The Ultimate Guide to Optimized Jade Stones for Jewelers
8 Common Optimization Treatments and Identification Methods for Jade Stones
Pendahuluan:
This article delves into the world of jade and gemstone enhancements, detailing treatments like dyeing, filling, and heat treatment. It distinguishes between A, B, and C grade jadeite, revealing how to authenticate their quality. Key takeaways include identification methods for natural versus treated stones, ensuring jewelry professionals source the highest quality materials for their creations.

Main Varieties of Quartz jade
Daftar Isi
Section I Jade
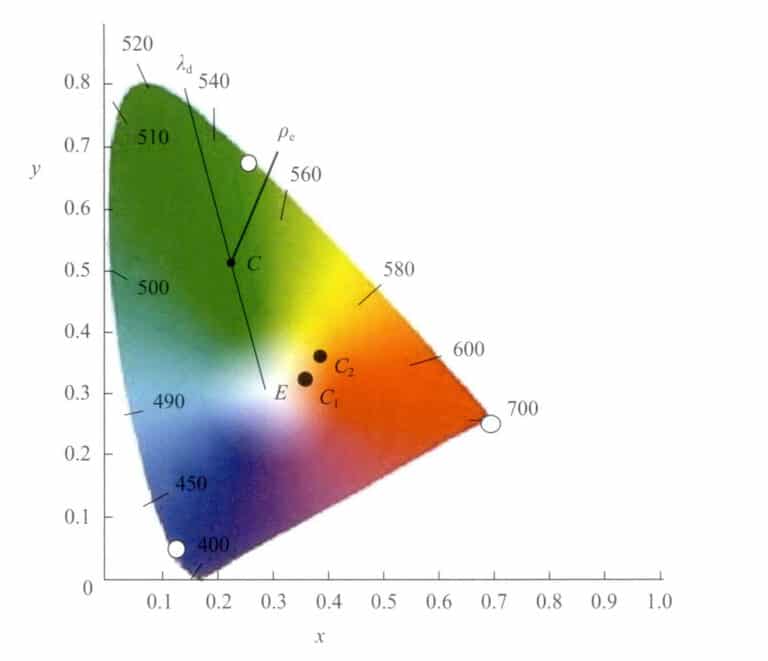
1. Gemological Characteristics and Classification of Jade
Jadeite mainly consists of jadeite or jadeite along with sodic (sodium chromium pyroxene) and sodic-calcic pyroxene (omphacite) and may contain amphibole, feldspar, chromite, limonite, etc. The chemical composition is NaAlSi2O6. Natural jadeite comes in various colors, such as green, purple, red, yellow, black, and white. Gem-quality jadeite is mostly semi-transparent to transparent, exhibiting a glassy luster after polishing, and can be completely clean (glass type) or contain inclusions such as white fibrous, white granular, and yellow-gray impurities. The finest jadeite is characterized by its pure, uniform, vibrant emerald green color and its delicate, warm, and transparent texture. The value of top-grade jadeite is comparable to that of emeralds of the same quality. Jadeite has a dense structure, often appearing as a microcrystalline or fibrous aggregate. Polarized light microscopy shows a granular mosaic or granitic metamorphic structure, and scanning electron microscopy presents a unique felt-like structure.
An A, B, and C grade jadeite are the common names for jadeite in the market. Grade jadeite refers to natural jadeite, B grade jadeite refers to jadeite that has undergone resin treatment, and C grade jadeite refers to dyed jadeite. The characteristics and differences among the three types of jadeite are as follows:
(1) A-grade jadeite
Grade A jadeite refers to natural jadeite. During the processing and polishing stages, cleaning or polishing with strong alkaline solutions and waxing after shaping are all permitted. A-grade jadeite’s color and transparency are natural and remain unchanged over time. The observable characteristics of A-grade jadeite are:
① Color:
The color of natural jadeite follows the direction of the texture, with the colored parts transitioning naturally to the colorless parts. The color has a beginning and an end, with a color root that is deep and not empty.
② Luster
The polished surface of jadeite has a glassy or sub-glassy luster, with a higher refractive index of 1. 66. High-quality jadeite, such as “a pool of autumn water, ” has bright colors, a delicate structure, and a transparent and dense texture.
③ Hardness
6. 5 to 7 is higher than other gemstones, and density is high, at 3. 34g/cm3.
④ No abnormalities on the surface:
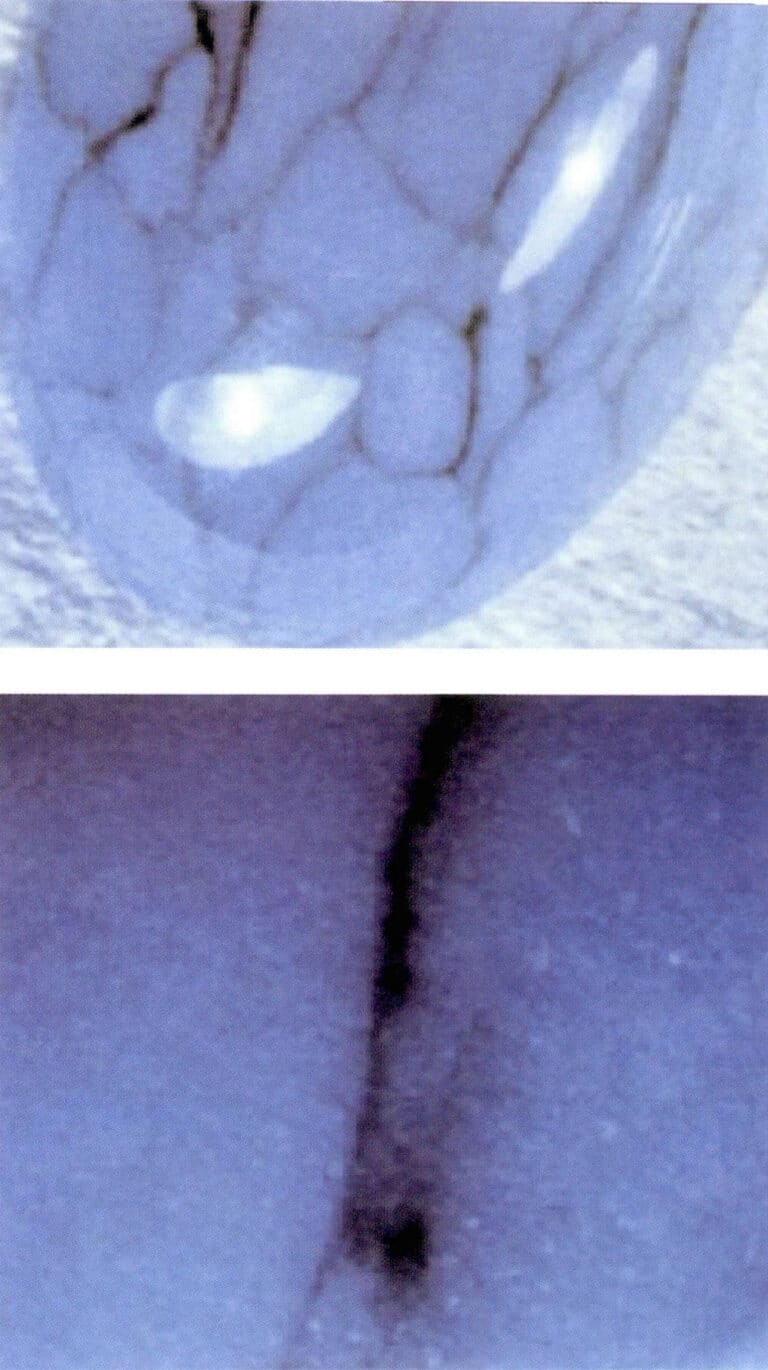
Although there are some rough and uneven patches or depressions on the surface, the areas that are not depressed are relatively smooth, with no pitting, network structure, or filling phenomena (Figure 6-1).

(2) B-grade jade
B-grade jade is natural jade artificially bleached and filled with resin after treatment. The color of B-grade jade is the original color of natural A-grade jade, but the base has been bleached, and the transparency has also been artificially treated. After treatment, the transparency of B-grade jade is unstable, and its structure may change accordingly, making the gemstone prone to cracking over time. The structural characteristics of B-grade jade are shown in Figure 6-2.

(3) C-grade jade
C-grade jade is a general term for dyed jade; as long as the color of the jade is artificially added, it is referred to as C-grade jade. C-grade jade is prone to fading. The production history of C-grade jade is long and frequently updated, with “new products” constantly emerging. C-grade jade has bright colors, and upon magnification, one can see that the structure is loose or has deeper colors in the fissures, while the dense areas appear lighter. Different dyes can achieve various colors, as Figure 6-3 shows.

