Hvordan identifisere optimaliserte edelstener?
En guide til instrumenter og utstyr som brukes i identifikasjons- og operasjonsprosessen
Etter optimaliseringsbehandling må smykker og edelstener fremvise en sertifisering av edelsteinsforbedringstesting fra en autoritativ institusjon når de selges. Formålet er klart: å avgjøre om edelstenen har blitt kunstig behandlet gjennom visuell inspeksjon og ulike testmetoder og instrumenter basert på de interne og eksterne egenskapene. De viktigste identifikasjonsmetodene og innholdet inkluderer følgende aspekter:
(1) Identifisering og bekreftelse av ulike egenskaper ved edelstener som har gjennomgått kunstig behandling.
Etter optimaliseringsbehandling vil edelstener endre farge, struktur, sammensetning osv. Funksjonene ved optimaliseringsbehandling av edelstener bestemmes gjennom visuell inspeksjon og instrumenttesting.
(2) Hva slags kunstige behandlingsmetoder kan brukes?
Basert på de interne og eksterne egenskapene og testdataene til edelstenen etter optimaliseringsbehandling, analyser hvilken optimaliseringsbehandlingsmetode edelstenen kan ha gjennomgått, og bestem edelstenens optimaliseringsbehandlingsmetode basert på optimaliseringsbehandlingsegenskapene.
(3) Stabilitet av optimaliserte behandlingsprodukters fysiske og kjemiske egenskaper.
Optimalisert behandlede edelstener må være vakre og trygge og ha stabile fysiske og kjemiske egenskaper, noe som øker den estetiske og økonomiske verdien av edelstenene for å komme inn på smykkemarkedet. Optimaliserte edelstener kan være umerket når de selges på markedet, men behandlede edelstener må være merket med hvilken type behandling de har gjennomgått, ellers vil det føre til forvirring i markedet og panikk blant forbrukerne.
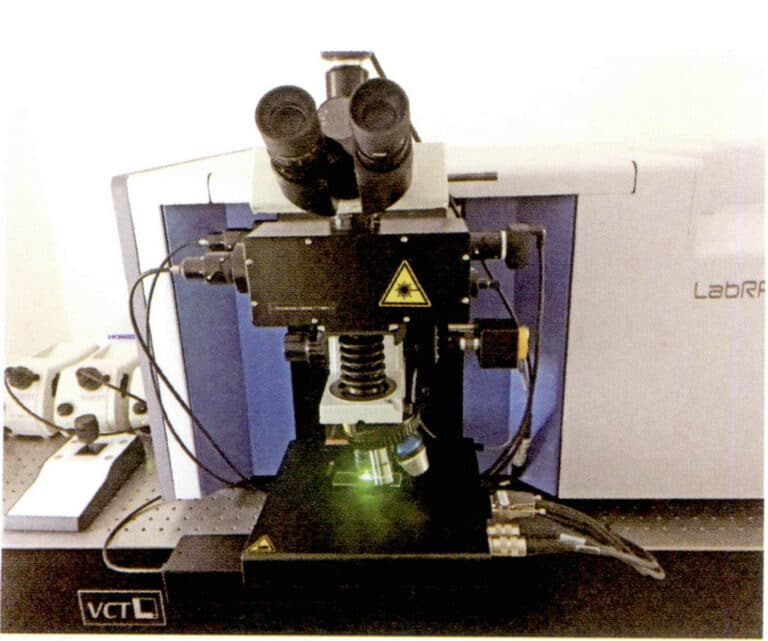
Raman-spektrometer
Innholdsfortegnelse
Del I Metoder og trinn for identifisering av optimaliserte behandlede edelstener
Det kreves mer enn å stole på visuell observasjon for å kunne identifisere optimaliserte, behandlede edelstener raskt og nøyaktig. Det er utviklet ulike instrumenter for å identifisere edelstener. Det er nødvendig med instrumenter for å identifisere edelstener for å observere de indre og ytre egenskapene til optimalisert behandlede edelstener og bestemme de spesifikke metodene for optimalisering av edelstener. I den faktiske identifikasjonen er ikke ett enkelt instrument alt - kraftig; flere instrumenter må brukes sammen for å bekrefte hverandre. Når du velger instrumenter for edelstener, bør de være enkle å bruke, gi raske målinger og ikke skade prøvene. Vanlige deteksjonsmetoder og -trinn er som følger:
(1) Utfør en detaljert visuell observasjon av edelstenen
Visse egenskaper ved edelstener kan bestemmes gjennom visuell observasjon, for eksempel farge, form, gjennomsiktighet, glans, spesielle optiske effekter, spalting, brudd og visse slipeegenskaper. Krystallformen bør brukes til å bestemme krystallfamilien eller krystallsystemet hvis det er en råkrystall. Under opplyst lys kan mer åpenbare inneslutninger i edelstenen observeres.
(2) Inspeksjon med forstørrelse
Rengjør prøven grundig, og bruk et forstørrelsesglass eller mikroskop for å observere edelstenens små indre og ytre trekk. Observer de ytre trekkene ved prøven med reflektert lys og de indre trekkene med gjennomlys eller en sterk lyskilde. I spesielle tilfeller kan man observere indre vekstmønstre og fargefordelinger ved hjelp av en spredningstavle eller nedsenking i olje. Observer fra ulike vinkler, og registrer observasjonene som bevis for å skille mellom naturlige, syntetiske eller kunstig forsterkede edelstener.
(3) Deteksjon av optiske egenskaper
Mål de optiske egenskapene til edelstenen, for eksempel brytningsindeks, polaritet, fluorescensegenskaper og absorpsjonsspekteregenskaper. Ulike edelstener har karakteristiske brytningsindekser eller områder av brytningsindekser. Ved å måle brytningsindeksen og dobbeltbrytningen kan man avgjøre om edelstenen er homogen eller ikke, om den er en enakset eller toakset krystall, osv. Noen edelstener som har blitt behandlet, kan også skilles ut ved hjelp av brytningsindeksen, for eksempel kan en komposittstein laget av to forskjellige edelsteinsmaterialer identifiseres basert på de to materialenes forskjellige brytningsindekser; brytningsindeksen til syntetisk spinell er større enn den til naturlig spinell.
(4) Påvisning av fysiske egenskaper og kjemisk testing
For eksempel vil rubiner eller smaragder som er behandlet med olje, avgi olje når de berøres med en varm nål; rav avgir en velduftende lukt når det brennes, mens plastkopier avgir en skarp lukt når de brennes; edelstener som er behandlet med kobbersaltfarge, kan skifte farge når de tørkes av; edelstener som har blitt fylt, har generelt en relativ tetthet som er lavere enn den naturlige edelstenens.
(5) Testing med store instrumenter
Noen optimalt behandlede edelstener kan ikke identifiseres ved hjelp av konvensjonelle instrumenter og metoder, men man kan bruke store instrumenttester, som infrarød absorpsjonsspektrometri, Raman-spektroskopi og ultrafiolett - synlig spektroskopi, for å finne ut hvilken type edelsten det er snakk om eller hvilken metode som er brukt for å optimalisere behandlingen.
Derfor er det viktig å forstå typer, strukturer, prinsipper og bruksmetoder for identifikasjonsinstrumenter for edelstener og deres forholdsregler, slik at man kan velge egnede identifikasjonsinstrumenter når man skal identifisere optimalt behandlede edelstener, og beherske bruksmetodene på riktig måte.
Del II Forstørrelsesglass
Forstørrelsesglasset er et av de mest brukte verktøyene ved identifisering av edelstener, med en forstørrelse på vanligvis ti ganger. Forstørrelsesglasset er lite, lett å ta med seg og mye brukt. Det brukes til å observere edelsteners overflate og mer åpenbare indre trekk, for eksempel overflatevekstmønstre, sprekker, brudd, indre vekstmønstre, mørke inneslutninger og så videre.
1. Håndholdt forstørrelsesglass Struktur
Det mest brukte forstørrelsesglasset ved identifisering av edelstener er en konveks linse (figur 2-1). Den enkleste strukturen er en enkelt linse, som vanligvis egner seg for lav forstørrelse. Mer komplekse strukturer er dublett- og triplettlinser, som gjennomgår to eller tre forstørrelser, noe som eliminerer problemet med økt krumning i konvekse linser, noe som kan forhindre sfærisk aberrasjon og forvrengning.
Når du skal kjøpe et forstørrelsesglass, kan du bruke millimeterpapir for å vurdere kvaliteten. Sjekk om det er noen forvrengning på kantene av millimeterpapiret under det håndholdte forstørrelsesglasset; jo mindre grad av forvrengning, desto bedre er kvaliteten på forstørrelsesglasset.
2. Forstørrelsesglassets funksjon
Forstørrelsesglass kan brukes til å observere de mer åpenbare trekkene i og utenfor edelstener, noe som gjør dem til et effektivt og praktisk verktøy for identifisering av edelstener. Etter å ha observert de grunnleggende egenskapene til edelstenen, som farge, gjennomsiktighet og glans, med det blotte øye, kan et forstørrelsesglass brukes til å undersøke de ytre og indre egenskapene til edelstenen, som sprekker, vekstmønstre og inneslutninger.
Observatørens kroppsholdning, vaner, lyskilde, bakgrunn og andre faktorer kan påvirke observasjonsresultatet. Når du bruker forstørrelsesglass, er den riktige metoden å holde forstørrelsesglasset så nær øynene som mulig for å kunne observere på nært hold. For å unngå at forstørrelsesglasset rister, bør hånden som holder edelstenen berøre hånden som holder forstørrelsesglasset, og albuene bør plasseres på bordet for å opprettholde en viss avstand mellom forstørrelsesglasset, øynene og edelstenen.
Del III Edelstensmikroskoper og deres bruksområder
Noen ganger er inneslutninger i edelstener små og kan ikke observeres med et vanlig forstørrelsesglass. I slike tilfeller kan man bruke et instrument med høyere forstørrelse - et mikroskop. Med et mikroskop kan man observere edelstener tydeligere enn med et forstørrelsesglass. Dette skyldes at mikroskoper ikke bare har et stort forstørrelsesområde, opptil 200 ganger, men også at man unngår rystelser som kan oppstå med håndholdte forstørrelsesglass. Ulempen er at det er stort og upraktisk å bære med seg. Mikroskopet brukes til å observere innvendige inneslutninger som er vanskelige å se under et ti ganger forstørrelsesglass, med høy forstørrelse og et bredt synsfelt, noe som gjør det mulig å observere noen typiske trekk ved optimalisert edelsteinsbehandling, for eksempel endringer i inneslutninger i varmebehandlede rubiner, "sollyset" som produseres av bobler som sprekker i varmebehandlet rav, og den blinkende effekten som er synlig i smaragder som er fylt med farget olje.
1. Typer og oppbygning av edelstenemikroskoper
Et edelstensmikroskop er et kikkertmikroskop med noe ekstrautstyr, for eksempel en edelstensholder, belysningssystem og nedsenkningsoljetank. Ved identifisering av optimalisert edelsteinsbehandling brukes det hovedsakelig til å observere indre og ytre trekk ved edelstener som er vanskelige å se med det blotte øye eller et ti meter stort forstørrelsesglass. Vanlige typer mikroskoper inkluderer vertikale mikroskoper og horisontale mikroskoper. De ulike mikroskopene velges ut fra edelstenens beskaffenhet og de ulike observasjonsmetodene.
(1) Vertikalt mikroskop:
Den vanligste og mest brukte typen mikroskop ved identifisering av edelstener (figur 2 - 2) . Det kjennetegnes ved at lyskilden og mikroskopsystemet er integrert, noe som gjør det mulig å observere edelstenen ovenfra.
(2) Det horisontale mikroskopet:
Det har et separat lyskilde- og forstørrelsessystem, med mikroskop, edelsten og lyskilde på samme horisontale linje, noe som gjør det mulig å observere edelstenen fra siden. Den viktigste egenskapen er at man kan bruke en beholder med olje for å observere edelstenens indre struktur.
2. Belysning av edelstensmikroskoper
Vertikale perlemikroskoper har vanligvis to lyskilder: en øvre og en nedre lyskilde. Den øverste lyskilden kan være en fluorescerende optisk lyskilde eller en glødelyskilde. Den nederste lyskilden er en glødelyskilde. Det finnes ni vanlige belysningsmetoder.
