O que torna os ornamentos de vidro e de vidro colorido tão únicos: Materiais, cuidados e artesanato
Desvendar a magia do vidro e dos ornamentos de vidro colorido: Artesanato, cuidado e criação
O vidro é um material mágico com uma transparência cristalina, uma solidez fria e propriedades reflectoras da luz. Estas propriedades tornam os efeitos da criação artística imprevisíveis. Para além de ser utilizado em artesanato decorativo, o vidro é agora também amplamente utilizado no fabrico de jóias.
O vidro colorido é um ornamento artesanal feito de cristal artificial utilizando a fundição por cera perdida. A história da arte chinesa do vidro colorido é longa, remontando às dinastias Shang e Zhou. Diz-se que os povos antigos criavam requintadas peças de arte em vidro colorido que eram cristalinas, húmidas e suaves para imitar pérolas e pedras preciosas. Atualmente, a aplicação bem sucedida de técnicas modernas de vidro colorido não só deu a este produto um novo estilo contemporâneo. No entanto, também permitiu que os ornamentos de artesanato em vidro colorido chinês entrassem na arena internacional dos ornamentos de artesanato.

anel incrustado em vidro colorido
Índice
Secção I Introdução ao vidro e aos materiais de vidro colorido
1. Vidro
1.1 Composição do vidro
O vidro refere-se geralmente ao vidro de silicato, um objeto amorfo obtido a partir da fusão sobrearrefecida. É geralmente frágil e transparente e possui propriedades mecânicas sólidas. Os tipos mais comuns são o "vidro de cal sodada", o "vidro de chumbo-bário" e o "vidro de potássio", que utilizam principalmente areia de quartzo, carbonato de sódio, feldspato e calcário como matérias-primas. Devido às diferentes formulações, o vidro pode ter propriedades variáveis, apresentando assim caraterísticas regionais e temporais distintas.
O vidro comum é constituído principalmente por dióxido de sílica amorfo (SiO2), a composição química do quartzo ou da areia. O quartzo puro tem um ponto de fusão muito elevado, pelo que são geralmente adicionados dois materiais ao fabrico do vidro: carbonato de sódio e carbonato de potássio. Isto baixa o ponto de fusão da sílica para cerca de 1000℃. No entanto, como o carbonato de sódio faz com que o vidro se dissolva na água, geralmente é adicionada uma quantidade adequada de óxido de cálcio para tornar o vidro insolúvel em água.
A maior caraterística do vidro é a sua transparência à luz visível. O vidro comum não é transparente à luz ultravioleta com um comprimento de onda inferior a 400 nm porque é adicionado carbonato de sódio durante o fabrico. Para permitir a penetração da luz ultravioleta, o vidro deve ser feito de sílica pura, que é mais cara e é geralmente designada por vidro de quartzo. O vidro puro é também transparente à luz infravermelha, o que permite criar fibras de vidro com vários quilómetros de comprimento para fins de comunicação.
O vidro comum também contém frequentemente outros componentes. Por exemplo, o vidro cristalino que tem um aspeto muito cintilante e deslumbrante é fabricado através da adição de chumbo para aumentar o índice de refração, resultando numa refração mais deslumbrante. O boro é adicionado ao vidro borossilicato para alterar as suas propriedades térmicas e eléctricas. O bário também pode aumentar o índice de refração. Ao vidro utilizado no fabrico de lentes ópticas é adicionado óxido de tório para aumentar significativamente o índice de refração. O ferro pode ser adicionado se o vidro precisar de absorver os raios infravermelhos; este tipo de vidro isolante térmico é encontrado em projectores. A adição de cério ao vidro absorve os raios ultravioleta.
A adição de vários metais e óxidos metálicos ao vidro também pode alterar a sua cor. Por exemplo, uma pequena quantidade de manganês pode alterar o verde claro causado pelo ferro no vidro, e quando a quantidade de manganês atinge um determinado nível, pode formar uma cor púrpura clara. O selénio tem um efeito semelhante. Uma pequena quantidade de cobalto pode criar uma cor azul. Os óxidos de estanho e os óxidos de arsénio podem formar um branco opaco, semelhante à cerâmica branca. Os óxidos de cobre formam uma cor turquesa, enquanto o cobre metálico cria um vermelho profundo opaco, semelhante ao rubi. O níquel pode formar-se em azul, roxo profundo ou mesmo preto. O titânio pode formar um amarelo acastanhado. Quantidades vestigiais de ouro (cerca de 0,001%) fazem com que o vidro tenha uma cor semelhante ao rubi. O urânio (0,1%~2%) faz com que o vidro tenha uma cor amarela ou verde. Os compostos de prata podem produzir vidro laranja a amarelo. A alteração da temperatura do vidro também altera as cores produzidas por estes compostos, mas os princípios químicos subjacentes são bastante complexos e ainda não foram totalmente clarificados.
1.2 Caraterísticas do material de vidro
O vidro é um material inorgânico não metálico com uma densidade de cerca de 2,46~2,5g/cm3, um coeficiente de dilatação linear de 9×10-6~10×10-6/℃(~350℃), e uma resistência à tração superficial de cerca de 50MPa. Estruturalmente, é um polímero termoplástico inorgânico que pode ser moldado a temperaturas acima de 650 ℃ e possui caraterísticas como transparência, resistência à corrosão, resistência ao desgaste e resistência à compressão após o resfriamento. O coeficiente de expansão térmica do vidro é menor do que o do aço, e é um mau condutor de eletricidade e calor, sendo uma substância amorfa frágil.
O vidro, um material inorgânico não cristalino obtido por aquecimento e fusão de matérias-primas, seguido de arrefecimento e solidificação, tem as seguintes caraterísticas básicas
(1) Força. A resistência do vidro depende da sua composição química, do teor e da distribuição das impurezas, da forma do produto, do estado da superfície, das propriedades, dos métodos de transformação, etc. O vidro é um material frágil, e a sua resistência é geralmente expressa em termos de resistência à compressão e à tração. A resistência à tração do vidro é relativamente baixa devido à fragilidade do vidro e às microfissuras na superfície do vidro. A resistência à compressão do vidro é cerca de 14 a 15 vezes superior à sua resistência à tração.
(2) Dureza. O vidro tem uma dureza elevada, com uma dureza de Mohs entre 5 e 7, apenas atrás de materiais como o diamante e o carboneto de silício. É mais duro do que os metais comuns e não pode ser cortado com facas e serras normais. Com base na dureza do vidro, podem ser selecionados abrasivos, ferramentas e métodos de processamento como a gravação, o polimento, o desbaste e o corte.
(3) Propriedades ópticas. O vidro é um material altamente transparente com determinadas constantes ópticas e caraterísticas espectrais, apresentando desempenhos ópticos importantes, como a absorção ou transmissão de luz ultravioleta e infravermelha, fotossensibilidade, fotocromismo, armazenamento de luz e visualização. É cristalino e pode ser submetido a coloração de corpo, coloração de superfície e tratamento de superfície, com infinitas variações de forma e forte expressividade. Geralmente, quanto mais luz passar, melhor será a qualidade do vidro. Devido à variedade de tipos de vidro, as propriedades dos diferentes vidros podem variar significativamente; por exemplo, alguns vidros de chumbo têm propriedades de proteção contra a radiação. Normalmente, ao alterar a composição e as condições de processamento do vidro, as suas propriedades podem variar bastante.
(4) Propriedades eléctricas. À temperatura ambiente, o vidro é um mau condutor de eletricidade. Com o aumento da temperatura, a condutividade eléctrica do vidro aumenta rapidamente e, no estado fundido, torna-se um bom condutor.
(5) Propriedades térmicas. O vidro tem uma fraca condutividade térmica e não consegue suportar mudanças rápidas de temperatura. Quanto mais espesso for o produto, pior será a sua capacidade de resistir a mudanças rápidas de temperatura. À medida que a temperatura aumenta, o vidro pode mudar gradualmente de um estado sólido duro para um estado sólido mole, um estado de gel e um estado líquido. Cada estado da matéria tem as suas propriedades, permitindo os métodos mais eficazes de moldar, modificar e colorir o vidro em diferentes gamas de temperatura, proporcionando um grande espaço criativo e possibilidades infinitas.
(6) Estabilidade química. As propriedades químicas do vidro são relativamente estáveis. A maioria dos vidros industriais resiste à corrosão dos ácidos, com exceção do ácido fluorídrico. O vidro tem pouca resistência à corrosão alcalina. Com o tempo, o vidro pode perder o brilho da sua superfície e até desenvolver manchas ou uma película de nevoeiro devido à erosão da atmosfera e da água da chuva, tornando-se baço e perdendo a sua transparência.
