De ce placarea cu paladiu este alternativa accesibilă la aur pentru bijuterii
Palladium Plating Guide for Jewelry: Cheaper Than Gold, Bright & Durable
Introducere:
This chapter provides a comprehensive guide to palladium (Pd) plating. It begins with an overview of palladium’s properties as a silver-white, ductile, and cost-effective precious metal. The content details electroplating processes for pure palladium, including solution compositions, and the effects of additives like crystal modifiers and organic acids. It further explores electroplating various Pd alloys such as Pd-Ni, Pd-Ag, and Pd-Co-In. The chapter also covers electroless (chemical) plating methods for Pd and its alloys, highlighting their application in the printed circuit board industry. Finally, it discusses the operational management of palladium plating solutions.

Tabla de conținut
Secțiunea I Prezentare generală
Palladium (Pd) has an atomic numbers 46 in the periodic table. Palladium metal is silver-white, with a melting point of 1554.9℃ and a boiling point of 3100℃. It is ductile and is one of the more affordable platinum group metals. Its crystal structure at room temperature and pressure is face-centered cubic. It readily absorbs hydrogen, absorbing about 935 times its volume of hydrogen, making it useful for manufacturing hydrogen-absorbing alloys. It also has catalytic properties. It is ductile, a soft white metal, and does not lose its metallic luster in air.
However, the corrosion resistance of pure palladium is poorer than that of other metals in the platinum group of elements, and it is susceptible to nitric acid leaching, which darkens the color, and discoloration in the air. To improve these shortcomings, electroplated white bright palladium-nickel alloys can be used. The density of palladium is 12g/cm3, about 2/3 of gold. palladium-nickel alloy plating contains 20% nickel, so if the palladium-nickel alloy plating instead of gold plating, then the cost of materials can be lower than the cost of gold plating.
Palladium plating is widely used in the electronics industry. It can save a significant amount of gold when used as an underlayer for hard gold plating. In recent years, the development of palladium-nickel alloy plating has partially replaced traditional palladium plating. It is not only saves the metal palladium but also reduces electroplating costs.
Since the density of palladium is lower than that of gold, the amount of metal saved compared to gold is about 40% the same thickness as gold and palladium.
At the same time, due to the allergic effects of Ni on the human body, Pd has also been used as a substitute plating for Ni. Table 4-1 shows some main parameters of Pd, and Table 4-2 shows some main demand quantities of Pd.
Table 4-1 Some Main Parameters of Palladium
| Parametrii caracteristici | Valoarea caracteristică | Parametrii caracteristici | Valoarea caracteristică |
|---|---|---|---|
|
Element name, element symbol, atomic number Clasificare Grup, Perioadă Densitate, duritate Culoare Masa atomică relativă Raza atomică Covalent bond radius
|
Palladium、Pd、46 Transition Metals 10(Ⅷ)、5 12023kg/m3, 4. 75 Silver White 106. 42 140pm 131pm |
Valoarea de oxidare Structura cristalină Punct de topire Punct de fierbere Căldura de vaporizare Heat of Melting Capacitatea termică specifică Electrical Conductivity Conductivitate termică |
-1、+ 1 face-centered cubic 1828. 05K (1554. 90℃) 3373K (3100℃) 357kJ/mol 16. 7kJ/mol 25. 9J/(kg • K) 10. 85X10-6m -Ω 75. 5W/(m • K) |
Table 4-2 Palladium Demand Unit: 1000 oz
| Articolul | 2000 | 2001 | 2002 | 2003 |
|---|---|---|---|---|
|
Automotive Catalyst:Total Recycling Chemical Dental Electronic Decorative Other Total |
5640 230 255 820 2160 255 60 8960 |
5090 280 250 725 670 230 65 6750 |
3050 370 255 785 760 260 90 4830
|
3460 410 250 725 895 340 90 5260 |
Section II Electroplating Palladium
1. Palladium Electroplating Solution
In 1885, the American company Pilot obtained the patent for the “White Pd Film Manufacturing Method”. This plating solution uses palladium chloride, ammonium phosphate, sodium phosphate, or ammonia water, and benzoic acid is added as needed. Since ammonia is volatile, the plating solution is alkaline. The purpose of adding benzoic acid is to reduce the plated parts and improve the adhesion to iron and steel surfaces.
There is a detailed description of bright palladium plating in Deuber’s U.S. patent (1978). The brightness can be adjusted by regulating the pH value by using the first and second types of organic brighteners within the pH range within 4.5~12. The Pd plating layer can improve the electrical conductivity of switch contacts and can achieve a bright white plating comparable to rhodium.
Besides Pd salts, there are conductive salts and brighteners in the plating solution, making the plating solution quite complex.
Table 4-3 shows the main components of a general Pd plating solution.
Table 4-3 Main Components of General Pd Plating Solution
| Pd compounds |
Palladium(II) ammonium chloride Pd(NH3)2Cl2 Diammonium palladium nitrite Pd(NH3)2(NO2)2 Tetraammonium palladium nitrite Pd(NH3)4(NO2)2 Diammonium palladium sulfate Pd(NH3)2SO4 Palladium tetraammonium chloride (NH3)2PdCl4 Diammonium palladium oxalate Pd(NH3)2C2O4 Tetraammonium oxalate palladium Pd(NH3)4C2O4 |
|
| Conductive salts | Ammonium chloride, ammonium citrate, ammonium nitrate, sodium nitrate, ammonium sulfonate, potassium citrate, ammonium sulfate, ammonium oxalate, potassium pyrophosphate | |
| Brighteners | Class 1 | Saccharin, sodium benzenesulfonate, ammonium benzenesulfonate, phenolsulfonic acid, naphthalenesulfonic acid |
| Class 2 | 1,4-butynediol, sodium benzyl alcohol-o-sulfonate, allyl sulfonate | |
Table 4-4 shows the process conditions for some typical Pd electroplating. When using the No. 4 plating solution in the table, the current efficiency gradually decreases as the plating proceeds. At this time, it is necessary to add sodium nitrite to the plating solution to promote the formation of Pd compounds, ensuring the continuous progress of the electroplating.
