A Comprehensive Guide to 10 Kinds of Improved Gemstones
Characteristics Of Various Improved Gemstones
Introduction:
Gemstone enhancement is a fascinating process where science and artistry combine to reveal the inner beauty of gemstones, transforming them into stunning pieces of jewelry and decorative art. This overview delves into the various techniques used to improve gemstones, such as heat treatment, chemical reactions, and physical modifications, which can enhance the color, clarity, and durability of rubies, sapphires, emeralds, and other gemstones. It also touches on the traditional and modern methods that have been used to bring out the inner brilliance of these gemstones, making them the stars of the jewelry world. Whether you’re a jewelry enthusiast, designer, retailer, or someone looking to add sparkle to their collection, this guide provides insights into the world of gemstone enhancement. It covers the methods like heat treatment, chemical reactions, and physical modifications that bring out the inner brilliance of rubies, sapphires, emeralds, and more, and how these methods can be used to improve the quality and value of gemstones. For those involved in the jewelry industry, this guide offers a comprehensive look at the techniques and processes used to enhance the beauty and value of gemstones, making them more desirable for jewelry and decorative purposes.
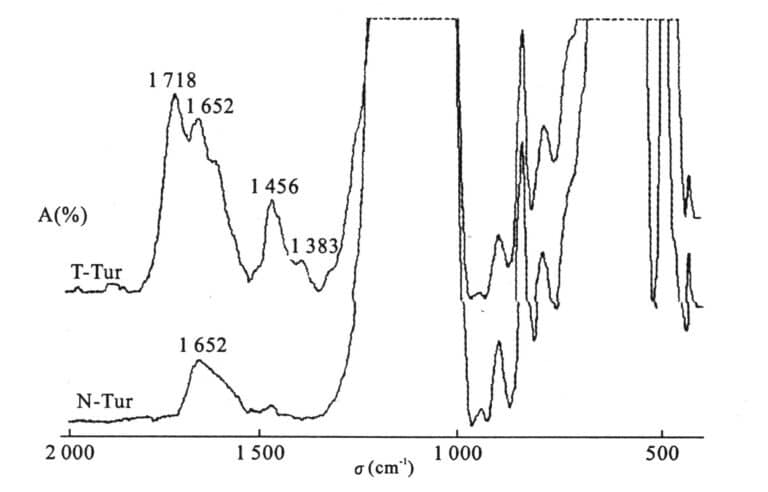
Infrared spectra of natural and filled turquoise N-Tur: Natural turquoise; T-Tur: Filled turquoise
Table of Contents
Section I Improving Diamonds Characteristics
Diamonds that are expensive and profitable often have various defects to some extent, such as low clarity, poor color, or small size. To increase their selling price, people seek various methods to improve diamonds.
1. Filling Diamonds
Filling diamonds cracks with colorless, transparent, high refractive index, hard, low melting point lead glass, and other amorphous materials can conceal the cracks, improve clarity, and thus pursue higher profits.
Foreign substance injection is a characteristic of filled diamonds, and its manifestation is:
(1) Flash Effect
After the filler is injected along the cracks, a rainbow-like bright flashing phenomenon can be seen along the direction of the cracks under a microscope. When the stage is rotated, or the diamond is slowly moved back and forth, the cracks also change, and the colors and areas of the flashes will change accordingly.
(2) Flow Structure
In some filled cracks or cavities, a glass-like substance can be seen flowing inside, and sometimes very fine transparent curved lines of flowing material can be observed within the filler. Since the flow patterns of the filler are not easily dissolved, they may only be observed in certain areas of the cracks. This sense of flow structure is produced when the filler is injected into the diamond cracks under high temperature and pressure, and its direction is consistent with that of the cracks.
(3) Inclusions of Gas Bubbles
Due to incomplete filling in the cracks or cavities of diamonds, gas often occupies these spaces, resulting in a high-contrast phenomenon. Bubbles may be distributed either on the walls of the cracks or within the filling material, appearing singly or in clusters, some visible to the naked eye while others are very small.
Additionally, when filling diamonds are cut and polished into loose diamonds, the filling material’s hardness is much lower than that of the diamond, leading to parabolic depressions and cracks on the facets. At the same time, because the refractive index of the filling material is lower than that of the diamond, Becke lines often appear along the cracks of the filled diamond. If the diamond is immersed in a high-refractive index oil, the Becke lines become more pronounced. If the diamond is immersed in gasoline and illuminated with strong light, flowing rainbows can be seen in the gasoline within the cracks.
When conducting a flame combustion test on filled diamonds, the filling material is leached out at high temperatures, and molten substances can be seen at the edges of the cracks, while the interior of the cracks or cavities appears misty.
(4) Detection Methods
① Observation Angle: The angle for detecting the flashing phenomenon in filled diamonds should be parallel to the cracks, while the optimal observation angle for unfilled diamonds should be perpendicular to the crack surface.
② Spotlight illumination: Using fiber optic lighting, the flash effect is particularly pronounced, revealing the filling range and exposing any hairline cracks in the filling. If a polarizing filter is placed between the microscope and the gemstone in conjunction with a transmitted light source, it can display the filling range and help distinguish the flash effect from natural iridescence.
③ Shadow method: Using an opaque, black, non-reflective light screen placed between the diamond and the microscope light source can help observe the flow structure.
④ Magnified observation: Filled diamonds are generally above 0.3 ct. To assess whether a diamond is filled, it should be carefully observed under a microscope at 6 x 10 or 8 x l0, while a 10x magnifying glass can only reveal some rough clues and signs.
2. Thermally irradiated diamonds
The color of diamonds is mainly caused by various color centers absorbing different ranges of visible light, and the formation of color centers is closely related to various defects in the diamond crystal structure. The elimination and formation of structural defects have special functions in the thermal-irradiation process.
There are various color centers that cause diamonds to be colored, such as the “canary yellow” characteristic of the N center; the N3 center is the most common among yellow diamond manufacturers, with an absorption line at 415nm; the N2 center is represented by 478nm, exhibiting bright yellow fluorescence under longwave ultraviolet light, and this diamond often appears as an enchanting amber yellow in sunlight; the H3 center (with an absorption line at 503 nm) along with the N3 and N2 centers are the main colorcausing factors for brown diamonds, while the H3 and H4 centers are the primary reasons why type I a colorless or light yellow diamonds exhibit a brighter yellow after heat irradiation. Additionally, diamonds generally the GRI heart produced by irradiation (such as particles, neutrons, high-energy electrons, protons, etc.) manifests as a very broad absorption band (from 741 nm- in the yellow-green visible light range), allowing diamonds to display various colors such as green, blue, blue-green, deep green, black, yellow, and more. The vacancies created by the substitution of boron for carbon atoms in type Ⅱ b diamonds are called B hearts, which make diamonds appear blue. However, B hearts are rare in natural diamonds. Therefore, the color change in diamonds mainly targets yellow diamonds.
3. Coated diamonds
Diamond coating is a method of growing a layer of polycrystalline diamond film on the surface of a diamond using chemical vapor deposition (DF), which has a distinct granular structure that is relatively easy to see under magnification. Raman spectroscopy determines that the characteristic peak of the diamond film is near 1332cm-1, with a full width at half maximum (FWHM); poor-quality diamond films have a significant peak shift and reduced intensity, and may even show a broad peak near 1500cm-1.
