Introduksjon av rent gullmateriale som brukes i smykkeproduksjon
Pure Gold Feature and its alloy materials for jewelry
Innledning:
Yellow gold has a beautiful color, good chemical stability, excellent aesthetic and collectible value, and a role in preserving and increasing value. It also has outstanding ductility and has been used as decorative materials and currency for jewelry, crafts, and commemorative coins since ancient times.

Innholdsfortegnelse
Section Ⅰ Basic Properties of Gold
1. Physical Properties of Gold
The physical property indicators of gold have multiple aspects, as shown in Table 3-1.
Table 3-1 Main Physical Properties and Index Values of Gold (Partially excerpted from Ning Yuantao and etc, 2013)
| Fysiske egenskaper | Indeksverdier | Fysiske egenskaper | Indeksverdier |
|---|---|---|---|
| Chroma | L* = 84.0, a* = 4.8, b*= 34.3 | Linear expansion coefficient (0 ~ 100℃) | 14.2 x 10-6/℃ |
| Density (18℃) | 19.31 g/cm3 | Resistivitet (25 ℃) | 2.125 x 10-6 Ω • cm |
| Smeltepunkt | 1064℃ | Spesifikk varmekapasitet (25℃) | 25.33 J/(mol • K) |
| Kokepunkt | 2860℃ | Fusjonsvarme | 12.5 kJ/mol |
| Vapor pressure(1064℃) | 0.012 Pa | Fordampningsvarme | 365.3 kJ/mol |
| Varmeledningsevne (25 ℃) | 315 W/(m • K) | Debye temperature ϴp | 178 K |
| Termisk diffusivitet (0 ℃) | 1.25 m2/s | Magnetisk susceptibilitet | -0.15x10-6 cm3/g |
Overall, the physical properties of gold have the following characteristics:
(1) Gold has a golden color and is one of the only two colored metals among all metallic materials (the other being copper).
(2) Gold has a high density and feels heavy. The density of gold decreases with increasing temperature, and when the temperature reaches its melting point (just about to start melting), the density drops to 18.2 g/ cm3; when it is completely melted into liquid (temperature remains constant at the melting point), the density drops to 17.3 g/ cm3.
(3) Gold has a moderate melting point, and its melting heat is relatively lower than platinum group metals, which is beneficial for thermal processing such as smelting, casting, and welding.
(4) Gold has good electrical and thermal conductivity. The electrical conductivity of gold is only second to silver and copper, ranking third. As the temperature increases, the resistivity increases. The thermal conductivity of gold is only second to silver, being 74% of silver.
(5) Gold has very low volatility. Between 1000-1300℃, the amount of gold vaporized is negligible. The rate of gold vaporization is related to the surrounding atmosphere and heating temperature. For example, when melting gold in atmospheric conditions at 1075℃,1125℃, and 1250℃, after 1 hour, the loss of gold is 0.009%, 0.10%, and 0.26%; in coal gas, the loss of evaporated gold is six times that in air; in carbon monoxide, the loss is two times that in air.
(6) The magnetic susceptibility of gold is negative, exhibiting diamagnetism.
2. The Chemical Properties of Gold
2.1 Gold has strong chemical stability.
(1) Antioxidant properties.
Gold has excellent antioxidant properties and does not undergo chemical reactions even in the presence of moisture in the atmosphere. Gold is the only metal that does not react with oxygen at high temperatures; at 1000℃, no weight loss was observed after placing gold in an oxygen atmosphere for 40 hours.
(2) Corrosion resistance.
Gold has a very high ionization potential and is chemically very stable. At room temperature, single inorganic acids such as nitric acid, sulfuric acid, hydrochloric acid, hydrofluoric acid, and other strong acids cannot react with it. Most organic acids (such as tartaric acid, citric acid, acetic acid, etc.) and alkaline solutions NaOH or KOH also cannot react with it. However, certain single acids, mixed acids, halogen gases, and salt solutions can cause varying degrees of corrosion to gold. For example, aqua regia (a 3:1 mixture of hydrochloric acid and nitric acid), chlorine water, bromine water, hydrobromic acid (HBr), iodine solution in potassium iodide (KI +I2), alcoholic iodine solution (C2H5OH + I2), iron chloride solution in hydrochloric acid(FeCl3 + HCl), cyanide solution(NaCN, KCN), chlorine (at temperatures above 420 K), thiourea(NH2⸳CS⸳NH2), acetylene (C2H2, at a temperature of 753 K), and mixed acids of selenic acid and telluric acid or sulfuric acid can all interact with gold. The effects of various corrosive media on gold are shown in Table 3-2.
Table 3-2 Behavior of gold in various corrosive media
| Etsende medier | Middels tilstand | Temperatur | Degree of Gold Corrosion | |||
|---|---|---|---|---|---|---|
| Etsende medier | Middels tilstand | Temperatur | Almost no corrosion | Slight corrosion | Moderat korrosjon | Alvorlig korrosjon |
