Unveiling other 8 kinds of single-crystal Optimized gemstones
Optimization and identification for Yellow Topaz, Tourmaline, Zircon and etc.
Gem crystals arranged in a periodic pattern according to certain rules by atoms or molecules are called single-crystal gems. Many single-crystal gems exist, such as rubies, sapphires, diamonds, emeralds, tourmaline, crystals, and zircon. Single crystal gems generally have high transparency and strong luster. The optimization treatment of single-crystal gems is mainly used to improve the color and transparency of allochromatic-colored gems. Most gems colored by trace elements can improve their color and increase transparency through optimization treatment. Different optimization treatment methods are selected based on single-crystal gems’ chemical composition, structure, and color mechanism. For example, natural emeralds and rubies with many fissures often use colorless or colored oil injection for filling. There are many optimization treatment methods for corundum gems, and almost all can be applied to corundum gems. The optimization treatment methods for other types of single-crystal gems should be chosen according to the color principle of the gems.
In addition, some single crystal gems colored by their components, such as garnet, malachite, and peridot, cannot use optimization treatment methods to change the color of the gems.

Irradiated blue topaz
İçindekiler
Section I Yellow Topaz
1. Gemological Characteristics of Yellow Topaz
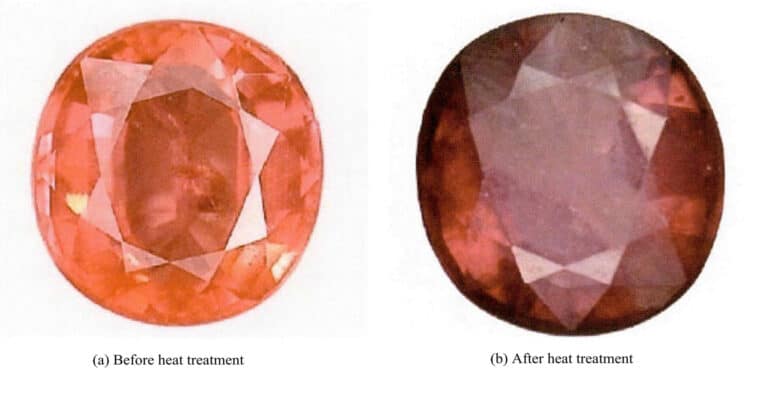
Yellow topaz, also known as topaz, has a chemical composition of Al2SiO4(K,OH)2 and may contain trace elements such as Li and Be, Ga. It commonly appears in colorless, light blue, blue, yellow, pink, rose, reddish-brown, green, and other colors; pink topaz may contain chromium ions.
According to different components, topaz is divided into F-type topaz and OH-type topaz. The colors of F-type topaz are mainly colorless, light blue, or brown, produced in pegmatite; the colors of OH-type topaz are mainly yellow, golden yellow, pink, red, etc. It is found in greisen or dike rocks, and the chromium-containing red OH-type topaz is a very precious variety. It is mainly produced in granite pegmatite, and greisen. The production areas are distributed worldwide, including Brazil, Myanmar, the United States, and Sri Lanka, and there are also outputs in Yunnan, Guangdong, and Inner Mongolia in China.
2. Changes in the color of topaz before and after improvement
Different types of topaz will produce different changes after optimization treatment. The main purpose of optimizing topaz is to improve its color. Depending on the type, the specific color changes are as follows:
(1) F-type topaz
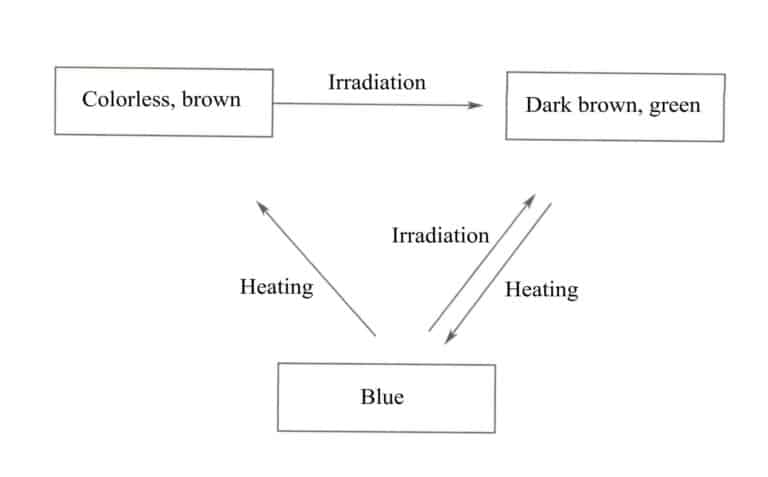
Colorless or brown F-type topaz, after radioactive irradiation, turns into dark brown or greenish-brown, and after heat treatment at around 200℃, beautiful blue topaz of varying shades can be obtained (Figure 5-27).
After improvement, F-type yellow topaz closely resembles aquamarine and has become a substitute for it. The blue color of the improved yellow topaz is stable, and excessive heating can restore it to its original state.
(2) OH-type yellow topaz
OH-type yellow topaz comes in various colors, with the most expensive being orange-yellow topaz, known as “Imperial Topaz.” Other colors of yellow topaz can also be optimized to achieve the color of “Imperial Topaz.”
Pink or purple-yellow topaz-containing chromium can turn orange-red and red after irradiation and can be restored to its original color after heating.
Brazilian pink and red topaz are made by heating yellow and orange topaz from the region. A type of Brazilian blue topaz turns black after radioactive irradiation, and sunlight exposure can restore it to its original color. If controlled heat treatment is applied, it can be transformed into pink, and with appropriate radiation, a golden color can be achieved, but blue will not appear. The color change of OH-type topaz after irradiation is shown in Figure 5-28.

3. Common optimization treatment methods for topaz
There are many optimization treatment methods for topaz; the most common and commercially valuable method is irradiation. Most blue topaz is first treated with irradiation from colorless topaz, followed by heat treatment to remove yellow and brown tones. This color change method results in vibrant colors that are very stable. F-type blue topaz that has undergone irradiation treatment is very popular in the market, but residual radioactivity must be below national standards before it can be sold. Other treatment methods, such as heat treatment, coating, and diffusion, are common optimization methods for topaz.
The stability of the blue color of blue topaz after color treatment has always been a major concern for the jewelry industry and consumers. Simulated fading and exposure experiments under sunlight for nearly 5 years show that irradiated blue topaz fades only 2%-3% in 5 years, meaning no significant fading can be observed within 5 years.
(1) Irradiation Technology and Equipment
The widely used treatment method for topaz in the market is irradiation treatment and irradiated topaz has gained high recognition over the years. Through irradiation and/or heat treatment, the pink, yellow, brown, and blue tones of topaz can be enhanced or produced. Any device that can generate radioactive rays can irradiate topaz. Commonly used equipment includes cobalt source irradiation devices, fast neutron reactors, and high and low-energy electron accelerators. The fast neutron reactor is currently the main equipment for improving topaz.
The characteristics of irradiation by fast neutron reactors are high efficiency and strong penetration ability, which can produce deep blue-finished topaz. Due to the reactor’s many channels and large volume, many samples can be irradiated at once.
High and low-energy electron accelerators can achieve deeper colors but must also undergo heat treatment to remove the yellow tones produced. This method can lead to residual radioactivity, so treated topaz cannot be immediately released to the market. Irradiating topaz with a reactor can turn it blue without requiring subsequent heating steps. The most typical reactor irradiation coloration is medium to deep gray-blue, often having an “ink” appearance. Sometimes, heat treatment is used to remove this ink appearance, resulting in a lighter and more saturated color (Figure 5-29). However, any gemstone treated with a reactor has residual radioactivity. Therefore, irradiated topaz must be stored for a certain period until the radioactivity decays to a certain level before it can be used commercially.

