O que é o revestimento químico a ouro e como funciona?
Revestimento químico de ouro: Técnicas, soluções e aplicações para joalharia
Introdução:
O que é o revestimento químico de ouro? É um processo utilizado para depositar uma fina camada de ouro em vários materiais, melhorando o seu aspeto e durabilidade. Como é que funciona? O revestimento químico de ouro envolve a utilização de soluções especiais com iões de ouro e agentes redutores. Os métodos tradicionais utilizam frequentemente cianeto, mas também existem opções sem cianeto. Porquê escolher o revestimento químico de ouro? Oferece um acabamento uniforme e de alta qualidade, ideal para jóias e outros artigos decorativos. Quer seja um fabricante de jóias, designer ou retalhista, compreender estas técnicas pode elevar os seus produtos.

Índice
Secção I Cianeto Químico Douradura
1. Visão geral
Com a elevada densidade dos componentes electrónicos, a complexidade da conceção das linhas, a microfabricação dos circuitos e a independência das propriedades eléctricas, o revestimento químico de ouro, que resolve a complexidade do processo de revestimento, tornou-se uma forma inevitável de revestimento de ouro. No entanto, o revestimento químico de ouro tem as seguintes desvantagens:
① Velocidade lenta;
② Condições de utilização e gama de funcionamento limitadas, aumentando a dificuldade de gestão;
③ A superfície do material deve estar completamente limpa;
④ A vida útil da solução de revestimento é curta (propensa a reacções auto-redox);
⑤ A espessura do revestimento é muito sensível às condições de agitação.
Por conseguinte, o revestimento químico de ouro deve ser efectuado com equipamento especial. A composição da solução de revestimento químico de ouro normalmente utilizada é mostrada no Quadro 1-24, preparada pela combinação de vários sais de coordenação de ouro e agentes redutores.
Tabela 1-24 Tipos de sais de coordenação de ouro e agentes redutores na solução química de douramento
| Agente redutor | KAu(CN)2 | KAu(CN)4 | Na3Au(SO2)2 | HAuCl4 - 3H2O | HAuCl4 ・ 3H2O | AuCN | KAu(CN)4 | KAu ・ O2 | KAu(OH)4 | AuI | Não específico |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hipofosfito de sódio | 57,58 | 71 | 77 | ||||||||
| Formaldeído | 71 | 78 | |||||||||
| Biazida | 59 | 71 | 76 | 78 | |||||||
| Borohidreto | 60,61 | 72 | 78 | 79 | |||||||
| Metilborano | 60 | 69 | 74 | 75 | 79 | 80 | |||||
| Hidroxilamina | 62 | ||||||||||
| Não publicado | 63 | 73 | 81 | ||||||||
| Tioureia | 64 | ||||||||||
| Hidróxido de amónio | 65 | ||||||||||
| Tartarato de sódio e potássio tetra-hidratado (combinado) | 66 | 70 | |||||||||
| N,N-Dimetilamida | 82 | ||||||||||
| N,N-Dimetilglicina | 67 | ||||||||||
| Nenhum | 68 |
Quadro 1-25 Funções dos componentes da solução química de douramento
| Componente | Objetivo | Nome do composto |
|---|---|---|
| Ião de ouro | Fornecimento de ouro metálico | KAu(CN)2、KAu(CN)4、AUCN、 Na3Au(SO2)2. HAuCl4 - 3H2O、KAu ・ O2 |
| Agente redutor | Agentes redutores para a deposição de ouro | Tartarato de sódio e potássio tetra-hidratado, borohidreto, amina borano, formaldeído, hipofosfito, N,N-dimetilglicina, ácido ascórbico |
| Agentes quelantes orgânicos | Ação tampão para evitar a rápida deposição de ouro | EDTA, citrato de potássio, ácido tartárico |
| Estabilizador | Mascarar os núcleos activos para evitar a decomposição da solução de galvanização | Tioureia, cianeto metálico, acetilacetona |
| Ativador | O efeito de abrandamento do agente quelante moderador | Ácido succínico |
| Agente tampão | Manter um determinado pH | Fosfatos, citratos, tartaratos |
| Surfactante | Tornar as peças revestidas fáceis de molhar | Sulfonatos alifáticos, sulfatos de álcool |
2. Solução de hipofosfito para douradura
Quando o metal de base e o ouro na solução de revestimento sofrem uma reação de deslocamento, o metal com um potencial eletroquímico mais baixo ioniza-se mais facilmente do que o ouro. Durante a reação, uma vez que a superfície do metal de base está completamente coberta por ouro, a reação pára imediatamente, pelo que só se pode obter uma camada fina de revestimento de ouro de 0,1~0,3μm. Esta solução de revestimento, ou solução de Brookshire, é uma solução de revestimento antiga e constitui a composição de base das soluções químicas de revestimento de ouro atualmente comercializadas. Caraterísticas da solução de revestimento: a camada de base para o revestimento de ouro deve ser de níquel para a reação de deslocamento; o ouro não pode ser revestido por deslocamento em material de cobre.