2. Optimization treatment and identification methods of jadeite
2.1 Methods and steps for heat treatment of red jadeite and identification
When natural red jadeite undergoes heat treatment, its color will change, resulting in varying degrees of improvement. There is not much red jadeite in nature, and heat treatment methods are needed to obtain better red jadeite. The heat treatment of jadeite is also known as firing. The heating promotes oxidation, transforming yellow, brown, and dark brown jadeite into vibrant red. Since this improvement method does not involve adding other materials, it is referred to as optimization and can be directly named jadeite.
(1) Steps for heat treating jadeite
Choose lighter jadeite raw materials, process them into the required shape through rough grinding, and set aside for treatment.
① Material Selection:
Only jadeite raw materials with coloring ions of iron can be changed to red. Jadeite containing iron ions can oxidize trace amounts of Fe2+ into Fe3+ under oxidative conditions, making the red color of jadeite more vivid. Generally, yellow, brown, and dark brown raw materials are selected. If the jadeite raw material does not contain iron ions, there will be no color change after heat treatment.
② Cleaning:
Clean the jadeite to be treated with dilute acid to remove the brown tones and other mixed colors from the jadeite.
③ Treatment:
Place the jadeite in a furnace for heat treatment. Gradually increase the temperature, and when the color changes to liver color, begin to slowly decrease the temperature. After cooling, the jadeite will show varying degrees of red. The time and temperature of the operation should be specifically adjusted to the different qualities of jadeite to achieve the desired color. The best heat treatment plan for red jadeite is generally in an oxidative atmosphere, with the highest temperature around 350℃ and isothermal treatment at 8-10h. Generally speaking, the smaller the sample size and the finer the texture, the lower the optimal isothermal temperature, so the experimental conditions should be adjusted according to the actual situation of the jadeite.
④ Post-treatment:
To achieve a more vivid red color, the jadeite can be further soaked in bleaching water for several hours for chlorination to enhance its brightness.
(2) Identification of heat-treated jadeite
Heat-treated jadeite is quite similar to natural jadeite. The similarity between natural jadeite and heat-treated jadeite lies in the same coloring principle; the red color in jadeite is caused by hematite in the gemstone, which is formed from the dehydration of limonite. The color of heat-treated jadeite is generally more vibrant.
The difference is that natural red jadeite is formed slowly under natural conditions, while heat-treated red jadeite is formed rapidly under heating conditions. Generally, there is no need to distinguish between them; they are directly named jadeite.
2.2 Production and identification of C-grade jadeite
The production history of C-grade jadeite is very long, and various dyeing agents can be used to dye colorless or light-colored jadeite into various colors. The dyeing method is simple, but the color is unstable and will gradually fade.
(1) The production steps of C-grade jadeite
① Select raw materials, choosing colorless or lightly colored jadeite raw materials, and ensure they have a certain porosity; those with particularly dense structures cannot be dyed. Roughly grind the jadeite into shape.
② Clean the jadeite to be dyed in an acidic solution to remove any unwanted color tones.
③ After drying, place it in a solution of dye or pigment; heating can accelerate the solution’s penetration into the jadeite’s pores. The soaking time depends on the quality of the jadeite; the denser the structure, the longer the soaking time. To ensure the color fully enters the jadeite’s pores, it should soak for at least 1 to 2 weeks.
④ Wax immersion: After soaking the partially colored jadeite and drying it, wax is applied to make the color distribution softer.
Dyed and colored green jade is sold as C-grade goods. The method for dyeing purple jade is similar, but the dye is changed to purple.
(2) Identification of C-grade jade
① Visual identification:
The color is bright, with high saturation, exaggerated, and unnatural tones.
② Magnified observation:
The color is attached to the surface of the jadeite mineral, with a thick surface color, which is noticeably deepened or accumulated in the fissures. The color often appears in a network-like clump distribution in the micro-gaps of the jade, with no color roots (Figure 6-4). It becomes clearer if soaked in water or in oil for observation.

③ Fading:
The color stability is poor; over time, it will fade or fade when hydrochloric acid drips on it.
④ Viewing through a color filter:
The color observed through a color filter appears dark brownish-red to brownish-pink. If there is no color change under the color filter, it does not necessarily mean it is A-grade jade; it could be B-grade or C-grade jade dyed using new methods.
⑤ Ultraviolet fluorescence reaction:
Natural jade does not exhibit or has very weak fluorescence under ultraviolet light, while dyed jade shows stronger fluorescence under ultraviolet light. Purple-dyed jade exhibits strong orange fluorescence under long-wave ultraviolet light.
⑥ Absorption spectrum:
There is a significant difference between the absorption spectra of C-grade green jadeite and natural green jadeite. The absorption spectrum of natural green jade has three step-like absorption lines in the red-light region at 630nm、 660nm、 690nm, and absorption lines in the violet region. Among the absorption lines in the absorption spectrum of natural green jade, the 437nm absorption line has diagnostic significance and can be used as a distinguishing feature. Dyed jade has a vague absorption band in the red spectrum region at 650nm, which is the absorption band of the dye (Figure 6-5).

The purple jade can be identified based on magnified observation and fluorescence response, and infrared spectroscopy can also provide identification evidence for various C- grade jade colors.
The color of natural jade is the color of the mineral itself, which is relatively stable. In contrast, dyeing involves artificially mixing dye into the tiny fissures of the crystals, which will fade over time and have poorer stability.
2.3 Production and identification of B-grade jade
(1) Steps for producing B-grade jade
① Material Selection:
Choose varieties that are originally green but have a yellow, gray, or brown base, with a structure that is not too dense, large, coarser granules, poor transparency, and inexpensive jade raw materials.
② Rough Processing:
Grind the raw materials of jade into bad pieces for bracelets or pendants, performing preliminary processing without polishing.
③ Acid Washing to Remove Yellow:
The acid washing is the most critical step in making B-grade jade. The selected samples are cleaned with strong acid and then soaked in a new acid solution for 2-3 weeks until the yellow color is mostly removed.
After removing yellow, the color of the jade is relatively bright, with green being prominent and the base color noticeably turning white. However, the transparency is poor, presenting a dry and cracked appearance, some resembling a chalk-like texture.
④ Alkaline Washing and Neutralization:
After taking out the samples soaked to remove yellow, they are placed in a weakly alkaline salt solution (such as a saturated solution of sodium carbonate) for soaking and cleaning for 1-2 days, neutralizing the acid solution from the yellow removal process, and then rinsed with clean water. Alkaline washing increases the internal voids of the raw jade materials, facilitating resin injection.
⑤ Drying:
Place the samples rinsed with clean water into the drying oven, and the drying temperature should not exceed 200℃.
⑥ Filling:
The jadeite that has undergone de-yellowing treatment has had its microstructure damaged. A hardening agent, generally epoxy resin, is used to restore strength for filling.
The method and steps for filling are as follows: immerse the sample in the adhesive, then place it in an oven or microwave for heating. The heating temperature should not exceed 200℃, allowing the resin to evenly penetrate the jadeite microfissures and cure.
⑦ Polishing:
Polish the cured jadeite samples according to their original shape, removing visible surface adhesive, thus completing the production of B-grade jadeite.
(2) Identification of B-grade jadeite
The B-grade jadeite that has undergone bleaching and filling treatment appears bright in color, clean, and impurities-free. Compared to natural jadeite, it has the following identification characteristics:
① The color, luster, and structure of the gemstone
- Color: A-grade jadeite has stable color, with color roots, and the color transitions naturally in depth; it does not change with the time it is placed. In contrast, B-grade jadeite generally has a brighter color; the base color looks very clean, feels somewhat unnatural, and sometimes does not completely lose its yellow tone, retaining a yellowish hue.
- Luster: Untreated natural A-grade jadeite has a glassy luster, while B-grade jadeite that has been bleached and filled often exhibits a resinous luster (Figure 6-6).

- Structure magnification inspection: A-grade jadeite has a granular mosaic or granitic metamorphic structure with a uniform surface reflection; B-grade jadeite has surface fissures or acid-etched pits, a loose structure, and misalignment between crystals, resulting in structural damage. Underside lighting, the white parts show rough white fibrous features and the surface exhibits uneven structural characteristics (Figure 6-7).

② Low relative density:
The relative density of B-grade jadeite is lower than that of A-grade jadeite, floating in a heavy liquid with a relative density 3. 32. This is because iron oxide in the jadeite structure was removed during acid washing and filled with resin or other adhesives.
③ Ultraviolet long-wave fluorescence test:
B-grade jadeite often exhibits a milky white fluorescence under long-wave light due to the added organic adhesive (such as epoxy resin) being fluorescent, with fluorescence intensity often increasing with the adhesive injected. If the added adhesive is not fluorescent, then B-grade jadeite will not show fluorescence.
④ Microscopic features:
Under a microscope at 30 -40 times magnification, the damaged microstructure of B-grade jadeite can be seen, with a darker luster and lower transparency in the filled areas. When the filling is large, it is also possible to observe adhesives such as resin filling the fissures, which will yellow over time.
⑤ Infrared spectroscopy testing
It can determine whether jadeite contains added components (resin or organic adhesives). Infrared spectroscopy can show the absorption peaks of glue within the 2800 -3000 cm-1 range.
⑥ Special methods:
- Fire burning: Burning the gemstone with fire, the glue contained in B-grade jadeite turns yellow and may even burn to a black char, while natural jadeite shows no reaction to fire.
- Liquid chromatography detection: Using organic solvents to dissolve the glue injected into jadeite, followed by detection with liquid chromatography, can identify the components of the injected glue (organic matter).
2.4 Bleaching and filling of jadeite
Bleaching is widely used in the optimization treatment of jade, aiming to remove surface discoloration and enhance the whiteness of light-colored jadeite. The treatment does not affect the durability of jadeite, is considered optimization, and does not require authentication; it is still in use in the current jade market. Jadeite particles often exhibit black, gray, brown, yellow, and other discolorations due to impurities such as iron and manganese, affecting the aesthetic quality and reducing the value of jadeite. To remove these discolorations, people often use chemical methods to bleach jadeite. The base color of jadeite after bleaching treatment is clean.
Bleaching involves placing jadeite in strong acid, which destroys the original structure of the jadeite. The bleached jadeite is often subjected to filling treatment to stabilize its structure. Filling refers to the solidification treatment of jadeite that has been acid-washed and bleached. During the bleaching process, while removing discolorations, the structure of the jadeite is also damaged, resulting in larger gaps between jadeite particles, some of which may even appear loose and crumbly. Such jadeite cannot be used directly, so it must be filled with organic polymers (such as resin, plastic, or glue) that can solidify, which not only strengthens the structure of the jadeite but also enhances its transparency. Jadeite that has been bleached and then filled is referred to as B-grade jadeite, and most jadeite in the sales market has undergone bleaching and filling treatment.
2.5 Waxing Method and Identification of Jadeite
Waxing is a commonly used process in jadeite processing. The method involves placing the finished jadeite into paraffin wax, allowing the wax to seep into fissures and gaps through heating and soaking, which not only fills the original gaps in the jadeite but also increases its transparency while enhancing the stability of the jadeite. It is a traditional method widely accepted by people. Waxing is an optimization directly named after jadeite and does not require identification.
(1) Purpose of Waxing
Mainly used for natural jadeite with many fissures, waxing can cover the fissures in the jadeite and increase its transparency.
(2) Treatment Method
① First, place the rough-textured, loosely structured jade semi-finished products into boiling water and cook for 5-6 minutes to remove the grease or adsorbed impurities left on the surface and in the fissures during the cutting and grinding process.
② Dry the samples to eliminate air and water between particles and micro-fissures.
③ Place the dried jade in melted wax, slightly heat it, and soak it so that the liquid wax seeps into the fissures and tiny gaps. Then, polishing can increase transparency and cover the original gaps.
④ Remove the excess wax accumulated on the wax-injected samples’ surface.
(3) Durability
This treatment method only temporarily masks the more obvious fissures, increases the ability of light refraction and reflection, and improves transparency. The wax will overflow if exposed to high temperatures, resulting in poor durability.
(4) Identification features
Wax immersion treatment is a common process in jadeite processing. Slight wax immersion does not affect the luster and structure of jadeite and is considered an optimization. However, excessive wax immersion can impact the luster and transparency of jadeite. The main identification features of wax-immersed jadeite are as follows:
① Visual observation: slight wax immersion does not affect the luster and structure of jadeite and is considered an optimization. Severe wax immersion reduces the transparency of jadeite and dulls its luster, presenting a distinct oily or waxy sheen;
② Under ultraviolet light, wax-immersed jadeite exhibits a blue-white fluorescence, with intensity increasing as the amount of wax immersion increases;
③ Hot needle detection, waxy liquid dissolution, and heating severely wax-impregnated jade slowly over an alcohol lamp can cause the wax to ooze out;
④ The infrared absorption peaks of organic matter are significant, with characteristic absorption peaks at 2854cm-1, 2920cm-1.
2.6 Other optimization treatment methods and identification
The main characteristics of jade optimization treatment currently are the transition from single-dyed jade (grade C) to dyed and resin-treated B+C-grade jade, from imitating high-end jade to replicating mid to low-end gray-green and blue-green jade, from uniform overall dyeing to imitating blue flower dyeing, resulting in dyed quartzite resembling glassy, ice seed, oily green, and light blue jade.
Due to certain defects in natural jade, the optimization treatment methods for jade are constantly being updated, and sometimes several methods are combined, leading to some characteristics of optimized jade being closer to natural jade, which brings certain difficulties to jade identification and causes confusion in the market. The summary of the identification of jade treated by different optimization methods is as follows:
(1) B+C-grade jade
Jade has been treated through bleaching, coloring, and resin filling. When identifying a jade, one must consider the characteristics of B-grade jade and C-grade jade, including color, structure, composition, and other aspects of the analysis. Upon magnified inspection, the loose structure of the jade shows that the filling resin is distributed in a filamentous manner, the color is also relatively concentrated, and there are no color roots (Figure 6-8).

