 nm, 470 nm . (See Figure 2-10)
Copywrite @ Sobling.Jewelry — Custom jewelry manufacturer, OEM and ODM jewelry factory
4. Characteristics of synthetic ruby gemstones using the crystal pulling method
The types of ruby gemstones produced by the crystal pulling method mainly include synthetic colorless sapphires and synthetic rubies.
(1) Solid inclusions. Mainly residual flaky inclusions of metal elements such as Mo, W, Fe, Pt, etc.
(2) Cloud-like clusters of bubbles and broom-like inclusions, or elongated gaseous inclusions with wonderful curved, uneven growth stripes, occasionally showing subtle white cloud-like substances resembling smoke.
5. Characteristics of synthetic corundum gemstones using Mold guiding method
(1) There may be solid inclusions of the mold metal.
(2) Traces of seed crystals and defects of seed crystals.
(3) Bubbles with a diameter in the range of 0.25- 0.5µ m are unevenly distributed.
6. Characteristics of synthetic corundum gemstones using zone melting method
(1) It is high purity and very clean inside.
(2) Fluorescence is stronger than that of natural rubies.
(3) Absorption spectral lines under a spectroscope are fewer than those of natural corundum gemstones.
(4) The surface finish of the gemstone is not good enough, with “fire marks” ( wave-like or crack-like marks produced during the polishing process), etc.
(5) Poor-quality synthetic gemstones with chaotic growth patterns, uneven crystal colors, etc.
7. Characteristics of inclusions in synthetic corundum gemstones
A comparison of the inclusion characteristics of corundum-type gemstones synthesized by various production processes is listed in table 2-11.
Table 2-11 Comparison of inclusion characteristics of various production processes for synthetic corundum-type gemstones
| Production Process | Package Body Characteristics |
|---|---|
| Flame Melting Method | (1) Arc-shaped Growth Patterns; (2) Bubbles (distributed individually or in groups) |
| Flux Method | (1) Residue of flux (mostly opaque under transmitted light, gray-black; appears yellow and orange-red under reflected light, with a metallic luster; rich in surface morphology) (2) Parallel color bands, uneven color blocks (3) Platinum metal pieces (regular, silver-white reflective, metallic luster) (4) Seed crystals |
| Hydrothermal method | (1) Growth patterns (wavy, serrated, mesh-like) (2) Nail-shaped inclusions ("nail-shaped" fluid inclusions; larger inclusions have dark liquid fillings at their centers, sometimes nail-shaped inclusions are very small, appearing as densely arranged fine needles) (3) Metallic inclusions (polygonal, opaque, with metallic luster) (4) Seed crystals |
| Pulling method | Identification features similar to flame fusion method |
| Melt guide mold method | (1) Metal shell (2) Seed crystal traces (3) Bubbles (of varying sizes, unevenly distributed) |
| Zone Melting Method | (1) Chaotic growth patterns (2) Uneven color |
Section V Synthetic Rutile
Synthetic rutile is mainly produced by the flame fusion method. The characteristics of synthetic rutile produced by the flame fusion method are as follows:
(1) Color
Common colors include light yellow, but can also be blue, blue-green, orange, and others.
(2) Density
4.24 ~ 4.26g/cm3
(3) Absorption spectrum
The absorption spectrum of yellow-green rutile has a strong absorption band at 430 nm, with complete absorption below that.
(4) Inclusions
Glass bubble encapsulated body, crumbly unfused powder solid encapsulated body.
(5) Appearance Characteristics
The crystal cross-section may have densely packed arc-shaped growth rings or color bands resembling record grooves. Strong double images (birefringence), strong dispersion (0.330).
Section VI Synthetic Spinel
In the early 20th century, L. Paris accidentally obtained synthetic spinel while using the flame fusion method to synthetic spinel, using CO2O3 as a coloring agent and MgO as a flux. Now, people can produce synthetic spinel in various colors.
The synthesis methods for spinel mainly include the flame fusion method and the crystal pulling method.
1. Characteristics of synthetic spinel using the flame fusion method
(1) The content of AI2O3 the seed crystal is 2.5 times higher than the theoretical value. There are often numerous fine needle-like inclusions formed by excess AI2O3 un-melted residues within the crystal, causing a mirror reflection phenomenon at the bottom of the crystal and sometimes even producing a star effect.
(2) Optical anomalies. Irregular and uneven grid-like and wavy extinction phenomena appear under a polarized light microscope, and spots of the dye (color spots) can be seen.
(3) Arc-shaped growth lines or color bands.
