How Much Do You Know About Diamond's Properties?
Diamond Guide: Types, Colors, Cuts & Sparkle for Jewelers

Diamonds in Various Crystal Forms
Πίνακας περιεχομένων
Section I Chemical Composition of Diamonds
Section II Classification of Diamonds
The most common trace element in diamonds is nitrogen. Nitrogen (N) replaces carbon (C) in the lattice in an isomorphic form; the content and form of occurrence of nitrogen (N) atoms have an important impact on diamond properties and are also the basis for diamond classification.
Depending on whether diamonds contain the trace element nitrogen (N), diamonds can be classified as Type I and Type II. Further, according to the different forms and characteristics of nitrogen (N) atoms in the lattice, Type I diamonds can be subdivided into Type Ia and Type Ib; according to whether diamonds contain the trace element boron (B), diamonds can also be classified into Type IIa and IIb.
1. Type Ia Diamonds
Type Ia diamonds contain nitrogen (N) atoms in an ordered aggregated state within the diamond structure. Specifically, they can be divided into the following situations.
(1) Paired nitrogen atoms. When nitrogen (N) simultaneously substitutes for two adjacent carbon atoms in the diamond as a paired form and forms a stable aggregated state, it is called a Type IaA diamond; this aggregated form is called an A aggregate. The characteristic absorption band of the A aggregate is an infrared-region absorption at 1282cm-1.
(2) Triatomic nitrogen. Prolonged exposure to high temperature and high pressure can cause the nitrogen (N) in a diamond to further aggregate into three nitrogen (N) atoms, which replace three adjacent carbon (C) atoms along the (111) direction of the diamond crystal. When a structural vacancy is left between the three nitrogen atoms, it is called triatomic nitrogen. The configuration of three nitrogen atoms plus a vacancy is called the N3 centre. The N3 centre can produce a strong absorption in the violet region of visible light at 415.5 nm; it is a major cause of diamonds exhibiting yellowish tints and blue-white fluorescence.
(3) 4~9 atomic nitrogen. When in the diamond crystal structure, 4~9 nitrogen (N) atoms occupy carbon (C) atom positions along certain structural directions (commonly four nitrogen atoms plus one vacancy), the diamond is called type IaB; this aggregated form is called the B (or B1) aggregate. A strong absorption band characterizes the B (or B1) aggregate in the infrared region at 1175cm-1.
(4) Platelet nitrogen. When the nitrogen content of type IaB diamonds reaches a certain level and aggregates into platelets of size of 50~100nm (usually flat layers a few atoms thick) that can be directly observed under an electron microscope, platelet nitrogen is typically produced. Small platelets are bilayer separations of trivalent nitrogen atoms connected by N-N bonds between atoms on adjacent planes along the (100) face of the diamond crystal, surrounded by C atoms. This platelet nitrogen is usually called the B2 centre, and its primary identifying feature is a strong absorption band in the infrared region at 1365~1370cm-1.
2. Type Ib diamonds
