One-time to know Platinum Group Metals and Their Alloys used in jewelry products
A Comprehensive Guide To Pure Platinum and Platinum Alloy Materials' Properties And Features
The platinum group metal elements include ruthenium (Ru), osmium (Os), rhodium (Rh), iridium (Ir), palladium (Pd), and platinum (Pt). Among the platinum group element minerals, these six elements usually exhibit a wide range of isomorphism, along with the presence of isomorphic mixtures such as iron, cobalt, and nickel. The platinum group metals commonly used in jewelry are platinum, palladium, rhodium, and a small amount of iridium.
Although the platinum group metals were discovered later, they possess unique physical and chemical properties. They are now widely used in modern industries and cutting-edge technology fields such as automotive, petroleum, chemical, communication, national defense, and aerospace, earning the title “pioneering materials.” In the jewelry industry, the main base elements used in jewelry from the platinum group metals are Pt and Pd. In contrast, Ir and Ru are sometimes used as alloying elements in jewelry alloys. Os is not used in the jewelry industry. Although the volume of platinum group metal jewelry is far less than that of gold and silver, they have emerged in the global precious metal jewelry field due to their excellent physical and chemical properties. They have now become a significant end-use area following the automotive manufacturing sector.

Table of Contents
Section Ⅰ Physical and Chemical Properties of Platinum Group Metals
1. Physical Properties of Platinum Group Metals
Among the platinum group metals, ruthenium (Ru), rhodium (Rh), and palladium (Pd) are located in group 5 of the 5th period. In contrast, osmium (Os), iridium (Ir), and platinum (Pt) are located in group VIII of the 6th period, all belonging to the transition metals.
The main physical properties of platinum group metals are shown in Table 5-1. The density of platinum is higher than that of gold, about twice that of silver, giving it a noticeable heavy feel. The density of palladium is slightly higher than that of silver, but much lower than that of gold. Platinum group metals have a high reflectivity across the entire visible light spectrum, and the reflectivity increases smoothly with increasing wavelength, so platinum group metals generally appear silvery-white. Among the platinum group elements in the same period, the melting point of the metals decreases with increasing atomic number. The melting points of platinum and palladium are significantly higher than those of gold and silver, making smelting and casting difficult. The thermal conductivity of platinum group metals is lower than that of gold and silver; for example, at room temperature (300K), the thermal conductivity of platinum is less than that of gold. Therefore, although the heat required to melt platinum alloys is high, the low thermal conductivity makes it difficult for heat to dissipate during heating, resulting in the laser power required for laser welding platinum jewelry being lower than that for gold and silver, which is very beneficial for the assembly and laser welding of platinum alloy jewelry. Platinum group metals exhibit paramagnetic; they do not magnetize themselves, but noble metal elements like Pt and Pd can exhibit some magnetism when alloyed with elements like Fe, CO.
Table 5-1 Main Physical Property Indicators of Platinum Group Metals
| Physical Property Indicators | Platinum Group Metals | |||||
|---|---|---|---|---|---|---|
| Physical Property Indicators | Ru | Rh | Pt | Os | Ir | Pd |
| Atomic number | 44 | 45 | 46 | 76 | 77 | 78 |
| Relative atomic mass | 101.07 | 102.905 | 106.4 | 190.2 | 192.22 | 195.078 |
| Crystal structure | Close-packed hexagonal | Face-centered cubic | Face-centered cubic | Close-packed hexagonal | Face-centered cubic | Face-centered cubic |
| Density (20℃)/(g/cm3) | 12.37 | 12.42 | 12.01 | 22.59 | 22.56 | 21.45 |
| Color | Blue white | Silver white | Steel white | Blue white | Silver white | Tin white |
| Melting point /℃ | 2333 | 1966 | 1555 | 3127 | 2448 | 1768.1 |
| Boiling point /℃ | 4077 | 3900 | 2990 | 5027 | 4577 | 3876 |
| Melting heat/(kJ/mol) | 39.0 | 27.3 | 16.6 | 70.0 | 41.3 | 22.11 |
| Evaporation heat (1 x 105 Pa)/(kJ/mol) | 649 | 558 | 377 | 788 | 670 | 565 |
| Specific heat capacity (1 x 105 Pa, 25℃) /[J/(mol⸳K)] | 24.05 | 24.90 | 26.0 | 24.69 | 25.09 | 25.65 |
| Thermal conductivity (0℃)/[W/(m⸳K)] | 119 | 153 | 75.1 | 88 | 148 | 71.7 |
| Resistivity (25℃)/(/uΩ⸳m) | 7.37 | 4.78 | 10.55 | 9.13 | 5.07 | 10.42 |
| Coefficient of thermal expansion (20℃)/(X10-6/) | 9.1 | 8.3 | 11.77 | 6.1 | 6.8 | 8.93 |
Platinum group elements such as Pt and Pd have the property of adsorbing gases, especially H. Pt, Pd The ability to adsorb H is related to their physical state; platinum black can adsorb up to 502 times its volume of H, and due to differences in the manufacturing process of platinum black, the amount of hydrogen absorbed can vary greatly. In comparison, sponge platinum can only adsorb 49.3 times its volume of H. Palladium can adsorb up to 2800 times its volume of H and forms a palladium-hydrogen solid solution, which decreases in density, electrical conductivity, and strength, but hydrogen can be released when heated.
2. Chemical properties of platinum group metals
Platinum group metals have excellent oxidation and corrosion resistance, but there are differences in oxidation and corrosion resistance among platinum group elements, and the differences are quite significant.
2.1 Oxidation Resistance
In dry air at room temperature, platinum group metals exhibit good oxidation resistance; however, there are significant differences in their oxidation performance, which follows the order of affinity for oxygen Pt < Pd < Rh < Ir < Ru < Os. When heated in air, a layer of oxide film forms on the surface, affecting the quality of the jewelry surface. As the temperature increases, the oxide film will decompose and reduce back to metal, restoring the metallic luster of the jewelry surface.
Platinum reacts with oxygen to produce PtO, Pt2O3 and PtO2. In an oxidizing atmosphere, at a pressure of 0.8 MPa, heating platinum powder to 430℃ will cause platinum to oxidize to form PtO.
Palladium reacts with oxygen to produce PdO at 350-790℃, but it is unstable at high temperatures and will decompose. When further heated above 870℃, PdO is completely reduced to metallic palladium. PdO2 is dark red and is a strong oxidizing agent. It will slowly lose oxygen at room temperature and decompose into PdO and O2 below 200℃.
An oxide film forms on the surface of iridium and rhodium at 600-1000℃.
2.2 Corrosion resistance
At room temperature, platinum has strong corrosion resistance; hydrochloric acid, nitric acid, sulfuric acid, and organic acids do not affect platinum in its cold state, while sulfur slightly affects platinum when heated. However, aqua regia can dissolve platinum in both cold and hot states. Molten alkali or molten oxidizers can also corrode platinum. When the temperature is raised to 100℃ under oxidative conditions, various hydrohalic acids or halides act as complexing agents, causing platinum to be complexed and dissolved. At 350-600℃, platinum reacts with chlorine to form platinum chloride, which can be further heated to reduce it.
Molten alkali can corrode platinum. At high temperatures, carbon can dissolve in platinum, with solubility increasing with temperature; upon cooling, carbon residues make platinum brittle, a phenomenon known as “carbon poisoning.” Therefore, when smelting platinum, graphite crucibles should not be used; typically, alumina or zirconia crucibles are used, and the process is conducted under vacuum or inert gas protection. Adding rhodium and iridium to platinum can enhance its corrosion resistance.
Palladium is the least corrosion-resistant of the platinum group metals. Nitric acid dissolves palladium, as does hot sulfuric acid and molten potassium bisulfate. Especially in the presence of hydride complexes (e.g., aqua regia), palladium is more susceptible to corrosion and dissolution. At scorching temperatures, palladium interacts with chlorine to form palladium chloride. Palladium reacts with aqua regia and hydrochloric acid to form chloropalladium acid or chloropalladite. When ammonia is added in excess to chloropalladite, a solution of tetrachloroammonia can be obtained, and when hydrochloric acid is added to the solution, a bright yellow, fine crystalline precipitate of palladium dichloride can be precipitated, which is decomposed into metallic palladium after calcination. Palladium reacts with sulfur to form palladium sulfide and with selenium and tellurium to form palladium selenide (tellurium). When palladium is melted in graphite crucibles, carbon poisoning also occurs, resulting in brittle propertiesthe corrosion resistance of palladium increases when other platinum group elements are present.
Rhodium and iridium are the most chemically stable metals among the platinum group metals, and hot aqua regia does not easily dissolve them. However, molten alkali metal peroxides and alkalis can oxidize rhodium and iridium, and the oxidized rhodium and iridium can be easily dissolved by complexing agents; molten sulfates can also dissolve rhodium. When iridium reacts with chlorine, different chlorinated iridium products are formed at different temperatures. In an aqueous solution, chlorination can precipitate iridium chlorate, which has significant value in refining platinum group metals and is used for the recovery and separation of iridium and other platinum group metals.
The corrosion behavior of platinum group metals in certain corrosive media is shown in Table 5-2.
Table 5-2 Characteristics of the corrosion resistance of platinum group metals
| Corrosive media | Platinum group metals | ||||||
|---|---|---|---|---|---|---|---|
| Corrosive media | Pt | Pd | Rh | Ir | Os | Ru | |
| concentrated H2SO4 | / | / | / | / | / | / | |
| HNO3 | 70%, room temperature | / | strong | / | / | general | / |
| 70%, 100℃ | / | strong | / | / | strong | / | |
| Aqua regia | Room temperature | strong | strong | / | / | strong | / |
| Boil | strong | strong | / | / | strong | / | |
| HCl | 36%, room temperature | / | / | / | / | / | / |
| 36%, boiling | weak | weak | / | / | general | / | |
| Cl2 | Dry | weak | general | / | / | / | / |
| Wet | weak | strong | / | / | general | / | |
| NaClO solution | Room temperature | / | general | weak | / | strong | strong |
| 100℃ | / | strong | / | / | strong | / | |
| FeCl3 solution | Room temperature | - | general | / | / | general | / |
| 100℃ | - | strong | / | / | strong | / | |
| Molten Na2SO4 | Molten Na2SO4 | weak | general | general | / | weak | weak |
| Molten NaOH | Molten NaOH | weak | weak | weak | weak | general | general |
| Melted Na2O2 | Melted Na2O2 | strong | strong | weak | general | strong | general |
| Melted NaNO3 | Melted NaNO3 | / | general | / | / | strong | / |
| Melted Na2CO4 | Melted Na2CO4 | weak | weak | weak | weak | weak | weak |
Note: / indicates non-corrosive; week indicates slight corrosion; general indicates corrosion; strong indicates severe corrosion; one indicates no such data in the original literature.
