Miten tehdä jalokivioptimointi? Avaa 5 menetelmää ja laitteiden opas
Gemstone Optimizatized Methods and main equipment Used
There are many optimization treatment methods for gemstones, and with the advancement of science and technology, these methods are continuously improved and updated. The most traditional optimization methods include heat treatment, dyeing and coloring, immersion in colorless oil, and surface coating. For example, in ancient times, people recognized early on that heating could enhance the color of agate, and by placing agate in different dyes, it could be dyed into various colors. Although these methods were known, they were often discovered by chance at the time. Only when people gradually mastered the physical properties and color-causing mechanisms of gemstone crystals (such as diamonds, rubies, sapphires, topaz, beryl, quartz, etc.) and organic gemstones (such as pearls, amber, etc.) could they break through traditional fields and develop new optimization treatment methods.
The main optimization treatment methods for gemstones currently include the following: physicochemical treatment, heat treatment, irradiation treatment, high-temperature and high-pressure treatment, and laser treatment. The most widely used method in gemstone optimization treatment is heat treatment, which improves the color of gemstones like rubies, sapphires, jadeite, and chalcedony, which are colored by trace impurity elements. The irradiation method mainly improves the color of gemstones with color centers, causing defects in the gemstone’s structural composition through irradiation, thus forming color centers and changing the gemstone’s color. Physicochemical treatment is a more traditional optimization method, such as dyeing, which commonly uses different dyes to color gemstones. The required equipment is simple, and the operation is convenient, but the improved gemstones are unstable and prone to fading. High-temperature and high-pressure treatment is currently a method for treating diamonds, changing their color through high temperature and pressure. Laser treatment is primarily used for localized treatment of diamonds to improve their color and clarity.

Stained quartzite
Sisällysluettelo
Section I Gemstone Chemical Treatment Method
The physical and chemical treatment methods for gemstones include common practices such as dyeing and coloring, bleaching, oil immersion, injection filling, bonding, coating, backing, layering, and encrusting, which have a long history. Among them, dyeing is a traditional method for improving gemstone color that dates back to ancient times. Historical records indicate that dyed red agate was found in Egyptian tombs around 1300 BC. Due to the simplicity of traditional improvement methods, they can be applied to most structurally loose cryptocrystalline or single-crystal gemstones with many cracks. Many dyed gemstones on the market impersonate natural gemstones, so we need to identify gemstones treated by dyeing and other coloring methods. They are classified into chemical and physical treatment methods based on the nature of the treatment methods.
Chemical treatment methods refer to the addition of a certain amount of chemical reagents, which react chemically with the components of the gemstone, allowing the coloring elements in the chemical reagents to enter the interior of the gemstone or seep into the cracks of the gemstone to change the appearance of the gemstone’s color. During the chemical treatment process, substances other than the gemstone’s components must be added. This optimization treatment method is a form of processing, and it must be labeled when the gemstone is sold. Common chemical treatment methods include dyeing, coloring, bleaching, and injection filling.
1. Dyeing and coloring
The processes and principles of dyeing and coloring differ only in the dyes used: dyeing uses organic dyes, while coloring uses inorganic pigments. The principles of dyeing and coloring are the same, involving the infiltration of coloring materials into the gemstone to enhance or change the gemstone’s color. Organic dyes are more vibrant but have poorer stability and will fade over time; the chemical reagents used in coloring are similar in color to natural gemstones and have good stability, making them less prone to fading. Currently, most gemstones are colored using inorganic pigments.
(1) Requirements for Materials, Dyes, and Solvents
The methods for dyeing and coloring are similar in processing, requiring minimal equipment; it is sufficient to soak in a container for some time. If you want the color to penetrate the gem, heating is necessary during the process, and the heating temperature is generally low. Dyeing and coloring are mainly used for gem materials that are light in color and have a loose structure. The effects of dyeing and coloring depend on the gem material, the selected dyes and pigments, and the colorant solvents, among other conditions, with specific requirements as follows.
① Requirements for gem materials
First, they must be resistant to acids, bases, and heat. The gem materials to be treated must be cleaned with acid or alkali before dyeing, and heating is required during the process, sometimes needing to be boiled for some time.
Secondly, the materials to be treated must also have a certain porosity to allow the colorant to penetrate the gem material. Materials such as jadeite, nephrite, chalcedony, agate, and marble are relatively easy to dye.
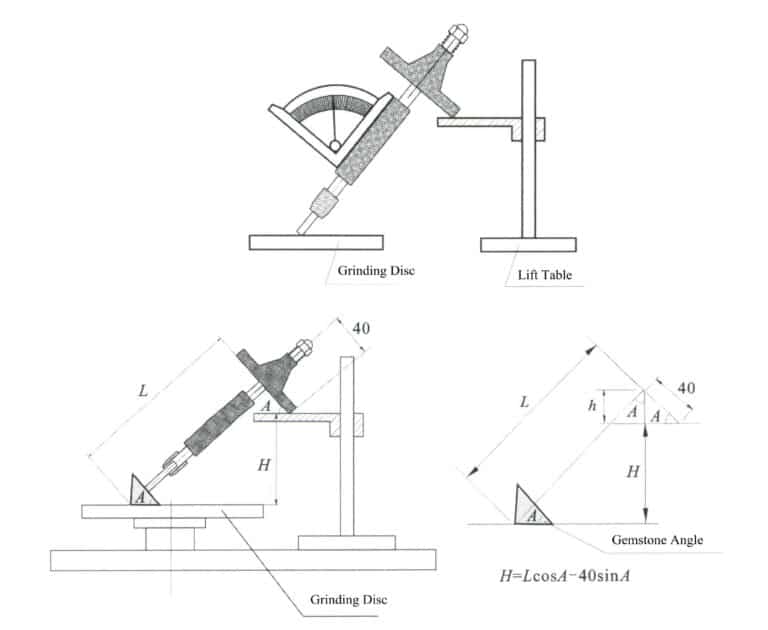
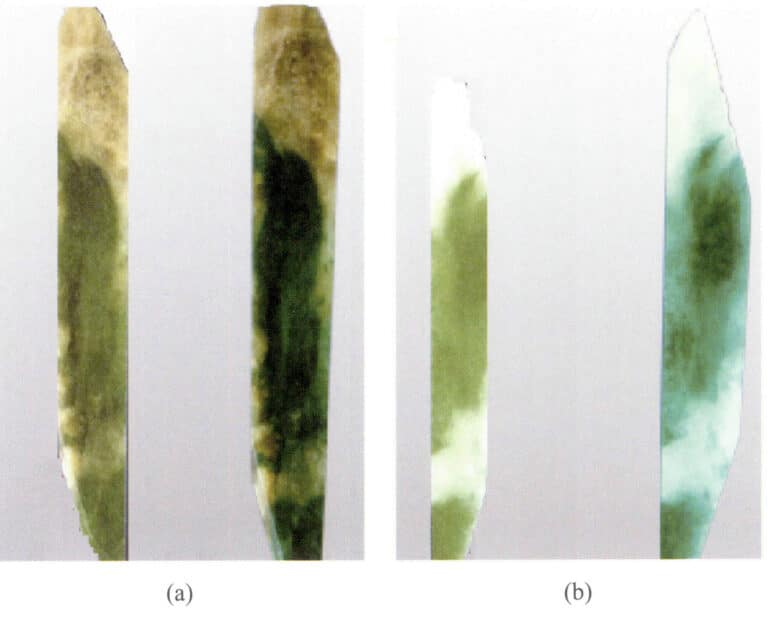
For those non-porous gems, artificial pores or cracks must be created to allow the colorant to enter the crystal. For example, the quartz explosion method requires first heating and quenching the quartz to create extremely small cracks, followed by dyeing or coloring, which can yield red or green quartz (Figure 4-1).

② Requirements for colorants (including dyes and pigments)
First, choose the appropriate dye or pigment based on the properties of the gemstone. When dyeing gemstones, the color of the colorant should be close to the natural color of the gemstone. Gemstones dyed with organic dyes have many colors and are very bright, but they give a “fake” feeling and have poor stability, easily fading; the color of inorganic pigments is often closer to natural gems, has better stability, and is not easy to fade, so people generally choose inorganic pigments. When selecting colorants, try to choose those that do not fade. Organic dyes, especially amine dyes, are prone to fading and should be cautiously used.
Secondly, select colorants that can chemically react with certain elements inside the gemstone or can be adsorbed by the pores of the gemstone material. Common dyes include chromium salts, iron salts, manganese salts, cobalt salts, copper salts, etc.
③ Requirements for colorant solvents
There are two types of dyeing with dyes (colorants): oil dyeing and water dyeing. Oil dyeing uses various oils to dissolve the dye, while water dyeing uses water or polar molecules like ethanol as solvents to dissolve the pigment. When dyeing, it is important to choose the appropriate solvent based on the type of dye (pigment) and the adsorption capacity of the gemstone material.
- Using non-polar molecular oil as a solvent is called oil dyeing. Colored oils (i.e., oils that dissolve organic dyes) are commonly used to soak rubies and emeralds, allowing the colored oil to penetrate the cracks in the gemstones.
- Water dyeing is mostly used for inorganic pigments, dissolving the pigments in water or alcohol, creating a saturated solution, and thensoaking the pre-treated gemstones. The soaking time is usually longer than that of oil dyeing, and sometimes chemical agents that react with the dye are used for reprocessing to achieve the desired color. For example, when dyeing agate, different chemical reagents are selected to induce a chemical reaction, and the resulting precipitate penetrates the cracks in the gemstone, stabilizing the color after dyeing.
(2) Factors Affecting the Dyeing Effect of Gemstones
In addition to considering the gemstone material and dye, other factors must also be considered, such as the acid-washing treatment of the gemstone before dyeing, the heating temperature during dyeing, and the duration of the dyeing process.
① Acid washing treatment
Before dyeing gemstone materials, acid washing is required to remove the yellow, brown, and other mixed colors from the gemstone’s surface, keeping the surface clean. After acid washing, a certain alkaline solution should be selected to neutralize the gemstone. If a chemical reaction method is chosen for dyeing, the conditions required for precipitation generation must be considered; otherwise, the reaction cannot proceed. After acid washing, it should be dried in an oven or air-dried before further treatment.
② Heating temperature and dyeing treatment time
During the dyeing process, heating is generally used to promote the penetration of the dye into the cracks of the gemstone. The heating temperature and dyeing treatment time will also affect the final color of the gemstone. A higher heating temperature results in a faster reaction rate, requiring a shorter dyeing time; conversely, a lower heating temperature requires a longer time to achieve a better dyeing effect.
The dyeing and coloring treatment process is simple, easy to operate, and widely used. It can be applied to single-crystal gemstones with cracks and polycrystalline or cryptocrystalline gemstone materials with loose structures. Commonly dyed and colored gemstones include rubies, emeralds, agates, chalcedony, nephrite, xiuyan jade, jadeite, pearls, ivory, opals, coral, quartzite, turquoise, and others.
