Cara Mencegah Noda pada Perak dan Menerapkan Pelapisan Perak Tanpa Listrik
Silver Plating and Anti-Tarnish Solutions for Jewelry
Pendahuluan:
This article explains how to prevent silver and silver-plated items from tarnishing, a common issue where surfaces darken due to reactions with sulfur or light. It details various anti-tarnish treatments, including inorganic methods like chromate or tin electrolysis, organic compound soaks, and surfactant applications. The text also covers what electroless silver plating is, exploring both displacement plating for substrates like copper circuits and reduction plating using agents like formaldehyde or hydrazine. Finally, it outlines why and how to analyze key components in cyanide silver plating solutions, such as silver ions and free cyanide.

Daftar Isi
Section I Silver Tarnish Prevention Treatment
Due to the high reactivity of the Ag plating surface, the silver-plated surface is prone to discoloration when in contact with corrosive media (such as H2S, SO2). Light also imparts excess energy to the plating surface, promoting the ionization of Ag and the reaction between Ag and the corrosive media.
Table 2-28 shows the effects of light wavelength and exposure time on the discoloration of Ag. Table 2-29 shows the effects of exposure time on color and chemical composition.
Table 2-28 Effect of Light Wavelength and Exposure Time on the Color Change of Ag
| Ray | Exposure time/h | ||||
|---|---|---|---|---|---|
| 6 | 12 | 18 | 24 | 48 | |
| 2527Å | No change in color | Yellow spots | Coklat kekuningan | Brownish-black | Coklat |
| 3650Å | No change in color | No change in color | No change in color | Kuning | - |
| Sunlight | No change in color | No change in color | No change in color | Yellow spots | - |
Table 2-29 Effect of Exposure Time on the Color and Chemical Composition of Silver-Plated Surface
| 2537Å Exposure color of light/h | 6 | 12 | 18 | 24 | 48 |
|---|---|---|---|---|---|
| Warna | Perak | Kuning | Coklat kekuningan | Brownish black | Hitam |
| Main chemical composition | Ag(metal) | Ag2O+AgO | Ag2O+AgO | AgO+Ag (Superfine) | Ag(Superfine) |
From these results, it can be seen that the discoloration products are silver sulfides, oxides, chlorides, or silver particles. The color of the Ag plating after discoloration varies depending on the chemical composition. This type of color change not only affects the decorative appearance of the silver plating, but also increases the electrical resistance of the Ag plating, which is detrimental to the electrical properties of the silver plating.
From these discoloration mechanisms of the Ag plating layer, it can be seen that it is only necessary to avoid contact between the plating surface and oxygen, light, and corrosive media to prevent discoloration of the silver plating layer. At the same time, forming a dense substance (film layer) on the surface that can absorb ultraviolet rays should have an inhibitory effect on the discoloration of the plating layer.
Among the traditional methods for preventing discoloration of Ag plating layers are inorganic compound methods, organic compound methods, surfactant methods, and combined use of these methods.
1. Inorganic Compound Treatment Method
To compare the effects of different anti-tarnish treatments, the test pieces were plated with 2~3μm Ag and then soaked in a concentration of 0.2% ammonium polysulfide solution [(NH4)2SX] for a certain period. Visual inspection was used for evaluation. When visual inspection was impossible, a digital gloss meter was used to measure the specular reflectance, and the difference △ before and after soaking in the ammonium polysulfide solution was calculated. At the same time, for indoor exposure tests, a gloss meter was also used for measurement.
As an anti-tarnish treatment for Ag, metal films or metal oxide films with the same tone as Ag, such as In, Zn, Cd, Cr, Pd, Rh, Sn, Be, Al, Th and Zr, were tested. Among them, the electrolytic treatment of chromate exhibited the most outstanding anti-tarnish function and was widely applied. Secondly, Rh and Be are also among the choices.
(1) Precious Metal Treatment
Electroplating of Rh and Pd alloys was conducted under optimal conditions, followed by their tone changes and discoloration resistance experiments. The results are shown in Table 2-30. The specular reflectance of Rh is 70%, while the specular reflectance of the alloy (Pd80:Ni20) is about 57%. Although the plating thicknesses differ, there may be some correlation. The plating thickness of Rh is 0.1μm, and the plating thickness of Pd-Ni is 0.3μm. When the plating thickness of other metals on Ag increases, the silver color of the underlying silver layer disappears. However, by visually comparing tone, brightness, and relative values of specular reflectance, it was found that when the specular reflectance is 80%, the difference from the silver color becomes obvious, and 70% is its limit. Below this, the silver color disappears and changes to a different metal color. Therefore, from the tone perspective, a Rh plating thickness below 0.1μm is more suitable. Still, its discoloration resistance is insufficient, while the Pd-Ni alloy plating layer is thicker and has good discoloration resistance. Using precious metals means increased cost, but they offer good drug and wear resistance. The better the discoloration resistance, the more the original silver color disappears. Hence, enhancing discoloration resistance without changing the tone and brightness of the silver plating is very difficult.
(2) Treatment of Tin and Tin Alloys
Alloys of Cu and Sn are called mirror alloys. When the Sn content is above 60%, their color is silver-white, and the specular reflectance is also high, with good corrosion resistance. Therefore, they have been studied since ancient times and used as mirror surfaces in reflecting telescopes. When used as silver anti-tarnish coatings, they perform quite well against tarnishing for 5~6h. However, the specular reflectance drops to about 65%, clearly losing the silver color. The usability of mirror alloys depends on the degree of tint. Although cheaper than precious metals, when the film thickness increases to several thousand angstroms, a potential difference arises between the precious metal Ag and the Cu-Sn alloy, which may cause corrosion resistance issues.
Besides Sn alloy plating, Sn metal or Sn compounds precipitated by electrolytic or soaking treatments of 50~100Å can also have tarnish resistance and improved weather resistance. As a tarnish treatment, it can maintain the stability of the contact resistance of contacts for a long time.
The experimental results are shown in Table 2-30. Compared with alkaline and acidic solutions, parts treated with acidic solutions have a misty white precipitate and cannot be used for decorative purposes. In contrast, parts treated with alkaline solutions and electrolytic treatments show no problems in the short term.
Table 2-30 Inorganic Compounds Resistance to Discoloration on Silver-Plated Surfaces
| Nomor seri | Solution composition | Processing conditions | Condition to the occurrence of apparent color | Catatan |
|---|---|---|---|---|
| 1 |
PNP (Nissin Kasei, Japan) Pd-Ni alloy plating |
DK lA/dm2 Suhu ruangan 1min
|
Above 6h | Loss of silver color, poor CN- resistance |
| 2 |
Pewter(Daiya Shokai) Cu-Sn alloy plating
|
3V 55℃ 40s
|
Above 6h | Loss of silver color |
| 3 |
Na2Cu(CN)3 24g/L Na2SnO3 90g/L Free NaCN 16g/L NaOH
|
Dk 2A/dm2 60℃ 30s
|
Above 6h | Loss of silver color, better than silver color of 1 and 2. |
| 4 |
Na2SnO3 90g/L CH3COONa 0g/L,5g/L,10g/L
|
DK 0. 5 A/dm2 Suhu ruangan 15〜90s
|
10 〜20s | Adding CH3COONa can stabilize the solution, and the longer the electrolysis time, the more stable it is, but it will lose its silver color. |
| 5 |
SnCl2 20 gram/L 0. 025NHCl 50mL/L
|
Suhu ruangan 1min
|
10min | |
| 6 |
SnCl2 20 gram/L 0.025NHCl 50mL/L
|
DK 0. 1 〜 2A/dm2 Room temperature, 10s
|
10min | Putih |
|
Room temperature ,1min, 10min |
Slightly yellowed | |||
| 7 | Tin fluoroborate 3% |
DK 0. 1 ~ 2 A/dm2 Room temperature , 10s
|
- | Putih |
| Room temperature ,1min, 10min | 10min | Slightly yellow | ||
| 8 |
Stannous sulfate 20g/L Iodic acid 30mL/L
|
DK 0. 1 〜 2 A/dm2 Room temperature ,10s
|
- | Putih |
| 9 |
Stannous fluoroborate 20mL/L Fluoric acid 30mL/L Fluoroboric acid 20g/L
|
Room temperature ,1min, 10min DK 0. 1 〜2 A/dm2 Room temperature ,10s |
10min - |
Slightly yellowish Putih
|
| Room temperature, 1min, 10min | 10min | Slightly yellow | ||
| 10 |
BeSO4 , 4H2O 2g/L pH = 5. 7〜5. 8
|
DK 0. 007 A/dm2 Room temperature 3〜10min
|
Above 1h | |
| 11 |
BeSO4 , 4H2O 2g/L pH = 5. 8
|
1 〜2. 4V 20s~4min
|
20min | |
| 12 | EverShine S(Tamura Chemical) |
6.5V Suhu ruangan 90s
|
15min | |
| 13 |
K2CrO4 15 gram/L NaOH 30g/L
|
2〜6V Room temperature 15〜120s
|
10 〜30s | The longer the electrolysis, the better |
| 14 |
K2CrO4 30g/L NaOH 40g/L
|
DK 4A/dm2 Room temperature 30〜40s
|
White fogging No silver color
|
|
| 15 |
K2CrO4 30g/L NaOH 40g/L Heating 20h
|
White fogging No silver color
|
||
| 16 |
Al2(SO)4)3 4 gram/L Ammonium oxalate 2g/L pH = 5. 8
|
DK 0.06A/dm2 Room temperature 30s〜1min
|
20 〜30s | Same time as without treatment |
| 17 | Sodium carbonate | |||
| 18 |
Na2SnO3 8. 5g/L CH3COONa 5g/L (No. 4)
|
DK 0,5A/dm2 15 〜30s Suhu ruangan
|
Above 6h | |
|
K2CrO4 15 gram/L KOH 30g/L (No. 13)
|
2〜3V 30s Suhu ruangan
|
|||
| 19 |
K2CrO4 15 gram/L KOH 30g/L K2CO3 50 gram/L
|
6V 15 〜60s
|
10 〜30min |
(3) Beryllium Treatment
Attaching a beryllium oxide film to Au, Cu and brass as a color-changing treatment can achieve good effect. The principle utilized by this method is: the isoelectric point of beryllium hydroxide is pH=5.8, and the following electrolytic reaction can occur:
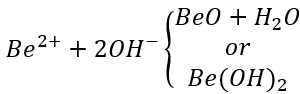
(4) Aluminum Treatment
There are reports of oxide film formation similar to Be; additional experiments showed no difference between treated and untreated samples, and oxide film could not be formed.
