Amit tudnia kell a fém előolvasztásról az ékszeröntési folyamatban
Techniques and Skills for Ingredient mixing, Torch melting, and Induction melting
The alloy materials for jewelry are made by mixing pure metals and intermediate alloys according to the required composition. When pure metals and intermediate alloys are directly melted and poured, it is easy to produce problems such as uneven composition, severe loss, and defects like holes. They must be pre-melted in production to create a uniform composition suitable for furnace charging. Common methods for pre-melting jewelry alloys include torch melting and induction melting. The melted metal is made into ingots or beads, decomposing the ingots for jewelry casting materials, while the beads can be used directly.
This project enables you to master the basic principles and operational skills of ingredient mixing, torch melting, and induction melting through three typical and post-class extension tasks.

Torches for melting
Tartalomjegyzék
I. szakasz Anyag előkészítése
1. Háttérismeretek
1.1 Types of Jewelry metal materials
Traditional jewelry materials mainly consist of precious metals such as gold, silver, platinum, and their alloys. Precious metals refer to valuable metals among colored metals that have high density, low production, and high prices, which include gold (Au), silver (Ag), ruthenium (Ru), rhodium (Rh), palladium (Pd), osmium (Os), iridium (Ir), and platinum (Pt).
With the improvement of living standards, jewelry has shifted from focusing on value preservation and appreciation to emphasizing fashion and decorative functions. The categories of jewelry alloy materials have become increasingly diverse, with non-precious metal materials such as copper, stainless steel, palladium, cobalt, and zinc alloys being widely used in jewelry production.
(1) Gold and its Alloys
Gold has a beautiful color, good chemical stability, and possesses great aesthetic and collectible value, as well as value preservation and appreciation functions. It also has excellent ductility and has been used as decorative and currency materials for jewelry, crafts, and commemorative coins since ancient times. The melting point of gold is 1063 ℃, and its density at room temperature is 19.3 g/cm3, giving it a noticeable weighty feel.
The quality of gold refers to the purity of gold, that is, the minimum quality content of gold. Traditionally, there are three methods to express the quality of gold: the percentage method, the thousandth method, and the K number method. The percentage method expresses the gold content in percentage (%); the thousandth method expresses the gold content in parts per thousand (‰); the K number method comes from the English word karat, which is the internationally recognized unit symbol for calculating the purity or quality of gold, abbreviated as K.
The quality of gold is divided into 24 parts, with the highest purity being pure gold at 24 K and the lowest purity at 1 K. Theoretically, the purity of pure gold is 100%, which can be calculated from 24K=100% to get 1K=4.16666666······%. Since the percentage value of 1 K is an infinitely repeating decimal, different countries and regions have slightly different regulations on the value of 1 K.
According to the quality of gold, jewelry gold can be roughly divided into two categories: pure gold and K gold. Currently, in terms of gold content, there are mainly three types of materials used to make pure gold jewelry in the Chinese market: “four-nine gold,” with a quality of 99.99%, which is 24 K gold; “three-nine gold,” with a quality of 99.9%, commonly known as “999 gold”; and “two-nine gold,” with a quality of 99%, commonly known as “nine-nine gold” or “pure gold.”
Pure gold has low strength and hardness, so adding a certain proportion of intermediate alloys to pure gold to form K gold of the corresponding quality can increase the strength and toughness of gold, making K gold a popular choice for jewelry internationally. These intermediate alloys added to pure gold or other precious metals are commonly known as “filler material,” and various types are available in the market. Figure 6-1 shows the appearance of several typical jewelry gold materials.

Table 6-1 Common Gold Grades for Jewelry in Different Countries and Regions
| Country or Region | Common Gold Grade | Corresponding minimum gold content |
|---|---|---|
| China | 24K gold, 18K | 24K gold:99.9%;18K:75.0% |
| India | 22K | 91.6% |
| Arab countries | 21K | 87.5% |
| United Kingdom | Mainly 9K, with a small amount of 22K and 18K | 9K:37.5%;22K:91.6%;18K:75.0% |
| Németország | 8K,14K | 8K:33.3%;14K:58.5% |
| Egyesült Államok | 14K,18K | 14K:58.5%;18K:75.0% |
| Italy, France | 18K | 75.0% |
| Russia | 9K〜18K | 37.5%〜75.0% |
| Egyesült Államok | 10K〜18K | 41.6%〜75.0% |
(2) Silver and its Alloys
Silver is widely used in jewelry production, with visible light reflectivity of 94%, making it the highest among all metallic elements. Silver has a melting point of 960.8℃ and density of 10.49 g/cm3 at room temperature.
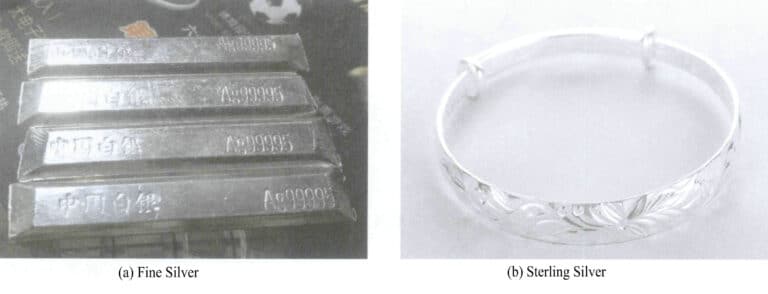
Jewelry-grade silver is classified into two main categories based on purity: fine silver and sterling silver. The former has silver content of above 99%. In contrast, the latter has several typical purities, the most widely used being 925 silver, which has a certain hardness and ductility, making it suitable for making rings, necklaces, brooches, hairpins, and other jewelry, and is conducive to gemstone setting. Additionally, 950 silver and 980 silver are sometimes also used. The typical appearance of pure silver and silver alloys for jewelry is shown in Figure 6-2.
(3) Platinum and its Alloys
The melting point of platinum is 1768.3℃, and its density at room temperature is 21.45g/cm3, which is higher than that of gold, about twice that of silver, giving it a noticeable weighty feel. Platinum has a high reflectivity across the entire visible light spectrum, and the reflectivity gradually increases with wavelength, thus appearing grayish-white.
Platinum can adsorb gases, especially hydrogen. The ability of platinum to adsorb hydrogen is related to its physical state; platinum black (a very fine powder of metallic platinum) can adsorb hydrogen equivalent to 502 times its volume.
Platinum has excellent oxidation resistance and corrosion resistance. Hydrochloric acid, nitric acid, sulfuric acid, and organic acids do not react with platinum at room temperature. Carbon can dissolve in platinum at high temperatures, and the solubility increases with temperature. Carbon precipitates upon cooling, making platinum brittle, a phenomenon known as carbon poisoning. Therefore, when melting platinum, graphite crucibles cannot be used; corundum or lead oxide crucibles are typically used, and melting is done under vacuum or inert gas protection.
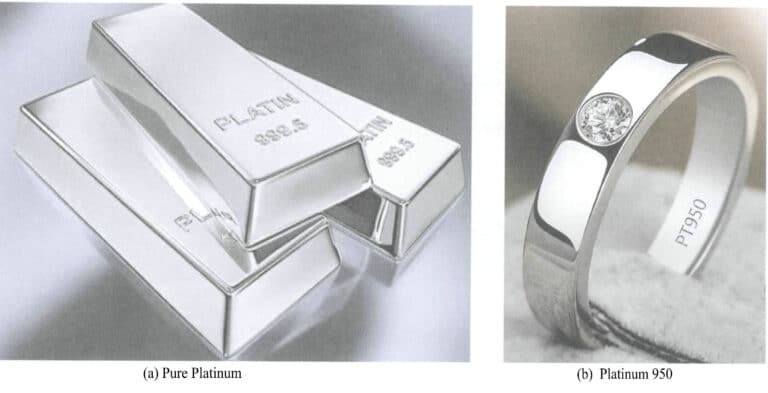
Platinum jewelry can be divided into two categories: pure platinum jewelry without gemstones and platinum alloy jewelry with gemstones. Pure platinum is soft, and due to material strength limitations, gemstones are usually not set when making jewelry. Adding alloying elements to platinum can enhance its strength. Many metallic elements are used for platinum alloying, and the strengthening effects of different alloying elements on platinum vary significantly. The amount of the same alloying element added can also lead to varying strengthening effects. The typical appearance of pure platinum and platinum alloy jewelry is shown in Figure 6-3.
(4) Copper and its Alloys
In popular jewelry, especially in imitation jewelry and many craft pieces, copper and copper alloy materials are used for production.
Pure copper is a rose-red metal. After forming an oxide film on its surface, it appears purplish-red; hence, it is called purple copper. Its density is 8.9 g/cm3, and its melting point is 1083℃.The characteristics of pure copper include low hardness, excellent plasticity, and the ability to withstand various forms of hot and cold pressure processing, forming wires, pipes, bars, and sheets. The tensile strength of pure copper is relatively low, making it unsuitable as a structural material. It has poor casting performance and easily absorbs gases such as carbon monoxide and sulfur dioxide when melted, forming gas holes.
There are many categories of copper alloys. For the copper alloys used in current jewelry, there are no specific technical standards domestically or internationally. Industrial copper alloy grades are typically used, and the application is quite chaotic, affecting product quality. Therefore, copper alloys for jewelry need further standardization. The main copper alloys used for jewelry are brass, nickel, silver, and bronze, with typical appearances shown in Figure 6-4.