(7) Desempenho ambiental. O vidro não é tóxico e é inofensivo. Não liberta substâncias nocivas para o corpo humano e para o ambiente, causando poluição. É um material "verde" e amigo do ambiente.
1.3 Cristal de vidro (diamante de cristal, strass)
O cristal de vidro é um termo coloquial na indústria, que se refere a um tipo de produto de imitação de diamante fabricado através da fusão de várias matérias-primas químicas em formas redondas de vidro cristalino com elevado teor de chumbo, que são depois moídas e polidas. O principal componente do cristal de vidro é o cristal de vidro. Este material é relativamente económico e visualmente impressionante, assemelhando-se a diamantes, o que o torna muito popular e uma das matérias-primas mais utilizadas atualmente na joalharia.
O vidro de cristal de chumbo tem a seguinte composição: RmOn-PbO-SiO2(B2O3). Na fórmula, SiO2(B2O3), o óxido de silício (óxido de boro) é designado por formador de rede e é a unidade básica que constitui a estrutura de rede do vidro. O RmOn representa os óxidos metálicos de metais alcalinos, alcalino-terrosos e de terras raras, que modificam a estrutura da rede do vidro para ajustar as suas propriedades. O PbO (óxido de chumbo) é um componente caraterístico que confere as propriedades básicas ao vidro. O Pb2+ em PbO podem formar unidades estruturais [PbO4] de pirâmides tetragonais, compondo uma estrutura de cadeia helicoidal [SiO4] que liga a estrutura do vidro em ângulos de vértice ou arestas partilhadas, formando uma rede especial. Esta rede tem uma gama de formação de vidro muito ampla, permitindo a formação de vidro mesmo quando o PbO (fração molar) atinge 80%. À medida que o teor de PbO aumenta, os valores dos indicadores de desempenho, como a densidade, o índice de refração, a dispersão, a constante dieléctrica e os coeficientes de absorção de raios X e raios γ aumentam, enquanto a dureza, a viscosidade a alta temperatura, a temperatura de amolecimento e a estabilidade química diminuem, resultando em cores brilhantes, aumento do brilho da superfície, um som nítido quando atingido e facilidade de escultura e polimento químico. Na segunda metade do século XVII, a Inglaterra produziu vasos de arte em vidro de cristal de chumbo. Devido às excelentes propriedades físicas do vidro de cristal de chumbo, como a sua elevada densidade e elevado índice de refração, tornou-se o material preferido para os ornamentos de vidro. Durante muito tempo, foi considerado insubstituível.
Devido ao fabrico global de vidro de cristal sintético em ambas as margens do rio Reno, os cristais de vidro são também designados por Rhinestones. Os que são produzidos na margem norte são designados por "cristais austríacos", abreviados como "Austrian". Os cristais austríacos podem ter mais de 30 superfícies de corte, o que resulta em bons efeitos de refração e uma sensação de profundidade na luz refractada; além disso, devido à sua elevada dureza e brilho duradouro, são os melhores entre os cristais de vidro, especialmente os cristais de vidro "Swarovski", que são famosos em todo o mundo. São sinónimo de produtos de cristal sintético e um símbolo de cultura. Atualmente, a Swarovski tem muitas fábricas em todo o mundo, pelo que a Swarovski representa apenas uma determinada qualidade e não é necessariamente produzida na Áustria. Os cristais produzidos na região sul são designados por "cristais checos", que têm geralmente cerca de uma dúzia de facetas, bons efeitos de refração e podem emitir uma luz mais deslumbrante. A sua dureza é relativamente forte e o seu brilho dura cerca de 3 anos, perdendo apenas para os cristais austríacos. Os cristais de vidro do Médio Oriente e os produzidos internamente são alguns dos que os fabricantes fizeram para satisfazer o mercado com uma produção de baixo custo, e a sua qualidade é inferior à dos cristais de vidro checos. Os cristais de vidro em geral têm 8 facetas, e a parte de trás do cristal de vidro é revestida com uma camada de mercúrio. A concentração da luz através das facetas confere-lhe um melhor brilho; quanto maior for o número de facetas, melhor será o brilho.
Os cristais de vidro podem ser divididos em cristais de vidro brancos, cristais de vidro coloridos (como rosa, vermelho, azul, etc.), cristais de vidro de fantasia e cristais de vidro AB de fantasia (como AB vermelho, AB azul, etc.) com base na cor. Os cristais de vidro podem ser divididos em duas categorias principais: cristais de vidro de fundo pontiagudo e cristais de vidro de fundo plano, de acordo com a forma do fundo. Com base na forma da mesa, os cristais de vidro podem ser divididos em duas categorias principais: cristais de vidro normais e cristais de vidro de forma extravagante. As formas dos cristais de vidro de fantasia podem ainda ser divididas em cristais de vidro em forma de diamante (marquise), cristais de vidro trapezoidais, pedras satélite, cristais de vidro em forma de lágrima, cristais de vidro ovais e cristais de vidro octogonais, entre outros. Com base no material, os cristais de vidro podem ser divididos em vidro, espinélio sintético, safira sintética, zircónio cúbico, etc.
Alguns cristais de vidro assemelham-se visualmente aos diamantes pela sua transparência impecável e pelas suas caraterísticas deslumbrantes. Por vezes, são utilizados para se fazerem passar por diamantes, levando as pessoas a acreditar que são verdadeiros. Os diamantes falsos feitos de vidro são fáceis de distinguir porque têm um índice de refração baixo e não têm a luz colorida cintilante dos diamantes verdadeiros, que alguém com um pouco de experiência consegue identificar facilmente. Estes "cristais de vidro" são frequentemente utilizados em jóias relativamente baratas. Antes do aparecimento da zircónia cúbica sintética, o zircão era frequentemente utilizado como substituto do diamante. O zircão tem uma forte birrefringência, o que significa que tem dois índices de refração, e a diferença entre eles é bastante grande. Isto resulta num fenómeno ótico muito especial; quando se observa uma gema facetada de zircão polido com uma lupa, podem ver-se imagens duplas distintas das facetas inferiores e dos bordos da superfície superior. Em contrapartida, os diamantes são "corpos homogéneos" e não apresentam o fenómeno da dupla imagem. A zircónia cúbica, ou "diamante soviético", é um composto sintético sem equivalente mineral natural. A zircónia cúbica está muito próxima dos diamantes naturais no que diz respeito ao índice de refração e à dispersão. No entanto, tem uma dureza inferior, uma densidade relativa que é 1,6~1,7 vezes superior à dos diamantes e a sua condutividade térmica é muito inferior à dos diamantes, permitindo que os instrumentos a distingam com precisão dos diamantes.
1.4 Vidro sem chumbo
Com o avanço contínuo da tecnologia e a crescente sensibilização para a proteção ambiental, a toxicidade do chumbo para os seres humanos e a sua poluição do ambiente estão a receber cada vez mais atenção de todas as partes. Durante a fusão do vidro de chumbo, uma grande quantidade de chumbo evapora-se para o ar; durante a utilização, o chumbo contido no vidro também se desprende gradualmente. Especialmente depois de ser deitado fora, devido ao contacto prolongado com a água e com substâncias ácidas, o chumbo pode infiltrar-se nas águas subterrâneas. Tudo isto representa um prejuízo significativo para a saúde das pessoas.
Pelas razões acima referidas, muitos países impuseram restrições ao chumbo nas jóias, levando as nações a acelerar o desenvolvimento de vidro sem chumbo. De acordo com as normas da UE e do Reino Unido, o vidro cristal de chumbo divide-se em dois tipos: vidro cristal de chumbo, contendo PbO≥24% com um índice de refração não inferior a 1,545, e vidro cristal de chumbo completo, contendo PbO≥30% com um índice de refração não inferior a 1,560. Ao desenvolver vidro cristalino sem chumbo, os óxidos de elevado índice de refração TiO2, ZrO2, BaO, SrO and ZnO are usually introduced to achieve a refractive index close to lead glass. However, considering the transparency, whiteness, and ease of long-term shaping and processing required for crystal glass, among these high refractive index oxides, although the refractive index of TiO2 is higher than that of PbO, it can easily cause the presence of Fe2O3 in the glass to form sodium iron coloration; and ZrO2 is difficult to melt, so the most commonly used is still crystal glass made of BaO and ZnO, as well as introducing BaO, SrO, ZnO and ZrO2 simultaneously and minimize the amount of ZrO2 and TiO2 under the required refractive index conditions to avoid adverse effects on melting and the color of the glass.