(3) Identification Characteristics of Dyed Gemstones
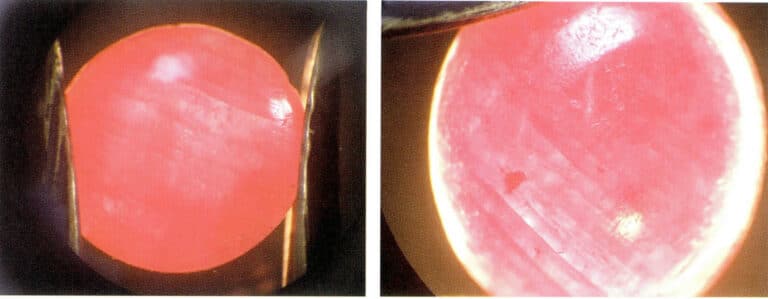
Dyed gemstones have bright colors, and upon magnification, the color can be seen along the cracks or between the particles, with lighter colors in dense structures and darker colors in loose structures. For example, dyed rubies (Figure 4-2) show color concentrated in the cracks of the ruby under a magnifying glass, with a clear color boundary phenomenon.
2. Bleaching
Bleaching is generally used for jade or organic gemstones with many surface color variations, such as jadeite, pearls, and coral. Bleaching agents typically include chlorine gas, hypochlorite salts, hydrogen peroxide, and sulfites. Sun exposure can also cause certain gemstones to fade, which can be a bleaching effect of sunlight. Hydrogen peroxide and hypochlorite salts are commonly used bleaching agents in gemstone optimization processes. Hydrogen peroxide and sunlight are often used to bleach natural or cultured pearls, allowing those particularly dark or greenish pearls to be whitened, making them closer to high-quality natural ones. Hydrogen peroxide and hypochlorite salts are commonly used to bleach jade, such as jadeite (Figure 4-3), which, after bleaching, removes the yellow and brown tones on the surface, allowing the green of the jadeite to be better showcased.
The structure of the jade is damaged after bleaching treatment, and it generally needs to be injected and filled to make its structure dense and stable. Organic gemstones such as pearls and corals can be sold after bleaching without filling treatment, and their colors are also very stable. Bleaching treatment is considered optimization and does not need to be labeled when selling gemstones; they can be named directly using the natural gemstone name. Gemstones used for bleaching are jadeite, nephrite, Xiuyan jade, quartzite, pearl, coral, chalcedony, siliceous wood, and tiger’s eye.
After bleaching treatment, the gemstones show an orange peel or channel-like structure under magnification, with fine micro-cracks visible on the polished surface, a loose internal structure, and a clean, bright color without impurities. Filling treatment is often used after bleaching to stabilize the gemstone structure.
3. Injection filling
Injection filling refers to a treatment method that injects liquid substances into the cracks of gemstones through certain technological means. It is mainly suitable for gem materials that are structurally loose or contain many cracks, filling the cracks and pores of the gemstones with materials such as colorless oil, colored oil, resin, wax, or plastic, making their structure more solid, improving the stability of the gemstones, or changing the color of the gemstones. Injection filling can be divided into colorless and colored, with the following main purposes.
(1) Covering cracks
Natural gemstones often contain many cracks when they are produced. The presence of numerous cracks affects both the appearance and stability of the gemstones. The cracks can be concealed by injecting colorless oil and other materials into the cracks, pores, or intergranular gaps of the gemstone material, making them less noticeable and increasing their usability and economic value. For example, natural emeralds and rubies often contain many cracks, and by injecting colorless or colored oil, their color appearance can be improved.
(2) Enhancing the stability of gemstones
For structurally loose gemstones, injecting and filling the pores to make them more solid, increasing hardness and stability, such as turquoise and emeralds.
(3) Improve the color brightness and economic value of gemstones
For gemstones with lighter colors, injecting colored oil, colored wax, and other materials not only strengthens their structure but also deepens the color of the gemstones.
Suppose a colored material is injected into the pores of turquoise. In that case, it can enhance its hardness and reduce light scattering, deepening its color and significantly improving its hardness.
Gemstones that can be improved using the injection filling method include rubies, sapphires, emeralds, turquoise, lapis lazuli, opal, beryl, quartz, and jade.
After injection filling, the gem shows reduced transparency and luster at the filling position under magnification. For example, a colorless oil-filled emerald (Figure 4-4) shows that the transparency and luster at the filling site are significantly lower than those of natural emeralds. If colored oil is used for filling, the color at the cracks deepens. Bubbles are visible at the filling site, and infrared spectroscopy tests reveal characteristic infrared absorption spectra of the filling material, with refractive index and density lower than those of natural gems.

Section II Physical Treatment Methods for Gems
Physical treatment methods for gems are also widely used, referring to modifying gems with other materials through bonding, splicing, and other techniques to create an overall impression. Common physical treatment methods include surface coatings, plating, encrusting, layering, backing, and splicing.
1. Surface coating
Applying a layer of colored foil (also known as “foil treatment”) on the surface or bottom of the gem or using paint as a coating on all or part of the gem’s facets changes its color and thus alters its appearance. Initially, this was commonly used for diamonds; for example, the simplest coating involves marking the surface of a diamond with blue ink, which can improve the diamond’s appearance due to the ink’s color. Applying a layer of blue film at the bottom of a light yellow diamond can enhance its color grade. This treatment method is commonly used for diamonds, topaz, crystals, coral, and pearls.
The currently common coating method is to apply a layer of colored coating on colorless or light-colored topaz or crystal, which can produce various color appearances. In most cases, the added color only exists on the gemstone’s surface. Gemstones with this coating are easy to identify, as the coated surface often presents a different color from the bottom, and due to the lower hardness of the surface coating, many scratches are often visible.
2. Surface Plating
With the development of science and technology, Pinta Plating has gradually evolved into applying a layer of colored film on the surface of colorless or light-colored gemstones to change the color appearance of the gemstones. This treatment method is commonly used for diamonds, topaz, crystals, etc. The diamond coating is often a diamond film, which is a very thin layer of synthetic diamond on top of the diamond; due to its strong luster and high hardness, it looks very similar to diamond. A layer of metal oxide film is often coated on light-colored topaz or crystals (Figure 4-5), which have a rainbow-like appearance on the surface. Still, scratches can be seen under magnification, and over time, the surface may be partially peeling.

3. Overgrowth
Overgrowth refers to a layer of gemstone grown on the surface of a synthetic or natural gemstone using synthetic methods. This Overgrowth gemstone can vary in thickness. It isn’t easy to strictly distinguish it from gemstones grown in aqueous solutions. For example, a layer of synthetic emerald can grow on a piece of emerald or beryl, exhibiting characteristics of both natural and synthetic emeralds. When identifying the Overgrowth gemstone, one should observe the junction area, color differences, and inclusion characteristics between the upper and lower layers of gemstones.
4. Interlayer and substrate
The Interlayer and substrate are bonded together using various methods to form a whole gemstone, enhancing the appearance, color, and look of natural gemstones. The substrate is mainly used to improve the color of lighter gemstones, such as diamonds with a yellowish tint; adding a layer of blue substrate at the bottom can enhance the color grade of the diamond. The layer is generally used in three-layer composite stones; for example, the upper layer is a natural light green emerald, and the lower layer is colorless or light green beryl, with a green layer in the middle, which enhances the emerald’s color.
5. Composite
Composite is the combination of several gemstones or materials in different ways. Common composite stones include two-layer and three-layer composite stones. Composite is a common physical enhancement method and is widely used. Through composite treatment, the color and appearance of gemstones can be improved. Common composite gemstones include emeralds, rubies, garnets, opals, diamonds, etc. (Figure 4-6). The identification of composite gemstones mainly uses magnified inspection, paying attention to the composite seams in the gemstones, the color and luster differences between different layers, and the bubbles between the composite seams.

Section III Heat Treatment Method
Heat treatment is one of the most widely used methods for gem optimization. Gems are placed in equipment that can control heating, with different heating temperatures and oxidation-reduction atmospheres selected for heat treatment, improving the gems’ color, transparency, and clarity. Heat treatment can enhance the aesthetic and economic value of gems, revealing the potential beauty within, making it an easy-to-operate and widely accepted method for gem optimization, classified as optimization. It can be directly named using the natural gem name in gem nomenclature.
1. Heat Treatment Equipment
To perform heat treatment on gems, certain equipment is first required to heat the gems. Based on its role in heat treatment, heat treatment equipment can be divided into two main parts: primary equipment and auxiliary equipment.
1.1 Primary equipments
The primary equipment for heat treatment is heating equipment, which includes two categories: heat treatment furnaces and heating devices. Commonly used heat treatment furnaces in laboratories include ordinary heat treatment furnaces (resistance furnaces, salt furnaces, fuel furnaces), controlled atmosphere furnaces, and vacuum heat treatment furnaces. Heating devices include laser heating devices and electron beam heating devices.
Auxiliary equipment includes controlled atmosphere devices (gas generators, ammonia decomposition devices, vacuum systems, etc.), power equipment (distribution cabinets, blowers, etc.), measuring instruments (temperature instruments, pressure gauges, flow meters, and automatic control devices, etc.), as well as crucibles and cleaning cooling equipment, etc.
(1) Ordinary heat treatment furnace
Ordinary heat treatment furnaces mainly refer to resistance furnaces, salt melting furnaces, fuel furnaces, etc., commonly used in heat treatment.
① Resistance furnace
A resistance furnace comprises heating elements (wires, silicon carbide, molybdenum silicide, cobalt oxide, etc.). The commonly used types in laboratories are box furnaces and tube furnaces.
- Box-type resistance furnace: The box-type resistance furnace has a box-shaped chamber (Figure 4-7), classified into high temperature, medium temperature, and low temperature based on working temperature. The box-type resistance furnaces manufactured in our country have been standardized, except for low-temperature applications where various constant-temperature boxes are used instead.

The high-temperature box-type resistance furnace is mainly used for color improvement of high melting point gemstones such as corundum, ruby, sapphire, and zircon, with a general heating temperature above 1000℃.
A medium-temperature box furnace is often used to heat-treat gemstones such as sapphire, topaz, crystal, and tanzanite that require medium-low temperature color modification, with the heat-treatment temperature typically ranging from 650°C to 1000°C.
The low-temperature heat treatment furnace is mainly used for organic gemstones and gemstones containing water in their structure, such as pearls, corals, opals, etc.
The box-type resistance furnace has a simple structure, is easy to operate, and has a low cost, making it an essential device in laboratories. The advantages of the box-type resistance furnace are its high heating temperature, large internal space, and the ability to accommodate multiple samples at once. However, this type of heat treatment furnace has drawbacks such as low thermal efficiency, slow heating, and uneven furnace temperature, which need to be improved during operation. For example, uneven furnace temperature can be pre-determined by measuring the thermal field and placing samples at specific temperature locations to overcome the uneven temperature.
- Tube resistance furnace: The tube resistance furnace generally uses resistance wire layered around high-temperature refractory materials (usually 99% alumina tubes) and can control temperature in segments. It can also use silicon carbide rods as heating elements arranged in a circle around the alumina tube. The tube resistance furnace can control the atmosphere, isolating the heating element from the furnace atmosphere with a casing, allowing different atmospheres (such as oxidizing or reducing atmospheres) to be introduced as needed, with waste gas expelled through exhaust holes on the furnace cover (Figure 4-8).

