The Ultimate Guide To Artificial Gemstones, Assembled gemstones and Reconstructed gemstones
Learn about the Manufacturing Methods, Processes and Characteristics
Artificial gemstones are crafted to mimic the beauty and properties of natural gems, made through advanced laboratory techniques like flame fusion, hydrothermal synthesis, and flux methods. Assembled gemstones are multi-layered structures bonded together to resemble natural gems, offering cost-effective alternatives. Reconstructed gemstones are refabricated from fragments, often used for decorative purposes, and jewelry, with processes like welding and sintering. These gemstones are valued for their affordability and ability to imitate the aesthetic qualities of natural gems, serving various industries including jewelry and decorative arts.

Innholdsfortegnelse
Section I Artificial gemstone
Artificial gemstone are an important part of the artificial gemstone series. Due to their beautiful colors, good transparency, and crystal sizes that meet gemstone processing conditions, they can achieve or even exceed the decorative effects of natural gemstones when used in jewelry, and their low cost makes them very popular among people.
Humans have been developing and utilizing Artificial gemstone for a long time. For example, 5,000 years ago, the ancient Egyptians fired glazed ceramics to imitate turquoise. With the development of social productivity and scientific technology, Artificial gemstone that appeared in the jewelry market include: in 1927, cellulose acetate was used to imitate pearls; in 1936, the acrylic resin was used to imitate amethyst, emerald, and ruby; in 1951, synthetic strontium titanate was produced using the flame fusion method; in 1958, Yttrium Aluminum Garnet(YAG) , Synthetic Yagallium Garnet (GGG) , and synthetic yttrium iron garnet (YIG) were produced using the flux method; in 1990, glass cat’s eye and rare earth glass were produced using high-temperature and atmospheric pressure methods; in 1994, synthetic star stone was produced using high- temperature and atmospheric pressure methods; in1995, glass porcelain cat’s eye was produced using microcrystalline glass methods; in 1999, low-pressure high-temperature synthetic luminescent gemstones appeared; as well as long-existing materials like Glass and plastic. All these Artificial gemstone were invented and created by scientists in laboratories based on social needs, with no corresponding natural counterparts. Besides imitating natural gemstones, they support other industries (such as machinery, aerospace, military, electronics, etc.) .
1. Methods of Manufacturing Artificial gemstone
The methods for manufacturing Artificial gemstone are often similar to those for manufacturing synthetic stones, meaning that the methods for producing synthetic stones can also be used to produce synthetic gemstones.
1.1 Flame Fusion Method
With the development of science and technology, the flame fusion method can not only be used to synthesize rubies, synthetic sapphires, synthetic colored spinel, Synthetic rutile, synthetic star rubies, and synthetic star sapphires but has also successfully manufactured synthetic strontium titanate(SrTiO3), synthetic Yttrium Aluminum Garnet (YAG) , and synthetic Yttrium Iron Garnet (YIG) and other gem-quality synthetic crystal materials.
1.2 Flux Method
The flux method for growing crystal materials has a history of a hundred years. Many crystals can now be grown using the flux method, which can synthesize rubies and emeralds and materials ranging from metals to chalcogens and halogens.
Compounds and synthetic crystal materials range from semiconductor materials, laser crystals, and nonlinear optical materials to magnetic materials, acoustics, and jewelry.
1.3 Crystal Pulling Method
The Czochralski method was first invented by J. Czochralski in 1917, so this Method is also called the Czochralski method. Our country began using this Method in the 1970s to develop Yttrium Aluminum Garnet and gadolinium garnet crystals, mainly used for laser materials and other necessities.
1.4 Melt-guided mold method
The melt-guided mold method is an advanced technique developed in the 1960s for growing single crystals of specific shapes, also known as the EBG method. This Method has grown various shapes such as sheets, rods, tubes, wires, and other special forms of synthetic ruby, gallium garnet, and other crystal materials.
1.5 Cold crucible melting method
The cold crucible melting method is used not only to produce cubic lead oxide. Still, it can also Yttrium Aluminum Garnet, dull mirror garnet, and strontium titanate.
1.6 Zone Melting Method
The zone melting Method is used to produce high-purity synthetic rubies, sapphires, and alexandrite and to grow synthetic crystal materials such as synthetic Yttrium Aluminum Garnet.
2. Characteristics of Artificial Gemstones
2.1 Artificial Strontium Titanate
Synthetic strontium titanate crystals were developed by Mike in the United States in 1951 using the Flame Fusion Method, but the grown crystals were prone to cracking and could not form large pieces. It wasn’t until 1955 that successful commercial production of large strontium titanate crystals was achieved.
(1) Production Process
Synthetic strontium titanate (SrTiO3) is mainly used to imitate diamonds, with raw materials being the common salt of strontium oxalate and Titanium oxalate. It is produced by the reaction of strontium chloride, ferric chloride, and oxalic acid SrTiO(C2O4) 2• 4H2O and is calcined under 750℃ to SrTiO3 deep blue to black anoxic crystals, which can then be obtained as colorless transparent crystals after 1200-1600℃annealing (in an oxidizing atmosphere) 2-4h; if annealed in a reducing atmosphere, blue crystals can be obtained. It can also undergo secondary annealing, first annealed under 1700℃ and then annealed under 800℃, to improve the crystal color.
Colored artificial strontium titanate crystals are obtained by adding coloring agents during their growth process. If vanadium, chromium or manganese are added to the powder, it turns red after annealing; adding iron or nickel gives yellow or brown color. (Table 3-1) .
Table 3-1 Relationship between synthetic strontium titanate color and coloring agents
| Farge | Coloring agent | Farge | Coloring agent |
|---|---|---|---|
| Yellow to yellow-brown | Fe | Yellow to dark red-brown | Cr |
| Yellow to dark red-brown | V | Light yellow to yellow | Ni |
| Light yellow to yellow | Mn | Light yellow and yellow | Co |
(2) Characteristics
- Crystalline state: Cubical system,
- Common colors: Colorless, green.
- Luster and cleavage: Glass luster to sub-adamantine luste No cleavage.
- Hardness and Density: Mohs hardness 5-6, density 5.13(±0.02) g/cm3.
- Optical Properties: Pleochroism: none, refractive index: 2.409, birefringence: none.
- Ultraviolet Fluorescence: generally none.
- Absorption Spectrum: not characteristic.
- Dispersion: strong ( 0.190) , very prominent.
- Magnification inspection: Occasionally, bubbles are seen, poor polishing quality, scratches can be seen at the waist of the facets, and fine scratches are visible on the table. Synthetic strontium titanate produced by the flame fusion method also shows arc-shaped growth rings or color bands, with un-melted powder solid inclusions densely distributed in small areas.
- Fire color: Extremely high dispersion is visible on its table, allowing each small facet to reflect a colorful fire color. It can be used to imitate bright-type diamonds.
2.2 Artificial Yttrium Aluminum Garnet
(1) Production process
① Flux Method
- Bottom seed crystal water cooling method
The raw materials are Y2O3 og Al2O3, with a flux agent of PbO—PbF2—B2O3 (in small amounts) . The ingredient ratio is Y2O3 (5.75%) , Al2O3 (5.53%) , Nd2O3 (1.16%) , PbO(38.34%, PbF2 ( 46.68% ) , B2O3(2.5%) . Seed crystal: YAG, with a bottom face of (110) crystal plane, height 8 mm, and bottom area of 16 mm x 16 mm. The powder is heated in a Pt crucible in the furnace to 1300℃, held at a constant temperature for 25 hours, and then cooled down to 1260℃ at a rate of 3℃/h. The bottom is cooled, and the seed crystal is immersed in the center of the cold zone at the bottom of the crucible, cooled down to 1240℃ at a rate of 20℃/h, and then to 0.3-2℃/h. The cooling rate is reduced to 950℃, and the growth ends.
- Spontaneous nucleation slow cooling method
There are two methods, one using PbO-PbF2 as a flux agent: weigh Y2O3 (3.4%) 、Al2O3 (7.0%) 、 PbO(41.5%) 、PbF2 (48.1%) according to the ratio, mix in a Pt crucible, heat in the furnace to 1150℃, hold at a constant temperature of 6-24h, and then cool down to 950℃ at a rate of 4.3℃/h. Remove, pour out the molten liquid, and return the crystal to the furnace, cooling to room temperature, and take out the crystal.
