Was ist Rutheniumbeschichtung und wie kann sie Ihre Produkte aufwerten?
Ruthenium Plating for Jewelry: Hard, Black, White Finishes Guide
Einleitung:
Ruthenium plating is an electroplating process that deposits a layer of ruthenium, a hard, silver-white platinum group metal. But what are its key benefits? This plating offers exceptional hardness (Hv 640), superior wear and corrosion resistance, and excellent heat resistance. It can produce both decorative white finishes and stable black finishes (using additives like thiourea). While the plating layer can have high internal stress, methods exist to reduce it. This article details the plating solutions, process conditions, and properties, explaining why ruthenium is used for jewelry, watches, and electronic components like relay contacts.
Inhaltsübersicht
Abschnitt I Überblick
Ruthenium has an atomic number of 44 in the periodic table, with the element symbol Ru. It is a silvery-white metal, hard and brittle, discovered by J.J. Berzelius and G.W. Osann in 1828. It dissolves in strong acids and reacts slowly with aqua regia.
Common parameters of ruthenium are shown in Table 7-1.
Table 7-1 Common Parameters of Ruthenium
| Charakteristische Parameter | Charakteristischer Wert |
|---|---|
|
Element Name, Element Symbol, Atomic Number Klassifizierung Gruppe, Zeitraum Dichte, Härte Farbe Relative Atommasse Atomradius Compound value Oxidation value Kristalline Struktur Schmelzpunkt Siedepunkt Wärme der Verdampfung Lösungswärme Spezifische Wärmekapazität Leitfähigkeit Wärmeleitfähigkeit |
Ruthenium、Ru、44 Transition metal 8(Ⅷ)、5 12370kg/m3、6. 5 Silber-weiß 101.07 130pm 126pm 2、3、4、6、8 Precision hexagonal structure 2607K (2334℃) 4423K (4150℃) 595kJ/mol 24kJ/mol 234J/(kg • K) 13. 7X106m •Ω 117W/(m • K) |
Ruthenium is widely used in industrial chemical catalysts, electronic components, dental alloys, decorative items, stationery, pharmaceuticals, etc. After adding alkali metals, ruthenium alloys are highly active catalysts for ammonia synthesis. To prevent global warming and control automobile exhaust emissions, various countries around the world are continuously developing different fuel cells. For example, 30%~50% ruthenium alloys added to platinum group metals can be used as catalysts for methanol fuel cells. In non-silicon series dye-sensitized solar cells, using ruthenium complexes as the dye layer has improved conversion efficiency, thus attracting much attention. Currently, the applications of ruthenium are developing in multiple fields and aspects, although its annual usage is still less than that of platinum and gold.
The characteristics of metallic ruthenium and comparisons with rhodium and palladium of the same platinum group are shown in Table 7-2.
Table 7-2 Characteristics of Metal Ruthenium, Rhodium, and Palladium
| Eigenschaften | Metal type | ||
|---|---|---|---|
| Ru | Rh | Pd | |
|
Atomic Number Relative Atommasse Kristalline Struktur Dichte (20℃)/(g/cm3) Melting point (20℃)/°C Boiling point/°C Hardness(after annealing)/Hv Resistivity (20℃)/μΩ-cm Redox potential/mV |
44 101.07 Densest pore square crystal 12. 45 2310 4052 240 6. 71 0. 68 |
45 102. 91 Face-centered cubic crystal 12.41 1960 3687 100 4. 33 0. 78 |
46 106. 4 Face-centered cubic crystal 12. 02 1552 2870 40 9. 93 0. 92 |
From the table, it can be seen that the melting point and boiling point of the pin are higher than those of rhodium and palladium, with greater heat resistance and arc resistance; the electrical resistivity is lower than that of palladium; the hardness after annealing is much higher than that of rhodium and palladium. In terms of electrochemical properties, the standard oxidation potential of the pin (0.68) is lower than that of rhodium. (0.78) and palladium (0.92); the electrode potential of the pin in an acidic medium is about (standard hydrogen electrode), and in an alkaline medium, is 0.4V (pH 12, standard hydrogen electrode).
Among the platinum group metals, the chlorinated overvoltage of the oxides of rhodium and ruthenium (RuO2) is very low, exhibiting excellent catalytic performance and good corrosion resistance.
