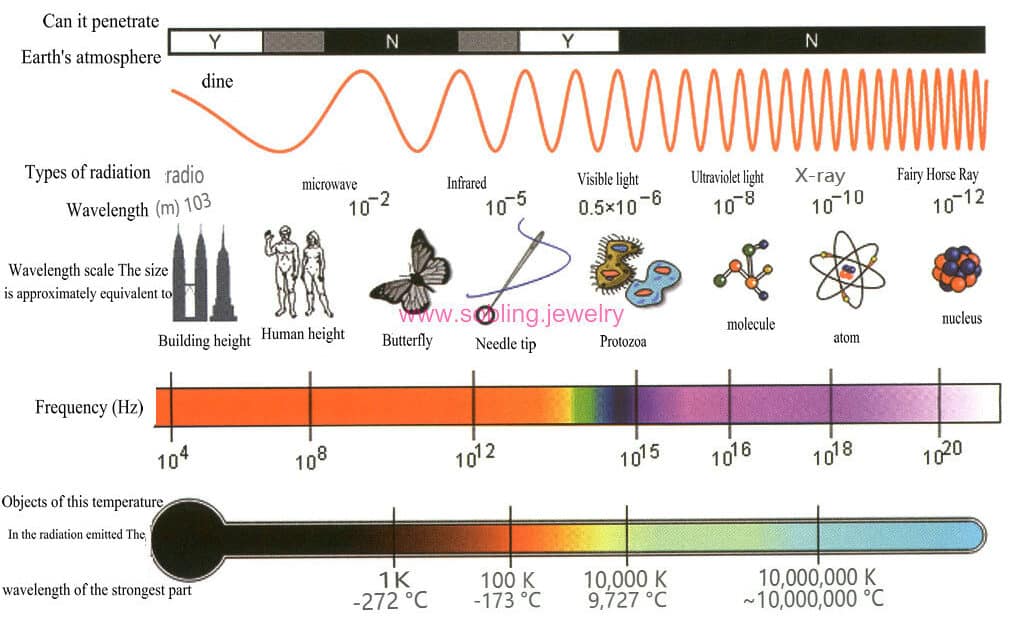
Comprehensive Guide to Crystal Optics, Mechanics, and Physical Properties
characristic including colors, luster, Transparency, Luminescence, Dispersion, cleavage, hardness, thermal properties
Giriş:
For jewelry aficionados, this guide decodes crystal optics, mechanical properties, and physical characteristics essential for gems. It’s a must for jewelry stores, brands, retailers, designers, and e-commerce platforms. Learn about color dispersion, pleochroism, and luminescence in crystal optics. Grasp the significance of transparency, luster, and refractive index. Dive into hardness, density, and toughness impacting a gem’s durability. This guide equips you with expertise to distinguish genuine gems, crucial for custom jewelers and celebrities seeking unique pieces. Enhance your collection with insights into the properties dictating a gem’s allure and value.

İçindekiler
Section I Definitions of Optical Terms Related to Crystals
In nature, the color or shape of crystals often immediately attracts our attention, guiding us to find them. Over the long years, we have discovered that crystals can have many forms and colors. With the development of modern technology, a discipline called crystallography has emerged. If you are more interested in crystals, you can read or study more specialized books.
This section will briefly discuss the phenomena observed when viewing crystal gemstones under light conditions and the professional terms used to describe these phenomena.
1. The Color of Crystals
1.1 Definition of Color
Color is a visual characteristic caused by light acting on the human eye, apart from spatial properties. This visual characteristic depends on the observer’s recognition of color and the lighting conditions (Figure 2-3-1).

Color in gemology is usually expressed as the color of the stone after absorption of visible light or can be described as the complementary color of the stone (Figure 2-3-2) after selective absorption of visible light in natural light (Figure 2-3-3).
In practical visual identification, clearly defining a gem’s hue can help us quickly distinguish between gems and their imitations, as well as help us differentiate certain natural gems from their enhanced versions.
1.2 Key Points for Observing Color
① Observe colors using reflected light. If there is an artificial light source, it can be done under a professional colorimetric lamp with a constant color temperature. If there is no artificial light source, it can be observed in the shade on a sunny day. It is generally recommended to observe in the morning, as it is best not to observe gemstone colors in the evening due to weaker light.
② Observe the environment against a neutral black, white, and gray background.
③ Other factors not mentioned do not affect the color observation results.
1.3 Methods of Describing Color
Gemology is an interdisciplinary subject, and the description of gem colors often draws on the methods used to describe mineral colors. Commonly used methods include standard colorimetric, binomial, and analogical methods. For certain gems with uneven color distribution, it is also necessary to specifically point out the phenomenon of color unevenness, which is usually referred to as color banding when the color is distributed in a striped or interlaced manner (in some gems, this phenomenon is directional and requires observation of the gem under transmitted light) (Figure 2-3-4 Figure 2-3-6).

Figure 2-3-4 Fluorspar with Color Banding
Figure 2-3-5 Tourmaline with Color Bands
Figure 2-3-6 Purple-red, with color bands, the color bands disappear after flipping (the top shows the color bands of ruby, the bottom shows the color bands of ruby after flipping)
(1) Standard chromatography
Using standard colors (red, orange, yellow, green, cyan, blue, purple) and white, gray, black, and colorless to describe the color of the mineral (Figure 2-3-7 ~ Figure 2-3-17)

Figure 2-3-7 Standard Red Reference Mineral Cinnabar

Figure 2-3-8 Standard orange reference mineral lead chromate

Figure 2-3-9 Standard yellow reference mineral orpiment

Figure 2-3-10 Standard green reference mineral malachite

Figure 2-3-11 Standard blue reference mineral azurite

Figure 2-3-12 Standard Purple Reference Mineral Amethyst

Figure 2-3-13 Standard Brown Reference Mineral Limonite

Figure 2-3-14 Standard black reference mineral tourmaline

Figure 2-3-15 Standard Gray Reference Mineral Bauxite

Figure 2-3-16 Standard White Reference Mineral Plagioclase

Figure 2-3-17 Standard Colorless Reference Mineral Ice Stone
(2) Binomial Method
When the color of a mineral is more complex, two colors can be used to describe it. For example, purplish-red is primarily red with a purple tone (Figure 2-3-18). For gemstones with uneven colors, a binomial method can also be used to describe each color category, but it must be noted that the colors are unevenly distributed (Figure 2-3-19).

Figure 2-3-18 Purple-red (Padma Sapphire)

Figure 2-3-19 Blue-green, rose-red, uneven color distribution (Tourmaline)
(3) Analogous Method
Gemstones can be compared with common objects to describe the mineral’s color, such as olive green (Figure 2-3-20).
The analogy method is a commonly used way to describe colors in the gemstone trading market, such as London Blue Topaz (Figure 2-3-21) and Swiss Blue (Figure 2-3-22).
Some of these comparative color terms represent the quality of gemstones, such as cornflower blue for sapphires (Figure 2-3-23) and royal blue (Figure 2-3-24). Pigeon blood red for rubies (Figure 2-3-25) and Pigeon blood red, etc.

Figure 2-3-20 Olive color (left is olivine, right is the color of olive trees and fruits)

Figure 2-3-21 London Blue Topaz

Figure 2-3-22 Swiss Blue Topaz

Figure 2-3-23 Cornflower Blue (left is cornflower blue sapphire; right is cornflower)

Figure 2-3-24 Royal Blue Sapphire. Royal Blue is the second most valuable color in sapphires after cornflower blue, it is the best-saturated blue, which can be pure blue or have a slight purple tint.

Figure 2-3-25 Pigeon Blood Red Ruby. Pigeon blood red is the most valuable color of ruby, referring to a rich, saturated, uniform pure red color without obvious other hues, such as blue or brown, but with a very slight hint of purple within an acceptable range. The gem's body color exhibits a strong fluorescence response under ultraviolet light.
On December 120, 2014, GRS (Swiss Gemological Laboratory) announced a new color, “Scarlet” (Imperial red), to describe the red color of Mozambican rubies. Scarlet rubies are certain Mozambican rubies with a vivid red color with an orange tinge, and the fluorescence of this ruby does not affect the color of the stone itself (type B rubies).
GRS classifies rubies into two types: Type A rubies and Type B rubies.
Type A rubies refer to those from Mozambique that exhibit significant fluorescence and are similar in color characteristics to Type B rubies, known as pigeon blood rubies. The naming is because these rubies have a color similar to the top-quality pigeon blood rubies from Myanmar.
Type B rubies are GRS type “Scarlet” (Imperial Red) rubies, with a certificate describing the Mozambique rubies (Type B) as vivid red on the main certificate and additional descriptions provided on supplementary certificates.
On November 5, 2015, SSEF and Gubelin Gem Lab announced a consensus on the professional terms for describing red and blue sapphires, pigeon blood red, and royal blue. Furthermore, these terms only describe color and clarity without any treatment (heating or filling), with no visible dark inclusions. They must exhibit uniform color and vivid internal reflections in red and blue sapphires.
2. The Luster of Crystals
2.1 Definition of Luster
The ability of a surface to reflect light and luster depends on the degree of surface polishing and refractive index. Terms like “shine” or “brightness” are often used in the market to replace the technical term luster.
In practical visual identification, luster can help us quickly distinguish between gemstones and their imitations, as well as help us differentiate certain natural gemstones from their treated counterparts.
2.2 Key points for observing luster
① Observe luster using reflected light.
② When observing crystals, pay attention to the effect of crystal face patterns on luster.
Generally, processed gemstones’ luster is better than their crystals (Figure 2-3-26).
③ In processing, the gem may be due to the difference in the hardness of the polishing material or the direction and difference in the hardness of the material itself, resulting in the difference in the luster of the same gems.
④ For crystal gemstones, under the same polishing conditions, the higher the refractive index of the gemstone, the stronger the luster. Aggregate gemstones may exhibit variations in luster due to their composition (Figure 2-3-27).
⑤ The absence of other factors does not affect the observation results of luster.


2.3 Methods of Describing Luster
This book discusses eight types of gemstone luster. The clusters that can be seen in crystals include metallic luster, sub-metallic luster, adamantine luster, vitreous luster, and greasy luster (which is easily seen in areas where the crystal is damaged). Other types of luster are more commonly found in aggregates or organic gemstones, which will be elaborated on in later chapters.
(1) Metallic Luster
When observing crystalline gemstones with reflected light, metals or a few gemstones can exhibit very strong reflections (most of the incident light undergoes specular reflection), such as gold, silver, and pyrite (Figure 2-3-28). This can be understood as having a reflection intensity similar to common metals.
(2) Elmas Parlaklığı
When observing crystalline gemstones with reflected light, the strongest reflective state appears in gemstones such as diamonds (Figure 2-3-29). In actual gemstone identification analysis, we consider gemstones with a refractive index (data observed under professional gemstone testing instruments like refractometers or reflectometers) greater than 2.417 to have diamond luster after polishing. Sub-diamond luster (Figures 2-3-30, 2-3-31) is between diamond luster and glass luster, with gemstones having a refractive index between 2.417 and 1.780 exhibiting sub-diamond luster after polishing.
(3) Glass luster
When observing crystal gemstones under reflected light, most crystal gemstones exhibit this type of luster, such as emeralds, crystal, tourmaline, etc. (Figures 2-3-32 and 2-3-34). In actual gem identification analysis, we consider gemstones with a refractive index between 1.45 and 1.78 to have a glassy luster after polishing, which can be understood as a reflection intensity similar to that of a glass surface. Under the same polishing conditions, the lower the refractive index, the weaker the glassy luster, which can be described as weak glassy luster; conversely, the higher the refractive index, the stronger the glassy luster, which is sometimes described as strong glassy luster.
(4) Greasy luster
When observing crystal gemstones with reflected light, a few gemstones can exhibit this phenomenon on their crystal faces. In contrast, most gemstones show this luster on uneven parts caused by external damage (this phenomenon can be described using professional terms such as fracture or undeveloped cleavage) (Figures 2-3-35 and 2-3-36). It can be understood as a reflection intensity similar to that of a greasy surface.

Figure 2-3-28 Metallic luster of pyrite crystals under reflected light

Figure 2-3-29 The diamond luster of diamond under-reflected light

Figure 2-3-30 The sub-diamond luster of cubic zirconia under reflected light

Figure 2-3-31 Sub-adamantine luster of artificial brazed aluminum garnet under reflected light
Figure 2-3-32 The weak glass luster of fluorite under reflected light
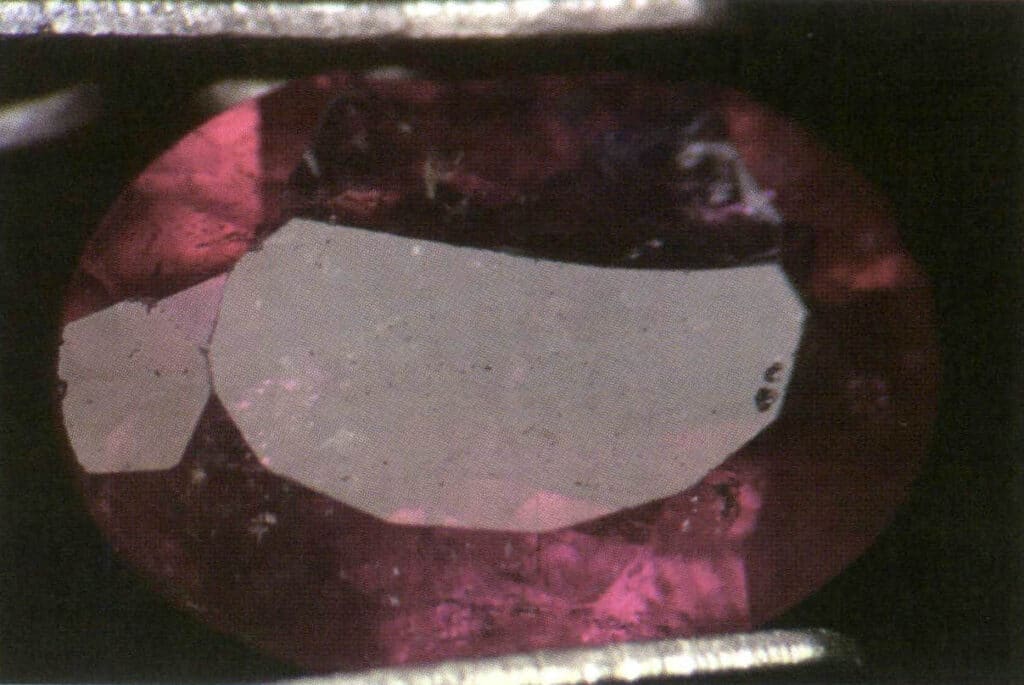
Figure 2-3-33 The glass luster of tourmaline under reflected light

Figure 2-3-34 The strong glass luster of ruby under reflected light
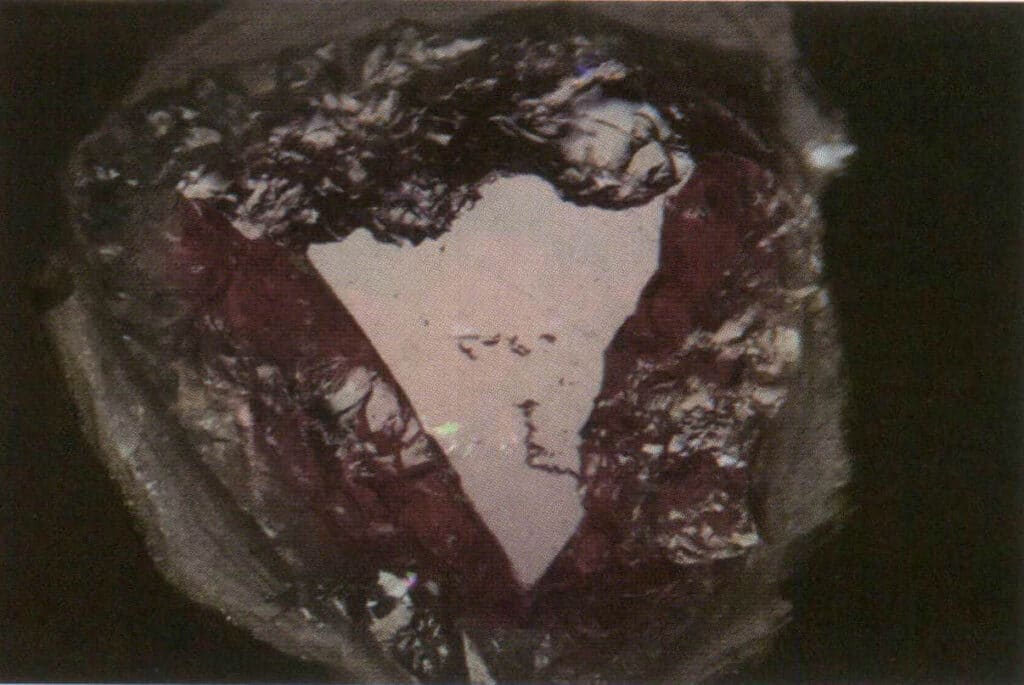
Figure 2-3-35 Comparison of the greasy luster (on the uneven edges) and glass luster (in the nearly triangular highlight area) of the broken surface of tourmaline under reflected light
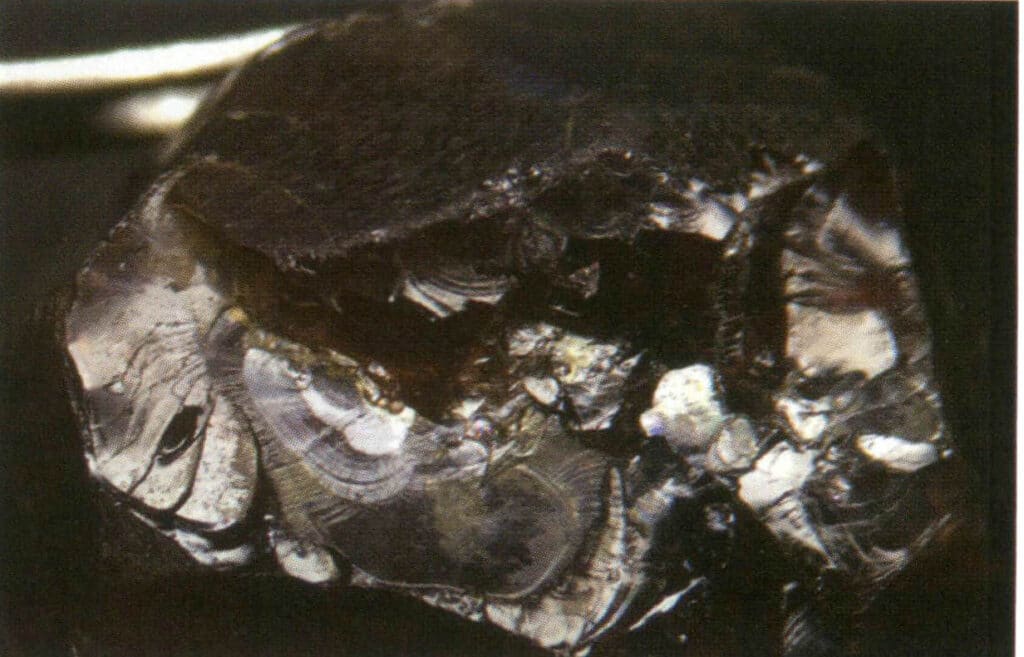
Figure 2-3-36 Greasy luster of garnet crystal fracture under reflected light
3. Transparency of Crystals
3.1 Definition of Transparency
The ability of an object to transmit visible light. The thickness and color of the crystal will affect the judgment of the gem’s transparency. Generally speaking, for colored gem crystals, the thicker the gem crystal, the worse its transparency.
In actual visual identification, transparency cannot be used as a standalone judgment factor to help us quickly distinguish between gems and their imitations; more often, it appears as a factor in evaluating gem quality.
3.2 Key points for observing transparency
① Use transmitted light to observe transparency; at this time, it is important to ensure that the intensity of the transmitted light is close to that of natural light. Misjudgment often occurs when there is a deviation between the observation light and natural light intensity.
② When the gem contains obvious inclusions (impurities), it will reduce or cause uneven transparency.
③ For stones of the same thickness, the darker the color, the less transparent; For stones of the same color, the thicker the thickness, the less transparent.
④ Other factors not mentioned do not affect the observation results of transparency.
3.3 Description of methods of transparency
Based on the degree of light transmission, transparency is divided into five levels: transparent, semi-transparent, translucent, micro-transparent, and opaque.
(1) Transparent
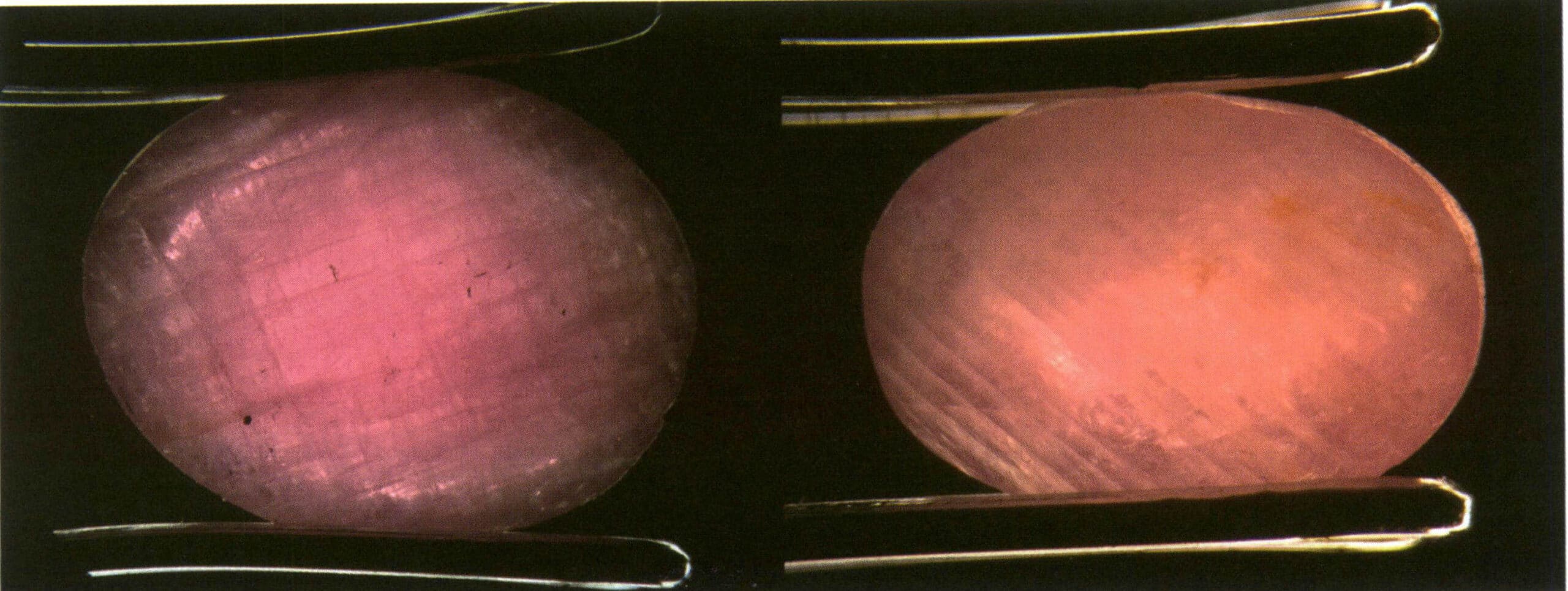
Observing the gem with transmitted light, the gem appears overall bright, and in comparison to the background, the brightness of the central part of the gem is either consistent with or slightly higher than the background. At the same time, the edge contours are darker (Figure 2-3-37 to Figure 2-3-39).
Objects on the same side as the transmitted light can be seen more clearly through the gem.
For faceted gems, the meaning of transparency is clearly seeing the pavilion’s facets and edges from the largest table (Figure 2-3-40).