The No. 5 plating solution uses a composition containing ammonium chloride palladium [Pd(NH3)2Cl2]. The anode reaction produces chlorine gas, chlorite, and other oxidation products and the decomposition of organic substances. Meanwhile, passivation of the substrate Ni also occurs during the plating process. Adding ammonium oxalate palladium can prevent substrate passivation.
Table 4-4 Process Conditions of Some Typical Pd Plating Baths
| Compoziția și condițiile de procesare | Nr. 1 | Nr. 2 | Nr. 3 | Nr. 4 | No. 5 |
|---|---|---|---|---|---|
| Palladium salt | Pd(NH3)2Cl2 | Pd(NH3)2(NO2)2 | Pd(NH3)4Cl4 | Pd(NH3)2(NO2)2 | Pd(NH3)2C2O4 |
| Conductive salts |
Ammonium sulfate 30g/L Potassium chloride 15g/L Ammonium hydroxide 8mL/L
|
Ammonium dichlorophosphate 95g/L Ammonium hydroxide 24g/L
|
Ammonium sulfate 25g/L |
Ammonium sulfate 90g/L Sodium nitrite 10g/L
|
Diammonium hydrogen phosphate 100g/L |
| Brightening agents | Sodium benzyl alcohol-o-sulfate 2g/L | Naphthalenesulfonic acid 35g/L |
Saccharin 1g/L Sodium allyl sulfate 3g/L
|
||
| Alloying metals | Nickel sulfate 0.2g/L | - | - | - | - |
|
рH Temperatura Current density Pd content
|
5. 5 〜7.0 50℃ 0.4〜1. 6A/dm2 2g/L
|
9.2 - 1. 1A/dm2 2g/L
|
7.5 50℃ 1.0A/dm2 1. 5g/L
|
8〜9 70℃ 1. 0A/dm2 50g/L [in the form of Pd(NH3)2(NO2)2 ]
|
7.5 50℃ 3A/dm2 10g/L
|
| Japanese Patent Showa 59-33674(1984) | Japanese Patent Showa 59-45758(1984) | Japanese Patent Showa 62-24517(1987) | Japanese Patent Showa 62-29516(1987) | Japanese Patent Showa 62-20279(1987) |
Palladium plating solutions are generally neutral or alkaline. In alkaline plating solutions, ammonia water is usually used to adjust the pH and ensure the stability of ammonium palladium salts. For processes with fast speed and large air contact area, the consumption rate of ammonia water is also fast, making the pH unstable. This may cause Pd to precipitate at the anode or absorb hydrogen, leading to increased tensile stress and, when plating thick Pd layers, may cause cracking.
F. Simon et al. proposed an acidic palladium plating solution. The pH of this solution is below 1, the Pd content is 20g/L, the sulfuric acid concentration is 100g/L, and 0.2~2g/L Pd in the plating solution exists in the form of sulfite complexes. When the current density is 1.0A/dm2, the cathode current efficiency is 97% and the electrodeposition rate is 0.26g/min. At high solution temperatures, the complexes are unstable, making them unsuitable for electroplating above 35℃. However, it is still considered better than alkaline plating solutions.
As mentioned above, the performance of the palladium plating layer is greatly affected by the amount of co-deposited hydrogen.
The amount of hydrogen contained in the palladium plating, expressed as the atomic ratio of H/Pd, is such that when this value is greater than 0.03, H atoms diffuse into the Pd lattice and the likelihood of cracking increases. This is because, when H/Pd < 0.03, the Pd-H compound is in the α-state and its lattice constant is close to that of pure Pd. However, when H/Pd>0.57, it is in the β state and its lattice constant is about 3.0% larger than that of pure Pd. Moreover, the β state is thermodynamically unstable and will transform into the α state and release hydrogen, which in turn causes the lattice to become smaller and cracks in the coating. When H/Pd is between 0.03~0.57, the crystallization is coexisted by α state and β state, and the existence of β state will cause the above mentioned problems, in order to avoid cracks in the plating layer, it is necessary to ensure that the H/Pd ratio is below 0.03.
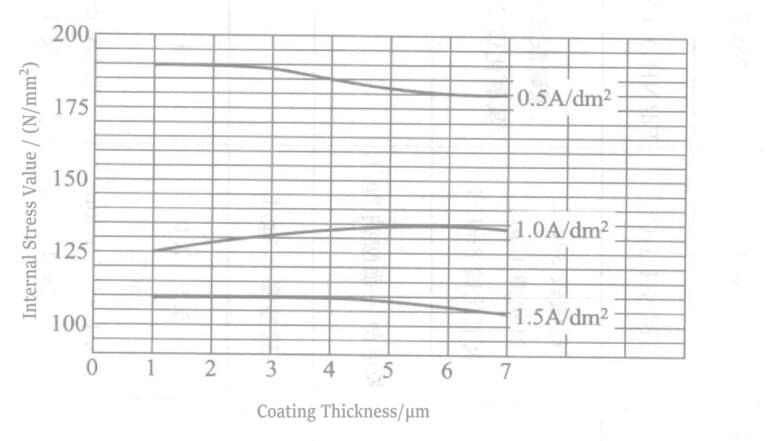
For the No.2 plating solution in Table 4-4, the H/Pd atomic ratio of the plated layer is about 0.2 when Pd=15g/L, conductive salt=100g/L, pH=8.0, temperature is 35°C, and the current density is 1~2A/dm2 At this time, the internal stress is about 2.25N/mm2 The H/Pd ratio of the plated layer obtained from the plating solution of F. Simon et al. can be as low as 0.0004, and the internal stress varies slightly with the current density, and the internal stress is about 135 N/mm2 for a plating thickness of 5 to 7 um at 1A/dm2 (see Fig. 4-1). This is the previous plating solution can not be achieved.