The other Method uses PbO-B2O3 as a flux agent: weigh PbO(185g) 、 B2O3(15g) and Al2O3(6g) 、 Y2O3(8g) according to the ratio, mix in a Pt crucible, heat in the furnace to 1250℃, hold at a constant temperature for 4 hours and then cool down to 950℃ at a rate of 1℃/ h(it can also be held at a constant temperature for 5 hours at 1250℃, and then cooled down to 1000℃ at a rate of 5℃/h ) . Pour the molten liquid from the crucible, return the crystal to the furnace, and continue cooling to room temperature. Use nitric acid solution to dissolve the flux agent.
② Pulling Method
Mix the raw material Y2O3 and the flux AI2O3 (if used to simulate emerald, coloring agent Cr2O3 can be added) , heat in a covered alumina crucible to 1300℃, maintain temperature at 5-10h, then take out the mixture, crush and mix, and press into sheets under 20 T pressure; then sinter under 1300℃, crush again, and press into sheets to form polycrystalline sheets. Finally, heat in a high-frequency furnace to 1950℃ (YAG melting point) , and protect with helium (Ar) . After the melt fully wets the seed crystal, slowly pull up and rotate the crystal rod, controlling the pulling speed (growth rate 1.22 mm/h) and rotation speed (10r/mim) .
③ Floating Zone Method
Weigh 55.35% of Y2O3 and the chemical pure 44.64% of AI2O3 and heat them at 500℃ temperature for a day and night, remove moisture, and cool to room temperature before weighing. Mix the powders of Al2O3 and Y2O3, press them into fine rods using static pressure, sinter at 1350℃ for 12 hours, then grind them, and press and sinter again, repeating this process three times. Finally, fix the sintered rod with a chuck and place it in an insulating tube; start heating, melt from one end, and rotate the heater or sintered rod to advance the melting zone to the other end, crystallizing from the melting zone to obtain crystals.
When growing Synthetic Yttrium Aluminum Garnet using the floating zone method, the amount of Al2O3 is more than the theoretical ratio. This is because the theoretical ratio should be: Y2O3 accounts for 57.05%、 Al2O3 as 42.95%, and if rods are made with this ratio, the crystals will change from a transparent state to an opaque state during the growth process, failing to reach gems quality, which is due to the generation of YAlO3.
(2) Characteristics
Colorless Yttrium Aluminum Garnet is often used to imitate diamonds, while green Yttrium Aluminum Garnet is commonly used to imitate emeralds. However, it has different characteristics from diamonds and emeralds.
- Crystal system: Cubical system, massive.
- Color: Colorless, green (can be color-changing) , blue, pink, red, orange, yellow, purplish-red, etc.
- Luster and cleavage: Glassy and sub-adamantine luster, no cleavage.
- Hardness and density: Mohs hardness 8, density 4.50-4.60 g/cm3.
- Optical properties: homogeneous body, no pleochroism, refractive index 1.833(±0.010, no birefringence.
- Ultraviolet fluorescence: colorless YAG: none to moderate orange (long wave) , none to red-orange (short wave) ; pink, blue YAG: none; yellow-green YAG: strong yellow, may exhibit phosphorescence; green YAG: strong, red (long wave) ; weak, red (short wave) .
- Absorption spectrum: light pink and light blue YAG have multiple absorption lines at 600-700 nm.
- Magnified inspection: clean, occasional bubbles. Due to different production processes, there may be inherent defects from different manufacturing methods.
2.3 Artificial Yagallium Garnet
Artificial Yagallium Garnet is part of a series that includes Yttrium Aluminum Garnet and synthetic yttrium iron garnet, belonging to the category of synthetic gemstones with a garnet structure. Because Synthetic Yagallium Garnet can be doped with Chromium, rare earth neodymium, and transition elements, it can exhibit a variety of vibrant colors. Synthetic Yagallium Garnet can be used as a synthetic gemstone, especially green and blue crystals; more importantly, it can also be used as a magnetic bubble material and laser matrix material needed in industry.
(1) Production Process
The production methods for Synthetic Yagallium Garnet (Gd3Ga5O12) include the cold crucible melting shell, guided mold, and crystal pulling Method.
The typical process for growing Synthetic Yagallium Garnet using the crystal pulling method involves: Medium frequency induction heating,Iridium Crucible,filling N2 + O2 gas, a pulling speed of 6 mm/h,and a seed crystal rod rotation speed of 30r/min.The seed crystal is oriented to grow along the (111) direction, resulting in a crystal length of 20-25 mm and a width of 60 mm.
(2) Crystal Characteristics
The gadolinium gallium garnet produced by different manufacturing methods not only has its process characteristics but also has the following common features:
- Crystalline state: Cubical system, massive crystalline body.
- Color: Usually colorless to light brown or yellow.
- Luster and cleavage: Glass luster to subadamantine luster; no cleavage.
- Hardness and density: Mohs hardness 6-7, density 7.05(+0.04, -0.10) g/cm3 .
- Optical properties: optically homogeneous, no pleochroism, refractive index 1.970 (+ 0.060) , no birefringence.
- Ultraviolet fluorescence: strong in shortwave, pink.
- Absorption spectrum: non-characteristic.
- Dispersion: strong (0.045) .
- Magnified inspection: may have bubbles, gas-liquid inclusions, or metallic plate-like inclusions.
2.4 Glass
Glass used as gemstones can be divided into natural Glass and artificial Glass. Natural Glass is formed under natural conditions (geological or cosmic processes) , such as volcanic obsidian, basalt glass, or meteorite glass that falls to the ground from space; artificial Glass is a gem-like material manufactured by people using melting and molding techniques. Glass can be classified by composition into crown glass made of silica, soda, and lime, and flint glass made of silica, soda, lead oxide, etc. It can also be classified by transparency into transparent Glass and semi-transparent to opaque Glass.
(1) Manufacturing Process
Now, China is a major glass producer, with a variety of glass types to meet various needs.
The Glass used for imitation gemstones is usually obtained through conventional melting techniques, and imitation gemstone glass products typically use molding techniques to achieve the desired gemstone shape, with tin oxide polishing applied to smooth out edges and facets that may have been caused by cooling shrinkage.
To obtain various colored glass imitation gemstone products, different coloring agents in the form of elemental ions are usually added to the glass raw materials. For example, adding Co2+ results in deep blue; adding Au gives a “golden red” color; adding Ag results in “silver yellow”; adding %, adding V2O5 produces a color-changing effect; adding Mn results in purple; adding Se gives red; adding Cu can yield red, green, or blue; adding Cr results in green; adding U gives yellow-green; adding antimony sulfide results in “antimony red”; when manufacturing colorless Glass, a “glass fertilizer” is added to eliminate the green caused by Fe; some colorless glass imitations have appropriate colors applied to the glass surface to present colors on the tabletop; or they may be treated with vacuum coating technology to create an iridescent effect; or a backing foil may be applied to the imitation gemstone product to exhibit strong flashes, and so on.
Different production processes control glass’s transparency. High-transparency Glass requires the addition of high-purity additives, while tin oxide should be added during the manufacturing process to obtain translucent or opaque Glass.
(2) Types of Imitation Treasures
① Transparent glass imitation gemstone
Transparent Glass can imitate gemstones, such as diamonds, crystals of various colors, topaz, emeralds, aquamarines, rubies, sapphires, and so on. High-lead Glass has a high refractive index, density, luster, and dispersion, making it suitable for imitating colorless diamonds; rare earth glass has a high refractive index, strong luster, and vibrant colors, closely resembling beryl, topaz, and others. However, despite their similar appearance, their essence is different, as Glass is ultimately an amorphous super-cooled liquid.
② Translucent to opaque Glass
The Glass used to imitate semi-transparent gemstones is made by adding certain oxides, phosphates, and other components to calcium-containing Glass, resulting in insoluble calcium compound that give the Glass a semi-transparent appearance. To imitate opaque gemstones like lapis lazuli, a larger amount of additives can be incorporated into the Glass.
- Artificial glass cat’s eye imitating cat’s eye stone
Its optical effect is achieved using various colors of optical fiberglass strands, each wrapped in a colorless glass tube. Hundreds to tens of thousands of these tubes are bundled, repeatedly heated, pressurized, and drawn into fibers, then cut and polished into a curved surface to reveal the cat’s eye effect. To ensure good fusion between the optical fiberglass strands and the colorless glass tubes, the refractive index and expansion coefficient of both must be the same, and the melting point of the tube should be slightly lower than that of the optical fiberglass. The heating temperature should be suitable for melting the colorless tube glass.