Brass is a copper-based alloy with zinc as the main alloying element, named for its common yellow color. Brass has an attractive color, good craftsmanship, and mechanical properties. It is corrosion-resistant in the atmosphere, freshwater, and seawater, easy to cut and polish, has good weldability, and is inexpensive, making it widely used in the jewelry industry. Based on the composition of brass can be divided into two main categories: simple brass and special brass. Simple brass is a binary alloy composed of copper and zinc. Special brass is a multi-element alloy formed by adding elements such as tin, aluminum, silicon, iron, manganese, and nickel to improve the performance of simple brass. It is named according to the added elements, such as tin brass, aluminum brass, manganese brass, and aluminum-manganese brass.
Brass is generally represented by the letter H, and the number following H indicates the copper content of the alloy. For example, H68 indicates the brass with a copper content of 68%. ZH represents brass used for casting. Among them, H62 and H68 brass have high plasticity and strength, good formability, and beautiful color, resembling 24K gold, making them the main varieties of brass for jewelry. The performance of brass is closely related to the zinc content; as the zinc content increases, its color gradually changes from purplish-red to yellow, golden yellow, and white. Generally, brass has a smaller solidification range, so the fluidity of the liquid metal is good, with excellent filling ability and a small tendency for shrinkage. During smelting, zinc generates a large vapor pressure, effectively removing gases from the copper liquid and making it difficult for brass to produce pores. The melting temperature of brass is lower than that of tin bronze, making casting more convenient; it can easily cast small jewelry pieces and is also commonly used for casting copper crafts.
White copper is named for its gray-white color, which is achieved by adding alloying elements that produce a bleaching effect to copper. The invention of white copper is an outstanding achievement in ancient Chinese metallurgy. The people of Yunnan invented and produced white copper, making it one of the earliest in the world, recognized by both domestic and international academic circles. The white copper produced in ancient Yunnan was also the most famous, known as “Yun white copper.” According to research, as early as the Qin and Han dynasties, white copper coins were cast in the Daxia Kingdom to the west of Xinjiang, with a nickel content of 20%. During the Tang and Song dynasties, Chinese white copper was already being exported to the Arab region, where Persians referred to white copper as “Chinese stone.” After the 16th century, Chinese white copper was sold worldwide, receiving widespread acclaim; it was exported from Guangzhou and sold in Europe by the British East India Company. The term “paktong” is a transliteration of the word “white copper” in the Cantonese, referring to the copper-nickel alloy produced in Yunnan. In the 17th and 18th centuries, white copper was widely introduced to Europe and regarded as a precious item, referred to as “Chinese silver” or “Chinese white copper,” which significantly impacted modern Western chemical processes. In 1823, the Heninger brothers in Germany successfully replicated Yunnan white copper. Subsequently, the west began large-scale industrial production and renamed this alloy “German silver” or “nickel silver.”
According to chemical composition, white copper can be divided into two main categories: simple white copper and complex white copper. Ordinary white copper is a binary alloy formed with nickel as the alloying element, where copper and nickel can infinitely solidify together, forming a continuous solid solution, meaning that regardless of their proportions, it is always a α single-phase alloy. When nickel is melted into purple copper and the content exceeds 16%, the resulting alloy becomes pure white like silver; the higher the nickel content, the whiter the color. Pure copper combined with nickel can significantly improve the metal’s strength, corrosion resistance, and hardness. Ordinary white copper is generally represented by the letter B, with the following number indicating the nickel content, such as B3\0 indicating a copper-nickel alloy containing 30% Ni. Models include B0.6, B19, B25, B30, etc. Complex white copper is made by adding alloying elements such as manganese, iron, zinc, and aluminum to ordinary white copper, represented by the letter B, and the alloying elements, such as BMn3-12, indicating a copper-nickel-manganese alloy containing 3% Ni and 12% Mn. Complex white copper includes categories such as iron white copper, manganese white copper, zinc white copper, and aluminum white copper.
White copper is a very good decorative material, widely used in the jewelry industry, often used to make imitation silver and imitation platinum jewelry. Due to the sensitization risk of nickel to human skin, there is a need to develop nickel-free white copper. Researchers have utilized manganese’s ability to bleach or fade copper and fully leveraged its advantages in enhancing alloy brightness, reducing redness, and improving casting performance, developing a series of multi-element nickel-free white Cu-Mn-Zn alloys, which are silver-white and have good hot and cold processing performance.
Bronze is a general term for copper alloys other than brass and white copper, and it is divided into two main categories: ordinary bronze and special bronze. Ordinary bronze is a binary alloy of copper and tin, also known as tin bronze. Its main characteristics include good wear resistance, high corrosion resistance (but poor acid resistance), sufficient tensile strength, and a certain degree of plasticity with a relatively low density. The grade of bronze is represented by the initial “Q” of the Chinese pinyin “qing”, plus the tin element and a number, such as QSn6.5-0.4, which indicates a tin content of 6.5% and a phosphorus content of 0.4% in the bronze.
Tin bronze is a great invention in human history; it is an alloy of pure copper with tin and lead and the earliest alloy in the history of metal casting. The solidification temperature range of tin bronze is quite large, up to 146%. Although its fluidity is not ideal, good filling performance can be achieved if the pouring temperature is well controlled. The liquid metal of tin bronze has a low tendency to oxidize, and the casting process is simple. Thin-walled castings use a vertical top-pouring method, and even with a large pouring drop, there are fewer oxidized impurities inside the castings. The shrinkage rate of tin bronze is smaller than that of brass, which prevents significant shrinkage deformation, thus ensuring the shape and dimensional accuracy of the castings. The solidification of tin bronze liquid metal follows a paste-like solidification method, which generally does not cause concentrated shrinkage cavities but may lead to dispersed shrinkage. The thicker the wall of the casting, the greater the tendency for shrinkage; conversely, the thinner the wall, the denser the metal structure, and the better the mechanical properties. Tin bronze tends to crack hot, so preventive measures against hot cracking must be taken in the casting process. The liquid metal of tin bronze tends to absorb gas, so the temperature and time of the alloy during the melting process must be controlled.
(5) Stainless Steel
Stainless steel is a general term for steel with certain chemical stability in solutions such as atmosphere, water, acids, alkalis, salts, or other corrosive media. Generally speaking, steel resistant to corrosion from weak media such as atmosphere, steam, and water is called stainless steel. In contrast, steel resistant to corrosion from acidic, alkaline, and saline corrosive media is called corrosion-resistant or acid-resistant steel. Stainless steel has rust resistance but is not necessarily corrosion-resistant, while corrosion-resistant steel generally has good rust resistance.
Various elements mainly determine Stainless steel’s performance and microstructure. Currently, there are over 100 known chemical elements, among which the elements that significantly affect the performance and structure of stainless steel include carbon, chromium, nickel, manganese, nitrogen, sodium, niobium, molybdenum, copper, aluminum, silicon, titanium, zirconium, boron, and more than a dozen others. The addition of these elements causes changes in the internal structure of the steel, thereby giving the steel special properties. Stainless steel can be divided into three categories based on alloy composition: chromium stainless steel, chromium-nickel stainless steel, and chromium-manganese-nitrogen stainless steel. Based on microstructure (metallographic structure), it can be divided into ferritic stainless steel, martensitic stainless steel, austenitic stainless steel, and other duplex stainless steel.
The stainless steel used for jewelry includes several typical grades, such as 304, 304L, 316, and 316L. 304 stainless steel is a versatile stainless steel with a melting point of 1454℃ and a density of 8g/cm3. It is widely used for equipment and components that require good overall performance (corrosion resistance and formability). Its variant is low-carbon 304L stainless steel. 316 stainless steel has a melting point of 1398% and a density of 8g/cm3. Its resistance to localized corrosion in marine and chemical industrial environments is significantly better than 304 stainless steel. Among them, the variants of 316 stainless steel include low-carbon stainless steel 316L, nitrogen-containing high-strength stainless steel 316N, and sulfur-rich easy-to-cut stainless steel 316F. As a jewelry material, it is best to choose 316L stainless steel to ensure good corrosion resistance, as shown in Figure 6-5.