Section II Electroplating Rhodium
Although Ru has superior physical and chemical properties, ruthenium plating has not been widely applied. This is mainly because Ru compounds are not stable enough, the current efficiency is low, and the internal stress of the ruthenium plating layer is also relatively high.
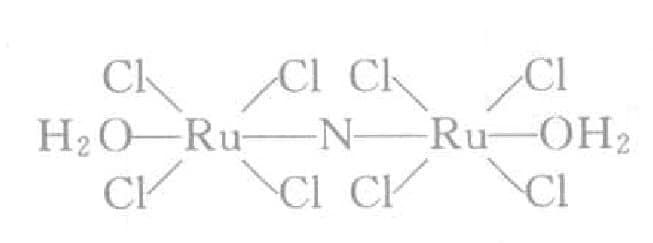
Later, after much research, many patents also emerged. Reid and others conducted a detailed comparison of various plating solutions. Ru belongs to the light platinum group and has special properties. Table 7-2 compares three light platinum metals (Note: heavy platinum metals are Os, Ir, Pt). In ruthenium plating, ruthenium salt nitro-chloride salts are mainstream, and the types of free acids vary. Conn considers the sulfonic acid plating solution in Table 7-3 best.
Table 7-3 Composition and Conditions of Ruthenium Plating Solution
| Zusammensetzung und Prozessbedingungen | Formulation and concentration of components | |||
|---|---|---|---|---|
| Nitrosyl chloride ruthenium plating solution | Potassium tetrachlorohydrate ruthenate plating solution | Ruthenium sulfate: white ruthenium plating | Ruthenium sulfate solution: black ruthenium plating | |
|
Nitrosoruthenium chloride/(g/L) Ruthenium concentration/(g/L) Potassium ruthenate tetrachlorohydrate/(g/L) Ruthenium concentration/(g/L) Potassium dihydrogen phosphate/(g/L) Ruthenium sulfate/(g/L) Ruthenium concentration/(g/L) Sulfamic acid/(g/L) Phosphoric acid/(mL/L) Sulfide/(g/L) pH-Wert Temperatur/°C Stromdichte/(A/dm2) |
10 3. 5〜4. 5 - - - - - 10〜20 - - - 50〜65 0. 5〜1. 5
|
- - 32. 5 10〜20 110 - - - 50 - 1. 7 70 1 |
- - - - - 4. 8〜12 3. 2〜5 100 - - 1. 2 65〜75 4〜6 |
- - - - - 4. 8〜12 2〜5 100 - 1 - 60〜75 3〜7 |
1. Metal Ruthenium Plating Process Specifications
Adding aminomethanesulfonic acid and others to inorganic salts such as ruthenium sulfate and ruthenium chloride forms very stable complex salts with these salts, suitable for plating solutions, industrially used for plating and decorative plating of functional electronic products such as magnetic wire switches, relays, and connectors.
The main reason for the instability of the usual tin plating solution is that the ruthenium ions are unstable and easily decompose in the plating solution to produce fine, powdery suspensions or precipitates. These co-deposits cause the surface of the plating layer to be rough, failing to meet the smoothness requirements of electronic product contacts; at the same time, ruthenium ions in the plating solution can also form oxide or hydroxide precipitates on the anode; during continuous operation, this leads to a low deposition rate. Some patents provide plating technology that achieves rapid deposition, color development by color developers, anode oxidation protection by anode sacrificial agents, and plating solution stability by adding reagents that increase the deposition rate in a stable plating solution of aminomethanesulfonic acid complex salts.
Tin plating can be divided into white tin plating and black ruthenium plating according to the color tone of the plating layer.
(1) Electroplating White Ruthenium
The parameters of the white plating solution are shown in Tables 7-4 and 7-5.
