Cum să deosebești diamantele adevărate de falsuri: Ghidul final de identificare
The Professional's Guide to Diamond Verification: From Luster to Lab-Grown
Introducere:
This guide provides jewelers with essential diamond identification techniques. Learn to examine luster, fire, and transparency, and use tools like thermal testers effectively. Discover key differences between natural diamonds and synthetic types (HPHT/CVD), plus identify treated diamonds through laser drilling, fracture filling, or color enhancement. The guide also covers spotting simulants like cubic zirconia and moissanite. Using methods from magnification to UV fluorescence observation, you’ll gain practical skills to verify diamond authenticity. Essential knowledge for jewelry professionals to ensure product integrity and maintain customer trust in today’s market.

CVD synthetic diamond and HPHT synthetic diamond
Tabla de conținut
Section I Basis for Diamond Identification
(1) Identification of Rough Diamonds
Identification of rough diamonds can be based on naked-eye observation of a strong adamantine luster, a “sparkling” dazzling surface appearance, distinctive crystal habit and face patterns (curved faces, triangular etch markings, stepped growth striations), very high hardness (H10), moderate relative density, and observation of UV fluorescence.
(2) Identification of Polished Diamonds
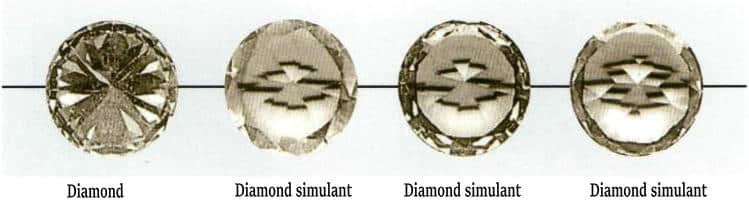
① Observe luster and fire. Diamonds have a high refractive index and a strong adamantine luster; well-polished diamonds reflect strongly and appear dazzling. They have a high dispersion value and good fire; a round brilliant with standard cutting presents a multicolored, lively yet soft diamond fire. The blue component is predominant in diamond fire, and true rainbow-like multicolored-fire diamonds are rare (Fig. 3-1). Cubic zirconia (CZ) has a higher dispersion than diamond, so its fire shows more varied colors and more orange, which is especially noticeable in sunlight (Fig. 3-2).

Figure 3-1 Fire (brilliance) of a diamond

Figure 3-2 Fire (brilliance) of cubic zirconia (CZ)
② Transparency test. Place the diamond with the table facing down and the culet (bottom point) up on a piece of paper marked with a black line; if it is a diamond, the black line will not be visible. Note, however, that high-refractive-index diamond simulants such as synthetic sodium strontium and synthetic rutile also hide the line. If the black line is visible, the stone is another simulant with a lower refractive index—the lower the refractive index, the more easily the line can be seen (Fig. 3-3). Because diamonds are usually cut to a standard round brilliant and, if proportions are proper, almost no light passes through the pavilion facets, the lines on the paper are not visible, except in improperly cut diamonds.
This identification method applies only to diamonds with round brilliant cuts; it is not suitable for diamonds with other cuts. If a liquid is attached to the diamond, it will be permeable to sight.
③ Oleo-philic test. Diamonds are oleophilic and resist being wetted by water; because diamonds strongly adsorb oils and fats, they appear to have an oily film after being touched by hand. When an oil-based pen is drawn across the diamond’s surface, it can leave clear, continuous lines. If the item is a diamond imitation, the surface will show separate small droplets rather than continuous lines.
(3) Features Observed Under Magnification
① Surface features. Because diamonds are extremely hard, after cutting and polishing, their facets are very flat and smooth, and the girdle edges between facets are straight and sharp. Most imitations, due to lower hardness, have facets that are relatively less smooth, with rounded or blunted edges, and may even show many knocks and chips.
② Girdle features. Diamond girdles fall into three types: rough-cut girdle, polished girdle, and faceted girdle. A rough-cut girdle has a coarse, non-glossy, frosted-glass appearance; this is the most common girdle shape on finished diamonds. A polished girdle is transparent and smooth; a faceted girdle shows multiple facets, smooth and transparent, though the facet sizes are usually uneven.
Imitation girdles can also be made to resemble those of diamonds, but because of different material properties, most are left untreated and have a coarse, frosted-glass girdle, and some show striations on the girdle; if striations are observed on the girdle, it is an imitation.
