Cam ve Renkli Cam Süsleri Bu Kadar Eşsiz Kılan Nedir? Malzemeler, Bakım ve İşçilik
Cam ve Renkli Cam Süslemelerin Büyüsünü Ortaya Çıkarın: Zanaat, Bakım ve Yaratım
Cam, kristal berraklığında şeffaflığı, soğuk katılığı ve ışığı yansıtma özellikleriyle büyülü bir malzemedir. Bu özellikler sanatsal yaratımın etkilerini öngörülemez hale getirir. Dekoratif el sanatları için kullanılmasının yanı sıra, cam artık mücevher üretiminde de yaygın olarak kullanılmaktadır.
Renkli cam, kayıp balmumu dökümü kullanılarak yapay kristalden yapılan bir zanaat süslemesidir. Çin renkli cam sanatının tarihi Shang ve Zhou hanedanlarına kadar uzanmaktadır. Eski insanların inci ve değerli taşları taklit etmek için kristal berraklığında, nemli ve pürüzsüz olan zarif renkli cam sanat eserleri yarattığı söylenir. Günümüzde modern renkli cam tekniklerinin başarıyla uygulanması bu ürüne sadece yeni ve çağdaş bir tarz kazandırmakla kalmamıştır. Aynı zamanda Çin renkli cam el sanatı süslemelerinin uluslararası el sanatı süsleme arenasına girmesini de sağlamıştır.