(6) Titanium Alloy
Iron has excellent properties such as low density, high specific strength, high temperature, and corrosion resistance. Iron alloys are good materials for making rocket engine casings, artificial satellites, and spacecraft, earning the nickname “space metal.” Due to its strong corrosion resistance and high stability, scandium does not cause allergic reactions after long-term contact with humans, and it is the only metal that does not affect human autonomic nerves and taste. It has unique medical applications and is called a “bio-friendly metal.” Thallium has a silver-gray tone, as shown in Figure 6-6. It performs well in mirror polishing, wire drawing, and sandblasting, making it one of the most suitable decorative metals besides precious metals, frequently used in modern jewelry design abroad.

The density of pure sodium is 4.51g/cm3, the melting point is 1668℃, and the boiling point is 3287℃. Due to sodium’s high melting point, it needs to be smelted at high temperatures, and its chemical properties become very reactive at high temperatures. Therefore, smelting must be conducted under inert gas protection, and using oxygen-containing materials must be avoided, which imposes high requirements on smelting equipment and processes.
1.2 The Color of Metal Materials
1.3 Electronic Scale
The quality of jewelry is generally very light. It involves gemstones and precious metals, so the instruments used for quality detection require high precision and must quickly and reliably obtain the desired results during production. Traditional mechanical weighing instruments cannot meet these requirements, and electronic balances are now used for weighing. Electronic scales utilize the principle of electromagnetic force, balancing the weight of an object. They connect the weighing pan to a powered coil in a magnetic field. When the object to be weighed is placed on the pan, the downward gravitational force generates an electromagnetic force in the coil that is equal in magnitude but opposite in direction to the weight of the object being weighed. At this point, the sensor outputs an electrical signal, which is rectified and amplified, changing the current in the coil until it returns to its original position. The strength of this current is proportional to the weight of the object being weighed. The weight, produced by the mass of the material, is then displayed after the generated electrical signal passes through the analog system. Compared to mechanical scales, electronic scales have the characteristics of fast weighing speed, high resolution, good reliability, simple operation, and diverse functions.
The precision of the scale used for weighing ingredients is generally 0.01 g, with a range determined by need, commonly 3200 g, as shown in Figure 6-7. When using an electronic scale, it should be placed on a stable workbench to avoid vibrations, air currents, and sunlight; before use, the bubble of the level should be adjusted to the center position; when weighing corrosive items, they should be placed in a sealed container to avoid corroding the electronic scale; do not overload the scale during weighing to prevent damage. The electronic scale should be periodically calibrated according to the metrology department’s regulations and managed and maintained by designated personnel to ensure it is in optimal condition. The main content of periodic calibration includes scale sensitivity and discrimination, maximum allowable error at each load point (weighing linear error), repeatability, eccentric load or corner error, balancing function, etc. After calibration, a calibration certificate or label should be issued based on the actual calibration results.

1.4 The Fineness and Control of Precious Metal Materials
For precious metal jewelry, the purity (i.e., fineness) of precious metals has always been a key concern for consumers. Different countries or regions have established standards for the fineness of precious metal jewelry, requiring that jewelry of a certain fineness guarantee the corresponding minimum content.
The purity of the materials for jewelry accessories should be consistent with the main body. Due to the strength and elasticity requirements, accessories with slightly lower purity are allowed. Still, it must meet the minimum requirements, such as gold jewelry with a fineness of no less than 22 K, platinum jewelry with a platinum content of no less than 950‰, and palladium jewelry with a palladium content of no less than 900‰. The gold, platinum, or palladium content in the accessories used must not be less than ; the silver content in sterling silver jewelry accessories must not be less than 925‰.
The content of gold, silver, platinum, and other precious metals in jewelry materials can be detected using chemical analysis methods or X-ray fluorescence spectroscopy (Figure 6-8). Chemical analysis is a destructive testing method with a longer cycle and relatively high precision; X-ray fluorescence spectroscopy is a non-destructive testing method that is convenient, quick, and widely used in quality control during jewelry production.