In round brilliant diamonds, if excessive pressure is applied during the rounding process, fine hairline cracks can form at the girdle, commonly called “bearded girdle.” During cutting and polishing, to maximize retained weight, some of the original crystal faces (natural facets) are often left below the girdle; these may show step-like or triangular growth lines or cleavage planes.
③ Inclusion characteristics. Diamonds commonly contain characteristic natural mineral inclusions, while simulants may contain round gas bubbles. Observing a diamond’s inclusion features can accurately distinguish diamonds from their imitations, natural diamonds from synthetic diamonds, and diamonds that have undergone enhancements. Double-image phenomena seen under a microscope can also separate diamonds from colorless zircon, sphene, synthetic silicon carbide (also called moissanite), and the like.
(4) Diamond Thermal Conductivity Tester
The diamond thermal conductivity tester is based on the principle that diamond conducts heat extremely rapidly; it was once able to accurately determine whether a tested gem was a diamond. In the mid-1990s, synthetic silicon carbide appeared as a diamond simulant with thermal conductivity similar to diamond; if a specimen passes a thermal tester and also shows double images of facet junctions, it is synthetic silicon carbide. The identifying features of these two gems are shown in Table 3-1.
Table 3-1 Identification Features of Diamonds and Synthetic Silicon Carbide
| Numele pietrei prețioase | Indicele de refracție | Birefringență | Gravitație specifică | Dispersie | Duritate | Other Characteristics |
|---|---|---|---|---|---|---|
| Diamant | 2.417 | Isotropic, exhibits anomalous | 3.52 | 0.44 | 10 | Adamantine luster, sharp facet edges and intersections |
| Synthetic Silicon Carbide(SiC) | 2.648 ~ 2.691 | High birefringence (0.043) | 3.22 | 0.104 | 9.25 | Obvious facet edge doubling, white thread-like inclusions, thermal conductivity close to diamond |
Section II Identification of Optimized Diamonds
(1) Identification of Color-Altered Diamonds by Irradiation
In the 1950s, with the use of reliable irradiation sources such as reactor neutrons and high-energy electrons from cyclotrons, several irradiated green diamonds appeared on the market. Finding methods to identify these diamonds thus became a research topic for gemologists, and most identification methods have been based primarily on abnormal color distribution characteristics. Because irradiation beams have limited penetration, diamonds treated by this method often exhibit the so-called “umbrella effect” near the culet (lower apex).
Irradiation can produce virtually any color in diamonds, but the colors are unstable and often require subsequent heat treatment. In irradiated diamonds, isolated vacancy defects are the main cause of green coloration. However, if the diamond contained little yellow before treatment, blue can result after treatment. When the temperature is heated to around 600℃, vacancies in the diamond lattice will migrate and may combine with nitrogen-related defects to form nitrogen-vacancy (N-V) centers. These N-V centers typically exhibit absorption lines (bands) in the visible range, which impart color to the diamond. Depending on the type and characteristics of the diamond before treatment, the irradiation source used, and the temperature and duration of any heat treatment, treated diamonds can display a variety of colors; certain spectral features of the diamond may also be produced or altered. By studying these spectral features, reliable identification methods can be developed. In particular, investigation of absorption spectral features from the ultraviolet through the mid-infrared is a typical approach for studying such treated diamonds, and spectroscopic studies of samples at liquid-nitrogen temperature can also provide more sensitive and critical identification characteristics.
Diamonds with low color grades or pale colored diamonds can be color-treated by irradiation and heating to produce more vivid colors, increasing the color saturation of the diamond and thereby raising its value. The most common colors produced by irradiation plus heating are: green, yellow, pink, and brown. Their main identification features are as follows.
① Color distribution characteristics. Naturally colored diamonds show color zones that are linear or triangular, with the color zones parallel to crystal faces. Artificially color-treated diamonds have color confined to the surfaces of the faceted gem; the position and shape of the color zones depend on the cut shape and the direction of irradiation. When the pavilion of a round brilliant diamond is bombarded, color produced by irradiation can be seen through the table, distributed around the pavilion in an umbrella-like pattern (Fig. 3–4).

