Why Gemstones have multiple Colors?
Secrets of colors formation and measure methods
The colors of gemstones are rich and varied, possessing a unique charm that people have always loved. The quality of gemstones largely depends on their color. The color of a gemstone is an important indicator in gemstone evaluation, and most optimization treatments for gemstones involve changing or improving their color. Therefore, understanding the causes of gemstone coloration is a crucial prerequisite for gemstone optimization treatment. Only by mastering how gemstones acquire their color can one determine whether a gemstone can be optimized, which optimization scheme to adopt, and which experimental plan to establish. There are five common theories of gemstone coloration: classical mineralogy theory, crystal field theory, molecular orbital theory, energy band theory, and physical optical effects. These theories constitute the coloration theories of common natural gemstones, and the following is a brief introduction to these five coloration theories.
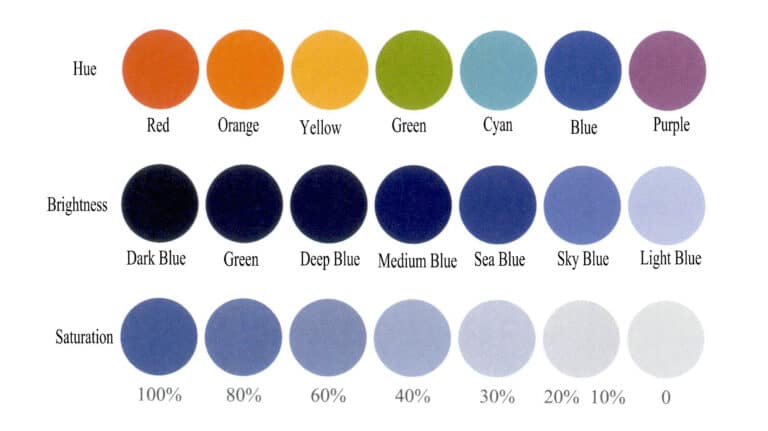
Schematic Diagram of the Three Elements of Color
İçindekiler
Section I Color and Measurement of Gemstone Color
The colors of gemstones are rich and varied, and determining the types of gemstone colors is crucial for evaluating their value. Different color grades also affect the value of gemstones; therefore, accurately assessing the colors of various gemstones is a fundamental prerequisite for determining their value. When evaluating colored gemstones, color is the most important factor. Generally speaking, the more attractive the color of a gemstone, the higher its value. Bright, rich, and intense colors are usually more appealing than those too dark or too light. Of course, there are exceptions, such as diamonds, where the higher the whiteness of the diamond’s color, the higher its value.
1. The Significance of Gemstone Colors
Since ancient times, people have loved gemstones for their unique charm, especially the rich and colorful hues of gemstones, such as the pigeon blood red ruby, the vivid green emerald, and jade, all of which leave a lasting impression. Color is an important indicator for evaluating the quality of gemstones and plays a crucial role in determining their quality, with significance mainly reflected in the following three aspects.
(1) The color of gemstone minerals is an important basis for evaluating gemstones
The color of a gemstone is fundamental to its evaluation and determines its value. For example, in diamonds, a one-grade difference in color can lead to a price difference of approximately 5%. The higher the whiteness, the higher the grade of the diamond; conversely, diamonds with yellow or brown tones have lower grades, and their prices plummet. The prices of colored diamonds vary, and different colored diamonds have different prices. However, generally speaking, the prices of rare-colored diamonds can multiply. Other colored gemstones, such as rubies, sapphires, and emeralds, are also classified into different grades based on color, and the value of gemstones in different grades can vary significantly.
(2) The optimization treatment of gemstones often involves improving their color
The optimization treatment methods for gemstones usually involve changing or improving the color of the gemstones, so the improvement of gemstones can also be referred to as gemstone color alteration. As the color of the gemstone improves, the transparency also changes accordingly. This is because transparency is a property related to color. For example, blue sapphires appear to have many opaque black colors to the naked eye, but transparent blue can be seen when cut into thin slices. The improvement in transparency often accompanies the improvement in color. Therefore, only by determining the cause of the gemstone’s color can one ascertain the methods for improving the gemstone. Understanding the cause of color is a prerequisite for studying gemstone optimization treatments.
(3) Studying the causes of gemstone color provides a theoretical basis for synthetic gemstones and gemstone improvement.
The colors of gemstones such as garnet, malachite, and olivine are due to their inherent components, and these gemstones cannot have their colors changed using conventional optimization treatment methods. Most gemstone colors are due to impurities caused by impurity ions, such as in rubies, sapphires, emeralds, jade, and agate. Based on the causes of gemstone color, during improvement, the content and valence state of certain color-causing impurity ions can be altered to change or improve the color of the gemstone, thereby enhancing the quality of the improved gemstone. Therefore, studying the causes of gemstone color is the theoretical basis for improving gemstones.
2. The Physics of Color
(1) Color and Light Waves
Photons carry the energy of light, and when photons reach the human eye, they create the sensation of color. Color is the perception of light by the eyes and the nervous system; the response generated by the signals formed on the eye’s retina stimulates the cerebral cortex. The formation of color perception has three main components: the light source, the object, and the human eye. Changing one or more of these three will alter the perception of color. Gems interact with light, and phenomena such as reflection, refraction, transmission, interference, and diffraction occurring on the surface of the gem result in different colors.
The energy range of the electromagnetic radiation spectrum is quite large, varying from very long radio wave photons to very short ray photons, with an energy range from less than one billionth of an electron volt to over one hundred million electron volts.
The visible light the human eye can accept and perceive is a very small part of the electromagnetic spectrum, with a wavelength range of 400-700nm and an energy of about 1.7-3.1eV. If the observation conditions are good enough, the range can be extended to 380-760nm (Figure 3-1). Visible light includes the colors we see, namely red, orange, yellow, green, cyan, blue, purple, and various other colors. Different wavelengths of visible light produce different colors; the visible light color with the longest wavelength and lowest energy is red, with a wavelength range of 647-760nm; the visible light with the shortest wavelength and highest energy is purple, with a wavelength range of 400-425nm. Other visible light colors fall between 425 and 647nm. The wavelengths of various colors of visible light and their complementary colors are shown in Table 3-1.
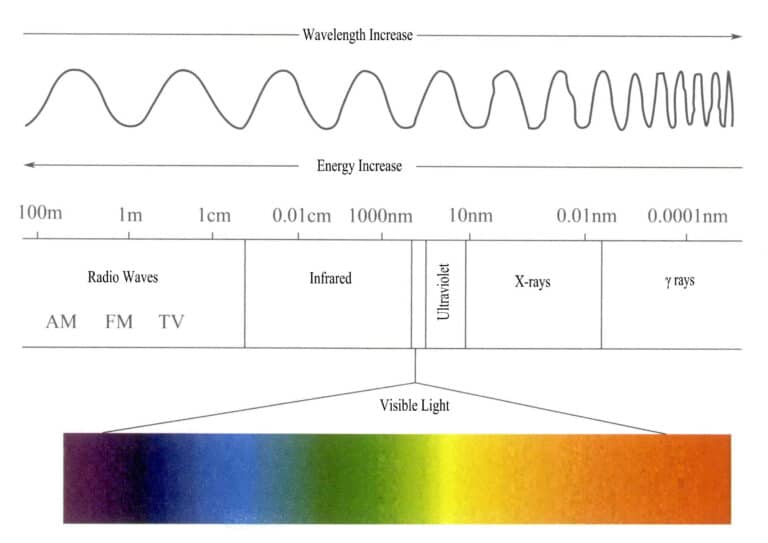
Table 3-1 Wavelengths of Various Colors of Visible Light and Their Complementary Colors
| Wavelength/nm | Spectral color | Complementary |
|---|---|---|
| 400 ~ 425 | Purple | Yellow-green |
| 425 ~ 455 | Mavi | Sarı |
| 455 ~ 490 | Green-blue | Orange |
| 490 ~ 500 | Blue-green | Kırmızı |
| 500 ~ 560 | Yeşil | Magenta |
| 560 ~ 580 | Yellow-green | Purple |
| 580 ~ 595 | Sarı | Mavi |
| 595 ~ 647 | Orange | Green-blue (cyan) |
| 647 ~ 760 | Kırmızı | Yeşil |
The essence of the color of an object is the result of the object’s selective absorption of different wavelengths of visible light. The essence of an object’s selective absorption of different wavelengths of visible light is the absorption of photons of visible light with different energies. When natural light shines on a gemstone, the gemstone absorbs part of the light and also transmits part of it. The color presented by the gemstone is the complementary color of the absorbed light, consistent with the color of the transmitted light (Figure 3-2). For example, in the case of ruby, when white light passes through the ruby, the chromium ions contained in the ruby gain energy by absorbing all the violet and green photons as well as most of the blue photons, while the other color photons, predominantly red, pass through the ruby, causing the gemstone to appear red.