The basic principle of X-ray fluorescence analysis is similar to that of electron probes, which determines the wavelength (or energy) and intensity of the characteristic X-ray spectral lines emitted by the excited sample. X-ray fluorescence analysis is similar to this but differs from electron probes in that the incident light is X-ray. The irradiated sample absorbs the primary X-rays and is excited to emit secondary X-rays. Various secondary X-rays are referred to as X-ray fluorescence, and by determining the wavelength (or energy) and intensity of these characteristic spectral lines, the content of the elements can be determined.
Several methods are available for detecting impurity elements in precious metal jewelry materials. Generally, the material must first be dissolved and then analyzed using flame atomic absorption spectrometry, direct current plasma atomic emission spectrometry, inductively coupled plasma spectrometry, mass spectrometry, and other methods.
In jewelry production, in addition to detecting the overall average content of materials, it is sometimes necessary to use electronic probes, spectrometers, etc., to focus on a specific part of the sample for localized testing. For example, if a jewelry piece has defects such as fractures or hard spots in a certain area, probes can be used to analyze the composition of those areas. This is particularly significant in practice because many harmful impurity elements tend to segregate at grain boundaries, lattice distortion areas, etc., resulting in impurity element content in those areas being many times higher than the average content, which may lead to product quality issues.
1.5 Material Dividing Tools
Figure 6-9 Bolt cutters

Figure 6-10 Power press machine
2. Feladat végrehajtása
This task uses pure gold ingots and 18K rose gold for patching.
(1) Preparation of Raw Materials
Due to the large size of the pure gold ingot must be broken down into smaller pieces for accurate weighing and melting in the furnace. Large bolt cutters can cut the pure gold ingot into smaller pieces of 30mm×30mm, as shown in Figure 6-11. Hold the bolt cutters sideways during operation, with one end fixed on the ground and the other lifted to open the jaws. Place the gold ingot into the jaws, determine the cutting position, and apply downward force on the upper handle to cut. If it does not cut through in one go, the gold ingot can be flipped and cut again at the original cut position, repeating this process until it is cut through. Note that fingers should not be placed within the range of the jaws during operation.
A rolling mill can also be used to thin the thickness of the pure gold ingot, and then small bolt cutters can be used to cut the gold sheet into small pieces, as shown in Figure 6-12.

Figure 6-11 Cutting material with large bolt cutters

Figure 6-12 Cutting material with a rolling press
(2) Mixing Materials
For 18K rose gold, the minimum fineness is gold content of 75%. To avoid potential fluctuations in composition during production, which causes the risk of substandard quality, companies will establish internal control standards during the formulation process, mixing according to the gold content of 75.2%~76.0%, that is, in the formulation of 100g new materials, adding 75.2~76.0 grams of pure gold per 100 grams of new material, with the rest as filler material, as shown in Figure 6-13.