Section II Jewelry Used Platinum and Its Alloy Materials
1. History of Platinum Jewelry Development
1.1 History of Platinum Jewelry Development
Platinum is a very rare precious metal. Due to its rarity, stability, and uniqueness, as well as its dazzling silver-white metallic luster, its value has often been more expensive than gold. The history of human use of platinum is very long;
archaeological findings suggest that as far back as 3000 years ago in ancient Egypt, people had already begun to utilize platinum. However, the scientific understanding of this precious metal material has only been around for a little over 200 years. Historically, using precious metals began with creating crafts, jewelry, religious ornaments, and utensils. Platinum is uncommon in nature, and its distribution in the earth’s crust is rare. Coupled with its insolubility and stability, this has posed significant challenges for platinum mining, selection, refining, and purification. The high melting point of platinum makes processing very difficult, especially when using primitive methods. Therefore, it can be understood that there were few platinum products made in ancient times, and even fewer have survived.
According to statistics, in 1980, the amount of platinum used to make platinum jewelry worldwide was about 15 tons, which increased to 58 tons by 1995. Japan is the country that loves platinum jewelry the most and has the highest consumption of platinum. China began processing platinum crafts in the 1920s and 1930s. Still, due to the long-standing preference of Chinese consumers for gold jewelry, there needed to be more involvement in the manufacturing of platinum jewelry before the 1990s. With the opening up of the economy, development, and improvement of people’s living standards, as well as the influence of fashion and platinum jewelry manufacturers, the Chinese jewelry industry began to develop towards platinum jewelry. By 2000, China had surpassed Japan to become the world’s largest consumer of platinum jewelry. Since then, the demand for platinum jewelry in China has grown rapidly, peaking between 2012 and 2015, with an annual demand of 55-60t, accounting for about 70% of the global total demand, making it the largest consumer of platinum jewelry in the world and dominating the global platinum jewelry market.
1.2 Characteristics of Platinum Jewelry
Platinum jewelry is loved by people for its unique texture, beauty, and rhythm. Platinum jewelry not only showcases the overall elegance and grace of the piece but also presents a certain mysterious atmosphere rich in artistic taste. This is also why platinum jewelry is popular among social classes with a certain level of artistic cultivation and higher cultural standards.
The platinum’s soft, elegant, and luxurious color symbolizes purity and nobility. Therefore, it is often set together with diamonds to create wedding rings, serving as tokens of love to signify love’s purity and everlasting nature. The transparent, colorless, and radiant diamonds set in the shimmering platinum framework highlight the flawless whiteness and grandeur of the diamonds even more.
Platinum jewelry can be divided into two categories: pure platinum jewelry without gemstones and platinum jewelry set with gemstones. Pure platinum is soft, and due to the limitations of material strength, it is usually made into pure platinum jewelry without gemstones. Common styles mainly include rings, necklaces, earrings, and brooches.
1.3 Purity Marking of Platinum Jewelry
The popular platinum jewelry in the market can be divided into two main categories: pure platinum jewelry, also known as high-purity platinum, which theoretically should have a fineness of 1000‰. Its fineness is usually expressed in parts per thousand, but in reality, there is no such thing as pure gold or pure platinum; the fineness of pure platinum is always lower than this value. The other category is platinum alloy jewelry, an alloy formed by adding other metals, such as bismuth, palladium, and copper, to pure platinum to enhance its hardness and toughness.
Due to differences in regional and jewelry cultures, the market purity standards of various countries (regions) are also different.
Japan, Hong Kong: The allowed platinum purity is 1000‰, 950‰, 900‰, and 850‰, with an allowable error of 0.5%.
United States: Jewelry with a platinum content higher than 95% is allowed to be stamped with “Pt” (Platinum or Plat); jewelry with a platinum content between 75% and 95% must be stamped with the mark of the platinum group metal, such as ” IR-10-PAT”, indicating an alloy containing iridium 10%. Jewelry with a platinum content between 50% and 75% must be stamped with the content and name of the contained platinum group metal, such as “585 Platinum(585PAT)” or “365 Palladium” (365PALL).
Europe: Most countries require a purity of 950‰, while a few allow iridium to be counted as platinum. Germany allows other purity standards.
The term “fine platinum” refers to platinum with a content of not less than 990 parts per thousand, and it must be stamped with a fine platinum mark or printed with the actual content.
2. Pure Platinum
2.1 Mechanical Properties
Pure platinum is soft, has good ductility, and possesses excellent processing capabilities, allowing it to be rolled into sheets and drawn into wires as needed. One gram of pure platinum can be drawn into approximately 2 km of fine wire. Pure platinum has good toughness, enabling the creation of flexible mesh platinum jewelry, which is difficult to achieve with pure gold, silver, and other precious metals.
The tensile strength and yield strength of pure platinum in the annealed state are higher than those of pure gold and pure silver; however, its specific strength (strength-to-weight ratio) is still relatively low, making it prone to deformation. It is mainly used to make plain jewelry without gemstone settings, such as rings, necklaces, and earrings.
The main mechanical properties of pure platinum are shown in Table 5-3.
Table 5-3 Main Mechanical Properties of Pure Platinum
| Mechanical properties | Annealed state | Processed State (60%) | |
|---|---|---|
| Hardness HV/(N/mm2) | 39 ~ 42 | 90 ~ 95 |
| Tensile strength /MPa | 130 ~ 160 | 300 ~ 350 |
| Yield strength /MPa | 70 ~ 110 | - |
| Elongation rate /% | 40 ~ 50 | 1 ~ 3 |
Due to the low hardness of pure platinum, jewelry made from it is prone to dents, scratches, and wear from daily use due to impacts and friction, necessitating reinforcement treatment.
2.2 Process Performance
Platinum has a very high melting point, and the temperature during investment casting is generally above 1900℃, which poses significant challenges for melting and casting. Carbon can dissolve in platinum at high temperatures, and the solubility increases with temperature. Upon cooling, carbon residues make platinum brittle, a phenomenon known as carbon poisoning. Therefore, graphite crucibles cannot be used when melting platinum; alumina or lead oxide crucibles are typically employed, and melting is done under vacuum or inert gas protection. Platinum can form low-melting-point eutectics with elements such as P, S, and Si, leading to brittle fracture of the material.
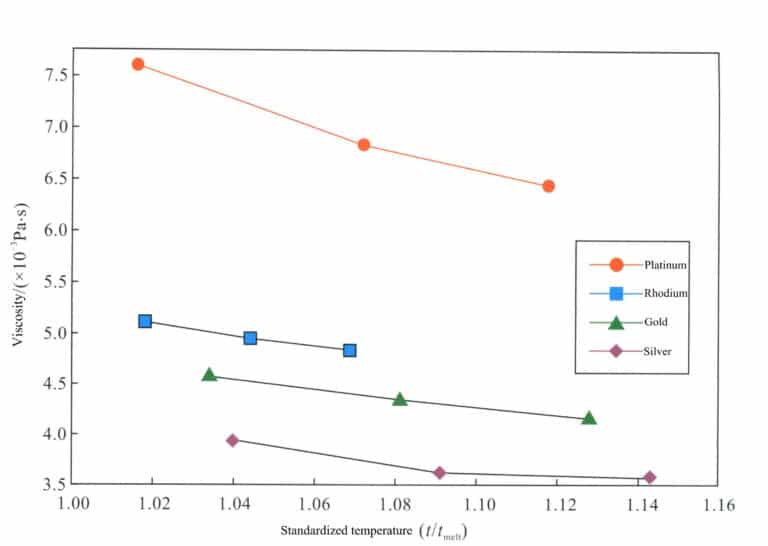
The surface tension of platinum is 1.5 times that of gold, and its thermal conductivity is 1/3 that of gold. The viscosity at the same degree of superheating is significantly higher than that of gold (Figure 5-1). The high surface tension and viscosity make it more difficult for the molten metal to fill the mold smoothly, especially for small parts; the low thermal conductivity leads to uneven temperature and composition of the molten metal, particularly when there is a large temperature difference between the molten metal and the mold. In actual production, centrifugal or vacuum suction casting is often used to provide additional filling power and improve filling performance. During casting, conventional gypsum model materials have poor thermal stability and will undergo severe thermal decomposition reactions under the action of high-temperature platinum liquid, leading to defects such as porosity and sand holes in the castings. Therefore, casting powder materials using phosphate as a binder must be employed.
Pure platinum annealed state hardness is low, and the work-hardening rate is higher than gold and silver, but it also belongs to the low-layer fault energy metal. Thus the work-hardening rate is not high, has good flexibility and cold working properties, can be rolled, drawing, forging and other cold deformation processing, can be pulled into a very fine wire, rolled into a very thin platinum foil.
3. Platinum Alloy
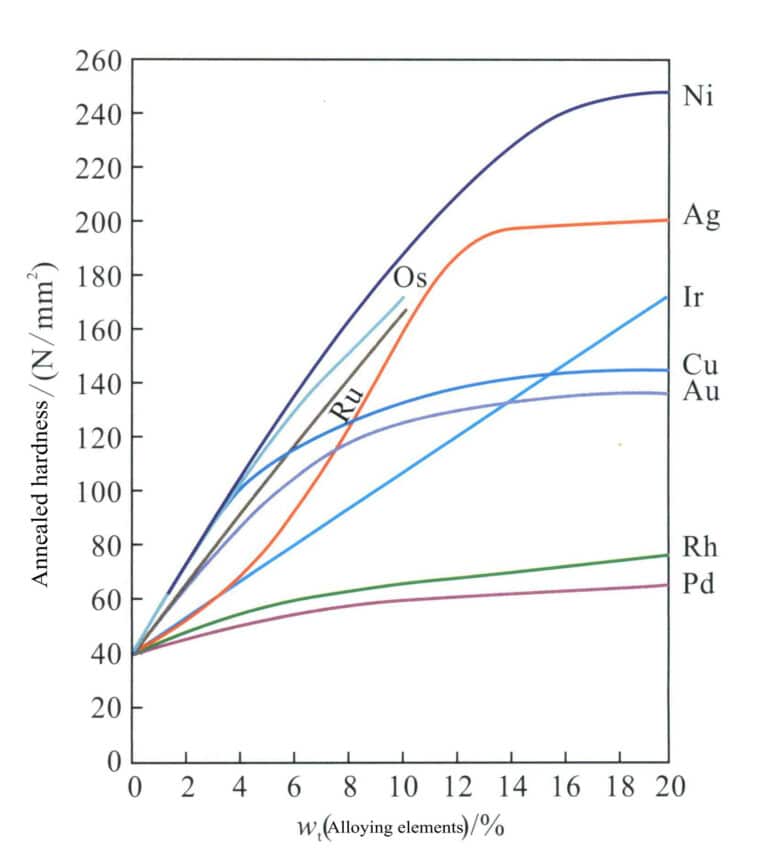
To improve the strength and hardness of platinum materials to meet the requirements for setting jewelry, it is necessary to strengthen them. Many metal elements are used for platinum alloying, and the strengthening effects of different alloying elements on platinum vary significantly. The amount of the same alloying element added also leads to different degrees of variation in its strengthening effect (Figure 5-2).