2. The Effect of Adding Crystal Modifiers to the Pd Plating Solution
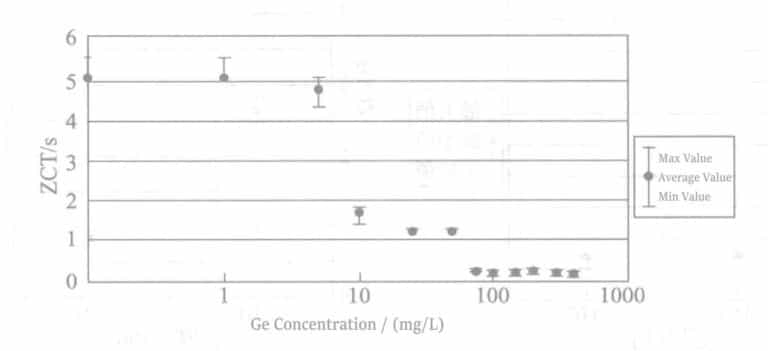
As can be seen from Figure 4-2, adding germanium to the Pd plating solution can improve the brazing wettability of the coating. The figure shows that when the germanium concentration in the plating solution is above 100×10-3g/L (0.1g/L), good wettability can be obtained.
The composition of the palladium plating solution is as follows:
Dichlorodiamine palladium (as palladium) 4g/L
Ammonia 20mL/L
Ammonium chloride 100g/L
Germanium oxide (as germanium) 10mg/L, 100mg/L, 500mg/L
рH 8.5
Plating solution temperature 55℃
Current density 0. 05A/dm2
In the experiment on palladium plating thickness, wetting tests were conducted by selecting palladium-plated workpieces with thicknesses of 0.01μm and 0.02μm. The results under different heat treatment conditions were compared.
As shown in Figure 4-3, under the heat treatment condition of 380℃, 1min, even with a plating thickness of 0.01μm, the ZCT was below 1 second, indicating good brazing wettability can be maintained even with thin palladium plating. When the heat treatment condition was 400℃, 30s (Figure 4-4), the ZCT of the 0.02 μm palladium plating layer was below 1 second, but the ZCT of the 0.01μm palladium plating layer was 2.66 seconds. Under the heat treatment condition of 430℃, the ZCT of the 0.01μm palladium plating layer was above 5 seconds, and the ZCT of the 0.02μm palladium plating layer was 1.84 seconds (Figure 4-5). Therefore, when the heat treatment degree is low, adding germanium to the plating solution may reduce the minimum thickness of the palladium plating layer.

Figure 4-3 Brazing wettability results for Pd plating thicknesses of 0.01μm and 0.02 μm
(Heat treatment condition: 380℃, 1min)
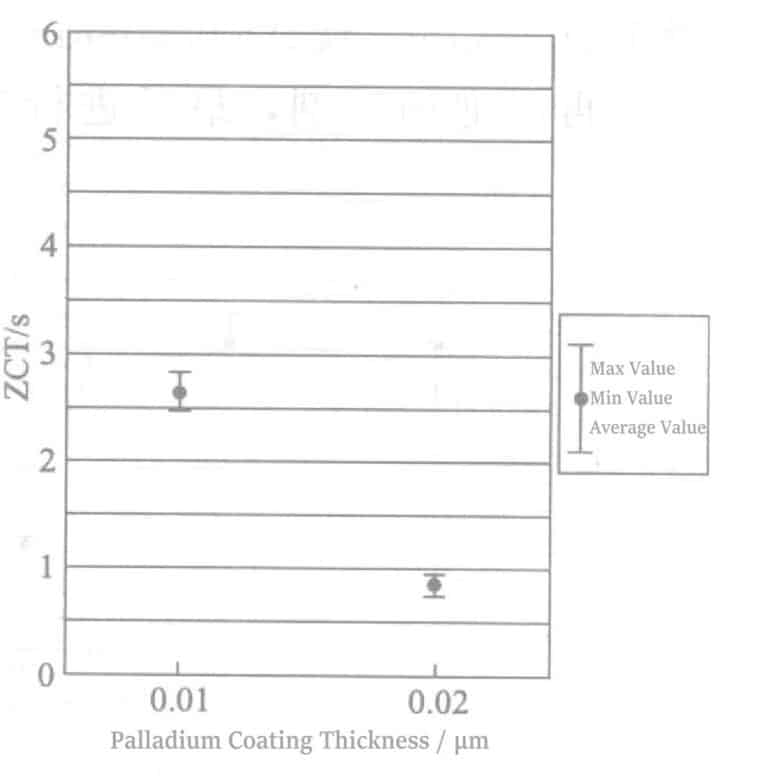
Figure 4-4 Brazing wetting results of Pd plating thickness of 0.01μm and 0.02μm
(Heat treatment conditions: 400℃, 30s)
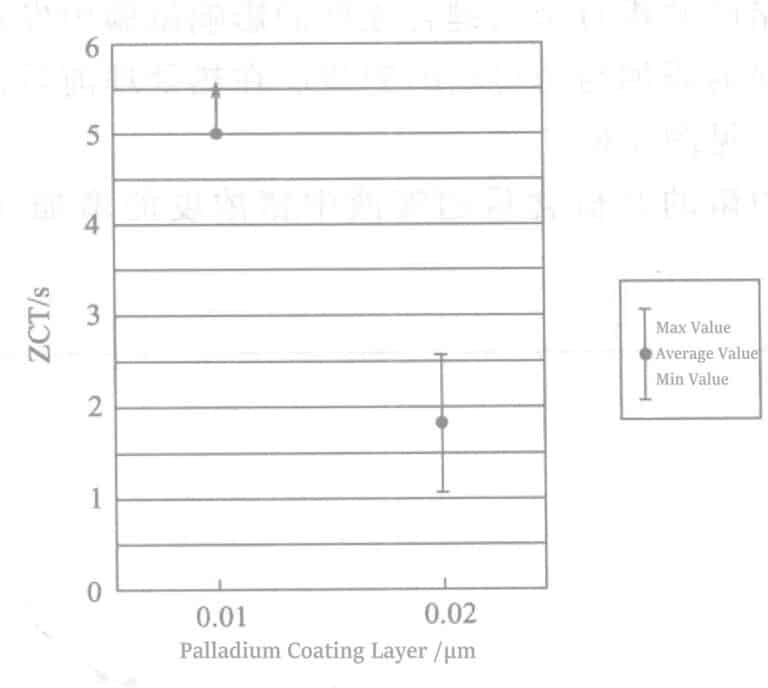
Figure 4-5 Wetting results of brazing with Pd plating thickness of 0.01μm and 0.02μm
(Heat treatment condition: 430℃, 30s)
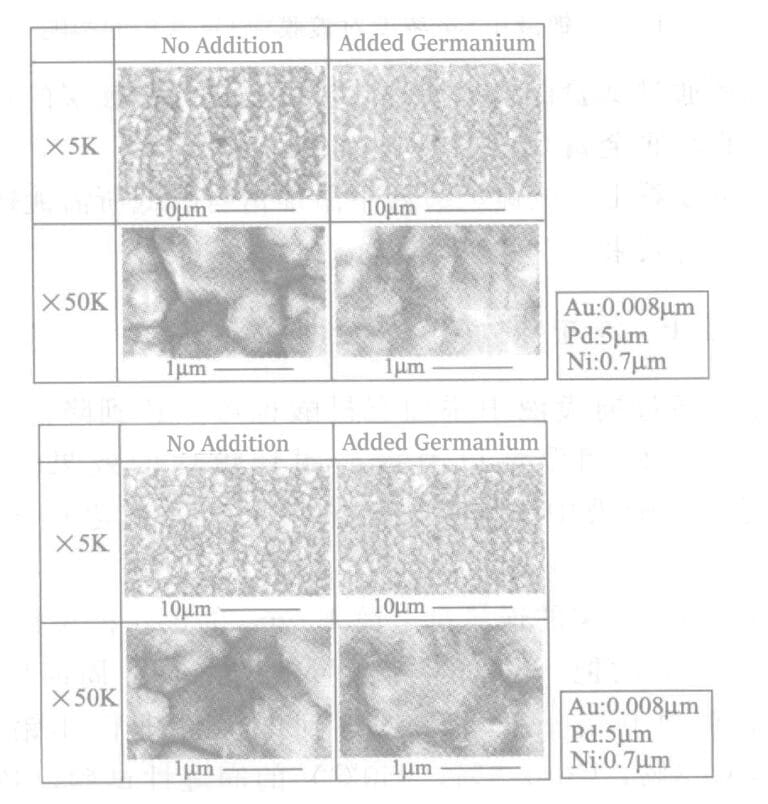
Figure 4-6 Surface photograph of the palladium plating layer
In the experiment confirming the effect of germanium eutectic on metal bonding strength, it was found that the effect was slight. At the same time, no crystallographic differences were observed in the palladium plating layer with or without the addition of germanium before and after heat treatment (see Figure 4-6).
The eutectic content of germanium in the palladium plating layer increases with the increase of germanium concentration in the plating solution (see Figure 4-7).
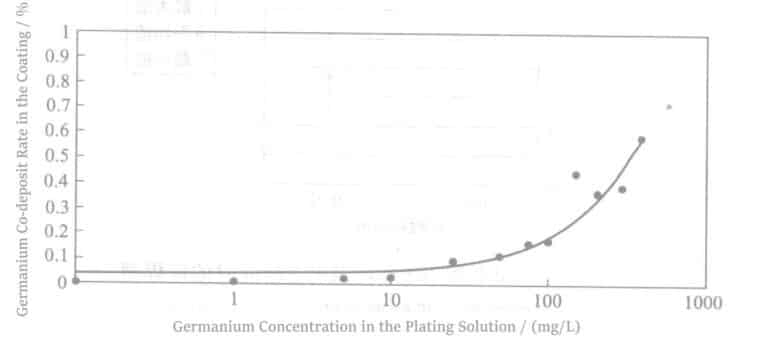