(2) “Dressed” jade
Select colorless or light-colored jade with high transparency or jade with a whitish surface, and cover its surface with a green organic film to change or improve the color of the jade.
Identification method:
① Appearance:
Appearance presents a beautiful uniform green with no color roots; the color is distributed on the surface, giving a hazy feeling. The luster is relatively weak, showing resinous luster.
② Magnified inspection:
The inspection shows no internal structure of the jadeite; the surface of the jadeite has a phenomenon of membrane shedding, and bubbles can sometimes be seen (Figure 6-9).
③ Others:
Low refractive index, hardness, wrinkling, and roughness on heated surfaces.

(3) High B-grade jadeite
B-grade jadeite made with nano-level filling materials has a luster and transparency close to natural jadeite. Judging using conventional identification methods is difficult, and large instruments are required to identify the organic components.
(4) Coated jadeite
The coating layer is generally thin and may sometimes peel off, revealing patchy areas. The gloss and hardness of the coating layer are lower than that of jadeite, and the surface may have scratches over time.
(5) Assembled treatment of jadeite
The treatment aims to imitate high-end jadeite varieties to increase their value.
Treatment method: Select fine-textured, transparent jadeite for the top and bottom, apply the green dye in the middle, and assemble them.
Identification features: When not set, check the assembly layer at the waist ridge; observe under magnification the assembly layer has bubbles; the green dye does not have the three-step absorption spectrum lines of natural green jadeite’s red light zone.
2.7 New Technologies and Identification Methods for Jade Optimization
(1) Spray Painting
In recent years, a new surface treatment method for jade has emerged in the market—spray painting treatment. This method is mainly used for small jade carvings, where a layer of colorless transparent varnish is sprayed on the surface of the jade to improve its appearance and enhance its commercial value.
Identification methods:
① Surface characteristics:
The color of spray-painted jadeite is mostly white, gray, lotus pink, brownish yellow, dark green, etc. , generally lacking particularly bright and vivid colors. The paint layer reduces the clarity of the jadeite, making its color lighter and duller and giving a strong sense of distance, presenting an obvious waxy, resinous, and oily luster. The surface of spray-painted jadeite has a strong sense of unevenness, showing an orange peel texture, with obvious bubbles visible inside, mostly in regular round shapes, sometimes in a beaded form; upon magnified inspection, various impurities wrapped in the paint layer can be seen, and the holes in the spray-painted jadeite are not round, with burrs left by the resin sometimes visible in the holes; occasionally, star-shaped shrinkage pits formed during the solidification of the paint layer can be seen.
② Relative density:
The density of treated jadeite is relatively low, below that of natural A-grade jadeite.
③ Others:
In the hot needle test, surface melting phenomena can be observed, accompanied by a distinctively pungent smell; when struck against each other, the sound is abnormally dull; there is a warm and smooth feeling when touched by hand; scratching the surface with a fingernail can leave marks.
(2) Color Pasting
The so-called “color pasting” refers to attaching small pieces of green or yellow jade to certain areas of light-colored jade, creating smart colors. It is commonly used for local jade treatment. The “pasted color” part blends seamlessly with the jade, making it difficult to identify with the naked eye.
Identification features of color-pasted jade:
① Magnified observation:
It reveals that the green areas have residual circular bubbles (Figure 6-10), which are caused by air being trapped by the adhesive used during the bonding of the jade. The green color patches, distributed in a vein-like pattern, show no gradient transition with the light green body color, and the boundary is distinct (Figure 6-11).

Figure 6-10 Bubbles in Colored Jade
Figure 6-11 Boundary of the Colored Part in Colored Jade
② Observation under long-wave ultraviolet light:
It shows that the main body of the sample has no fluorescence. Still, the area around the “pasted color” displays strong blue-white fluorescence (Figure 6-12) caused by the organic materials used during the pasting process.

Section II Nephrite
The main mineral composition of nephrite is tremolite, which belongs to the amphibole group, specifically the series of tremolite and actinolite, along with trace amounts of diopside, chlorite, serpentine, calcite, graphite, and magnetite as associated minerals. The mineral particles are fine, exhibiting felt-like interwoven and microcrystalline structures. Upon magnification, the felt-like structure and black solid inclusions can be observed. Nephrite has a dense and fine texture, and the interweaving of fine fibers enhances the bonding ability between particles, resulting in good toughness and resistance to fracture, especially in pebbles formed through weathering and transportation.
1. Gemological Characteristics and Classification of Nephrite
The chemical formula of the main mineral component of nephrite, tremolite, is Ca2(Mg, Fe)5(Si)4O11)2(OH)2. In most cases, nephrite is usually an intermediate product of tremolite and actinolite, the two end-member components. According to the naming scheme of the amphibole group by Nick (B. E. Leake), the classification of tremolite and actinolite is based on the different proportions of Mg2+ and Fe2+ in the unit cell: 0. 5≤Mg2+ / (Mg2+ + Fe2+) < 0. 9 is actinolite, and 0. 9≤Mg2+/(Mg2+ + F2+) ≤ 1 is tremolite.
The color of nephrite depends on the color of the minerals that compose it. Iron-free tremolite appears white or light gray while iron-containing tremolite appears green. As iron substitutes for magnesium in the tremolite molecule, nephrite can exhibit varying shades of green; the higher the iron content, the deeper the green.
The mineral composition of nephrite varies, and so does its color. Generally, it can be white, grayish-white, yellow, yellow-green, gray-green, dark green, ink green, black, etc. Actinolite is green, yellow-green, and dark green. Graphite and magnetite are black.
The raw materials for nephrite include jade from mountain, nephrite pebble, and slope jade.
(1) Jade from mountain
Extracted from primary ore deposits, the characteristics of jade from mountain are that it varies in size, has angular shapes, is of mixed quality, lacks rounding and skin, and its luster and structural fineness are generally [Figure 6-13 (a)].
(2) Nephrite pebble
Hetian jade pebble is mainly produced in rivers. Nephrite pebble is the original ore eroded, washed, and transported into the river. Its characteristics include a smaller size, often oval shape, smooth surface, generally good texture, relatively warm, and a denser structure. Nephrite pebble is further divided into naked nephrite pebble and skin-colored nephrite pebble. Naked nephrite pebble is generally collected from the river water, while skin-colored nephrite pebble is usually collected from the riverbed’s soil. Skin-colored nephrite pebble is older, and some precious varieties of nephrite pebble, such as jujube red, black skin, autumn pear yellow, yellow wax skin, sprinkled gold yellow, and tiger skin, all come from skin-colored seed jade.
(3) Slope jade
The jade stone is formed by the weathering and collapse of primary jade ore, which is then washed into the river’s upper reaches by river water. Its characteristics include: being close to the original mine, having a larger size, slightly rounded edges, a smoother surface, and being somewhat older than nephrite pebble.

2. Optimization treatment and identification methods of nephrite
The optimization treatment of nephrite mainly includes waxing, rounding, dyeing, filling, and assembling.
(1) Waxing nephrite and identification
Paraffin or liquid wax is used to fill the surface of soft jade to cover fissures and improve luster. It generally improves nephrite with loose structure and surface fissures. Waxed nephrite has a waxy luster, may sometimes contaminate packaging, can melt when touched with a hot needle, and shows organic absorption peaks in infrared spectroscopy tests.
(2) Rounding and Dyeing of Nephrite and Identification
The nephrite material used to imitate ancient or nephrite pebble must be rounded before dying. The specific method involves: placing the coarsely ground raw material into a drum, adding pebbles and water, and continuously rolling until the edges of the nephrite material become smooth. Nephrite with better rounding has a higher surface gloss, but new fissures may sometimes occur due to the rolling process.
There are many methods for dyeing; some use chemical methods with agents like potassium permanganate, while others use direct burning, and some combine both methods. All nephrite or parts are dyed to cover imperfections or imitate nephrite pebble or ancient jade. Common colors include reddish-brown, brown, and yellow.
① Nephrite Dyeing Process
The jade raw material to be dyed is placed into a container filled with a pre-prepared dye solution, left to sit for a certain period, then taken out, washed, and dried. The jade is then heated to a specific temperature and maintained at that temperature for a certain time, after which it is left in the air to cool naturally to room temperature and finally treated with paraffin or other surfactants on the surface.
During the above operation, the content of Fe2+ and Fe3+ in the coloring solution and the process control conditions can be adjusted as needed to regulate the dye color tone, allowing gray-white or light-colored jade to be dyed red, brown, yellow, reddish-brown, yellow-brown, and other colors. The depth of color depends on the material properties.
② Identification characteristics of dyed nephrite
- Color: Dyed nephrite can be yellow, brownish-yellow, red, reddish-brown, etc. Dyed nephrite has bright colors, often found on the surface and in fissures. The dyeing starts from the skin, penetrating the jade along fissures and weak areas, but its color is dull and lacks layers. In contrast, the color of ancient jade is formed over hundreds of years, with its extension, diffusion, and infiltration being very natural and smooth. Dyeing is a short-term action, and they cannot be completely similar.
- Magnified inspection: Dyed nephrite has an overall color that is vivid and unnatural, with a single tone, and the color “floats” on the surface; the dye agent is concentrated along fissures or edges; the transition at the edges is obvious, with clear boundaries; As the surface has been bleached, traces of acid corrosion, frosting, and polishing are sometimes visible (Figure 6-14).
- Fluorescence: Under long-wave and short-wave ultraviolet fluorescence, the edges of dyed nephrite exhibit fluorescence, generally showing a strong blue-white fluorescence. The fluorescence intensity is related to the composition of the dye; some dyes do not fluoresce.
- Fading Experiment: Using cotton balls soaked in acetone or anhydrous ethanol to wipe the surface of the jade can remove some of the color, causing the surface color of the jade to lighten. This is because some dyes dissolve in acetone or anhydrous ethanol.
- Component Analysis: Using component analysis instruments (such as XRF, etc. ), the surface of dyed jade sometimes shows detectable elements that are rarely present in the jade (such as Pb, Cu, Co, etc. ).