The compositions of several types of lead-free crystalline glass developed internationally are shown in Table 8-1, and their properties are shown in Table 8-2.
Table 8-1 Composition of Lead-Free Crystalline Glass
| Número | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Crystalline glass type | Potassium crystalline | Barium crystalline | Zirconium crystalline | Mixed crystalline | Mixed crystalline | Mixed crystalline | Mixed crystalline |
| SiO2 | 77.0 | 58.0 | 64.0 | 59.5 | 55.7 | 57.2 | 54.2 |
| A12O3 | 1.0 | ||||||
| CaO | 5.0 | 9. 5 | |||||
| SrO | 10. 5 | 10. 5 | 10. 5 | 12.0 | |||
| BaO | 18.0 | 10. 5 | 10. 5 | 10. 5 | 12.0 | ||
| ZnO | 5.0 | 4.7 | 7.5 | 7.5 | 7.5 | 7.5 | |
| K2O | 9.0 | 16.0 | 3.0 | 5.4 | 6.4 | 5.4 | 5.4 |
| Na2O | 9.0 | 3.0 | 12.6 | 3.6 | 4.2 | 3.6 | 3.6 |
| Li2O | 1.7 | 1.7 | 1.7 | 1.7 | |||
| ZrO2 | 6.2 | 3.0 | 1.0 | ||||
| TiO2 | 3.0 | 2.0 | |||||
| Sb2O3 | 0.3 | 0.1 | 0. 3 | 0.4 | 0.4 | ||
| Como2O3 | 0.1 | 0.3 | |||||
| (Wang Chengyu et al., 2006) | |||||||
Table 8-2 Performance of Lead-Free Crystalline Glass
| Número | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Density/(g · cm-3) | 2. 898 | 2. 977 | 2. 914 | 3.046 | |||
| Índice de refração | 1.510 | 1.534 | 1. 549 | 1.545 | 1. 566 | 1.571 | 1. 575 |
| Linear expansion coefficient/(10-7·℃-1) | 87.2 | 91.4 | 87.4 | ||||
| Softening point/℃ | 674 | 674 | 675 | 673 | |||
| (Wang Chengyu et al., 2006) | |||||||
2. Colored Glass
2.1 The Origin of Colored Glass
2.2 Types of Colored Glass
The types of colored glass commonly referred to generally fall into three categories.
(1) Tradition Chinese colored glass. It is made by adding “colored glass stone” to “colored glass mother” and firing. Tradition Chinese colored glass has a murkiness, looks like jade but not jade, lacks transparency, and fundamentally differs from modern colored glass. The shapes are mostly colored glass beads, imitation jade discs, tablets, and the like. Ancient colored glass art experienced a brief prosperity during the Tang and Qing dynasties. Due to the fragility of such artworks during this period, very few artifacts survived, and the craftsmanship techniques have not been able to form a significant legacy for future generations. Therefore, there are still many mysteries regarding ancient Chinese colored glass craftsmanship.
(2) Modern colored glass. Typically represented by Taiwanese colored glass, modern colored glass evolved from Western colored glass art and originated from the ancient Egyptian “faience” craft. The analysis results from “Research on Ancient Chinese Glass” indicate that the proportion of silica in “faience” significantly is 92%~99%, which differs from the colored glass of the Zhou Dynasty in China. However, due to their similar forms, some refer to it as Western colored glass. Modern colored glass is more transparent and has diverse shapes than traditional Chinese colored glass.
(3) Water colored glass. Many low-priced “water colored glass” products have appeared on the market in recent years. In fact, this is a type of “imitation colored glass” product, not real colored glass. The term “water colored glass” arises from the deliberate actions of merchants and the misunderstandings of consumers. Water colored glass is a resin product made from transparent resin glue mixed with pigments. Its characteristics include being lightweight, lacking the metallic sound of real colored glass when struck, and easily changing color and becoming cloudy over time, with no collectible value. However, it has low cost, low technical content, simple craftsmanship, and is easy to mass produce.
2.3 The Properties of Colored Glass
Colored glass is a valuable craft artwork, and its price is higher than that of crystal for two reasons: first, the materials for traditional Chinese colored glass are special, and the production process is quite complex, involving dozens of steps to complete, with some pieces taking 10~20 days just for the production process, and mainly relying on manual labor. Each stage is quite difficult to control, and the difficulty of mastering the heat can rely half on skill and half on luck. Second, glass is not just a material but also a cultural product. More importantly, colored glass products are unique, and no two are identical.
(1) The difference between colored glass and crystal. First, there is a clear distinction in historical texts, as noted in the “Diamond Sutra.” Among all Buddhist scriptures in China, the first five categories of the seven treasures of Buddhism are universally recognized: gold, silver, colored glass, conch shell, and agate. The last two categories are said to be crystal, amber, or glass, among others, indicating that colored glass is recognized as a Buddhist treasure; colored glass is distinctly different from crystal and glass. Second, the chemical composition is different. The main component of natural crystal, colored glass, and glass is silicon dioxide. The contemporary national-level authoritative work “Research on Ancient Chinese Glass” records that the proportion of silicon dioxide in ancient Egyptian “faience” (the ancestor of Western crystal glass) is 92%(not transparent)~99%. In contrast, the colored glass from China’s Zhou Dynasty has a proportion of silicon dioxide that is just slightly greater than 90%(transparent). This distinction of 9% is the biggest difference between colored glass and crystal. The ancient colored glass was made by adding colored glass stone to a colored glass mother for firing. Colored glass stone is a colored crystal material, primarily composed of silicon dioxide. In contrast, colored glass mother is a traditional formula derived from natural sources and refined by human hands, which can alter crystal structure and physical properties, resulting in significant differences in shape, color, and transparency. The colored glass grade depends on the colored glass mother’s raw materials and preparation methods, which has been a closely guarded secret since ancient times. It is precisely because of the existence of the colored glass mother that ancient Chinese colored glass 9% differs in composition from crystal and even Western crystal glass, which is called “faience.”
(2) The differences between water colored glass and colored glass mainly lie in the following three aspects: first, the refractive index of resin is low, resulting in different product textures; water colored glass lacks the luster of lead crystal glass; second, the weight difference, the weight of water colored glass is about 30% of that of colored glass; third, colored glass is prone to aging, and its color is unstable.
For the water colored glass products that have appeared on the market, it is important to grasp their distinguishing features from colored glass, which is briefly introduced in Table 8-3.
Table 8-3 Identification Features of Water Colored Glass and Traditional Chinese Colored Glass
| Identify features | Water colored glass | Traditional Chinese colored glass |
|---|---|---|
| Color and luster | Obvious chemical dyes, the same as plastic products | Mixed in various colors, yet still transparent as before |
| Densidade | Equivalent to plastic, much lighter than real colored glass | The density of traditional Chinese colored glass is significantly higher than that of glass and slightly higher than that of crystal, and it has a smooth feel |
| Sound | The same as plastic products | Gently tapping the traditional Chinese colored glass will produce a metallic sound |
| Transport agency | Obvious turbidity, not transparent | Between glass and crystal, there are occasional small bubbles produced during the firing and flowing process |
| Storage time | Fades within one to two years, the transparency decreases over time, and the longer it lasts, the more it resembles plastic. | Indefinitely, from the perspective of materials, the traditional Chinese colored glass never changes color |
Section II Glass and Colored Glass Ornaments
1. Glass Ornaments
There are many kinds of glass ornaments.
(1) Glass ring face.
(2) Glass bracelet.
(3) Glass ring.
(4) Glass earrings.
(5) Glass pendant.
(6) Glass bracelet.
(7) Glass necklace.
(8) Glass crystals.
The typical examples of the above glass ornaments are as follows.