Table 7-4 General Parameters for Plating White Ruthenium with Sulfate Ruthenium Plating Solution
| Zusammensetzung und Prozessbedingungen | Control value | Beschreibung |
|---|---|---|
| Ruthenium sulfate/(g/L) | 1〜25 | Optimum value: 2~10g/L |
| Sulfamic acid/(g/L) | 5〜100 |
Stabilizer and concentration: sulfuric acid or sulfate 60~200g/L, sulfamic acid or sulfamate 20~60g/L |
| Halogenated elements or halides of anions | Above 100mL/L |
Deposition speed promoter: Ammonium halide, metal halide, alkali metal halide, etc. 100mL/L~10g/L; or halogen (one of F, Cl, Br, I) that can produce the same effect can be added directly to the plating solution, so that it reacts with the components of the plating solution to form anions. |
| pH-Wert | 0. 5〜2 | |
| Temperatur/°C | <70 | |
| Stromdichte/(A/dm2) | 2〜10 |
Table 7-5 Examples of White Ruthenium Plating Applications
| Composition and Process Conditions | Nr. 1 | Nr. 2 | Nr. 3 | Nr. 4 |
|---|---|---|---|---|
| Plating solution composition | After dissolving ruthenium concentration of 5g/L (as ruthenium sulfate) in pure water, add ammonium sulfamate 100g/L, chloride ion 1.0g/L (as ammonium chloride) and adjust pH=1.3 with sulfuric acid. | Replace ammonium chloride with ammonium bromide, all other conditions are the same as No.1 for plating. | Replace ammonium chloride with ammonium iodide, all other conditions are the same as No.1 for plating. | Plating without ammonium chloride, all other conditions are the same as No.1. |
| Betriebsbedingungen |
Anode: white gold; cathode: gold-plated brass. Temperature:70℃; Current density:5A/dm2; Time:50min |
|||
| Deposition rate/(μm/min) | 0. 0472 | 0. 0440 | 0.0508 | 0. 0315 |
| Thickness of plating layer/μm | 2.36 | 2.20 | 2.54 | 1.58 |
(2) Electroplate Black Ruthenium
The black plating solution is easy to manage, with good adhesion between the plating layer and the bright substrate and good corrosion and wear resistance. During the plating process, the sacrificial oxidant ammonium sulfate (hydroxy) suppresses the anodic oxidation and decomposition reactions of the color developer thiol compounds, ensuring the strong blackness and stability of the plating layer. Tables 7-6 and 7-7 show some parameters of the black plating solution.
Table 7-6 Some Parameters of Black Ruthenium Plating in Sulfuric Acid Ruthenium Plating Solution
| Zusammensetzung und Prozessbedingungen | Control value | Explanation |
|---|---|---|
| Ruthenium concentration/(g/L) | 1〜10 | Used in the form of ruthenium sulfate |
| Ammonium sulfamate/(g/L) | 5〜150 | Stabilizer, inhibits anodic oxidation and enhances cathodic reduction of ruthenium ions. |
| Thiourea/(g/L) | 1.0〜5.0 |
Colorant: Thiourea, thiourea derivatives, thiourea compounds, mercaptans, dibutyric acid, ammonium thiocyanate, etc. are used. Thiourea is the most suitable coloring agent considering the stability and price of the drug. |
| Ammonium (combined) hydroxyl(base) sulfate/(g/L) | 1〜100 |
Sacrificial oxidizing agent: one of the following: ammonium sulfate, formaldehyde, vitamin C, etc. Among them, ammonium sulfate (combined) hydroxyl (base) is the most effective, and oxides do not affect the plating layer |
| pH-Wert | <2 | |
| Temperatur/°C | Above 40 | |
| Stromdichte/(A/dm2) | 5〜15 |
Table 7-7 Application Examples of Black Ruthenium Plating
| Composition and Process Conditions | Nr. 1 | Nr. 2 | Nr. 3 | ||
|---|---|---|---|---|---|
| Plating solution composition |