The advantages of the tube resistance furnace are its fast heating speed, segmented temperature control, and accurate temperature control; its drawbacks are that it can handle a smaller amount of samples and is not easy to extract.
② Salt melting furnace:
The salt melting furnace is a heat treatment device that uses molten salt as the heating medium, characterized by its simple structure and fast, uniform heating speed. The melting temperature of the salt in the salt melting furnace ranges from 150~1300℃, depending on the composition of the salt solution, generally allowing a heating temperature range suitable for low-temperature and medium-temperature heat treatment of gemstones. The disadvantages are high power consumption, difficulty cleaning samples after treatment, and certain corrosive and contaminating effects on gemstones. Common types of salt melting furnaces include electrode-type and electric heating-type.
- Electrode salt melting furnace: This electric furnace inserts electrodes into the furnace chamber and passes low-voltage high current, generating strong electromagnetic circulation when the current flows through the molten salt, promoting the swirling of the molten salt to heat the sample. Our country’s electrode salt melting furnaces are mostly large for industrial production and unsuitable for laboratories. In laboratories, small furnaces can be designed using series-produced salt-melting furnace transformers.
- Electric heating salt melting furnace: This furnace consists of a crucible containing molten salt and a furnace body that heats the crucible. The heat source is often electric energy, but other fuels are also used. It is commonly used for the thermal treatment of self-colored gemstones caused by chemical components. Its characteristics include no heat source restrictions and no need for transformers, but the lifespan of the crucible is low, and the temperature distribution inside the furnace is uneven. Many models of this type of furnace are produced in our country, but only some are suitable for gemstone optimization treatment laboratories.
③ Fuel furnaces:
Fuel furnaces can be classified into solid fuel furnaces, gas fuel furnaces, and liquid fuel furnaces based on the type of fuel used. According to the shape of the heating chamber, they can also be divided into chamber furnaces, table furnaces, well-type furnaces, etc. The most common solid fuel furnace is the bottom-fired chamber furnace, with coal as the main fuel. The advantages are simple structure and low cost; the disadvantages are poor temperature uniformity and difficulty controlling temperature.
Gas fuel furnaces use combustible gases (such as coal gas, natural gas, liquefied petroleum gas, etc.) as fuel. Because combustible gases easily mix with air and burn completely, the furnace temperature is more uniform than solid fuel furnaces, making it suitable for routine laboratory gemstone processing. However, the accuracy of temperature measurement inside the furnace could be improved.
Liquid fuel furnaces use diesel or heavy oil as fuel, and their structure is similar to gas furnaces. The only difference between the two is in the structure of the combustion device.
(2) Controlled Atmosphere Furnace
Oxygen or reducing gas is injected into the controlled atmosphere furnace to improve the color and appearance of gemstones by controlling the oxidation or reducing atmosphere. The controlled atmosphere furnace usually comprises two parts: the controlled atmosphere working furnace and the controlled atmosphere generating device.
① Controlled atmosphere working furnace:
This type of furnace is generally an improved version of a resistance furnace, and both box-type and tube-type furnaces can be used as controlled atmosphere furnaces. A controlled atmosphere furnace can be formed by adding a controllable atmosphere attachment that allows gas to enter and seal the furnace chamber on a resistance furnace. It is commonly used to control the heat treatment atmosphere, such as oxidation, reduction, or neutral. The oxidizing gases introduced generally include oxygen, air, etc.; the reducing gases generally include H2, CO, N2, CH4, etc., and some of these gases are flammable, so extra caution is required during operation. To prevent explosions, the best method is to purge the furnace chamber with N2 (or CO2) gases before introducing gas or shutting down the furnace, with the amount of gas introduced generally being 4~5 times the volume of the furnace chamber. Additionally, the gas introduced sometimes has a high CO content, which can easily poison operators, so it is important to ensure good ventilation and regularly check the sealing of the furnace body and pipelines. The discharged waste gas should be ignited or released outdoors.
② Controlled atmosphere generating device
- Reducing atmosphere generating device (also known as endothermic atmosphere generator): This device mixes raw gases (natural gas, liquefied petroleum gas, coal gas, etc.) with air in a certain proportion. Under the action of an external heat source and catalyst, it is produced through incomplete combustion and a series of reactions. The generated gas is a good reducing atmosphere that is strictly controlled and stable, but the equipment structure is complex, and the cost is relatively high.
- Ammonia decomposition generator: In the heat treatment process, different atmospheres must be introduced according to the color formation causes of gemstones, such as oxidizing atmosphere, reducing atmosphere, etc. The commonly used reducing atmosphere is achieved through an ammonia decomposition generator.
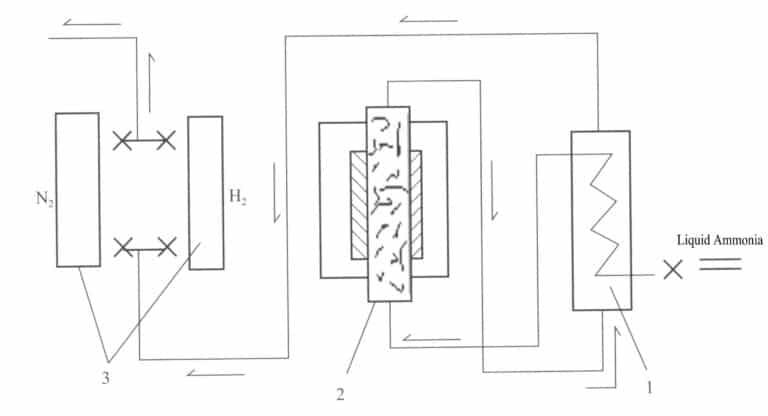
A reducing atmosphere is generated using a device that decomposes ammonia gas into nitrogen and hydrogen, as shown in Figure 4-9. Liquid ammonia from the ammonia bottle flows into vaporizer 1, where it is heated and vaporized, then enters reaction tank 2, where it decomposes under high temperature and the action of a catalyst. The cooled ammonia decomposition gas is purified in purification device 3, where residual oxygen and water vapor are removed and can then be introduced into the heat treatment furnace for use. The gas after decomposition H2:N2 is 3:1, which is a reducing atmosphere.
(3) Vacuum heat treatment furnace
Vacuum heat treatment is a heat treatment method where the heating or cooling process of the sample occurs in a vacuum (negative pressure) state, and the furnace used for this treatment is called a vacuum heat treatment furnace.
Vacuum heat treatment is used for special heat treatment conditions, such as the processing of black cubic zirconia, and the temperature in a vacuum furnace is also relatively high. Due to concerns about the oxidation of non-heating elements, high-temperature metals such as aluminum, wolfram, tantalum, and graphite products can be used as heating elements. Still, it is less widely used in gemstone optimization processes than in controlled atmosphere furnaces.
(4) Laser and electron beam heat treatment device
Laser and electron beam heat treatment technologies have developed in recent years. They are characterized by fast heating speed, high temperature, and no oxidation, making them particularly suitable for localized heat treatment. However, due to this equipment’s uneven heating, fast cooling rate, and high investment costs, they are used less frequently in gemstone heat treatment and are often applied to treating dark inclusions in diamonds.
An electron beam refers to a high-energy density beam of electrons emitted from a heated cathode filament, accelerated by an “anode,” and focused by a magnetic lens. When this electron beam contacts the surface of a sample, it immediately converts the energy of the electrons into thermal energy, heating the sample and even melting metals. The device that generates the electron beam is called an electron beam gun. This device is generally used for localized enhancement of the thermal treatment of gemstones.
1.2 Auxiliary Instruments and Devices for Heat Treatment
(1) Thermocouple
Thermocouples are the most widely used temperature sensing elements in temperature measurement. They have a simple structure, are easy to use, possess high accuracy and stability, and have a wide temperature measurement range, playing an important role in temperature measurement.
① The measurement principle of a thermocouple:
It involves connecting two metal wires (A and B) with different chemical compositions to form a closed loop, which is a thermocouple. When the temperatures at the two junctions of these wires are different, an electromotive force, known as thermoelectric potential, is generated in the circuit.
The magnitude of the thermoelectric potential of a thermocouple is related to the material properties of the conductors and the temperatures at the two junctions. When the conductor material is fixed, the greater the temperature difference between the two junctions, the larger the thermoelectric potential. Temperature can be measured by measuring the magnitude of the thermoelectric potential.
② The structure and types of thermocouples:
A thermocouple consists of two different conductive wires, A and B, called thermos electrodes. The welded end is called the working end, also known as the hot end, and is placed in the measured medium; the other is called the reference end, also known as the free or cold end, and is connected to the instrument.
When the hot and cold end temperatures differ, the thermoelectric potential generated by the thermocouple can be indicated or recorded by the instrument according to the temperature scale. A schematic diagram of the thermocouple is shown in Figure 4-10.
The two thermocouple wires are covered with insulating tubes to prevent short circuits, and they are protected by ceramic or heat-resistant steel tubes to prevent corrosion from harmful substances. The structure of the thermocouple is shown in Figure 4-11.
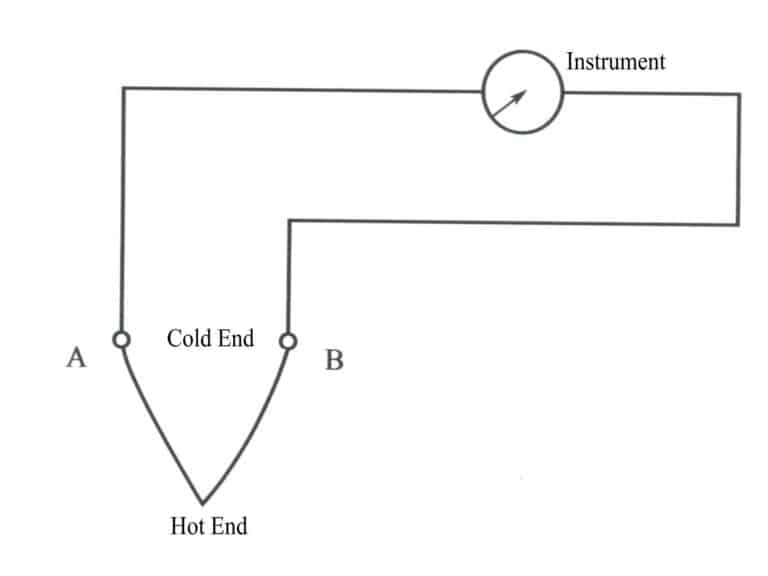
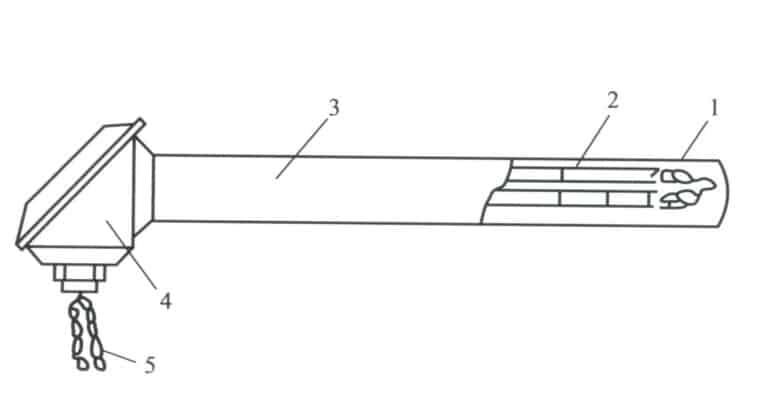
Figure 4-11 Structure of the Thermocouple
1—Thermocouple wires; 2—Insulating tube; 3—Protective tube; 4—Junction box; 5—Compensating lead wire
③ Thermocouple compensation wire:
The thermoelectric potential generated by the thermocouple can only directly reflect the temperature at the hot end when the cold end is maintained at 0℃.