renkli cam kakmalı yüzük
İçindekiler
Bölüm I Cam ve Renkli Cam Malzemelere Giriş
1. Cam
1.1 Camın Bileşimi
Cam genellikle aşırı soğutulmuş eriyikten elde edilen amorf bir nesne olan silikat camı anlamına gelir. Genellikle kırılgan ve şeffaftır ve katı mekanik özelliklere sahiptir. En yaygın türleri "soda-kireç camı", "kurşun-baryum camı" ve "potasyum camı" olup, hammadde olarak çoğunlukla kuvars kumu, soda külü, feldispat ve kireçtaşı kullanılır. Farklı formülasyonlar nedeniyle cam farklı özelliklere sahip olabilmekte, dolayısıyla farklı bölgesel ve zamansal özellikler gösterebilmektedir.
Sıradan cam esas olarak amorf silika dioksit (SiO2), kuvars veya kumun kimyasal bileşimi. Saf kuvars çok yüksek bir erime noktasına sahiptir, bu nedenle cam yapılırken genellikle iki malzeme eklenir: sodyum karbonat ve potasyum karbonat. Bu, silikanın erime noktasını yaklaşık 1000°C'ye düşürür. Bununla birlikte, sodyum karbonat camın suda çözünmesine neden olduğundan, camı suda çözünmez hale getirmek için genellikle uygun miktarda kalsiyum oksit eklenir.
Camın en büyük özelliği görünür ışığa karşı şeffaflığıdır. Sıradan cam 400 nm'den daha kısa dalga boyuna sahip ultraviyole ışığa karşı şeffaf değildir çünkü üretim sırasında sodyum karbonat eklenir. Ultraviyole ışığın nüfuz edebilmesi için camın daha pahalı olan ve genellikle kuvars cam olarak adlandırılan saf silikadan yapılması gerekir. Saf cam aynı zamanda kızılötesi ışığa karşı da şeffaftır ve bu sayede iletişim amacıyla birkaç kilometre uzunluğunda cam fiberler oluşturulabilir.
Sıradan camlar genellikle başka bileşenler de içerir. Örneğin, çok parlak ve göz kamaştırıcı görünen kristal cam, kırılma indisini artırmak için kurşun eklenerek yapılır, bu da daha göz kamaştırıcı kırılma ile sonuçlanır. Borosilikat cama termal ve elektriksel özelliklerini değiştirmek için bor eklenir. Baryum da kırılma indisini artırabilir. Optik lens yapımında kullanılan cama, kırılma indisini önemli ölçüde artırmak için toryum oksit eklenir. Camın kızılötesi ışınları emmesi gerekiyorsa demir eklenebilir; bu tür ısı yalıtımlı camlar projektörlerde bulunur. Cama seryum eklenmesi ultraviyole ışınlarını emecektir.
Cama çeşitli metaller ve metal oksitler eklemek de rengini değiştirebilir. Örneğin, az miktarda manganez camda demirden kaynaklanan açık yeşili değiştirebilir ve manganez miktarı belirli bir seviyeye ulaştığında açık mor bir renk oluşturabilir. Selenyum da benzer bir etkiye sahiptir. Az miktarda kobalt mavi oluşturabilir. Kalay oksitler ve arsenik oksitler, beyaz seramiklere benzer şekilde opak beyaz oluşturabilir. Bakır oksitler turkuaz rengi oluştururken, metalik bakır yakuta benzeyen opak koyu kırmızı bir renk oluşturacaktır. Nikel mavi, koyu mor ve hatta siyah renk oluşturabilir. Titanyum kahverengimsi sarı bir renk oluşturabilir. Eser miktarda altın (yaklaşık 0,001%) camın yakut benzeri bir renge sahip olmasına neden olur. Uranyum (0,1%~2%) camın ateş böceği sarısı veya yeşil olmasına neden olur. Gümüş bileşikleri turuncu ila sarı cam üretebilir. Camın sıcaklığının değiştirilmesi de bu bileşikler tarafından üretilen renkleri değiştirecektir, ancak altta yatan kimyasal ilkeler oldukça karmaşıktır ve henüz tam olarak açıklığa kavuşturulmamıştır.
1.2 Cam Malzemenin Özellikleri
Cam, yaklaşık 2,46~2,5 g/cm yoğunluğa sahip metalik olmayan inorganik bir malzemedir.3doğrusal genleşme katsayısı 9×10-6~10×10-6/℃ (~350℃) ve yaklaşık 50MPa yüzey gerilme mukavemetine sahiptir. Yapısal olarak, 650 ℃ üzerindeki sıcaklıklarda şekillendirilebilen ve şeffaflık, korozyon direnci, aşınma direnci ve soğuduktan sonra basınç dayanımı gibi özelliklere sahip inorganik termoplastik bir polimerdir. Camın termal genleşme katsayısı çelikten daha düşüktür ve kırılgan amorf bir madde olarak zayıf bir elektrik ve ısı iletkenidir.
Hammaddelerin ısıtılıp eritilmesi ve ardından soğutulup katılaştırılmasıyla elde edilen kristal olmayan inorganik bir malzeme olan cam, aşağıdaki temel özelliklere sahiptir.
(1) Güç. Camın mukavemeti, kimyasal bileşimine, safsızlıkların içeriğine ve dağılımına, ürünün şekline, yüzey durumuna, özelliklerine, işleme yöntemlerine vb. bağlıdır. Cam kırılgan bir malzemedir ve mukavemeti genellikle basınç ve çekme mukavemeti olarak ifade edilir. Camın çekme mukavemeti, camın kırılganlığı ve cam yüzeyindeki mikro çatlaklar nedeniyle nispeten düşüktür. Camın basınç dayanımı, çekme dayanımının yaklaşık 14~15 katıdır.
(2) Sertlik. Cam, 5~7 Mohs sertliği ile elmas ve silisyum karbür gibi malzemelerden sonra ikinci sırada yer alan yüksek bir sertliğe sahiptir. Sıradan metallerden daha serttir ve normal bıçak ve testerelerle kesilemez. Camın sertliğine bağlı olarak aşındırıcılar, aletler ve gravür, parlatma, taşlama ve kesme gibi işleme yöntemleri seçilebilir.
(3) Optik özellikler. Cam, ultraviyole ve kızılötesi ışığın emilmesi veya iletilmesi, ışığa duyarlılık, fotokromizm, ışık depolama ve görüntüleme gibi önemli optik performanslar sergileyen, belirli optik sabitlere ve spektral özelliklere sahip oldukça şeffaf bir malzemedir. Kristal berraklığındadır ve formda sonsuz çeşitlilik ve güçlü ifade gücü ile gövde renklendirmesi, yüzey renklendirmesi ve yüzey işlemine tabi tutulabilir. Genel olarak, içinden ne kadar çok ışık geçerse, cam kalitesi o kadar iyi olur. Cam türlerinin çeşitliliği nedeniyle, farklı camların özellikleri önemli ölçüde değişebilir; örneğin, bazı kurşun camlar radyasyondan korunma özelliklerine sahiptir. Tipik olarak, camın bileşimi ve işleme koşulları değiştirilerek, özellikleri büyük ölçüde değişebilir.
(4) Elektriksel özellikler. Oda sıcaklığında cam zayıf bir elektrik iletkenidir. Sıcaklık yükseldikçe camın elektrik iletkenliği hızla artar ve erimiş haldeyken iyi bir iletken haline gelir.
(5) Termal özellikler. Camın ısı iletkenliği zayıftır ve hızlı sıcaklık değişimlerine dayanamaz. Ürün ne kadar kalın olursa, hızlı sıcaklık değiĢimlerine dayanma kabiliyeti de o kadar kötü olur. Sıcaklık yükseldikçe cam kademeli olarak sert katı halden yumuşak katı hale, jel haline ve sıvı hale geçebilir. Maddenin her halinin kendine has özellikleri vardır ve farklı sıcaklık aralıklarında camı şekillendirmek, değiştirmek ve renklendirmek için en etkili yöntemlere izin vererek büyük bir yaratıcılık alanı ve sonsuz olanaklar sağlar.
(6) Kimyasal stabilite. Camın kimyasal özellikleri nispeten kararlıdır. Endüstriyel camların çoğu hidroflorik asit dıĢındaki asitlerin korozyonuna karĢı dayanıklıdır. Camın alkalin korozyonuna karşı direnci zayıftır. Zamanla cam yüzey parlaklığını kaybedebilir ve hatta atmosferin ve yağmur suyunun aşındırması nedeniyle lekeler veya sisli bir film oluşturabilir, matlaşabilir ve şeffaflığını kaybedebilir.
(7) Çevresel performans. Cam toksik değildir ve zararsızdır. İnsan vücuduna ve çevreye zararlı maddeler salmaz ve kirliliğe neden olmaz. "Yeşil" çevre dostu bir malzemedir.
1.3 Cam Kristal (Kristal Elmas, Rhinestone)
Cam kristali, endüstride çeşitli kimyasal hammaddelerin eritilerek yüksek kurşunlu kristal cam yuvarlak şekillere dönüştürülmesiyle yapılan ve daha sonra taşlanıp parlatılan bir tür taklit elmas ürününe atıfta bulunan konuşma dilinde kullanılan bir terimdir. Cam kristalin ana bileşeni kristal camdır. Bu malzemenin nispeten ekonomik ve görsel olarak çarpıcı olması, elmasa benzemesi onu çok popüler ve günümüzde mücevherat için en yaygın kullanılan hammaddelerden biri haline getirmektedir.