Sometimes, several treatment methods are combined to produce deeper colors without the ink-like appearance of topaz. This combined treatment uses reactor irradiation, electron acceleration, and heat treatment, resulting in bright, highly saturated topaz.
After irradiation treatment, the color of blue topaz is stable, widely used in the gemstone field, and loved by many.
(2) Heat treatment
The purpose of heat treatment is to remove poorly colored and unstable color centers, leaving good color and stable color centers. Heating removes the brown and brownish color centers in F-type topaz, revealing the blue color center.
The commonly used equipment for heat treatment is an oven or muffle furnace, with a heating temperature of 180-300℃, which must be controlled accurately. The blue color center of topaz appears at a specific moment temperature; below this temperature, the color remains unchanged, and above this temperature, the blue fades to colorless.
(3) Yüzey filming
Surface filming is a common treatment method for topaz, where a layer of colored film is applied over colorless or light-colored topaz to produce different color appearances. The surface filming is generally colored, with a very thin film, and the most commonly used is metal oxide film.
(4) Diffusion Treatment
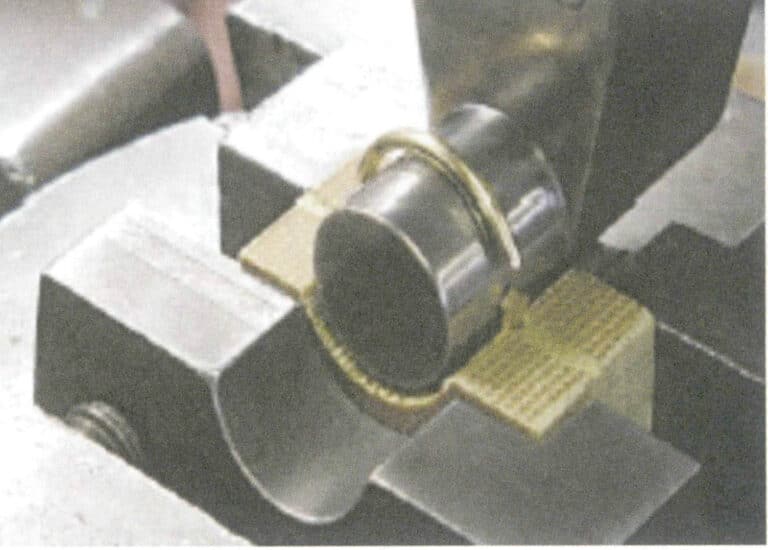
Generally, diffusion treatment using Co2+ can produce blue topaz. Its diffusion process is similar to sapphire diffusion, using high-temperature heating. Colorless or light-colored topaz can produce cobalt blue topaz after diffusion.
4. Identification characteristics of optimally treated topaz
After optimization treatment, topaz should be distinguished based on its characteristics. Except for heat treatment, which is considered optimization, all others are classified as treatments, and the treatment method should be noted in the naming. The identification characteristics of treated topaz are summarized as follows.
(1) Identification methods for irradiated topaz
Most irradiated topaz exhibits varying shades of blue. Although this blue color intensity and depth have not been found in nature, there is currently no non-destructive method to accurately prove whether the color of blue topaz has been irradiated. However, if it is confirmed to be irradiated, it should be noted on the identification certificate. Additionally, some yellow and brown topaz, whether naturally or artificially colored, may fade under light exposure.
The color formation of F-type blue topaz is due to external irradiation, creating a blue color center. The difference from natural topaz is that irradiated specimens are formed through artificial high-dose, short-term irradiation, and heating; natural specimens result from low-dose, long-term irradiation and light exposure in nature. The color of irradiated blue topaz is stable, so generally, there is no need to identify whether it is natural, but residual radioactivity testing should be conducted on irradiated topaz.
Samples irradiated with a neutron reactor inevitably produce residual radioactivity. Therefore, a longer cooling and placement time is required to reduce residual radioactivity. Irradiated topaz must be placed for at least one year before being released to the market, as the residual radioactivity of topaz has a half-life of about one hundred days, and it must wait until three half-lives have passed to ensure it does not harm the human body before being marketed.
Currently, the standards for the maximum residual radioactivity of irradiated topaz vary by country. Most countries and regions adopt 70 Bq as the standard, meaning that the residual radioactivity in the gemstone must be below 70Bq to be marketed, with the standards in the United States and Hong Kong being even lower.
(2) Identification characteristics of filmed
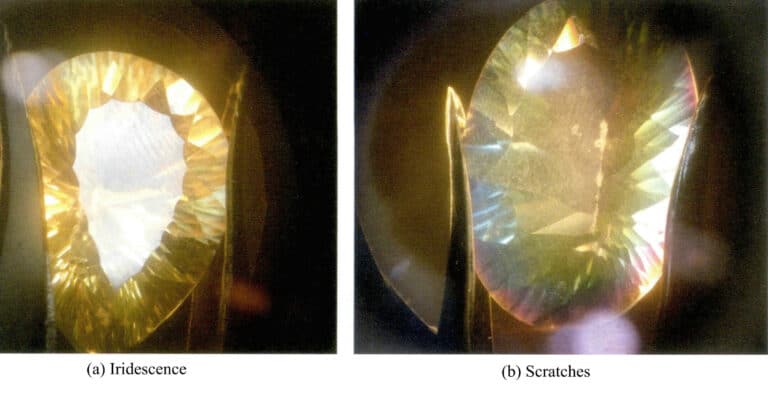
The topaz treated with a filming shows very bright rainbow colors on its surface [Figure 5-30(a)]. Upon magnified inspection, scratches can be seen on the surface, which are caused by the low hardness of the filming material.
(3) Identification characteristics of diffused treated topaz
The diffusion treatment of topaz is similar to that of diffusion-treated blue sapphire, both involving introducing coloring ions into the lattice or fissures of the gemstone under heating conditions. After diffusion treatment, the main identification features of topaz are as follows:
① The color of the topaz shows a characteristic blue-green hue of Co2+, and the blue-green color is limited to the surface, with a general thickness of no more than 5 μm.
② Upon magnified inspection, the surface color of topaz appears uneven, often showing clusters of brownish-green spots, which are more apparent when the gemstone is observed in immersion liquid.
③ Due to a large amount of Co2+ in diffusion-treated yellow sapphire, it appears orange-red under a Chelsea filter.
④ The absorption spectrum can show the Co2+ absorption spectrum.
Section II Tourmaline
1. Gemological Characteristics of Tourmaline
Gem-quality tourmaline is called tourmaline, and its chemical composition is complex. Tourmaline belongs to a complex boron silicate mineral with the chemical formula Na(Mg, Fe, Mn, Li, A1)3A16 (Si6O18)(BO3)3(OH, F)4. Depending on the components, it is mainly divided into four varieties: dravite, schorlite, elbaite, and tsilaisite. Trace elements such as iron, magnesium, lithium, manganese, and aluminum can substitute for each other, and the varying ion content can affect the color and type of tourmaline.
There are two complete solid solution series between dravite and schorlite and between schorlite and elbaite. At the same time, there is an incomplete solid solution between dravite and elbaite. Those with bright colors and clear transparency can be used as gemstones. Iron-rich tourmaline appears black and green; the higher the iron content, the darker the color; magnesium-rich tourmaline shows yellow or brown; lithium, manganese, and cesium tourmaline display rose red, pink, red, or blue; chromium-rich tourmaline shows green to deep green. Among them, the best colors are sky blue and bright rose red tourmaline, and high-quality, heavy tourmaline is priced similarly to rubies of the same grade.
In the same tourmaline crystal, the unevenness of the distribution of the components also tends to lead to variations in color, with bicolor tourmaline, multicolor tourmaline, or watermelon tourmaline with internal infrared green appearing along the tourmaline. The varieties of tourmaline are mainly classified by color into red series, blue series, green series, and bicolor series. The varieties and color causes of tourmaline are shown in Table 5-8.
Table 5-8 Varieties of tourmaline and their color causes
| Gemstone Name | Main Chemical Composition | Renk | Color Cause |
|---|---|---|---|
| Red Tourmaline | Na(Li,Al)3Al6B3(Si6O27) | Pink to Red | Lithium Ion and Manganese Ion |
| (OH, F)4NaMn3Al6B3(Si6O27)(OH, F)4 | |||
| Green Tourmaline | Na(Mg, Fe)3Al6B3 (Si6O27)(OH, F)4 | Yellow-Green to Dark Green as well as Blue-Green and Brown Green | Small amount of iron ions, more iron ions can cause black color |
| Blue Tourmaline | Na(Fe, CU)3Al6B3 (Si6O27)(OH, F)4 | Light blue to dark blue | Iron ions and a small amount of copper ions |
| Paraíba Tourmaline | Na(Cr, Mn)3Al6B3 (Si6O27)(OH, F)4 | Green to Blue | Copper ions and manganese ions |
The tourmaline is rich in inclusions and has developed fissures. Generally, in the processing of semi-precious stones, factories will inject resin before cutting to avoid the breakage of raw materials and increase yield. This serves to enhance adhesion and also increases transparency. Even after resin injection, the yield is only 10%-20%; without resin injection, the yield may be less than 5%. Almost all tourmaline undergo resin injection before cutting to reduce costs and improve yield.
2. Optimization Treatment and Identification Methods for Tourmaline
Common optimization treatments for tourmaline include heat treatment, filling treatment, dyeing treatment, filming treatment, irradiation treatment, and diffusion treatment.
(1) Heat Treatment
Heat treatment can be used to improve the color of tourmaline, generally heating darker tourmaline to lighten its color, thereby enhancing transparency and increasing the quality of the gemstone.
Due to the numerous fissures in natural tourmaline, pre-treatment is required before heating and shaping the tourmaline into the desired form without fine grinding and polishing. The heating temperature should not be too high, and the heating speed should be gradual to prevent the gemstone from cracking. After thermal treatment, tourmaline will exhibit the following characteristics:
① The heat treatment of tourmaline is classified as optimization in the national standard and may not be specified in the certificate. Heat treatment can change the color of tourmaline and improve its purity.
② Color changes can lighten the blue-green color after heating, increase transparency, enhance green, and eliminate blue; remove the red tones from tourmaline color; some brown turns pink or colorless; purplish-red tones turn blue; orange tones turn yellow, etc. The color is relatively stable after heat treatment.
③ After heat treatment, the internal inclusions of tourmaline often show significant changes, and magnified inspection reveals some gas-liquid inclusions that have ruptured, resulting in darkening.
(2) Filling treatment
Due to the many fissures in natural tourmaline, filling them can increase the yield of tourmaline and enhance the stability of the gemstones. Therefore, filling treatment is a widely used optimization method for tourmaline.
① The purpose of a filling is to prevent the raw stone from cracking during processing, making its structure more solid. Generally, organic materials or glass are filled into the rich fissures of tourmaline.
② Common filling materials include organic substances and glass, subdivided into colorless glue, colorless oil, colored glue, colored oil, colorless glass, and colored glass, among others.
Filling treatment is commonly used for mid to low-grade tourmaline, often found in bracelets, carvings, and decorative items. In the market, over 90% of mid to low-grade tourmaline jewelry has undergone varying degrees of filling (Figure 5-31). High-quality tourmaline may also undergo filling treatment, but the amount is generally very small and difficult to identify.