- Imitation jade glass
Also known as Devitrified glass. “Malaysian jade” (abbreviated as Malaysian jade) is created by adding a green coloring agent to the molten Glass, which forms some crystallization during the cooling process, resulting in a network-like or speckled structure resembling green jade’s appearance.
- Imitation Opal Glass
It involves irregularly mixing some rainbow-colored metallic foil pieces between layers of silicate glass, creating an effect similar to the “color-changing effect.”
- Imitation Pearl Glass
It is usually made of a “pearl core” made of transparent to opaque white lead silicate glass, coated with a shiny film of pearl essence (guanine) , consisting of these two parts. The surface has colors like cream, rose, and wine, similar to seawater cultured pearls. This “glass pearl” is most famously produced by the Spanish company Majorica S.A. and is very popular in Europe and America.
- Imitation Lapis Lazuli Glass
It is made by melting glass with copper or mica powder and coloring agent. The copper powder is used to imitate pyrite, while the mica powder imitates the calcite in lapis lazuli.
- Imitation starlight gemstone glass
It is made using lamination technology on a red or blue curved semi-transparent glass base, with several fine lines engraved, or with metal foil pieces engraved with fine lines attached to the bottom of the Glass, creating a “starlight effect,” used to imitate star light rubies and starlight sapphires, where the star lines appear just like natural starlight gemstones.
- Imitation Emerald Glass
Using raw materials with the chemical composition of emerald and the coloring element chromium, prepare Be3Al2Si6O18 + Cr, and then after melting and cooling; you can obtain green Glass used to imitate emerald.
(3) Characteristics
Glass can imitate various gemstones, but its essence is primarily an amorphous silicate based on SiO2. Its composition, structure, and optical properties completely differ from the gemstones it imitates, making it easy to identify. The specific characteristics of imitation gemstones are shown in Table 3-2.
Table 3-2 Common Characteristics of Glassy Materials
| Type | Chemical composition (%) | Brytningsindeks | Tetthet (g/cm3) |
|---|---|---|---|
| Melting Glass | SiO2 : 100 | 1.46 | 2.2 |
| Ordinary Glass | SiO2 : 73, B2O3 : 12, CaO : 12 | 1.5 | 2.5 |
| Toughened Glass | SiO2 :72, B2O3 :12,Na2O : 10, Al2O3 : 5 | 1.5 | 2.4 |
| Leaded Glass | SiO2 :54, PbO : 37, K2O :6 | 1.6 | 3.2 |
| Heavy leaded glass | SiO2 : 34, PbO : 34, K2O : 3 | 1.7 | 4.5 |
| Extra heavy leaded glass | SiO2 : 18, PbO : 82 | 1.96 | 6.3 |
- Crystalline state: amorphous body, can be crystallized.
- Color and Luster: The colors are diverse, with a glassy luster.
- Hardness and Density: Hardness ranges between 5-6, usually 5.5; density is 2.30 -4.50 g/cm3 , typically less than2.65 g/cm3.
- Optical Characteristics: Homogeneous body, usually exhibits anomalous extinction under orthogonal polarized light. Faceted melted crystals show a black cross interference pattern. Glass spheres can display colorful double arcs and alternating black cross interference colors; no pleochroism; refractive index 1.47-1.700 (including rare earth element glass 1.80±) ; no birefringence. Devitrified glass can show full brightness under orthogonal polarizing filters.
- Ultraviolet Fluorescence: Weak to strong, varying by color, generally shortwave is stronger than longwave. Common fluorescence is chalky white.
- Absorption Spectrum: Non-characteristic, varies by coloring elements.
- Appearance characteristics: rounded faceted edges, surface with cavities, bottom with condensation shrinkage pits; the eye line is too straight, sharp, and glaring, and usually presents 1-3 lines of eye line.
- Magnified inspection: bubbles, various solid inclusions, elongated hollow tubes, flow lines, “orange peel” effect, swirling or flowing structures.
- Special optical effects: goldstone effect, cat’s eye effect, color change effect, luster effect, halo effect, starlight effected.
- Optimization treatment: film treatment, whole or partial film covering, to imitate natural gemstones or enhance color and luster, often with visible partial film peeling; sharp objects can scrape the film.
2.5 Plastic
Plastic is a soft, heat-resistant synthetic organic material. It is commonly produced using heating and molding methods to imitate organic gems such as Amber, jet, ivory, coral, pearls, shells, and tortoiseshell. It can also imitate inorganic gems like opal, turquoise, jade, and nephrite. The most important limitation is amber.
(1) Manufacturing Process
Plastic products imitating gems are mostly made using injection molding, and some also use film lamination, mirror backing, and surface coating techniques.
① Plastic Amber
Crush an appropriate amount of acrylic sheet (formaldehyde acrylic ester) into small particles or powder and place it in a covered glass container; add chloroform (trichloromethane) , seal the container tightly, and dissolve it into a transparent liquid. Then, inject the organic liquid into the mold, where various paintings, portraits, flowers, birds, fish, insects, or souvenirs can be placed in advance. Finally, place the mold in a clean, dust-free, quiet place and wait for it to harden to obtain a satisfactory product. If pigments are added to the organic liquid, the imitation can also be colored. (Figure 3-1) .
② Plastic Opal
Plastic imitation opal products were made by Japanese scientists in the 1980s by slowly depositing 150-300mm polystyrene spheres in the laboratory, which were tightly stacked to form a three-dimensional diffraction grating. The plastic opal has a two-layer structure: polystyrene on the inside and an acrylic resin on the outside. Plastic opal has a two-layer structure: the inside is polystyrene, and the outside is coated with acrylic resin.
Making polystyrene into tightly packed small spheres and adding another type of plastic with a slightly different refractive index between the spheres for consolidation can display a color-changing effect similar to that of opal.
③ Plastic Pearl
Imitation pearl plastic comes in two types: one is made by mixing pearl essence or fish scale essence into a plastic nitrocellulose paint to create a liquid coating applied to translucent plastic beads. After the coating dries, several layers are applied until a pearlescent sheen is achieved; the other type involves adding materials like mica flakes and copper carbonate crystals into the paint, which is then applied to the plastic beads, sometimes with an additional layer of guanine coating on top.
④ Plastic Goldstone
It is made by adding metallic copper to colorless transparent plastic.
⑤ Plastic Tortoiseshell
Plastic imitation tortoiseshell is mainly used as a material for eyeglass frames, combs, and shoe horns. It is made by adding black pigment to plastic liquid.
(2) Characteristics
- Chemical composition: C, H, and O are constituent elements.
- Crystalline state: Amorphous non-crystalline.
- Color and luster: Can have various colors, commonly red, orange-yellow, yellow, etc.;
- Transparency: Transparent to opaque.
- Hardness and density: Hardness 1-3, density generally 1.05-1.55 g/cm3.
- Optical characteristics: Homogeneous body, no pleochroism, refractive index generally between 1.460-1.700, strong dispersion (0.190) . Snake-skin-like bands of anomalous birefringence and interference colors are commonly observed due to stress under crossed polarizers.
- Magnification inspection: Often has streamlines and bubbles, with bubbles commonly appearing spherical, oval, elongated, tubular, etc. The surface is often uneven or has small pits. Shell-like fracture.
- Special inspection: The hot needle test may have camphor, carbonic, acid, formaldehyde, fishy, yogurt, or sweet fruit smells; rubbing will generate static electricity and noticeable warmth when touched.
2.6 Imitation Gemstone Ceramics
Ceramics can imitate many types of gemstones, such as imitation opal, lapis lazuli, imitation coral, imitation turquoise, imitation malachite, etc.
Earthenware is made from clay (clay minerals) sintered; porcelain is made from Ceramic clay (feldspar, quartz, mica, pearl clay) sintered. Both are opaque to semi-transparent.
(1) Manufacturing Process
Silicate mineral raw materials are ground into powder or adhesives and pigments are added, then heated, roasted, or hot-pressed to form. Sometimes, glaze is applied to the surface to enhance brightness and aesthetics.
- Opal-like ceramics are a type of chemically bonded ceramics produced by the Japanese in the 1980s, featuring a color-changing effect and long-lasting stability.
- Lapis lazuli-like ceramics: made from polycrystalline spinel materials, containing star-like yellow opaque inclusions (containing cobalt) that resemble pyrite, and are very similar in appearance to lapis lazuli. Refractive index 1.728,density 3.64 g/cm3 . The yellow star points are very soft and can be pierced with a needle.
- Coral-like ceramics: made by adding additives to calcium carbonate(CaCO3) powder and sintering, available in white and red.
