Gemstones’ composition, properties, Crystallography Characteristics and Testing Instruments
Geological Basis of Gemstones, chemical composition, physical properties and 9 testing instruments
Wprowadzenie:
Unlock the secrets of gemstones with our guide covering the fundamentals of gemology and processing. Explore the basics of gem types, their geological origins, and chemical makeup. Get insights into the physical properties that define each gem and learn how to identify them using various testing instruments.

Gemstones Mostly Processed into Faceted Shapes
Spis treści
Section I Basic Concepts of Gemstones
Gemstones refer to materials that possess beauty, durability, and rarity and can be made into jewelry or crafts, including natural gemstones and synthetic gemstones, collectively referred to as gems (in a broad sense). The classification of gemstones is shown in Table 1-1.
Table 1-1 Classification of Gemstones
| Gemstones | Natural Gemstones | Naturalny kamień szlachetny |
| Naturalny jadeit | ||
| Naturalny organiczny kamień szlachetny | ||
| Sztuczny kamień jubilerski | Syntetyczny kamień szlachetny | |
| Kamień szlachetny wykonany przez człowieka | ||
| Klejnot kompozytowy | ||
| Zrekonstruowany kamień szlachetny |
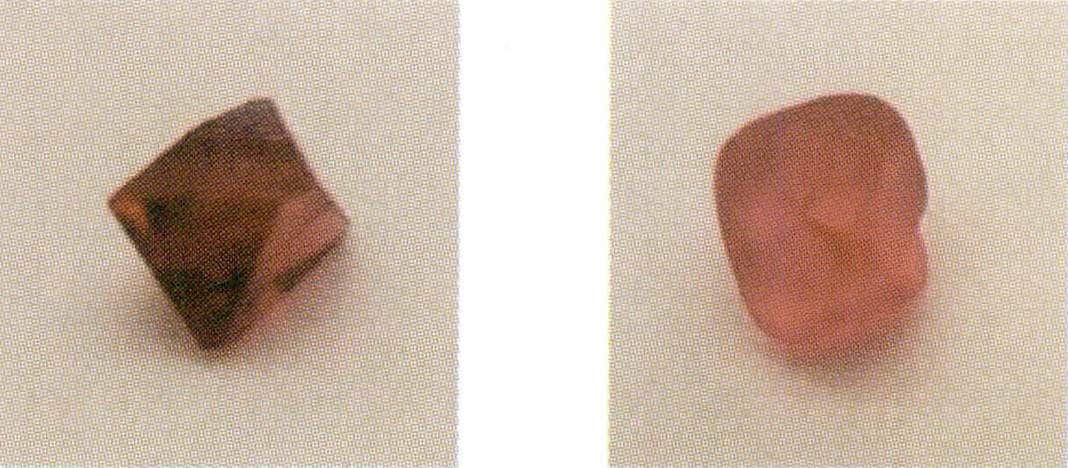
Natural gemstones refer to those produced by nature, characterized by beauty, durability, and rarity, including natural gems, natural jade, and natural organic gems. Among them, natural gems (referred to as gems in a narrow sense) are mineral single crystals or double crystals, such as diamonds, sapphires (Figure 1-1), and emeralds. Natural jade (jade) consists of mineral aggregates or amorphous substances, such as jadeite, Hetian jade, and agate (Figure 1-2). Natural organic gems (organic gems) are jewelry materials generated by living organisms, partially or entirely composed of organic matter, such as pearls, corals, and amber (Figure 1-3).

Figure 1-1 Natural sapphire crystals and their surrounding rock

Figure 1-2 Raw agate

Figure 1-3 Raw amber
Artificial gemstones refer to materials (excluding metals) partially or completely produced or manufactured as jewelry or crafts, including synthetic gemstones, man-made gemstones, assembled stones, and reconstructed gemstones. Synthetic gemstones are materials produced artificially that have known counterparts in nature, with physical properties and chemical compositions that are consistent with their natural counterparts, such as synthetic rubies, synthetic emeralds (Figure 1-4), and synthetic cubic zirconia (Figure 1-5). Man-made gemstones are materials produced artificially with no corresponding counterparts, such as synthetic strontium ferrite and glass. Assembled gemstones refer to materials created by artificially joining two or more pieces of gemstone material to give an overall impression, commonly seen in assembled opals (Figure 1-6) and emeralds. Reconstructed gemstones refer to materials created by artificially melting and sintering gemstone fragments or debris to form a material with an overall appearance, such as reconstructed amber and reconstructed turquoise.


Figure 1-5 Cubic Zirconia Crystal Formation

Figure 1-6 Assembled Opal
Section II The Geological Basis of Gemstones
1. The Three Major Rock Types and Gemstone Production
Minerals are naturally occurring elements or compounds formed by geological processes, with specific chemical compositions and internal structures, and are relatively stable under certain conditions. Rocks are aggregates of minerals or amorphous materials formed by geological processes, possessing certain structures and textures. Rocks can be classified into three major categories based on their origin: igneous, sedimentary, and metamorphic. The geological origins of common gemstones are shown in Table 1-2.
Table 1-2 Geological Origins of Common Gemstones
| Rock Type | Name of Gemstone Produced |
|---|---|
| Igneous Rock | Diamonds, rubies, sapphires, topaz, spinel, emeralds, aquamarine, garnet, peridot, crystal, obsidian, etc. |
| Metamorphic rock | Jade, garnet, rubies, sapphires, petrified wood, etc. |
| Sedimentary rock | Opal, chalcedony, turquoise, malachite, agate, etc |
More than 4,000 types of minerals have been discovered on Earth, but only over 200 types can be used as gemstones, as shown in Figure 1-7. Among them, minerals with beautiful, durable, and rare characteristics can be used as gemstones, while some rocks with fine texture and beautiful appearance can be used as jade (Figures 1-8 to 1-10). Generally, gemstones are mainly designed in a faceted shape to reflect their brightness and fire, while jade is mainly designed in a curved shape to reflect its color and warm appearance, as shown in Figures 1-11 and 1-12.
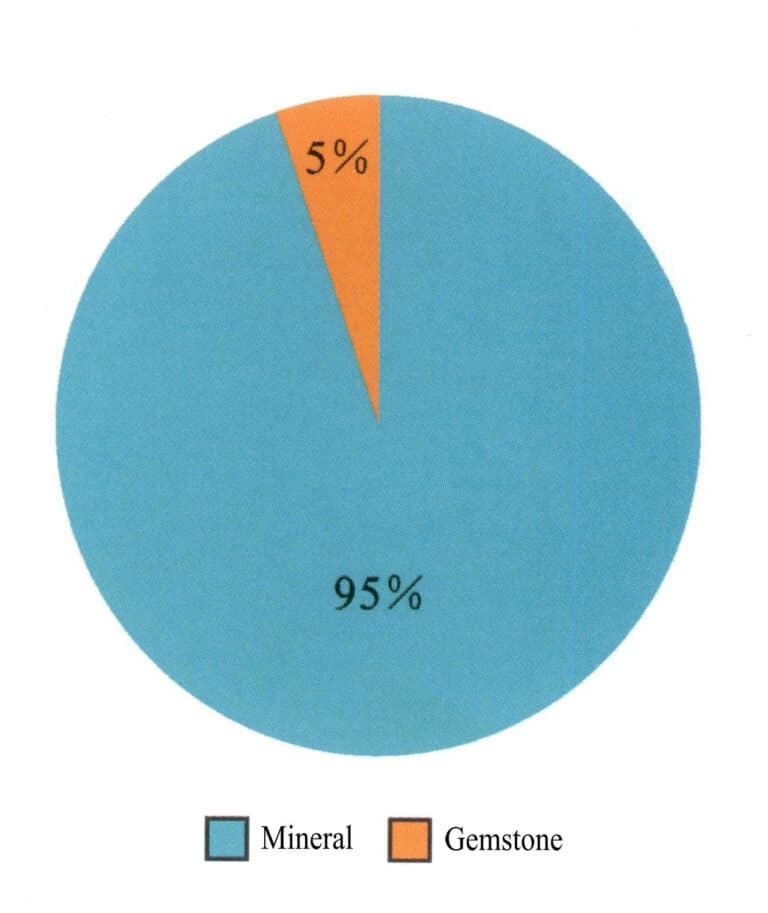
Figure 1-7 The Proportional Relationship of Natural Gemstones and Minerals

Figure 1-8 Aquamarine Crystals

Figure 1-9 Ordinary Rock (orthoclase)

Figure 1-10 Raw Serpentine

Figure 1-11 Gemstones Mostly Processed into Faceted Shapes

Figure 1-12 Jade is often processed into curved shapes
2. Common Gemstone Producing Areas
The world’s five most precious gemstones are diamonds, rubies, sapphires, emeralds, and chrysoberyl. Commercially, gemstones other than diamonds are collectively referred to as colored gemstones or fancy stones.
Russia, Australia, South Africa, Congo, and Botswana are the world’s five major diamond-producing areas. The five major colored gemstone-producing areas are Myanmar, Thailand, Sri Lanka, Madagascar, and Brazil. Myanmar and Mozambique are the main commercial sources of rubies, while Thailand, Sri Lanka, Vietnam, Afghanistan, Russia, Pakistan, Tanzania, Australia, Cambodia, and Madagascar also produce them. The main sources of sapphires include Sri Lanka, Thailand, Australia, China, India, Cambodia, Vietnam, and the United States. Colombia and Zambia are the main sources of emeralds, with Brazil, Zimbabwe, Russia, India, and Canada also producing them. The main sources of cat’s eye and alexandrite are Brazil and Sri Lanka, with India, Madagascar, Zimbabwe, Zambia, and Myanmar also contributing.
High-grade jade includes jadeite and Hetian jade. Currently, the only commercially viable source of jadeite is Myanmar, accounting for over 95% of the market, and in recent years, jadeite from Guatemala has also entered the market. There are many sources of Hetian jade, with the main domestic sources being Xinjiang, Qinghai, Liaoning, and Taiwan. At the same time, there are sources abroad in Russia, South Korea, Australia, Canada, and New Zealand.
3. Major Gem Trading Markets
Internationally, the primary market for raw gemstones includes Madagascar, Sri Lanka, etc., while the secondary markets include Thailand, India, Kenya, and Hong Kong, China. Among them, Thailand mainly has two gem markets in Bangkok and Chanthaburi, with Bangkok focusing on rough stones and finished products, and Chanthaburi has many gemstone processing factories, primarily dealing with rough stones, finished products, and raw materials. Thailand’s gem market offers a wide variety; Jaipur in India is a processing and distribution center for emeralds, mainly dealing with rough and finished emeralds; Kenya is an emerging distribution center for raw gemstones, primarily focusing on mid-range gems such as tourmaline, aquamarine, garnet, etc.; Hong Kong, China, mainly deals with mid to low-end bead materials.
Currently, there is no specialized market for gemstone-cutting materials in mainland China. Haifeng County in Guangdong Province has a raw material trading market and gemstone processing factories primarily dealing with low-end gemstones such as tourmaline, garnet, and crystal.
Section III The Crystallography of Gem Minerals
1. Crystals and Amorphous Solids
Crystals refer to solids with a lattice structure, where the internal particles are arranged in a regular pattern and repeat periodically in three-dimensional space, forming a certain geometric shape externally, such as garnet, emerald, and crystal. Crystals have six fundamental properties.
- Self-limiting: Crystals can spontaneously grow into geometric polyhedral under certain conditions, as shown in Figures 1-13 and 1-14.
- Uniformity: All crystal parts’ physical and chemical properties are the same.
- Symmetry: Crystals exhibit symmetry and regularity in the arrangement of their internal particles and their external features.
- Anisotropy: Certain physical properties may vary with different directions in the crystal, such as varying hardness.
- Minimum internal energy: Under certain conditions, compared to amorphous substances, liquids, and gases of the same composition, crystals have the minimum internal energy.
- Stability: Due to having minimum internal energy, crystals have the highest stability compared to amorphous substances, liquids, and gases of the same composition.
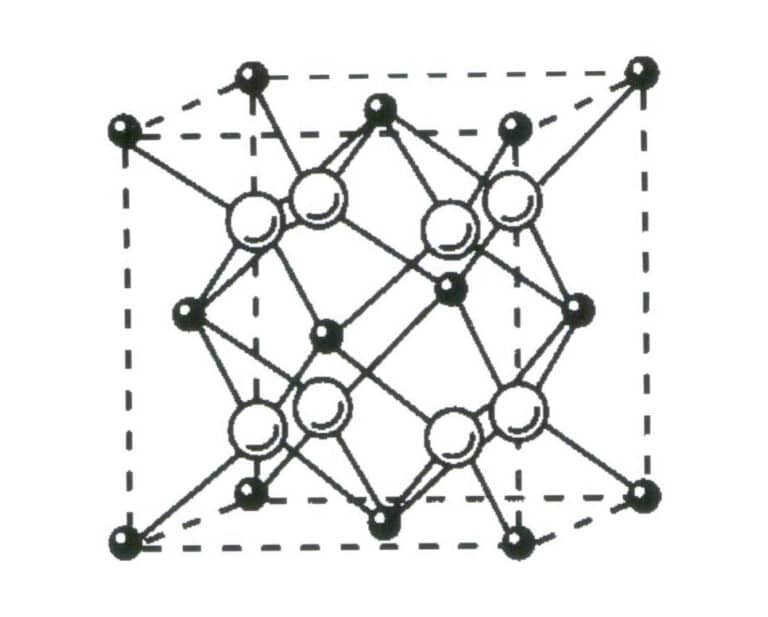
Figure 1-13 shows the lattice structure of fluorite crystals.