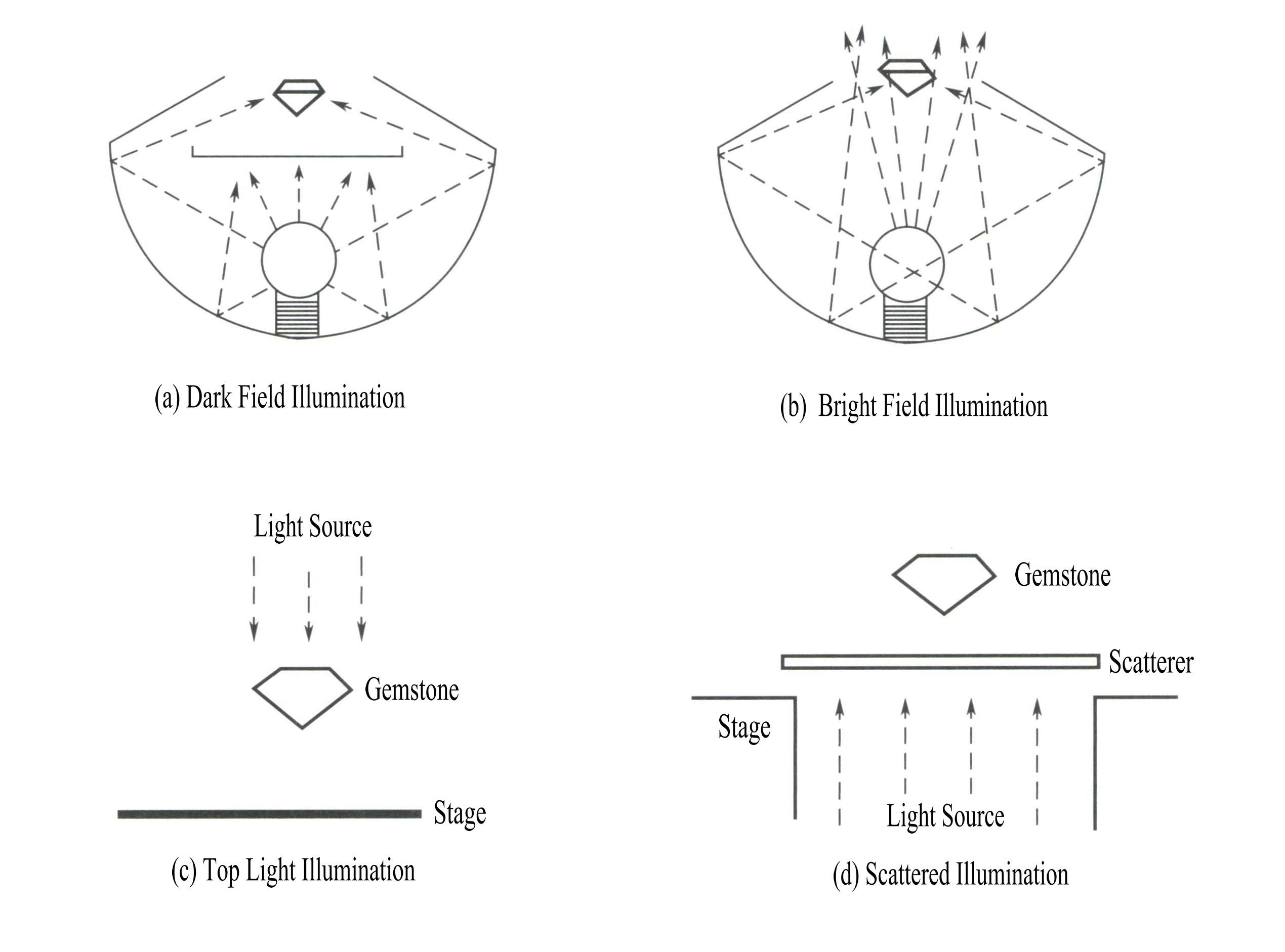
(1) Belysning av mørke felt
En svart plate plasseres mellom edelstenen og lyskilden, uten reflekterende bakgrunn. Lyset diffrakterer fra kantene, noe som skaper en tydelig kontrast mellom de lyse, lyse inneslutningene og den svarte bakgrunnen. Denne typen er den mest brukte [Figur 2-3 (a)]. Den brukes hovedsakelig til å observere lyse inneslutninger og vekststrukturer i transparente edelstener, for eksempel krystallinneslutninger og vekstmønstre.
(2) Lysfeltbelysning
Lyset skinner direkte på edelstenen fra undersiden, noe som ofte låser blenderåpningen til et punktlys. Dette skaper en tydelig kontrast mellom de mørke inneslutningene i edelstenen og det lyse feltet, og er også egnet til å observere buede striper eller lavt utstikkende inneslutninger [Figur 2-3(b)].
(3) Vertikal belysning (ved hjelp av den øverste lyskilden)
Lyset skinner ovenfra, og ved hjelp av reflektert lys kan man observere edelstenens overflate [Figur 2-3(c)]. Det brukes hovedsakelig til å se etter sprekker, riper og ujevnheter på edelstenens overflate.
(4) Diffus belysning
Plasser en overflatefiber eller et annet gjennomskinnelig materiale mellom edelstenen og lyskilden for å spre og myke opp lyset, noe som gjør det lettere å observere edelstenens fargetoneringer og fargebåndstruktur [Figur 2-3(d)].
(5) Horisontal belysning (med hvilken som helst lyskilde)
En smal lysstråle rettes fra kanten mot edelstenen, sett ovenfra, noe som gjør det lettere å se lyse nåler - som krystaller og bobler (blyantlysteknikk).
(6) Belysning av nålens lyskilde
Lås lysringen mellom edelstenen og lyskilden, slik at bare vertikalt lys skinner på edelstenen, noe som gjør det lettere å observere buede striper og fargebånd, spalting, skillelinjer og andre strukturer.
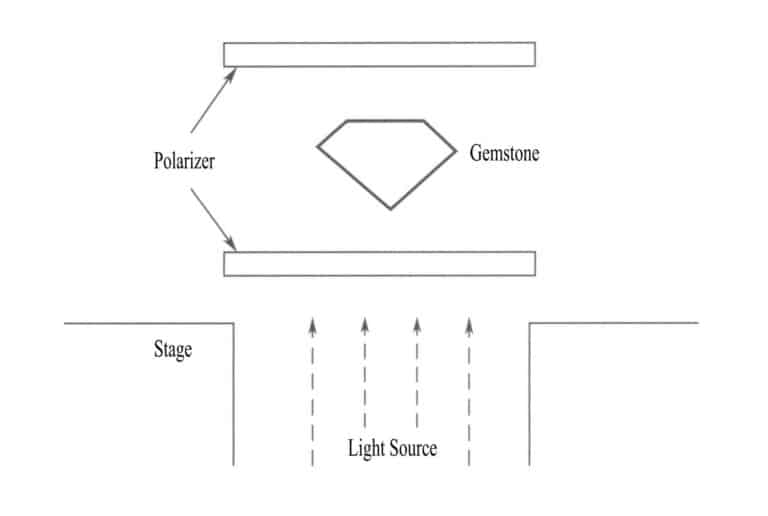
(7) Polarisert belysning (ved bruk av polarisator og analysator)
Plasser edelstenen mellom to kryssede polarisatorer for å observere om den er en homogen kropp og for å se etter pleokroisme, avvikende ekstinksjon og andre effekter som kan observeres med et polarisasjonsmikroskop (figur 2-4).
(8) Skrå belysning (ved hjelp av en hvilken som helst fiberlyskilde)
I en skrå vinkel skinner en smal lysstråle på edelstenen, ettersom vinkelen mellom vertikal og horisontal belysning gjør det lettere å observere tynne lag-effekter forårsaket av væskeinneslutninger i spalting (for eksempel irisering) .
(9) Mørkefeltteknikk
Sett inn en delvis ugjennomsiktig baffel mellom edelstenen og lyskilden for å hindre direkte lys i å skinne på edelstenen, slik at inneslutningene får en tydelig tredimensjonal effekt, noe som gjør det lettere å observere posisjonen til vekststrukturer, for eksempel buede striper og tvillingdannelse (Figur 2-5) .
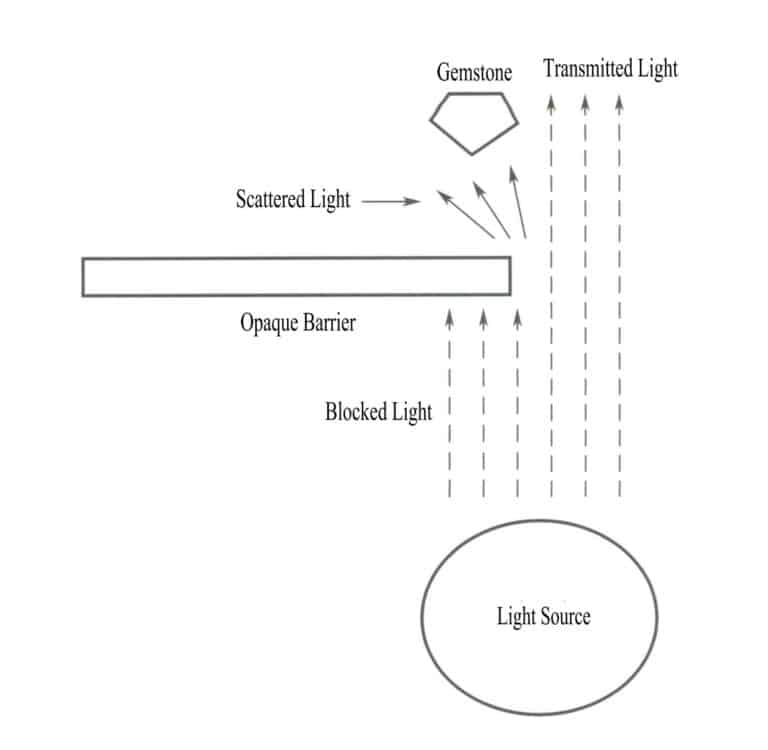
3. Vanlige nedsenkningsvæsker som brukes i perlemikroskopi
(1) Vanlige nedsenkningsvæsker
Den vanligste nedsenkningsvæsken for edelstener er en oljeaktig væske som er utstyrt med en nedsenkningstank i både stående og liggende mikroskoper. Ved å senke edelstenen ned i væsken kan man observere innvendige inneslutninger, vekstmønstre og andre særtrekk, noe som reduserer interferens fra refleksjoner på overflaten eller små fasetter og muliggjør effektiv observasjon av indre egenskaper. Ved å legge edelstenen i en nedsenkningsvæske med en brytningsindeks som ligger nær edelstenens egen, får man et tydeligere resultat. Den ideelle nedsenkningsvæsken bør ha god flyktighet og høy transparens, og den bør være giftfri og luktfri. Den kan også formuleres slik at den har en tetthet eller brytningsindeks som ligner på den observerte edelstenen. Vanlige nedsenkningsvæsker som brukes i edelstensmikroskoper inkluderer glyserin, flytende parafin, naftalenklorid og diiodmetan, med brytningsindeksverdier som vist i tabell 2-1.
Tabell 2 - 1 Brytningsindekser for ulike nedsenkningsvæsker
| Navn på nedsenkningsvæske | Brytningsindeks |
|---|---|
| Vann | 1.33 |
| Terpentin | 1.47 |
| Glyserin | 1.47 |
| Naftalenklorid | 1.63 |
| Flytende parafin | 1.47 |
| Diiodometan | 1.74 |
(2) Forholdsregler ved bruk av nedsenkningsløsningen
Mange typer nedsenkningsvæsker kan brukes i edelstensmikroskoper, og den valgte nedsenkningsvæsken varierer for ulike edelstener. Kravene til valg av nedsenkningsvæske omfatter følgende aspekter:
① Ved valg av nedsenkningsvæske er det viktig at væskens brytningsindeks ligger nær edelstenens brytningsindeks, noe som er en fordel for å kunne observere edelstenens indre egenskaper.
② Porøse edelstener, organiske edelstener og sement av sammensatte edelstener skal ikke legges i nedsenkningsvæsken.
Naftalenklorid og diklormetan har en sterk lukt, og edelstener som har vært nedsenket i disse stoffene, bør rengjøres etter at de er fjernet.
④ Når du justerer brennvidden, må du unngå at objektivlinsen kommer i kontakt med nedsenkningsvæsken eller blir påvirket av væskedamp på grunn av at linsen er for lav.
⑤ I det oppreiste mikroskopet er nedsenkningstanken plassert under objektivlinsen og over lyskilden, og observasjonstiden bør være overkommelig lang.
4. Forholdsregler ved bruk av edelstenemikroskop
Når du observerer edelstener, er det viktig å bruke mikroskopet riktig for å unngå feil i observasjonsresultatene eller skade på mikroskopet på grunn av betjeningsfeil. Vær oppmerksom på følgende aspekter når du bruker det:
(1) Når du skal observere edelstenens indre og ytre trekk, må du velge en passende lyskilde. Vanligvis brukes gjennomskinnelig lys til å observere indre trekk, mens reflektert lys brukes til ytre trekk.
(2) Når du justerer brennvidden på objektivlinsen, må du heve og senke tuben sakte for å unngå et plutselig fall som kan skrape eller knuse objektivlinsen mot perlen.
(3) Hold mikroskopet rent; ikke ta på linsen med fingrene, og bruk linsepapir til å tørke av den.
(4) Etter bruk av mikroskopet må du slå av strømmen, justere objektivlinsen til laveste posisjon og dekke til mikroskopet etterpå.
5. Edelstensmikroskopets rolle i identifisering av edelstener
Edelstensmikroskoper brukes i stor utstrekning til identifikasjon av edelstener, først og fremst for å observere edelstenens overflate og indre egenskaper. Vanlige ytre kjennetegn inkluderer overflatedefekter (riper, slitasje, vekstmønstre, syreetsingsmønstre osv.) og slipestil (fasettformer, symmetri osv.); vanlige indre kjennetegn inkluderer typer og fordelingskarakteristikker av inneslutninger, fargefordeling, vekstmønstre, om det er dobbel brytning, og om det er en sammensatt stein laget av forskjellige materialer.
Ved å observere noen typiske trekk under mikroskopet kan man avgjøre om edelstenen har blitt kunstig behandlet. For eksempel kan man i mikroskopet se forskjeller i farge, glans og gjennomsiktighet på fyllingsstedet sammenlignet med smaragdens hoveddel, når det gjelder smaragder som har gjennomgått en fyllingsbehandling.
(1) Forskjeller mellom edelstenens overflate og innvendige inneslutninger
Det er svært viktig å skille mellom overflatetrekk og indre trekk ved edelstener for å kunne identifisere dem. Generelt sett har overflatetrekk mindre innvirkning på edelstenens kvalitet enn indre trekk. For eksempel er innvendige inneslutninger av større betydning for diamantens klarhet enn groper på overflaten, vekstlinjer og andre faktorer. I et edelstensmikroskop kan man skille mellom overflatetrekk og indre trekk ved hjelp av metoder som refleksjonslys, fokalplan og svingende metoder.
① Refleksjonslysmetoden
Lyset belyses fra den retningen man observerer edelstenen, og mikroskopets fokus justeres til posisjonen til den reflekterende overflaten, som er edelstenens overflate. Hvis det er en innvendig inneslutning, vil inneslutningen være uklar når overflaten er klar; hvis det er en ekstern funksjon, vil begge være klare samtidig.
② Fokalplanmetoden
Juster fokusknappen slik at det meste av edelstenens overflate blir klar samtidig. I likhet med refleksjonsmetoden ovenfor er de indre inneslutningene uklare når edelstenens overflate er klar. Omvendt må overflaten klargjøres når de indre inneslutningene er klare.
③ Svingende metode
Juster fokuset til en bestemt posisjon og observer amplituden til de indre og ytre trekkene mens du svinger, samtidig som du roterer edelstenen, der amplituden til de indre inneslutningene er mindre enn amplituden til et bestemt trekk på overflaten.