- Imitation turquoise ceramics: made from aluminum ore (aluminum trihydrate) materials sintered with green coloring agents. The color is dull, the structure is denser than natural turquoise, and the refractive index and density are usually greater than those of natural turquoise.
(2) Ceramic characteristics
- Composition: various mineral salts and additives.
- Color: commonly found in white, green, and blue.
- Hardness and Density: The hardness is usually higher than that of the simulated gemstones, and the density is also relatively high.
- Optical Properties: The luster is dull, the optical properties are variable, and the refractive index has a wide range of variation; the refractive index of simulated lapis lazuli ceramics reaches 1.728.
- Magnified Inspection: A uniform distribution of powder particles is visible, lacking the unique structure of the simulated gemstones.
2.7 Artificial Luminescent Pearls
There are more than a dozen types of minerals in nature that can emit light, commonly including Diamond, fluorite, apatite, scheelite, calcite, copper-uranium mica. If large particles of luminescent gemstones are ground into “spheres,” they are commonly referred to as “Luminescent Pearls,” but they are extremely rare.
For nearly half a century, some have mixed luminous powder with mineral powder or plastic to create spherical bodies, or coated the surface of spherical bodies with luminous powder to imitate the natural gem “luminous pearl.”
(1) Manufacturing Process
① Raw material formulation: including raw material activators and additional activators
- Raw materials: weigh SrCO3: 71.69 g, Al2O3: 50.5 g, H3BO3: 0.3 g; weigh activator and additional activator EU2O3: 0.88 g, Nd2O3: 0.84 g and Dy2O3: 0.93 g. Crush these raw materials and activator and mix them evenly into crucible.
- Sintering of raw materials: put the crucible containing raw materials into the electric furnace, heated to 800-1400℃ under the reducing conditions, constant temperature for 3 hours; after that, cooled down to 1300℃, constant temperature for 2 hours; and then naturally cooled down to 200℃, removed from the furnace, that is, to obtain the luminescent material.
② Luminescent stone synthesis
- The prepared luminescent material (fine powder or block) in the crucible.
- The crucible is buried in the pressure furnace in the carbon powder (as a reducing atmosphere) within the heating. Furnace temperature after 5-8h slowly rise to 1550-1700℃, at the same time add more than two atmospheres, constant temperature and pressure 2-3h, natural cooling to 200℃.
- Remove the sintered body from the pressure electric furnace and cool it to room temperature.
- Polish (or carve) the sintered body to make luminous gemstones.
(2) Characteristics and Uses
① Uses of luminous powder
- Luminous powder is added to coatings, inks, and other materials to create luminous coatings and inks, which can be used in fields such as home decoration, textiles, paper printing, calligraphy, and painting works, stage design, playing a beautifying role and adding a mysterious color to these items.
- Luminous powder is used in road traffic indicator lights, daily necessities, and emergency equipment, marking their location and preventing dangers.
② Characteristics of glow gemstones
- Color light: green, cyan, white, red, purple. The body color is bright and diverse.
- Texture: bubbles, particles.
- Hardness: The smaller the raw material particle size, the greater the hardness of the gemstone and the better its durability; when the temperature exceeds 1700℃, the gemstone becomes brittle. The Mohs hardness can reach 6.5.
- Density: 3.54g/cm3; the smaller the raw material particle size, the higher the density of the gemstone.
- Optical properties: Chemically stable structure, strong acid, and alkali resistance, with a refractive index of 1.65, can emit different light colors depending on the composition.
Section II Assembled gemstones
Assembled gemstones, Their production process is completely different from that of synthetic gemstones and artificial gemstones. They are combinations made from various solid materials bonded or fused with adhesives and appear like natural gemstones.
Assembled gemstones have been around for a long time. As early as the Roman Empire, jewelry craftsmen could use Venetian turpentine to bond three different colored gemstones together to create larger gemstones, and they would also melt glass to cover garnets, processing them into Assembled gemstone jewelry through cutting, polishing, and setting techniques.
Assembled gemstone jewelry has remained popular due to its good quality and low price, especially before the mass production of synthetic gemstones. The reason Assembled gemstones are still popular today is that they can imitate high-end gemstones, allowing small, difficult-to-process gemstone materials to be utilized through bonding, better revealing their potential beauty while also making the surface of the gemstones more wear-resistant and enhancing their luster, and providing reinforcement for fragile, thin-layered gemstones with a hard backing.
1. Production Process
The key point in producing Assembled gemstones is that the combined materials should have an overall appearance. Generally speaking, when processing faceted Assembled stones, the joints are often placed at the waist edges, reflecting the overall appearance through the pavilion’s reflection; if processing round brilliant or emerald-shaped Assembled gemstones, the number of facets at the pavilion should be increased. For example, when polishing round brilliant Assembled stones, two layers of 16 main facets can be polished at the pavilion; for emerald-shaped Assembled stones, several layers should be polished at the pavilion. This way, the Assembled stones’ color and other optical properties can be reflected.
1.1 Type of Craft
According to the materials, structural construction, and artistic features used in Assembled gemstone, they are internationally classified into three main types: Two-layered stone, Triple-layered stones, and substrate stones.
(1) Two-layered stone
Two-layer stone refers to the two materials (natural jewelry and jade, synthetic or artificial stones) by bonding or fusion together to give the impression of a whole piece of jewelry and jade (Figure 4-1). According to the similarities and differences in the materials used, they can be categorized into homogeneous two-layered stone, similar two-layered stone and heterogeneous two-layered stone.
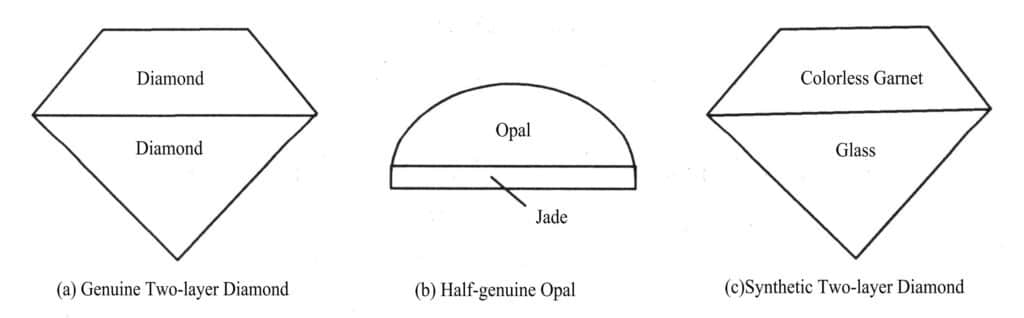
① Homogeneous Two-layered stone
Homogeneous Two-layered stone is composed of two pieces of the same material. One of the good quality of a piece of the crown, the other poor quality of a piece of the pavilion, giving people a large and beautiful overall vision. This is the case with two rubies, or two opals, which form a bilayer. The stone is also known as a true diorite. Homogeneous Two-layered stone is also known as true Two-layered stone [Figure 4-1(a)].
② Similar Two-layered stone
The homogeneous two-layer stone, is composed of a piece of natural jewelry and jade and a corresponding synthetic gemstone, improve the composition of the stone. The natural stone is the crown and the synthetic stone is the pavilion, giving the impression of a natural stone. Such as opal and synthetic opal two-layer stone, jadeite and dyed jadeite combination of two-layer stone. Class texture two layer stone, also known as half true two layer stone [Figure 4-1 (b).
③ Heterogeneous Two-layered stone
Heterogeneous Two-layered stone, is composed of two different materials dolomite. Such as colorless synthetic cubic zirconia and glass combination of diopside imitation diamond, colorless garnet and colorless glass combination of diopside imitation diamond, this type of diopside is also known as false Two-layered stone [Figure 4-1 (c)].