Kurşun kristal cam aşağıdaki bileşime sahiptir: RmOn-PbO-SiO2(B2O3). Formülde, SiO2(B2O3), silisyum oksit (bor oksit) ağ oluşturucu olarak adlandırılır ve cam ağ yapısını oluşturan temel birimdir. RmOn, özelliklerini ayarlamak için cam ağ yapısını değiştiren alkali, toprak alkali ve nadir toprak metallerinin metal oksitlerini temsil eder. PbO (kurşun oksit) cama temel özelliklerini kazandıran karakteristik bir bileşendir. Pb2+ PbO içinde yapısal birimler oluşturabilir [PbO4] sarmal bir zincir yapısı oluşturan tetragonal piramitlerin [SiO4] cam çerçeveyi tepe açılarında veya ortak kenarlarda birbirine bağlayarak özel bir ağ oluşturur. Bu ağ çok geniş bir cam oluşum aralığına sahiptir ve PbO (mol kesri) 80%'ye ulaştığında bile camın oluşmasına izin verir. PbO içeriği arttıkça, X-ışınları ve γ-ışınları için yoğunluk, kırılma indisi, dağılım, dielektrik sabiti ve soğurma katsayıları gibi performans göstergelerinin değerleri artarken sertlik, yüksek sıcaklık viskozitesi, yumuşama sıcaklığı ve kimyasal kararlılık azalır, bu da parlak renkler, artan yüzey parlaklığı, vurulduğunda keskin bir ses ve oyma ve kimyasal cilalama kolaylığı ile sonuçlanır. İngiltere, 17. yüzyılın ikinci yarısında kurşun kristal cam sanat kapları üretmiştir. Kurşun kristal camın yüksek yoğunluğu ve yüksek kırılma indisi gibi mükemmel fiziksel özellikleri nedeniyle, cam süs eşyaları için tercih edilen malzeme haline geldi. Uzun süre yeri doldurulamaz olarak kabul edildi.
Ren Nehri'nin her iki yakasında sentetik kristal camın küresel olarak üretilmesi nedeniyle cam kristallere Rhinestones da denmektedir. Kuzey yakasında üretilenler "Avusturya kristalleri" olarak adlandırılır ve "Avusturya" olarak kısaltılır. Avusturya kristalleri 30'dan fazla kesme yüzeyine sahip olabilir, bu da iyi kırılma etkileri ve kırılan ışıkta derinlik hissi sağlar; ayrıca yüksek sertlikleri ve uzun süreli parlaklıkları nedeniyle cam kristaller arasında en iyisidir, özellikle de dünya çapında ünlü olan "Swarovski" cam kristalleri. Sentetik kristal ürünlerle eşanlamlıdırlar ve bir kültür sembolüdürler. Şu anda Swarovski'nin dünya çapında birçok fabrikası vardır, bu nedenle Swarovski sadece belirli bir kaliteyi temsil eder ve mutlaka Avusturya'da üretilmesi gerekmez. Güney bölgesinde üretilenlere "Çek kristalleri" denir ve bunlar genellikle bir düzine kadar fasete, iyi kırılma etkilerine sahiptir ve daha göz kamaştırıcı bir ışık yayabilir. Sertlikleri nispeten güçlüdür ve parlaklıkları Avusturya kristallerinden sonra ikinci olarak yaklaşık 3 yıl sürer. Orta Doğu ve yurt içinde üretilen cam kristaller, üreticilerin düşük maliyetli üretimle pazara hitap etmek için yaptıkları bazı ürünlerdir ve kaliteleri Çek cam kristallerinden daha düşüktür. Genel cam kristallerin 8 faseti vardır ve cam kristalin arkası bir cıva tabakası ile kaplanmıştır. Işığın fasetler boyunca yoğunlaşması ona daha iyi parlaklık verir; ne kadar çok faset varsa parlaklık o kadar iyi olur.
Cam kristaller renklerine göre beyaz cam kristaller, renkli cam kristaller (pembe, kırmızı, mavi, vb.), fantezi cam kristaller ve fantezi AB cam kristaller (kırmızı AB, mavi AB, vb.) olarak ayrılabilir. Cam kristal, tabanın şekline göre sivri tabanlı cam kristaller ve düz tabanlı cam kristaller olmak üzere iki ana kategoriye ayrılabilir. Tablonun şekline göre cam kristaller iki ana kategoriye ayrılabilir: normal cam kristaller ve süslü şekilli cam kristaller. Fantezi şekilli cam kristallerin şekilleri, diğerlerinin yanı sıra elmas şekilli cam kristaller (markiz), trapez cam kristaller, uydu taşları, gözyaşı damlası şekilli cam kristaller, oval cam kristaller ve sekizgen cam kristaller olarak ayrılabilir. Malzemeye bağlı olarak cam kristaller cam, sentetik spinel, sentetik safir, kübik zirkonya vb. olarak ayrılabilir.
Bazı cam kristaller şeffaf kusursuzlukları ve göz kamaştırıcı özellikleriyle görsel olarak elmasa benzerler. Bazen elmasları taklit etmek için kullanılırlar ve insanları gerçek olduklarına inandırırlar. Camdan yapılmış sahte elmasları ayırt etmek kolaydır çünkü düşük bir kırılma indeksine sahiptirler ve biraz tecrübesi olan birinin kolayca tespit edebileceği gerçek elmasların ışıltılı renkli ışığından yoksundurlar. Bu "cam kristaller" genellikle nispeten ucuz mücevherlerde kullanılır. Sentetik kübik zirkonya ortaya çıkmadan önce, zirkon genellikle elmas yerine kullanılırdı. Zirkonun güçlü çift kırılma özelliği vardır, yani iki kırılma indisi vardır ve aralarındaki fark oldukça büyüktür. Bu da çok özel bir optik olguya yol açar; cilalı bir zirkon yüzlü mücevheri büyüteçle incelerken, alt yüzlerin ve kenarların üst yüzeyden farklı çift görüntüleri görülebilir. Buna karşılık elmaslar "homojen cisimlerdir" ve çift görüntü fenomeni sergilemezler. Kübik zirkonya veya "Sovyet elması", doğal mineral karşılığı olmayan sentetik bir bileşiktir. Kübik zirkonya, kırılma indisi ve dağılım açısından doğal elmasa çok yakındır. Bununla birlikte, daha düşük bir sertliğe, elmasın 1,6 ~ 1,7 katı olan bir bağıl yoğunluğa sahiptir ve termal iletkenliği elmasınkinden çok daha düşüktür, bu da cihazların onu elmastan doğru bir şekilde ayırt etmesini sağlar.
1.4 Kurşunsuz Cam
Teknolojinin sürekli ilerlemesi ve çevre koruma bilincinin artmasıyla birlikte, kurşunun insanlar üzerindeki toksisitesi ve çevreyi kirletmesi her taraftan giderek daha fazla dikkat çekmektedir. Kurşun camın eritilmesi sırasında büyük miktarda kurşun buharlaşarak havaya karışır; kullanım sırasında da camın içerdiği kurşun yavaş yavaş dışarı sızar. Özellikle atıldıktan sonra, su ve asidik maddelerle uzun süreli temas nedeniyle kurşun yeraltı sularına sızabilir. Tüm bunlar insan sağlığına önemli ölçüde zarar verir.
For the above reasons, many countries have imposed restrictions on lead in jewelry, prompting nations to accelerate the development of lead-free glass. According to EU and UK standards, lead crystal glass is divided into two types: lead crystal glass, containing PbO≥24% with a refractive index of no less than 1.545, and full lead crystal glass, containing PbO≥30% with a refractive index of no less than 1.560. When developing lead-free crystal glass, high refractive index oxides TiO2, ZrO2, BaO, SrO and ZnO are usually introduced to achieve a refractive index close to lead glass. However, considering the transparency, whiteness, and ease of long-term shaping and processing required for crystal glass, among these high refractive index oxides, although the refractive index of TiO2 is higher than that of PbO, it can easily cause the presence of Fe2O3 in the glass to form sodium iron coloration; and ZrO2 is difficult to melt, so the most commonly used is still crystal glass made of BaO and ZnO, as well as introducing BaO, SrO, ZnO and ZrO2 simultaneously and minimize the amount of ZrO2 and TiO2 under the required refractive index conditions to avoid adverse effects on melting and the color of the glass.
The compositions of several types of lead-free crystalline glass developed internationally are shown in Table 8-1, and their properties are shown in Table 8-2.
Table 8-1 Composition of Lead-Free Crystalline Glass
| Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Crystalline glass type | Potassium crystalline | Barium crystalline | Zirconium crystalline | Mixed crystalline | Mixed crystalline | Mixed crystalline | Mixed crystalline |
| SiO2 | 77.0 | 58.0 | 64.0 | 59.5 | 55.7 | 57.2 | 54.2 |
| A12O3 | 1.0 | ||||||
| CaO | 5.0 | 9. 5 | |||||
| SrO | 10. 5 | 10. 5 | 10. 5 | 12.0 | |||
| BaO | 18.0 | 10. 5 | 10. 5 | 10. 5 | 12.0 | ||
| ZnO | 5.0 | 4.7 | 7.5 | 7.5 | 7.5 | 7.5 | |
| K2O | 9.0 | 16.0 | 3.0 | 5.4 | 6.4 | 5.4 | 5.4 |