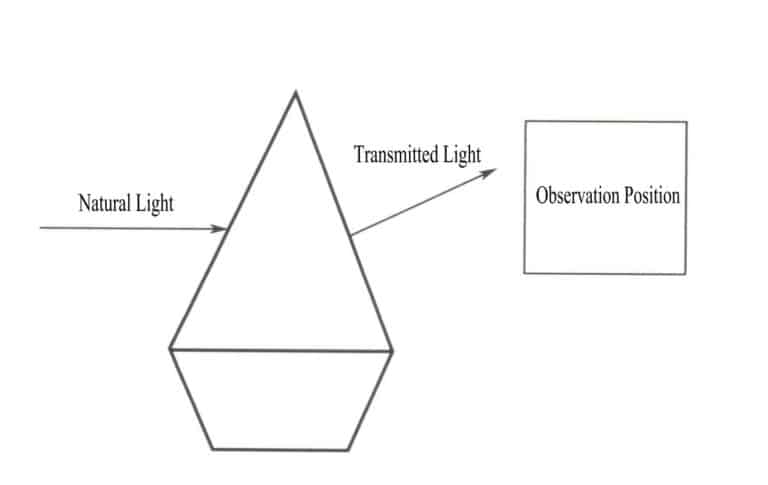
A single wavelength of light does not produce the color formed by an object; rather, the object’s radiation is a mixture of photons with different energies, where the energy band with the largest proportion determines the object’s color. A uniform mixture of various colors of light forms white light. The generation of gemstone colors results from the selective absorption of photons of different wavelengths of visible light. When white light passes through a gemstone, the light absorbed and transmitted by the gemstone is a mixture, and the color presented by the gemstone depends on the one with the largest proportion of the transmitted light. For example, in the case of a ruby, when white light shines on the ruby, the light that passes through is predominantly red with a small amount of bluish-purple. Thus, rubies often appear red with a bluish-purple tint.
(2) Types and properties of light sources
The color of gemstones has a certain subjectivity, which is related to the observer’s environment and is most influenced by the light source. Observing the color of gemstones under different light sources can show differences; for example, a alexandrite appears green in sunlight but red under incandescent light. Generally, people consider the color seen in natural sunlight the standard, commonly referred to as white light.
The initial gemstone market operations were conducted at specific times to obtain a relatively accurate gemstone color. For instance, gemstone shops in Ratnapura, Sri Lanka, have operated for many years from 10 AM to 12 PM, as the light source during this time is closest to white light. Sunlight and daylight are not isotropic light sources, and the relative differences in the relative amounts of radiation in different wavelengths among different light sources can be very large due to different observation conditions. Figure 3-3 lists the energy distribution curves of five common light sources, and the energy differences among these light sources are very large.
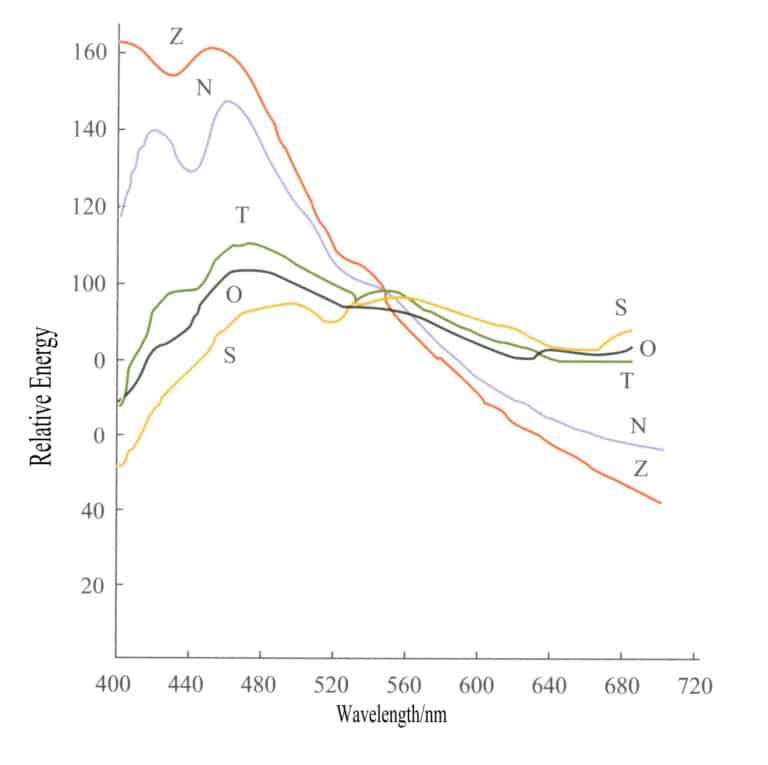
S – Direct sunlight; O – Illumination of overcast sky light on the horizontal plane; T – Illumination of sunlight and clear sky on the horizontal plane; N – Light from the clear northern sky; Z – Zenith light;
The color characteristics of direct sunlight are often expressed in terms of color temperature (measured in K). The same color temperature indicates similar colors of light sources. The currently recognized daylight color temperatures are D6500K, D5500K, and D7500K. Three lamps are internationally designated as standard light sources for color measurement work. SA represents the average artificial illumination of an incandescent tungsten lamp, with a color temperature of 2854K;SB represents average sunlight, with a color temperature of 4900K; SC represents average daylight, with a color temperature of 6700K. In gem testing, the Sc light source is used as the standard light source.
(3) Sensitivity to light and color effects
The observation of gem colors is subjective; in addition to objective conditions, the gem’s color is also related to the observer’s sensory perception. Under the same light source, human eyes have different sensitivities to different colors, and different people have varying sensitivities to colors. The observation of color is subjective; to achieve objectivity as much as possible, colors need to be characterized and expressed relatively objectively.
① Photoreceptive Effect:
Under normal conditions, the wavelength range of visible light sources the human eye can observe is 400-700nm. The sensitivity range can be extended by improving observation conditions to 380-780nm. The human eye has different sensitivities to light waves of different wavelengths. For daytime vision, the eye is most sensitive to green light with a wavelength of 555nm, while the most sensitive wavelength for twilight vision shifts to 507nm. The traffic lights on the road are designed based on the colors to which the human eye is most sensitive.
② Color Sensation:
Color is the sensation caused by the different spectral components of radiation energy within the visible light range. A normal person’s vision can distinguish more than 150 shades of pure spectral colors. Although there is a one-to-one correspondence between light waves and colors, the correspondence between colors and light waves is not singular; often, one color of light can be formed by the combination of two or more other colors of light. The three basic independent colors are red, green, and blue, known as the primary colors. Other colors are formed by mixing two or more primary colors in different proportions, and the human eye is very sensitive to colors, capable of distinguishing many different colors.
3. Three Elements Representing Color
Color results from selective absorption of light by gemstones, and different gemstones exhibit different colors. Current color theory suggests that color characteristics depend on brightness, hue, and saturation (Figure 3-4). The types of gemstone colors can be determined by describing the three elements of different colors.

(1) Brightness
Brightness is the degree of brightness caused by light acting on the human eye, referring to the degree of light and dark colors, and can also be called luminance. Brightness largely depends on the eye’s perception of the light and dark levels of the light source and the object’s surface, primarily determined by the intensity of the light. Brightness depends on the object’s illumination level and the reflectivity of the object’s surface.
Brightness can be understood as the brightness of a color; different colors have different brightness levels, and any color exhibits variations in light and dark. Brightness has two characteristics: the same object can exhibit changes in brightness due to different lighting, and different colored lights of the same intensity can also have different brightness levels.
In the absence of color, the color with the highest brightness is white, and the color with the lowest brightness is black, with a grayscale between light and dark. In color, any degree of purity has its brightness characteristics. For example, yellow has the highest brightness, while purple has the lowest. Green, red, blue, and orange have similar brightness levels, representing medium brightness. Additionally, within the same hue, there are variations in brightness from light to dark, such as light green, pale green, and emerald green in the green spectrum.
(2) Hue
Hue refers to the differences between different colors and is the most prominent characteristic of color. Hue is determined by the spectral components of light that pass through an object and reach the human eye after being incident on it, depending on the wavelength of the transmitted light. The hue of an object is determined by the spectrum of the incident light source and the light reflected or transmitted by the object itself.