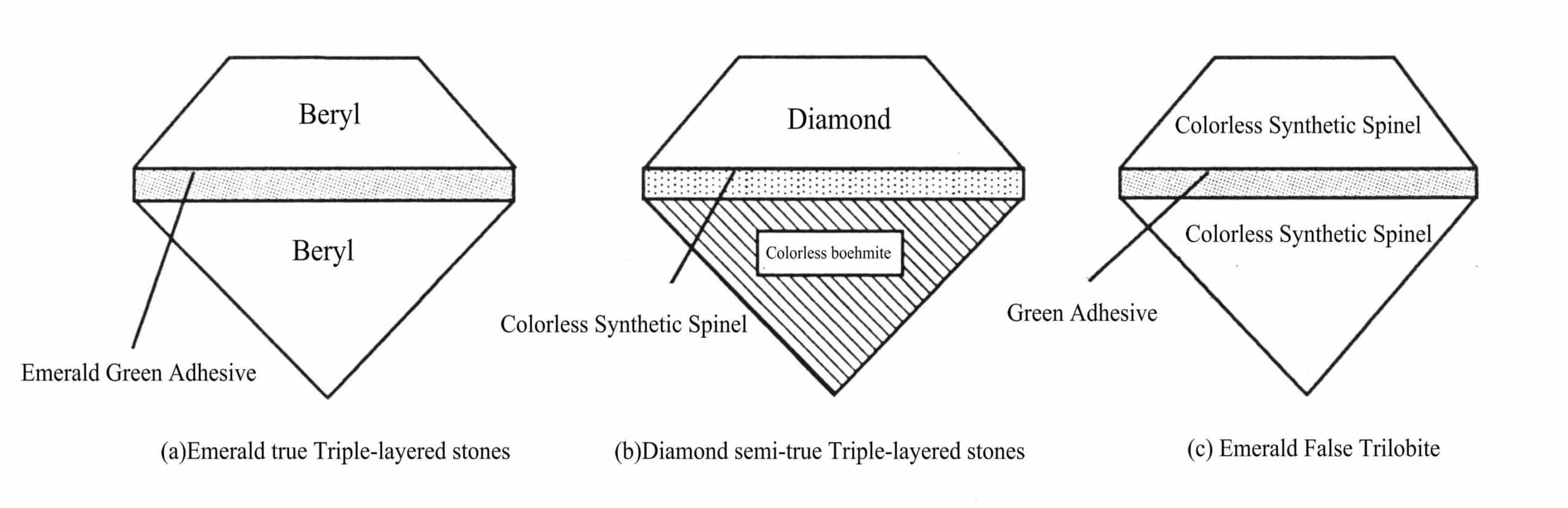
(2) Triple-layered stones
Triple stone as the name suggests refers to three kinds of gemstone materials or by a colored substance and the other two gemstone materials bonded or fused together to form a whole patchwork stone (Figure 4-2).
Copywrite @ Sobling.jewelry - Tilpasset smykkeprodusent, OEM og ODM smykkefabrikk
According to the composition of the three layers of stone material differences and similarities, can be divided into homogeneous Triple-layered stones, class quality Triple-layered stones and heterogeneous Triple-layered stones three kinds.
① Homogeneous Triple-layered stones
Homogeneous Triple-layered stones, is composed of three pieces of the same kind of material with the imitation of gemstones bonded into a whole Triple-layered stones. Such as three jadeite composed of three layers of stone [Figure 4-2 (a).
② Similar Triple-layered stones
A trilobite is a combination of a natural stone and two corresponding synthetic or improved stones, or a trilobite consisting of a natural stone, a corresponding synthetic stone, and a colored adhesive bonded to imitate a natural stone [Figure 4-2(b)].
③ Heterogeneous Triple-layered stones
As the name suggests, heterogeneous Triple-layered stones is a combination of three different materials or two of the same material and a different material composition of the Triple-layered stones. Such as a layer of synthetic ruby, the second layer of red spinel, the third layer of red glass composed of three layers of stone, imitation ruby; or by the natural ruby, synthetic ruby and red glass combination of three layers of stone, imitation ruby [Figure 4-2 (c).
(3) Substrate Stone
This is a special form of Assembled stone, using opaque materials as the substrate, bonded or coated on the back of the gemstone or the pavilion. Depending on the substrate material, it is divided into two types: foil stone and coated stone.
① Foil Substrate Stone
This is a metal foil made of an opaque material pasted on the back or pavilion of a gemstone to enhance its light reflection ability, improving the star effect, color, and other aesthetic qualities of the Assembled stone.
There are many types of Assembled stones. Common ones include pasting a blue reflective mirror on the back of a star effect fuchsite, which can produce colors and special optical effects similar to star-fuchsite; engraving “star lines”” on metal foil and pasting it on the back of curved transparent gemstones or transparent glass or other transparent materials to imitate star gemstones; some paste metal foil between two layers of gemstones to create special optical effects.
② Coated Substrate Stone
This involves applying a layer of colored substance on the back of a gemstone to enhance its color or cover some defects of the gemstone; this type of Assembled stone is also called coated stone.
For example, to enhance the blue of blue diamonds, a transparent and wear-resistant colored fluoride film is applied to the reflective part at the bottom of the diamond; a layer of green film is applied to the bottom of non-gem-quality beryl to imitate emerald.
1.2 Production Process
As mentioned earlier, the production process of assembled gemstones is a type of manual modification. Regardless of the type of Assembled gemstone, its basic characteristic is a layered structure, which means that several materials are bonded together layer by layer to form a whole.
(1) Two-layered stone Production
Two-layered stone are generally formed by bonding two pieces of gemstone material with a colorless adhesive. Common varieties include:
① Garnet glass Two-layered stone
Made from garnet and glass of the same color. To achieve more benefits, garnet is only used as part of the crown top cover, while the majority is made of cheap glass. The purpose of using garnet is to enhance the hardness and durability of the Assembled gemstone. This Two-layered stone is often used to imitate colored gemstones such as garnet, sapphire, ruby, emerald, and amethyst colorless can imitate diamonds.
The general production method is to punch several holes about 1.3 cm in diameter in steel plate approximately 2.5 cm thick, fill the holes with glass powder, and then cover the holes filled with glass powder with thin slices of garnet. Then the prepared steel plate is then placed in a heater to heat it, causing the glass powder to melt and cool. The garnet bonded with glass is then removed. It is processed and polished to form a Garnet glass Two-layered stone.
② Corundum Two-layered stone
(a) Sapphire Two-layered stone and ruby Two-layered stone
The materials used are mainly natural and synthetic sapphires or natural and synthetic rubies. The crown part is made of flat or wedge-shaped thin slices of natural material, or part of the crown, or even just the tabletop. The pavilion part is made of synthetic material bonded with adhesive. The seams are below the waist or tabletop.
The cut of this Two-layered stone is primarily a mixed cut, with the crown part using a brilliant cut and the pavilion part using a step cut. It is used to imitate natural sapphires or rubies.
(b) Imitation star sapphire and imitation star ruby Two-layered stone
There have historically been two methods for making this Two-layered stone.
- The top cover is made of natural star fuchsite with a curved cut, and the bottom is a mirror-reflective metal film or a metal backing engraved with star lines or blue (or red) glass, bonded together as one.
- The top cover is made of synthetic star sapphire or synthetic star ruby with a curved cut, and the bottom is made of blue or red glass, both bonded into one.
③ Jadeite Two-layered stone
The jadeite Two-layered stone mainly consists of a high-quality natural green jadeite top cover with a curved cut. At the same time, the bottom is made of inferior jadeite or glass and other imitation jadeite materials, with the joint seam hidden beneath the curved surface and embedded with a precious metal frame.
④ Diamond Two-layered stone and imitation diamond Two-layered stone
- Diamond Two-layered stone: Two smaller natural diamonds are used for the crown and pavilion, bonded together with colorless adhesive at the waist to form a larger diamond [Figure 4-1(a)].
- Imitation diamond Two-layered stone: The crown part uses natural diamonds; the pavilion part uses colorless crystals, colorless synthetic sapphires, colorless synthetic spinel, or colorless glass bonded together with colorless glue; or the crown part is made of synthetic cubic lead oxide, colorless synthetic sapphires, or colorless synthetic spinel, and the pavilion part is made of artificial synthetic strontium titanate, bonded together with colorless glue at the waist.
(2) Triple-layered stones production
The production process of Triple-layered stones usually consists of two gemstones and a colored adhesive or three pieces of gemstone material bonded together with a colorless adhesive. Common varieties of Triple-layered stones include:
① Imitation emerald Triple-layered stones
There are four methods for making imitation emerald Assembled stones:
(a) Made of two pieces of natural green tourmaline for the crown and pavilion, bonded with green adhesive to form a Triple-layered stones. [Figure 4 – 2(a)].
(b) Made of two pieces of colorless crystal for the crown and pavilion, bonded in the middle with green adhesive.
(c) Made of colorless crystal for the crown and pavilion, with a layer of green lead glass in the middle, bonded with colorless adhesive.
(d) Made of two pieces of colorless synthetic spinel for the crown and pavilion, bonded in the middle with green adhesive; green glass can also be used instead of green adhesive, with colorless adhesive bonding the three together.