Ruthenium concentration 3g/L (as ruthenium sulfate) Sulfamic acid 100g/L Thiourea 1.5g/L Hydroxylammonium sulfate 10g/L |
Ruthenium concentration 3g/L (as ruthenium sulphate), Sulfamic acid 100g/L Thiourea 1.5g/L Hydroxyl ammonium sulfate 50g/L |
Ruthenium concentration 3g/L (as ruthenium sulfate). Sulfamic acid 100g/L Thiourea 1.5g/L |
||
| Betriebsbedingungen |
Anode: Platinum Cathode: Brush gold plated nickel plated plate Temperature:50℃ Cathode current density:5A/dm2 pH=0.1 Time:30min |
Anode: Platinum Cathode: Brush gold-plated nickel-plated plate Temperature:50℃ Cathode current density:5A/dm2 pH=0.1 Time:30min |
Anode:Platinum Cathode: Brush gold-plated nickel-plated plate Temperature:50℃ Cathode current density:5A/dm2 pH=0.1 Time:30min
|
||
| Plating material | Brush gold plating/watt-hour liquid nickel plating/brass plate | ||||
| Close | Energization | 5A ・ h/L | Schwarz | Schwarz | Light black gray |
| 10A • h/L | Schwarz | Black, Supplemental Ammonium Hydroxyl Sulfate 5g/L | Light black gray, supplement thiourea 1.5g/L | ||
| 15A ・ h/L | Schwarz | Black, Supplemental Ammonium Hydroxyl Sulfate 5g/L | Light black gray, supplement thiourea 1.5g/L | ||
| 20A ・ h/L | Schwarz | Black, Supplemental Ammonium Hydroxyl Sulfate 5g/L | Light black gray, supplement thiourea 1.5g/L | ||
| 25A ・ h/L | Schwarz | Black, Supplemental Ammonium Hydroxyl Sulfate 5g/L | No bright gray-brown color, turbid plating solution, supplement thiourea 3g/L. | ||
| 30A ・ h/L | Schwarz | Black, Supplemental Ammonium Hydroxyl Sulfate 5g/L | No bright gray-brown color, turbid plating solution, supplement thiourea 3g/L. | ||
2. Characteristics of the Plating Solution
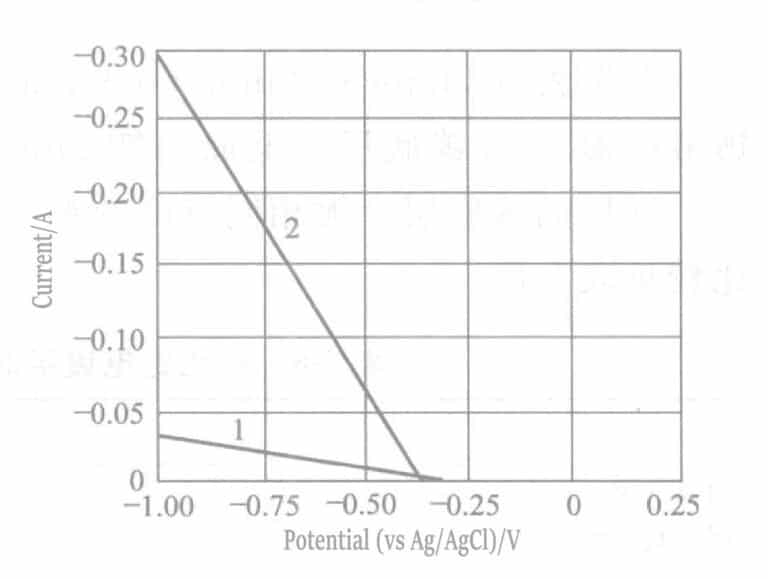
Figure 7-2 Current-potentials of aqueous solutions of ruthenium sulfate and aqueous solutions of sulfamic acid added to ruthenium sulfate.
Curve 1 - aqueous solution of ruthenium sulfate; 2 - aqueous solution of ruthenium sulfate with added sulfamic acid
3. Applications of Ruthenium Plating Layers
Table 7-7 Adding aminomethanesulfonic acid to sulfuric acid ruthenium plating solution can produce a white ruthenium plating layer with a metallic tone, suitable for magnetic wire switches, relay contacts, and anodes for electrolytic salt production. After adding additives, a stable black ruthenium plating layer can be obtained, which can be used for decorative plating on glasses, watches, pins, necklaces, earrings, collar pins, etc., and can replace black chromium to improve the added value of decorative items. Due to environmental protection, the use of chromium has gradually decreased in recent years, and the use of black ruthenium plating layers has been continuously increasing.
Rhodium and other platinum group metal coatings are extremely difficult to peel off from the substrate coating. However, ruthenium plating layers are easily peeled off during reverse electrolysis in alkaline stripping solutions.
4. Methods for Evaluating the Properties of Ruthenium Plating Layers
Table 7-8 Characteristics Evaluation of White Ruthenium Plating Layer
| Eigenschaften | Ru | Rh | Pd |
|---|---|---|---|
|
Hardness Hv Abrasion resistance(wear amount)/mg Contact resistance(after plating)/mΩ Contact resistance(after SO2 gas corrosion)/ma Heat resistance(300℃,30min) Solderability(Zero-crossing time)/s Plating internal stress/(kgf/mm2) H2 content/x10-6 |
640 0. 2 7. 4 8.8 O 6. 2 85,Tension 1590 |
830 0. 1 7. 4 8.8 O 5. 6 31,Tension 130
|
280 3. 6 4. 1 4. 4 △ 4 81,Tension 220
|
The evaluation items are as follows.