| Svovelsyre | 98% | Room temperature - 100℃ | Ja | |||
| Salpetersyre | 70% | Room temperature - 100℃ | Ja | |||
| Salpetersyre | Smoky > 90% | Romtemperatur | Ja | |||
| Saltsyre | 36% | Room temperature - 100℃ | Ja | |||
| Hydro fluoric acid | 40% | Romtemperatur | Ja | |||
| kongevann | 75%HC1 + 25%HNO3 | Romtemperatur | Ja | |||
| Perchloric acid | 70-72% | Room temperature -100℃ | Ja | |||
| Fosforsyre | > 90% | Room temperature - 100℃ | Ja | |||
| Klor | Tørt klor | Romtemperatur | Ja | |||
| Klor | Våt klor | Romtemperatur | Ja | |||
| Sitronsyre | Romtemperatur ~ 100 ℃ | Ja | ||||
| Selenic acid | Room temperature - 100℃ | Ja | ||||
| Kvikksølv | Romtemperatur | Ja | ||||
| Iron(III) chloride solution | Romtemperatur | Ja | ||||
| Sodium hydroxide solution | Romtemperatur | Ja | ||||
| Ammoniakkløsning | Romtemperatur | Ja | ||||
| Kaliumcyanidoppløsning | Room temperature - 100℃ | Ja | ||||
| Smeltet natriumhydroksid | 350℃ | Ja | ||||
| Smeltet natriumperoksid | 350℃ | Ja | ||||
| Iodine solution in alcohol | Romtemperatur | Ja | ||||
2.2 Gold can form various compounds, and exist in the compounds as either +1 or +3 oxidation states.
Gold chlorides include gold trichloride (AuCl3) and monochloride (AuCl). The anhydrous AuCl3 is red, and AuCl3⸳2H2O is orange-yellow. Heating gold powder in chlorine at 140-150℃ can produce AuCl3. Dissolving gold in aqua regia or chlorine-containing aqueous solutions also generates AuCl3. AuCl3, which easily forms complexes with other chlorides, such as M[AuCl4], H[AuCl4], allowing gold to exist in a stable AuCl4 form. This is the basis for the chlorination method of gold extraction. Gold can be precipitated from gold-containing chloride solutions using ferrous salts, sulfur dioxide, oxalic acid, etc.
Gold cyanides include gold cyanide (AuCN), gold dicyanide [Au(CN)2], etc. Heating hydrochloric acid or sulfuric acid with potassium gold cyanide [KAu(CN) 2] can yield AuCN. It is a lemon-yellow crystalline powder that can dissolve in ammonia, ammonium polysulfide, alkali metal cyanides, and thiosulfates. Simple gold cyanides easily react with alkali metal cyanides to form gold cyanide complexes, such as Na[Au(CN)2], K[Au(CN)2], etc.; in the presence of oxygen, gold in the cyanide solution can also form the above complexes, so that the gold to stabilize the Au(CN) 2, exists in the solution. This is very important for cyanide gold extraction, Au(CN) 2, the gold in the solution is easy to be precipitated by the reducing agent
Gold sulfides include gold(II) disulfide (Au2S) , gold(II) disulfide (Au2S2) , and gold(II) trisulfide (Au2S 3) . Au 2S can dissolve in KCN solution and alkali metal sulfides.
Gold oxides include gold(II) oxide (Au2O) and gold(III) oxide (Au2O 3). Since gold does not react directly with oxygen,
gold oxides can only be obtained from gold-containing solutions. Treating cooled diluted gold chloride with caustic soda can produce a deep purple powder, a hydrate of gold oxide, and heating it generates Au 2O. When Au 2O comes into contact with water, it decomposes into Au2O 3.
The hydroxides of gold have trivalent [Au(OH) 3] and monovalent (AuOH), with the former being more stable.
2.3 Gold compounds are quickly reduced to elemental gold.
The strongest metals that can reduce gold are magnesium, zinc, and aluminum. This property is utilized in the cyanide process for gold extraction, where zinc powder is used for replacement. Organic substances such as formic acid, oxalic acid, hydroquinone, hydrazine, acetylene, etc., can also reduce gold. There are many reducing agents for gold compounds, including hydrogen under high pressure, metals with a potential series before gold, as well as hydrogen peroxide, stannous chloride, ferrous sulfate, ferric chloride, lead oxide, manganese dioxide, strong bases, and peroxides of alkaline earth metals.
3. Mechanical properties of gold
3.1 Low hardness
In the annealed state, the hardness of gold is only HV 25-27. In the cast state, its hardness is also only around HV30. When the deformation rate is 60% in the cold deformed state, its hardness is about HV60.
3.2 Poor wear resistance
Due to its low hardness, scratches from nails and bites from teeth can leave marks. Gold jewelry can quickly develop dents, scratches, and wear issues due to impacts and friction during daily wear.
3.3 High elongation rate, good ductility
The elongation rate in the cast state reaches 30%, while the elongation rate in the annealed state can reach 45%.
3.4 Low strength, small elastic modulus, easy to deform
The yield strength of high-purity gold at room temperature is only 3.43 MPa, and the elastic modulus is only 79 GPa.
4. The process performance of gold
4.1 Good casting performance
The melting point of gold is moderate, and the casting temperature of the molten metal generally does not exceed 1200℃, making it suitable for precision casting processes using gypsum molds, which are not prone to casting defects such as shrinkage and vacuum. The volatility of gold is extremely low; when smelting between 1100℃-1300℃, the volatilization loss of gold is only 0.01% 0.025%, and the amount of volatilization loss is related to the content of volatile impurities in the charge and the smelting atmosphere. The evaporation loss of gold in gas is six times that in air, and the loss in carbon monoxide is 2 times that in air.
4.2 Good cold working performance
Due to the low strength of gold, it is easy to shape it at room temperature through processes such as rolling, drawing, and forging—ancient artifacts. The materials contain countless gold ornaments and gold items made using cold working techniques such as filigree, weaving, hammering, and engraving. 1 g of pure gold can usually be drawn into a wire 320 m long. With modern processing technology, 1 gram of pure gold can even be drawn into a fine wire 3420m long. Pure gold can be hammered into gold foil with a thickness of 0.1 x 10-3 mm, which appears very dense even under a microscope. However, when impurities such as lead, bismuth, tellurium, cadmium, antimony, arsenic, and tin are present, it can become brittle; for instance, gold foil containing bismuth at 0.05% can be crushed by hand. The effect of lead is even more pronounced; when pure gold includes 50 x 10-6 of lead, it affects the plasticity of gold, and when the lead content reaches 0.01%, its ductility is wholly lost.