(5) Chromate Treatment
Silver plating is the most common method for the anti-tarnish treatment of brass plating. There are two chromate treatment methods: cathodic electrolysis and soaking, but the cathodic electrolysis method is much superior.
Additives used in chromate electrolytes include: K2CO3, Na2CO3, KCN, NaS2O3, KCNS, NaCl, KI, EDTA, etc. Among them, the K2CO3 experimental results found that it can also act as a conductive salt, thereby shortening the electrolysis processing time compared to when it is not added. The effects of the other additives showed no significant differences.
The disadvantage of chromate electrolysis treatment is that the film has poor UV resistance and will turn brown after being left for a long time.
2. Treatment with Organic Compounds and Surfactants
Table 2-31 Anti-discoloration Experiment of Organic Compounds
| Categorization | Nomor seri | Organic compounds | Sifat Fisik | Solubility | Processing conditions | Appearance | Hydrophobicity | Anti-discoloration effect |
|---|---|---|---|---|---|---|---|---|
| Aliphatic amine | 1 |
Ethylamine (70% aqueous solution) CH3CH2NH2
|
Mw 45. 09 d15 0. 6892 Melting point 83.3℃ Boiling point 16℃ |
(Aqueous) alcohols Ether
|
0. 1mol/L (0. 65mL/L) 0. 01mol/L (6. 5mL/L) Room temperature,20min,60min |
pH 10〜11 Colorless Transparent O |
X | X |
| 2 |
Dodecylamine CH2(CH2)11NH2 |
Mw 18. 5 Melting point 25℃ Boiling point 247℃ |
Acetone (Ethanol) |
0. 1mol/L (18. 5g/L) Room temperature, 20min,60min |
Colorless Transparent Water stain |
X | X | |
| 3 |
Tributylamine (CH3CH2CH2CH2)3N |
Mw 185 d 0. 7782 Boiling point 216.5℃ |
(Ethanol) Ether
|
0. 1mol/L (24mL/L) Room temperature, 20min, 60min |
Colorless Transparent O |
X | X | |
| Aromatic amines | 4 | O-Toluidine |
Mw 107. 16 d20 0. 9989 Boiling point 199. 7℃ |
Water 1. 5(25) (Ethanol) Ether |
0. 1mol/L (24mL/L) Room temperature, 20min, 60min |
Reddish brown transparent O |
X | X |
| 5 | Diphenylamine |
Mw 169.23 d 1.159 Boiling point 302℃ |
(Ethanol) 56 Ether |
0. 1mol/L (17g/L) Room temperature, 20min, 60min |
Colorless transparent Water stain |
X | X | |
| Diamines, Polyamines | 6 | H2N(CH2)2NH2 |
Mw 45.09 d35 0.892 Melting point 8.5℃ Boiling point 117℃ |
(water) Etanol |
0.1mol/L (6. 7mL/L) Room temperature, 20min, 60min |
Colorless Transparent pH 10〜11 O |
X | X |
| Diamine, polyamine | 7 |
Diethylenetriamine H2 N(CH2 )2 NH(CH2 )2 NH2 |
Mw 103 Boiling point 208℃ |
(water) Etanol |
0. 1mol/L (10mL/L) Room temperature, 20min,60min |
Colorless Transparent pH 10〜11 O |
X | X |
| 8 |
Trietilen tetramina H2 N(CH2 )2 NH(CH2 )2 NH2 (CH2 )2 NH2 |
Mw 146 Melting point 208℃ Boiling point 174℃ |
(water) |
0.1mol/L (15mL/L) Room temperature, 20min,60min |
Colorless Transparent pH 10〜11 O |
X | X | |
| Amino alcohols | 9 |
Trietilen tetramina H2N(CH2)2NH(CH2)2 NH(CH2)2NH2 |
Mw 189 | (water) |
0. 1mol/L (19mL/L) Room temperature, 20min, 60min |
Colorless Transparent pH 10〜11 O |
X | X |
| 10 |
Ethanolamine HO(CH2)2NH2 |
Mw 61.09 d27 1.0111 Boiling point 171℃ |
(water) Etanol |
0. 01mol/L (0.61mL/L) 0.1mol/L (6.1mL/L) Room temperature, 20min, 60min |
Colorless Transparent pH 9〜10 O |
X | X | |
| 11 |
Diethanolamine (HOCH2CH2)2NH |
Mw 105.14 d20 1.0916 Melting point 28℃ Boiling point 268℃ |
(water) Etanol |
0. 01mol/L (0.96mL/L) 0.1mol/L (9.6mL/L) Room temperature, 20min, 60min |
Colorless Transparent pH 9〜10 O |
O | X | |
| 12 |
Triethanolamine (HOCH2CH2)3NH |
Mw 149.19 d20 1.124 Melting point 21.2℃ Boiling point 227℃ |
(water) Etanol |
0. 01mol/L (1.3mL/L) 0.1mol/L (13mL/L) Room temperature, 20min, 60min |
Colorless Transparent pH 9〜10 O |
O | X | |
| 13 |
2N-Diethylamine (CH2)2NCH2CH2OH |
Mw 93.1 Boiling point 39.5℃ |
(water) |
0. 1mol/L (0.89mL/L) 0. 01mol/L (8.9mL/L) Room temperature, 20min, 60min |
Colorless Transparent pH 9〜10 O |
O | X | |
| 14 | 2-Amino-2-ethyl-1,3-propanediol | Mw 105 | (water) |
0.01mol/L (1.1g/L) 0. 1mol/L (11g/L) Room temperature, 20min, 60min |
Colorless Transparent pH 9〜10 O |
O | X | |
| Amide | 15 | Acetamide |
Mw 59.07 d 1.159 Melting point 52.62℃ Boiling point 221℃
|
(water) Etanol |
0. 1mol/L(5.1mL/L) Room temperature, 20min, 60min |
Colorless Transparent O |
△ | X |
| 16 | Acrylamide |
Mw 71.1 d 1.122 Melting point 85℃ |
(water) Etanol Ether Trichloromethane
|
0. 1mol/L(7.1mL/L) Room temperature, 20min, 60min |
Colorless Transparent O |
△ | X | |
| 17 | Benzylamine |
Mw 121.14 d 1.341 Melting point 128℃ Boiling point 290℃ |
(water) Etanol
|
0. 1mol/L(12g/L) 30℃ Room temperature, 20min, 60min |
Colorless Transparent O |
O | X | |
| Oxime | 18 | Butanedione oxime |
Mw116.12 Melting point 240℃ |
(Ethanol) Ether (Water) |
0. 01mol/L (0.12g/L) 0. 001mol/L (1.2gmL/L) Room temperature, 20min, 60min |
Colorless Transparent O |
X | X |
| 19 | Benzohydrin oxime |
Mw227 Melting point 154℃ |
(Ethanol) (Ammonia)
|
0.01mol/L (0.23g/L) 0. 001mol/L (2.3g/L) Room temperature, 20min, 60min |
Colorless Transparent △ |
X | X | |
| Pyridine | 20 | Pyridine |
Mw 79.10 d20 0.977 Boiling point 115.5℃ |
(water) Etanol |
0. 001mol/L (0.1mL/L) 0.1mol/L (8mL/L) Room temperature, 20min, 60min |
Colorless Transparent O |
X | X |
| Quinoline | 21 | Quinoline |
Mw 127.16 d20 0.938 Melting point -15℃ Boiling point 238℃ |
Hot water, dilute acid, ethanol, ether, carbon disulfide (6mol/L HCl) 2~20mol/L |
0.1% (1g/L) 1% (10g/L) Room temperature, 20min, 60min |
Colorless Transparent pH 2~4 O |
O | X |
| 22 | Carboxyquinoline |
Mw 145 Melting point 75~76℃ |
Ethanol, acetone, trichloromethane, benzene (6mol/L HCl) 2~20mol/L |
0.1% (1g/L) 1% (10g/L) Room temperature, 20min, 60min |
Yellow Transparent pH 2~4 O |
X | X | |
| Quinoline | 23 | Butanedione oxime | Mw 256 |
Inorganic acid (6mol/L HCl) 40mol/L Etanol |
0.1% (1g/L) 1% (10g/L) Room temperature, 20min, 60min |
Yellow Transparent pH 10~11 O |
X | X |
| Diazo compounds | 24 | p-Ethoxy-2,4-diaminoazobenzene | Mw 256 |
Inorganic acid (6mol/L HCl) 40mol/L Etanol |
0.1% (1g/L) 1% (10g/L) Room temperature, 20min, 60min |
Red Transparent O |
O | X |
| Hydroxycarboxylic acid | 25 | Tannic acid | Yellowish white, light color, powder |
(water) Etanol Acetone |
0.1% 0.1% 5% |
Colorless Transparent pH 6 Yellow transparent pH 4 Yellowish brown transparent pH 2 |
X | X |
| Thiourea | 26 | 1-Acetyl-2-thiourea |
Mw 118.16 Melting point 165℃ |
(water) Alcohol |
10 gram/L Room temperature, 20min, 60min |
Colorless Transparent pH 7 O |
O | X |
| Thiourea | 27 | Aminothioureas |
Mw 95.6 Melting point 81~183℃ |
(6mol/L HCl) 50mL/L |
10 gram/L Room temperature, 20min, 60min |
Colorless Transparent pH 1 O |
X | X |
| 28 | Dithizone | Mw 256 |
(Trichloromethane) Karbon tetraklorida |
10 gram/L Room temperature, 20min, 60min |
Blue water stain Turns yellow |
O | X | |
| Monosaccharide | 29 | Ascorbic acid (vitamin C) |
Mw 176 Melting point 190~192℃ |
(water) |
10 gram/L Room temperature, 20min, 60min |
Colorless Transparent pH 1 O |
X | X |
| Imidazoles | 30 | 1-Acetyl-2-thiourea |
Mw 155 Decomposition 287~288℃ |
Methanol Water 45mL
|
1g/L, 10g/L Room temperature, 20min, 60min |
Colorless Transparent pH 1 O |
X | X |
| 31 | 2-Heptadecylimidazole |
Methanol 55mL Water 45mL Dissolution Alcohol, acid |
1g/L, 10g/L Room temperature, 20min, 60min |
White suspension Uneven White adherence |
O | O | ||
| Benzimidazoles | 32 | 2-mercaptopyridine |
Mw 150 Melting point 301~302℃ |
Methanol 15mL Water 45mL Hot water Etanol NaOH |
1g/L, 10g/L Room temperature, 60℃ 20min, 60min |
Colorless transparent O |
