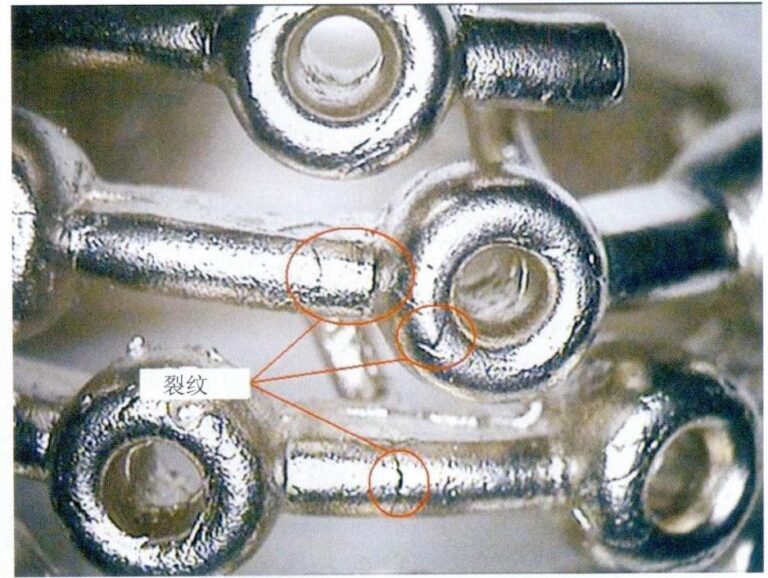
Vilka är de viktigaste metoderna och tillämpningarna för platinabeläggning i modern industri?
Tekniker för platinaplätering: Lösningar, legeringar och tillämpningar för smycken
Inledning:
Undrar du om platinaplätering? Den här guiden täcker allt från grunderna till avancerade tekniker. Lär dig mer om olika pläteringslösningar, inklusive klorid- och sulfatbaserade alternativ, och upptäck hur du kan förbättra dem. Utforska lösningar för tunn- och tjockplätering för olika tillämpningar. Är du nyfiken på platina-legeringar? Vi går igenom populära legeringar som Pt-Au, Pt-Co och Pt-Ir. Dessutom kan du dyka in i kemisk plätering för unika tillämpningar. Oavsett om du är smyckesdesigner, återförsäljare eller specialtillverkare kommer den här omfattande översikten att hjälpa dig att förbättra dina produkter med platinaplätering.

Innehållsförteckning
Avsnitt I Översikt
Platina har atomnummer 78 i det periodiska systemet, grundämnessymbol Pt, relativ atommassa 195,7, densitet 21,09 g/cm3 (20℃) och en smältpunkt på 1768℃.
Några huvudparametrar för platina visas i tabell 3-1.
Tabell 3-1 Några huvudparametrar för platina
| Karakteristiska parametrar | Karakteristiskt värde |
|---|---|
|
Grundämnesnamn, grundämnessymbol, atomnummer Klassificering Grupp, Period Densitet, hårdhet Färg Relativ atommassa Atomradie Radie för kovalent bindning Kemisk valens Kristallin struktur smältpunkt kokpunkt Förångningsvärme Värme vid upplösning Specifik värmekapacitet Konduktivitet Termisk ledningsförmåga |
Platina、Pt、78 Övergångsmetall 10(Ⅷ),6 21090kg/m3, 3.5 Gråvit 195.084 135 pm 128pm 2、4 Ansiktscentrerad kubisk 2041. 4K( 1768.3℃) 4098K (3825℃) 510 kJ/mol 19:6 kJ/mol 130J/(kg - K) 9. 66X 106m ・Ω 71. 6W/(m ・ K) |
Avsnitt II Elektroplätering av platina
Tabell 3-2 Industriella tillämpningar av Pt-pläteringsbeläggningar
| Produkt | Material | Pläteringstjocklek/μm | Produkt | Material | Pläteringstjocklek/μm |
|---|---|---|---|---|---|
|
Komponenter för flyg- och rymdindustrin Komponenter för luftfart Säkerhetsskottslådor Elektroder |
Niob-innehållande superlegeringar SUS347 Titan SUS316 |
10 10 5 10 |
Elektroder Elektroder Elektroder - |
Titan Titan-nät Tråd av volfram - |
2〜7 2〜7 10 - |
Tabell 3-3 Typiska platinasalter
| 2, 4-värdiga salter | Typiska platinasalter |
|---|---|
| Pt(II)-salter |
Kloroplatinsyra :H2PtCl6 - 6H2O Diammin platinanitrit :Pt(NH3)2(NO2)2 Platina nitrit sulfat :H2Pt(NO2)2SO4 |
| Pt(Ⅳ)-salter | Natriumhydroxyplatinat :Na2Pt(OH)6 - 2H2O |
1. Olika lösningar för platinaplätering
Tabell 3-4 Olika sammansättningar av Pt-pläteringslösningar och processförhållanden
| Sammansättning och processförhållanden | Klorid | Diammoniumsulfit | DNS | Hydroxibasiska salter | Fosforsyra | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nr 1 | Nr 2 | Nr 3 | Nr 4 | Nr 5 | Nr 6 | Nr 7 | Nr 8 | Nr 9 | Nr 10 | Nr 11 | Nr 12 | Nr 13 | Nr 14 | |
| Kloroplatinsyra H2PtCl6/(g/L) | 10 〜50 | |||||||||||||
| Ammoniumkloroplatinat (NH4)2PtCl6/ (g/L) | 15 | |||||||||||||
| Diammin platina nitrit Pt(NH3)2(NO2)2/(g/L) | 8~16. 5 | 20 | 6~20 | 8 | 6~20 | 16.5 | ||||||||
| Platina nitrit sulfat H2Pt(NO2)2SO4/ (g/L) | 10 | |||||||||||||
| Natriumhydroxyplatinat Na2Pt(OH)6 ・ 2H2O/(g/L) | 20 | 18.5 | ||||||||||||
| Hydroxyplatinsyra H2Pt (OH)6/ (g/L) | 20 | |||||||||||||
| Kaliumhydroxyplatinat K2Pt(OH)6/ (g/L) | 20 | |||||||||||||
| Platinaklorid PtCl4- 5H2O/(g/L) | 7.5 | |||||||||||||
| Ammoniak(28%)/(g/L) | ||||||||||||||
| Saltsyra/(g/L) | 180~300 | |||||||||||||
| Natriumcitrat/(g/L) | 100 | 20~25 | ||||||||||||
| Ammoniumklorid/(g/L) | 4~5 | |||||||||||||