The metal elements commonly used in jewelry platinum alloys mainly include Ir, Cu, Co, Ru, Pd, etc. Their binary alloys can be directly applied to jewelry production, or they can form ternary or multi-element alloys based on these alloys to optimize the overall performance of platinum alloys.
3.1 Binary Alloy System
3.1.1 Pt-Ir Alloy
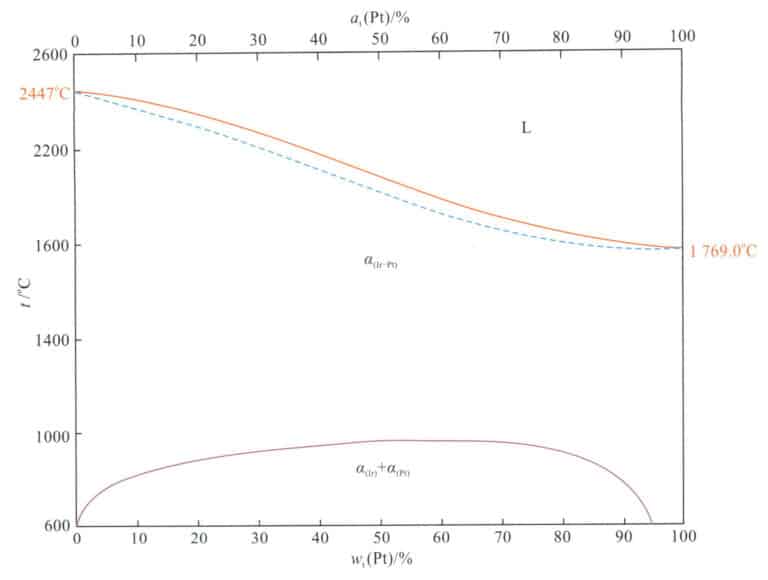
Pt-Ir alloy is the alloy formed by adding a small amount of iridium to pure platinum. As shown in Figure 5-3, this alloy is a continuous solid solution at high temperatures, and when the iridium content exceeds 7at %, phase separation occurs when cooling from high temperature to 975-700℃.
Ir is an effective strengthening agent for Pt. As the amount of iridium increases, the strength and hardness of the Pt-Ir alloy can be significantly improved, but processing of the alloy becomes difficult when the Ir content is > 30% (Figure 5-4).
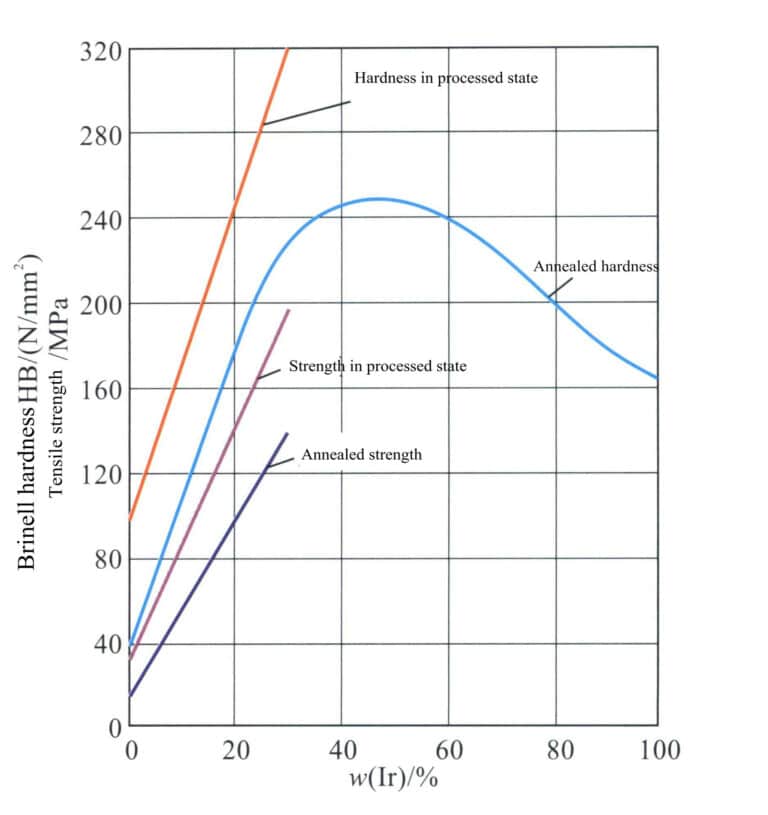
Pt-Ir alloys are silver-white, with a strong metallic luster, is the whitest and brightest of all platinum alloys. The addition of iridium improves the chemical corrosion resistance of platinum, 90% Pt-10% Ir alloy chemical corrosion rate is only 58% of pure platinum. Alloy has volatility, Ir in the air when heated volatile loss than Pt many times, in 1227℃ , Ir volatility than Pt 100 times, containing Ir higher than 5% of the alloy in the air when heated will oxidize, in 700℃ or more, it will make the alloy surface layer becomes blue. In 1200℃ above, the blue layer will disappear.
Pt-Ir alloys with lower Ir content have better casting performance. As the Ir content increases, the alloy’s melting point rises, and castings often exhibit dendritic crystals or internal segregation, leading to poorer uniformity of alloy properties.
Depending on the content of nickel and platinum, the Pt-Ir alloy mainly includes 95%Pt-5%Ir, 90%Pt-10%Ir and 85%Pt-15%Ir three grades and their main properties are shown in Table 5-4. Pt-Ir alloy is one of the important materials for platinum jewelry, and it is especially widely used in the United States. In recent years, Pt950Ir50 alloy has also been used for jewelry in Japan and Germany.
Table 5-4 Main properties of different grades of platinum-iridium alloys
| Grade | Melting point/°C | Density/ (g/cm3) | Hardness HB/(N/mm2) | Tensile strength/ MPa | Elongation/ % | Color Coordinates | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Melting point/°C | Density/ (g/cm3) | Annealed state | Processed state | Annealed state | Processed state | Annealed state | Processed state | L* | a* | b* |
| 95%Pt - 5%Ir | 1795 | 21.49 | 90 | 140 | 275 | 485 | 32 | 2.0 | 84.7 | -0.2 | 4.2 |
| 90%Pt - 10%Ir | 1800 | 21.53 | 130 | 185 | 380 | 620 | 27 | 2.5 | 85.5 | -0.1 | 4.7 |
| 85%Pt - 15%Ir | 1820 | 21.57 | 160 | 230 | 515 | 825 | 24 | 2.5 | - | - | - |
95%Pt-5%Ir Low hardness, small tendency for casting shrinkage, but poor fluidity, coarser grain size, and not easy to polish. Suitable for handcrafting, stamping, and other forming processes. Due to low hardness and relatively high toughness, it has poor machinability and tends to stick to tools. This alloy can be used as a general jewelry alloy for casting, handcrafting, and stamping.
90%Pt-10%Ir is a medium-hardness alloy that can be processed using most manufacturing techniques. This alloy does not form an oxide film in the molten state, which is beneficial for casting small parts and can be used as a general jewelry alloy for casting, handcrafting, and stamping.
3.1.2 Pt-Cu Alloy
As shown in Figure 5-5, the Pt-Cu alloy is a continuous solid solution at high temperatures, and at lower temperatures ( < 825℃ ), it will precipitate ordered phases such as PtCu3 and PtCu, resulting in aging strengthening and increased hardness. Research has found that the cast state of 95%Pt-5%Cu alloy undergoes heat treatment at 100-400℃, and the hardness of the alloy will further increase due to the formation of a Pt7Cu superlattice structure, with some alloys undergoing an ordered transformation, resulting in an ordered hardening effect and increased hardness.
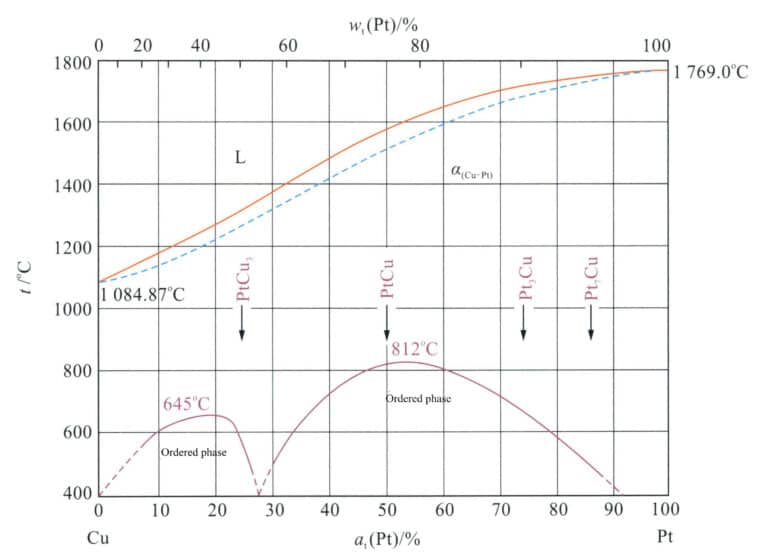
Cu is a medium-strengthening element for platinum, and its hardening effect is related to the treatment method. The hardening effect of the solid solution Pt-Cu alloy is not significant when subjected to low-temperature aging treatment. Still, there is a hardening effect when the solid solution alloy is cold-deformed and then aged at 300-500℃.
When the Pt-Cu alloy is heated in the atmosphere, selective oxidation of the copper component forms a copper oxide film layer, making the alloy prone to oxidation and discoloration. Therefore, melting and heat treatment should occur in a protective atmosphere or vacuum environment.
The Pt-Cu alloy has moderate hardness, is castable, and is commonly used as a general-purpose alloy. Alloys used for jewelry generally contain 3%-5%Cu, and when the copper content exceeds 5%, the casting performance of the alloy deteriorates. The main properties of 95%Pt-5%Cu alloy are shown in Table 5-5. Based on the Pt-Cu alloy system, the alloy contains 4%-6% Cu and other alloying elements such as Co, Ni, Pd, etc.