The so-called hue refers to the name that can more accurately represent a certain color category, such as rose red, orange-yellow, lemon yellow, cobalt blue, purple-red, emerald green, etc. From the perspective of physical optics, various hues are determined by the spectral components of the light that enters the human eye. For monochromatic light, the types of hue completely depend on the wavelength of that light; for mixed color light, it depends on the relative amounts of various wavelengths of light. The color of an object is determined by the spectral components of the light source and the characteristics of the light reflected (or transmitted) by the object’s surface. It is related to the wavelength of light. For example, a color with a dominant wavelength of 470nm is called blue of 470nm wavelength, commonly seen as the blue of sapphires.
(3) Doygunluk
The saturation of a color refers to the purity and vividness of the color, indicating the proportion of chromatic components contained in the color. The greater the proportion of chromatic components, the higher the purity of the color; the smaller the proportion of chromatic components, the lower the purity of the color. The saturation changes when a color is mixed with black, white, or other colors. When the proportion of the mixed color reaches a significant level, the original color will lose its original brilliance to the eye, and the color seen will become that of the mixed color. Of course, this does not mean that the original color no longer exists, but rather that the original color has been assimilated due to the many other colors mixed in, making it unnoticeable to the human eye.
The monochromatic lights in the visible spectrum have the highest saturation and are the most vivid. Monochromatic light is usually considered as 100/100 = 1, and as the color fades, the value gradually decreases, with pure white having zero saturation. Taking pure blue ink as an example, the saturation of pure blue ink is 1, and as it gradually dilutes to completely colorless, the saturation becomes zero.
4. Measurement of Gemstone Color
The system that quantitatively represents color is called a color system. There are two commonly used types of color systems: one is a color system based on standard color samples for comparison, and the other is a set of color standard systems measured using modern color measurement instruments.
(1) Color System of Standard Color Samples
This color system consists of various “color cards” made from paper that are standard color samples compiled into a book. They are compared with gemstone samples to select the “color card” that matches the gemstone’s color.
① Munsell Color System
The Munsell Color System is one of the earliest and most classic color representation systems. It is still used by some organizations today. It was established in 1905 by American educator and color theorist Albert Munsell, who was named directly after him. It is a way of representing colors through a color-solid model. The “Munsell Color Atlas,” published by the Optical Society of America (OSA), includes glossy and matte versions.
The glossy version includes 1,450 color samples, accompanied by a set of 37 achromatic samples; the matte version includes 1,150 color samples and 32 achromatic samples.
In the Munsell Atlas, each color is represented by a set of symbols. The symbols provide equidistant indicators for the three elements of color representation: hue, value, and chroma, represented as HV/C = hue value/chroma.
Hues are divided into five primary tones: red (R), yellow-red (YR), yellow (Y), green-yellow (GY), green (G), blue-green (BG), blue (B), purple-blue (PB), purple (P), and red-purple(RP), along with five intermediate tones. Each tone is further divided into ten levels (1 to 10), with the fifth level being the intermediate color of that tone (Figure 3-5).
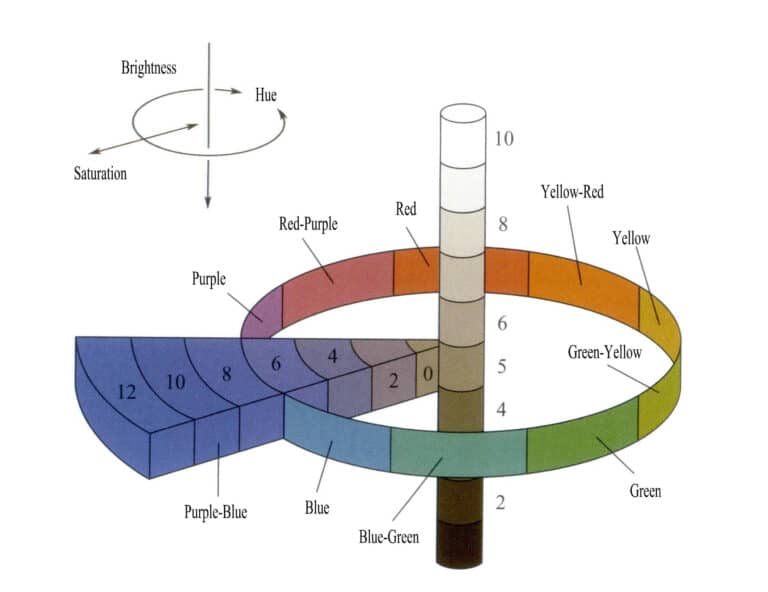
Value is divided into 11 levels, with higher values indicating higher lightness; the minimum value is 0 (black), and the maximum is 10 (white). Chroma is divided into 12 levels. The entire color atlas includes 40 types of hue samples. For example, 5GY 8/7 represents a yellow-green with a value of 8 and a saturation of 7. The naming convention for non-color (black, white, gray) series is NV/= neutral lightness value; for instance, a color labeled N5/ indicates a gray with a lightness value of 5.
② DIN 6164 Color System
Germany’s DIN 6164 manual is also an important color system. Many gemologists in Europe and the UK use this system. This color system was developed based on the Munsell system.
The DIN 6164 color card has 24 colors, with the back of each color marked with the corresponding Munsell color notation. The representation is in the format of hue: saturation: lightness. For example, 6:6:2 represents hue 6 (red), saturation 6 (vivid), and lightness 2 (light) of the standard color card.
③ ISCC-NBS Color System
ISCC (Inter-Society Colour Council) was established in 1931 as a domestic color association in the United States, and its color system aims to develop a color naming system. It collects 18 hues at the same hue and lightness positions in Munsell and DIN 6164.
ISCC-NBS (National Bureau of Standards) has very few color samples in the American National Standard system but has collected some uncommon samples. Structurally, it also differs from the Munsell system, as colors are not arranged according to perceptual equidistant metrics. The most important contribution of the ISCC-NBS system to color science is that it defines color names.
④ OSA Color Standard
OSA (Optical Society of America) has prepared a practical set of acrylic gloss color cards, including 558 colors, of which 424 colors make up a set known as the OSA color standard. The drawback of the OSA color standard is that the color cards are made of paper or plastic, which differs in texture from gemstones, and the surface gloss of the color cards differs from the light reflected by faceted gemstones, requiring careful use. Due to fading, most color cards have a usage time of 4 to 5 years.
(2) Chromatic Coordinates and Chromatic Diagram
① 1931 CIE-XYZ Color Space System
This color space system is based on the RGB system and uses mathematical methods to select three ideal primary colors to replace the actual primary colors. Using virtual primary colors as axes, it projects different wavelengths of visible light. The values of the primary colors are called tristimulus values. This system requires that the tristimulus values are not negative and that y equals the luminous flux. Using the x as horizontal axis and y as the vertical axis, it forms chromatic coordinates, projecting the chromatic coordinate values of each monochromatic light to obtain the chromatic diagram. Figure 3-6 is the standard chromatic diagram of the (XYZ) system established internationally in 1931.
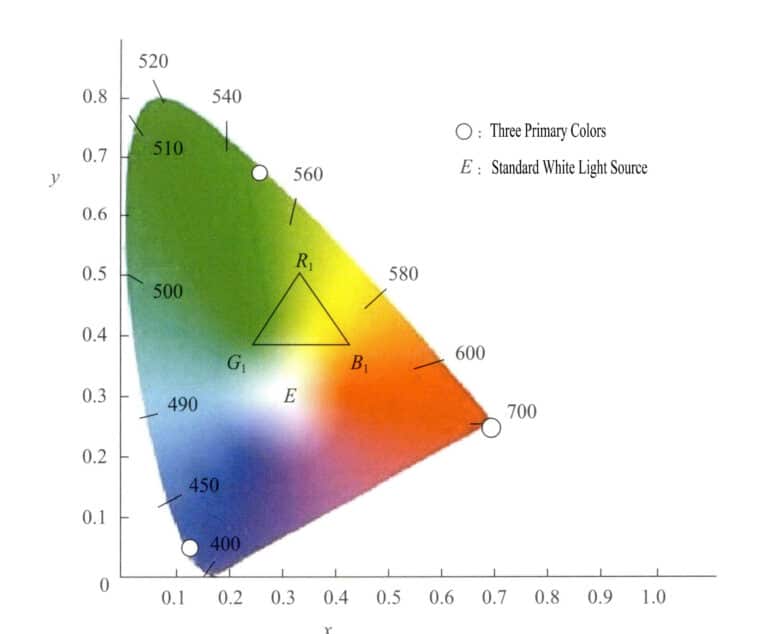
The chromatic diagram has the following characteristics:
- The tristimulus values of this system’s primary colors are virtual.