However, in the practical use of thermocouples, due to the heat conducted by the thermocouple itself and the influence of the surrounding environmental temperature, the cold end temperature often varies, leading to inaccurate temperature readings by the measuring instrument.
To overcome this effect, compensation wires are often used to extend the cold end of the thermocouple to a location with a more constant temperature, allowing for compensatory measures to be taken.
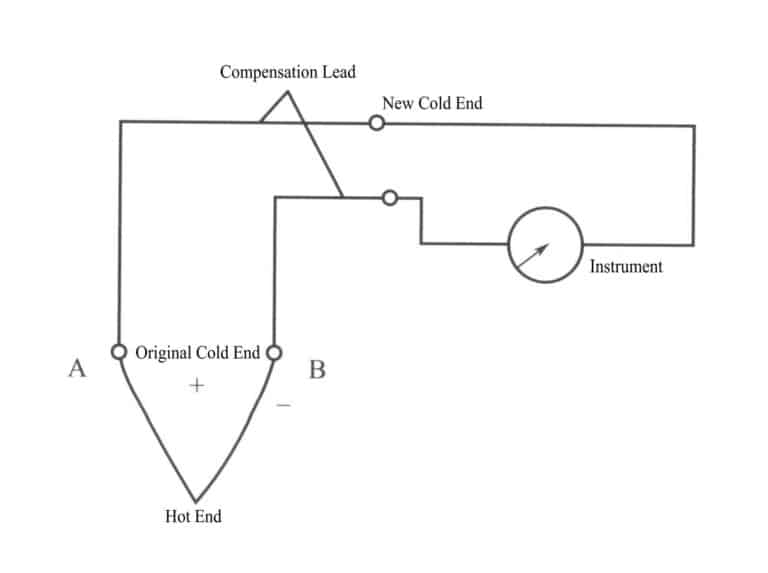
Compensation wires are a pair of metal wires with different chemical compositions. They have the same thermoelectric properties as the thermocouple they are connected to within the range of 0-100℃ but are much cheaper. The connection of the compensation wires is shown in figure 4-12.
Compensation wires are dual-core, single-strand, or multi-strand, and different colors distinguish their inner insulation layers to indicate positive and negative polarity. When using, it should be noted that various thermocouples should use corresponding compensation wires for connection; the temperature at the connection ends of the compensation wire and thermocouple should be maintained below 100℃; the new cold end extended through the compensation wire should still be compensated using methods such as constant temperature or calculation; the positive terminal of the compensation wire should connect to the positive terminal of the thermocouple, and the negative terminal to the negative terminal, to avoid incorrect connections.
(2) Radiation thermometers and optical thermometers
① Radiation Thermometer:
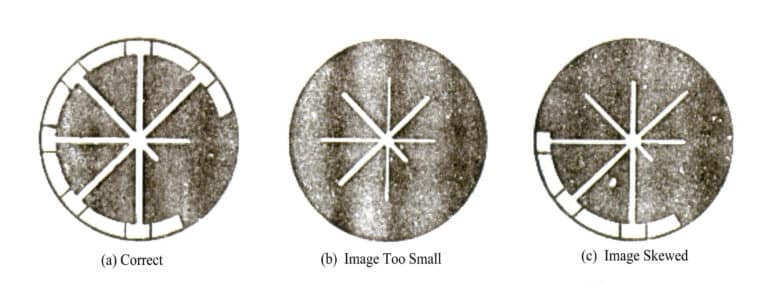
The radiation thermometer consists of a radiation temperature sensor and a display instrument. During use, the image of the measured object seen through the eyepiece must completely cover the thermopile [Figure 4-13 (a)] to ensure that the thermopile adequately receives the thermal energy radiated by the measured object. If the image of the measured object is too small or skewed, the measured value will be lower than the actual.
② Optical Pyrometer:
An optical pyrometer is a portable temperature-measuring instrument. The commonly used type is the filament extinction optical pyrometer. It operates on the principle that there is a corresponding relationship between the brightness of the glowing object and its temperature, using a method of comparing brightness to measure temperature.
When in use, aim the pyrometer at the measured object and move the eyepiece back and forth. Compare the brightness of the filament until the brightness of the filament is the same as the brightness of the measured object; that is, the filament image disappears in the image of the measured object [Figure 4-14 (b)], then the temperature of the measured object can be obtained, the temperature indicated by the immediate degree.
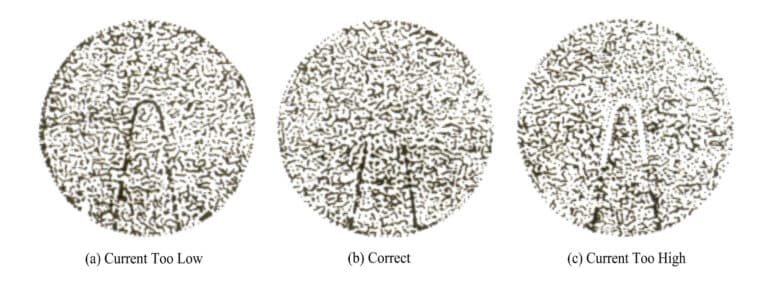
Figure 4-14 Aiming condition of the optical pyrometer (Wu Ruihua, 1994)
(a) If the measured object is brighter than the filament, the indicated temperature is low; (c) If the measured object is darker than the filament, the indicated temperature is high.
(3) Crucible
Crucibles are commonly used containers in the heat treatment process of gemstones. Since heat-treated gemstones are often completed at higher temperatures and come into direct contact with the crucible, the choice of the crucible is a crucial factor for the success of heat treatment. During the heat treatment process, the selection of the crucible must meet the following conditions:
① The crucible material must have sufficient strength at working temperatures and should not develop cracks over extended periods at high temperatures.
② Under working atmospheres, the crucible material must be quite stable concerning gemstones. It should not chemically react with them, and special attention must be paid to the purity of the crucible material to avoid introducing harmful impurities into the gemstone crystals.
③ The crucible material should have low porosity and high density to maintain a certain pressure after the crucible is sealed.
④ Since crucibles are commonly used containers in the thermal processing of gemstones, the crucible material should be easy to process and inexpensive.
Copywrite @ Sobling.Jewelry - Custom korujen valmistaja, OEM ja ODM korut tehdas
2. Principles of Thermal Treatment to Improve Gemstones
Heating natural gemstones at certain temperatures can improve their color, transparency, and appearance. The reason for this is mainly that through heat treatment, the structure and composition of the gemstones change, thereby enhancing their appearance characteristics and increasing their aesthetic and economic value. Therefore, to understand the changes in the appearance characteristics of gemstones, it is necessary to analyze the principles by which heat treatment improves gemstones.
Heating is the process of tapping into the potential of gemstones and maximizing the beauty within them. Treated gemstones have no differences in physical and chemical properties compared to natural gemstones. The principle is that heating causes changes in the content and valence state of the coloring ions contained within the gemstone, or creates some structural defects that lead to changes in the physical properties of the gemstone, such as color and transparency.
Most gemstones containing trace element impurities change color or transparency after heat treatment. The equipment commonly used for heat treatment is simple and easy to operate, suitable for most allochromatic-colored gemstones, such as rubies, sapphires, emeralds, tourmalines, zircon, jade, and agate. This method applies to gemstones whose color is caused by transition element components or transition element impurities, and it is also suitable for gemstones whose color changes are caused by charge transfer. Organic gemstones can also have their color and transparency altered through heat treatment; for example, amber can become clear and transparent after heat treatment by removing internal bubbles.
According to the physical and chemical properties of gemstones and their coloring mechanisms, the principles of commonly treated gemstones are summarized as follows:
(1) Change the content or valence state of chromophore ions in gemstones through heat treatment
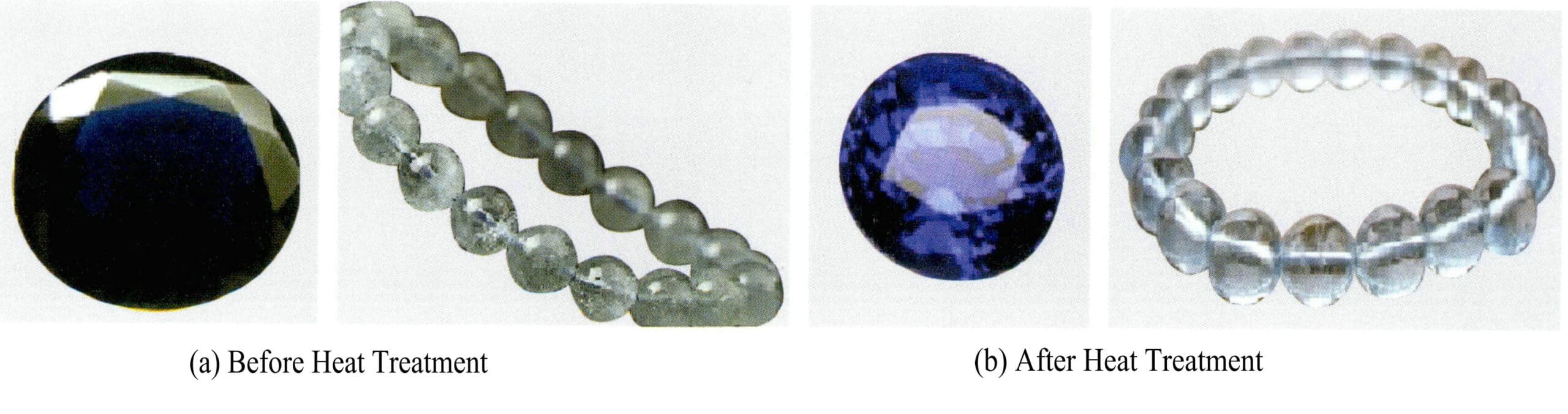
Trace impurity ions, color some gemstones, and use heat treatment to oxidize the low-valence cations in the gemstones into high-valence cations, changing the color of the gemstones. For example, red agate is primarily colored by Fe3+. Through heat treatment, Fe 2+ in the agate can be oxidized to Fe3+, increasing the content and ratio of trivalent iron ions, thereby enhancing the red tone of the agate. The heat treatment of rubies and red jadeite also strengthens the color of the gemstones through this principle. Aquamarine with a green tint can also have its green tone removed through heat treatment, enhancing the blue tone of the aquamarine. Figure 4-15 shows that aquamarine (a) has a significantly deepened blue and weakened green tone after heat treatment.
(2) Changing the composition of organic gemstones through heat treatment
For organic gemstones such as pearls, ivory, coral, and amber, heat treatment can oxidize the organic matter within them. If the temperature is too high, it can produce black coloration, resulting in the “carbonization” phenomenon of the organic matter. This type of heat treatment can mimic “ancient jade” in the gemstone industry, commonly known as “aging” treatment, often called color roasting, and is frequently used for amber, coral, and others.