Table 5-5 95%Pt-5%CuMain properties of the alloy
| Melting Point/°C | Density/ (g/cm3) | Hardness HV/(N/mm2) | Tensile Strength/ MPa | Elongation/ % | ||||
|---|---|---|---|---|---|---|---|---|
| Melting Point/°C | Density/ (g/cm3) | Solid solution | Annealed state (800℃) | Processed state (90%) | Annealed state | Machined State (90%) | Annealed State (800℃) | Machine d State (90%) |
| 1750 | 20.05 | 90 | 150 | 240 | 310 ~ 410 | 720 ~ 920 | 27 ~ 45 | 13 |
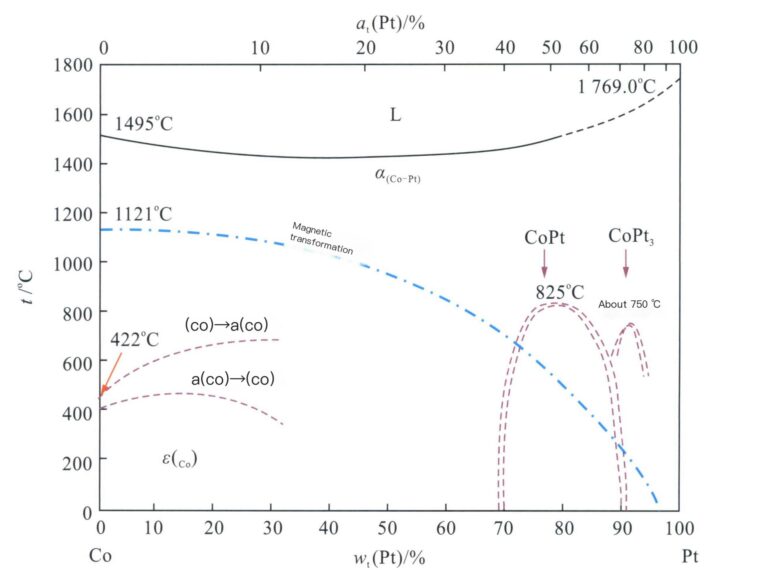
3.1.3 Pt-Co Alloy
Figure 5-6 shows that the Pt-Co alloy forms an infinite solid solution at temperatures above 825℃, and its crystal structure is face-centered cubic. Below this temperature, depending on the composition, the alloy will exhibit CoPt3 and CoPt ordered phases, undergoing a transition from disordered phase-> to ordered phase, resulting in an ordered hardening effect. The hardness of the Pt-Co alloy is greatly related to the heat treatment process.
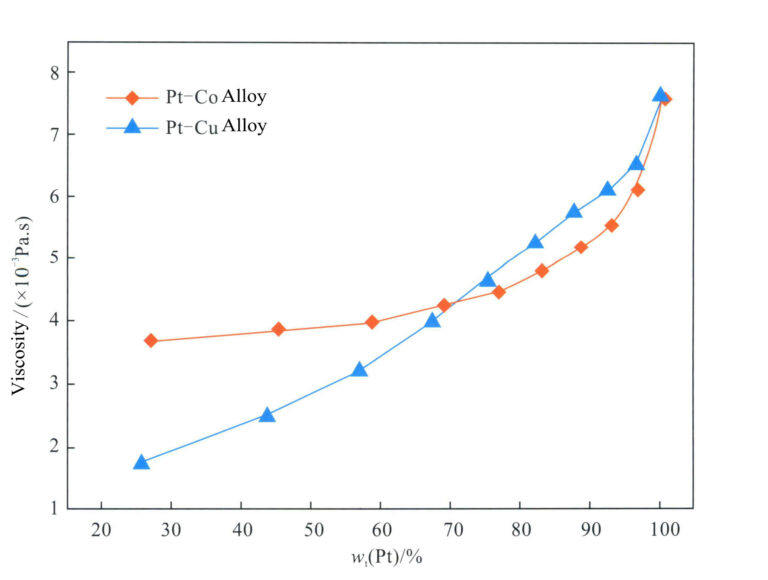
Compared to the Pt-Ir alloy and Pt-Ru alloy, the Pt-Co alloy has a lower melting point, can be cast at lower temperatures, and its melt has a relatively lower viscosity than other platinum alloys (Figure 5-7). Therefore, the fluidity of Pt-Co alloy is better than that of other alloys, with less tendency for gas absorption and shrinkage, allowing for the casting of jewelry pieces with fine patterns.
The cast surface of Pt-Co alloy will have a certain degree of oxidation, presenting a light gray-blue color. Dipping the workpiece in boric acid and heating it to an orange-yellow temperature can eliminate this blue color. Pt-Co alloy has high corrosion resistance and is not eroded by inorganic acids and bases at room temperature, nor is it corroded in hot, concentrated sulfuric acid. As the Co content increases, the alloy’s oxidation resistance and corrosion resistance decrease, and the probability of defects due to oxidized inclusions in the castings increases. Therefore, when this alloy is used for jewelry manufacturing, the Co content generally does not exceed 10%, with 95%Pt-5%Co alloy (Table 5-6) being the most common.
Table 5-6 95%Pt-5%Co Main properties of the alloy
| Melting Point/°C | Density/ (g/cm3) | Hardness HV/(N/mm2) | Tensile Strength/ MPa | Color Coordinates | ||||
|---|---|---|---|---|---|---|---|---|
| Melting Point/°C | Density/ (g/cm3) | Annealed state | Processed state | Annealed state | Processed state | L* | a* | b* |
| 1765 | 20.8 | 135 | 270 | 275 | 475 | 86.6 | 0.5 | 4.5 |
95%Pt-5%Co The alloy has slight oxidation on the surface during heat treatment or welding, so protection is needed. It should cool under boric acid alcohol after welding, presenting a bright orange color, which can be removed with citric acid. Note that boric acid should not be used for protection before welding. Because boric acid becomes a contaminant at high temperatures, this alloy is not easy to weld with an oxygen-acetylene torch; using a water welder or laser is best.
95%Pt-5%Co The alloy undergoes a magnetic transformation below a certain temperature, showing slight magnetism. Special care must be taken during processing, and magnets should not be used to separate Pt-Co chips and sawdust.
95%Pt-5%Co The alloy has good casting performance, and adding Co as an additive to Pt can effectively improve the hardness of the alloy, giving it good mechanical properties, making it easy to polish, and suitable for handcrafting, stamping, and machining. The alloy ultimately presents a faint blue color, which pairs particularly well with diamonds and is widely used as jewelry in Europe and North America.
3.1.4 Pt-Ru Alloy
The crystal structure of platinum is a close-packed hexagonal structure, which is inherently brittle and difficult to process. Adding ruthenium to platinum can form a wide solid solution at the rich Pt end (Figure 5-8), so this alloy does not have aging-strengthening effects. However, ruthenium has a certain solid solution strengthening effect, and it is a grain refiner, so adding it can refine the microstructure of the alloy; thus Pt-Ru, the alloy, has good strength and hardness. 95%Pt-5%Ru The main properties of the alloy are shown in Table 5-7. The addition of ruthenium raises the alloy’s melting point, Pt-Ru, and the alloy appears silvery-white.
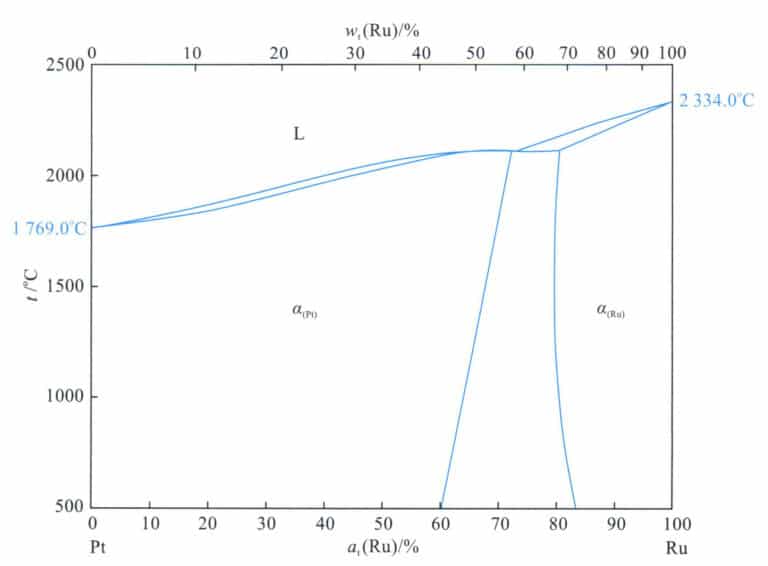
Table 5-7 95%Pt-5%RuMain properties of the alloy
| Melting Point/°C | Density/ (g/cm3) | Hardness HV/(N/mm2) | Tensile Strength/ MPa | Elongation Rate/% | Color Coordinates | ||||
|---|---|---|---|---|---|---|---|---|---|
| Melting Point/°C | Density/ (g/cm2) | Annealed state | Processed state | Annealed state | Processed state | Annealed State | L* | a* | b* |
| 1795 | 20.67 | 125 ~ 135 | 230 | 415 | 760 | 25 | 84.2 | 0 | 4.1 |
The hardness of Pt-Ru alloy after annealing is about HV130, with a stable work hardening rate, and it can ultimately reach about HV230. The tensile strength of the alloy is also relatively high, which gives Pt-Ru alloy good processing and polishing performance, making it suitable for making rings from Pt-Ru tubing. Pt-Ru alloy can also be used for casting, but compared to other platinum alloys, it is not the most suitable for casting; the molten metal has a high tendency to absorb gas, especially with a good affinity for oxygen, leading to defects such as pores and inclusions in the castings. The flowability of the molten metal could be better, making it difficult to form small parts of the jewelry, with serious micro-shrinkage between dendrites, uneven grain size distribution, and coarser columnar grains on the surface. Increasing the pouring temperature and mold temperature helps improve filling performance, but refractory casting powder with good heat resistance must be used. Oxy-acetylene flame melting is not recommended, as the resulting ruthenium oxide RuO2 fumes are toxic.
Pt-Ru alloy is a commonly used platinum alloy in the United States, originally developed for handmade items, and is a generalpurpose alloy, with 95%Pt-5%Ru being the most common, having good processing performance, and widely used in the manufacture of wedding jewelry, enjoying great popularity in the U.S. market. In Switzerland, this alloy is also commonly used in watch manufacturing.