Type Ib diamonds are rare in nature; they contain nitrogen (N) as isolated single atoms, randomly occupying carbon (C) positions in the crystal structure. In the infrared spectrum, there is a strong absorption band at 1130cm-1, and the absorption band at 1130cm-1 is significantly stronger than the absorption band at 1280cm-1. These diamonds are often vivid yellow. Under certain temperatures, pressures, and long periods, type Ib diamonds can convert into type Ia diamonds.
Type Ia diamonds can be preserved for long periods in the upper mantle at a temperature of 1000~1400℃. Under the same conditions, Type Ib diamonds do not last more than 50 years before converting to Type Ia. Therefore, natural diamonds are mainly Type Ia, while synthetic diamonds are mainly Type Ib.
3. Type IIa diamonds
4. Type IIb diamonds
Type IIb diamonds can contain small amounts of boron (B), which gives the diamond a blue colour. In the infrared spectrum, there are strong absorption bands at 2800cm-1. Type IIb diamonds are semiconductors and are the only natural diamonds that can conduct electricity.
The classification and characteristics of diamonds are shown in Table 1–1.
Table 1–1 Overview of the classification and characteristics of diamonds
| Classification & Basis | Type I: Contains a certain amount of Nitrogen impurities | Type II: Contains no Nitrogen, Boron or other impurities | |||||
|---|---|---|---|---|---|---|---|
| I a | I b | II a | II b | ||||
| Nitrogen exists in aggregated form | Nitrogen exists in single atomic form | No nitrogen, carbon atom displacement causes defects | No nitrogen, contains a small amount of boron | ||||
| Form of Impurity Element & Subtype | Diatomic Nitrogen I aA | Triatomic Nitrogen I aAB | 4 〜 9 atom Nitrogen I aB | Platelet Nitrogen I aB2 | Isolated Nitrogen | Dispersed boron substitutes for carbon | |
| Crystal Defect Center | N2 / A Center | N3 Center | B/B1 Center | B2 Center | N/C Center | B Center | |
| IR Absorption Spectrum / cm-1 | 1282 | 1175 | 1365 〜1370 | 1130 | No absorption between 1100 〜1400 | 2460、 2800 | |
| Visible Absorption Spectrum / nm | N2, N3 centers absorb blue/violet light. N3 characterized by 415 nm absorption, also 423, 435, 465, 475 nm. B1, B2 do not absorb visible light. | 503, 637 weak absorption. UV ~270 nm - Blue-green absorption. | Does not absorb visible light. | No distinct absorption peaks in the visible region. | |||
| UV Absorption Spectrum / nm | Transmits UV light down to 330 nm. | Same as Type I a | Transmits UV light down to 220 nm. | Same as Type II a | |||
| Color Characteristics | Colorless - Light Yellow (Typically, natural yellow diamonds belong to this type) | Colorless - Yellow, Brown (All synthetic diamonds and a small number of natural diamonds) | Colorless - Brown, Pink (Extremely rare) | Blue (Extremely rare) | |||
| UV Fluorescence | Often blue fluorescence; rarely green, yellow, red, or no fluorescence. | Same as Type Ia | Mostly no fluorescence. | Same as Type IIa | |||
| Phosphorescence | Those with strong blue-white fluorescence may have phosphorescence. | No phosphorescence. | Has phosphorescence. | ||||
| Ηλεκτρική αγωγιμότητα | Non-conductive | Non-conductive | Non-conductive | Ημιαγωγός | |||
| Αλλος | Accounts for 98% of natural diamond production. | Vast majority are synthetic diamonds; extremely rare in natural diamonds. | Quantity is very small, but large diamonds often belong to this type. | Rare, often blue. | |||
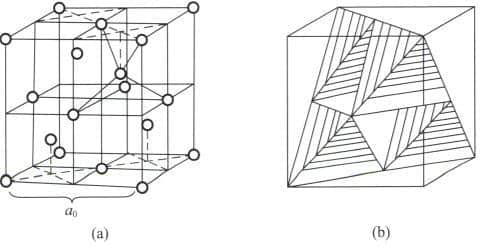
Section III The Crystal Structure of Diamonds
Section IV Crystal Forms of Diamond