(2) Observation of Surface Features
In gem identification, the first step is to observe the surface features of the gem, such as surface luster, cracks, and fracture characteristics, to make a preliminary judgment about the type of gem. If observing a rough gem, focus on features such as the gem’s crystal form, crystal face patterns, and cleavage.
① Surface features of mineral crystals or raw stones
- Crystal face stripes appear as linear stripes on the surface of mineral crystals, reflecting the growth and development of the crystal faces. Different mineral crystal forms have different growth stripes on their surfaces. For example, α – quartz crystals have horizontal stripes on their surfaces; diamonds have typical triangular stripes; tourmaline crystals have firm stripes (Figure 2 – 6) .

- Twinning A continuous body formed by two or more identical crystals arranged according to a certain symmetry relationship is called twinning, also known as twin crystals. According to how twin individuals are connected, they can be classified into contact twins, interpenetrating twins, and cyclic twins. Contact twins are further divided into simple contact twins and aggregate contact twins. Twin stripes are linear stripes that appear on the crystal face, cleavage plane, or gemstone cutting and polishing plane at the twin junction. Twinning is a distinguishing feature of gemstone minerals, such as the interpenetrating twins of crystal, the triangular thin slice twins of diamonds (Figure 2 – 7) , the three – fold chrysoberyl, and the contact twins of spinel, etc.

- Cleavage and Fissures: Cleavage is how minerals split along certain directions under external force, forming smooth planes. The cleavage directions and the number of cleavages vary among different crystals. The fissure surfaces are irregular and not smooth, unrelated to the type of crystal but only related to the external forces applied.
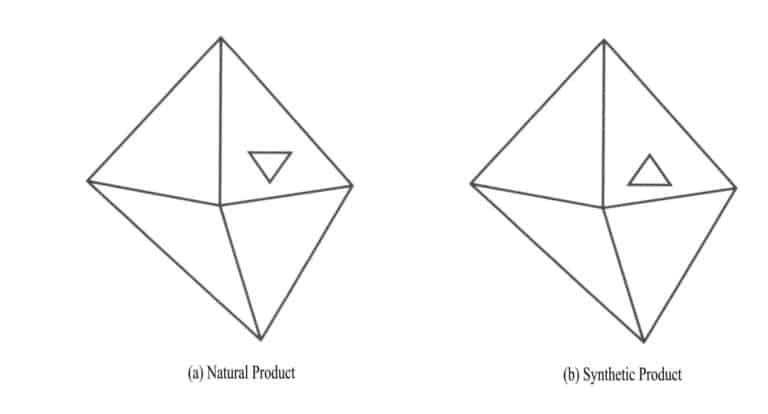
- Growth Hillock: The geometric shapes that form during the growth process of crystals, which have a regular shape and slightly rise above the crystal surface, are called growth hillocks. The characteristics of growth hillocks in natural diamonds and synthetic diamonds are significantly different (Figure 2 – 8) .
② Polished Gemstone
After optimization treatment, the cutting style of gemstones will differ from that of natural gemstones. Compared to natural gemstones, the cutting ratio of optimized gemstones is poorer, and the surface may exhibit unevenness. For optimized gemstones, the main observations include the cutting ratio, the matching of edges, polishing quality, scratches, and surface defects.
③ Composite Stone (Combination Stone)
Composite gemstones can also improve the processing of gemstones formed by combining two or more different material gemstones. Through observation under a microscope, composite gemstones exhibit the following characteristics:
- The junction seam of the composite stone A distinct junction seam appears at the junction of different materials in the composite gemstone, with color and luster differences observed above and below the seam.
- Variations in the luster of the composite stone’s parts Since the composite stone is made up of different materials, which have different refractive indices and transparencies, variations in luster caused by different materials can be observed under a microscope (Figure 2 – 9).
- Are there any bubbles in the bonding area? For example, in the case of a joined stone with garnet on top, magnified inspection will reveal bubbles at the bonding layer and the red ring effect caused by the color difference between garnet and glass.

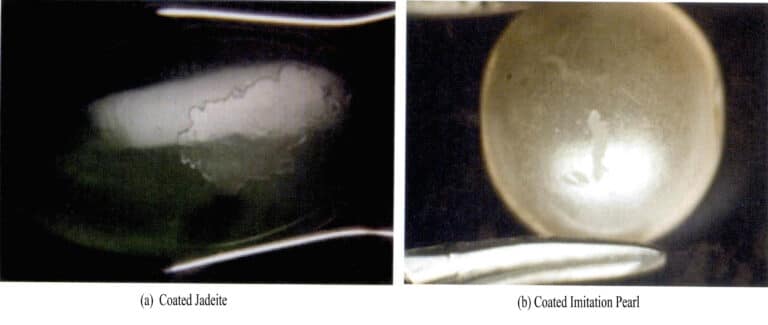
④ Coatings, films, and encrustations
Gems that have been coated or filmed generally have a thin surface layer and lower hardness. Gems treated at high temperatures can also observe surface differences under a microscope, such as scratches, collision marks, bubbles, and partial peeling of the coating (Figure 2 – 10) ; after being subjected to high temperatures, the gemstones can also be found to have high – temperature characteristics. The surface of coated gems is generally a polycrystalline film with lower transparency and luster; the surface of encrusted gems is that of synthetic gems, typically exhibiting characteristics of synthetic gems, such as growth lines and bubbles.
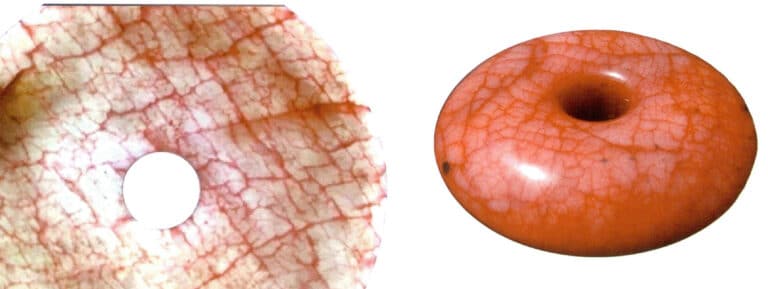
⑤ Dyed and colored products
Gems that have been dyed or colored generally have many natural fissures. Under a magnifying glass or microscope, the dye and coloring agents can be observed in the fissures and pits of the gems. The presence of these colorants increases the variety of colors in the gems, and under a microscope, the color distribution is extremely uneven; the color is darker in the fissures and lighter in the dense structures (Figure 2-11) .
(3) Observation of internal features
① Color observation
The color of natural gemstones is not necessarily evenly distributed; the color distribution of dyed gemstones is related to the structure of the gemstone. For example, the color of dyed jadeite is distributed along the fibrous structure, with deeper colors in areas where the structure is loose and lighter colors in denser areas. Due to the many fissures in natural rubies, dyed rubies often have deeper colors in the fissures.
② Observation of growth lines
The growth patterns of natural gemstones differ from those of synthetic gemstones. Generally, the growth lines of natural gemstones are straight, such as the angular growth color bands of natural sapphires, while the growth lines of sapphires synthesized by the flame fusion method are arc – shaped. Of course, there are different situations, such as the growth lines in rubies synthesized by the flux method being straight while the growth lines of natural pearls being concentric circles.
③ Observation of inclusions
The characteristics of inclusions are the most important identification criteria for distinguishing natural gemstones, synthetic gemstones, and optimally treated gemstones. The types of inclusions vary under different growth environments.
Natural gemstones contain a wealth of inclusions. The types of inclusions (referred to as inclusions) are related to the genesis of the gemstones.
- Gemstones found in basic and ultrabasic rocks mainly include solid dark minerals such as goethite, hematite, magnetite, and rutile.
- Gemstones in pegmatites contain many gas and liquid inclusions, generally appearing in teardrop shapes, oval shapes, or parallel tubular forms. For example, the aquamarine cat’s eye from Altay, Xinjiang, is caused by densely packed fine tubular inclusions.
- Gemstones related to hydrothermal activity often have gas, liquid inclusions, and solid mineral inclusions; sometimes, two – or three – phase inclusions coexist. For example, three – phase inclusions are developed in Colombian emeralds (Figure 2 – 12) .
- The origin marks of inclusions and their effects. Due to differences in the conditions of gem formation, inclusions in gems show significant differences. Some gems also have their characteristic inclusions. For example, tubular inclusions in tourmaline, two – phase immiscible liquid inclusions in topaz, three – phase inclusions, and mineral inclusions in emeralds, etc.
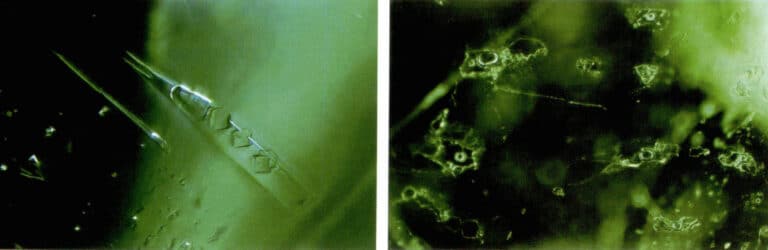
Inclusions in synthetic gems
- Flame fusion method: This method can synthesize rubies, sapphires, spinels, rutiles, and strontium titanate, among others. The synthesized gems generally show arc – shaped growth lines due to the accumulation and crystallization process and may also exhibit unmelted raw material powder and round bubbles (Figure 2 – 13) .
- Flux method: This method can synthesize rubies, emeralds, and chrysoberyl. Due to the use of platinum containers, there may be platinum inclusions. If the temperature is not controlled properly, inclusions of the raw materials may appear, typically in the form of broomstick – like or cloud – like bubble aggregates, such as the veil – like inclusions in synthetic emeralds (Figure 2 – 14) .
- Hydrothermal method: It was Initially used for synthesizing optical crystals, later for synthesizing rubies and amethysts, and recently for synthesizing emeralds. A typical example is inclusions with crystal seeds inside, such as the needle – like beryllium oxide solid inclusions in synthetic emeralds and liquid and gas inclusions (Figure 2 – 15) .
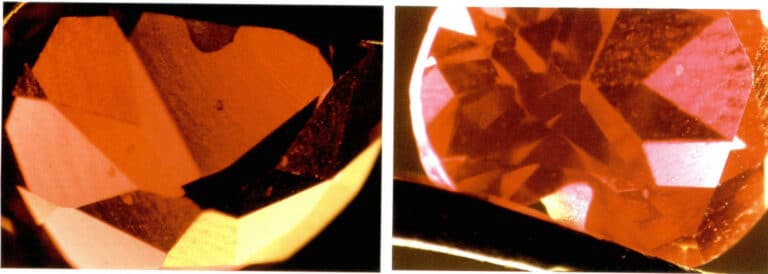
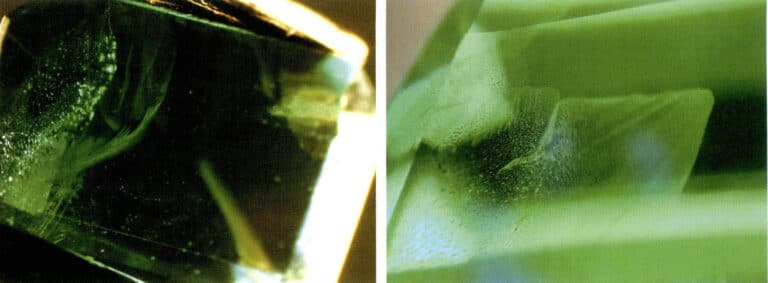
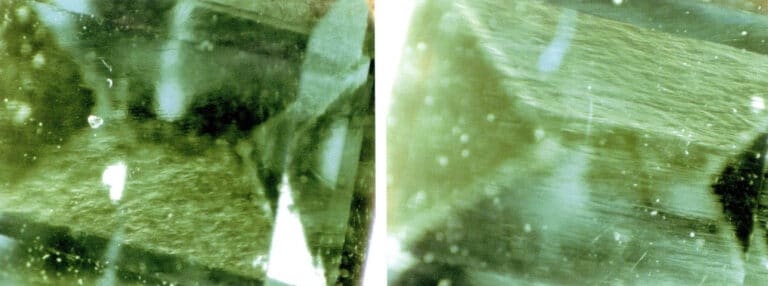
Artificial improvement of gemstones
- Colorless material filling. When the refractive index and luster of filled gemstones are observed under a microscope, bubbles and uneven distribution of luster and refractive index may sometimes appear. For example, bubbles can be observed in treated rubies caused by the difference in refractive index between the filling material and the ruby, resulting in differences in luster and brightness on the gemstone surface (Figure 2 – 16) .
- Dyeing and coloring. Dyeing treatment can be applied to many types of gemstones, such as rubies, jade, agate, pearls, and crystals. Since natural gemstones often have many fissures, using brightly colored organic dyes or inorganic pigments for dyeing can improve the color of natural gemstones. After dyeing treatment, gemstones can be observed under a microscope to determine whether coloring substances or color distribution exist in the gemstone fissures or between grains. For example, in dyed crystals (Figure 2 – 17) , under magnification, the color can be seen concentrated in the cracks of the gemstone; wiping the surface of the gemstone with white paper or cotton will show that poorly dyed gemstones will leave the presented color on the white paper or cotton.
- Coating, Adhering, and Backing Coating is a common treatment method, such as using vacuum coating to apply a layer of synthetic diamond film on the surface of crystals, topaz, or other colorless gemstones to imitate diamonds. Under a microscope, the surface appears with a adamantine luster. Since synthetic diamonds are polycrystalline, cracks or wear may develop on the surface over time. A layer of metal can be coated on the table or pavilion of the gemstone, providing a better reflective effect and vibrant colors. Under magnification, a rainbow surface can be observed. Adhering is commonly used for colorless or lightly colored beryl. A layer of green synthetic emerald is grown on the surface of beryl using synthetic methods to act as an emerald. Due to different thermal expansion, cracks will likely form at the interface between the synthetic emerald layer and the beryl, which can be observed under a microscope. Backing is often applied to lightly colored gemstones, such as creating a black backing under a thinner opal to deepen its overall color. Color differences between layers can be observed under a microscope.