(3) End of Work
After mixing the ingredients, submit the precious metal materials, turn off the electronic balance, and clean the workplace.
Section II Torch Melting
1. Háttérismeretek
1.1 Melting Point and Melting Temperature Range of Jewelry Metal Materials
The melting point refers to the temperature at which a substance transitions from solid (melting) to liquid, while the temperature at which it transitions from liquid to solid is called the freezing point. Under certain environmental conditions, pure metals’ melting points are fixed. Different categories of pure metal materials generally have varying melting points, and the differences can be significant; for example, the melting point of pure silver differs from that of pure platinum by more than 800℃. When other alloying elements are added to pure metal materials to form alloy materials, the atoms of the alloying elements enter the lattice of the base material, causing lattice distortion, which increases the overall internal energy of the metal, leading to a melting point that differs to varying degrees from that of pure metals. The types and amounts of alloying elements added influence the differences in melting points. When the added alloying elements are low melting point materials or can undergo eutectic reactions with the base material, the melting point of the alloy material will decrease. Generally speaking, alloys do not have a fixed melting point but possess a certain range of melting temperatures.
The melting point is of guiding significance for the production of metal jewelry. Metal materials need to be prepared through melting, and the viscosity and fluidity of molten metal are closely related to its temperature, with the temperature of the molten metal being determined by the melting point of the alloy. Most jewelry forming uses gypsum mold investment casting. Still, gypsum has poor thermal stability and can undergo thermal decomposition at high temperatures, leading to defects such as porosity and sand holes in the castings. Therefore, the gypsum mold casting process requires the melting point of metals; when the melting point of materials (such as platinum and palladium) is too high, this casting process is unsuitable. In jewelry production, defects are often repaired by welding or assembling components together, and the melting points of the base material and welding material are also important process parameters. Generally speaking, the lower the melting point of the metal, the easier it is to refine, cast, and weld.
1.2 Melting Conditions
(1) Burner
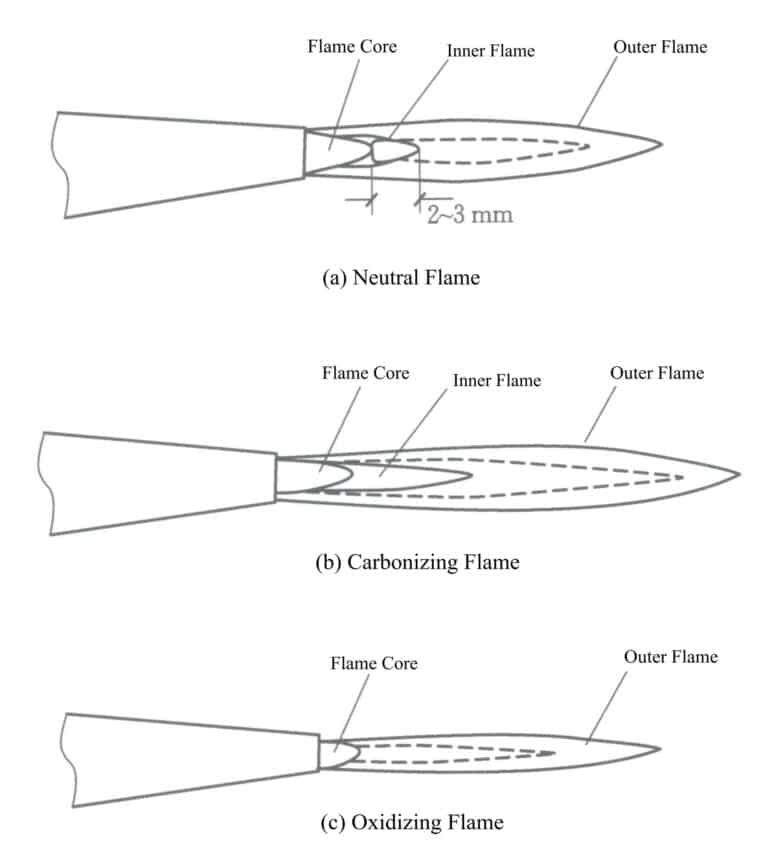
Melting generally uses the traditional flame melting method, and the torch is the basic tool for flame melting. The torches used for melting are generally suction-type torches divided into single-tube and double-tube types, with their shape and components shown in Figure 6-14. The single-tube torch is the most commonly used, mostly using liquefied natural gas as fuel, suitable for melting jewelry materials with medium to low melting points such as gold, silver, and copper; the double-tube torch uses acetylene as fuel, mainly for melting high melting point jewelry alloys like platinum and palladium. By filler materially adjusting the gas and oxygen valves, the flame’s size, nature, and shape can be controlled.
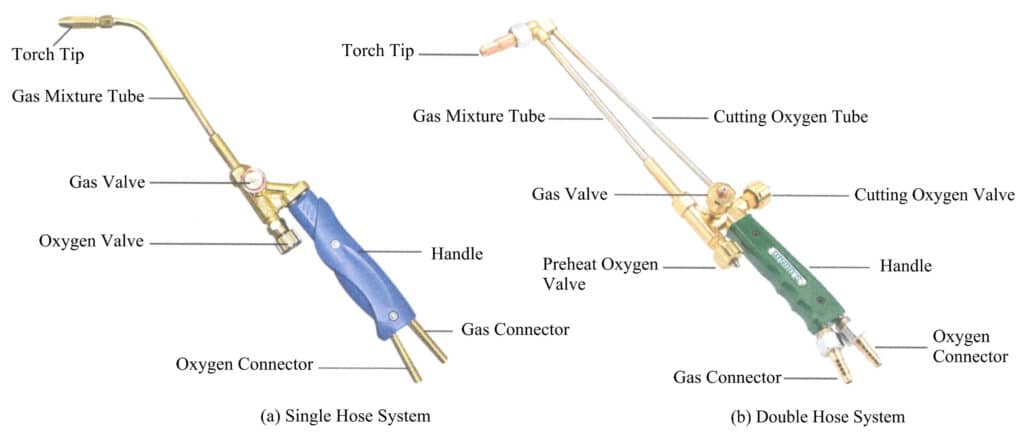
(2) Gas
The properties and flow of gas, as well as the purity and flow of oxygen, will affect the nature of the flame. In jewelry production, the gases used for melting metal materials with a torch mainly include two types: acetylene; the other is liquefied petroleum gas. Acetylene is an organic compound with the chemical formula being C2H2, known as carbide or acetylene gas. It is the smallest member of the alkyne compounds, colorless at room temperature and pressure, flammable, and poses a risk of explosion in liquid and solid states or in gaseous form under certain pressures. Factors such as heat, vibration, and electric sparks can trigger explosions, so they cannot be stored or transported after being pressurized and liquefied. Liquefied petroleum gas is a byproduct of oil field development or the cracking of petroleum in refineries, with its main components being propane (C3H8), butane (C4H10), and some other minor hydrocarbons. At room temperature and pressure, the hydrocarbons that makeup liquefied petroleum gas exist in gaseous form, but when pressure of 0.8~1.5Mpa is applied, they turn into liquid, making it convenient for storage and transportation in bottles. Liquefied petroleum gas forms an explosive mixture with air or oxygen. Still, the range of explosive mixture ratios is much smaller than that of acetylene, and its ignition point is higher than that of acetylene, making it safer to use. The physical and chemical properties of the main components of acetylene and liquefied petroleum gas are shown in Table 6-4. When the flame is neutral, the decomposition of propane in the flame core area is an endothermic process. The decomposition in the flame core consumes the energy produced by the inner flame, and the heat of the flame core and inner flame accounts for 9% of the total heat, compared to only 49% of the heat of acetylene in the inner flame and flame core, while the length of the outer flame is 2.3 times that of the outer flame of an oxy-acetylene flame. At this time, the volume of the outer flame is large, and the range is wide, but the temperature is very low. Therefore, the flame length should be adjusted by increasing the proportion of preheated oxygen to make the combustion of the outer flame become part of the premixed atmospheric diffusion combustion process.
Table 6-4 Physical and Chemical Properties of the Main Components of Acetylene and Liquefied Petroleum Gas
| Indikátor | Acetylene | Propane | Butane |
|---|---|---|---|
| Molecular formula | C2H2 | C3H8 | C4H10 |
| Molecular weight | 26 | 44 | 58 |
| Density(15.6℃)/(kg·m -3) | 1.099 | 1.818 | 2.460 |
| Relative density to air(15.6℃) | 0.906 | 1.520 | 2.010 |
| Total calorific value/(kJ·kg-1) | 50 208 | 51 212 | 49 380 |
| Oxygen consumption of neutral flame/m3 | 2.5 | 5 | 6.5 |
| Neutral flame temperature (with oxygen combustion) /℃ | 3100 | 2520 | - |
| Flame burning speed (with oxygen burning) /(m • s-1) | 8 | 4 | - |
| Ignition temperature at 0.1 MPa (in oxygen) /℃ | 416〜440 | 490〜570 | 610 |
(3) Gas Cylinder
In jewelry production, the gases mainly used for torch melting are liquefied petroleum gas and acetylene, with oxygen as an oxidizer. They all need to be stored and transported in gas cylinders.
An oxygen cylinder is a specialized high-pressure container for storing and transporting oxygen, usually made of high-quality carbon steel or low-alloy structural steel, rolled into seamless cylindrical containers, as shown in Figure 6-16. The commonly used cylinder volume is 40 L, with an internal oxygen pressure of 15MPa, capable of storing 6m3 oxygen. Before leaving the factory, in addition to strict inspections of all components of the oxygen cylinder, a hydrostatic test of the cylinder body is also required, generally at a test pressure of 1.5 times the working pressure. At the upper spherical part of the cylinder body, a clear mark indicates the cylinder number, working pressure, test pressure, next test date, inspector’s stamp, manufacturer’s inspection department’s stamp, cylinder capacity and weight, manufacturer, and date of manufacture, etc. Additionally, the oxygen cylinder must undergo regular internal and external surface inspections and hydrostatic tests during use. The surface of the oxygen cylinder is sky blue, with the word “Oxygen” marked in black paint.

The acetylene cylinder is a special container for storing and transporting acetylene gas. Its shape is similar to that of an oxygen cylinder. Its construction is more complex than that of an oxygen cylinder, mainly because acetylene cannot be compressed into a regular gas cylinder at high pressure but must utilize the property of acetylene dissolving in acetone. First, porous materials such as asbestos are filled in the steel cylinder, allowing the porous material to absorb acetone, and then acetylene is compressed for storage and transportation. The body of the acetylene cylinder is made of high-quality carbon structural steel or low-alloy structural steel through rolling and welding. The volume of the acetylene cylinder is 40 L, and generally, it can dissolve 6~7kg acetylene. The working pressure of the acetylene cylinder is 1.5MPa, and the pressure for the hydraulic test is 6MPa. The surface of the acetylene cylinder is white, marked with red words “Acetylene” and “Keep Away from Fire,” and the rubber gas hose is generally black, as shown in Figure 6-17.
The liquefied petroleum gas cylinder is a special container for storing liquefied petroleum gas. Depending on the usage and method, the storage capacity of the cylinder varies in multiple specifications, including 10kg, 15kg, and 36kg. The cylinder is made of 16Mn, A3 steel or high-quality carbon steel No. 20. The maximum working pressure of the cylinder is 1.6MPa, and the pressure for the hydraulic test is 3MPa. After passing the test, the cylinder also needs to display similar content on its metal nameplate as on the surface of the oxygen cylinder. The color of the cylinder’s surface is not limited, and it has the words “Liquefied Petroleum Gas,” as shown in Figure 6-18.