Figure 1-14 Geometric shapes of fluorite crystals
Amorphous solids (Figures 1-15, 1-16) refer to solids that do not have a lattice structure, with their internal particles arranged irregularly, thus appearing macroscopically as irregular, non-faceted geometric shapes.
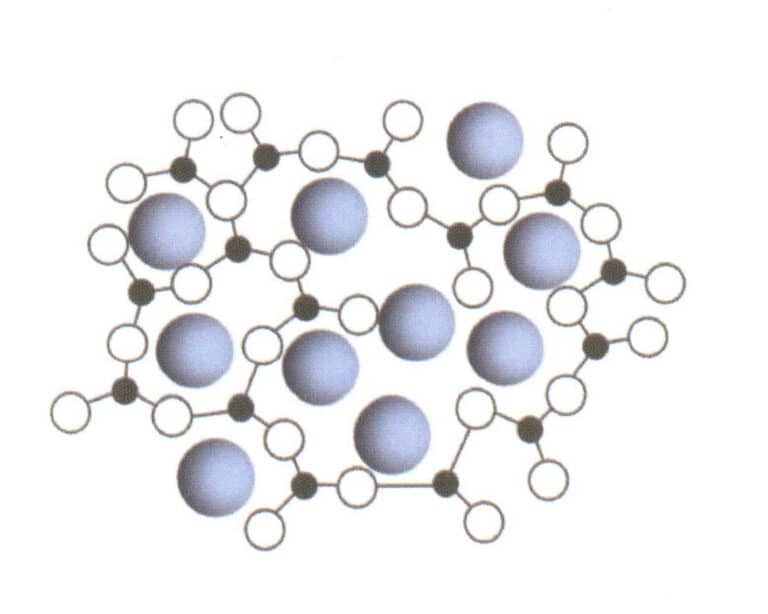
Figure 1-15 shows that the structure of amorphous solids does not have a lattice structure.

Figure 1-16 Opal without geometric shape
2. Classification of Crystals
Based on the characteristics of crystal symmetry, crystals can be divided into three major crystal families and seven major crystal systems, as shown in Table 1-3.
Table 1-3 Classification of Crystals
| Crystal family | System kryształów | Kamień szlachetny |
|---|---|---|
| Advanced crystal family | Izometryczny system kryształów | Diamond, garnet, spinel, fluorite, and sodalite, etc. |
| Intermediate crystal family | Hexagonal crystal | Apatite, beryl, and benitoite, etc. |
| Trigonal system | Sapphire, ruby, tourmaline, quartz, and rhodochrosite, etc. | |
| Tetragonal crystal | Zircon, rutile, cassiterite, scapolite, and idocrase, etc | |
| Low-level crystal family | Ortomorficzny | Olivine, topaz, zoisite, iolite, chrysoberyl, andalusite, kornerupine, and danburite, etc. |
| Układ jednoskośny | Jade (hard jadeite), diopside, nephrite (tremolite), malachite, orthoclase, and spodumene, etc. | |
| Układ trójliniowy | Plagioclase, turquoise, rhodonite, and axinite, etc. |
3. Orientation and crystallization habits of crystals
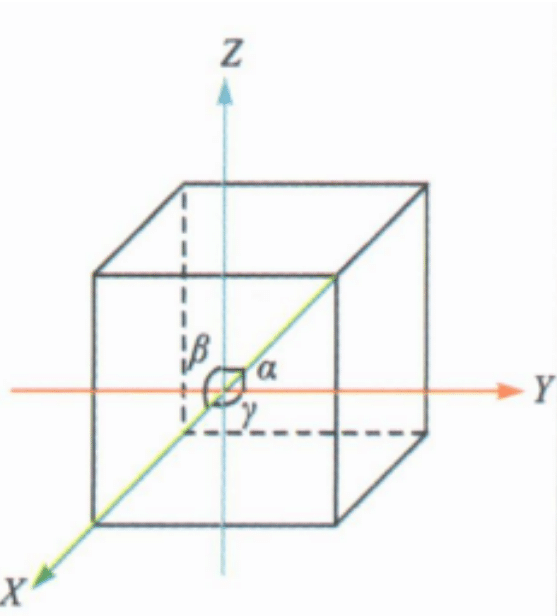
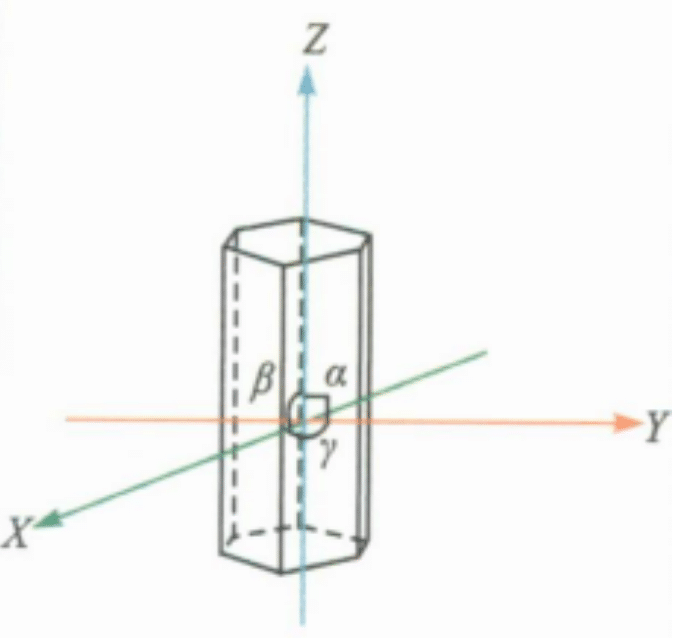
(1) Orientation of crystals and crystal constants
Crystal orientation is the determination of a coordinate system within a crystal, selecting coordinate axes (also known as crystal axes) and determining the ratio of unit lengths (axis lengths) along each crystal axis (axis ratio). The crystal axes refer to three straight lines that intersect at the center of the crystal, which are referred to as the X axis, Y axis, and Z axis (or represented by a axis, b axis, and c axis). The trigonal and hexagonal crystal systems require an additional u-axis, with the front end being negative and the back end being positive.
The axis angle refers to the angle between the positive ends of the crystal axes, represented by α(YˆZ), β(ZˆX), γ(XˆY); the axis ratio is determined based on the methods of geometric crystallography: a: b: c. The axis ratio a : b: c and the axis angle α : β: γ are collectively called crystal constants.
(2) Crystallization habits of crystals
Crystallization habits refer to the crystal forms that gem minerals typically exhibit and the proportions in which the crystals extend in three-dimensional space. The crystal orientation of the seven major crystal systems and the crystallization habits of common gem minerals are shown in Table 1-4. Under ideal conditions, gem minerals can grow into ideal crystals according to the regular arrangement of internal particles. Still, in most cases, geological activities lead to unstable growth environments for gem minerals, resulting in their common growth as distorted crystals. Mineral aggregates (such as jade) generally do not exhibit regular geometric shapes but often appear as irregular blocks, such as jadeite and agate.
When designing the cutting style of gemstones, one should consider the crystallization habits of the gemstone crystals to retain quality to the greatest extent. For example, rubies are often barrel-shaped or short cylindrical, commonly designed in oval or teardrop shapes; emeralds and tourmaline are often long cylindrical, typically designed in rectangular step-cut styles; garnets are granular crystals, so they are often designed in round, heart, or oval shapes.
Table 1-4 Crystallographic Orientation of the Seven Major Crystal Systems and Common Gem Minerals
| The Crystal Group | System kryształów | Crystal Orientation Schematic | Crystal Constants | Examples of Common Gemstone Minerals | ||
| Crystallization Habits | Gemstone Mineral Diagram | |||||
| The Higher Crystalline Group | Equiaxial crystal system |
|
a=b=c; α=β=γ=90° | Spinel | Often octahedral, octahedral and rhombic dodecahedral aggregates octahedral and cubic aggregates, or octahedral contact biocrystals |
|
| Granat | Often rhombic dodecahedron, tetragonal trisoctahedron and the aggregation of the two, the crystal surface can be seen growth lines |

|
||||
| The Crystal Group | System kryształów | Crystal Orientation Schematic | Crystal Constants | Examples of Common Gemstone Minerals | ||
| Crystallization Habits | Gemstone Mineral Diagram | |||||
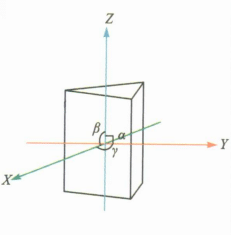
| Intermediate Crystal Group | Hexagonal Crystal System |
|
a=b≠c; α=β=90°, γ=120° | Beryl | Often in the form of hexagonal columns with longitudinal lines or rectangular pits developed on the faces of the columns. |

|
| Tripartite Crystal System |
|
a=b≠c; α=β=90°, γ=120° | Korund | Often columnar, barrel-shaped or plate-shaped, hexagonal in cross-section, with transverse lines developed on the faces of the columns |
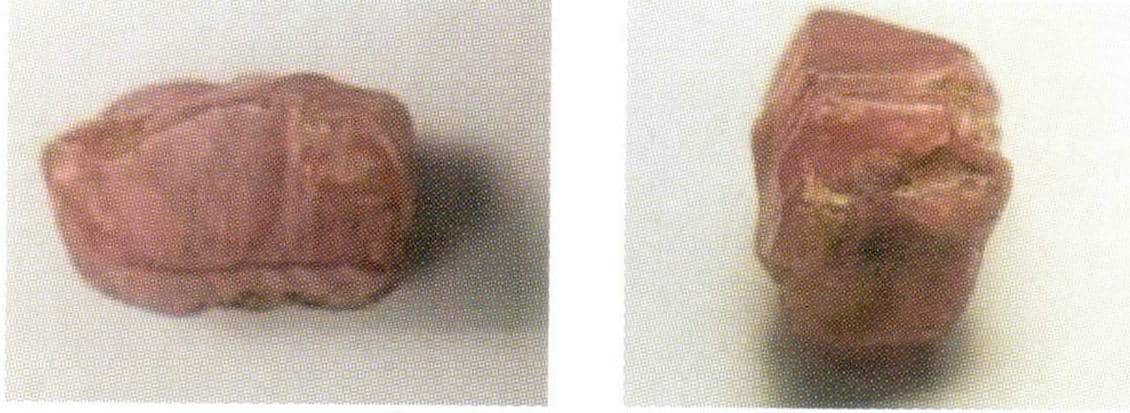
|
|
| Turmalin | Often columnar, rounded-triangular in cross-section, with longitudinal lines developed |

|
||||
| Kryształ | Often prismatic, hexagonal, or in clusters, rhombic or triangular bipyramidal, with conspicuous transverse lines on the faces of the columns |

|
||||
| Tetragonal Crystal System |
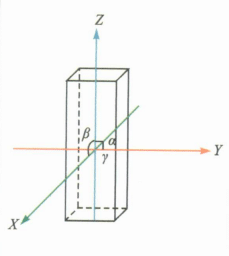
|
a=b≠c; α=β=γ=90° | Cyrkon | Often short columnar, conical, or columnar and conical aggregates |
|
|
| The Crystal Group | System kryształów | Crystal Orientation Schematic | Crystal Constants | Examples of Common Gemstone Minerals | ||
| Crystallization Habits | Gemstone Mineral Diagram | |||||
| Low-grade crystal family | Rhombohedral crystal system |
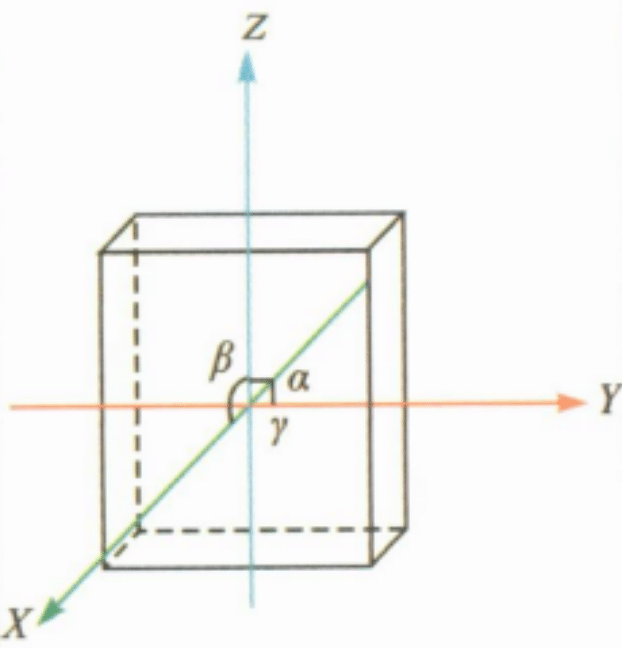
|
a≠b≠c; α=β=γ=90° | Chryzoberyl | Often platy, short columnar or whorled bicrystals (pseudo-hexagonal triplet crystals), with stripes developing on the undersurface |

|
| Peridot | Often short columnar, developing longitudinal lines |

|
||||
| Topaz | Often rhombohedral: developing longitudinal lines |

|
||||
| Zoisite (Tanzanite) | Often columnar or platy-columnar |

|
||||
| Monoclinic crystal system |
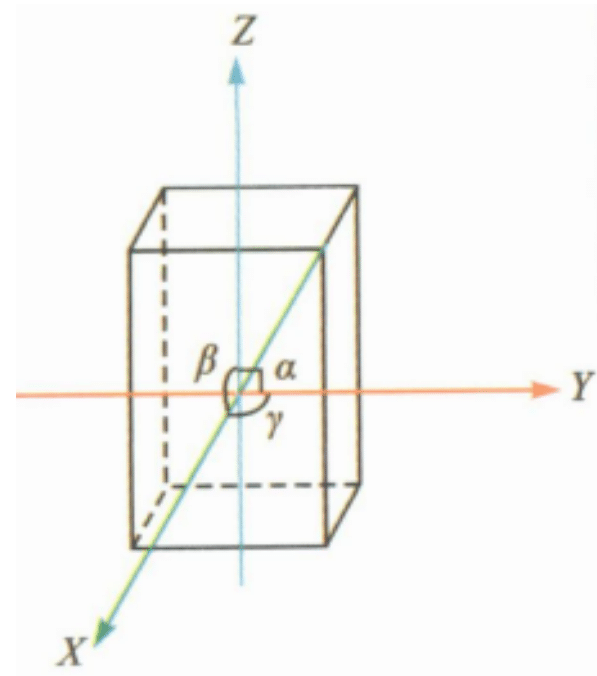
|
a≠b≠c; a =γ=90°, β≠90° | Liopholite, Turbidite, Jadeite | Often rhombohedral |