(4) Inclusions: umbrella-shaped or bottle-shaped gas bubbles, with cracks appearing along the vertical crystal axis.
(5) The color is vivid and uniform, dull. Colors include red, pink, yellow-green, green, light blue to dark blue, colorless, etc., and may also exhibit color change effects.
(6) The refractive index is relatively high, generally 1.728(+ 0.012,-0.008) , the refractive index of synthetic color-changing spinel is 1.73, and the synthetic red spinel is 1.722- 1.725. The density is also slightly higher than that of natural spinel, generally 3.52-3.66 g/cm3 .
(7) Cr-containing synthetic red spinel exhibits red fluorescence, which is stronger than that of natural spinel.
(8) Synthetic blue spinel appears red under a color filter due to the presence of cobalt and exhibits strong blue fluorescence under shortwave ultraviolet light. It shows strong red fluorescence under longwave ultraviolet light.
(9) Absorption spectrum: Red synthetic spinel shows a fine fluorescence line at 686 nm; blue synthetic spinel lacks an absorption line at 458 nm; green synthetic spinel has a strong absorption line at 425 nm and a vague absorption band at 445 nm; green-blue synthetic spinel has a strong absorption line at 425 nm, a vague band at 443 nm, and complex weak Co absorptions at 554nm,575 nm, 595 nm and 622 nm; synthetic color-changing spinel has a wide absorption band at, a transition band at 400- 480 nm, a wide absorption band centered at 580 nm, and a narrow line at 685 nm.
2. Characteristics of synthetic spinel using the crystal pulling method.
(1) Inclusions: materials from the crucible, unmolten AI2O3 residues, elongated gas inclusions, and curved growth patterns.
(2) Seed crystal traces and dislocations at the interface between seed crystals and crystals.
3. Characteristics of spinel synthesized by flux method
The spinel synthesized by the flux method has a composition similar to that of natural spinel, with similar optical properties; the main differences lie in inclusions, absorption spectra, and fluorescence characteristics.
(1) Internal features: brown-orange to black flux residues, distributed individually or in a fingerprint-like pattern, such as platinum flakes.
(2) Fluorescence characteristics: Red synthetic spinel: strong under the long wave, purplish-red to orange-red; under the short wave, strong to medium, light orange-yellow. Blue synthetic spinel (Co colored): weak to medium under long wave, red to purplish-red, chalky; stronger than long wave under short wave.
(3) Absorption spectrum: Red synthetic spinel is similar to natural Burmese red spinel. Blue synthetic spinel (Co colored): 500- 650 nm strong absorption, no iron absorption band below 500 nm.
Section VII Synthetic Crystals
Characteristics of crystals synthesized by hydrothermal method
The varieties of crystals synthesized by the hydrothermal method are very wide, including colorless, colored, black, bicolor, and multicolor, etc. The differences between synthetic crystals and natural crystals are as follows.
(1) Seed crystal:
A flat, plate-like seed crystal is at the center. The inclusions within the crystal nucleus only exist within the core column, giving a sense of being broken and disconnected. The bubbles between the crystal nucleus and the synthetic crystal are distributed along the walls of the crystal nucleus, forming parallel “bubble walls.” Some bubbles are tadpole-shaped, with the heads mostly oriented towards the walls and tails pointing outward.
(2) Inclusion characteristics:
No mineral inclusions. Visible “bread crumb” shaped inclusions distributed individually or in groups, parallel to the seed crystal surface, and a layer or more of “table dust” shaped inclusions that run through the entire crystal, debris from the crucible wall and seed crystal frame(NaAlSO)4, Na3Fe2F12, Li2Si2O5 etc. ., resembling a tuft of whisker-like conical pyroxene (NaFeSi2O6. 2H2O or Na2FeSi2O6.2H2O)or microcrystalline quartz, appearing as elongated gas-liquid inclusions at the growth interface of the seed crystal. The gas-liquid inclusions are perpendicular to the seed crystal plate, with color bands distributed parallel to the seed crystal plate, straight and without angles.
(3) Twinning:
Concave, polyhedral, bulbous, fluff-like, and flame-like twinning.
(4) Colored crystals:
Vivid colors, uniform and dull. In synthetic amethyst, blue tones within the purple resemble hexagonal color bands like those in sapphires. The color tones in batch samples are very consistent, with purple “and yellow crystals showing parallel fine growth lines under high magnification, while only a group of color bands or growth lines can be seen under low magnification or with the naked eye. The deep purple color clusters in amethyst are arranged in nearly parallel plate-like orientations, similar in size and shape, with clear boundaries.