4.3 Good welding performance
Due to the good high-temperature chemical stability of gold, its welding performance is excellent, and it does not form an oxide film layer during welding that affects the metal connection, nor is it prone to forming inclusions.
4.4 Gold has very low volatility
Under 1000℃, gold was placed in an oxygen atmosphere for 40 hours, and no weight loss was observed. Under 1075℃, 1125℃, and 1250℃, gold was melted in air, and after 1 hour, the loss of gold was only 0.009%, 0.10%, and 0.26%; this loss is due to volatilization rather than oxidation.
Section II The Purity and Measurement Units of Gold
1. The Purity of Gold
1.1 Methods of Indicating Purity
The purity of gold refers to the content of gold, that is, the minimum quality content of gold. Traditionally, there are three methods to indicate the purity of gold: percentage method, per thousand method, and K number method. The percentage method expresses the gold content in percentage (%); the per thousand method expresses the gold content in per thousand (‰); the K number method originates from the English word “karat,” which is the internationally recognized unit symbol for calculating the purity or quality of gold, abbreviated as K.
K number method: divides the purity of gold into 24 parts, with the highest purity, pure gold, being 24K, and the lowest purity being 1 K. Theoretically, the purity of pure gold is 100%, derived from 24K = 100%, which can be calculated as K = 4.16666666 %. Since the percentage value of 1 K is an infinitely repeating decimal, different countries and regions have slightly different regulations on the value of 1 K.
1.2 Purity of Gold for Jewelry
According to the purity of gold for jewelry, it can be roughly divided into two categories: pure gold and K gold.
(1) Pure Gold Category
The gold content of the pure gold category is at least 99%. The commonly referred to pure gold, total gold, 999 gold, 9999 gold, red gold, and 24K gold in the market belong to the pure gold category.
Pure gold refers to gold with a purity of one thousand parts per thousand. In reality, achieving one thousand parts per thousand pure gold is impossible. As the saying goes, “Gold cannot be completely pure, and no person is perfect.” Absolute pure gold does not exist. According to the current world’s most advanced technology level,
The purest gold can only reach 99.999999%, specifically used as “reagent gold” for standard reagents. Producing reagent-grade high-purity gold requires a large amount of raw materials and fuel, so its price is many times higher than that of pure gold in the international precious metals trading market. Even in particular industries, reagent-grade gold is used with a grain of salt to avoid increasing costs and causing waste. Furthermore, from the perspective of jewelry usage value, it has no practical significance.
Currently, in the market, there are mainly three types of gold used for making pure gold jewelry based on gold content:
“Four Nine Gold,” with a fineness of 99.99%, which is 24K gold;
“Three Nine Gold,” with a fineness of 99.9%, commonly known as 999 gold;
“Two Nine Gold,” with a fineness of 99%, is widely known as “99 Gold” or “Pure Gold.”
(2) K Gold Types
The strength and hardness of pure gold are too low, so an alloy is created by adding a certain proportion of alloying elements to pure gold, forming K gold of corresponding fineness, which can increase the strength and toughness of gold, becoming the internationally famous gold for jewelry.
Due to the differences between Eastern and Western cultures, the gold content used for making jewelry and decorative items varies among different countries and regions. However, as jewelry-grade gold, the standards adopted by countries around the world stay below 8K, and they must ensure the minimum gold content for each grade, as shown in Table 3-3.
Table 3-3 Common gold grades for jewelry in different countries and regions
| Land eller region | Vanlig gullkvalitet | Corresponding Gold Content |
|---|---|---|
| Kina | Pure gold, 18K | 99.9% , 75% |
| India | 22K | 91.6% |
| Arabiske land | 21 K | 87.5% |
| Storbritannia | Hovedsakelig 9K, med en liten mengde 22K og 18K | 37.5%, 91.6%, 75.0% |
| Tyskland | 8K , 14K | 33.3% , 58.5% |
| USA | 14K , 18K | 58.5% , 75.0% |
| Italia, Frankrike | 18K | 75.0% |
| Russland | 18K - 9K | 75.0% ~ 37.5% |
| USA | 10K - 18K | 41.6% ~ 75.0% |
The International Organization for Standardization (ISO) sets requirements for the purity of gold used in jewelry, which is consistent with the purity recommended by the Their characteristics are roughly as follows:
22K gold,
with a hardness slightly higher than pure gold, can be used to set larger single gemstones. However, due to the material’s weaker strength, the jewelry designs should be simple, and it is not widely used in the jewelry industry.
18K gold,
with moderate hardness and ideal ductility, is suitable for setting various gemstones, and the finished product is not easily deformed, making it the most widely used K gold material in the jewelry industry.
14K gold,
with a harder texture, high toughness, and strong elasticity, can set various gemstones, has good decorative properties, and is moderately priced.
9K gold,
with high hardness and poor ductility, is only suitable for making simple-shaped jewelry that sets single gemstones. It is inexpensive and often used to create trendy jewelry, medals, and plaques.
1.3 Jewelry Purity Marks and Labels
For gold jewelry, the purity is expressed in parts per thousand ( K number) and a combination of gold, Au, or G. For example, for gold with a purity of 18K, the mark can be one of the following: Gold 750 18K Gold, Au750 (Figure 3-1), Au18K, G750, G18K.