② Absorption spectrum characteristics. The absorption spectra of yellow and brown diamonds treated by irradiation and heat show an absorption line in the yellow region (594nm) and several absorption lines in the blue-green region (504nm, 497nm). Irradiation-treated red-series diamonds often exhibit orange-red UV fluorescence, with a 570nm fluorescence line (bright line) in the visible spectrum and a 575nm absorption line; in most cases, these are accompanied by an absorption line at 610nm, 622nm, and 637nm.
③ Conductivity characteristics. Natural blue diamonds are conductive due to boron impurities, while blue diamonds produced by irradiation are not conductive.
(2) Identification of Filled Diamonds
Filling diamond fractures with high-refractive-index glass can improve the diamond’s clarity grade and thus increase its value. The filling process is carried out in a vacuum, injecting high-refractive-index lead glass into fractures that extend to the diamond’s surface, which can, to some extent, conceal internal fractures. The main identification characteristics are as follows.
① Microscope observation. Filled fractures can exhibit a distinct flashing effect; under dark-field illumination, the most common flash colors are orange-yellow, purplish red, and pink, followed by pinkish-orange (Fig. 3–5). Under bright-field illumination, the most common flash colors are bluish-green, green, yellow-green, and yellow. Different parts of the same fracture may show different flash colors, and the flash color of a filled fracture can change as the sample is rotated. Sometimes, flow-like textures and flattened gas bubbles can also be seen within the fracture.

② X-ray radiography. X-ray radiography can provide a definitive conclusion about a filled diamond, and it can also determine the extent of the filling treatment and the filling material, as well as locations damaged by heating during jewelry repair. Under X-ray exposure, the diamond appears highly transparent, while the filling material is nearly opaque (containing elements such as Pb, Bi, etc.). The filled area appears with a white outline in the transmitted-light photograph.
(3) Identification of Laser-Drilled Diamonds
Laser-drilled diamonds are diamonds that have undergone laser drilling to remove black or dark inclusions, to improve clarity. Under 10x loupe or microscope examination, such treated diamonds are generally not difficult to identify; their main distinguishing features are as follows.
① Observe the uneven “pit” at the laser entry hole on the diamond surface.
② Rotate the diamond and observe the linear laser channels (Fig. 3–6). The laser channels stand out due to contrast, since the refractive index, transparency, and color of the filling material differ from those of the diamond.
③ The color and luster of the material filling the laser holes differ from the surrounding diamond.

(4) Identification of Surface-Coated Diamond Films
Diamond films are polycrystalline materials composed of carbon atoms with a diamond-like structure and with the physical, chemical, and optical properties of diamond. Natural diamonds are single crystals, whereas diamond films are polycrystalline; their thickness is generally tens to hundreds of micrometers, and can be as thick as a millimeter. The main identifying features are as follows.
① Observe the surface characteristics of diamonds with coated diamond films. Under microscope magnification, they show a granular structure, which does not occur in natural diamonds. If a colored coating has been applied, the diamond can be placed in methylene iodide for observation; an interference color will appear on the diamond’s surface layer.
② Raman spectrometer measurement. The characteristic absorption peak of natural diamond is at 1332cm-1; because diamond is a single crystal, the peak’s half-height width is narrow. High-quality diamond films have characteristic absorption peaks near 1332cm-1, with a relatively wider half-height width. Poor-quality diamond films show large peak frequency shifts and reduced intensity.
(5) Multi-Process Treated Colored Diamonds
Multi-process treated diamonds actually use multiple treatment methods combined on the same diamond to alter its color and clarity. Multi-process treatments of diamonds first appeared in the 1990s, initially involving multiple clarity treatments, such as laser drilling followed by glass filling along the laser channels. From the late 1990s to the early 2000s, with the emergence and maturation of high-pressure high-temperature (HPHT) treatment and irradiation plus high-temperature techniques, multi-process composite methods were gradually used to change diamond color. Since 2002, foreign gemological laboratories have successively reported studies on yellow diamonds, pink–red diamonds, and orange to orange-red diamonds that HPHT first treated and then subjected to irradiation plus low-temperature annealing. The types of multi-process treated diamonds seen domestically and internationally at present are roughly as follows.
