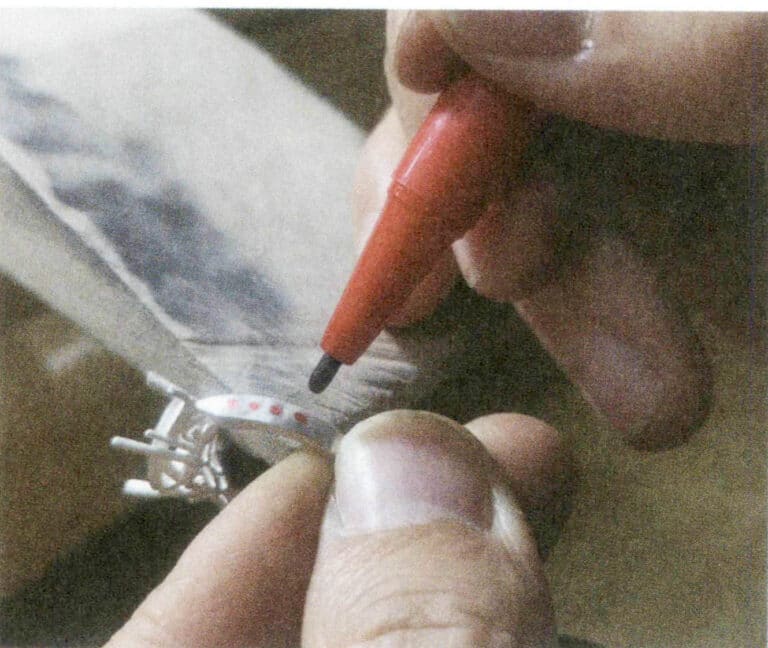
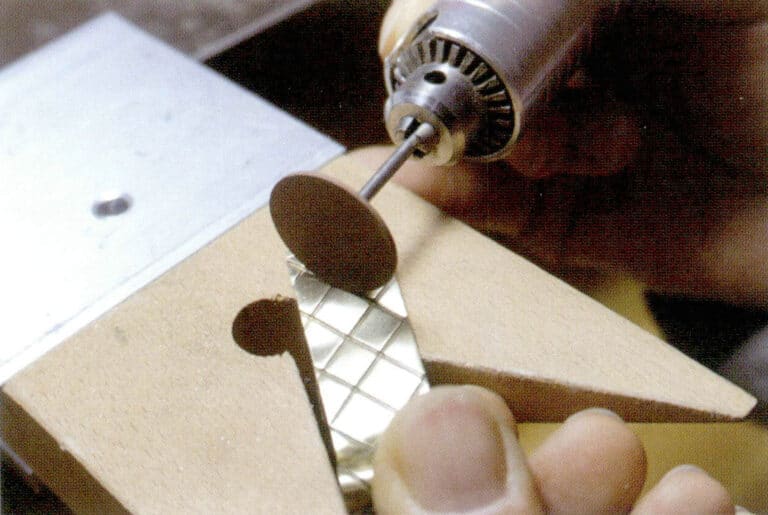
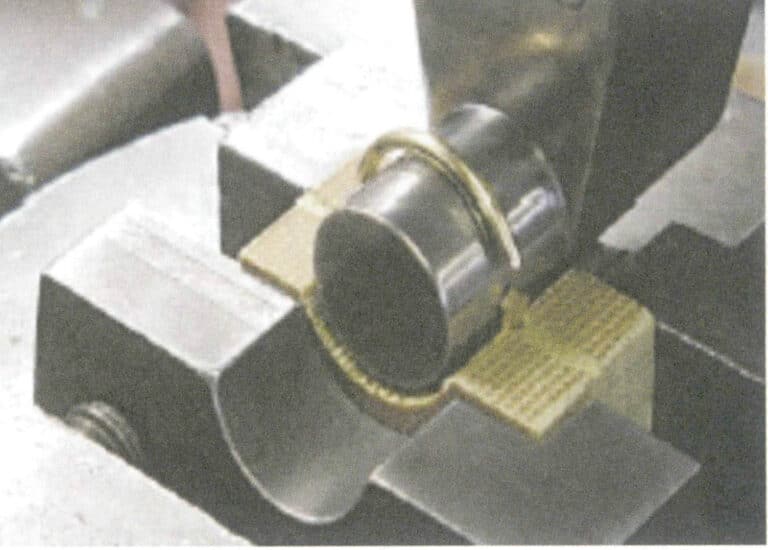
Jak optimalizovat perly a jiné organické drahokamy? jak identifikovat optimalizované organické drahokamy?
Optimalizace zpracování a metody identifikace perel a jiných organických drahých kamenů
Úvod:
Tento článek se zabývá světem perel a dalších organických drahých kamenů, jejich úpravami a identifikačními metodami. Zkoumá chemické složení a barevné variace perel a nabízí pohled na jejich přirozenou krásu a účinky úprav, jako je bělení, barvení a ozařování. Příručka se rovněž zabývá rozdíly mezi přírodními a kultivovanými perlami a poskytuje klenotníkům znalosti pro ověřování pravosti a oceňování těchto drahokamů. Kromě toho se zabývá optimalizací jantaru, korálu a slonoviny a vybavuje čtenáře nástroji, které jim umožní rozeznat skutečnou kvalitu a zpracování organických drahých kamenů.

Perly různých barev
Obsah
Oddíl I Perla
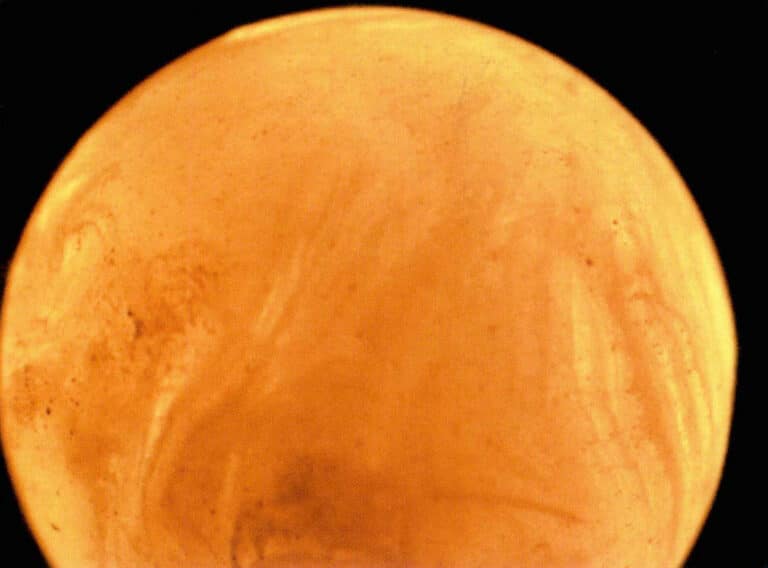
Chemické složení perel zahrnuje: uhličitan vápenatý tvoří více než 80%, organické látky 10% 〜14%, voda 2% 〜4% a další stopové prvky. Barva perel zahrnuje barvu těla a podtón. Tělesná barva je základní barva perly, kterou vytvářejí organické látky a stopové prvky. Podtónem se rozumí jedinečné barvy perel, které vznikají odrazem, interferencí a dalšími účinky světla na povrchu a vnitřních vrstvách perly a které se překrývají s barvou těla. Iridescence perel se týká duhových barev vytvořených na povrchu nebo těsně pod povrchem perly, které jsou komplexním odrazem optických jevů, jako je lom, odraz, difuzní odraz a difrakce způsobené perlou. K tělovým barvám perel patří černá, bílá, růžová, žlutá a další, zatímco k podtónům patří růžová, modrá, zelená atd (obrázek 7-1). Při zvětšeném pohledu je na povrchu perly patrná struktura podobná šindelům, zatímco uvnitř je soustředná vyzařující vrstevnatá struktura.

Existují tři hlavní oblasti produkce perel: oblast Perského zálivu, kde mají perly silný lesk s nádechem zelené duhovky a bílou nebo krémově bílou barvu těla; oblast Srí Lanky, kde mají perly bílou nebo krémově bílou barvu těla se zelenou, modrou nebo fialovou duhovkou; oblast jihovýchodní Asie, kde jsou perly z Jižního moře velké, kulaté, bílé a se silným leskem.
V současné době se na trhu prodávají především přírodní perly, kultivované perly, upravené perly a imitace.
1. Identifikační znaky přírodních perel a kultivovaných perel
(1) Identifikační znaky přírodních perel
Přírodní perly jsou většinou kulaté, na průřezu jsou vidět vrstvy soustředných kruhů perleťových vrstev, které jsou poměrně silné. Jádro cizího tělesa není pouhým okem viditelné.
① Barva:
Přírodní perly mají jednotnou barvu, převážně bílou a růžovou, občas šedočernou, doplněnou různými barvami duhy.
② Struktura:
Struktura je osvětlena silným světelným zdrojem a odhaluje rovnoměrně strukturovanou průsvitnou kouli.
③ Povrchové papuly:
Na povrchu perly jsou zřetelné různě velké výstupky a při jemném tření zubem nebo při tření dvou malých perel o sebe je patrný pocit zrnitosti (obrázek 7-2).
(2) Identifikační charakteristiky kultivovaných perel s jádry
Jádrové perly jsou zpravidla kulaté a jejich barvy zahrnují bílou, žlutou a malé množství černé. Typickým znakem je přítomnost vazebných linií a vnitřních pruhů jádra.