For the product labels of 24K gold jewelry, to avoid exaggerating the product’s purity and misleading consumers, whether labeled as “24K gold,” “999 gold,” or “9999 gold,” it must be labeled as “24K gold.” Suppose the nominal gold content needs to be indicated. In that case, it can be clearly stated in other positions on the label (not before or after the product name) based on the registered enterprise standards.

Figure 3-1 Color stamp on the ring
2. Measurement Units for Gold
2.1 Measurement Units for Gold Weight
The internationally recognized gold measurement units include gram, kg, ounces, troy pounds, pennyweights, etc. The commonly used measurement units for gold are listed in Table 3-4.
Table 3-4 Common Gold Measurement Unit Conversion Table (with internationally recognized abbreviation symbols)
| Kvalitet | Gold Balance (gr.) | penny weight (dwt.) | Troy ounce (t. oz.) | Avoirdupois ounce (av. oz.) | Avoirdupois pound (average lb.) | gram(g) |
|---|---|---|---|---|---|---|
| 1 gold ounce | 1 | 0.041666 | 0.0020833 | 0.00228571 | 0.000142857 | 0.0648 |
| 1 penny weight | 24 | 1 | 0.05 | 0.0548571 | 0.00342857 | 1.5552 |
| 1 troy ounce | 480 | 20 | 1 | 1.0971428 | 0.0685714 | 31. 1035 |
| 1 troy pound | 5760 | 240 | 12 | 13.165714 | 0.822857 | 373.248 |
| 1 avoirdupois ounce | 437.5 | 18.2292 | 0.911458 | 1 | 0.0625 | 28.35 |
| 1 avoirdupois pound | 7000 | 291.666 | 14.58333 | 16 | 1 | 453.6 |
| 1 mg | 0.015432 | 0.000643 | 0.00003215 | 0.000035274 | 0.0000022046 | 0.001 |
| 1 g | 15.432 | 0.643 | 0.03215 | 0.035274 | 0.0022046 | 1 |
| 1 kg | 15432 | 643 | 32.15 | 35.274 | 2. 2046 | 1000 |
2.2 International Gold Price Measurement Units
Before 1933, gold was priced in various currencies, including the US dollar, British pound, French franc, etc. By 1944, countries reached the Bretton Woods system, directly linking the dollar to gold. The dollar gradually became the world currency, with a fixed exchange rate of 1 troy ounce of gold equal to 35 dollars, allowing countries to exchange their dollars for gold. Until the 1970s, the US’s loose monetary policy ultimately led to the collapse of the Bretton Woods system, and the gold price was no longer fixed at 35 dollars per troy ounce, allowing central banks to print money without restrictions. However, as the US became the world’s largest economic and military power, the dollar became the currency for pricing gold. To date, the international gold price measurement unit is dollars per ounce.
Section III Materials and Modifications for Decorative pure gold
1. The Market Position and Common Issues of Solid Gold Jewelry
According to the views passed down over thousands of years in China, gold and silver jewelry represent wealth and the embodiment of nobility. At the same time, ancient emperors recognized yellow as the color representing status, and rewards in the palace were often replaced with various gold and silver jewelry. Therefore, gold jewelry continues to carry the deep meaning of nobility and wealth, especially as it embodies the beautiful connotation of a harmonious marriage. In traditional wedding customs, gold ornaments are almost indispensable. As a result, solid gold jewelry has been loved by the masses in various countries since ancient times and still occupies a major share of the jewelry market today.
However, traditional pure gold jewelry also has some issues in production, processing, and wearing, with common problems as follows.
1.1 Purity Guarantee
The category of pure gold in the jewelry industry is relatively vague; commonly referred to as 24K gold, 999 gold, and pure gold are all classified as pure gold. The gold content of 24K gold is not less than 99.99%, and the “9999 pure gold” claimed in the market in recent years belongs to 24K gold; the gold content of pure gold is not less than 99%; the gold content of thousand pure gold is not less than 99.9%.
Jewelry companies generally purchase pure gold ingots as raw materials when producing pure gold jewelry. Legitimate commercial pure gold ingots must have marks on the surface indicating the manufacturer, quality, purity, and serial number, etc. (Figure 3-3).

Figure 3-3 Pure Gold Ingot
International Organization for Standardization (ISO) restricts the impurity elements in pure gold nuggets, as shown in Table 3-5.
Table 3-5 Impurity content requirements for pure gold ingots.
| Karakter | Au content % | Impurity content / X 10-6 | Total Impurity content X 10-6 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Karakter | Au content % | Ag | Cu | Fe | Pb | Bi | Sb | Pd | Mg | Sn | Cr | Ni | Mn | Total Impurity content X 10-6 |
| IC - Au99. 995 | ≥99.995 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤3 | ≤3 | ≤3 | < 50 |
| IC - Au99. 99 | ≥99.99 | ≤50 | ≤20 | ≤20 | ≤10 | ≤20 | ≤10 | ≤30 | ≤30 | - | ≤3 | ≤3 | ≤3 | ≤100 |
| IC - Au99. 95 | ≥99.95 | ≤200 | ≤150 | ≤30 | ≤30 | ≤20 | ≤20 | ≤200 | - | - | - | - | - | 500 |
| IC - Au99. 50 | ≥99.50 | - | - | - | - | - | - | - | - | - | - | - | - | 5000 |
During the production process, impurities may be mixed in during smelting, casting, welding, cold processing, etc. Using solder with a lower melting point during welding will affect the quality of gold. Taking Au999 (pure gold) jewelry, which has the largest market share in the market, as an example to ensure its quality, in addition to strengthening the production process and control, the purchased gold raw materials are generally recommended to be IC – Au99.99.