Microhardness: measured using a microhardness tester (Teraoka type M-2), with a force of 5gf, duration 30 s, using Vickers hardness.
Abrasion resistance: Measured and evaluated concerning JIS H 8503 “Methods for measuring precious metal plating layers.” The abrasion resistance test uses an abrasion tester with a force of 500 gf, contact area of 3.75cm2, and 1500# water sandpaper rubbed back and forth 200 times. Evaluation is based on the reduction in friction mass.
Contact resistance: The contact resistance was measured using a contact resistance meter (MS880 made by KS Parts Research Institute) with a force of 0.1 to 100gf, a measurement current of 1mA, a sliding distance of 0 mm, and a K625R probe. The exposure test was conducted in the same way after exposure to SO2 gas at 40°C, 80% humidity, and a volume fraction of 10-5 for 24h, after which the contact resistance was measured as described above.
Heat resistance: Using a uniform electric furnace to heat the atmosphere at 300℃ for 30 minutes. After heating, the plated layer is visually inspected.
Solderability: Evaluated using the Solder-Checker tester. The solder is a lead-tin alloy, and the flux is rosin methanol. The test conditions are 230℃, immersion depth of 6 mm, force of 5gf, time 5 s, speed of 16mm/s. Zero cross-time evaluation is conducted according to the meniscus test method.
Internal Stress:The internal stress of the electrodeposits was measured by using a spiral wire slip method stress gauge and plating a 2μm layer under each plating standard condition.
Copywrite @ Sobling.Jewelry - Hersteller von kundenspezifischem Schmuck, OEM- und ODM-Schmuckfabrik
5. Mechanical and Electrical Properties of the Ruthenium Plating Layer
From Table 7-3, it can be seen that the Vickers hardness of the plating layer is 640, which is much higher than that of the palladium plating layer, slightly lower than that of rhodium (830 Hv), and three times that of the annealed metal plating in Table 7-2.
The wear resistance of ruthenium plating is comparable to that of rhodium plating used in magnetic wire switches, relays, sliding switches, and other wear-resistant applications. It is even superior to general rhodium plating, making it more suitable for electronic components with high wear resistance requirements and decorative parts.
The contact resistance on the surface of the ruthenium plating was 7.4mΩ, which was slightly higher than that of the palladium 4.1mΩ. The probe tip plating was similar to the rhodium plating. The contact resistance changed slightly after exposure to SO2 gas with a volume fraction of 10-5 for 24 h. However, similar to the rhodium plating of the contact probes, the contact resistance was very stable.
In terms of heat resistance, ruthenium has excellent heat resistance. After heating in the atmosphere at 300℃, the surface oxidation discoloration of the plating layer shows that palladium and rhodium exhibit slight discoloration, while ruthenium shows no discoloration. Palladium forms oxides at 300~350℃, and literature records indicate that rhodium and ruthenium remain stable under 700℃.
Using a tin-lead alloy plating solution, the solderability is evaluated using the meniscus test’s zero-crossing time method. Compared to the wetting speed of gold plating with a zero-crossing time of 1 second, platinum group metals are about 4~6s slower. Compared to palladium, which is the fastest at 4 seconds, ruthenium is 6 seconds. Therefore, appropriate flux and soldering conditions must be selected when soldering on ruthenium plating.
The deposition stress of the plating layers of pins, rhodium, and palladium are all tensile stresses. Among them, the plating layer of pins and palladium is similar, with high tensile stress, which is one of the causes of crack formation.
The hydrogen absorption amount in the plating layer after plating pins, compared with palladium’s 220X10-6, rhodium’s 130X10-6, and ruthenium’s 1590X10-6, is quite large. Many literatures record that palladium is widely used as a hydrogen absorption material, so pins are expected to become a new material to replace palladium.