Round faceted stone made of glass

Glass bracelet


Glass earrings

Glass bracelet

Glass Pendant

Glass necklace

2. Colored Glass Ornaments
Colored glass ornaments are colorful, with an ancient style, reasonable structure, and rich traditional national characteristics of our country. They possess the beauty of imitating pearls and jade, infused with creativity and ingenuity. Some have praised glazed items with such flattering words: ethereal and noble, subtle yet profound, capable of absorbing brilliance, crystal clear, able to dazzle the world yet can be instantly destroyed, can transform into myriad forms yet remain eternally tranquil. In Buddhism, colored glass ornaments are spiritual objects for warding off illness and evil. Placing or wearing glazed ornaments can bring three blessings: removing illness, resilience, and inspiration.
Colored glass integrates the advantages of several art forms, such as carving and painting, gradually becoming a popular, fashionable accessory material. There are many categories of colored glass accessories, with the following seven being common: ① colored glass rings; ② colored glass pendants; ③ colored glass necklaces; ④ colored glass bracelets; ⑤ colored glass bangles; ⑥ colored glass earrings; ⑦ colored glass ornaments.
The typical examples of the above glass ornaments are as follows.


colored glass bracelet

colored glass bangles

colored glass earrings

colored glass ornaments
3. Maintenance of Colored Glass Ornaments
When wearing and maintaining colored glass ornaments, attention should be paid to the following matters due to the characteristics of the material:
(1) Do not collide or rub during movement to avoid surface scratches.
(2) Keep it at room temperature; the real-time temperature difference should not be too large. Do not heat or cool it by yourself.
(3) It is not advisable to place the cushion directly on the table on smooth surfaces; it is best to use a cushion.
(4) It is advisable to use purified water for wiping. If tap water is used, it should be left to stand for more than 12 hours to maintain the luster and cleanliness of the colored glass surface. It should not come into contact with oil stains or foreign objects.
(5) Avoid contact with sulfur gas, chlorine gas, etc.
Section III Glass and Colored Glass Ornament Production Process
1. The Production Process of Glass Craft Ornaments
1.1 The Production Process of Glass Craft Ornaments
The production process of making glass craft ornaments mainly includes materials preparation, melting, forming, annealing, and decorative treatment, as described below.
(1) Materials Preparation
According to the designed formula, weigh various raw materials and mix them evenly in the mixer. The main raw materials for glass are quartz sand, limestone, feldspar, soda ash, and boric acid.
(2) Melting
The prepared raw materials are heated at high temperatures to form a uniform, bubble-free glass melt. The melting of glass takes place in a melting furnace. There are two main types of melting furnaces. The first is the crucible furnace, where the glass batch is placed in a crucible and heated from the outside. Small crucible furnaces hold only one crucible, while larger ones accommodate up to 20. Crucible furnaces are used for batch production, and currently, only optical and colored glass are produced using crucible furnaces. The other type is the tank furnace, where the glass batch is melted in the furnace tank, with the flame heating the upper surface of the glass melt. Glass melting is a complex physical and chemical reaction process, which can be roughly divided into the following five stages.
① Silicate formation stage. The silicate generation reaction largely occurs in the solid state. The various components of the raw powder undergo a series of physical and chemical changes, and during the solid-phase reaction, a large amount of gaseous substances is released. At the end of this stage, the mixture transforms into an opaque sintered material composed of silicates and silica. For most glasses, this stage is completed at 800~900℃.
② Glass formation stage. Continue heating; the sintered material and the low melting point mixture begin to melt first. During the process, silicates and the remaining silicon dioxide melt into each other, and the sintered material becomes a transparent body. There are no unreacted raw materials, but many bubbles and streaks still exist in the glass, and the chemical composition and properties are very uneven. The temperature during the glass formation stage is approximately between 1200~1250℃.
③ Clarification stage. As the temperature rises, the viscosity gradually decreases, and visible bubbles in the glass liquid slowly escape into the furnace gas, known as the clarification process. The temperature during the clarification stage is 1400~1500℃, and the glass’s viscosity is maintained at around 100 P.
④ Homogenization stage. The components of the glass melt, which has been at high temperatures for a long time, gradually become consistent due to molecular thermal motion and mutual diffusion, and the stripes disappear. Homogenization is the stage in which the chemical composition and refractive index of the glass melt tend to be consistent. The temperature of the homogenization stage is slightly lower than that of the clarification stage.
⑤ Cooling stage. After the above four stages, the quality of the glass has reached the requirements. The glass liquid is then cooled to reduce 200~300℃, and the viscosity increases to the value required for feeding to the feeder (103P). The temperature after cooling is approximately 1200℃.
(3) Forming
The molten glass is transformed into solid products with fixed shapes. The forming process must occur within a certain temperature range, a cooling process where the glass continuously transfers heat to the surrounding medium. Due to cooling and hardening, the glass transitions from a viscous liquid to a plastic state to a brittle solid. The viscosity and its variation with temperature, surface tension, plasticity, elasticity, and other rheological properties of glass and their changes with temperature are all crucial during the forming process. The forming methods can be divided into two main categories: manual and mechanical.
① Manual molding. Manual molding uses the thermal processing method, which is the method of making glass between the melting point (1450℃) and the annealing point (450℃). The six commonly used methods are as follows:
- a. Blowing. Originating in the year 1 AD in the Roman Empire, it remains the most important, widely used, and varied glassmaking method today. During blowing, a blowpipe is dipped into molten glass paste, and the air is blown to form a small bubble, which is then shaped using tools through heat (see Figure 8-1). A second blowpipe takes a small amount of glass to create a bridge for the base, and the work is struck off to cool slowly. It primarily shapes glass bubbles, bottles, spheres (for making lenses), etc.
- b. Pulling. After blowing into small bubbles, stick with the top plate and pull while blowing (Figure 8-2).
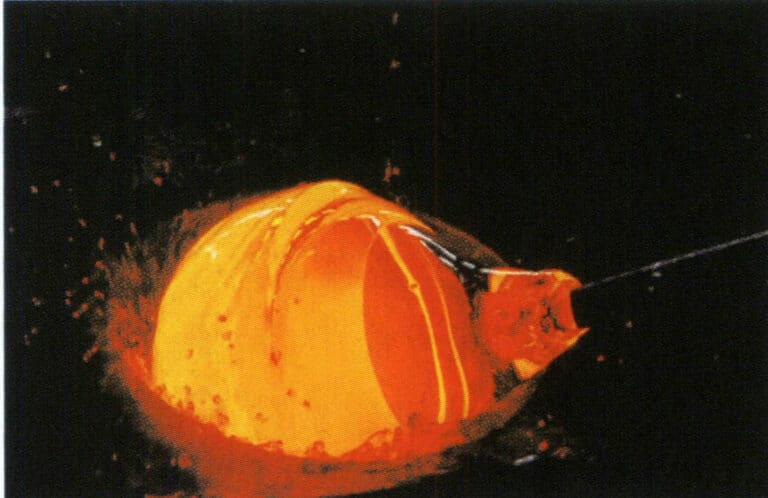
Figure 8-1 Glass Blowing