- Composite Stone: The process of organically bonding two or more materials together using an adhesive to form the appearance of a whole gemstone is called composite. Composite gemstones are used for diamonds, opals, emeralds, rubies, sapphires, and garnets. Under magnification, one can observe whether there are boundary interfaces in the composite stone, adhesive present between layers, differences in inclusion characteristics in various parts of the upper and lower layers, and bubbles present on the composite surface.

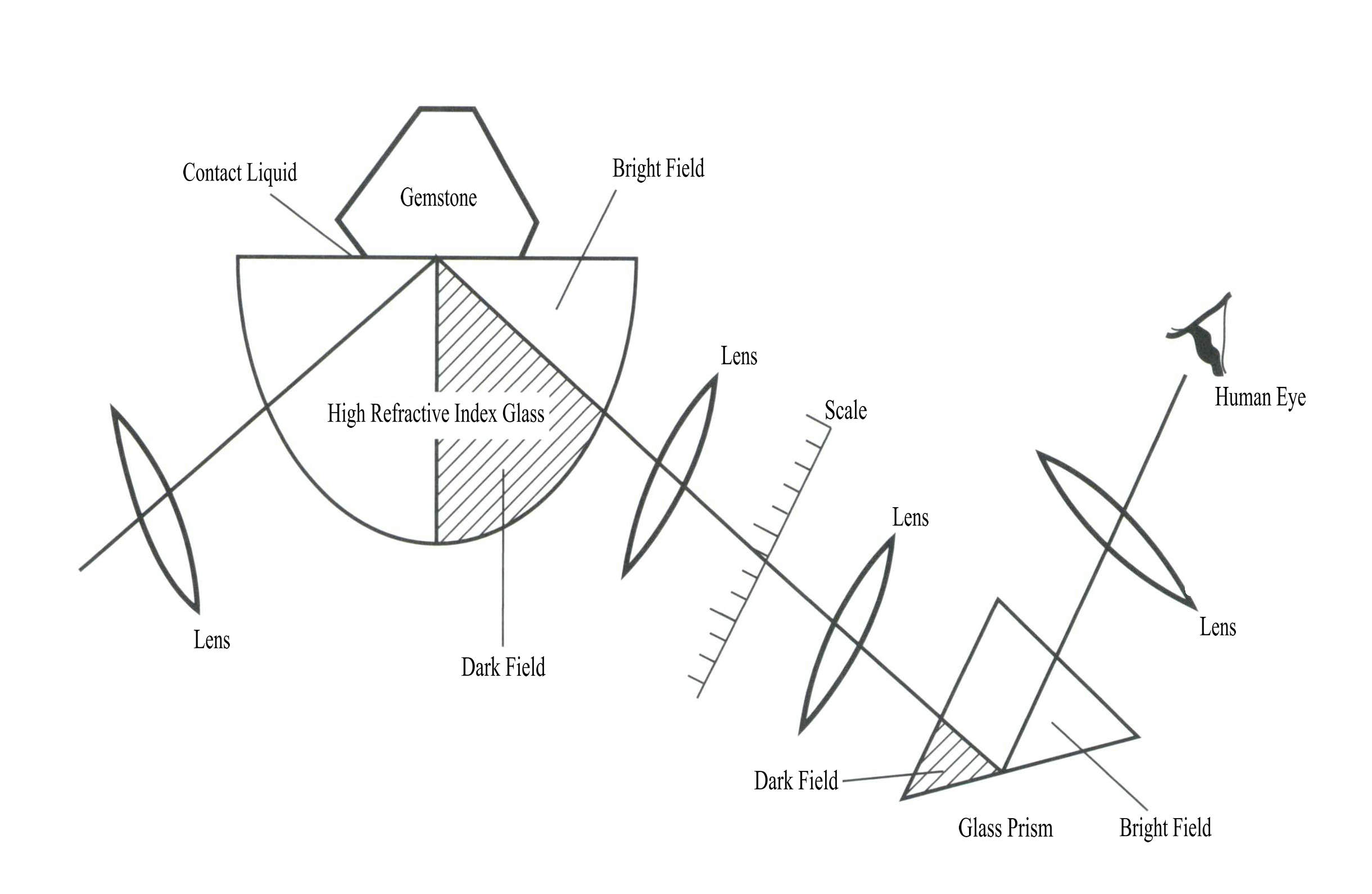
Section IV Refractometer
The gemstone refractometer is designed and manufactured based on the law of total internal reflection. When light waves propagate from a dense medium to a less dense medium, total internal reflection occurs when the angle of incidence reaches a certain degree. The size of the critical angle for total internal reflection is related to the refractive index of the medium. When light shines from the front of the refractometer onto high – lead glass, it passes through the high – lead glass hemisphere to the contact area with high refractive index immersion oil and the gemstone, resulting in total internal reflection. The light reflects the other side of the normal high – lead glass, lens, scale, and prism, reaching the eyepiece, where the observer can directly read the refractive index value of the measured gemstone (Figure 2 – 18) .
The refractometer is suitable for gemstones with smooth surfaces. Samples must have smooth surfaces, be too small, or have insufficient contact area with the refractometer to measure their refractive index and birefringence. Organic gems, porous gems, and samples with a refractive index greater than 1.78 also cannot have their refractive index and birefringence tested.
1. Prerequisites and limitations for using the refractometer
In addition to the refractometer, two conditions are also required for measuring the refractive index: one is the illumination light source, which is generally a yellow light source at 589nm, obtainable through a sodium lamp or by adding a yellow filter to the light source or eyepiece; the second is the contact liquid, which is necessary for good contact between the glass table and the gemstone sample, requiring its refractive index to be greater than that of the gemstone sample. It is worth noting that the contact liquid used in the refractometer is toxic. To avoid the sample floating or causing unnecessary harm to the observer, the amount of contact liquid used should be minimized, and the bottle should be tightly closed after use. Pay attention to the following points when using:
(1) The immersion oil selected must have a refractive index close to that of high – lead glass, generally around 1.80 – 1.81.
(2) The refractive index of the gemstone must be less than that of the immersion oil and the glass hemisphere to produce total internal reflection, thus allowing its refractive index to be measured. If the refractive index of the gemstone is greater than that of the immersion oil, the refractive index value of the gemstone cannot be measured on the refractometer.
(3) The critical angle of various gemstones is fixed, so based on the different areas of total internal reflection of the light, different refractive index values of gemstones can be described (that is, regardless of how the angle of incidence changes, there is only one maximum angle of incidence for total internal reflection; all light exceeding this maximum value will not be reflected) . This creates bright and dark areas in the field of view. By rotating the sample and the polarizer in all directions and observing the scale at the boundary between light and dark in the eyepiece, the refractive index of the gemstone can be determined.
2. Steps for Operating the Refractometer
(1) Clean or wipe the sample to be measured, and place an appropriate amount of contact oil on the measuring stage.
(2) Place the polished surface or crystal face of the sample facing down gently onto the contact oil on the measuring stage.
(3) Rotate the sample and polarizer in all directions and read the light and dark boundary scale value from the observation eyepiece, which is the refractive index.
(4) A homogeneous body can only measure one refractive index value. In contrast, a non – homogeneous body can measure a maximum and a minimum value, and the difference between these two values is the birefringence of the sample.
(5) The optical characteristics of the sample can be determined Based on the changes in the light and dark boundary.
3. Uses of the refractometer
The refractometer plays an important role in gem identification. It can help identify optimally treated gems. For example, the refractive indices of two materials in a composite gem are often different. It can also determine the anisotropy or isotropy of the gem. It is mainly used in the following aspects of gem identification:
(1) Determine the isotropy and anisotropy of gemstones and measure the refractive index of isotropic gemstones.
(2) Measure the maximum and minimum values of the refractive index of anisotropic gemstones and the birefringence.
(3) Determine the axial nature of anisotropic gemstones, whether they are uniaxial or biaxial, and the optical sign.
(4) Determine composite gemstones. Due to the different materials in the upper and lower layers of assembled gemstones, there may be differences in refractive index, which can help determine if there is an assembly phenomenon.
Section V Gemstone spectroscope
A spectroscope can be used to observe the absorption spectrum of gemstones, helping to identify the variety of gemstones, infer the coloring elements within the gemstones, especially for those with typical spectra, it can be used to determine the subspecies of gemstones, and it can also be used to distinguish whether the gemstones have been treated. The spectroscope is particularly useful in identifying treated gemstones, such as distinguishing irradiated diamonds from natural diamonds, natural corundum from improved corundum and synthetic corundum, natural jade from dyed jade, and distinguishing various composite gemstones can also be accomplished using a spectroscope.
1. Principle of the spectroscope
A spectroscope identifies gemstones by observing the light that passes through the gemstone or is reflected from its surface, which absorbs light waves of certain wavelengths. Each gemstone has its unique internal structure; even gemstones with the same coloring ions can produce very different colors due to their different internal structures. For example, emeralds and rubies are colored due to the presence of the coloring element chromium in the crystal, one being green and the other red. Each gemstone has its characteristic absorption spectrum, which forms the basis for testing and identifying gemstones. The color of transparent gemstones results from their selective absorption of light.
(1) Dispersion
When a beam of white light passes through the slanted surface of a transparent object (such as a prism) , it is decomposed into its constituent wavelengths, producing spectral colors, namely red, orange, yellow, green, cyan, blue, and violet. The wavelengths of commonly seen colors in visible light are as follows: red 770–640nm; orange 640–595nm; yellow 595– 575nm; green 575–500nm; cyan 500–450nm; blue 450 – 435nm; violet 440 – 400nm.
(2) Selective Absorption
All objects have varying degrees of absorption for visible light. The absorbed wavelengths can be seen when the light passing through these objects is decomposed. When all light waves are absorbed, they appear black in the spectrum; when they pass through, they show spectral colors. If the object absorbs some light waves, the material presents a specific color, and this absorption is often related to specific elements within the material.
2. Types and Functions of Spectroscopes
Both raw stones and set gemstones can be tested using a spectroscope. The reasons for the coloration of gemstones can be studied by examining their absorption spectrum. Using a spectroscope for the identification of certain gemstones is convenient and quick, especially for those that cannot be identified by methods measuring density and refractive index, such as set gemstones where density cannot be measured and gemstones with a refractive index above 1.81 where refractometers become ineffective. Therefore, using a spectroscope for observation and testing to identify gemstones is particularly important.
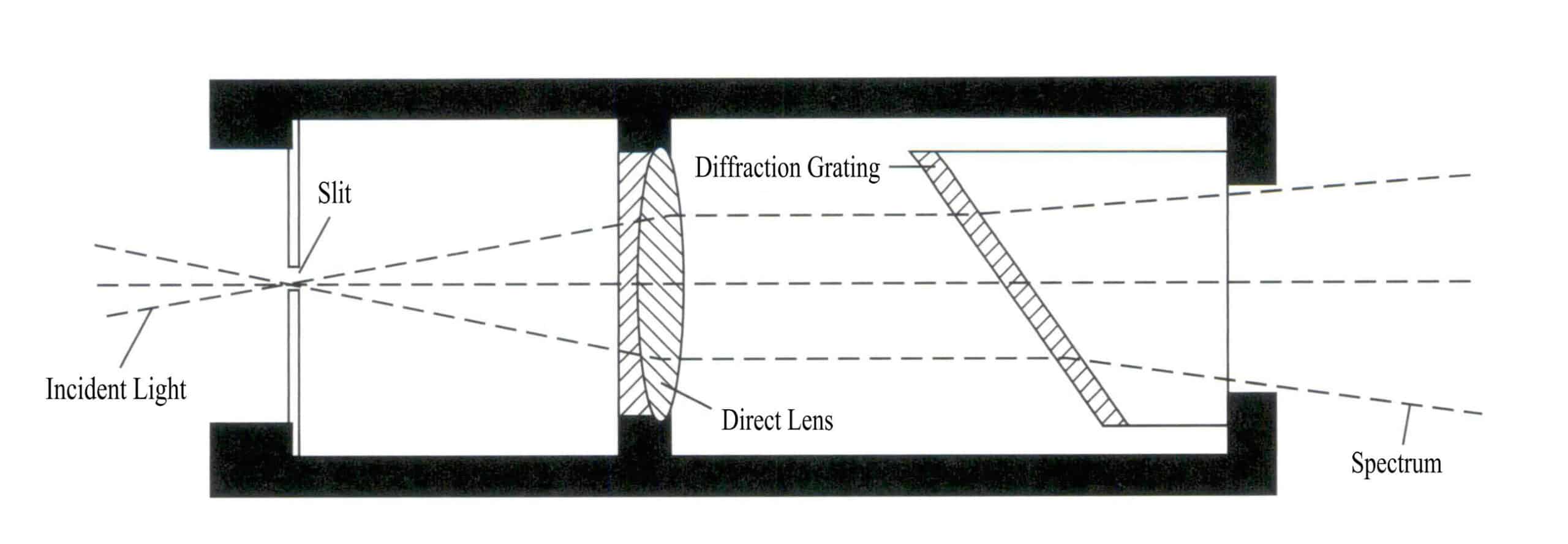
The spectroscope used for identifying gemstones is generally quite simple in structure, being tubular and easy to carry (Figure 2 – 19) . Spectroscopes can be divided into two types based on their structure: prism type and diffraction grating type.

3. Structure and Characteristics of Spectroscopes
(1) Prism Spectroscope
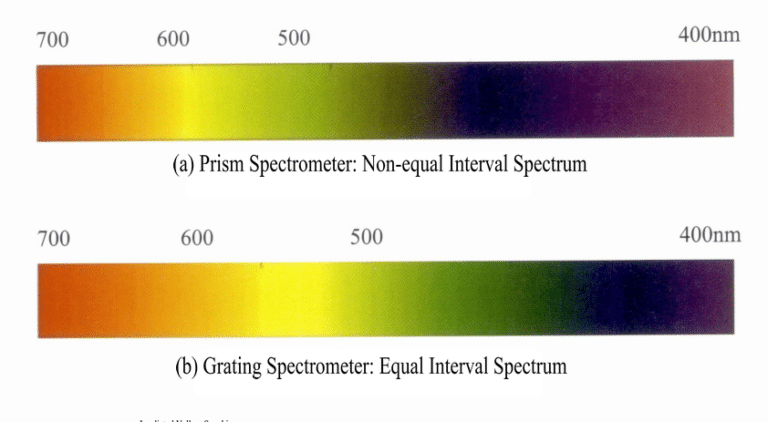
The prism spectroscope consists of a series of prisms, producing a relatively straight light path, with these prisms in optical contact. The characteristic of the prism spectroscope is that the blue – violet light region is relatively broadened. In contrast, the red light region is relatively compressed, resulting in an uneven distribution of color zones in the spectrum. The advantage is good light transmission, allowing a bright segment of the spectrum to appear, which is beneficial for observing the blue – violet light region spectrum.