Experiments also showed that the co-deposition of germanium can improve the heat resistance of the palladium plating layer and inhibit the diffusion of substrate metals Cu or nickel to the surface.
From the above, adding germanium to the palladium plating solution significantly reduces the minimum thickness of the required palladium plating.
3. The Effect of Adding Organic Acid to the Pd Plating Solution
Additionally, adding organic acids to the plating solution can reduce the Pd plating thickness to achieve improved wettability of the lead frame and enhance soldering resistance. Shigeki Kiyomizu et al. reduced palladium plating thickness by adding sulfonic or sulfonic acid to the plating solution.
1.0μm Nickel was plated on the copper lead frame’s bonding pad, followed by plating 0.03μm palladium under Table 4-5 (plated with both newly prepared plating solution and plating solution after three cycles), and finally, 0.005μmgold was plated. The resulting plated parts were subjected to wettability tests with lead-free solder (Sn 96.5%, Ag 3%, Cu 0.5%, 250℃), each tested 3 times. The results are shown in Table 4-6.
Table 4-5 Composition of Plating Solution and its Process Conditions
| Compoziția și condițiile de procesare | Nr. 1 | Nr. 2 |
|---|---|---|
|
Tetraammonium palladium dichloride (as palladium) Diammonium palladium vinyl chloride (as palladium) Sodium 2-naphthalenesulfonate Disodium 1,5-naphthalenedisulfonate Ammonium nitrate Sodium sulfate Ammonium acetate Sodium succinate Ammonium chloride рH Plating solution temperature Cathode current density |
3. 0g/L - 3. 0g/L - 125g/L - 50g/L - 10g/L 7. 5 〜 8. 5 60℃ 0. 5A/dm2 |
- 3. 0g/L - 3. 0g/L - 125g/L - 50g/L 10g/L 7. 5 ~ 8. 5 60℃ 0. 5A/dm2
|
Table 4-6 Wetting Test Results of Plated Parts [ZCT (s)]
| Numărul de serie | New plating solution | After three cycles of plating | ||||
|---|---|---|---|---|---|---|
| N=1 | N=2 | N=3 | N=1 | N=2 | N=3 | |
| Nr. 1 | 0.85 | 0.90 | 0.77 | 0.85 | 0.90 | 0.77 |
| Nr. 2 | 1.00 | 1.23 | 0.98 | 0.99 | 1.35 | 1.03 |
Section III Electroplating Palladium Alloys
1. Electroplating Palladium-Nickel Alloy
(1) Composition of Palladium-Nickel Alloy Plating Solution
Table 4-7 shows the composition of some Pd-Ni alloy plating solutions and their process conditions. In Pd-Ni alloy plating, the most important thing is to ensure a certain ratio of Pd-Ni precipitation. Especially in ammonium plating solutions, the stable management of the deposition ratio is particularly important due to large pH value changes. In recent years, with the implementation of high current density and high-speed plating in roll-to-roll plating, the deposition ratio is an extremely important factor.