| Na2O | 9.0 | 3.0 | 12.6 | 3.6 | 4.2 | 3.6 | 3.6 |
| Li2O | 1.7 | 1.7 | 1.7 | 1.7 | |||
| ZrO2 | 6.2 | 3.0 | 1.0 | ||||
| TiO2 | 3.0 | 2.0 | |||||
| Sb2O3 | 0.3 | 0.1 | 0. 3 | 0.4 | 0.4 | ||
| As2O3 | 0.1 | 0.3 | |||||
| (Wang Chengyu et al., 2006) | |||||||
Table 8-2 Performance of Lead-Free Crystalline Glass
| Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Density/(g · cm-3) | 2. 898 | 2. 977 | 2. 914 | 3.046 | |||
| Kırılma indisi | 1.510 | 1.534 | 1. 549 | 1.545 | 1. 566 | 1.571 | 1. 575 |
| Linear expansion coefficient/(10-7·℃-1) | 87.2 | 91.4 | 87.4 | ||||
| Softening point/℃ | 674 | 674 | 675 | 673 | |||
| (Wang Chengyu et al., 2006) | |||||||
2. Colored Glass
2.1 The Origin of Colored Glass
2.2 Types of Colored Glass
The types of colored glass commonly referred to generally fall into three categories.
(1) Tradition Chinese colored glass. It is made by adding “colored glass stone” to “colored glass mother” and firing. Tradition Chinese colored glass has a murkiness, looks like jade but not jade, lacks transparency, and fundamentally differs from modern colored glass. The shapes are mostly colored glass beads, imitation jade discs, tablets, and the like. Ancient colored glass art experienced a brief prosperity during the Tang and Qing dynasties. Due to the fragility of such artworks during this period, very few artifacts survived, and the craftsmanship techniques have not been able to form a significant legacy for future generations. Therefore, there are still many mysteries regarding ancient Chinese colored glass craftsmanship.
(2) Modern colored glass. Typically represented by Taiwanese colored glass, modern colored glass evolved from Western colored glass art and originated from the ancient Egyptian “faience” craft. The analysis results from “Research on Ancient Chinese Glass” indicate that the proportion of silica in “faience” significantly is 92%~99%, which differs from the colored glass of the Zhou Dynasty in China. However, due to their similar forms, some refer to it as Western colored glass. Modern colored glass is more transparent and has diverse shapes than traditional Chinese colored glass.
(3) Water colored glass. Many low-priced “water colored glass” products have appeared on the market in recent years. In fact, this is a type of “imitation colored glass” product, not real colored glass. The term “water colored glass” arises from the deliberate actions of merchants and the misunderstandings of consumers. Water colored glass is a resin product made from transparent resin glue mixed with pigments. Its characteristics include being lightweight, lacking the metallic sound of real colored glass when struck, and easily changing color and becoming cloudy over time, with no collectible value. However, it has low cost, low technical content, simple craftsmanship, and is easy to mass produce.
2.3 The Properties of Colored Glass
Colored glass is a valuable craft artwork, and its price is higher than that of crystal for two reasons: first, the materials for traditional Chinese colored glass are special, and the production process is quite complex, involving dozens of steps to complete, with some pieces taking 10~20 days just for the production process, and mainly relying on manual labor. Each stage is quite difficult to control, and the difficulty of mastering the heat can rely half on skill and half on luck. Second, glass is not just a material but also a cultural product. More importantly, colored glass products are unique, and no two are identical.
(1) The difference between colored glass and crystal. First, there is a clear distinction in historical texts, as noted in the “Diamond Sutra.” Among all Buddhist scriptures in China, the first five categories of the seven treasures of Buddhism are universally recognized: gold, silver, colored glass, conch shell, and agate. The last two categories are said to be crystal, amber, or glass, among others, indicating that colored glass is recognized as a Buddhist treasure; colored glass is distinctly different from crystal and glass. Second, the chemical composition is different. The main component of natural crystal, colored glass, and glass is silicon dioxide. The contemporary national-level authoritative work “Research on Ancient Chinese Glass” records that the proportion of silicon dioxide in ancient Egyptian “faience” (the ancestor of Western crystal glass) is 92%(not transparent)~99%. In contrast, the colored glass from China’s Zhou Dynasty has a proportion of silicon dioxide that is just slightly greater than 90%(transparent). This distinction of 9% is the biggest difference between colored glass and crystal. The ancient colored glass was made by adding colored glass stone to a colored glass mother for firing. Colored glass stone is a colored crystal material, primarily composed of silicon dioxide. In contrast, colored glass mother is a traditional formula derived from natural sources and refined by human hands, which can alter crystal structure and physical properties, resulting in significant differences in shape, color, and transparency. The colored glass grade depends on the colored glass mother’s raw materials and preparation methods, which has been a closely guarded secret since ancient times. It is precisely because of the existence of the colored glass mother that ancient Chinese colored glass 9% differs in composition from crystal and even Western crystal glass, which is called “faience.”
(2) The differences between water colored glass and colored glass mainly lie in the following three aspects: first, the refractive index of resin is low, resulting in different product textures; water colored glass lacks the luster of lead crystal glass; second, the weight difference, the weight of water colored glass is about 30% of that of colored glass; third, colored glass is prone to aging, and its color is unstable.
For the water colored glass products that have appeared on the market, it is important to grasp their distinguishing features from colored glass, which is briefly introduced in Table 8-3.
Table 8-3 Identification Features of Water Colored Glass and Traditional Chinese Colored Glass
| Identify features | Water colored glass | Traditional Chinese colored glass |
|---|---|---|
| Color and luster | Obvious chemical dyes, the same as plastic products | Mixed in various colors, yet still transparent as before |
| Yoğunluk | Equivalent to plastic, much lighter than real colored glass | The density of traditional Chinese colored glass is significantly higher than that of glass and slightly higher than that of crystal, and it has a smooth feel |
| Sound | The same as plastic products | Gently tapping the traditional Chinese colored glass will produce a metallic sound |
| Transport agency | Obvious turbidity, not transparent | Between glass and crystal, there are occasional small bubbles produced during the firing and flowing process |
| Storage time | Fades within one to two years, the transparency decreases over time, and the longer it lasts, the more it resembles plastic. | Indefinitely, from the perspective of materials, the traditional Chinese colored glass never changes color |
Section II Glass and Colored Glass Ornaments
1. Glass Ornaments
There are many kinds of glass ornaments.
(1) Glass ring face.
(2) Glass bracelet.
(3) Glass ring.
(4) Glass earrings.
(5) Glass pendant.
(6) Glass bracelet.
(7) Glass necklace.
(8) Glass crystals.
The typical examples of the above glass ornaments are as follows.