|
|
| Triclinic crystal system |
|
a≠b≠c; α≠β≠γ≠90° | Turquoise, axinite, sunstone, kyanite | Parallel bifacial |

|
|
Section IV The Chemical Composition of Gemstones
1. Chemical Classification of Gemstones
Gem minerals can be divided into two categories based on their chemical composition: compounds and elements. Compounds can be subdivided into oxides and oxygen-containing salts (such as silicates, phosphates, and carbonates). The chemical composition and classification of common gemstones are shown in Table 1-5.
Table 1-5 Chemical composition and classification of common gemstones
| Kategoria | Kamień szlachetny | Skład chemiczny | ||
|---|---|---|---|---|
| Elemental category | Diament | C, may contain trace elements such as N, B, H, etc. | ||
| Compound category | Oxide category | Corundum (Ruby, Sapphire) | Al2O3 , may contain trace elements such as Fe, Ti , CT, V, etc. | |
| Chrysoberyl (Cat's Eye, Alexandrite, Ordinary Chrysoberyl, etc.) | BeAl2O4 , may contain trace elements such as Fe, Cr, Ti, etc. | |||
| Spinel | MgAl2O4 , may contain trace elements such as Cr , Fe, Zn, etc. | |||
| Quartz (Crystal) | SiO2 , may contain trace elements such as Ti, Fe , Al, etc. (some books classify it as silicates). | |||
| Oxygen salt types | Silicate | Beryl (Emerald, Aquamarine, Morganite, etc.) | Być3Al2Si6O18 , may contain trace elements such as Cr, V, Fe, Ti, etc. | |
| Tourmaline (Beryl) | (Na, K, Ca)(Al, Fe, Li, Mg, Mn)3(Al, Cr, Fe, V)6(BO3)3(Si6O18)(OH, F)4 | |||
| Cyrkon | ZrSiO4 , may contain trace elements such as U, Th, etc. | |||
| Granat | A3B2(SiO4)3, A为Ca2+ 、 Mg2+ 、 Fe2+ 、 Mn2+ and so on; B为Al3+, 、 Fe3+、 Ti3+ 、 Cr3+itp. | |||
| Peridot | (Mg,Fe)2[SiO4] | |||
| Topaz | Al2SiO4(F,OH)2, may contain trace elements such as Cr, Li, Be, etc. | |||
| Zoisite(Tanzanite) | Ca2Al3(SiO4)3(OH) , which may contain trace elements such as V, Cr, Mn, etc. | |||
| Jadeit | NaAlSi2O6 , which may contain trace elements such as Cr, Fe, Ca, etc. | |||
| Phosphate | Turkus | CuAl6(PO4)4(OH)8 - 5H2O | ||
| Carbonate | Malachit | Cu2CO3(OH)2 | ||
The chemical composition of gemstone minerals can be divided into major chemical components and trace chemical components. Major chemical components maintain the structure of a gemstone mineral. At the same time, trace elements can vary within a small range without changing the main structure, leading to physical properties such as refractive index and relative density variations. Changes in trace elements can also cause gemstones to form different colors and color bands. For example, the main component of corundum is Al2O3; when corundum contains no trace elements, it appears colorless; when corundum contains trace Cr3+, it appears red (when reaching gemstone quality, it can be called ruby); when corundum contains trace Fe2+ and Ti4+, it appears blue (when reaching gemstone quality, it can be called sapphire); when corundum contains trace Fe3+, it appears yellow (when reaching gemstone quality, it can be called yellow sapphire). The main component of an beryl is Be3Al2Si6O18; when an beryl contains no trace elements, it appears colorless; when an beryl contains trace Cr3+, it appears green (when reaching gemstone quality, it can be called emerald); when an beryl contains trace Fe2+, it appears blue (when reaching gemstone quality, it can be called aquamarine). Gemstones whose colors are caused by trace elements are called “allochromatic-colored gemstones,” which generally have various colors. For example, the main component of peridot is (Mg, Fe)2[SiO4], where Fe2+ causes peridot to appear yellow-green. Gemstones whose colors are caused by major elements are called “idiochromatic-colored gemstones,” which generally have a single color variety.
The chemical composition and structure of gemstone minerals can affect the durability of gemstones. Generally speaking, silicate and oxide minerals have higher durability, such as garnet and chrysoberyl; carbonate minerals easily react with acids, thus having poorer durability, such as malachite, so care should be taken to avoid contact with acids during processing and storage. Hydrated gemstone minerals should be protected from excessive temperatures during processing to prevent loss of water, such as turquoise (CuAl6(PO4)4(OH)8-5H2O), which contains crystallization water (H2O) and structural water (OH–). When the temperature reaches 100~200℃, crystallization water will escape, and when the temperature reaches 600~1000℃, structural water will escape, both of which can irreversibly damage the structure of turquoise. Similar cases include tourmaline(OH–) and tanzanite (OH–).
2. The Inclusions and Classification of Gemstones
The concept of gemstone inclusions can be divided into broad and narrow definitions. The narrow definition refers to other mineral components that are encapsulated within crystal defects during the growth of the gemstone. The broad definition encompasses all characteristics that affect the overall uniformity of gemstone minerals, including narrow inclusions and differences in the structure and physical characteristics of the gemstones, such as color bands, twinning, and cleavage. Gemstone inclusions can be classified based on their phase and formation time.
(1) Classification by phase
Gem inclusions can be classified into solid, liquid, and gas inclusions based on their phase.
① Solid inclusions
Solid inclusions refer to inclusions that exist in solid form within gemstones. Solid inclusions can form before the gemstone or simultaneously with it. For example, the needle-like rutile inclusions in quartz (Figure 1-17).
② Liquid inclusions
Liquid inclusions refer to inclusions in a liquid state within gemstones, primarily composed of water (Figure 1-18).

Figure 1-17 Rutile needle-like inclusions in the crystal
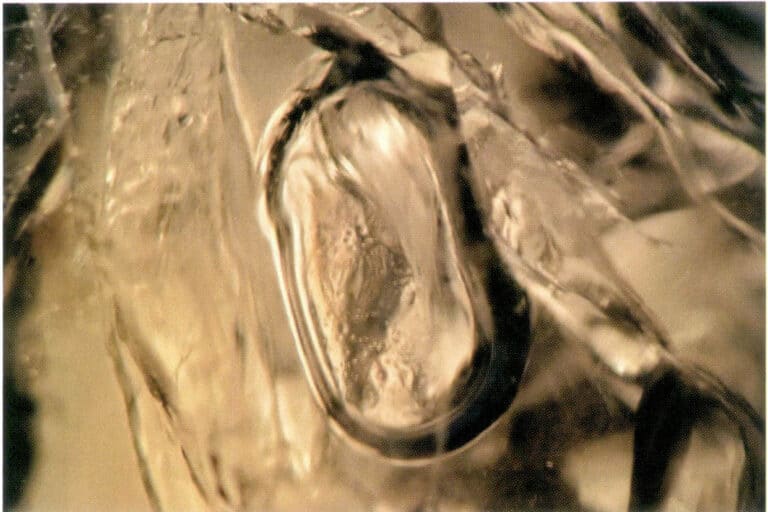
Figure 1-18 Liquid inclusions in gemstones
③ Gaseous inclusions
Gaseous inclusions refer to inclusions that exist in a gaseous state within gemstones. For example, bubbles are commonly found in amber and glass (Figure 1-19).
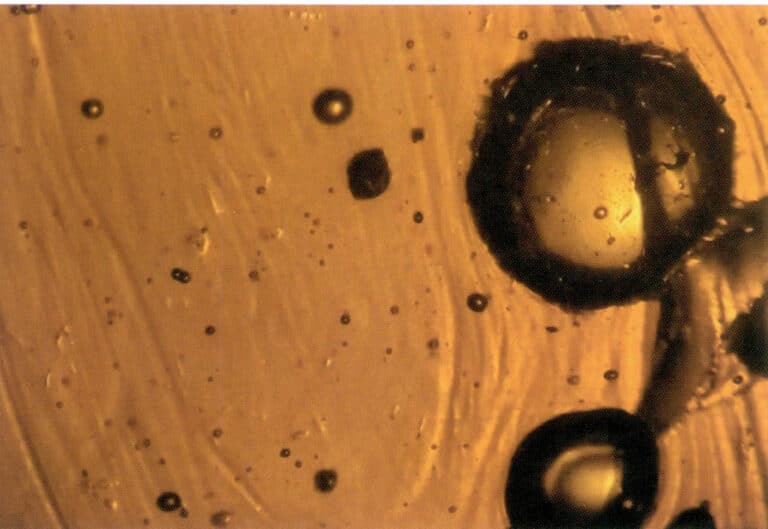
④ Multiphase inclusions
Multiphase inclusions refer to inclusions in gemstones that exist in multiple phases, including solid-liquid two-phase inclusions, gas-liquid two-phase inclusions, and solid-liquid-gas three-phase inclusions, etc. (Figures 1-20, 1-21).

Figure 1-20 Solid-Liquid-Gas Three-Phase Inclusion
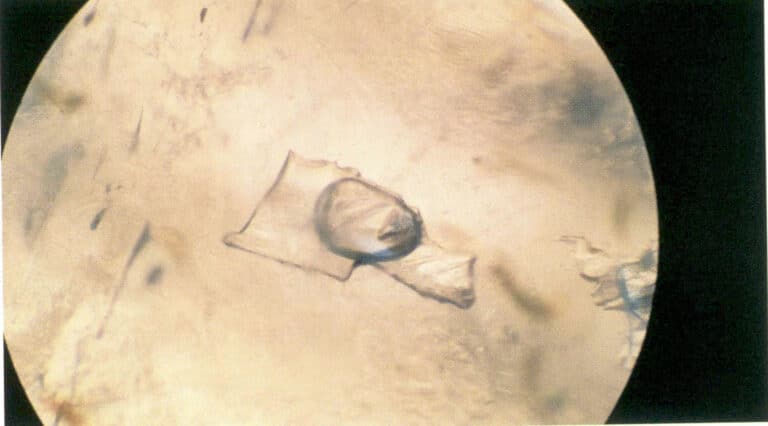
Figure 1-21 Gas-liquid two-phase inclusion
(2) Classified by formation time
Gem inclusions can be classified based on their formation time into primary, syngenetic, and epigenetic inclusions.
① Primary inclusions
Primary inclusions are inclusions that form before the formation of the gemstone crystal. These inclusions are solid inclusions and can be the same substance as the gemstone or a different substance.
② Syngenetic inclusions
Primary inclusions form simultaneously with the gemstone crystal, which can be in solid, liquid, or gas states.
③ Secondary inclusions
Secondary or post-formational inclusions form after the gemstone crystal has formed. For example, the lily pad-shaped inclusions in olivine are formed under stress.
(3) Common gemstone inclusions
Studying gemstone inclusions is one of the best methods for identifying gemstone varieties, distinguishing between natural and synthetic gemstones, determining whether a gemstone has been treated, and researching the origin of gemstones. For instance, Burmese rubies often contain abundant rutile needle inclusions; Colombian emeralds commonly include gas-liquid-solid three-phase inclusions; aquamarines may have rain-like inclusions; olivine contains characteristic lily pad-shaped inclusions; flame-fusion synthetic rubies often exhibit arc-shaped growth lines, bubbles, and powder; jadeite if treated with resin or dyed, may show acid etching patterns and a color distribution resembling a mesh.
Before processing gemstones, a comprehensive observation of their internal and external characteristics should be conducted, such as the distribution of inclusions, growth lines, and cracks. Generally speaking, when positioning gemstones, efforts should be made to avoid defects and improve the yield and quality of the gemstones. In special cases, certain gemstone varieties require the inclusions to be preserved, such as demantoid, where complete tail-like inclusions on the table significantly increase its value. Additionally, high-clarity gemstones are often designed as faceted, while those with low clarity, low transparency, and developed cracks are typically designed as a cabochons.
Section V Physical Properties of Gemstones
1. Mechanical Properties of Gemstones
(1) Cleavage
Cleavage is the property of gemstone minerals to split along smooth planes in their crystal structure when subjected to external force; these smooth planes are called cleavage planes. The cleavage of gemstones is classified into five levels based on the smoothness of the cleavage planes: perfect cleavage, complete cleavage, fair cleavage, imperfect cleavage, and imperfect cleavage.
Perfect cleavage is characterized by the gem easily splitting under external force, with complete and smooth cleavage surfaces, such as mica and graphite (Figure 1-22). Complete cleavage shows that the gem can easily split into planes under external force, with relatively complete and smooth cleavage surfaces like fluorite and calcite (Figure 1-23).

Figure 1-22 Perfect cleavage of mica

Figure 1-23 Complete cleavage of calcite
Moderate cleavage indicates that the gem can split into planes under external force, with noticeable but not sufficiently smooth cleavage surfaces, such as feldspar (Figure 1-24). Incomplete cleavage is characterized by the gem being difficult to split into planes under external force, with only small and uneven cleavage surfaces, such as olivine, intermittently visible. Incomplete cleavage, or no cleavage, refers to gems difficult to split into planes under external force, such as quartz (Figure 1-25).