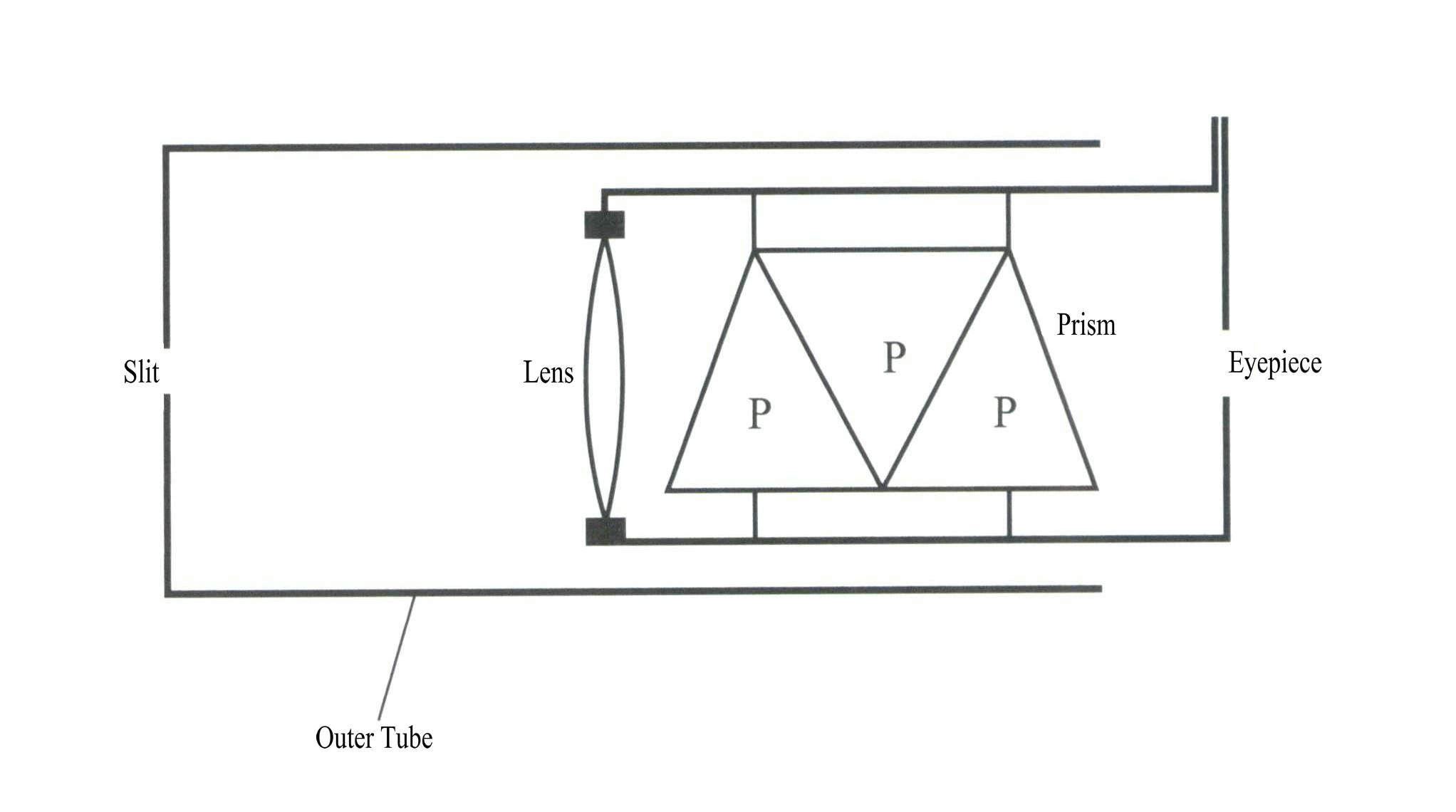
① Construction:
The prism spectroscope is composed of a slit, a lens, a set of prisms, a scale, and an eyepiece (Figure 2 – 20) .
② Prism Materials:
The selection of prism materials must meet three conditions: they must not absorb visible light at specific wavelengths; the dispersion color cannot be too wide or too narrow; it must be uniaxial. Otherwise, two sets of spectra will be produced.
Prisms are usually made from leaded or unleaded glass, preferably using a combination of triangular or pentagonal prisms, and they must be interlocked.
③ Slit:
A window used to control the amount of backlight. For transparent gems, the slit is almost completely closed; for semi – transparent or weakly translucent gems, the slit should be opened slightly wider.
④ Focusing Sliding Tube Eyepiece:
Adjusts the eyepiece’s focal length according to the different focal lengths of each person’s eyes.
⑤ Spectral Characteristics:
The spectrum is bright, belonging to a non – uniform spectrum, with uneven wavelength scales; the purple and blue regions are relatively widened, while the red and yellow regions are narrowed, suitable for darker – colored gems, facilitating the observation of gems that absorb blue – violet light.
(2) Grating Spectrometer
The grating spectrometer is mainly composed of a diffraction grating group. The characteristic of a grating spectrometer is that the spectral regions are approximately equal in size, and the resolution of the red light region is higher than that of the prism spectrometer. Compared to the prism spectrometer, it has a lower transmission rate and requires a stronger light source (Figure 2 – 21) .
① Struktur:
The grating spectrometer comprises a collimating lens, a diffraction grating, and an eyepiece (Figure 2 – 22) .
② Spectral characteristics:
Compared to prism spectrometers, the spectra of grating spectrometers are slightly darker, more uniform, and have a uniform wavelength scale. They are suitable for gemstones with good transparency and those with absorption lines in the red region.
4. Precautions for using spectrometers
(1) The light source used for the spectroscope must be a strong, focused white light source (incandescent lamp) , typically using a spotlight flashlight, microscope light source, or the light source of a polarizer.
(2) The light source has thermal radiation; samples should be kept under the light source for a short time to avoid overheating the gems, which can affect the spectrum. Prolonged exposure may cause absorption lines to blur or even disappear.
(3) Do not hold the gems directly with your hands, as human blood can produce an absorption line at 592nm.
(4) The absorption of certain gems may be directional, and careful observation must be made from various angles. Gems with strong pleochroism may show differences in absorption spectra depending on the direction.
(5) For composite gems, careful observation must be made from different directions, as the absorption spectra of different parts may vary.
(6) People wearing photochromic glasses should remove their glasses during spectral testing to avoid confusion between the absorption lines of neodymium in the glasses and the absorption lines of the test gemstones.
5. Color - causing ions in gemstones and their applicable range
When white light passes through transparent gemstones containing color – causing ions or reflects off the surface of opaque gemstones, part of the light is absorbed, causing us to observe the gemstone displaying color.
The color of a gemstone is related to the color – causing ions it contains. Gemstones colored by different metal ions have different absorption spectral characteristics. However, gemstones colored by the same metal ions have similar absorption spectral characteristics. The characteristic absorption spectral lines of metal ions can help determine the variety of the gemstone or whether the gemstone has been treated.
Spectrometers are very broad; they can be used to determine the color – causing elements in gemstones, mainly applicable to colored gemstones. Colorless gemstones, except for zircon, diamonds, and enstatite, do not have significant absorption spectra. In identification, they are only applicable to gemstones with typical spectra. Gemstones with typical spectra can serve as diagnostic identification features and should be mastered with emphasis.
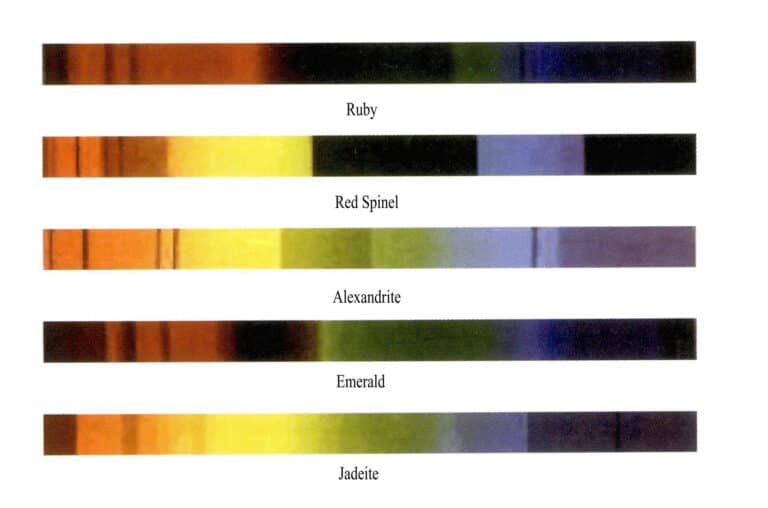
(1) Absorption Spectrum of Chromium – Ion Colored Gemstones
Chromium ions are the most important coloring elements in common precious gemstones. Common gemstones colored by chromium ions include rubies, red spinels, alexandrites, emeralds, and jade, and the characteristic absorption spectra of these gemstones are shown in Figure 2 – 23 (observed under a grating spectrometer) .
Although the gemstones in Figure 2 – 23 are all colored by chromium ions, their absorption spectra are similar but not identical. The absorption spectrum of ruby has three absorption lines in the red region, a broad absorption in the yellow – green region, three absorption lines in the blue region, and full absorption in the purple region; the absorption spectrum of red spinel has an absorption line in the red region, an absorption band in the yellow – green region, and full absorption in the purple region; the absorption spectrum of alexandrite has an absorption line in the red region, an absorption band in the yellow – green region, one absorption line in the blue region, and full absorption in the purple region; the absorption spectrum of emerald has an absorption line in the red region, a weak absorption band in the orange – yellow region, a weak absorption line in the blue region, and full absorption in the purple region; the absorption spectrum of jade has three step – like absorption lines in the red region ( 630 – 690nm) ) , and an absorption line in the purple region at 437nm (the 437nm absorption line may be missing when the green is bright and pure) .
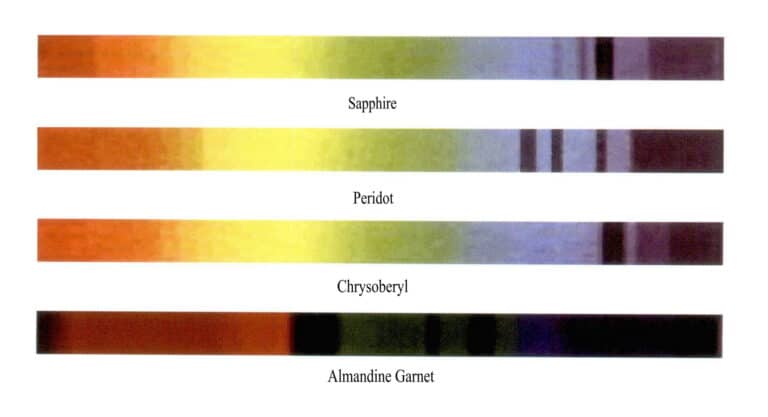
(2) Absorption spectra of iron ion colored gemstones
Common gemstones colored by iron ions include sapphires, olivine, chrysoberyl, and almandine, and the characteristic absorption spectra of these gemstones are shown in Figure 2 – 24 (observed under a grating spectrometer) .
Sapphire, olivine, chrysoberyl, and almandine are all colored by iron ions, but their absorption spectra differ. The absorption lines of sapphire are three narrow absorption bands in the blue region at 450nm, 460nm, and 470nm; the absorption lines of olivine are three narrow absorption bands in the blue region at 453nm, 473nm, and 493nm; the absorption line of chrysoberyl has a strong absorption narrow band at 444nm in the blue region; the absorption lines of almandine have three strong absorption narrow bands in the yellow – green region (505nm, 527nm, 576nm) , with weak bands in the blue and orange – yellow regions.
(3) Absorption spectrum of cobalt ion colored gemstones
Common gemstones colored by cobalt ions include synthetic blue spinel and cobalt glass. The absorption spectrum lines of these gemstones are shown in Figure 2 – 25. The absorption spectrum of synthetic blue spinel has three strong absorption bands in the green, yellow, and orange – yellow regions, with the narrowest absorption band in the green region; the absorption spectrum of cobalt glass has three strong absorption bands in the green, yellow, and orange – yellow regions, with the narrowest absorption band in the yellow region.

(4) Absorption spectra of other common gemstones
Other common gemstones include diamond, zircon, and spessartine, among others. The absorption spectra of these gemstones are shown in Figure 2 – 26.
The absorption spectrum of a colorless diamond is a line at 415nm in the violet region; the red region absorption line at 653.5nm is a diagnostic absorption line for colorless zircon; the absorption lines of colored zircon are uniformly distributed in various color zones from 1 to 40, with the red region absorption line at 653.5nm; the purple region absorption narrow band at 432nm is a diagnostic absorption band for spessartine.

Copywrite @ Sobling.jewelry - Tilpasset smykkeprodusent, OEM og ODM smykkefabrikk
6. Optimization of the absorption spectrum of treated gemstones
(1) Heat – treated gemstones
After natural gemstones undergo heat treatment, their coloring elements change valence state or are transformed into other coloring ions, thereby altering the color of the gemstones or increasing their transparency.
For example, more than 90% of Australian sapphires undergo heat treatment; before treatment, the absorption lines at 450nm, 460nm, 470nm are almost connected, while after treatment, the absorption line at 470nm is separated, and the three lines are relatively distinct; in the absorption band of the tourmaline, the strongest is at 595nm, and after heat treatment, the one at 595nm may not be the strongest.
(2) Irradiated gemstones
Irradiation can color gemstones, mainly by causing defects in the gemstones, forming color centers. Gemstones colored by this method generally do not have characteristic absorption spectra, with only a few showing absorption spectra. For example, diamonds colored by neutron bombardment show a pair of absorption lines at 498nm and 504nm.