4. GE processing of diamonds
This method mainly targets Ⅱ a -type brown diamonds with a certain level of clarity, moving external color centers under high temperature and pressure to present the best internal color. Brown series diamonds are believed to be caused by lattice defects resulting from plastic deformation of the diamond crystal lattice, which occurs during the process of diamond formation from the mantle to the surface due to pressure changes. Therefore, it should be possible to repair this deformation through pressurization or depressurization. However, only about 1% of diamonds can actually be treated, as characterized below.
① The vast majority show weak to obvious white or rare brown morphology. Half exhibit a slightly blurred morphology, which may be due to the scattering effect of growth lines on other light.
② Cleavage or feather-like cracks near the surface.
③ Many near-surface cleavages exhibit “partial healing,” similar to the “fingerprint-like inclusions” commonly found in sapphire gemstones. Other cleavages show a frosted or granular appearance near the surface but become glassy at greater depths. A black area (feathery graphite inclusion) can be seen in some cleavages.
④ Inclusions are often surrounded by stress fractures, such as graphite inclusions surrounded by translucent halos radiating outward from tiny fractures, and some graphite inclusions are encircled by a network of fine fractures. This radiating fracture pattern may be caused by the differing thermal expansion of the inclusion and diamond after high-temperature heating. A set of circular fractures is distributed along the octahedral shape, resulting from the release of internal stress around the inclusions in the diamond. Some solid opaque inclusions do not exhibit the aforementioned radiating or circular fractures but display a flowing and melting structure, and sometimes cloud-like or mist-like substances are observed.
⑤ Under a polarized light microscope, medium to strong stress patterns and a cross-shaped “Tammie” can be observed, arranged in bands and spots. The stress interference colors are mostly first and second-order gray, blue, or orange, while natural type Ⅱ a diamonds usually show lower-intensity gray and brown interference colors.
5. Coated, dyed, and laser-treated diamonds
(1) Coated diamonds
Coating and spraying a very thin layer of colored organic material on the surface of diamonds can both improve the color of the diamond and enhance its “fire.”
(2) Colored Diamonds
Applying red, blue, pink, and other colors to the diamond’s girdle, which can be difficult to detect after metal inlay, can give the diamond a red or blue hue. To reduce the yellow tone of the diamond, yellow complementary colors (blue or purple) can be used to dye the surface of the diamond, making it appear whiter.
(3) Laser Cleaning Diamonds
Using laser drilling technology, “blemishes” in the diamond can be vaporized or corroded away with strong acid, and then the cavities can be filled with glass to improve the diamond’s clarity.
Section II Improving Beryl Gemstones
Beryl gemstones include emerald, aquamarine, golden beryl, cesian beryl, and Max-ixe (Max-ixe type) beryl, among others. Emeralds are 0.15%-0.5%beryl replaced by aluminum and are green; Aquamarine is made of A small amount of beryl Al3+ and Be2+ replaced by Fe3+ and Fe2+, respectively, in a charming sky blue and blue-green; The color of golden beryl is yellow to brownish yellow, which is caused by Fe3+replacing Al3+ into the octahedron in the form of isomorphism. Pink and purple red cesium beryl, the color ions are mainly Mn2+ and Mn3+, in addition to Cs1+and Fe3+ so on; Maxisi beryl is a dark blue beryl that is colored by a lighter color center.
Common methods for improving beryl gemstones include low to medium-temperature heat treatment, irradiation treatment, and infusion methods. For example, certain green and blue-green beryl can undergo heat treatment (400-450℃), which can eliminate yellow tones from light blue to sky blue aquamarine, turn some golden beryl into colorless stones, and transform orange-red beryl into pink cesian beryl, as well as change cesian beryl into red or purplish-red. Irradiation treatment can turn colorless, light green, and light blue beryl into yellow, green, or blue, while some colorless cesian beryl can become pink or orange-red. Some colorless or pink beryl may turn deep blue after irradiation but will quickly fade in sunlight. The infusion method is the main technique for improving natural emeralds, which involves soaking in strong acid, followed by repeated washing with clean water and dilute alkaline solution, drying, and then infusing with Canadian balsam using hot infusion or high-pressure infusion (vacuum infusion) methods, sealing with wax, and polishing. Some also use colored dyes or pigments for infusion.
Improved beryl gemstones have different characteristics due to the varying improvement processes. However, as it stands, distinguishing between beryl gemstones treated with low to medium-temperature heat and irradiation from their natural counterparts remains quite challenging.
1. Improving Emeralds
(1) Emerald treated by the injection method
There are three types of injection agents: colorless oil, colored oil, and resin filling, each with its own characteristics.
① Injecting colorless oil: The main purpose is to cover existing cracks and holes without changing the color of the gemstone. It is recognized by the jewelry industry and consumers as an optimization of the gemstone. During identification, the emerald can be placed in water or another colorless solution and observed under reflected light. By rotating the gemstone, interference colors caused by the colorless oil or liquid inclusions can be seen in one direction; heating experiments may show oil flow, commonly referred to as “sweating.”
② Injecting colored oil: Under magnification, green oil can be seen distributed in a thread-like manner within the cracks, and some oils exhibit fluorescence. After the oil dries, it leaves behind green dye in the cracks.
③ Injecting resin: Bubbles may remain in the cracks, sometimes appearing misty or having a flowing structure. Under reflected light, web-like filling materials can be seen on the surface of the gemstone.
(2) Surface coating methods include two types: the backing method and the coating method.
① Backing method: A layer of green film or green foil is placed at the bottom of the emerald ring, which is often not easily noticeable after being set in a question-setting style. During identification, the joining seam can be seen, and there may be bubbles remaining in the seam. Sometimes, the film may wrinkle, crack, or fall off, and the backing shows no dichroism.
② Coating method: It is easy to see a network of interwoven cracks, and when immersed in water, the color can be seen concentrated at the edges. From the side, a layered distribution phenomenon can be observed.
2. Improving Maxixe blue beryl
The main method for improving Maxixe blue beryl is irradiation. After γ ray or short wave ultraviolet irradiation, it is cobalt blue, and its visible light absorption spectrum is 695nm, 655nm strong absorption bands, 628nm, 615nm, 581nm, 550nm weak absorption bands.
Section III Improving corundum gemstones
1. Improvement of Ruby
The corundum gemstone with a red color is called ruby. The colors of rubies include light red, medium red, deep red, and red with other hues. In the Bible, it is listed as one of the most precious gemstones. Today, improved rubies account for the vast majority of the ruby market, and they have characteristics different from natural rubies due to the different types of improvement processes.
(1) Thermal Treatment
① Rubies treated at high temperatures often have uneven colors, and the clarity of the original color bands may change to varying degrees.
② Inclusions will also change to varying degrees. (For example, solid inclusions with low melting points may partially melt, edges may become rounded, and fibrous inclusions may become intermittent; liquid inclusions may rupture due to volume expansion, even entering newly formed fracture lines.)