③ Characteristics of filling treatment identification: After filling, the surface gloss of the exposed part of the filled tourmaline is different from that of the main gemstone, and flashes and bubbles can be seen at the filling site.
- Under conventional gem testing instruments, the filling material in filled tourmalinecan be observed as white fibrous substances, yellow fibrous substances, blue flashes, and flowing structures within the t
- The filling material is filled in open fissures. When identifying oil and glue-filled tourmaline, it is important to observe the difference between the tourmaline’s surface gloss and the filling material’s gloss; generally, the yellow-brown filling material can be seen. When identifying glass-filled tourmaline, a flashing effect will appear during the shaking process of the tourmaline (Figure 5-32).

In addition to conventional instruments, large instruments such as infrared spectroscopy can reveal the absorption spectrum of filling material characteristics, and luminescence image analysis (such as ultraviolet fluorescence observation instruments) can observe the distribution state of the filling material.
④ Classification of filling degree levels: It is divided into extremely light, light, moderate, and severe based on the amount of filling in the market, with the identification characteristics of each level shown in Table 5-9.
Table 5-9 Classification and identification characteristics of filling amounts in the market
| Özellikler | Extremely light | Light | Moderate | Severe |
|---|---|---|---|---|
| Filling characteristics | Very small and very shallow area | Relatively small and shallow area | Small and shallow area | Larger and deeper area |
| Fissure gland filling characteristics | Fissure is very shallow, difficult to distinguish the filling material | Fissure is relatively shallow, the filling part is smaller than the sample's 1/2 | Obvious fissures, the filled part closed to sample 1/2 | Obvious fissures, the filled part exceeds sample 1/2 |
| Filling position | No restrictions | Mostly at the edges of the sample | No obvious open fissures | There is a noticeable crack in the center |
| Gem microscope | Extremely difficult to detect | Not easy to detect | Relatively easy to detect | Easily detectable |
| Kızılötesi spektrum | Cannot be identified | Cannot be identified | Identifiable partial features | Can identify all features |
(3) Dyeing treatment
Dyeing treatment is commonly used for tourmaline, which has many fissures and is often seen in red, green, and blue beads. Generally, lighter colors are dyed darker, or colorless ones are dyed colorful. During the dyeing process, heating is usually applied to ensure the color evenly penetrates the fissures in the tourmaline.
Identification features of dyed tourmaline: Observed with the naked eye or a tenfold magnifying glass, the color distribution of dyed tourmaline is uneven, often concentrated in fissures or surface depressions, with no obvious pleochroism. The uneven color phenomenon is even more pronounced under a gem microscope.
(4) Irradiation treatment
Colorless or lightly colored, multi-colored tourmaline is treated with high-energy radiation, which presents different colors depending on the irradiation time, radiation dose, and other factors. Electron bombardment can also turn colorless or pink tourmaline into bright red tourmaline, producing many fissures.
(5) Coating treatment
This treatment is generally suitable for colorless or nearly colorless tourmaline. After the coating treatment, various bright colors can be formed, and sometimes, a layer of colored film is also applied (Figure 5-33).

Identification features: Magnified inspection reveals abnormal luster and local film peeling. Most coated tourmaline shows only one reading on the refractometer, and the RI variation range increases, even exceeding 1.70, with no obvious pleochroism. Infrared or Raman spectroscopy tests can reveal characteristic peaks of the film layer. After coating, a halo effect can be observed floating on the surface.
(6) Diffusion treatment
① Diffusion treatment is the latest proposed method, first appearing in African-produced tourmaline.
② It generally appears more in blue tourmaline, diffusing the light-colored surface into a darker color, noting that there may be cracking due to uneven heating in the tourmaline.
This treatment method mostly appears in high-end tourmaline, and conventional instruments need help distinguishing diffusion-treated tourmaline from natural tourmaline, requiring large instruments to test its surface composition. Due to the high concentration of chromophore ions produced by the dye, ion mass spectrometry can detect a higher content of chromophore ions than in natural tourmaline.
Section III Zircon
1. Gemological characteristics of zircon
Zircon is a medium to low-grade gemstone primarily composed of zirconium silicate. In addition to containing zircon, it often includes rare earth elements, niobium, tantalum, and thorium. Natural zircon comes in various colors, including colorless, blue, yellow, red, orange-yellow, green, bright green, dark green, brown-yellow, and brown. Among gemstones, colorless, blue, and orange-yellow are the most common, and the color tones are generally darker(Figure 5-34). When the content of ZrO2, SiO2 is relatively low, its physical properties also change, with hardness and relative density decreasing. Zircon generally has weak radioactivity, and some zircons exhibit stronger radioactivity and amorphization due to the presence of U, Th, etc. , which can reduce the hardness to 6 and the relative density to 3.8, thus forming various varieties.