Figure 6-17 Acetylene Cylinder

Figure 6-18 Liquefied Petroleum Gas Cylinder
(4) Crucibles and Accessories
The main types of crucibles for oxy-fuel melting are clay-based and high-purity quartz-based. The former has poor high-temperature and thermal shock resistance, making it prone to cracking, leading to molten metal invading the inner wall of the crucible. Therefore, it is now used less frequently, with high-purity quartz being the primary choice. Quartz melting crucibles can be used for melting metals such as gold, silver, platinum, and copper, made from selected fused quartz as the main raw material, SiO2 content greater than 99%, produced using modern ceramic processing technology, capable of withstanding high temperatures of 1800℃, resistant to corrosion, and having high strength, with a normal temperature compressive strength above 70MPa, it has strong thermal shock resistance, and it does not crack even after multiple rapid quenching under 1100℃, resulting in a long service life. The crucible is generally bowl-shaped, with a rounded pouring spout for easy pouring, making it difficult for the molten metal to spill. The crucible comes in various specifications, as shown in Figure 6-19.


(5) Fluxing Agent
A small fluxing agent is generally sprinkled on its surface when the metal approaches melting. It not only aids in melting but also forms a protective layer on the surface of the molten metal to prevent oxidation and gathers the slag on the surface of the molten metal. Borax, which is sodium tetraborate decahydrate (Na2B4O7·10H2O). It is a good fluxing agent for melting jewelry alloys such as gold, silver, and copper. It has a low melting point and loses its crystalline water, causing it to become a porous substance when calcined to 320℃. After heating and melting, it has good fluidity, covers the surface of the molten metal, prevents gas absorption and metal oxidation, and can separate boric anhydride (B2O3). Boric anhydride is extremely unstable at high temperatures and reacts violently with metal oxides when separated. The chemical reaction equation is as follows:
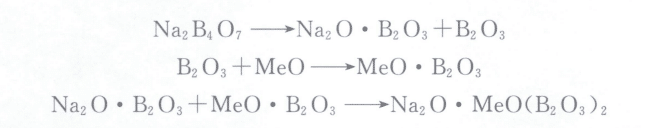
Copywrite @ Sobling.Jewelry - Egyedi ékszergyártó, OEM és ODM ékszergyár
1.3 Oil Tank

1.4 Safety Precautions
(1) Safety Precautions for Oxygen Cylinders
Oxygen cylinders should be transported using dedicated vehicles, and oxygen cylinders, acetylene cylinders, or other flammable gases should not be transported. When loading, the oxygen cylinders should be fitted with caps and anti-vibration rubber rings, laid flat in the same direction, and secured to avoid collisions between cylinders and severe vibrations. It is forbidden to roll the oxygen cylinders on the ground.
In production areas, the distance between oxygen cylinders and acetylene generators, flammable materials, or other sources of open flames should generally not be less than 10 meters. When environmental conditions do not allow this, it should be ensured that the distance is not less than 5 meters, and additional protection measures should be taken. The gas cylinders should be placed vertically and secured with brackets to prevent tipping when used.
When installing a pressure reducer on the cylinder valve, the nut connecting to the valve opening must be tightened to prevent it from falling off when the gas is opened, and the body should avoid the direction of the valve’s gas discharge. It is prohibited to tighten the cylinder valve screw while under pressure or to use methods such as striking the adjustment screw of the pressure reducer to handle leaking cylinders.
In summer, it is important to prevent gas cylinders from sunlight exposure. When used outdoors, temporary shelters or covers should be set up. Additionally, direct radiation from high-temperature heat sources should be avoided to prevent the gas inside the cylinder from expanding and causing an explosion.
The valve should be opened before installing a pressure reducer on the cylinder valve, and impurities in the gas outlet should be blown out. Then, the valve should be gently closed. After installing the pressure reducer, the valve should be opened slowly; opening it too quickly can easily cause the pressure reducer to catch fire or explode. Oxygen cylinder valves, oxygen pressure reducers, blowtorches, oxygen hoses, etc., are strictly prohibited from contaminating flammable substances and oils to prevent fire or explosion.
The oxygen in the gas cylinder must not be completely used up; at least 0.1~0.2Mpa of residual gas should be left to identify the nature of the gas during oxygen refilling and to prevent air or combustible gases from flowing back into the oxygen cylinder. The gas cylinder should be placed in a well-ventilated area, away from heat sources and electrical equipment.
During the use of the oxygen cylinder, regular inspections should be conducted by the “Regulations on the Safety Technical Supervision of Gas Cylinders” (TSG R0006-2014). Non-compliant cylinders must not be used.
(2) Safety Precautions for Acetylene Cylinders
Acetylene cylinders should not be subjected to severe vibrations and impacts to avoid the risk of explosion. Acetylene cylinders should be kept upright during use and must not be laid down to prevent the outflow of acetone, which could lead to combustion and explosion. The connection between the acetylene regulator and the acetylene cylinder valve must be secure, and using it in case of gas leakage is strictly prohibited. When opening the acetylene cylinder valve, it should be done slowly, and the valve should not be turned more than 1.5 turns; generally, only 3/4 turns are needed. The surface temperature of the acetylene cylinder should not exceed 40℃, as the solubility of acetylene in acetone decreases at high temperatures, causing a rapid increase in pressure inside the cylinder. The acetylene in the cylinder must not be completely used up; at least 0.03MPa of gas must be left. The cylinder valve should be tightly closed to prevent leakage.
(3) Safety Precautions for Liquefied Petroleum Gas Cylinders
Liquefied petroleum gas cylinders produced by regular manufacturers with product qualification certificates should be selected and regularly inspected. Using unqualified cylinders or those not inspected for an extended period is strictly forbidden. Liquefied petroleum gas cylinders should be handled gently, and knocking or colliding with the cylinders is prohibited. After connecting the cylinder to the torch, check for gas leaks at the pressure regulator and hose connections with soapy water before the first use. If a leak is found, repairs should be made promptly. The cylinder’s valve opens clockwise and closes counterclockwise; do not confuse them. Cylinders must be used upright and are strictly prohibited from being placed horizontally or upside down. Cylinders must not be used in the same room as other open flames. Cylinders are strictly prohibited from being exposed to sunlight and should not be placed in excessively high-temperature areas. Cylinders should be stored in explosion-proof cabinets to ensure air circulation at the bottom. If gas leakage is detected during use, the cylinder valve should be closed immediately, and windows and doors should be opened for ventilation. Hoses should avoid contact with high-temperature objects and heat radiation and should generally be replaced every two years.
(4) Safety Precautions for Melting Operations
Operators must undergo specialized training and strictly adhere to operating procedures, wearing protective gear during operations. Appropriate types and quantities of firefighting equipment and leak emergency response devices should also be equipped near the melting area. Smoking is strictly prohibited in the workplace.
Check the power before connecting the gas hose when using a suction torch. The method of checking is to connect only the oxygen hose, open the gas valve and (preheat) the oxygen valve on the torch, and place a finger over the gas inlet of the torch. If suction is felt, it indicates good suction power. Then, check if the gas flows normally from the gas hose before connecting the gas hose to the torch.
Before igniting the torch, check for gas leaks at the connections and each gas valve. After the oxygen and gas valves are opened, do not block the torch nozzle with hands or other objects to prevent oxygen from flowing back into the gas supply system, which could cause a backfire.
When igniting, first open the gas valve, and after it ignites, open the oxygen valve to adjust the flame. If backfire signs are detected, the oxygen valve can be immediately closed to extinguish the flame. The downside is that black smoke is produced at the beginning. If the oxygen valve is slightly opened first, then the gas valve is opened, and then ignition occurs, black smoke can be avoided. However, in the working environment of a suction-type torch, if there is a gas leak inside the torch or the nozzle is blocked, backfire is likely to occur. Before ignition, the torch should be pointed slightly downward to prevent the flame from injuring the body after ignition. A special ignition gun or lighter should be used for ignition. A lit torch cannot be placed casually on the workpiece or the ground.
Once backfire occurs, the gas valve should be immediately closed, followed by the oxygen valve. After the backfire stops, release the pressure regulator, and only after identifying the cause of the backfire can ignition be attempted again. Before ignition, the smoke and ash in the hose and the mixed gas pipe of the torch should be blown out, and the torch should be placed in water to cool down.
When extinguishing a suction-type single-tube torch, the oxygen valve should be closed first, followed by the gas valve. For a suction-type double-tube torch, the cutting oxygen valve should be closed first, then the gas valve, and finally the preheating oxygen valve.
When the torch is not in use, it should not be placed in a pit, trench, under a workpiece, or locked in a toolbox to prevent gas leakage from the valve due to poor sealing, which could mix with air and cause an explosion when encountering sparks. At the end of each workday, the pressure regulator and torch should be disassembled, and the valves of the gas cylinder and gas line should be closed.
2. Feladat végrehajtása
This task uses a torch, liquefied petroleum gas, and oxygen to smelt 18 K platinum.
(1) Előkészítő munka
Clean the oil tank used for pouring, ensuring no water, metal waste, impurities, or slag are mixed in the tank. According to the required size of the ingot, place the steel blocks used to adjust the ingot size in the oil tank, and preheat the oil tank to around 200℃ with a torch. Pour a small amount of vegetable oil into the tank, with a depth of about 3 mm, as shown in Figure 6-23, so that after pouring in the molten metal, the top surface of the molten metal can be submerged in oil, reducing oxidation on the surface of the ingot.
(2) Adjusting the Flame
Connect the torch, and according to safety operation regulations, open the pressure relief valves of the gas and oxygen cylinders. Open the preheating oxygen valve to expel any impurities in the torch gas line, then close the oxygen, open a small amount of gas, ignite the gas with a lighter, and then increase the gas flow while also increasing the oxygen flow, alternating between the two until the flame from the torch has an outer flame, inner flame, and flame core structure, accompanied by a noticeable airflow sound.
(3) Melting Pure Gold
Aim the outer flame at the gold material to heat it, and when the pure gold starts to melt, sprinkle a small spoonful of borax powder onto the pure gold, continuing to heat until the pure gold is completely melted.
(4) Melting the Filler material
Remove the flame, add the filler material from the furnace material into the molten pure gold, and then aim the flame at the metal material to heat it. To effectively protect the molten metal and reduce the oxidation of metal elements, a yellow neutral flame is required, and sprinkle 1~2 spoonfuls of borax powder onto the surface of the molten metal. Use a glass rod to stir the molten metal, checking whether the filler material is completely melted while continuously stirring the molten metal to accelerate the melting of the filler material and ensure uniform composition and temperature, and let the slag flow to the wall of the crucible, as shown in Figure 6-24.