Figure 1-2 Octahedral Diamond

Figure 1-3 Rhombic Dodecahedral Diamond

Figure 1-4 Aggregated Diamond Crystal

Εικόνα 1-5 Διαμάντια σε διάφορες κρυσταλλικές μορφές

Section V Mechanical Properties of Diamond
(1) Diamond Hardness
Diamonds are the hardest substance in nature; on the Mohs scale, they rank 10, and they have an extremely strong ability to resist mechanical forces such as scratching, indentation, and abrasion. The Mohs hardness indicates relative hardness among gemstones; the absolute hardness of diamond is far greater than that of the other minerals on the Mohs scale — about 140 times that of a mineral with Mohs hardness 9 (corundum) and 1,000 times that of a mineral with Mohs hardness 7 (quartz).
(2) Cleavage of Diamonds
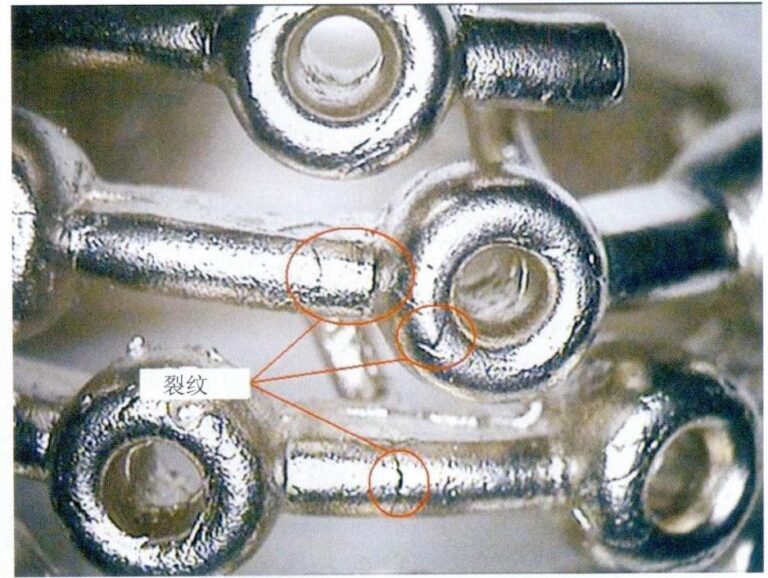
When struck by an external force, diamonds often fracture along octahedral directions, producing four sets of distinct cleavage planes. Finished diamonds commonly show “feather”-like features at the girdle and small, “V”-shaped nicks, which are mainly caused by diamond cleavage. When cleaving rough diamonds during cutting, this property of diamonds is also exploited.
Although diamond is the hardest natural substance discovered by humans, it is very brittle — it is susceptible to heavy blows, which can readily produce cracks or even cause it to shatter.
(3) Relative Density of Diamonds
The relative density of diamonds is 3.52. Because their composition is simple, the relative density is fairly stable. Transparent diamonds have a more stable relative density, while colored diamonds tend to have a slightly higher relative density; diamonds with more impurities and inclusions show slight variations in relative density.
Section VI Optical Properties of Diamonds
1. Colour of Diamonds
Diamond colour can generally be divided into three series: the colourless to light yellow (grey) series, the brown series, and the fancy colour series.
(1) Colourless to light yellow series diamonds. This includes colourless, near colourless, slightly yellow-white to distinctly light yellow diamonds; the vast majority of naturally occurring diamonds belong to this series.
(2) Brown series diamonds. This includes a range of diamonds from light brown to deep brown (Figure 1–7).
(3) Fancy colour series diamonds. These are diamonds that exhibit characteristic hues. Fancy colour diamonds can display all hues in the visible spectrum, including yellow, pink, blue, orange, red, green, purple, etc., with red being the rarest (Figures 1–8 to 1–10). Most fancy colour diamonds have subdued tones; vividly colored fancy diamonds are extremely rare. The colours of fancy diamonds arise from small amounts of impurity elements—nitrogen (N), boron (B), and hydrogen (H)— incorporated into the diamond’s crystal structure, forming various colour centres. Another cause is plastic deformation of the crystal, which produces dislocations and defects that absorb certain light energies and impart colour to the diamond. Fancy colour diamonds are very rarely found in nature and are highly valuable.