Table 4-7 Pd-Ni Alloy Plating Solution and its Process Conditions
| Compoziția și condițiile de procesare | Nr. 1 | Nr. 2 | Nr. 3 | Nr. 4 |
|---|---|---|---|---|
|
Palladium salt Nickel salts Conductive salts - - Brightening agents |
Pd(NH3)2Cl2 40g/L NiSO4 ・ 6H2O 45g/L NH4OH 90mL/L (NH4)2SO4 50g/L - Adequate amount |
Pd(NH3)2Cl2 (as Pd ) 10g/L Ni(NH3)2Cl2 (as Ni ) 12g/L NH4Cl 30g/L Ammonium citrate 10g/L H3BO3 15g/L — |
Pd(NH3)4Cl2 -H2O (as Pd ) 25g/L Ni(CH3COO)2 - 4H2O (as Ni ) 10g/L — - - — |
PbSO4 • H2O (as Pd ) 7. 1g/L NiSO4· 6H2O (as Ni) 29g/L Glycine 10g/L (NH4)2SO4 50g/L Benzoic acid sulfide 5g/L Polyethylene polyamines 0. 1g/L |
|
рH Temperatura Current density Pd/Ni molecular ratio - |
8.5 30°C 1A/dm2 80/20 - |
9. 0(Adjustment with ammonia) 50°C 2A/dm2 - Te Kung Chao 60-9116 (1983)
|
8. 0(Adjustment with NaOH) 30°C 1 A/dm2 86/14 Te Kung Chao 59-29118(1984) |
8. 25(Adjustment with NH4OH) 40°C 0. 2〜2A/dm2 70/30 Te Kung Chao 58-30395(1983) |
Table 4-8 Composition and Process Conditions of Low-Speed and High-Speed Plating Baths
| Compoziția și condițiile sale de procesare | Low-speed plating solution | High-speed plating solution |
|---|---|---|
|
Pd/(g/L) Ni/(g/L) NH4Cl/(g/L) Temperatură/°C рH Densitatea curentului/(A/dm2) Agitation/(cm/s) Cathodic current efficiency/% Additive 1/(mL/L) Additive 2/(mL/L) |
6〜8 2〜4 80〜120 35 8.0 1 5 92 2〜25 0. 1〜10 |
15〜25 15〜25 50〜100 35 8. 0 10 50 92 2〜50 0. 1〜20 |
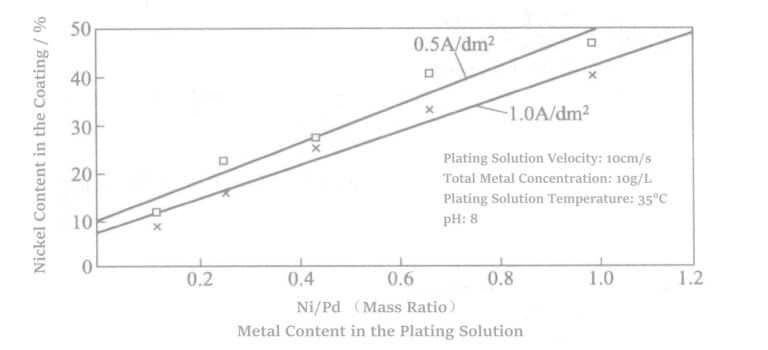
Table 4-9 Comparison of Various Coatings
| Proprietăți | Hard plating | Pure Pd plating | Pd-Ni (20%) plating |
|---|---|---|---|
|
Hardness HV Curing agent Crystalline size/Å Density/(g/cm 3) Elongation/% Volatile component(mass fraction)/% Thermal stability/°C Internal Stress/(N/mm 2) |
160 Co 200〜250 17. 3 2. 3〜3. 5 <1. 5 150 500〜700 |
315 Aditiv 50〜200 11. 75 >9 <0. 5 >450 700〜900 |
520 Ni+additive 50〜200 10. 73 >9 <0. 1 380 250〜350 |
|
Drug resistance Nitric acid gas SO2 gas NH3 gas Sweat H2 gas |
- O O O O O |
- X O O O △ |
- X O O O △ |
|
Culoare Solderabilitate
|
Golden color - |
White (slightly black) O
|
Alb O
|
| Resistance/mΩ | 7. 3 After H2S gas exposure 80 | 8. 6 After H2S gas exposure 13. 7 | 10 |
Copywrite @ Sobling.Jewelry - Producător de bijuterii personalizate, fabrică de bijuterii OEM și ODM
(2) Corrosion Resistance of Palladium-Nickel Alloy Plating
P. Wilkinson believes that although it is impossible to find other metals or alloys with all the characteristics of, in terms of corrosion resistance, wear resistance, and electrical resistivity, the Pd-Ni20% alloy has characteristics comparable to those of gold.
K. J. Whitlaw also conducted detailed research. According to his report, data on the alloy composition, conditions, and coating composition analysis can be found in Tables 4-10 to 4-12.