1.2 Rust Spot Issues
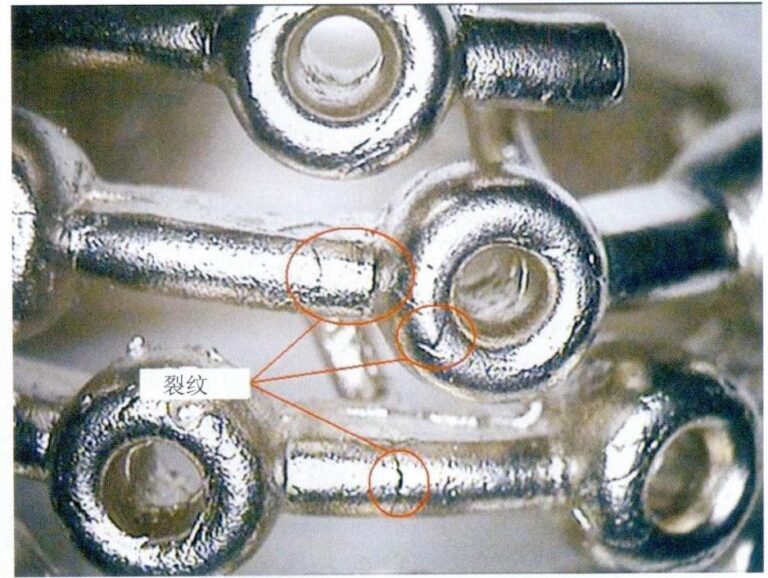
Au999 has excellent corrosion resistance, but reports of rust issues on gold jewelry surfaces are not uncommon. Figure 3-4 shows the “rust spots” on the surface of Au999 gold jewelry (Figure 3-4). Several serious “rust spot” areas have appeared on the surface of the gold jewelry. The distribution of “rust spots” is uneven, varying in size, with most spots visible to the naked eye or under low magnification microscopy. The color of “rust spots” varies in different areas, mainly including.
Red, brown, dark brown, and black contrast the Au999 pure gold background. Most spots have a reddish-brown color ring, and the more severely discolored spots are connected, forming rust spots that tend to expand outward.

Figure 3-4 The "rust spots" on the surface of Au999 gold jewelry
Under scanning electron microscopy, micro-holes in the central area of the “rust spot” vary in number. In larger areas of the “rust spot,” there are more or larger micro-holes, as shown in Figure 3-5.

Figure 3-5 Micro-holes in the center of the "rust spot" area
A chemical analysis of the gold jewelry shows that its overall gold content meets the Au999 standard. Using XPS photoelectron spectroscopy to detect the rust spot area, it was found that besides Au, there were also Ag2S and NaCl impurity contaminants, and trace amounts of SiO2 contaminants appeared on the inner walls of the micro-holes. Therefore, the issue of gold jewelry’s surface rust spot is largely due to inadequate management at the production site. For example, the layout of the site is not reasonable enough, and there is insufficient distinction between the production areas and processes for gold and silver products; the smelting and acid treatment processes are not isolated, and even high-speed rotating grinding tools are used to repair molds in the finished oil pressure area; the site hygiene is not clean enough, and production workers do not strictly follow the process requirements for cleaning gold bars and mold surfaces during operation. Since the production process of gold jewelry involves multiple processes such as smelting, rolling, cutting, oil pressure, and grinding, and sometimes pure silver products are also produced in the same production facility, it is inevitable that silver debris or particles may be pressed onto the surface of pure gold products, causing discoloration. Over a long period of production processing, dust or dirt inevitably accumulates in the production area. During the rolling and stamping processes, if the working area is not cleaned properly, especially when grinding operations are conducted nearby, dust or dirt can easily be stirred up and pressed onto the surface of the gold bar, forming heterogeneous spots. When the gold jewelry is treated with acid, the acid will corrode the heterogeneous spots into micro-holes. If the acid-washing products cannot be completely removed during the cleaning of the workpiece, or if there is residual acid in the micro-holes, it will continue to erode the heterogeneous spots. Metal impurities not removed by acid washing can easily form micro-batteries with the gold substrate under certain conditions, leading to electrochemical corrosion as they act as anodes. During the storage of gold jewelry, corrosion products will slowly migrate outward, ultimately causing “rust spots” and discoloration.
1.3 Deformation Issues
The strength of pure gold is very low. Jewelry made from pure gold using conventional techniques is prone to deformation during production and wear and is unsuitable for setting gemstones. To improve the anti-deformation capability of the jewelry, it is often necessary to increase its wall thickness, which increases the weight of the gold and makes each piece more expensive.
1.4 Wear and tear issues
The hardness of pure gold is very low. Jewelry made from pure gold using conventional techniques is easily bumped and scratched during wear, leading to dents and scratches on the surface, gradually causing the jewelry to lose its luster.
1.5 Style issues
Due to pure gold’s low strength and hardness, it isn’t easy to create jewelry with complex shapes, intricate patterns, high processing precision, and gemstone settings. This results in traditional pure gold jewelry being in an awkward position of being rough and lacking artistic value, which imposes certain limitations on the development and expansion of jewelry, restricting its artistic value as a high-end consumer product.