O | X |
| Triazoles | 33 | 3-Amino-1,2,3-triazole |
Mw 159 Melting point 159℃ |
(water) Etanol Trichloromethane |
Room temperature, 60℃ 1min, 10min, 60min |
O | - | X |
| Benzotriazole | 34 | Benzotriazole |
Mw 119.13 Melting point 99℃ |
Hot water Alcohol |
12g/L, pH 6 60℃ 1min, 3min, 10min
|
Colorless Transparent O |
- | X |
| Triazine | 35 | Triethylenediamine |
Mw 140 Sublimation above 230°C |
(water) Hydrate (CN2)2N Trichloromethane Alcohol |
10 gram/L Room temperature, 20min, 60min |
Colorless Transparent pH 8 O |
X | X |
| Oxazole | 36 | 2-Oxobenzazole |
Mw 1151 Melting point 143.2℃ |
Amonia 6mol/L ammonia 200mL/L Acetic acid Ether |
10 gram/L Room temperature, 20min, 60min |
Colorless Transparent pH 10 O |
O | X |
| (Oxo)zines | 37 | Morpholine |
Mw 87.12 d13 1.0007 Boiling point 128℃ |
(water) Alcohol Ether |
10 gram/L Room temperature, 20min, 60min |
Colorless Transparent pH 9~10 O |
X | X |
| Thiazole | 38 | Ortanin |
Mw 264 Melting point 200~246℃ (Decomposition) Red needle-like crystals
|
Strong acids Dense Trichloromethane Ether Benzene (acetone)
|
1g/L Room temperature, 20min, 60min |
Red transparent (Red precipitate) Brown water stain |
O | X |
| Benzothiazole | 39 | Benzothiazole |
Mw 135.39 d12 1.2349 Boiling point 231℃ |
(Ethanol) Ethyl ether |
1g/L Room temperature, 3min, 60min |
Reddish brown Transparan O |
△ | X |
| 40 | 2-Hydrophobic benzothiazole |
Mw 167 Melting point 177℃ |
Etanol Ethyl ether Benzene Acetic acid Potassium carbonate Potassium hydroxide Sodium hydroxide 2g/L |
1g/L Room temperature, 3min, 60min |
White turbid pH 10 O |
X | O | |
| Naphthalenes | 41 | Naphthalene sparing agent |
Mw 217 Melting point 109.5℃ |
Water (20℃) 0.01g/L |
0.2g/L (Precipitation) Room temperature, 2min, 10min, 30min |
O | - | X |
| Isoacetone |
10 gram/L Room temperature, 10min, 60min |
Yellow transparent Water stain
|
O | O | ||||
|
Isoacetone 50% Water 50% |
5 gram/L Room temperature, 10min, 60min |
Yellow transparent Water stain
|
O | O |
Table 2-32 Surfactants’ Anti-Discoloration Experiment
| Nomor seri | Name (company name) | Status | Concentration | Processing conditions | Appearance | Hydrophobicity | Inhibition effect | Keterangan |
|---|---|---|---|---|---|---|---|---|
| 1 | Rust Preventive Agent MC- 501 (Japan Lion Grease) |
Light red transparent, d 1.06 pH 8. 0〜8. 5 |
2%,10% | Room temperature 20min, 60min | O | O | Ag:x | |
| 2% | Room temperature 15s, 10min | O | O | Cu:Tap water immersion X, exposure test △ | 15s better | |||
| 2 | Enajiko-ru CNS (Japan Lion Grease) |
Amphoteric pH 8〜9 |
1% | Room temperature 20min, 60 min (Ag)15s, 10min(Cu) | O | O | Ag:x Cu:Tap water immersion X, exposure test: | 1% 15s good |
|
15% Sodium benzene sulfonate 15% (Neutral)
|
Room temperature 20min,60min (Ag)15s, 10min(Cu) | Brown, white precipitate adhesion | O | |||||
|
10% Potassium pyrophosphate 5% (Alkaline) |
Room temperature 20min,60min (Ag) 15s, 10min(Cu) |
White turbidity O |
O | |||||
| 3 | Ripa-ru OH- 104P (Japan Lion grease) | 0.1% | Room temperature 20min,60min |
Colorless and transparent O Yellowish-white turbid O
|
O △
|
Ag:x | ||
| 1% | ||||||||
| 10% | ||||||||
| 4 | Dyuomin CD (Japan Lion Grease) | 0.1% | Room temperature 20min,60min |
White cloudy O Yellow muddy streaks O
|
O | Ag:x | ||
| 1% | ||||||||
| 10% | ||||||||
| 5 | Dyuomin CDA-50 (Lion Grease, Japan) | 0.1% | Room temperature 20min,60min |
Colorless and transparent O
|
△ O
|
Ag:x | Slightly better at 60min | |
| 1% | ||||||||
| 10% |
Turbid yellow O |
|||||||
| 6 | FC-98 (Kanto Chemical, Japan) | Anionic white powder containing fluorine |
0.1% 1%
|
Room temperature 20min,60min |
Colorless and transparent pH 5~7 Mottled marks
|
O | ×(Ag) | |
| 7 | FC-134 (Kanto Chemical, Japan) | Fluoride-containing oxygen ion tea-brown powder |
0.1% 1% (Ethanol) |
Room temperature 20min, 60min 5min, 30s |
Yellow transparent, Transparent yellowish-brown pH 6 Mottled marks
|
O | ○(Ag) | Marks disappear when temperature is lowered |
| 8 | Soft-data-jento W (Japan Lion grease) | Cationic White powder | 1% | Room temperature 20min |
White turbid pH 7 O
|
X | X |
OH RCH2CH(CH2)nSO3Na (MIX) α-Allylsulfonate
|
| 9 | Ripomin COH (Japan Lion Grease) | Amphoteric liquid | 1% | Room temperature 20min |
White turbid pH 8 O
|
O | X | Imidazolyl |
| 10 | Perettex TR (Kao, Japan) |
Cationic Colorless transparent
|
1% | Room temperature 20min |
White turbid pH 10 O
|
O | X |
RODC—CH—SO3N4R—OOC—CH3 Sodium dipropylsulfosuccinate
|
| 11 | Ema-ru 20C (Kao, Japan) |
Cationic Light yellow liquid
|
1% | Room temperature 20min |
Colorless turbidity pH 7 O
|
X | X |
R—O(CH2 OH)nSO3Na Polyoxyethylene alkyl acids
|
| 12 | Koutamin 24P (Kao, Japan) |
Anionic White solid
|
1% |
Colorless and transparent pH 7 O
|
O | X | Trimethylamine | |
| 13 | Perettex # 1222 (Kao, Japan) | Nonionic | 1% | Room temperature 20min |
White cloudy O
|
O | X | |
| 14 | Perettex # 1265 (Kao, Japan) | Nonionic | 1% | Room temperature 20min |
Colorless and transparent O
|
O | X | |
| 15 | Soft-kurin QA-1 (Miyoshi, Japan) |
Amphoteric White Solid
|
1% | Room temperature 20min |
Colorless turbidity pH 7 O
|
O | X | β-alanine type |
| 16 | Soft-kurin MA-3-70 (Miyoshi, Japan) | Amphoteric | 1% | Room temperature 20min |
Colorless and transparent pH 8 O
|
O | X | |
| 17 | Marusenokku AgT (Japan Ma-ruseru) | Silver anti-tarnish agent |
3mL/L 30mL/L
|
Room temperature 30s, 60min |
White cloudy pH 8 Adhesion
|
O | △ | Refer to the instruction manual |
| 18 | Dainshiruba-SS (Yamato Kasei, Japan) | Silver anti-tarnish agent |
10% Isopropyl Ketone 50% Water 10%
|
25~30℃ 4min, 20min, 60min
|
Colorless and transparent pH 8 O
|
O | X | Refer to the instruction manual |
| 19 | T611 (Uemura Kogyo, Japan) | Silver anti-tarnish agent |
Liquid 10% 90%
|
Room temperature 30s, 2min, 10min, 60min | Colorless transparent spots | O | O | Refer to the instruction manual |
| 20 | Roukorinsu- eido (Aikorouko, Japan) | Silver anti-tarnish agent | 1% | Room temperature 31min, 5min, 30min |
White cloudy pH 7 O
|
O | X | For use in electronic factories |
Among organic compounds, those with an anti-Ag discoloration effect when soaked in 0.2% ammonium polysulfide solution are 2-17 alkanimidazole, 2-mercaptobenzimidazole, and complexing agents.
Organic compounds with these anti-discoloration effects share the following characteristics:
① Treatment is carried out in an alkaline region near pH 7.
② Contains hydrophobic groups (long-chain alkyl, phenyl).
③ Contains dimethylamine groups or —SH groups or contains double bonds.
④ Insoluble in water.
⑤ Colorless, close to solid.
However, some cases meet the above conditions but do not have anti-discoloration effects, such as 2-mercapto benzimidazole and 2-thiazolyl benzimidazole.
The mutual relationship between them is still not very clear.
The effects of FC-134 and T611 have been confirmed regarding the anti-discoloration effect of surfactants. At the same time, experiments were conducted on the combined effect of organic compounds and surfactants (FC-134), and no significant effect was found (Table 2-33). The discoloration resistance of 0.2% polysulfide ammonium solution lasts only about 10 minutes at most. Meanwhile, the solvent (diluent) resistance is also relatively weak. For discoloration resistance of decorative items due to the presence of stains, considering these issues comprehensively, the only possible usage is 2-mercapto benzimidazole with FC-134 (see Table 2-34).