③ The surface of gemstones often shows some “pockmarks” or pits.
(2) Injection treatment
① Light-colored rubies are soaked in organic dye (immersion) and heated to solidify the dye and color them.
② Colored oil is filled into the cracks of the gemstone, sometimes producing colorful interference colors.
③ Borax, water glass, paraffin, plastic, silica, high-lead glass, etc., are filled into the cracks of rubies, or chromium oxide coloring agents are added to enhance the red color of the ruby.
The main purpose of injection along the fissure is to improve the color and transparency of the ruby. Its characteristic is that all the injectors are located in the split god of the gemstone, the refractive index of the injectors is different from that of the ruby, and the absorption spectrum of the ruby can be different from that of the infrared spectral analysis. Raman spectroscopic analysis shows that elements that do not appear in natural rubies, such as lead, boron, silicon, phosphorus, calcium and so on.
(3) Thermal diffusion treatment
① Chromium diffusion
High temperatures are used to allow external chromium elements to enter the surface layer of light-colored rubies by isomorphous substitution, occupying the aluminum lattice and forming a red diffusion layer.
Rubies treated with thermal diffusion often exhibit varying shades of red, are uneven, or appear mottled. If such rubies are immersed in dibromomethane and observed under diffuse reflected light, a concentration of red can be seen at the girdle, facet edges, and fissure surfaces. Additionally, thermally diffused rubies can have an anomalous refractive index of up to 1.80.
② Beryllium diffusion
Beryllium diffusion can give corundum gemstones a yellow, orange, or brown hue, and beryllium elements can penetrate from the surface of the ruby to the inside of the gem, or even the entire stone. The outer layer is orange-red, and the center is pink and red.
Beryllium diffusion ruby, can also appear crystal growth, but the difference is that the new crystal in the shape of a small plate exists in the gem surface cavity, but does not cover the entire gem surface. The random growth of the attached crystals can gradually grow into a flat and hexagonal shape, and a large number of aggregates can form a solid layer composed of irregular blocks. The phenomenon of attached crystals on the surface of the gem is usually easy to observe in the dark area lighting, and it is easy to see with transmitted light, and the appearance is murky.
Another characteristic of beryllium diffusion is that the cavities inside the gemstone are filled with a glassy substance and contain spherical bubbles.
Natural rubies (chromium-induced color) exhibit strong fluorescence under ultraviolet light and even in natural light. The fluorescence of treated rubies is not obvious, and it appears to be a very weak light green. To the naked eye, they generally have an orange-red hue, with noticeable pleochroism, showing clear orange-yellow and orange-red tones.
2. Improving sapphires
Sapphires, recognized as one of the four major precious gemstones in the world, have high economic and aesthetic value. Their colors are stunningly diverse and unpredictable. Two sapphires often differ in price due to minute differences in color. Currently, about 95% of sapphires on the market have been treated, with heating and surface heat diffusion being the most common methods. Filling with oil, resin, glass, or high molecular polymers for gaps or blemishes or surface coating and dyeing are currently less commonly used treatment methods.
(1) Thermal energy process
Heating sapphires to between 450-900℃ and maintaining that temperature for 7 hours to 14 days, followed by gradual cooling to room temperature, will yield various results: increased blue, lightening of dark colors, reduction of green, filling of cracks, disappearance of dark silk, etc., thereby improving the gemstone’s color, clarity, and transparency, and even producing a star effect. For example, Geuda milk stone appears colorless or brownish tea due to containing Ti , Fe, and heating it to 1600℃ can change its Ti , Fe state, greatly improving its color and turning it into a valuable blue sapphire while also enhancing its transparency and luster.
(2) Heat diffusion treatment
① Surface diffusion treatment
The sapphire is placed in a crucible containing alumina and sodium oxide and heated to near its melting point, allowing the compound to penetrate the shallow layers of the gem, forming a thin blue layer (0.5 mm) to achieve color enhancement, characterized similarly to diffusion rubies.
② Deep beryllium diffusion
This often refers to sapphires from Madagascar and Tanzania as Red song, using heat diffusion to infuse beryllium into the sapphire, even throughout the entire gem, resulting in a vibrant orange-yellow to reddish-orange, sold as high-end rare natural orange sapphires from Sri Lanka.
To determine the beryllium diffusion in sapphire, the beryllium content can be measured using SIMS (Secondary Ion Mass Spectrometry). Natural sapphire contains beryllium at 1.5-5PPM±, while the beryllium content after diffusion can be between 10-35PPM.
For the identification of beryllium diffusion-treated sapphires, the immersion method using dibromo methane allows for the observation of color zones around the gemstone. Additionally, if a beryllium diffusion method is used to “lighten” the body color of dark sapphires (basalt host), after immersion in dibromo methane, a faint layer of colorless to yellow color zone can be seen surrounding the blue body color at the periphery, enveloping the entire gemstone.
Due to the different temperatures during the beryllium diffusion treatment process, the results also vary. If the diffusion treatment temperature is at 400-600℃, the color of the sapphire improves, appearing significantly more yellow or brown compared to iron-colored Citrine. If the beryllium diffusion occurs in a high-temperature oxidation environment, beryllium can diffuse into the deeper layers of the gemstone; if the heating time is long, it can diffuse throughout the entire gemstone.
For sapphires subjected to extremely high-temperature beryllium diffusion, the peripheral color zones are no longer visible when identified with dibromo methane. At this point, internal inclusions can be observed to make judgments, such as whether there are healed feather-like cracks, whether the gemstone surface has burn pits, and whether there are new growths of crystals (synthetic corundum). Yellow and red corundum have been treated with beryllium diffusion, and sometimes the internal diffusion of blue color gamut formed by TiO2 due to the release of Ti element at high temperature can be seen.
In summary, the identification of beryllium diffusion sapphires can be comprehensively analyzed and judged based on the above characteristics.
3. Diffusion Star Light
Corundum gemstones treated by heat diffusion can produce star sapphires and star rubies. There are two causes for the star lines: one is that during heat treatment, the originally disordered inclusions in the gemstone become ordered due to heat; the other is formed by surface diffusion. The former is located inside the gemstone, while the latter is on the surface of the gemstone (surface layer).
(1) Heat-treated starlight
Heating Ti-rich sapphires or rubies to 1600-1900℃ causes the disordered Ti-rich inclusions (cloudy) to melt, allowing Ti to enter the corundum lattice. After maintaining the heat for a period of time and then gradually cooling down, TiO2 will dissolve again, forming directionally arranged rutile needle-like inclusions, thus producing the starlight effect. Alternatively, maintaining heat at medium-high temperatures (1100-1300℃) and slowly cooling can also reveal potential starlight effects.
(2) Surface diffusion starlight-
Star rubies and star sapphires formed by surface diffusion methods have already been marketed in our country. After surface diffusion treatment, the refractive index, density, and other physical parameters, as well as the characteristics of inclusions, are the same as those of natural corundum gemstones. The difference between diffusion starlight and natural starlight gemstones is:
① Color: Surface diffusion starry blue sapphire, with a deep blue tone of black and gray, the surface of the gemstone, especially at the bottom of the curved gemstone or on the fracture surface, has red patchy substances.