2. Modified Pure Gold Materials and Production Processes
2.1 Electroformed Hard Pure Gold Jewelry
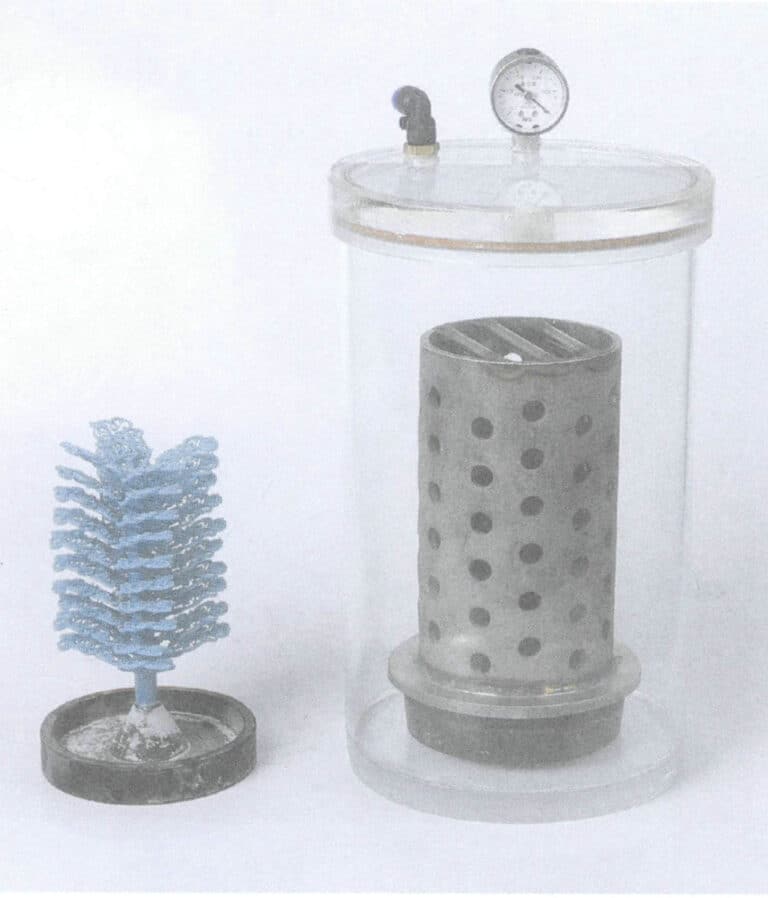
Against the backdrop of increasingly prominent decorative jewelry functions and the continuous surge in international gold prices, hollow, thin-walled pure gold jewelry has considerable market competitiveness due to its large form, lightweight, and low price per piece. Conventional jewelry forming processes such as casting and stamping need help to meet this demand. Therefore, electroforming has become the main forming process for hollow gold jewelry. However, pure gold jewelry made using traditional electroforming processes is very prone to deformation and collapse, making it suitable only as display items rather than wearable jewelry. Over a decade ago, the industry began adopting the electroformed hard pure gold process, which utilizes the principle of electrodeposition. By adjusting the formulation of the electroforming solution and improving the electroforming process conditions, gold ions are migrated to the conductive cathode mold under the influence of an electric field. After removing the core, thin-walled hollow, hard, pure gold pieces are produced, as shown in Figure 3-6.

Figure 3-6 Typical electroformed hard gold jewelry
2.1.1 Characteristics of Electroformed Hard Pure Gold Jewelry
Compared to traditional pure gold jewelry, electroformed hard pure gold jewelry has the following characteristics:
(1) High purity.
The gold content exceeds 99.9%, usually fully complying with the relevant international standards for the purity of gold while also meeting the market demand for gold purity reaching Au999. Three samples of electroformed hard gold jewelry were randomly selected for chemical composition testing, and the results are shown in Table 3-6.
Table 3-6 Chemical composition of electroformed hard gold (2012)
| Chemical elements | Content /% | Chemical Element | Content /% |
|---|---|---|---|
| Ag | 0.001 ~ 0.0036 | Pd | < 0.0003 |
| Cu | 0.0025 ~ 0.0046 | Mg | < 0.0003 |
| Fe | 0.0003 ~ 0.0012 | Som | < 0.0003 |
| Pb | 0.0003 ~ 0.0004 | Sn | < 0.0003 |
| Bi | < 0.0005 | Cr | < 0.0003 |
| Sb | < 0.0003 | Ni | < 0.0003 |
| Si | < 0.0020 | Mn | < 0.0003 |
(2) High hardness.
Depending on the composition of the electroforming solution, the electroforming process, and the thickness of the coating, the hardness in the cast state can generally reach above HV80, with some even reaching HV140-160, which is equivalent to the hardness of 18K gold, more than four times that of traditional pure gold.
(3) Wearable.
As the hardness significantly increases, the deformation resistance of the jewelry improves, allowing it to be worn as accessories, solving the problem that traditional hollow gold jewelry can only serve as ornaments.
(4) Wear-resistant.
It breaks through the limitation of the softness of traditional pure gold jewelry, with wear resistance far superior to that of traditional pure gold items.
(5) Light weight.
Using a hollow electroforming process, the wall thickness is generally within 220μm, significantly reducing the weight compared to traditional pure gold jewelry of the same appearance and volume.
However, although electroformed hard gold has a relatively high hardness, it is relatively brittle in nature. Since it is hollow, care must be taken to avoid collisions with sharp objects during wear. In addition, there are still certain limitations regarding style and product structure for electroformed hard gold.