(1) Composição e condições da solução química de revestimento de ouro com hipofosfito
O quadro 1-26 apresenta a composição típica da solução química de revestimento de ouro com hipofosfito.
Quadro 1-26 Composição e condições da solução química hipofosfito para douradura
| Composição e condições de funcionamento | Número de série | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Cianeto de potássio e ouro/(g/L) | 2 | 2 | 20 | 2 | 2 |
| Hipofosfito de sódio/(g/L) | 10 | 10 | 10 | 10 | 10 |
| Cianeto de potássio /(g/L) | 0.2 | 0.2 | 80 | 0.4 ① | 0.2 |
| Temperatura/°C | 96 | 96 | 96 | 96 | 96 |
| pH | 13.5 | 7.5 | 13.5 | 13.5 | 7.1 |
| Taxa de carga/(cm2/cm3) | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Taxa de galvanização/[mg/(cm2- h)] | 9.8 | 9.85 | 12.3 | 8.2 | 3.86 |
(2) O efeito do níquel na taxa de deposição da solução de revestimento de hipofosfito
Na solução química de revestimento de ouro com hipofosfito como agente redutor, os iões de metal ativo (Ni2+) são adicionados. O metal (níquel) e o ouro são depositados em quantidades vestigiais na superfície das peças revestidas, criando uma grande força motriz química e aumentando a velocidade de revestimento a ouro. O princípio é apresentado no diagrama da Figura 1-25.
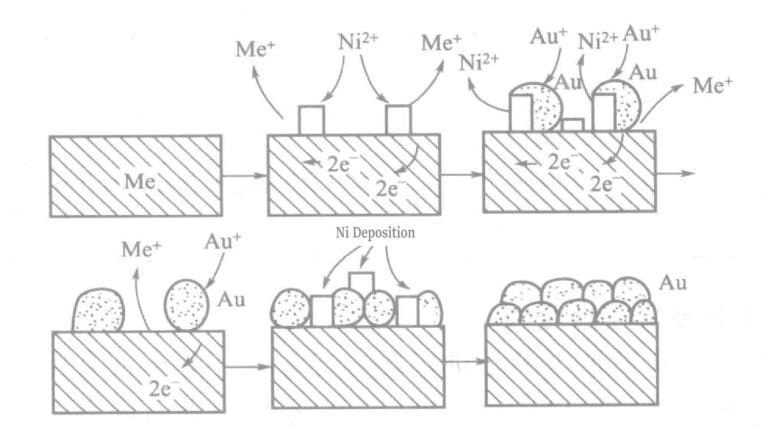