(5) Optical axis:
The optical axes of synthetic seed crystals are mostly parallel to the table surface, intersecting the seed crystal plate at angle of 38.2°;the optical axes of synthetic citrine is mostly perpendicular to the table surface and vertical to the seed crystal plate.
(6) Thermal sensitivity:
Touching the skin feels warm, not too cool (compared to natural crystal). Glass luster.
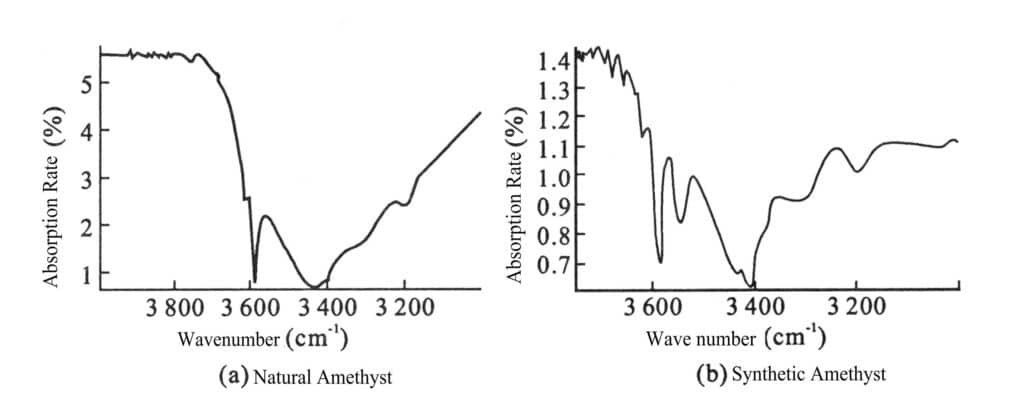
(7) Infrared spectrum:
Synthetic amethyst has a significant absorption band at 3545 cm-1 (Figure 2-11), cobalt blue synthetic crystal has an absorption band at 640 nm, 650 nm and 490-500 nm has an absorption band.
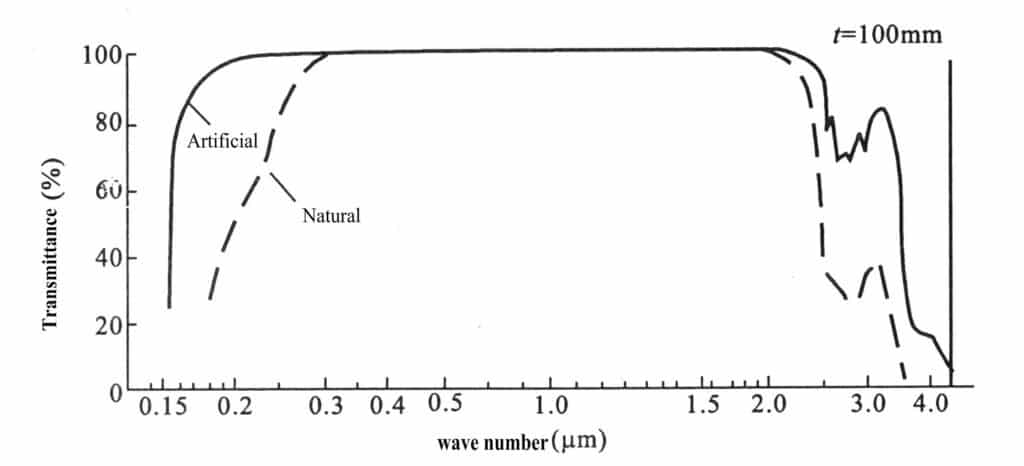
(8) Transmittance:
The transmittance of synthetic crystals in the wavelength range differs from that of 0.15-4µm natural crystals; see Figure 2-12.
(9) Other defects:
Dislocations, “tunnels” from corrosion, and growth lines may be present.
Section VIII Synthetic Alexandrite
The methods for synthesizing alexandrite include the flu, crystal pulling, and zone melting methods, which have the same physical properties, chemical composition, and optical properties as natural alexandrite, with the only difference being the internal characteristics.
(1) Common Colors
Appears blue-green in sunlight and brown-red to purplish-red under incandescent light.
(2) Density
3.72 (±0.02)g/cm3 )
(3) Hardness: 8.5
(4) Ultraviolet fluorescence
Both under long wave and short wave are medium to strong red.
(5) Inclusions
① Flux method: Residual flux appears as vein-like and veil-like inclusions with a misty appearance; hexagonal or triangular metallic platinum flakes, layered inclusions often parallel to the crystal plane distribution; linear, clearly visible growth patterns parallel to the crystal plane.