(3) Dyed gemstones
Natural green jade has three absorption lines at 630nm, 660nm, and 690nm, while dyed jade shows a broad absorption band at 630 – 670nm. After fading, the spectral lines may appear shallower and narrower, or only one absorption line may appear; dyed jadeite has a vague absorption band in the red light region at 650nm (Figure 2 – 27) , a typical identification feature.

(4) Filled gemstones
Filling treatment is commonly used for structurally porous gemstones, such as turquoise, which is often filled with colored plastic due to its lighter color and soft texture. The filled turquoise does not exhibit characteristic absorption spectral lines. In contrast, natural turquoise shows a weak absorption line at 460nm and a strong one at 432nm when observed with reflected light.
Section VI Determination of Gemstone Density
Density is an important physical parameter in gemstone identification, and each type of gemstone has its fixed density value. Therefore, gemstones can be identified based on their density. Different gemstones have different densities or density ranges due to variations in chemical composition and crystal structure, and even the same type of gemstone can have certain differences in density due to variations in chemical composition or the presence of impurities.
Density testing is also a relatively effective identification method for optimized treated gemstones. Most gemstones that have undergone filling treatment have a lower density than natural gemstones, such as filled turquoise, which has a lower density than natural turquoise. However, some optimized treated gemstones, such as organic and composite gemstones, cannot be identified using density testing. Currently, commonly used methods for measuring density include balance weighing and heavy liquid methods.
A balance is a tool for measuring the mass of objects. In gemology, it is used not only for weighing gemstones but also for determining their density. For weighing the quality (weight) of gemstones, national standards require the balance to be accurate to one ten – thousandth of a gram. The quality (weight) of gemstones and their density are important bases for identifying and evaluating gemstones, so using the balance correctly is an important skill.
The commonly used balance is electronic. Regardless of the type of balance, to ensure the accuracy of weighing, the following two points must be observed: it should be calibrated and set to zero before use; during weighing, the environment must be kept relatively still, such as preventing vibrations of the balance platform and air convection.
1. Method for determining the relative density of gemstones
(1) Testing principle
The commonly used unit for the density of gemstones is g/㎝³, which represents the mass of a gemstone with a volume of 1㎝³. Density determination is quite complex because the relative density is very close to the density value, with a conversion factor of only 1.0001. In gemology, the measured relative density value is usually taken as an approximate density value, and the relative density in gemstones is commonly represented by d.
The method for determining relative density (also known as the hydrostatic weighing method) is based on Archimedes’ principle. When an object is immersed in a liquid, the buoyant force exerted by the liquid on the object equals the weight of the liquid displaced. If the liquid is water, the effect of water temperature on the mass of a unit volume of water is negligible. According to Archimedes’ principle, the density of the sample (p) can be calculated using the mass of the sample in air (m) and the mass(m1) in the liquid medium (p0) according to formula (2 – 1) .
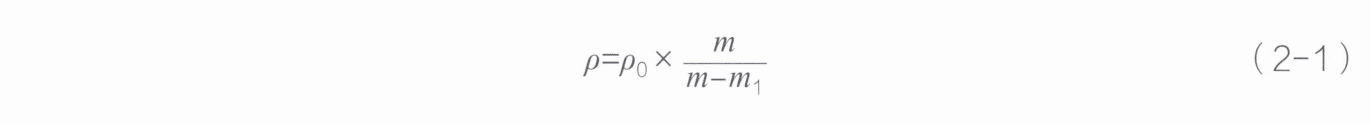
In the formula,
ρ— the density of the sample at room temperature, g/cm3
m—the mass of the sample in air, g;
m1—the mass of the sample in the liquid medium, g;
ρ0—the density of the liquid medium, g/cm3.
The commonly used liquid is water; as the density of water is approximate, the buoyancy of air on the gem can be ignored, and the mass of the gem is the same as the mass of the object in the air. To obtain the density value, weigh the object in air and water.
(2) Test Steps
The equipment required to test relative density includes a balance, glass beaker, wooden stand, and copper wire.
① Clean the gemstone to ensure there are no impurities on its surface.
② Adjust the balance to a level position and measure the mass(m) of the gemstone in the air.
③ Place a beaker filled with water on the stand, put the gemstone in a wire basket, and weigh the mass(m1) of the gemstone in water.
④ Calculate the relative density of the gemstone(d) = the mass of the gemstone in air(m) / (the mass of the gemstone in air(m) – the mass of the gemstone in water(m1) ) .
(3) Forholdsregler
The static water weighing method for determining relative density is suitable for testing a single variety of gemstone materials. Pay attention to the following points during measurement:
① The gem to be tested must be non – absorbent; filled gems, organic gems, etc. cannot be tested for relative density using this method.
② When measuring in water, it should be stable, and bubbles should be avoided as much as possible.
③ Use tweezers to gently handle the gem, and try not to shake it.
④ The surrounding environment should be quiet to avoid affecting measurement accuracy.
⑤ If the sample is too small, the measurement error will be larger; if the sample is too large and exceeds the weighing range of the balance, its relative density cannot be determined.
⑥ The test results retain two decimal places.
When weighing the mass of gemstones in water, it is important to eliminate the influence of surrounding objects on the weighing data. For example, no bubbles should be attached around the gemstone, the support and beaker should not touch the balance pan, the copper wire should not contact the beaker, etc.
2. Determination of the relative density of gemstones using the heavy liquid method
In gem identification, the distribution state of gemstones in heavy liquids (immersion oil) is often used to estimate the relative density range of the gemstones. The relative density of different heavy liquids is determined based on the relative density of the gemstones.
This method is the simplest and most convenient way to measure the relative density of a substance, without the need for a balance scale, but rather by comparing the substance’s relative density with a set of heavy liquids of different relative densities. By placing the gem into a liquid of known relative density and observing the sinking or floating phenomenon, if it sinks to the bottom of the liquid, it indicates that the gem’s relative density is greater than that of the liquid; if it floats on the surface of the liquid, the gem’s relative density is less than that of the liquid; only when it is suspended in the liquid do the two relative densities become similar. Commonly used heavy liquids include bromoform, tetrabromoethane, Duriel’s solution, diiodomethane, and Clerici’s solution, all of which have fixed relative densities. They need to be diluted with different solutions to create a series of heavy liquids, as shown in Table 2 – 2.
Table 2 – 2 Relative Densities of Common Heavy Liquids
| Heavy liquid name | Relativ tetthet | Diluent | Dilution range |
|---|---|---|---|
| Bromomethane | 2.89 | Benzene, dimethylbenzene, bromonaphthalene | 2.5 - 2.88 |
| Tetrabromoethane | 2.95 | Dimethylbenzene | 2.67 - 2.95 |
| Duriel's solution | 3.19 | Vann | 2.2 - 3.19 |
| Diiodometan | 3.34 | Benzene, dimethylbenzene | 3.1 - 3.3 |
| Clerici's solution | 4.15 | Vann | 3.33 - 4.15 |
Heavy liquid can determine the relative density of some optimally treated gemstones; for example, filled gemstones’ relative density is lower than that of natural gemstones. When determining the relative density of gemstones, the following points should be noted:
① Heavy liquid is often toxic; the measurement time should not be too long and should be sealed and stored away from light after use.
② Try to avoid evaporation and contamination. Otherwise, it will cause errors in the relative density of the heavy liquid.
③ Avoid using heavy liquid measurement for easily soluble substances such as natural organic gemstones, synthetic plastics, artificial coatings, and two – layer and three – layer stones.
The heavy liquid method is commonly used to measure gemstones with significantly different relative densities, such as diamonds and their imitations. It is one of the most effective identification methods in a flowing environment.
3. Heavy liquid (immersion oil) testing optimization for gemstone characteristics
Heavy liquid can be used to test the characteristics of partially optimized gemstones, mainly in the following aspects.
(1) Detecting assembled stones
Place the assembled gemstones in the immersion liquid and observe them in a direction parallel to the girdle plane. Various characteristics of the assembled gemstones can be seen, such as the bonding seams of the assembly layers, color changes between the upper and lower layers, etc.
(2) Observing gemstone structure in conjunction with a microscope
When the refractive index of the gemstone is close to that of the immersion oil, the reflected light and diffuse reflected light on the gemstone’s surface decrease, which is beneficial for observing and studying the internal features of the gemstone, such as growth lines, color bands, inclusions, etc.
(3) Detection of composite growth treatment and diffusion treatment
Using heavy liquid (immersion oil) allows observation of the composite growth layers and diffusion – treated gemstones of synthetic emeralds, etc.
Section VII Identification of Long - wave and Short - wave Ultraviolet Light
Ultraviolet fluorescence lamps (referred to as UV lamps) are an important auxiliary identification instrument mainly used to observe the luminescent characteristics of gemstones. Some gemstones emit visible light when irradiated with ultraviolet light, called ultraviolet fluorescence. Although fluorescence reactions are rarely decisive
evidence for determining the species of gemstones, they can quickly distinguish between different types of gemstones in certain aspects, such as identifying diamonds from their imitations like cubic zirconia, rubies from garnets, etc. Ultraviolet fluorescence characteristics can also determine whether a gemstone has undergone optimization treatment.
Ultraviolet light is outside the visible light range, with a wavelength range of approximately 100nm – 380nm. Different gemstones exhibit different colors under ultraviolet light. Some optimally treated gemstones produce specific colors under ultraviolet light, which helps identify whether a gemstone has undergone optimization treatment. Ultraviolet light is divided into long – wave ultraviolet light and short – wave ultraviolet light, with long – wave ultraviolet light ranging from 380 to 300nm and short – wave ultraviolet light ranging from 300 to 200nm.
1. Working principle of the UV lamp
Long – wave ultraviolet lamps typically emit light with a wavelength of 365nm, while short – wave ultraviolet lamps emit light with a wavelength of 253.7nm (Figure 2 – 28) .
Ultraviolet lamp tubes can emit ultraviolet light waves within a certain wavelength range. After passing through specially designed filters, they only emit long – wave ultraviolet light with a wavelength of 365nm or short – wave ultraviolet light at 253.7nm. The fluorescence characteristics of gemstones under long – wave and short – wave ultraviolet light can help identify gemstones.

2. How to Use Ultraviolet Lamps
Currently, there are various types of ultraviolet lamps on the market, all with the same internal structure and working principle, consisting of three parts: ultraviolet light source, dark box, and observation window. Some also come with eye protection goggles to prevent eye damage from ultraviolet light.
Place the gem to be tested under a UV lamp, turn on the light source, select long wave (LW) or short wave (SW) , and observe the gem’s luminescence. In addition to noting the strength of the fluorescence, please pay attention to the color of the fluorescence and the area from which it emanates. The strength of the fluorescence is often categorized into four levels: none, weak, medium, and strong. Sometimes, due to the reflection of UV light on the gem’s facets, a false impression of purple fluorescence may occur; in this case, change the gem’s orientation slightly. Furthermore, fluorescence is the light emitted by the gem as a whole, while facet reflection is localized, with uneven light intensity, and appears rigid. The fluorescence intensity of the gem under a long wave is usually greater than that under a short wave. If you need to observe the sample’s phosphorescence, turn off the switch and continue to observe.
3. The Role of UV Lamps in Gem Identification
(1) UV fluorescence is used to identify gem varieties
Some gem varieties are similar in color appearance, such as rubies and garnets, certain emeralds and green glass, sapphires, and benitoite. Still, their fluorescence characteristics have significant differences, so fluorescence testing can help distinguish them.
(2) Helps to differentiate some natural gems from synthetic gems
Natural rubies contain some iron elements to varying degrees, and their fluorescence color under ultraviolet light is less bright and vivid than synthetic ones. The fluorescence color of natural emeralds is often not as bright as that of synthetic emeralds; flame – fusion synthetic yellow sapphires appear inert or emit red fluorescence under long – wave light, while some natural yellow sapphires exhibit yellow fluorescence; flame – fusion synthetic blue sapphires show light blue – white or green fluorescence, while the vast majority of natural blue sapphires appear inert.
(3) Help identify diamonds and their imitations
The fluorescence intensity of diamonds varies greatly, ranging from none to strong, and can display various colors. Diamonds with strong blue fluorescence usually have yellow phosphorescence. Common imitations, such as synthetic cubic zirconia, appear inert or emit yellow fluorescence under long – wave ultraviolet light. In contrast, yttrium aluminium garnet exhibits yellow fluorescence, and gadolinium gallium garnet often appears pink. Under short – wave light, synthetic colorless spinel emits blue – white fluorescence, and colorless synthetic corundum shows light blue fluorescence. Therefore, ultraviolet light is very useful for identifying diamond clusters, as if they are all diamonds, their fluorescence intensity and color will not be uniform, while synthetic cubic zirconia, yttrium aluminium garnet, etc., have more consistent fluorescence intensity.
(4) Help determine whether gemstones have undergone artificial enhancement
Optimized gemstones sometimes have different fluorescent characteristics than natural gemstones. For example, the glue layer of some split stones will fluoresce, the filling of oil and glass – filled gems may fluoresce, silver nitrate – treated black pearls do not fluoresce, and some natural black pearls can fluoresce.
B – grade jadeite sometimes emits a strong fluorescence (Figure 2 – 29) . Natural jadeite may also produce localized fluorescence, while treated B – grade jadeite or B + C grade jadeite can produce uniform overall fluorescence. If it is eroded by strong acid and then dyed with resin, the dye may cover the fluorescence, making it invisible. Other methods should be used in conjunction during detection for comprehensive judgment.

4. Notes on Fluorescence Observation
Observing the fluorescence of gemstones is very convenient, and the color and intensity of the fluorescence can help determine the type of gemstone and whether it has been treated. During the observation process, the following points should be noted:
(1) Short – wave ultraviolet light can cause harm to the eyes and skin, and in severe cases, it can lead to blindness. Directly looking at fluorescent light tubes should be avoided. Additionally, do not place your hands under short – wave ultraviolet light; using tweezers instead of hands is best to prevent burns.