Spojovací linie je hnědá linie mezi perletí a perleťovou vrstvou, jasně viditelná od vrtného otvoru směrem dovnitř; pruhy jádra jsou různě průhledné pruhy na perleti kultivovaných perel; podobně jako přírodní perly mají i kultivované perly na povrchu rýhy.
(3) Identifikační charakteristiky nejaderných kultivovaných perel
Kultivované perly bez jader mají různé tvary: téměř kulaté, oválné, hruškovité, slzovité a nepravidelné. Vyrábějí se také v různých barvách, například bílé, žluté, růžové, fialové a šedočerné. Nejtypičtějším znakem je centrální dutina, což znamená, že při pohledu z vrtaného otvoru je střed prázdný. Na povrchu perly se také nacházejí pockmarky s nápadnými malými výstupky.
Přírodní perly a sladkovodní kultivované perly bez jader mají obecně silnou perleťovou vrstvu, přičemž přírodní perly mají v jádře malé množství cizích látek, zatímco jádro sladkovodních kultivovaných perel bez jader je duté. Naproti tomu perlová vrstva kultivovaných perel s jádrem je velmi tenká, přičemž jádro zabírá převážnou většinu a jádro je navrstveno paralelně.
(4) Rozdíly mezi přírodními a kultivovanými perlami
① Vzhled:
Charakteristika Přírodní perly mají jemnou strukturu, vysokou průhlednost a jemný lesk a jsou většinou nepravidelně kulaté, jednotlivé velikosti jsou menší.
Uměle pěstované perly mají kratší dobu tvorby a relativně nižší jemnost textury a jejich průhlednost a lesk jsou horší než u přírodních perel. Mají většinou kulatý nebo oválný tvar, větší velikost a často se na jejich povrchu objevují znaky, jako je pás a vrásky.
② Zvětšená kontrola:
Perleťová vrstva přírodních perel je silná a zasahuje hluboko do středu perly, má jemné vrstvy a zpravidla nemá žádné zjevné mezery. Při pozorování vnitřního povrchu vyvrtaného otvoru u kultivovaných perel je v blízkosti otvoru vidět zřetelná hnědá linie, která je mezerou mezi vrstvou lastury a perlovým jádrem. Při míchání jehlou může dojít k odpadnutí prášku připomínajícího šupinky.
③ Kontrola přenosu světla:
Při použití silného bodového zdroje světla, který propouští světlo skrz zadní stranu perly, lze při natočení perly do vhodného úhlu na jádru kultivované perly slabě odhalit efekty paralelních pruhů, které se projevují ve vnitřních vrstvách jádra.
④ X Radiografická metoda:
Přírodní perly mají soustřednou vrstevnatou strukturu od středu k vnějšímu plášti. Hranice mezi jádrem a perlovou vrstvou je u perel kultivovaných jádrem zřetelná. Naproti tomu perly bez jádra vykazují vnitřní dutou strukturu a vnější koncentrickou vrstevnatou strukturu.
⑤ Metoda rentgenové difrakce:
Perleťová vrstva přírodních perel je silná a má soustřednou radiální strukturu, přičemž její rentgenový Laueův difrakční obrazec ukazuje šestinásobně symetrický difrakční obraz; jádro kultivovaných perel je větší a má paralelní vrstevnatou strukturu, přičemž její Laueův difrakční obrazec ukazuje čtyřnásobně symetrický difrakční obraz. Pokud je rovnoběžný směr vrstevnatého jádra v souladu se směrem uspořádání krystalů aragonitu vnější perlové vrstvy, lze prezentovat 6násobně symetrický difrakční obraz (obrázek 7-3).
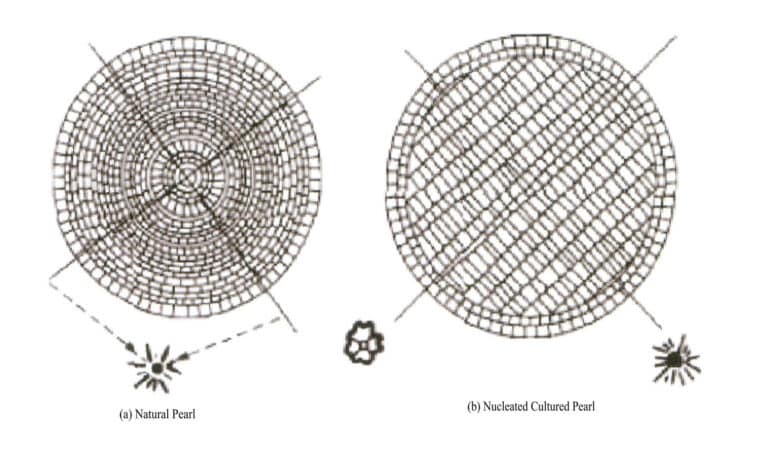
⑥ Metoda rentgenové fluorescence:
Většina přírodních perel pod rentgenovým zářením nefluoreskuje; většina kultivovaných perel s jádry vyzařuje zelenožlutou fluorescenci, kterou způsobují perleťové malé kuličky; kultivované perly bez jader mohou rovněž vyzařovat světlo.
⑦ Pozorování perlovým endoskopem:
Perleťový endoskop má dvě zrcadla směřující k sobě pod úhlem 45°, přičemž vnitřní zrcadlo odráží světlo směrem nahoru a vnější zrcadlo je na dně jehlové trubice.
Vložte endoskop do otvoru pro perlu. Když se jehla nachází ve středu perly, silné světlo z jednoho konce ozáří světelný paprsek a pronikne do soustředných vrstev přírodní perly a odrazí se do trubice jehly. Na zrcadle na druhém konci je vidět mihotání světla. Když světelný paprsek dopadne na jádro kultivované perly, zalomí se mimo jádro, což znemožní pozorování odraženého jasného mihotání na druhém konci perlového otvoru.
Z hlediska vzhledu a struktury se tedy přírodní a uměle pěstované perly zřetelně liší. Přesto jsou v "Názvech šperků a drahých kamenů (GB/T 16552-2017)" jak kultivované, tak přírodní perly označovány jako perly.
(5) Rozdíly mezi mořskými a sladkovodními kultivovanými perlami
Kromě rozdílů ve vzhledových vlastnostech, vnitřní struktuře, hustotě atd. se perly pěstované v mořské vodě a perly pěstované ve sladké vodě liší také obsahem organických látek a stopových prvků.
Sladkovodní kultivované perly mají ve srovnání s mořskými nižší nutriční a léčebnou hodnotu. Obecně platí, že stopové prvky jako S, Na, Mg, Sr jsou v mořských perlách relativně obohaceny, zatímco Mn je relativně ochuzen; u sladkovodních perel je tomu naopak.
Většina mořských kultivovaných perel jsou perly s jádrem, zatímco většina sladkovodních kultivovaných perel jsou perly bez jádra. Lze je identifikovat podle záblesku perlového jádra v silném světle nebo podle struktury perlové vrstvy v místě vrtání.
Hlavní identifikační znaky přírodních a kultivovaných perel jsou uvedeny v tabulce 7-1.
Tabulka 7-1 Hlavní identifikační znaky přírodních a kultivovaných perel
| Metody identifikace | Přírodní perly | Kultivované perly |
|---|---|---|
| Empirical method | The texture is fine, the transparency and luster are better than cultured pearls, and the shape is mostly irregular with a smaller diameter. | The shape is mostly round, with a larger size, but the luster is not as strong as that of natural pearls. |
| Density difference identification method | There is 80% floating in a heavy liquid with a density of 2.713 g/cm3. | There is a 90% sinking in the same heavy liquid. |
| Observation method under strong light source | Uniform structure, good transparency, with strong iridescence and halo, surface has fine lines, delicate texture, smooth surface, thicker nacre layer | Parallel gray-white striped layers of prominent mother-of-pearl nucleus can be seen, with a semi-transparent, greasy appearance, surface often has pits, loose texture, and luster not as strong as natural pearls |
| X-ray diffraction method | Hexagonal pattern spots appear on the Laue photograph, with small nuclei | The pearl layer is thick, with square patterns of spots, and has a large nucleus; the pearl layer is thin. |
| X-ray radiography | It can be displayed as a complete series of concentric circles from the outside to the center. | Cultured pearls with a nucleus will show a strong line around the nucleus in the concentric circular structure; non-nucleated cultured pearls also display a series of concentric lines, but an irregular hollow part appears in the center. |
| Polarizing microscope observation method | Almost fully transparent, with little difference in brightness | The transparent layer is whiter, with a more noticeable difference in brightness |
| Light transmission method | Cannot see the pearl nucleus or the core layer stripes, no striping effect | Most show striping effects, and the pearl nucleus and core layer stripes can be seen |
2. Methods and Identification Features of Pearl Optimization Treatment
The optimization treatment of pearls mainly aims to improve their luster and color, including pre-treatment, bleaching, whitening, coloring, polishing, and repair. The color is improved through physicochemical methods, thereby enhancing the practical value of pearls. The main optimization treatments for pearls are bleaching, dyeing, and irradiation.
2.1 Bleaching
The bleaching of pearls refers to treating pearls in an oxidative solution to remove discoloration or whiten-colored substances. The methods of bleaching pearls include chemical bleaching, light exposure, thermal decomposition, and decolorization.