(5) Pouring the Ingot
Observe the condition of the molten metal surface; when it is bright like a mirror, carefully lift the crucible and gently swirl the molten metal to observe its viscosity and fluidity. Tilt the crucible slightly towards the pouring spout while moving the flame to heat the spout. Aim the crucible’s spout at one end of the oil trough, and further tilt the crucible to allow the molten metal to flow smoothly into the oil trough while along the length of the oil trough to move the crucible evenly to shorten the flow path of the molten metal in the trough. After the molten metal is poured cleanly, use the torch to heat the top surface of the ingot back and forth one to two times, making the top surface of the ingot smoother and denser after solidification, as shown in Figure 6-25. Check if there are any residual metal beads on the inner wall of the crucible; if so, use the torch to melt the metal beads, blow them toward the spout, and recover them. After smelting is complete, close the oxygen and gas valves and extinguish the flame.

(6) Disassemble the Ingot
Please wait for the ingot to solidify and cool down below 300℃, then use pliers to remove the ingot and quench it in water. Clean the ingot’s surface with detergent, then dry it with a hairdryer. Use scissors to cut the ingot into small pieces for convenient batching and feeding into the furnace.
(7) Calculate the Loss Rate
Use an electronic scale to weigh the cut ingots and the melting tailings and calculate the melting loss rate based on the mass of the initial batch.
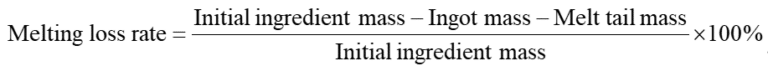
(8) Testing the Fineness
Randomly select a casting ingot segment, use an X-ray fluorescence spectrometer to detect the gold content, determine whether the quality of the ingot meets standards, and assess its uniformity.
(9) A munka vége
After completing the pre-melting task, submit all materials, close the liquefied petroleum gas and oxygen cylinders, properly store the torch and gas pipes in the designated location, turn off related power sources, and clean the workplace.
Section III Induction Melting
1. Háttérismeretek
1.1 Principle of Induction Melting
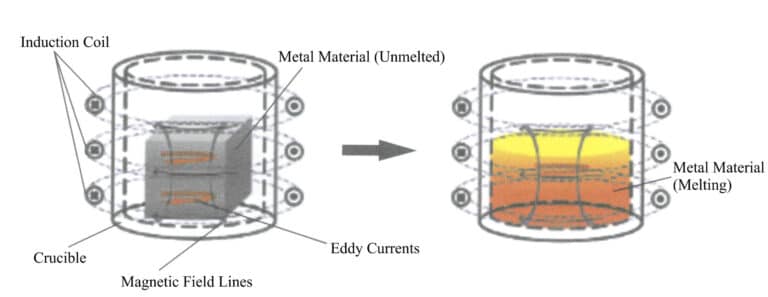
During the induction melting process, the distribution of the induced current in the metal is uneven, with the current density being highest at the surface of the charge and decreasing towards the interior, resulting in the so-called skin effect. The skin effect is closely related to the current frequency; the higher the current frequency, the more pronounced the skin effect. When the crucible capacity is large, severe skin effect is detrimental to melting. Therefore, there is a certain correspondence between crucible capacity and current frequency. For materials with lower melting points, such as gold, silver, and copper, the melting amount is generally relatively large, mainly using medium-frequency induction power, while for high melting point platinum materials, the single melting amount is small, often using high-frequency induction power.
In induction melting, the metal liquid generates an electrodynamic effect under the action of the electromagnetic field, promoting the circulation of the metal liquid and producing an electromagnetic stirring effect, which is beneficial for the uniformity of the temperature and composition of the metal liquid, as well as for the rising of non-metallic inclusions in the metal liquid. The lower the current frequency, the stronger the electromagnetic stirring effect.
1.2 Induction Melting Furnace