| Ammoniumnitrat/(g/L) | 100 | |||||||||||||
| Natriumnitrit/(g/L) | 10 | |||||||||||||
| Fluoroborsyra/(g/L) | 50~100 | |||||||||||||
| Natriumfluoborat/(g/L) | 80~120 | |||||||||||||
| Sulfonsyra/(g/L) | 20~100 | |||||||||||||
| Fosforsyra/(g/L) | 80 | 10~100 | ||||||||||||
| Svavelsyra/(g/L) | 10~100 | pH2 | ||||||||||||
| Natriumacetat/(g/L) | 70 | |||||||||||||
| Natriumkarbonat/(g/L) | 100 | |||||||||||||
| Natriumhydroxid/(g/L) | 10 | 5.1 | ||||||||||||
| Natriumoxalat/(g/L) | 5.1 | |||||||||||||
| Natriumsulfat/(g/L) | 30.8 | |||||||||||||
| Kaliumhydroxid/(g/L) | 15 | |||||||||||||
| Ammoniumvätefosfat)(g/L) | 20 | |||||||||||||
| Natriumvätefosfat/(g/L) | 100 | |||||||||||||
| Kaliumsulfat/(g/L) | 40 | |||||||||||||
| Pläteringslösningens temperatur/°C | 45~90 | 80~90 | 90~95 | 70~90 | 65~100 | 75~100 | 75~100 | 80~90 | 30~70 | 75 | 65~80 | 75 | 70~90 | 70~90 |
| Strömtäthet/(A/dm²2) | 3.0 | 0.5~1.0 | 0.3~2.0 | 2~5 | 0.2~2 | 0.5~0.3 | 0.5~0.3 | 0.5 | 2.5 | 0.8 | 0.8 | 0.75 | 0.3~1 | 0.3~1 |
| Strömverkningsgrad/% | 15~20 | 70~10 | 10 | 14~18 | 15 | 15 | 15 | 35~40 | 10~15 | 100 | 80 | 100 | 10~50 | 15~50 |
(1) Kloridpläteringslösning
Den första tekniskt framgångsrika Pt-pläteringslösningen använde kloroplatinsyra (H2PtCl6・6H2O) som bassalt. En löslig Pt-elektrod användes och dess förhållanden var 10 ~ 15 g / l kloroplatinsyra, 180 ~ 300 g / l saltsyra, pläteringslösningstemperatur på 45 ~ 90 ℃, strömtäthet på 2,5 ~ 3,5A / dm2och katodströmseffektivitet på 15%~20%. Den pläteringsfilm som erhålls från denna lösning kan nå 20 μm utan sprickor och god duktilitet. pH-värdet måste dock kontrolleras inom ett smalt intervall för att förhindra hydrolys av pläteringslösningen. När pH-värdet i pläteringslösningen börjar hydrolyseras når det 2,2.
(2) Diamminonitrit Pläteringslösning
För att säkerställa koncentrationen av divalent Pt och förhindra att den oxideras till Pt(Ⅳ) måste en lämplig mängd aminföreningar tillsättas för att bilda ett komplex med Pt (II). Den grundläggande komponenten i denna pläteringslösning är diamminonitritoplatina Pt(NH3)2(NO2)2, ofta benämnt Pt-P-salt (II). Pläteringslösningen som använder detta salt upptäcktes av W. Keitel 1931 (pläteringslösning nr 3 i tabell 3-4). När nitritkoncentrationen i lösningen ökar, påverkar det dissociationen av Pt-komplexet och påverkar därmed pläteringslösningens beteende. Efter kokning har NH4OH tillsätts för att reagera med NaNO3 för att generera NH4NEJ2 för att återställa den ursprungliga strömeffektiviteten, som sedan sönderdelas till kväve- och vätgas. På detta sätt blir nästan alla icke-metalliska komponenter i Pt-P-saltet i pläteringslösningen gaser och försvinner, vilket gör pläteringslösningens livslängd längre än för kloridpläteringslösningar. Fördelen med denna pläteringslösning är att det är relativt enkelt att justera dess komponenter.
A. B. Triper och andra använde PR som strömkälla och uppnådde en elektropläteringshastighet på 5 μm/h. Förhållandena var: 5~6A/dm2, katodelektrolystid på 5 s och anodelektrolystid på 2 s. Pläteringslösning nr 4 i tabell 4-3 föreslogs i Lacroix patent från 1967 i Frankrike. Denna pläteringslösning kan producera en beläggningstjocklek på upp till 7,5 μm. Pläteringslösning nr 5 kommer från ett amerikanskt patent (US PAT. 2984603, 2984604), som föreslogs 1961 och som innebär att sulfonsyra tillsätts till Pt-P-saltpläteringslösningen. Nr 6 innehåller fosforsyra, medan nr 7 använder fosforsyra-svavelsyra som baslösning, vilket föreslogs i ett franskt patent från 1960 (Fr PAT. 1299226). De använde olösliga anoder och tillämpade flexibelt viktiga metoder som omrörning och skakning.