(3) Heat treatment produces color centers
The color of some gemstones is mainly due to color centers. Gemstones can produce color centers that absorb certain light and generate color by heat treatment. Heat treatment is usually applied after gemstone irradiation treatment to remove unstable color centers and retain stable ones. For example, topaz treated with heat removes unstable brown color centers while preserving stable blue ones. The goal of improving gemstone color can be achieved by mastering the heating temperature and duration of heat treatment. Amethyst turning yellow or green and smoky quartz turning yellow-green or colorless are also results of using heat treatment to change color centers.
(4) Heat treatment causes color changes in hydrous gemstones due to dehydration effects
Some gemstones contain adsorbed water and structural water. Some gemstones can improve their color during heat treatment without damaging the structural water. For example, beryl contains structural water, and orange-yellow beryl containing iron and manganese can be transformed into beautiful pink beryl through heat treatment. Opal contains structural water, and if opal is heated to around 300℃, the color-changing effect will disappear due to water loss. Tiger’s eye loses structural water through heat treatment, resulting in deep brown or reddish-brown colors.
(5) Heat treatment causes changes in the crystal structure
Heat treatment can reorganize the internal structure of crystals, improving their crystallinity and thus affecting the color of the crystals. Common types of zircon include low-type zircon, medium-type zircon, and high-type zircon. Through heat treatment, low-type zircon can be transformed into medium-type zircon, and medium-type zircon can be transformed into high-type zircon, etc. At the same time, the color of the crystals will also change; under different atmospheres, they can transform into different colors. For example, under reduced conditions, heat treatment can improve brownish-red zircon and turn it into colorless zircon.
(6) Heat treatment improves silk-like inclusions and starlight effect in gemstones
Common gemstones, such as sapphires, contain titanium ions as rutile (TiO2), which produces a white silk or star effect. The formation of rutile is controlled by the geological conditions when the gemstone was formed. In some natural sapphires, the distribution of star lines is uneven, and the star effect is poor. Through heat treatment, rutile in sapphires can be melted and rearranged, thereby improving the starlight effect of natural gemstones. The starlight effect in synthetic gemstones is also produced using this principle.
3. Conditions for heat treatment
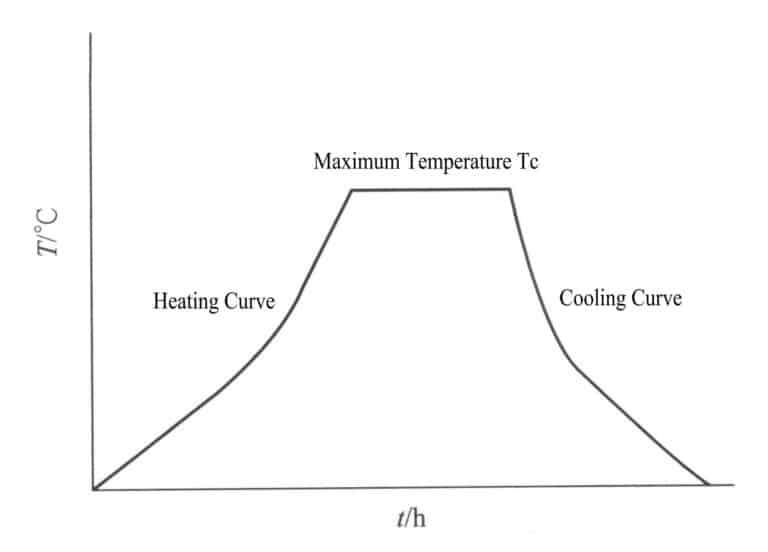
During the heat treatment process, it is necessary to master various factors such as heating speed, the highest temperature reached under experimental conditions, holding time, cooling speed, and the atmosphere and pressure inside the heating furnace. These conditions need to be considered comprehensively.
(1) The speed of heating to a higher temperature
Due to the poor thermal conductivity of most gemstones, the heating speed during heat treatment can be a little slow to avoid cracks caused by a large temperature difference between the inside and outside of the gemstone. If the heating speed is plotted as a curve, it represents the heating curve of the treated gemstone, which requires smoothness, meaning that most of the heating should be done slowly to prevent the gemstone from cracking.
(2) The highest temperature reached during heat treatment
The highest temperature reached during heat treatment is the maximum temperature that can improve the color or transparency of the gemstone, and it is also the optimal temperature for changing the color or transparency of the treated gemstone. This is the most important condition that needs to be explored repeatedly.
(3) Holding Time
The time during which the gem is maintained at the highest temperature, commonly referred to as holding time, with a temperature curve that is a straight, constant temperature curve. To ensure the entire gem is stable and uniform, it often needs to be held for a period to allow for uniform internal changes. The optimal holding time must be determined through extensive experimentation.
(4) Cooling Curve
The cooling rate from the highest temperature and the gradient of temperature maintained during cooling is known as the cooling curve. In most cases, cooling is relatively slow to prevent the gem from cracking, but sometimes there are special requirements for rapid cooling, such as eliminating needle-like inclusions in corundum; quartzite and serpentine jade may sometimes require rapid cooling to create crack patterns before dyeing.
(5) Atmosphere in the Furnace
The atmosphere in the furnace refers to the control of oxidation-reduction conditions during the heat treatment process and the roasting with useful components. Some experiments require adding chemical agents for roasting or heating samples immersed in certain liquid reagents. For example, to eliminate the purple hue of rubies, it is necessary to oxidize Fe2+ in the ruby to Fe3+ under an oxidizing atmosphere, reducing the purple hue’s impact on the ruby; for instance, the red burning of agate involves oxidizing Fe2+ in the agate to Fe3+ under an oxidizing atmosphere, enhancing the red color of the agate.
(6) Pressure in the furnace
Some gemstone heat treatment experiments require controlling a certain pressure. For example, diamond heat treatment often uses high pressure, while the heat treatment of ordinary gemstones such as rubies, aquamarines, and agates is done under normal pressure conditions. During the experiment, whether to use normal, reduced, or increased pressure must be explored, as the pressure conditions required for each type of gemstone are different.
In gemstone heat treatment, these six factors are obtained through repeated exploration in experiments. The experimental conditions for each type of gemstone are different. Among the conditions for gemstone heat treatment, it is most important to determine the heating rate, cooling rate, maximum temperature reached, and holding time (Figure 4-16). Both heating and cooling during the heat treatment process must be slow; otherwise, cracks may occur, reducing the quality of the gemstone. The optimal combination of these factors can often be achieved in a specific process.
The improved gemstones are natural materials from different origins, contain different impurity components, or have undergone different histories. The historical environment and geological conditions are quite complex, and even gemstones that appear the same may have very different heat treatment methods. Moreover, most heat treatment processes are kept strictly confidential, and no ready-made experimental conditions are available; one must explore on their own.
For example, sapphires that are the same brownish-yellow appear when subjected to the same heat treatment conditions; the sapphires from Hainan turn blue, while those from Shandong turn orange-yellow. Experiments must be conducted under various conditions to achieve a specific color through heat treatment. Care must be taken with all samples to avoid damaging the material.
To prevent gemstones from cracking during heat treatment, in addition to strictly controlling the temperature rise and fall conditions, one must also prevent the expansion of cracks. The specific method is to appropriately remove all areas with cracks before heat treatment, then re-polish after heating; raw stones can be heated for small particles of flawless gemstone material.
4. Thermal effects in heat treatment
There are various thermal effects in heat treatment. However, among the common gemstones, the most important thermal effects on gemstone materials are the nine types summarized by American scholar Nassau, as shown in Table 4-1.
Table 4-1 Mechanism and Examples of Thermal Effects
| Effect | Mechanism | Esimerkki |
|---|---|---|
| Darkening | Slowly oxidizing and turning black in the air | "Aging" amber and ivory |
| Color change | Destruction of color heart | Blue or brown topaz turns colorless; pink topaz turns yellow; amethyst turns yellow or green; smoky quartz turns yellow-green or colorless |
| Color change | Changes due to hydration or condensation | Pink chalcedony turns orange, red, or brown; tiger's eye heated produces deep brown to reddish-brown |
| Homogeneous polyhedral body | Structural changes caused by radiation | "Low-type" zircon changes to "High-type" zircon |
| Color change | Change in atmosphere, related to oxygen concentration | Green aquamarine turns blue; amethyst turns into dark yellow topaz; colorless, yellow, and green sapphires turn blue; brown or purple rubies turn red. |
| Structural changes. | Temperature changes, crystal precipitation or melting. | The generation or elimination of silky or starlight effects in corundum. |
| Color overlay | Impurity diffusion | Blue and star-like diffusion on the surface of sapphire |
| Fracture | Sudden temperature change, internal structure fracture | "Halo" around inclusions in sapphire, "exploding" quartz |
| Regeneration and purification | Rheology under heat and pressure | Regeneration and purification of amber; regeneration of tortoiseshell |
Table 4-1 omits those thermal effects that are completely reversible or metastable. For example, when ruby is heated to a red-hot state, it turns green, and when cooled to room temperature, it returns to its original color; smoky quartz turns blue-green when heated and reverts to yellow when cooled to room temperature.
The darkening effect in Table 4-1 is sometimes used to “age” amber and ivory. This effect is equivalent to the process of slow charring. Research shows that amber will darken even when placed in a dark storage room, indicating that the organic materials are easily oxidized. Therefore, it is reasonable to expect that the oxidation process speeds up during slow heating.
Table 4-1 shows that the color center damage caused by heating can lead to the fading or disappearance of gemstone colors. For example, brown topaz, yellow sapphire, and red tourmaline can all become colorless after heat treatment; some amethyst, citrine, and smoky quartz can also become colorless.
The destruction of color centers may sometimes result in color changes. For instance, irradiated brown topaz can turn blue after heat treatment; amethyst can become citrine under controlled heat treatment temperatures; certain brown topaz can turn pink after heat treatment. These color changes can be restored to their original colors through radiation treatment.
The color changes caused by hydration or condensation, as shown in Table 4-1, generally involve impurities such as iron. Heating limonite can yield deep orange, brown, or red hematite.
In some iron-containing quartz materials ranging from gray to yellow and brown, such as agate, chalcedony, and tiger’s eye, heating produces deep brown to reddish-brown colors based on this principle.
The homogeneous polycrystalline bodies in Table 4-1 are changes in gem structure caused by the transformation of homogeneous polycrystalline bodies under heat treatment conditions. For example, graphite can be converted into diamond under high temperature and pressure; “low-type” zircon can transform into “high-type” at high temperatures, etc.
The color changes of gems in Table 4-1 caused by changes in the oxidizing or reducing atmosphere in the environment are mainly related to the oxygen concentration in the environment. For instance, green aquamarine turns blue under reducing conditions; amethyst turns into dark citrine under oxidizing conditions; colorless, yellow, and green sapphires turn blue under oxidizing conditions; brown or purple rubies turn red, etc.
The structural changes in Table 4-1 lead to physical optical effects in gems. For example, under heat treatment conditions, the rutile inclusions in starlight sapphires melt, causing the starlight effect to disappear. Upon cooling, rutile crystals precipitate, and the starlight effect is regenerated.