② Starry light: The surface diffusion of starry light is perfect, with uniform star lines resembling synthetic starry light. Upon magnified inspection, it can be seen that the starry light is limited to the surface of the gemstone. Under a microscope, the surface of the curved gemstone has a very thin layer of fluff formed by tiny white dots, while inside the gemstone, no three groups of directionally arranged golden-red needle-like rutile are visible.
③ Fluorescence: Under SW and LW ultraviolet light, there is no fluorescence, and occasionally, red fluorescent spots can be seen on the surface of the gemstone.
④ Red circle phenomenon: Due to the gemstone surface Cr2O3 content can be as high as 4%; when observed in oil, the surface of the gemstone appears red and has a clearly defined, highly raised red color circle.
Section IV Improving jadeite
1. Heat-treated jadeite
Jade heat treatment, commonly known as color treatment. It involves heating jadeite samples to remove grayish-yellow, brownish-yellow, and other colors, changing them from orange to reddish-brown. Experiments show that yellow and brown jadeite is caused by the dehydration of brown iron ore under natural conditions, resulting in the mineralization of hematite coloring. Hematite dissolves in dilute acid and can be removed. Therefore, after acid washing the sample, it is placed on an iron plate covered with fine sand and evenly heated in a furnace to around 200℃. When the jadeite turns liver-colored, it is cooled, resulting in red, and finally soaked in bleaching water for several hours to ensure full oxidation and color fixation. The identification characteristics are as follows:
① Heated red jadeite: The red has a dry feel and is not easy to distinguish.
② Infrared spectral characteristics: Natural jadeite has a strong absorption band near 1500-1700cm-1, 3500-3700cm-1, while heat-treated products do not.
2. Wax-immersed jadeite
The wax immersion process involves washing the sample with dilute acid. The structural damage is not severe, but it can increase the porosity of jadeite, leading to more paraffin filling into the stone. If the wax-immersed jadeite is left for a long time, it will age and produce white spots, causing a decrease in the transparency of the stone.
Identification features:
① Exposure to high temperatures will cause the wax to ooze out (commonly known as “sweating”), indicating poor durability.
② A blue-white fluorescence can be seen under ultraviolet light.
③ Infrared spectral characteristics: The organic peaks are prominent, exhibiting 2854cm-1, 2920cm-1 characteristic spectrum.
3. Bleached and filled jade
(1) Luster
Often has a resinous luster, waxy luster, or a mixture of glassy luster with resinous and waxy luster.
(2) Color
Lacks depth, with a very white base, green floating on the surface, and color lacking directionality, making it look very uncomfortable.
(3) Structure
Under transmitted light, internal interwoven cracks are visible; under reflected light, surface etching pits or spider web-like patterns can be seen.
(4) Surface Features
Sometimes, more pronounced grooves can form at the native cracks, and even cementing materials or residual bubbles may be visible within them.
(5) Density and Refractive Index
The density of the majority decreases to 3.00-3.43g/cm3, with a refractive index of around 1.65.
(6) Fluorescence
No or weak to strong ultraviolet fluorescence, with a mottled distribution. Under shortwave, weak, appears yellow-green or blue-green (blue-white); under longwave, medium to strong, appears yellow-green or blue-white.
(7) Carbonization
After heating to 200-300℃, the gel undergoes carbonization.
(8) Identification of Large Instruments
Under the Cathode luminescence microscope, its fluorescence colors are mainly yellow, yellow-green, and bluish-green. The color distribution is relatively uniform, and the edge rings appear uneven or incomplete due to erosion. Greenish and deep blue colloidal substances are present in the erosion patterns and cracks (Figure 6-7).
4. Dyed Jadeite
The dyeing process is mostly confidential, usually selecting rough grains of jade with a certain porosity, which are then treated with dilute acid to remove impurities, dried, heated, and subsequently soaked in a dye solution, boiled for several days, allowing the dye to penetrate and fix in the pores (green, purple, etc.). Identification features:
(1) Color
It is distributed in a silk network, and the precipitation or aggregation of dyes can be seen in the larger lock cracks into color spots and spots to imitate natural jadeite.
(2) Spectral characteristics
Appearance of 650 nm broad absorption band. Green colour changes to red under colour filter. Yellow-green or orange-red fluorescence under UV fluorescent lamp. Absorption peaks at 2854cm-1 and 2920cm-1 appear in the infrared spectrum. Appears blue-green and yellow-green fluorescence under cathode rays.
5. Coated Jadeite
The process of applying a colored film is rarely reported. The commonly used material is a green gel-like, highly volatile polymer.
Identification Characteristics:
(1) Color
Evenly distributed, consistent tone, fully colored. The front and back are the same, with no natural product’s mottled, striped, fine vein, or silk-like color distribution characteristics.
(2) Refractive Index
About 1.65 (Refractive index of film).
(3) Luster
The surface gloss is weak, mostly resinous, with no grainy feel.
(4) Package
Bubbles are visible in some areas.
(5) Surface Features
Visible film peeling mostly occurring at the edges; feels soft to the touch; has a sticky feel when touched by hand. On closer inspection, there are small hair-like scratches on the surface. The orange peel effect and granular structural features (intergranular boundaries) of natural products are not visible.
Copywrite @ Sobling.Jewelry — Custom jewelry manufacturer, OEM and ODM jewelry factory
Section V Improving Agate
Natural agate is beautiful, but improved agate is even more beautiful, not only in color but also in the permanence of its color after improvement. This is due to agate’s characteristics of being micro-transparent and having good permeability, making it easy to improve. We know that agate is a collection composed of microcrystalline quartz, forming various structures (fibrous, radial, filamentous, granular) and textures (banded, fine-threaded, moss-like, striped, lichen-like, branching, and shape-like), creating countless beautiful and captivating patterns. However, there are also many agates with unclear shapes and dull, monotonous colors that require manual improvement. Common methods of improvement include:
(1) Heat treatment
The uneven light brown agate semi-finished product is heated in an air electric furnace to 700-1000℃ for a period of time. After finishing the dehydration of the limonite, it is slowly cooled to prevent cracking, ultimately achieving a bright red color. The heat treatment does not change the composition of the agate; it only oxidizes the iron content.
The heat-treated red agate is called fire agate or burnt agate, and its transparency and hardness are slightly reduced compared to natural agate, with increased brittleness.
Tiger’s eye, which is similar to agate, can change from brownish-yellow to brown-red when heated under oxidizing conditions and to gray-yellow or gray-white under reducing conditions. It can be used to imitate the cat’s eye effect of chrysoberyl.
(2) Dyeing
Most agate products on the current market have undergone dyeing treatment, especially natural white, gray, and gray-white agate, which have all been dyed. There are two methods of dyeing.
① Chemical precipitation reaction for coloring
When natural agate (chalcedony) is rich in iron, heat treatment can improve its color. However, most agate contains little or no iron oxides, so only chemical reaction methods can be used to infiltrate colored inorganic substances into the pores of the agate, changing the body color of the agate. There are two specific treatment methods.