Figure 2-3-37 On the left is citrine, in the middle is synthetic

Figure 2-3-38 Transparent (Yellow Crystal, Transmitted Light)

Figure 2-3-39 Transparent (garnet, transmitted light).

Figure 2-3-40: Transparent (artificial brazed aluminum garnet, transmitted light). The key point for judging the transparency of high refractive index gemstones like diamonds is the ability to see the facets and surfaces on the other side of the gemstone.
(2) Sub-transparent.
Observing the gemstone with transmitted light, the gemstone appears bright overall. Compared to the background, the brightness of the gemstone is consistent with the background. Objects observed on the same side as the transmitted light are more pronounced, while the objects appear somewhat hazy, as if a layer of dense white gauze has been added between the transparent gemstone and the light source (Figures 2-3-41, 2-3-42).

Figure 2-3-41 Powder crystal (reflected light)

Figure 2-3-42 Sub-transparent (powder crystal, transmitted light)
(3) Translucent
When observing the gem with transmitted light, it appears relatively bright overall, but its brightness is weaker than the background. Objects on the same side as the transmitted light are more apparent, but it is impossible to determine what the object is; one can only know that there is an object (Figures 2-3-43, 2-3-44).
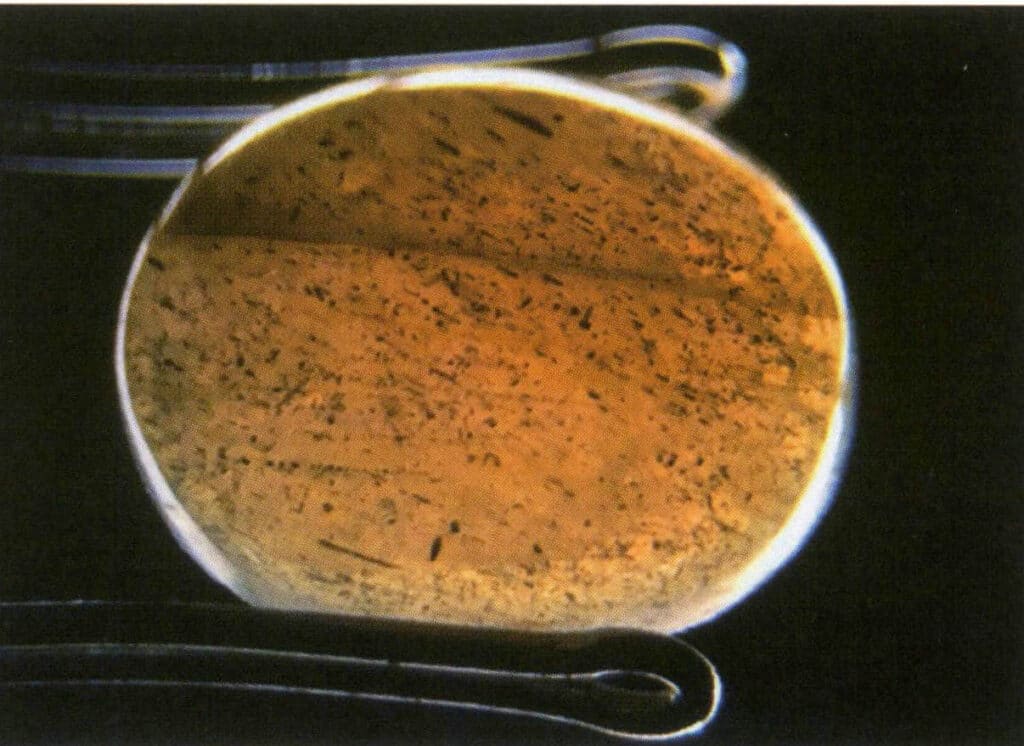
Figure 2-3-43 Translucence (pulled feldspar, transmitted light)
Figure 2-3-44 Translucent (sunstone, transmitted light)
(4) Subtranslucent
There are two situations for semi-transparency.
One situation is observing the gemstone with transmitted light, where the brightness of the gemstone appears black in the center due to low light transmission, but the edges appear bright due to high light transmission.
Another situation is to observe the gem with transmitted light. The gem appears black overall due to its opacity, but the internal features of the gem can be seen under reflected light (Figure 2-3-45).

(5) Opaque
Observing the gem with transmitted light, the gem is opaque, and compared to the relatively bright background, the edges of the gem are bright, while other areas appear black or do not allow light to pass through (Figures 2-3-46, 2-3-47).

Figure 2-3-46 Opaque (crystal: tourmaline)

Figure 2-3-47 Opaque (Crystal: ruby)
4. The Pleochroism of Crystals
4.1 Definition of Pleochroism
The phenomenon where certain translucent to transparent colored crystals appear to have different colors when observed from different angles is called pleochroism.
The different colors here refer to the differences in color hue, lightness, and darkness.
It is important to note that not all gemstones exhibit this phenomenon; only some gemstones from the intermediate or lower crystal families can show pleochroism. Typically, intermediate crystal family gemstones may display two colors, dichroism; lower crystal family gemstones may show three colors, known as trichroism, collectively referred to as pleochroism.
In practical visual identification, pleochroism can help us quickly distinguish between gemstones and their imitations, such as sapphire and its imitation, iolite (Figures 2-3-48 to 2-3-50).

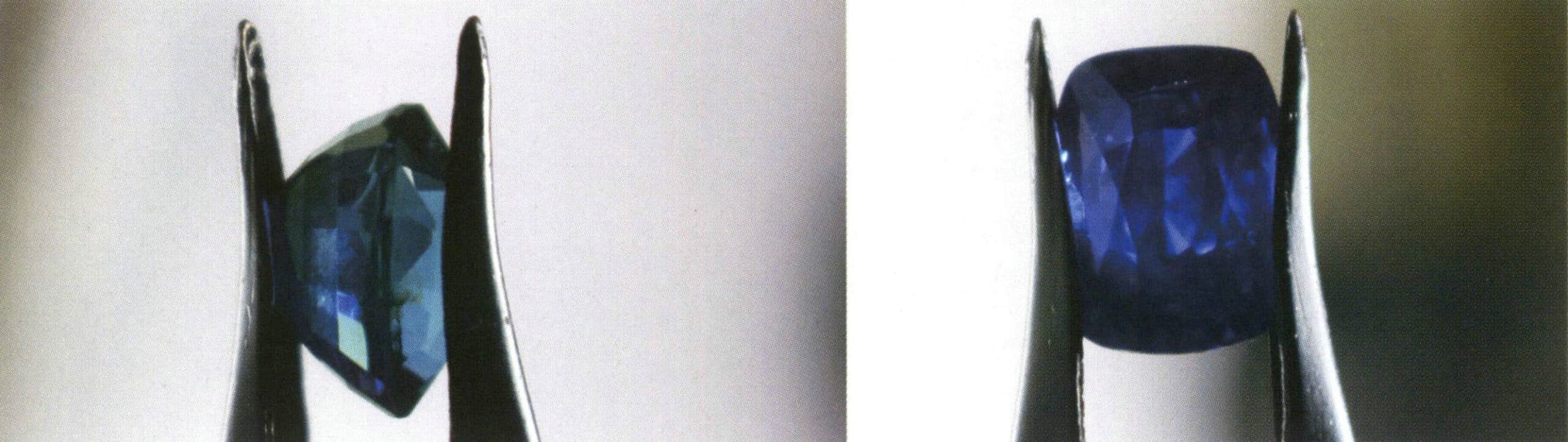

4.2 Key points for observing pleochroism
① Use transmitted light to observe the pleochroism of gemstones. It is important to note that the pleochroism of most gemstones can only be seen with the aid of a dichroscope; it is very difficult to observe with the naked eye.
② When there are obvious inclusions (impurities) inside the gemstone, reducing the transparency of the gemstone may affect the observation of pleochroism.
③ Other factors not mentioned do not affect the results of pleochroism observation.
4.3 Description of methods of pleochroism
The format for describing pleochroism observed with the naked eye is present and absent.
The description format for observing the pleochroism phenomenon of gemstones using a dichroscope includes the following: The number of pleochroic colors; The strength of pleochroism; The description of pleochroic colors. For example, Gemstones with dichroism can be described as dichroism, strong, red/purple-red; for gemstones with trichroism, it can be described as trichroism, strong, deep blue-purple/light blue-purple/light yellow.
5. Luminescence of Crystals
5.1 Definition of Luminescence
Gemstones with luminescence are even more enchanting. Apart from rubies, which exhibit asterism easily, and fluorspar, which show phosphorescence easily, most gemstones’ fluorescence or phosphorescence can only be observed under ultraviolet light. Therefore, in practical visual identification, the fluorescence of rubies can help us quickly distinguish rubies from most natural imitations (Figure 2-3-51).
(1) Luminescence
When stimulated by external energy, the property of crystals emitting visible light is called luminescence. External energy includes friction, ultraviolet light, X-rays, and other high-energy radiation.
Ultraviolet light is one of the easiest external energy sources for us to obtain; sunlight contains ultraviolet light, and in real life, ultraviolet light is used in currency verification machines and hospital ward disinfection.
(2) Fluorescence and phosphorescence
In gemology, different wavelengths of ultraviolet light sources are often used to observe the luminescence of gemstones, divided into two types: fluorescence and phosphorescence.
Fluorescence is when a gemstone emits light when excited by ultraviolet light, and the emission ceases when the external energy disappears (Figures 2-3-52, 2-3-53).
Phosphorescence refers to the phenomenon where a gem emits light when excited by ultraviolet light and continues to glow for some time after the external energy has dissipated (Figure 2-3-54).

Figure 2-3-51 Fluorescence of gemstones (left is tourmaline, right is ruby) under strong reflected light; the left red tourmaline without fluorescence shows uneven color, while the right red ruby with strong fluorescence shows uniform color. This is an important visual identification difference between strongly fluorescent rubies and their non-fluorescent imitations.

Figure 2-3-52 Fluorescence of red spinel

Figure 2-3-53 Fluorescence of Ruby (Compared to non-fluorescent blue sapphires, fluorescent rubies are more attractive)

Figure 2-3-54 Plastic (phosphorescence of artificial boron strontium aluminate)
(3) Influencing factors
The intensity of fluorescence is related to the types and amounts of impurities and defects in the gem, which is why the fluorescence of the same type of gem can vary. When a gem contains iron, it often suppresses the appearance of fluorescence, which is why iron is also referred to as a fluorescence quencher (Figures 2-3-55 to 2-3-57).

Figure 2-3-55 Imitation diamond under a normal light source.
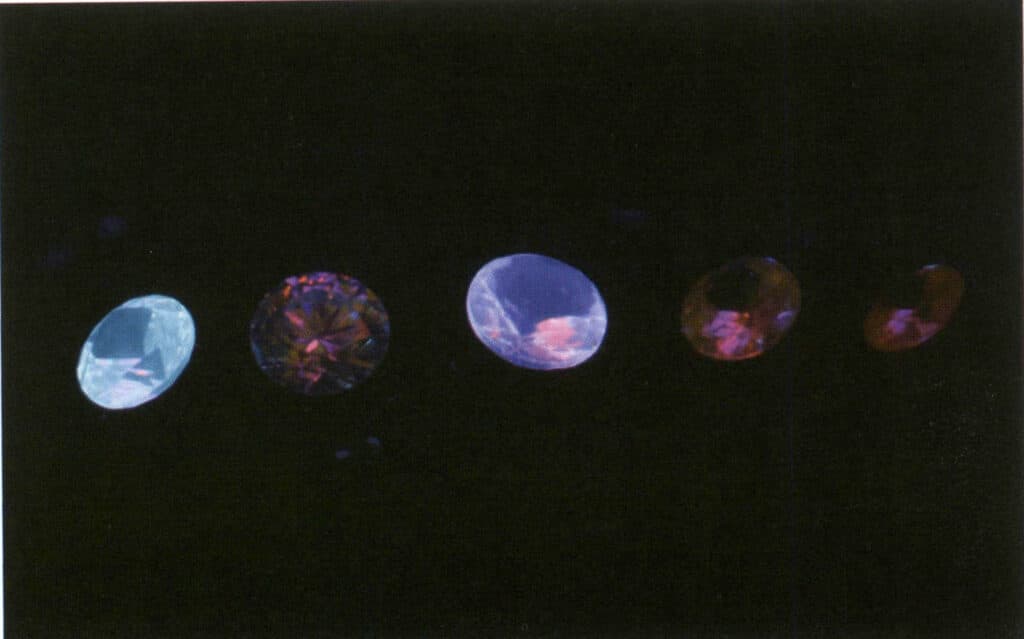
Figure 2-3-56 Fluorescence of synthetic diamonds under long-wave ultraviolet light, not observable to the naked eye.
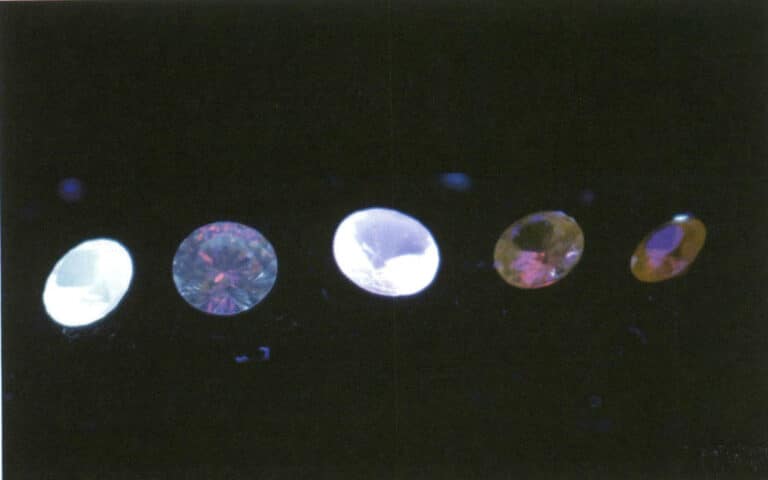
5.2 Key points for observing luminescence
① Except for a few gemstones like rubies and red spinels, the fluorescence observation in most gemstones requires specific energy ultraviolet light.
② Observing the luminescence of gemstones using specific energy, ultraviolet light must be used against a dark background.
③ The observation time is the phenomenon of the gemstone after the external energy excitation until the end of the external energy.
④ The luminescence of crystalline gemstones is characterized by changes in the overall brightness of the gem rather than a point, a line, or the reflection of the surface.
⑤ The fluorescence color of most gemstones under external energy excitation differs from those observed in natural light. The fluorescence color of the same gemstone can vary under different intensities of energy excitation, and the luminescence and fluorescence of the same gemstone may differ.
⑥ The absence of other factors does not affect the observation results of luminescence.
5.3 Description of methods of luminescence
Use the naked eye to observe the gem’s luminescence description format: present, absent.
Use a special ultraviolet fluorescent lamp to observe the gem’s luminescence. Description format: test the type of ultraviolet light, the gem’s luminescence intensity, and color, for example, long-wave ultraviolet light, strong, blue. For intensity, the following terms can be used: strong, medium, weak, none. It should be noted that the term “chalky” is often used when describing the blue-white fluorescence color.
6. Special Optical Phenomenon of Crystals
6.1 Definition of Special Optical Phenomenon
When light hits the surface of a gemstone, the colors or phenomena of star-like or banded bright areas displayed by the gemstone will flicker, move, and change as the light source or the gemstone moves relative to each other (Figure 2-3-58). Special optical phenomenon can only show color changes under two different lighting conditions.


Şekil 2-3-94 Gelişmiş kristal sisteminin değerli taşları (elmas)

Figure 2-3-95 Amorphous solids (natural glass)

Figure 2-3-96 Organic gemstone (yellow transparent amber)
6.2 Key points for observing special optical phenomenon
① The vast majority of special optical phenomenon in gemstones require reflected light for observation, and it is best to use a flashlight to illuminate the gemstone to make the phenomena more apparent.
② The color change effect in special optical phenomenon must be observed under different light sources, such as natural light during the day and artificial light at night.
③ The absence of other factors does not affect the observation results of special optical phenomenon.
6.3 Description of methods of special optical phenomenon
The special optical phenomenon of gemstones include the cat’s eye effect, star effect, color change effect, sand gold effect, color change effect, moonlight effect, and halo effect, totaling seven types. In some textbooks, the color change effect, moonlight effect, and halo effect are collectively called the halo effect.
Among those above special optical phenomenon, only the cat’s eye effect, star effect, and color change effect are involved in gemstone naming; the other special optical phenomenon are not involved in naming.
This book will cover the common cat’s eye effect, star effect, color change effect, sand gold effect, moonlight effect, and color change effect in crystals.
(1) Cat’s Eye Effect
Definition: It refers to the phenomenon where a bright band appears on the surface of a curved gemstone when illuminated, and the light band moves parallel on the gemstone’s surface as the light source and gemstone are moved (Figures 2-3-59, 2-3-60).

Figure 2-3-59 The cat's pupils appear linear under strong light.

Figure 2-3-60 shows a gem with a cat's eye phenomenon (sillimanite).
Cause: The cat’s eye effect can be observed in gemstones only if the three conditions are curved shape, directional cutting, and a set of directional dense parallel inclusions inside the gemstone (Figure 2-3-61 ~ Figure 2-3-64). This phenomenon has nothing to do with whether the gem is a crystal group or crystal system and whether the gem is a crystal. This phenomenon will also appear in the aggregate and amorphous solids.

Figure 2-3-61 shows the dense parallel arrangement of inclusions observed in a gem with a cat's eye effect after magnifying the bright band section.

Figure 2-3-62 shows the dense parallel arrangement of inclusions observed in a gem with a cat's eye effect after magnifying the bright band section.