The color enhancement in Table 4-1 is due to the addition of coloring ions, which deepen the color of the gems. For example, in diffusion sapphires, the addition of coloring ions like iron and titanium deepens the color of light-colored sapphires.
The fractures in Table 4-1 are changes in the internal structure of gems under heat treatment conditions, such as stress lines generated around inclusions in sapphires and the cracking patterns that occur in artificially heat-treated quartzite under quenching conditions.
The regeneration and purification in Table 4-1 are internal changes caused by gas-liquid interactions under heat and pressure. For example, bubbles inside amber burst under heat treatment conditions, increasing transparency; turtle shells can regenerate under hydrothermal conditions, etc.
5. Redox and Gas Diffusion
In the process of gem heat treatment, redox conditions are very important and are a key factor in the success of gem heat treatment. Controlling the oxidizing or reducing atmosphere during the heat treatment can change the gem’s color. The oxidizing or reducing atmosphere during heat treatment is related to the temperature of the gem and the oxygen concentration inside the container at that temperature.
(1) Redox
① Standard Oxygen Partial Pressure (Po2) : When oxygen-containing gems are heated in air, the gems stabilize at the same concentration as the oxygen in the atmosphere. This concentration is the standard oxygen partial pressure of the gem at this temperature.
② In an oxidizing atmosphere, the partial pressure of oxygen in the furnace is greater than the standard partial pressure of oxygen for this gem at the same temperature Po2.
③ In a reducing atmosphere, the partial pressure of oxygen in the furnace is less than 002.
In addition to using air, a strong oxidizing atmosphere uses pure oxygen; sometimes, compressed air increases oxygen density. Chemically inert gases (such as nitrogen) are generally considered neutral, forming a neutral atmosphere. If it can dilute the atmosphere and reduce the oxygen content, it can also be viewed as a reducing gas, although its reducing ability is very weak.
Similarly, the atmosphere can be improved by burning fuels. For example, by using natural gas, propane, gasoline, etc., and controlling the amount of air or oxygen blown in, a carbon reduction can occur, but this isn’t easy to control.
Another type of drip protection atmosphere is to directly drip organic liquid into the furnace to react with oxygen to control the atmosphere.
(2) Gas diffusion
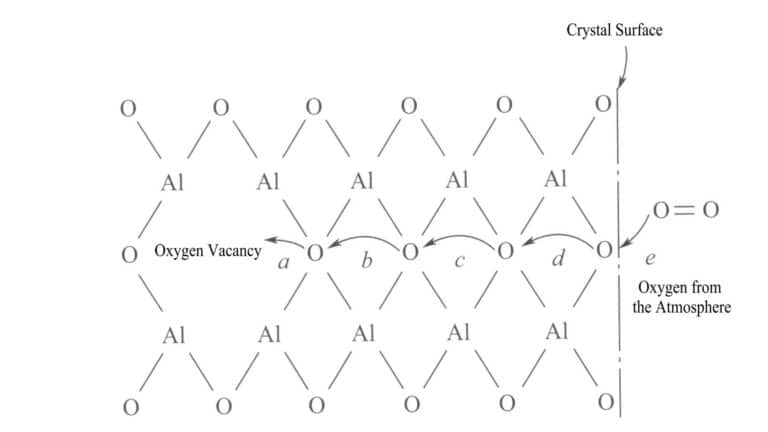
The redox reaction is achieved through the diffusion of gases. For it to act on the entire sample, oxygen must diffuse into the interior of the gem sample along a certain path, usually over a distance of more than 1 cm. The diffusion temperature must exceed 1000℃, and the time must be several hours.
Due to the characteristics of the oxide gem structure, oxygen does not need to move the entire distance to produce the desired effect, allowing this diffusion to occur rapidly. For example, the diffusion process of oxygen in the atmosphere into the oxygen vacancies of corundum aluminum oxide shown in figure 4-17.
6.Classification of Heat Treatment Methods
According to the type and method of heat treatment, there are three common heat treatment methods:
(1) Ordinary Heat Treatment Method
The ordinary heat treatment method involves directly heating the gemstone, causing changes in the coloring ions’ content and valence state. Sometimes, it can also change the crystal’s internal structural defects, altering the gemstone’s physical properties, such as color and transparency.
For example, Sri Lankan milky white, brownish, and light yellow Geuda stones turn into sapphires, aquamarines change from green to aquamarine blue, tanzanites turn blue after heat treatment, etc.
(2) Chemical Reagent Roasting Method
The chemical reagent roasting method, also known as the diffusion method, refers to using chemical reagents to destroy the crystal structure of the gemstone’s surface, causing the chemical composition of the surface layer to change as intended. The coloring ions within the gemstone can also exchange through the surface layer (diffusing outward or inward), resulting in valence state or content changes.
The popular diffusion sapphire, diffusion topaz, and diffusion tourmaline in the international market are obtained using this method. Gemstones improved by this method can lighten dark gemstones, turning light gray gemstones into blue gemstones, and so on.
(3) Molten Salt Electrolysis Method
After mixing the molten salt, please place it in a graphite crucible and proceed with the electrolysis process. A platinum (Pt) wire is used as the anode, wrapping the gemstone sample with the platinum wire anode so the gemstone becomes the anode and the graphite crucible serves as the cathode.
After the electrolyte melts in the furnace, place the anode and the gemstone together in the electrolytic cell for electrolysis, as shown in Figure 4-18. The control tank voltage is set to 3.0V, and the electrolysis time is 40-45 min. Then, remove the anode and the sample. The electrolysis process changes the valence state and content of the coloring ions in the gemstone, thereby altering the color and transparency of the gemstone. The disadvantage of this method is that if the molten salt is improperly selected, it can be excessively corrosive to the gemstone.
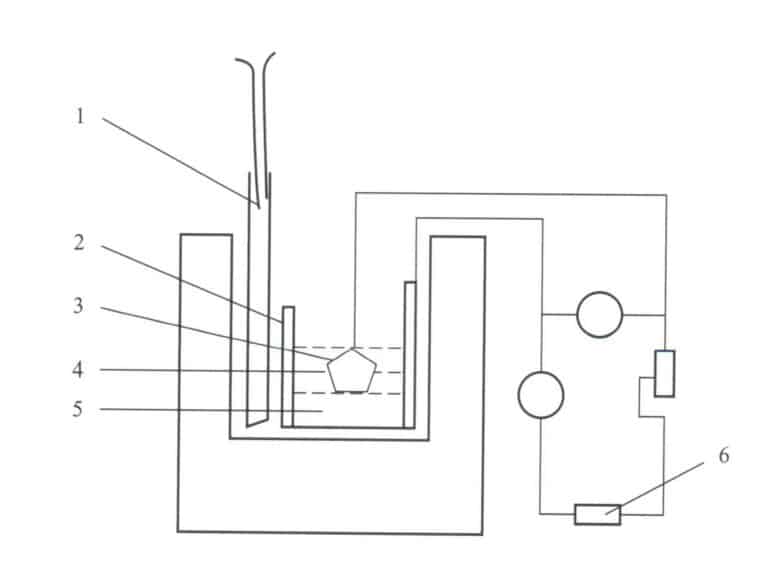
Figure 4-18 Schematic diagram of the molten salt electrolysis experiment
1—Thermocouple; 2—Graphite crucible; 3—Pt anode and sample; 4—Electrolyte; 5—Aluminum melt; 6—Direct current power supply
7. Common heat treatment methods to improve gemstone conditions
There are many types of gemstones suitable for improvement through heat treatment, and the required heat treatment temperatures vary for different gemstones. For example, sapphires require a high heat treatment temperature, generally above 1300℃; rubies require a relatively lower heat treatment temperature, around 1000℃; other gemstones such as aquamarine, crystal, and chalcedony require temperatures around 700℃. The controlled temperatures can be roughly divided into four segments: low heat 200-400°C; medium heat 400-700℃; high heat 800 ~1300℃; and strong heat above 1300℃. The heat treatment conditions for common gemstones are shown in Table 4-2.
Table 4-2 Conditions for Heat Treatment of Common Gemstones
| Jalokivi | Purpose of Heat Treatment | Final Color | Lämpötila | Usage |
|---|---|---|---|---|
| Ruby | Remove mixed colors (brown, purple) to exclude or reduce filamentous substances and increase transparency. | Punainen | Around 1000℃ | Often |
| Blue sapphire | Deepen the color of iron and titanium-containing corundum, lighten the deep blue of corundum | Sininen | Strong heat | Often |
| Yellow sapphire | Heating suitable light-colored or colorless iron-containing corundum | Deep yellow | High fever | Often |
| Various colors of sapphires | Heat suitable corundum to eliminate "fibrous" or "star-like" inclusions | Increase | Strong high heat | Often |
| Diffuse starlight ruby, sapphire | Impurities are diffused to the surface of the gemstone by heating ( TiO2 ), presenting star light | Ruby, sapphire starlight | Strong heat first, then high heat for a long time | Not often used |
| Diffusion of ruby and sapphire | Coloring ions diffuse to the surface of the gem through heating, presenting color | Various colored corundum | Strong heat | Commonly used for blue |
| Aquamarine (colorless or green) | Exclude yellow tones in green | Sea blue | Low fever | Commonly used |
| Orange-yellow beryl | Exclude yellow tones from green | Vivid red | Low heat | Not often used |
| Deep blue or green tourmaline | Color lightens | Blue or green | Medium heat | Commonly used |
| Dark Red Tourmaline | Remove black tones | Vaaleanpunainen | Low heat | Commonly used |
| Smoky Green Tourmaline | Remove Brown Tone | Bright Green | Low Heat | Commonly used |
| Smoky quartz | Color lightens | White or yellow | Low heat | Commonly used |
| Some amethyst | Brown heating | Orange-yellow or green | Low heat | Commonly used |
| Green or brown zircon | Brown treatment | Colorless or blue | High fever | Commonly used |
| Agate, chalcedony, etc. | Iron ion varieties | Punainen | Medium-High Fever | Commonly used |
| Iris quartz | Heated Quartz Crystal Quenching | Can Be Dyed in Various colors | Medium heat | Use less |
| Tanzanite | Heating transforms the transparent zoisite into blue | Purple Blue | Medium heat | Widespread |
Section IV Radioactive Irradiation Methods
Irradiation is the process by which microscopic particles propagate from a radiation source in all directions through space, which can cause changes in the physical and chemical properties of materials. This section mainly introduces the equipment required for radioactive irradiation, precautions, and the formation and elimination process of gemstone color centers after irradiation.
1. Types of Irradiation Rays and Radiation Sources
A radiation source is a material or device that can produce ionizing radiation. Common types of radiation sources include the following:
(1) Rays emitted by radioactive elements
Radioactive elements emit β rays and γ rays through decay, among which seven are mainly used for the irradiation treatment of gemstones. For example, the radioactive isotope 60Co can serve as γ ray source, emitting two types of rays at 1.17MeV and 1.33MeV, with a half-life of 5.3 years, commonly used as a radiation source for gemstone irradiation; additionally, isotope 137Ce and spent nuclear fuel elements can also be used as γ ray radiation sources.