- Soak the agate in a soluble metal salt dye for a certain period, then take it out, dry it, and place it in a heating furnace to heat, allowing the metal salt to infiltrate into the agate and decompose into colored insoluble oxides, coloring the agate.
- Soak the agate in a dye, take it out after a period, and then place it in a second solvent for soaking, allowing the two solvents to undergo a chemical reaction, precipitating insoluble colored compounds, thus dyeing the agate red, green, blue, yellow, or black.
To dye the agate red, the white agate can be soaked in iron nitrate solution, taken out and dehydrated, then heated in a furnace to about 300℃, at which point the iron nitrate infiltrating the agate pores turns into hematite, or the agate can be soaked in iron chloride solution and then placed in ammonia water for soaking, and after the two undergo a chemical reaction, it is taken out and heated, producing the precipitation of limonite, can yield red agate.
To obtain green agate, one can soak the agate in chromic acid (H2CrO4) or potassium chromate (K2CrO4) solution for a period of time, then take it out and heat it or soak the agate (white) in a solution made from potassium dichromate, an appropriate amount of ferrous sulfite, and dilute sulfuric acid, take it out after a while, and heating can also yield green.
Using two coloring elements, Fe and Co, can turn agate blue. If using Fe ions for coloring, one can first soak the white agate in a solution of potassium ferrocyanide (Ⅱ)K4[Fe(CN)6] at a concentration of 20% for 10-15 days, then take it out and soak it in ferrous sulfate solution for several weeks to generate Prussian blue or Turnbull’s blue K4[Fe(CN)6]3; or using cobalt salts or copper salts with ammonium salts can also yield blue agate.
There are many methods to dye white agate black; a common method is to soak the agate in a sugar solution for several weeks, then take it out and soak it in concentrated sulfuric acid, appropriately heating it for 30 minutes to 2 hours, then take it out, rinse, and dry to complete.
Yellow agate is dyed with potassium dichromate (K2Cr2O7) and can also be soaked in mercury chloride solution and potassium iodide solution to form it. The reaction between the two solvents can result in the formation of an iodine spring (Hg2I) yellow precipitate.
② Dyeing with dyes
The process of dyeing agate with dyes has a history of hundreds of years. Due to the relatively simple process, dyed agate can often be seen in the market. Currently, the dyes used include amines, azo compounds, or sulfide organic dyes. Before dying, the agate undergoes certain chemical pretreatments for bleaching and impurity removal, and then it is soaked in the dye solution. After a period of time, it is taken out and dried, allowing the water-soluble dye to precipitate on the pore walls of the agate, coloring it.
(3) Water injection treatment
When water chalcedony has many cracks or develops cracks during processing, the water inside will slowly flow out until it dries up. If water chalcedony loses moisture, it loses its craft value and economic value. At this point, water injection treatment can be performed. There are two methods for water injection treatment.
① Water-filled agate: Soak the water-filled agate that has lost moisture in water, using capillary action to refill the water or use injection methods to refill the water, and then seal the small gaps with glue or other materials.
② Agate water injection: Agate originally does not contain water (water-filled). To turn it into a water-filled agate product, a small incision can be made in an inconspicuous part of the agate product, hollowing out the inside, injecting water, and then covering the incision with agate pieces, it can be tightly sealed.
(4) Improving agate inspection
① Heat treatment of agate is considered optimization and does not require testing.
② Dyed agate detection is relatively simple. The colors of most blue, green, yellow, and black agate do not appear in natural agate. Currently, there is no simple and reliable detection method for agate treated by chemical precipitation, and it is often unnecessary. Sometimes, a spectroscope can reveal fine Cr absorption lines appearing at the end of the red region in Cr-colored agate; under a color filter, green agate appears red.
③ Water-injected agate can be examined for signs of artificial treatment on the water chamber wall. At suspicious points, scratching with a needle tip can reveal holes or cracks filled with gelatinous or waxy substances.
Section VI Improving Opal
1. Mechanism of Opal Improvement
Colorful and beautifully patterned opal, known as the “palette” of gemstones, is famous worldwide for its unique color-changing effect.
(1) Opal Composition
Natural opal is a sub-microscopic aggregate composed of AG- opal (SiO2 spherical particles are amorphous) and/or CT-opal (a mixture of quartz and feldspar layers) and contains varying amounts of water (generally 4%-9%, up to a maximum of 20%). Its chemical formula is SiO2 • nH2O.
(2) Types of Opal
There are many varieties of opal, which can be broadly classified into four categories: black opal, white opal, fire opal, and “crystalline” opal.
2. Opal Improvement Process
The artificial improvement of natural opal is mainly approached from two angles: first, by attempting to deepen the body color of the opal to highlight the play-of-color effect; second, by injecting foreign substances to fill the voids, thereby producing and enhancing the play-of-color effect.
(1) Dyeing
Natural opal is composed of countless small spheres of diameter 150- 400 nm and SiO2, tightly packed spheres.
There are countless voids between the particles, which provides favorable conditions for the dyeing process. Dyeing can deepen the body color of the opal, making the play-of-color effect more pronounced and making the appearance of the opal more vibrant and enchanting. There are several dyeing methods, as follows:
- Sugar Acid Treatment
The purpose is to enhance the body color to black. This method began in 1960. The process involves first washing, then drying the opal at a low temperature below 100℃, soaking it in a hot sugar solution for several days; after slowly cooling, quickly wiping off the excess sugar juice from the surface of the opal and soaking it in hot concentrated sulfuric acid (100℃±) for one or two days; after cooling, rinsing thoroughly multiple times, then quickly rinsing in carbonate solution, and finally rinsing clean. At this Point, the hydrogen and oxygen in the sugar are removed, leaving carbon in the cracks and voids of the opal, thus creating a dark background.
- Smoke Treatment
The purpose is to make the opal turn black, imitating black opal. The smoke treatment process involves wrapping the opal in paper and then heating it until the paper smokes. After being smoked, the opal’s surface develops a black background.
- Silver Nitrate Exposure Method
The purpose is to imitate black opal. After cleaning the opal and drying it at a low temperature, soak it in a silver nitrate solution, allowing the silver solution to fully penetrate the opal’s pores and cracks, then take it out for exposure; the silver black makes the opal turn black.
- Aniline dyeing method
The purpose is to imitate black opal. Soak the opal in black aniline dye, and once the opal has turned black, take it out and let it dry (or bake it).
(2) Foreign substance injection
The foreign substance injection method is mainly used for porous water protein stones and low-quality protein stones (colorless, black, or red) to create a color-changing effect, conceal flaws, and improve transparency.
- Injection Molding Treatment
The opal is first dried, the water in the pores is removed, and then pumped into a vacuum, and then soaked in a hot (below 100℃) injector, and the injection agent is pressed into the deep hole god by the outside atmospheric pressure to cover the cracks and make the opal (Opal) present a dark background.
- Oil Injection Treatment
This method uses oil injection and waxing to cover the cracks of inferior opals, improving the appearance of the gemstone and making it comparable to high-quality opals.
3. Improving the Characteristics of Opal
(1) Dyed Opal
- Sugar Acid Treated Opal
Upon magnified observation, the color spots appear as fragmented small pieces limited to the surface of the opal, with a granular structure, and small black dot-like carbon dye is visible accumulating in the gaps of the color flakes or granules.