② Opal Triple-layered stones
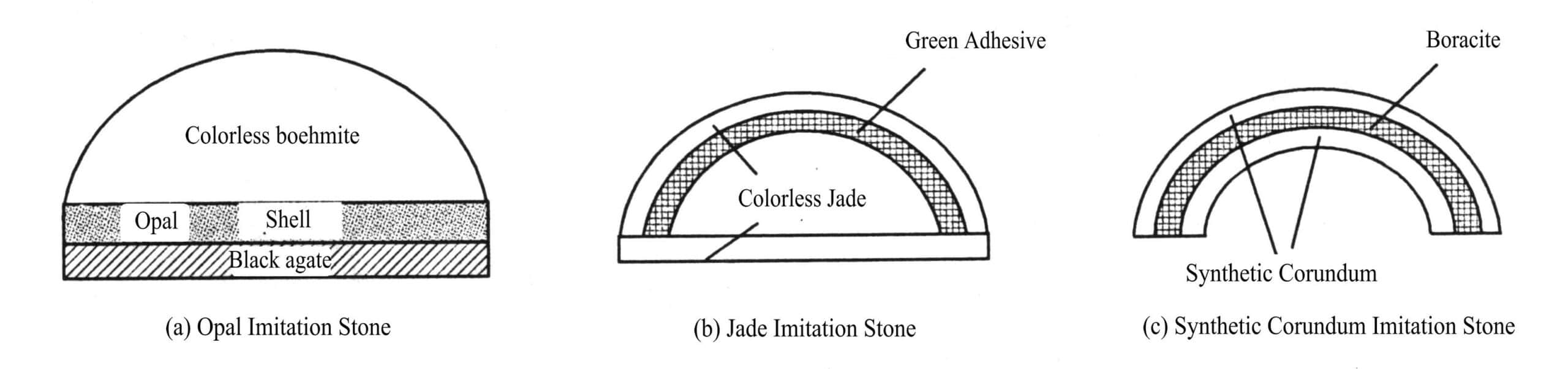
The Opal Triple-layered stones consists of a layer of colorless transparent glass, or colorless crystal, synthetic spinel, synthetic sapphire, etc., forming the pavilion, with opal slices in the middle and the bottom made of black agate or black glass, all bonded together with colorless adhesive. Because materials like crystal, spinel, or sapphire have high hardness, they can enhance the durability of the Assembled gemstone [Figure 4-3(a)].
③ Jade Triple-layered stones
This Assembled gemstone is made of three pieces of translucent colorless jade. First, an oval-shaped jade is inserted into a hollow round cap-shaped jade, with a green gel-like substance filling the space between them, and then the third flat-bottomed jade is glued to it. In this way, the green gel-like substance reflects images through the round cap, giving the surface of the Assembled gemstone a high-quality emerald green [Figure 4-3(b) ].
④ Imitation red (blue) gemstone Triple-layered stones
Made of synthetic red (blue) gemstones, two hollow oval-shaped shell layers of matching sizes are created, with Fibrous sodium borate calcium stone added in between and glued together [Figure 4-3(c)].
2. Characteristics of Assembled Gems
2.1 Layered Structure
All forms of Assembled stones, whether they are Two-layered stone, Triple-layered stones, or Substrate Stone, are composed of two or more identical or different materials that are layered and bonded to create a cohesive appearance and are set with a metal (precious or ordinary) framework to cover the seams of the interlayer bonding.
(1) Shape of the Structural Layer
① Planar Shape
Generally, the structural layers of faceted Assembled stones are flat and panel-like, with the layers that make up the Assembled stone presenting a horizontally integrated structure between them.
② Curved Surface Shape
Whether circular, elliptical, or hollow, the curved surface Assembled stones have each structural layer presenting curved, arc-shaped thin layers, with layers in arc-shaped parallel contact. The cross-sectional shapes of these curved surface Assembled stones can be single convex, double convex, concave-convex, and concave.
(2) Hierarchy of Structural Layers
① Bilayer construction
- Colorless cemented bilayer construction: The Assembled stone is made up of two layers of materials, with the top layer often being transparent or semi-transparent durable natural or synthetic gemstones, while the bottom layer consists of inferior and inexpensive materials, bonded together with a colorless adhesive. This Assembled stone is composed of three materials.
- Colored cemented bilayer construction: This involves applying color or a colored film to the bottom or pavilion of transparent or semi-transparent gemstones of two materials.
② Multilayer construction
A multilayer construction refers to constructing Assembled stones made of three or more different types of gemstone materials. It can be further divided into:
- Colorless cemented three-layer structure: A Assembled stone bonding three pieces of the same or different types of gemstone materials with colorless adhesive. This structure is composed of five layers of materials.
- Colored adhesive three-layer structure: two pieces of gemstones of the same or different varieties, bonded together with colored adhesive to form a Assembled stone, which has only three layers in its structure.
2.2 Different materials and their identification characteristics
Whether it is a Two-layered stone, Triple-layered stones, or substrate stone, they are all composed of different materials. Due to the different combinations of materials, the structural layers’ chemical composition, internal structure, and physical properties vary. The Assembled stones listed in this section have different identification characteristics based on the differences in their structural layers.
(1) Types of Two-layered stone
① Garnet glass Two-layered stone
- Red ring effect: Placing it on a white paper surface, the red ring phenomenon of garnet appears on the paper under light.
- Observing the facets or girdle of the Assembled gemstone crown with reflected light, the bonding line and its sides show different luster and colors.
- Red flag effect: When observing with a refractometer, the refractive index on both sides of the bonding seam differs. If the eyepiece is removed, it can also be seen the image of the bottom of the gemstone appears with a red reflection on the scale.
- Different fluorescence: Garnet has no fluorescence, while glass may have a fluorescence of any color.
- Inclusion differences: Garnets may contain needle-like rutile or other crystal inclusions, while glass contains bubbles.
② Corundum Two-layered stone
(a) If composed of natural red (blue) gemstones and synthetic red (blue) gemstones, in addition to observing the presence or absence of bonding lines (surfaces), one should also observe the inclusions, colors, and fluorescence differences of the red (blue) gemstones on both sides of the bonding line.
- Inclusions: The inclusions of natural corundum gemstone are minerals with straight growth lines. In contrast, the inclusions of synthetic corundum gemstone are “”un-melted powder”” and bubbles with growth lines that can be arc-shaped.
- Fluorescence: Natural rubies’ fluorescence intensity is lower than synthetic rubies; natural sapphires have no fluorescence, while synthetic sapphires may exhibit weak blue-white fluorescence.
- Color: Natural red (blue) gemstones have an uneven color intensity that appears more natural, while synthetic red (blue) gemstones appear overly pure and bright, glaring and artificial.
(b) If a Two-layered stone is composed of synthetic red (blue) gemstones and red (blue) glass, it is usually the synthetic red (blue) gemstone on the upper part (crown or top) and the glass on the lower part (pavilion, bottom). Its identification characteristics are obvious:
- Optical properties: Synthetic red (blue) gemstones are heterogeneous, while glass is homogeneous. When rotated 360°under a polarizing microscope, synthetic red (blue) gemstones show four bright and four dark areas, while glass appears completely dark or anomalously disappears.
- Inclusions: Synthetic red (blue) gemstones contain “un-melted powder” and arc-shaped growth lines, while glass contains numerous bubbles and swirl structures.
- Refractive index: The refractive index of synthetic red (blue) gemstones is 1.76-77, while the refractive index of glass is lower, generally 1.46-1.70.
(2) Triple-layered stones type
① Characteristics of imitation emerald Triple-layered stones
- If the top layer is made of beryl, crystal, or spinel and the bottom layer is made of the same, with a green adhesive in between, the Assembled gemstone can be placed in water. When observed along the direction parallel to the waist surface, it can be found that the crown and pavilion of the Triple-layered stones are colorless, while there is a thin color layer between the two.
- If the top layer is made of crystal or spinel and the bottom layer is made of green glass, a color layer can be observed at the parallel waist plane under a gem microscope, containing round bubbles, swirling structures, and irregular intertwined color bands.
② Characteristics of opal Triple-layered stones
It is a Assembled stone bonding three different materials (layers). Its identification can be approached from the following four aspects.
- Observing from the side, the colorless transparent material can be seen on top, with a color-changing layer in the middle and a black opaque layer at the bottom.
- The two bonding layers between the layers contain bubbles or dry cracks.