Figure 8-2 Drawing glass crafts
- c. Pressing. Take a lump of molten hot glass paste, inject it into the cavity of a mold that has already been engraved with patterns, and then press it with a punch. While it takes shape, the patterns are also pressed, mainly used to form cups, plates, etc. (Figure 8-3).
- d. Free forming. After selecting the material, use tools such as pliers, scissors, and tweezers, and heat with a small spray gun or torch, also known as torch thermoplastics. Various colored borosilicate or soda-lime glass rods can stretch, twist, and wind, continuously combining to create shapes suitable for delicate and intricate expressions, such as glass beads, animals, plants, and various shapes (Figure 8-4). Additionally, the glass rods used can be categorized as solid, hollow, and drawn thermoplastics and paired with painting to enhance the interest of the work.

Figure 8-3 Suppressed Glass Plate

Figure 8-4 Free-form glass crafts
- e. Lost-wax casting. After covering the wax mold with refractory gypsum, the raw glass materials, and the empty mold are placed in the furnace for heating. The glass slowly flows into the mold at high temperatures to take shape. It is then placed in the furnace to remove the wax and allowed to cool gradually, and the gypsum mold is removed before grinding and polishing to complete the process (Figure 8-5).
- f. Powder casting. The glass blocks and powder are placed into a pre-designed mold and put into a furnace to be heated and melted into a complete glass piece.

② Mechanical forming. Manual forming has high labor intensity, temperatures, and poor conditions, so except for lamp torch thermoplastics, some craft ornaments have been replaced by mechanical forming in addition to free-forming (Figure 8-6). Mechanical forming includes pressing, blowing, drawing, rolling, casting, centrifugal casting, and sintering.
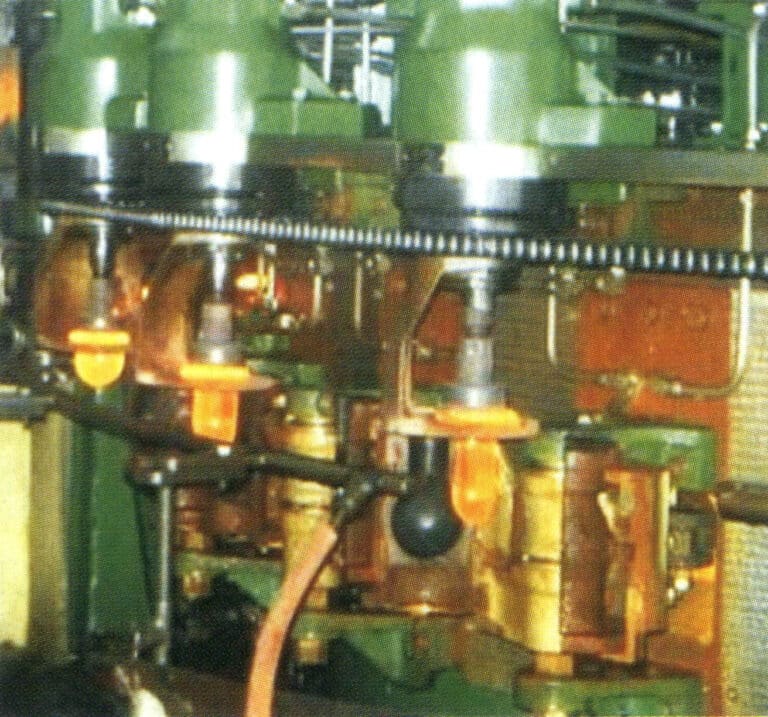
(4) Annealing
During the forming process, glass undergoes intense temperature and shape changes, which leave thermal stress in the glass. This thermal stress can reduce the strength and thermal stability of glass products. Cooled directly, it is likely to self-fracture during the cooling process or later storage, transportation, and use (commonly known as glass cold breakage). Glass products must be annealed after forming to eliminate the cold breakage phenomenon. Annealing is maintaining a certain temperature range or slowly cooling for a period to eliminate or reduce the thermal stress in the glass to an acceptable level.
In addition, certain glass products can undergo tempering treatment to increase their strength, including physical tempering (quenching) and chemical tempering (ion exchange). The principle of tempering is to create compressive stress on the surface layer of the glass to enhance its strength.

Copywrite @ Sobling.Jewelry - Fabricante de jóias personalizadas, fábrica de jóias OEM e ODM
(5) Decorative Treatment
Craft ornaments emphasize surface effects, so they generally undergo surface decoration treatment after forming. There are various methods for this treatment, including the following.
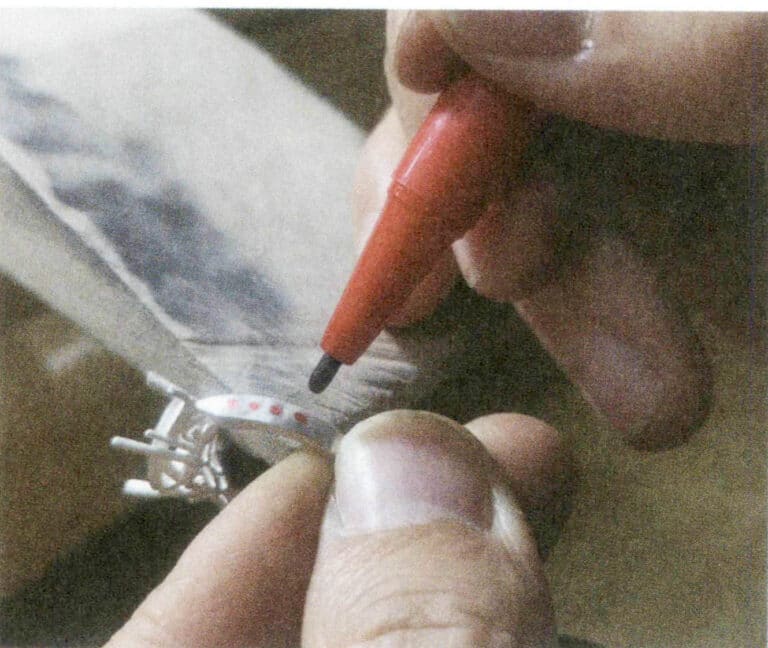
① Painting. Using colored paint, drawings are made on the surface of glass ornaments at room temperature (Figure 8-8). Some need to be heated to fix, while others do not. During this process, gold leaf and silver leaf melted into metallic paint can also be added, referred to as gilded painting.