(3) Filling and Identification of Nephrite
Artificial methods, such as organic glue, resin, and plastic, fill loose or cracked Hetian jade. The nephrite after filling treatment has the following characteristics:
① when observed with a magnifying glass or microscope, the filled jade shows a difference in surface gloss between the filled parts and the main jade; sometimes bubbles can be observed at the filling sites.
② Infrared spectroscopy testing often reveals characteristic peaks of the filling material; using luminescence image analysis (such as ultraviolet fluorescence observation), the distribution state of the filling material can be observed.
③ If the filling material is wax, using a heated needle to probe the surface of the nephrite may result in wax being exuded from the surface.
(4) Assembly and Identification of Nephrite
The assembly of nephrite is mainly used for surface or decorative carving parts. The main body of assembled nephrite is usually made of white jade material with a oily luster and weak glassy luster. Nephrite can be carved and generally has a brownish skin.
The surface after assembly is semi-transparent, with relatively weak luster. However, due to its small volume, it is not easily noticed by people, resembling high-quality nephrite pebble with a sugar color. When paired with exquisite carving techniques, it has an aesthetically pleasing shape.
Upon careful observation of the exquisite carving parts, the color boundary at the junction of the surface and the main body is obvious, with the surface color distributed along the boundary between the main body and the surface (Figure 6-15).

Section III Quartz jade
1. Gemological Characteristics and Classification of Quartz jade
The main component of quartz jade is SiO2, which often contains trace amounts of iron oxide, organic matter, and other substances, giving the jade various colors. There are many types of quartz jade , with the main varieties being agate, chalcedony, aventurine, and quartzite (Figure 6-16). Agate generally appears in blocky, nodule, or vein forms, has a fine texture, belongs to the cryptocrystalline structure, and has a hardness of 6. 5 ~ 7. It comes in various colors, including red, green, blue, orange-red, gray, and white. Chalcedony is similar to agate, but agate has a typical banded structure.

Different varieties of jade have different inclusions; the most typical inclusion in agate is its banded structure, which sometimes contains brown substances and chlorite, distributed in a staining manner; chalcedony has white vein-like inclusions; aventurine contains green chromium mica flakes, rutile, zircon, chromite, pyrite, etc. (Figure 6-17).

2. Optimization Treatment and Identification Methods of Quartz jade
The common optimization treatment methods for quartz jade mainly include heat treatment and dyeing. Due to the stability of jade after heat treatment and dyeing, it is classified as optimized and named directly with the jade name. Another type is agate containing water bladder, and the common treatment method is water injection treatment.
2.1 Agate
The common methods for agate include heat treatment and dyeing. Heat treatment, also known as color modification, is commonly referred to as “burning red” and is the most frequently used optimization treatment method for agate. Agate with heat treatment has bright colors and good stability, classified as optimized and named directly as agate.
(1) Agate Heat Treatment
① Principle: The red color of agate is mainly due to trace components Fe3+ that cause coloration. At high temperatures, the coloring ions Fe2+ are oxidized to Fe3+, increasing the ratio of Fe3+ and making the red color of agate more vivid.
② Equipment: The most important equipment for agate heat treatment is heating equipment; commonly used heating devices are coal furnaces and electric furnaces. Choose the appropriate heating equipment based on the agate material; the pros and cons of coal furnaces and electric furnaces are as follows:
- Coal Furnace: It is not easy to control the temperature, which may lead to cracking, melting, and insufficient flame, but it has a good insulation effect.
- Electric Furnace: It is easier to operate, and the temperature can be manually controlled for heating and cooling; the time at the highest temperature can also be controlled, but it is generally not convenient for batch production and has a smaller capacity.
③ The heat treatment temperature of agate is relatively high, generally requiring 1300 -1600℃. Heating should be done slowly to prevent fissures caused by excessive heating speed.
When heat treating agate, the “timing” should be based on the agate’s original color, and the heat treatment’s maximum temperature must be controlled accurately. The process is not complicated; as long as the “timing” (optimal heat treatment temperature) is mastered, agate with varying degrees of red tones can be fired into bright red colors of different depths.
Heat treatment of agate belongs to optimization and does not require identification. Heat-treated agate is directly named using the name of natural gemstones. Compared to natural agate, heat-treated agate has more vivid colors and higher saturation, but the overall texture of the agate is dry, with poorer moisture content.
(2) Agate Dyeing
The dyeing of agate involves immersing dye materials into the pores of the agate, resulting in overall coloration. The dye does not react with the components of the agate SiO2 but is merely a mechanical deposition. There are several requirements during the dyeing of agate:
① Raw Materials:
Before dyeing agate, selecting raw materials that are easy to dye is necessary. The agate used for dyeing must meet the following requirements:
- Structure: The structure of the agate raw materials used for dyeing should have a low density and micro-pores. Dyes are not easily absorbed into the fissures of high-density agate, making it difficult to achieve vibrant colors. an electron microscope study on agate structure and proposed the principle of “three dyeing, five not dyeing” for agate dyeing.
“Three-color” refers to the fact that agate has the following three easily dyed structures: herringbone-shaped fibrous structure, wavy fibrous structure, and multi-generational slender fibrous structure.
“Five-colorless” refers to the fact that agate has the following five structures that are not easily dyed: non-directional short fibrous granular structure; flower-like speckled granular structure; quartz’s allotriomorphic uneven granular structure; central and core quartz particles; coarse crystallization, clear boundaries at the edge of the grains, close intergranularity, and no microporosity cannot form a channel grain.
- Color: The raw material requirement is for light-colored or white varieties that must be cleaned thoroughly. The color of the agate raw material to be dyed black should be a bit darker.
- Thermal history: The agate to be dyed must have been removed, as roasted agate is difficult to color.
② Equipment:
The equipment required for agate dyeing is simple, as it immerses the dye. A glass container for soaking, a thermometer, a drying oven, and a muffle furnace are needed.
③ Dye
- Easily soluble in water or other reagents.
- Can react with some chemical reagents (fixatives) to form insoluble precipitates in water and alcohol, and the residues are colored.
- The generated colored substances should have good stability and not be decomposed or destroyed by sunlight, air, water, oxidants, or reductants.
④ Methods of dyeing and common dyeing agents
- Traditional methods: Previously, organic dyes were commonly used. In recent years, inorganic pigments have gradually replaced organic dyes due to their bright colors and stable physical properties.
For black agate, the sugar-acid process is still used to dye the agate black, known as “Black Anils. ” The sugar-acid process involves soaking sugar into the pores of the agate and then heating it with concentrated sulfuric acid to carbonize the sugar and form a black color.
- Some current methods abroad: Red: Soak the agate in a Fe (NO3)3 solution for about four weeks, allow it to dry slowly, then heat to decompose, producing Fe3+ that turns the agate red.
Prussian blue: Soak the agate in potassium ferrocyanide K4[Fe (CN)6] solution for about two weeks, then place it in iron sulfate [Fe2(SO)4)3] solution, soaking for about five days, where Fe3+ reacts with potassium ferrocyanide to generate Prussian blue precipitate in the agate fissures. The reaction formula is as follows:
4Fe3+ + 3[Fe(CN)]64- 一 Fe4[Fe (CN)6]3 ↓ (6-1)
This reaction is very sensitive, and the generated blue is very bright.
Tururnbull’s Blue: Soak white agate in potassium ferricyanide K3[Fe (CN)6] solution for about two weeks, then take it out to dry and place it in FeSO4 solution for 3-5 days. The Tururnbull’s Blue residue generated from the reaction between Fe2+ and potassium ferricyanide settles in the fissures of the agate, but the color is darker.
3Fe2+ + 2[Fe(CN)]63- 一 Fe3[Fe (CN)6]2 ↓ (6-2)
Prussian Blue and Tururnbull’s Blue have similar colors, but Prussian Blue is slightly lighter than Tururnbull’s Blue.
Blue-green: Soak agate in chromate (Na2CrO4, K2CrO4) or dichromate (K2Cr2O7 or Na2Cr2O7) for1-2weeks, then take it out and place it in a container containing (NH4)2CO3, gently heat it, maintain for about two weeks, and then heat again, the agate turns blue-green. The reaction formula is as follows:
K2Cr2O7 + (NH4)2CO3 →(NH4)2Cr2O7 + K2CO3 (6-3)
(NH4)2Cr2O7 →Cr2O3 + N2 ↓ +4H2O (6-4)
Black: Soak agate in silver nitrate solution for 1-2 weeks, then place it in (NH4)2S solution to soak; the resulting black precipitate Ag2S makes the agate appear black. The reaction formula is as follows:
2AgNO3 + (NH4)2S →Ag2S ↓+2NH4NO3 (6-5)
- The methods used domestically: the methods used domestically for agate dyeing technology are relatively mature, allowing agate to be dyed in different colors. In addition to the commonly seen red, green, and purple, agate can be dyed in other colors, such as brown, cherry red, peach red, and apple green. The operation method is similar to the abovementioned methods, but the chemical reagents used differ. Dyeing is a major optimization treatment for agate, and it can enhance or change the color of agate.
According to the principles of agate dyeing, there are three types of dyeing methods:
The coloring agent is immersed in agate, followed by heating, decomposition, or redox reactions to generate colored oxides. For example, to dye agate apple green, a nickel nitrate solution can be used to soak the agate, followed by heating to allow nickel ions to penetrate the fissures in the agate.
Two chemical reagents that can react chemically to generate coloring agents are sequentially immersed in agate in two stages. The generated coloring agents are subjected to heat treatment, which can decompose into colored oxides. For instance, in the blue-green dyeing method, potassium dichromate reacts with ammonium carbonate to generate ammonium dichromate, which decomposes upon heating to produce chromium trioxide as the coloring agent.
Two chemicals that can react chemically to produce a dye are applied to agate in two separate treatments. The dye formed by the reaction is then subjected to heat treatment, which can break it down into a pigmenting oxide. For example, the blue-green dyeing method involves the reaction of potassium dichromate with ammonium carbonate to produce ammonium dichromate, which is then heat-treated to produce chromium(III) oxide, the pigment.
First, immerse a dye into the interior of the agate and then soak it in a fixing agent, allowing the dye to react with the fixing agent to produce a poorly soluble colored compound, thereby coloring the agate.
This method does not require high-temperature heating, and the generated precipitate has good stability.
⑤ Identification of dyed agate
- Finding differences in color: Different color tones: organic dyes are bright and prone to fading. In contrast, inorganic pigments’ colors are closer to natural products, but careful observation can also reveal differences. Below are distinctions based on three common colors:
Natural red agate is a pure red color. In contrast, artificially dyed red agate has iron ion compounds added, resulting in a red color with a yellowish tint.
Natural blue agate is produced in very small quantities, mostly in gemstone blue, and often exhibits varying degrees of banding. Artificially colored blue agate appears violet (cobalt blue) due to the addition of cobalt salts, which gives it a bluish-purple hue, with very few cases showing gemstone blue coloring.
The colored green agate is very close in color to the natural variety. However, upon closer inspection, the natural variety is a soft onion green, while the colored green agate is a bright emerald green with higher saturation.
- Finding differences in structure: Since dyed agate is colored by soaking and drying it with pigments, the pigments settle in the pores of the agate, and under magnification, uneven color spots can be found in the fissures and pores.
Generally, a tenfold magnifying glass is sufficient for identification, while fine-dyed products need to be observed under a gem microscope. Dyed and heat-treated agate can be observed to have “nail marks” on the surface.
Natural red agate does not have “nail marks, ” and the coloring particles in the agate are red, dot-like iron inclusions, with diffusion phenomena not being obvious or absent. The dyed and heat-treated red agate surface can show “nail marks, ” concentrated in specific areas with varying degrees of color, structure, and transparency, showing uniform color distribution and blurred band boundaries (Figure 6-18).
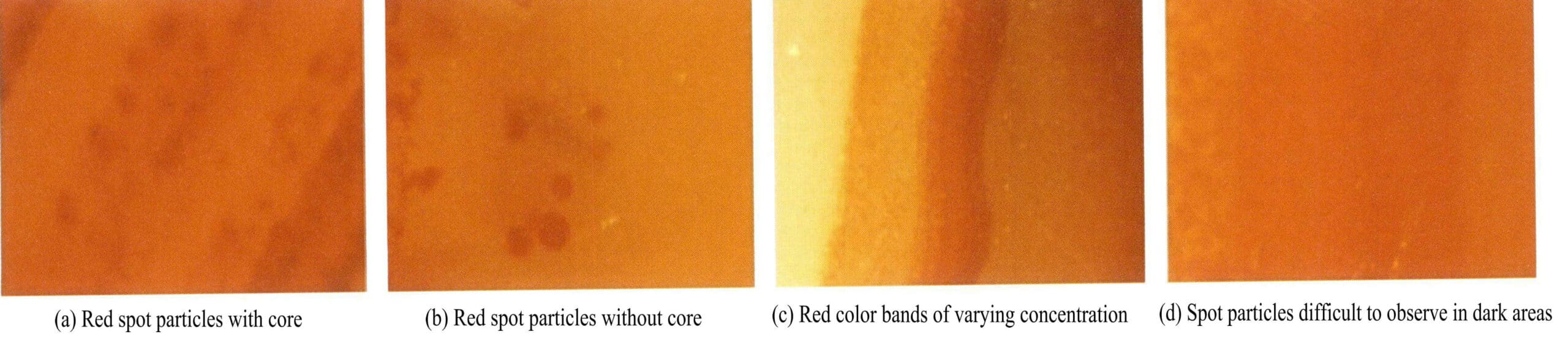
(3) Water-filled agate treatment
Water-filled agate is a type of agate that contains water. When there are many fissures in the water-filled agate or when fissures occur during processing, the water in the cavity will slowly leak out until it dries up, causing the entire water-filled agate to lose its artistic value.
The treatment method is to soak the water-filled agate in water, using capillary action to refill the water to its original position or to use an injection method to refill the water and seal the small fissures with glue or other materials.
Identification features after water-filled agate treatment: Carefully observe the water-filled walls for any signs of artificial treatment. In suspicious areas, use a hot needle to probe; water-injected water-filled agate will have gelatinous or waxy materials precipitate out.
2.2 Chalcedony
Chalcedony is a cryptocrystalline quartz jade, with the main chemical component being SiO2, and it may contain trace elements such as Fe, Al, Ti, Mn, and V. The crystallization state is a cryptocrystalline aggregate, appearing dense and massive, and can also present as granular, radiating, or fine fibrous aggregates. Chalcedony comes in various colors, and common enhancement methods include heat treatment and dyeing.
(1) Heat treatment
Yellow to brown chalcedony contains a large amount of iron, which forms a deep reddish-brown color after heat treatment. Since this treatment method only involves heating without adding any components other than natural chalcedony, and the color after heat treatment is stable, it does not need to be labeled commercially and is directly named after natural gemstones.
(2) Dyeing method
The dyeing materials for chalcedony are generally selected from colorless or light-colored stones and can be dyed into different colors as needed. Sometimes, dark-colored materials can also be dyed into black chalcedony.
① Sugar and sulfuric acid treatment: Sugar and sulfuric acid treatment of light-colored chalcedony or gray chalcedony is processed into black chalcedony; almost all black chalcedony is treated this way.
② Swiss lapis lazuli: Dyed jasper (variegated chalcedony), is used to imitate lapis lazuli, commonly referred to as “Swiss lapis” in the market [Figure 6-19 (a)]. However, the dyed jasper lacks the granular structure of lazurite and contains no pyrite; wiping with a cotton swab dipped in acetone will cause it to fade.
③ Green chalcedony: Chalcedony is dyed with chromium salts, which can be used to imitate green chalcedony; the treated chalcedony turns red under a color filter [Figure 6-19 (b)]. A blurry absorption band can be seen under a spectroscope in the red light region.