- Smoke Treated Opal
The black color is limited to the surface, with reduced density (1.38-1.39g/cm3)
- Silver nitrate treatment of opal
Upon magnified inspection, a silver-black precipitate can be seen in the pores; acetone or dilute hydrochloric acid can be used to wipe off the discoloration, and chemical analysis can detect silver.
- Aniline-stained opal
The dye precipitates in the pores or cracks, forming speckled pigment clusters as if “pepper powder” has been sprinkled.
(2) Foreign substance injection opal
- Injection-molded opal
Bright colors, stable properties, and high transparency. Upon magnification, bubbles, flow patterns, and flashes can be seen; infrared spectroscopy shows plastic absorption spectral lines; a hot needle test reveals an odor; acetone wipes result in color fading; opal density decreases, and the refractive index diminishes.
- Oiling (or waxing) opal
Greasy or waxy luster may appear, and when tested with a hot needle, oil or wax is extracted.
(3) Heat treatment of opal
Whether it is dyeing treatment or foreign substance injection treatment, opal must be purified and heated to remove impurities, discoloration, and adsorbed water. If the heating temperature is relatively high (300℃), most of the moisture in the opal can be extracted, allowing the dye and injected agents to occupy the moisture position. This indicates that when the opal is heated to 300℃, some isolated water molecules are lost, and all liquid water is lost. Therefore, when improving natural opal, heating should be done at a stable low temperature.
Section VII Improving turquoise
With a unique sky blue turquoise, it is mainly composed of water-containing copper aluminophosphate composed of cryptocrystalline aggregates, which are often Eloite, kaolinite, quartz, mica, limonite, phosphoaluminite and other symbiosis. These symbiotic minerals affect the quality of turquoise.
The pure color of turquoise is determined by the presence of Cu2+ ions, which define its blue base color, while the presence of iron and the loss of copper and water will affect its color changes and structural variations.
In addition, turquoise color, under the action of alcohol, aromatic oil, soapy water and some other organic solvents, can occur fading phenomenon.
Therefore, lower-grade turquoise needs to be artificially improved to enhance its aesthetic and economic value, satisfying the preferences and wear of people from both ancient and modern times, as well as from around the world.
1. Improvement process
Since turquoise has a certain porosity (especially sponge turquoise), various improvement methods can greatly enhance some turquoise that has a poor appearance, loose structure, and undesirable color.
(1) Foreign object injection
- Oil injection
Soaking turquoise in liquids like gasoline to change its color and luster. However, samples soaked in this way are prone to fading. This is a traditional improvement method that is now rarely used.
- Waxing
Boiling turquoise in paraffin (insect wax, Sichuan wax) can deepen the color of the turquoise and seal fine pores.
- Injection molding
Soak turquoise in colorless or colored plastic liquid for infusion, sometimes adding coloring agents. Once the plastic fully penetrates the pores or cracks, remove it and clean off the excess plastic from the surface. This method can enhance the stability of turquoise, increase surface smoothness, reduce surface light scattering, and give turquoise a medium blue tone, improving its appearance.
- Water glass
Soak turquoise in a water glass (sodium silicate) to allow the water glass to penetrate the pores or cracks of the turquoise, condensing and solidifying to enhance the stability of the turquoise and improve its transparency.
(2) Dyeing
Using the porous nature of turquoise, it is immersed in inorganic or organic dyes to dye light-colored or near-white turquoise to the desired color. After the dyeing liquid penetrates into the inside of the gem, the water is heated to make the dyeing liquid undergo a chemical reaction, so that the blue dye (or pigment) is deposited in the pores, making the gem colored.
2. Improving the characteristics of turquoise
Compared to natural turquoise, the improved turquoise has the following characteristics:
(1) Oiled turquoise
Oiled turquoise is very prone to fading and is rarely used now. It smokes when burned, and when probed with a hot needle, it “sweats.”
(2) Wax-impregnated turquoise
A hot needle touches it, and it “sweats”; it fades after exposure to sunlight or heat.
(3) Injection-molded turquoise
Refractive index less than 1.61 density less than 2.76g/cm3(hardness is generally only 3-4, the surface is prone to scratches. Zoom in and see bubbles. Hot needle test, there is a special spicy smell, and there are burn marks. In the infrared spectrum, there is a strong absorption spectrum line caused by plastics (1450-1500cm-1), and in the new injection variety, there is a strong absorption band of 1725 cm-1. X-ray diffraction analysis, there is a block of phosphonamidite phase. (Figure 6-8).
(4) Water glass turquoise
Density decreases, usually 2.40-2.70g/cm3; magnified observation reveals bubbles.
(5) Dyed turquoise
The color is unnatural, deep blue-green or deep green, with an overly uniform distribution; the color darkens at cracks due to dye accumulation; the color layer is very thin, generally around 1 mm; at the peeling areas on the surface of the sample and in the pits behind, an undyed light-colored core may be exposed; wiping with a cotton ball dipped in ammonia can make the cotton ball appear blue-green.

Section VIII Improving Amber
Amber is an organic mixture formed from the resin of coniferous plants from the Mesozoic Era, specifically the Cretaceous to the Cenozoic Era, through geological processes. It is formed from the resin of coniferous trees buried underground, undergoing petrification and diagenesis. It comes in various colors, among which light yellow and honey yellow are called honey wax, red ones are called blood amber, golden yellow ones are called golden amber, those containing biological remains are called insect amber, those that appear blue under ultraviolet light are called blue amber, highly petrified and hard ones are called stone amber, and those with fragrance are called fragrant amber, etc.
Amber is prone to oxidation, which can cause color changes and brittleness, and it often contains impurities such as sand, stones, insects, and grass, so it often needs to be improved and updated. Common types include compressed amber and coated amber.
1. Coated Amber
In recent years, the commonly seen coated amber can be divided into colorless and colored coatings, with the colored coatings further classified into full coating and partial coating.
These coating methods enhance the luster of amber, partially improve its color, and enhance the three-dimensional effect of “sunlight” in light-colored amber, thereby increasing the grade of amber.
(1) Colorless Coated Amber
Due to the low hardness of amber, it is easy to carve and difficult to polish. Now the amber products sold on the market about 99%of its surface are covered with a colorless transparent light film to achieve the purpose of enhancing luster and polishing, and played a certain anti-scratch role. Compared with natural amber, the characteristics of colorless coated amber are as follows:
① Strong luster can reach a bright resin luster.
② There are bubbles in the film; when the coating is thick, a large number of bubbles can be trapped in the depressions of the product, and when pricked with a needle, the film will peel off in sheets.
③ When scratched with a needle, its surface is mostly concave, has a sticky and soft feel, is not easy to crack, and feels similar to scratched plastic products.
④ Infrared spectroscopy detection shows that the composition of the colorless film is complex and varied.
(2) Colored Coated Amber
The commonly seen colored coated amber in the market mainly comes in two types: one has a colored film coated on the bottom of the amber product to enhance the three-dimensional effect of “too much light obstruction” in light-colored amber; the other is a spray of a colored glossy film on the surface of the amber product, making the amber present different shades of red blood amber or brownish-yellow “old beeswax.”