Figure 2-3-63 cat's eye phenomenon is caused by vertical cat's eye bright bands with dense parallel inclusions.
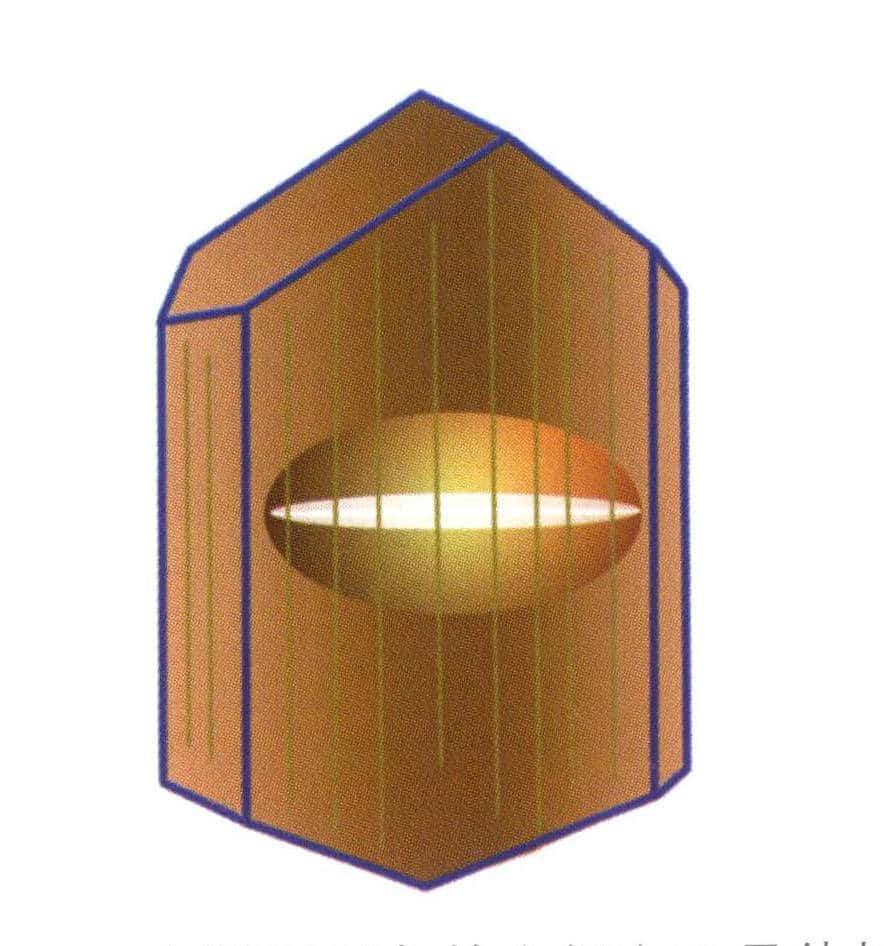
Figure 2-3-64 The bottom plane of the curved surface of the cat's eye phenomenon in the crystal of the
Identification method: By illuminating the raised part of a curved gemstone with reflected light, a bright band can be observed, and this bright band will move with the relative movement of the light source or the position of the gemstone (Figure 2-3-65).
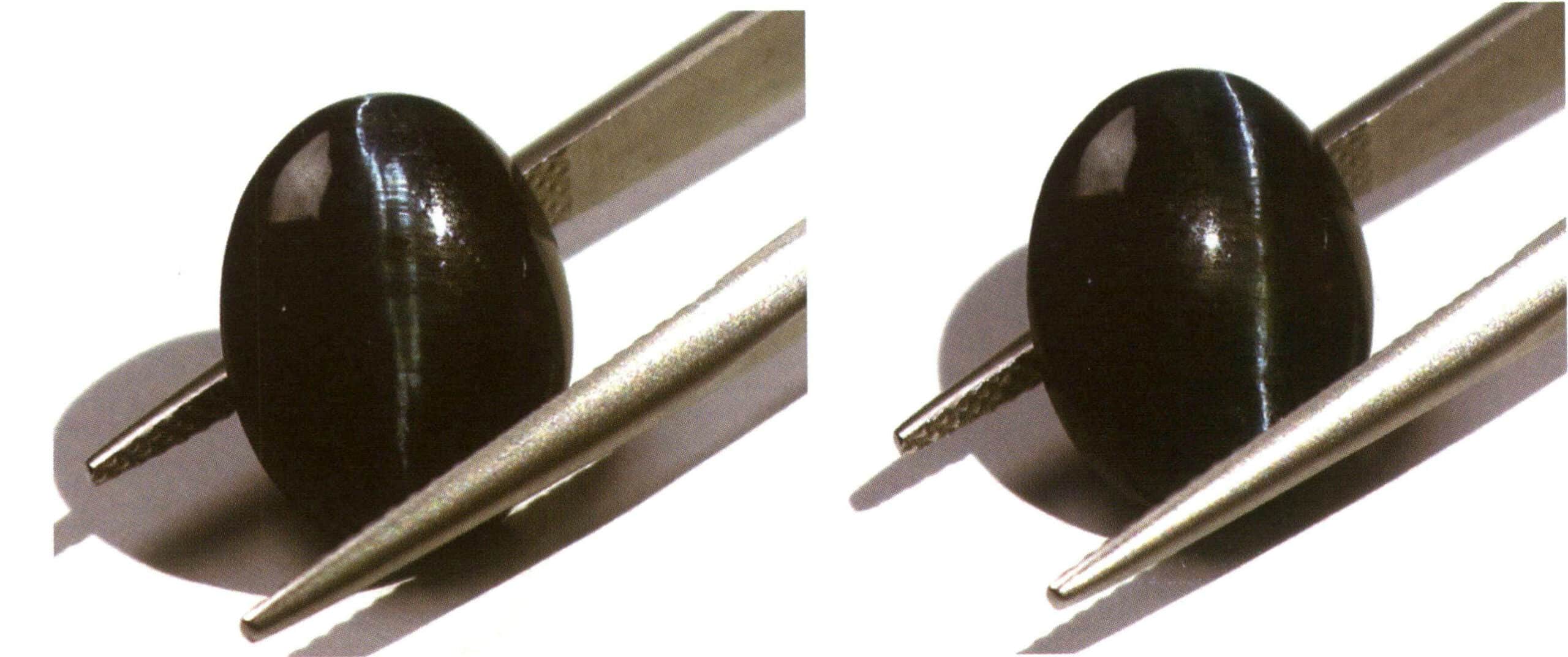
(2) Star Light Effect
Definition: The phenomenon where a curved gemstone exhibits two, three, or six intersecting bright bands when illuminated. If two bright bands intersect, it is called a four-ray starlight; if three bright bands intersect, it is called a six-ray starlight; and if six bright bands intersect, it is called a twelve-ray starlight. The bright bands in the starlight effect are also referred to as star lines.
Cause: For the gemstone to observe the starlight effect, it must be curved and directionally cut, and there are two, three, or six groups of directionally dense parallel inclusions inside the gemstone (Figure 2-3-66). Figure 2-3-67). This phenomenon occurs more often in crystalline gemstones, especially in intermediate and low crystalline gemstones.
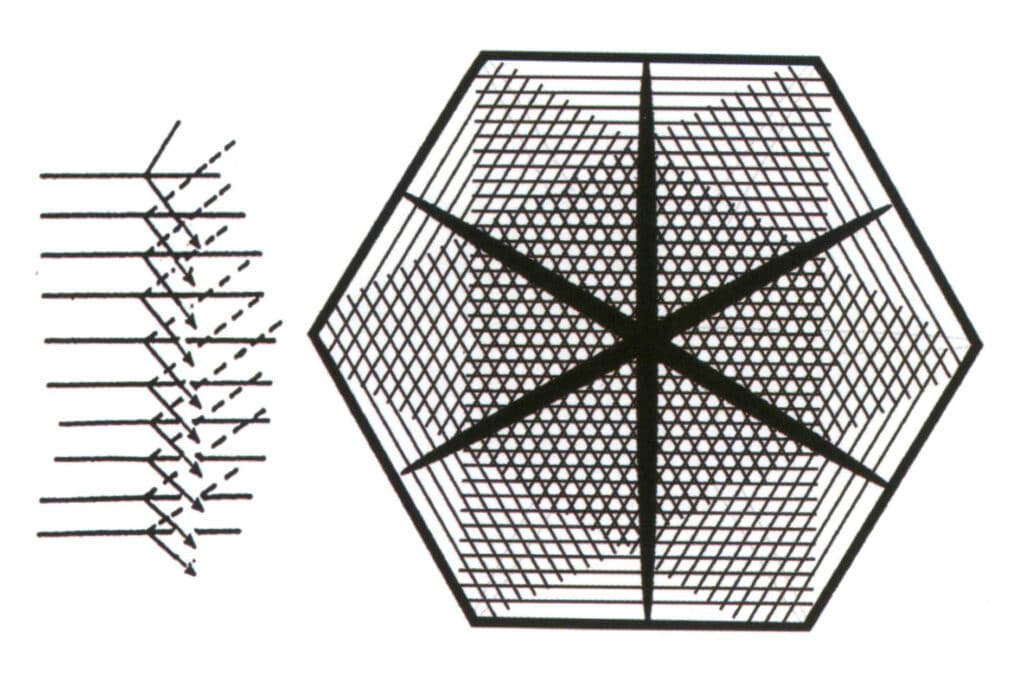
Figure 2-3-66 Depiction of factors contributing to the starlight effect

Figure 2-3-67 Three Groups of Directionally Dense Parallel Inclusions in Starlight Sapphire ( 30 x, Dark Field Illumination Method)
Identification Method: Shining reflected light on the raised part of a curved stone will reveal two, three, or six bright bands, which will move with the relative movement of the light source or the stone position (Figure 2-3-68). Figure 2-3-69) Some special gemstones require transmitted light to pass through the curved gemstone to observe the starlight effect, also called transparent starlight.
Figure 2-3-68 Starlight Sapphire Under Constant Light

Figure 2-3-69 Movement of Star Lines When the Light Source of Starlight Sapphire Moves
Due to the presence of multiple sets of oriented inclusions, quartz can exhibit asterism in different directions (Figure 2-3-70). Figure 2-3-66 Starlight Effect Factor Diagram.

Three situations in crystal gemstones can easily be confused with the asterism effect, and the common point of these phenomena is that the “star lines” are fixed. The first is called Trapiche, also known as dead asterism, which looks very similar to the asterism effect, but instead of crossing bright bands, it features six rays composed of white or black minerals spaced 60° apart, and these six rays do not move with the light source. This phenomenon usually occurs in gemstones with hexagonal prism crystal habits, such as emeralds, rubies, and quartz (Figures 2-3-71, 2-3-72). The second is a similar star-like phenomenon caused by oriented inclusions, such as rutilated quartz (Figure 2-3-73). The third is due to the inclusion of black carbonaceous materials like carbon and clay during the growth of crystal gemstones, resulting in special patterns; for example, the characteristic of empty quartz in red beryl is the oriented arrangement of black carbonaceous inclusions, appearing cross-shaped in cross-section (Figure 2-3-74).
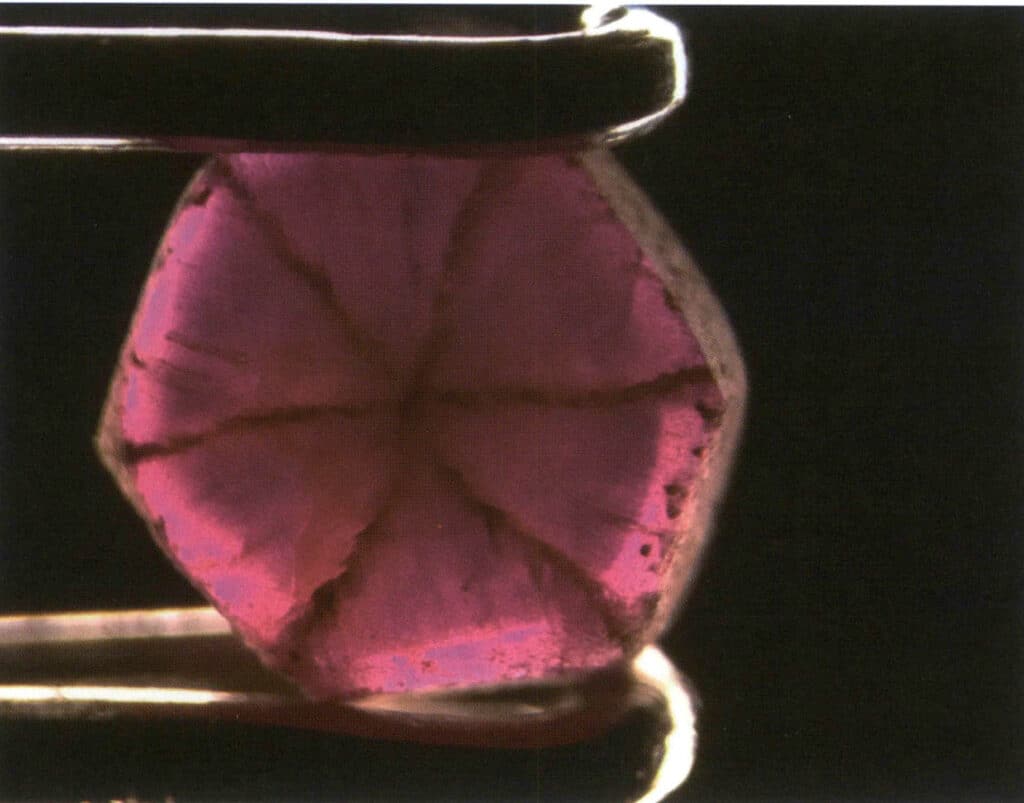
Figure 2-3-71 Trapiche Ruby
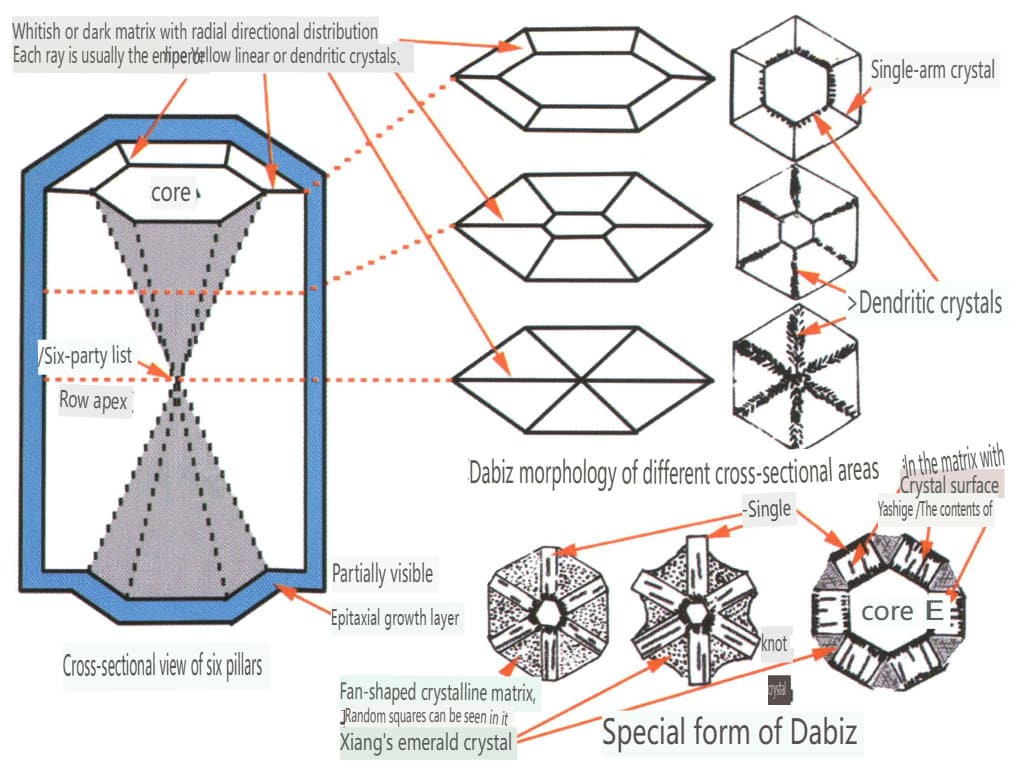
Figure 2-3-72 The form of Dabbiz (Isabella Pignatelli et al. 2015)

Figure 2-3-73 Rutilated Quartz

Figure 2-3-74 Andalusite crystal (orthorhombic gemstone, cross-section often square)
(3) Color change effect
Definition: The phenomenon where gemstones exhibit different colors under different light sources.
Cause: When gemstones contain an appropriate amount of chromium (Cr) or vanadium (V), this phenomenon can occur, which is unrelated to the naturalness of the gemstone and whether the gemstone has been cut or polished; the color change effect can be seen in both crystal roughs and synthetic gemstones.
Identification method: Illuminate the gem with two different color temperatures of reflected light (usually natural daylight and candlelight at night), and the gem will display two distinctly different colors (Figure 2-3-75).

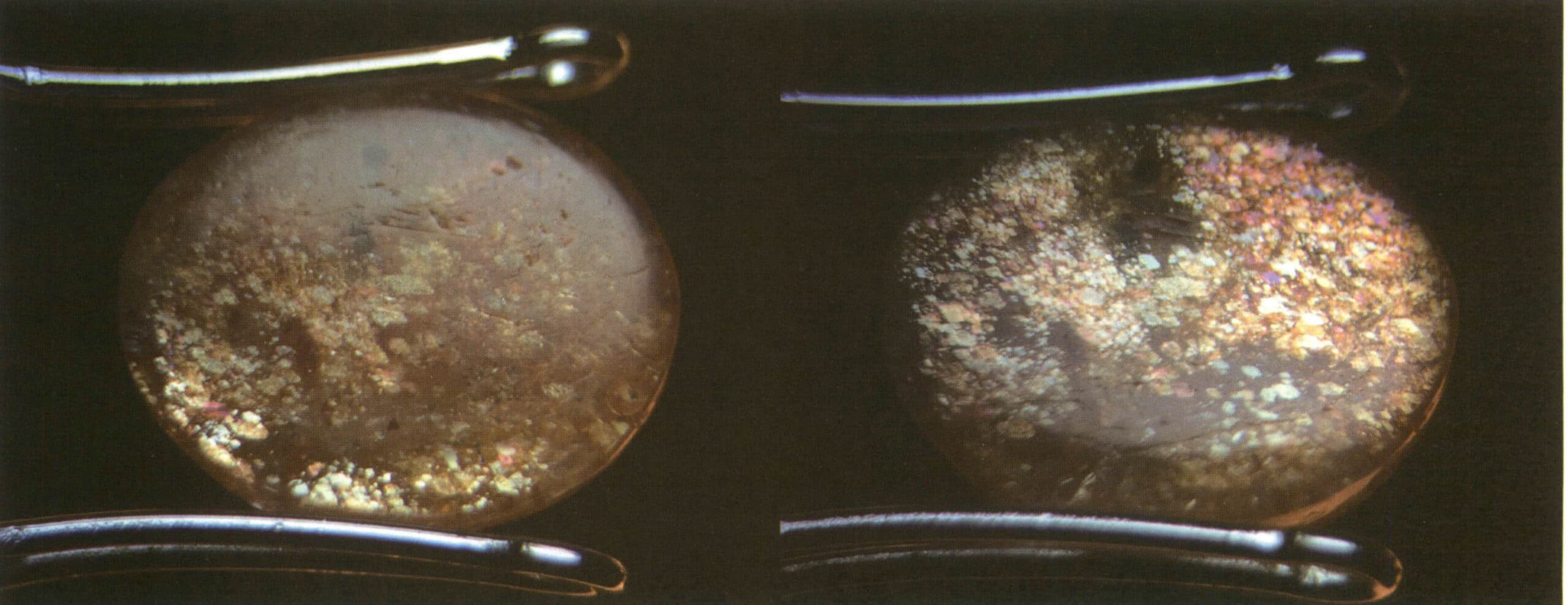
(4) Sand gold effect
Definition: When a transparent gemstone contains opaque, flaky solid inclusions, it produces a star-like reflection phenomenon due to the reflection of light by the opaque, flaky solid inclusions (Figures 2-3-76, 2-3-77).

Figure 2-3-76 Sunstone (orange-red, translucent).