(2) The fluorescence reaction of gemstones serves only as an auxiliary identification evidence. If a sample glows locally, especially in jade composed of multiple minerals, the fluorescence may originate from one of those minerals. For example, calcite in lapis lazuli exhibits fluorescence; sometimes, it is due to oil or wax on the surface of the gemstone, so the sample should be cleaned and retested
(3) When assessing the fluorescence of gemstones, the transparency of the sample should be considered, as there are differences in fluorescence between transparent and opaque samples.
(4) The fluorescence color of a gemstone may differ from the color of the gemstone itself, and there may be significant differences in fluorescence among different samples of the same type of gemstone.
(5) When observing fluorescence, the gemstone should be placed in a dark environment; a black background is beneficial for observing the fluorescence of the gemstone.
5. Characteristics of some gemstones under long - wave ultraviolet light
(1) Diamond
High – quality colorless diamonds often exhibit a blue hue when observed in natural light. Due to different impurities, diamonds can display fluorescence in pink, blue – white, yellow, green, orange, and other colors.
Diamonds with a yellow – brown color mostly have weak fluorescence, with murky colors or no fluorescence at all. High temperature and high – pressure treated Novo diamonds have strong yellow – green fluorescence, and some diamond composite stones also emit different fluorescence than natural diamonds.
(2) Emerald
The emerald exhibits different optical characteristics due to its varying origins. Colombian emeralds with inclusions often display a dark red fluorescence, while those with fewer inclusions tend to show a bright red fluorescence; some emeralds from other origins may not exhibit fluorescence or have very weak fluorescence.
Synthetic emeralds generally exhibit a strong, bright red fluorescence. The fluorescence of synthetic emeralds is usually stronger than that of natural emeralds. Most oil – filled emeralds show strong fluorescence under long – wave light, and the fluorescence intensity depends on the filling oil’s nature; some may have weak or no fluorescence.
(3) Ruby
Natural rubies typically exhibit bright red fluorescence under long – wave ultraviolet light, and their optical characteristics may vary slightly based on quality and color; lower – quality or lighter – colored rubies may show weaker fluorescence. Synthetic rubies display a more vivid red fluorescence; dyed rubies, colorless oil – filled, or colored oil – filled rubies may also exhibit different fluorescence phenomena
(4) Sapphire
Most natural sapphires do not exhibit asterism, but yellow, light – colored, and nearly colorless sapphires from Sri Lanka can show orange, pink, and dark red asterism.
Synthetic sapphires and pink, orange, violet, and color – changing sapphires exhibit red asterism, nickel – colored synthetic yellow sapphires generally do not fluoresce, and blue synthetic sapphires have no asterism.
6. Characteristics of some gemstones under short - wave ultraviolet light
(1) Corundum gemstones
Natural rubies exhibit a dark red fluorescence under short – wave ultraviolet light, while synthetic rubies show a bright red fluorescence; natural sapphires generally do not fluoresce, whereas synthetic sapphires typically exhibit a milky white fluorescence; heat – treated natural sapphires display a milky white fluorescence, and dyed rubies show a bright red fluorescence under short – wave ultraviolet light.
(2) Diamond
Natural diamonds show no fluorescence or exhibit weak red fluorescence under short – wave ultraviolet light; synthetic diamonds produce different fluorescence effects under short – wave ultraviolet light, depending on their color.
(3) Imperial Topaz
Imperial topaz displays a murky yellow – green or blue – white fluorescence under short – wave ultraviolet light.
(4) Zircon
Colorless natural zircon exhibits a cloudy light yellow fluorescence under short – wave ultraviolet light, while brown zircon shows a strong turbid yellow fluorescence. The “white zircon” and other mid – range gemstones available in the market are all artificially synthesized cubic zirconia, which do not possess the same optical properties, making it easy to distinguish zircon from diamonds using these characteristics.
Section VIII Chelsea Color Filter
The filter is commonly used to detect certain gemstones that exhibit different colors due to special selective absorption. It can detect certain green, blue, and dyed gemstones and serve as an auxiliary instrument for identification. The Chelsea filter consists of two gel filter plates that only allow deep red and yellow – green light to pass through (Figure 2 – 30) . When incident light reflects off the gemstone onto the filter plates, a small amount of green light can pass through when the wavelength is 560nm. At the same time, a large amount of near – infrared light passes through when the wavelength is 700nm, and light in other wavelength ranges is absorbed and filtered out by the filter plates.

In transparent gemstones, most gems colored by chromium ions appear in bright red and green. When detecting emeralds, most naturally produced emeralds appear red under a Chelsea filter; if the original gemstone has good color, it shows a beautiful ruby – like color under the filter; if the original gemstone is light in color, it appears light red. Synthetic emeralds show a deep red or bright red under the Chelsea filter. The Chelsea filter is very effective in detecting green, blue, and red gemstones, and it is especially successful in identifying emeralds, sapphires, jade, spinels, and Burmese rubies. When using the Chelsea filter for inspection, the eyes and the filter should be as close as possible to avoid interference from external light.
1. How to Use the Chelsea Filter
(1) Clean the sample.
(2) Place the sample on a blackboard (non – reflective or not affecting the observation background) .
(3) Place the sample in a well – lit area or under strong incandescent light, allowing light to reflect off the surface of the tested gemstone sample.
(4) Hold the color filter as close to the eyes as possible, observing from about 30cm away from the sample.
2. Application of Chelsea Color Filter
In the 1990s, as people’s love for jadeite grew in China, imitation natural high – quality colored jadeite entered the market. Most dyed jadeite is colored with chromium salts, and due to the presence of chromium ions inside the gemstone, it appears red under the Chelsea color filter. This characteristic can be used to distinguish it from natural jadeite. Therefore, the Chelsea color filter is sometimes called the jadeite color filter. It is emphasized that not all dyed jadeite appears red under the color filter; jadeite dyed with nickel salts does not change color under the Chelsea filter.
The Chelsea color filter mainly identifies green and blue gemstones and certain dyed gemstones. Jadeite, opal, green tourmaline, aquamarine, natural blue spinel (Fe – colored) , sapphire, blue topaz, and certain emeralds generally do not change color under the filter. Some emeralds, demantoid, chrome vanadium grossular, hydrogrossular, lapis lazuli, and aventurine change to red under the filter. Green or blue gemstones treated with chromium salts turn red under the filter.
3. Precautions for Using Chelsea Color Filters
Color filters are small in size, easy to carry, and can distinguish certain natural green and blue gemstones and dyed gemstones. The following points should be noted when using them:
(1) Choose an appropriate light source for observation; weak flashlights and incandescent lamps are unsuitable, and direct sunlight is also ineffective.
(2) The depth of color observed through the color filter depends on the sample’s size, shape, transparency, and inherent color.
(3) Due to differences in the dyes’ type and content, each sample’s reaction may vary.
(4) The color filter identification is only an auxiliary means and needs to be combined with other identification results for judgment.
Section IX Application of Large Instruments in the Identification of Gemstone Optimization Treatment
With the development of modern science and technology, new optimization treatment methods and varieties of gemstones are constantly emerging. Some gemstones that have undergone optimization treatments have surface and internal characteristics very similar to natural ones, leading to challenges in identification and making it difficult for conventional gemstone identification instruments to distinguish them. In recent years, introducing and applying some large analytical instruments have solved many problems that cannot be identified with conventional instruments. Therefore, large instruments are playing an increasingly important role in the identification of optimized gemstones.
1. Fourier Transform Infrared Spectroscopy
An infrared spectrometer typically consists of a light source, monochromator, detector, and computer information processing system (Figure 2 – 31) . Depending on the type of spectroscopic device, it can be classified as dispersive or interferometric. For a dispersive dual – beam optical zero – balance infrared spectrophotometer, when the sample absorbs infrared radiation at a certain frequency, the vibrational energy levels of the molecules undergo transitions, resulting in a reduction of the corresponding frequency of light in the transmitted beam. This creates a difference in intensity between the reference beam and the sample beam, allowing for the measurement of the infrared spectrum of the sample.

Infrared spectroscopy can be used to study the structure of molecules and chemical bonds, and it can also serve as a method for characterizing and identifying chemical species. Infrared spectroscopy, abbreviated as FTIR, has a high degree of specificity and can be analyzed and identified by comparing it with the infrared spectra of standard compounds. Several collections of standard infrared spectra have been published, and these spectra can be stored in a computer for comparison and retrieval for analysis and identification.
(1) Basic Principles
The infrared light at 4000 – 400cm – 1 causes molecules to undergo transitions in vibrational and rotational energy levels during the vibrational and rotational processes; when the molecular vibration changes with the dipole moment, the charge distribution within the molecule changes, generating an alternating electric field. Infrared absorption occurs only when the frequency of this field matches the frequency of the incident electromagnetic radiation. Therefore, there are two conditions for generating infrared spectra: the radiation must have enough energy to induce vibrational transitions in the substance, and the molecule must have a dipole moment.
Infrared spectral lines are divided into three categories based on wave – number: far infrared, 50 – 400cm – 1; mid – infrared, 400 – 4000cm – 1; near – infrared, 4000 – 7500cm – 1. The absorption spectrum of minerals refers to the different frequencies of infrared light irradiating the mineral, resulting in different transmission ratios. The vertical axis represents transmittance, and the horizontal axis represents frequency. This forms a curve representing the mineral’s changes, which is called the infrared absorption spectrum of that mineral. Qualitative and quantitative analysis of substances can be performed based on the absorption bands of ionic groups in the infrared range.
(2) Testing Methods
The gem infrared spectroscopy testing methods are divided into transmission and reflection methods.
① The transmission method (powder tablet method) is a destructive identification method, mainly studying water, organic matter, and impurities in gemstone minerals. The preparation method is the potassium bromide (KBr) tablet method, so to reduce the impact on the measurement, KBr should preferably be of optical reagent grade or at least analytical grade. It should be appropriately ground (below 200 mesh) before use and placed in a desiccator for at least 4 hours after drying at 120℃ or above. If clumping is found, it should be dried again. The prepared empty KBr tablet should be transparent, and the transmittance should be above 75%. The sample taken for the tablet method is generally 1 – 2mg, and the KBr used is around 200 mg.
② The reflection method is currently the most commonly used method in identifying optimized gemstone treatment. Based on the infrared reflection spectral characteristics of transparent or opaque gemstones, it helps in the identification of filling treatment materials, dyes, and other organic polymer materials, making it an accurate and non – destructive identification method.
(3) Application in gemology research
The infrared spectral characteristics depend on the material composition and structure of the gemstone; no two gemstones have completely identical infrared spectra. Infrared spectral analysis does not damage the sample, the instrument operation is simple, the response is sensitive, and the testing structure is accurate. The infrared spectral characteristics of gemstones can determine the type of gemstone, whether it is synthetic or optimized.
① Distinguishing natural gemstones from synthetic gemstones: Natural and synthetic gemstones are the same in composition and physicochemical properties. Still, different changes occur in the structure due to differences in growth environments. For example, natural amethyst and synthetic amethyst, apart from differences in color, transparency, and internal inclusions, also have different infrared spectra; the infrared spectrum of synthetic amethyst has an absorption peak at 3450cm – 1, while natural amethyst does not have this absorption peak (Figure 2 – 32) .
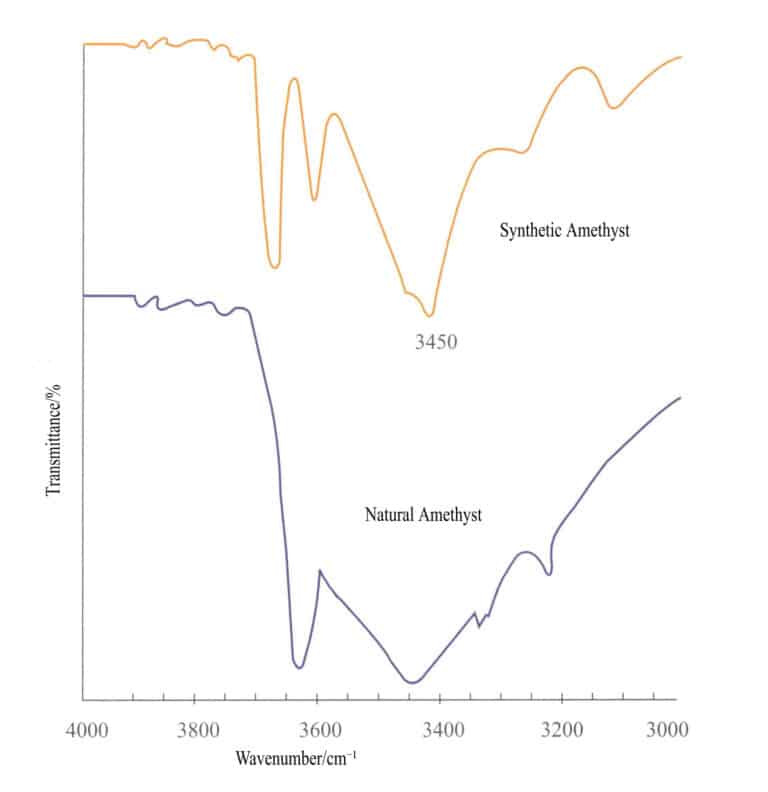
② The identification method of artificial filling treatment has two or more epoxy groups, uses aliphatic, alicyclic, or aromatic functional groups as the skeleton, and reacts with a curing agent to generate a three – dimensional network structure of polymer epoxy resin, mostly in the form of filling, widely used in artificial filling treatment of jade, turquoise and emerald and other precious jade. Many kinds of epoxy resins exist, and new varieties are still emerging. Common varieties are epoxidized polyolefin, peracetic acid epoxy resin, epoxy olefin polymer, epichlorohydrin resin, bisphenol A resin, epichlorohydrin – bisphenol A condensation polymer, bisepichlorohydrin resin and so on.
By obtaining the molecular vibrations of substances, FTIR can effectively analyze water molecules, hydroxyl groups, resins, or oils in crystals. For example, testing the filled emeralds using a Fourier transform infrared spectrometer is generally done by reflection method, placing the gem’s table face down on the sample stage, with light entering from the pavilion of the gem, passing through the entire gem, reflecting off the mirror, and then passing through the gem again to the detector. When inspecting the sample, the gem should be rotated on the mirror 360°, as the resin or oil filling in the cracks occupies only a small part of the gem, and the light produced must penetrate the filled area.