- All points representing spectral colors fall on the tongue-shaped curve called the spectral color. Connecting the ends of the curve with straight lines, all actual colors are contained within the area surrounded by the tongue-shaped curve and the straight lines.
- The coordinate value of point E at x=0.333, y=0.333 represents theoretical white. Different white light sources have slightly different spectral components. The commonly used white light is divided into SA, SB, SC, SE, and so on.
- The chromatic coordinates of colors are higher in saturation the closer they are to the spectral color trajectory. Points on the spectral color trajectory have the highest saturation; the white point has the lowest saturation. A line drawn from the white point to the chromatic coordinates of a color, extended to intersect the spectral color trajectory, has points along this line that share the same hue.
- A graphical method can be used to derive the composite color of any two colors. By inputting the chromatic coordinates of the two colors into the chromatic diagram, the composite color must lie on the line connecting the two color coordinate points. The distance projected to the two color points is related to the intensity of the two colors, determined by the centroid distribution law.
- Points on the straight line between the endpoints of the spectral color curve do not represent spectral colors but rather various mixed colors obtained by mixing purple at 380nm and red at 780nm in different proportions.
- Select any three points within the spectrum color trajectory to create a color. For example, if you choose R1, G1, B1 as the three colors for matching, then all the various colors formed by these three hues are contained within the triangle with vertices at the three points R1, G1, B1.
② Representation of dominant wavelength and saturation
In the chromatic diagram, colors can be represented not only by chromatic coordinates (x, y). Another method proposed by Helmholtz is to represent them using the dominant wavelength λd and saturation (stimulus purity) Pe. λd and Pe are specific values derived from the chromatic coordinates in the diagram (Figure 3-7). The dominant wavelength roughly represents the color sensation perceived by the human eye.
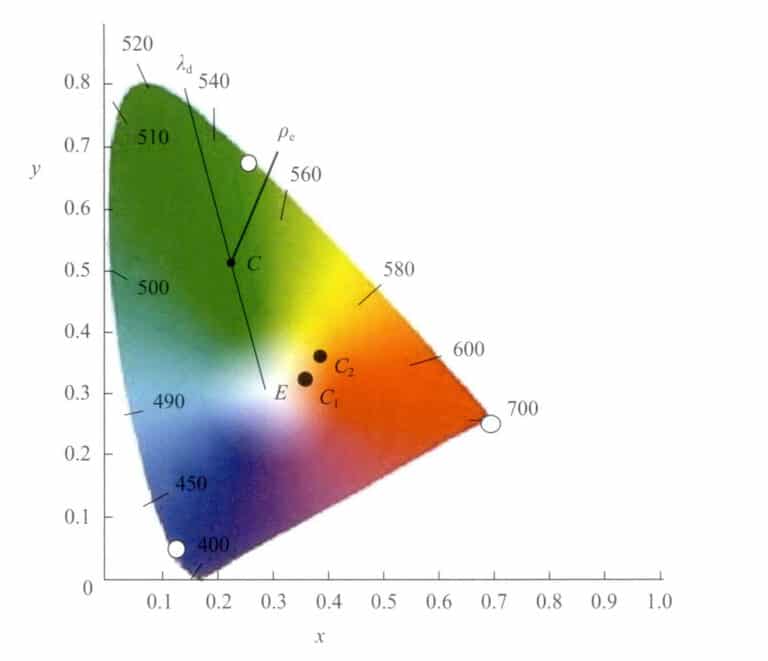
Let the position of this point in the chromatic diagram be c(x, y). Connect point c with white E and extend it to intersect the spectrum trajectory at λd . Then, the wavelength number of point λd on the spectrum trajectory is the dominant wavelength of this color light.
The point C is located on the line connecting points W and λd , representing the saturation of this color. Point W is pure white (E) or a specific white light source. When the chromatic coordinates of a certain color gradually move along a direction towards the spectral trajectory from the white point W, the color’s saturation gradually increases until it reaches the spectral trajectory, with a maximum saturation of 1.
Colors are represented using dominant wavelength and saturation, making it easy to compare color differences. The slight color differences, represented by the numerical changes in λd and ρe, can be displayed.
For two colors, C1, C2, the chromatic coordinates, dominant wavelength, and saturation under the same illumination are shown in Table 3-2. It can be seen that the dominant wavelength of color C1, C2 differs by 0.052nm, and the saturation differs by 7%.
Table 3-2 Comparison of color data between C1 and C2
| Color projection point | X | y | λd | ρe |
|---|---|---|---|---|
| C1 | 0.368 | 0.416 | 0.592 | 0.35 |
| C2 | 0.392 | 0.355 | 0.540 | 0.28 |
5. Instruments for Measuring Gemstone Color
(1) Spectrophotometer
Working principle: Because the spectral energy distribution of the standard light source inside the instrument is known, the tristimulus values of an object can be obtained by measuring its spectral reflectance.
(2) Tristimulus Value Colorimeter
It is a photoelectric integrative instrument that directly measures the three stimulus values of an object’s color. It mainly simulates the standard observer’s three responses to color through an appropriate combination of filters and photoelectric converters.
Currently, the judgment of gemstone color mainly relies on human eye observation. For example, emeralds, rubies, and diamonds are affected by environmental factors, leading to low accuracy in instrument measurements; therefore, the application of instrument color measurement in gemstone identification is relatively rare.
Section II Theoretical Origins of Gemstone Mineral Color
Gemstone minerals come in various colors, but the reasons for their coloration also differ. According to classical mineralogy theory, the origins of gemstone mineral color can be classified into idiochromatic-color, allochromatic-color, and pseudo-color.
1. Classification of Classical Mineralogy Color Origin Theory
The classic theory of mineralogy is the most fundamental theory for studying the colors of gem minerals. According to whether the color of the mineral is due to the mineral itself, it can be divided into three categories: idiochromatic-colored, allochromatic-colored, and pseudo-colored.
(1) Idiochromatic-colored
The color of a gem is formed by the inherent chemical components of the mineral it is composed of, known as idiochromatic-colored. Such gems are called idiochromatic-colored gems. Their inherent components cause the color produced by idiochromatic-colored gems; thus, they have good color stability and are not easily altered. For example, the chemical composition of turquoise isCuAl6(PO4)4(OH)8-5H2O, and the blue or blue-green it presents is caused by Cu2+; the chemical composition of azurite is 2CuCO3 • Cu(OH)2, and Cu2+ also causes its blue color”; the green of olivine is produced by iron ions in its chemical composition (Figure 3-8).

There are not many types of self-colored gems in natural gem minerals, with the main varieties being turquoise, malachite, azurite, olivine, garnet, and rhodochrosite. The common colors, coloring elements, and chemical compositions of self-colored gems are shown in Table 3-3.
Table 3-3 Common coloring elements, chemical composition, and colors of colored gemstones
| Coloring elements | Gemstone Name | Kimyasal bileşim | Renk |
|---|---|---|---|
| Demir | Olivine | (Mg, Fe)2 (SiO4) | Yeşil |
| Almandine | Fe3Al2 (SiO4)3 | Kırmızı | |
| Krom | Uvarovite | Ca3Cr2 (SiO4)3 | Yeşil |
| Cuprum | Malakit | Cu2Co3(OH)2 | Yeşil |
| Chrysocolla | (CuAl)2H2Si2O5(OH)4 • nH2O | Green - Blue | |
| Turkuaz | CUAl6(PO4)4(OH)8.5H2O | Sky blue - Green | |
| Azurite | 2CuCO3 • Cu(OH)2 | Mavi | |
| Manganez | Spessartine-Garnet | Mn3Al2(SiO4)3 | Orange |
| Rodokrozit | MnCO3 | Pink - Red | |
| Rhodonite | (Mn, Ca, Fe) - 5(Si5O15) | Pink - Red |
Copywrite @ Sobling.Jewelry - Özel takı üreticisi, OEM ve ODM takı fabrikası
Some colored gemstones can change color under certain conditions. For example, turquoise and malachite can change color when exposed to high temperatures due to the evaporation of water molecules in their composition; rhodochrosite, a carbonate gemstone, can decompose when it encounters acids (such as hydrochloric acid or sulfuric acid), and its color will also change accordingly.
(2) Allochromatic-colored
The color of a gemstone is caused by trace or minor impurity elements outside of the inherent chemical composition of the constituent minerals, known as allochromatic-color. Such gemstones are referred to as allochromatic-colored gemstones.
In gemstone minerals, there are many varieties of gemstones with different colors. When there are no impurities in the gemstone’s chemical composition, it is colorless and transparent; when it contains different coloring element impurities, it can produce different colors.