Zircon is widely distributed in China and is mainly found in various places along the southeastern coast, such as Wenchang in Hainan, Mingxi in Fujian, and Liuhe in Jiangsu.
Natural zircon is classified into high-type and low-type in mineralogy, with those in between referred to as intermediate-type. There are differences in the physical properties of these three types of zircon: high-type, low-type, and intermediate-type.
High-type zircon is well-crystallized, with a higher refractive index, hardness, and density than the other two zircon types. Gem-quality zircon is mostly high-type zircon.
Low-type zircon often contains some U3O8, HfO2 radioactive impurities, which reduce the relative content of ZrO2 and SiO2, damage the internal lattice, cause the crystal to become amorphous, and lead to a decrease in refractive index, relative density, hardness, etc. Completely low-type zircon can reach an amorphous state and is generally unsuitable for gemstone use.
The content of radioactive impurity elements in medium-type zircon is not too high, the damage to the internal crystal lattice is insignificant, and the crystal has not reached the amorphous state of low-type zircon. Medium-type zircon is often yellow-green or brown-green.
The physical properties of the three types of zircon, such as hardness, density, and refractive index, have significant differences; specific physical parameters can be seen in Table 5-10.
Table 5-10 Comparison of the physical properties of the three types of zircon
| Kategoriler | High-type | Intermediate type | Low type |
|---|---|---|---|
| Kristal sistemi | Tetragonal kristal sistemi | Tetragonal kristal sistemi | Amorphous solids |
| Output form | Square columnar and square double-cone gravel shapes, etc. | Columnar or gravelly | |
| Sertlik | 7 ~ 7.5 | 6.5 ~ 7 | 6.5 |
| Yoğunluk/ (g/cm3) | 4.60 ~ 4.80 | 4.10 ~ 4.60 | 3.90 ~ 4.10 |
| Kırılma | Kabuk şeklinde | Kabuk şeklinde | Kabuk şeklinde |
| Kırılma indisi | 1.925 ~ 1.984 | 1.875 ~ 1.905 | 1.810 ~ 1.815 |
| Birefringence | 0.054 | 0.008 ~ 0.043 | 0 ~ 0.008 |
| Dispersion value | 0.039 | 0.039 | 0.039 |
| Polychromaticity | Blue has a distinct dichroism, while others have weak dichroism | Weak dichroism | Weak dichroism, completely low-type without polychromatism |
Natural zircon belongs to mid-range gemstones, with colorless and blue zircon being the most common in the market. Both zircon colors are in nature but in limited quantities; most are obtained through artificial heat treatment. Zircon has a refractive index second only to diamond among natural gemstones and has a very high dispersion value. Colorless transparent zircon resembles diamond and is the gemstone variety most similar to diamond in nature, often used as a substitute for diamonds. Zircon is frequently heat-treated to enhance its quality, change its color or alter the type of zircon. Since no other substances are added during the optimization process, it is still recognized as a natural gemstone during jewelry appraisal.
2. Distinguishing features of zircon and diamond
Zircon is a very good diamond substitute with similar appearance and properties. The main differences between the two have the following characteristics:
(1) Exhibits double refraction:
Gem-quality zircon is high-grade zircon. Zircon is a heterogeneous material with a double refraction rate of 0.054. When observing the crown facets of zircon, one can see the double image at the adjacent facets; diamond is a homogeneous material and does not exhibit the double image phenomenon.
(2) The characteristic absorption spectrum of zircons:
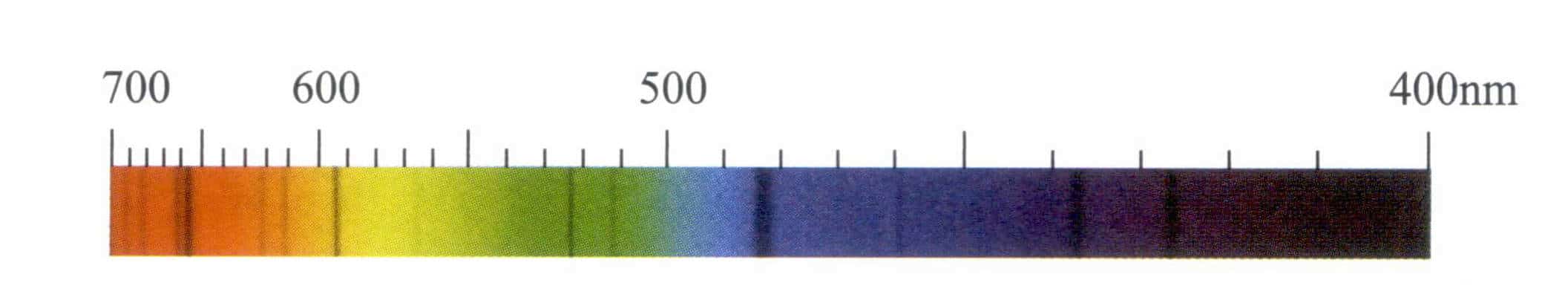
They often show two very distinct red spectral lines, with a strong one at 653.5nm, and a frequently visible accompanying spectral line at 659nm (Figure 5-35).
(3) Relative density:
The relative density of colorless zircon is 4.70, while the relative density of diamond is about 3.52.
(4) Line experiment:
Diamonds and zircon can be distinguished based on their visibility on a straight line. Place the zircon and diamond with their table faces down on a piece of white paper with a straight line drawn and observe from above perpendicular to the paper. The diamond on the left shows total internal reflection, so the line is not visible, while the zircon on the right shows a curved line (Figure 5-36).

3. Optimization Treatment and Identification Methods of Zircon
(1) Heat Treatment of Zircon
Heat treatment can change the color and type of zircon. The color modification experiments for zircon began in the 1980s. Due to the low cost of heat treatment and the stable color of zircon after treatment, it has become the most common optimization method for zircon. Almost all blue zircon is obtained through heat treatment.
① Change of Color
Heat treatment under reducing conditions can produce blue or colorless zircon. Zircon from different origins will exhibit different colors after heat treatment. For example, brown-red zircon raw materials from Vietnam can produce colorless, blue, and golden-yellow zircon after heat treatment; red and brown zircon from Hainan Province in China can turn colorless. Colorless and blue are the most common color types of zircon.
The heat treatment steps are as follows: First, the sample is placed in a closed crucible and put into the furnace, heated to 900-1000℃ under reduced pressure and reduction conditions, which allows the sample to achieve gem-quality color. Heat treatment removes the brown tones in zircon to produce colorless zircon while also creating a white misty effect.
Heat treatment under oxidation conditions can produce golden yellow and colorless zircon when the temperature reaches 900℃. Some samples may appear red, and samples that do not reach gem-quality color can also be heat-treated under oxidation conditions to become colorless or golden yellow zircon.
Heat treatment can yield colorless and blue zircon. The remaining blue zircon, which is poor in color but has good clarity, can be further heated to produce colorless, yellow, and orange-red zircon. The optimization process of zircon heat treatment does not involve the addition of any other substances, and it is still recognized as a natural gemstone during jewelry identification.
② Change type
Heating zircon raw materials to 1450℃ for an extended period can cause recrystallization of silicon and zircon, transforming low-type zircon into high-type zircon. Through this treatment, low, medium, and high-type zircon can all increase in density (up to 4.7 g/cm3 ), have a higher refractive index and clear absorption lines, and improve transparency and brightness. The recrystallization caused by heat treatment can also produce fibrous microcrystals, forming a cat’s eye. For example, most zircon from Sri Lanka is green low-type zircon, which becomes significantly lighter in color after heat treatment, turning into high-type zircon gemstones.
(2) Zircon Irradiation Treatment
Due to the darker color of natural zircon is often irradiated to produce colorless and blue zircon with higher brightness.
The irradiation treatment of zircon is a reverse reaction process to heat treatment. Almost all high-grade zircon improvements obtained through heat treatment can be restored to their pre-heat treatment color through irradiation (X-rays, γ rays, high-energy electrons, etc. ), and the color may even become deeper. Natural zircon also undergoes color changes under irradiation; for example, colorless zircon can turn deep red, brownish-red, or purple, orange-yellow zircon under X-ray irradiation; blue zircon can turn brown to reddish-brown zircon under X-ray irradiation. However, the color change process of these irradiated zircons is reversible and can return to its original state under extremely high temperatures and pressure.
Copywrite @ Sobling.Jewelry - Özel takı üreticisi, OEM ve ODM takı fabrikası
Section IV Crystal
Quartz is the most abundant mineral in the Earth’s crust and is also the gem family with the richest variety. Quartz gemstones can be classified into various crystalline forms, such as macrocrystalline and microcrystalline, among which single-crystal quartz is called crystal in gemology. The main chemical component of crystal is SiO2, and pure crystal is colorless and transparent. It contains different trace elements such as iron, manganese, titanium, etc. , which can produce different colors (Figure 5-37). When trace elements like aluminum or iron are present, irradiation causes these trace elements to form different types of color centers, resulting in various colors such as smoky, purple, yellow, etc.