② Glaze coloring. This is a glazing technique that requires additional heating. Patterns are drawn on the surface of glass ornaments with glaze color pigments and then placed in a furnace for heating to fix the pigments and prevent peeling.
③ Setting. This technique uses lead strips with grooves as a frame to assemble thousands of pieces of colored glass panels. It requires drawing small floor plans, creating sketches of the same size based on the floor plans, determining the shape and size of each color, correctly cutting the glass panels, and welding them into large surface mirrors with lead wire.
④ Printmaking. Using sandblasting or engraving techniques to engrave the image on a glass plate, making a model, coloring with a printing press or roller, and pressing it onto cotton or watercolor paper to create a print.
⑤ Relief engraving. Glass ornaments are embossed with a three-dimensional pattern of double or multiple layers of colored materials, revealing the background color and creating a relief effect (Figure 8-9).

⑥ Cutting. Use a cutting wheel to cut patterns, surfaces, lines, and other decorations on glass ornaments or to shape large surfaces. Sometimes, two-color layered glass is used to achieve a special effect that shows different colors inside and outside.
⑦ Engraving. A technique of decorating the surface of glass ornaments with lines, patterns, and designs using engraving tools such as diamonds, metal carving tools, or engraving pens (Figures 8-10). Depending on the tools used, it can be divided into several techniques, such as wheel engraving, dot engraving, and flat engraving.

⑧ Acid etching. Patterns are drawn on glass ornaments, lines are outlined, and staged corrosion with chemical acids creates designs with varying depths (Figure 8-11).
⑨ Sandblasting. First, cover the entire glass ornament with tape, then use a carving knife to etch away the unwanted parts of the pattern. Place the ornament in the sandblasting machine and use the high jet force of the diamond sand to create a sanded surface effect on the glass (Figures 8-12).

Figure 8-11 Glass Etching Pattern

Figure 8-12 Crystal glass sandblasted surface
⑩ Grinding. Use a rotating disc as the grinding table, mix water with diamond powder, and polish glass works.
⑪ Polishing. Use a rotating leather wheel as a platform to place the glass ornaments on it and polish large flat surfaces.
⑫ Gluing. Place the ornament in the furnace to heat, using the properties of glass to heat and melt the surface to produce brightness.
⑬ Composite materials. Creating works by combining glass with other materials.
1.2 The Production Process of Glass Crystals
Glass crystals are widely used in popular jewelry; they belong to high-lead crystalline glass, but the processing method has particularities.
(1) Materials preparation. The raw materials must undergo strict quality screening and be mixed according to the formula to produce high-quality glass crystals blanks. Glass crystals generally use crystalline glass materials containing at least 25% lead or more to achieve a high refractive index.
(2) Melting material. The furnace lining should be made of high-quality zirconia refractory materials, which will reduce the contamination of the lining material on the glass crystal. The melting material should be used either in flame heating or electric furnaces.
(3) Bad material forming. The glass liquid is poured and formed using specialized machines and molds to obtain glass crystal blank material (Figure 8-13).

(4) Glass crystal grinding. The basic process of grinding crystal includes fixture preheating→loading blank material→ bevel grinding→bevel polishing→docking feeding→bevel grinding→bevel polishing→flat grinding→flat polishing→unloading.
(5) Cleaning. Wash the polished glass crystal to remove dirt.
(6) Inspection. Place the cleaned glass crystal under the light for inspection to obtain qualified finished products.
(7) Screening. Sort the glass crystal by size and model.
(8) Chemical plating. A layer of silver is plated on the bottom of the glass crystal to increase its reflectivity, that is, refractive brightness.
(9) Protective film. A layer of protective film is sprayed on top of the already coated silver film to prevent the silver layer from discoloring when exposed to the atmosphere, maintaining the brightness and longevity of the rhinestones.
(10) Embalagem. Avoid scratches during inventory transportation.
2. The Production Process of Colored Glass Ornaments
2.1 The Lost-Wax Casting Process of Colored Glass Ornaments
Most colored glass ornaments are formed using the lost-wax casting process, which is the most difficult method in colored glass processing. However, this method also best allows the author to express their artistic concepts freely in colored glass art creation.
The lost-wax casting method has been widely used in the industrial production of metal materials and artistic creation, and it is a well-known casting technique in the industry. Its application in crystal and colored glass casting has only existed for about a hundred years. In mainland China and Taiwan, it has only been in the past 20 years that crystal and colored glass art enthusiasts have begun to use the lost-wax casting method for colored glass art creation. Although the time is short, due to continuous investment, considerable achievements have been made. In certain craft operations, they have surpassed the level of their counterparts in Europe and America, attracting international attention and exchanging invitations.
The process of lost-wax casting colored glass ornaments is similar to that of lost-wax casting metal ornaments, and its main procedures are as follows.
(1) Creative design. Transform the creative concept into graphic design drawings.
(2) Prototype making. Based on the sketch, sculpt a three-dimensional prototype using materials such as clay or wax. To master perfect proportions, elegant lines, and intricate patterns, every stroke and cut must be precise and delicate.
(3) Make a silicone mold. There are two methods for making a silicone mold: applying the silicone in layers. After the sculpture is completed, silicone mixed with silicone oil in an appropriate ratio is evenly brushed in layers onto the sculpture using a paintbrush, ensuring that the sculpture is fully coated with silicone (Figure 8-14).