(1) Purpose
Bleaching is the most important step in the pearl optimization process. Its main purpose is to remove the dirt and black spots on the surface of the pearl and the yellow pigments in the pearl layer, making the color whiter. The reagents used for pearl bleaching mainly consist of bleaching agents, solvents, and surfactants. The main bleaching agent is hydrogen peroxide, and the solvents include organic solvents and distilled or deionized water, primarily to dilute the concentration of hydrogen peroxide and to enhance its penetration into the pearl. Surfactants are very important additives; their main function is to reduce the surface tension of the bleaching solution, disperse the bubbles that form on the surface of the pearl during the bleaching process and gradually accumulate, and achieve uniform and rapid wetting, emulsification, dispersion, and penetration. The main role of bleaching is to remove the mixed colors often carried by organic gems due to the presence of shell material or other organic substances. Bleaching treatment does not require labeling and is considered optimization.
(2) Process
① Pre-treatment:
The treatment of pearls mainly includes sorting, drilling, swelling, and dehydration. The purpose is to make it easier for subsequent improvement processes. For example, drilling aims to facilitate the penetration of chemical liquids for degreasing, bleaching, whitening, and dyeing into the pearl. Due to the tight layered structure of the pearl, the bleaching solution makes it difficult to penetrate the inner layer of the pearl; swelling uses a swelling agent to make the pearl structure “looser” without causing obvious damage to the pearl, and then the pearl is bleached.
② Dehydration treatment:
Dehydration refers to the removal of residual water in the gaps of the pearl from the above processes, often using anhydrous ethanol as a dehydrating agent, and pure glycerin can also be used to remove the adsorbed water in the internal gaps of the pearl.
③ Formula:
The formula uses hydrogen peroxide as a bleaching agent and solvents, surfactants, pH stabilizers, and other reagents. The pearls are immersed in the prepared solution and heated at 70 〜80℃. The treatment time depends on the depth of the color. The more pronounced the color variations in the pearls, the longer the soaking time.
(3) Identification characteristics of bleached pearls
Bleached pearls have the following characteristics:
① Loose structure:
After bleaching treatment, the pearls have a clean surface color, and the gaps between the layers of the pearls increase, making the structure looser, which may damage the luster.
② Acid etching:
Gems bleached with acid treatment will show acid-etched structures. The surface of bleached pearls often has a very clean base color, and upon magnified inspection, acid etching patterns can be observed on the surface.
2.2 Dyeing
By using different chemical reagents, white or light-colored pearls can be dyed into various colors.
(1) Black dyeing process
Soak the pearls in a dilute silver nitrate and ammonia solution, then place the soaked pearls in sunlight or expose them to hydrogen sulfide gas for reduction, turning the color of the pearls to black. The black tones of dyed pearls are very similar to those of naturally colored pearls, and the treated color is stable to light and heat.
(2) Brown dyeing process
Using potassium permanganate solution as a coloring agent can turn pearls brown.
(3) Pink dyeing process
Placing pearls in a solution made of alkali and cobalt salts can make the pearls appear pink.
(4) Central dyeing method
Dye is injected into the drilled holes of artificially cultivated pearls to color them, with the dye chosen based on the desired color.
(5) Identification features of dyed pearls
① Barva:
Dyed black pearls exhibit a deep gray-black tone with uneven color distribution on the surface. Especially at the holes, a clear color of inhomogeneity can be seen (Figure 7-4 ).
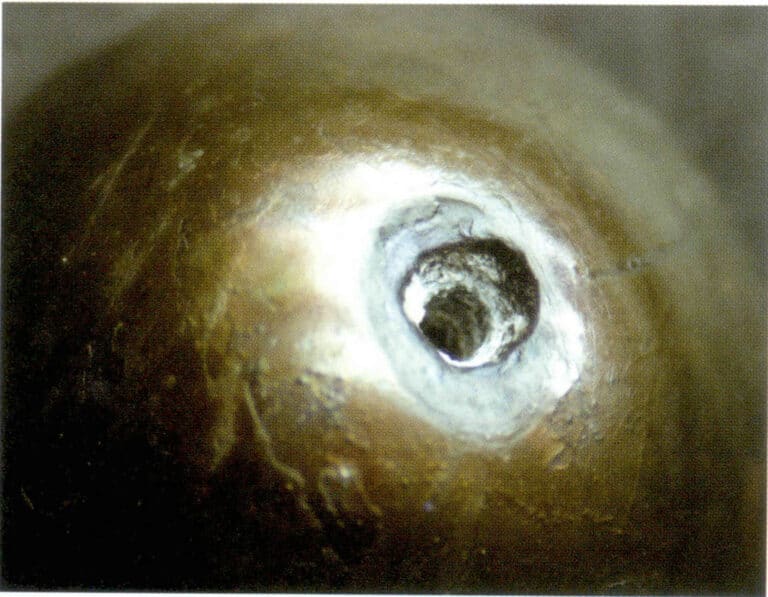
② Internal feature:
The phenomenon of interference halos can be seen under internal characteristic reflected light beneath the thin layer of the pearl layer.
③ Chemical method:
Wipe the pearl with a cotton swab dipped in dilute nitric acid with a concentration of 2%. The blackened pearl with silver nitrate will stain the cotton swab black. A cotton swab dipped in acetone can also cause the colored pearls (red, blue, yellow) to fade.
Methods such as ultraviolet fluorescence, X-ray photography, Raman spectroscopy, and ultraviolet-visible spectrophotometry can also distinguish dyed black pearls from natural black pearls. The main identification features of dyed black pearls and natural pearls are shown in Table 7-2.
Table 7-2 Main identification features of dyed black pearls and natural black pearls
| Charakteristika | Natural black pearl | Dyed black pearl |
|---|---|---|
| Appearance characteristics | Deep blue-black with slight rainbow-style shimmer or black with a bronze tint (not pure black) | Pure black, uniform color, poor luster, radiance, unnatural accompanying colors |
| Kontrola zvětšení | Surface is delicate and smooth or has growth textures. No color accumulation at surface flaws or fissures | The color is concentrated in the fissures and surface flaws or fissures, with visible signs of corrosion and fine wrinkles on the surface nacre. Pearls with dyed nuclei show distinct parallel stripes of the nucleus under strong light transmission, or when viewed under reflected light through the pearl hole, the nucleus appears very dark in color while the surface is colorless nacre.s nacre. |
| Ultraviolet fluorescence characteristics | Generally appear dark red-brown or red fluorescence under long-wave ultraviolet light. | Inert or dark green fluorescence; pearls with dyed nuclei exhibit ultraviolet fluorescence from the dye. |
| Rentgenová fotografie | A distinct connection band can be seen between the nacre layer, hard protein, and pearl nucleus on the substrate. | Due to silver often depositing in the hard organic protein layer between the pearl layer and the nucleus, the photo presents white stripes. |
| Acetone wipe | Does not fade | Fades |
| Nitric acid experiment | Does not fade | If a cotton swab dipped in dilute nitric acid concentration of 2% turns black, it indicates that the pearls are dyed using the silver nitrate staining method. |
| Ramanova spektroskopie | Has absorption lines of aragonite and organic porphyrin | Has a strong fluorescent background, with only the absorption peak of aragonite or the dye's absorption peak |
| Ultraviolet- visible absorption spectrum | Absorption peaks around 400nm, 500nm, and 700nm | Typical absorption peak without pearls |
| Powder | White powder | Black or gray-brown powder |
2.3 Irradiation Method
(1) Irradiation Source
Light-colored pearls can turn black through X-rays and γ rays of irradiation. The general method is to place the pearls in a cobalt source of 3.7 x 1013 Bq intensity, irradiating for 20 minutes at a distance of 1 cm from the irradiation source at room temperature. The color of the irradiated black pearls is similar to that of natural pearls, and their stability is relatively good.
(2) Sample Requirements
Limited to freshwater pearls containing manganese elements and the pearl layer of shallow-water mussels, natural pearls grown in seawater, and the pearl layer attached to the outer layer of nucleated pearls cannot change color.
(3) Identification Features
① Iridescence:
The black pearl that has changed color due to radioactive irradiation exhibits an intense spectrum of colors, accompanied by a strong metallic luster.
② Granularity:
Cultivated black pearls rarely have a diameter smaller than 9 mm, and round, nucleated black pearls smaller than 8 mm are generally products that have been color-treated by radioactive irradiation.
The surface color distribution of irradiated pearls is uniform, but from the cross-section, the internal color is lighter, while the outermost pearl layer is usually darker. The thickness of irradiated black pearls can reach 3 〜 4 mm.
2.4 Other Treatments of Pearls
(1) “Peeling” treatment
Peeling treatment involves carefully removing the unattractive surface layer of the pearl using a very fine tool, revealing a better layer underneath to serve as the surface. This operation is very difficult and requires highly skilled personnel; sometimes, even after peeling several layers, a better layer can only be found once the pearl’s substance is completely stripped away.
(2) Surface crack filling method
Treatment method: Soak the pearl in a high refractive index oil, such as olive oil, to fill the fissures with oil. To ensure even filling, heat to around 150℃ and maintain for a period to allow the oil to fully penetrate the fissures. Pearls filled with oil after this process exhibit a noticeable oily luster, and oil can be extracted with a heated needle.
(3) Surface coating
For some pearls with fissures, a thin layer of a colorless and transparent adhesive is applied to the surface of the pearl to fill the fissures. This method often gives the pearl a yellowish tint, making it easy to detect.