① Orange-red, pink–red diamonds treated by multiple processes of high-pressure, high-temperature, plus irradiation annealing. Since 2005, a type of orange-red, pink–red diamond has appeared successively on foreign jewelry markets. The treatment method for these colored diamonds differs from traditional HPHT treatment and irradiation-plus-annealing methods; they undergo a combined, superimposed treatment of high-pressure, high-temperature, and irradiation annealing. In the international market, the so-called multi-process treated colored diamonds usually refer to this type. Their treatment mainly consists of two steps: the first step is HPHT treatment of Ia-type and IIa-type brown diamonds, intended to generate isolated nitrogen atoms under HPHT conditions and to remove brown tones caused by plastic deformation and other factors related to the brown coloration; the second step is irradiation annealing of the diamonds with isolated nitrogen atoms to produce centers such as N-V, H3, H4 and other lattice defects. Diamonds treated by multiple processes, because they contain strong defects such as the N-V center, exhibit pink, orange-red, or red colors (the specific color varies according to the diamond type before treatment and the treatment conditions).
The main identification features of this type of treated diamond are: under the microscope one can sometimes see color concentration in the pavilion, and their mineral inclusions often have disk-like fractures; under ultraviolet illumination, orange, orange-red, or orange-yellow fluorescence can be observed, especially when the pavilion is oriented upward, making this phenomenon more obvious (Figure 3–7); spectroscopically, characteristic peaks of the absorption peaks (1344cm-1) and the GR1 center (741 nm) absorption peak and the N-V center (637, 575nm), etc. caused by isolated nitrogen, can often be detected.

② Colored diamonds treated by multiple processes of HPHT and fracture filling. The main identification features of this type of diamond are: under microscopic observation, obvious fractures are present; under darkfield illumination, green and purple flashes can be seen in the fractures, indicating the presence of glass filling in the fractures; under long-wave ultraviolet light, the emitted fluorescence is stronger than that under short-wave ultraviolet light, and spectroscopic features detect the isolated nitrogen absorption peak (1344cm-1) produced by HPHT treatment.
③ Colored diamonds subjected to multi-step treatments of surface coating and fracture filling. In recent years, a new type of “colored diamond” treated by coating with nanoparticles of metals or compounds that participate in the coloration has appeared on the international diamond market; these colors are relatively uniform and reasonably stable. The main identification features of these diamonds: under a microscope, coating traces can sometimes be seen on the pavilion; under differential interference contrast microscopy, scratches, white spots, stains, areas of film detachment, and other coating traces can be clearly observed; using X-ray fluorescence chemical composition analysis, metal elements such as Au, Ag, Al, Ti and Fe, and the element Si, can be detected. For the identification of this type of diamond, the most important thing is to carefully inspect the pavilion for coating traces and to check fractures for any sparkling phenomenon under microscopic dark-field illumination.
Copywrite @ Sobling.Jewelry - Producător de bijuterii personalizate, fabrică de bijuterii OEM și ODM
Section III Identification of Synthetic Diamonds

(1) Identification of Synthetic Diamonds
Because synthetic diamonds and natural diamonds have identical chemical composition, crystal structure, and physical properties, they cannot be distinguished by the naked eye. They can only be differentiated in laboratories equipped with advanced specialized instruments through careful testing. Because synthetic diamonds and natural diamonds form under different conditions, there are certain differences in some gemological characteristics compared with natural diamonds. Therefore, identification is possible when conditions permit. See Table 3-2 for specific identification features.