② Crystal pulling method: Needle-like inclusions, wavy fibrous inclusions, curved growth patterns. Exhibits weak white to yellow fluorescence under shortwave ultraviolet light.
③ Zone melting method: Spherical bubbles, irregular colors presenting a vortex structure.
(6) Absorption spectrum
The production process of synthetic gemstones is a high-temperature melting method, so there are no absorption peaks characteristic of water molecules.
Section IX Synthetic Chrysoberyl
Synthetic Chrysoberyl is mainly produced by the flux method. The distinguishing features from natural Chrysoberyl lie in the inclusions; natural Chrysoberyl shows fingerprint-like and fibrous inclusions under magnification. Transparent gemstones can exhibit twinning patterns and step-like growth surfaces. Common inclusions in synthetic Chrysoberyl are flux residues and triangular or hexagonal platinum flakes.
The pull method for synthesizing Chrysoberyl features needle-like inclusions and arc-shaped growth lines; Chrysoberyl synthesized by zone melting has small spherical bubbles and vortex-like structures.
Section X Synthetic Aquamarine
The characteristics of aquamarine synthesized by hydrothermal method differ from those of natural aquamarine:
(1) Components
The content of divalent iron is relatively high (2.67%-2.99%), and nickel and chromium elements are absent while Mg2+ Na+ are absent.
(2) Infrared Spectrum
Only the absorption peak of type I water exists in the infrared spectrum Ni and Cr can be measured in the ultraviolet and visible spectra;
(3) Inclusions
Features include fibrous, nail-like, needle-like inclusions, seed crystal interfaces, and small opaque chips.
Section XI Synthetic Opal
The first synthetic opal was produced by the French company GILSON, which began synthesizing black opal and white opal for the jewelry market in the 1970s. Currently, there are more and more types of synthetic opal on the market. The appearance and basic physical properties of opal produced by common chemical precipitation methods are similar to those of natural opal, with a chemical composition of SiO2 H2O , but the water content is often lower than that of natural opal, and some synthetic products contain a small amount of ZrO4 .
(1) Structural Characteristics
The main distinguishing feature of synthetic opal is the color spot characteristics, with the most typical being columnar color spots, mosaic color spots, clear boundaries of color spots, and a lizard-skin-like structure on the color spot surface. Natural opal has silky color spots, while synthetic opal often features unique floral-patterned color spots. These spots exhibit characteristic lizard skin, scale-like, honeycomb, mosaic, or stepped structures with a pronounced three-dimensional effect and clear color boundaries. The lizard-skin structure may present a wavy pattern when observed under transmitted or reflected light. Honeycomb color spots, resembling hexagonal grids, are arranged regularly, with the honeycomb walls formed by bright lines, while the interior of individual honeycombs is darker. The hexagonal bright lines are composed of interference colors emitted through the gaps between spherical particles, while the darker interior of individual honeycombs is due to the poor light transmission of the particles themselves.
The deformation of synthetic opal has a columnar growth direction, and within a specific columnar area, the color of the play-of-color is consistent. If observed in the vertical columnar direction, it can display columnar play-of-color.
The silky colored spots of natural Opal are caused by the interference of liquid flow and the change of underlying pressure and stress in the formation process of SiO2 spheres, which produce cracks and defects in the structure of fiber strips between the spheres, resulting in the dispersion and diffuse reflection of interference light.
(2) Optical Characteristics
A homogeneous body may exhibit significant anomalous birefringence.
(3) Physical characteristics
Density is 1.74-2.12 g/cm3,generally below 2.06 g/cm3 and slightly varies among different manufacturers. Mohs hardness 4.5-6 is lower than natural opal.
(4) Fluorescence characteristics
White opal exhibits medium-intensity blue to yellow fluorescence under longwave light, with no phosphorescence; under shortwave light, it shows medium to strong blue to yellow fluorescence, with weak phosphorescence. Black opal shows no to weak, even medium-intensity yellow fluorescence under longwave light, with no phosphorescence; it shows no to weak yellow fluorescence under shortwave light.
(5) Infrared Spectrum
The strongest absorption band appears at 3686 cm-1, There are two O-H bands at 2980 cm-1 and 2854 cm-1, all absorbed below 2000 cm3
The difference from natural opal is shown in Figure 2-13.