During melting, the metal is heated by induction and then conducted to the slag, so the temperature of the slag is relatively low, and the structure of the furnace type determines that the molten pool and interface are small. These factors do not affect the physical and chemical reactions between the molten pool and the slag. Therefore, the refining effect through the slag in induction melting is not good, and it is advisable to use better raw materials for melting.
For jewelry materials that use base metals as alloying elements, especially those containing reactive metals such as iron and rare earths, direct melting in the atmosphere can easily lead to oxidation losses and reduced metallurgical quality. Therefore, adding vacuum protection measures based on induction melting is a widely adopted method in the industry, which involves first evacuating the melting chamber before melting, and then heating and melting in a vacuum or filling the chamber with protective gases such as pure argon or pure nitrogen after evacuation. This can greatly reduce the oxidation losses of reactive metal elements, decrease the amount of gas absorbed by the molten metal, lower the content of gases and non-metallic inclusions in the molten metal, and improve metallurgical quality.
1.3 Granulator

1.4 Melting Crucible
Depending on the properties of the jewelry materials and the method of pouring the molten metal, different materials and structures of crucibles can be used for melting. The requirements for crucible materials in melting mainly focus on aspects such as refractoriness, density, thermal stability, and reactivity with molten metal. Common crucible materials include two categories: graphite and ceramic.
(1) Graphite Crucible
Graphite crucibles are widely used in jewelry casting, featuring high refractoriness, good thermal conductivity, high thermal efficiency, low thermal expansion rate, good thermal shock resistance, and resistance to slag erosion, providing a certain protective effect on molten metal and achieving better metallurgical quality. Graphite crucibles are suitable for melting materials such as gold, silver, and copper, with molten metal exhibiting good non-wettability on their surface, preventing adhesion. Graphite crucibles are conductive, and when the induction current passes through, the graphite generates heat due to its resistance, assisting in heat transfer to the metal materials. Graphite will oxidize when heated, so a quartz outer shell is needed during melting, as shown in Figure 6-29; the quartz outer shell provides a certain protective effect. For crucibles used for pouring, the bottom is closed; for bottom-pour crucibles, the bottom has openings, utilizing a graphite plug to control the opening and closing of the bottom pour hole.

The quality of graphite crucibles is related to their material, density, and other factors. Crucibles made from high-purity graphite are dense, oxidize uniformly when heated, have a long service life, and are not prone to metal adhesion, resulting in low precious metal loss; those made from ordinary graphite have coarse particles, uneven density, shorter service life, and high precious metal loss. High-purity graphite crucibles should be prioritized during production.
(2) Ceramic Crucible
When melting platinum, palladium, stainless steel, and other jewelry alloys, graphite crucibles are not suitable because these metal materials will react with carbon; ceramic crucibles must be used. To meet melting requirements, ceramic crucibles should perform well in refractoriness, density, thermal shock resistance, and reactivity with molten metal. Currently, the most widely used in the industry are quartz crucibles. Depending on the melting amount and pouring method, crucibles come in various structures and specifications, as shown in Figure 6-30.

1.5 Melting Atmosphere
2. Feladat végrehajtása
An induction melting granulator was used to produce 18 KY gold beads.
(1) Előkészítő munka
Wrap thermal insulation cotton around the outer wall of the graphite crucible, then insert the crucible into the quartz outer shell, check the fit of the two, ensuring the graphite crucible does not wobble, as shown in Figure 6-31, then place the crucible into the induction coil.
Insert the thermocouple into the center hole of the graphite plug rod, then place the plug rod into the crucible. Turn on the air compressor, activate the cylinder, press the plug rod tightly, and completely seal the bottom pouring hole of the crucible, as shown in Figure 6-32.

Figure 6-31 Place the graphite crucible into the quartz outer shell
Figure 6-32 Install the graphite plug rod

(2) Melting Pure Gold
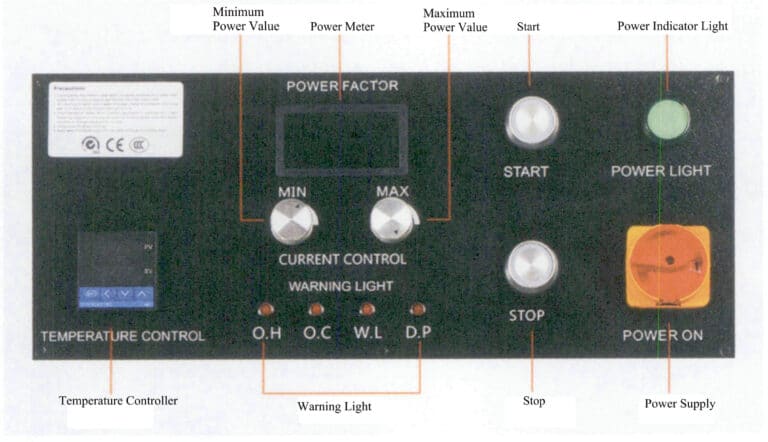
Set the temperature to 1150 ℃ using the temperature controller on the equipment dashboard, and adjust the current control knob to the minimum. Use the charging hopper to add the pure gold ingot into the crucible, then press the “Start” button. Adjust the current clockwise, and the heating power will be displayed on the LCD screen, as shown in Figure 6-34. Note: Do not set the power to maximum simultaneously to avoid overheating.
(3) Melting the Filler material
Add the filler material to the molten metal when the pure gold is completely melted. After the filler material is fully melted, set the temperature to 1050℃ on the temperature control gauge to maintain that temperature for 1~2 min and stir thoroughly.
(4) Pouring and Granulation
Open the plug rod, letting the molten metal leak into the granulation bucket. When quenching into cold water, the liquid flows into small droplets due to the surrounding cold water’s vaporization, boiling, and cavitation forces. The droplets form granules under the action of surface tension, as shown in Figure 6-35 and Video 6-1.
Video 6-1 Water granulation
(5) Drying Beads
Remove the receiving hopper from the granulation barrel; water leaks from the gap. Take out the beads from the granulation barrel, as shown in Figure 6-36 and Video 6-2. Place the beads in the drying oven to dry.
Video 6-2 Extracting beads
(6) Calculating Loss Rate
Remove the residual metal from the crucible, weigh the beads and the residual metal separately, compare with the amount of materials used, and calculate the loss rate.
(7) Testing the Purity
Randomly sample from the beads and use an X-ray fluorescence spectrometer to test the purity.
(8) End of Work
After the pre-melting task is completed, submit all materials. Keep the chiller in the on position until the temperature of the induction melting granulator is shown below 100℃; then, you may turn off the chiller. Turn off the air compressor and related power supply. Store various tools in the designated location and clean the equipment and workplace.
