Figure 2-3-77 Sunstone (light orange-red, transparent)
Cause: When a transparent or semi-transparent gem contains opaque or semi-transparent flaky solid inclusions (Figures 2-3-78, 2-3-79), the sand gold effect is visible, commonly found in sunstone and cordierite. This phenomenon is unrelated to the naturalness of the gem and whether the gem has been cut or polished.
Figure 2-3-78 Magnified features of inclusions in moonstone ( 10 x , vertical illumination method)
Figure 2-3-78 Magnified features of inclusions in moonstone ( 10 x , vertical illumination method) Figure 2-3-79 Magnified features of inclusions in sunstone ( 40 x, dark field illumination method)
Identification method: Illuminate the gem with reflected light, and the interior of the gem will display star-like reflections. The star-like reflections will flicker as the light source or the gem’s position moves relatively (Figure 2-3-80).
(5) Moonlight effect
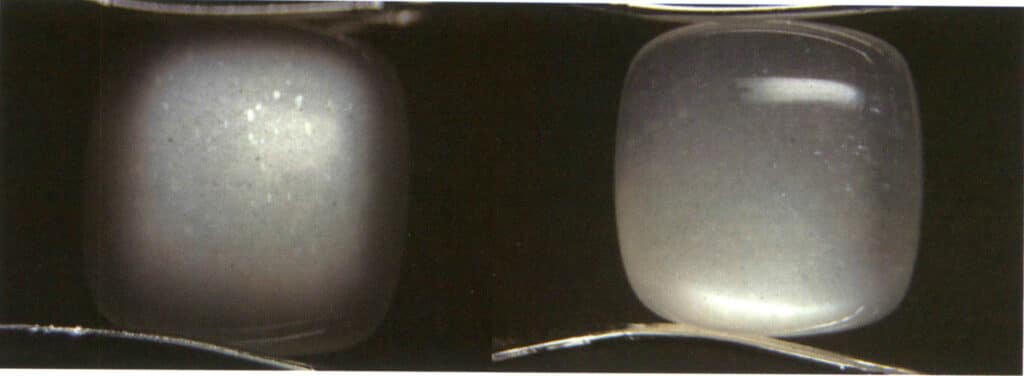
Definition: The phenomenon where incident light scatters inside the gemstone, resulting in bright blue or milky white light in localized areas on the gemstone’s surface. The moonlight effect can occur simultaneously with other special optical phenomenon, such as cat’s eye moonstone, spectral moonstone, etc. (Figure 2-3-81)

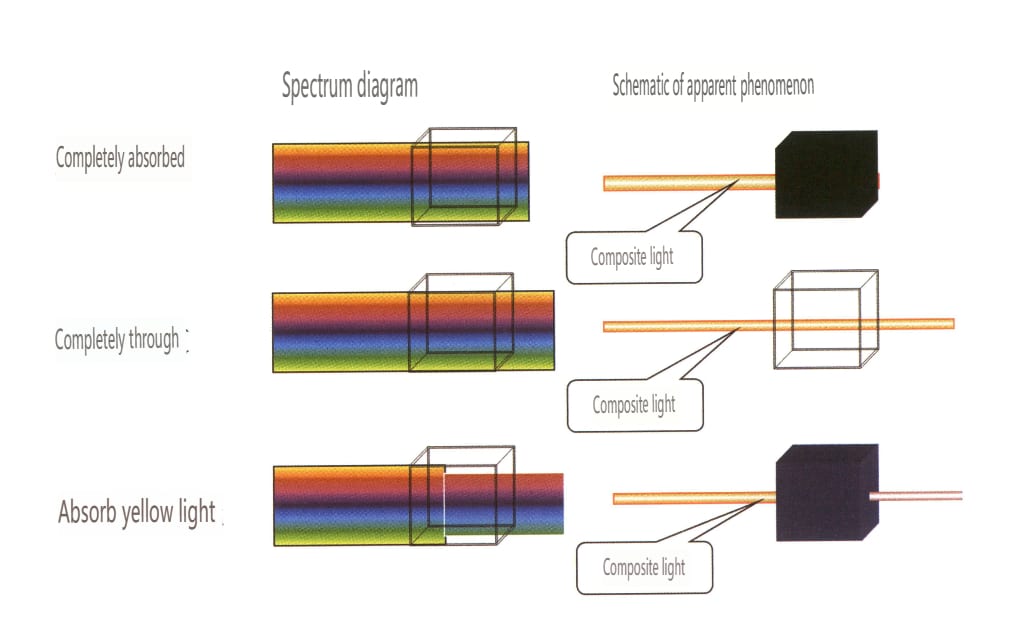
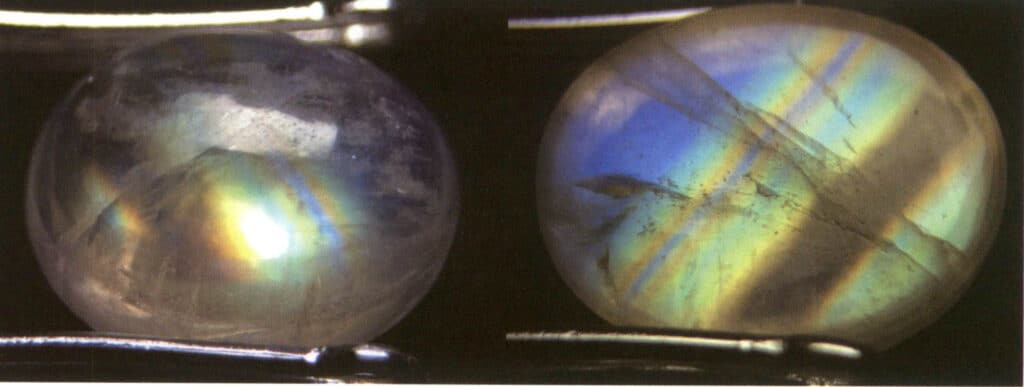
Cause: The moonlight effect is common in moonstone, a gem mineral with alternating layers of albite and potassium feldspar, and the thickness of the parallel layers of each component is between 50 and l00nm. This layered crossbedding structure scatters incoming light, creating a wandering color on the gem’s surface. The thicker the parallel layer, the lower the saturation of the wandering color and the more obvious the grayish white. For example, the blue moonlight effect can be observed from the front under the reflected light due to the strong scattering of blue and violet light. The scattering degree of other color light is small, and most of the composite light through the sample into the complementary color of blue and violet light – orange and yellow light (Figure 2-3-82).
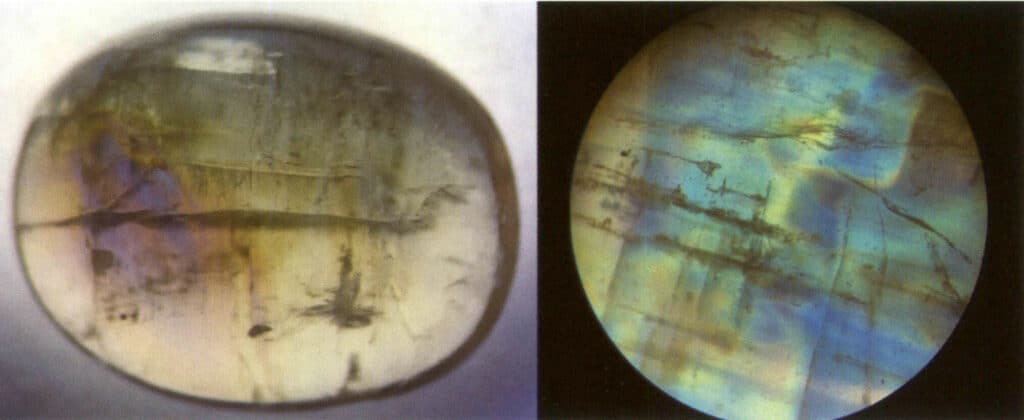
Identification method: Illuminate the gem with reflected light; a hazy color appears in a specific direction on the gem’s surface. The hazy color will shift as the relative position of the light source or the gem moves. When making slight rotations near the area where the moonlight effect occurs, there will be no change in the hue of the moonlight effect; however, if the rotation is too large, the moonlight effect will not be visible (Figures 2-3-83 to 2-3-86).
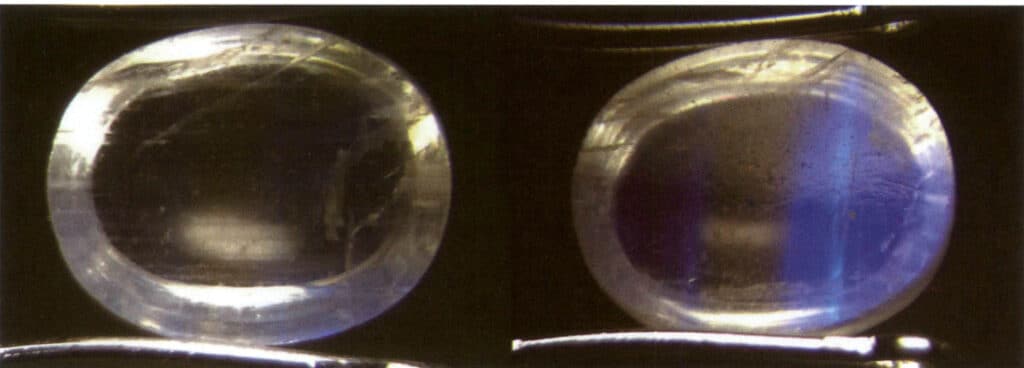
(6) Color-changing effect
The color change is also known as the play of color.
Definition: The color change exhibited by gemstones due to different light sources or observation angles is called the color change effect. Gemstones that can produce the color change effect include labradorite (Figure 2-3-87).

Cause: When light reflects or transmits through gemstones with specific structural compositions, the colors change due to diffraction and interference effects, depending on the direction of illumination or observation angle.
Identification method: Suppose the reflected light is used to illuminate the gem, even if the illumination direction and observation Angle do not change, as long as the gem is moved. In that case, it will see its color gradually transition to another color.
On the same gemstone, the parts with different colors are called color patches, which vary in shape and size. Their edges are often irregular and transition from one color patch to another (the color patches of opal-like color-changing glass, plastic, or synthetic opal often have regular serrated edges).
The spectrum presented by color change can be a full-color change from purple to red or a dichroic or trichroic color change from purple to green.
7. Dispersion of crystals
7.1 Definition of dispersion
Dispersion is the phenomenon where white composite light is decomposed into different wavelength spectra when passing through materials with prism properties. It can be described as the ability of gemstones to break white light into seven colors or understood as the colorful phenomenon visible inside faceted gemstones when shaken under a light source (Figure 2-3-88). It is commonly referred to as “fire” or “fire color” in the market, a technical term often discussed concerning diamonds.

Dispersion is a phenomenon unique to faceted crystal-type gemstones. Dispersion is unrelated to the naturalness of the gemstone; synthetic gemstones can also exhibit dispersion phenomena, such as synthetic strontium titanate, synthetic rutile, synthetic cubic zirconia, synthetic silicon carbide, and synthetic aluminum garnet (Figure 2-3-89). Dispersion is not related to the crystal system of the gemstone; for example, dispersion can be observed in diamonds of the isometric crystal system and synthetic silicon carbide of the hexagonal crystal system.

In actual gemstone identification, the colors and areas of dispersion presented by different gemstones in “total internal reflection” faceting vary, which can help us quickly distinguish diamonds from their imitations (Figures 2-3-90, 2-3-91).

Figure 2-3-90 Dispersion of diamond

Figure 2-3-91 Dispersion of synthetic silicon carbide (one of the common diamond simulants)
7.2 Key points for observing dispersion
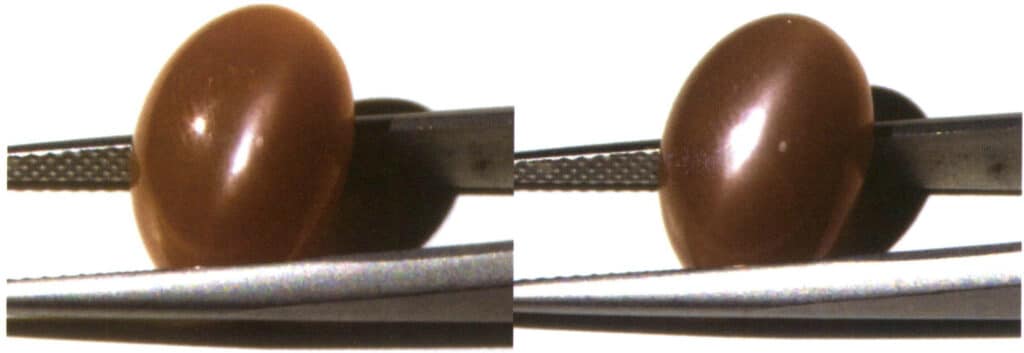
① Use transmitted light to observe the dispersion of the gem in a specific direction. To make the phenomenon more apparent, it is recommended to observe from the pavilion’s tip towards the crown’s table (Figure 2-3-92).
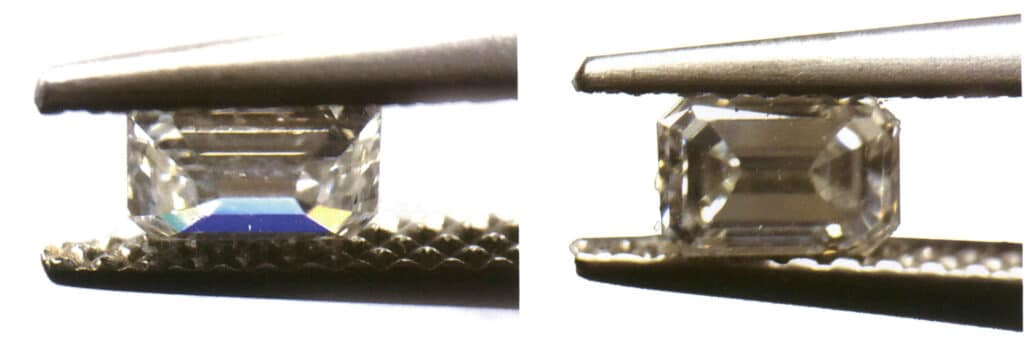
② When the gem contains obvious inclusions (impurities), reducing the gem’s transparency may affect the observation of dispersion.
③ Gems with the same degree of dispersion (which can also be described as having the same dispersion rate) are more difficult to observe if they are darker in color compared to lighter-colored gems under the same other conditions (Figure 2-3-93).
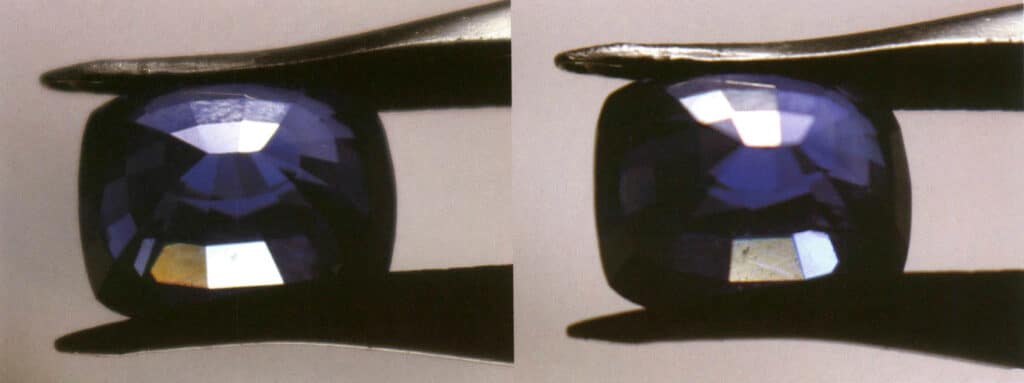
④ Dispersion is one of the common phenomena in faceted gemstones, and the quality of the cut (specifically, whether the cut can achieve “total internal reflection” of the light entering the gemstone) will affect the visibility of dispersion.
⑤ The omission of other factors does not affect the observation results of dispersion.
7.3 Description of methods of dispersion
We usually describe the dispersion phenomenon’s observation difficulty, such as obvious or not obvious.
8. Definitions of optical terms related to crystals when using conventional laboratory identification instruments
8.1 Isotropic and Non-homogeneous materials
(1) Isotropic body
Definition: A type of gemstone with isotropic optical properties. This includes gemstones of the isometric crystal system and some amorphous and transparent to translucent organic gemstones (Figures 2-3-94 to 2-3-96).
Identification method: Isotropic bodies before processing, can be preliminarily judged by their shape. Most isotropic bodies after processing can only be distinguished by instruments, such as observing whether the gemstone exhibits single refraction in a refractometer, magnifying to check for the absence of ghosting and whether it appears completely dark or shows abnormal extinction under polarized light.

Şekil 2-3-94 Gelişmiş kristal sisteminin değerli taşları (elmas)

Figure 2-3-95 Amorphous solids (natural glass)

Figure 2-3-96 Organic gemstone (yellow transparent amber)
Copywrite @ Sobling.Jewelry - Özel takı üreticisi, OEM ve ODM takı fabrikası
(2) Non-homogeneous body
Definition: A type of optical anisotropy in gemstones and minerals. Includes gemstones belonging to the trigonal system (Figure 2-3-97), tetragonal system (Figure 2-3-98), hexagonal system (Figure 23-99), orthorhombic system (Figure 2-3-100), monoclinic system (Figure 2-3-101), and triclinic system (Figure 2-3-102).
Identification method: The non-homogeneous body, before processing, can be accurately identified by its shape. After processing, some gemstones of the non-homogeneous body can be accurately identified if they exhibit visible pleochroism, but most non-homogeneous bodies need to be distinguished using a refractometer, microscope, polarizer, or dichroscope.

Figure 2-3-97 Intermediate crystal family trigonal system tourmaline

Figure 2-3-98 Zircon of the Intermediate Crystal Family in the Tetragonal System

Figure 2-3-99 Emerald of the Intermediate Crystal Family in the Hexagonal System

Figure 2-3-100 Topaz of the Low-Level Crystal Family in the Orthorhombic System

Figure 2-3-101 Spodumene of the Low-Level Crystal Family in the Monoclinic System