No. 8 använder natriumacetat och natriumkarbonat för att ersätta ammoniumsalter och därigenom uppnå maximal strömeffektivitet och förbättra pläteringslösningens stabilitet. Beläggningen som erhålls från denna lösning är slät och plan, med en pläteringstjocklek på upp till 10 μm utan pinholes eller sprickor.
I Japan används denna pläteringslösning i stor utsträckning industriellt. Nedan visas ett exempel:
|
Platina(diammineplatinumnitrit) Ammoniumnitrat Natriumnitrit Ammoniumhydroxid |
10g/L 100 g/L 10g/L 35 g/L |
Lösningens temperatur Aktuell densitet Nuvarande effektivitet - |
90~92℃ 1A/dm2 10%~20% - |
(3) Pläteringslösning av nitrosvavelsyra för platina
Denna pläteringslösning innehåller inte ammoniak eller aminkomponenter utan använder platina nitrosvavelsyra[H2Pt (OH)6 - 2H2O]som grundingrediens. Vid framställningen av pläteringslösningen används nitrosalter, kaliumsalter av platinaklorid eller platinasvavelsyra ([K2Pt(NO2)3Cl, K2Pt (NO2)2Cl2 eller K2Pt (NO2)2SO4]). En låg strömtäthet används för blankplätering och svavelsyra tillsätts för att justera pH-värdet till under 2,0. Representativa kompositioner visas i tabell 3-4, nr 9. Denna pläteringslösning kan producera relativt tjocka pläteringsskikt.
(4) Pläteringslösning av metallsalt med alkalisk hydroxiplatinsyra
I en typisk alkalisk pläteringslösning används ett natrium- eller kaliumsalt av hydroxiplatinsyra, såsom Na2Pt(OH)6 eller K2Pt(OH)6 används. Representativa sammansättningar av pläteringslösningar visas i tabell 3-4, nr 11. Pläteringslösningens temperatur på 75 ℃, strömtäthet på 0,8A / dm2, och strömeffektiviteten kan nå 100%, och anoden använder Ni eller rostfritt stålmaterial.
Nr 10 föreslogs av A.R. Powell 1913 och ett brittiskt patent erhölls (Brit PAT. 363569). En ljus beläggning som är jämförbar med Rh-pläteringslösningen kan
kan erhållas från denna pläteringslösning. När Pt-koncentrationen är under 3g/L sjunker strömeffektiviteten kraftigt. En strömtäthet kan vara upp till 2,5A/dm2 när koncentrationen är hög (12 g / L ). Vid en lösningstemperatur på 65 ~ 70 ° C kan den nuvarande effektiviteten nå cirka 80%. Ytterligare ökning av temperaturen förbättrar dock inte effekten avsevärt.
(5) Lösning för fosfatplätering
Redan 1855 föreslog Roseleuer ett fosfatschema. Denna pläteringslösning använder ett tetravalent Pt-kloridsamordningssalt, alkalimetallfosfatsalter och ammoniumsalter som ledande salter. År 1949 föreslog W. Pfanhauser pläteringslösningen No. 14, som under dessa förhållanden kan producera en beläggning på 0,5 μm.
Druve rapporterade experimentella resultat med samma pläteringslösning. Den största nackdelen med denna pläteringslösning är svårigheten att justera. Utfällningar som bildas när nyberedd pläteringslösning måste lösas upp under lång tid. Ammoniumfosfat måste användas för att undvika porösa och svampiga beläggningar. Ammoniumfosfat hjälper till att lösa upp platinakomplexet. Under vissa förhållanden bildas ett olösligt gult salt på anodytan i pläteringslösningen, vilket blir ett isolerande skikt som uppskattas vara ammoniumhydroxyplatinatsalt.
(6) Sulfatbaserad platinabeläggning
Plätering av platina på titan eller tantal är inte problematiskt även om det inte är ljust, men när man pläterar platina på dekorativa föremål blir ljusstyrkan en viktig fråga, och sprickor är också ett problem som inte kan ignoreras. Masashi och andra föreslog att man skulle använda en sulfatpläteringslösning för att ta itu med detta problem. Egenskaperna hos denna pläteringslösning är att lösa upp platinasalt i sulfat, tillsätta sulfit till lösningen och justera pH-värdet till mindre än 2 med svavelsyra. Eftersom tillsatsen av sulfit kan göra platinapotentialen mer negativ än vätejoner, säkerställer den en låg vätehalt i platinapläteringsskiktet, vilket resulterar i låg inre spänning och ljusstyrka i pläteringsskiktet. Men om sulfitkoncentrationen är för hög kan platina reduceras. Om pH>2 hydrolyseras sulfiten lätt. Även pH<2 hjälper till att stabilisera platinakomplexet.
Förbehandlingen för plätering är alkalisk→elektrolytisk avfettning→syradippning och 2min katodisk elektrolys.
Pläteringsprocessen visas i tabell 3-5.