2.1.2 Material strengthening mechanism of electroformed hard gold
The electroforming process of hard gold uses IC – Au9.99 pure gold as raw material, preparing it into an electroforming solution containing complex alloy ions. By improving the additives in the electroforming solution and the conditions of the electroforming process, the crystallization method of the gold layer is enhanced, resulting in a cast structure with fine grains and dense structure. The crystal structure of electroformed hard gold also differs from that of ordinary gold (Figure 3-7). This fine and dense structure is the fundamental reason for the high hardness of electroformed hard gold.
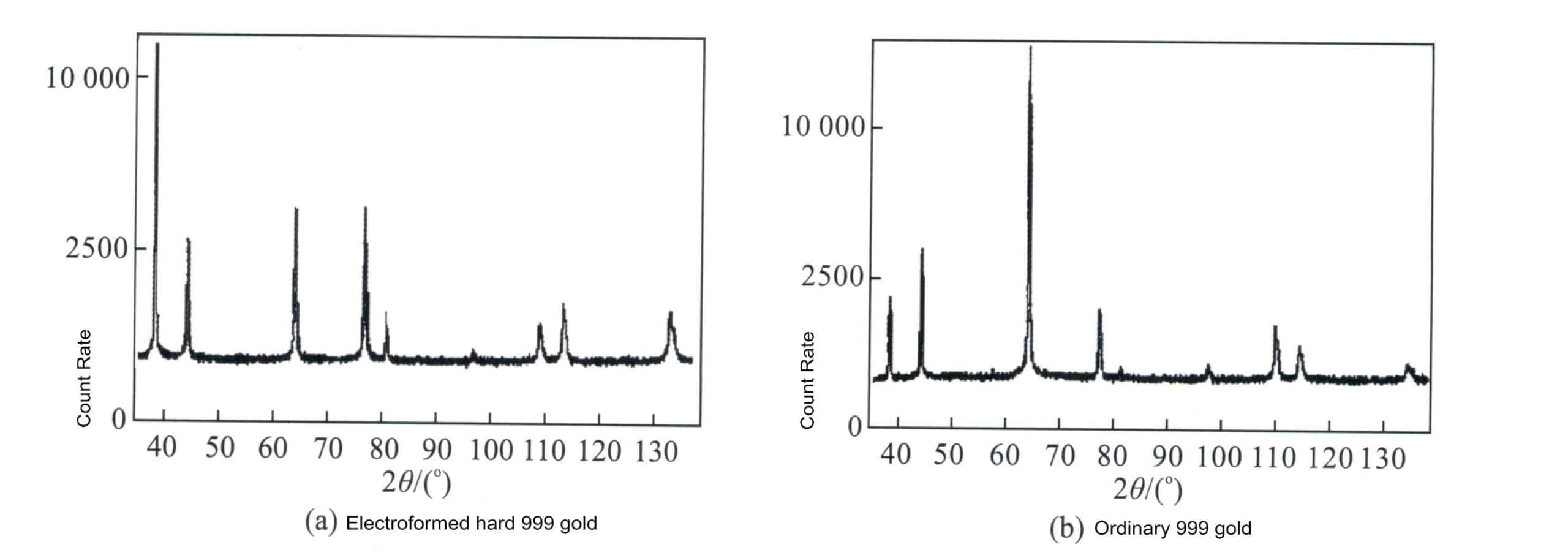
Figure 3-7 Comparison of X-ray diffraction between electroformed hard 24K gold and ordinary 24K gold
2.2 Micro-alloyed high-strength 24K gold
Due to the low strength and hardness of 24K gold materials, it isn’t easy to create jewelry with complex shapes, intricate patterns, high processing precision, and embedded gemstones. Additionally, jewelry is prone to deformation during wear and can easily become worn and lose its luster. With the improvement of material and cultural living standards, consumers have higher expectations for 24K gold jewelry than before, requiring high purity and higher expectations for the jewelry’s structure, style, and performance. Therefore, the research and development of micro-alloyed high-strength 24K gold materials and production processes have become a hot topic in the industry.
2.2.1 Strengthening methods for micro-alloyed 24K gold
As mentioned earlier, the strengthening methods for precious metal materials include solid solution strengthening, fine grain strengthening, deformation strengthening, precipitation strengthening, dispersion strengthening, and phase transformation strengthening. In the development of micro-alloyed gold, it is also necessary to select appropriate methods from the strengthening above methods, and due to the very small amount of alloying elements added, a comprehensive effect of multiple strengthening pathways is required to achieve good strengthening results.
From the perspective of metallurgical principles, micro-alloying elements are pretty broad. Except for alkali metals, some refractory metals and low melting point metals, simple metals, transition metals, light metals, and metalloids can all serve as micro-alloying elements for Au, and even those elements considered harmful at conventional concentrations can also serve as major micro-alloying elements. When selecting alloying elements, the following factors are generally considered.
(1) The effect of solid solution strengthening.
The solid solution strengthening effect of alloying elements in pure gold is related to factors such as the atomic size difference, electronegativity difference, crystal structure differences between them, and the content of the alloying elements. The solid solution strengthening effect of alloying elements on Au can be measured using solid solution strengthening parameters; the larger the parameter value, the better the solid solution strengthening effect. Generally speaking, light metal elements with smaller atomic weights, such as Li, Be, Na, K, Mg, Ca, and Sr, as well as rare earth elements with larger atomic sizes, have higher solid solution strengthening parameter values.
(2) Fine grain strengthening effect.
The grain refinement of pure gold includes both the primary grain refinement during the solidification crystallization process of the molten metal and the suppression of recrystallization and grain growth during the heat treatment process. Some alloying elements, such as rare earth elements and some high melting point alloying elements, can act as effective grain refiners or modifiers during solidification crystallization. Rare earth elements, which have a strong affinity for oxygen, can purify the molten metal and also serve as effective grain refiners during solidification crystallization; cobalt can increase the recrystallization temperature of gold alloys and suppress the occurrence of recrystallization.