Figure 2-3-102 amazon stone of the low-level crystal system (triclinic)
8.2 Uniaxial refraction, birefringence, birefringence index
Uniaxial refraction refers to the phenomenon where the angle of incidence changes when light enters a transparent to semi-transparent homogeneous medium, and the light does not split.
Birefringence refers to the phenomenon that after the light enters the transparent to slightly transparent heterogeneous body, the incident angle changes, and the light is divided into two beams (Figure 2-3-103). The two beams of light that follow the refraction law of light are called normal light, and those that do not are called extraordinary light.
Birefringence is one of the phenomena of non-homogeneous gemstones, and certain gemstones with particularly high birefringence can exhibit double vision observable to the naked eye (Figure 2-3-104 to Figure 2-3-105)
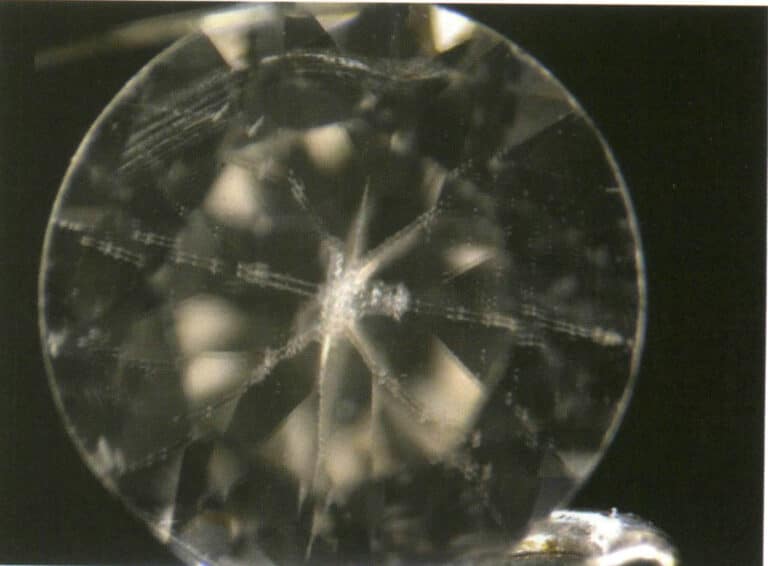
Figure 2-3-104 The phenomenon of double refraction in gemstones
Figure 2-3-105 The phenomenon of double refraction in gemstones (the double refraction index of synthetic silicon carbide on the left is 0.043, and the double refraction index of synthetic rutile on the right is 0.287)
8.3 Optical axis, optical indicatrix, uniaxial crystal, biaxial crystal
(1) Optical Axis
When light enters an non-homogeneous medium, it usually undergoes double refraction. However, in uniaxial crystals, there is one direction in which the incident light does not split; in biaxial crystals, there are two directions in which the incident light does not split. We refer to these in one or two directions in which the incident light does not split as the optical axis, represented as OA in crystal optics.
(2) Optical Indicatrix
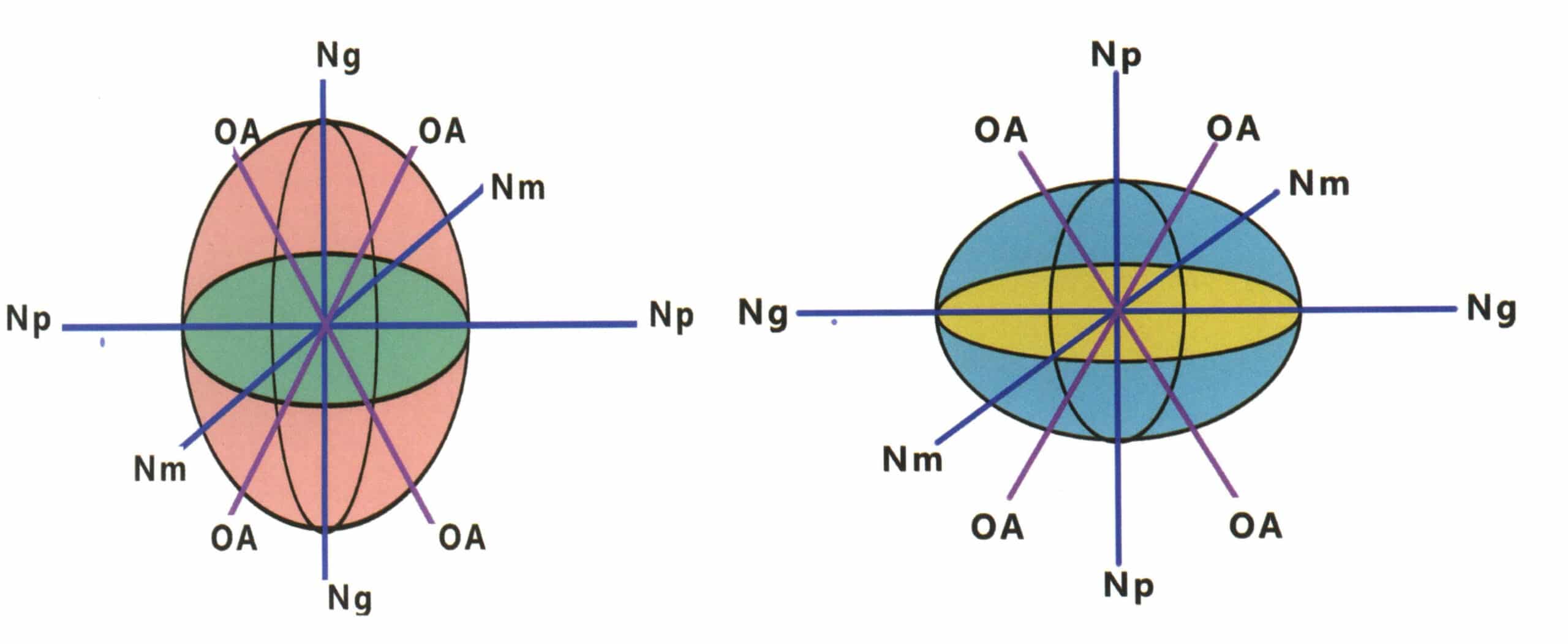
A hypothetical closed sphere whose radius is equal to the refractive index of the measured gemstone in all directions. Although the refractive index of the measured gemstone varies, the overall shape of the light-rate body has only two forms: a sphere and a rough sphere.
The light rate body of a isotropic body is a sphere. Any cross-section through the center of the sphere in any direction is a circular cross-section, and its radius represents the refractive index value of the isotropic gemstone (Figure 2-3-106). The light rate body of a non-homogeneous body is an ellipsoid, where the intermediate crystal family light rate body has a circular cross-section ellipsoid (Figure 2-3-107), and the lower crystal family light rate body has an elliptical cross-section ellipsoid (Figure 2-3-108).
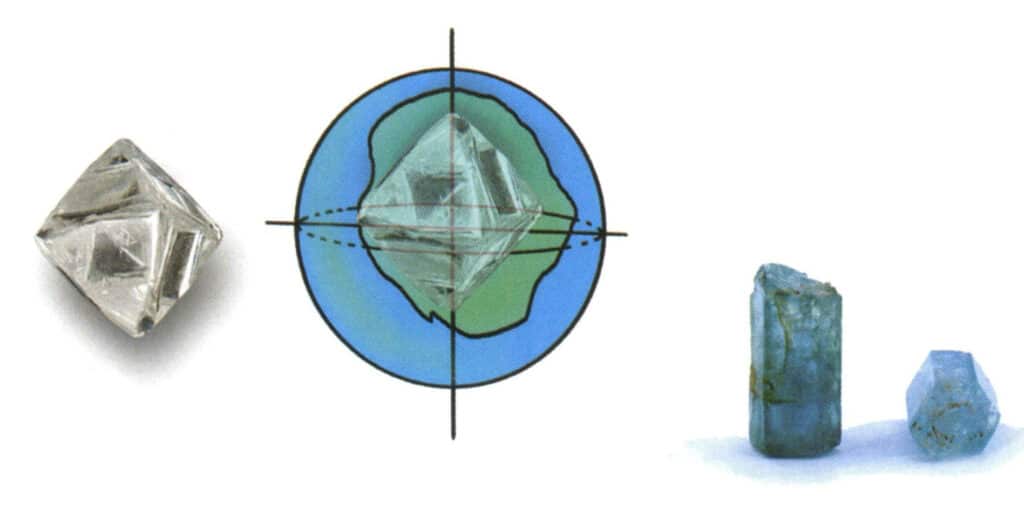
Figure 2-3-106 Luminosity of a homogeneous body
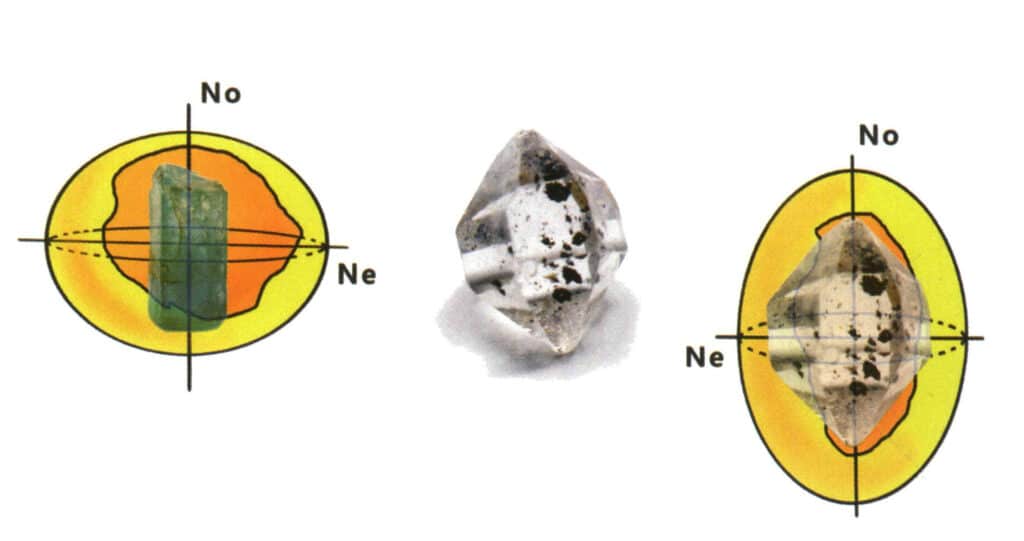
Figure 2-3-107 one-axis crystal light rate body (No is the direction of light refraction that follows optical laws, Ne is the direction of light refraction that does not obey optical laws, also known as the direction of extraordinary light, OA direction coincides with No, the cross-section is circular, OA indicates the direction of optical axis)
(3) Uniaxial crystal
A non-homogeneous gemstone with one optical axis is called a uniaxial crystal. Gemstones of the intermediate crystal family are all uniaxial crystal gemstones (Figure 2-3-109). For example, all trigonal system gemstones such as tourmaline, crystal, ruby, and sapphire, and all tetragonal system gemstones such as zircon, as well as all hexagonal system gemstones such as the beryl family and apatite.
Gemstones with a relatively perfect crystal form can be directly identified as uniaxial crystals based on their shape.
The imperfect crystal shape and processed gemstones cannot be determined to be uniaxial crystals based solely on their appearance (Figure 2-3-110). Only by observing the corresponding phenomena under a refractometer (Figure 2-3-111) or polarizing microscope (Figure 2-3-112) can a determination be made.

Figure 2-3-109 Intermediate crystal group tourmaline, the crystal form is relatively perfect and can be directly judged by the shape of the one-axis crystal.

Figure 2-3-110 The processed gemstone cannot be judged by its appearance (left emerald, right tourmaline)
Figure 2-3-111 refractometer

Figure 2-3-112 polarizer
(4) Biaxial crystals
Non-homogeneous gemstones with two optical axes are called diaxial. Gemstones of the lower crystal group are all biaxial gemstones (Figure 2-3-113). For example, topaz, olivine , and all other rhombic gems, diopside, monoclinic gems, lapidite, sunstone, moonstone, and triclinic gems.
Gemstones with a relatively perfect crystal form can be directly identified as biaxial crystals based on their shape (Figure 2-3-114).
Gemstones with imperfect crystal forms and those processed cannot be identified as biaxial crystals based on their shape; they can only be determined by observing the corresponding phenomena under a refractometer or polarizing microscope.

Figure 2-3-113 Topaz of the lower crystal group has a relatively perfect crystal shape and can be directly judged as a biaxial crystal by its shape.

Şekil 2-3-114 Bitmiş taş görünüşüne göre değerlendirilemez.
8.4 Dispersion rate, total internal reflection
(1) Dispersion rate
The difference in refractive index was measured for the B line (686.7 nm) and G line (430.8 nm) in the solar spectrum. Alternatively, it can be understood more simply as the difference between two specific refractive indices of the same gemstone, with each specific refractive index measured under light of a specific energy.
The dispersion rate of gemstones is rarely memorized; it is mainly used for reference and comparison.
Generally speaking, the higher the dispersion rate of a gemstone, the more likely it is to exhibit dispersion phenomena among faceted gemstones with the same total internal reflection degree (Figure 2-3-115). The dispersion rate of gemstones is rarely memorized; it is mainly used for reference and comparison.