Table 4-10 Composition of Au Plating Solution, Process Conditions, and Coating Performance
| Compoziția și condițiile de procesare | Composition and performance of plating layer |
|---|---|
|
Au 8. 0g/L Ni 0.65g/L pH 4. 6 SG 1. 10 Temperature 38℃ Current density 1A/dm2 Agitation Cathode up and down vibration 3. 5m/min Deposition rate 1μm/2. 5min |
Au 99.0%(mass fraction) Ni 0. 14% (mass fraction) C 0. 27% (mass fraction) K 0. 30% (mass fraction) Density 17. 5g/cm3 Hardness 160VPN Poor ductility (50μm foil) |
Table 4-11 Composition, Process Conditions, and Performance of Pd-Ni Electroplating Solution
| Compoziția și condițiile de procesare | Composition and performance of plating layer | ||
|---|---|---|---|
| Main plating bath | Impact plating bath | ||
|
Pd Ni pH SG |
5. 0g/L 6. 5g/L 8. 2 1.09 (12°Be) 1A/dm2 |
2. 0g/L — 5. 5 1.05 (7°Be) 0. 3 A/dm2 |
Pd mass ratio 73.0% (60% atomic) Ni mass ratio 27.0% (atomic ratio 40%) C 0 K 0 Density:11g/cm3 Ductility: good (elongation 6%) (50μm foil)
|
| Agitation:Slow cathodic vibration and continuous filtering cycle Plating speed 5min, μm, 1.5min/0.lμm | |||
Table 4-12 Comparison of Six Electroplating Combinations Used in the Test and Their Relative Costs
| Plating layer | Cost factor |
|---|---|
|
Au plating on Cu 2.5μm thick Pd-Ni plating on Cu 3.5μm thick Pd-Ni plating on Cu 3.0μm + Au plating 0.25μm Plating Ni on Cu 5.0μm + Plating Au 1.0μm Plating Ni on Cu 5.0μm + Plating Pd-Ni 2.0μm Plating on Cu Ni 5.0μm + Plating on Pd-Ni 1.5μm + Plating on Au 0.25μm |
100 35 38 40 20 23 |
Table 4-13 Contact Resistance after Exposure to Industrial Atmosphere (21 days)
| Plating Matching No. | Contact resistance | Plating Matching No. | Contact resistance |
|---|---|---|---|
|
1 2 3 |
1. 7mΩ 2. 2mΩ Cannot be measured |
4 5 6 |
Cannot be measured 2. 8mΩ 10. 0mΩ |
From the visual results of the test pieces, the electroplated Au, Ni on the Pd-Ni intermediate plating layer of Cu plated did not undergo corrosion, nor were there any pinholes. However, the Au plated on Ni had a few pinholes, but their presence could not be confirmed in the staining test.
On the two types of Pd-Ni alloy sheets, the corrosion at their ends is relatively severe and can be considered as:
① Corrosion in the Ni-rich region under high current density;
② Corrosion creep at the unprotected ends.
Among these plating solutions, flash plating of 0.25μm Au on Pd-Ni 30μm is the best alternative to acid hard 2.5μm Au plating. The plating layer has the following characteristics:
① No pinholes;
② Stable contact resistance;
③ Good corrosion resistance;
④ Good wear resistance;
⑤ Good resistance to Cu diffusion at high temperatures.
At the same time, to prevent corrosion caused by pinholes, sealing treatments apply an organic film on the coating surface, such as the good effect when using N-methylglycine.
2. Electroplating Palladium-Silver Alloy
Used as a junction, Pd-Ag plated is used to replace gold. Keisuke Kishimoto chose to use amido polycarboxylic acid as a complexing agent for Pd-Ag alloy plating. Amido compounds are also used as stabilizers. The typical plating conditions are (adjusted with NaOH), the plating solution temperature is 20~60℃, and current density is 0.5`10A/dm2 .
The composition is as follows:
Pd(NO3)2(Pd salt) 1.0〜30g/L
AgNO3(Ag salt) 0. 01 〜15g/L
Acylamino polycarboxylic acid (Stabilizer) 1〜300g/L
Acylamino compound (Stabilizer) 1〜100g/L
Plating solution temperature 20〜60 ℃
Cathode current density 0.5〜10A/dm2
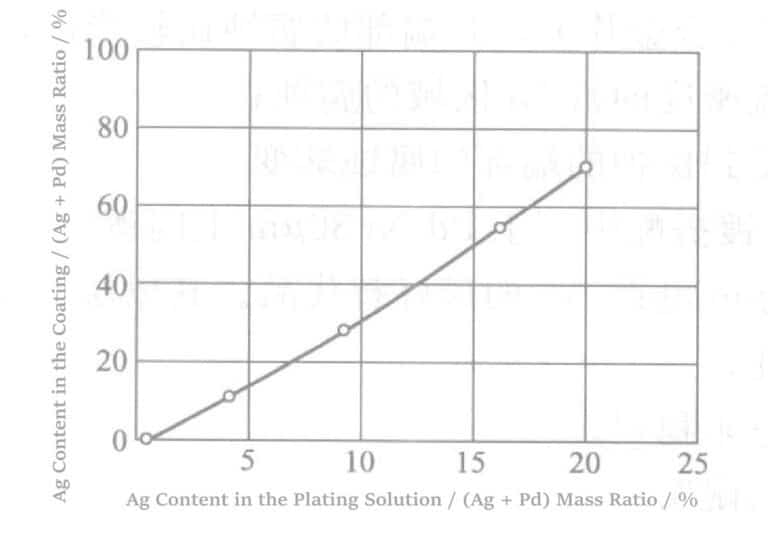
This results in a uniform coating with a metallic luster and good adhesion. However, because the deposition ability of Ag in this plating solution is relatively strong, the Ag content in the coating is much higher than the Ag ion content among the metal ions in the plating solution, which leads to difficulties in controlling the plating solution.