Figure 1–7 Brown diamond

Figure 1–8 Yellow diamond

Figure 1-9 Pink diamond

Figure 1-10 Blue diamond
2. Lustre, Transparency and Refractive Index of Diamonds
3. Diamond Fire


Figure 1-12 Diagram of diamond fire

Figure 1-13 Diamond fire
Copywrite @ Sobling.Jewelry - Κατασκευαστής προσαρμοσμένων κοσμημάτων, εργοστάσιο κοσμημάτων OEM και ODM
4. Absorption Spectrum of Diamonds
5. Luminescence of Diamonds
Diamonds exhibit different luminescent characteristics when irradiated by ultraviolet, cathode rays, and X-rays.
(1) Ultraviolet fluorescence and phosphorescence. Under ultraviolet illumination, diamonds usually emit stronger fluorescence under long-wave than under short-wave ultraviolet. Under long-wave UV irradiation, fluorescence can range from none to strong, and the colours may appear light blue, blue, yellow, orange-yellow, pink, yellow-green, and white. Type I diamonds predominantly show blue to light-blue fluorescence, while Type II diamonds predominantly show yellow to yellow-green fluorescence. Colourless to light-yellow series diamonds often exhibit blue-white fluorescence; brown diamonds exhibit yellow-green fluorescence; vivid yellow diamonds exhibit yellow fluorescence. Diamonds with distinctly strong blue-white fluorescence often have light yellow phosphorescence. It can be determined that, under equal-intensity UV irradiation, non-fluorescent diamonds are the hardest, those emitting yellow fluorescence are next in hardness, and those emitting pale blue fluorescence are the softest. This characteristic can be fully utilized during diamond cutting.
(2) Cathode-ray fluorescence. The phenomenon in which a diamond emits visible light when excited by high-energy cathode electrons is called cathode luminescence. It specifically manifests as yellow-green and blue of varying intensities; the distribution patterns of luminescent and non-luminescent zones within the diamond and of differently colored luminescent areas differ, making this an important factor in distinguishing natural diamonds from synthetic diamonds.
(3) X-ray fluorescence. Regardless of the type of diamond, it can fluoresce under X-ray irradiation, and the fluorescence colour is consistent, usually appearing bluish-white. The X-ray diamond sorter designed using this characteristic is very effective in diamond sorting, being both sensitive and accurate.
Section VII Other Properties of Diamonds
(1) Thermal properties of diamonds. Diamonds have excellent thermal conductivity; the thermal conductivity of diamond is times higher than that of silver and copper, and it is the highest among transparent gemstones, far exceeding all other gems. In other words, diamonds conduct heat very rapidly, which is why they feel cool to the touch. People have used this property to design and manufacture a specialized instrument—the thermal conductivity tester—to distinguish diamonds from diamond simulants.
(2) Wettability of diamonds. Diamonds have a pronounced affinity for oils and repel water. The wetting property of a diamond refers to its oil-loving and water-repellent nature; diamonds have a strong ability to adsorb oils. In diamond beneficiation, an oil-shaking table can be used to adsorb and capture diamonds. The hydrophobicity of diamonds means that water cannot form a thin film on the diamond surface and can only exist as droplets. A diamond tester pen uses this oil-affinity and water-repellency to identify diamonds: it contains a special oily ink that leaves a continuous mark when drawn across a diamond’s surface, whereas on diamond imitations it leaves a discontinuous trace.
Section VIII Mineral Inclusion Characteristics of Diamonds