When pure corundum is colorless, it becomes ruby when it contains a small amount of chromium ions; when it contains a small amount of iron and titanium, it turns into blue or green gemstones. Similar gemstones include emerald, spinel, tourmaline, jadeite, chalcedony, nephrite, etc. The common coloring elements, chemical compositions, and allochromatic-colored gemstones are shown in Table 3-4.
Table 3-4 Common coloring elements, chemical composition, and allochromatic-colored gemstones
| Coloring elements | Gemstone Name | Kimyasal bileşim | Renk |
|---|---|---|---|
| Krom | Ruby | Al2O3 | Kırmızı |
| Emerald | Olmak3Al2(Si6O18) | Yeşil | |
| Alexandrite | BeAl2O4 | Red-Green | |
| Spinel | MgAl2O4 | Kırmızı | |
| Chalcedony | SiO2 | Yeşil | |
| Demir | Aquamarine | Olmak3Al2Si6O18 | Mavi |
| Turmalin | (Na, K, Ca) (Al, Fe3+, Cr)6(BO3)3Si6O18(OH)4 | Green-Brown | |
| Spinel | MgAl2O4 | Sarı | |
| Nephrite | Ca2(Mg, Fe2+) 5 (Si4O11) 2 (OH)2 | Yeşil | |
| Vanadium | Tanzanite | Ca2Al3(SiO4)3(OH) | Purple-blue |
| Green beryl | Olmak3Al2Si6O18 | Yeşil | |
| Titanium | Benitoite | BaTiSi3O9 | Mavi |
| Safir | Al2O3 | Mavi | |
| Manganez | Red beryl | Olmak3Al2Si6O18 | Kırmızı |
| Rodokrozit | MnCO3 | Pembe | |
| Cobalt | Natural Spinel | MgAl2O4 | Mavi |
| Synthetic Spinel | MgAl2O4 | Mavi | |
| Nickle | Green chalcedony | SiO2 | Yeşil |
(3) Pseudo-colored
The colors produced by pseudo-colors are unrelated to the chemical composition of gemstone minerals. Still, they are caused by structural and compositional changes resulting from mechanical mixtures or the formation of minerals. Pseudo-colors are not the colors of the minerals themselves but rather colors caused by special structures formed by external influences. For example, beautiful interference colors produced by the interference of reflected and incident light, such as labradorite’s Fluorescence effect and opal’s play-of-color effect. Coloration caused by inclusions also falls under pseudo-colors, such as black diamonds, due to many black opaque graphite inclusions within the diamond.
2. Color-causing Ions in Gem Minerals
The chemical elements that produce gem colors can be the main or minor components. Transition metal elements, especially the 4th-period transition metals titanium, vanadium, chromium, manganese, iron, cobalt, nickel, and copper, are often referred to as chromophores or coloring ions. These eight elements occupy consecutive positions in the periodic table, with atomic numbers ranging from 22(Ti) to 29(Cu). The basic properties of these elements are shown in Table 3-5.
Table 3-5 Basic properties of eight transition elements
| Element Name | Titanium | Vanadium | Krom | Manganez | Demir | Cobalt | Nikel | Bakır |
|---|---|---|---|---|---|---|---|---|
| Element Symbol | Ti | V | Cr | Mn | Fe | Co | Ni | Cu |
| Atomic number | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 |
| Main oxidation states | +2, +3, +4 | +2, +3, +4, +5 | +2, +3, +6 | +2, +3, +4, +6 | +2, +3, +6 | +2, +3 | +2, +3 | +1, +2 |
| Valence electron configuration | 3d24s2 | 3d34s2 | 3d54s1 | 3d54s2 | 3d64s2 | 3d74s2 | 3d84s2 | 3d104s1 |
These eight transition metal elements have the following characteristics:
① Valence electrons are filled in the d orbitals of the penultimate shell in sequence, and the general formula for the valence electron configuration of transition element atoms is (n-1)d1-10nS1-2, so these elements are also called d block elements.
② In transition metals, because the d orbitals of the penultimate shell are connected to the outermost s orbitals, and the d orbitals have not yet reached a stable structure, both s electrons and d electrons can participate in bonding partially or completely, resulting in a series of variable oxidation states for transition metals, with different oxides presenting different colors in gemstones.
③ Ions generally exhibit color because there are unpaired single electrons in the d orbitals, and the energy levels of these electrons in the excited and ground states are relatively close, allowing visible light energy to excite them. Different excitation conditions may also cause gemstones to exhibit different colors.
If the spin electrons in the ion are all paired, such as ions with a valence electron configuration of d0, d10, d10S2, the electrons are in a stable state and are not easily excited, resulting in the ion having no color; thus, C+, Cr6+ and others have no color and cannot produce color in gemstones.
These eight transition metal elements constitute the color formation in common natural-colored gemstones. Different chromophores produce different colors in different gemstones, and the same chromophore may also produce different colors. Common natural gemstones and chromophores are shown in Table 3-6.
Table 3-6 Colors presented by transition metal ions in common natural gemstones and gemstone varieties
| Chromophore | Common colors | Gem varieties |
|---|---|---|
| Titanium (Ti) ion | Mavi | Sapphire, benitoite, Topaz |
| Vanadium (V) ion | Yeşil | Synthetic Color-Change corundum, Essonite, Emerald |
| Chromium (Cr) ion | Red, Green | Ruby, Corundum, Emerald, Pyrope, Jade |
| Manganese (Mn) ion | Pink, Red | Spessartine, Rhodolite, Red Beryl |
| Iron (Fe) ion | Blue, green, yellow | Sapphire, olivine, aquamarine, tourmaline, spinel |
| Cobalt (Co) ion | Mavi | Synthetic spinel, cobalt-colored staurolite |
| Nickel (Ni) ion | Yeşil | Green chalcedony |
| Copper (Cu) ion | Blue, blue-green | Malachite, turquoise, azurite |
Different chromophore ions produce different colors in gemstones, resulting in absorption spectra with distinct characteristics. For common chromophore ions, the absorption spectra have typical identification significance.
(1) The absorption spectrum of chromium ions
The absorption spectrum of chromium ions is mainly characterized by many narrow absorption lines in the red region, with the two strongest located in the deep red region and another two in the orange region. The yellow-green region has a wide absorption band, and the width, position, and intensity are related to the depth of the gem’s color. There can be several narrow bands in the blue region, while the purple region is fully absorbed. Chromium ions mainly produce red and green colors, which vary in different gems, and the absorption spectra show some differences. For example, rubies have three absorption lines in the red region, a wide absorption band in the yellow-green region, three absorption lines in the blue region, and full absorption in the purple region; emeralds have absorption lines in the red region, a weak absorption band in the orange-yellow region, weak absorption lines in the blue region, and full absorption in the purple region; and alexandrite has absorption lines in the red region, an absorption band in the yellow-green region, one absorption line in the blue region, and full absorption in the purple region. The absorption spectra of these three gems are shown in Figure 3-9.

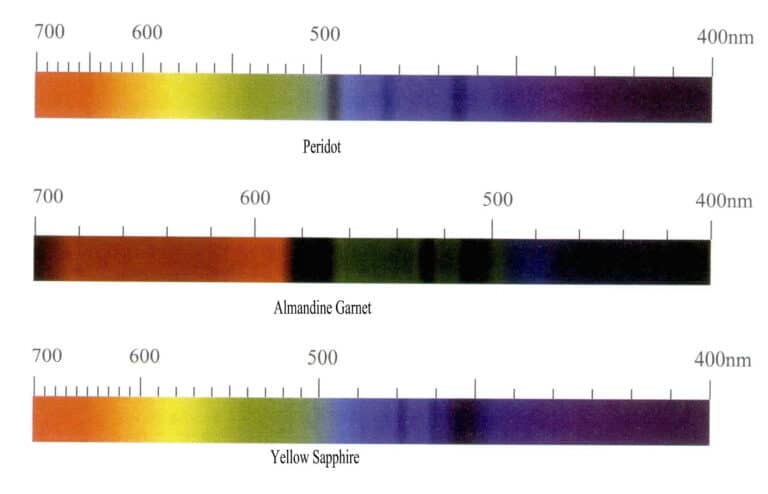
(2) Characteristics of the iron ion absorption spectrum
Iron ions produce different colors in different gems and have a strong coloring effect, but the absorption spectrum of iron ions varies greatly. When the gemstone is green, it produces a red-zone absorption, and when it is red, it produces an absorption feature dominated by the blue zone, with the main characteristic absorption line located in the green and blue zones. For example, iron ions in olivine appear olive green, with the absorption spectrum mainly showing three narrow absorption bands in the blue region at 453nm, 473nm, and 493nm; red almandine has a typical iron absorption spectrum, with three strong narrow absorption bands at 504nm, 520nm, and 573nm in the yellow-green region, commonly referred to in the industry as the “iron window.” Additionally, there are weak absorption narrow bands at 423nm, 460nm, 610nm, and 680~690nm; the absorption spectrum of yellow sapphire has three narrow absorption bands in the blue region at 450nm, 460nm, 470nm.(Figure 3-10).