In addition, Yasuyuki Matsumura et al. filed a patent for the use of ammonia as a complexing agent as Pd-Ag alloy plating for hydrogen separation membranes.The stabilizers used are nitrate ions or sulfate ions. The main process conditions are as follows:
Palladium salt[Pd(NH3)4Cl2] 5〜200mmol/L
Silver salt Ag2SO4 0. 5〜20mmol/L
Complexing agent [(NH4)2SO4 ,NH4H2PO4 ,(NH4)2SO4] 20〜2000mmol/L
pH adjusting agent [NH4OH] pH = 9〜12
Plating solution temperature 20〜50℃
Voltage -0. 7〜-1. 0V(vs. Ag/AgCl Standard electrode)
Anode Inert Anode (Ti-Pt)
3. Electroplating of Palladium-Cobalt-Indium Alloy
Table 4-14 Composition and Process Conditions of Pd-Co-In Alloy Plating Bath
| Nr. 1 | Nr. 2 |
|---|---|
|
Sodium tartrate 150g/L Sodium sulfate 60g/L Pd(NH3)4Cl2 (as palladium) 30g/L Cobalt sulfamate (as cobalt) 40g/L Indium sulfate (Indium) 5g/L Saccharin (as brightener) 4g/L 1,4-Butynediol 0.3g/L pH 10 Plating solution temperature 50℃ Cathode current density 1.5A/dm2 Plating White alloy |
Citric acid 180g/L Sodium sulfite 100g/L Pd(NH3)2Cl2 (as palladium) 3g/L Cobalt chloride (as cobalt) 5g/L Indium sulfamate (Indium) 20g/L Saccharin (as brightener) 4g/L Formic acid 0.5ml/L pH 6.5 Plating solution temperature 25℃ Cathode current density 0.5A/dm2 Light gray alloy |
Section IV Chemical Palladium Plating and Its Alloys
1. Electroless Palladium Plating Using Hypophosphite as a Reducing Agent
Murakado Akihiko et al. significantly improved the stability of the plating solution by adding stabilizers to the plating bath, ensuring good brazing properties and metal wire bonding connectivity even with long-term use of the plating solution.
Table 4-15 shows the formula and process conditions proposed by Murakado Akihiko et al. Table 4-16 shows the pretreatment conditions.
Table 4-15 Electroless Palladium Plating Formula and Process Conditions
| Compoziția și condițiile de procesare | Nr. 1 | Nr. 2 | Nr. 3 | Nr. 4 | No. 5 | No. 6 | No. 7 | No. 8 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Chemical palladium plating solution | Pd salt | Palladium chloride/(mol/L) | 0.03 | 0.03 | 0.03 | 0.03 | ||||
| Tetraammonium palladium chloride/(mol/L) | 0. 005 | 0. 005 | 0. 005 | 0. 005 | ||||||
| Complexing agent | Ethylenediamine/(mol/L) | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | |
| EDTA/(mol/L) | 0.03 | 0.03 | 0.03 | 0.03 | ||||||
| Glycine/(mol/L) | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | |||||
| Ammonia(28%)/(mol/L) | Appropriate amount | Appropriate amount | Appropriate amount | Appropriate amount | Appropriate amount | Appropriate amount | Appropriate amount | Appropriate amount | ||
| Agent reducător | Sodium Hypophosphite/(mol/L) | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | ||
| Ammonium hypophosphite/(mol/L) | 0.2 | |||||||||
| Unsaturated hydroxy acid | Isobutenoic acid/(mol/L) | 0.3 | ||||||||
| Isobutenoic acid/(mol/L) | 0.3 | |||||||||
| Maleic acid/(mol/L) | 0.2 | |||||||||
| Fumaric acid/(mol/L) | 0.2 | |||||||||
| Itaconic acid/(mol/L) | 0.2 | |||||||||
| Citraconic acid/(mol/L) | 0.2 | |||||||||
| Mesoacetic acid/(mol/L) | 0.2 | |||||||||
| Cinnamic acid/(mol/L) | 0.2 | |||||||||
| Temperatură/℃ | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | ||
| pH | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | ||
| Coating Characteristics | Separation speed/(μm/h) | New solution | 0.4 | 0.4 | 0.5 | 0.5 | 0.7 | 0.6 | 0.6 | 0.7 |
| After 50h continuous plating | 0.4 | 0.4 | - | - | 0.7 | 0.6 | - | - | ||
| Appearance of plating | New solution | Bun | Bun | Bun | Bun | Bun | Bun | Bun | Bun | |
| After 50h continuous plating | Bun | Bun | - | - | Bun | Bun | - | - | ||
| Solderabilitate | New solution | Bun | Bun | Bun | Bun | Bun | Bun | Bun | Bun | |
| After 50h continuous plating | Bun | Bun | - | - | Bun | Bun | - | - | ||
| Wire solderability | New solution | Bun | Bun | Bun | Bun | Bun | Bun | Bun | Bun | |
| After 50h continuous plating | Bun | Bun | - | - | Bun | Bun | - | - | ||
| Liquid Stability | 50°C continuous plating | No decomposition after 50h | No decomposition after 50h | No decomposition after 50h | No decomposition after 50h | No decomposition after 50h | No decomposition after 50h | No decomposition after 50h | No decomposition after 50h | |
| 80°C heating | No decomposition after 30h | No decomposition after 30h | No decomposition after 30h | No decomposition after 30h | No decomposition after 30h | No decomposition after 30h | No decomposition after 30h | No decomposition after 30h | ||
| Temperatura camerei | No change in 6 months | No change in 6 months | No change in 6 months | No change in 6 months | No change in 6 months | No change in 6 months | No change in 6 months | No change in 6 months | ||
Table 4-16 Substrate Pretreatment Conditions in Table 4-15
| Processing | Solution | Temp./℃ | Processing time/min | |||
|---|---|---|---|---|---|---|
| Pre-treatment | (1) | Clean Processing | ACL-009 | Uemura Industrial Products | 50 | 5 |
| (2) | Weak Etching | 100g/L SPS | 25 | 2 | ||
| (3) | Pickling | 10% H2SO4 | 1 | |||
| (4) | Pre-impregnation | 3% H2SO4 | 1 | |||
| (5) | Activation treatment | MNK-4 | Uemura Industrial Products | 30 | 2 | |
| Chemical Plating | (6) | Ni-P Plating | NPR-4 | Uemura Industrial Products | 80 | 30 |
| (7) | Pd Plating | See Table 4-17 | 5 | |||
| (8) | Replacement plating Au | TAM-55 | Uemura Industrial Products | 80 | 10 | |
Table 4-17 Chemical Plating Pd-P Process Conditions
| Compoziția și condițiile de procesare | Formulation and component concentration | Compoziția și condițiile de procesare | Formulation and component concentration |
|---|---|---|---|
|
PdCl2 Ethylenediamine Thiodiacetic acid |
0. 01mol/L 0. 08mol/L 30mg/L |
Na2HPO3 pH Temperatura |
0. 02 〜1.0mol/L 6 60℃ |
It is believed that similar to hypophosphite as a reducing agent; phosphite can also obtain Pd-P alloy plating from ethylenediamine complex salts. It also has an autocatalytic effect. Moreover, as the the concentration of hypophosphite in the plating solution increases, and the phosphorus content in the coating also increases.