Tabell 3-5 Processförhållanden för platinabeläggning i svavelsyraserie
| Sammansättning och processförhållanden | Nr 1 | Nr 2 |
|---|---|---|
|
HAuCl4 (räknad som Au) K2SO4 K2SO3 pH (justerat med svavelsyra) temperatur Aktuell densitet Plätering tid Pläteringens tjocklek Pläteringsskikt |
10g/L 50 g/L 1,0 g/L 1.0 75℃ 2A/dm2 60 minuter 7 μm Ljusstyrka |
10g/L 100 g/L 2. 0g/L 2.0 65℃ 1 A/dm2 100 minuter 5/μm Vackert utseende, bra bindning |
I tabell 3-5 nr 1 kan en dikroisk beläggning av Pt-Au erhållas genom förplätering av flashguld på substratet, tjock plätering av 7 μm platina och plätering av 2 μm guld på platinan.
2. Lösning för tunnplätering
3. Lösning för tjock plätering
(1) Dekorativ plätering
Som tidigare nämnts har platinapläterade produkter som glasögonbågar och klockfodral kommit fram på grund av betoningen på själva platinamärket. Pläteringstjockleken på platinapläterade produkter är i allmänhet under 5 μm.
Nyligen har en annan ny teknik dykt upp inom området dekorativa föremål, som är elektroformning.
Tjockleken på elektroformade produkter är i allmänhet 100~150 μm, och genom att göra dem ihåliga kan man minska vikten och sänka kostnaderna. Vid plätering med vanliga pläteringslösningar med konventionella galvaniseringsmetoder kommer sprickor att uppstå när pläteringstjockleken överstiger 10 μm, vilket gör det tekniskt utmanande.
(2) Industriella tillämpningar
Pt-plätering av delar i rostfritt stål för flygindustrin har börjat användas i praktiken. Processen är som följer:
Tabell 3-6 Prestanda för Pt-anodmaterial
| Fastigheter | Pt | Ti | Nb | Ta |
|---|---|---|---|---|
|
Densitet(20℃)/(g/cm3) Smältpunkt/°C Hårdhet (efter värmebehandling) Termisk ledningsförmåga/[W/(m-K)] Resistivitet/μΩ-cm Koefficient för linjär expansion (x105)/[mm/(mm-K)]
|
21. 45 1769 37〜42 (Vickers) 71. 6 10. 6 9. 1 |
4. 54 1668 120 (Brennel) 16.8 48 8. 5 |
8. 57 2468 84 (Vickers) 67. 4 13. 1 7. 1 |
16. 6 2996 E-60 (Rockwell) 54. 8 12.4 6. 5 |
I allmänhet är tjockleken på Pt-pläteringsskiktet cirka 2 μm, så strömtätheten är hög. Under förhållanden som kortslutningar när katoden kontaktas och operationer som involverar ammoniumbifluorid, fluoroborsyra, starka alkalier och lösningar med hög cyanid accelererar förbrukningen av Pt. Därför är det nödvändigt att förlänga dess livslängd så mycket som möjligt, vilket kan uppnås genom att öka förhållandet mellan anod- och katodarean. Vid plätering av Pt på Ti-elektroder kan Ti först ruggas upp genom sandblästring, sedan syraaktiveras för att avlägsna ytoxidfilmen, följt av Pt-elektroplätering.
Den typiska åldringsprocessen för Pt-pläterade Ti-anoder är: ① Ti-oxidfilm vid pinhålet för Pt-plätering förstörs; ② Ti börjar lösa upp; ③ Pt-Ti-gränssnittet upplever gropfrätning när upplösningen fortskrider och Pt-filmen skalar av. Vid denna tidpunkt, om det inträffar under guldplätering, kommer det att orsaka en plötslig ökning av avvikelsen från guldpläteringstjockleken. När man stöter på sådana problem i praktiken är det bäst att inspektera anoden.
4. Andra förbättringar av pläteringslösningen
(1) Förbättringar av förbehandlingen
Det finns också metoder för att förbättra vidhäftningen mellan natrium och dess legeringar och platinaskiktet genom att förbättra förbehandlingsprocessen. Kamata föreslog i ett patent att en syraplätering utförs i en pH=1 syrapläteringslösning, följt av plätering av den erforderliga tjockleken på platinaskiktet i en alkalisk pläteringslösning. Huvudkomponenterna i den sura pläteringslösningen är 0,3~3g/L kloroplatinsyra (beräknat som platina) och 5%~15% halogenidjoner (massfraktion). pH-värdet måste kontrolleras under 1; annars minskar titanets aktivitet, vilket leder till dålig vidhäftning. Anta att halidjonkoncentrationen är för låg. I så fall kan borttagningen av den passiva filmen på titanytan vara ofullständig, vilket i sin tur påverkar vidhäftningen av pläteringsskiktet. Förutsättningarna för strejkplätering är pläteringslösningens temperatur på 40 ~ 80 ℃ och strömtäthet på 5 ~ 25A / dm2. Pläteringsförhållanden och resultat för platinaplätering visas i tabell 3-7.