Section IX Diamond Cutting Styles
The shape of a diamond after cutting, faceting, and polishing is called the diamond’s cut style, i.e., the style of the finished diamond. It generally includes two elements: first, the geometric outline of the diamond’s girdle as seen from the top view, such as round, heart, marquise, oval, etc.; second, the geometric shape of the diamond’s facets and their arrangement, mainly including brilliant, step, and mixed cuts. The facets of a brilliant-cut diamond are mainly triangular and kite-shaped, arranged radiating outward from the small table facets at the pavilion; the facets of a step-cut diamond are mainly trapezoidal, rectangular, and triangular, arranged in parallel layers on both the upper and lower sides of the girdle; when a diamond displays characteristics of both brilliant and step cuts, it is called a mixed cut.
The most common diamond cut is the standard round brilliant cut (also called the round brilliant style).
1. Standard Round Brilliant Cut
The standard round brilliant cut evolved gradually from earlier table cuts.
The table cut roughly appeared in the early 15th century. This simple cut made full use of the shape characteristics of octahedral diamond rough, merely grinding off one corner tip of the octahedral diamond rough, thereby forming a table with one relatively large square facet on the crown and four naturally inclined pavilion facets. The table cut was the first regular cut form to appear after diamond cutting (Fig. 1–17). Considering the productivity level at the time, the primitive polishing machines were very crude; the diamond’s culet was noticeably worn, creating a relatively large culet, which resulted in low brilliance. At the same time, the existence of the culet greatly reduced the degree of diamond breakage.
With continuous improvements in productivity and advances in science and technology, the machinery used for diamond processing was also continuously improved, and diamond outlines began to evolve from irregular to regular. In 1919, Marcel Tolkowsky (1899–1991), the founder of the standard round brilliant cut, using optical principles and mathematical calculations, proposed a cut with a total of 58 facets, as well as the standard proportions that can fully display diamond fire and brilliance, which he called the American Brilliant Cut, and published the famous book Diamond Design (1919). This was the first book to calculate diamond proportions based on optical principles.
Because no universally accepted common standard was established for the proportions of round diamonds, different “ideal cuts” emerged in different countries, regions, and institutions. Summarised, the representative types are the following.
(1) American Brilliant Cut. The standard proportions proposed for this cut are: table width ratio of 53%, crown height ratio of 16.2%, crown angle of 34°30’, pavilion depth ratio of 43.1%, pavilion angle of 40°45’[Figure 1–18(a)].
(2) Practical Fine Cut. Designed and invented by the German W. F. Eppler in 1949. The standard proportions proposed for this cut are: table width ratio of 56%, crown height ratio of 14.4%, crown angle of 33°10’, pavilion depth ratio of 43.2%, pavilion angle of 40°50’ [Figure 1–18(b)]. Today in Europe, higher-quality diamonds are often cut into this style. Therefore, this cut is also called the European Fine Cut.
(3) IDC Cut. Designed and promoted by the International Diamond Council. The standard proportions proposed for this cut are: table width ratio of 56%~66%, crown height ratio of 11.0%~15.0%, crown angle of 31°0’~37°0’, pavilion depth ratio of 41.0%~45.0%, pavilion angle of 39°40’~ 42°10’ [Figure 1–18(c)].
(4) Scan. D. N. Cut. Introduced in 1970 by the Scandinavian Diamond Committee. The standard proportions released for this cut are: table width ratio of 57.5%, crown height ratio of 14.6%, crown angle of 34°30’, pavilion depth ratio of 43.1%, pavilion angle of 40°45’ [Fig. 1–18(d)].
.jpg)
.jpg)
2. Fancy Cuts

3. Mixed Cut

4. New Cuts
(1) The “Eight Hearts and Eight Arrows” (also called Cupid) cut. The “Eight Hearts and Eight Arrows” cut belongs to the round brilliant family; this cut requires very high cutting proportions and symmetry, and produces a visual phenomenon observable through a special viewer (Firescope). The standard “Eight Hearts and Eight Arrows” pattern consists of two parts: hearts and arrows. From the pavilion (bottom view), eight symmetrical hearts are visible, called the “Eternal Hearts”; from the crown (top view), eight symmetrical arrows are visible, called the “Arrows of Cupid.” Both radiate outward in eight directions; the overall image is complete and clear, proportions are appropriate, and strict symmetry is maintained (Fig.1-21).

(2) The “Nine Hearts One Flower” cut. The “Nine Hearts One Flower” cut (also called Estrella), from Spanish meaning “a shining star in the sky.” This cut consists of 100 facets, including 37 crown facets and 63 pavilion facets; the pattern effect must also be seen with a special magnifier. Looking straight at the table, a floral pattern composed of nine facets appears at the table centre, while from the crown, nine main facets can be seen, evenly arranged around the table perimeter. After refraction, light can produce the “Nine Hearts One Flower” phenomenon (Fig.1-22).