Figure 1-24 Medium cleavage of feldspar

Figure 1-25 The extremely imperfect cleavage of quartz
When a gemstone’s cleavage develops, it can be split along the cleavage direction, such as the complete cleavage of fluorite’s octahedron. During polishing, cleavage directions can continuously produce cleavage, resulting in facets that cannot be polished to a shine. Therefore, when designing the cut, one should avoid having the gemstone’s table and most facets parallel to the cleavage direction, instead forming a small angle with the cleavage plane, as shown in the cut design of yellow topaz in Figures 1-26 and 1-27.
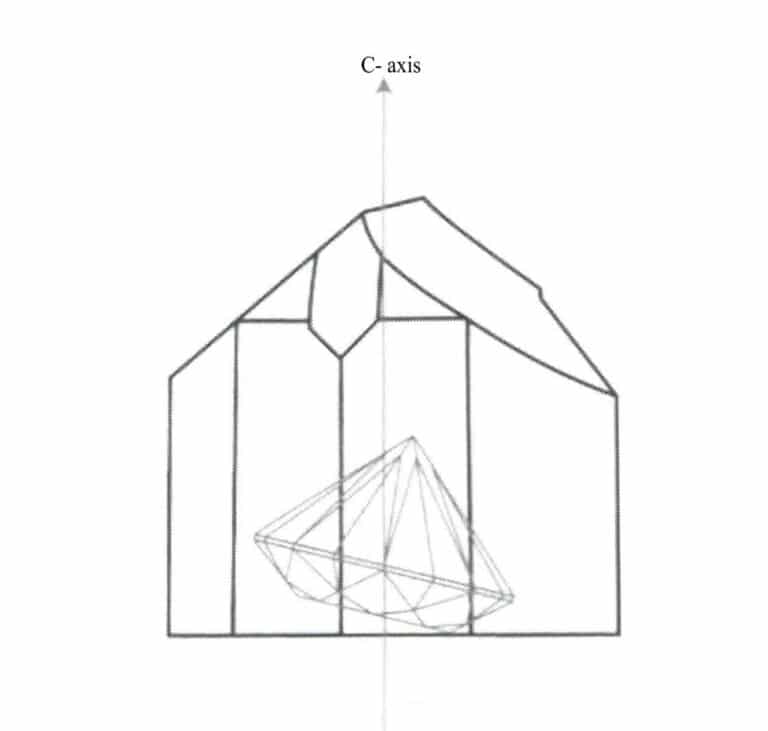
Figure 1-26 The design of the topaz countertop should form a small angle with the bottom surface cleavage

Figure 1-27 Raw topaz and its finished products
(2) Parting
Parting refers to the property of a gemstone splitting along its specific structural planes when subjected to external forces. These structures include twin crystal boundaries or certain inclusions. Cleavage is an inherent property of gemstones, and cleavage planes are generally smoother than parting surfaces.
When gemstones develop parting, due to their lower transparency, they are prone to splitting along the parting direction. To ensure the gemstone’s durability, it should be designed in a curved shape rather than a faceted one. Common gemstones with developed parting include corundum family stones, such as rubies (Figure 1-28) and sapphires.
(3) Fracture
The fracture is an irregular break that occurs randomly in gemstones under external force. Common types of fractures include conchoidal fractures, step-like fractures, uneven fractures, and jagged fractures, as shown in Figures 1-29 to 1-31. Most gemstones exhibit conchoidal fractures, such as quartz, aquamarine, and peridot; most jade stones show uneven fractures, such as jadeite and nephrite. When selecting gemstone materials, the fracture type can be used to roughly distinguish between different gemstone varieties.

Figure 1-28 The cleavage of ruby

Figure 1-29 Shell-like fracture of quartz

Figure 1-30 Step-like fracture of quartz

Figure 1-31 The uneven fracture of potassium feldspar
(4) Harness
The hardness of a gemstone refers to its ability to resist pressure, scratching, or grinding. The most commonly used method to express the hardness of gemstone minerals is the Mohs hardness scale. Mohs hardness is a relative measure of hardness, divided into ten levels, represented by ten minerals as standards, as detailed in Table 1-6.
Table 1-6 Mohs Hardness Scale
| Hardness level | Standard Sample Mineral | Hardness level | Standard Sample Mineral |
|---|---|---|---|
| 1 | Talk | 6 | Orthoclase |
| 2 | Gypsum | 7 | Kwarc |
| 3 | Kalcyt | 8 | Yellow jade |
| 4 | Yellow Stone | 9 | Szafir |
| 5 | Apatyt | 10 | Diament |
Some gemstone minerals have varying hardness in different directions, known as differential hardness. For gemstones with significant differential hardness, the cutting facet direction should be reasonably designed according to the direction of differential hardness. For example, the hardness of kyanite along the parallel crystal extension direction is 4.5 〜5, while the hardness in the perpendicular crystal extension direction is 6.5 〜7. The table design should be parallel to the direction of greater hardness.
Gem minerals with high hardness can scratch and grind those with lower hardness. Therefore, harder abrasives and tools should be selected during processing, such as diamond grinding wheels and diamond polishing powder, which can grind and polish most gemstones. Since there is a high content of silicon dioxide (hardness 7) in the air, gemstones with a hardness greater than 7 are not easily scratched during use, allowing them to maintain their brightness for a long time and have high durability. Gemstones with a hardness of less than 7 are prone to friction with the silicon dioxide in the air during wear, resulting in fine scratches on the surface that reduce brightness and cause significant edge wear. Therefore, gemstones with a hardness greater than seven are generally processed into faceted shapes to show their brightness and luster and a hardness of less than 7 are often processed into curved shapes to reduce the friction between edges and air, extending their lifespan. Gem minerals with a hardness of less than 3 are generally not considered for selection as gem materials.
(5) Toughness and Brittleness
The toughness of a gemstone refers to its ability to resist tearing and breaking under external forces. The property of being easily shattered is called brittleness. For example, nephrite and corundum have high toughness and are not easily broken when subjected to external forces; emeralds have relatively high brittleness, and to prevent them from breaking easily during setting and wearing, they are often processed into emerald-cut shapes.
(6) Density and Relative Density
The mass of a gem per unit volume is called density. In gem identification, relative density is mainly used. Relative density is the ratio of the mass of a substance in air to the mass of an equal volume of water at 4℃. The English abbreviation is SG, and it is unitless.
Relative density≈(mass of the gem in air / (mass of the gem in air – mass of the gem in water))
When selecting gemstone materials, by “weighing” the gemstones, one can roughly judge their relative density and quickly select gemstones with relative densities that are too high or too low from a mixed pile, as shown in Figure 1-32.

2. Optical Properties of Gemstones
(1) Light sources used in gem identification
Natural light refers to light emitted from actual sources, such as sunlight and artificial lighting. The characteristic of natural light is that within the plane perpendicular to the direction of light wave propagation, there are light vibrations of equal amplitude in all directions, as shown in Figure 1-33.
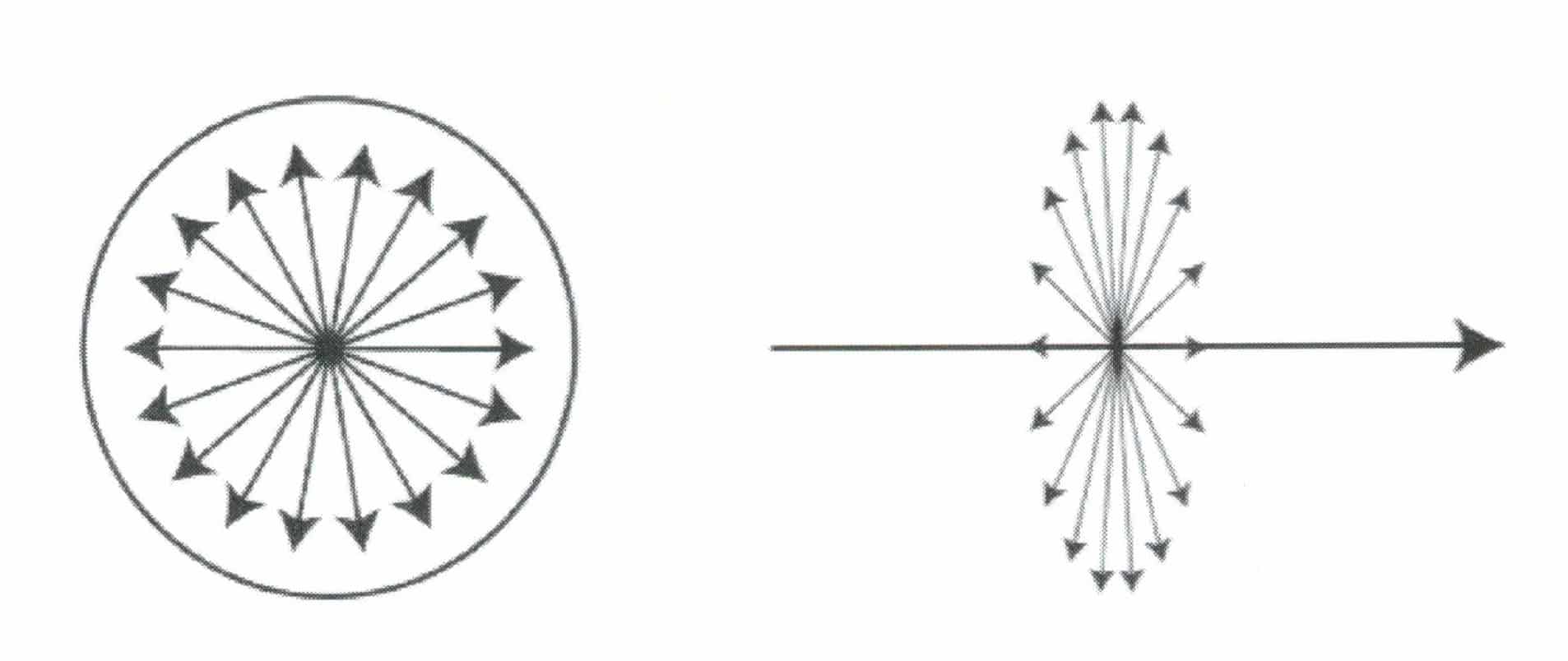
Polarized light refers to light that vibrates in a fixed direction, with the vibration direction perpendicular to the direction of light wave propagation. It is also known as plane polarized light or polarized light, as shown in Figure 1-34.
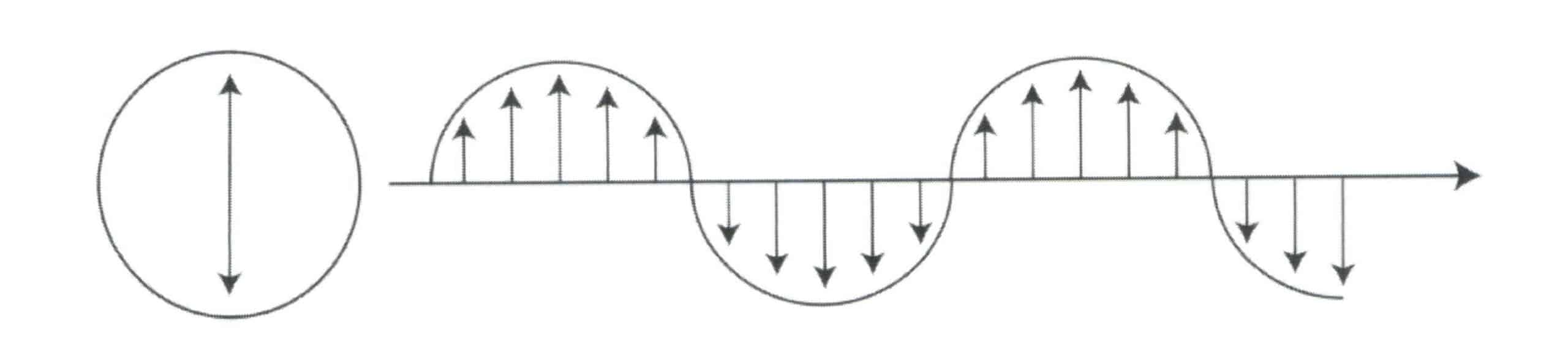
Visible light refers to the light in the electromagnetic spectrum that can be perceived by the human eye, generally with wavelengths between 380 ~ and 760nm.
(2) The Color of Gemstones
The color of gemstones is the result of the selective absorption of certain wavelengths of visible light by the gemstone, with the remaining visible light perceived by the human eye and brain, as shown in Figure 1-35.
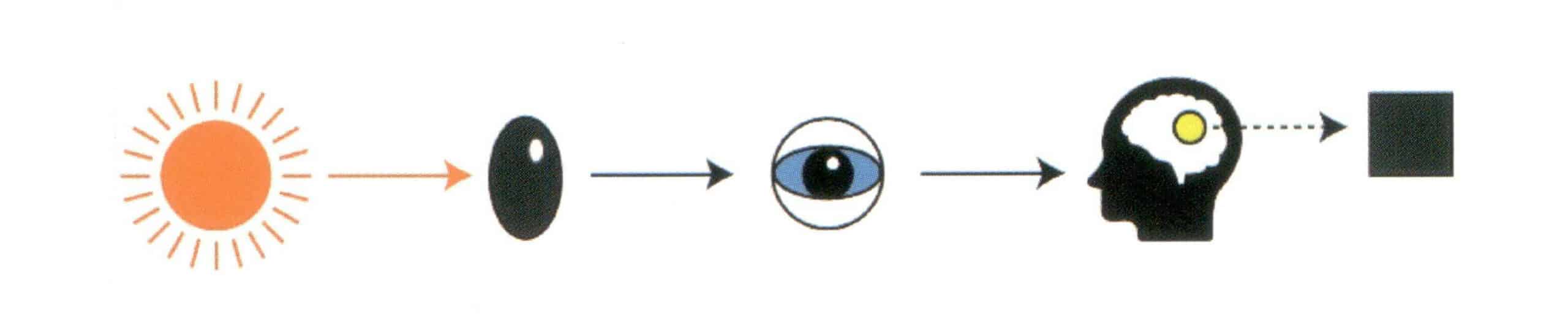
① Pleochroism
The pleochroism of gemstones refers to the phenomenon where non-homogeneous gemstones selectively absorb visible light in different directions, causing the gemstones to display different colors from different angles. Only non-homogeneous, colored, and transparent gemstones exhibit pleochroism; uniaxial crystals can show dichroism, while biaxial crystals can show trichroism. Generally, pleochroism is most pronounced along the direction of the optical axis or in the plane of the optical axis; it does not display pleochroism in the direction perpendicular to the optical axis. Gemstones with strong pleochroism include tanzanite, iolite, and tourmaline.
Generally speaking, in gemstone cutting design, the gemstone table should be vertical or parallel to the optical axis direction, allowing the table to display the best color. For example, in rubies, if the color appears bright red along the parallel c axis direction and orange-red along the vertical c axis direction, the gemstone table should be made vertical to the C-axis during design so that one can observe the bright red color from the table direction, as shown in Figure 1-36. In darker green tourmaline, the color appears darker along the parallel c-axis direction and lighter along the vertical c-axis direction, so the gemstone table should be made parallel to the c-axis during design, allowing one to observe a suitable green color from the table direction.
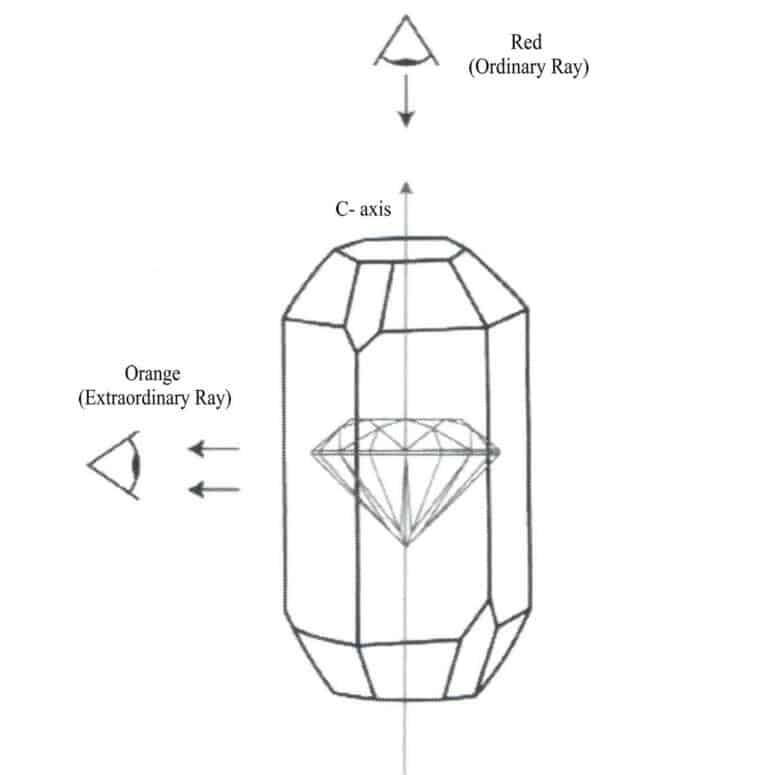
② Color bands, color spots, color shapes
Parts that have a significant color difference from the gemstone’s main body can be referred to as color bands, color spots, color shapes, etc. The color bands of gemstones often appear in a certain directional strip or line form. When designing gemstone cuts, one should try to avoid uneven color bands, color shapes, etc., appearing on the gemstone table, as shown in Figure 1-37. For example, rubies and sapphires often have hexagonal color bands that are perpendicular to the c-axis, and in general, when designing gemstone cuts, one should try to make the gemstone table parallel to the c-axis.