Copywrite @ Sobling.Jewelry - Produsen perhiasan khusus, pabrik perhiasan OEM dan ODM
2.3 Aventurine
Aventurine is a type of quartz jade that exhibits a sand-gold effect, often displaying different colors due to the presence of other colored minerals. Those containing chromium mica appear green, known as green aventurine (the green aventurine produced in Xinjiang, China, contains green fibrous actinolite); those containing dumortierite appear blue, known as blue aventurine; and those containing lepidolite appear purple, known as purple aventurine.
The quartz grains in aventurine are relatively coarse, and the flaky minerals within are relatively large, which can exhibit a noticeable sand-gold effect under sunlight.
The most common type in the domestic market is green aventurine (Figure 6-16), often used as a substitute for green jadeite. The main difference from natural jadeite is the internal characteristics; under a magnifying glass, large fuchsite flakes can be seen arranged in a directional pattern, and under a color filter, it appears reddish-brown.
2.4 Quartzite
The dyeing treatment method for quartzite involves heating the quartzite, quenching it to form micro-fissures, and then dyeing it. It is mainly dyed green, and dyed quartzite is commonly referred to in the market as “Malaysian jade, ” used to imitate high-end jadeite.
Quartzite is dyed with inorganic dyes, often turning green. Under a gem microscope, common green substances are distributed in a net-like pattern in the gaps between particles, with deeper colors in loose structures and lighter colors in dense structures (Figure 6-20). An absorption band can be seen at 650nm in the red light region under a spectroscope (Figure 6-21). Under short-wave ultraviolet light, it may exhibit a dark green luster.

Bagian IV Opal
People, especially those revered in Europe, have always loved opal. The literary giant Shakespeare called opal the “queen of gems. ” The “Light of the World” — black opal from Lightning Ridge, Australia, has a rough weight of 273ct ( lct = 0. 2g ). After grinding, it weighs 242ct and is currently housed in the Smithsonian Institution in Washington, USA. High-quality opal can gather various colors in one, with brilliant hues that provide a beautiful illusion. Thus, opal is the birthstone for October, known as the “stone of hope. “
1. Gemological Characteristics of Opal
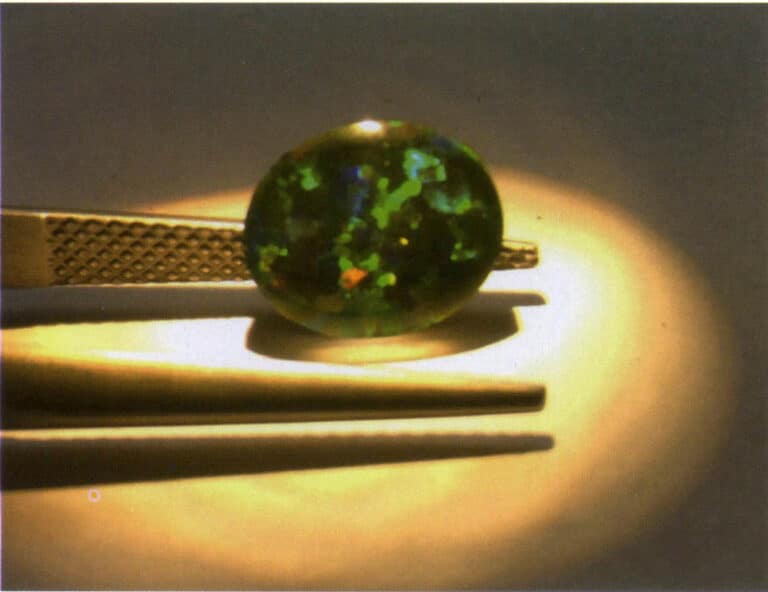
The mineral composition of opal is primarily opal, with small amounts of quartz, pyrite, and other minor minerals. The English name is opal, referring to opal or precious opal that exhibits color-changing effects. Opal is an amorphous solid lacking a crystalline shape and often appears in plate-like, vein-like, and irregular forms. The chemical composition is SiO2 - nH2O, with variable water content, generally 4% -9% , and can reach up to 20% . Opal has a wide variety of colors, with body colors including black, gray, white, brown, pink, orange-yellow, yellow, green, light blue, and green. It has a glassy to resinous luster, ranging from transparent to opaque. It exhibits a typical color-changing effect (Figure 6-22), and rotating the opal under a light source reveals colorful spots.