1. Main Varieties and Identification Features of Crystals
According to the color of the crystal, it can be divided into different gemstone varieties: colorless crystal, amethyst, citrine, smoky quartz, rose quartz, etc. According to the characteristics of the inclusions (called “inclusions”) inside the crystal, it can also be divided into varieties such as rutilated quartz and water-in-crystal, as shown in Table 5-11.
Table 5-11 Main Types and Features of Crystals
| Renk | Characteristic | Color-causing ion |
|---|---|---|
| Colorless Crystal | The chemical composition is a single SiO2, produced under pure conditions, and is completely colorless and transparent | Hiçbiri |
| Ametist | The color ranges from light purple to dark purple, with deep purple being the best, characterized by a strong and bright color, and high transparency. | Contains trace elements of iron, which produces [FeO4]5- color center causing color due to irradiation. |
| Sitrin | Also known as citrine stone, it appears in light yellow, yellow, and orange-yellow, with bright and deep colors being the best. Natural citrine is extremely rare and expensive. | The main color-causing ion is Fe2+ |
| Smoky Crystal | Smoky colored to brownish crystal, with uneven color, also known as "tea-coloured citrine," relatively low in value | Al3+ replaces Si4+ , producing [AlO4]5- vacancy color centers after irradiation |
| Rose Crystal | Light pink to mauve pink quartz, usually with a lighter tone, also known as "Ross Crystal" | The main color-causing ions are manganese and titanium ions |
| Blue Crystal | Light blue, dark blue; natural blue crystals are rare and are generally synthetic | Iron and titanium ions |
| Green crystal | Green to yellow-green; natural green crystals are rare and are generally synthetic | The color-causing ions are mainly Fe2+ |
| Quartz Rutilated | Colorless, light brown, light yellow, with different mineral inclusions that produce different colors | Inclusions that cause color |
(1) Colorless crystal
Colorless, transparent, and pure silicon dioxide crystals can contain rich inclusions, commonly including negative, fluid, and solid inclusions. The types of solid inclusions in crystals are diverse, with common solid inclusions being rutile, tourmaline, and actinolite.
(2) Amethyst
The color of amethyst ranges from light purple to deep purple, and it may have varying degrees of brown, red, and blue tones. High-quality amethyst from Brazil exhibits a deeper purple color, while amethyst from Africa tends to have a strong blue tone. Amethyst produced in places like Henan, China, is lighter in color, sharing color characteristics with Brazil’s lighter amethyst, both being light purple with a slight brownish tone and high transparency.
The color distribution of amethyst is uneven, with the most common feature being color bands. The purple color bands are arranged parallel to each other, and sometimes two sets of color bands intersect at a certain angle; color patches can also be seen, with straight edges at the borders, forming irregular geometric shapes.
When irradiated, crystals containing trace amounts of iron have electrons in the Fe3+ electronic layer excited, producing vacancy color centers [FeO4]5-. The vacancy color centers primarily absorb light at 550nm in the visible spectrum, causing the crystal to appear purple. Under heating or sunlight exposure, the color centers in amethyst can be damaged, leading to fading.
(3) Citrine
Citrine refers to yellow-colored crystals commonly found in light yellow, yellow, golden yellow, and brownish yellow. The chemical composition contains trace amounts of iron and structural water. The color may be related to the paired occupancy of Fe2+ in the crystal. Citrine generally has high transparency, and its internal features resemble amethyst’s. Citrine is relatively rare in nature and is often found in association with amethyst and quartz clusters. Most of the citrine available on the market is heat-treated from amethyst or synthetic citrine.
(4) Smoky Kristal
A type of crystal that ranges from smoky to brownish, with uneven coloration, also known as “tea-coloured citrine.” The chemical composition contains trace amounts of Al3+, Al2+ replacing Si4+, and upon irradiation, it produces [A104]5- vacancy color centers, resulting in the smoky appearance of the crystal. Smoky quartz can turn into colorless crystal when heated.
(5) Rose Kristal
A type of light pink to rose red crystal, also known as “Rosy Crystal,” which gets its color from trace amounts of Mn and Ti in its composition. Rose Crystal has relatively low transparency, is often found in massive form, and its color is not very stable; it can fade when heated, and if exposed to sunlight for a long time, its color will gradually lighten.
(6) Blue Crystal
Blue Crystal mainly refers to crystals that are light blue to dark blue. Natural blue crystal is rare, and almost all is artificially synthesized.
(7) Green Crystal
The color of green crystal ranges from green to yellow-green. The color formation is related to Fe2+, and there is almost no naturally occurring green crystal in the market; it is usually an intermediate product formed during the heating of amethyst to citrine.
(8) Quartz Rutilated
The common colors of quartz rutilated include colorless, light yellow, light brown, etc. It can appear golden yellow or reddish-brown due to the presence of rutile and gray-black due to tourmaline; it often appears gray-green when containing actinolite.
2. Optimization Treatment and Identification Methods of Crystals
The commonly used optimization treatment methods for crystals mainly include heat treatment, irradiation treatment, dyeing treatment, and filming treatment.
(1) Heat Treatment
Heat treatment is often used for poorly colored amethyst; heating it to 400-500℃ can transform it into citrine or the transitional product green quartz. After heat treatment, the citrine can have color bands (the color bands can remain unchanged during the heating process) and does not exhibit pleochroism.
Another type of heat-treated product is ametrine. The purple and yellow form their respective color spots or patches, often without clear boundaries, and sometimes form distinct color zones related to the growth areas of the rhombohedron. Natural ametrine is only found in Bolivia, but this color feature can be achieved through heat treatment of amethyst (or synthetic amethyst), and there is currently no effective method to distinguish treated ametrine from natural ametrine.
This heat treatment has been widely accepted and is considered optimization, named directly after the natural gemstone.
(2) Irradiation treatment
Irradiation treatment is used to transform colorless quartz into smoky quartz or amethyst. In this case, colorless quartz is first irradiated to turn deep brown or black and then heat-treated to change its color to achieve the desired hue. The principle is that quartz forms vacancy color centers through irradiation. The principle is that crystal is colored by the formation of vacancy color centers through irradiation. In colorless crystals, impurity Al3+ must be present, and when Al3+ replaces Si4+, some alkali (such as Na+ or H+) must be present around Al3+ to maintain the crystal’s electrical neutrality.
When the crystal is irradiated by sources such as x rays and γ rays, the energy of the adjacent oxygen atoms to Al3+ increases and one of the electrons in its pair can be ejected from its normal position. If the irradiation intensity is high and there is enough Al3+ in the crystal, the crystal can turn black after irradiation. A schematic diagram of the vacancy color center of smoky quartz is shown in Chapter 3, Figure 3-18.
The main coloring principle of amethyst is the presence of trace amounts of iron and manganese ions. Amethyst can also be formed through irradiation and heat treatment, but the formation principle differs slightly from smoky quartz. Amethyst has the same color centers for vacancy, but its impurity is iron instead of aluminum. Crystals containing impurity iron ions undergo irradiation, and the electrons in Fe3+ are excited to produce vacancy color centers, causing the crystal to appear purple. When irradiated amethyst is heated, the vacancy color centers disappear, and purple fades. After heat In treatment, the purple amethyst can regenerate color centers through irradiation and restore the purple color.
When amethyst is heated, its color changes to yellow or green. At this point, the color is no longer caused by color centers but by the position and valence state of the transition metal iron. Irradiated crystals are classified as optimized by national standards and do not need to be marked on identification certificates.
(3) Dyeing treatment
The dyeing process of crystals involves first heating and quenching the colorless crystals, then immersing them in a prepared colored solution, allowing the colored solution to seep into the fissures formed during quenching, thus dyeing the crystals in various colors. Dyed crystals have obvious fracture lines, with colors concentrated in the fissures, making them easy to identify under a magnifying glass or microscope. Another situation involves immersing the heated and quenched colorless crystals in a colorless solution, where the colorless solution fills the fissures, and due to the interference effect of the liquid film within the fissures, this originally colorless crystal takes on a colorful iridescence.
(4) Coating treatment
Generally, a layer of colored film is coated on colorless crystals to enhance the luster of the crystal surface; another method is to coat a layer of colored film on the pavilion of light-colored crystals to enhance the color of the crystal. Coated crystals are generally easier to identify; sometimes, the rainbow-like thin film on the surface is visible to the naked eye. Crystals with coatings on the pavilion are not easy to identify and usually require magnification to observe the changes in color and luster between the pavilion and the crown (Figure 5-38).

Section V Spinel
1. Gemological Characteristics of Spinel
The chemical composition of spinel is MgAl2O4. Pure spinel is colorless, but when it contains trace elements Cr, Fe, Zn, and Mn, it can produce colors such as red, orange-red, pink, purple-red, yellow, orange-yellow, brown, blue, green, and purple (Figure 5-39). Chromium ions can produce a bright red color, and the finest red spinel is similar to pigeon blood red rubies, making it very expensive. The refractive index of spinel is generally around 1.718, gradually increasing to above 1.78 with the increase of iron, zinc, and chromium elements.