Depending on the size of the work, the curing time of silicone varies, generally around 2~4 hours, while larger works may even require 10~24 hours. Silicone and silicone oil must be in the proper ratio to achieve good flexibility and strength. Conversely, suppose excessive silicone oil or hardener is added to complete the silicone mold quickly. In that case, it can significantly shorten the curing time but will result in insufficient elasticity of the silicone. When removing the wax mold, it is easy to cause the wax piece to break, making it impossible to create fine works. Moreover, silicone molds are prone to brittleness and do not have a long usage life, so patience is required to wait for the silicone mold to cure naturally. Another key factor affecting the flexibility and elasticity of the silicone mold is that the prepared silicone oil must be evenly brushed around the sculpture in layers. Although the work has height differences, the silicone mold must be evenly formed; after one layer dries, the second layer and third layer can be brushed on until a certain thickness of the silicone layer is achieved, which is a suitable and durable mold for creation.
Another method is to inject liquid glue. First, fix the wax mold on a glass surface and surround it with sandpaper, leaving a certain distance between the mold and the sandpaper tube. The silicone that has been mixed and stirred evenly is first vacuumed, then injected into the sandpaper tube, and vacuumed again, depending on the actual situation for injecting glue. After filling it with silicone, please place it in a vacuum machine to vacuum, and put the sandpaper tube that has been vacuumed last in a suitable and stable position to let it dry naturally.
(4) Reduplicating the resin mold. Use a silicone mold to cast epoxy resin and create a permanent model. Due to the aging and deformation of the silicone mold during high-temperature casting of the wax mold, it is necessary to use this resin mold to create a new silicone mold.
(5) Copying the negative mold. The sculpture is called the positive mold. The resin mold is removed from the silicone mold, polished, and adjusted to achieve the required shape, size, and better surface finish. Then, the resin mold replicates the silicone mold, reinforcing it with plaster. Appropriate dividing lines are chosen to segment the plaster. After the plaster cures, it is removed. A utility knife cuts open parts of the silicone mold, removing the resin mold. The dividing lines of the silicone mold are aligned to form a hollow mold. Tape strengthens the fixation of the plaster outer mold, creating a silicone negative mold and preparing to make a wax mold.
(6) Pouring wax molds. Pour the molten wax into the silicone mold in an appropriate amount, filling the mold, and then let it sit to allow the wax to cool and set naturally. Sometimes, the wax in the mold can be vacuumed to remove as much gas as possible to obtain a high-quality wax mold. The wax used must have a certain hardness; both too hard and too soft are unsuitable, as they will not achieve the delicate beauty of the original work. Wax that is too hard is difficult to modify, while wax that is too soft cannot hold its shape. If the wax liquid temperature is insufficient, it is prone to defects in the wax mold, and if the temperature is too high, it can lead to air bubbles, so the melting wax temperature needs to be properly controlled.
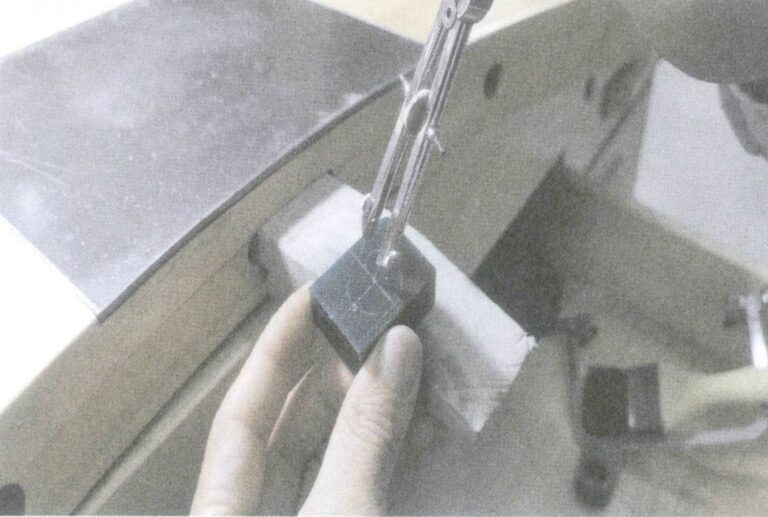
(7) Removing the wax mold. Carefully remove the cooled wax mold from the silicone mold. When opening the silicone mold, it must be pulled apart gradually, especially in areas such as hollow parts and chamfers where the wax is easily damaged; caution and dexterity are required (Figure 8-15).
(8) Adjusting the wax mold. If the wax mold has deformed, it needs to be reshaped. If there are defects on the surface of the wax mold, such as parting lines or air holes, tools are also needed for repair. When the wax mold breaks, it needs to be welded properly, ensuring that the dimensions of the wax mold are maintained during welding (Figure 8-16).

Figure 8-15 Removing the wax mold
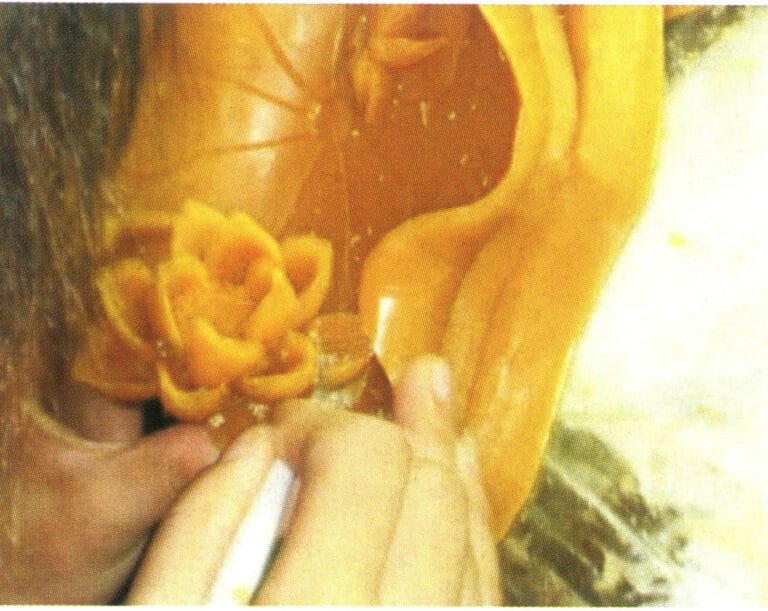
Figure 8-16 Adjusting the wax mold
(9) Making a gypsum mold. Once the wax mold is finished, you can pour the gypsum model. The gypsum casting powder is made from refractory aggregates, gypsum binders, and other modified additives. It is important to choose high-quality casting powder, as inferior casting powder often fails to ensure quality. When preparing the gypsum slurry, the water-to-powder ratio is an important parameter. If the water-to-powder ratio is too low, the gypsum slurry will be difficult to fill the fine details of the wax mold, making it hard to guarantee the quality of the work; if the water-to-powder ratio is too high, the strength of the gypsum mold will be insufficient, leading to cracks or peeling. Therefore, water should be appropriately added to prepare the gypsum slurry. The poured gypsum mold should be placed in a cool place to naturally harden (Figure 8-17).

(10) Steam wax removal. Place the naturally hardened plaster mold with the pouring gate facing down in the steam barrel, and close the door tightly to prevent high-temperature steam from spraying out and causing injury. Adjust the gas valve to introduce steam, using the high temperature of the steam to gradually melt the wax model in the plaster, slowly draining it out until there is no residual wax left in the plaster mold, at which point you can stop. The operation time varies depending on the size of the work. As mentioned above, it is also important to pay attention to the water-to-powder ratio of the plaster mixture; if the ratio is incorrect, the plaster mold can easily crack and become unusable under the high-temperature steam operation.
(11) Selected raw materials. Specific colors and sizes of colored glass materials are chosen based on the design, and each piece of raw colored glass material is carefully cleaned. The lost-wax casting of colored glass mainly uses kiln casting. In addition to the aforementioned wax removal and gypsum mold making, the suitability of the colored glass material is also an important factor affecting the success or failure of kiln-cast colored glass. Ordinary soda-lime colored glass, potash colored glass, etc., have a relatively light specific gravity, making them unsuitable for kiln casting. In 1676, England invented colored glass containing lead oxide (PbO), and the effects presented vary with different ratios of metal oxides. In addition to the suitability of crystal colored glass, attention must also be paid to the “compatibility” of the colored glass materials. The different origins of glass quartz sand or the varying ratios of added metal oxides can lead to different internal expansion coefficients, which means that stress may differ, resulting in repulsion. Once melted together, even if the heating and cooling curves of the kiln casting are calculated quite accurately, the work may still crack from the inside when the kiln is opened, during mold removal and cold processing, or even after the work is completed and placed indoors, it may suddenly and silently crack from the inside, which requires special attention.
(12) Preparing the colored glass material. Before firing colored glass ornaments, there is a very important coloring process, which is the key step in achieving the colorful effect of colored glass products. The color of colored glass ornaments is achieved by placing various colored glass materials in the mold (Figure 8-18). For example, when a flower needs to be red, then put in the red color, and if green is needed in a certain position, it should be the main color, so place it properly when arranging. To accurately control the proportions of various colors and the beauty of their flow, the positions of the colored glass raw material color blocks must be precisely arranged according to the shape and design. Once the colors are arranged, they can be put into the furnace for high-temperature firing.
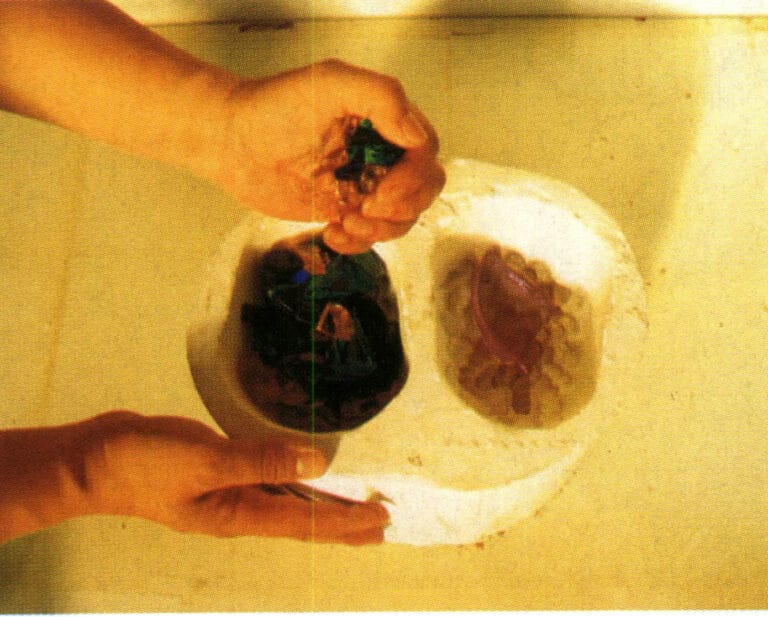
(13) Sintering in the furnace. Clean the gypsum mold thoroughly, use a dust extraction machine to remove dust from the surface of the cavity, then place the entire gypsum mold along with the prepared colored glass material into the furnace and slowly heat it to around 850℃, causing the molten glass material to soften like maltose and slowly flow into the gypsum mold to take shape (Figure 8-19). Glass casting utilizes “kiln casting,” which means completing the casting in a high-temperature furnace, rather than casting at room temperature as is common. Kiln casting is a key process in the entire procedure, with heating methods including gas furnaces, natural gas furnaces, and electric furnaces, among which electric furnaces are the cleanest and easiest to control. The difficulty of kiln firing lies in temperature control; even a slight miscalculation can lead to failure, resulting in a high failure rate. This process controls the heating and cooling (slow cooling) curves. Based on the size of the gypsum mold, precise calculations are made to start heating the electric furnace from room temperature, gradually increasing the temperature so that both the gypsum and colored glass are heated simultaneously, allowing the glass to melt rhythmically as the temperature rises, flowing slowly into the internal cavity of the mold along the curved lines of the hollow gypsum mold, filling every branch and corner, whether it is a simple bas-relief, brick shape, block, or high-difficulty features like chamfers, hollowing, and interlacing. At this moment, through the kiln casting process, the wonderful wax-free casting method of colored glass is fully expressed, unfolding the creativity of the artwork.