Table 2-33 Combined Effects of Organic Compounds and Surfactants on the Anti-Discoloration of Silver-Plated Layers
| Nomor seri | Komposisi | Processing conditions | Appearance | Hydrophobicity | Anti-color change property | Keterangan |
|---|---|---|---|---|---|---|
| 1 |
2-Heptadecamidazolelg/L FC-134 1g/L EtOH : H2O = 1 :1 |
Suhu ruangan 1min, 60min |
White precipitate pH7 Adhesion | O |
O Less effective than when used alone
|
|
| 2 |
2-Mercaptobenzimidazole 1g/L FC-134 1g/L NaOH 2g/L
|
Suhu ruangan 1min, 60min |
O | X |
O Better than when used alone |
Reduced foaming power, the longer the better |
| 3 |
Mercaptone 5g/L FC-134 0. 5g/L Isoacetone:H2O = 1 :1 |
Suhu ruangan 1min, 60min |
O |
O Better than when used alone
|
Reduced foaming power, the longer the better |
Table 2-34 Effect of Solvents on Anti-Discoloration Film
| Organic compounds | Processing conditions | Solvent | Appearance | Anti-discoloration effect |
|---|---|---|---|---|
| 2-Heptadecamidazole |
1g/L (C2H5OH : HO = 1 : 1) 20min
|
Stains | O | |
|
Diluent (5min)
|
Stain disappears O
|
X | ||
|
Alcohol (5 min)
|
Stain disappears O
|
X | ||
| Coarse spray | Scratch |
O Only the scratched part is discolored
|
||
| 2-Mercaptobenzimidazole |
10 gram/L NaOH 2g/L Room temperature 20min
|
O | O | |
|
Thinner (5min)
|
O | X | ||
|
Alcohol (5min)
|
O | △ | ||
| Coarse spray | Scratch |
O Only the scratched part is discolored
|
The above experimental results are summarized as follows.
① It is difficult to ensure that Ag’s unique tone and luster are not damaged when applying other precious metals on Ag for anti-tarnish treatment.
② Using Sn or Sn alloy methods, when the thickness of Sn is 50~10Å, the anti-tarnish effect can be achieved, but the effect of this method alone is limited.
③ Treatments with Be and Al have little effect.
④ Classical treatments like chromate treatments have relatively good effects. However, their biggest drawback is that the treated film has weak UV resistance and may turn brown over long-term protection.
⑤ Among organic compounds, those with anti-discoloration properties include 2-heptadecylimidazole, 2-alkyl benzimidazole, and mercapto naphthalene agents. However, other organic compounds with the same structure do not necessarily have the same anti-discoloration ability.
⑥ The effectiveness of FC-134 in surfactants sold on the market has been confirmed.
3. Anti-Discoloration Effect of Combined Treatment with Tin Electrolysis and Chromic Acid Electrolysis
(1) Discoloration Promotion Test and its Discoloration Measurement Method
The conditions for the discoloration promotion test are shown in Table 2-35.
Table 2-35 Conditions for Anti-Discoloration Promotion Test
| Larutan |
Ammonium sulfide solution (yellow) (Showa Chemical) (NH4)2Sx(2%) |
| Suhu | 20℃ |
| Soaking time | 2h |
△(%) = L1 – L2 (2-1)
In the formula,
L1— reflectance before the color change test, %;
L2— reflectance before the color change test, %.
(2) Tin Electrolyte
Through experiments on the effects of treatment conditions of the tin electrolyte, it was determined that the concentration of sodium stannate, electrolysis time, current density, and treatment temperature could be ignored regarding their impact on the discoloration resistance characteristics. At the same time, sodium acetate was added as a stabilizer in the alkaline Sn plating. The composition and optimal conditions for Sn electrolysis are shown in Table 2-36.
Table 2-36 Composition and Optimal Conditions for Sn Electrolysis
| Composition of plating solution |
NaSnO3 • 3H2O 8. 5g/L CH3COONa ・ 3H2O 5g/L |
| Treatment conditions |
Current density 0. 5A/dm2 Electrolysis time 15s (10〜30s) Temperature 20℃ (7〜30℃) Anode material Stainless steel plate |
(3) Relationship Between Emissivity and Electrolysis Time
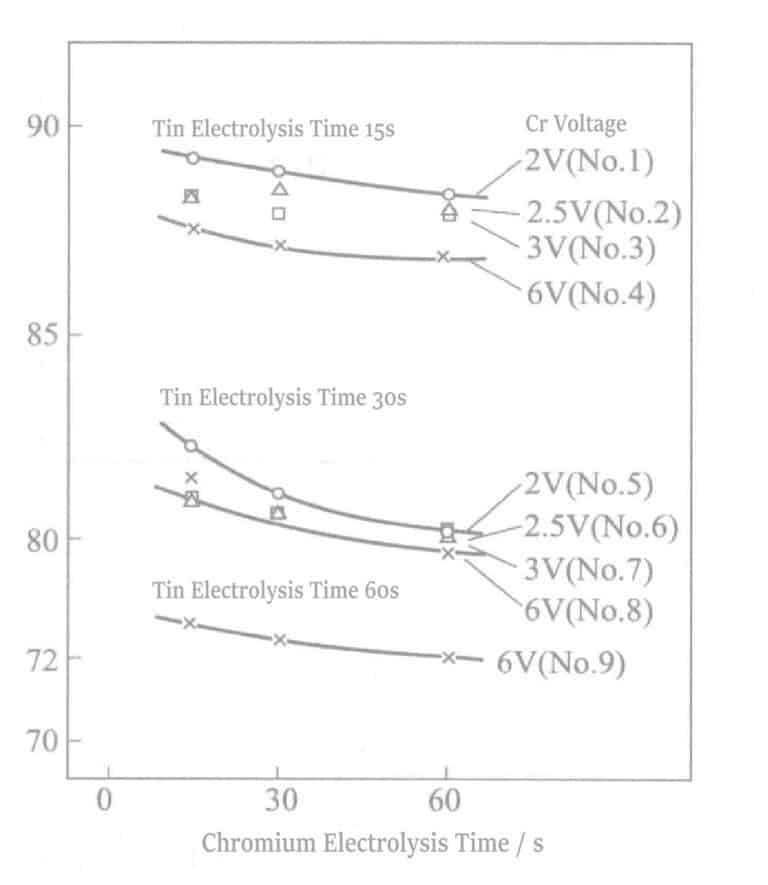
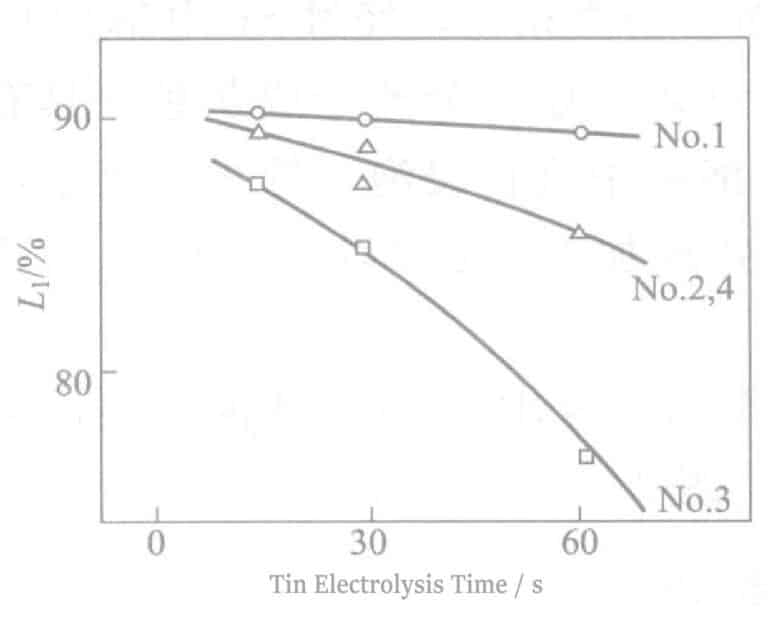
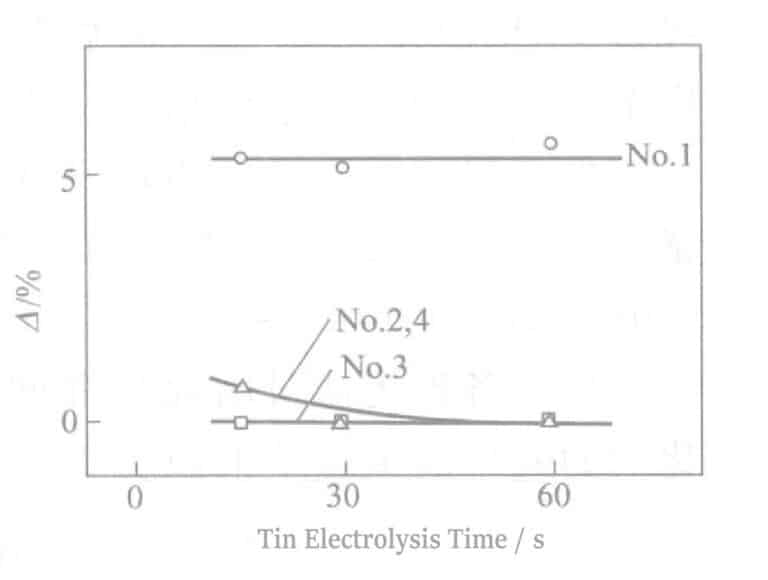
The relationship between reflectivity and electrolysis time is shown in Figure 2-8. As the electrolysis time increases, the reflectivity decreases sharply, which detracts from the hue of Ag. An electrolysis time of about 90 seconds will show a tin-white color, and at about 180 seconds of electrolysis, light brown streaks appear on the surface. At the same time, when the electrolysis time exceeds 60 seconds, the electrical properties also deteriorate. The experimental results obtained according to equation (2-1) are shown in Figure 2-9. As the electrolysis time increases, the △ value decreases.