6. Composition, Structure, and Configuration of the Ruthenium Plating Layer
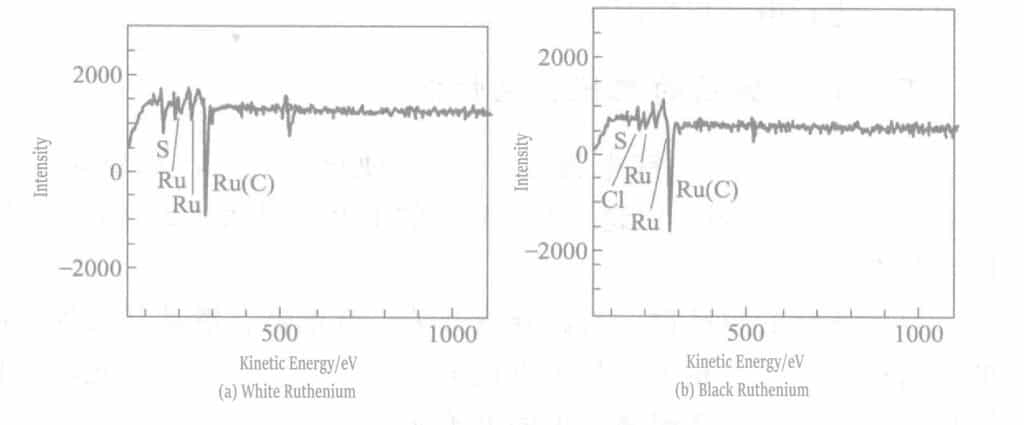
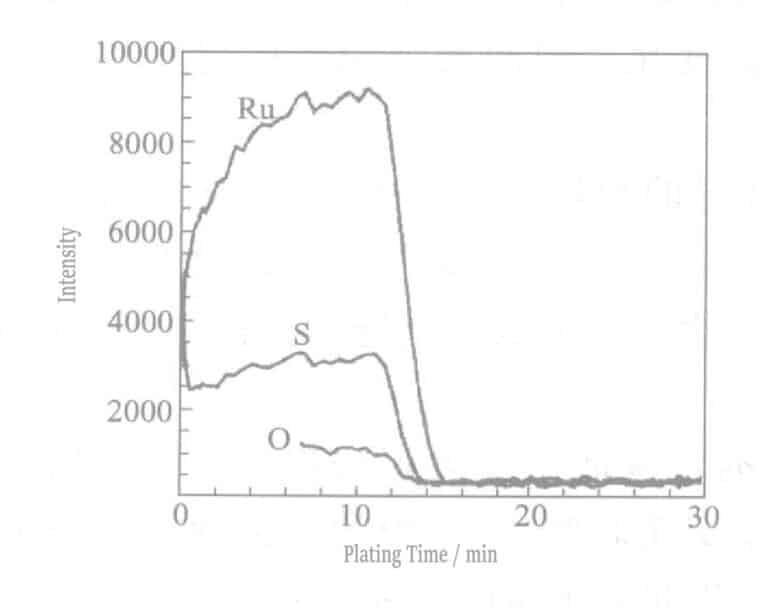
O and S are not only present on the very surface of the plating layer but also distributed inside the plating layer. Sulfides were added to the black ruthenium plating solution, and sulfur co-deposition occurred due to the decomposition of this compound. As shown in Figure 7-3(a), traces of sulfur were also detected in the white ruthenium, which can be assumed to be co-deposited with the ruthenium after decomposition of the complex sulfamic acid. From this, it can be inferred that the source of S in the black ruthenium plating layer is the sulfide and aminomethanesulfonic acid during blackening.
To better understand the ionic states in the black ruthenium plating layer, XPS (X-ray photoelectron spectroscopy) was used to detect the S 1s peaks in the white ruthenium and black ruthenium plating layers. S 1s was not detected in the white ruthenium but in the black ruthenium, mainly because sulfides were added to the black ruthenium plating solution. Based on the relative sensitivity factor and peak area, the sulfur content was calculated to be approximately 10% (atomic ratio). As shown in Figure 7-5, the S 1s peak of the black ruthenium plating layer has two peaks at about 162 eV and about 164 eV, corresponding to two different sulfur bonding states.

Section III Attempts to Improve the Ruthenium Plating Layer

In der Formel,
σ— internal stress of the plating layer, N/mm2;
t— Coating thickness, mm;
E— Elastic modulus of the test strip, N/mm2;
d— Length of the test strip, mm;
x— Length change, mm;
L— Sample peel length, mm.
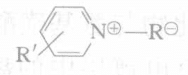

Preparation of the main components of the plating solution (preparing a 50g/L Ru solution): Take 200 g of amino sulfonic acid, add it to a reflux apparatus containing 400 mL of deionized water (the reflux apparatus is placed in a constant temperature bath), then add 120 mL of ammonia water, and heat to 50℃. Add 50 g of Ru (added as ruthenium chloride hydrate), boil the solution for 4 hours, then filter with a 1μm filter. Finally, the obtained solution is diluted to make 1 L, resulting in a solution containing Ru. When in use, dilute it 10 times (i.e. Ru 5g/L, ) for use in experiments.