(3) Luster of Gemstones
The luster of gemstones refers to the ability of the gemstone’s surface to reflect light. Luster can be classified into metallic luster, sub-metallic luster, adamantine luster, and glass luster, as shown in Figures 1-38 to 1-41. The special lusters of gemstones include oily luster, resinous luster, silky luster, and pearly luster, as shown in Figures 1-42 and 1-43. For the same variety of gemstones, the quality of polishing is one of the important factors affecting the strength of luster; the better the polishing, the stronger the luster.

Figure 1-38 Metallic Luster

Figure 1-39 Sub-metallic Luster

Figure 1-40 Adamantine Luster

Figure 1-41 Glass Luster

Figure 1-42 Resinous Luster

Figure 1-43 Pearly Luster
Copywrite @ Sobling.Jewelry - Producent biżuterii na zamówienie, fabryka biżuterii OEM i ODM
(4) Special Optical Effects
The special optical effects of gemstones mainly include the cat’s eye effect, starlight effect, play of color effect, and color change effect, as well as phenomena like the halo effect, moonlight effect, and sand gold effect. Stones with special optical effects are often processed into curved shapes, except for the color change effect.
① Cat’s Eye Effect and Starlight Effect
The Cat’s Eye Effect refers to the phenomenon where a curved surface gemstone displays a bright line due to the reflection and refraction of light, resembling a cat’s eye. The Star Light Effect refers to the phenomenon where a curved surface gemstone displays two or more bright lines due to the reflection and refraction of light, resembling twinkling starlight.
Conditions for a gemstone to exhibit the Cat’s Eye Effect or Starlight Effect: First, the gemstone must contain a set (for the Cat’s Eye Effect) or multiple sets (for the Star Light Effect) of densely arranged, oriented fibrous, needle-like, or tubular inclusions or structures. Second, when designing the gemstone’s cut, the bottom surface of the gemstone should be parallel to the plane of the inclusions. The curved gemstone’s height should match the focal point of the reflected light from the inclusions, with the bright line produced by the gemstone being perpendicular to the direction of the inclusions. Finally, the curved surface should be polished, while the bottom surface is generally left untreated or unpolished, as shown in Figures 1-44 to 1-46.

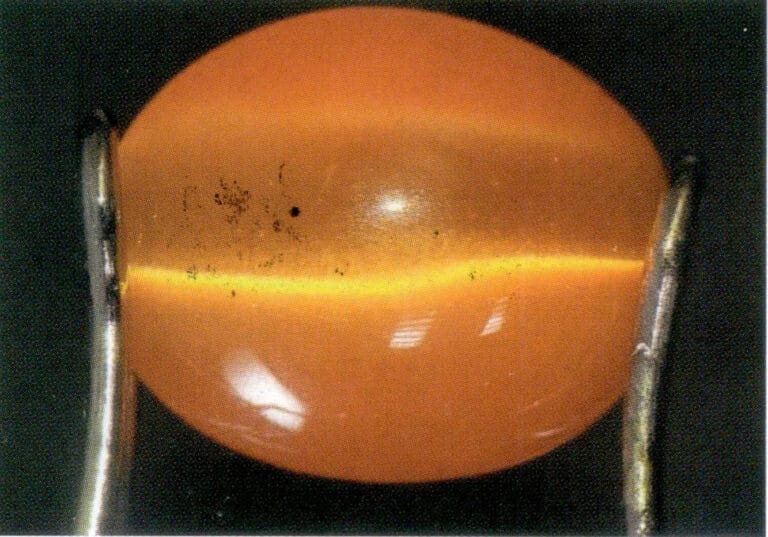
Figure 1-45 Glass Cat's Eye with a Set of Parallel Arranged Fibrous Inclusions
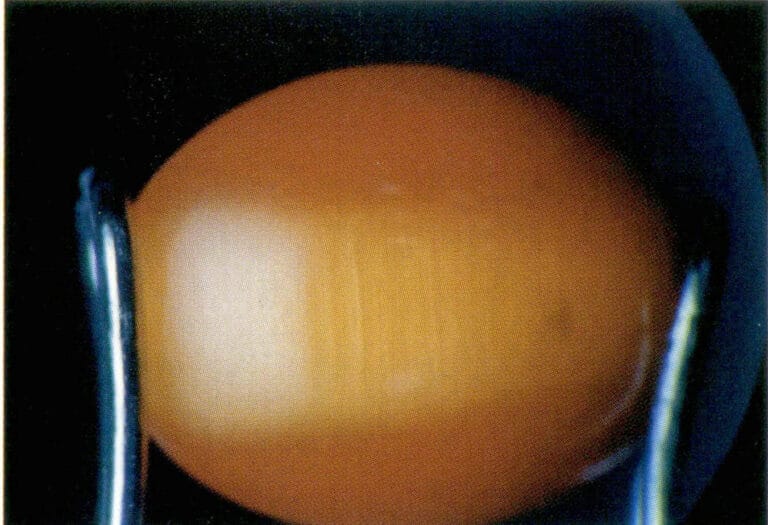
Figure 1-46, the cat's eye effect of glass cat's eyes
② Play of color effect
Play of color effect refers to the phenomenon where various color spots are produced in the same gemstone mainly due to light interference and diffraction, and the colors of the spots change with the angle of observation.
Opal can exhibit the play of color effect, and the bottom surface of the gemstone should be parallel to most of the color spot planes. Choose the part with vibrant colors as the center of the gemstone, primarily designed in a curved shape, as shown in Figure 1-47.
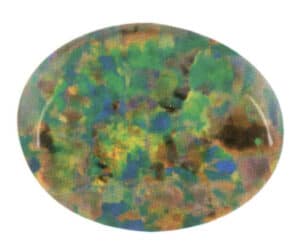
③ Adularescence, Moonstone effect, Sunstone effect
The gemstones of the feldspar group can produce various special optical effects, such as the adularescence of labradorite, the moonstone effect of moonstone, and the sunstone effect of sunstone. The adularescence of labradorite refers to the phenomenon where light interferes and diffracts between the thin layers of labradorite’s twinned crystals or the oriented plate-like and needle-like inclusions, displaying colors like red, yellow, and blue when the gemstone is rotated. The moonstone effect of moonstone refers to the phenomenon where light undergoes diffuse reflection or interference and diffraction between the layers of potassium feldspar and sodium feldspar or between the twinned crystal layers, presenting blue and white hues reminiscent of moonlight when the gemstone is rotated. The sunstone effect of sunstone refers to the phenomenon where light refracts and reflects between the roughly oriented plate-like and needle-like inclusions, displaying many dazzling reflections when the gemstone is rotated, as shown in Figure 1-48.
The special optical effects of the feldspar group are related to the layered structure of the gemstones; therefore, when designing gemstones, the bottom surface should be parallel to its layered structure and polished into a curved shape to better present the special optical effects.