Table 3-2 Identification Table of Natural Diamonds and Synthetic Diamonds
| Feature | Natural Diamond | HPHT Synthetic Diamond | CVD Synthetic Diamond |
|---|---|---|---|
| Culoare | Colorless, with yellow tones, brown tones, gray tones, pink, red, yellow, blue, green, etc. | Near-colorless, light yellow, yellow to brown, even blue | Dark brown and light brown, near-colorless with a slight grayish tone (G-H color), blue |
| Obiceiul de cristal | Common octahedron, rounded edges, crystal faces often have rough, curved surfaces; trigonal growth patterns, surface etch features or growth steps can be seen. | Cuboid-octahedral combinations; crystal faces often show leaf-vein-like, dendritic, or nodular surface features; some crystals may show seed crystals. | Tabular crystals |
| Includeri | Natural mineral crystal inclusions such as diamond, chromian diopside, pyrope, graphite, olivine, spinel, etc. (Figure 3-9) | Plate-like, rod-like, needle-like metallic inclusions (Figure 3-10) | Irregular dark inclusions, pinpoint inclusions, feather-like fractures (Figure 3-11) |
| Growth Patterns | Straight, linear patterns | "Hourglass-shaped" growth patterns, irregular color zoning. Uneven color distribution. | Parallel color bands, layered growth textures characteristic of this synthesis method |
| Fluorescență UV | Can show various colors, mostly blue-white, green, yellow, or inert; fluorescence is stronger under Long Wave UV than Short Wave UV (Figure 3-12) | Often inert under Long Wave UV; shows distinct zoning under Short Wave UV, with none-to-medium patchy yellow, orange-yellow, green-yellow fluorescence; may have phosphorescence. | Typical orange, or yellow, yellow-green fluorescence under both Long and Short Wave UV; Short Wave fluorescence is stronger than Long Wave |
| Diamond View™ Fluorescence | Fluorescence is blue, with closed growth patterns (Figure 3-13) | Fluorescence is green, with mottled geometric patterns (Figure 3-14) | Fluorescence is light greenish-blue, showing internal series of parallel structural growth lines (Figure 3-15) |
| Cathodoluminescence | Shows relatively uniform medium-strong blue - gray-blue fluorescence, and displays regular or irregular growth zoning (Figure 3-16) | Has regular geometric patterns; different growth zones emit different fluorescence colors (Figures 3-17, 3-18), predominantly yellow-green fluorescence; zoned growth textures are often visible within the growth sectors. | |
| Magnetism | Not attracted to magnets | Some with larger metallic inclusions can be attracted to magnets | |
| Absorption Spectrum | Most colorless-to-fancy light yellow diamonds show the 415 nm absorption line. | Lacks the 415 nm absorption line. | |
| Anomalous Birefringence | Complex, irregular banding, mosaic, or cross-shaped patterns. | Very weak, relatively simple, appearing as cross-shaped bright bands | Strong anomalous extinction. |
| Diamond Type | Mostly Type I a (containing aggregated nitrogen) | Most synthetic diamonds are Type I b (containing single nitrogen atoms) | Type II a (contains no nitrogen) |


Figure 3-10 Iron-nickel fluid inclusions in HPHT synthetic diamond

Figure3-11 Black carbonaceous inclusions in CVD synthetic diamond
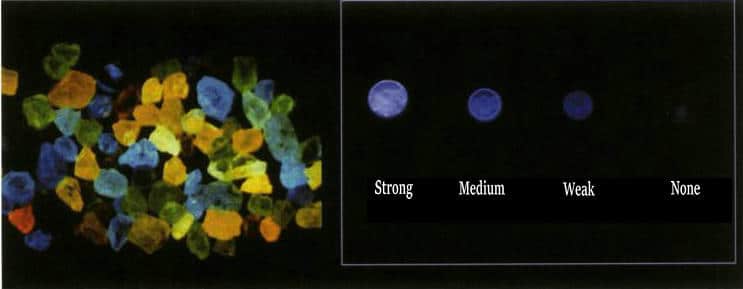

Figure 3-13 Fluorescence and closed-type textures of natural diamonds under Diamond ViewTM
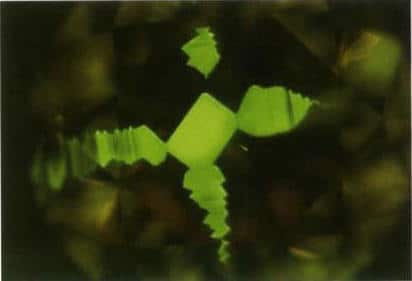
Figure 3-14 Fluorescence and mottled geometric banding of HPHT under Diamond ViewTM
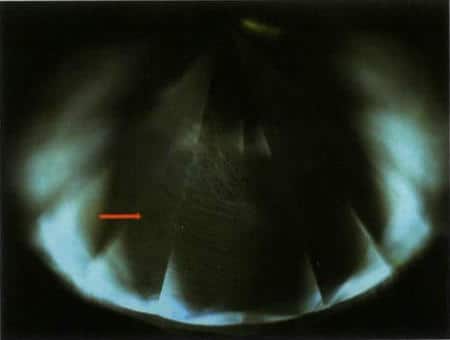
Figure 3-15 Fluorescence and layered growth textures of CVD synthetic diamonds under Diamond ViewTM
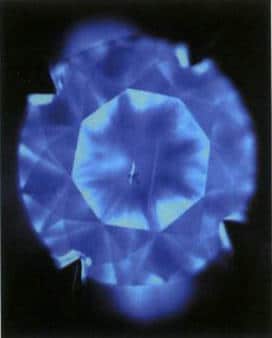
Figure 3-16 Cathodoluminescence image of a natural diamond

Figure3-17 Cathodoluminescence image of an HPHT synthetic diamond
Nearly square geometric patterns or different growth zones emit fluorescence of different colors
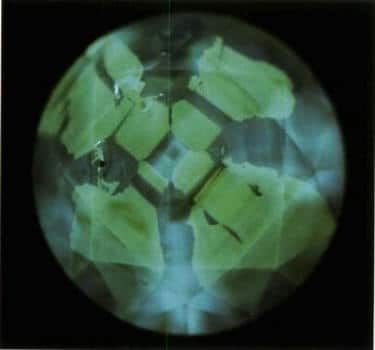
Figure 3-18 Cathodoluminescence image of an HPHT synthetic diamond
Octahedral growth zones form a cruciform intersection
(2) Color Alteration Treatments of Synthetic Diamonds
In recent years, pink-treated CVD synthetic diamonds have appeared on the market. The starting material for these pink diamonds is CVD synthetic diamond; their color is produced by high-pressure, high-temperature treatment or irradiation treatment. Their grains are generally smaller than 1ct, and the vast majority are 0.02~0.30ct.