3. Methods for Identifying Treated Pearls
(1) Ultraviolet Fluorescence Method
Natural black pearls appear bright red to dark reddish-brown under long-wave ultraviolet light; dyed black pearls show little fluorescence or appear dark green under long-wave light.
(2)X-ray Fluorescence Spectroscopy Method
X-rays are used to irradiate and measure the wavelength of its fluorescence with a spectrometer. This method can detect silver elements in pearls dyed with various silver salts, but the pearls may turn dark brown using this method.
(3) X-ray photography method
The principle of distinguishing between natural and cultured pearls is that different materials have varying degrees of transparency in X-rays, resulting in different colors on the developed film.
Pearls treated with silver have silver deposited in the hard protein layer between the pearl nacre and the nucleus, which does not transmit X-rays, causing the hard protein layer to appear white in X-ray photographs. In treated black pearls, the annular blank area surrounding the nucleus is also known as the reversal ring.
(4) X-ray diffraction method
① The patterns of natural pearls in transmission and diffraction have 6 points because the calcite crystal axis is arranged radially.
② The diffraction patterns of non-nucleated cultured pearls are the same as those of natural pearls.
③ Nucleated cultured pearls can produce a diffraction pattern with 4 points when transmitted in most directions, but when transmitted from two angles that are mutually 90°, a diffraction pattern with 6 points can be obtained. If the pearl layer is thick, the diffraction pattern will be the same as natural pearls when illuminated from any direction.
4. Identification of Pearls and Imitations
As early as the 17th century, France produced imitation pearls by applying “pearl essence” extracted from fish scales onto glass balls. Currently, the main types of imitations on the market include: plastic imitation pearls, wax-filled glass imitation pearls, solid glass imitation pearls, bead nucleus-coated imitation pearls, and coated treatment pearls.
(1) Plastic Imitation Pearl
A “pearl essence” layer is applied to the milky white plastic. At first glance, it looks beautiful, but upon closer inspection, the color is monotonous and dull, and the size is uniform.
Identification features: Light to the touch, with a warm sensation. There are depressions at the drilled holes; if you poke it with a needle, the coating will flake off in pieces, revealing the new pearl nucleus. Under magnification, the surface shows a uniformly distributed granular structure. No fluorescence under UV light, and it is insoluble in hydrochloric acid.
(2) Glass Imitation Pearl
Divided into hollow glass filled with wax and solid glass imitation pearls.
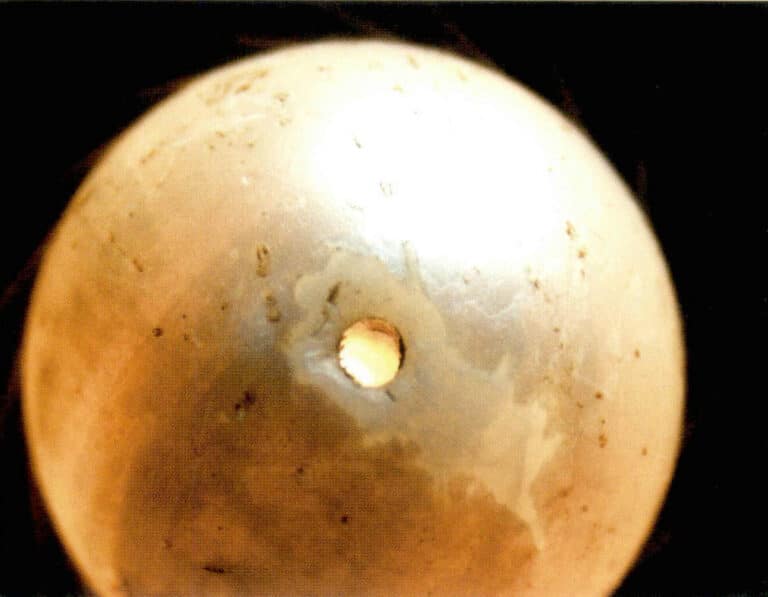
Common points: It feels warm, cannot be marked with a needle, and the surface peels off in sheets. The pearl core has a glassy luster, and whirlpool patterns and bubbles can be found. Under polarized light, it shows homogeneity, is insoluble in hydrochloric acid, and has no fluorescence.
Differences: Hollow glass filled with wax imitation pearls is lightweight, has a 1.5 g/cm3 density, and feels soft when a needle is inserted into the drilled hole. Solid glass imitation pearls have a density of 2.85 〜3.18 g/cm3. It is noticeably heavier when held, and the surface of the glass imitation products has a very thin layer of pearl essence that forms the imitation pearl layer, often scratched (Figure 7-5).
(3) Shell imitation pearls
Made by grinding the pearl layer on thick shells into round balls or other shapes, then coated with a layer made from “pearl juice.”
Identification features: Good simulation effect, with a noticeable pearly luster on the surface. The main difference is that when observed under magnification, the unique growth spiral patterns of the pearl’s surface cannot be seen, and it only appears as a monotonous rough surface similar to that of an eggshell, exhibiting a “flame-like” structure characteristic of shells.
(4) Coated pearls
① Polymer-coated pearls:
A layer of thick colorless polymer (plastic) is applied to the surface of the less lustrous black nucleated cultured pearls from the Taqi River. The identification feature is that the luster does not come from the surface like natural pearls but from the bottom of the polymer layer; the color of the pearl appears inconsistent in tone when viewed from the top and the side; bubbles and uneven surfaces are visible; lower hardness with more surface scratches.
② Silica-coated pearls:
A layer of polydimethylsiloxane is applied to the surface of the pearls. The surface is smooth and feels slippery to the touch. Upon magnified inspection, it is difficult to observe the edges of the pearl’s overlapping platelets, and sometimes the colorless coating layer and surface scratches can be seen.
Section II Amber
Amber contains organic substances such as succinic acid and amber resin. Amber is a common organic gemstone, with a chemical composition of C10H16O, containing a small amount of hydrogen sulfide, and trace elements such as Al, Mg, Ca, Si, Cu, etc. Different ambers have certain differences in their composition. Amber is a type of resin fossil formed from tree resin buried underground tens of thousands of years ago after undergoing certain chemical changes. It is an organic mineral that has been completely petrified over tens of thousands or even hundreds of millions of years.
Amber has a wide variety of colors: light yellow to honey yellow, yellow-brown to brown, dark brown, and orange, while blue, light green, and pale purple are rare. Amber comes in various shapes, and its surface often retains the patterns created during the initial flow of the resin. The interior of amber contains many types of inclusions, which can be seen under magnification, including animals, plants, gas-liquid inclusions, spiral patterns, impurities, fissures, and other internal inclusions (Figure 7-6). The refractive index of amber is 1.54, and its density is approximately 1.08 g/cm3, allowing it to float in a saturated salt solution.
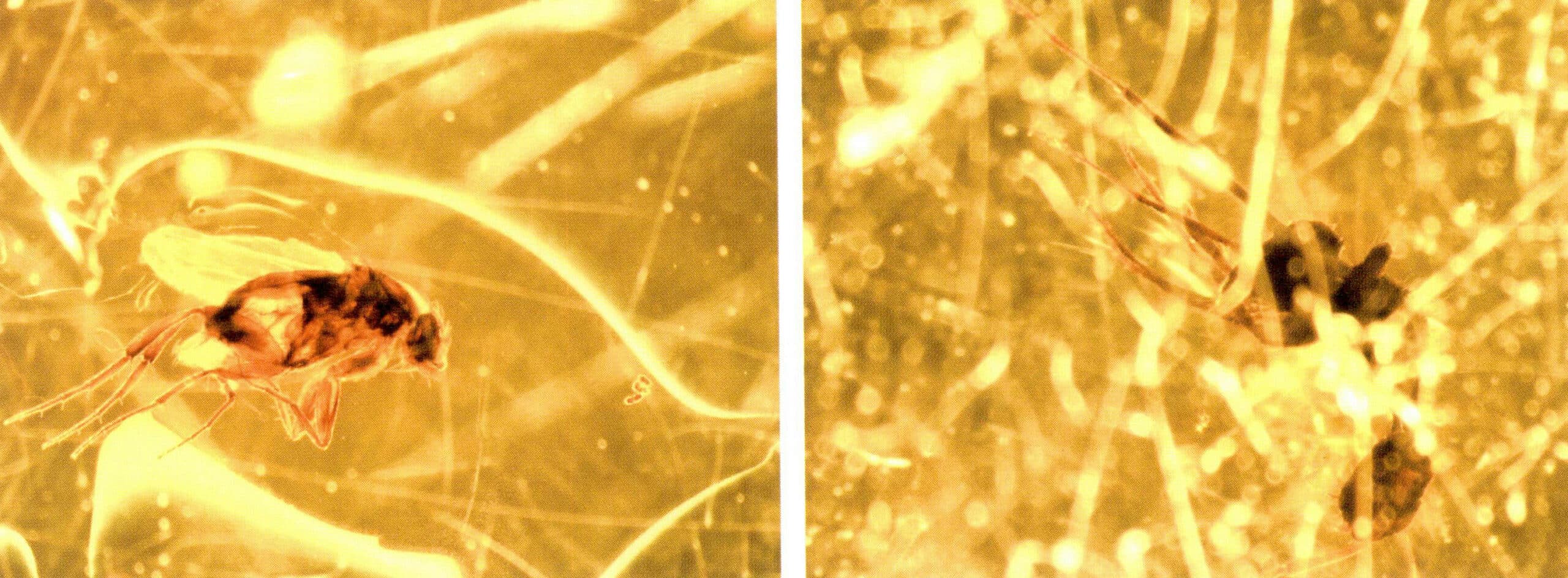
Amber is an organic gemstone that is very popular as a jewelry accessory. Amber has a rich color and comes in various types, suitable for different groups of people to wear. Natural amber often has many imperfections, such as lighter colors and lower transparency, leading people to begin optimizing it during use. The initial optimization method was heating, which increased the transparency of amber. As people’s understanding of amber grew, many optimization methods emerged, such as pressure treatment, color roasting, irradiation, reconstruction, dyeing, and coating. The optimization methods for amber are divided into two main categories: optimization and treatment.