Round faceted stone made of glass

Glass bracelet


Glass earrings

Glass bracelet

Glass Pendant

Glass necklace

2. Colored Glass Ornaments
Colored glass ornaments are colorful, with an ancient style, reasonable structure, and rich traditional national characteristics of our country. They possess the beauty of imitating pearls and jade, infused with creativity and ingenuity. Some have praised glazed items with such flattering words: ethereal and noble, subtle yet profound, capable of absorbing brilliance, crystal clear, able to dazzle the world yet can be instantly destroyed, can transform into myriad forms yet remain eternally tranquil. In Buddhism, colored glass ornaments are spiritual objects for warding off illness and evil. Placing or wearing glazed ornaments can bring three blessings: removing illness, resilience, and inspiration.
Colored glass integrates the advantages of several art forms, such as carving and painting, gradually becoming a popular, fashionable accessory material. There are many categories of colored glass accessories, with the following seven being common: ① colored glass rings; ② colored glass pendants; ③ colored glass necklaces; ④ colored glass bracelets; ⑤ colored glass bangles; ⑥ colored glass earrings; ⑦ colored glass ornaments.
The typical examples of the above glass ornaments are as follows.


colored glass bracelet

colored glass bangles

colored glass earrings

colored glass ornaments
3. Maintenance of Colored Glass Ornaments
When wearing and maintaining colored glass ornaments, attention should be paid to the following matters due to the characteristics of the material:
(1) Do not collide or rub during movement to avoid surface scratches.
(2) Keep it at room temperature; the real-time temperature difference should not be too large. Do not heat or cool it by yourself.
(3) It is not advisable to place the cushion directly on the table on smooth surfaces; it is best to use a cushion.
(4) It is advisable to use purified water for wiping. If tap water is used, it should be left to stand for more than 12 hours to maintain the luster and cleanliness of the colored glass surface. It should not come into contact with oil stains or foreign objects.
(5) Avoid contact with sulfur gas, chlorine gas, etc.
Section III Glass and Colored Glass Ornament Production Process
1. The Production Process of Glass Craft Ornaments
1.1 The Production Process of Glass Craft Ornaments
The production process of making glass craft ornaments mainly includes materials preparation, melting, forming, annealing, and decorative treatment, as described below.
(1) Materials Preparation
According to the designed formula, weigh various raw materials and mix them evenly in the mixer. The main raw materials for glass are quartz sand, limestone, feldspar, soda ash, and boric acid.
(2) Melting
The prepared raw materials are heated at high temperatures to form a uniform, bubble-free glass melt. The melting of glass takes place in a melting furnace. There are two main types of melting furnaces. The first is the crucible furnace, where the glass batch is placed in a crucible and heated from the outside. Small crucible furnaces hold only one crucible, while larger ones accommodate up to 20. Crucible furnaces are used for batch production, and currently, only optical and colored glass are produced using crucible furnaces. The other type is the tank furnace, where the glass batch is melted in the furnace tank, with the flame heating the upper surface of the glass melt. Glass melting is a complex physical and chemical reaction process, which can be roughly divided into the following five stages.
① Silicate formation stage. The silicate generation reaction largely occurs in the solid state. The various components of the raw powder undergo a series of physical and chemical changes, and during the solid-phase reaction, a large amount of gaseous substances is released. At the end of this stage, the mixture transforms into an opaque sintered material composed of silicates and silica. For most glasses, this stage is completed at 800~900℃.
② Glass formation stage. Continue heating; the sintered material and the low melting point mixture begin to melt first. During the process, silicates and the remaining silicon dioxide melt into each other, and the sintered material becomes a transparent body. There are no unreacted raw materials, but many bubbles and streaks still exist in the glass, and the chemical composition and properties are very uneven. The temperature during the glass formation stage is approximately between 1200~1250℃.
③ Clarification stage. As the temperature rises, the viscosity gradually decreases, and visible bubbles in the glass liquid slowly escape into the furnace gas, known as the clarification process. The temperature during the clarification stage is 1400~1500℃, and the glass’s viscosity is maintained at around 100 P.
④ Homogenization stage. The components of the glass melt, which has been at high temperatures for a long time, gradually become consistent due to molecular thermal motion and mutual diffusion, and the stripes disappear. Homogenization is the stage in which the chemical composition and refractive index of the glass melt tend to be consistent. The temperature of the homogenization stage is slightly lower than that of the clarification stage.
⑤ Cooling stage. After the above four stages, the quality of the glass has reached the requirements. The glass liquid is then cooled to reduce 200~300℃, and the viscosity increases to the value required for feeding to the feeder (103P). The temperature after cooling is approximately 1200℃.
(3) Forming
The molten glass is transformed into solid products with fixed shapes. The forming process must occur within a certain temperature range, a cooling process where the glass continuously transfers heat to the surrounding medium. Due to cooling and hardening, the glass transitions from a viscous liquid to a plastic state to a brittle solid. The viscosity and its variation with temperature, surface tension, plasticity, elasticity, and other rheological properties of glass and their changes with temperature are all crucial during the forming process. The forming methods can be divided into two main categories: manual and mechanical.
① Manual molding. Manual molding uses the thermal processing method, which is the method of making glass between the melting point (1450℃) and the annealing point (450℃). The six commonly used methods are as follows:
- a. Blowing. Originating in the year 1 AD in the Roman Empire, it remains the most important, widely used, and varied glassmaking method today. During blowing, a blowpipe is dipped into molten glass paste, and the air is blown to form a small bubble, which is then shaped using tools through heat (see Figure 8-1). A second blowpipe takes a small amount of glass to create a bridge for the base, and the work is struck off to cool slowly. It primarily shapes glass bubbles, bottles, spheres (for making lenses), etc.
- b. Pulling. After blowing into small bubbles, stick with the top plate and pull while blowing (Figure 8-2).
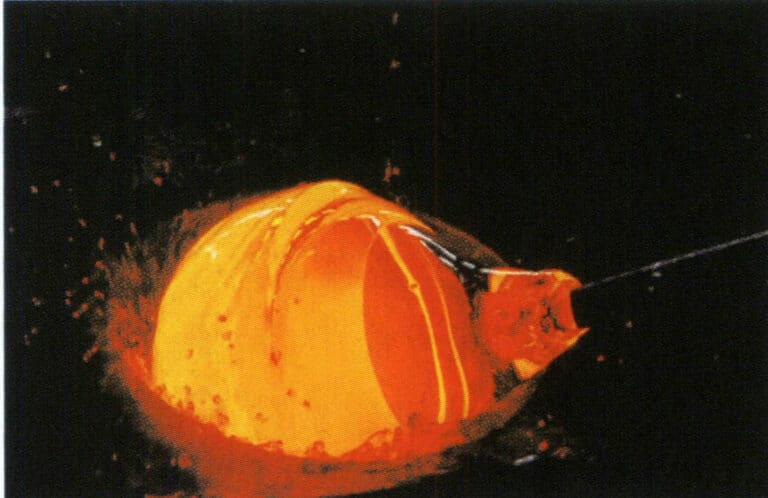
Figure 8-1 Glass Blowing

Figure 8-2 Drawing glass crafts
- c. Pressing. Take a lump of molten hot glass paste, inject it into the cavity of a mold that has already been engraved with patterns, and then press it with a punch. While it takes shape, the patterns are also pressed, mainly used to form cups, plates, etc. (Figure 8-3).
- d. Free forming. After selecting the material, use tools such as pliers, scissors, and tweezers, and heat with a small spray gun or torch, also known as torch thermoplastics. Various colored borosilicate or soda-lime glass rods can stretch, twist, and wind, continuously combining to create shapes suitable for delicate and intricate expressions, such as glass beads, animals, plants, and various shapes (Figure 8-4). Additionally, the glass rods used can be categorized as solid, hollow, and drawn thermoplastics and paired with painting to enhance the interest of the work.

Figure 8-3 Suppressed Glass Plate

Figure 8-4 Free-form glass crafts
- e. Lost-wax casting. After covering the wax mold with refractory gypsum, the raw glass materials, and the empty mold are placed in the furnace for heating. The glass slowly flows into the mold at high temperatures to take shape. It is then placed in the furnace to remove the wax and allowed to cool gradually, and the gypsum mold is removed before grinding and polishing to complete the process (Figure 8-5).
- f. Powder casting. The glass blocks and powder are placed into a pre-designed mold and put into a furnace to be heated and melted into a complete glass piece.