2. Optimization Treatment and Identification Methods of Spinel
Common optimization treatment methods for spinel include heat treatment, filling, dyeing, and diffusion treatment.
(1) Heat Treatment
Few spinels can be used for heat treatment, and they are limited to improving pink spinel. Pink spinel from Tanzania, through heat treatment, changes color from light pink to dark pink or from pink to red, but the overall color adjustment tends to be darker (Figure 5-40). After high-temperature treatment at 1400℃, the color of the spinel noticeably darkens. If the heating temperature is below 1400℃, it can only change the clarity of the spinel, not its color.
(2) Filling
The filling method of spinel is similar to that of rubies and emeralds, using colorless oil, colored oil, or materials like plastic and wax for filling. After filling, the fissures in natural spinel are reduced, improving its color and transparency.
The filling of the spinel is completed under vacuum conditions, with pre-processing and rough grinding of the spinel to shape it as needed, followed by acid washing to remove impurities from the fissures. Then, the dried spinel is placed with the filling material into a heating device for filling, and after filling, it undergoes fine grinding and polishing.
Identification features of filled spinel: Magnified inspection reveals differences in surface luster between the exposed parts of the filling and the main gemstone, with flash effects visible at the filling sites, and sometimes bubbles can be seen. Infrared spectroscopy testing shows characteristic infrared absorption peaks of the filling material.
(3) Dyeing
The dyeing of spinel is mainly used for light-colored natural spinels with many fissures, most of which are dyed red to imitate rubies. The dyeing agent is chromium salt, which can penetrate the spinel fissures fully under heating conditions.
Identification characteristics of dyed spinel: Under magnification, the color distribution of dyed spinel is uneven, often concentrated in fissures or surface depressions; under ultraviolet fluorescence light, the fluorescence is strong, and infrared spectroscopy tests reveal the presence of the dyeing agent.
(4) Diffusion Treatment
The diffusion treatment of spinel generally uses cobalt ions for coloring, allowing cobalt ions to enter the surface lattice of the spinel through heating, forming a characteristic cobalt blue, which is used to improve the color of light and heavily cracked blue spinel.
Identification features of diffusion-treated spinel: magnified inspection reveals healing fissures caused by heat and partially melted crystalline inclusions; magnified inspection or oil immersion observation shows color enrichment in the fissures, with lighter gem colors in dense structural areas and darker colors in fissure areas; component analysis indicates a high concentration of chromophore ions in the diffusion layer (surface layer) and a low concentration of chromophore ions internally; appears red under a Chelsea filter; absorption spectrum shows characteristic cobalt ion absorption lines, and laser photoluminescence (such as UV-visible spectrum) can also distinguish diffusion spinel from natural spinel.
Section VI Garnet
There are many isomorphic substitution phenomena among garnet group gem minerals, which can be divided into several garnet varieties based on different chemical compositions, resulting in significant variations in color, chemical composition, and physical properties for each type of garnet.
1. Gemological characteristics of the garnet group
The general chemical composition formula for garnet is A3B2(SiO4)3, where A represents divalent cations, primarily Mg2+, Fe2+, Mn2+, Ca2+, etc. ; B represents trivalent cations, mostly Al3+, Cr3+, Fe3+, Ti3+, V3+, and Zr3+. Due to the significant differences in the radii of the cations entering the lattice, this isomorphic substitution is divided into two major series: one series is dominated by trivalent cation Al3+ at the B position, while the A position consists of smaller radius divalent cations such as Mg2+, Fe2+, Mn2+, forming the aluminum series, which is also known as the red series, with common varieties including pyrope, almandine, and spessartite (Figure 5-41); the other series is dominated by the largest radius divalent cation Ca2+ at the A position, while the B position consists of trivalent cations such as Al3+, Cr3+, Fe3+, forming the calcium series, with common varieties including essonite, andradite, and uvarovite (Figure 5-42). Additionally, some garnets have lattice inclusions of OH–, forming hydrous varieties, such as hydrogrossular.


1.1 Aluminum Series Garnet
(1) Pyrope
Gem-quality pyrope is commonly purple-red, pink, brown-red, orange-red, etc. The main chemical component is Mg3Al2(SiO4)3. The variation in color depth is related to the iron ion content in pyrope; the higher the iron ion content, the deeper the color. The orange tone in pyrope is related to the presence of Cr2O3; when the Cr2O3 content is high, the red tone deepens, and when the Cr2O3 content is low, the orange tone deepens. The absorption spectrum of pyrope: a wide absorption band at 564nm, an absorption line at 505nm, and chromium-containing pyrope has characteristic chromium absorption in the red region, with absorption lines at 685nm, 687nm, and absorption bands at 670nm, 650nm (Figure 5-43). Common internal needle-like and mineral inclusions.