There are two main causes for diamonds to appear pink: an absorption band at 550 nm and the formation of N-V centers in the diamond that produce pink. The absorption band mainly causes the color of natural pink diamonds at 550 nm, whereas N-V centers in the diamond mainly produce the color of treated pink diamonds. Various treatment techniques can make diamonds pink to red. When there are isolated nitrogen atoms in the diamond, high-energy irradiation (such as electron irradiation) plus lower-temperature thermal treatment can produce centers. Whether HPHT synthetic diamonds or CVD synthetic diamonds, they commonly contain small amounts of isolated nitrogen atoms; these synthetic diamonds can also be treated to obtain pink–red diamonds. By controlling the density of N-V centers, a range of weak pink to deep pink diamonds can be produced. Most of these treated diamonds carry a purplish–orange-yellow tone; pure pink or even red is rare. These pink diamonds are unstable under ultraviolet light and show photochromic characteristics: when exposed to a UV light source, the diamonds exhibit pronounced fading. This fading mostly recovers to its original color after being removed from the UV source and heated for several minutes. Some pink diamonds, once faded by UV irradiation, are more difficult to recover. Therefore, during identification, UV light sources should be avoided as much as possible. The most typical identifying features of this type of diamond are: ultraviolet–visible absorption spectrum, infrared absorption spectrum and the photoluminescence spectrum, relatively strong 595nm, GR1 centers (741nm) and N-V centers (637, 575nm) and relatively weak H1a center (1450cm-1), lone nitrogen (1344cm-1) and other absorption peaks can be seen; when the high-temperature high-pressure treatment temperature exceeds 2200℃, an absorption peak related to hydrogen can be seen in its infrared spectrum at 3107cm-1, and sometimes an absorption peak at 3123cm-1 can be observed. In addition, CVD synthetic diamonds sometimes show [Si-V] defects, manifested as a double absorption peak at 736.6nm and 736.9nm in the photoluminescence spectrum.
Section IV Identification of Diamonds and Diamond Simulants
(1) Main Types of Diamond Simulants
Modern materials used to counterfeit diamonds and make simulated diamond jewelry are mainly various kinds of man-made synthetic materials. They appeared in the jewelry market in the early 20th century. The artificial crystals used to imitate diamonds were colorless sapphires and spinels synthesized by the flame-fusion method. Both of these simulants have relatively high hardness, but their refractive indices and dispersion values are lower than those of diamond; when cut into diamond faceting styles, their surface luster is relatively weak, and their fire is also weak. They are now rarely used as diamond simulants. However, colorless synthetic sapphire has found a new use in the watchmaking industry and is called “never-wearing watch crystal.”
In 1947, flame-fusion synthetic rutile was produced, possessing a very high refractive index and dispersion; its dispersion is six times that of diamond. After cutting and polishing, it shows extremely strong fire and is very beautiful, but it differs greatly from a diamond. Its biggest drawback is its low hardness, with a Mohs hardness of only about 6.5, making it unsuitable for jewelry. As a result, this material did not become an important diamond simulant. In today’s jewelry market, synthetic rutile simulants are now hard to find.