No.1: Add 2g/L of pyridinium propane sulfonate to the solution prepared above.
No.2: Add 2g/L of pyridinium hydroxypropanesulfonate to the solution prepared above.
The preparation of plating solution No. 3 is as follows: take 150g of amino sulfonic acid, add 400mL of deionized water and 25g of Ru (added in the form of ruthenium chloride hydrate) into a reflux apparatus (the reflux apparatus is placed in a constant temperature liquid bath) and heat to boil for 4 hours, then filter with a 1μm filter. Finally, the obtained solution is diluted to make 500mL of solution, resulting in a solution containing 50g/L Ru. When in use, dilute it 10 times (i.e., Ru 5g/L) for use.
The test results of No. 1 to No. 3 are shown in Table 7-9.
Table 7-9 Comparison Results of Stress in Electroplated Ruthenium
| Composition and its process conditions | Nr. 1 | Nr. 2 | Nr. 3 |
|---|---|---|---|
|
Ruthenium concentration/(g/L) pH-Wert Temperatur/°C Stromdichte/(A/dm2) Stromausbeute/% Beschichtungsdicke/μm Internal stress/(N/mm2) |
5 1. 5 ~ 1. 7 70 1. 68 1. 0 250 |
5 1.5 ~ 1.7 70 1 69 1. 0 252 |
5 1. 5 ~ 1. 7 70 1 70 1. 0 489 |
From the table, it can be seen that the internal stress of No. 1 and No. 2, which contain stress relievers, is significantly reduced, indicating that pyridine and N-alkyl pyridinium salts indeed have the effect of reducing the internal stress of the rhodium plating layer.
Additionally, there are reports of blowing air into the plating solution to increase crack-free plating layers. The preparation process of this plating solution is to add 160 g of amidosulfonic acid to 83 g of ruthenium chloride solution. Boil for 3 hours, cool to room temperature, and cool to 5℃ to obtain 56 g of ruthenium-containing compound. Dissolve 36.5 g of the complex in 1 L of water and adjust the pH to 9.0 with ammonia water, obtaining 24 g of the nitrogen-hydroxide compound of the ruthenium. Dissolve this 24 g compound in 300 mL of pure water, add 15 mL of 98% sulfuric acid, boil for 1 hour, and cool to room temperature.