Tabell 3-7 Platineringsförhållanden och deras resultat (koncentrationsvärden inom parentes)
| Serienummer | Lösning för slagmetallisering | Lösning för platinaplätering | Pläteringstjocklek/μm | Strippningstest | ||
|---|---|---|---|---|---|---|
| Platinajon/(g/L) | Halogenjon (massfraktion)/% | Platinajon/(g/L) | pH-värde | |||
|
1 2 3 4 5 6 7 8 9 |
H2PtCl6 (0. 1) H2PtCl6 (0. 1) H2PtCl6 (0. 1) H2PtCl6 (1. 0) H2PtCl6 (1. 0) H2PtCl6 (1.0) H2PtCl6 (5.0) H2PtCl6 (5.0) H2PtCl6( 5. 0) |
HCl (5) HCl (5) HCl (5) HCl (10) HCl (10) HCl (10) HCl (20) HCl (20) HCl (20) |
K2Pt(OH)6 (5) K2Pt(OH)6 (10) K2Pt(OH)6 ⑸ Platina dinitrat (5) Platina dinitrat (10) Platina dinitramid (20) K2Pt(OH)6 ⑸ K2Pt(OH)6 (10) K2Pt(OH)6 (20) |
12. 0 13. 0 13. 5 12. 0 13. 0 13. 5 12.0 13. 0 13. 5 |
10 15 20 10 15 20 10 15 20 |
Bra Bra Bra Bra Bra Bra Bra Bra Bra |
(2) Plätering av platina med hjälp av en neutral pläteringslösning
Att använda en nästan neutral pläteringslösning är fördelaktigt för mönsterplätering, eftersom det undviker att använda alkalimetaller som Na, vilket förhindrar de negativa effekterna som orsakas av ackumulering av alkalimetaller. Den platinapläteringslösning som Otani föreslår uppfyller detta villkor. Tabell 3-8 visar sammansättningen av pläteringslösningen och dess processvillkorstester.
Tabell 3-8 Sammansättning och processförhållanden för test av neutral platinabeläggningslösning
| Ingredienser och deras processförhållanden | Nr 1 | Nr 2 | Nr 3 |
|---|---|---|---|
|
Dinitrodiammine platinum(Pt-koncentration)/(g/L) Glycin/(mol/L) Iminodiättiksyra/(mol/L) Diaminotriättiksyra/(mol/L) рH Temperatur/°C Strömtäthet/(A/dm²2) Nederbördshastighet/(μm/min) Strömverkningsgrad/%
|
12 0. 57 - - 5.0 70 1. 0 0. 3 80 |
12 - 0. 3 - 5. 0 70 1. 0 0. 2 65 |
12 - 0. 1 0. 1 5. 0 70 1. 0 0. 1 65 |
Eftersom denna pläteringslösning är nära neutral är den gynnsam för mönsterplätering och kommer inte att påverka motpläteringsfilmen negativt.
Kamata från Japan studerade också effekten av alkaliska jordartsmetaller som vitmedel. Det visade sig att alkaliska jordartsmetaller, såsom Ca, Ba, Mg etc., har en uppljusande effekt på alkaliska pläteringslösningar. Den lämpliga koncentrationen av jordalkalijoner är (2×100)×10-6. Ljusstyrkan styrs också genom att variera koncentrationen av tillsatta jordalkalimetalljoner.
De viktigaste komponenterna och driftsförhållandena för pläteringslösningen är följande:
| Huvudkomponenter i pläteringslösningen |
KOH 40g/L Pt [tillsatt i form av K2Pt(OH)6] 20g/L Ca [tillsätts i form av CaCl2 vattenlösning] Tillräcklig mängd |
| Driftförhållanden |
рH 13,5 Temperatur 80 ℃ Strömtäthet 3A/dm2 Basmetall Kalandrerad kopparplåt Pläteringstjocklek 20 μm |
Tabell 3-9 Effekt av Ca-jonkoncentration på Pt-pläteringsskiktets ljushet
| Ca-jonkoncentration/x10-6 | Utseende | Ca-jonkoncentration/x10-6 | Utseende |
|---|---|---|---|
|
0 0. 1 0. 3 0. 5 0. 7 1. 0 |
Icke-glänsande Icke-glänsande Icke-glänsande Icke-glänsande Icke-glänsande Halvblank |
1. 5 2. 0 2. 5 3. 0 5. 0 - |
Halvblank Halvblank Halvblank Halvblank Spegel ljus - |
Copywrite @ Sobling.smycken - Anpassad smyckestillverkare, OEM och ODM smyckesfabrik
Avsnitt III Plätering av platinalegeringar
(1) Platina-Iridium-legering
Elektropläterad Pt-Ir-legering kan användas på elektroder för sodaproduktion och elektroplätering.
De pläteringsprocessförhållanden för legeringen som föreslagits av Kamada et al. visas i tabell 3-10.
Tabell 3-10 Processförhållanden för elektroplätering av Pt-Ir-legering
| Sammansättning och processförhållanden | Nr 1 | Nr 2 |
|---|---|---|
|
Natriumiridiumhexaklorid Borsyra Dinatriummalonat Natriumtetrakloroplatinat Kaliumoxalat Natriumtetrabromoplatinat рH Temperatur Aktuell densitet |
10g/L 40 g/L 0. 02mol/L 0. 5~3g/L - - 5 85℃ 0. 5 A/dm2 |
10g/L 40 g/L - - 0. 02mol/L 0. 5〜3g/L 2 85℃ 0. 5 A/dm2 |
Elektropläteringsstegen är att först flashplätera 1 μm guld på mässingsarket, sedan plätera bort guld och slutligen plätera en Pt-Ir-legering ovanpå. Beläggningen som erhålls med denna metod har god hårdhet, vidhäftning, värmebeständighet och metalltrådsbindningsförmåga, med en strömeffektivitet som når 100%.
När det gäller denna pläteringslösning, om pH är för lågt, är strömtätheten för liten för att vara praktisk; om pH är för högt, bildas hydroxidutfällningar lätt. Om temperaturen är för låg är legeringen svår att deponera; om temperaturen är för hög avdunstar pläteringslösningen snabbt, vilket är ogynnsamt för att bibehålla pläteringslösningen. Om strömtätheten är för låg är deponeringshastigheten för långsam; om strömtätheten är för hög är den katodiska reaktionen huvudsakligen väteutveckling.