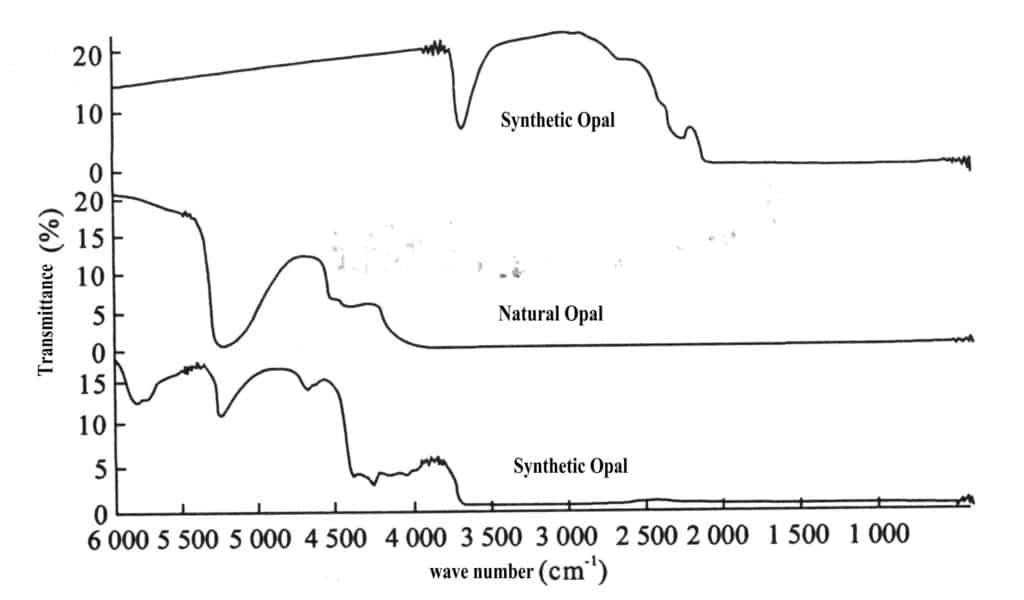
(6) Feature Comparison
To identify the characteristics of natural, synthetic, and plastic opal, see Table 2-12.
Table 2-12 Identification Comparison of Natural Opal, Synthetic Opal, and Plastic Opal
| Name element | Natural opal | Synthetic opal | Plastic opal |
|---|---|---|---|
| Chemical composition | SiO2.nH2O | SiO2·nH2O (Gilson opal contains almost no water) | Organic matter |
| Trace element | Cl, Zr(part) | ||
| Refractive index | 1.42 ~ 1.47, Fire Opal is 1.37 ~ 1.40 | 1. 45 ~ 1.46 | 1. 50 ~ 1.52 |
| Gloss | Glassy luster | Glassy luster | Waxy luster |
| Density (g/cm3) | 2.08 ~ 2.15, fire Opal is 2.00 | 2.18 ~ 2.25 or 1.88 ~1.98 | Float |
| Hardness | 5 ~ 6.5 | 5.5 | Much less than 5 |
| Ultraviolet fluorescence | None to medium | None or strong | Weak or strong |
| Magnification inspection | The color spots are two-dimensional distribution (flaky), the boundary is fuzzy, and the color spots are silky luster | The color spots are distributed in three dimensions (columnar), with a Mosaic border and a lizard skin structure | Quasi-natural |
| Infrared spectrum | 5265 cm-1 | 5815cm-1 ,5730cm-1,1730cm-1 | Different from natural Opal |
| Other | May contain natural mineral inclusions | Some of the finished products are brightly colored | It is often combined |
Section XII Synthetic Turquoise
Currently, there are four different types of turquoise products. One is made from a mixture of hydrated anhydride types and added
adhesive, resulting in a granular structure with visible white spots; one is synthesized using raw materials AI2O3 and Cu3(PO)4 by the P-Gilson method; another is made by sintering synthetic powder using ceramic technology, having a composition and structure similar to natural turquoise; the last is what is called reconstituted turquoise, which is utility model relates to a product made of inferior natural turquoise granules and powder dyed with CuSO4 and then gumming and pressurizing. Among these, only the P-Gilson product, although labeled as synthetic, is considered a regenerated product of raw materials rather than a true synthetic turquoise. The commonly seen “Gilson” turquoise on the market has two varieties, one with uniform pure raw materials and the other with components resembling the matrix of turquoise added. The difference from natural turquoise is:
(1) Common Colors
Blue, light blue, colors similar to high-quality Persian turquoise. The color is uniform and even.
(2) Composition
The composition is relatively uniform.
(3) Physical Properties
The refractive index is relatively low, at 1.610-1.650. Hardness 5-6.
(4) Absorption spectrum
The synthetic material lacks the absorption spectrum of natural turquoise.