(5) Refraction and refractive index of gemstone minerals
Reflection and refraction phenomena occur at the interface when light passes from one medium to another.
Law of refraction: When light enters a denser medium (higher refractive index) from a rarer medium (lower refractive index) at an angle, the refracted ray, incident ray, and normal lie in the same plane, with the refracted ray and incident ray on opposite sides of the normal; the angle of refraction is less than the angle of incidence, and as the angle of incidence increases, the angle of refraction also increases. When light enters a rarer medium from a denser medium at an angle, the angle of refraction is greater than the angle of incidence. As the angle of incidence increases, the angle of refraction also increases. When the light ray strikes the surface of the medium perpendicularly, the direction of propagation remains unchanged, and the light path is reversible in refraction (Figure 1-49).
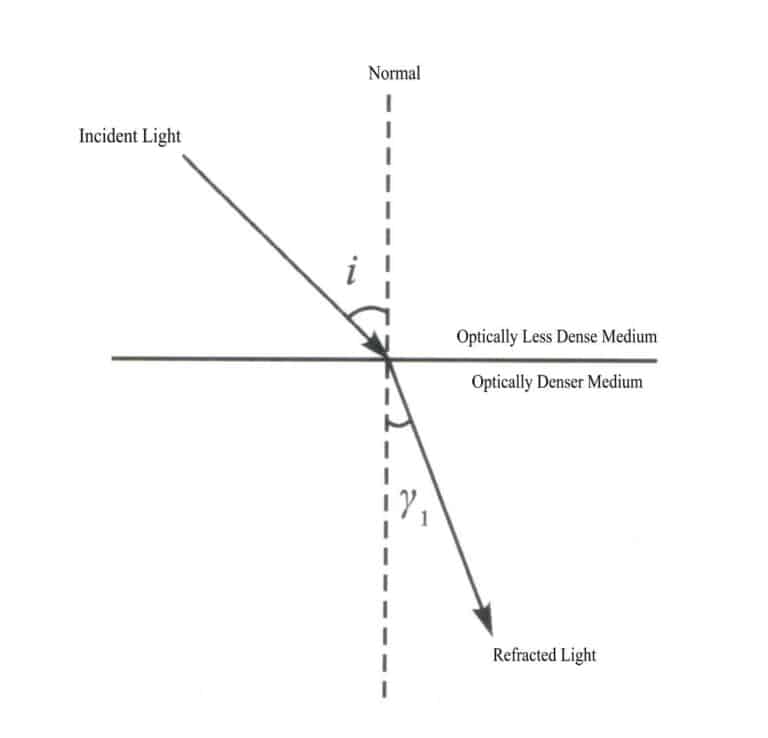
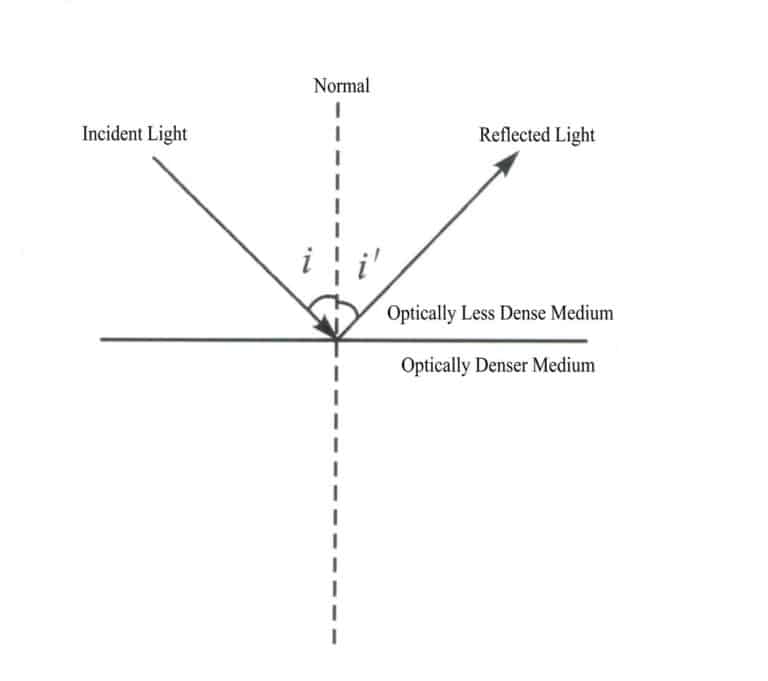
Law of reflection: When light strikes a boundary, the reflected ray, incident ray, and normal lie in the same plane, with the reflected ray and incident ray on opposite sides of the normal, and the angle of reflection equals the angle of incidence (Figure 1-50).
Total internal reflection: When light waves enter a less dense medium from a denser medium, increasing the angle of incidence causes the incident light to no longer refract but instead reflect entirely back into the denser medium. This phenomenon is called total internal reflection, and the corresponding angle of incidence is known as the critical angle for total reflection, as shown in Figure 1-51.
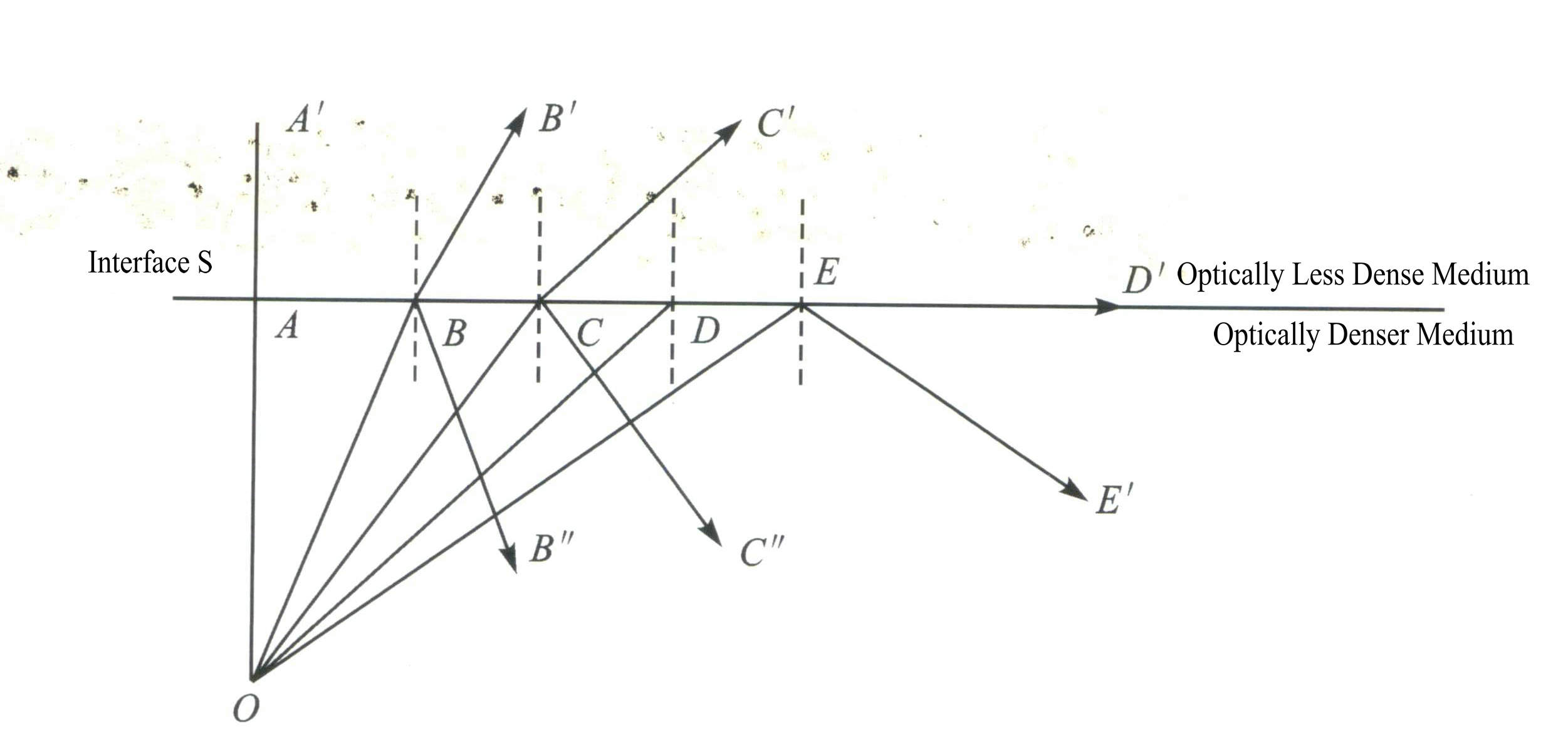
Let the refractive index of the less dense medium be n1, the refractive index of the denser medium be n2 (n2 > n1), and the critical angle for total reflection be ɸ, sinɸ=n1/n2.
Birefringence is the difference between heterogeneous gemstones’ maximum and minimum refractive indices. For gemstones with high birefringence, the design of the cut should ensure that the table is perpendicular to the optical axis. When viewed along the optical axis, the gemstone does not exhibit birefringence, preventing noticeable facet edge ghosting that could affect the gemstone’s appearance, as shown in Figures 1-52 and 1-53.

Figure 1-52 Olivine rough (left) and its finished product (right)

Figure 1-53 Faceted double refraction of olivine 6. Dispersion of gemstone minerals
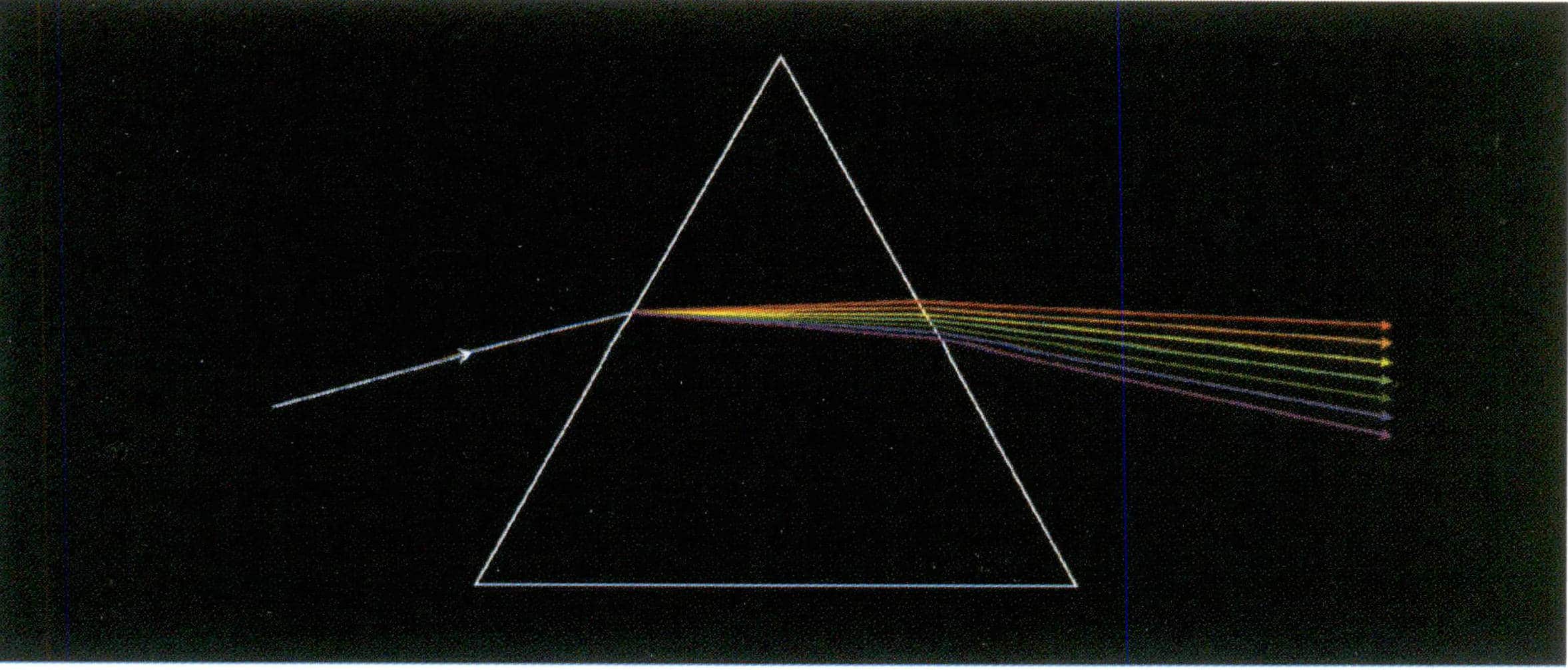
(6) Dispersion of gemstone minerals
The phenomenon where white light is decomposed into different wavelengths of colored light when passing through a material is called dispersion. For example, a white light beam is decomposed into constituent colors due to different refractive indices, as shown in Figure 1-54. Gemstones with high dispersion include spessartine 0.027, zircon 0.039, diamond 0.044, sphene 0.051, demantoid 0.057, and cubic zirconia 0.065.
Gemstones with high refractive indices and dispersion values, such as rubies, garnets, and olivines, are often designed in bright-cut styles to showcase their brightness and fire. Those with lower refractive indices or dispersion values are often designed in step-cut styles highlighting the gemstone’s color, such as emeralds and aquamarines.
(7) Other physical properties of gemstones
① Thermal Conductivity
Thermal conductivity refers to the ability of a material to conduct heat. Metals have the strongest thermal conductivity, followed by crystals, while amorphous materials have the poorest thermal conductivity. For example, gold has strong thermal conductivity, and touching it feels cool, while plastic has poor thermal conductivity, and touching it feels warm. Among gemstone crystals, diamonds have the best thermal conductivity; therefore, a thermal conductivity meter was invented to distinguish diamonds from other similar gemstones.
② Electrical Conductivity
Electrical conductivity refers to the ability of a material to conduct electric charge. Generally, metals have higher electrical conductivity than non-metals. Among common gemstones, natural blue diamonds are semiconductors, while irradiated blue diamonds do not conduct electricity, which can help identification. At the same time, semiconductors can be used to develop electronic components, such as type IIb diamonds (diamonds), which can be used as semiconductors.
③ Piezoelectricity
Piezoelectricity refers to the property of a material that generates an electric charge when subjected to external force. Minerals with piezoelectric properties can be applied in radio technology and quartz electronics, such as quartz crystals.
④ Thermoelectricity
Thermoelectricity refers to the property of a material that generates an electric charge when heated. For example, tourmaline has thermoelectric properties.
⑤ Electrostatics
Electrostatics refers to the property of a material that generates static electric charge when subjected to friction. For example, amber and plastic have electrostatic properties.
⑥ Magnetism
The presence of metal elements such as iron, cobalt, and nickel in gemstone minerals mainly causes magnetism. For example, a significant amount of magnetite inclusions in labradorite can assist in identification.
Section VI Gemstone Testing Instruments
1. Gemstone 10x Magnifier
(1) Structure of the Gemstone 10x Magnifier
The commonly used gemstone 10x magnifier is a three-component lens consisting of three parts: an upper and a lower concave-convex lens and a middle biconvex lens, as shown in Figure 1-55.