(2) Total Internal Reflection
Refraction occurs when light passes through materials with different actual optical densities. When light moves from a dense medium to a less dense medium, the refracted ray deviates from the normal direction, and the refracted angle is greater than the incident angle. The angle of incidence when the refracted angle is 90° is called the critical angle; all incident light rays greater than the critical angle cannot enter the less dense medium and are reflected within the dense medium, following the law of reflection (Figure 2-3-116).
When using this principle in faceted cutting and grinding, it can still exhibit a noticeable dispersion phenomenon even if the gem’s dispersion rate is very low (Figure 2-3-117).
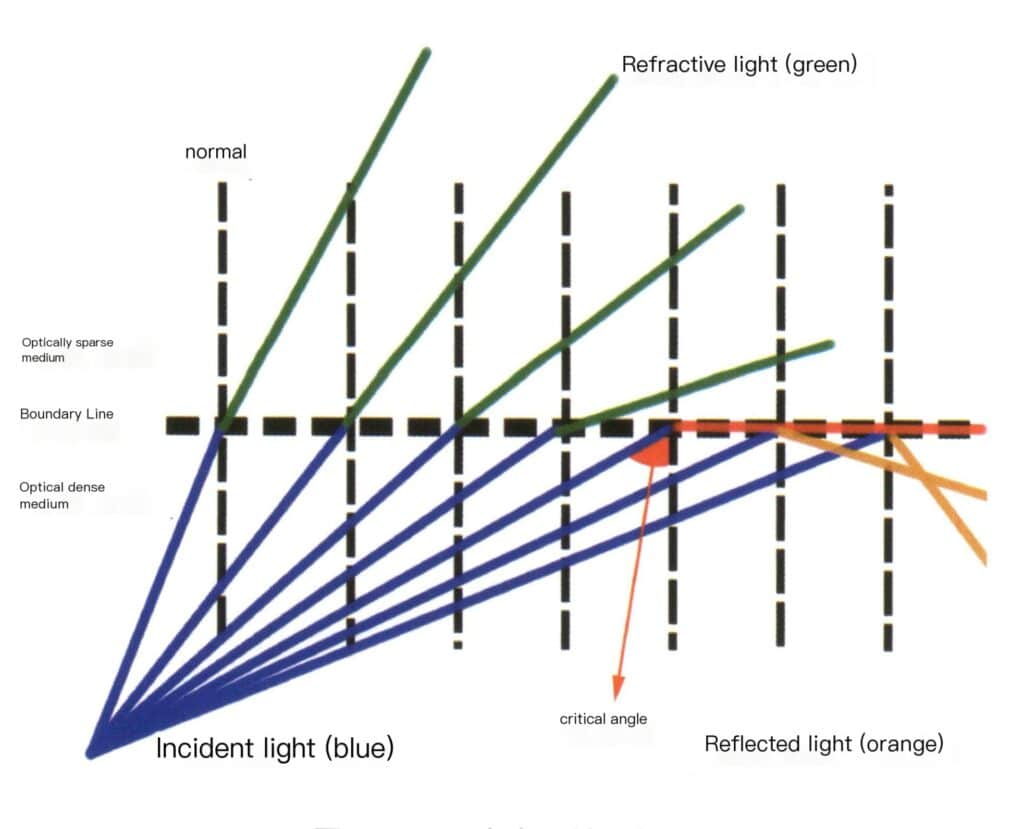
Figure 2-3-116 Schematic Diagram of Total Internal Reflection
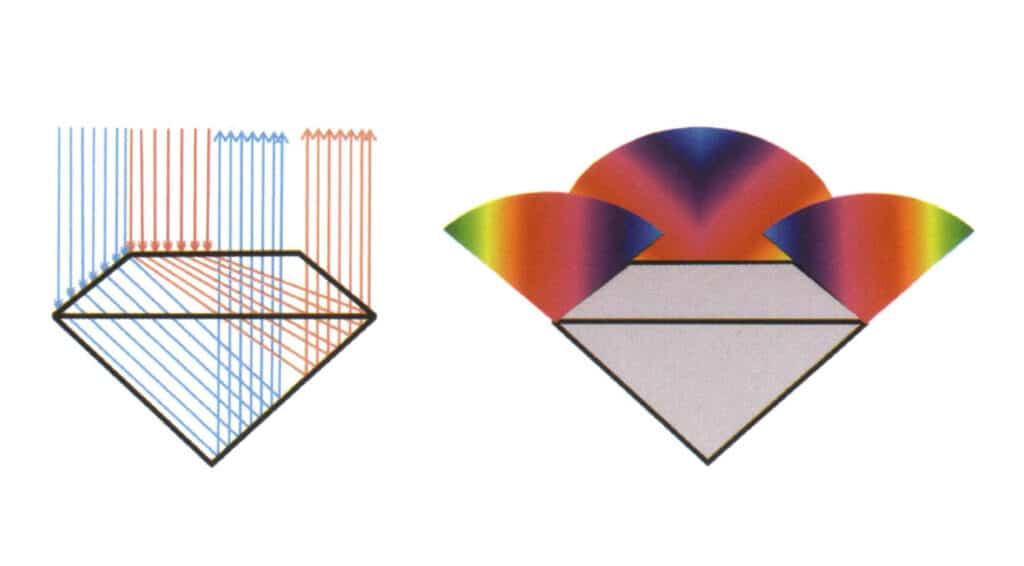
Figure 2-3-117 Schematic diagram of the light path of a standard round brilliant cut diamond with total internal reflection.
This principle is also applied in identifying diamonds and imitation diamonds, commonly called the line test. This experiment’s steps and analysis results are as follows: Place the gem with the largest face facing down and the pointed end facing up on a piece of paper with straight lines drawn on it. If lines can be seen through the gem, it indicates that the gem is an imitation diamond; otherwise, it is a diamond. It is particularly important to note that the experimental judgment is incorrect if the tested gem’s waist length-to-width ratio deviates from 1 1 or if the tested gem exhibits a sub-diamond luster or diamond luster (Figures 2-3-118 to 2-3-121).
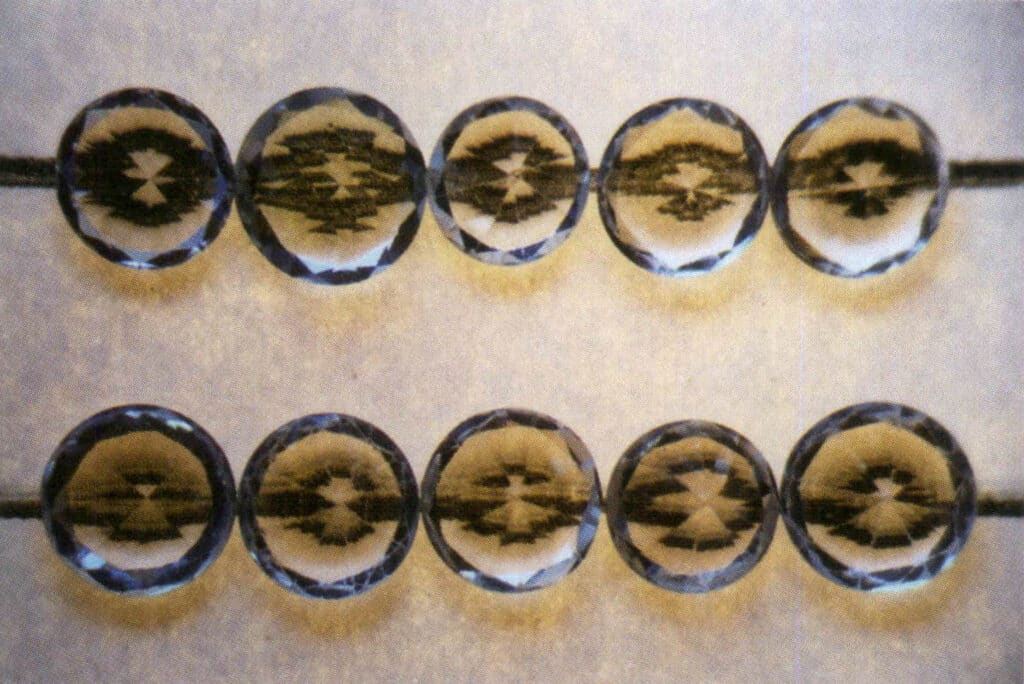
Figure 2-3-118 A straight line can be seen below the gemstone through the imitation diamond, and the straight line is divided into two.
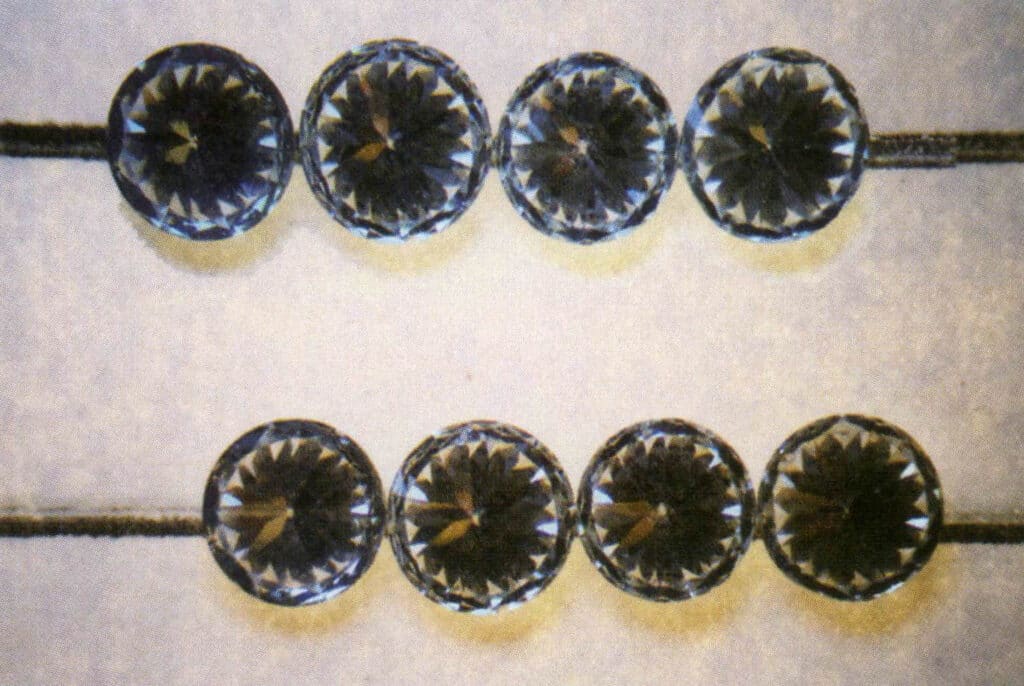
Figure 2-3-119 Straight lines cannot be seen through the diamond.
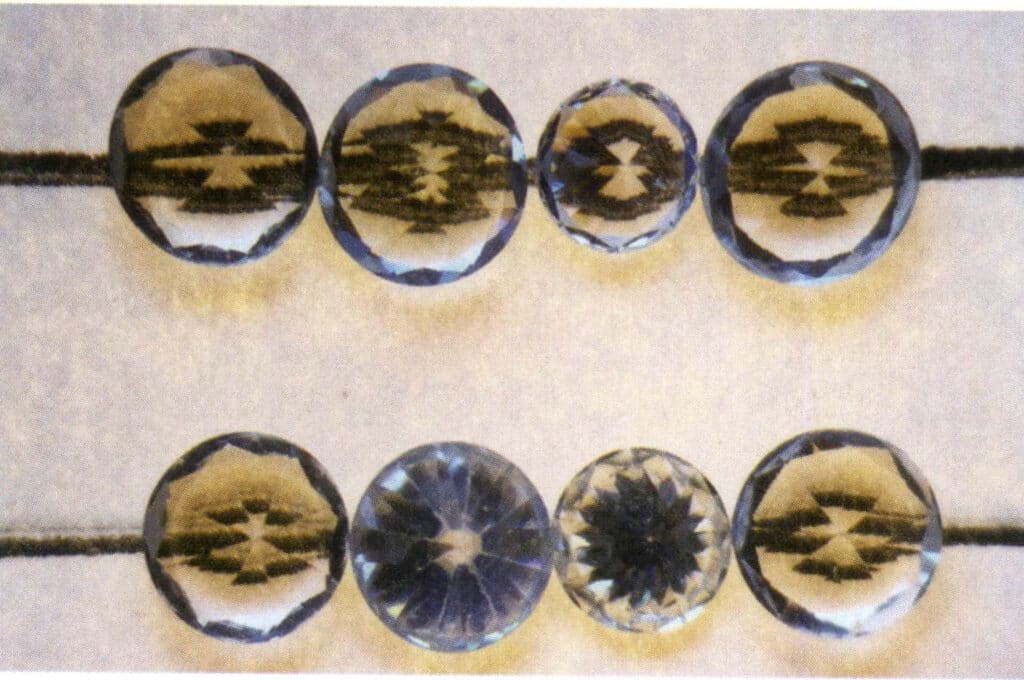
Figure 2-3-120 Some imitation diamonds exhibit phenomena similar to diamonds and cannot show the underlying lines through the gem (the two imitation diamonds in the middle of the second row).
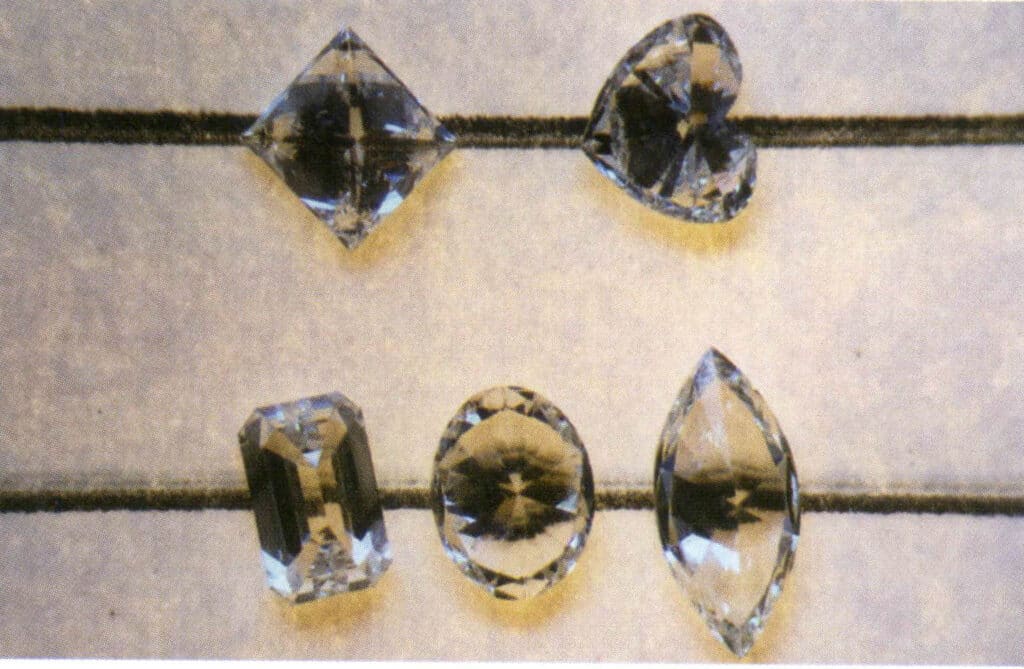
Figure 2-3-121 For diamonds with a length-to-width ratio not equal to 1:1, straight lines can also be seen through the gem (the diamond in the second row).
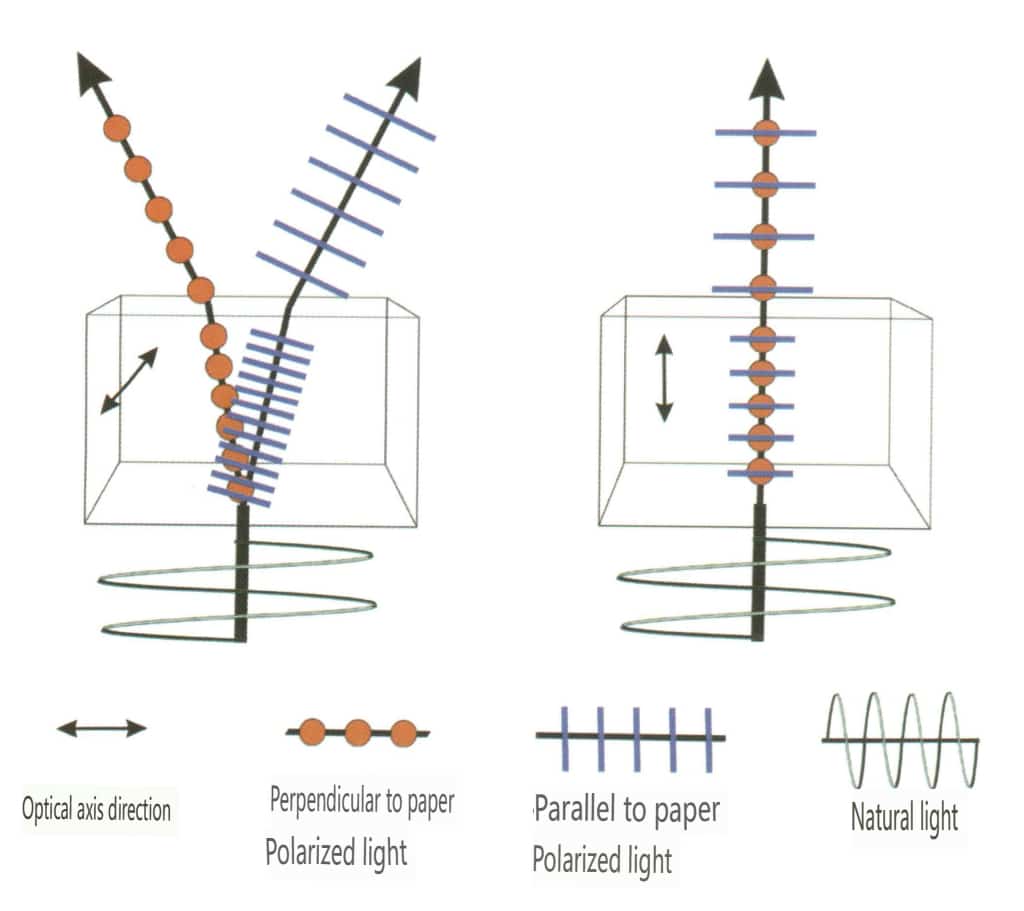
8.5 Natural Light, Polarized Light
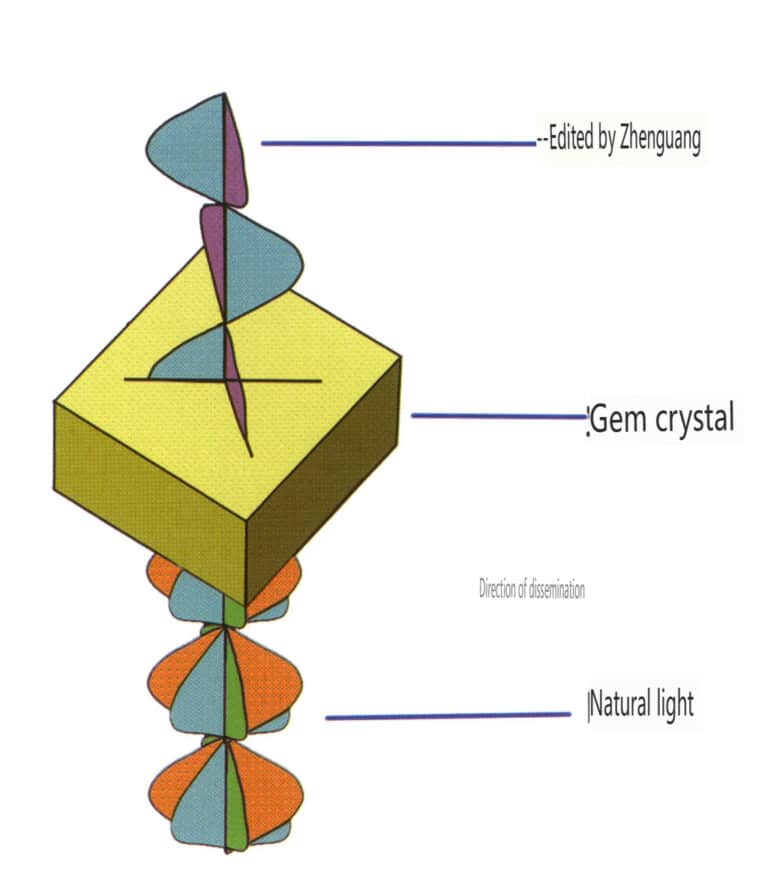
(1) Natural Light
The light emitted from a general light source contains light vectors in all directions, with equal amplitudes in all possible directions (axially symmetric). This type of light is called natural light. Natural light is represented by two mutually perpendicular, independent (without a definite phase relationship), equal amplitude light vibrations, each possessing half of the vibrational energy (Figure 2-3-122).
Natural light is one of the important light sources for observing gemstones with the naked eye, and there are many ways to obtain it, such as light in the shade on a sunny day, light from a flashlight, and light from specific color temperature lamps.
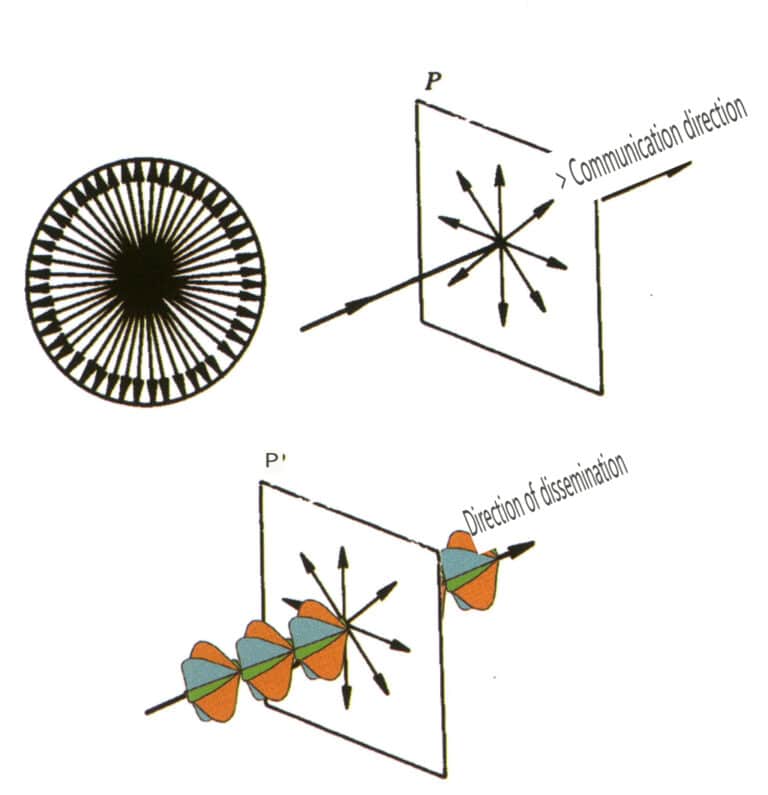
(2) Polarized Light
Light that vibrates only in a fixed direction is called polarized light. Polarized light will be specifically noted; if not noted, it is assumed to be natural (Figure 2-3-123).
The main way to obtain polarized light is to let natural light pass through a special polarizer or to let natural light pass through non-crystalline gemstones to produce polarized light.
Polarized light can be used to explain the appearance of gem color diversity, and the phenomenon of double refraction in gems is also the design principle of polarizing filters.
9. Summary of Crystal Optics Terminology Relationships
Many specialized terms are involved in crystals, and the relationships between optical terms can take time for beginners to understand. Therefore, this book summarizes the relationships between some optical terms involved in crystals (Table 1).
The optical term mentioned last exists as a separate phenomenon and has no relation to other optical terms.
Table 1: Summary table of crystal optics terminology relationships.
| Kristal | Can it be judged by the naked eye? | Common observation instruments | |||
|---|---|---|---|---|---|
| Crystal Classificat ion | Gelişmiş kristal ailesi | Ara kristal ailesi | Düşük seviyeli kristal ailesi | The typical shapes of crystals can be observed with the naked eye, usually requiring instruments for assistance. | Refractometer, polarizer , dichroscope, microscope |
| İzometrik kristal sistemi | Trigonal crystal system, tetragonal crystal system, hexagonal crystal system | Orthorhombic crystal system, monoclinic crystal system, triclinic crystal system | |||
| Optical property | Isotropic body | Non-homogeneous body | |||
| Uniaxial crystal positive or negative birefringence | Uniaxial crystal positive or negative birefringence | × | Refractometer Polarizer | ||
| Light refraction | Uniaxial refraction | Birefringence exhibits uniaxial refraction in a certain direction | Birefringence Exhibiting single refraction in certain two directions | High birefringence can be observed with the naked eye, but generally requires the aid of instruments. | Refractometer, polarizer, microscope. |
| Polychro maticity | No pleochroism | Strong to weak dichroism | Trichroism from strong to weak or dichroism from strong to weak | A few gemstones can, but most require the use of instruments | Dichroscope |
| Renk | It is unrelated to whether it is a crystal and the classification of crystals; the color of the crystal depends on the impurity elements and lattice defects within the crystal. | √ | × | ||
| Parlaklık | It is unrelated to whether it is a crystal and its classification; the polishing degree of any type of gemstone will affect its luster | √ | × | ||
| Şeffaflık | It is unrelated to whether it is a crystal and the classification of crystals; the transparency of a crystal often depends on the content of inclusions within the crystal. | √ | × | ||
| Luminescence | It is unrelated to whether it is a crystal and the classification of crystals; it depends on the impurity elements and lattice defects within the crystal | A few gemstones can, but most require the use of instruments | Ultraviolet fluorescent lamp | ||
| Special optical phenomenon | Possible color change effects, etc. | Possible cat's eye effect, starlight effect, color change effect, etc. | Possible effects include cat's eye effect, starlight effect, color change effect, gold dust effect, moonlight effect. | √ | × |
| Dağılım | This phenomenon is common in crystal gemstones but is unrelated to crystal classification; the visibility of dispersion depends on the crystal's dispersion rate and the degree of total internal reflection of the facets. | √ | × | ||
Section II Why do gemstones have color
1. Traditional causes of gemstone color
In field mineral identification, there is a very important piece of evidence called streak color, which involves rubbing the obtained natural material on an unglazed white porcelain plate to leave mineral powder, using the color of the mineral powder to identify certain characteristic minerals (Table 2).
Table 2: The relationship between mineral color, streak color, transparency, and luster
| Renk | Streak color | Şeffaflık | Parlaklık |
|---|---|---|---|
| Renksiz | Colorless or white | Şeffaf | Glass luster |
| Light color | Colorless or white | ||
| Dark color | Light or colorful | Semi-metallic luster | |
| Metallic color | Dark or metallic color | Opaque | Metallic luster |
According to literature records, as early as the Eastern Jin period, people were already able to use streak color to distinguish between silver-gold ore and natural gold.
The streak color is of great significance for the identification of minerals.
① The streak color of minerals eliminates pseudo colors; in powdered form, minerals will lose all interfaces that affect light, and the pseudo colors of minerals disappear.
② The streak color of the mineral has weakened allochromatic color.
③ The streak color of the mineral highlights idiochromatic color.
The powder cannot reflect light and is not transparent for opaque minerals (mainly those with metallic luster), so the streak is gray-black. Semi-transparent minerals absorb some light, so the streak color is not very different from the bulk minerals. Due to their good light transmission and almost no absorption of visible light, transparent minerals appear white.
Pyrite and bornite belong to minerals with a metallic luster, so their streak is black; the crystalline hematite is generally called specular hematite, which has a sub-metallic to metallic luster and absorbs some wavelengths of light, thus presenting a certain color, namely red; At the same time, rhodochrosite is a transparent mineral, so its streak is white.
To explain the color differences between the color of large solid mineral pieces and their streak color, mineralogy classifies mineral colors into three types: idiochromatic color, allochromatic color, and pseudo color, based on the hypothesis of chromophore elements (Table 3). This hypothesis also applies to gemstones within minerals.
Table 3: Common coloring elements in gemstones
| Coloring elements | Atomic number | Gemstone color | Gemstone Examples |
|---|---|---|---|
| Iron Fe | 26 | Colors such as red, blue, green, yellow, etc. | Blue sapphire, peridot, aquamarine, tourmaline , blue spinel, jade, almandine, olivine, diopside, idocrase , kyanite, etc. |
| Chromium Cr | 24 | Green and red | Ruby, emerald, jade, alexandrite, uvarovite, red spinel, demantoid, pyrope, tourmaline and others |
| Manganese Mn | 25 | Pink, orange | Red beryl, rhodochrosite, rhodonite, Spessartine-Garnet , charoite, certain red tourmalines, etc. |
| Diamond Co | 27 | Pink, orange, blue | Blue synthetic spinel, synthetic alexandrite, etc. |
| Lanthanum Pr, Neodymium Nd | Praseodymium 59 Neodymium 60 | Praseodymium and neodymium often coexist to form yellow and green | Apatite, light purple synthetic cobalt oxide, etc. |
| Uranium U | 92 | Causes the original gem color | Zirkon |
| Key V | 23 | Green, purple, or blue | Essonite, Zoisite, synthetic corundum (imitation alexandrite), etc. |
| Copper Cu | 29 | Green, blue, red, etc. | Malachite, silicon malachite, turquoise, azurite, etc. |
| Selenium Se | 34 | Kırmızı | Certain red glass, etc. |
| Nickel Ni | 28 | Yeşil | Chrysoprase, green opal, etc. |
| Scandium Ti | 22 | Mavi | Sapphire, benitoite, topaz, etc. |
(1) Idiochromatic color
The color is caused by elements that are basic chemical components of gemstone minerals, most of which are transition metal ions. The color of self-colored gemstones is stable (Table 4).
Table 4: Common self-colored gemstones and their coloring elements
| Değerli Taş Adı | Kimyasal bileşim | Gemstone color | Coloring elements |
|---|---|---|---|
| Uvarovit | Ca3Cr2 (SiO4) | Yeşil | Krom |
| Olivine | (Fe,Mg)2SiO4 | Yellow-green | Demir |
| Malakit | CU2(CO3)(OH)2 | Yeşil | Bakır |
| Rodokrozit | MnCO3 | Pembe | Mn |
| Turkuaz | CUAl6((PO4)4(OH)8 •4H2O | Mavi | Bakır |
| Spessartine-Garnet | Mn3Al2(SiO4) | Orange | Mn |
| Rhodonite | (Mn,Fe,Mg,Ca)SiO3 ve SiO3 | Magenta | Mn |
| Almandine | Fe3Al2(SiO4) | Kırmızı | Demir |
(2) Allochromatic color
The color is caused by chromophore elements contained in gemstone minerals. The color of other gemstones is stable.
① When pure-colored gemstones are colorless, they can produce colors when containing trace coloring elements, with different trace coloring elements producing different colors. For example, spinel and tourmaline (Table 5).
② Different valences of the same element can produce different colors, such as those containing Fe³⁺ often appearing brown, while those containing Fe²⁺ often appear light blue, such as aquamarine.
③ The same element in the same oxidation state can also cause different colors in different gemstones, such as Cr³⁺, producing red in corundum and green in emerald.
Table 5: Colors of Some Other Gemstones and Their Coloring Elements
| Değerli Taş Adı | Kimyasal bileşim | Gemstone color | Coloring elements |
|---|---|---|---|
| Spinel | MgAI2O4 | Renksiz | - |
| Mavi | Fe or Zn | ||
| Kahverengi | Fe, Cr | ||
| Yeşil | Fe | ||
| Kırmızı | Cr | ||
| Turmalin | (Na,Ca)R3Al3Si16O18 (O,OH,F) , where R mainly refers to elements such as Mg , Fe , Cr, Li, Al , Mn | Renksiz | - |
| Kırmızı | Mn | ||
| Mavi | Fe | ||
| Yeşil | Cr, V, ,Fe | ||
| Brown, yellow | Mg |
(3) Pseudo-color
Pseudo-color does not have a direct effect on the chemical composition of gemstones. Gemstones with pseudo-color often contain tiny parallel arranged inclusions, such as dissolved crystal chips and fissures. They refract, reflect, interfere, and diffract light, thus producing pseudo-color. Certain special gemstone cuts can also cause pseudo-color in gemstones (Table 6).
Pseudo colors are not inherent to the gemstone but can add charm.
Table 6: Classification of Causes of Pseudo RenkS
| Classification of Causes | Definition | Example |
|---|---|---|
| Dağılım | The phenomenon where white composite light is decomposed into different wavelength spectra when passing through materials with prism properties. | Diamond, zircon, synthetic cubic zirconia, synthetic silicon carbide, sphalerite, artificial strontium titanate, synthetic rutile, etc. |
| Scattering | The phenomenon where light beams deviate from their original direction and disperse during propagation in a medium due to the presence of uneven clumps in the material. | (1) The color changes of gemstones that can be explained by scattering include blue moonstone, blue quartz, opal, purple fluorite, and white milk quartz. (2) Special optical phenomenon that can be explained by scattering include cat's eye effect, star effect, and sand gold effect. (3) One type of luster that can be explained by scattering is the pearly luster. |
| Interference | The phenomenon of the superposition of two monochromatic light sources emitting two columns of light waves that are in the same direction, have the same | (1) Can be used to explain the iridescence caused by the presence of fissures or cleavage, such as iridescent quartz (Figure 2-3-124). (2) Can be used to explain the color-changing effect in special optical phenomenon, such as opal. (3) Can be used to explain the opaque surface of bornite and the bronze color produced by the oxidation of synthetic silicon carbide. No gemstone has a bronze color (figure 2-3-125). |
| Diffraction | The phenomenon of light waves deviating from their geometric path when encountering obstacles during propagation. |