(3) Absorption spectrum characteristics of manganese ions
Manganese ions mainly form pink, orange, and red in gemstones, with the absorption spectrum primarily showing strong absorption in the violet region, extending into the ultraviolet region, and some absorption in the blue region. For example, the absorption spectrum characteristics of pink rhodochrosite have three absorption bands at 410nm, 450nm, 540nm; the absorption spectrum lines of spessartine mainly have 410nm, 420nm, 430nm three absorption bands and absorption lines at 460nm, 480nm, and 520nm. Sometimes, there may be 504nm, 573nm two absorption lines (Figure 3-11).

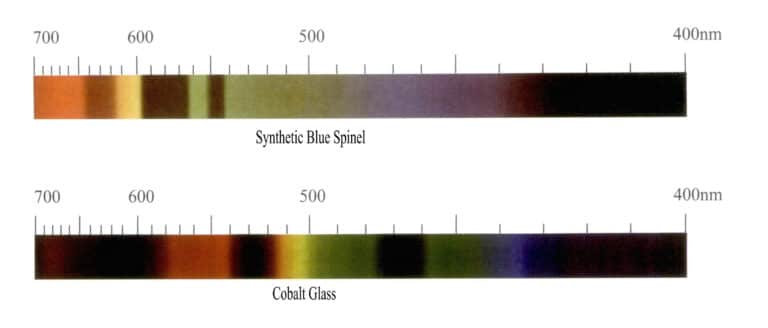
(4) Absorption spectrum characteristics of cobalt ions
Cobalt ions have a strong coloring effect, usually appearing in a bright blue color in gemstones, with the absorption spectrum mainly showing three strong and broad absorption bands in the yellow-green region. Due to the low abundance of cobalt in the Earth’s crust, there are very few natural gemstones colored by cobalt ions. The absorption spectrum of cobalt ions also indicates synthetic gemstones, such as synthetic blue spinel and cobalt glass. Synthetic blue spinel has three strong absorption bands in the yellow-green and orange-yellow regions, with the green region absorption band being the narrowest; the absorption lines of cobalt glass mainly have three strong absorption bands in the yellow-green and orange-yellow regions, with the yellow region absorption band being the narrowest (Figure 3-12).
3. Rare Earth Element Coloring
Research on the influence of trace rare earth elements on the color of gemstones is becoming increasingly in-depth. The colors of rare earth elements are more vivid, and their physicochemical properties are also very stable. Rare earth elements like apatite and fluorite can also color natural gemstones. Different rare earth elements can be added to synthetic and optimally treated gemstones to obtain different colored gemstones, such as yellow from cerium and blue from neodymium.
The rare earth elements that color gemstones are mainly the lanthanide and actinide elements in the periodic table of chemical elements and the colors they can produce are shown in Table 3-7.
Table 3-7 Rare Earth Elements and Their Colors in Common Gemstones
| Element Symbol | La | Ce | Nd | Pr | Dy | Sm | Er | Tm | U |
|---|---|---|---|---|---|---|---|---|---|
| Full Name | Lanthanum | Cerium | Neodymium | Praseodymium | Dysprosium | Samarium | Erbium | Thulium | Uranium |
| Renk | Renksiz | Sarı | Mavi | Yeşil | Light yellow | Light yellow | Pembe | Light Green | Silver-white |
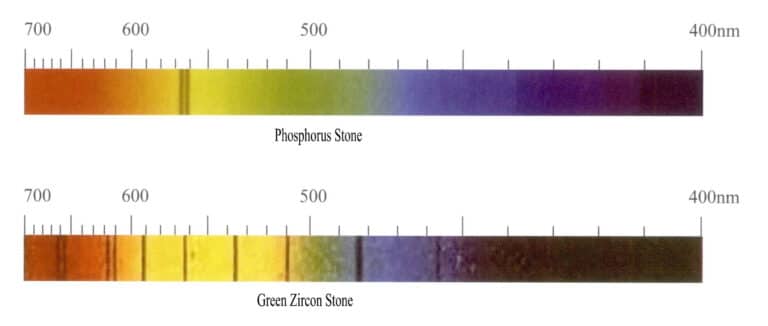
Rare earth elements have characteristic absorption spectra, often forming unique fine lines. For example, yellow apatite often contains the rare earth element Ce, with characteristic absorption fine lines in the yellow region. However, uranium does not produce a bright yellow; it can generate distinct absorption spectral lines. For instance, more than ten absorption lines can appear in green zircon in various color zones (Figure 3-13).
Section III Colors Produced by Crystal Defects and Color Centers
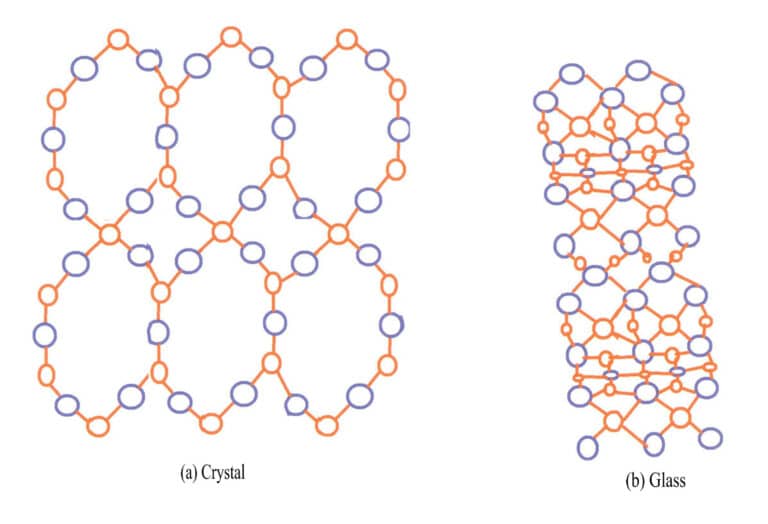
In nature, minerals are divided into crystals and non-crystals based on different degrees of crystallization. Most gemstones, such as rubies, sapphires, diamonds, emeralds, and quartz, are crystals; some organic gemstones, such as amber and coral, are non-crystalline. Crystal structures have a lattice-like structure, where their internal particles (atoms, ions, or molecules) are arranged in a regular periodic pattern in three-dimensional space, and crystals can spontaneously form polyhedral shapes; non-crystals are amorphous bodies with non-lattice structures that cannot form polyhedral shapes, such as glass, rosin, and resin.
The most typical example of the difference between crystals and amorphous solids is quartz and glass. Natural quartz is formed from molten material SiO2 in magma that cools in cavities within the Earth’s crust. The outer layer of common quartz spheres is agate, which does not show the crystal shape, while the inner layer presents the crystal shape of quartz. The main chemical components of both glass and quartz are SiO2; quartz is a crystal with silicon and oxygen ions arranged in an orderly manner, while glass is an amorphous solid with silicon and oxygen ions arranged chaotically, lacking regularity, as shown in Figure 3-14.
Most gemstone crystals are colored due to impurity ions, such as rubies, emeralds, and tourmaline. Some gemstones, although lacking chromophore ions, are colored due to defects in their crystal structure. Natural gemstones produced in nature can change color due to external conditions, such as irradiation and ionization, which alter the crystal structure. The most common example is smoky quartz, which develops color due to the formation of color centers from radiation-induced vacancies. Artificially irradiated smoky quartz has a similar formation principle to natural smoky quartz, except that artificial radiation creates color quickly.
1. Crystal Defects and Types
The phenomenon in which the arrangement of particles deviates from the lattice structure rules (particles undergo periodic translational repetition in three-dimensional space) within a local range of the gemstone crystal structure is called a lattice defect. The causes are related to the thermal vibrations of particles within the gemstone crystal, external stress, high temperature and pressure, irradiation, diffusion, ion implantation, and other conditions.
For example, diamonds crystallized in the high-temperature and high-pressure environment of the upper mantle when rapidly carried to near the Earth’s surface by the host magma (kimberlite or lamproite), the rapid change in temperature and pressure conditions and the mutual collisions between the crystals and surrounding rocks can easily lead to local changes in the structure of the intrusive diamond crystals, resulting in lattice defects that change the color of originally colorless diamonds, forming brownish- yellow, brown, and pink diamonds.