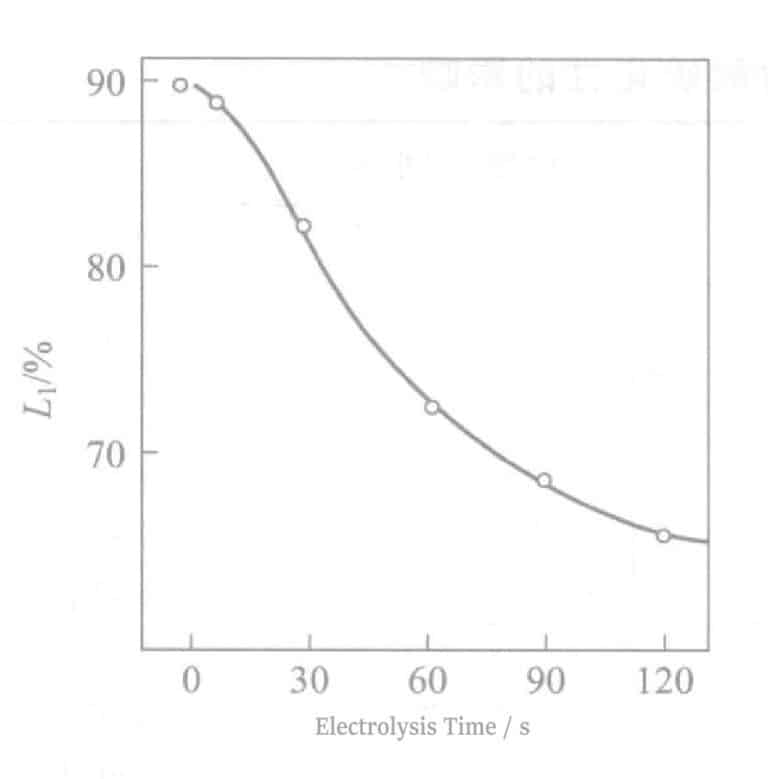
Figure 2-8 Relationship between reflectivity and electrolysis time
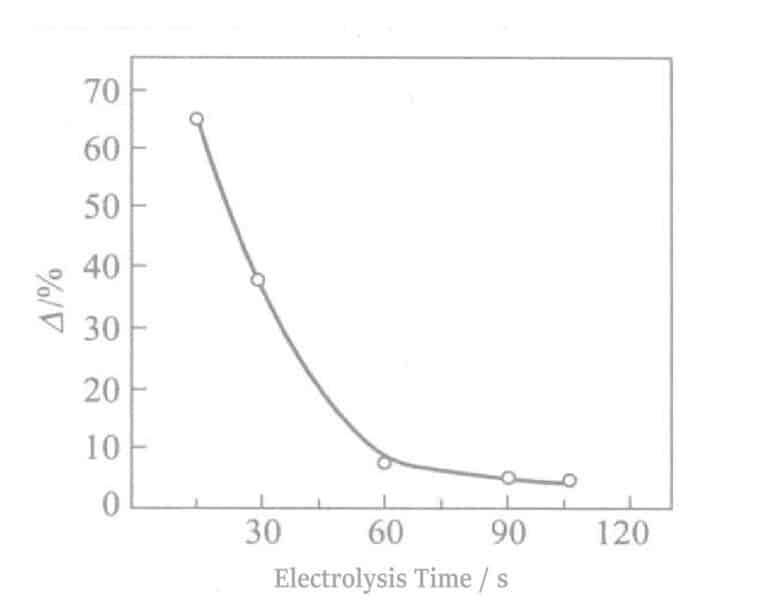
Figure 2-9 Electrolysis Time and Discoloration Resistance
(4) Electrolytic Tin Film Thickness and Electrolysis
Considering the cathode current efficiency according to Faraday’s law, the relationship between electrolysis time and film thickness is shown in Figure 2-10. The tin thickness is about several tens of angstroms.
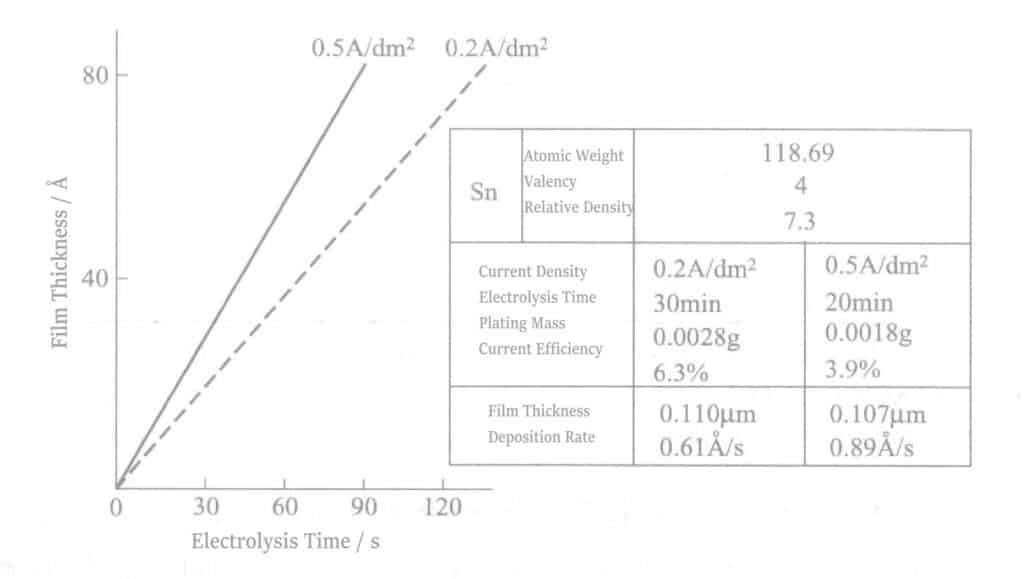
(5) The Influence of Impurities
The effects of possible impurities (CN, Ag, Cu, Ni, etc.) that may be mixed into the electrolyte in Table 2-36 on the appearance and sulfide resistance of the electrolytic membrane were confirmed through experiments. The results are shown in Table 2-37.
Table 2-37 Effect of Impurities on Sulfur Resistanc
| Impurities | Concentration/×10-6 | Electrolysis Time /s | ||
|---|---|---|---|---|
| 30 | 60 | 120 | ||
| Tidak ada | 0 | O | O | △(white) |
| CN |
20 200 2000 20000 |
O O O △,white |
O △,white △,white △,white |
△,white △,white △,white △,white |
| Ag |
20 50 100 150 200 400 |
O O △ X X X |
O △,red X, red-black X,red-black X,red-black X, black |
△,red △,red-black X, red-black X, red-black X, black X, black |
| Cu |
20 200 300 400 500 600 1000 |
O O O △,red △,red x,red x,red |
O O △,white △,red X,red X,red X,red |
△,white △,white △,red △,red X,red X,red X,red |
| Ni |
20 200 1000 |
O O O |
O O O |
O O O |
|
Note: 1. Compounds used: CN—NaCN;Ag—KAg(CN)2;Cu—KCu(CN)2; Ni—Ni (CN)2 • 2KCN - 2H2O. 2. Degree of color change: ○ no appearance change; △ slight color change; × color change.
|
||||
(6) Chromate Electrolysis
Besides chromate, inorganic salts in the electrolyte, such as (Na2CO2, KCN, KI, Na2S2O3, etc.), are used as additives. These aqueous solutions have long been reported as anti-tarnish agents for silver. The basic composition of the electrolyte is shown in Table 2-38.
Table 2-38 Cr Electrolytic Process Conditions
| Electrolyte Composition |
K2 CrO4 15 gram/L NaOH 30g/L |
| Used after 30h heating and maturing process | |
| Treatment Conditions |
Voltage 6V (2 〜 6V) Electrolysis time 15s (60s) Temperature 20℃ Anode material Stainless steel plate |
(7) The Relationship between Reflectivity and Electrolysis Time
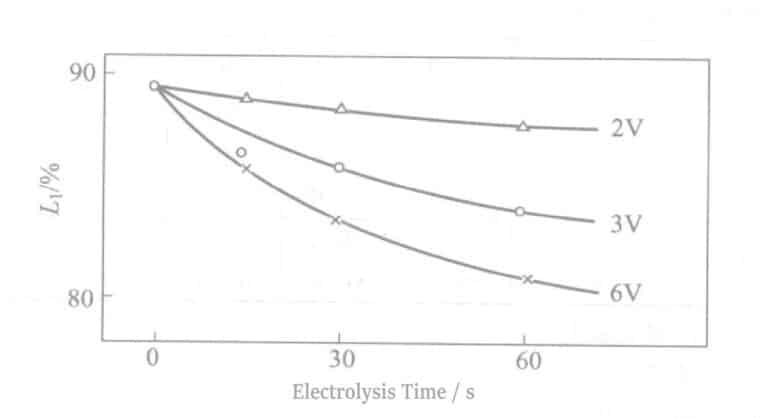
The relationship between reflectivity at a voltage of 2~6V and electrolysis time is shown in Figure 2-11. As the voltage increases, the thickness of the Cr film increases while the reflectivity decreases. Unlike the case with Sn electrolyte, under the same voltage conditions, the increase in electrolysis time does not affect reflectivity.
7. The Relationship between Reflectivity and Electrolysis Time
The relationship between reflectivity at a voltage of 2~6V and electrolysis time is shown in Figure 2-11. As the voltage increases, the thickness of the Cr film increases while the reflectivity decreases. Unlike the case with Sn electrolyte, under the same voltage conditions, the increase in electrolysis time does not affect reflectivity.