Figure 1-55 The physical object of the gemstone 10x magnifier and its optical structure
(2) How to use the gemstone 10x magnifier
- Clean the specimen.
- Hold the magnifier close to your eyes, keeping both eyes open to avoid fatigue in a short time.
- Use gem tweezers to pick up the sample and lean it against the hand holding the magnifying glass, observing at a distance of about 2.5 cm from it.
- First, observe the external and internal features of the gem as a whole, then focus on specific observations.
(3) The use of a 10x magnifying glass for gems
A 10x magnifying glass can be used to observe gems’ internal and external features, such as the distribution of inclusions, color bands, growth lines, cleavage, and processing quality.
(4) Środki ostrożności
- The specimen must be cleaned before use to avoid mistaking surface stains and dust for surface features.
- Observing the specimen from multiple angles is necessary to comprehensively observe various phenomena.
- When using a gem magnifier, achieving the “three supports” is important: elbows on the table, hands together, and the hand holding the magnifier against the cheek to ensure maximum stability.
- Glass lenses have a relatively low hardness and should be promptly retracted and covered with a protective case after use.
2. Gem Microscope
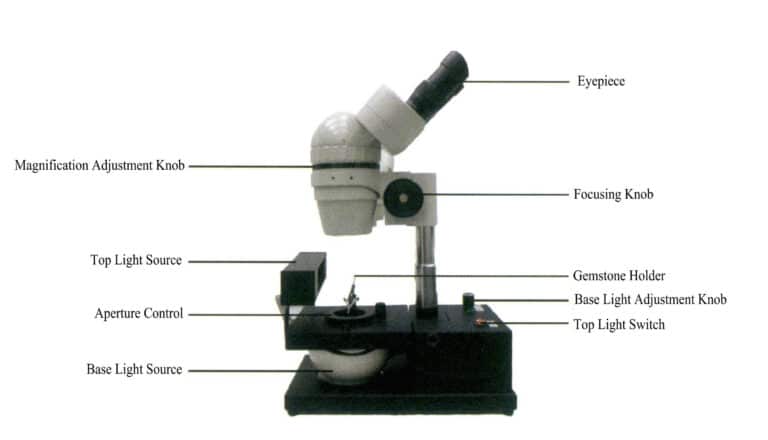
(1) Structure of the gem microscope (Figure 1-56)
Optical system: includes eyepiece system, objective system, zoom system, etc.
Lighting system: includes a bottom light source, top light source, power switch, light intensity adjustment knob, etc.
Mechanical system: includes bracket, base, focal length adjustment knob, aperture lock, gem holder, etc.
(2) Method of using a gem microscope
- Clean the specimen and place it on the gem clip.
- Adjust the lens to the lowest position and turn on the microscope illumination light.
- Adjust the eyepiece according to the interpupillary distance; the field of view will become a complete circle, indicating that the adjustment is complete.
- First, adjust the focal length to make the field of view of the fixed-focus eyepiece clear, then adjust the focal length of the variable-focus eyepiece to make the field of view clear, and finally adjust the focus knob for focusing.
- Choose the appropriate lighting method as needed, first observe the overall condition of the specimen, and then continue to increase the objective magnification for local observation.
- After observing, neatly store the gemstones, reset the microscope, and put on the cover.
(3) The illumination methods of gemstone microscopes
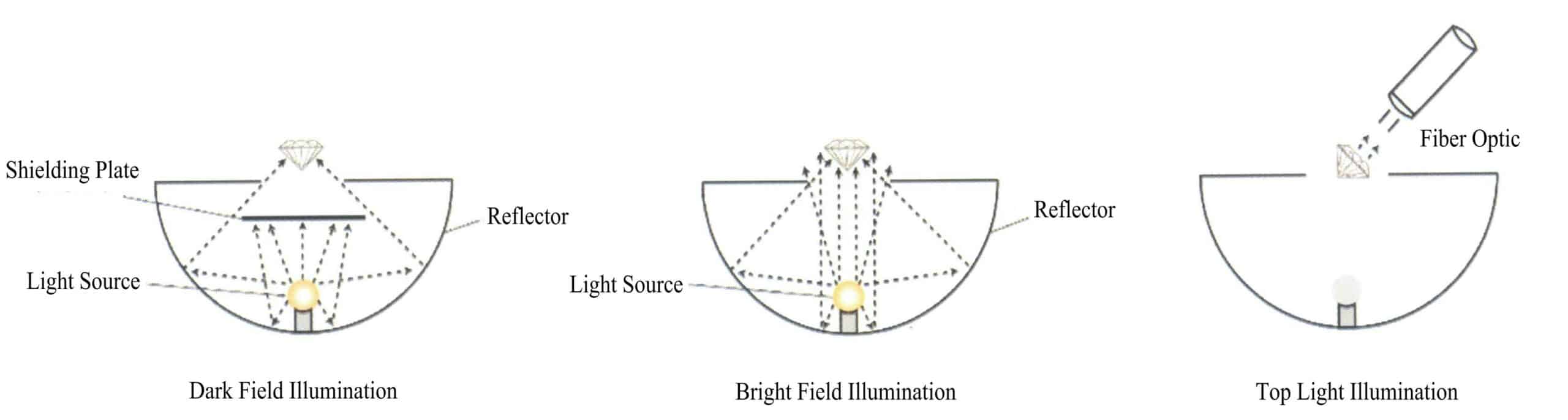
The main illumination methods for gemstone microscopes include reflected illumination, dark field illumination, and bright field illumination. Reflected illumination uses a top light source and is mainly used to observe the external features of gemstones. Darkfield illumination uses a bottom light source along with a black shield, primarily for observing the internal features of gemstones. Bright-field illumination uses the built-in bottom light source of the microscope and removes the shield, which is used for observing internal inclusions or growth lines in darker gemstones. In addition to the abovementioned methods, scattering light illumination, point light illumination, horizontal illumination, masking illumination, and polarized light illumination are also used, as shown in Figure 1-57.
(4) The uses of gemstone microscopes
Using a gem microscope, one can comprehensively observe the internal and external features of gem materials, including cracks, inclusions, color bands, and growth lines.
(5) Precautions
- When using the microscope, handle the mechanical parts gently.
- Do not touch the eyepiece or objective lens with your hands; use specialized lens paper for cleaning.
- After using the microscope, adjust the brightness of the light source to the lowest setting and turn off the power.
- After use, the objective lens tube should be promptly adjusted to the lowest position to prevent the adjustment knob from loosening.
3. Refractometer
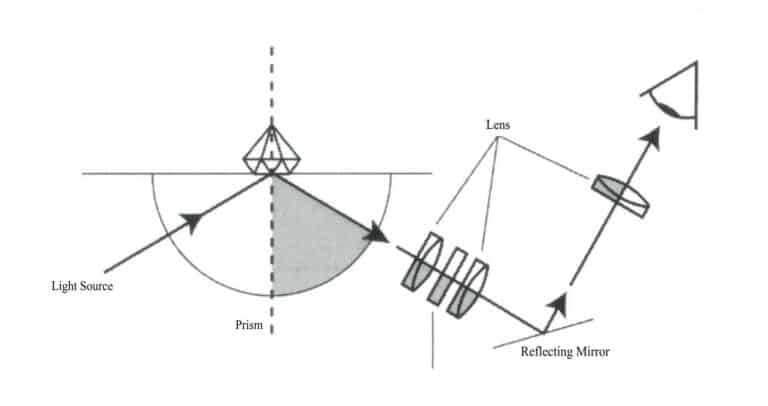
(1) Principle of the Refractometer
The principle of the gem refractometer is based on the law of refraction and the principle of total internal reflection, as shown in Figure 1-58.
(2) Structure of the Refractometer
The gem refractometer mainly consists of a high refractive index prism, mirrors, lenses, polarizers, light sources, and scales, as shown in Figure 1-59. Currently, most refractometer prisms on the market are made of lead glass, and the light source generally uses yellow light with a wavelength of 589.5nm. Since there is a thin layer of air film between the gem and the prism, a contact liquid (refractive oil) is needed to ensure good optical contact between the two.

(3) Method of Using the Refractometer
Depending on the specific situation of the gem, either the near-sighted method or the far-sighted method can be chosen. Generally speaking, faceted gems are mostly measured using the near-sighted method, while small facets or curved gems are mostly measured using the far-sighted method.
① Myopia method
- Clean the specimen and the testing platform.
- Turn on the power and drop the refractive oil in the center of the prism testing platform, with a droplet diameter of approximately 1 〜2 mm.
- Select the largest facet that has been polished, and gently push it onto the oil droplet in the center of the prism testing platform.
- Bring your eyes close to the eyepiece, rotate the gem, observe the movement of the shadow line up and down, and read and record the measurements.
- After testing is completed, specimens and the testing platform should be cleaned promptly, specimens should be collected, and the power should be turned off.
② Hyperopia method
- Clean the specimens and the testing platform.
- Turn on the power and drop an appropriate amount of refractive oil onto the metal surface near the testing platform.
- Place the gemstone with the curved surface facing down so that the curved surface of the gemstone contacts the appropriate amount of refractive oil.
- Place the gem with an appropriate amount of refractive oil in the center of the testing table.
- Move your eyes back and forth to observe the outline of the gem.
- Move your eyes up and down to observe the changes in light and dark within the outline of the gem, and record the readings at the boundary where it is half light and half dark.
- After testing, promptly clean the specimen and testing table, recover the specimen, and turn off the power.
(4) Purpose of the refractometer
It can test the refractive index, birefringence, axial properties, and optical properties of gemstones.
(5) Precautions
- The gemstone should have a good polished surface; if the bottom surface of a curved gemstone is well polished, the facet method can be used for testing.
- Organic gemstones and porous gemstones should not be tested for refractive index using a refractometer.
- Clean the testing table and the gemstone before testing.
- To obtain accurate values of the double refraction index, several facets need to be measured.
- Pay attention to distinguishing between the refractive index of gemstones and the refractive index of refractive oil.
- Be careful to protect the refractometer testing platform to avoid scratches from gemstones or tweezers that could affect the lifespan of the testing platform. The accuracy of the test results depends on various factors, such as the polishing condition of the gemstone, the amount of refractive oil used, and the precision of the refractometer itself.
- After the test, promptly wipe off any residual contact liquid on the testing platform to prevent corrosion.
4. Polarizing Filter
(1) The Principle of Polarizers
When natural light passes through the lower polarizer, it produces polarized light parallel to the lower polarizer. When the vibration directions of the upper and lower polarizers are parallel, the view is brightest; when the vibration directions are perpendicular, the view is darkest, as shown in Figure 1-60.

(2) The Structure of Polarizers
The main structure of the polarizer includes the upper polarizer, lower polarizer, gem stage, and light source, as shown in Figure 1-61.
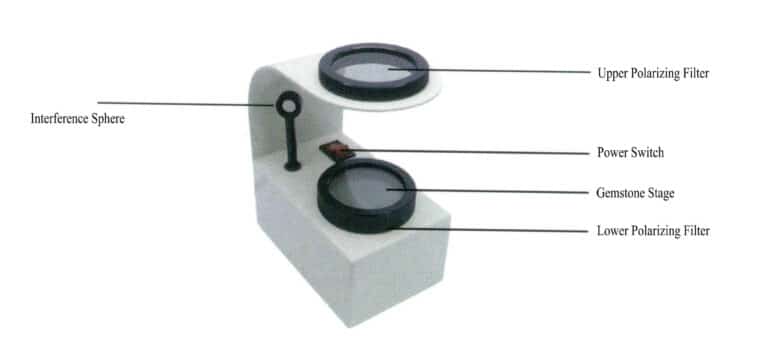
(3) How to Use a Polarizer
- Clean the gemstone to be tested.
- Turn on the light source, rotate the upper polarizer to make the vertical and horizontal polarized light perpendicular, and observe the field of view from above to find the darkest point.
- Place the gemstone to be tested on the stage.
- Rotate the gemstone (stage) 360°, observe the changes in brightness of the gemstone, record and conclude the phenomena observed with the polarizing microscope, and conclusions are shown in Table 1-7.
- Protect the gemstone to be tested and turn off the power.
Table 1-7 Phenomena observed with the polarizing microscope and conclusions
| Operation | Phenomenon | Conclusion |
|---|---|---|
| Under crossed polarizers, rotate the gemstone 360° | Four bright and four dark | Optical heterogeneous body |
| Under crossed polarizers, rotate the gemstone 360° | Completely dark/abnormal extinction | Optical Homogeneous Body |
| Rotating the Gemstone under Orthogonal Polarized Light 360° | Fully Bright | Optical heterogeneous aggregate |
(4) Uses of Polarizing Filter
Using a gem polarizing filter, you can test the optical characteristics and axial properties and observe the gem’s pleochroism.
(5) Precautions
- Gems that are opaque, too small, or have many cracks or inclusions are unsuitable for testing.
- During testing, the gem should be observed from several directions to avoid affecting the conclusion.
5. Electronic Balance
The principle of using an electronic balance to test the relative density of gemstones
(1) The principle of using an electronic balance to test the relative density of gemstones is Archimedes’ principle.
Relative density (d)≈ the mass of the gemstone in air / (the mass of the gemstone in air – the mass of the gemstone in water).
(2) Structure of the electronic balance
The electronic balance consists of a weighing pan, leveling feet, and a display, as shown in Figure 1-62.

(3) Method of Using the Electronic Balance
① Method of Measuring Mass
- Adjust the leveling feet so that the bubble in the level is centered in the ring.
- Use tweezers to place the gem on the scale pan, wait for the data to stabilize, then read and record the measurement.
- Weighing completed, put away the gemstones, and turn off the instrument.
② Testing relative density using the clean water weighing method.
- Clean the gemstone to be tested.
- Turn on the electronic balance and calibrate it to zero.
- Place the gemstone on the scale and record its mass G空 in the air.
- Use tweezers to remove the gem and adjust the balance to zero.
- Gently place the gem into the metal basket with tweezers, ensuring that both the gem and the metal basket are completely submerged in water, and measure the mass of the gem in water G水.
- Substitute the measured value into the formulaSG≈G空/ (G空 – G水), to obtain the relative density of the gem.
- Take out the gem, dry it, store it, and turn off the power.
(4) Uses of electronic balances
The commonly used electronic balance can read accurately to the fourth decimal place, and it is mainly used for weighing gemstones and determining relative density.
(5) Precautions
- Porous gemstones with many cracks or are too small (less than 0.3 ct) should not be tested for relative density using the clean water weighing method.
- Air bubbles must be eliminated when the metal scoop and the gemstone to be tested are submerged in water.
- The electronic balance should be placed on a stable surface, with doors and windows closed to avoid interference.
6. Dichroscope
(1) The Principle of the Dichroscope
When natural light enters a heterogeneous gemstone, it splits into two beams of polarized light with perpendicular vibration and different propagation directions. The heterogeneous gemstone absorbs light differently based on the vibration direction, separating these two types of light, which may reveal different colors. Only colored, transparent (light-permeable) heterogeneous gemstones can exhibit pleochroism.
(2) The Structure of the Dichroscope
The dichroscope mainly comprises an objective lens, calcite, and an eyepiece, as shown in Figures 1-63 and 1-64.
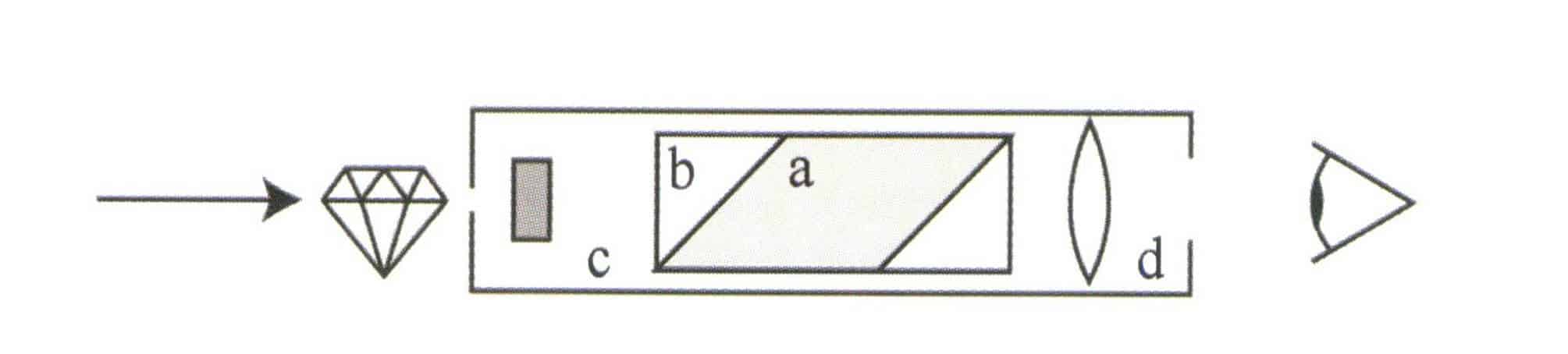