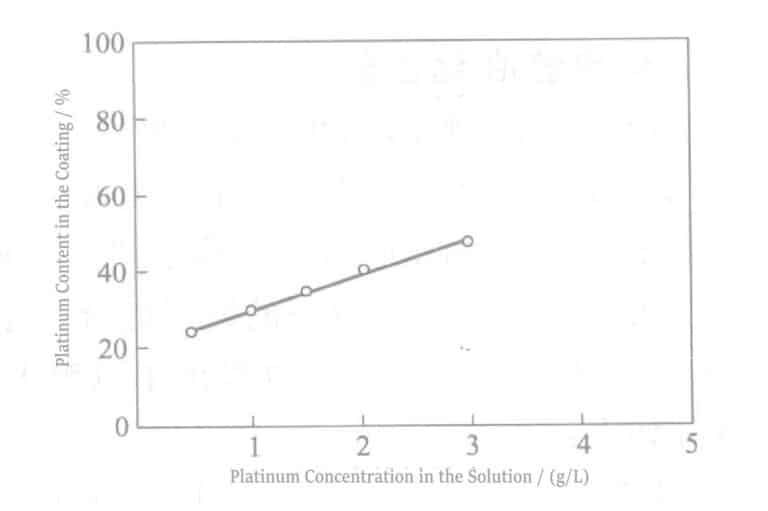
Samtidigt kan legeringssammansättningen i pläteringsfilmen också kontrolleras genom att justera metallkoncentrationsförhållandet i pläteringslösningen. Figur 3-1 visar variationen av legeringsbeläggningens sammansättning med metallkoncentrationsförhållandet i pläteringslösningen.
Som framgår av figuren har Pt-Ir-kompositionskvoten i pläteringsskiktet inom det experimentella koncentrationsområdet ett linjärt förhållande till metalljonkoncentrationskvoten i pläteringslösningen.
(2) Elektroplätering av platina-järnlegering
Legeringar som innehåller Fe används i allmänhet som magnetiska material. Ju högre inspelningstäthet, desto bättre. Platina-järnlegeringar har hög magnetisk anisotropi, god korrosionsbeständighet och slitstyrka och förväntas förbättra prestandan hos magnetiska filmer.
Katsutsugu Koda föreslog en pläteringslösningsformel med god stabilitet som möjliggör kontinuerlig elektroplätering. Eftersom trevärda järnjoner i pläteringslösningen tenderar att bilda geler är detta skadligt för pläteringsskiktets utseende och minskar koncentrationen av tvåvärt järn, vilket påverkar pläteringslösningens stabilitet negativt. Trivalent järn genereras baserat på följande reaktion:
Pt4+ + 2e–→ Pt2+
2Fe2+ → 2Fe3+ + 2e–
Från ovanstående formel, från perspektivet att överväga stabiliteten hos järnjoner, spelar tetravalenta platinajoner en negativ roll, vilket ledde till uppfinningen av divalent platina för att ersätta tetravalent platina. Övningen har visat att divalent platina kan användas för galvanisering.
Tabell 3-11 visar processförhållandena och resultaten av binär Pt-Fe-legeringselektroplätering. Från tabellen kan man se att metallatomförhållandet för Pt-Fe-legeringsbeläggningen erhålls i nr 1 ~ nr 3. Det är nära. När legeringens atomförhållande är 50% är det optimalt som en magnetisk film för inspelning.
Tabell 3-11 Processförhållanden för plätering av Pt-Fe Binary Alloy och deras resultat
| Sammansättning och processförhållanden | Nr 1 | Nr 2 | Nr 3 | Nr 4 | Nr 5 | |
|---|---|---|---|---|---|---|
| Platina salt | Typ | Pt(NH3)2(NO2)2 | [Pt(NH3)4]Cl2 | Pt(NH3)2(NO2)2 | Pt(NH3)2(NO2)2 | Na[Pt(C2O4)2 |
| Innehåll | 5 g/L | 5 g/L | 5 g/L | 5 g/L | 10g/L | |
| Järnsalt | Typ | FeSO4 • 7H2O | FeSO4 • 7H2O | FeSO4 • 7H2O | FeSO4 • 7H2O | FeSO4 • 7H2O |
| Innehåll | 2g/L | 30 g/L | 30 g/L | 10g/L | 20 g/L | |
| Antioxidanter | Typ | Natriumsulfit | Klorid av hydroxiammoniak | L-askorbinsyra | Citronsyra hydrerar | Hydroxiammoniaksulfat |
| Innehåll | 5 g/L | 3g/L | 3g/L | 40 g/L | 50 g/L | |
| Komplexbildande medel | Typ | Triammoniumcitrat | EDTA-2Na | Triammoniumcitrat | EDTA-2Na | Natriumoxalat |
| Innehåll | 50 g/L | 10g/L | 15 g/L | 2g/L | 30 g/L | |
| Tillsatser | Typ | - | Kaliumdivätefosfat | Kaliumdivätefosfat | Kaliumaskorbylfosfat | - |
| Innehåll | - | 15 g/L | 15 g/L | 5 g/L | - | |
| Temperatur på pläteringslösningen | 40℃ | 30℃ | 60℃ | 50℃ | 70℃ | |
| рH | 8 | 2 | 3 | 4 | 8 | |
| Aktuell densitet | 1A/dm2 | 2A/dm2 | 1A/dm2 | 1A/dm2 | 1,5A/dm2 | |
| Pläteringskomposition (atomisering) | Pt | 51% | 49% | 55% | 72% | 37% |
| Fe | 49% | 51% | 45% | 28% | 63% | |
| Utseende på det pläterade skiktet | O | O | O | O | O | |
(3) Elektroplätering av platina-koboltlegering
Pt-Co-legeringsfilmen har en mycket hög magnetisk inspelningstäthet, vilket är mycket attraktivt för den stora kapaciteten hos magnetiska inspelningsmedier. Speciellt när dess atomförhållande är 1:1 är prestandan optimal.