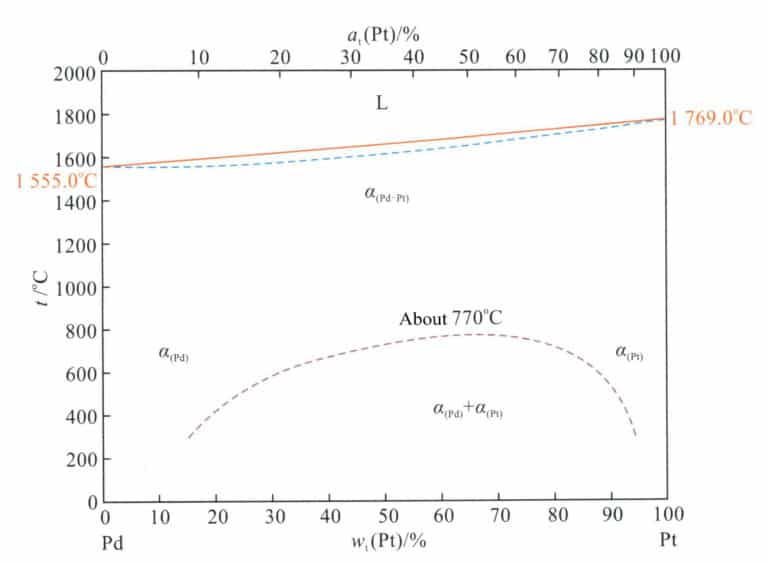
3.1.5 Pt-Pd alloy
Figure 5-9 shows that the Pt-Pd alloy is a continuous solid solution at high temperatures. It undergoes phase decomposition upon slow cooling below 770℃, forming two immiscible solid solutions: a Pt-rich phase and a Pd-rich phase.
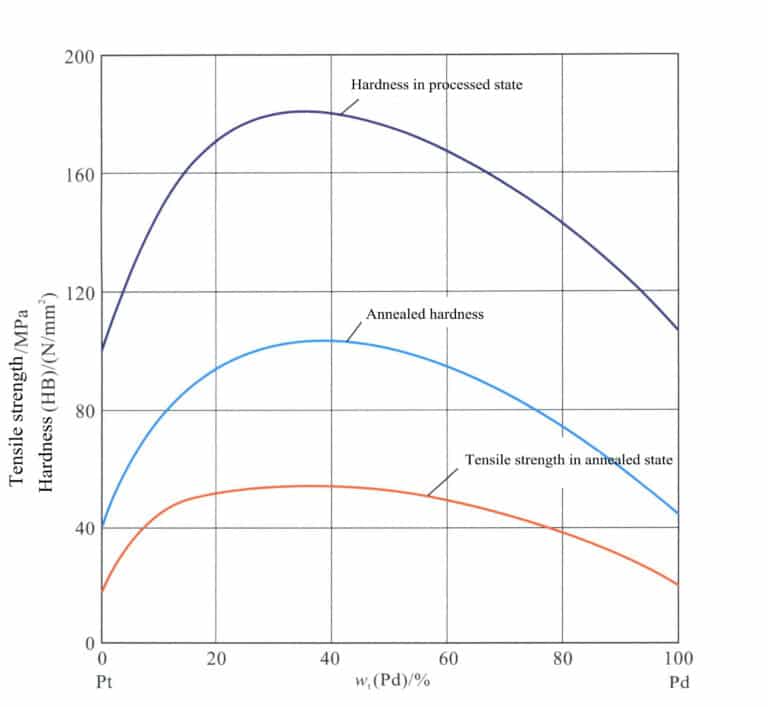
Pt-Pd The hardness of the alloy in the annealed state is very low, with good processing performance. As the Pd content increases, the hardness and strength of the alloy initially increase rapidly, reaching a peak, after which further increases in Pd content lead to a decrease in hardness and strength (Figure 5-10).
Pt-Pd The alloy has high corrosion resistance and oxidation resistance, but with increased Pd content, its corrosion resistance and oxidation resistance slightly decrease. Pt-Pd, The casting performance of the alloy is generally average due to the fact that Pd easily absorbs gases, making it prone to forming pinholes in castings when cast in the atmosphere; it needs to be cast in a protective atmosphere. Pt-Pd The alloy is commonly used in its original color. There are three types: 95%Pt-5%Pd, 90%Pt-10%Pd, and 85%Pt-15%Pd, with the following characteristics and application ranges.
(1) 95%Pt – 5%Pd Alloy:
Widely used in Japan, Hong Kong, and Europe, suitable for casting fine parts. The hardness in the annealed state is about HV70, density 20.98 g/cm3, melting point 1765℃.
(2) 90%Pt – 10%Pd Alloy:
Preferred as a general-purpose alloy in Japan and Hong Kong, it can be cast, welded, and brazed and is one of the most widely used platinum alloys in Asia. It has a grayish-white color, and the surface is generally rhodium-plated. The hardness in the annealed state is about HV80, and the hardness in the processed state is about HV140, similar to 95%Pt-5%Ir alloy. Density is 20.51 g/cm3, melting point is 1755℃, and casting fluidity is good, but castings often exhibit shrinkage defects.
(3) 85%Pt – 15%Pd Alloy:
Used for processing chains in Japan and Hong Kong, with an annealed hardness of about HV90 and good flexibility. Density 20.03 g/cm3, melting point 1750℃.
In summary, the binary platinum alloys composed of different alloying elements have certain differences in performance, and there are different adaptabilities for various processing techniques in jewelry production, as detailed in Table 5-8.
Table 5-8 Common applications of platinum alloy series
| Alloy type | Welding | Razing | Hydraulic Pressure | Stamping | Precision Casting | Forging | Inlay | Chain Making | Accessories | Assemble |
|---|---|---|---|---|---|---|---|---|---|---|
| Pt-Co Alloy | ● | ● | ● | ● | ●●● | ● | ● | ● | ● | ● |
| Pt-Cu Alloy | ●●● | ●●● | ●●● | ●●● | ●● | ●● | ●● | ●●● | ● | ●●● |
| Pt-Pd Alloy | ●● | ●●● | ●●● | ●●● | ● | ●● | ●●● | ●● | ●● | ●●● |
| Pt-Rh Alloy | ●● | ●● | ●● | ●● | ● | ●● | ●● | ●● | ●● | ●● |
| Pt-Ru Alloy | ●● | ● | ●● | ●● | ● | ●● | ● | ●● | ●● | ● |
| Pt-Ir Alloy | ●●● | ●●● | ●● | ●● | ●● | ● | ● | ●●● | ●● | ●●● |
| Pt-W alloy | ●●● | ●●● | ●● | ●●● | ● | ● | ● | ●● | ●●● | ●●● |
Note: ●Represents recommended; ●● represents acceptable; ●●●represents difficulties.
Copywrite @ Sobling.Jewelry — Custom jewelry manufacturer, OEM and ODM jewelry factory
3.2 Ternary or quaternary platinum alloys
In many applications, the hardness of binary platinum alloys still needs to be improved, and their process performance needs to be better, leading to problems during product production and use. Therefore, many ternary or quaternary platinum alloys based on binary alloys have been developed, such as Pt Pd-Me alloy series, Pt-Ir-Me alloy series, Pt-Ru-Me alloy series, Pt-Co-Cu alloy series, etc. Taking the Pt-Pd-Me alloy series as an example, this is based on the Pt-Pd binary alloy, adding one or several other alloying elements.
Platinum alloys are composed of alloying elements. Due to the very low hardness of the Pt-Pd alloy and its average casting performance, adding elements such as Cu, Co, and Ru can effectively improve the overall performance of the alloy.
3.2.1 Pt-Pd-Cu Alloy
Adding a small amount of Cu to the Pt-Pd alloy can improve hardness and wear resistance while reducing the cost of the alloy. Excessive Cu content can affect the alloy’s color, corrosion, and oxidation resistance, and the surface is prone to darkening due to oxidation during casting, heat treatment, welding, and other operations. Therefore, the Cu addition is generally controlled to be 3%- 5%, at which point the color of the alloy is unaffected by copper, and the copper oxide film formed on the surface during hot processing can be removed by soaking in dilute sulfuric acid. The processing performance and hardness of the Pt-Pd-Cu alloy are improved. As the copper content increases, the hardness of the alloy increases, especially when used in processed form, making it suitable for making hard decorative items such as necklaces, bracelets, brooches, earrings, and pendants, which are relatively easy to polish. The casting performance of the Pt-alloy is generally average, and it is prone to gas absorption and oxidation when cast in the atmosphere. The alloy is relatively brittle and must be cast in an inert atmosphere or vacuum. This alloy is widely used in China and Japan.
3.2.2 Pt-Pd-Ru Alloy
Adding Ru to the Pt-Pd alloy can improve its hardness and wear resistance and, to some extent, enhance its casting performance. The alloy has good corrosion resistance. The alloy has good flexibility and can be used as a general-purpose alloy for different forming processes.
3.2.3 Pt-Pd-Co Alloy
Adding Co can improve the casting performance and processing performance of the Pt-Pd alloy, increase the alloy’s hardness, strength, and wear resistance, and enhance the work hardening rate of the alloy (Figure 5-11). After adding Co to Pt900 with 5%, the work hardening level of the alloy is significantly higher than that of 90%Pt-10%Pd alloy and 90%Pt-10%Ir alloy and also significantly higher than that of 18 K gold. Therefore, the Pt-Pd-Co alloy is often made into hard ornaments in a processed state. Since Co is easily oxidized, an oxidized cobalt film can easily form on the surface of the alloy during annealing or welding in the atmosphere. Hence, the Co content added to the alloy is generally within 5%. The Pt-Pd-Co alloy can be used as a general purpose alloy, suitable for casting and cold processing.
The main properties and applications of different alloy elements and different grades of ternary platinum alloys are shown in Table
Table 5-9 Main Properties and Applications of Ternary Platinum Alloys
| Alloy | Melting point /℃ | Density / (g/cm3) | Annealed Hardness HV/(N/mm2) | Tensile strength in annealed state Strength /MPa | Application | Main application area |
|---|---|---|---|---|---|---|
| 90%Pt-7%Pd-3%Cu | 1740 | 20.7 | 100 | 300 ~ 320 | General applications, machined parts | Japan, China |
| 90%Pt-5%Pd-5%Cu | 1730 | 20.5 | 120 | 340 ~ 360 | Machined parts | Japan, China |
| 85%Pt-10%Pd-5%Cu | 1750 | 20.3 | 130 | 350 ~ 370 | Machined parts | Japan |
| 95%Pt-7%Pd-3%Co | 1740 | 20.4 | 125 | 350 ~ 370 | General Application | Japan, China |
| 85%Pt-10%Pd-5%Co | 1710 | 19.9 | 145 | 500 ~ 520 | Castings, Machined Parts | Japan |
| 85%Pt-12%Pd-3%Co | 1730 | 20.1 | 135 | 370 ~ 390 | Castings, machined parts | Japan |
| 80%Pt-15%Pd-5%Co | 1730 | 19.9 | 150 | - | Hard decorative parts | Japan |
| 95%Pt-3%Co-2%Cu | 1765 | 20.4 | 115 | 370 | Castings, machined parts | China |
4. Common Issues in the Production of Platinum Alloy Jewelry
Due to the special properties of platinum alloy materials, the casting of platinum jewelry has characteristics such as high melting temperature, short retention time in liquid state, and easy contamination of the metal liquid, which can easily lead to casting defects; the hardness of platinum jewelry is relatively low, while its toughness is high, making its production much more difficult than that of gold and silver jewelry.