1. Optimization of Amber and Its Identification Features
Common optimization methods for amber include pressure clarification, firing, and heat treatment.
(1) Pressure Clarification
Natural amber usually contains bubbles inside; too many bubbles can make the amber appear cloudy. Pressure clarification refers to the heating and pressurizing treatment of opaque amber materials to allow the internal bubbles to escape, making it clear and transparent. After pressure clarification, the transparency of amber can be increased, enhancing its appearance and economic value. This method is mainly used for amber with poor transparency to improve its clarity. The treated amber has good stability and can be sold as a natural product.
(2) Firing
The firing of amber refers to imitating the natural aging process of amber by using heat to produce a darker reddish-brown color on the surface. Sometimes, it is a partial firing, resulting in a deeper color after treatment. Firing is an accelerated oxidation process; truly ancient amber requires oxidation in a natural environment for over a decade or even decades. However, using roasting equipment, natural amber can be heated and oxidized quickly, achieving decades of oxidation effects in about half a month to a month. This firing technique originated in Europe and has about four hundred years of history. The color of amber after firing is stable and can be sold as a natural product.
(3) Heat Treatment
The purpose of heat treatment is to increase the transparency of amber. Heating cloudy amber in vegetable oil makes it more transparent. During the process, internal bubbles may burst, resulting in leaf-like fissures, which typically feature inclusions resembling “water lily leaves” or “sunlight rays.”
Identification features: Natural amber can also crack due to geothermal heat, but under natural conditions, the heat is uneven, and not all bubbles can burst. In treated amber, all bubbles have burst, so there are no bubbles present, and it is common to see disc-like fissures resembling “sunlight rays” due to heating (Figure 7-7).

Kopírování @ Sobling.Jewelry - Výrobce šperků na zakázku, továrna na šperky OEM a ODM
2. Treatment of Amber and Its Identification Features
Common processing methods for amber include reconstituting, dyeing, and coating.
(1) Reconstituting (pressing) amber and identification
Since some pieces of amber are too small to be directly used for making jewelry, these amber fragments are sintered at appropriate temperatures and pressures to form larger pieces of amber, known as reconstituted amber, also referred to as pressed amber, melted amber, or molded amber. To ensure pure color and high transparency, the amber must first be purified during the production of reconstituted amber.
The process involves: crushing the amber to a certain particle size, removing impurities through gravity flotation, and pressing it into shape under a pressure of about 2.5MPa and 200 〜300℃ temperature. Different temperatures and times during pressing can yield different products, with certain variations in their internal characteristics. Additionally, other organic materials such as dyes, fragrances, and binders can be added during the pressing process. This type of pressed amber requires higher temperatures and longer times to achieve a uniform, transparent product without obvious flow structures.
The naked eye can observe that there are some dark red areas inside the pressed amber, resembling capillaries, appearing thread-like, misty, or grid-like. Due to long-term exposure to air, the surface of the amber oxidizes over time, forming a thin red oxide film; the closer to the surface, the more pronounced the oxidation, and the redder the color, while the interior of the amber retains its original color. During the pressing process, traces of deeper red, thread-like particles can be seen on the surface, which are clearer under ultraviolet light. Natural amber can sometimes crack due to temperature, humidity, and other conditions, and the resulting fissures can also oxidize to red, but they distribute in a branching pattern along the fissures rather than along the edges of the particles.
There are a large number of bubbles in natural amber, but the bubbles in pressed amber are more abundant. In addition to the bubbles in the amber itself, new bubbles are formed between particles and during the stirring process, with irregularly distributed bubbles throughout the piece of amber. Dense, small bubbles undergo heat treatment and can also burst into water-lily-shaped amber flowers. Still, they are particularly small and mostly arranged directionally, layer upon layer, very densely. This is due to the fact that pressed amber is often subjected to directional pressure during the condensation process, causing tighter contact between particles.
Some reconstructed amber has other substances added during the pressing process, showing functional group characteristics not present in amber in the infrared spectrum, allowing it to be distinguished from natural amber.
Reconstructed amber without additives cannot be distinguished using infrared spectroscopy; at this time, conventional instruments such as microscopes, polarizing microscopes, and ultraviolet fluorescence lamps can be used for detection. The main identification features are summarized as follows (Table 7-3).
Table 7-3 Identification features of natural amber and reconstructed amber
| Identifikační prvky | Natural Amber | Reconstructed Amber |
|---|---|---|
| Barva | Yellow, orange, reddish-brown, etc. | Mostly orange-yellow or orange-red |
| Struktura | Smooth surface | Granular structure, surface appears uneven |
| Tectonic | Has annual ring-like or radial texture | Early products have a fluid-like structure, while the new pressing has a syrupy and blood-like swirling structure. |
| Hustota/ (g/cm3) | 1.05 ~ 1.09 | 1.03 〜 1.05 |
| Ultraviolet fluorescence characteristics | Light blue or light yellow fluorescence | Strong chalky blue fluorescence |
| Aging | The color darkens, appearing slightly red or brown | Over time, the color turns white |
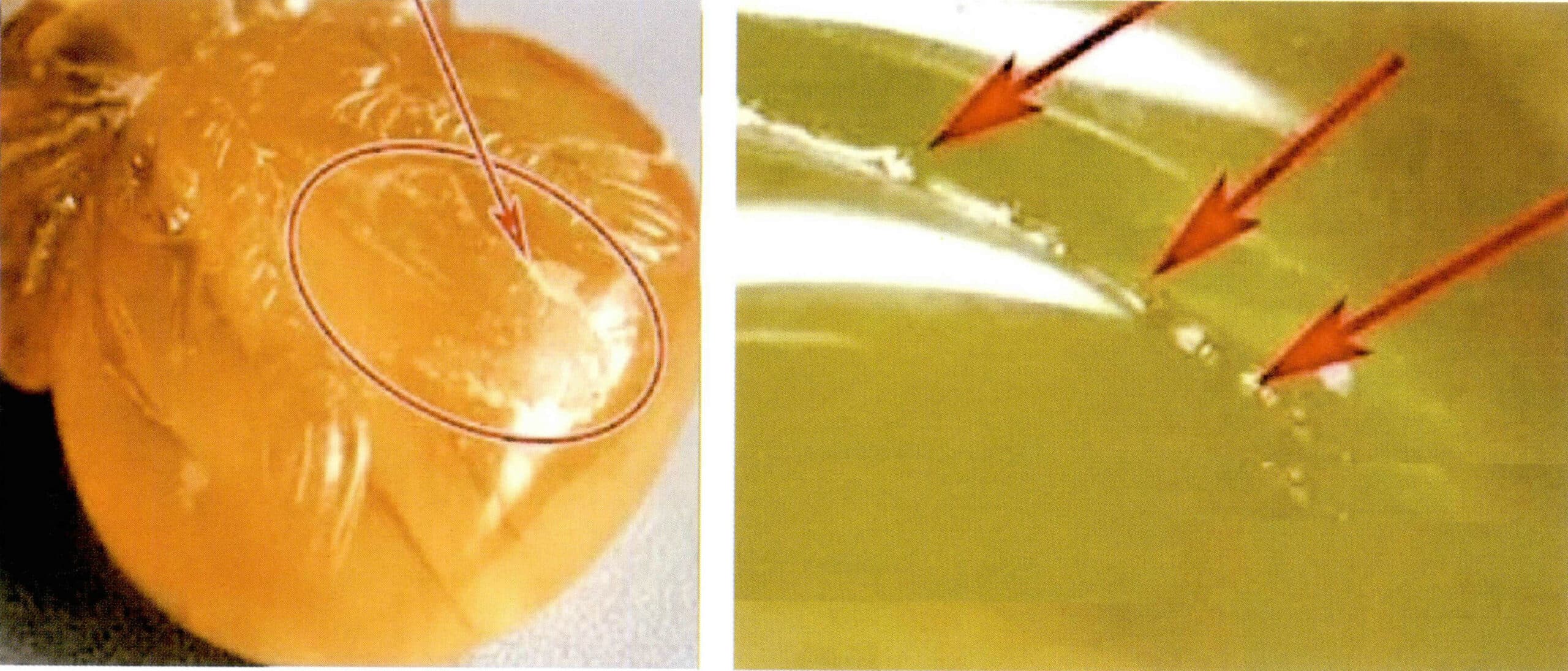
① Magnified inspection:
Magnified inspection under a microscope reveals “blood vessel”-like structures and the cracking patterns distributed along the “blood vessels,” as well as unmelted particles and contact surface boundaries, with uneven particle boundaries visible on the surface (Figure 7-8).