(14) Slow cooling. During the sintering process, to ensure uniform cooling of the colored glass inside and outside and to avoid uneven stress release that leads to cracking, it is necessary to set a cooling curve and control the slow cooling time. The cooling time varies depending on the different structural shapes. This differs greatly from lost-wax casting metals, where metal materials generally need to solidify to form. However, for colored glass, after casting and forming, it must be immediately de-molded and placed in a slow cooling furnace, with different slow cooling times assigned according to the size of the cast piece, allowing the internal stress of the colored glass material to be completely released, to prevent sudden cracking after the casting solidifies.
(15) Removing the gypsum mold. After allowing it to cool slowly, remove it from the furnace and carefully remove the gypsum mold with tools to obtain the rough blank of the colored glass (Figure 8-20).

(16) Cutting the pouring gate. After demolding, the rough colored glass blank needs to have the pouring port cut off, and the residual casting port should be removed using tools and equipment such as grinding wheels and grinding discs (Figure 8-21).
(17) Polishing. Use diamond sand rods, sandpaper, and other tools to polish the surface of the colored glass ornaments from coarse to fine (Figure 8-22).
(18) Grinding. Place the work on a cloth wheel and continuously grind and polish to bring out the luster of the glass. This will present the translucent texture of crystal material, completing the jewelry.

Figure 8-21 Polishing the gate area

Figure 8-22 Polishing the surface of colored glass ornaments
2.2 The Craft Characteristics of Lost-Wax Casting Colored Glass Ornaments
The lost-wax casting process has been widely used in producing glass ornaments. The ornaments made using this process have strong expressiveness and a wide range of adaptability. For delicate and small earrings and pendants, it can intricately express textures, sharp angles, and smooth surfaces according to the original sculpture. For medium-sized works with hollow internal carvings and intersecting branches, it is equally effortless to handle. This process can also showcase large-scale works’ grandeur and magnificent momentum, such as ancient cast bells and large relief walls. Additionally, the colors within the glass are formed by high-temperature sintering of various metal oxides, which prevents fading, oxidation, and other aging phenomena. This is why the lost-wax casting method is the most challenging among all glass processing techniques and can fully express areas that other cold and hot processing techniques cannot reach. Lost-wax casting is integrated with colored glass art creators, resembling a free-spirited horse galloping and expressing itself freely.
However, the lost-wax casting process of colored glass ornaments presents some difficulties, mainly reflected in the following aspects.
(1) The production process is complicated. From conception, design, sculpting, firing, fine-tuning, and polishing, dozens of intricate and meticulous steps are required to complete the work.
(2) Handmade. Workers must master exquisite skills to operate, as each process has uncertain variable factors, and repeated experiments are required. The works are rich in color and extremely difficult to produce.
(3) One mold, one product. A mold can only create one piece and cannot be reused. Large and complex works may even require multiple mold openings and firings. The success rate is low, making the work even more precious.
(4) High-temperature firing. Selected raw materials are melted at high temperatures to create various colored crystal glass, then repeatedly selected and cleaned. According to the material ratio of the work, it is placed in a mold, and strict heating and cooling curves are set to ensure the shaping and appearance quality of the work.
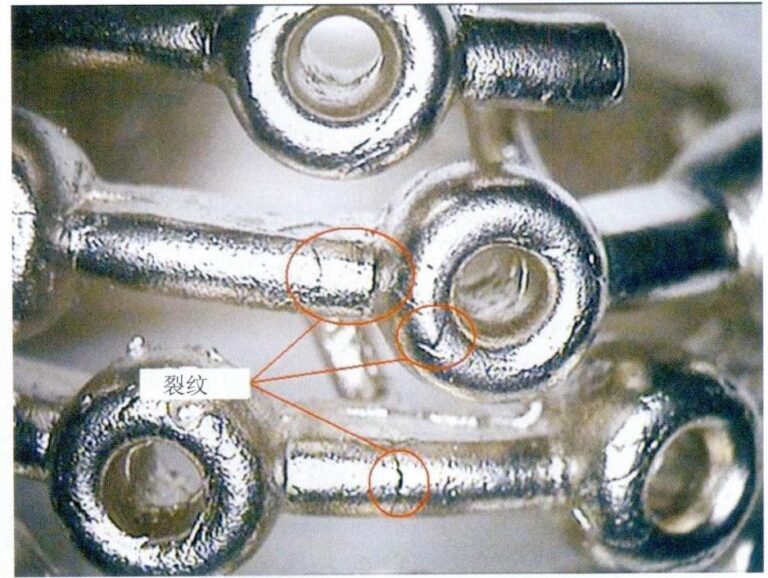
(5) Bubbles. Bubbles often appear in lost-wax cast glass works because lost-wax casting is mostly done at medium temperatures (about 900℃) during sintering. When the colored glass slurry flows into the mold cavity, it is like magma slowly flowing in, creating bubbles. At this time, the colored glass is in a thick state, and the bubbles formed in the gaps between glass pieces are not easy to rise, thus resulting in bubbles (Figure 8-23). Since bubbles often affect the visual perception of the work, especially when they appear on the surface, making the flaws more apparent, measures should be taken to reduce bubbles.