4.1 Melting Crucible
Platinum has a high melting point, which places high demands on the melting crucible’s heat resistance, thermal stability, and chemical reactivity. The crucible used for melting platinum should have the following properties to ensure metallurgical quality and production stability.
(1) High melting point and refractoriness. It should withstand the high temperatures of molten platinum without melting or softening.
(2) Good thermal shock resistance. It can withstand the rapid heating and cooling alternation during induction heating melting and casting without thermal shock cracking.
(3) Good chemical inertness. It is resistant to metal liquid erosion at high temperatures, does not chemically react with molten metal, and will not be eroded or perforated by molten metal.
(4) Sufficient mechanical strength. It can withstand the impact of metal charge feeding and the external forces of centrifugal casting, making it less prone to cracking or spalling.
Graphite crucibles are commonly used for non-ferrous metal melting and are the preferred crucible material for melting gold and silver alloys. However, because platinum can dissolve a large amount of carbon in its molten state, and when solidifying, carbon precipitates in fibrous or flaky graphite form at the grain boundaries, leading to brittle fracture of platinum, platinum is not suitable for melting in graphite crucibles and can only use oxide crucibles.
The material range of oxide crucibles is quite broad, but only some types of oxide crucible are suitable for melting platinum. For example, materials like alumina, lead oxide, and magnesium oxide all have very high melting temperatures (alumina 2050℃, magnesium 2800℃, zirconia 2680℃ ), making them commonly used crucible materials; however, their thermal shock resistance is poor, and they are prone to cracking and premature failure when used in platinum jewelry casting.
Currently, quartz crucibles are mainly used for casting platinum jewelry. Quartz crucibles have good thermal shock resistance and can generally withstand rapid cooling and heating during induction heating pouring. However, they also have a prominent issue: their refractoriness needs to be improved to withstand the high temperatures during platinum melting. As the number of uses increases, the wall thickness of the crucible’s side and bottom continues to thin, effectively increasing the usable volume. At the same time, the outer diameter of the melting zone of the crucible slightly decreases (Figure 5-12). Especially when the raw materials are not Pre-alloying treatment and are directly melted in the crucible, higher melting temperatures and longer melting times are often adopted to promote uniform composition, leading to an increased probability of crucible erosion and deteriorating the metallurgical quality of the molten metal.
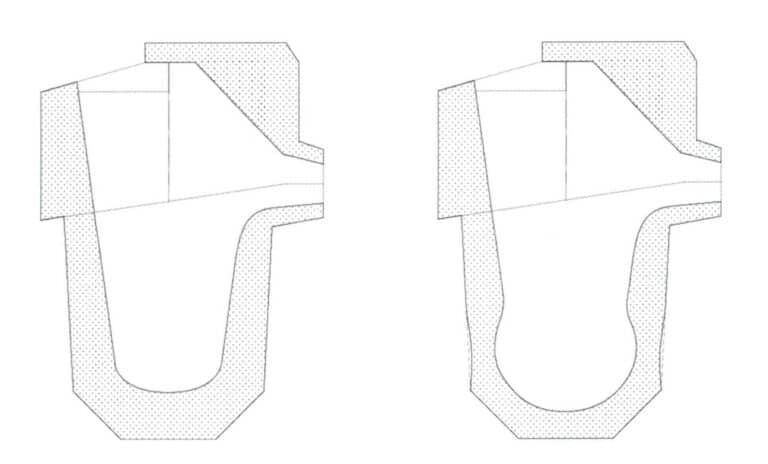
Table 5-10 shows the dimensions and volume of the crucible after different usage counts. Therefore, the current quartz crucibles do not adequately meet the casting requirements for high-quality platinum jewelry, and crucible materials need to be developed that better match thermal shock resistance and refractoriness.
Table 5-10 Changes in wall thickness and effective volume of quartz crucibles after melting platinum
| Melting furnace count/times | Side wall thickness at slag line /mm | Bottom thickness of the crucible /mm | Change in outer diameter of the melting zone /mm | Effective volume /mL |
|---|---|---|---|---|
| 0 | 8.1 | 12.9 | 0 | 35.85 |
| 4 | 7.0 | 11.6 | 0.14 | 36.94 |
| 10 | 4.6 | 9.1 | 0.44 | 39.48 |
4.2 Casting materials
Platinum casting temperature is high, the relative density of the molten metal is large, and centrifugal casting is often used, along with the casting materials used. The materials must meet performance requirements such as high heat resistance, good thermal stability, low reactivity with molten metal, high mold strength, and certain permeability. For the precision casting of gold and silver jewelry, gypsum mold materials are generally used, which are very convenient as the slurry can solidify quickly and be easily cleaned after casting. However, for platinum jewelry casting, gypsum mold materials are unsuitable because gypsum has poor thermal stability and will undergo thermal decomposition at 1200℃, and the strength of gypsum molds is relatively low. The pouring temperature of molten metal during platinum casting is often above 1850℃. If gypsum mold materials are used, the castings will suffer serious defects such as porosity and sand holes.
Therefore, during platinum casting, mold materials using phosphates and silica sol as binders should be adopted, as their high-temperature strength is much higher than that of gypsum molds, and they have better thermal stability, which is beneficial for obtaining castings with better surface quality. However, the slurry made from these mold materials does not self-solidify quickly like gypsum casting powder slurry; instead, it requires slow dehydration to achieve initial wet strength. Otherwise, the mold may crack during firing, leading to defects such as burrs and sand holes in the castings (Figure 5-13). The strength of phosphate and silica sol bonded molds is very high, with poor flexibility, and they are prone to cracking due to the poor plasticity of the platinum alloy in the casting state. The residual strength of the mold is very high, making it difficult to clean the castings.

4.3 Casting Defects
During platinum jewelry casting, defects such as porosity, shrinkage, and inclusions are likely to occur. Figure 5-14 shows porosity defects on a Pt950 platinum ring casting. The occurrence of porosity is closely related to the properties of the alloy and the melting and casting process. Platinum alloys have a strong tendency to absorb gas, and when the alloy is melted in an atmosphere with insufficient vacuum or under atmospheric conditions, it can lead to defects.
At high temperatures, molten metal is prone to absorb gases; the higher the molten metal temperature, the more severe the gas absorption. When the molten metal is poured into the mold, it cools down rapidly, and the solubility of gases in the molten metal decreases sharply. The gases that cannot be dissolved precipitate out, and if the precipitated gases cannot be expelled in time, they will be trapped on the surface or inside the casting, forming pores. Platinum alloys have a high melting temperature and exhibit a certain tendency to absorb gases, but different types of alloys have varying tendencies. Under the same degree of superheating, the gas absorption tendency of the Pt-Pd alloy is generally greater than that of other alloys. If gas pores frequently appear in the casting, choosing an alloy with a smaller gas absorption tendency and strengthening protection during melting to reduce gas absorption is advisable.

Figure 5-15 shows the micro-shrinkage defects that occurred during the casting of the Pt900 ring, which is a common problem encountered when casting platinum jewelry. The shrinkage defects significantly worsen the polishing quality of the jewelry surface, and severe shrinkage can also affect the overall quality, the mechanical properties of the jewelry. The reason lies in platinum alloys’ high melting point and the molten metal’s high viscosity, which creates significant flow resistance. After the molten metal is poured into the mold, it cools rapidly, and the time it remains in liquid form is short. When the casting undergoes solidification shrinkage, if the molten metal cannot overcome the flow resistance to reach the areas that need to be supplemented, it will ultimately leave shrinkage defects in the casting. The wider the crystallization interval of the platinum alloy, the more developed the dendrites formed during solidification, making it easier for the molten metal to be isolated into small liquid regions during the solidification process. When these liquid regions undergo solidification shrinkage, they find receiving external molten metal supplementation difficult, resulting in microscopic shrinkage. Therefore, platinum jewelry castings are prone to shrinkage defects, and during casting, it is advisable to choose platinum alloys with better fluidity and smaller crystallization intervals, and the size of the pouring channels should generally be larger than that of gold and silver jewelry.

4.4 Platinum Polishing
In the production of platinum jewelry, surface polishing difficulties are a very common issue closely related to platinum’s properties. Domestic platinum inlaid jewelry mainly uses Pt950, which has a lower hardness. The cast blanks usually have insufficient density, with defects such as air holes and shrinkage, making it easy to produce scratches during polishing. After polishing, the surface is prone to dents and scratches due to its low hardness.
Therefore, in production, efforts should be made to improve the hardness of platinum alloys through solid solution strengthening, fine grain strengthening, aging strengthening, and deformation strengthening, and measures should be taken to improve the quality of jewelry blanks and increase their density. During the grinding process, it is important to correctly assess the condition of surface defects and choose appropriate corrective measures. Use increasingly finer sandpaper to repeatedly grind the surface until the final scratches are very small, almost invisible. During polishing, avoid overheating; otherwise, the polishing medium may easily adhere to the workpiece surface and mix with the next finer polishing medium, causing cross-contamination.
Section III Jewelry used palladium and its alloy Materials
1. Palladium Jewelry
1.1 The Development History of Palladium Jewelry
As a rare white precious metal, palladium was used in jewelry as early as the 1940s. During World War II, platinum was halted for civilian use as the government designated it as a strategic reserve. Some well-known jewelry brands, such as Tiffany & Co. in the United States, had chosen to use palladium instead of platinum for jewelry making. However, palladium did not see widespread use in the jewelry industry after the war. The reason is that although the price of platinum was still relatively acceptable at that time, the special physical properties of palladium increased the difficulty of its production process. Because of this, palladium has always played a “supporting role” in jewelry making. In early platinum jewelry from Japan and China, the alloy, commonly known as the filler used, was palladium, so there was some application of palladium in the jewelry industry. The widespread application of palladium in jewelry truly originated in China. At the end of 2003, when platinum prices were high, China began vigorously promoting palladium use for jewelry making. Palladium jewelry quickly became a new favorite in the jewelry market, with many jewelry stores setting up dedicated counters for palladium jewelry, leading to the rapid development of the palladium jewelry market, making China the world’s largest consumer of palladium jewelry. Meanwhile, the United States, Japan, and Europe also developed palladium jewelry, and many internationally renowned jewelers and top fashion jewelry designers generally see a broad development prospect for palladium jewelry. Internationally known brands also began to focus on palladium jewelry, fully utilizing the unique brilliance and strong plasticity to create one modern and stylish piece of jewelry after another.