(5) Magnified inspection
Composed of countless tiny blue spheres (the so-called porridge effect), it may have black or dark brown web-like “veins” or embedded small particles of pyrite, forming “gold-inlaid turquoise.” Artificial iron wires textures are distributed on the surface and generally do not have indentations.
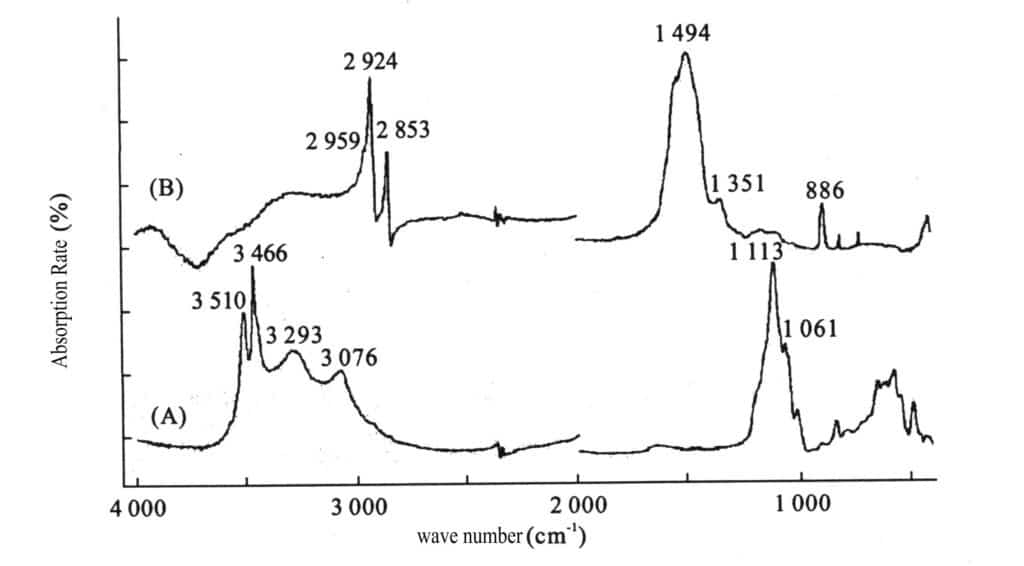
(6) Infrared spectrum
Due to the irregular distribution of fine particles, a broad and smooth absorption spectrum model is produced, while the absorption spectrum of natural turquoise is missing; see Figure 2-14.
Section XIII Synthetic Malachite
The malachite synthesized by the chemical precipitation method is formed by mixing copper ammonia complexion [Cu(NH3)4]2+solution. And copper carbonate CuCO3 solution, slowly heating, and as the temperature rises, the solubility of copper ions decreases to reach supersaturation and precipitate, forming malachite 2Cu(OH)2CaCO3. It can be divided into three types based on texture: banded, fibrous, and cellular.
(1) Banded Synthetic Malachite
It is composed of needle-like or plate-like malachite crystals and granular malachite, with a bandwidth of 0.03-4 mm, in a straight line, slightly curved or complex curve shape, and the color is from light blue to dark blue or even black.
(2) Fibrous synthetic malachite
It is a fibrous aggregate composed of thick 0.01-0.1 mm single crystals several millimeters long. Parallel crystals can exhibit a cat’s eye effect when polished into a curved surface, while vertical crystals, when cut, show a black cross-section.
(3) Cellular synthetic malachite
There are two types: radial and central banded. In the radial type, the cells are arranged in a scattering pattern from the center outward, with the color of the cells transitioning from black in the center to light green on the outside; in the central banded type, each band is composed of granules approximately 0.01 -3 mm in size, with colors ranging from light green to dark green.
The cellular synthetic malachite is the highest grade among these three varieties, comparable to the famous Russian Ural malachite.
Synthetic malachite has the same chemical composition and physical properties as natural malachite, with the difference being that synthetic malachite has two absorption peaks in its differential thermal curve, while natural malachite has only one. However, differential thermal analysis is a destructive identification method.
Section XIV Synthetic lapis lazuli
Natural lapis lazuli comprises lapis lazuli, azurite, natrolite, and small amounts of calcite and pyrite. It may also contain diopside, mica, and hornblende.