The presence of crystal defects has a significant impact on the properties of the crystals. In reality, crystals have more or less defects. A moderate amount of certain point defects can greatly enhance semiconductor materials’ conductivity and luminescent materials’ luminescence, playing a beneficial role. In contrast, defects such as dislocations can make materials prone to fracture, reducing the tensile strength of crystals with almost no lattice defects to a fraction of that.
In an ideally perfect crystal, atoms are strictly arranged in a regular, periodic lattice at specific points in space. However, during the actual growth and formation of crystals, due to the influence of growth environments such as temperature, pressure, and medium component concentration, the crystal morphology after growth may sometimes deviate from the ideal crystal structure. Any deviation from the ideal crystal structure can be called a crystal defect. Crystal defects significantly impact the physical and chemical properties of crystals, and many disciplines are related to crystal defects, such as ionic doping in materials science. The color of gemstones is largely related to the crystal defects within them. This is one of the causes of color in gemstones—color centers.
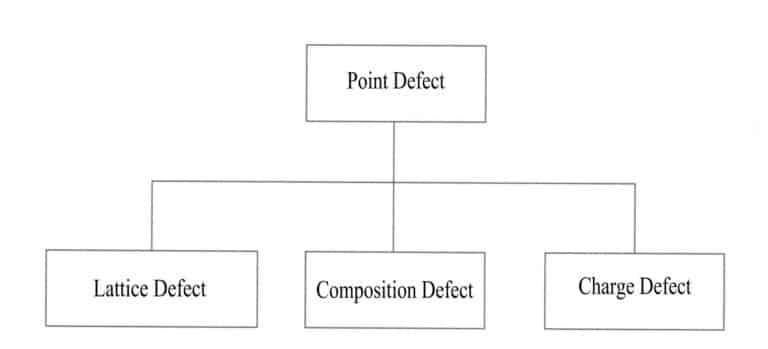
There are many types of crystal structure defects, which can be classified into four categories based on the extent of their distribution in three-dimensional space: point defects, line defects, surface defects, and volume defects.
(1) Point defects
Other atoms replace some atoms in an ideal crystal, some are doped in, or vacancies are created. Some atoms in the crystal are replaced or missing due to external atoms, and these changes disrupt the periodic arrangement of the crystal’s regular lattice, causing changes in the potential field of the particles and resulting in the incompleteness of the crystal structure, limited to certain positions, affecting only a few nearby atoms. The impact of point defects on the crystal is minimal, and common types of point defects include lattice position defects, compositional defects, and charge defects (Figure 3-15).
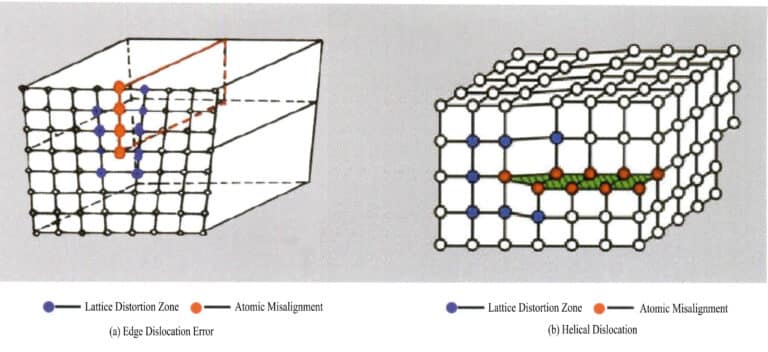
(2) Line Defects
Line defects are very small in size in two directions and mainly extend longer in another direction. They are also known as one-dimensional defects and primarily consist of various dislocations. Dislocations can be seen as the product of local lattice slip along a certain atomic plane. The slip does not penetrate the entire lattice; the crystal defect terminates within the lattice, causing a disordered arrangement of particles at the boundary between the slipped and unslipped parts of the lattice, known as dislocation. This boundary, the intersection line between the slipped and unslipped areas, is called the dislocation line. There are two basic types of dislocations: when the crystal is subjected to compressive forces, the particle slip plane forms a dislocation line with the unslipped plane, and the dislocation line is perpendicular to the slip direction, known as edge dislocation, also called wedge dislocation; due to shear stress, slip occurs between planes, and the intersecting dislocation lines in the slipped part of the crystal are parallel to the slip direction, referred to as screw dislocation (Figure 3-16).
(3) Surface Defects
The simplest surface defect is the stacking fault, divided into intrinsic stacking faults (where a crystal plane is removed) and extrinsic stacking faults (where an atomic layer is inserted into the crystal). These defects occur within a range of a few atomic spacings on either side of a certain plane along the lattice or between grains. They mainly include stacking faults and interfaces within and between crystals, such as small-angle grain boundaries, domain walls, and twin and intergranular boundaries.
(4) Volume Defects
Volume defects refer to defects that exist to varying degrees in all three directions, which are three-dimensional defects, such as embedded cracks, mesh structures, familial structures, twins, and various gemstone inclusions.
2. Color Centers in Gemstones
Color centers are a special case of lattice defects, generally referring to lattice defects in gemstones that can selectively absorb visible light energy and produce color, belonging to the most typical structural color types. In some cases, the unpaired electrons that produce color can also appear in non-transition element ions or in crystal defects formed due to a lack of electrons, which is what color centers are. Point defects in ionic crystals can cause the absorption of visible light, resulting in originally transparent crystals appearing colored; these types of point defects that can absorb visible light are usually referred to as color centers. Many types of natural gemstones produce color from color centers, such as purple fluorite, smoky crystal, and green diamonds.
In the optimization treatment process of gemstones, some natural and artificial gemstones can also have color centers generated by radiation, such as blue, yellow, red, green diamonds, and blue topaz that change color due to radiation, and blue topaz, among which some colors are relatively stable and only disappear when heated; some colors are unstable and may fade even at room temperature. This type of color-causing color center is closely related to the crystal structure of the gemstone, such as green diamonds, where the cause of color is the presence of vacancies in the crystal structure, but this structural defect can also be removed by irradiation, turning the diamond colorless. The common types of color centers in gemstones are “electron color centers” and “hole color centers.”
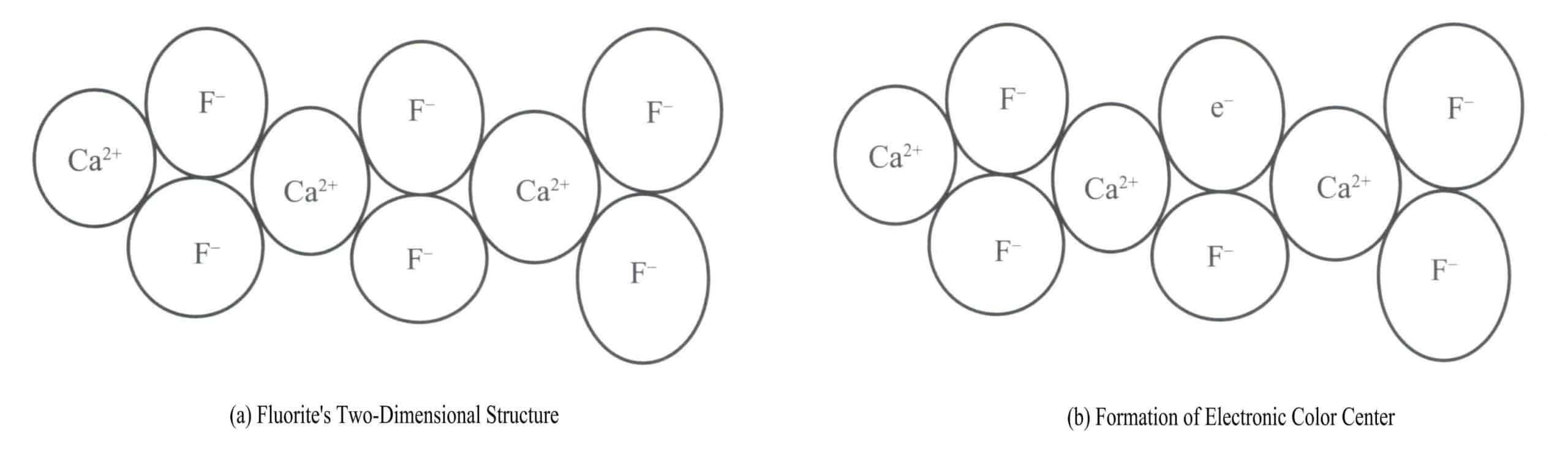
(1) Electron color center (F center)
Electron color centers are color centers formed when electrons exist in vacancies of crystal defects caused by anionic vacancies in the gemstone’s crystal structure. When the anion is absent, the vacancy becomes a positively charged electron trap that traps electrons. If a vacancy captures an electron and binds it to that vacancy, the electron becomes excited and selectively absorbs a certain wavelength of energy to become colored. Therefore, an electron color center consists of an anionic vacancy and an electron bound by the electric field of that vacancy.