Quadro 1-27 Composição e condições de funcionamento da solução de revestimento de ouro com hipofosfito
| Composição e condições de funcionamento | I | II | Composição e condições de funcionamento | I | II |
|---|---|---|---|---|---|
| Cianeto de potássio e ouro/(g/L) | 2, 5, 7 | 2, 5, 7 | Cianeto de potássio /(g/L) | - | 2 |
| Citrato de sódio/(g/L) | 75 | 75 | H2NNH2 - H2O /(g/L) | - | 2 |
| Hipofosfito de sódio/(g/L) | 15 | 15 | pH | 8.5 | 4.3 |
| EDTA-2Na/(g/L) | - | - | Temperatura/℃ | 70, 90 | 70, 90 |
| Cloreto de sódio/(g/L) | - | 5 |
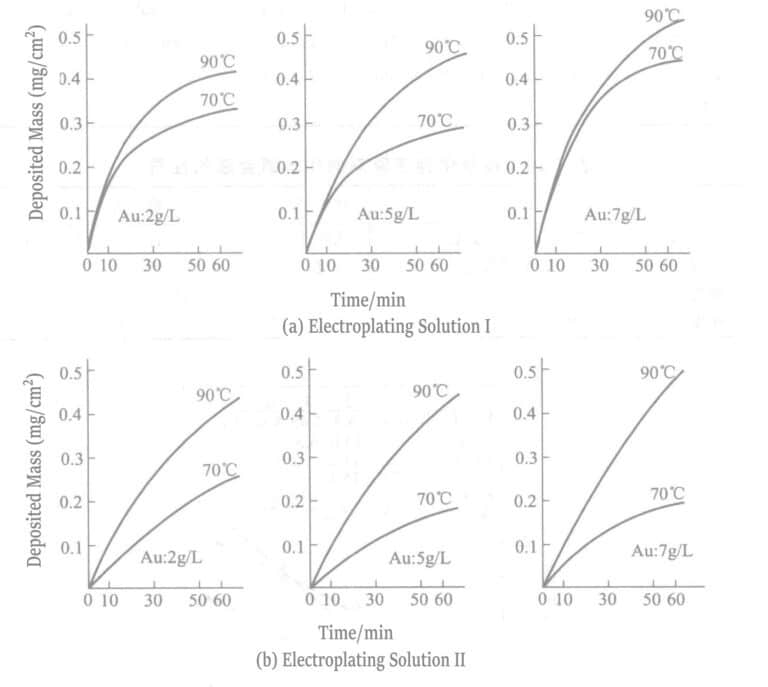
3. Solução de revestimento com sal de borohidreto
(1) Composição e Propriedades da Solução Química de Douradura de Borohidreto de Potássio
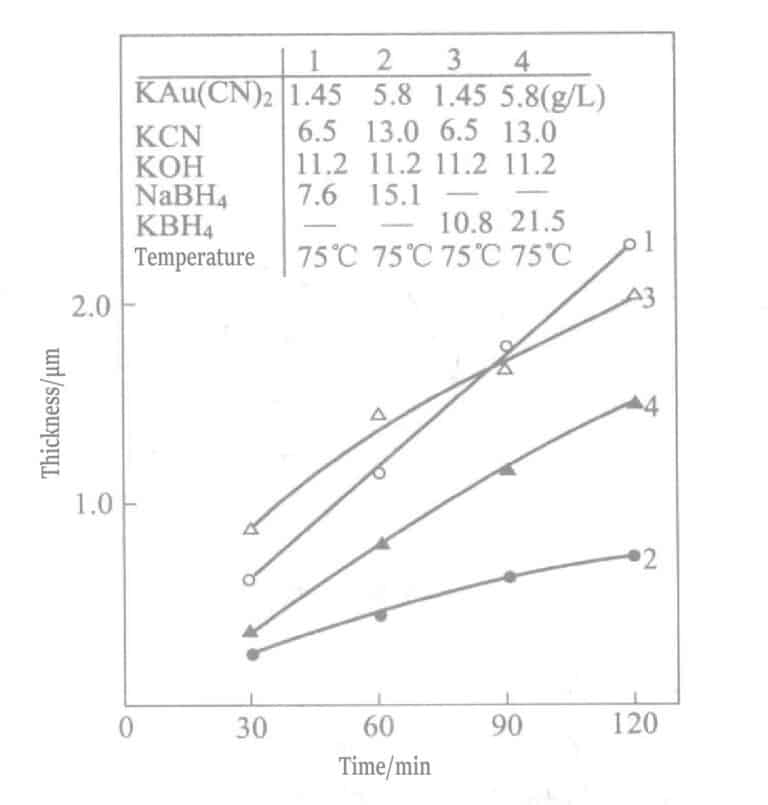
Em 1969, Okinaka et al. propuseram a utilização de uma solução de revestimento com compostos de borohidreto como agente redutor. A composição e as condições da solução química de revestimento de ouro com borohidreto são apresentadas nos Quadros 1-28, e as propriedades das camadas de ouro revestidas por estas soluções são apresentadas nos Quadros 1-29. A espessura das camadas de revestimento revestidas em tempos diferentes com várias soluções de revestimento é indicada na Figura 1-27. Os resultados resumidos dos agentes redutores borohidreto de potássio e borano dimetilamina são apresentados na Tabela 1-30.