In 1953, flame-fusion synthesized strontium titanate, a material with both high refractive index and dispersion, which was introduced and called “Fabulite.” Its refractive index is 2.40 and its dispersion is 0.19, about four times that of diamond; after cutting, its appearance as a diamond simulant was closer to diamond than synthetic rutile. However, strontium titanate’s hardness remained too low, with a Mohs hardness of only about 5.5. It is now rarely seen on the market.
Yttrium aluminum garnet (YAG) appeared in the jewelry market in 1960 and was a common diamond simulant at the time. Its Mohs hardness is about 8, which is relatively high. Still, its refractive index is only 1.83 and its dispersion only 0.028, both of which are lower than diamond, so its brilliance and fire after cutting fall far short of diamond. Currently, it is relatively uncommon on the market.
Gadolinium gallium garnet (GGG) has a refractive index of 1.970 and a dispersion of 0.045, which is quite close to that of diamond. When faceted into a standard brilliant cut, it has an appearance similar to a diamond; its Mohs hardness is 6.5. However, GGG turns brown and develops snowflake-like white inclusions when exposed to ultraviolet light. This phenomenon can also be induced by the ultraviolet component of sunlight, which limits the use of this material for manufacturing diamond simulants.
Cubic Zirconia (CZ) has a refractive index of 2.15 and a dispersion of 0.060, close to diamond; it is also relatively hard, with a Mohs hardness of 8.5, and it polishes and cuts well. In 1976, the Soviet Union introduced colorless cubic zirconia to the market as a diamond simulant, and it quickly replaced other types of simulants to become the most popular diamond imitation. When cut into a standard brilliant, CZ’s brilliance and fire are similar to a diamond’s, making it one of the best diamond simulants. Simulants made from cubic zirconia are sometimes improperly called “Russian diamond” or “Soviet diamond.”
Synthetic silicon carbide (SiC), commercially known as moissanite, has a refractive index of 2.648~2.691 and a dispersion of 0.104, both higher than those of cubic zirconia. Its surface exhibits the same adamantine luster as diamond, and it is even harder, with a Mohs hardness of up to 9.25. When cut into a standard brilliant, its fire is stronger, and its appearance is closer to diamond than any previous simulant. In June 1998, the U.S. company C3 introduced it to the market as a new diamond simulant. Because synthetic silicon carbide’s various properties are closer to diamond than cubic zirconia— particularly its excellent thermal conductivity, which causes it to react like diamond on diamond thermal testers—synthetic silicon carbide (moissanite) is currently the best diamond simulant available on the jewelry market.
Although there are many varieties of diamond simulants, they still differ from diamonds in many ways. For an experienced jeweler or appraiser, it is not difficult to distinguish them under 10x magnification or with the aid of simple methods. Even when already set into jewelry, this is still not hard to do. However, accurately identifying the specific type of simulant material is not easy and requires further study.
(2) Identification of Diamond Simulants
In fact, each new simulant material shares certain properties that resemble aspects of diamonds. If one is unfamiliar with the properties and characteristics of simulant materials and lacks the skills to recognize them, it is easy to confuse them with diamonds. Identification is mainly based on their physical and optical properties. The characteristics of diamonds and simulant materials are shown in Table 3-3.