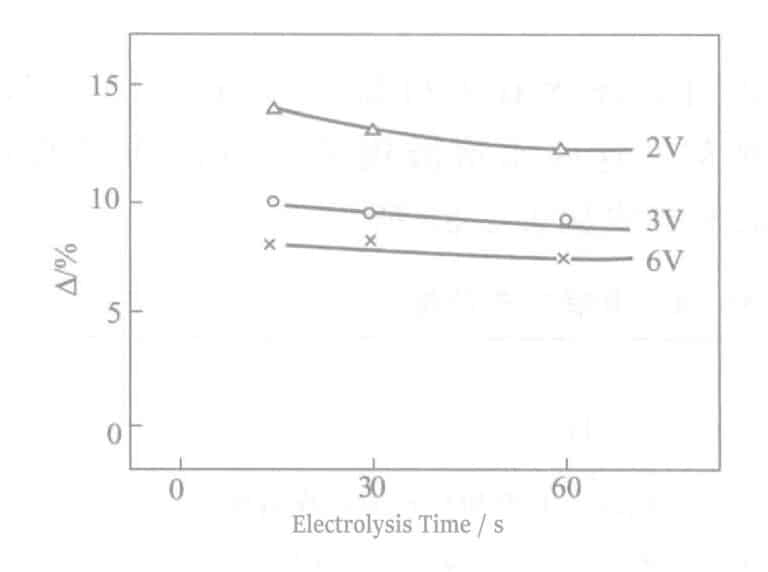
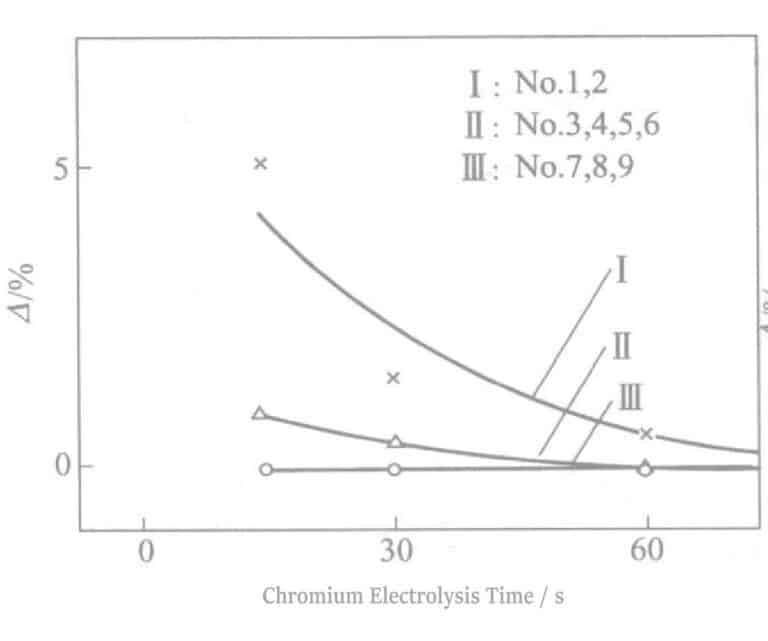
(8) Sulfide Resistance
The comparison △ results under the condition of soaking in 0.2% (NH4)2Sx solution for 2 hours are shown in Figure 2-12. The higher the voltage, the better the sulfur resistance, mainly due to the thickening of the chromium oxide film. If the electrolysis time is prolonged without improvement, it is because there are too many pinholes in the electrolytic film, resulting in an increase in chromic acid mixed into the pinholes.
(9) Discoloration Caused by ultraviolet light
The discoloration and sulfur resistance results of Cr electroplated films caused by ultraviolet irradiation are shown in Table 2-39 (Cr electroplating conditions: 6V, 30s). After ultraviolet irradiation, the Cr electroplated film is completely damaged, turning reddish-purple and bluish-purple. At the same time, the sulfur resistance also sharply decreases, dropping to the same level as without anti-discoloration treatment. There is little difference between the anti-discoloration-treated samples and those exposed to sunlight due to the difference in ultraviolet intensity compared to general ultraviolet irradiation experiments.
Table 2-39 Color Change Caused by Ultraviolet Irradiation
| Nomor seri | Ultraviolet | L1/% | L2/% | L3/% | △1/% | △2/% | Keterangan | |
|---|---|---|---|---|---|---|---|---|
| 1 | - | - | 86. 3 | - | 84. 8 | 0 | 1.5 | (Turns yellow) |
|
2 3 4 |
UV① |
0.5 1 3 |
86. 0 86. 2 86. 5 |
86. 0 83. 2 58〜65 |
67 53 — |
0 3 21〜28 |
19 33 — |
Biru - Violet |
|
5 6 |
Sunlight② |
3 6 |
86.6 86.4 |
86.6 86.4 |
85.6 85.5 |
0 0 |
0.8 0.9 |
(Turns yellow) - |
|
① The photoelectric gloss meter uses a hydrogen discharge tube. ② Outdoor exposure during the daytime: L1 —— Reflectance before irradiation; L2 —— Reflectance after irradiation; L3 —— Reflectance after soaking in 0. 2% (NH4 )2 Sx for 30 minutes following irradiation. △1 =L1 - L2 △2 =L1 - L3
|
||||||||
Copywrite @ Sobling.Jewelry - Produsen perhiasan khusus, pabrik perhiasan OEM dan ODM
(10) The Impact of Impurities
The test results of impurity effects in the basic composition are shown in Table 2-40.
Table 2-40 Effects of Impurities
| Impurities | Concentration /×10-6 | Appearance |
|---|---|---|
| CN |
200 400 600 800 1000
|
O O O O O |
| Ag |
10 20 30 40 50 |
O O O X(black) X(black) |
| Sn |
200 400 600 800 1000
|
O O O O O |
(11) Sn Electrolysis Treatment Plus Cr Sulfur Resistance during Electrolytic Treatment
The methods introduced above each have pros and cons and cannot be considered complete anti-discoloration methods. Therefore, the following approach is considered: immediately performing electrolytic Sn treatment after plating Ag, followed by electrolytic Cr treatment.
Under constant current conditions ( DK = 0. 5A/dm2 ), the electrolysis time of Sn was changed, and during the subsequent Cr electrolysis treatment, its voltage and electrolysis time were varied. The results are shown in Figure 2-13. The reflectivity reduction under each condition is caused by changes in Sn electrolysis time. The electrolysis time must be kept within 30 seconds to maintain the silver tone.
When in the same Sn electrolysis treatment time state, extending the Cr electrolysis time causes the color of the Cr underlying Sn to show through, resulting in a white or gray uneven state. At the same time, when Cr electrolysis is at a high voltage (6V), this phenomenon appears earlier; at 6V, it can be seen in 5 seconds, whereas at 2V, it can last more than 60 seconds. The extension of Sn electrolysis time shows the same tendency. The longer the electrolysis time, the more likely whitening occurs.
This phenomenon appears when the charge passed during the Cr treatment of a certain Sn electroplated layer reaches a certain value. The redox reaction during Cr electroplating plays a certain role on the Sn electroplated layer, causing changes in the crystallization morphology of Sn or causing changes in the contrast between the Sn electroplated layer and the Cr electroplated layer.
When no color change occurs, it is related to the extension of Sn electrolysis time. The electrolysis time is 15~30s+ electrolysis time over 30 seconds especially has good sulfur resistance, but there will be appearance issues. The combination of Sn electrolysis at 0.5A/dm2 for 15~30s, plus Cr electrolysis at 2~3V for 30s was the optimal condition.
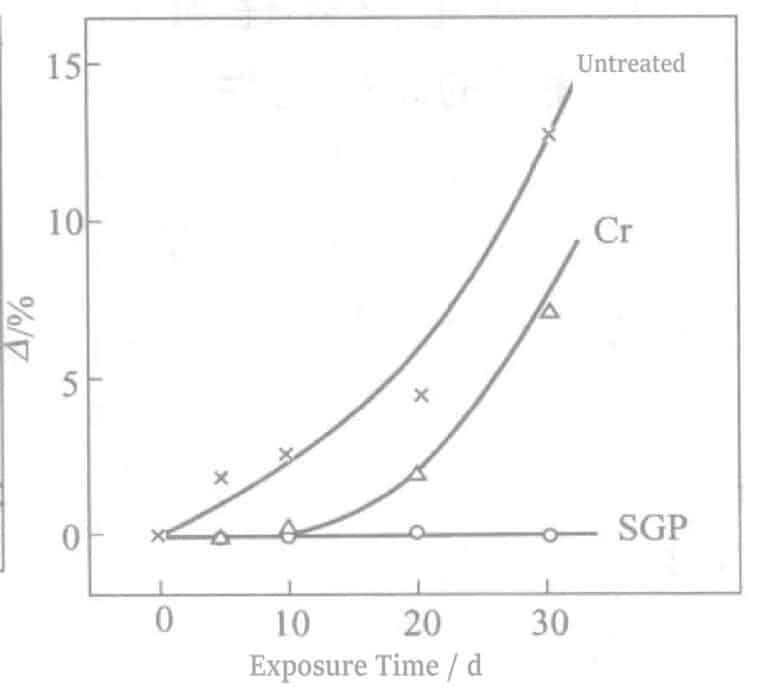
The results of the indoor exposure test are shown in Figure 2-15. Yellowing after 1~2d for untreated and 7~10d for Cr electrolytic treatment. In contrast, the parts treated with Sn electrolysis followed by Cr electrolysis only began to partially turn gray after 60~100d, showing good effectiveness. Soaking was conducted using a 0.2% (NH4)2Sx solution equivalent to one month of indoor exposure testing. The Sn electrolysis treatment followed by the Cr treatment is abbreviated as SGP (silver guard process).
① The effect of various tin salts on sulfur resistance
The test results of the effects of various tin salt surface activators in the SGP method are shown in Table 2-41 and Figure 2-16.