2. Main Optimization Treatment Methods for Opal
The main optimization treatment methods for opal include heat treatment, oil treatment, sugar acid treatment, colorless filling treatment, and dyeing treatment. The opal’s color can be changed through optimization treatment, enhancing the color-changing effect. Some non-gem quality opals can be improved to gem quality through treatment, increasing their economic and aesthetic value.
(1) Heat Treatment
Due to the presence of water in the composition of opal, heat treatment is generally not used for improvement. For opals with color-changing effects, heat treatment will cause water loss, resulting in a uniform refractive index, and the color-changing effect will also disappear. If it is re-immersed in water, the color cannot be restored. Opals can regain color and color change under special conditions of dehydration, provided that the conditions during restoration are consistent with those during the growth of the opal. After water treatment, opals can restore their color change. Natural opals generally do not display color-changing effects during water permeation treatment. Heat treatment can be used on inferior opals that do not have color-changing effects to improve their color and appearance.
(2) Oil Treatment
The oil treatment of opal is a traditional treatment method with a long history. In ancient times, people began using this method to improve the color-changing effect of opal or change the color of opal.
① Treatment Object: Porous water opal.
② Method One: Wrap the opal in wrapping paper, cover it with aluminum foil, soak the opal in waste lubricating oil, then wrap it in the paper and heat it at high temperature to carbonize the paper and enter the fissures of the opal.
③ Method Two: Place the opal in a ceramic pot, bury it with combustible fertilizer, and roast the ceramic pot with charcoal.
Due to the large amount of oily or tar-like substances seeping into the opal during processing, the opal exhibits a color-changing effect. The oil treatment process requires heating, which is usually referred to as smoke dyeing. The color will only be recovered if the heat treatment temperature is lowered.
Oil and water treatment can mask the fissures and pores of the opal, resulting in color or color change. However, the color and color change is unstable; over time, the color fades or the color change disappears.
(3) Colorless filling treatment
Colorless filling is usually done with plastic, filling the fissures of chalky, low-quality opal with plastic to make the opal transparent and produce color. The specific filling process includes several steps: cleaning, drying, vacuum filling, and polishing. The filling materials include silica, silane, and silicate polymers.
(4) Dyeing treatment
The history of sugar acid dyeing of opal is very long and is the main method for dyeing black opal in history. The specific dyeing process is as follows:
① Pre-cleaning, drying at a temperature below 100℃ ;
② Place the opal in a hot sugar solution (commonly a solution of 2 cups of sugar and 3 cups of distilled water), heat to boiling, and soak for several days;
③ After cooling the opal, quickly wipe off the excess surface sugar syrup, soak it in about 100℃ concentrated sulfuric acid for about 1-2days, and then slowly cool it down;
④ After carefully rinsing the opal, rinse it in a carbonate solution and then clean it.
(5) Substrate, assembly, and coating
Natural opal has a loose and porous structure, and high-quality opal is often relatively thin, usually combined with other materials to enlarge the opal and enhance its color-changing effect.
① Substrate: Stick refractive oil or margarite underneath the transparent opal to enhance the color change.
② Assembly (two-layer stone or three-layer stone): The upper part of the two-layer assembly stone is generally opal, while the lower part is plastic or glass, or the upper part is colorless crystal and the lower part is opal pieces, bonded with colorless glue; the three-layer stone generally has a top layer of colorless transparent glass or plastic, a middle layer of natural opal, and a bottom layer of black material.
③ Surface Coating: Mainly to protect the opal’s surface, but the coating’s hardness is not high. Some fully plastic imitation opals (such as softer polystyrene) are often protected with acrylic coatings.
3. Optimization of Opal Identification
(1) Identification characteristics of dyed opal
Under a gem microscope, particles of carbon or dye can be seen in the opal, and dye can also be found aggregated in the fissures. After dyeing, the color spots are fragmented and limited to the granular structure on the gem’s surface (Figure 6-23).

Gambar 6-23 Opal yang dicelup
The two black dyed opals are located at the bottom left of the image.
(2) Identification characteristics of injection-molded opal
After injection molding, the opal has poor transparency, ranging from translucent to opaque, with a relatively low specific gravity of about 1. 90, often containing black fibrous or fingerprint-like inclusions and opaque metallic inclusions.
(3) Main identification features of the assembled opal doublet.
The glued surface is visible in the unmounted doublet; under strong light magnification, bubbles in the glued surface, hemispherical pits in the adhesive, and near-surface bubbles can be seen, along with changes in the luster of the iron ore near the boundary; a hot needle can reveal the presence of the adhesive; color spot structures distinguish the top layer material [Figure 6-24(a)]. If the upper layer is opal and the lower layer is plastic or glass, magnification reveals differences in color and luster between the two layers, with the color change effect occurring in the upper part of the gemstone; if the upper part of the doublet is colorless crystal and the lower part is opal, the color change effect of the opal occurs in the lower layer.
(4) Identification features of the three-layer assembled opal.
The top layer does not exhibit color change, and the refractive index is usually higher than that of opal; bubbles and swirl patterns are visible in the glass top layer; a bubble layer is visible at the bonding surface; pits, bubbles, and changes in luster may be present at the bonding surface boundary; the opal layer is distinguished based on the location of structural color spots of different materials [Figure 6-24(b)]. In three-layer doublets, the top layer is generally colorless transparent material, with color spots located in the middle layer of opal, and the color change effect occurs within the gemstone at a certain depth from the gemstone surface.

(5) Methods and Identification Features of Synthetic Opal
Currently, most synthetic opals are synthesized using the Gilson synthesis method. The main synthesis process is as follows:
① Formation of Silica Spheres: Add a medium-strength alkali (such as ammonia) to the organic silicon compounds that diffuse into small droplets in a mixed solution of alcohol and water, turning the organic silicon compounds into silica spheres. The purity, concentration of the reagents and stirring speed must be carefully controlled to generate spheres of the same size and to obtain different types of opal varieties as required, with a diameter between 200 and 300nm.
② Precipitation: They continuously precipitate After forming silica spheres. Once precipitated, these spheres automatically arrange themselves tightly. This stage is relatively slow and may take more than a year.
③ Compaction and bonding: This process is the most difficult and key to producing high-quality opal materials. Silica spheres are covered with liquid, and equal hydrostatic pressure is applied to the spheres in all directions to avoid structural changes; finally, the silica spheres may be bonded with added colloidal material, or the materials are sintered at a certain temperature.
Finally, the formed opal is cut and polished to display a better play of color effect.
Identification of synthetic opal versus natural opal:
① Structure:
The color spots of natural opal are two-dimensional, with a silky appearance, elongated in one direction; they are irregular thin sheets; the color spots have a gradient relationship with blurred boundaries; the color spots have fibrous or striped structures in one direction (Figure 6-25).

Synthetic opal has typical columnar color spot characteristics, mosaic-like color spots, and clear color spot boundaries, exhibiting a three-dimensional form. Looking through the synthetic opal column, the boundaries are distinct, with jagged edges divided by closely arranged intersecting lines, creating a mosaic-like structure. Each mosaic piece may contain snake skin (also known as scorpion skin) patterns, honeycomb structures, or step-like structures (Figure 6-26).

② Luminescence:
The reactions under ultraviolet light can also be an auxiliary means to distinguish between natural and synthetic opal. For example, natural black and white opal may exhibit weak to moderate intensity white, blue-green, or yellow fluorescence. In contrast, fire opal may show weak to moderate intensity green-brown fluorescence. Most natural opals have persistent phosphorescence; synthetic white opals have almost no fluorescence or phosphorescence, and synthetic opals are more transparent than natural opals when exposed to long-wave UV light.
③ Infrared spectrum:
In identifying infrared spectra, there are significant differences in the molecular vibration spectra of water between synthetic and natural opal, providing a basis for distinguishing between them.
Section V Serpentine Jade
1. Gemological Characteristics of Serpentine Jade
Serpentine is a layered hydrous magnesium silicate mineral with the chemical formula Mg6Si4O10(OH)8. In it, Mg can be replaced by trace elements such as Mn, Al, Fe, and Ni, and sometimes small amounts of Cu and Cr ions are mixed in. Serpentine is generally green but can also be white, yellow, bluish-green, brown, and dark black; green and emerald green often contain chromium and nickel. The main mineral composition of serpentine jade is serpentine, with secondary minerals including dolomite, magnesite, chlorite, tremolite, calcite, and chromite. The chemical composition of serpentine is influenced by its mineral composition. Generally, the chemical composition of pure serpentine jade is close to the theoretical content of various components of serpentine minerals. When the content of tremolite in the jade increases, the chemical composition becomes high in silicon, high in calcium, and low in magnesium. When the content of chlorite in the jade significantly increases, the chemical composition is relatively low in magnesium, low in silicon, and rich in aluminum.
2. Optimization Treatment and Identification Methods of Serpentine Jade
Visually, it appears as a uniform dense mass, and under high magnification microscopy, it shows fine granular and fibrous mineral aggregates. Upon magnification, pale green chlorite and dark chromite inclusions can be seen distributed within (Figure 6-27), and water wave patterns are visible. Common optimization treatments for serpentine jade include dyeing and filling.