A Fourier transform infrared spectrometer can distinguish between natural jadeite and filled jadeite. Natural jadeite exhibits very broad absorption peaks, while the spectrum of filled jadeite shows distinct infrared absorption peaks of resin in a very narrow band (3200~ 2800cm – 1) (see figure 2-33).
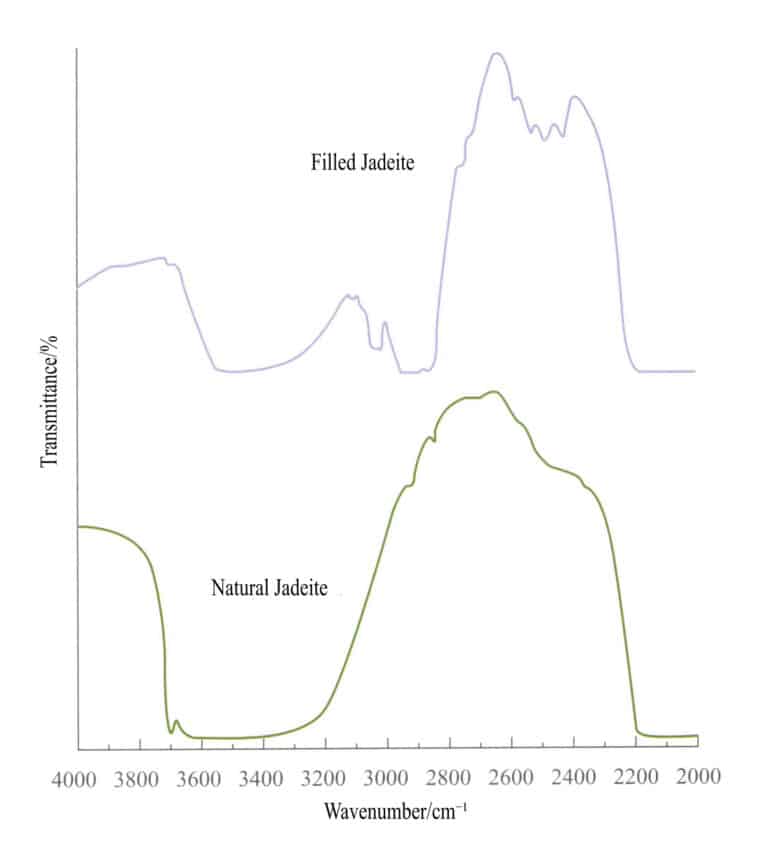
2. Raman Spectroscopy Analysis
(1) Basic Principles
Raman spectroscopy is a type of scattering spectroscopy. The Raman spectroscopy analysis method is based on the Raman scattering effect discovered by Indian scientist C.V. Raman, analyzing the scattered light spectrum that differs in frequency from the incident light to obtain information about molecular vibrations and rotations, and is used as an analytical method for molecular structure research. By analyzing the Raman spectrum, we can know the substance’s vibration and rotational energy level to identify the substance and analyze its nature. Raman spectroscopy has the advantages of non – destructive, extremely fast detection speed and low cost. It is also sensitive to highly symmetric covalent bonds with little or no natural dipole motion. Figure 2 – 34 shows the Raman spectrometer’s basic structure.
Raman spectroscopy can identify gemstone chemical properties and origins by comparing the Raman spectral IDs from different sources. The Raman spectrometer produces precise and unique spectral data for all types of borates, carbonates, halides, native elements, oxides, phosphates, silicates, sulfates, and sulfides.

(2) Applications of Raman spectroscopy in gemology
① It can be used to distinguish diamonds from their imitations, such as from moissanite and quartz, as different gemstones have different Raman spectral characteristics. Diamonds have a single C—C Raman shift at 1332cm – 1; the strongest Raman peak of moissanite is at 788cm – 1, followed by a characteristic peak at 965cm – 1, 766cm – 1; quartz’s main Raman feature peak is the absorption peak at 475cm – 1. The differences in Raman spectra between diamonds, moissanite, and quartz are shown in Figure 2 – 35.
② Imitations of natural oriental jasper. There is an essential difference between the Raman spectra of natural oriental jasper and imitated oriental jasper: the former is mainly the Raman spectrum of dickite and cinnabar. At the same time, the latter is mainly the Raman spectrum of organic materials, which can be distinguished using Raman spectroscopy. The main component of natural oriental jasper “earth” is dickite, and the sample of natural oriental jasper “blood” contains both cinnabar and dickite, essentially a composite of cinnabar and dickite. The main component of imitated oriental jasper “earth” is polystyrene – acrylonitrile, and “blood” is a red organic dye.
(3) Application in the identification of gemstone optimization treatments
① Raman spectroscopy can identify gemstones treated with fillers, such as jadeite treated with synthetic resin, emeralds, turquoise, rubies, and diamonds treated with lead glass. The various filling materials in gemstone cracks pose certain challenges for gem identification, and using Raman spectroscopy analysis testing technology helps accurately identify the types of fillers.
- Identification of filled rubies Low – temperature filling is generally applied to rubies with cracks reaching the surface, and it involves low – melting – point substances. If it is glue or wax, Raman spectroscopy analysis can be used, and the organic components can be observed showing C—H bond stretching vibration absorption peaks at 2800 – 3000cm – 1. (Figure 2 – 36) .
- Identification of filled emeralds. Raman spectroscopy can distinguish between natural emeralds and filled emeralds. Natural emeralds exhibit very broad absorption peaks, while the spectra of filled emeralds show significant infrared absorption peaks of resin and oil in a very narrow wavelength range ( 3200 – 2400cm – 1) (Figure 2 – 37) .

② Distinction between natural red coral and dyed coral. The Raman spectral peaks of natural red coral are 1129cm – 1 and 1517cm – 1, while dyed red coral has a single high – intensity spectral peak at 1089cm – 1 (Figure 2 – 38) , showing significant differences in their Raman spectra.
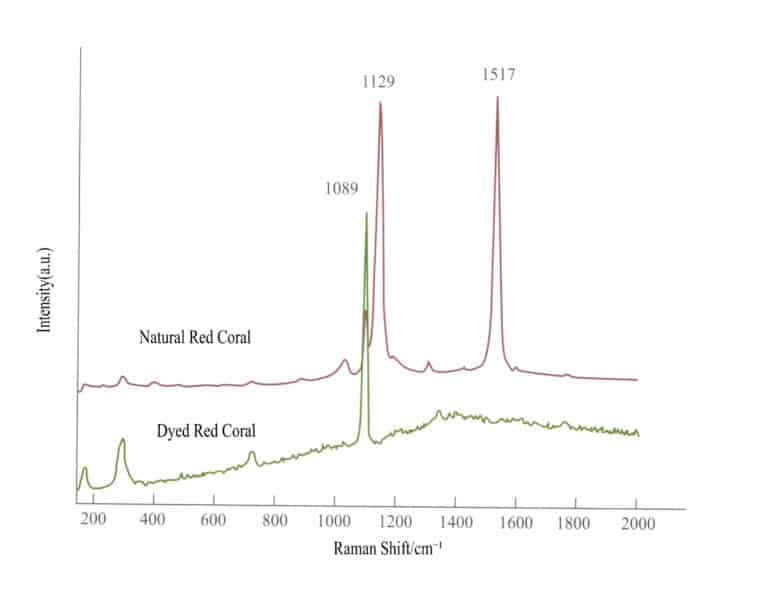
3. Ultraviolet - Visible Spectrophotometric Analysis
(1) Basic Principles
The ultraviolet – visible absorption spectrum is a molecular absorption spectrum generated by the transitions of valence electrons and electrons in molecular orbitals of atoms, ions, and molecules in gemstones under electromagnetic radiation. Various colored gemstones with different crystal structures have color – causing impurity ions that selectively absorb incident light of different wavelengths to varying degrees, resulting in different absorption spectral lines. Based on the wavelength region of the absorbed light, ultraviolet – visible spectrophotometry is divided into ultraviolet and visible spectrophotometry.
In gemstone crystals, electrons exist in different states and are distributed across different energy level groups. Suppose the energy difference between the ground state and the excited state of an impurity ion in the crystal exactly equals the energy of the monochromatic light passing through the crystal. In that case, the crystal will absorb that wavelength of monochromatic light, causing an electron in the ground state to transition to the excited state energy level, resulting in an absorption band in the crystal’s absorption spectrum, thus forming the ultraviolet – visible absorption spectrum.
(2) Testing Methods
Testing methods for gemstones can be divided into two categories: direct transmission method and reflection method.
① Direct Transmission Method
Place the polished surface or ring face of the gemstone sample (allowing the light beam to pass through the side of the ring’s waist) directly on the sample stage to obtain the ultraviolet – visible absorption spectrum of natural gemstones or certain artificially treated gemstones. Although the direct transmission method is a non – destructive testing method, the information obtained about the gemstones is quite limited, especially when dealing with opaque gemstones or jewelry with bottom inlays, making it difficult to measure their absorption spectrum. This limits the further application of the ultraviolet – visible absorption spectrum.
② Reflection Method
Utilizing the reflection device of the ultraviolet – visible spectrophotometer (such as mirror reflection and integrating sphere devices) helps to address the issues encountered during testing with the direct transmission method, thereby expanding the application range of the ultraviolet – visible absorption spectrum.
(3) Application in Optimizing Gemstone Detection
① Distinguishing natural diamonds from irradiated diamonds
It is possible to effectively distinguish natural blue diamonds from artificially irradiated blue diamonds using ultraviolet – visible absorption spectroscopy. The color of natural blue diamonds is caused by impurity B atoms, characterized by ultraviolet – visible absorption spectra ranging from 540nm to longer wavelengths, with an increasing absorption rate in the visible absorption spectrum. Irradiated blue diamonds exhibit a characteristic GR1 (741nm) color center (Figure 2 – 39) .
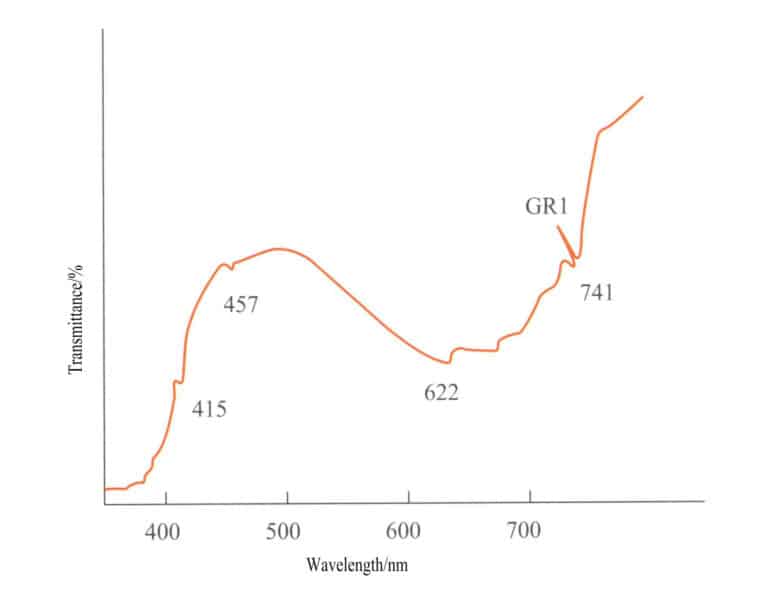
② Distinguishing natural yellow sapphires, heat – treated yellow sapphires, and irradiated yellow sapphires
Ultraviolet – visible absorption spectroscopy can also effectively distinguish natural yellow sapphires, heat – treated yellow sapphires, and irradiated yellow sapphires. The color mechanism of natural yellow sapphires is due to the electronic transitions of trivalent iron ions, with absorption bands in the ultraviolet – visible light at 375nm, 387nm, and 450nm; the heat – treated yellow sapphires show almost no absorption in these three bands; the irradiated yellow sapphires have very weak absorption at 387nm and 450nm, as the color mechanism of these sapphires is mainly due to color centers (Figure 2 – 40) .
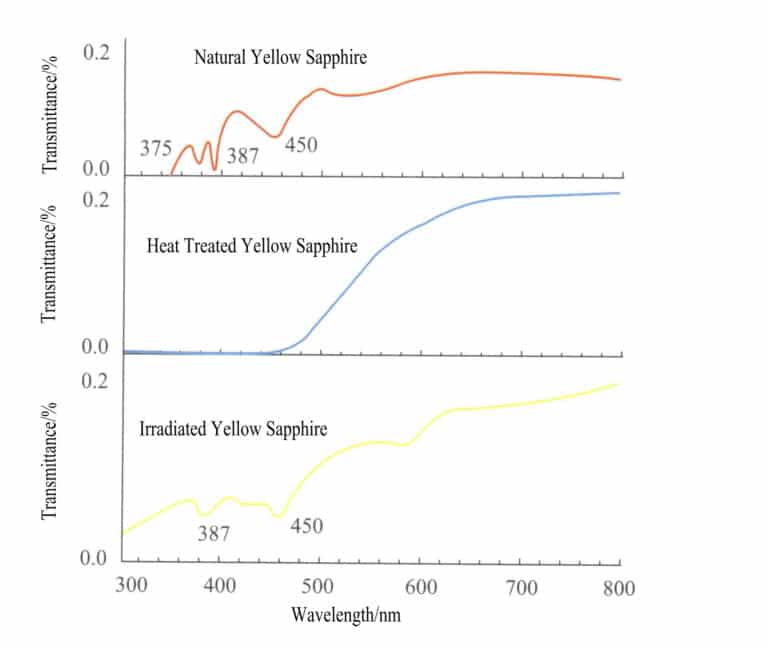
With the development of science and technology, the methods and techniques for optimizing gemstones are also increasing daily. It has become difficult to distinguish between optimized and natural gemstones using conventional identification methods. New methods and techniques for gemstone optimization continue to emerge and update, and for some optimization methods that cannot be distinguished by conventional instruments, large – scale instrument testing can be used to determine them. Therefore, large – scale instrument testing plays a very important role in gemstone identification. These common instruments can only provide preliminary observation and identification of gemstones. Large – scale instruments often provide us with more detailed information and data, helping us observe and understand gemstones more deeply and accurately.