Table 2-41 Comparison of the Effects of Various Tin Salts
| Nomor seri | Komposisi dan kondisi prosesnya | Sn electrolysis time /s | Cr Electrolysis time/s | L1/% | △/% | Keterangan |
|---|---|---|---|---|---|---|
| 1 |
SnCl2 4 gram/L HCl 0.025mol/L (immersion) |
13 30 60 |
15 |
90. 2 89. 8 89. 2 |
5. 4 4. 8 5. 8 |
Yellowing Yellowing Yellowing |
| 2 |
Na2SnO3 ·3H2O 8. 5g/L CH3COONa·3H2O 5g/L 3. 5V(0. 1A/dm2) |
15 30 60 |
15 |
89. 7 87. 3 85. 2 |
0. 7 0. 1 0
|
Partial yellowing - Tin color |
| 3 |
No. 2 plus Peretekkusu# 1232① 0. 1g/L 3. 5V (0. 15A/dm2) |
15 30 60 |
15 |
87. 4 85.0 76. 6
|
0 0. 1 0 |
- - Tin color |
| 4 |
K2SnO3·3H2O 8. 5g/L CH3COONa·3H2O 5g/L 3. 5V (0. 1A/dm2) |
15 30 60 |
15 |
89. 6 88. 9 85. 4 |
0. 9 0 0 |
Partial yellowing - Tin color |
|
① Peretekkusu# 1232 is a product of Miyoshi Oil & Fat in Japan. Note: Cr electrolyte 3 V.
|
||||||
Adding a non-ionic surfactant (No. 3) to the sodium stannate electrolyte significantly improves current efficiency, accelerates the electrolysis rate (No. 2), reduces reflectivity, and enhances sulfur resistance.
Although the results of the potassium stannate electrolyte (No. 4) are the same as those of the sodium stannate electrolyte, there are certain differences between the effects of sodium salts and potassium salts in alkaline tin plating solutions.
② The effect of chromium electrolyte on sulfur resistance was mainly studied from aspects such as electrolyte concentration, the effect of surfactants, and the influence of current density on the appearance after treatment.
Table 2-42 Effect of Concentration on Sulfur Resistance
| Concentration /(g/L) | Electrolysis time /s | Current value at constant voltage (6V)/A | ||
|---|---|---|---|---|
| 5 | 15 | 30 | ||
|
K2CrO4 15 NaOH 30 |
X | X | X |
4. 0 (40A/dm2) |
|
K2CrO4 7.5 NaOH 15 |
O | X | X |
2.4 (24A/dm2) |
|
K2CrO4 1.5 NaOH 3.0 |
O | O | X |
0.6 (6A/dm2) |
|
Note: O indicates no change in appearance; × indicates a change in appearance (whitening).
|
||||
- Effect of concentration: The standard solution was diluted to 1/2, 1/10, and the electrolysis time at which a tin color appeared during electrolysis at 6 V was investigated. The results are shown in Table 2-42.
- Effect of Surfactants: Standard solutions of surfactant (Perettekusu#1265, a product of Miyo-shi Oils and Fats Co., Ltd., Japan) were added with ionic surfactant at concentrations of 1/1 and 1/2, and the electrolysis time was investigated at the time when it began to show a tin color. The results are shown in Table 2-43. Compared to no addition, the effect was better at high current density when surfactants were added. At low current density, there was little difference from the case without addition, but gas hindered the progress of electrolysis. When surfactants were present, their concentration and current density affected the time for whitening to start, increasing management difficulties. At the same time, bubbles were produced due to the large amount of hydrogen generation. It was easier to manage without addition.
Table 2-43 Effects of Surfactants
| Concentration/(g/L) | Perettekusu# 1265/(mL/L) | Voltage/V | DK/(A/dm2 | Electrolysis Time/s | ||
|---|---|---|---|---|---|---|
| 5 | 10 | 15 | ||||
|
K2CrO4 15 NaOH 30
|
0 |
6 5 6 3 |
40 29 19 7 |
X X X X |
X X X X |
X X X X |
| 0.01 |
6 5 6 3 |
40 29 19 7 |
O O O O |
X X O O |
X X X X |
|
| 0.1 |
6 5 6 3 |
40 29 19 7 |
O O O O |
O O O X |
X X X X |
|
| 1 | 6 | 40 | O | X | X | |
|
K2CrO4 7.5 NaOH 15
|
0 |
6 5 6 3 |
24 17 10 3 |
X X X O |
X X X O |
X X X X |
| 0.01 |
6 5 6 3 |
24 17 10 3 |
O O O O |
O O X X |
X X X X |
|
- Influence of Current: Density The time when the tin color first appears at various current densities during electrolysis is shown in Figure 2-18. At the same time, the E-I curve is shown in Figure 2-19. The current density is more appropriate when the electrolysis time is 0.5A/dm2 around 30 seconds. Meanwhile, the time without the appearance of tin color is also affected by electrolyte concentration, anode current density, electrode spacing, and other factors. Concentration management is done by calculating the size of the tank, and it is possible to derive the intrinsic power of the tank that does not appear tin-colored at a certain voltage, for ease of management.
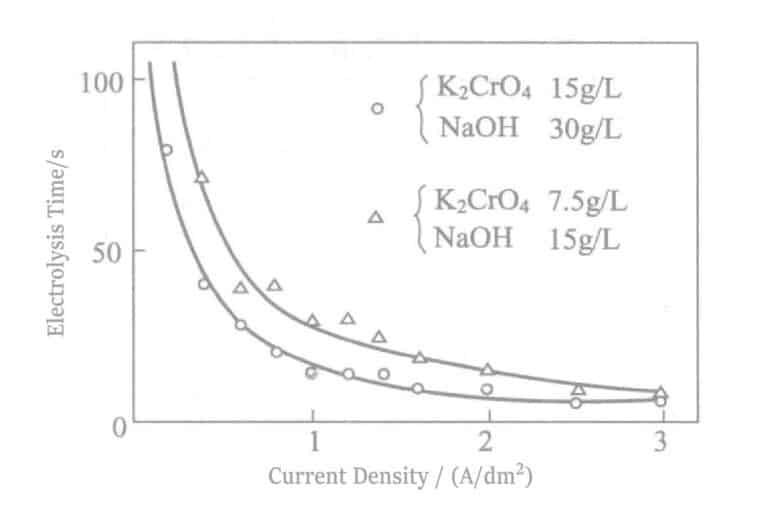
Figure 2-18 Relationship between current density and electrolysis time
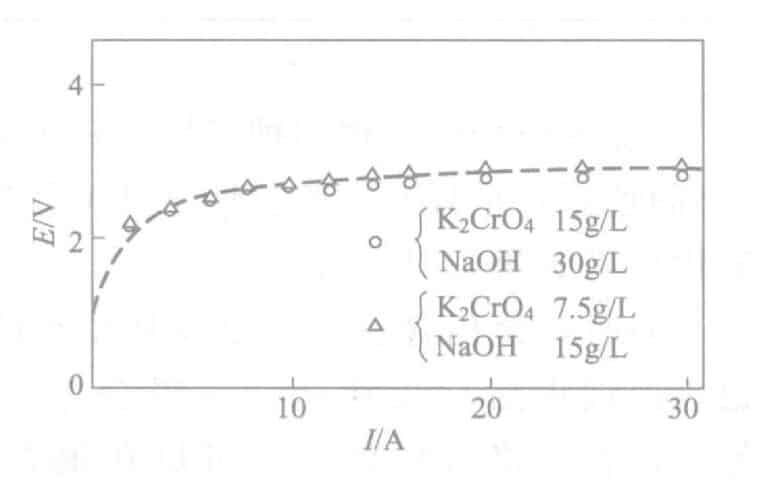
Figure 2-19 E-I curve of the electrolyte solution
Figure 2-20 Chromium Electrolyte Treatment Process

Figure 2-21 Comparison of Sulfur Resistance of Various Treatment Methods
At the same time, using detergents (surfactants) containing α-diketone and its salts to wash or soak silver (or silver-plated items) and its alloys (tin alloy, zinc alloy, indium alloy, palladium alloy, etc.) can greatly inhibit surface discoloration or corrosion.
The basic structure of its additive is shown in Figure 2-22.
Table 2-44 Formulation Examples of Diketo Surfactants for Silver Tarnish Prevention
| Komposisi | Nomor 1 | Nomor 2 | Nomor 3 | Nomor 4 | Nomor 5 | Nomor 6 | Nomor 7 | Nomor 8 |
|---|---|---|---|---|---|---|---|---|
| Chlorovanillic acid | 10 | |||||||
| Polyoxyethylene alkyl ether | 0.5 | |||||||
| Sodium dihydrogen phosphate | 5 | |||||||
| Polyethylene glycol | 1 | |||||||
| Tretinoin | 5 | |||||||
| Pyrazole chloride | 5 | |||||||
| Sodium bicarbonate | 5 | |||||||
| Protease | 1 | |||||||
| Potassium iodide | 0.05 | |||||||
| Tetrahydroxy-1,4-benzoquinone | 0.5 | |||||||
| 2-Alkyl-N-carboxymethyl-N-hydroxymethylimidazoline | 10 | |||||||
| Thiourea | 0.5 | |||||||
| Glycerol | 1 | |||||||
| Sodium 3-ethyl-2-hydroxy-2-cyclopentane-1-carboxylate | 0.05 | |||||||
| Potassium alkyl sulfonate | 5 | |||||||
| Isocyanuron salt | 5 | |||||||
| N-Acylated amines | 1 | |||||||
| 3-Hydroxy-2-methyl-4-pyrone | 20 | |||||||
| Asam sitrat | 5 | |||||||
| Sodium benzoate | 5 | |||||||
| Menthol | 0.1 | |||||||
| Calcium rosmarinic acid | 2 | |||||||
| 3-Methyl-1,2-Cyclopentanaminium | 20 | |||||||
| Polyoxyethylene castor oil | 5 | |||||||
| Steroidal Glycosides | 1 | |||||||
| 3-Hydroxy-1,2-dimethyl-4(1H)-pyridine | 6 | |||||||
| Mercaptoalanine | 2 | |||||||
| Glucoamylase | 5 | |||||||
| Sorbitol | 1 | |||||||
| 2,5-Dihydroxy-1,4-benzoquinone | 10 | |||||||
| Alkyl benzyl ammonium chloride | 30 | |||||||
| Potassium sodium tartrate | 5 | |||||||
| Papain | 1 |
Section II Electroless Silver Plating
Because silver has low resistivity and is much cheaper than other precious metals, silver plating is widely used in the electronics field.
As a precious metal, silver has a more positive redox potential than other metals, allowing it to be displaced and deposited on metals such as copper, iron, and zinc, forming a silver plating layer on these metals. It will be mentioned later that this type of reaction is used on printed circuit boards to replace the copper with silver so that the copper pattern is covered by a layer of silver, thereby increasing solderability and reducing contact resistance. However, this displacement deposition generally results in a relatively thin plating layer, about 2μm or less. In contrast, the plating layer obtained through a reduction reaction can be somewhat thicker.