- Under strong light, magnified inspection reveals two bonding seams.
③ Characteristics of jade stone Triple-layered stones.
It is a colorless, translucent jade with two layers bonded in the middle with green adhesive. When observing the joined stone from a vertical or curved surface, it appears green, while from a parallel waist view, the upper and lower sides are colorless, with green in the center.
2.3 Characteristics of the adhesive layer
Various types of joined stones are all bonded together by adhesives, forming a whole. This creates an extremely thin liquid adhesive layer between the solid layers. The adhesive layer has the following characteristics:
(1) The color of the adhesive is variable, either colorless or in various colors. Colorless ones do not form a structural layer, while colored ones serve as the structural layer of the joined stone.
(2) The adhesive layer often contains bubbles. The bubbles are spherical or tubular.
(3) After the adhesive in the bonding layer solidifies, its volume shrinks and causes dry cracking, forming shrinkage cracks.
(4) When exposed to fire, it turns to ash. The adhesive in the bonding layer is prone to aging and ash formation when exposed to fire, appearing black.
The various types of Assembled gemstones should be carefully examined for their seams, bonding traces, and bubbles, as well as the refractive index, color, luster, transparency, and inclusion characteristics of various materials during identification. Observe from multiple angles and test carefully.
Section III Reconstructed Gems
In manufacturing processes, reconstructed gemstones (synthetic gemstones) belong to transformed gemstones. That is, the original gemstone fragments (or pieces) and decorative gemstone ornaments (or remnants) that have lost their decorative function are crushed, purified, heated, and pressurized to reconstitute them into a gemstone material with an overall appearance, which is then cut, polished, and processed into various ornaments. Common varieties include reconstructed turquoise, reconstructed Amber, and reconstructed lapis lazuli. In the past, there were reconstructed rubies (known as Geneva rubies); recently, Reconstructed yellow Nephrite jade, nephrite, and even reconstructed synthetic gemstones have appeared.
1. Reconstructed Processes
1.1 Welding Process
Dr. E. D. Clarke first developed the welding process in 1819, which used a newly invented hydrogen-oxygen flame blowpipe to melt and combine two ruby crystals into a spherical ruby on charcoal. Later, Fufulai, Feier, and Uze collaborated to melt natural ruby fragments using a hydrogen-oxygen flame. They added a small potassium chromate reagent to deepen its red color, creating a regenerated ruby.
This welding process later evolved into the “flame fusion method.” However, the method of growing crystals by flame fusion has far exceeded the scope of the welding process. The distinction between the two mainly lies in whether the crystal itself is the raw material for growing crystals. In other words, if the raw material for growing crystals is fine from the crystal itself, it belongs to the welding method for regenerated gemstones; if made from other chemical raw materials through melting, it is classified as synthetic gemstones by the flame fusion method.
1.2 Sintering Process
The sintering process is similar to producing bricks or tiles in a kiln. Materials are placed in a container and pressed together to form a cohesive whole without altering their physical or chemical properties. A small amount of binder and coloring agent can be added during the sintering process. To ensure a strong bond, a certain temperature is often applied, but it should not exceed the melting point of the materials.
1.3 Molding Process
The molding process is similar to the sintering process. First, the crushed materials of gemstones are purified and then placed into a designed mold. Under certain temperature conditions, pressure is applied to directly form the materials into jewelry. This includes items like reconstructed nephrite and reconstructed yellow Nephrite jade.
2. Characteristics of Reconstructed Gemstones
2.1 Reconstructed Amber
Amber is a unique natural treasure. It is both a natural organic gemstone and an important traditional Chinese medicine. It is cherished even more in countries along the Baltic Sea, where Amber is abundantly produced. For example, in the early 18th century, Frederick William I, the founding emperor of the Prussian Hohenzollern dynasty in Germany, hired a famous Danish jeweler to spend ten years processing over 100 pieces of Amber, carving more than 150 amber statues and creating an “Amber Room.” Besides being processed into cabochon gemstones for use in rings, pendants, and other jewelry, a large quantity is also made into various decorative items for people to adorn and appreciate.
Due to the presence of organic compounds such as succinic acid and amber resin in Amber, it is prone to oxidation, turning red, aging, and cracking, becoming loose and friable, and containing many impurities. Therefore, it must be artificially improved and recreated to enhance its quality and utility.
(1) Production Process
① Fusion method
- Crush the amber fragments into fine powder, use a heavy selection method to remove impurities, and purify the powder.
- Place the purified powder into a container and heat it to 200-250℃ under inert gas using far-infrared heating, causing the powder to melt into liquid.
- After the powder melts, control the constant temperature, stop heating, and slowly cool down. Once it condenses into a block, remove it to obtain reconstituted Amber. It can also be cast into a shaped mold to condense into the desired shape of jewelry.
- During the welding process, various animal images, plant, or other decorative patterns can be added during the welding process to enhance its aesthetic appeal.
② Sintering method
- Pour the pure amber powder into a container (or mold).
- Apply pressure to about 2.5 MPa and maintain a temperature below the melting point of Amber to form blocks (or shapes).
- During sintering, binders, colorants, or fragrances may also be added.
- Sintered Amber requires a lower temperature and a longer sintering time to achieve uniform, transparent amber jewelry without flowing structures.
(2) Process characteristics
If no other chemical substances are added during the reconstruction process, Reconstructed Amber is basically the same as natural Amber because neither the chemical composition nor the internal structure has changed. If foreign substances are added or certain defects in the production process during reconstruction, reconstructed Amber may differ from natural Amber (Table 5-1).
Table 5-1 Comparison of Characteristics between Reconstructed Amber and Natural Amber
| Kjennetegn | Natural Amber | Reconstructed Amber |
|---|---|---|
| Farge | Yellow-orange and brown-red are both present | Mostly orange-yellow or orange-red |
| Pause | Shell-shaped, with grooves perpendicular to the shell pattern | Skallformet |
| Struktur | Smooth surface | Granular structure with a surface exhibiting an uneven orange peel effect |
| Density (g/cm3 ) | 1.05 ~ 1.09 | 1.03 ~ 1.05 |
| Capsule | Plant and animal remains, mineral impurities, round bubbles | Clean and transparent, with aggregated un-dissolved substances, bubbles arranged in a flattened elongated orientation |
| Struktur | Has tree-like growth rings or radial textures | Early with a flowing structure, new style with syrup-like swirling structure |
| Ultrafiolett fluorescens | Light blue-white, light blue, or pale yellow fluorescence | Bright white-serious blue fluorescence |
| Soluble | No reaction when placed in diethyl ether | Becomes soft after a few minutes in diethyl ether |
| Aging characteristics | Darkens due to aging, appearing slightly red or brownish | Turn white due to aging |
① Welded Amber
Reconstructed Amber was produced using the welding method. Due to the amber powder melting at a higher temperature and becoming a viscous liquid will generate a vortex-like flow and many bubbles during the manual mixing. This phenomenon is retained during condensation, becoming a distinguishing feature of welded Amber.
Suppose certain additives, bonding agent, colorants, and insects, plants, or sand fragments are added during the welding process. In that case, it will complicate the composition of reconstructed Amber and diversify the inclusions. Therefore, the differences between welded Amber and natural Amber is:
- Color: golden yellow, yellow-orange, and various other colors.
- Fluorescence: Exhibits a distinct chalky blue fluorescence.
- Inclusions: Upon magnified inspection, fused Amber often shows obvious flow structures, with clear layers interspersed, containing blurred outlines of un-melted materials and bubbles of varying sizes that are oval, round, or elongated, irregularly distributed throughout the Amber, dense and small. Bubbles can also explode during heat treatment, forming lily pad-like inclusions inside the Amber.
- Transparency: Fresh reconstructed Amber is all transparent.
- Imitation of insect amber: In the molten state of reconstructed Amber, people often add some insects to imitate insect amber. However, the insects included showing no signs of “a dying struggle.”
② Sintered amber
Reconstituted Amber produced by the pressing method has a special deformed granular structure because the amber powder is pressed and formed under high pressure and low temperature (below the melting point of Amber), resulting in only plastic deformation of the powder, which tightly aggregates together, or adheres to each other due to the addition of a binder. The identification characteristics of Sintered amber are as follows:
- Color: Mostly orange-yellow and orange-red.
- Density: 1.03-1.05 g/cm3 , lower than natural Amber.
- Fracture: Shell-like fracture.
- Structure: Granular structure, with a surface exhibiting an uneven orange peel effect.