(1) Treatment methods and identification of dyed serpentine jade
Heat the serpentine jade to create fissures, then soak it in dye. The dye concentrates in the fissures of the dyed serpentine jade, and upon magnified observation, the presence of dye can be seen in the fissures (Figure 6-28). Dyed serpentine jade is sometimes sold under “golden silk jade. “

(2) Wax-filled serpentine jade and identification
Fill the fissures or gaps in serpentine with wax, oil, or resin to change the appearance of the sample or improve stability. When filled with wax, magnification reveals a distinct waxy luster at the filling site, and a hot needle probing the crack shows wax flow, while the smell of wax can also be detected; when filled with oil, magnification shows lower transparency and luster at the crack, and oil may be exuded when probed with a hot needle.
Filling with a small amount of colorless wax or colorless oil can be classified as optimization while filling with colored wax, colored oil, glass, or artificial resin is classified as treatment, which must be noted when sold.
Section VI Turquoise
1. Gemological Characteristics of Turquoise
Turquoise varies in color due to its different elements; it appears blue when containing copper and green when containing iron. Natural turquoise is mostly sky blue, light blue, greenish-blue, green, or pale with a hint of green. The uniform color, soft luster, and absence of brown iron veins indicate the best quality. Color is an important factor affecting the quality of turquoise. Sky blue or slightly greenish blue turquoise is commonly regarded as high quality.
Turquoise is a hydrated copper aluminum phosphate mineral with the chemical formula CuA16(PO)4)4(OH)8-5H2O. The texture of turquoise is very uneven, with colors ranging from deep to light, and it may even contain light-colored stripes, spots, and dark brown iron lines. The density also varies significantly; those with many pores are loose, while those with fewer are dense and hard. After polishing, it has a soft, glassy luster to a waxy luster. Most belong to a cryptocrystalline structure, with very few showing visible crystals. The surface of turquoise often contains irregular white textures and patches, as well as brown matrix textures and color spots.
the famous turquoise-producing area in Iran produces the highest quality porcelain turquoise and iron line turquoise, known as Persian turquoise. In addition, countries such as Egypt, the United States, Mexico, Afghanistan, India, and Russia also produce turquoise.
2. Classification of Turquoise Varieties
The quality of turquoise is mainly related to factors such as its color and structure. Based on the color and texture of turquoise, it is internationally classified into four categories: porcelain turquoise, green turquoise, iron line turquoise, and foam turquoise (Figure 6-29).
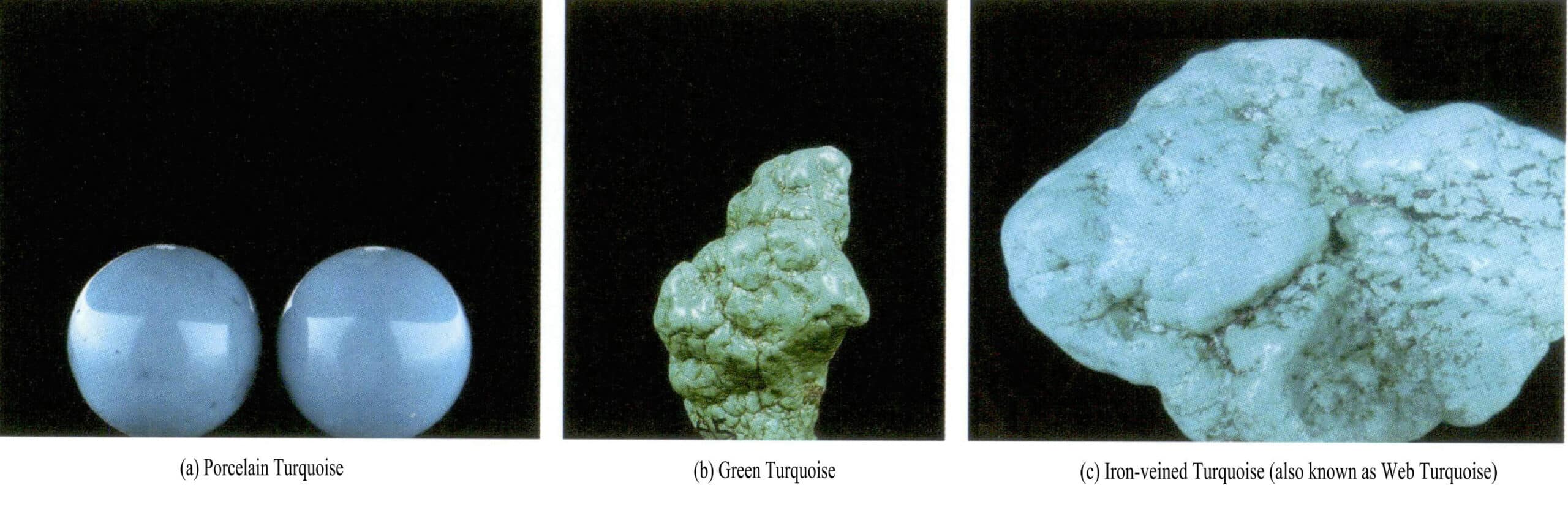
(1) Porcelain Turquoise
Porcelain turquoise is the highest quality and hardest type of turquoise, with a hardness that is the greatest among all varieties of turquoise, ranging between 5. 5 -6. The color of porcelain turquoise is usually pure sky blue or blue-green, with a dense structure, and it has a porcelain-like finish after polishing, exhibiting a strong porcelain luster. Porcelain turquoise is a premium type of turquoise.
(2) Green Turquoise
Green turquoise is a relatively common variety, with colors generally ranging from blue-green to pea-green. It has a high hardness, second only to porcelain turquoise, with a strong luster, fine texture, and quality just below that of porcelain turquoise.
(3) Iron Wire Turquoise
This variety is sky blue, blue-green, and bean green. In turquoise, fine black-brown iron ore veins are distributed in a net-like pattern, making the blue or green turquoise exhibit a black net pattern or vein-like texture known as iron wire turquoise. The limonite veins are referred to as “iron wire. ” The clearer and more distinct the pattern of the iron wire, the better, creating natural patterns on the turquoise that resemble ink lines, beautiful and unique. Turquoise with beautiful spider web patterns can also be considered a fine product.
(4) Foam Turquoise
After weathering and losing moisture, it becomes moon white, has a low value, and a hardness below 4. 5, which can be scratched with a small knife. Because this type of turquoise is soft and loose, only larger pieces have any practical value, making it the lowest quality turquoise. It is often treated with injection molding, waxing, and dyeing to improve its quality and appearance, allowing it to be used as a gemstone.
3. Optimization Treatment and Identification Methods for Turquoise
Due to the loose structure of natural turquoise, it is generally reinforced by methods such as filling with resin or wax, which also improves its stability. Some light-colored turquoise can also have its color improved through dyeing. Common optimization methods for turquoise include dyeing, resin filling, wax filling, molding, reconstruction, and density optimization.
(1) Dyeing treatment
Purpose of treatment: To change the color appearance and enhance the color of turquoise. After losing moisture, turquoise becomes lighter in color and has a loose structure, making it easier to dye. Light green and light blue turquoise can be dyed to enhance their color using aniline dyes.
The identification method for dyed turquoise mainly involves magnified inspection. Dyed turquoise is unnatural; dyed turquoise on the market often appears deep blue-green or deep green, with overly vivid colors concentrated in the fissures. After dyeing, the surface color is deep, while the internal color is lighter. The color distribution after dyeing is more pronounced for turquoise with iron veins, and magnified inspection reveals color concentration at the iron vein locations. (Figure 6-30).
Dyed turquoise colors are unstable and will fade over time. If a drop of ammonia is applied to an inconspicuous area of dyed turquoise, it will fade, revealing the original green and white colors.

(2) Injection filling treatment
① Injection of resin and wax:
The injection of resin and wax is mainly aimed at turquoise with loose structures. Treating it with resin or wax makes the natural turquoise structure dense, enhancing its stability. The identification characteristic is that the color of turquoise treated with filling is not durable; over time, it will fade, and after a few seconds of probing with a hot needle, the resin and wax will seep out to the surface, showing a distinct resinous or waxy luster (Figure 6-31).