(3) How to use the dichroscope
- Transmit white light through the gem sample.
- Place the dichroscope close to the gemstone to ensure that the light entering the dichroscope is transmitted light.
- Bring your eyes close to the dichroscope and observe the color differences in the two windows of the dichroscope while rotating it.
- Record and analyze the results.
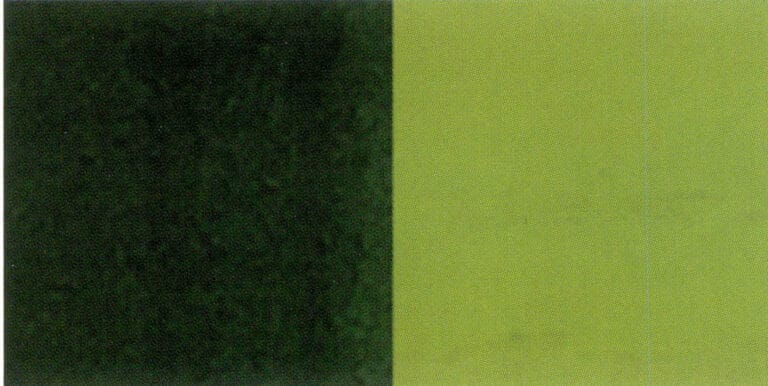
(4) Uses of the dichroscope
Observe the pleochroism of the gemstone, as shown in Figure 1-65.
(5) Precautions
- Only colored and transparent gemstones can exhibit pleochroism.
- Observations should be made from multiple directions.
- Refrain from hastily concluding on gemstones with weak pleochroism; other methods should be used for verification.
- Avoid mistaking uneven color distribution in gemstones for pleochroism.
7. Ultraviolet Fluorescent Lamp
(1) Principle of Ultraviolet Fluorescent Lamp
The ultraviolet fluorescent lamp can emit long-wave ultraviolet light with a main wavelength of 365nm and short-wave ultraviolet light with a wavelength of 253.7nm, allowing observation of the luminescent characteristics of gemstones under long-wave and short-wave ultraviolet light.
(2) Structure of Ultraviolet Fluorescent Lamp
The ultraviolet fluorescent lamp mainly consists of long-wave and short-wave ultraviolet light sources, a dark box, and a power switch, as shown in Figure 1-66.
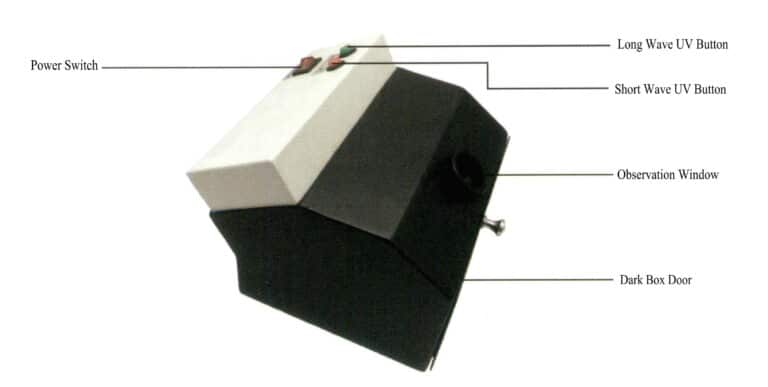
(3) How to Use the Ultraviolet Fluorescent Lamp
- Clean the gemstone to be tested, place it under the ultraviolet fluorescent lamp, and close the dark box.
- Turn on the light source, select long-wave or short-wave ultraviolet light, and observe the gemstone’s luminescent characteristics.
- Record the phenomena, mainly the fluorescence’s intensity, color, and location.
(4) Uses of ultraviolet fluorescent lamps
Observing the luminescent characteristics of gemstones can assist in identifying the variety, origin, and whether they have been treated or optimized.
(5) Precautions
- Short-wave ultraviolet light can cause harm to the eyes, and in severe cases, it can lead to blindness; direct viewing of ultraviolet fluorescent lamps should be avoided.
- Short-wave ultraviolet light can cause harm to the skin; it is prohibited to place hands directly under the ultraviolet fluorescent lamp during operation.
- Attention should be paid to distinguishing between purple fluorescence and the illusion of purple fluorescence. Purple fluorescence is the light emitted by the gemstone, while the illusion of purple fluorescence is the gemstone’s reflection of ultraviolet light.
8. Diamond Thermal Conductivity Meter
(1) Principle of the Diamond Thermal Conductivity Meter
The diamond thermal conductivity meter is designed based on diamonds’ extremely high thermal conductivity, serving as an instrument to quickly differentiate diamonds from similar gemstones.
(2) Structure of the Diamond Thermal Conductivity Meter
The diamond thermal conductivity meter mainly consists of metal contacts, a display screen, and a power switch, as shown in Figure 1-67.

(3) How to Use the Diamond Thermal Conductivity Meter
- Clean and dry the gemstone to be tested and place it in the appropriate position on the metal plate.
- Turn on the thermal conductivity meter switch, adjust to the appropriate mode based on room temperature and gemstone size, and preheat.
- Hold the detector, touch the metal plate with your fingers, align it at a right angle to the test gem, apply a certain amount of pressure, and the instrument will display light and sound signals to obtain the test results.
- When the thermal conductivity instrument gives a sound prompt in the diamond zone, the test sample may be diamond or synthetic silicon carbide, which can be further distinguished using a magnifying glass. Diamonds are homogeneous without facet edge ghosting, while synthetic silicon carbide has obvious facet edge ghosting.
(4) Uses of the diamond thermal conductivity instrument
The diamond thermal conductivity instrument can quickly distinguish diamonds from similar gemstones.
(5) Precautions
- During the testing process, care should be taken to protect the metal contacts and the protective cover should be put on immediately after use.
- The battery should be replaced promptly with low power to avoid affecting the test results.
9. Introduction to Large Instruments
(1) Fourier Transform Infrared Spectrometer
Fourier transform infrared spectrometer is the use of infrared light waves irradiation gem material so that the material vibration energy level jumps, absorption of the corresponding infrared light and the resulting spectrum, to carry out the instrument’s material analysis. The testing methods include transmission and reflection, providing convenient, accurate, and non-destructive testing.
In gemology, the differences in infrared spectra can be used to identify gem varieties. It can detect artificial materials in gems, thus identifying whether there is any filling treatment, such as epoxy resin in jadeite C grade. It can distinguish between natural and synthetic crystals by testing the hydroxyl and water molecules in gems. The presence of impurity atoms in diamonds can be tested to classify diamond types, as shown in Figures 168 and 1-69.

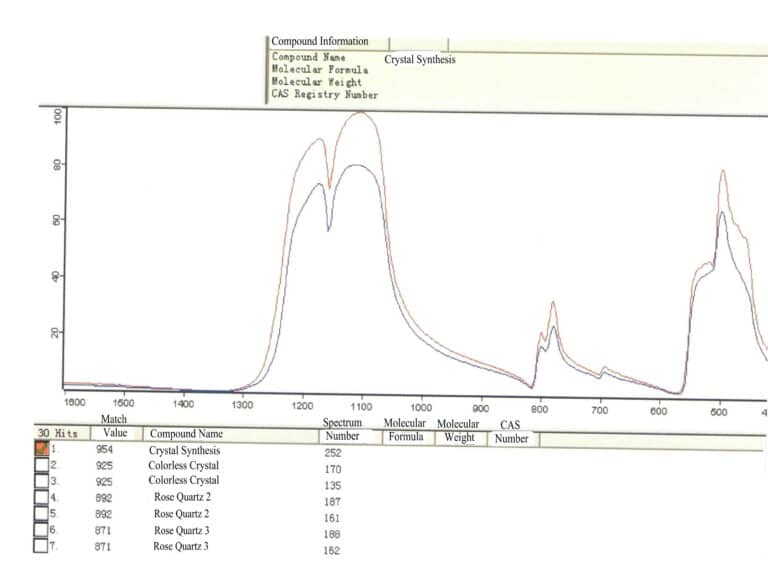
(2) Laser Raman Spectrometer
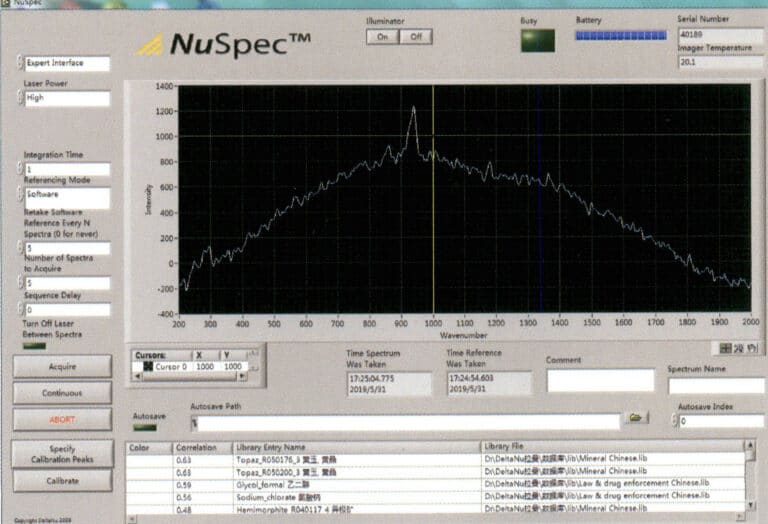
The laser Raman spectrometer is an instrument that analyzes materials by utilizing the inelastic collision between laser photons and material molecules, producing molecular scattering spectra. It features high resolution, sensitivity, and fast non-destructive analysis.
Gemology can detect the composition of inclusions in gemstones, especially by studying single fluid inclusions of size 1 μm and various solid mineral inclusions within the gemstone to analyze their genesis types. It can detect filling materials in gemstones and distinguish dyed black pearls (rich in silver) from seawater-cultured black pearls. Gem species can be identified based on spectra, as shown in Figures 1-70 and 1-71.
(3) Ultraviolet-Visible Spectrophotometer
The UV-visible spectrophotometer is an instrument that uses ultraviolet-visible electromagnetic waves to irradiate materials, causing electronic transitions between energy levels and producing absorption spectra for material analysis, as shown in Figure 1-72.
In gemology, gemstones can be identified based on the characteristics of their absorption spectra. It can detect artificially treated gemstones, such as natural blue diamonds and irradiated blue diamonds; it can distinguish between some natural gemstones and synthetic gemstones, such as natural red beryl and synthetic red beryl; it can also study the coloring mechanisms of gemstones.
(4) Cathodoluminescence instrument
The cathodoluminescence instrument uses a cathode ray tube to emit high-energy electron beams, exciting the surface of gemstone materials to make them luminescent. It also conducts material research based on the luminescent characteristics.
In gemology, natural and synthetic rubies, natural and synthetic diamonds, natural jade, and treated jade can be classified based on the luminescent characteristics of the gems, as shown in Figure 1-73.
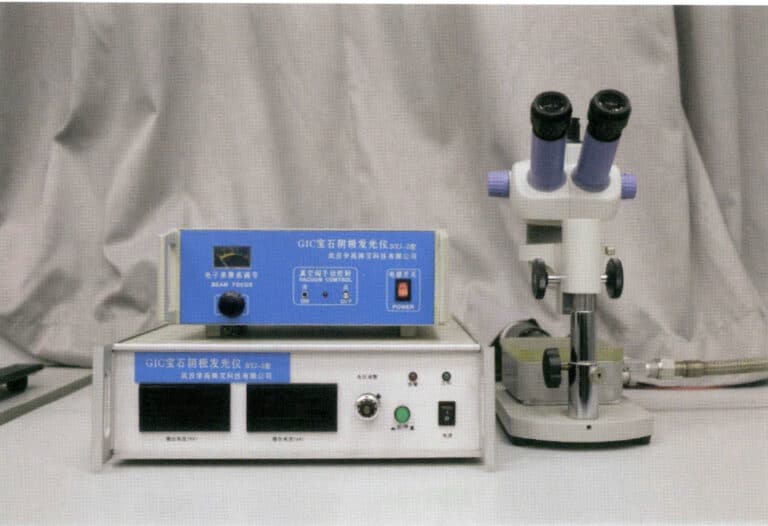
(5) Gem Proportion Analyzer
The gem proportion analyzer is a conventional instrument for measuring gem proportions, which measures the proportions and main symmetry deviations of finished gems through the relationship between the projected image and the standard graphics and scale on the screen, as shown in Figures 1-74 and 1-75.

















Jedna odpowiedź
Merci pour la qualité de l’information