(2) Almandine
The common colors of gem-quality almandine are brownish-red, pink, and orange-red; the main chemical composition is Fe3Al2(SiO4)3, in which Fe2+ is often replaced by Mg2+, Mn2+, forming a solid solution series. The chromophore ions of almandine are mainly ferrous, and the absorption of Fe2+ causes the characteristic absorption spectrum of almandine. The absorption spectrum of almandine shows a strong absorption band at 573nm, and two narrower strong absorption bands at 504nm and 520nm are referred to as the “almandine window.” There may also be weak absorption bands in the red and blue-violet regions. (Figure 5-43). The strength of the absorption lines of almandine is related to the solid solution replacement of Mg2+; the more Mg2+ replaces Fe2+, the weaker the absorption becomes. Internally, needle-like inclusions may be visible, and when arranged regularly, they can produce a star effect, and mineral inclusions may also appear.
(3) Spessartite
The common colors of gem-quality spessartite include brownish-red, rose-red, yellow, and yellow-brown. The main chemical composition is Mn3Al2(SiO4)3, in which Mn2+ is usually partially replaced by Fe2+, and Fe3+ often replaces Al3+. The absorption spectrum of spessartite shows three strong absorption bands at 410nm, 420nm, and 430nm and three weak absorption bands at 520nm, 480nm, and 460nm (Figure 5-43). Internally, there may be wavy, rounded, or irregularly shaped crystals or liquid inclusions.
1.2 Calcium Series Garnet
Common types include essonite, andradite, and uvarovite. In addition, some garnets have additional OH– in their lattice, forming hydrous varieties, such as hydrogrossular.
(1) Essonite
The colors of essonite are diverse, mainly including green, yellow-green, yellow, and brown-red. Essonite is the most common type of garnet in the calcium series, with its main chemical composition being Ca3Al2(SiO4)3. Essonite and andradite form a complete solid solution series, meaning that Al3+ and Fe3+ can completely substitute. When the quantity of Al3+ exceeds Fe3+, it is called essonite.
Essonite usually does not have characteristic absorption spectra. Still, when it contains components of almandine, it can also show weak absorption spectral features. There are two absorption bands at 407nm and 430nm.
(2) Andradite
The common colors of gem-quality garnets include yellow, green, brown, and black. The main chemical component is Ca3Fe2(SiO4)3, in which Mg2+ and Mn2+ often replace Ca2+, and Al3+ often substitutes Fe3+; when Cr3+ replaces part of Fe3+, it is called demantoid. Demantoid has very characteristic tail-like inclusions, which are composed of fibrous asbestos. The most important source is the Ural Mountains in Russia, where black garnet with a higher content of Ti is referred to as black garnet.
(3) Uvarovite
Uvarovite is similar to demantoid, commonly found in bright green and blue-green colors, often called emerald green garnet. The main chemical component of uvarovite is Ca3Cr2(SiO4)3, in which a small amount of Fe3+ usually replaces Cr3+. Pure uvarovite has bright colors, and the blue tones intensify with the increase of iron ions.
Due to extensive isomorphic substitution, the chemical composition of garnet is usually quite complex, with the main gem species classification shown in Table 5-12. The composition of natural garnet is typically a transitional state of isomorphic substitution, and there are rarely garnets with end-member components.
Table 5-12 Classification of Garnet Group Gemstones
| İsim | Renk | Kırılma indisi | Kimyasal Bileşim | Color-causing Ions | |
|---|---|---|---|---|---|
| Aluminum Series | Pyrope | Purple-red, brown-red, pink, orange-red, etc. | 1.740 ~ 1.760 | Mg3Al2(SiO4)3 | Fe2+, Mn2+, Cr3+ |
| Almandine | Brown-red, pink, orange-red, etc. | 1.760 ~ 1.820 | Fe3Al2(SiO4)3 | Fe2+ , Mn2+ | |
| Spessartine | Brownish-red, rose-red, yellow, and yellow-brown, etc | 1.790 ~ 1.814 | Mn3Al2(SiO4)3 | Mn2+, Fe2+, Fe3+ | |
| Calcium Series | Essonite | Green, yellow-green, yellow, brown-red, and milky white, etc. | 1.730 ~ 1.760 | Ca3Al2(SiO4)3 | A small amount of Fe3+ replaces Al3+ |
| Andradite | Yellow, green, brown, black, etc. | 1.855 ~ 1.895 | Ca3Fe2(SiO4)3 | Fe3+, Cr3+, Ti3+ | |
| Uvarovite | Bright green, blue-green | 1.820 ~ 1.880 | Ca3Cr2(SiO4)3 | Cr3+, Fe3+ | |
| Hydrogrossular | Commonly green, with small amounts of blue-green, white, and pink | 1.670 ~ 1.730 | Ca3Al2(SiO4)3-x(OH)4x | Fe2+, Cr3+ | |
2. Optimization treatment and identification methods of garnet
Due to the color-causing mechanism of garnet being attributed to its mineral components, there currently needs to be more optimization treatments for garnet, mainly including heat treatment, diffusion, and combination optimization methods.
(1) Heat Treatment
The purpose of heat treatment for garnet is to improve its color. After optimization, the garnet color can change from light yellow to orange-yellow or green. After heat treatment, the surface of pyrope, almandine, and spessartine changes from yellow to orange-yellow; after heat treatment of essonite and demantoid, their color and transparency improve, and slight melting of the internal tail-like inclusions occurs. The ability of heat treatment to improve the color of garnet is due to the presence of trace impurity ions in the fissures of the garnet, which can alter the content and valence state of the impurity ions through heating, thereby improving the color of the garnet.
Identification features of heat-treated garnet: After heat treatment, the internal inclusions of garnet will change, such as the rupture of bubbles in the garnet and partial melting of mineral inclusions.
(2) Diffusion Treatment
The diffusion treatment of garnet targets light essonite. Iron ions and chromium ions are used as coloring agents, and diffusion is carried out through heating, allowing light yellow garnet to improve to orange-yellow; using cobalt ions as coloring agents can improve light yellow garnet to green or yellow-green.
The identification characteristics of diffusion-treated garnet: The color after diffusion treatment exists only on the surface of the garnet. The surface color is deep, while the internal color is light, concentrated on the surface and in the fissures. If re-cut or polished, the diffused color becomes less noticeable.
(3) Composite treatment
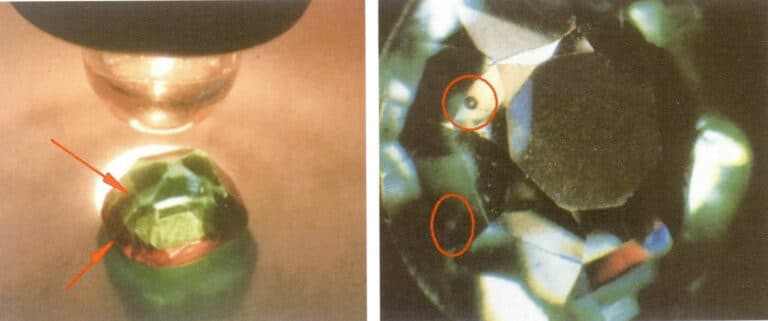
Composite treatment is a common optimization method for garnet. The typical composite method involves two layers of stone. The upper layer is usually garnet, and the lower layer is glass, referred to as garnet top composite stone. A common composite stone has a red garnet on the top and green glass on the bottom, which is used to imitate natural emeralds.
The primary identification characteristic of a garnet composite stone is to observe the presence of a “red ring” effect (Figure 5-44). The observation method involves placing the gemstone with its pointed end against a white background and illuminating it with a point light source. If a red ring is visible around the waist of the stone, it can be confirmed as a composite stone. Additionally, careful examination of the composite area may reveal the seam, and air bubbles may also be present within the seam.
Section VII Tanzanite
The mineralogical name of Tanzanite is Zoisite, belonging to the Epidote group in mineralogy. In 1962, George Kruchiuk first discovered tanzanite, which was initially used mainly as a decorative material. After discovering blue-violet transparent crystals in Tanzania in 1967, they gradually found applications in the gemstone field. Later, this gemstone was named tanzanite after its origin in Tanzania.
1. Gemological Characteristics of Tanzanite
Tanzanite is a hydrous calcium aluminum silicate with Ca2Al3(SiO4)3(OH) chemical composition, containing trace elements such as V, Cr, and Mn. The V element replaces 41 in the lattice, giving tanzanite its blue-violet color, while the pink opaque variety containing Mn is called Manganese Zoisite. Additionally, granular aggregates of Zoisite coexisting with opaque rubies and black hornblende are marketed as “Ruby-Zoisite,” while those coexisting with plagioclase are referred to as “Dushan Jade.”
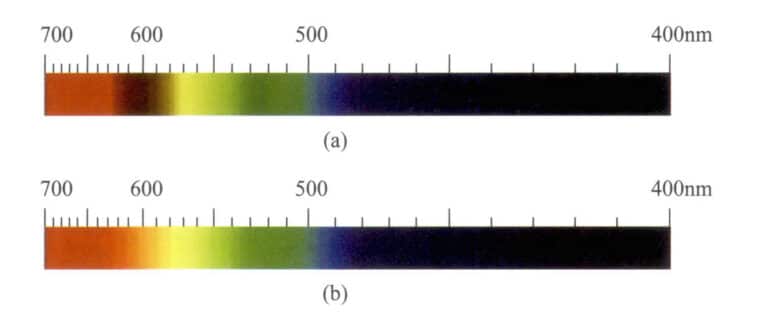
The vanadium-containing Zoisite is of the orthorhombic crystal system, with crystals often elongated along the c-axis, appearing columnar or platy, featuring parallel columnar stripes, and having a cross-section close to hexagonal. Other varieties of Zoisite often appear as granular aggregates, with common hues including green-blue with brown tones, as well as gray, brown, yellow, green, and light pink. After heat treatment, the brown-green to gray-yellow can be removed, resulting in blue and blue-violet colors. Blue Zoisite has a strong absorption band at 595nm and a weak absorption band at 528nm. Yellow Zoisite has an absorption spectral line at 455nm (Figure 5-45).
2. Optimization Treatment and Identification Methods for Tanzanite
Due to the varied colors of natural tanzanite, which rarely exhibits the enchanting bright blue-purple color, it is often subjected to artificial heat treatment. Common methods include low-temperature or medium-temperature heating, followed by filming, while diffusion treatment is less common.
(1) Heat Treatment
Approximately 95% of the violet-blue tanzanite on the market has undergone heat treatment at 600-650 C. This heat treatment temperature can transform tanzanite’s brown, yellow, and green colors into blue. Data analysis shows that tanzanite loses water and denatures from 965°C, changing its internal structure. Therefore, the heat treatment temperature for tanzanite should be below 965℃ to ensure that the treatment occurs within the stable phase range of tanzanite, preventing structural changes.
The vanadium is trivalent within brown and other types of zoisite crystals, while it is tetravalent in tanzanite. By heating at medium to low temperatures, the valence state of the vanadium changes from trivalent to tetravalent, producing a violet-blue color, which is stable. However, green gem-quality zoisite is generally sold directly in the market without heat treatment.
Due to the heat treatment temperature of tanzanite being in the medium-low range, the internal inclusion characteristics of tanzanite generally do not show very obvious changes, unlike the common molten crystal inclusions and broken, bent rutile needles found in high-temperature treated corundum. Additionally, there are no significant changes in the infrared and Raman spectra of tanzanite before and after heat treatment, exhibiting natural untreated tanzanite characteristics.
However, for tanzanite with strong trichroism and significant color differences, the change in trichroism after heating is the most pronounced, shifting from yellow-green-purple-blue to purple-blue.
(2) Filming Treatment
Filming is a treatment in gemstone optimization, a physical modification method in gemstone optimization treatment, where thin film materials are evaporated or sputtered in a vacuum using thermal evaporation or cathodic sputtering and deposited as a thin layer on the gemstone surface. The purpose of filming tanzanite is to enhance its blue hue.
The application of filming on tanzanite is far less common than heat treatment. Shane F. McClure and others reported in 2008 the detection of coated tanzanite containing elements such as cobalt (Co), zinc (Zn), and tin (Sn); Amy Cooper and Nathan Renfro reported in 2014 on filmed tanzanite containing titanium (Ti) elements.
Identification characteristics of tanzanite after filming treatment:
① The body color is vibrant but not dynamic, with a clear boundary of colors;
② The differences before and after treatment are obvious, with a strong luster at the filmed areas accompanied by rainbow colors;
③ The edges are prone to wear, caused by the shedding of the surface coating (Figure 5-46);