② Injection Molding:
Injection molding treatment is divided into colorless plastic and colored plastic injection, injecting light-colored or white turquoise to change its color and structure, making its structure denser and its color more vibrant.
The detection method can be tested with a hot needle in inconspicuous places. Look for fissures and pits, and probe with a hot needle; certain plastics will emit a pungent smell when heated, and this type of turquoise generally has a relative density of less than 2. 76; the hardness of injection-molded turquoise is relatively low, and the surface is prone to scratches; infrared spectroscopy testing may show strong absorption caused by plastic at 1450 cm-1 and 1500 cm-1, while in newer injection-molded varieties, strong absorption at 1725 cm--1 may appear during infrared spectroscopy testing.
(3) Reconstructed Turquoise
Reconstructed turquoise is made from broken pieces of turquoise, turquoise microparticles, blue powder materials, and some binding agents pressed together at a certain temperature and pressure. Strictly speaking, reconstructed turquoise should be referred to as a turquoise imitation. Reconstructed turquoise is mainly identified through the following aspects:
① Structure and Color:
The reconstructed turquoise surface has a distinct porcelain-like luster, and under magnification, there is a noticeable fine-grained structure. The distribution of iron lines is irregular, and sometimes, the color distribution is also uneven (Figure 6-32).
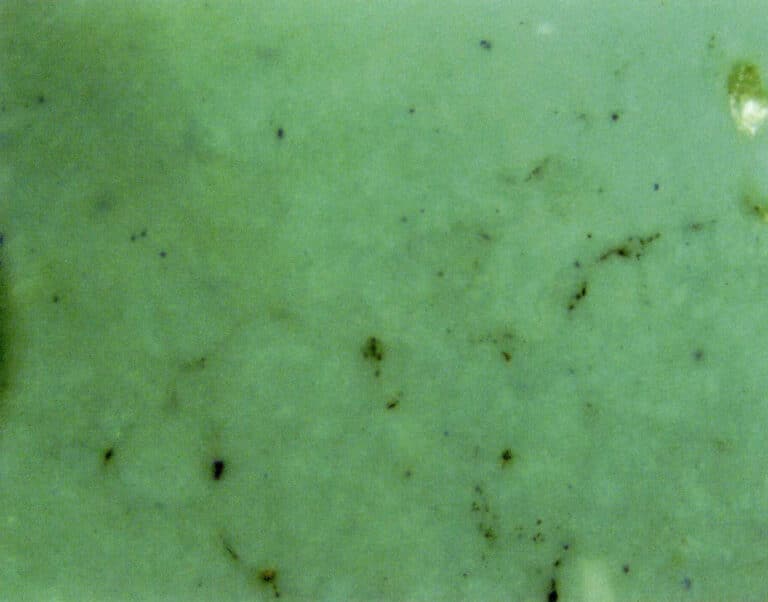
② Acid Experiment:
The reconstructed turquoise appears blue due to the presence of copper compounds. Copper salts can dissolve in hydrochloric acid; the reconstructed turquoise will fade when acid is dripped onto the surface and wiped with a white cotton ball.
(4) Density Optimization
Density optimization mainly targets natural turquoise with many pores and a loose structure to improve its density, enhancing the texture, luster, and hardness of the turquoise near and on the surface.
The most widely used technology for density optimization is the electrochemical treatment method. Most of the “Sleeping Beauty” turquoise appearing in the domestic jewelry market has undergone electrochemical optimization treatment. Early on, turquoise treated with electrochemical methods had bright surface colors, limited to a very shallow surface layer. If subjected to multiple electrochemical treatments, the color can penetrate the turquoise’s interior.
The electrochemical treatment method improves turquoise based on the changes in its structure during the electrolysis process. During electrolysis, the crystallization water and adsorbed water in turquoise are electrolyzed to produce many hydroxyls (-OH), and the hydroxyls (-OH) in the electrolytic cell can also slightly permeate into the turquoise. These hydroxyls (one OH) will combine all the isolated octahedra in the turquoise structure into octahedral pairs, making the turquoise structure denser and the color more vibrant.
4. Identification of turquoise and similar gemstones
(1) Identification characteristics of natural turquoise
Natural turquoise has a cryptocrystalline structure, with no granular structure observed under magnification, and the surface often has pyrite particles and limonite found in veins. The refractive index of turquoise is 1. 62, with a relative density of 2. 60 -2. 70, and there are two absorption lines in the blue region at 432nm and 420nm under the spectroscope.
(2) Identification characteristics of synthetic turquoise
Most synthetic turquoise on the market is produced using the Gilson synthesis method. The structure of synthetic turquoise is fine-grained, and when magnified 50 times, it shows a granular structure (Figure 6-33). The refractive index is 1. 60, the relative density is 2. 70, and there are no absorption lines in the blue region under the spectroscope. Applying acid to inconspicuous areas of synthetic turquoise can change blue synthetic turquoise to green, as synthetic turquoise often contains copper compounds, which can dissolve in hydrochloric acid.
(3) Identification characteristics of chrysocolla
The color of chrysocolla is blue, sky blue, and green with mottling. The refractive index is 1. 50, the relative density is 2. 0 to 2. 5, and the Mohs hardness is 4. Therefore, chrysocolla’s low refractive index, low density, and color characteristics distinguish it from turquoise.
(4) Identification characteristics of dyed magnesite
The structure of dyed magnesite is dense and blocky, significantly different from the granular structure of turquoise. Upon magnification, the dye is observed along the fissures.
The gaps are concentrated, appearing light brown under a Charles filter. The refractive index varies greatly, around 1. 60, with a relative density of 3. 00 -3. 12.
(5) Identification characteristics of dyed chalcedony
Dyed chalcedony has a layered structure and a mottled color. Under magnification, the dye in the dyed chalcedony is concentrated in the fissures, appearing red or light brown under a Charles filter. The refractive index is 1. 53, and the relative density is between 2. 60 and 2. 63.
(6) Identification characteristics of glass
Glass does not have the granular structure of turquoise. Under magnification, bubbles can be seen reaching the surface in small hemispherical holes, and shell-like fractures are visible at the breakpoints. The refractive index varies significantly, ranging from 1. 40 to 1. 70, and the relative density can reach 3.30.
Section VII Lapis Lazuli
The English name for lapis lazuli is “lapis, ” derived from Latin. According to sources, lapis lazuli was introduced to China from Afghanistan via the “Silk Road. ” It is usually found in aggregate form, presenting a dense, blocky, and granular structure. The colors are dark blue, violet-blue, sky blue, greenish blue, and so on. Lapis lazuli is also the main raw material for natural blue pigments. In ancient Greece and Rome, wearing lapis lazuli was considered a symbol of wealth. During the Qing Dynasty in China, lapis lazuli became an ornament for the court officials’ hats, and it was used to flaunt their identity and status.
1. Gemological Characteristics of Lapis Lazuli
Lapis lazuli is a rock primarily composed of lapis lazuli minerals, containing small amounts of impurities such as pyrite and calcite, forming a cryptocrystalline aggregate. Due to a small amount of calcite, the surface color often appears with white spots. Cleavage is not developed, the fracture is uneven, and the streak is light blue. It emits orange points of light under long-wave ultraviolet light and white fluorescence under short-wave ultraviolet light. Under a Charles filter appears light red, with a glassy to waxy luster, a refractive index of 1. 502 ~ 1. 505, and a specific gravity of 2. 7 to 2. 9.
The sources of lapis lazuli include Afghanistan, the United States, Mongolia, Myanmar, and Chile, among which Afghan lapis lazuli is the most famous. Lapis lazuli generally appears blue, with the best quality being a deep, pure, and uniform blue. White lines or white spots in the color will reduce the color’s concentration, purity, and uniformity.
2. Optimization Treatment and Identification Methods of Lapis Lazuli
The main optimization treatment methods for lapis lazuli are wax filling, dyeing, and bonding treatment.
(1) Waxing filling
Wax is applied to the surface fissures of lapis lazuli to improve appearance and fill the fissures.
Main identification features: After wax filling, lapis lazuli has a waxy luster, the waxed areas have lower hardness, and the surface has scratches; in places where the wax layer has peeled off, there is a buildup of wax in the depressions, which can be scraped off with a steel needle (Figure 6-34).
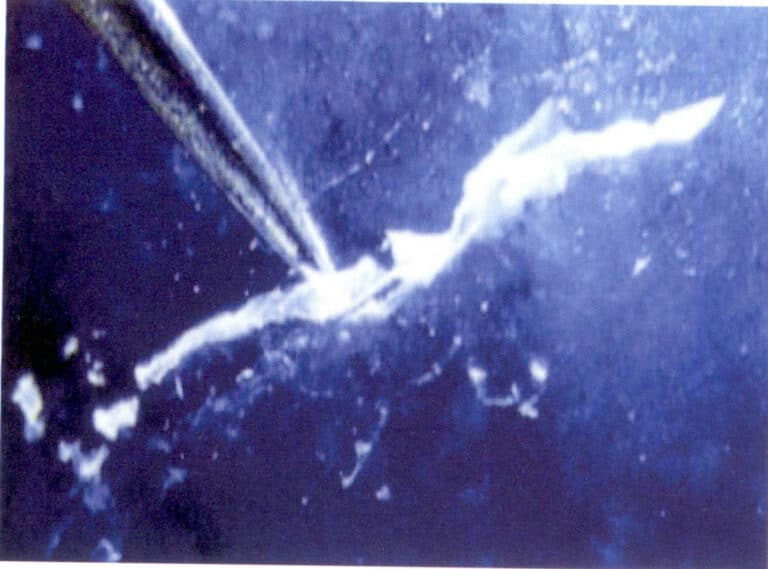
(2) Dyeing Treatment
Blue dye is used to change the color appearance of inferior lapis lazuli, enhancing the quality and commercial value of natural lapis lazuli.
Main identification features: dyed lapis lazuli is darker, with color concentrated in surface fissures. Wiping with a cotton swab dipped in acetone can turn the swab blue. If it appears waxed, the wax layer should be removed before wiping the surface of the dyed lapis lazuli with a cotton swab.
(3) Bonding Perawatan
Crush inferior lapis lazuli and bond it with plastic to form a large overall appearance of lapis lazuli.
Main identification features: The adhesive lapis lazuli shows a distinct granular structure with uneven color distribution under magnification. When touched with a hot needle, it emits a pungent plastic smell.
(4) Identification features of synthetic lapis lazuli and natural lapis lazuli
The appearance of synthetic lapis lazuli is similar to that of natural lapis lazuli, with the main identification features as follows:
① Color:
The distribution is relatively uniform, lacking the characteristic mottled distribution found in most natural lapis lazuli.
② Structure:
Fine granular structure; if there are pyrite particles in synthetic lapis lazuli, the edges of the pyrite particles are generally very straight and evenly distributed throughout the gemstone; in natural lapis lazuli, pyrite is randomly distributed, and the particle shapes are irregular.
③ Density:
The relative density of synthetic lapis lazuli is lower than that of natural lapis lazuli, with a relative density 2. 70.
3. Identification of characteristics of Lapis lazuli and common Imitations
(1) Sodalite
Sodalite is similar in color to lapis lazuli but can be distinguished structurally. Sodalite has a coarse crystalline structure, while lapis lazuli is mostly a cryptocrystalline aggregate with a fine-grained structure; sodalite may sometimes show cleavage and has a higher transparency than lapis lazuli; the relative density of sodalite ( 2. 15 -2. 35 ) is significantly lower than that of lapis lazuli ( 2. 7 -2. 9 ), which is enough to differentiate them. Sodalite often contains white mineral spots or patterns and rarely has pyrite inclusions (Figure 6-35).

(2) Dyed jasper (Swiss lapis lazuli)
The color distribution of dyed jasper is uneven, enriched in stripes and patches, with no pyrite present, and the fracture is shell-like; it usually does not show reddish-brown under a polarizing filter; it has a higher refractive index and lower density; in streak tests, the streak of natural lapis lazuli is light blue while jasper does not leave a streak.
(3) Glass
The blue glass used to imitate lapis lazuli does not have the granular structure of lapis lazuli. It may contain bubbles and swirling textures, with a shell-like fracture visible on the broken surface.
(4) Dyed marble
Under magnification, it can be observed that the color of dyed marble is concentrated at the fissures and grain boundaries, and the dye can be wiped off with acetone. Dyed marble has a lower hardness and can be easily scratched with a knife.
Section VIII Fluorite
1. Gemological Characteristics of Fluorite
Fluorite, or fluorspar, is a relatively common mineral that can coexist with other minerals. It belongs to the isometric crystal system, with octahedral and cubic common crystal forms. The crystals have a glassy luster, are brittle, have a Mohs hardness of 4, and a melting point of 1360℃, with perfect cleavage. Some samples can fluoresce under friction, heating, or ultraviolet light exposure. It is called fluorite because it fluoresces like a firefly when exposed to ultraviolet or cathode rays. When fluorite contains some rare earth elements, it will emit phosphorescence, meaning that after the ultraviolet or cathode ray exposure, the fluorite can continue to glow for some time. The production of phosphorescent fluorite is not large.
Fluorite comes in various colors, including purple-red, blue, green, and colorless (Figure 6-36). The main chemical component of fluorite is calcium fluoride ( CaF2 ). Pure fluorite is colorless but often appears in different colors due to various impurities. Calcium is often replaced by rare earth elements such as Y and Ce, and it also contains small amounts of Fe2O3, SiO2, and trace amounts of Cl, O, He.

2. Optimization treatment and identification methods of fluorite
Common optimization treatment methods for fluorite include heat treatment, filling, and irradiation.
(1) Heat Treatment
Heat treatment is the most common optimization method for fluorite. By heating, the dark blue to black fluorite can be transformed into a better blue, and the color after treatment is very stable. This treatment is considered optimization and does not require identification.
(2) Filling
Generally, plastic or resin is used to fill the fissures in fluorite, with the main purpose of healing surface fissures to prevent them from appearing during processing or wearing. The identification characteristics of filled fluorite mainly include the following points:
① Under magnification with a magnifying glass or microscope, the fissures in the fluorite are not obvious, and the fissures often exhibit a resinous luster.
② Using a hot needle for detection, resin or plastic may be precipitated.
③ Observing under ultraviolet fluorescence, the plastic and resin in the filled areas may exhibit characteristic fluorescence.
(3) Iradiasi
Colorless fluorite can produce a purple color through irradiation. Irradiated fluorite is extremely unstable and will fade when exposed to light, so this treatment method has no practical or commercial value.