Koda forskade också på Pt-Co-legeringar (se tabell 3-12).
Tabell 3-12 Processförhållanden och resultat vid plätering av Pt-Co binära legeringar
| Sammansättning och processförhållanden | Nr 1 | Nr 2 | Nr 3 | Nr 4 | Nr 5 | |
|---|---|---|---|---|---|---|
| Platina salt | Typ | Pt(NH3)2(NO2)2 | [Pt(NH3)4]Cl2 | Pt(NH3)2(NO2)2 | Pt(NH3)2(NO2)2 | Na[Pt(C2O4)2 |
| Innehåll | 2g/L | 5 g/L | 5 g/L | 2g/L | 10g/L | |
| Järnsalt | Typ | CoSO44 • 7H2O | CoSO44 • 7H2O | CoSO44 • 7H2O | CoSO44 • 7H2O | CoSO44 • 7H2O |
| Innehåll | 30 g/L | 30 g/L | 2g/L | 45 g/L | 20 g/L | |
| Buffert(1) | Typ | EDTA-2Na | Triammoniumcitrat | Triammoniumcitrat | Borsyra | Ammoniumoxalat |
| Innehåll | 30 g/L | 5 g/L | 50 g/L | 30 g/L | 30 g/L | |
| Buffert(2) | Typ | Triammoniumcitrat | - | - | EDTA-2Na | - |
| Innehåll | 5 g/L | - | - | 2g/L | - | |
| Konduktivt salt | Typ | Sulfaminsyra | Ammoniumsulfat | Ammoniumsulfat | Sulfaminsyra | Ammoniumsulfat |
| Innehåll | 15 g/L | 15 g/L | 15 g/L | 20 ml/L | 15 g/L | |
| Antiprecipitant | Typ | - | Ammoniak | - | - | - |
| Innehåll | - | 3g/L | - | - | - | |
| Temperatur på pläteringslösningen | 60℃ | 50℃ | 40℃ | 50℃ | 70℃ | |
| рH | 3 | 2 | 4 | 3 | 4 | |
| Aktuell densitet | 1A/dm2 | 2A/dm2 | 4A/dm2 | 3A/dm2 | 4A/dm2 | |
| Pläteringskomposition (atomisering) | Pt | 65% | 49% | 30% | 40% | 37% |
| Fe | 35% | 51% | 70% | 60% | 63% | |
| Utseende på det pläterade skiktet | O | O | O | O | O | |
Legeringens atomförhållande för beläggningen som erhålls från nr 2 i tabell 3-11 är cirka 50%.
Hu Zhongmin et al. föreslog också en formel för plätering av Pt-Co-legering. Dess huvudkomponenter är följande:
|
Pt(NH3)2(NO2)2 (som Co) 0,2~15g/L CoSO44 (som kobolt) 5~70g/L (bibehålla Co:Pt=30:1) |
|
pH 1,2 (justerat med NH2SO3H) Temperatur 70 ℃ Strömtäthet 2A/dm2
|
(4) Platina-rodium-legering
Eftersom Pt-W-legeringsbeläggning har högre oxidationskatalytisk förmåga än Pt-beläggning har människors intresse för Pt-W-legeringsplätering väckts. Matsunori Sawada et al. föreslog en platina-volframlegeringsformel som kan uppnå ett enhetligt utseende, god katalytisk förmåga och god pläteringslösningsstabilitet.
En stabil pläteringslösning erhålls genom att tillsätta organiska syror eller organiska syrasalter till huvudkomponenterna och sedan åldra blandningen.
De organiska syror som används kan vara ättiksyra, citronsyra, oxalsyra, vinsyra etc. Representativa komponenter och koncentrationer är följande:
H2PtCl4 2g/L(som Pt)
Na2WO4 - 2H2O 25g / L (som W)
Natriumcitrat 5g/L
Citronsyra 5g/L
Natriumsulfat 15g/L
Åldringsförhållanden 60 ℃ × 8h
Pläteringsförhållanden 65°C ,6mA/cm2 , 10min
Pläteringsmaterial Trådnät av rostfritt stål med en diameter på 0,3 mm
Behandlingar före plätering är:
Elektrolytisk avfettning→Vattensköljning→Hydroklorsyrablötläggning→Vattensköljning→Flashguldplätering→Svavelsyrablötläggning→Vattensköljning→Elektroplätering Pt-W Legering
Anta att ingen åldringsbehandling tillämpas och att pläteringen sker omedelbart med den förberedda pläteringslösningen. I det fallet kommer samdepositionen av volfram att vara instabil, särskilt eftersom den initiala volframdepositionen kommer att vara låg. Pläteringslösningen kommer gradvis att stabiliseras vid fortsatt användning och samdepositionen av volfram kommer att öka. Ett stabilt volframinnehållande pläteringsskikt kan erhållas om ovanstående åldringsbehandling används.