② Mechanical forming. Manual forming has high labor intensity, temperatures, and poor conditions, so except for lamp torch thermoplastics, some craft ornaments have been replaced by mechanical forming in addition to free-forming (Figure 8-6). Mechanical forming includes pressing, blowing, drawing, rolling, casting, centrifugal casting, and sintering.
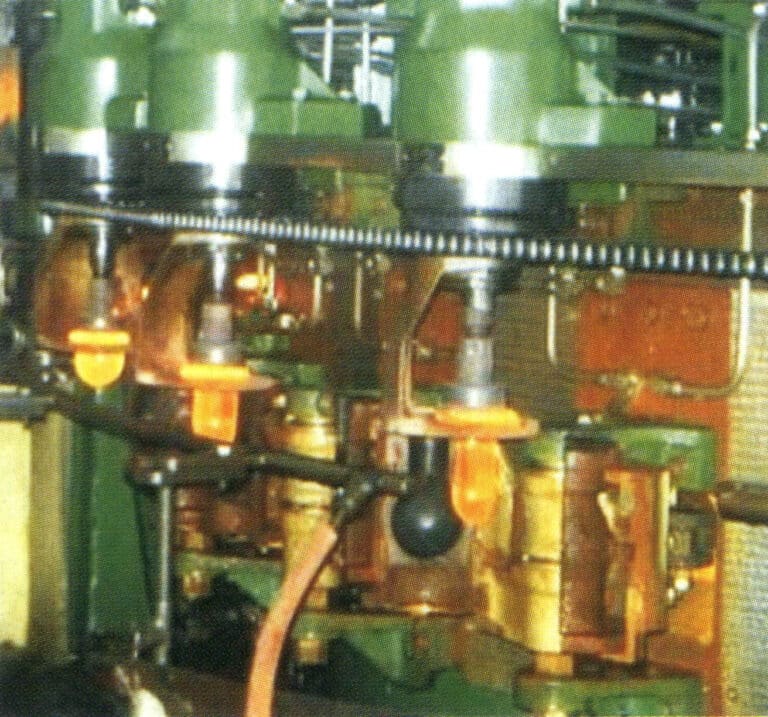
(4) Annealing
During the forming process, glass undergoes intense temperature and shape changes, which leave thermal stress in the glass. This thermal stress can reduce the strength and thermal stability of glass products. Cooled directly, it is likely to self-fracture during the cooling process or later storage, transportation, and use (commonly known as glass cold breakage). Glass products must be annealed after forming to eliminate the cold breakage phenomenon. Annealing is maintaining a certain temperature range or slowly cooling for a period to eliminate or reduce the thermal stress in the glass to an acceptable level.
In addition, certain glass products can undergo tempering treatment to increase their strength, including physical tempering (quenching) and chemical tempering (ion exchange). The principle of tempering is to create compressive stress on the surface layer of the glass to enhance its strength.

Copywrite @ Sobling.Jewelry - Özel takı üreticisi, OEM ve ODM takı fabrikası
(5) Decorative Treatment
Craft ornaments emphasize surface effects, so they generally undergo surface decoration treatment after forming. There are various methods for this treatment, including the following.
① Painting. Using colored paint, drawings are made on the surface of glass ornaments at room temperature (Figure 8-8). Some need to be heated to fix, while others do not. During this process, gold leaf and silver leaf melted into metallic paint can also be added, referred to as gilded painting.

② Glaze coloring. This is a glazing technique that requires additional heating. Patterns are drawn on the surface of glass ornaments with glaze color pigments and then placed in a furnace for heating to fix the pigments and prevent peeling.
③ Setting. This technique uses lead strips with grooves as a frame to assemble thousands of pieces of colored glass panels. It requires drawing small floor plans, creating sketches of the same size based on the floor plans, determining the shape and size of each color, correctly cutting the glass panels, and welding them into large surface mirrors with lead wire.
④ Printmaking. Using sandblasting or engraving techniques to engrave the image on a glass plate, making a model, coloring with a printing press or roller, and pressing it onto cotton or watercolor paper to create a print.
⑤ Relief engraving. Glass ornaments are embossed with a three-dimensional pattern of double or multiple layers of colored materials, revealing the background color and creating a relief effect (Figure 8-9).

⑥ Cutting. Use a cutting wheel to cut patterns, surfaces, lines, and other decorations on glass ornaments or to shape large surfaces. Sometimes, two-color layered glass is used to achieve a special effect that shows different colors inside and outside.
⑦ Engraving. A technique of decorating the surface of glass ornaments with lines, patterns, and designs using engraving tools such as diamonds, metal carving tools, or engraving pens (Figures 8-10). Depending on the tools used, it can be divided into several techniques, such as wheel engraving, dot engraving, and flat engraving.

⑧ Acid etching. Patterns are drawn on glass ornaments, lines are outlined, and staged corrosion with chemical acids creates designs with varying depths (Figure 8-11).
⑨ Sandblasting. First, cover the entire glass ornament with tape, then use a carving knife to etch away the unwanted parts of the pattern. Place the ornament in the sandblasting machine and use the high jet force of the diamond sand to create a sanded surface effect on the glass (Figures 8-12).

Figure 8-11 Glass Etching Pattern

Figure 8-12 Crystal glass sandblasted surface
⑩ Grinding. Use a rotating disc as the grinding table, mix water with diamond powder, and polish glass works.
⑪ Polishing. Use a rotating leather wheel as a platform to place the glass ornaments on it and polish large flat surfaces.
⑫ Gluing. Place the ornament in the furnace to heat, using the properties of glass to heat and melt the surface to produce brightness.
⑬ Composite materials. Creating works by combining glass with other materials.
1.2 The Production Process of Glass Crystals
Glass crystals are widely used in popular jewelry; they belong to high-lead crystalline glass, but the processing method has particularities.
(1) Materials preparation. The raw materials must undergo strict quality screening and be mixed according to the formula to produce high-quality glass crystals blanks. Glass crystals generally use crystalline glass materials containing at least 25% lead or more to achieve a high refractive index.
(2) Melting material. The furnace lining should be made of high-quality zirconia refractory materials, which will reduce the contamination of the lining material on the glass crystal. The melting material should be used either in flame heating or electric furnaces.
(3) Bad material forming. The glass liquid is poured and formed using specialized machines and molds to obtain glass crystal blank material (Figure 8-13).

(4) Glass crystal grinding. The basic process of grinding crystal includes fixture preheating→loading blank material→ bevel grinding→bevel polishing→docking feeding→bevel grinding→bevel polishing→flat grinding→flat polishing→unloading.
(5) Cleaning. Wash the polished glass crystal to remove dirt.
(6) Inspection. Place the cleaned glass crystal under the light for inspection to obtain qualified finished products.
(7) Screening. Sort the glass crystal by size and model.
(8) Chemical plating. A layer of silver is plated on the bottom of the glass crystal to increase its reflectivity, that is, refractive brightness.
(9) Protective film. A layer of protective film is sprayed on top of the already coated silver film to prevent the silver layer from discoloring when exposed to the atmosphere, maintaining the brightness and longevity of the rhinestones.
(10) Paketleme. Avoid scratches during inventory transportation.
2. The Production Process of Colored Glass Ornaments
2.1 The Lost-Wax Casting Process of Colored Glass Ornaments
Most colored glass ornaments are formed using the lost-wax casting process, which is the most difficult method in colored glass processing. However, this method also best allows the author to express their artistic concepts freely in colored glass art creation.
The lost-wax casting method has been widely used in the industrial production of metal materials and artistic creation, and it is a well-known casting technique in the industry. Its application in crystal and colored glass casting has only existed for about a hundred years. In mainland China and Taiwan, it has only been in the past 20 years that crystal and colored glass art enthusiasts have begun to use the lost-wax casting method for colored glass art creation. Although the time is short, due to continuous investment, considerable achievements have been made. In certain craft operations, they have surpassed the level of their counterparts in Europe and America, attracting international attention and exchanging invitations.
The process of lost-wax casting colored glass ornaments is similar to that of lost-wax casting metal ornaments, and its main procedures are as follows.
(1) Creative design. Transform the creative concept into graphic design drawings.
(2) Prototype making. Based on the sketch, sculpt a three-dimensional prototype using materials such as clay or wax. To master perfect proportions, elegant lines, and intricate patterns, every stroke and cut must be precise and delicate.
(3) Make a silicone mold. There are two methods for making a silicone mold: applying the silicone in layers. After the sculpture is completed, silicone mixed with silicone oil in an appropriate ratio is evenly brushed in layers onto the sculpture using a paintbrush, ensuring that the sculpture is fully coated with silicone (Figure 8-14).