④ The color of the re-polished area will noticeably lighten;
⑤ Under magnification with a microscope, the surface has many tiny holes and a large number of chaotic scratches;
⑥ X-ray fluorescence spectroscopy testing shows abnormal content of metal elements such as Ti or Co;
⑦ Ultraviolet-visible spectroscopy analysis: the absorption peaks of natural blue tanzanite are at 528nm and 595nm, while the tanzanite filmed with Ti element is missing the absorption band at 528nm of natural blue tanzanite, and the absorption band at 595nm has shifted to 620nm.
Infrared spectroscopy of Ti-coated samples did not show peaks of other substances, so it is impossible to identify titanium-coated tanzanite by infrared spectroscopy; Raman spectrometers, and Diamond View are unsuitable for detecting tanzanite treated with titanium coating. Coated tanzanite may fade after prolonged ultrasonic cleaning.
(3) Diffusion treatment
In gemstone optimization, diffusion treatment is a common method for improving gemstones by infiltrating color-causing ions into the gemstone, enhancing the purple-blue color of tanzanite. However, this optimization treatment is rare in tanzanite; a deep blue-purple diffusion-treated tanzanite was discovered in New York in 2003. Unlike ordinary diffusion-treated gemstones, this diffusion tanzanite does not exhibit the “spider web” phenomenon under immersion observation. However, it can still be tested for abnormal elemental content using large instruments like electron probes to determine if the tanzanite has undergone diffusion treatment.
Bölüm VIII Feldspat
Feldspar minerals are found in rocks of various origins, accounting for about 50% of the crust’s mass, and are one of the most important rock-forming minerals. Feldspar belongs to the aluminum silicate mineral group. Its general chemical formula can be represented as XAlSi3O8, where X is Na, Ca, k, Ba along with small amounts of Li, Rb, Cs, Sr, etc. , which are monovalent or divalent alkali metal ions with larger ionic radii, Si can be replaced by AI and small amounts of B, Ge, Pe, Ti, etc. , which are mostly tetravalent or trivalent ions with smaller ionic radii.
1. Common feldspar gemstone varieties and their gemological characteristics
The feldspar group of minerals is diverse, and any brightly colored, high transparency, free of fissures, and relatively large can be used as gemstones. Important feldspar gemstones, such as moonstone, sunstone, and labradorite, also exhibit special optical effects. Feldspar gemstones are widely found in nature. Upon magnified inspection, small solid inclusions, twinned crystals, cleavage inclusions, twin patterns, gas-liquid inclusions, and needle-like inclusions can be seen in feldspar. The main varieties of feldspar gemstones include moonstone, amazonite, labradorite, and sunstone.
(1) Moonstone
Moonstone is a gem mineral composed of two components, orthoclase (KAISi3O8) and albite (NaAlSi3O8), arranged in a layered intergrowth. It usually appears colorless to white but can also be red-brown, green, dark brown, and other colors, transparent or semi-transparent, commonly exhibiting blue, colorless, yellow, and other iridescence, with a characteristic moonlight effect (Figure 5-47).
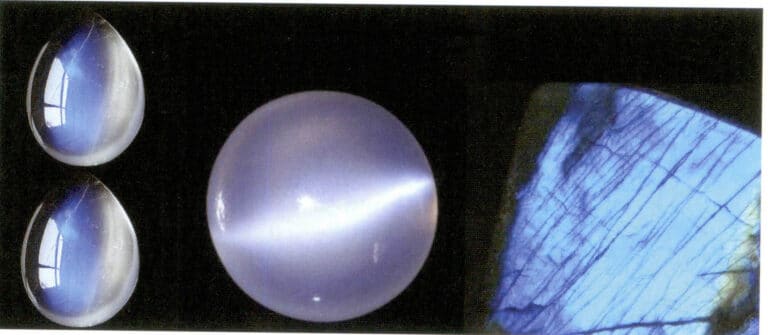
Moonstone exhibits well-developed cleavage, with two sets of cleavage intersecting nearly perpendicularly, forming “centipede” inclusions, fingerprint-like inclusions, needle-like inclusions, etc. A luminescent effect ranging from white to blue can be seen at a certain angle, resembling hazy moonlight. This is due to the albite dissolved in orthoclase being oriented within the orthoclase crystal, with the layered microcrystals of the two feldspars intergrown parallel. The slight difference in refractive index causes visible light scattering, producing a physical optical effect. When cleavage planes are present, interference or diffraction phenomena may accompany it, and the combined effect of feldspar on light creates a blue floating light on the surface of the feldspar.
(2) Amazonite
Amazonite, or “Amazon Stone,” is a microcline containing rubidium (Rb). Its common colors range from green to bluish-green, and the gemstone’s surface can reflect the cleavage planes. Amazonite is a variant of microcline that appears green to bluish-green (Figure 5-48).

The chemical composition of Amazonite is KAISi3O8, containing Rb and Cs, with the general content of Rb2O being 1.4%-3.3% and Cs2O being 0.4%-0.6%. One theory for its coloration is that it is due to Rb. In contrast, others believe that trace amounts of Pb substituting for K in the structure cause structural defects, resulting in color centers. Amazonite has relatively high transparency, generally from transparent to translucent, often containing plagioclase aggregates or intergrowths, presenting green and white checkerboard, striped, or mottled patterns with visible flashes from the cleavage planes. It exhibits yellow-green fluorescence under long-wave ultraviolet light, no reaction under short-wave, and a weak green color after prolonged exposure to X-rays.
(3) Sunstone
Sunstone, also known as “solar stone,” is the most important variety of sodium feldspar, commonly found in colors ranging from golden red to reddish-brown, and is generally semi-transparent. The most typical feature of sunstone is its sunstone effect, also known as aventurescence, which is caused by roughly oriented metallic mineral flakes (such as hematite and goethite) within the stone (Figure 5-49). As the gemstone rotates, it can emit red or golden reflections.
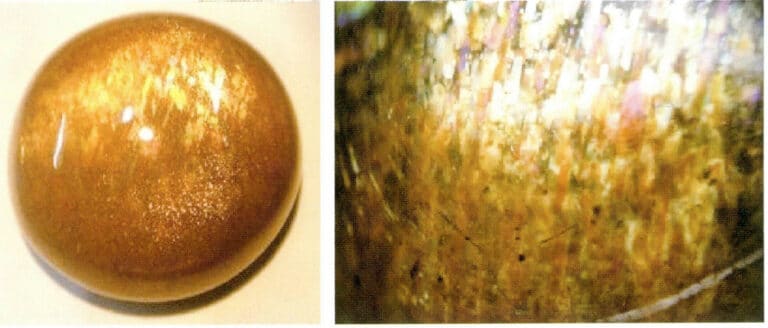
(4) Labradorite
Labradorite, also known as spectrolite, has a chemical composition consisting of albite (NaAlSi3O7) and anorthite (CaAl2Si2O8), belonging to the banalsite group. The most typical identification feature of labradorite is its blue and spectral color-changing effect (Figure 5-50).

When the gemstone sample is rotated to a certain angle, it can display blue, green, orange, yellow, golden, yellow, purple, and red iridescence. The cause of the iridescence is the interference of light between the thin layers of the plagioclase twin crystals or the fine, flaky hematite inclusions and some needle-like inclusions within the plagioclase, causing interference inside the plagioclase. Due to the needle-like inclusions, plagioclase can appear dark, producing blue iridescence. Cut and polished in a certain way can sometimes produce a cat’s eye effect.
2. Optimization Treatment and Identification Methods for Feldspar Gemstones
Feldspar gemstones often have cleavage or fractures, and the main purpose of optimization treatment is to conceal these fissures, making the gemstone’s structure more robust and enhancing stability. Common optimization treatment methods include filling and coating, wax immersion, irradiation, and diffusion.
(1) Filling and Filming
Due to the developed cleavage of moonstones, special layered fissures often form, affecting their appearance. Colorless oil or resin is used for filling, and then a layer of resin-like film is applied to the surface. The identification method checks whether the interference colors formed in the fissures have special reflections and, then, is a coating phenomenon on the surface. Since the refractive index of resin and feldspar is very close, it is necessary to see if any special phenomena are occurring in birefringence. A blue or black film is coated on the surface of other types of feldspar gemstones to produce iridescence, and upon magnified inspection, the film can be seen peeling off. If the characteristics of these treatment methods are clear, infrared spectroscopy can be used for identification.
(2) Waxing
For feldspar with many fissures, colorless or colored wax can fill the surface cleavage gaps. The stability of the filled gemstone is generally average, and a wax phenomenon can be detected by probing with a hot needle; the composition of the wax can also be measured using infrared spectroscopy.
(3) Irradiation
White microcline can be transformed into blue amazonite through irradiation treatment. This treatment of gemstones is rare and difficult to detect.
(4) Diffusion
Gem-quality red feldspar belongs to the plagioclase group and is a new type of gemstone in recent years. The color is often related to copper and iron. Currently, most red feldspar is formed under high-temperature oxidation conditions with diffusion of copper and iron elements. The identification characteristics include a high content of copper and iron elements, and the gemstone surface shows signs of high-temperature sintering.