The characteristics of colored coated amber can serve as a basis for identification.
① Characteristics of amber with a colored film on the bottom
- Under magnification, the color layer of the coated amber is shallow, with no transition and uneven coloring.
- The coated surface often retains traces of spraying.
- Using a needle to pry, the film may sometimes peel off in sheets.
- The spectrum in the red area can detect the composition of the film, which is different from amber.
② Characteristics of amber with a colored film on the surface.
- Upon magnified observation, the color layer of the coated amber is shallow, with no transition and uneven coloring.
- Due to the large amount of spraying, there may sometimes be a concentration of color in the recessed areas of the coated amber.
- Due to uneven spraying, there may sometimes be uncolored areas in the recessed parts of the coated amber.
- After pricking with a needle or soaking in acetone, the film may sometimes peel off in sheets.
- Infrared spectroscopy can detect film components in amber that should not be present.
For coated amber, according to the national standard (GB/T16552), the definition of a film is “a film applied to the surface of gemstones using methods such as coating, plating, or lining to improve the luster, color, or produce special effects,” which should be classified as a type of “treatment” for gemstones and must be noted in the identification certificate.
2. Heat Treatment of Amber
To improve the transparency, clarity, color, and size of amber, oil boiling, and reconstruction methods are often used for optimization.
(1) Oil-Boiled Amber
Cloudy amber is heated and boiled in vegetable oil to increase the transparency of amber. This type of heat-treated amber often has leaflike cracks resembling “water lily leaves” and “sunlight rays.”
(2) Reconstructed Amber
The reconstruction of amber has been discussed in the chapter on synthetic gemstones, but during the reconstruction process, thermal energy plays an important role. Therefore, to some extent, reconstructed amber also falls under the category of thermal energy processes.
Reconstructed amber can be divided into three types: fused amber, compressed amber, and molded amber.
Compressed amber is a type of reconstructed amber made from natural amber as raw material, formed into an overall organic gemstone through medium to low-temperature heating and pressure.
Compressed amber has characteristics that are different from natural amber and fused amber, and the obvious indicators for identification are:
① Dark red fibrous bodies
There are dark red filaments, clouds and lattice-like bloodlines visible to the naked eye in the pressed amber. This is a thin red oxide film formed by oxidation of the aged amber raw material, which is more clearly seen under ultraviolet fluorescence. Natural amber is sometimes blasted to form cracks due to temperature, humidity and other effects, and is oxidized to be red, but it is distributed along the cracks in a dendritic shape rather than along the edges of the particles.
② Animal and plant inclusions
In compressed amber, complete and intact animal or plant inclusions are not seen, nor is there any introduction of foreign substances.
③ Bubbles
Compressed amber contains abundant gaseous inclusions; these bubbles not only come from the original natural amber but also form new bubbles between particles and, during stirring, are irregularly distributed throughout the amber and densely small. Although they may burst during heating to form “amber flowers” resembling water lilies, they are particularly small and often arranged in layers of space.
④ Flow structures
Although pressed amber sometimes shows flow structures that are either obvious or not, it is accompanied by indistinct boundaries between particles, appearing very uniform internally; however, this structure can also be found in natural amber.
⑤ Luminescence
Under ultraviolet fluorescent light, pressed amber exhibits the luminescent properties of natural amber, which often reveals the edges and contours of amber particles, allowing clear observation of individual connections and the shapes of the particles. In samples with dark red thread-like bodies, the boundaries of the particles can be seen distributed along the thread-like bodies.
(3) Dyed Amber
The practice of dyeing amber has a long history, with ancient methods using natural plant dyes to color amber in various shades (red, green, purple, etc.) to mimic the characteristics of aged amber. Modern dyeing, some jewelry manufacturers also use organic dyes, because amber is also organic matter, and the two are easy to react, so that the dye chromophore penetrates into the interior of amber, resulting in different colors of amber dyes.
Section IX Improvement of pearls
Pearls are known as the queen of gemstones. They are round, have soft colors, and their luster is captivating. They are pure and beautiful, highly cherished by people. Pearls have a unique body color, accompanying colors, and a combination of iridescence, making them easily distinguishable from any other jewelry or gemstones.
Beautiful pearls undergo optimization treatment, which will enhance their color and increase their commercial value. The methods for improving pearls are divided into two main types: optimization and treatment.
1. Optimized pearls
The optimization process of pearl is generally divided into pretreatment, purification, bleaching, whitening and polishing.
(1) Pretreatment
The quality of pearl pretreatment directly affects the effectiveness of subsequent processes. Pretreatment mainly includes sorting and drilling stages.
① Sorting
Grading of Cultured Pearls,” sorting is done based on the size, shape, luster, color, and thickness of the pearl layer so that they can be treated separately. This not only benefits the economic value utilization but also, due to the different thicknesses of the pearl layers and the varying organic pigment clusters and impurities in different types of pearls, the reagents, dosage, concentration, and time parameters used will differ, making sorting beneficial for optimizing the improvement of effects.
② Drilling
Drilling the sorted pearls, according to processing requirements, can be done as half-drilling or full-drilling. Drilling can also reduce or eliminate surface defects, such as pits on the pearls, and promote purification and whitening effects.
(2) Purification
Purification is the process of using purifying agents to remove dirt and moisture from the surface of the pearls, which involves the following steps:
① Expansion
Soak the pearls in a mixture of benzene (C6H6) and ammonia water (NH4OH) at low temperature (35-50℃) for several hours, then take them out and rinse them several times with deionized water. The purpose of swelling is mainly to enhance the connectivity of the pores in the pearl structure, making it a bit “looser.”
② Dehydration
After swelling and cleaning the pearls, proceed to dehydration. Soak the pearls in a detergent solution for a while, then rinse them several times with clean water and let them dry; use anhydrous ethanol or pure glycerin as a dehydrating agent to remove the adsorbed water in the pores and cracks of the pearl structure.
③ Sunlight
After the pearl is puffed and dehydrated, it is exposed to the sun and dried.
(3) Pearl Bleaching
The pearl bleaching process, which began in 1924, is the most important part of pearl optimization, as pearls often exhibit undesirable colors due to the presence of organic pigment clusters and impurity ions, affecting the color grade of the pearls. Pearl bleaching is essentially a chemical reaction the bleaching solution is a mixture of bleaching agents (hydrogen peroxide), solvents (organic solvents, water), surfactants (alcohols, ketones, ethers, etc.), and pH stabilizers (triethanolamine or sodium silicate)]. Currently, the jewelry industry mainly uses two methods: hydrogen peroxide bleaching and chlorine bleaching.
① Hydrogen Peroxide Bleaching Method
The pearl is soaked in a solution of hydrogen peroxide (H2O2) with a concentration of 2%-4%, the temperature is controlled at 20-30℃, the PH value is between 7-8, and it is exposed to sunlight or ultraviolet light, after about 20 days of bleaching, the pearl will become gray or silver white, and it is best to become pure white.