Table 7-10 Effect of Air Blowing on Crack-Free Plating Thickness of Plating Solution
| Seriennummer | Blowing air volume/(mL/L) | Plating speed/(μm/min) | Crack-free plating thickness/μm |
|---|---|---|---|
|
1 2 3 4 5 |
0 100 200 500 1000 |
0. 100 0. 100 0. 098 0. 097 0. 095 |
5.2 7. 6 7.5 7. 3 7.2 |
Section IV Equipment for Plating Ruthenium
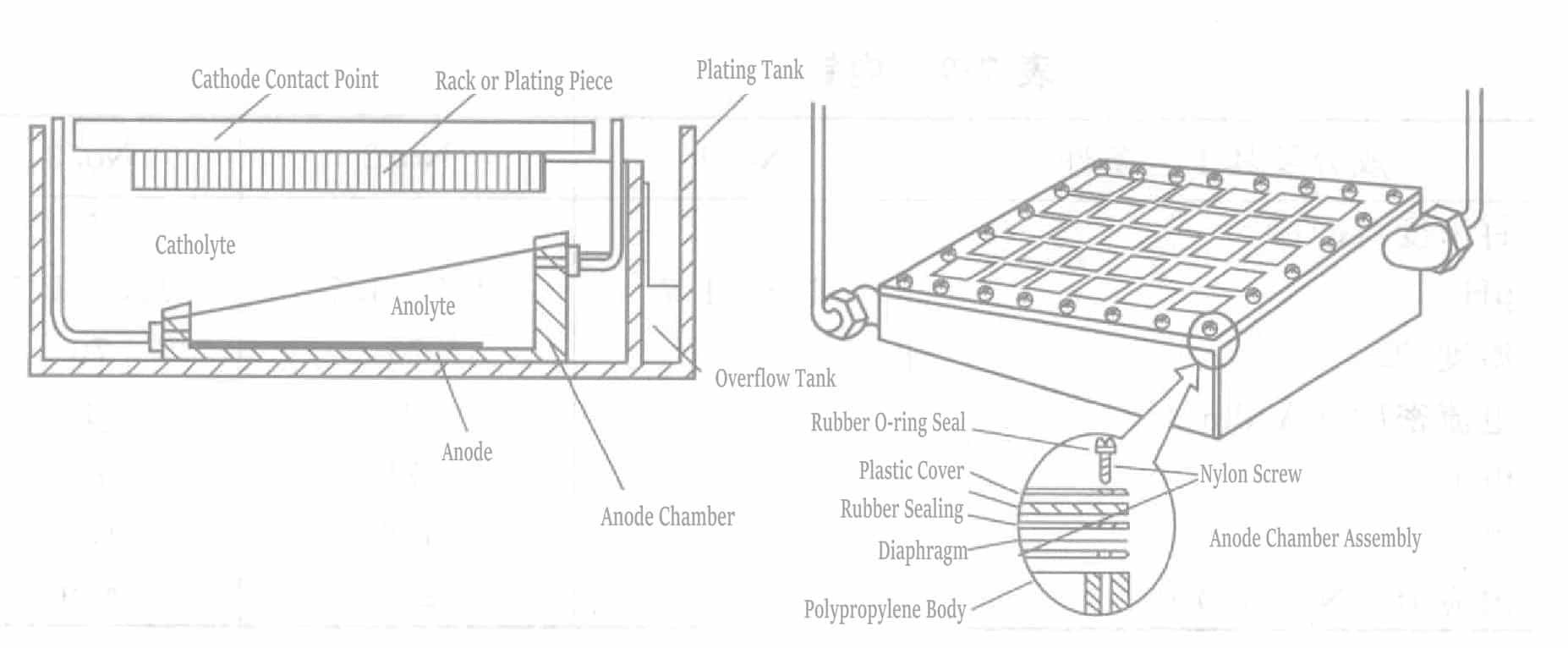
However, Ru transforms into RuO4-type compounds in actual plating solutions, which adversely affect plating. Therefore, T. A. Palumbo pointed out that placing a diaphragm between the anode and cathode and using the plating solution composition and operating conditions shown in Table 7-3 can achieve high current efficiency ruthenium plating layers.
Figure 7-6 shows the plating tank structure for plating ruthenium. This tank uses a shielded wiring design (material is Co: 49%, Fe: 49%, Ni: 2%), which has proven practical.
Section V Outlook on Ruthenium Plating
The plating layer of ruthenium and hooks is significant to the platinum group metal industry and energy-related fields such as fuel and solar cells. The ruthenium plating solution introduced in this chapter has excellent stability and is suitable for industrial production. The plating layer has excellent hardness, wear, and contact resistance, making it widely applicable in electronic and decorative plating. In particular, its excellent heat resistance and arc resistance can be used in products such as magnetic wire switches and relays. Ruthenium has a greater hydrogen absorption capacity than rhodium, palladium, and other platinum group metals. After blackening treatment using sulfides, its excellent properties can be applied to selective solar heat-absorbing black electroplating layers, chromium removal, nickel phase transformation reactions, and other new fields.
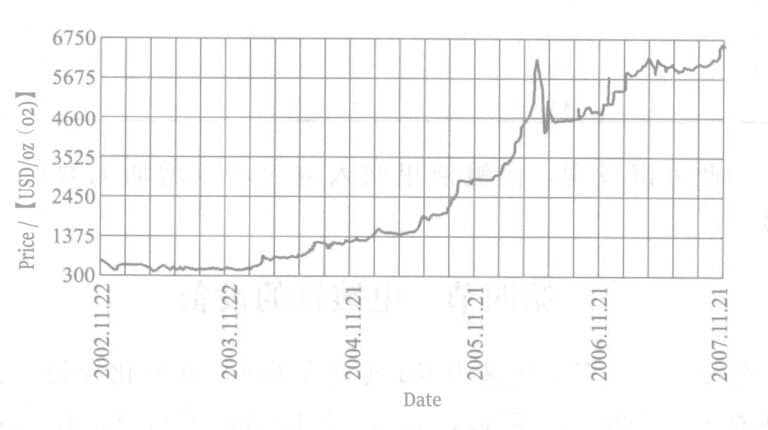
However, the market price of ruthenium has surged dramatically (see Figure 7-7), which is expected to affect its applications.