Purple fluorite is a color produced by electronic color centers. Fluorite (CaF2) belongs to the isometric crystal system, with each Ca2+ connected to two F– [Figure 3-17 (a)]. In some cases, the F– in the fluorite may leave its normal position. A vacancy appears at the original F– position, and to maintain the electrical neutrality of the crystal, a negatively charged entity must occupy this vacancy. An electron from a certain atom in the crystal becomes the negatively charged entity occupying this vacancy [Figure 3-17 (b)]. This creates a “color center,” known as an electronic color center. In fluorite, electronic color centers absorb visible light, producing purple.
(2) Hole color centers (V centers)
The vacancy color center is formed by external factors, where cations create electron vacancies. This means that electrons are ejected from their original positions, leaving behind an unpaired electron. The reason for the color is that when cation vacancies form in the crystal, to achieve charge balance, the anions near the cation vacancy release electrons under the influence of external energy, forming unpaired electrons that absorb visible light and produce color. For example, in irradiated diamonds and blue topaz, irradiation provides energy to activate electrons, causing ions or atoms in the lattice to displace, thus forming structural defects and color centers due to irradiation.
A classic example of a color center in a crystal is the coloring of smoky crystal. The crystal structure of quartz is a silicon-oxygen tetrahedron, with silicon in a four-coordinate state, as shown in the two-dimensional structural diagram in Figure 3-18(a). Every 10,000 Si4+ atoms have one replaced by Al3+, and when Al3+ substitutes for Si4+ in the crystal, Al3+ must be surrounded by some alkali ions (such as Na+ or H+) to maintain electrical neutrality. Still, these ions are often at a certain distance from Al3+.
When quartz is irradiated by X-rays, γ rays, and other radiation sources, the energy of the adjacent oxygen atoms to Al3+ increases, allowing one of the electrons in its pair to be ejected from its normal position. H+ will capture this electron to form H . High-energy radiation causes the O2- with more valence electrons to release an electron, forming a [A1O4]4- vacancy color center, and the [A1O4]4- atomic cluster absorbs visible light to produce color, forming smoky quartz.
[A1O4]5-→ [A1O4]4-+e– (3-1)
H+ +e–→ H (3-2)
H+ captures electrons to become H, which is colorless and does not absorb visible light. If the irradiation intensity is high and there is enough Al3+ in the crystal, the quartz can be irradiated to black. Since there is often a vacancy at the position where an electron is ejected, this type of color center is referred to as a “vacancy color center.”
During geological history, natural smoky crystal is mostly formed through long-term low-dose radiation from radioactive materials. Heating can eliminate the color; when this smoky crystal is heated to around 400℃, the ejected electrons will return to their original positions, all the electrons will pair up, and the quartz will become colorless again; if it is irradiated again, it can turn smoky again [Figure 3-18 (b)].
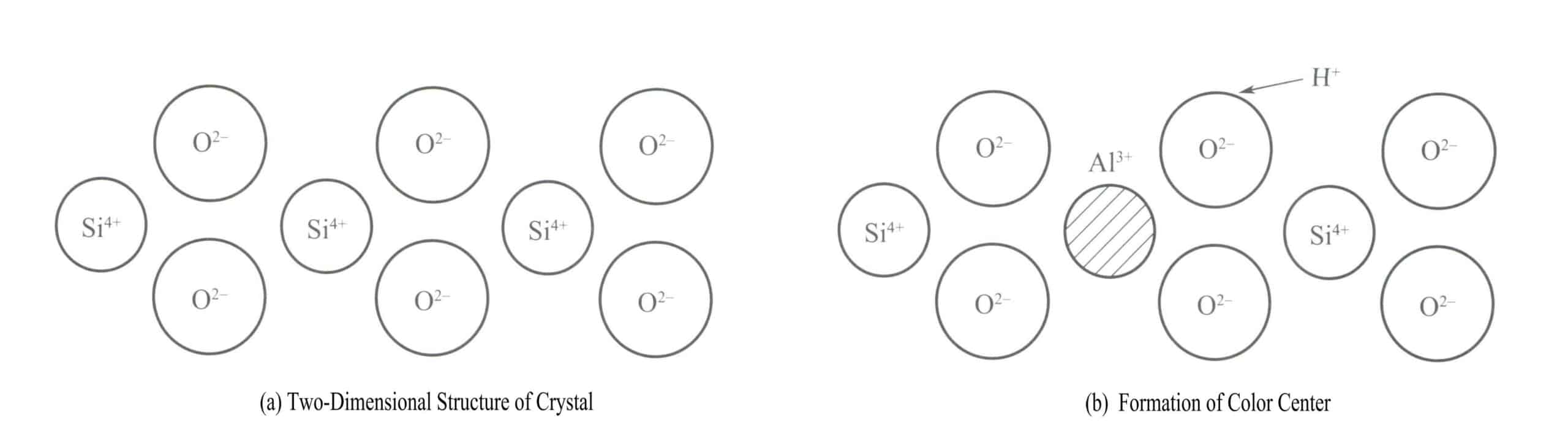
Amethyst has the same color centers for vacancy, but its impurity is iron instead of aluminum. When the impurity Fe 3+ replaces Si4+ in the quartz, the following changes occur when irradiated with high-energy rays:
[FeO4]5- → [FeO4]4- +e– (3-3)
H+ +e–→ H (3-4)
Similar to the formation principle of smoky crystal, it produces a purple color due to the formation of [FeO4]4- hole color centers after being irradiated. When this amethyst is heated, it turns yellow, becoming citrine, and with further heating, it fades to colorless. Generally, the heating temperature is relatively low, around 400℃. The purple color of heat-treated amethyst can be restored by irradiating the color centers again. Synthetic amethyst is also synthesized based on this principle.
In some gemstones, a few impurity atom clusters can also form color centers and exhibit color. For example, during the growth of beryl and under radioactive irradiation, can lose an electron to become , forming a red-green absorption band that produces a blue color. There are many color centers in diamonds, most of which are caused by the formation of vacancies or dislocations in the structure under external conditions, and the colors are generally very stable.
The difficulty of studying color center-induced color is relatively high, requiring various techniques such as spectroscopy and electron paramagnetic resonance. Previous research shows some typical characteristics of color center-induced color are relatively clear. Table 38 summarizes the colors and causes of color center-induced color in common gemstones.
Table 3-8 Colors and Causes of Color Center-Induced Color in Common Gemstones
| Types of Gemstones | Renk | Cause |
|---|---|---|
| Elmas | Yeşil | Carbon vacancy GR1 color center in diamond |
| Sarı | Missing structure of diamond N3 aggregates | |
| Orange | Natoms and H3, H4 color center defects | |
| Kristal | Smoke color | Vacancies generated by Al3+ substituting Si4+ , related to radiation |
| Sarı | Related to Al3+, it can also be generated by radiation | |
| Purple | Fe3+ replaces the vacancy created by Si4+ | |
| Corundum gemstone | Sarı | Color is unstable, the reason for structural defects is unknown |
| Topaz | Mavi | Color is stable, the reason for structural defects is unknown |
| Sarı | Color is stable, the cause of structural defects is unknown | |
| Brownish-red | Color is unstable, the cause of structural defects is unknown | |
| Turmalin | Kırmızı | Related to Mn3+ , can also be caused by radiation |
| Beryl | Mavi | Related to CO32- , can also be caused by irradiation |
| Florit | Purple | Electronic e- replaces F- to produce |
The principle of color centers is also used to improve the color of natural gemstones in gemstone enhancement. Most methods use irradiation to change the color of the gemstones. Some color centers are relatively stable, while some gemstone varieties fade quickly, making this enhancement method less significant for those gemstones. Table 3-9 lists some colors produced by color centers, including stable color centers, unstable color centers, and colors produced by other possible factors.
Table 3-9 Colors produced by color centers
| Basically stable in light | Amethyst, fluorite(purple-red), irradiated diamonds (green, yellow, brown, black, blue, pink); some natural or irradiated topaz (blue) |
|---|---|
| Fades quickly in light | Lithium cesium green beryl (deep blue); some irradiated topaz (brown or tan); irradiated sapphire (yellow); ultraviolet irradiated purple sodalite (purple-red) |
| Other colors that color centers may produce | Sylvine (blue); halite(blue or yellow); zircon (brown); calcite (yellow); barite, celestine (blue); amazonite (blue to green) |