Depending on the size of the work, the curing time of silicone varies, generally around 2~4 hours, while larger works may even require 10~24 hours. Silicone and silicone oil must be in the proper ratio to achieve good flexibility and strength. Conversely, suppose excessive silicone oil or hardener is added to complete the silicone mold quickly. In that case, it can significantly shorten the curing time but will result in insufficient elasticity of the silicone. When removing the wax mold, it is easy to cause the wax piece to break, making it impossible to create fine works. Moreover, silicone molds are prone to brittleness and do not have a long usage life, so patience is required to wait for the silicone mold to cure naturally. Another key factor affecting the flexibility and elasticity of the silicone mold is that the prepared silicone oil must be evenly brushed around the sculpture in layers. Although the work has height differences, the silicone mold must be evenly formed; after one layer dries, the second layer and third layer can be brushed on until a certain thickness of the silicone layer is achieved, which is a suitable and durable mold for creation.
Another method is to inject liquid glue. First, fix the wax mold on a glass surface and surround it with sandpaper, leaving a certain distance between the mold and the sandpaper tube. The silicone that has been mixed and stirred evenly is first vacuumed, then injected into the sandpaper tube, and vacuumed again, depending on the actual situation for injecting glue. After filling it with silicone, please place it in a vacuum machine to vacuum, and put the sandpaper tube that has been vacuumed last in a suitable and stable position to let it dry naturally.
(4) Reduplicating the resin mold. Use a silicone mold to cast epoxy resin and create a permanent model. Due to the aging and deformation of the silicone mold during high-temperature casting of the wax mold, it is necessary to use this resin mold to create a new silicone mold.
(5) Copying the negative mold. The sculpture is called the positive mold. The resin mold is removed from the silicone mold, polished, and adjusted to achieve the required shape, size, and better surface finish. Then, the resin mold replicates the silicone mold, reinforcing it with plaster. Appropriate dividing lines are chosen to segment the plaster. After the plaster cures, it is removed. A utility knife cuts open parts of the silicone mold, removing the resin mold. The dividing lines of the silicone mold are aligned to form a hollow mold. Tape strengthens the fixation of the plaster outer mold, creating a silicone negative mold and preparing to make a wax mold.
(6) Pouring wax molds. Pour the molten wax into the silicone mold in an appropriate amount, filling the mold, and then let it sit to allow the wax to cool and set naturally. Sometimes, the wax in the mold can be vacuumed to remove as much gas as possible to obtain a high-quality wax mold. The wax used must have a certain hardness; both too hard and too soft are unsuitable, as they will not achieve the delicate beauty of the original work. Wax that is too hard is difficult to modify, while wax that is too soft cannot hold its shape. If the wax liquid temperature is insufficient, it is prone to defects in the wax mold, and if the temperature is too high, it can lead to air bubbles, so the melting wax temperature needs to be properly controlled.
(7) Removing the wax mold. Carefully remove the cooled wax mold from the silicone mold. When opening the silicone mold, it must be pulled apart gradually, especially in areas such as hollow parts and chamfers where the wax is easily damaged; caution and dexterity are required (Figure 8-15).
(8) Adjusting the wax mold. If the wax mold has deformed, it needs to be reshaped. If there are defects on the surface of the wax mold, such as parting lines or air holes, tools are also needed for repair. When the wax mold breaks, it needs to be welded properly, ensuring that the dimensions of the wax mold are maintained during welding (Figure 8-16).

Figure 8-15 Removing the wax mold
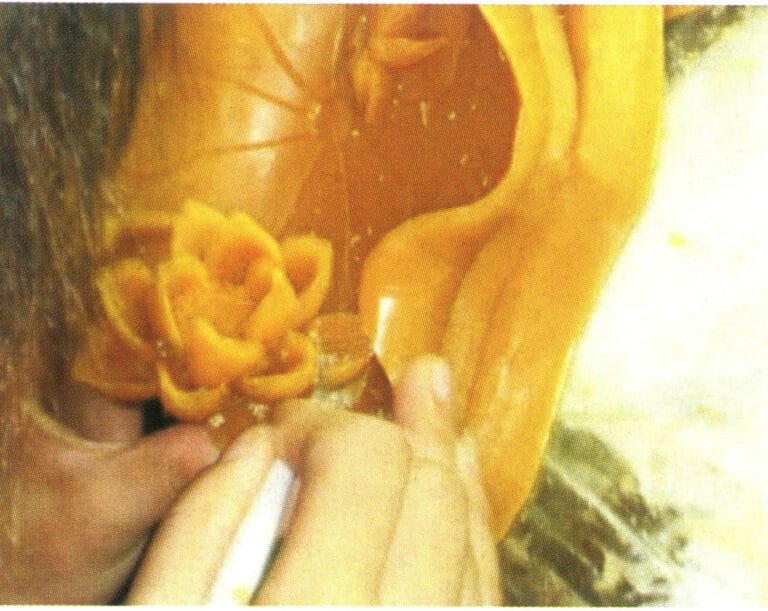
Figure 8-16 Adjusting the wax mold
(9) Making a gypsum mold. Once the wax mold is finished, you can pour the gypsum model. The gypsum casting powder is made from refractory aggregates, gypsum binders, and other modified additives. It is important to choose high-quality casting powder, as inferior casting powder often fails to ensure quality. When preparing the gypsum slurry, the water-to-powder ratio is an important parameter. If the water-to-powder ratio is too low, the gypsum slurry will be difficult to fill the fine details of the wax mold, making it hard to guarantee the quality of the work; if the water-to-powder ratio is too high, the strength of the gypsum mold will be insufficient, leading to cracks or peeling. Therefore, water should be appropriately added to prepare the gypsum slurry. The poured gypsum mold should be placed in a cool place to naturally harden (Figure 8-17).