② Characteristics under cross-polarization:
Extinctions under cross-polarization exhibit a distinct partition phenomenon, with clear boundaries and a strong granular feel, and sometimes are accompanied by abnormally influenced colors.
③ Ultraviolet Fluorescence:
Characteristics The ultraviolet fluorescence characteristics of some reconstructed amber display bright chalky blue fluorescence, and the edges of amber particles may sometimes show stronger fluorescence, often consistent with the distribution direction of “blood streaks” observed under a microscope.
(2) Dyeing Treatment
Amber will turn red after being exposed to air for several years. To mimic this aging characteristic, amber can be dyed red with dyes, and it can also be dyed green or other colors.
The main identification features can be observed with a microscope or magnifying glass, checking whether the quality of the amber is uniform and if there are any fine impurities mixed in during the polymerization process. Additionally, the color can be examined for uniformity and whether it is darker or accumulated in fissures. If color gathers in the fissures or pits of the amber, it indicates that it is dyed amber.
Amber that is only dyed on the surface is relatively easy to identify; simply puncturing a discreet area with a needle can reveal whether the inside matches the outside. Wiping dyed amber with a cotton swab soaked in acetone will cause the sample to fade, and color will appear on the swab.
(3) Coating Treatment
Generally, a colored film is applied to the bottom to enhance the three-dimensional effect of “sunlight” in light-colored amber. Upon careful observation under a microscope, the color of the oxidized surface of natural amber transitions naturally with the color produced after firing, whereas the color layer of coated amber is shallow, lacks transition, is unevenly colored, and often shows signs of spraying. Due to the thinness of the film layer and its lower hardness, there are often instances of partial peeling, and bubbles can sometimes be seen at the junction between the film and the amber surface (Figures 7-9).
3. Identification of Amber and Similar Materials
Gemological gemstones similar to amber include carnelian, colophony, copal resin, and plastic.
(1) Carnelian
Carnelian is (red), orange-red, or brownish-red, with visible color bands, a cryptocrystalline aggregate, and a luster ranging from oily to glassy. It is translucent to slightly transparent, feels cool to the touch, and has a hardness greater than amber. It needs to be cuttable. The refractive index of carnelian is the same as that of amber.
(2) Colophony
Colophony is a type of resin that has not undergone geological processes, appearing light yellow to orange-yellow, with poor transparency, generally opaque to slightly translucent, and has a resinous luster (Figure 7-10). It has a low density and hardness and can be crushed into powder by hand. The surface of colophony has many oil droplet-like bubbles, has poor thermal conductivity, and exhibits strong green-yellow fluorescence under shortwave ultraviolet light. It has a fragrant smell when burned.

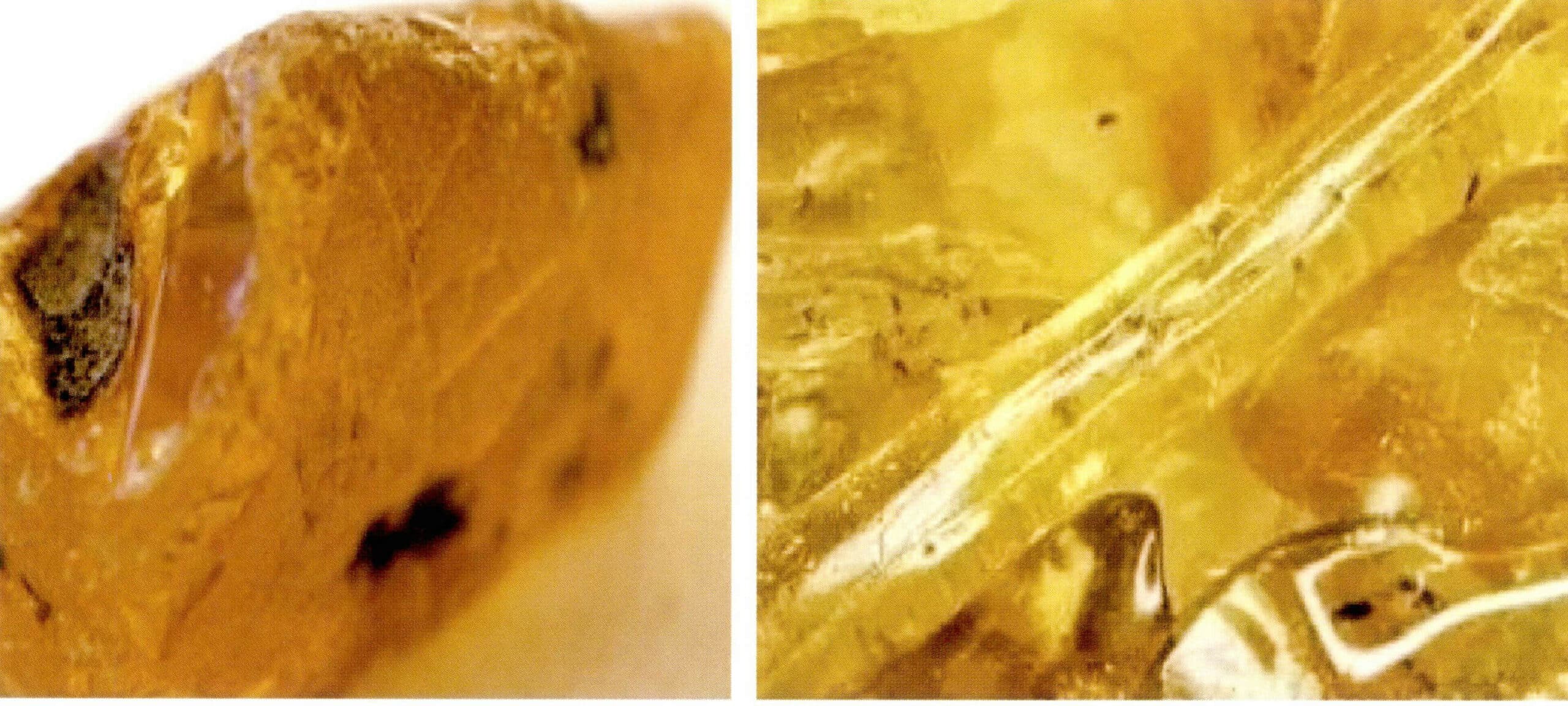
(3) Copal resin (natural hard resin)
Copal resin, also known as copal, is a hard and transparent, amber-colored substance secreted from certain trees’ sapwood and inner bark. Copal resin can be collected from the trees or accumulated in the soil beneath the trees, and if deeply buried underground, it can also be mined. It mainly makes varnishes, natural lacquers, inks, and oils. The hard and dense copal can be used for fine carving and is often confused with amber.
The structural composition of copal resin is the same as that of amber, and it can also contain inclusions of plants and animals, but it is younger than amber. Its basic properties and physical parameters are as follows:
① The physical parameter refractive index is 1.54 (point measurement), and the relative density is 1.060.
② Under ultraviolet florescence, the luminescence characteristics show blue-white fluorescence in the long wave and a weak light purple in the short wave.
③ Hot needle reaction: The hot needle probing produces a resinous aromatic smell.
The physical parameters of copal resin and the hot needle reaction are similar to amber. The main identification basis is that their infrared spectra are completely different, and they can also be assisted by solubility and ultraviolet light characteristics. A small drop of ether is placed on the surface of the copal resin and rubbed with the hand; the resin will soften and become sticky. Ethanol can also be used to distinguish amber from copal resin. After applying ethanol to the surface of amber, there is no reaction. Still, if ethanol is applied to the copal resin’s surface, the surface of the copal resin will become sticky and opaque (Figure 7-11).
(4) Plastic amber imitations
Plastic amber imitations include phenolic resin, celluloid, polystyrene, and acrylic glass, among others. The relative density of amber is the lowest among gemstones, allowing for the separation of amber from phenolic plastic (bakelite) (refractive index 1.61-1.66, relative density 1.25) and celluloid (refractive index 1.49-1.52, relative density 1.38). The initial plastic amber imitation products had a distinct flowing structure, and to resemble amber, they often contained disc-shaped fissures inside (Figure 7-12).
The density of plastic imitations is greater than that of amber, and saturated salt water can distinguish amber from plastic imitations. Amber floats in saturated saltwater, while phenolic plastic, celluloid, and other plastics sink. Polystyrene (refractive index 1.59, relative density 1.05) has a relative density close to amber and can have animal inclusions added inside.
When detected with a hot needle, amber emits a pine resin smell, while polystyrene gives off an unpleasant, spicy odor of burning plastic. Plastic is cuttable; when sliced with a small knife in inconspicuous areas of the sample, it will flake off in sheets, while amber produces small notches. When burned, plastic melts, while amber can burn and smoke, leaving only burn marks but not melting.
The main differences between amber, copal, and synthetic resin are shown in Table 7-4.