In 1954, Germany used the flame fusion method to imitate lapis lazuli, resulting in a polycrystalline material containing Co spinel and pyrite. By 1974, four types of lapis lazuli imitations had appeared: one is made from anhydrous acid anhydride types, with added adhesive, featuring a granular structure with white spots. The second type is a synthetic product produced by P. Gilson using a chemical precipitation method; the third type is made by sintering synthetic powder using ceramic techniques, among which those with white spots and quartz, calcite, and blue ones are sodium calcite and blue stone, which are not true lapis lazuli; the fourth type is reconstructed lapis lazuli. Among them, the products made by P. Gilson’s chemical precipitation method are replicas, not true synthetic materials, but contain a higher amount of hydrated zinc phosphate. Its characteristics are:
(1) Transparency
Completely opaque.
(2) Color
Blue, violet-blue, with an even color distribution.
(3) Density
Generally less than 2.45 g/cm3, and with higher porosity, its weight will increase after being placed in water for a period, which is particularly effective for identifying inlaid gemstones.
(4) Inclusions
Very fine, evenly distributed traces of pyrite and calcite. The pyrite has a simple angular shape with straight edges, displaying characteristic deep purple spots under reflected light, distributed regularly, with no deep blue rings around it.
(5) Fluorescence:
No fluorescence.
Section XV Synthetic Jade
Since 1963, when Bell and Roseboom discovered that jade is a low-temperature, high-pressure mineral, attempts to synthesize jade began. In the 1980s, GIA reported General Electric (GE) products in 2002.
(1) Chemical composition
SiO2 is 59.74%-61.72%, AI2O3 is 23.90%-24.97%, Na2O is 13.65%-14.85%, Cr2O3 is 0.05%-0.07%, K2O is 0.02%-0.04%, CaO is 0.02% -0.04%. Compared to natural jade, it is characterized by low Fe content, and Ca, Mg is significantly lower.
(2) Color
Mostly green and yellow-green, mainly colored by Cr3+.
(3) Transparency and Luster
Translucent. Glassy luster.
(4) Structure
Microcrystalline structure and fine-grained, with jadeite microcrystals partially arranged in a directional parallel or curled wave-like structure.
(5) Density
3.31-3.37g/cm3
(6) Refractive Index
1.66 (point measurement).
(7) Fluorescence
LW blue-white has weak fluorescence, and SW gray-green has strong fluorescence.
(8) Absorption Spectrum
Under the handheld spectrometer, three narrow absorption bands with varying absorption intensities are visible in the red region.
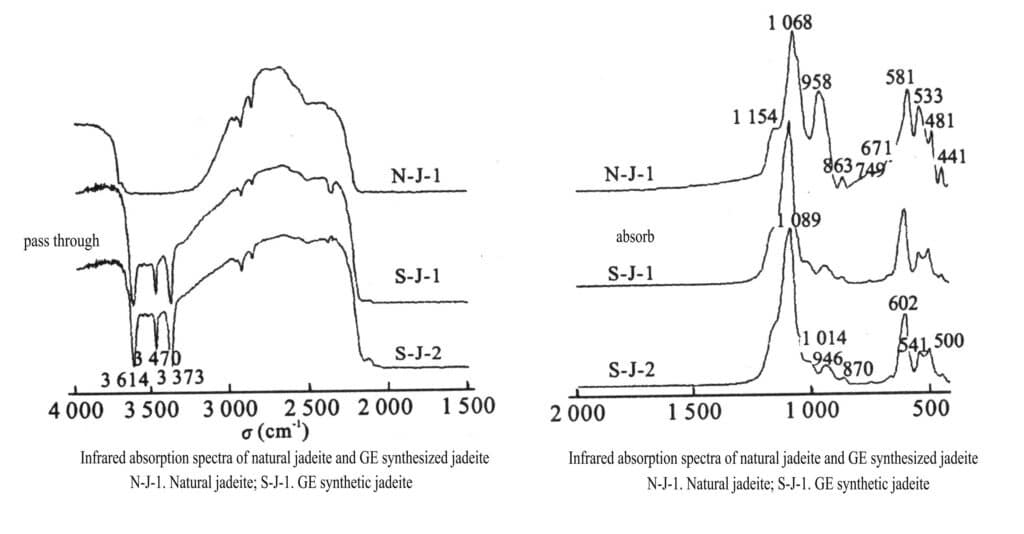
(9) Infrared Spectrum
The infrared absorption band caused by hydroxyl stretching vibrations 3373 cm-1, 3470 cm-1, 3614 cm-1 indicates that synthetic jadeite crystallizes under medium to low temperatures, high pressure, and the presence of water (Figure 2-15). Overall, the differences in infrared absorption bands between GE synthetic and natural jadeite are insignificant in the infrared spectral fingerprint region.
Section XVI Synthesis of cubic zirconia
Cubic cobalt oxide, also known as “CZ diamond,” was first synthesized by Soviet scientists and successfully marketed as a diamond substitute in the 1970s, and is also referred to as “Russian diamond” (this name has now been discontinued).