(3) They are aging-strengthening effects.
If the solubility of alloying elements in Au decreases with decreasing temperature, then through solid solution aging treatment, metastable or stable second phases can precipitate, resulting in precipitation strengthening of the alloy. Many elements can produce effective precipitation in Au, such as small amounts of Ti, REE, Co, Sb, Ca, etc., which can all lead to aging precipitation-strengthening effects in gold.
(4) The role of strain hardening.
This is a necessary way for micro-alloyed gold to achieve significant strengthening effects. The processing hardening rates of different alloying elements in gold vary, fundamentally due to the differences in the hindrance to dislocation slip, which depends on the interactions between grain boundaries and dislocations, solute atoms, and dislocations, second-phase particles and dislocations, and dislocations with each other.
2.2.2 The quality of micro-alloyed high-strength gold
lThe quality of Au999 gold is maintained above 99.9%, meeting the market’s acceptance of gold quality. By adding trace alloying elements and combining them with cold deformation processing, significantly higher strength and hardness can be achieved than traditional 24K gold. The market’s so-called “5G hard gold” belongs to micro-alloyed 24K gold. Figure 3-8 shows a “5G” hard 24K gold hollow bracelet with a wall thickness of only 0.2 mm, formed through tube drawing, bending, and welding, featuring lightweight, high hardness, and good elasticity.

Figure 3-8 "5G" hard 24K gold hollow bracelet
Due to the insufficient introduction of alloying elements 0.1%, depending on the added alloying elements, the as-cast hardness generally ranges from HV40 to HV60. After cold deformation processing such as rolling and drawing, the hardness generally ranges from HV80 to HV120. In some cases, the hardness of certain alloys is even better. Foreign countries have also developed and commercialized micro-alloyed Au999, which significantly improves hardness and strength compared to ordinary Au999, as shown in Table 3-7.
Table 3-7 Properties of micro-alloyed high-strength Au999 (partially excerpted from Christopher W. Corti, 1999)
| Materialer | Manufacturer | Renhet | Cast Hardness HV/(N/mm2) | Annealed Hardness HV/(N/mm2) | Processing Hardness HV/(N/mm2) | Tensile Strength / MPa | Suitable Craft |
|---|---|---|---|---|---|---|---|
| 5G Hard Gold | Kina | 99.9% | 40 ~ 60 | - | 80 ~ 110 | - | Castable |
| High-intensity pure gold | Japan Mitsubishi | 99.9% | - | 55 | 123 | 500 | Castable |
| TH Gold | Japan Tokuriki Honten | 99.9% | - | 35 ~ 40 | 90 ~ 100 | - | Castable |
| Ordinary pure gold | - | 99.9% | - | 30 | 50 | 190 ~ 380 |
2.2.3 Micro-alloyed high-strength Au995
Since the alloy element content of Au995 is slightly higher than that of Au999, there are more alloy elements to choose from. By using a combination of several strengthening mechanisms, a significant strengthening effect can be achieved. Table 3-8 lists some properties of micro-alloyed Au995, and the hardness of some alloys after comprehensive treatment can reach 22K gold or even 18K gold.
Table 3-8 Performance of micro-alloyed Au995 (according to Christopher W. Corti, 1999)
| Materialer | Manufacturer | Renhet | Cast Hardness HV/(N/mm2) | Annealed Hardness HV/(N/mm2) | Processing Hardness HV/(N/mm2) | Aging state Hardness HV/(N/mm2) | Suitable Craft |
|---|---|---|---|---|---|---|---|
| 24K Hard Gold | Africa Mintek | 99.5% | - | 32 | 100 | 131 ~ 142 | Can be aged |
| Pure gold | Japan Three O Co. | 99.7% | - | 63 | 106 | 145 ~ 176 | Can be aged & Castable |
| Uno-A- Erre 24K Gold | Uno-A- Erre Italy | 99.6% | - | 33 | 87 | - | Cold processing |
| Uno-A- Erre 24K gold | Uno-A- Erre Italy | 99.8% | - | 62 | 118 | - | Cold processing |
| DiAurum 24 | British Titan | 99.7% | 60 | - | 95 | - | Castable |
2.2.4 99%Au – 1% Ti Hard Gold
In the 1980s, the World Gold Council funded research on hard gold, successfully developing Au990 hard gold, which uses 1% Ti as an alloying element, leveraging the fine grain strengthening effect of Ti, as well as the aging precipitation strengthening effect of Ti diffusing from the supersaturated solid solution an Au to form the second phase, significantly improving the strength and hardness of the alloy, as shown in Table 3-9.
Table 3-9 99%Au – 1%Ti Performance of Hard Gold According to Christopher W. Corti, 1999
| Ytelse | Solid solution state (800℃, 1 h , quenching) | Cold working state (processing rate 23%) | Aging state (500℃, 1h , quenching) |
|---|---|---|---|
| Hardness HV/N/mm2 | 70 | 120 | 170- ~ 40 |
| Strekkgrense /MPa | 90 | 300 | 360 ~ 660 |
| Strekkfasthet /MPa | 280 | 340 | 500 ~ 700 |
| Forlengelseshastighet /% | 40 | 2 ~ 8 | 2 ~ 20 |
99%Au – 1% Ti is a promising micro-alloyed high-strength gold material. However, due to the presence of Ti, this alloy system needs to be melted in a vacuum, which makes the process more difficult, and the color differs slightly from traditional gold, limiting its applications.