Table 7-4 Differences between amber, copal resin, and synthetic resin
| Charakteristika | Amber | Copal resin | Synthetic resin (plastic) |
|---|---|---|---|
| Gas-liquid inclusions | Circular or irregular bubbles | Visible bubbles | Round bubbles |
| Plant and animal inclusions | Struggling animal inclusions | Struggling animal body | Contracted insect body |
| Vortex pattern | Annual ring or radial | Annual ring or radial | Intertwined, wavy flow structure |
| Ultraviolet florescence characteristics | Medium blue-green florescence | Strong white florescence | Weak or no florescence |
| Cuttable | Non-cuttable | Non-cuttable | Cuttable |
| Soluble | Ether is insoluble | Kneading can change its viscosity | Ether can corrode surfaces |
| Ostatní | Has a fragrant smell, flammable | Has a fragrant smell, flammable | Has a spicy or plastic taste |
Section III Coral
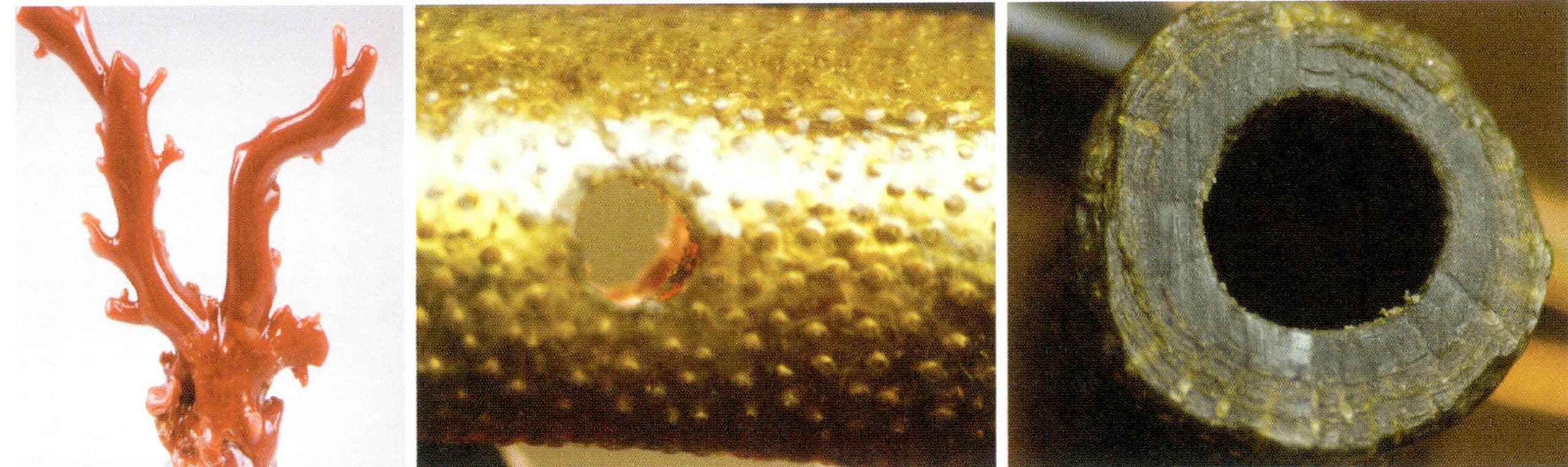
Coral is divided into calcareous and keratinous corals based on internal composition and structure. Calcareous coral mainly comprises inorganic components, organic components, and water; keratinous black coral and golden coral are almost entirely made of organic matter, with little or no calcium carbonate. Calcareous coral commonly appears in white, cream, light pink to deep red, orange, and occasionally blue and purple; the common colors of keratinous coral are golden yellow and black. The refractive index of calcareous coral is 1.486 〜1.658, while keratinous coral’s is about 1.56. The density of calcareous coral is 2.60 〜2.70 g/cm3, and that of keratinous coral is 1.30 〜1.50 g/cm3.
1. Internal and External Characteristics of Coral
Coral has regular growth characteristics, with different longitudinal and cross-sect growth structures.
(1) In the longitudinal section, the coral polyp cavity exhibits parallel wavy stripes with slight variations in color and transparency.
(2) The cross-section shows a radial and concentric circular structure. Black coral and gold coral cross-sections display concentric ring structures surrounding the primary branch axis, with a surface appearance of small bumps (Figure 7-13).
2. Optimization treatment of coral and its identification characteristics
(1) Bleaching (optimization) of coral and identification
Bleaching is a common optimization treatment for coral. The purpose of bleaching is to remove surface discoloration, making the main color of the coral more vibrant. After the coral is processed into fine pieces, it is usually bleached with hydrogen peroxide to remove its murky colors, such as brownish-yellow. In contrast, unbleached coral often appears murky yellow.
Different coral materials can achieve different colors after bleaching. Dark-colored coral can be bleached to obtain light-colored coral, such as black coral being bleached to golden yellow, while dark red coral can be bleached to pink. This optimization treatment is difficult to detect and can be directly named after the coral.
(2) Dyed coral and identification
Dyeing is commonly used for calcareous coral, where white or light-colored coral is soaked in red or other colored organic dyes to achieve the corresponding color.
Identification characteristics of dyed coral: Wipe with a cotton swab soaked in acetone, the cotton swab becomes stained, and the wiped area shows a fading phenomenon; the color of dyed coral is monotonous and inconsistent inside and outside. Under magnification, dye is concentrated in small fissures and holes between calcite particles, with deeper color outside, lighter inside, and uneven coloring (Figure 7-14). Dyed coral can easily show color phenomenon or lose luster after prolonged wear.

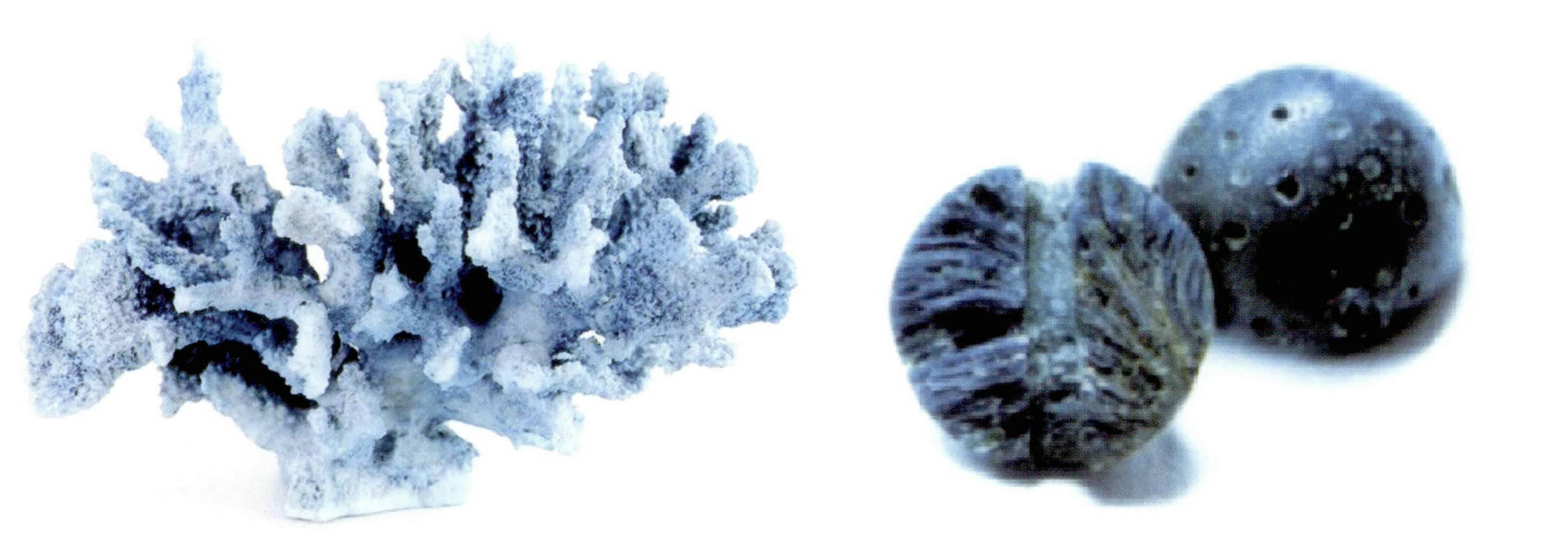
(3) Filling treatment of coral and identification
Filling porous inferior coral with substances like epoxy resin is commonly used for structurally loose calcareous coral (Figure 7-15). The density of filled coral is lower than that of natural coral; in the hot needle test, substances like resin may be precipitated from filled coral.
(4) Coating treatment of coral and identification
For corals with loose texture or poor color, a coating treatment is commonly applied with black and golden coral materials. The coated black coral has a strong luster, and the papule-like protrusions are relatively flat (Figure 7-16). Wiping with acetone shows signs of color fading.

3. Identification of corals and similar products
Similar products to coral include dyed bone products, dyed marble, and conch pearls.
(1) Barvené výrobky z kostí
Dyed bone products are a common type of coral imitation, typically made from animal bones such as cow bone, camel bone, or elephant bone that have been dyed or coated to resemble coral.
Cross-sectional features: In cross-section, coral has a radial and concentric circular structure, while bone products have a round hole structure; in longitudinal section, coral has continuous wavy textures, while bone products have intermittent straight textures and hollow tubular structures (Figure 7-17).
① Color features:
Coral is uniformly red; dyed bone products have inconsistent colors inside and out and may fade, with colors that can lighten.
② Fracture:
Coral is brittle with a relatively flat fracture; bone products are tough with jagged, uneven fractures.
③ Reaction with hydrochloric acid:
Coral reacts with dilute acid, while bone products do not react with acid.
④ Sound:
When struck, coral produces a crisp and pleasant sound; bone products sound dull and murky.

(2) Barvený mramor
Dyed marble does not have the appearance characteristics and structural features of coral. Dyed marble has a granular structure with layered textures, and the color is distributed along the edges of the grains (Figure 7-18). The swab will be stained when wiped with a cotton swab dipped in acetone.