Figure 2-3-124 Colorful Quartz

Figure 2-3-125 Rust color
2. The Modern Causes of Gemstone Color
Each hypothesis has its limitations. In the study of modern gemstone minerals, traditional color-causing mineralogists and gemologists have found that the appearance or change of color in certain gemstone minerals cannot be explained, such as the color causes of diamonds and the changes in gemstone color before and after irradiation treatment.
Modern physics and chemistry development has compensated for the shortcomings of traditional color genesis hypotheses. It is based on crystal field theory, molecular orbital theory, band theory, and physical optics theory, combined with spectroscopic methods to explain the colors of gemstones.
Modern theories of material structure suggest that matter is composed of atoms, which consist of a nucleus and electrons, with electrons moving outside the nucleus. Quantum mechanics describes the motion of electrons and other microscopic particles. In 1913, Bohr proposed the hypothesis that atoms exist in stable states with definite energy, known as stationary states. Each type of atom can have many stationary states with different energy values, and these stationary states are arranged for energy to form energy levels, with the lowest energy stationary state being called the ground state, and the other states being called excited states. Generally, atoms or ions are in a stable state, that is, in the ground state, at which point there is no radiated energy. If an atom or ion is subjected to external thermal energy, electrical energy, or other forms of energy, the outer electrons will absorb energy and transition to an excited state. However, electrons in the excited state are unstable, and after about 10⁻⁸ seconds, the electrons return to the ground state, simultaneously radiating a portion of energy in the form of light.
The above viewpoint can be understood in gemology as the appearance of color in gemstones being due to the effect of external energy, such as light, on the electrons in the atomic composition of the gemstone. This causes electrons to transition from the ground state to the excited state, selectively absorbing specific wavelengths of light. The types of electron transitions and the differences in absorbed energy during this process result in the different colors that gemstones ultimately present. Table 7 is a comprehensive summary by Russian and American scholars, categorizing gemstone colors into 12 types belonging to 4 main theories.
Table 7: Modern Color Types of Gemstones
| Corresponding traditional color causes | Modern color cause theory models | Modern color causes types | Typical gemstones |
|---|---|---|---|
| Idiochromatic color, Allochromatic color | Crystal Field Theory | Transition Metal | Malachite, garnet, turquoise, etc. |
| Transition Metal Impurities | Emerald, Citrine, Ruby, etc. | ||
| Colour center | Amethyst, Smoky Quartz, Fluorite, etc. | ||
| Molecular Orbital Theory | Charge Transfer | Sapphire, Lapis Lazuli, etc. | |
| Organic dyeing | Amber, coral, etc. | ||
| Band theory | Conductor | Copper (Cu ) , silver (Ag ) , etc. | |
| Semiconductor | Galena, proustite, etc. | ||
| Impure semiconductor | Blue diamonds, yellow diamonds, etc. | ||
| Pseudo color | Theory of Physical Optics | Dağılım | "Fire" of Faceted Diamonds, etc. |
| Scattering | Moonstone, etc. | ||
| Interference | Coloring chalcopyrite and others etc. | ||
| Diffraction | Opal, chalcopyrite surface color, etc. |
Section III Explanation of Mechanical Properties Related to Crystals
The mechanical properties of gemstones are divided into four major categories and seven phenomena: cleavage, fracture, and breakage belong to one category, while the other three categories are hardness, density, and toughness. Here, we will discuss cleavage, fracture, breakage, hardness, and relative density related to crystals.
Cleavage, fracture, and breakage are the properties of crystals that occur under external force, and their breaking characteristics and causes differ. They are one of the important physical properties for identifying and processing gemstones.
1. Cleavage of Crystals
1.1 Definition of cleavage
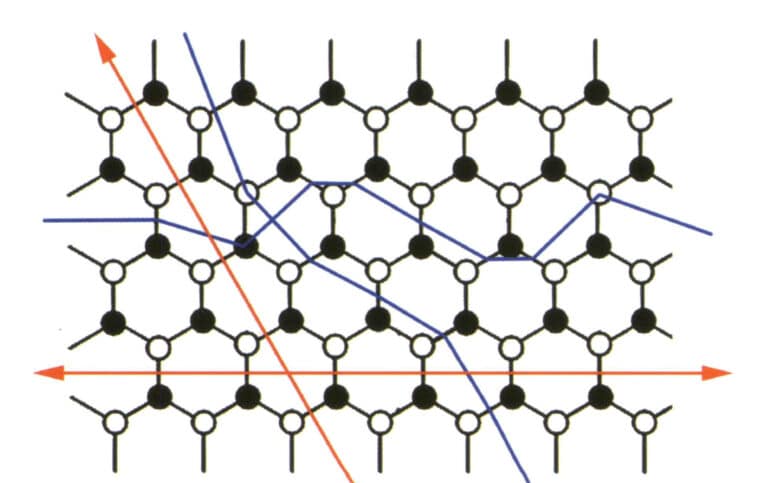
The phenomenon where a crystal breaks along certain crystallographic directions into smooth planes under external force is called cleavage and these smooth planes are referred to as cleavage planes (Figure 2-4-1).

Cleavage can be used to distinguish between different crystals. The integrity degree of the cleavage plane, cleavage direction, and cleavage Angle of different crystals are different. Cleavage is one of the important features reflecting crystal structure (figure 2-4-2) and has a more general significance than crystal morphology. No matter how close the crystal is to the ideal level, as long as the crystal structure does not change, the characteristics of the cleavage remain unchanged, which is an important characteristic basis for the identification of crystals.
1.2 Key points for observing cleavage
Observing the fracture surface in a crystal or gemstone from a certain direction with reflected light, if the fracture surface is flat and shows a mirror-like flash during shaking, then this fracture surface is called cleavage.
Cleavage surfaces can appear not only in crystals but also in processed gemstones, such as the feather-like waist of a finished diamond and the centipede-like cleavage in a moonstone.
When observed with reflected light, cleavage surfaces sometimes exhibit a pearly luster (Figure 2-4-3), and interference colors can also be seen between the cleavage layers (Figures 2-4-4, 2-4-5).
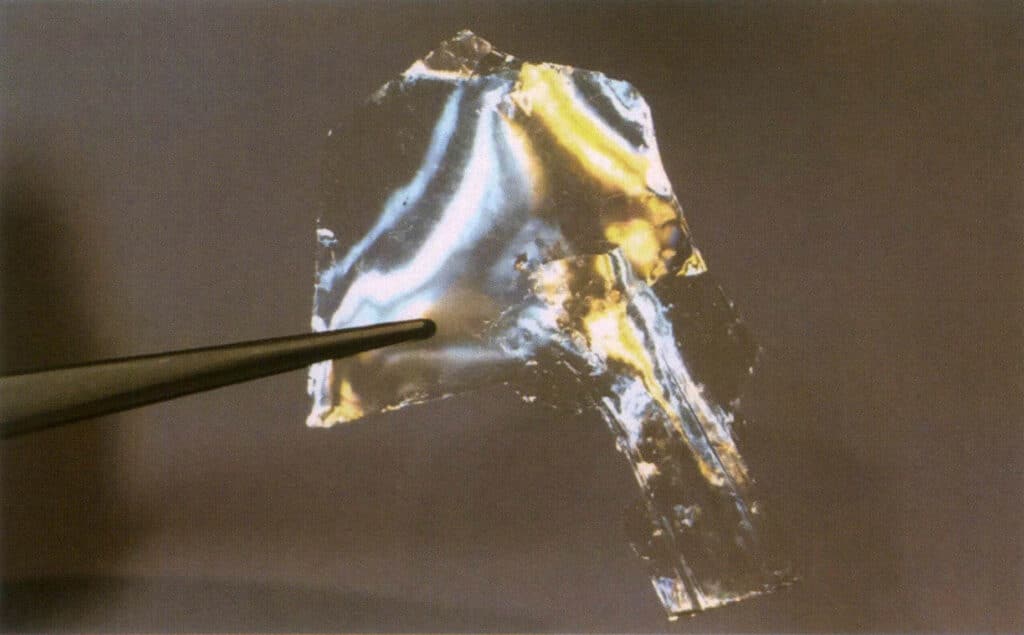
Figure 2-4-3 Mica with perfect cleavage showing pearly luster

Figure 2-4-4 Interference Colors Between Completely Cleaved Layers of Gypsum

Figure 2-4-5 Interference colors between layers of gypsum with perfect cleavage
1.3 Description Methods of Cleavage
The description of cleavage is divided into three aspects: the completeness of the cleavage plane, the cleavage’s direction, and the cleavage’s angle.
(1) Completeness of cleavage surfaces
Based on the presence or absence of cleavage and the degree of smoothness (also known as the degree of development), cleavage can be divided into four categories: complete cleavage, complete cleavage, moderate cleavage, and incomplete cleavage (Table 1).
Table 1: Cleavage Levels and Observation Characteristics
| Cleavage level | Difficulty Level | Characteristics of Cleavage Surface Observation | Example |
|---|---|---|---|
| Perfect cleavage | Easily split into thin sheets | Smooth and flat thin sheets | Mica, graphite, etc. |
| Complete cleavage | Easily splits into planes or small pieces, with difficult fracture surfaces. | Smooth, flat, and shiny surfaces that can present a stepped appearance. | Diamond, topaz, fluorite, calcite, etc. |
| Moderate cleavage | Can split into planes, with fractures appearing more easily | A relatively flat surface, not very continuous and somewhat rough. | Chrysoberyl, moonstone, etc. |
| Incomplete cleavage | Not easy to split into planes, with many fractures | Discontinuous, uneven, with a greasy feel | Apatite, zircon, olivine, etc. |
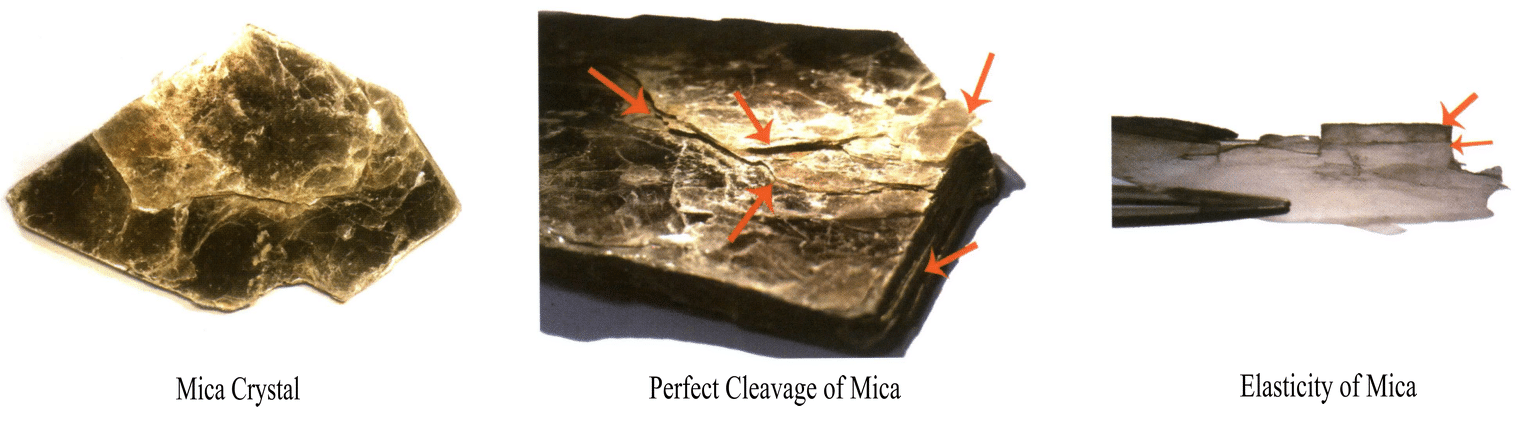
Crystals with perfect cleavage are unsuitable for jewelry due to their durability and poor workability. For example, Mica (Figure 2-4-6) and graphite.
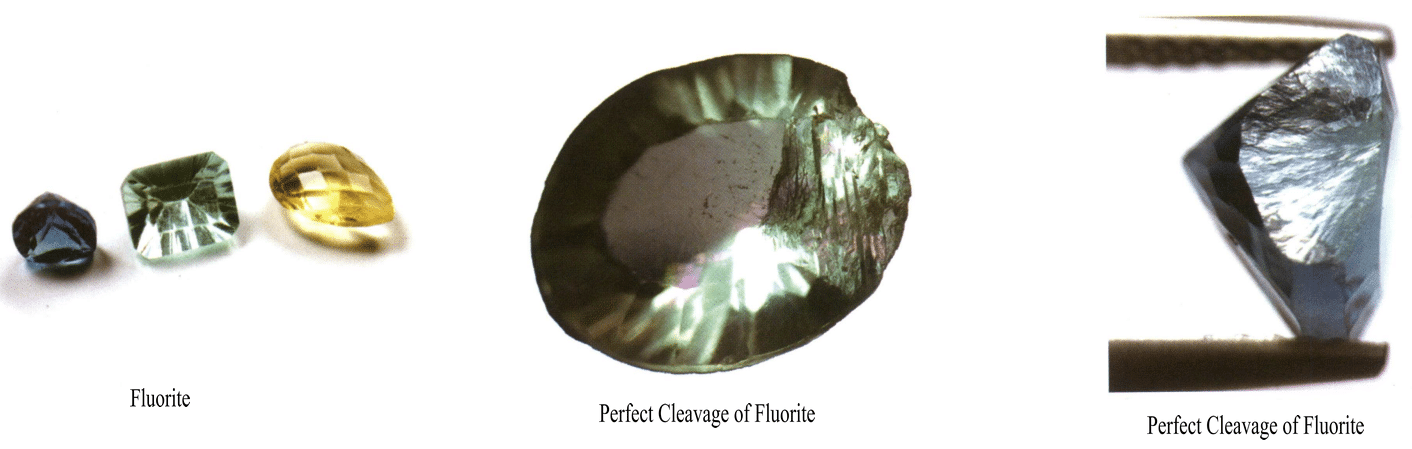
Crystals of other degrees of cleavage other than the very perfect cleavage can be used as gemstones, such as perfectly cleavage diamonds and fluorite (Figure 2-4-7). Topaz (Figure 2-4-8), etc.
The word development is often used when describing or discussing cleavage, and it can be understood to mean predisposition, such as cleavage development, which means that cleavage tends to occur.

(2) Cleavage direction
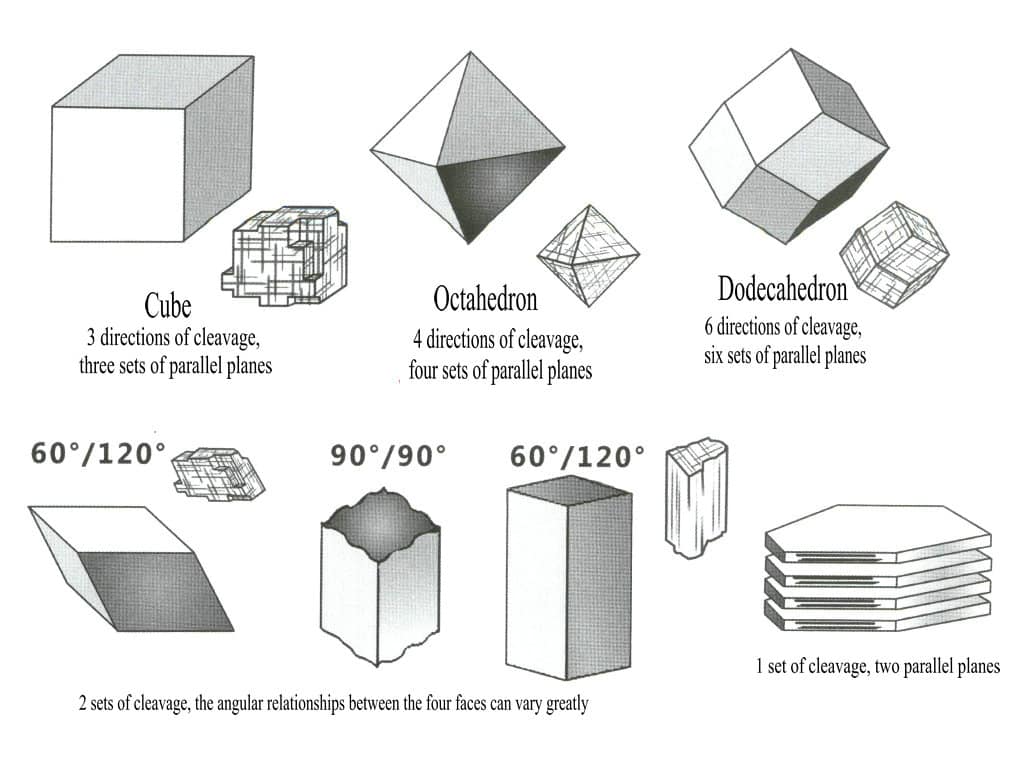
Different minerals may have one cleavage direction or multiple directions.
Commonly, there is one direction (graphite, Mica, etc.), two directions (hornblende, etc.), three directions (calcite, etc.), and additionally four directions (such as fluorite) and six directions (such as sphalerite) cleavage (Figure 2-4-9).
Since cleavage is a directional phenomenon, it is important to ensure that the plane of the gemstone being processed is not parallel to the cleavage plane. It must be offset by at least 5° degrees; otherwise, there will be a phenomenon where the facets cannot be polished smoothly and brightly no matter what.
(3) Cleavage intersection angle
For crystals or gemstones with two or more cleavage directions, the multiple cleavage directions are at certain angles, and this angular relationship is called the intersection angle (Figures 2-4-10, 2-4-11).
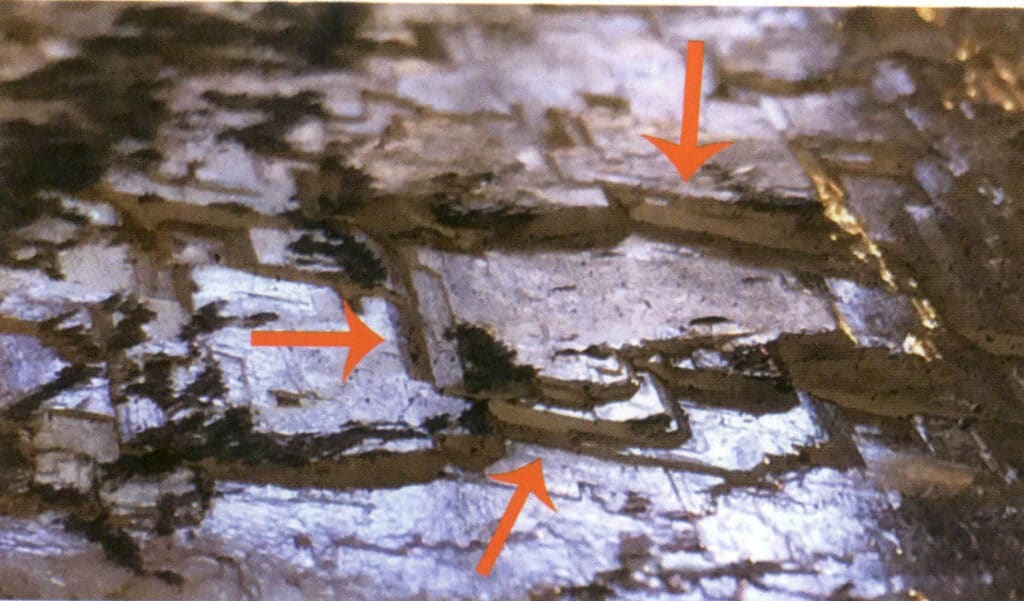
Figure 2-4-10 Tri-directional cleavage of gypsum (the red arrows indicate the three different directions of the step-like perfect cleavage)