When radioactive elements decay, they can emit two energy-close γ rays. γ rays have strong penetrating power and can change the color of gemstones; with a long half-life, they can be used for irradiation treatment for a long time.
(2) Rays produced by electron accelerators
An electron accelerator is an electrical device that accelerates charged particles to high energy through electromagnetic fields. Electron accelerators mainly obtain very high energy through electromagnetic fields, and different types of electron accelerators can produce electron beams ranging from several mega-electron volts to 300MeV, including electron static accelerators, X-ray tubes, microwave electron accelerators, etc.
(3) Rays produced by nuclear reactors
A nuclear reactor is a device or material that produces ionizing radiation through nuclear transformation. The neutrons produced in nuclear reactors are generally used for gemstone irradiation, and the common reaction is the interaction of α particles with beryllium ( 9Be + 4He —> 12C + n) ). Therefore, mixing natural α particle radiation sources with beryllium powder can yield a neutron source with energy distributed around 0-13MeV, and the most abundant neutron energy is about 4MeV. Thus, when treating gemstones with irradiation, it is best to use the fission process of a nuclear reactor as the neutron source.
2. Common Equipment for Irradiating Gemstones
Common equipment for irradiation includes reactors, electron accelerators, and cobalt source irradiation devices. Different types of irradiation equipment are used for different types of gemstones.
(1) Reactor
The commonly used type is a research reactor, which can utilize the radioactivity of reactor components to irradiate gemstones. There are four common types of research reactors: Heavy Water Research Reactor (HWRR), Swimming Pool Reactor (SPR), Mini Neutron Source Reactor, and Fast Neutron Reactor. The Mini Neutron Source Reactor is generally not used for gemstone irradiation treatment.
Gemstone samples are placed in the reactor for irradiation, with the irradiation time and dose determined by the desired color improvement. The commonly used reactors include the following types:
① Heavy Water Research Reactor (HWRR)
The heavy water research reactor is a device for conducting isotope irradiation, fuel, and material testing, neutron doping of single crystal silicon, neutron activation analysis in the reactor, irradiation for electronic device modification, and various physical research. Irradiation of gemstones is just one application area it has developed. Different heavy water reactors have different parameters.
② Swimming Pool Reactor (SPR)
Swimming pool reactors are widely used, with advantages such as high flux, flexible layout, and low underwater irradiation temperatures. In addition to scientific research, they can provide irradiation technology for agriculture, medicine, aviation, electronics, etc., for irradiating gemstones and freshwater pearls, electronic devices, and more.
③ Fast Neutron Reactor
Fast neutron reactors are a relatively advanced type of nuclear reactor. The utilization rate of nuclear fuel is very high, reaching 60%-70%, while the utilization rate of uranium fuel in our pressurized water reactor nuclear power plants is only 1%-2%; fast neutron reactors use industrial plutonium- 239 produced by pressurized water reactors as the initial charge, converting non-fissile uranium-238 into fissile plutonium fuel, also known as neutron breeding reactors.
(2) Electron Accelerators
Electron accelerators have a wide range of applications in physics. The electrostatic accelerator is commonly used for irradiating gemstones.
① High Voltage Multiplier
High voltage multipliers are mainly used for nuclear data measurement, neutron and charged particle nuclear reactions, neutron activation analysis, and electron beam irradiation of various materials, such as modifying wires and cables and preserving food and fruits.
Its accelerated particles include protons, hydrogen, oxygen, nitrogen, etc. Injection below 5keV, N+ can changes the material properties.
② Electron Linear Accelerator
The electron linear accelerator is used to study transient irradiation effects, irradiation modification of semiconductor materials (including gems), food preservation, etc. The advantages are high energy (10 ~ 14MeV) and high penetration rate.
③ Electrostatic Accelerator
The particles that can be accelerated include protons, deuterons, helium, electrons, oxygen, and nitrogen. Its energy range is adjustable, mainly used for nuclear data measurement, neutron and charged particle nuclear reaction experiments, electron beam irradiation, ion implantation, etc., and is only suitable for irradiating surface-modified gems such as pearls.
④ Cyclotron
The cyclotron is a fixed-energy accelerator mainly used for experiments involving charged particle nuclear reactions and for the activation analysis of charged particles and testing material properties, with rare applications in gem research.
(3) Cobalt Source Irradiation Device
The cobalt source irradiation device is a tool that uses the radiation emitted by the radioactive isotope 60Co and the seven rays to study the effects of radiation on materials (minerals, crystals, organic materials, and living organisms, etc.) and to perform irradiation treatment on these materials.
This irradiation source has low energy consumption, minimal pollution, and no radioactive residue. It has been applied early in the irradiation of gems, and it is particularly suitable for the irradiation of smoky quartz.
3. Irradiation Technology
When irradiating gemstones, the gemstones are placed in a sample box at the physical center of the reactor. A motor must continuously rotate the sample box, and there must be water inlet and outlet devices to cool the samples, with the water temperature not exceeding 50℃. The irradiation equipment and process are shown in Figure 4-19.
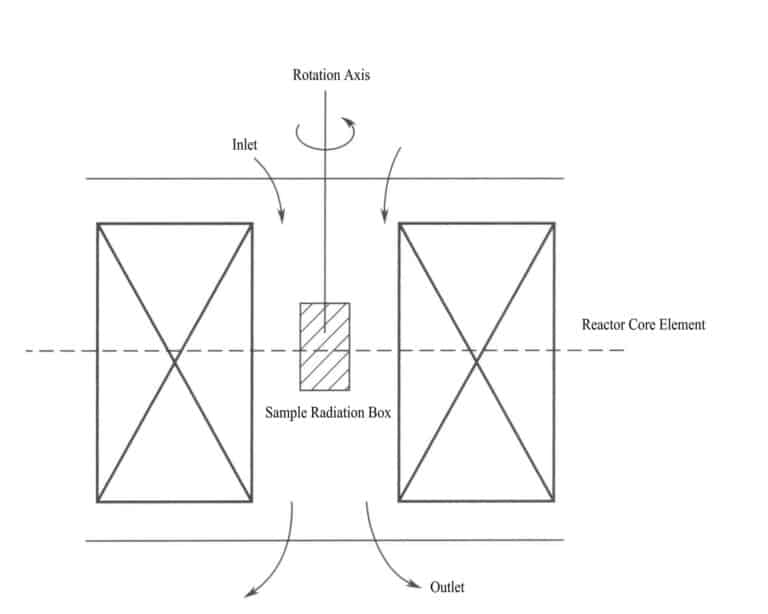
During the irradiation process, to achieve uniformly colored gemstones with appropriate shades, the following four key technical issues must be adhered to when irradiating gemstones:
(1) To ensure uniform product color, uniform irradiation must be achieved, and the gemstones should be rotated at a constant speed or flipped repeatedly during irradiation.
(2) To avoid the samples from cracking or overheating due to excessive temperature during irradiation, appropriate cooling measures should be taken. This can include adding circulating cooling water or periodically exposing the samples to air for cooling.
(3) The color depth must be controlled with sufficient radiation dosage. Repeated irradiation is necessary if a deeper color is required for the gem. Before the irradiation dosage is saturated, the depth of the gem’s color is proportional to the irradiation dosage; the longer the irradiation time, the deeper the color of the gem.
(4) The color improved by irradiation is sometimes unstable and prone to fading when exposed to light and heat. A low-temperature heating method can remove unstable color centers while retaining stable ones. However, there are often color changes after low-temperature heating. For example, topaz may change from brown to blue and crystal from brown to yellow. If the heating temperature is poorly controlled, it may completely fade and revert to the color before irradiation.
4. Formation and elimination of color centers during irradiation
Irradiation can cause colorless crystals to produce vacancy color centers, resulting in smoky or purple colors. The color and depth formed in the crystal after irradiation depends on the type and content of impurities contained in the crystal. If the colorless crystal contains Al3+ impurities, it will turn smoky to black after irradiation; if it contains Fe3+ impurities, it will turn purple.
The depth of color after irradiation is related to the impurity content in the gemstone. A higher impurity content results in a deeper color, while a lower impurity content results in a lighter color.
(1) The formation and elimination process of color centers
After irradiation treatment, gemstones generate color centers internally, causing a color change. For example, in smoky quartz, the formation and elimination process of color centers can be seen in the energy level diagrams from Figure 4-20 (a) to Figure 4-20 (d). When forming a color center, electrons are excited from state A to state D and then to state B, which requires much energy. When eliminating the color center or fading, electrons move from state B to state C and then to state A, which also requires significant energy. These color centers, which require a lot of energy for formation and elimination, are stable color centers in visible light.
There is also another situation as shown in Figure 4-20 (e). The system forms a color center by exciting from state / to state D and then to state B, which requires a lot of energy; however, moving from state B to state C back to state A requires very little energy. Figure 4-20 ( f ) shows that forming a color center from state A to state D and state B requires very little energy, and moving from state B to state C back to state A also requires very little energy.
This energy is within the visible light range. The system can overcome the energy barrier C and fade when visible light shines. The properties of absorbing light and transitioning to excited states E and F remain unchanged, but these colors can all fade in visible light. Therefore, the color centers in Figures 4-12 (e) and (f) are called unstable color centers.
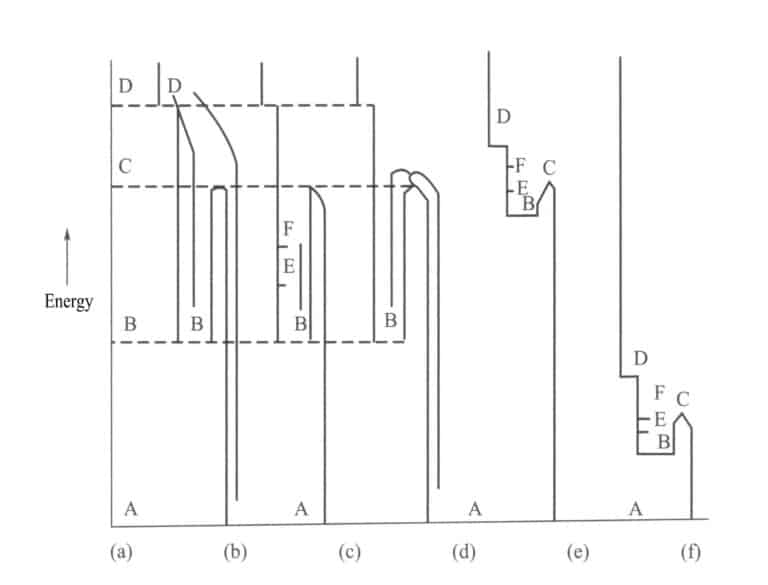
(2) Stability of color centers
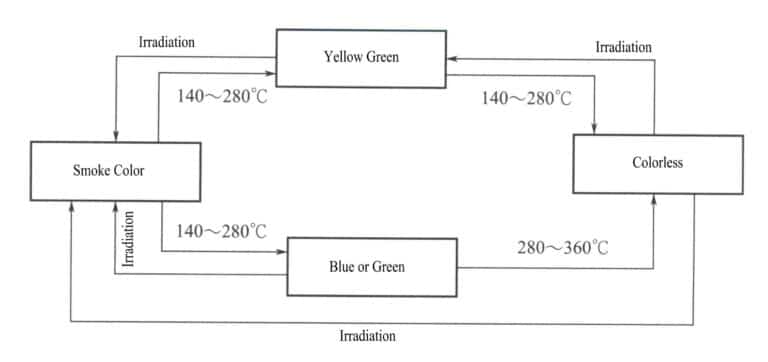
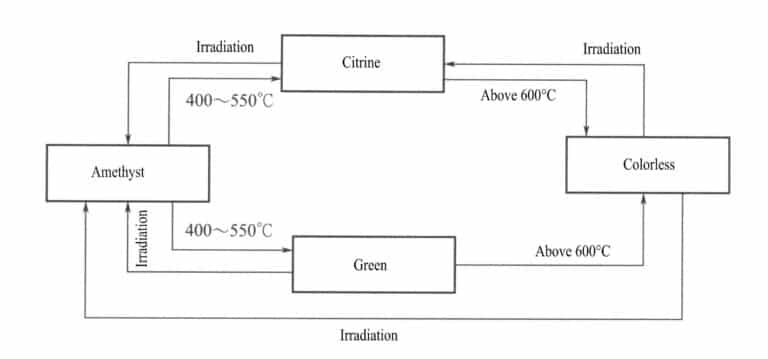
Generally, the color of gemstones after irradiation treatment can be restored to its original color through heating. Gemstones with stable color centers require higher heat treatment temperatures, while those with unstable color centers require lower heat treatment temperatures. For example, smoky quartz generally requires a heat treatment temperature of 140-280℃ to eliminate the smoky color (Figure 4-21), while amethyst requires a higher heat treatment temperature, generally above 400℃ (Figure 4-22). Therefore, irradiated amethyst is more stable than smoky quartz.