This process mainly includes five steps: soaking, washing, liquid replacement, pearl selection, and decontamination. The required equipment mainly consists of a light and temperature control device, a bleaching container, and a vacuum washing device. The formula for the bleaching solution is confidential; a Japanese research institute proposed a formula in 1930: 3% of H2O2 1000ml, 10 ml of benzene, 10 ml of ether, neutralized with ammonia water, adding an appropriate amount of PH stabilizer, temperature below 30-50℃, with the surfactant being dioxane and the stabilizer being triethanolamine.
② Chlorine Bleaching Method
The bleaching ability of chlorine is stronger than that of hydrogen peroxide. Improper use can make pearls brittle and fragile or leave a chalky, powdery surface on the pearl’s surface. Therefore, this bleaching method is usually not commonly used.
(4) Pearl Whitening
The bleaching method cannot completely eliminate organic pigment clusters, resulting in pearls not turning completely white. After bleaching, the base color of the pearls is primarily white. To enhance the whiteness and luster of the pearls, a fluorescent whitening treatment is still needed. The fluorescent whitening method is an optical whitening method that utilizes the principle of complementary colors in optics to achieve the goal of removing yellow and discoloration from pearls to enhance their whiteness.
Is the whitening agent that makes pearls whiter a special fluorescent coating? It emits blue fluorescence that is complementary to yellow, resulting in a blue-white appearance of the pearls. Commonly used whitening agents include AT, DT, VBL, PBS, WG, RBS, etc., with a typical dosage of around 0.5%-3%.
There are two types of fluorescent whitening agents: direct dye type (water-soluble) and dispersive type.
① Direct dye whitening method
During the bleaching process, the whitening agent can be used simultaneously with the bleaching solution, or it can be used alone.
If used alone, the pearls should be purified in advance and then soaked in the whitening solution. In the whitening solution, in addition to the whitening agent, there are also solvents (water and organic solvents) and surfactants as auxiliaries. This method requires high water quality, free from metal ions such as iron and copper, and generally requires softening treatment.
② Dispersive whitening method
The use of solid powder to whiten the color of pearls is the third-generation whitening process currently adopted in Japan. The specific process is not detailed, but it is likely that some method is used to permeate and fill a certain fluorescent whitening agent into the inner layer of the pearls.
(5) Polishing
Polishing, or buffing. Pearl polishing is also a very important process. Good polishing can enhance the bleaching and whitening effects. The polishing materials currently used include small bamboo pieces, small stones, and paraffin, as well as sawdust, granular salt, and diatomaceous earth.
After polishing the pearl, wash it with detergent and let it dry in the sun.
2. Processing Pearls
(1) Dyed Pearls
Currently, in the market, most colored pearls (black, silver-gray, pink, red, orange-yellow, etc.) are dyed, except for white pearls.
The dyeing process of pearls is similar to the bleaching process. After pre-treatment and purification, the pearls are placed in a vacuum filtration bottle, then immersed in the dye solution (at a temperature below 30 〜40℃ ) for one to two days until the desired color is achieved.
The dye solution consists of dyes (mostly organic dyes), solvents (pure water, organic solvents), and penetrants (potassium iodide or pyridine). Commonly used dyes include peach pink, pink, and magenta.
The dyeing of pearls can be divided into two methods: chemical dyeing and center dyeing.
① Chemical dyeing method
Soak the pearls in certain special chemical solvents to dye them. For example, using dilute silver nitrate and ammonia solution as dye, soaking the pearls turns them black; using cold potassium permanganate as dye can turn them brown.
② Central dyeing method
First, after swelling and removing impurities from the pearls, inject specific dyes into the pores and holes of the pearls to make them show color.
Regardless of the dyeing method, there is a certain level of deception. Dyed pearls have bright colors and uniform luster. Dyes often concentrate in the pores and cracks of the pearls.
(2) Irradiated pearls
The radiation irradiation method is a pearl improvement process that began in the 1960s and is currently widely used. The radiation source used is 60 Co , with an intensity of 3.7 x 1013 Bq , a radiation distance of 1 cm, and an irradiation time of about 30 minutes. Irradiated pearls can produce blue-gray and black colors, with seawater pearls being somewhat darker. Additionally, neutron irradiation of certain freshwater pearls can produce silver-gray colors.
The color of irradiated pearls is stable to light and heat, and it is easy to distinguish from silver nitrate dyeing in tone, but irradiation may cause radioactivity, and not all pearls can utilize irradiation to change color.
(3) Filling Pearls
The surface of pearls often has some small cracks and bumps, affecting the luster and smoothness of the pearls, which must be repaired and healed. There are two treatment methods.
① Peeling and Smoothing
Use very fine tools to carefully peel off the unsightly surface layer of the pearl to achieve a smooth and even surface, hoping a better layer of pearls appears beneath the surface, achieving the goal of transforming it into jade.
② Filling the pores
The small cracks on the surface of the pearl, or the marks left by peeling and polishing, must be repaired and filled. The specific method is to soak the peeled, polished, and cleaned pearls in hot olive oil. The oil’s penetration gradually heals and repairs the cracks and wounds on the pearl’s surface, achieving a smooth, rounded surface with a bright color. If the olive oil is heated to 150℃ , a deep brown color will appear on the surface of the pearl.
3. Improving pearl identification
After the above optimization or treatment, the pearls become bright in color, smooth, and round. The distinguishing features of dyed pearls compared to natural pearls are as follows:
(1) Color characteristics
① Dyed pearls
Dyed black pearls have a uniform color, but in areas with lesions or cracks, the color is deeper, resulting in uneven local color distribution. For drilled dyed pearls, there is often color concentration and small color spots near the hole, on surface cracks, and at peeling areas. On the string of beads, traces of color fading can be seen. If a cotton ball soaked in diluted nitric acid is used to wipe a dyed black pearl, the cotton ball will turn black. Other brightly colored dyed pearls have the same color distribution as dyed black pearls; if they are strung together, their tones and shades are consistent.
② Core
The dyed black nucleated pearls show a strong color difference between the white nucleus and the black nacre when viewed through the drilled hole; nucleated pearls dyed in other colors have both the nucleus and the nacre layer dyed, revealing a black inner core. Nucleated pearls that have been color-changed through irradiation show a black nucleus, while the nacre layer is nearly colorless and transparent.
③ Accompanying colors
Black pearls that have been color-changed through irradiation exhibit vibrant hues in the spectral color, along with a metallic luster, but the color is uniform and lacks the diversity of accompanying colors found in cultured pearls.
(2) Ultraviolet fluorescence
Dyed pearls are often emotional; freshwater pearls often exhibit yellow-green fluorescence, while seawater cultured pearls often show weak blue-white fluorescence.
In addition, generally speaking, dyed black pearls have a diameter greater than 9 mm, while dyed or irradiated pearls are mostly less than 8 mm.
Section X Other improvements for gemstones
In the current jewelry market, almost all natural gemstones can be improved, and even synthetic gemstones have improvement products.
The characteristics of common gemstone improvement products are summarized in Table 6-1, for more details, please visit the website: https://sobling.jewelry/improving-gemstones-the-art-and-science-of-enhancing-jewels/