(10) Steam wax removal. Place the naturally hardened plaster mold with the pouring gate facing down in the steam barrel, and close the door tightly to prevent high-temperature steam from spraying out and causing injury. Adjust the gas valve to introduce steam, using the high temperature of the steam to gradually melt the wax model in the plaster, slowly draining it out until there is no residual wax left in the plaster mold, at which point you can stop. The operation time varies depending on the size of the work. As mentioned above, it is also important to pay attention to the water-to-powder ratio of the plaster mixture; if the ratio is incorrect, the plaster mold can easily crack and become unusable under the high-temperature steam operation.
(11) Selected raw materials. Specific colors and sizes of colored glass materials are chosen based on the design, and each piece of raw colored glass material is carefully cleaned. The lost-wax casting of colored glass mainly uses kiln casting. In addition to the aforementioned wax removal and gypsum mold making, the suitability of the colored glass material is also an important factor affecting the success or failure of kiln-cast colored glass. Ordinary soda-lime colored glass, potash colored glass, etc., have a relatively light specific gravity, making them unsuitable for kiln casting. In 1676, England invented colored glass containing lead oxide (PbO), and the effects presented vary with different ratios of metal oxides. In addition to the suitability of crystal colored glass, attention must also be paid to the “compatibility” of the colored glass materials. The different origins of glass quartz sand or the varying ratios of added metal oxides can lead to different internal expansion coefficients, which means that stress may differ, resulting in repulsion. Once melted together, even if the heating and cooling curves of the kiln casting are calculated quite accurately, the work may still crack from the inside when the kiln is opened, during mold removal and cold processing, or even after the work is completed and placed indoors, it may suddenly and silently crack from the inside, which requires special attention.
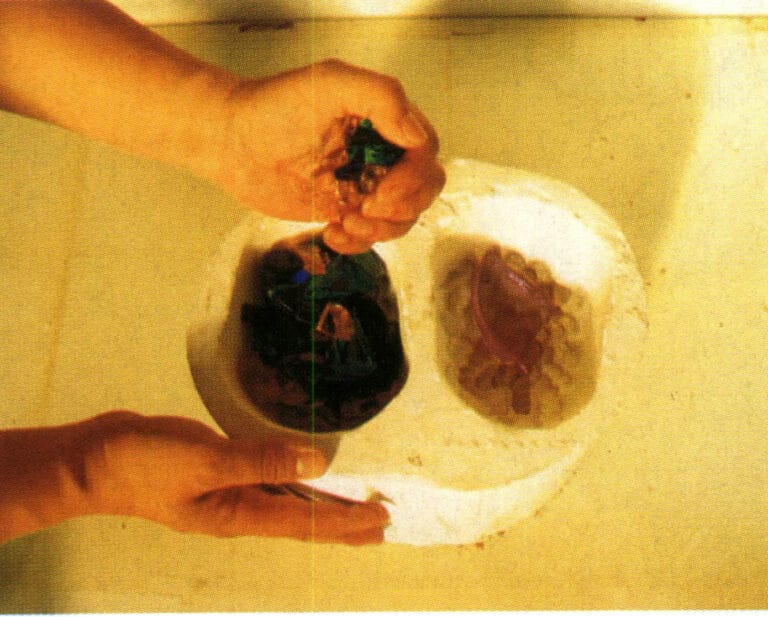
(12) Preparing the colored glass material. Before firing colored glass ornaments, there is a very important coloring process, which is the key step in achieving the colorful effect of colored glass products. The color of colored glass ornaments is achieved by placing various colored glass materials in the mold (Figure 8-18). For example, when a flower needs to be red, then put in the red color, and if green is needed in a certain position, it should be the main color, so place it properly when arranging. To accurately control the proportions of various colors and the beauty of their flow, the positions of the colored glass raw material color blocks must be precisely arranged according to the shape and design. Once the colors are arranged, they can be put into the furnace for high-temperature firing.
(13) Sintering in the furnace. Clean the gypsum mold thoroughly, use a dust extraction machine to remove dust from the surface of the cavity, then place the entire gypsum mold along with the prepared colored glass material into the furnace and slowly heat it to around 850℃, causing the molten glass material to soften like maltose and slowly flow into the gypsum mold to take shape (Figure 8-19). Glass casting utilizes “kiln casting,” which means completing the casting in a high-temperature furnace, rather than casting at room temperature as is common. Kiln casting is a key process in the entire procedure, with heating methods including gas furnaces, natural gas furnaces, and electric furnaces, among which electric furnaces are the cleanest and easiest to control. The difficulty of kiln firing lies in temperature control; even a slight miscalculation can lead to failure, resulting in a high failure rate. This process controls the heating and cooling (slow cooling) curves. Based on the size of the gypsum mold, precise calculations are made to start heating the electric furnace from room temperature, gradually increasing the temperature so that both the gypsum and colored glass are heated simultaneously, allowing the glass to melt rhythmically as the temperature rises, flowing slowly into the internal cavity of the mold along the curved lines of the hollow gypsum mold, filling every branch and corner, whether it is a simple bas-relief, brick shape, block, or high-difficulty features like chamfers, hollowing, and interlacing. At this moment, through the kiln casting process, the wonderful wax-free casting method of colored glass is fully expressed, unfolding the creativity of the artwork.

(14) Slow cooling. During the sintering process, to ensure uniform cooling of the colored glass inside and outside and to avoid uneven stress release that leads to cracking, it is necessary to set a cooling curve and control the slow cooling time. The cooling time varies depending on the different structural shapes. This differs greatly from lost-wax casting metals, where metal materials generally need to solidify to form. However, for colored glass, after casting and forming, it must be immediately de-molded and placed in a slow cooling furnace, with different slow cooling times assigned according to the size of the cast piece, allowing the internal stress of the colored glass material to be completely released, to prevent sudden cracking after the casting solidifies.
(15) Removing the gypsum mold. After allowing it to cool slowly, remove it from the furnace and carefully remove the gypsum mold with tools to obtain the rough blank of the colored glass (Figure 8-20).

(16) Cutting the pouring gate. After demolding, the rough colored glass blank needs to have the pouring port cut off, and the residual casting port should be removed using tools and equipment such as grinding wheels and grinding discs (Figure 8-21).
(17) Polishing. Use diamond sand rods, sandpaper, and other tools to polish the surface of the colored glass ornaments from coarse to fine (Figure 8-22).
(18) Grinding. Place the work on a cloth wheel and continuously grind and polish to bring out the luster of the glass. This will present the translucent texture of crystal material, completing the jewelry.

Figure 8-21 Polishing the gate area

Figure 8-22 Polishing the surface of colored glass ornaments
2.2 The Craft Characteristics of Lost-Wax Casting Colored Glass Ornaments
The lost-wax casting process has been widely used in producing glass ornaments. The ornaments made using this process have strong expressiveness and a wide range of adaptability. For delicate and small earrings and pendants, it can intricately express textures, sharp angles, and smooth surfaces according to the original sculpture. For medium-sized works with hollow internal carvings and intersecting branches, it is equally effortless to handle. This process can also showcase large-scale works’ grandeur and magnificent momentum, such as ancient cast bells and large relief walls. Additionally, the colors within the glass are formed by high-temperature sintering of various metal oxides, which prevents fading, oxidation, and other aging phenomena. This is why the lost-wax casting method is the most challenging among all glass processing techniques and can fully express areas that other cold and hot processing techniques cannot reach. Lost-wax casting is integrated with colored glass art creators, resembling a free-spirited horse galloping and expressing itself freely.
However, the lost-wax casting process of colored glass ornaments presents some difficulties, mainly reflected in the following aspects.
(1) The production process is complicated. From conception, design, sculpting, firing, fine-tuning, and polishing, dozens of intricate and meticulous steps are required to complete the work.
(2) Handmade. Workers must master exquisite skills to operate, as each process has uncertain variable factors, and repeated experiments are required. The works are rich in color and extremely difficult to produce.
(3) One mold, one product. A mold can only create one piece and cannot be reused. Large and complex works may even require multiple mold openings and firings. The success rate is low, making the work even more precious.
(4) High-temperature firing. Selected raw materials are melted at high temperatures to create various colored crystal glass, then repeatedly selected and cleaned. According to the material ratio of the work, it is placed in a mold, and strict heating and cooling curves are set to ensure the shaping and appearance quality of the work.
(5) Bubbles. Bubbles often appear in lost-wax cast glass works because lost-wax casting is mostly done at medium temperatures (about 900℃) during sintering. When the colored glass slurry flows into the mold cavity, it is like magma slowly flowing in, creating bubbles. At this time, the colored glass is in a thick state, and the bubbles formed in the gaps between glass pieces are not easy to rise, thus resulting in bubbles (Figure 8-23). Since bubbles often affect the visual perception of the work, especially when they appear on the surface, making the flaws more apparent, measures should be taken to reduce bubbles.


















Bir Yanıt
Amazing platform! I especially liked the product categories—they are well-structured and easy to find. Perfect for gadget lovers.