- Optical properties: Abnormal birefringence often appears under a polarizing microscope.
- Fluorescence: There is often uneven blue-white fluorescence, with granular structures visible under ultraviolet light. When observing samples with dark red thread-like distributions, filamentous bodies can be seen along the boundaries of the particles.
- Inclusions: Contains bubbles and blurred outlines of un-melted powder grains.Dark reddish filaments are characteristic of Sintered amber, and their morphology is similar to that of capillaries, which are filamentous, nebulous, and lattice-like. This red color is a thin layer of red oxide film formed on the surface of Amber due to oxidation. Although natural amber can also have fissures that are oxidized and red, they are dendritic along the fissures rather than along the edges of the grains.
- Aging characteristics: It appears whitish, unlike natural Amber, which darkens due to oxidation and presents a slight red or brownish color.
2.2 Reconstructed turquoise
The elegant and stunning turquoise is a traditional gemstone loved by people from ancient to modern times, domestically and internationally. Because it resembles a pine cone and is close in color to pine green, it is also called “pine stone.”
There are many varieties of turquoise. They can be classified by color into sky blue, deep blue, light blue, blue-green, green, yellow-green, light green and colorless varieties; by production state, they can be divided into crystal turquoise, dense block turquoise, block turquoise, dyed turquoise, and veinlet turquoise. It is also called iron line turquoise if it contains fine vein-like black iron or carbon. The turquoise produced in ancient Persia is called “Turkish jade” in the West.
(1) Reproduction process
There are two types of reconstructed turquoise on the market.
① Sintering method
The reconstructed turquoise produced by Gilson was introduced in 1972. It is made by crushing some natural turquoise scraps or low-quality turquoise and mixing them with copper salts or blue metal salts, then pressing them at a certain temperature. There are two types of reconstructed turquoise produced by the sintering method available on the market: one is made from relatively pure turquoise powder, and the other is made by adding a matrix containing turquoise from surrounding rocks to the turquoise powder.
② Welding method
The production of reconstructed turquoise using the welding method involves a ceramic firing process. The turquoise powder is formed through sintering. This reconstructed turquoise is very similar to natural turquoise.
(2) Craft Characteristics
① Structure
It looks very much like blue ceramic, with a typical granular structure. Under a magnifying glass, clear particle boundaries and deep blue dye particles in the matrix can be seen.
② Density
The density of reconstructed turquoise is not fixed; its density depends on the amount of binder contained. According to the American Gemological Institute, its density can be one of three values: 2.75 g/cm3, 2.58 g/cm3, 2.06 g/cm3.
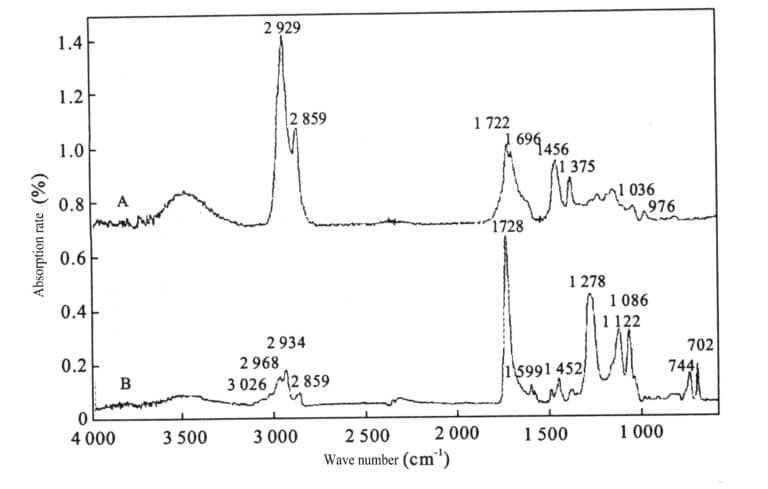
③ Infrared Spectroscopy
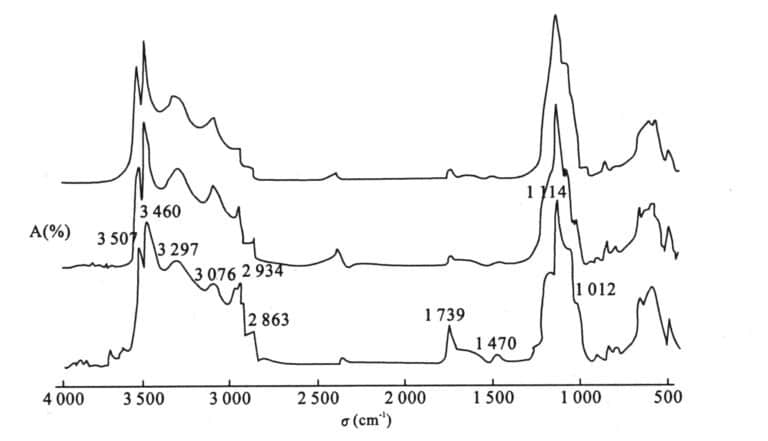
It has a typical 1725 cm-1 absorption peak. 1470 cm-1, 1739 cm-1, 2863 cm-1, 2934 cm-1 These peaks may be caused by synthetic resin materials used as binders. (See Figure 5-1)
④ Micronization tests
Part of the recycled turquoise contains blue copper salts, can be dissolved in hydrochloric acid, the blue color will soon become a light greenish-blue, cotton ball dipped in hydrochloric acid can be stained white cotton ball is blue. In 2002, a type of imitation turquoise product appeared on the market. Tests showed that it was made from magnesium ore (MgCO3) as the matrix, pressed with organic dyes and adhesives at 500-600 atmospheric pressure. The dye was originally organic but is now replaced by inorganic coloring agents.
2.3 Reconstructed nephrite
In recent years, the “White Jade Carving Brand” has appeared in the market and is very popular, with buyers flocking to it. Its appearance is indistinguishable from white jade, and its price is not high; it belongs to reconstructed nephrite.
(1) Production Process
White tremolite is crushed, mixed with a binder, and formed into a solid appearance through heating and pressing. It can also be molded in a die.
(2) Process Characteristics
① Magnification Inspection
Reconstructed nephrite has a fine, powdery, granular structure different from natural nephrite. The color is uniform, the interior is clean.
② Density and Hardness
Both are slightly lower than natural nephrite.
③ Infrared Absorption Spectrum
There is an absorption peak of the binder.
2.4 Reconstructed Jade
In the Guangzhou jewelry market in 2002, a kind of jade pieces and beads and necklaces accessories appeared. After detailed inspection, it was found to be a reconstructed jade product made from green opaque jade fragments bonded with glass glue. The identification features are as follows:
(1) Appearance Characteristics
① Colorless root
Green, emerald green, or dark green, evenly distributed, with a chaotic color direction, lacking a “color root.”
② Micro-transparent
Almost opaque, only weakly translucent at the edges of the sample and in thinner areas.
③ Agglomeration of fragments
Has a distinct angular granular structure, with varying particle colors and disordered aggregation.
④ Pockmarked Surface
The surface of reconstituted jade pieces is usually well-polished, presenting a glassy luster, but often has small round Pockmarked Surface that differ from the “orange peel effect.”
⑤ Irregular Fracture
The overall fracture is irregular, but it contains shell-like fractures within the irregular fractures.
(2) Internal Features
① High refractive index: Measured at 1.66-1.68, higher than jade.
② Low density: Density is 3.00 g/cm3(static water weighing method), far lower than jade.
③ Fracture structure: Composed of fragments of varying sizes and cementing material, clearly visible under reflected light, resembling sedimentary rock with high-luster jadeite fragments and low-luster cementing material, and small bubbles can be seen in the cementing material.
④ Foreign substance addition: Chemical analysis contains PbO, ZnO component, with PbO content reaching around 7%.
2.5 Other reconstructed gemstones
Various types of reconstructed jewelry and gemstones have appeared in the market. These include reconstructed lapis lazuli, reconstructed Alabaster, reconstructed siliceous jade, and reconstructed synthetic spinel, among others.
For example, synthetic spinel particles are fused into a whole appearance using the welding method to imitate lapis lazuli. It presents a bright blue color, with an even color distribution and a granular structure, which may contain small yellow spots resembling pyrite. This reconstructed synthetic spinel that imitates lapis lazuli has a luster stronger than that of lapis lazuli, good polish ability, and appears bright red under a Charles filter, with a refractive index of 1.72, density 3.52 g/cm3, and typical cobalt absorption spectra visible in the red, green, and blue regions when observed with a spectroscope.