The mechanism of the reducing agent’s dehydrogenation reaction in the first stage is as follows:
| Dehydrogenation | HPO32- → ·PO32- + H | (4-1) |
| Oxidation | PO32- + OH- → HPO32- + e- | (4-2) |
| Recombination | H + H → H2 | (4-3) |
| Oxidation | H + OH- → H2O + e- | (4-4) |
| Metal precipitation | Pd2+ + 2e- → Pd | (4-5) |
| Hydrogen precipitation | 2H2O + 2e- → H2 + 2OH- | (4-6) |
| Co-precipitation of P | HPO32- + 2H2O + 3e- → P + 5OH- | (4-7) |
2. Electroless Palladium-Nickel Alloy Plating
Hideo Honma et al. proposed a patent for plating Pd-Ni alloys using hydrazine as the reducing agent. This allows for alloy plating without the need for electrical current and without shape constraints. At the same time, since phosphorus-containing reducing agents are not used, contamination of P in the plating layer can be avoided. The presence of P increases the contact resistance of electrical contacts and reduces the wettability of brazing, which may cause reliability issues.
The pretreatment conditions for the substrate to be plated are shown in Table 4-18.
Table 4-18 Pretreatment Process Conditions for Electroless Pd-Ni Alloy Plating
| Tratament | Temperatură/℃ | Soaking time/min |
|---|---|---|
|
Alkaline Degreasing Water washing Acid treatment Water washing Activation treatment |
10 ~ 100 10 〜100 10 〜 100 10 〜100 10 〜100 |
1〜10 1〜5 1〜10 1〜5 1〜10 |
Table 4-19 Composition and Process Conditions of Pd-Ni Alloy Chemical Plating Solution
| Compoziția și condițiile de procesare | Formulation and component | Compoziția și condițiile de procesare | Formulation and component |
|---|---|---|---|
|
Palladium chloride Nickel sulfate Hydrazine monohydrate Ethylamine |
0. 01mol/L 0. 0501mol/L 1. 001 mol/L 0. 201mol/L |
Complexing agent (carboxylic acid) Lead sulfate Temperatura рH |
0. 301 mol/L 0. 005g/L 60℃ 9. 0 |
3. Chemical Plating of Palladium-Molybdenum Alloy
Table 4-20 Composition and Process Conditions of Pd-Mo Electroless Plating Alloy
| Ingredients and their process conditions | Nr. 1 | Nr. 2 | Nr. 3 | Nr. 4 | No. 5 |
|---|---|---|---|---|---|
|
PdCl2 (as Pd) Potassium formate Sodium hypophosphite Trimethylamine borane Ammonia Diethylamine Sodium citrate Triethylenetetramine Boric acid Hydroxyethylenediamine triacetate Fumaric acid Lead acetate (as Pb) Potassium succinate Sodium thiosulfate Sodium molybdate(as molybdenum) рH Plating solution temperature Wire bonding test Brazed joint test |
2g/L 0. 1mol/L - - 2mol/L - - - 0. 5mol/L - - 1X10-6 - - 0. 05g/L 7 70℃ Above 8g No flaking Above 1.5kg |
2g/L - 0. 5mol/L - - 0. 1mol/L 0. 25mol/L - - - - - - 25X10-6 0. 5g/L 7 60℃ Above 8g No flaking Above 1.5kg |
2g/L - 0. 3mol/L - - - - 0. 05mol/L - - - - 0. 1mol/L 40X10-6 5g/L 8 60℃ Above 8g No flaking Above 1.5kg |
2g/L - - 0. 02mol/L - - - - - 1mol/L 0. 1mol/L - - 40X10-6 20g/L 8 70℃ Above 8g No flaking Above 1. 5kg |
2g/L 0. 1mol/L - - 2mol/L - - - 0. 5mol/L - - 1X10-6 - - 20g/L 7 70℃ Above 8g No flaking Above 1. 5kg |
4. Electroless Plating of Palladium-Silver Alloy
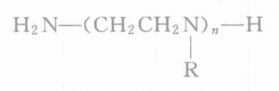
In the formula, n is an integer of 1~5, and R is H or a functional group of -CH2-CH2-NH2.
The plating solution stability is evaluated by continuously adding metal concentration and other components during plating and repeatedly performing Pd-Ag plating. After using one cycle, the plating solution is heated to 90℃ and maintained for 24 hours to confirm whether there is any decomposition of the plating solution and any metal deposition in the tank. The film thickness is tested using a fluorescent X-ray thickness gauge.
The hydrogen permeability coefficient is calculated by passing hydrogen through a porous ceramic tube plated with an alloy and heated to 500℃, then analyzing the permeated hydrogen using gas chromatography.
Section V Operation Management of Palladium Plating Solution
Pure palladium or palladium alloy plating solutions generally consist of palladium and its complexes, conductive salts, additives, etc. Additives may be organic, inorganic, or a mixture of organic and inorganic substances.
Generally speaking, the analytical instruments used for palladium plating are as follows.
Atomic absorption spectroscopy or ICP: analysis of palladium and other metal ion concentrations;
Ion spectroscopy or electrophoresis: analysis of conductive salts and their complexing agents;
HPLC or electrophoresis or potentiometric titration: analysis of organic additives.
The anode is generally reactive. Oxidation reactions occur at the anode, which may accelerate the aging of the plating solution. The oxidation phenomenon when using nail or sheet anodes is weaker than the oxidation reaction when using Pt-Ti anodes.
When using ammonia water to adjust pH, the volatilization of ammonia gas can cause pH instability. Ammonia gas can be directly introduced into the plating solution.