In the application of silver plating, the most famous should be the silver mirror reaction. The silver mirror reaction was developed by Drayton in 1830 and was applied to the mirror manufacturing industry. In addition, chemical silver plating (especially reduction silver plating) is widely used in electronics, thereby expanding the industrial applications of silver plating. Chemical silver plating can be applied not only to metals but also to non-metals.
1. Displacement of Silver Plating and its Applications
Displacement silver plating utilizes the potential difference between the base metal and silver.
The conductor patterns on printed circuit boards are usually made of copper, but during storage, before component mounting, an oxide film forms on the copper surface. This oxide film affects the solderability of the circuit board. High-end products or even Ni/Au, Ni/Pd, Ni/Pa/Au plating are generally used to enhance the brazing ability of copper circuits, which naturally increases the cost. However, Sn plating lacks sufficient rust resistance. Silver plating is close in price to Sn and not only prevents rust and is suitable for brazing but can also be used for metal wire connections, meaning silver can be displacement-plated on copper circuits. However, the thickness must be increased to compensate if silver is plated directly on copper due to uneven plating. This makes the plating fail the tape test, with plating peeling off easily, especially after storage. However, this problem can be solved by two-step plating. The method is to first plate a layer of metal, which is more expensive than copper, on the copper, then displacement plate silver. The first metal layer can be Pd or Ag. The thickness is so thin, on the order of nanometers (i.e., several nanometers thick), that the presence of the plating cannot be recognized with the naked eye. The displacement reaction is:
The silver displacement solution must also contain a complexing agent for monovalent copper to stabilize the displaced copper ions. When the first coating is displacement silver, its displacement rate must be much slower than that of the second Ag plating layer, equivalent to less than 15% of its speed. If it is too fast, it is easy to cause a decrease in coating adhesion, resulting in coating peeling during the tape test. The following is an example of silver plating.
When the first plating layer is Pd, the Pd plating solution is
PdSO4 50mg/L (accounted as Pd)
H2SO4 5%
Temperature Room temperature
Time About 30s
Table 2-45 Composition and Process of Displacement Silver Plating (first plating layer)
| Komposisi dan kondisi proses | Formulasi dan komponen | Komposisi dan kondisi proses | Formulasi dan komponen |
|---|---|---|---|
| Silver methanesulfonate (as Ag) | 1g/L | Lurotex A25 | 20mg/L |
| NaBr | 320g/L | pH | 5.5 |
| Diethylenetriamine pentylenephosphonic acid | 30mL/L | Suhu | 50℃ |
| Polyethylene glycol 400 | 80mL/L | Time | 5 min |
| 2,2'-Bipyridine | 90mg/L |
After plating, the plated parts undergo heat treatment at 150℃ for 4 hours, followed by a wettability test, which is qualified.
When the first plating layer is Ag, the plating solution and its process are shown in Table 2-46.
Table 2-46 Composition and Process of Displacement Silver Plating (Second Coating)
| Komposisi dan kondisi proses | Formulasi dan komponen | Komposisi dan kondisi proses | Formulasi dan komponen |
|---|---|---|---|
| Silver methanesulfonate (as Ag) | 1g/L | Polyethylene glycol 400 | 80mL/L |
| NaBr | 320g/L | 2,2'-Bipyridine | 90mg/L |
| Diethylenetriamine pentylenephosphonic acid | 30mL/L | pH | 5.5 |
The plating solution for the first and second plating layers can have the same composition, but the temperature and immersion time differ. The conditions for the first plating layer are as follows: 25℃, 30s. The conditions for the second plating layer are as follows: 50℃, 6min. The resulting plating layers are uniform, silver bright, and have good brazing weldability.
Hutchinson et al. proposed a plating solution method using alkyl polyethylene glycol ether as a brightener, ethylenediamine, and 1-hydroxyethylene-1,1-diphosphonic acid as copper complexing agents, and the obtained plating layer can meet the soldering conditions of printed circuit boards.
ITO (a transparent indium tin oxide) is widely used in liquid and plasma crystal displays. As the size of liquid crystal displays and plasma displays has increased in recent years, the resistance of ITO itself has become relatively high. To solve this problem, a silver layer with lower conductivity can be added to the ITO. This cannot be implemented by silver electroplating directly because it is difficult to directly deposit catalytically active metals on ITO. Therefore, a layer of tin is first deposited on the ITO, followed by a catalytically active metal deposition, and finally, silver plating. The composition of the plating solution and process conditions for silver plating on ITO are shown in Table 2-47.
Table 2-47 Composition of the Plating Solution and Process Conditions for Silver Plating on ITO
| Immersion Tin Liquid |
SnCl2 HCl
|
70g/L 230mL/L |
| Activation Fluid | AgNO3 | 10 gram/L |
| Silver plating solution |
AgNO3 NH4OH (NH4)2SO4 Co(SO4)2 Mg(OH)2 Ag thickness Specific resistance Specific resistance (250℃, 30min after treatment) |
0. 03〜0. 08mol/L 7〜10mol/L 0. 3〜0. 8mol/L 0. 1〜0. 2mol/L 0. 01〜0. 05mol/L 2900〜3200Å 3〜3. 5μΩ• cm 1. 6〜2. 5μΩ• cm |
2. Reduction of Silver Plating and its Applications
The composition of the reduction silver plating solution is similar to that of general chemical plating, with the main components being: Ag salt, complexing agent, reducing agent, pH adjuster, stabilizer, etc. The reducing agents for silver include glucose, saccharin, glyoxal, ascorbic acid, gluconic acid, sorbitol, hydrazine, HCHO, KBH4, NaBH4, DMAB and others.
The reactions of some representative silver-reducing agents are as follows.
(1) HCHO (when using ammonia solution as a complexing agent)
2AgNO3 + 2NH4OH → Ag2O + 2NH4NO3 + H2O
Ag2O + 4NH4OH → 2[Ag(NH3)2]OH + 3H2O
2[Ag(NH3)2]OH + HCHO → 2Ag + 4NH3 + HCOOH + H2O
4AgNO3 + 4NH3 + C4H4O6NaK + H2O → 4Ag + 4NH4NO3 + C3H2O6NaK + CO2
4[Ag(NH3)2]NO3 + N2H4 → 4Ag + 4NH4NO3 + 4NH3 + N2
N2H4BH3 + 3Ag+ + 4OH– → 3Ag + N2H4 + B(OH)4–+ 3/2H2
Table 2-48 Formulations Using Organic Solvents for Electroless Silver Plating
| Bahan-bahan dan kondisi prosesnya | Nomor 1 | Nomor 2 |
|---|---|---|
|
Dimethyl sulfoxide/mL Ethylene glycol/ml Ethanol/mL d-(+)Glucose/mL Triethylamine/mL Suhu/°C Time/min Plating materia |
300 200 - - 15 70 20 Al2O3 powder |
200 - 300 5g 2. 5mL/min 60 10 Nylon cloth
|
Among them, diols and glucose are used as reducing agents. At the same time, since dimethyl sulfoxide has a slight reducing ability, the plating solution does not require aging treatment. The resulting plated parts have silver-like metallic luster.
Organic compounds containing two monothiol groups are used as complexing agents, with the structural formula as follows:
In the formula, R1 and R2 are alkylene groups containing 1~5 carbon atoms; R3 are an alkylene group containing 2~8 carbon atoms X and Y can be carboxyl, sulfonic acid, amino, alkyl groups, etc.
The reducing agents include aldehydes, hydrazines, borohydride compounds, ascorbic acid, etc. Table 2-49 shows examples of the use of this series.
Table 2-49 Process Conditions of Silver Reduction Plating Solution Using Two Monothiol Organic Compounds as Complexing Agents
| Komposisi dan kondisi prosesnya | Nomor 1 | Nomor 2 | Nomor 3 |
|---|---|---|---|
|
Silver nitrate/(g/L) 1,2-Bis(2-carboxyethylthio)ethane/(g/L) 1,4-bis(2-carboxyethylthio)butane/(g/L) 1,2,2'-(Ethylthio)diethyl mercaptan/(g/L) Hydrazine hydrate/(g/L) Formalin/(g/L) Sodium hypophosphite/(g/L) pH (adjusted with NaOH) Suhu/°C |
0. 17 10 - - 8 - - 10 35 |
0. 31 - 15 - - 4 - 11 50 |
0. 34 - - 20 - - 10 10. 5 50 |
Section III Analysis of Cyanide Silver Plating Solution
1. Analysis of Silver
Table 2-50 Silver Ion Analysis Method for Silver Plating Solution
| Operation sequence | Notes and Instructions |
|---|---|
|
(1) Take 5ml of plating solution into 300 beaker, add 20mL of sulfuric acid and 5mL of nitric acid while observing. (2) Heat until white smoke is produced (3) Add 100mL of water and 3mL of 10% ammonium iron sulfate. (4)Titrate with 0.1mol/L potassium thiocyanate. Calculation formula: Ag(g/L) = mL × 2. 158× f AgCN(g/L) = Ag(g/L) ×1. 2402 In the formula, mL——0. 1mol/L titer of potassium thiocyanate; f——0. 1mol/L potassium thiocyanate solution coefficient |
Due to the generation of toxic hydrogen cyanide gas, it is necessary to operate in a local ventilation area. Change from brown smoke to white smoke Titration endpoint: light red color 0. 1mol/L KCNS 1mL = 0. 01079g Ag |
2. Methods for Analyzing Free Cyanide
Table 2-51 Analytical Methods for Free Cyanide
| Operation sequence | Notes and Instructions |
|---|---|
|
(1)Take 5ml of plating solution into 300m beaker, add water 50mL (2)Add 10% potassium iodide solution 1~2mL (3)Titrate with 0.1mol/1 silver nitrate solution Calculation formula. Free KCN (g/L) = mL×2.60×f Free NaCN (g/L) = mL×1.96× f In the formula, f - coefficient of 0.1mol/L silver nitrate solution |
Titration endpoint: light red color 0. 1mol/L AgNO3 1mL = 0. 0130g KCN = 0. 0098g NaCN |