The solution of dyed marble after reacting with dilute acid is red, while the solution of red coral after reacting with dilute acid is white.
(3) Conch Pearl
The color of conch pearls has distinct layered pink and white patterns, resembling the appearance of a amazonite, and the luster has a certain directionality. It features a characteristic flame-like structure, with a relative density (2.85) greater than corals.
(4) Rhodochrosite
Rhodochrosite is pink to red, with distinct banded layers, and the boundaries between layers are mostly serrated, with clear adjacent boundaries. Its relative density is 4, much greater than that of coral.
(5) Red Jasper
The main component of red jasper is SiO2, containing impurities of iron oxide and clay. It has a cryptocrystalline structure, lacks the coral’s ridge-like structure, and fine particles of clay and iron oxide can be seen under magnification. Red jasper’s relative density is greater than coral’s and has a stronger luster.
(6) Gilson Coral
Gilson coral is a material made by binding calcite powder with a small amount of dye under high temperature and high pressure, and its color variation range is quite large. Gilson coral has a uniform color, and under magnification, a granular structure can be seen, lacking the ridge-like appearance of coral, with a relative density of 2.45, which is smaller than that of natural coral.
(7) Red Glass
An opaque glass material on the market—red glass, which can imitate coral. The main difference between red glass and coral is that they do not have coral’s appearance, characteristics, or special structure. Red glass has a distinct glassy luster, develops shell-like fractures, and sometimes shows pores on the surface. Its Mohs hardness is higher than coral’s and does not fizz when encountering hydrochloric acid.
(8) Red plastic
Plastic does not have the appearance, color distribution characteristics, and special structure of coral and often shows marks left by molds. The relative density is 1.05 ~ 1.55, and common bubbles can be seen upon magnification; the surface is uneven, and a hot needle test may produce a spicy smell, with no bubbling when encountering hydrochloric acid.
(9) Dyed shell
Common colors of shells are white, light yellow, and light brown. Light-colored shells can be dyed red, and the dyed shells are often used to imitate pink coral. Identification characteristics of dyed shells: the surface of the shell has a pearly luster and a layered structure, and the color accumulates between the layers after dyeing (Figure 7-19). It can be tested by wiping it with a solvent or dropping it in dilute acid.

Currently, imitation sea bamboo coral products on the market resemble the color appearance and coral structural features. Dyed sea bamboo coral imitates the radial patterns of coral cross-sections, also known as “sun heart,” and has a rough structure with very prominent textures (Figure 7-20).
Section IV Ivory
The chemical composition of ivory is hydroxyapatite and organic matter. Ivory generally has a curved horn shape, with almost half being hollow. The cross-section of ivory is mostly circular or near-circular, with the diameter varying depending on the species, growth period, and growth location of the ivory in different regions. The diameter of the cross-section of the same ivory gradually increases from the tip to the root. The color of ivory is generally white, yellow, light brown, and other shades, with a fine texture and soft luster.
For many years, ivory has been used as a gemstone decoration or for craft displays. However, many elephants are hunted for ivory today, leading to strict restrictions and prohibitions on ivory trade under agreements such as the Washington Convention and the Convention on International Trade in Endangered Species of Wild Fauna and Flora. Today, the ivory trade is resisted and prohibited to protect elephants.
1. Classification and Structure of Ivory
African ivory is generally longer, relatively harder, and is milky white, mainly sourced from Tanzania, Cameroon, Ghana, and Ivory Coast. The highest quality ivory bracelets come from Ivory Coast. Asian ivory is generally shorter and white but prone to yellowing, with the best ivory coming from Sri Lanka.
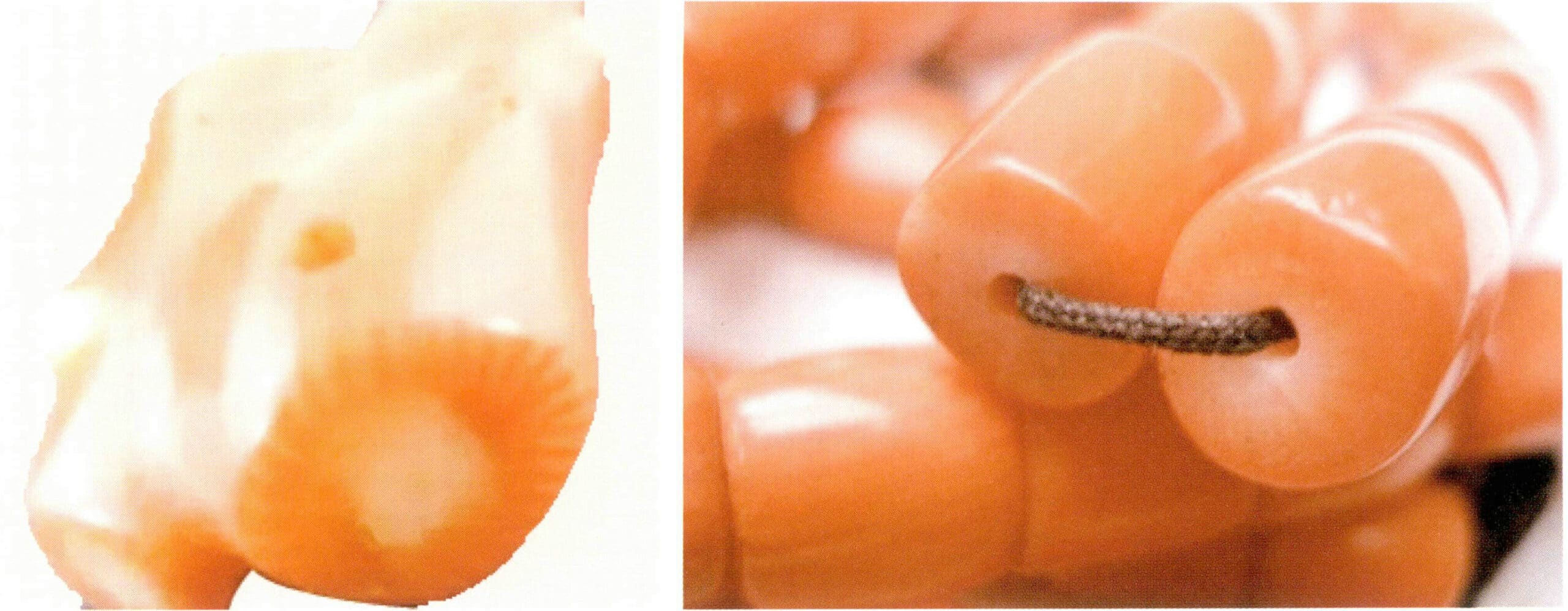
The cross-section of ivory has a layered structure with clear boundaries, generally divided into four layers from outside to inside (Figure 7-21):
Layer I is dense or concentric, resembling the growth rings of trees.
Layer II is a coarse Schreger line layer, with a large angle between the texture lines, up to 124 °, and the spacing between the texture lines is about 1 to 2.5 mm wide.
Layer III is the fine reticulated line layer, with a smaller angle between the texture lines than Layer II, averaging around 120°, and the spacing between the texture lines is very narrow, about 0.1 〜0.5 mm.
Layer IV is dense or cavity-like.
The ivory starts from the tooth tip, with a small black dot that extends to the center of the hollow tube opening, referred to as the core. If the tip of the ivory is cut cross-sectionally, the core of the ivory can be roughly divided into three types: sun core, sesame core, and rotten core. The sun core is the best, followed by the sesame core, and the rotten core is the worst.

2. Optimization treatment and identification characteristics of ivory
The main optimization methods for ivory are bleaching and dyeing.
(1) Bleaching treatment
Ivory that has yellowed over time or has a yellowish tint is soaked in hydrogen peroxide or other oxidative solutions to remove the yellow, aiming to enhance the quality and value of the ivory. Bleaching is an essential optimization treatment for most ivories.
(2) Dyeing treatment
Dyeing involves soaking ivory with undesirable colors in various dyes to achieve the desired color. It is often used in the production of carvings.
Identification features: Under magnification, the dye can be seen distributed along the fissures; when wiped with a cotton swab containing acetone, the sample fades.
3. Common Imitations and Identification Features
(1) Bone Products
Dense bone products are very similar to ivory in appearance, refractive index, relative density, and other aspects, but their structures are different. Animal bones have a hollow tubular structure, with these fine tubes appearing circular or elliptical in cross-section and line-like in longitudinal section. When dirt seeps into the hollow tubes, these structures become more pronounced.
(2) Ivory of Plant
Ivory of plant grow in South America and Africa, with brown skin and a hard shell the size of an egg, which is white or egg-white in color. Its hardness, refractive index, and fluorescence characteristics are similar to ivory.
The cross-section has a honeycomb structure, while the longitudinal section shows parallel, coarse, straight lines with cellular structures. The relative density of the nuts is about 1.4, which is lower than ivory’s.
When soaked in sulfuric acid, ivory does not fade, while ivory of plant present a rose color and are easily dyed. The ivory of plants’ toughness is better than ivory’s, allowing them to be cut with a blade and easily processed.
(3) Plastic
Celluloid is the most common and effective material for imitating ivory. Plastic is pressed into thin sheets to mimic the stripes of ivory’s longitudinal section, but these stripes are much more regular than ivory ones and cannot produce the Lutz pattern.