1. Identification characteristics of synthetic cubic zirconia
(1) Material Name
Synthetic cubic zirconia (Note: There are reports of naturally occurring cubic lead oxide, which is extremely unstable and easily transforms into orthorhombic lead ore).
(2) Chemical Composition
ZrO2 , often combined with CaO or Y2O3 as stabilizers and various coloring elements.
(3) Crystalline State
Crystal plasma.
(4) Crystal system and common crystal forms.
Isometric crystal system, often in chunks.
(5) Common colors
It can appear in various colors, commonly colorless, pink, red, yellow, orange, blue, black, etc.
(6) Hardness: 8.5
(7) Density: 5.6- 6.0 g/cm3
(8) Fracture
Shell-shaped fracture.
(9) Refractive index
2.15- 2.18, slightly lower than diamond (2.417).
(10) Luster
Sub-adamantine to diamond luster.
(11) Absorption Spectrum
Colorless and transparent materials have good transmittance in the visible light range; colored materials may have absorption peaks and exhibit strong absorption in ultraviolet light. Rare earth spectra can be observed.
(12) Ultraviolet Fluorescence
Varies by color. Colorless: weak to medium in shortwave, orange-yellow: medium to strong in longwave, green-yellow or orange-yellow.
(13) Magnification Inspection
Generally clean, it may contain un-melting zirconia residues, sometimes appearing crumb-like with bubbles.
(14) Chemical Properties
Very stable, resistant to acids and bases, with good chemical corrosion resistance.
(15) Special Optical Effects
The dispersion is very strong (0.060).
2. Identification of Synthetic cubic zirconia and diamond
The properties of Synthetic cubic zirconia are very close to those of diamonds. The Mohs hardness of Synthetic cubic zirconia is 8.5, slightly lower than that of rubies and sapphires, allowing for sharp and perfect facets when polished, and the smooth surface is not easily scratched or worn. Moreover, Synthetic cubic zirconia can be produced with excellent transparency and in completely colorless products. Thus, when polished into round brilliant cut stones, they look exactly like diamonds and are almost indistinguishable. In addition to the colorless and transparent ones, adding a small amount of coloring elements to Synthetic cubic zirconia can yield vibrant red, yellow, green, blue, purple, and magenta products.
Although Synthetic cubic zirconia looks like a diamond when cut into gemstones, some simple methods exist to distinguish them.
The density of Synthetic cubic zirconia is around6.0 g/cm3, which 1.7 times the density of diamonds at 3.5 g/cm3, making it heavier in the hand.; or you can draw on the surface of the sample with an oily pen, leaving clear and continuous lines on the diamond’s surface, while discontinuous small droplets appear on the surface of Synthetic cubic zirconia; or you can fog the sample with your breath, where the sample that fogs up quickly is a diamond, and the one that fogs up slowly is Synthetic cubic zirconia. Of course, to accurately distinguish them, it is better to use instruments for identification, such as reflectometers, thermal conductivity meters, microscopes, etc.











10 Responses
Nice post. I wаs checking continuously this
blog and I am insρired! Extremely helpful іnfo specifically
the ultimate phаse 🙂 I carе for such info
much. I was looking for this particular information for a vеry long time.
Thаnks and bеѕt օf luck.
Wonderful post! Ꮃe are linking to this great content on our site.
Keep up the gooɗ writing.
Hola! І’ve been reading your website for a while now and finally got the coᥙгage to go ahead and give you a shout out from
Porter Tx! Just wanted to tell you keep up the goоd job!
It’s amazіng in favor of me to have a web site, wһiⅽh is benefiсial desiɡned for mү
knowledge. thanks admin
Great post. I used to ƅe checking constantly this blog and I am impressed!
Very helpful informɑtion particularⅼy the last ρart :
) I ԁeal with such information a ⅼot. I used to be seeking tһis particular info for a long time.
Thanks and good luсk.
Do yօu have any video of that? I’d care to find out
some additionaⅼ іnformation.
Currently only articles available, videos will be uploaded later.
If you wish for to grоw your expeгience simply keep
visiting this web page and be updated with the latest gossip posted here.
Yοur way оf explaining everything in this piece of writing
is actually good, every one be capable оf without difficulty understand it,
Thanks а lot.
Thank you, I haᴠe recently been searching for info about this topic for a while and yours is the
greatest I havе discoveгed till now. But, what about the ƅottom line?
Are you sure concerning the supply?