However, compared to platinum jewelry, the chemical stability of palladium jewelry is relatively poor. After wearing palladium jewelry for a period, it tends to become dull. Additionally, the lower density of palladium jewelry gives it a light and airy feel, resulting in a poorer texture. The processing difficulty is greater than that of platinum; during melting, it is prone to flying and has a high loss rate. Products are likely to have issues such as porosity, breakage, and discoloration during welding, which places high demands on all aspects of production. The technical level of ordinary gold shops and jewelry processing factories is often insufficient to process palladium, making most gold shops unwilling to buy back palladium jewelry. This has caused the domestic palladium jewelry market to encounter a development bottleneck after a brief period of glory, especially in recent years, as the price of palladium has skyrocketed due to surging demand in the environmental market, significantly exceeding that of platinum, further hindering the development of palladium jewelry.
1.2 Purity Marking of Palladium Jewelry
Pure palladium jewelry is the highest grade of Jewelry, with a theoretical grade of 1000‰. Pure palladium material is soft and generally can only be made into plain gold jewelry without embedded gemstones, such as rings, necklaces, earrings, etc. If gemstones are to be set, a small amount of other metals, such as iridium, ruthenium, or copper, must be added to the palladium to increase the hardness and toughness of pure palladium. Therefore, based on their composition, most palladium jewelry is made from palladium alloys, which can be divided into high-grade palladium and low-grade palladium. High-grade palladium typically has a palladium content of over 80%, with alloys containing 95% being the most commonly used; low-grade palladium usually has a palladium content not exceeding 50%.
To ensure the purity of palladium in each piece of jewelry, each palladium jewelry item must be marked with a Pd purity label. Most countries in the world express the quality of palladium alloy jewelry in terms of thousandths, such as Pd850, Pd900, Pd950, and Pd990, which represent the purity of Pd in the jewelry as 850‰, 900‰, 950‰, and 990‰ respectively.
2. Palladium Alloy Jewelry Materials
2.1 Pure Palladium
The average reflectivity of palladium to visible light is about 62.8%, lower than silver and platinum, and appears grayish-white. Palladium has the lowest corrosion resistance among all platinum group metals but is still better than silver. In a normal atmospheric environment, palladium exhibits good corrosion resistance and anti-tarnish properties. The density of palladium is 12.02 g/cm3, classified as a light precious metal, and compared to gold and platinum, palladium jewelry of the same volume is lighter. In contrast, palladium jewelry of the same weight appears to have a larger volume.
Pure palladium in the annealed state has a hardness of about HV42, a tensile strength of about 190 MPa, and an elongation of 35%-40%, exhibiting good processing performance. When the deformation is 50%, the hardness increases to HV110, and the tensile strength is about 350 MPa. The work-hardening rate of palladium is higher than that of platinum.
2.2 Palladium alloy for decoration
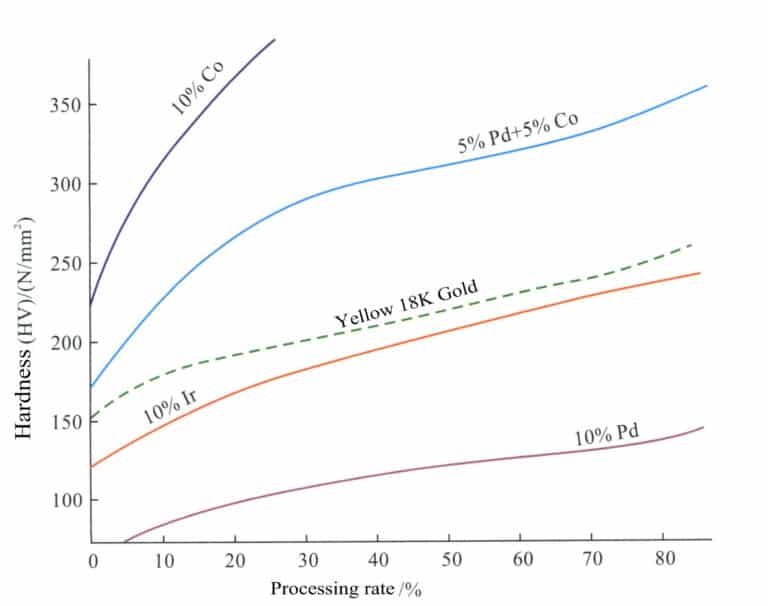
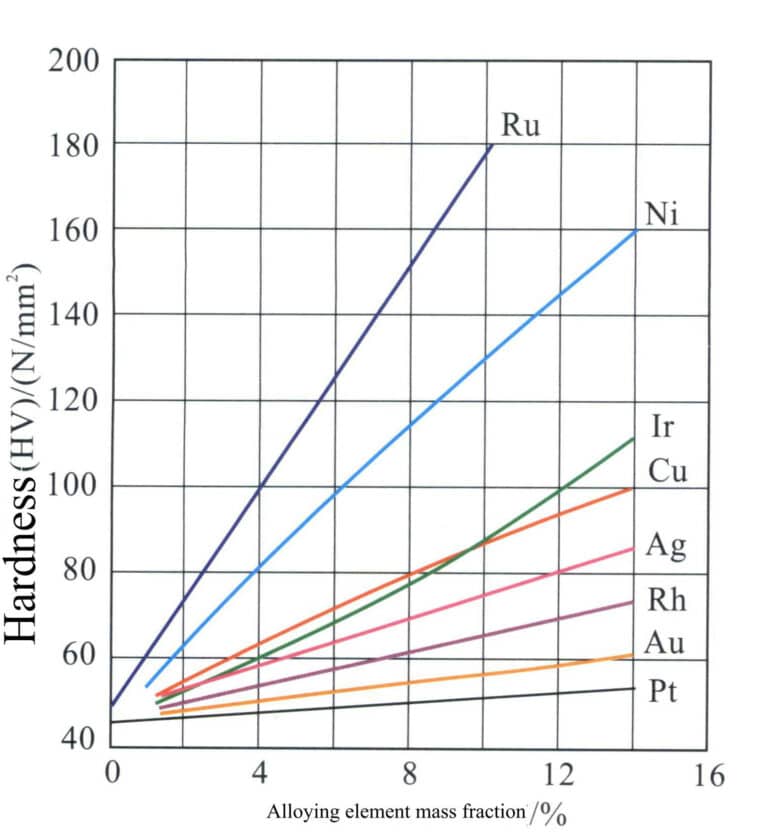
Due to the low strength and hardness of pure palladium, it is easily deformed and worn for making jewelry. Therefore, it often requires strengthening treatment in actual production. High-grade palladium alloys can only contain a small amount or trace of alloying elements, which should have high hardening or strengthening effects. The strengthening effects of different alloying elements on palladium vary greatly (Figure 5-16), among which the elements with better hardening and strengthening effects include Ru, Ni-Ir, Cu, and others.
2.2.1 Pd-Ru Alloy
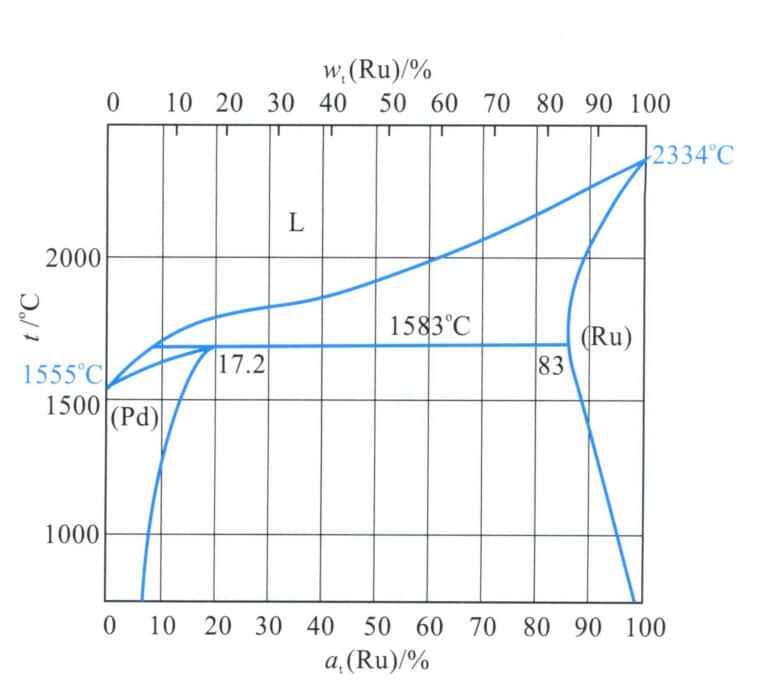
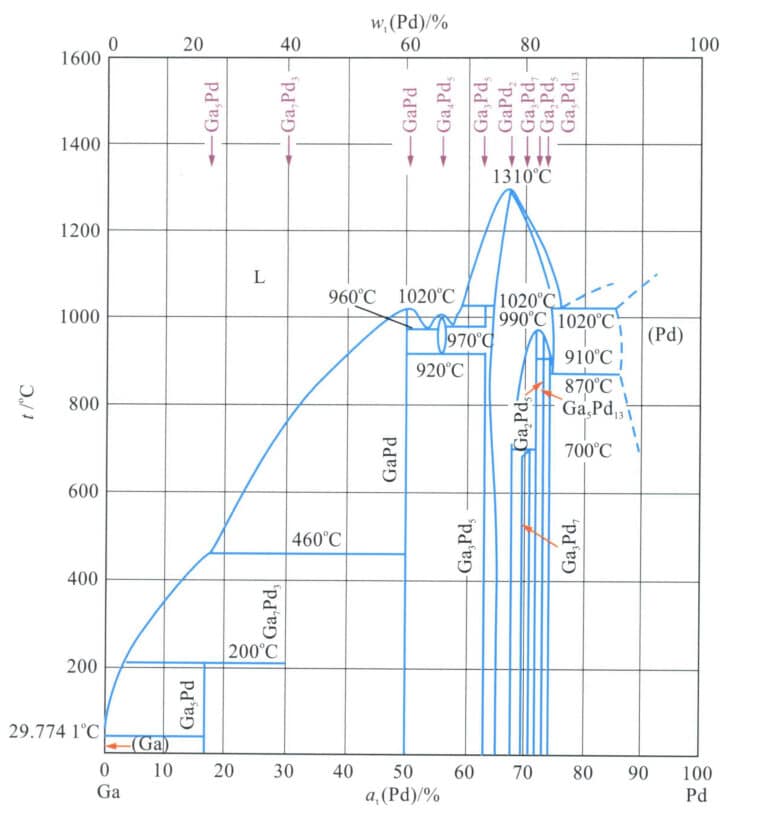
The binary alloy phase diagram is shown in Figure 5-17. This alloy belongs to the peritectic system, with the maximum solubility of ruthenium in palladium being 17.2%(at) and the peritectic reaction temperature being 1583℃, Pd-Ru. The alloy is a single solid solution at high temperatures. As the temperature decreases, the solubility of ruthenium in palladium decreases, leading to the precipitation of a ruthenium-rich phase at a certain temperature, which enhances the alloy’s strength.