The color centers of gemstones are not fixed; the temperature at which samples fade after irradiation varies with different radiation sources. The stability of the same material’s color center, formed by different causes, also differs. For example, the yellow color center of sapphire, formed by artificial irradiation, is very unstable and fades quickly in visible light. However, the yellow color center of naturally occurring sapphire is stable in visible light and does not easily fade.
Artificial irradiation is of high dose and short duration, while irradiation in nature is of low dose and long duration, resulting in different heights of energy barriers C.
5. Color Changes in Gemstones Caused by Irradiation
Irradiation produces different effects on gemstones, causing various changes in different types of gemstones. When irradiated particles enter a gemstone, they interact with the atoms or ions within it, altering its structure or ionic charge, thereby changing its color. The changes in gemstones caused by radiation include the following aspects.
(1) It causes the gemstone to form natural, already-discovered color centers.
Irradiation can produce the color centers already present in natural gemstones, but they are not commonly found in nature due to the scarcity of natural gemstones. For example, natural blue topaz is rare. In contrast, the color of blue topaz produced through irradiation is stable against light, heat, and other factors, with a formation mechanism similar to that of natural blue topaz. Therefore, irradiated blue topaz has commercial value, and currently, no effective identification method has been found to distinguish between natural blue topaz and irradiated blue topaz, except for a small amount of radioactive residue; it has the same utility value as natural blue topaz.
(2) Strengthening existing color centers
Irradiation treatment can enhance the color centers formed in natural gemstones, making the colors of the gemstones more vibrant. For instance, natural quartz can produce green and purple colors after irradiation treatment. By controlling the dosage and duration of irradiation, the desired color can be achieved, which remains stable at room temperature and does not affect usage and wear.
(3) Restoring color centers that have faded due to heating and light exposure
Irradiation and heat treatment are reversible reactions; generally, the colors formed by irradiation can be restored to their pre-irradiation colors through heat treatment. Similarly, further irradiation can also yield the desired colors.
(4) Improve and remove colors unrelated to the color core
Generally, when gemstones undergo irradiation treatment, the color of the irradiated gemstones can be changed by controlling irradiation conditions such as dosage and time. The stability of the color after irradiation is an important factor affecting the value of the gemstone, and efforts are made to achieve a stable color core while eliminating unstable color cores in the gemstone.
(5) Forming natural color cores that have not been discovered before
As people’s understanding of the causes of gemstone colors deepens, the types of gemstones that can undergo irradiation treatment are continuously increasing, and the color variations of gemstones are becoming more diverse. It is believed that irradiation can produce color cores that natural gemstones do not possess, thereby creating new varieties and forming new mechanisms of gemstone color.
Currently, many types of gemstones are commonly used for irradiation treatment, with diamonds, sapphires, topaz, beryl, zircon, crystal, tourmaline, and pearls being relatively common. The color changes of these gemstones after irradiation treatment are shown in Table 4-3.
Table 4-3 Common Types of Irradiated Gemstones and Color Changes
| Types of Gemstones | Color Changes Before and After Irradiation |
|---|---|
| Timantti | Colorless, Light Yellow, Green, Blue or Black, Brown, Pink, Red |
| Sapphire | Colorless-Yellow (Unstable) |
| Beryl | Colorless-Yellow, Pink, Golden Yellow, Blue-Green, etc |
| Aquamarine | Blue - Green, Light Blue - Dark Blue |
| Topaz | Colorless - Brown (unstable), Blue; Yellow - Pink, Orange Red |
| Turmaliini | Colorless, Light Yellow, Brown, Pink, Red, Green, Blue, etc |
| Zirkoni | Colorless to brown, light red |
| Kristalli | Colorless to yellow, yellow-green, green, smoky, purple |
| Marble | White, yellow, blue, purple |
| Pearl | Colorless to gray, brown, blue or black |
6. The impact of irradiation treatment on gemstones
When irradiating gemstones, it is important to consider the effects of irradiation dose and time on them. Different irradiation sources should be used for different types of gemstones, and the irradiation time depends on the desired color. The following points should be noted during the irradiation process:
(1) Excessive irradiation energy and prolonged irradiation time can adversely affect the formation of color centers in the gemstone crystals. They may sometimes lead to vacancy aggregation, causing the gemstone to appear gray or black.
(2) The effect of irradiation is from the surface to the interior, with the gemstone’s color deepening gradually from the outside. When the irradiation energy is too high, the ions on the gemstone’s surface can absorb enough energy to detach from the surface, resulting in surface damage.
(3) When the irradiation energy is too high, it may cause localized high temperatures in the gemstone quickly, leading to surface chipping.
(4) The radioactive residues produced after gemstone irradiation treatment are related to the type of irradiation rays, irradiation dose, and the half-life of radioactive isotopes. Radioactive residues must meet national standards before being put on the market.
After irradiation, the residual radioactivity on the surface of the gemstone is related to the type of radiation exposure, the amount of irradiation, the types and content of impurities in the sample, and the half-life of radioactive elements. Irradiated gemstones must be placed for some time, and their residual radioactivity must be below national standards before being marketed. According to the “Radiation Protection Standards” established by the International Commission on Radiological Protection, the exemption value for the specific activity of natural radioactive materials is the same across countries. The specific activity of natural radioactive materials must be less than 350Bq/g per gram; the exemption limits for artificial radioactive materials vary, with the UK’s limit being less than 100Bq/g, while Japan, France, and Italy set their exemption limits for artificial radioactive materials at less than 74Bq/g. The standard set by the United States is the lowest, at 15Bq.
Section V High-Temperature and High-Pressure Treatment Method
The color optimization treatment of diamonds mainly includes irradiation treatment and high-temperature, high-pressure treatment. Since 1930, commercial treatment methods using high-energy radiation to improve the color of gem-quality diamonds have been used. Since the residual radiation of irradiated diamonds has potential harm to the human body, which limits consumers’ acceptance of irradiated gemstones, gemologists have been working to find a harmless and feasible diamond color treatment method. The high-temperature, high-pressure method was initially used for synthetic diamonds, and later, it was discovered that simulating the growth conditions and environment of diamonds could improve their color.
1. History of High-Temperature High-Pressure Color Modification
In nature, most diamonds are type Ia brown diamonds, and naturally occurring high-quality colorless and colored diamonds are rare. The rarity of diamonds, color, and brilliance have intensified the demand for high-quality diamonds. The modification of diamond color has always been a topic of research for gem researchers.
Since the 1960s, countries such as the United States, Japan, and Russia have successively conducted research on the high-temperature and high-pressure color modification of diamonds. General Electric was the first to propose a possible prediction of diamond color changes. Subsequently, Nikitin et al. (1969) used high-temperature and high-pressure treatment methods to transform type Ia light yellow diamonds into yellow and yellow-green diamonds.
General Electric and De Beers have published a series of global natural brown diamond color modification methods. However, most of these brown diamonds are of type IIa, and the instruments used are two-sided presses, resulting in treated diamonds that are mostly close to colorless with a slight gray tone. By the end of the 20th century, using a prismatic press, Nova Company successfully treated type Ia brown diamonds into yellow-green, green-yellow, blue-green, and pink-colored diamonds. In the 21st century, some scholars and businesses have applied high-temperature and high-pressure treatment methods to improve or alter the color of diamonds synthesized by chemical vapor deposition, mainly treating them into yellow and light brown tones. Gem companies in countries such as Russia and Sweden have also successfully adopted high-temperature and high-pressure methods to improve the color of diamonds.
The technology for high-temperature and high-pressure color modification of diamonds in our country started relatively late, with relevant research only beginning at the end of the 20th century. Our country has successfully conducted experimental research on high-temperature and high-pressure color modification of diamonds. The commonly used domestic equipment is a six-sided press, and the pressure conditions are still lower than those of advanced experimental conditions abroad; however, as long as the conditions are controlled properly, it is still possible to convert brown diamonds into colorless diamonds.
2. Main Types Improved by High Temperature and High Pressure
The high-temperature and high-pressure color modification method is similar to the conditions for synthetic diamonds; the pressure of the samples usually needs to reach 6GPa, the temperature is around 2100℃, and the duration is very short, not exceeding 30 minutes.
Two common types of diamonds have undergone color treatment in the market: brown diamonds of type IIa with low nitrogen content that are transformed into white diamonds, with color lightening after treatment, and can even be changed to color grades E, F, G, etc. These are usually marked with the inscription “GE-POL” on the diamond’s girdle using laser and are commonly referred to as GE-POL diamonds or GE-treated diamonds; the other type is Nova diamonds, which transform brown or impure yellowish-white diamonds of type Ia containing nitrogen into colored diamonds. The treated diamonds exhibit a distinct green component or vibrant yellow, mostly falling within the greenish-yellow to yellow-green spectrum, with a small number being yellow or brownish-yellow, often retaining octahedral growth patterns in brown to yellow. The conditions and main identification features of these two types of high-temperature and high-pressure treated diamonds can be found in section III (2) of diamond optimization treatment methods with website: https://sobling.jewelry/unveiling-single-crystal-gemstones-like-sapphire-beryl-and-diamond/
Since 2010, some large jewelry companies have begun conducting experimental research on color modification of sapphire gemstones using high-temperature and high-pressure methods. The pressure required for sapphire gemstones is relatively low compared to diamonds, generally around 100MPa, which can make the color of blue sapphires more vibrant. A German company was the first to use a low pressure of 2.5MPa to treat sapphire gemstones. Meanwhile, beryl can achieve more vibrant colors through low-temperature and low-pressure heating.