Table 3-3 Characteristics of Diamonds and Simulants
| Numele pietrei prețioase | Indicele de refracție | Birefringență | Gravitație specifică | Dispersie | Duritate | Other Characteristics | Observații |
|---|---|---|---|---|---|---|---|
| Diamant | 2.417 | Isotropic, exhibits anomalous birefringence | 3.52 | 0.044 | 10 | Adamantine luster, sharp facet edges and point intersections | |
| Synthetic Moissanite (SiC) | 2.648~2.691 | 0.043 | 3.22 | 0.104 | 9.25 | Obvious facet edge doubling, white thread-like inclusions, thermal conductivity close to diamond | |
| Zirconiu cubic (CZ) | 2.09~2.18 | Isotropic | 5.60~6.0 | 0.060 | 8.5 | Strong dispersion, gas bubbles or flux inclusions; fluoresces orange-yellow under Short Wave UV | High specific gravity |
| Barium Titanate | 2.409 | Isotropic | 5.13 | 0.190 | 5.5 | Extremely strong dispersion, low hardness, easily scratched, contains gas bubble inclusions | |
| Gadolinium Gallium Garnet (GGG) | 1.970 | Isotropic | 7.00~7.09 | 0.045 | 6.5~7 | Very high specific gravity, low hardness, occasionally contains bubbles | |
| Yttrium Aluminum | 1.833 | Isotropic | 4.50~4.60 | 0.028 | 8~8.5 | Weak dispersion, may contain bubbles | |
| Scheelite | 1.918 ~ 1.934 | 0.016 | 6.1 | 0.026 | 5 | High specific gravity, low hardness | |
| Zircon | 1.925 ~ 1.984 | 0.059 | 4.68 | 0.039 | 7.5 | Obvious facet edge doubling, worn facet edges, 653.5 nm absorption line | Obvious facet edge doubling can be observed. |
| Rutile sintetic | 2.616 ~ 2.903 | 0.287 | 4.6 | 0.330 | 6.5 | Extremely strong dispersion, relatively low hardness, very obvious birefringence, may contain gas bubble inclusions | |
| Colorless Sapphire | 1.762 ~ 1.770 | 0.008 ~ 0.010 | 4.00 | 0.018 | 9 | Birefringence not obvious | Refractive index and birefringence can be measured using a refractometer. |
| Spinel sintetic | 1.728 | Isotropic, exhibits anomalous birefringence | 3.64 | 0.020 | 8 | Irregularly shaped gas bubble inclusions; fluoresces blue-white under Short Wave UV | |
| Topaz | 1.610 ~ 1.620 | 0.008 ~ 0.010 | 3.53 | 0.014 | 8 | Weak dispersion, birefringence not obvious | |
| Sticlă | 1.50 ~ 1.70 | Isotropic, may exhibit anomalous birefringence | 2.30 ~ 4.50 | 0.031 | 5 ~ 6 | Gas bubble inclusions and swirl marks; low hardness, easily scratched; some types luminesce |
Because diamonds are extremely precious, it is very important to be familiar with the basic characteristics of diamonds and simulants and to carry out comprehensive analysis, comparison, and research. Although the basic characteristics and identification criteria of diamonds cannot be applied entirely to all gems similar to it and their imitations, there are always 1~2 items that play a leading role or have been proven in practice to be effective identification features. Therefore, by comparing different features of various imitations, one can always distinguish those imitations from diamonds.
The difference between diamonds and cubic zirconia (CZ) is that the latter has lower hardness, higher density, and much lower thermal conductivity than diamonds, so they are easily distinguished with a diamond thermal tester. For mounted stones, the breath test can separate them from diamonds: after breathing on cubic zirconia, the “fog” evaporates more slowly than on a diamond.
Diamonds are most similar to synthetic silicon carbide (moissanite); both can register on a diamond thermal tester, but synthetic silicon carbide (moissanite) has strong birefringence and can be differentiated from diamonds by observing double images and white linear inclusions.
Diamonds also share many similarities with zircon, but zircon is uniaxial and shows distinct double images, has lower dispersion than diamond, exhibits relatively weaker fire after cutting, lower hardness than diamond, and a pronounced “paper-etching effect.”
Both diamond and spinel belong to the isometric crystal system and are homogeneous, but they differ in that spinel has lower hardness, refractive index, and dispersion than diamond, producing noticeably weaker fire than diamond.
The difference between diamond and synthetic rutile is that rutile contains spherical gas bubble inclusions; its density, refractive index, and dispersion are all higher than diamond’s—especially its very high dispersion value—so after cutting, it shows much stronger fire than diamond.
The difference between diamond and strontium titanate is that under a loupe, strontium titanate lacks the brilliance of diamond, appears almost buttery in look, and shows spherical gas bubble inclusions; under UV light, strontium titanate shows no fluorescence, but its dispersion value is higher than diamond’s, producing strong fire after cutting. Its hardness is much lower than diamond’s, so after being worn for some time, the facet edges become rounded, and its density is greater than diamond’s.
Diamond and yttrium aluminum garnet (YAG) look similar, but YAG has lower hardness, lower refractive index, and lower dispersion than diamond, so its fire after cutting is relatively weak, and its density is much higher than diamond’s. The difference between diamond and gadolinium gallium garnet (GGG) is that GGG emits strong orange to orange-red fluorescence under short-wave UV light, and shows no fluorescence under long-wave UV. It also has lower hardness, higher density, and contains triangular tabular inclusions and tiny spherical gas bubble inclusions.