(5) Elektroplätering av platina-nickellegering
Hu Zhongmin föreslog huvudkomponenterna i formeln för elektroplätering av Pt-Co-legering enligt följande:
(5) Elektroplätering av platina-nickellegering
Hu Zhongmin föreslog huvudkomponenterna i formeln för elektroplätering av Pt-Co-legering enligt följande:
|
Pt(NH3)2(NO2)2 (som Pt) 0,2~15g/L Nickelsulfamat (som Ni) 5~70g/L (bibehålla Ni:Pt=30:1) Sulfaminsyra Tillräcklig mängd |
|
pH 1~1,4 (justerat med sulfaminsyra) Temperatur 70°C Strömtäthet 2A/dm2 |
Avsnitt IV Kemisk platinagalvanisering
Förutom att användas i smycken, katalys och värmebeständiga material kan platina också användas som en tunnfilmselektrod för halvledarkomponenter. Att erhålla tunna platinafilmer genom kemisk plätering är ett nytt tillvägagångssätt. Reduktionsmedlen är i allmänhet hydrazin eller hydrazinhydrat; hypofosfit används ibland.
Raitian raffinerar platinasalter genom att leda koldioxid in i en lösning av hexaammineplatinumkomplexet [Pt(NH3)6(OH)4], vilket gör att platinasaltet fälls ut och uppnår stabil och höghastighets platinagalvanisering.
Den specifika raffineringsmetoden är att leda koldioxid in i en lösning av hexaammineplatinumkomplexet [Pt(NH3)6(OH)4] i cirka 3 timmar för att erhålla en platinasaltfällning. Därefter filtreras, tvättas och torkas fällningen och karbonatet löses upp med en organisk syra för att erhålla raffinerat platinasalt för elektroplätering. Syftet med att använda organiska salter är att undvika kontaminering av oorganiska joner. Halidjoner tenderar att adsorberas på de pläterade delarna, vilket minskar deponeringshastigheten och gör att platinafilmen mörknar. Förekomsten av sulfat- och nitratjoner kan också orsaka utseendemässiga problem med pläteringen. De organiska syror som används är sulfonsyror, t.ex. metansulfonsyra eller etansulfonsyra, eller organiska karboxylsyror med låg molekylvikt, t.ex. ättiksyra eller propionsyra.
För att underlätta förångningen och avlägsnandet av koldioxid kan lösningen hållas under reducerat tryck när platinakarbonatfällningen löses upp med organisk syra.
Pläteringslösning och processförhållanden:
Pt(NH3)6(CH3COO)4 (som Pt upplöst i ättiksyra) 3g/L
Hydrazinhydrat 3mL/L
Glycerolester (utjämningsmedel) 20×10-6
pH (justerat med ammoniak) 11
Temperatur 60 ℃
Pläterade delar Aluminiumoxidplatta (aktiverad)
Deponeringshastighet 1,8 μm/h
Utjämningsmedlet kan vara polyoxietylendodecyleter och reduktionsmedlet kan ersättas med hypofosfit.
Koslov Alexanders formel, som också använder hydrazinhydrat som reduktionsmedel, är:
Pt(NH3)2(NO2)2 (som Pt) 2g/L
Hydrazinhydrat (reduktionsmedel) 3g/L
NH2OH - HC1 (som stabilisator) Tillräcklig mängd
pH (justerat med ättiksyra) 3
Temperatur 50 ℃
Deponeringshastighet 0. 1μm/h
Tabell 3-13 Kemisk plätering Pt-test
| Föremål | Test 1 | Test 2 | Test 3 |
|---|---|---|---|
| Testkarakteristik | Jonbytarmembranet som dränkts i 5% (NH4)4PtCl2 lösning placerades i en lösning av 1g/L natriumhydroborid+1mg/L magnesiumkarbonat vid 50℃ under 1h. | Jonbytarmembran indränkt i 5% (NH4)4PtCl2 lösning placerades i en lösning av 1g/L natriumhydroborid + 10mg/L magnesiumsulfat vid 30℃ under 1h. |
HPtCl4 1g/L Natriumhydroborid 1g/L Kalciumkarbonat 10ml/L 80℃,1h Reaktion av plåten Al i ovanstående lösning genom nedsänkning |
| Basmaterial | Katjonbytarmembran | Katjonbytarmembran | Aluminiumplåt |
| Platinas tjocklek | 0. 1 mm | 0. 1 mm | 0. 1 mm |
| Platinapartikelns diameter | Under 10 μm | Under 10 μm | Under 10 μm |
| Ytmotstånd | 10Ω/cm | 10Ω/cm | 10Ω/cm |
I denna reaktion krävs alkaliska jordartsmetaller; de kan lösas upp med reduktionsmedlet (som i Experiment 1, Experiment 2) eller tillsättas till pläteringslösningen (som i Experiment 3). Verkningsmekanismen för alkaliska jordartsmetaller är dock oklar. Ju bättre pläteringsskiktets kompakthet är, desto färre defekter, t.ex. sprickor i pläteringsskiktet, vilket kan säkerställa ett relativt lågt motstånd och därmed garantera elektrodens kvalitet.
Kenji Takahashi föreslog ett kemiskt pläteringssystem som använde tetravalent platinaammoniumsalt som huvudsalt. Den allmänna formen av platinasaltet är [Pt(NH3)6X]. I formeln kan X vara en halogenidjon, OH– grupp, SO42-, etc.
Dess sammansättning är:
Platinasalt (tetravalent platinaammoniumsalt) (i platina) 0. 5〜5,0g/L
Ammoniak (28%) 10〜100 g/L
Vatten och hydrazin (reduktionsmedel) 0. 5〜5g/L
рH 10〜12. 5
Pläteringslösningens temperatur 50〜70°C