Figure 2-4-11 Gypsum cleavage intersection angle 120°
2. Cleavage of Crystals
2.1 Definition of Cleavage
The phenomenon where a crystal breaks along certain crystallographic directions under external force, resembling cleavage, but with a smoother surface than cleavage.
Fracture and cleavage have different causes; fractures often occur at the twin boundary, especially in certain aggregate twin gemstones, and in gemology, they only appear in corundum (Figure 2-4-12)

2.2 Key points for observing fractures
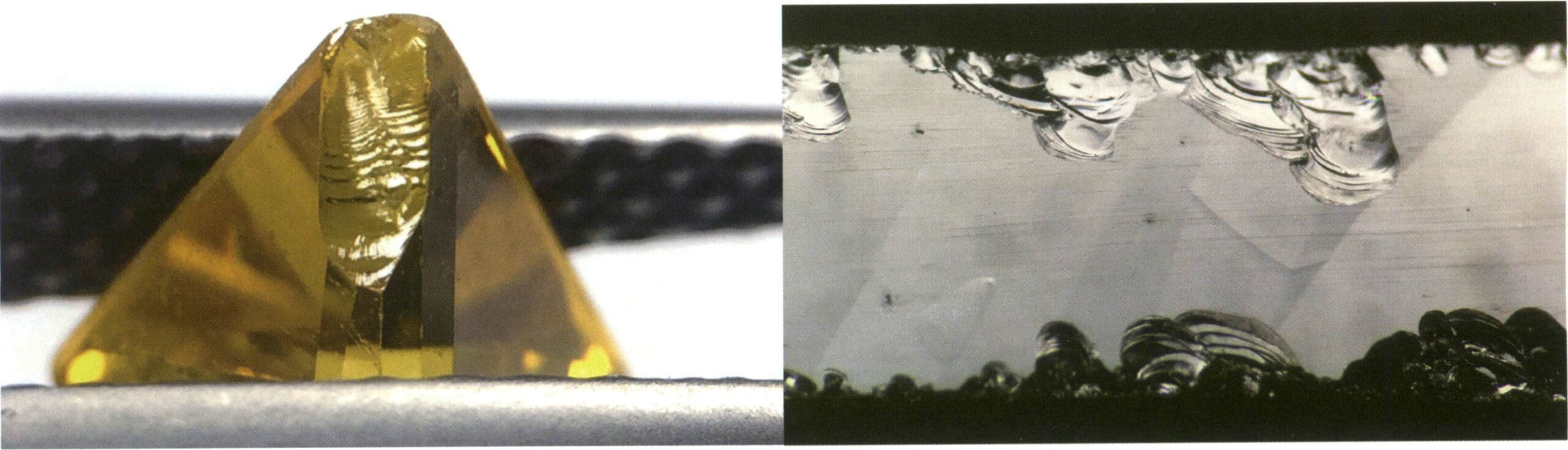
① Crystals before processing can be observed for fractures using reflected light, revealing one to three directions of step-like fracture surfaces on the gemstone, similar to cleavage (Figures 2-4-13, 2-4-14).
② Processed gemstones can be observed for fractures using transmitted light, revealing one to three directions of parallel, smoother fracture surfaces inside the gemstone (Figure 2-4-15).

Figure 2-4-13 The cleavage of corundum (parallel lines on a reflective plane)

Figure 2-4-14 The cleavage of corundum under reflected light (left shows parallel lines on the reflective plane, right shows a stepped fracture surface)
3. The fracture of crystals
3.1 Definition of fracture
The phenomenon where minerals do not break in a specific direction after being stressed, resulting in fracture surfaces with various uneven and irregular shapes, is called a fracture (Figure 2-4-16). The occurrence of fractures is unrelated to the naturalness of gemstones; this phenomenon can be seen in natural, synthetic, and artificial gemstones. The occurrence of fractures is also unrelated to the classification of gemstones; this phenomenon can be observed in crystals, aggregates, organic gemstones, and amorphous solids.

3.2 Key points for observing fractures
Observing the fracture surface of the crystal or gemstone in a certain direction using a reflective light tube. If the fracture surface is uneven and shows reflective flickering during movement, then this fracture surface is called a fracture.
Fractures can occur in raw crystal stones and gemstones with intact shapes after processing, especially after falling or being subjected to external forces (Figure 2-4-17). Shell-like fractures often exhibit a greasy luster.

3.3 Methods for describing fractures
Fractures differ from smooth and flat cleavage surfaces; they are generally uneven and curved. We often use analogies to describe the morphology of fractures, employing terms commonly found in everyday life, such as shell-like and irregular.
The common fracture shape in crystals is shell-shaped fractures, which can be easily observed in many gemstones where cleavage is poorly developed. For example, in quartz, tourmaline, and synthetic yttrium aluminum garnet(Figures 2-4-18, 2-4-19).

4. Hardness of Crystals
4.1 Definition of Hardness
Hardness, a term in physics, refers to a material’s ability to resist a hard object’s penetration into its surface. It indicates various materials’ comparative softness or hardness based on their local resistance to external intrusion. Due to the establishment of different testing methods, various hardness standards exist. The mechanical meanings of these hardness standards differ and are usually compared using experimental results; however, Vickers hardness and Mohs hardness can be converted through formulas.
There are many methods for testing hardness, including indentation, penetration, grinding, and rebound methods, among which the first two methods are widely used.
The indentation method uses a cone-shaped indenter made of alloy or diamond, applying a certain load (weight) on the mineral’s polished surface. The relationship between the load and the indentation’s area (or depth) is used to determine the mineral’s hardness. The hardness measured with a rhombic-shaped indenter is called Knoop hardness. The hardness measured with a square-shaped indenter is called Vickers hardness (HV), also known as absolute hardness (Figures 2-4-20, 2-4-21). In mineralogy and gemology studies, the Vickers hardness is usually tested.

Figure 2-4-20 Microhardness testing instrument
Figure 2-4-21 Calculating absolute hardness through the diameter of surface indentations
The scratching method evaluates a mineral’s resistance under external forces such as scratching, pressing, or grinding. This method has been consistently used in mineralogy with the Mohs hardness scale (Friedrich Mohs, 1822) (Figure 2-4-22). The Mohs hardness scale is a ranking table of 10 common high-purity minerals in nature, arranged according to their resistance to scratching. The recorded results of this ranking are called Mohs hardness (HM), also known as relative hardness.

The hardness in the gem identification parameter table refers to Mohs hardness.
Vickers hardness and Mohs hardness can be converted through a formula, and the conversion results show that the relationship between Mohs hardness is a nonlinear growth relationship (Figure 2-4-23).

4.2 Observations on Mohs hardness
① The hardness of the vast majority of minerals is tested in crystallography by characterizing standard minerals on the Mohs hardness scale against the minerals being tested. In gem identification, it is strictly prohibited for gems to scratch each other (the presence of scratches can affect the value of the gem).
② For certain gemstones and their imitations that have been cut into faceted shapes, we can distinguish between the gemstones and their imitations by observing the sharpness of the facet edges due to their different hardness, such as the distinction between diamonds and diamond simulants (Figure 2-4-24 to Figure 2-4-25), and the distinction between rubies and synthetic rubies (Figure 2-4-26).
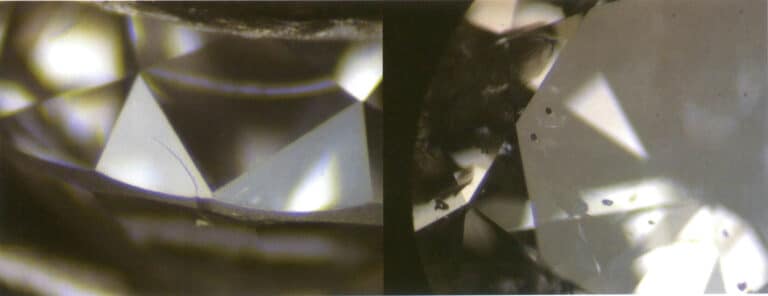


4.3 Description method of Mohs hardness
If a mineral can scratch Apatite (i.e., its hardness is greater than Apatite) but can be scratched by orthoclase(i.e., its hardness is less than orthoclase), then the hardness of that mineral is between 5 and 6, which can be written as 5-6. In practice, simpler methods can be used instead of a hardness tester; for example, the hardness of a fingernail is 2.5, and the hardness of a knife is 5.5, so the mineral hardness can be roughly divided into less than a fingernail (< 2.5), between a fingernail and a knife (2.5-5.5), and greater than a knife (> 5.5). A common steel needle (HM=5.5~6) can also be used. A table of common gemstones and everyday items with Mohs hardness is shown in Table 2.
Table 2: Common gemstones and household items Mohs hardness table
| Sertlik | Representative object | Yaygın Kullanımlar |
|---|---|---|
| 1 | Talc, Graphite | Talc is the standard mineral for the Mohs hardness scale, and it is known to be the softest mineral. Common applications include talcum powder, but due to its very low Mohs hardness, it cannot be used as a gemstone. |
| 2 | Alçı | Standard mineral for the Mohs hardness scale; due to its very low Mohs hardness, it cannot be used as a gemstone. It appears in the market as seal stone and collectible items |
| 2 ~ 3 | Ice cube | One of the common items in daily life |
| 2.5 | Nails, amber, Ivory | Amber and ivory are common organic gemstones |
| 2.5 ~ 3 | Gold, silver, aluminum | Gold and silver are commonly used for jewelry, while aluminum is often found in industrial applications |
| 3 | Calcite, copper, pearls, copper needles. | Calcite is the standard mineral for the Mohs hardness scale and can be used as a carving material; it is also an important component of dichroscopes used in gem identification. Copper was first used for decoration and is commonly used in alloy production and as a transmission medium in the electronics industry. Pearls are common organic gemstones. |
| 3.5 | Shells. | Common organic gemstones; smaller shells can be directly inlaid for decoration, while larger shells can be cut and polished into beads and other decorative materials, such as tridacna gigas. |
| 4 | Fluorspar | A standard mineral for the Mohs hardness scale, also known as fluorite, it can be used as a carving material and is one of the common gemstones. Due to its relatively low hardness, it often appears in some more unique handmade jewelry. |
| 4 ~ 4.5 | Platin | Rare metals, and also the hardest among precious metals. Platinum is often used in military industry or jewelry processing |
| 4 ~ 5 | Demir | Commonly used in steelmaking and other industrial applications. |
| 5 | Apatit | Mohs hardness scale standard minerals, one of the common gemstones |
| 5 ~ 6 | Stainless steel, small knife, steel needle, glass slide | One of the tools commonly used in geology to characterize minerals and rocks, and to preliminarily assess the Mohs hardness of minerals and rocks |
| 6 | Orthoclase, tanzanite, pure titanium | Feldspar is the standard mineral for the Mohs hardness scale, and Tanzanite is one of the common gemstones. |
| 6 ~ 7 | Teeth (outer layer of the crown), porcelain pieces. | The main component is hydroxyapatite. |
| 6 ~ 6.5 | Nephrite | One of the common types of jade. |
| 6.5 | Pyrite | The crystal has strong ornamental value and is rarely cut and polished into gemstones. |
| 6.5 ~ 7 | Jadeite | One of the common types of jade. |
| 7 | Quartz, Amethyst | Mohs hardness scale standard mineral, one of the common gemstones |
| 7.5 | Tourmaline, zircon | One of the common gemstones |
| 7 ~ 8 | Garnet | One of the common gemstones |
| 8 | Topaz | Mohs hardness scale standard minerals, one of the common gemstones |
| 8.5 | Heliodor | One of the common precious gemstones |
| 9 | Korundum | Mohs hardness scale standard minerals, one of the common gemstones |
| 9.25 | Synthetic Silicon Carbide | One of the common diamond simulants |
| 10 | Elmas | Mohs hardness scale standard minerals, one of the common gemstones |
| Greater than 10 | Polymer diamond nanorods | German scientists developed a material harder than diamond in 2005, which has broad industrial application prospects |
5. Relative Density of Crystals
5.1 Definition of Relative Density
Density is one of the important properties of gemstones, as it reflects their chemical composition and crystal structure. The density of a gemstone refers to the mass of the gemstone per unit volume, usually measured in g/cm³.
Gemstones’ relative density and density are numerically the same, but the former is easier to measure. The relative density of a gemstone refers to the ratio of its weight in air to the weight of an equal volume of water at 4℃, where at 4℃, the mass of 1cm³ of water is almost precisely 1 g.
The relative density of a gemstone depends on its chemical composition. The relative density of the same type of gemstone can vary due to changes in chemical composition, isomorphous substitution, mechanical inclusions, the presence of inclusions, and the adsorption of air in cavities and cracks. For example, the average relative density of diamonds is 3.52 g/cm³, but the relative density of Australian diamonds is 3.54; some yellow diamonds from Africa have a relative density of 3.52, and some brown diamonds from Brazil have a relative density of 3.60.
5.2 Relative Density Testing Methods
The hydrostatic weighing method and the heavy liquid method are commonly used methods for determining the relative density of gemstones. The former method can measure the relative density of gemstones more accurately, while the latter can quickly distinguish between two similar gemstones with different relative densities.
The relative density of gemstones generally ranges from 1 to 7. Those below 2.5 (such as amber) are considered low relative density, those between 2.5 and 4 (such as quartz) are of medium relative density, and those above four are regarded as high relative density. Most gemstones have a relative density between 2.5 and 4.
(1) Hydrostatic Weighing Method
According to Archimedes’ principle, when an object is immersed in a liquid, the buoyant force exerted by the liquid on the object is equal to the weight of the liquid displaced by the object. By measuring the weight of the gem in air based on the weight of the liquid displaced by the object, we can calculate the relative density of the gem (abbreviated as SG, also known as specific gravity). (Figure 2-4-27 to Figure 2-4-29).
Figure 2-4-27 Clean Water Weighing Attachment

Figure 2-4-28 State of water purification weighing accessories on the balance after combination (net suspension bracket is placed on the balance weighing disc, beaker bracket is at both ends of the balance weighing disc, other attachment combinations refer to the following figure)

The calculation method is the weight of the gem in air divided by the difference between the weight of the gem in air and water. The calculated value is usually retained to two decimal places, that is, relative density = weight of the gem in air ÷ (weight of the gem in air – weight of the gem in water) x density of water =weight of the gem in air÷ weight of water of the same volume as the gem x density of water.
Using the above formula, suppose a gem weighs 5.80 g in air and 3.50 g in water, with the density of water being 1 g/cm³; the calculation process is as follows:
SG = 5.80 ÷ (5.80 – 3.50) x 1 g/cm³
=5.80 4÷2.30 x 1 g/cm³
=2.50 g/cm³
Thus, we calculate that the relative density of this gem is 2.50 g/ cm³.
It is important to note that unless otherwise specified, the density of water is generally taken at 4℃ at g/cm³.
(2) Heavy Liquid Method
The state of the clean water weighing accessory assembly is placed on the balance (the net bag hanging support is placed on the balance weighing pan, and the beaker support is at both ends of the balance weighing pan; other accessory assemblies refer to the diagram below).
The heavy liquid method is a simple and effective way to indirectly determine the relative density of a gemstone by placing the sample in a known heavy liquid (see Table 3) and observing whether the gemstone sinks or floats. Heavy liquids are one of the organic volatile, mildly toxic solutions and are used less frequently in modern gemstone testing.
Table 3: Four Common Heavy Liquids and Indicator Minerals
| Common Heavy Liquids | Density of Common Heavy Liquids | Suspended Indicator Minerals in Common Heavy Liquids |
|---|---|---|
| Diluted Tribromomethane CHBr₃ | 2.65 | Clean crystal without cracks |
| Trichloromethane CHBr₃ | 2.89 | Clean green beryl without cracks |
| Diluted diiodomethane CH₂I₂ | 3.05 | Clean pink tourmaline without cracks (the density of tourmaline varies slightly with different colors, and the relative density of pink tourmaline is relatively stable) |
| Diiodomethane CH₂I₂ | 3.32 | Clean jade without cracks |
6. Toughness of crystals
The toughness of a crystal includes both flexibility and Brittleness. The phenomenon where gemstones have poor resistance to breaking (wear, stretching, pressing, cutting) is called Brittleness.
Brittleness has nothing to do with the optical properties of the gemstone and other mechanical properties such as cleavage, cleavage, fracture, hardness, density, etc. The crystal’s brittleness is related to how the crystal elements are connected, which we cannot observe with the naked eye. It can only be felt and seen in gemstone processing and wearing (Figure 2-4-30). It is often found in the early sale of finished pieces of faceted stone that the edge of the faceted stone is damaged due to loose wrapping paper, and the damage is reduced after using separate soft cotton paper packaging. Faceted edge breakage due to brittleness is also common in gems picked and observed for a long time (Figure 2-4-31).
The common gemstone crystal brittleness from strong to weak is as follows: fluorite, chrysoberyl, moonstone, topaz, emerald, olivine, aquamarine, quartz, diamond, sapphire, ruby.
Figure 2-4-30 Brittleness of diamonds (damage to the edges)

Figure 2-4-31 Synthetic rutile (damage caused by long-term observation)
Section IV Other physical properties of crystals
1. Electrical properties of crystals
(1) Conductivity
The ability of gemstone minerals to conduct electricity is called conductivity. Most gemstones are non-conductive, but gems like hematite, synthetic rutile, and natural blue diamonds (Type IIb) can conduct electricity. The semiconductor properties of natural blue diamonds are particularly important, as they are one of the distinguishing features of artificially colored diamonds, while artificially colored blue diamonds are non-conductive.
(2) Thermoelectric effect
When quartz and tourmaline are repeatedly heated and cooled, they expand or contract, generating voltage or charge at both ends of the crystal. This phenomenon is called the thermoelectric effect. This is also why tourmaline absorbs dust when heated by sunlight or artificial light.
(3) Piezoelectric Effect
The phenomenon where equal amounts of opposite charges appear at both ends of crystal materials like quartz when compressed or stretched in a certain direction.
2. Thermal Properties of Crystals — Thermal Conductivity
The ability of a material to conduct heat is called thermal conductivity, and different gemstones have different thermal conductivity capabilities. Comparing thermal conductivities can effectively distinguish gemstones. Although thermal properties assist in identifying many gemstones, the most important and obvious is diamond, which has a thermal conductivity far greater than that of the second highest, corundum. This is also one of the design principles of gemstone thermal conductivity testing instruments.
3. Radioactivity of Crystals
Radioactive elements, such as U, Th, Ra, etc., can spontaneously emit particles or rays from the nucleus while releasing energy. This phenomenon is called radioactivity, and this process is called radioactive decay. If scientists know the rate of radioactive decay and have instruments capable of measuring the presence of different isotopes, they can very accurately calculate the age of an object. For example, studying the radioactive isotope content of rare metals osmium (Os) and rhenium (Re) in diamonds can determine the age of billions of years old diamonds.
Radioactivity in natural gemstone minerals, such as diamonds, contains radioactive elements. The impact of radioactivity on gemstone properties is reflected in two aspects: it causes the natural coloration of gemstones and improves gemstone color. It is important to note that excessive radioactivity can harm the human body.
4. Surface Properties of Gemstones
The surface properties of gemstone minerals are related to the surface crystal structure of the gemstone minerals. The surface structure of gemstone minerals varies with the specific type of gemstone, and the surface properties determined by the surface structure will inevitably differ.
The surface properties of gem minerals are prominently manifested in their adsorption effects on external substances, such as hydrophobicity and lipophilicity. Hydrophobicity is a term in chemistry that refers to the physical property of a molecule (hydrophobic substance) that repels water. Hydrophobicity is often called lipophilicity, but these two terms are not entirely synonymous. At the same time, most hydrophobic substances are usually lipophilic; there are exceptions, such as silicone rubber and fluorinated compounds.
The property involved in gemology is diamond, and the identification of diamonds and their imitations and the diamond selection process often utilize this property.