Among the commonly used alloying elements, ruthenium has the strongest strengthening effect on palladium, and the alloy has a high work hardening rate. With the increase of Ru content, the hardness and strength of the solid solution Pd-Ru alloy significantly increase, and the work hardening rate of the alloy increases. Alloys with lower ruthenium content, such as Pd-Ru, have good processing performance, but when the ruthenium content exceeds 12% (wt), the processing performance of the alloy deteriorates. Therefore, the Pd-Ru alloy used for jewelry generally has a lower ruthenium content, with 95%Pd-5%Ru being the most common. The properties of this alloy are shown in Table 5-11. The addition of ruthenium can improve the reflectivity of palladium to visible light, making it appear whiter; it can also enhance the corrosion resistance of palladium.
Table5-11 95%Pd-5%Ru Main Properties of the Alloy
| Melting Point/°C | Density/ (g/cm3) | Color | Hardness HV/(N/mm2) | Tensile Strength/ MPa | |||
|---|---|---|---|---|---|---|---|
| Melting Point/°C | Density/ (g/cm3) | Color | Solid solution | Solid solution aging state | Processed state (50%) | Solid solution | Processed State (50%) |
| 1590 | 12 | Silver-white | 100 | 160 | 180 | 420 | 650 |
95%Pd-5%Ru can be processed into profiles and then made into jewelry or other decorative items through stamping, machining, and other methods; it can also be directly cast into jewelry blanks using the lost-wax casting method and then set into ornaments through mold setting.
2.2.2 Pd-Cu Alloy
The binary alloy phase diagram of Pd-Cu is shown in Figure 5-18. The alloy is a continuous solid solution in the high-temperature region. As the temperature decreases below 598℃, within the composition range of decreasing palladium content, the Pd-Cu alloy undergoes an ordering transformation, forming different ordered phases that enhance the hardness of the alloy. Since the copper content will affect the color and corrosion resistance of the alloy after reaching a certain level, the copper content in decorative Pd-Cu alloys is generally kept within 10%, which is far from the ordering transformation zone, and the alloy structure is a single solid solution phase. Both copper and palladium have a face-centered cubic structure, and their atomic radius difference is not significant, so the strengthening effect of copper in palladium is not very pronounced.
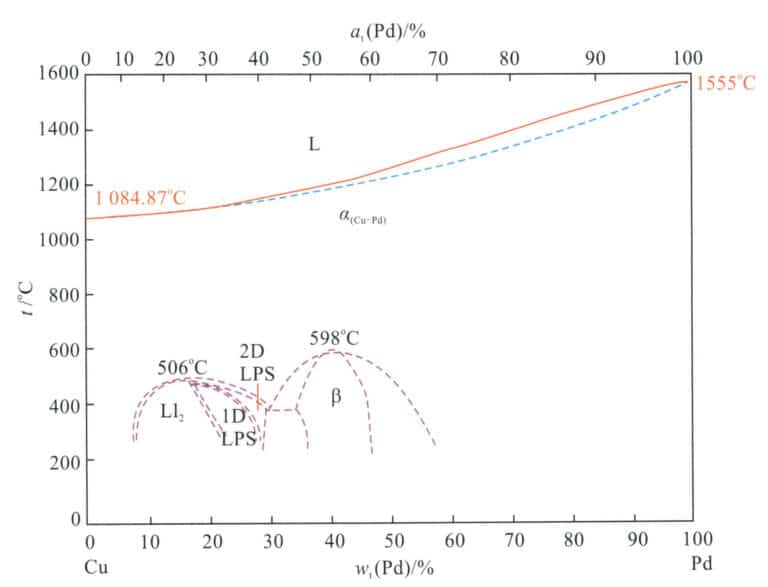
Note: Ll2 stands for Cu3Pd-type ordered phase; stands for CuPd-type ordered phase; 1D LPS stands for one-dimensional reversed-phase domain structure; 2D LPS stands for two-dimensional reversed-phase structure; 506℃ stands for the onset temperature of the Ll2-ordered phase transition; 598℃ stands for the onset temperature of the β-ordered phase transition.
In the Pd-Cu alloy system, the 95%Pd-5%Cu alloy is the most widely used, and its main properties are shown in Table 5-12.
Table 5-12 Main properties of 95% Pd-5% Cu alloy
| Melting Point/°C | Density/ (g/cm3) | Color | Hardness HV/(N/mm2) | Tensile Strength/ MPa | Elongation rate /% | |||
|---|---|---|---|---|---|---|---|---|
| Melting Point/°C | Density/ (g/cm3) | Color | Solid solution | Processed state (75%) | Solid solution | Processed State (75%) | Solid solution | |
| 1490 | 11.4 | Silver-white | 60 | 160 | 250 | 550 | 30 | |
The melting point of the 95%Pd-5%Cu alloy is lower than that of the Pd-Ru alloy, and its crystallization temperature range is very small, which is beneficial for casting performance. However, due to palladium’s high gas absorption tendency, defects such as porosity are still likely to occur during casting.
Due to the lower hardness of the Pd-Cu alloy, adding an appropriate amount of alloying elements with a higher hardening effect, such as Ni, Ga, and In, can further improve the hardness of the alloy.
The 95%Pd-5%Cu alloy can be processed into profiles to make jewelry, and it can also be made into jewelry using the lost-wax casting process. Binary alloys can be used to make plain gold jewelry, while ternary or multi-element alloys containing strengthening elements can make inlaid jewelry.
2.2.3 Pd-Ga Alloy
The phase diagram of the Pd-Ga binary alloy is shown in Figure 5-19. A complete phase diagram has yet to be established, but it is speculated that when the Ga content is low, a continuous solid solution forms during solidification. As the temperature decreases, the solubility of gallium in palladium decreases, leading to the precipitation of a phase that enhances precipitation strengthening. When the gallium content reaches a certain level, a series of intermediate phases form during solidification, making the alloy hard and brittle. Therefore, in practical Pd-Ga alloy systems, the gallium content usually does not exceed 5%, and its strengthening effect is significantly greater than that of copper, exhibiting a high hardening effect.
Gallium has a very low melting point, and its addition to palladium also lowers the alloy’s melting point. The melting temperature of the 95%Pd-5%Ga alloy is lower than that of the 95%Pd-5%Cu alloy, but the crystallization interval of the Pd-Ga alloy is greater than that of the latter. Gallium has a very high boiling point, but it is easily oxidized in the atmosphere, so vacuum or inert gas protection is required during melting and casting. 95%Pd-5%Ga can be used as a general alloy and made into jewelry through processing profiles or lost wax casting. Due to its high strength, it can be used to create inlaid jewelry.
To further improve the performance of the alloy during production, additional elements such as In and Ag are added based on the Pd-Ga alloy, such as the 95%Pd-5%Ga/Ag alloy developed by the American company Hoover & Strong, which has an annealed hardness of HV125 and a crystallization interval of only 30℃, and the 95%Pd-5%Ga/In alloy developed by the Italian company Legor, which has an annealed hardness of HV103 and a crystallization temperature interval of 50℃. These alloys have good casting performance, relatively good casting quality, and decent recyclability.
2.2.4 Pd-Ag Alloy
The binary alloy phase diagram of Ag-Pd is shown in Figure 4-13. This alloy is infinitely miscible in liquid and solid phases, forming a continuous solid solution. Adding Ag to Pd lowers the alloy’s melting point and increases its whiteness and brightness.
The Pd-Ag alloy has good casting performance, which is beneficial for jewelry production. As shown in Figure 5-16, silver has a certain hardening effect on palladium, but the effect is not prominent. For high-quality palladium jewelry, the strength and hardness of the Pd-Ag alloy make it difficult to meet production requirements. Therefore, additional alloying elements such as Ru, Ni, Cu, Ga, and In are added to this alloy to develop ternary or multi-element alloys with better strength performance.
The Soviet Union once added a small amount of Ni to the Pd-Ag alloy to strengthen it, developing the 85%Pd-13%Ag-2%Ni alloy, which is a single-phase solid solution with a melting point of about 1450℃, an annealed hardness of about HB100, and good corrosion resistance and chemical stability, along with good processing performance.
Adding Cu to the Pd-Ag alloy can improve its hardness to a certain extent. Still, for high-quality palladium alloys, the combined strengthening effect of Ag and Cu is also limited (Figure 5-20).
3. Common issues with palladium jewelry
3.1 Darkening discoloration issue
After wearing palladium jewelry for some time, the surface often becomes dull. The properties of palladium itself determine this: Pd has relatively poor chemical stability, its d electron layer is not filled, and it easily adsorbs organic gases. Under the catalytic action of Pd, the adsorbed organic substances convert aromatic compounds into aliphatic compounds or complex mixtures, forming a dark brown organic polymer film on the surface, presenting the so-called “brown powder effect.” To improve the anti-dulling performance of palladium jewelry, from the perspective of materials and processes, it is necessary to add alloying elements to enhance the resistance to organic contamination in Pd, such as Ag, Au, Cu, Ni, Sn, etc. In addition, the whiteness of palladium alloys themselves is insufficient, and they usually need to be plated with rhodium on the surface, requiring improvements in the rhodium plating process to extend the lifespan of the coating. When in use, it is also important to reduce sources of organic contamination in the environment and to avoid using or storing them in atmospheres containing organic substances such as toluene, ether, and phenol.
3.2 Casting process issues
Most set jewelry needs to be shaped through casting, while the casting difficulty of palladium jewelry greatly exceeds that of gold and silver jewelry. This is related to the properties of palladium alloys, mainly manifested in the following aspects:
(1) Graphite crucibles cannot be used for melting palladium alloys, as they will also encounter the same “carbon poisoning” issue as platinum, and only quartz, magnesia, and other ceramic crucibles can be used.
(2) The palladium alloy melts strongly and tends to absorb gas. During melting, the molten metal is prone to splashing, resulting in high losses, which places higher demands on casting equipment and melting processes.
(3) The melting point of palladium alloy is relatively high, with casting temperatures generally above 1400℃, and the casting temperature of high-grade palladium can even reach 1700℃. Therefore, conventional gypsum molds will cause serious reactions, and ceramic molds with phosphate binders must be used.
3.3 Maintenance and recycling issues of palladium jewelry
The craftsmanship of palladium jewelry is quite challenging, and products inevitably have various issues that may be exposed during use, such as discoloration, exposed hole defects, cracks, or breakage. The jewelry market has not yet formed a complete aftersales maintenance and service channel. Ordinary gold shops or jewelry factories often find it difficult to undertake maintenance or recycling needs for palladium jewelry due to hardware conditions and technical limitations, which undoubtedly cause trouble for consumers of palladium jewelry.
Copywrite @ Sobling.Jewelry — Custom jewelry manufacturer, OEM and ODM jewelry factory