Quadro 1-28 Composição e condições de funcionamento da solução química de revestimento a ouro com borohidreto de potássio
| Composição e funcionamento | Número de série | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Cianeto de potássio e ouro/(g/L) | 0. 02mol/L(5,8g/L) | 0. 03mol/L(8,6g/L) | 0. 005mol/L(1. 45g/L) |
| Cianeto de potássio | 0. 2mol/L(13,0g/L) | 0. 2mol/L(13,0g/L) | 0. lmol/L(6,5g/L) |
| Hidróxido de potássio | 0. 2mol/L(11,2g/L) | 0. 2mol/L(22,4g/L) | 0. 2mol/L(11,2g/L) |
| Borohidreto de potássio | 0. 4mol/L(21,6g/L) | 0. 8mol/L (43,1g/L) | 0. 2mol/L(10,8g/L) |
| Temperatura/°C | 75 | 75 | 75 |
Quadro 1-29 Propriedades da solução química de douradura com o agente redutor borohidreto de potássio
| Natureza | Dados | Natureza | Dados |
|---|---|---|---|
| Força de ligação | Excelente força de ligação da camada metálica | Porosidade | Sem poros com mais de 1μm na superfície uniforme |
| Aparência | Amarelo baço | Pureza | 99. 9% |
| Densidade | Teor de ouro 19. 3g/cm3 | Valor da resistência | 0. 03Ω/cm2 ( Au: 1 μm) |
| Dureza | Dureza Nup 60〜80 | Soldabilidade | Excelente |
Quadro 1-30 Funções dos componentes da solução química de revestimento a ouro com borohidreto de potássio
| Factores de influência | Solução de revestimento de borohidreto de potássio | Solução de revestimento DMAB (Dimetilaminoborano) |
|---|---|---|
| Efeito da concentração de ouro | Quando a concentração de ouro atinge 0,002mol/L, a taxa de precipitação aumenta acentuadamente e diminui acima disso. | A taxa de precipitação aumenta linearmente a partir de uma concentração de ouro de 0,01 mol/l, mas não tem qualquer efeito acima desse valor. |
| Efeitos do cianeto de potássio |
Solução de revestimento instável quando inferior a 0,005 mol/l. O ouro não precipita acima de 0,2 mol/l. |
Igual à esquerda |
| Efeito do borohidreto de potássio | O BH não é estável à temperatura ambiente. A adição de hidróxido de potássio melhora a estabilidade do banho, e a taxa de precipitação diminui com o aumento da concentração. | O DMAB é estável à temperatura ambiente, a taxa de precipitação aumenta com o aumento do hidróxido de potássio. |
| Concentração do agente redutor | Aumento da taxa de precipitação com o aumento da concentração | |
| Efeito da temperatura do banho de revestimento | Aumentar a taxa de precipitação em 10℃, e decompor a 85℃. |
(2) Influência da velocidade de revestimento e dos iões de metais pesados
A adição de quantidades vestigiais de iões de metais pesados (chumbo, tálio, etc.) à solução química de revestimento de ouro aumenta significativamente a taxa de deposição. Mcintyre explicou este efeito da seguinte forma. Devido ao baixo potencial de deposição, o efeito catalítico dos átomos de chumbo adsorvidos, como demonstrado nos mecanismos (1-4) e (1-5), promove a nucleação.
Pb2+ + 2e– → Pbanúncio (1-4)
2Au(CN)2– + Pbanúncio → 2Au + Pb2+ + 4CN– (1-5)
Devido à grande diferença nas funções de trabalho entre o cobre, os materiais de ouro e os iões de chumbo, o chumbo é reduzido e depositado a um potencial mais positivo do que o potencial redox termodinâmico, formando uma monocamada. Devido à reação catalítica dos átomos de chumbo adsorvidos, como em (1-4) e (1-5), a reação de redução do Au(CN) ocorre rapidamente, promovendo eficazmente a deposição de ouro.
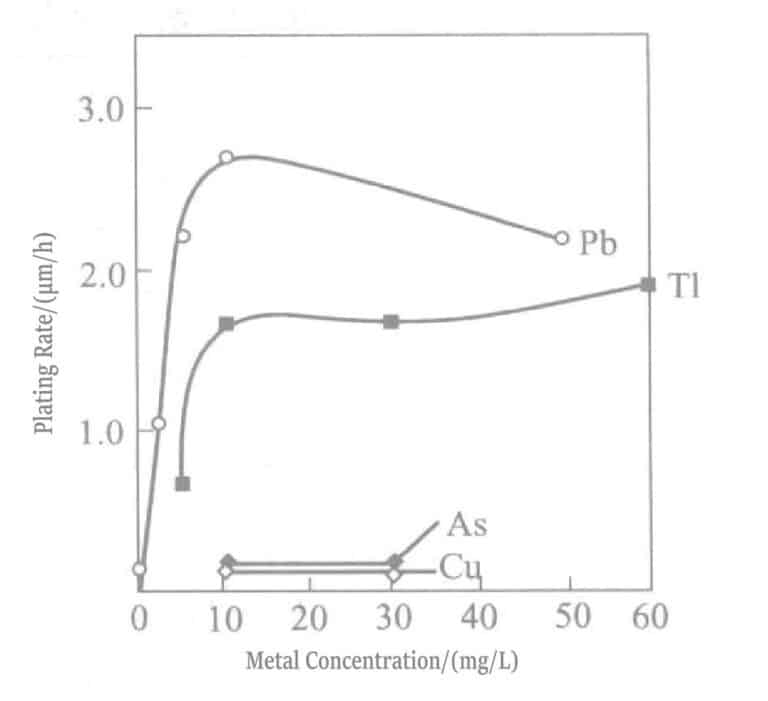
A relação entre a concentração de chumbo e a taxa de deposição numa solução de revestimento de ouro redutora de borohidreto de potássio é mostrada na Figura 1-28, e a composição da solução de revestimento é mostrada na Tabela 1-31. O chumbo é adicionado sob a forma de nitrato de chumbo. A Figura 1-28 mostra que a concentração de adição de chumbo na solução de revestimento só pode ser de cerca de 20 ml/L, e a adição de mais não tem qualquer efeito.
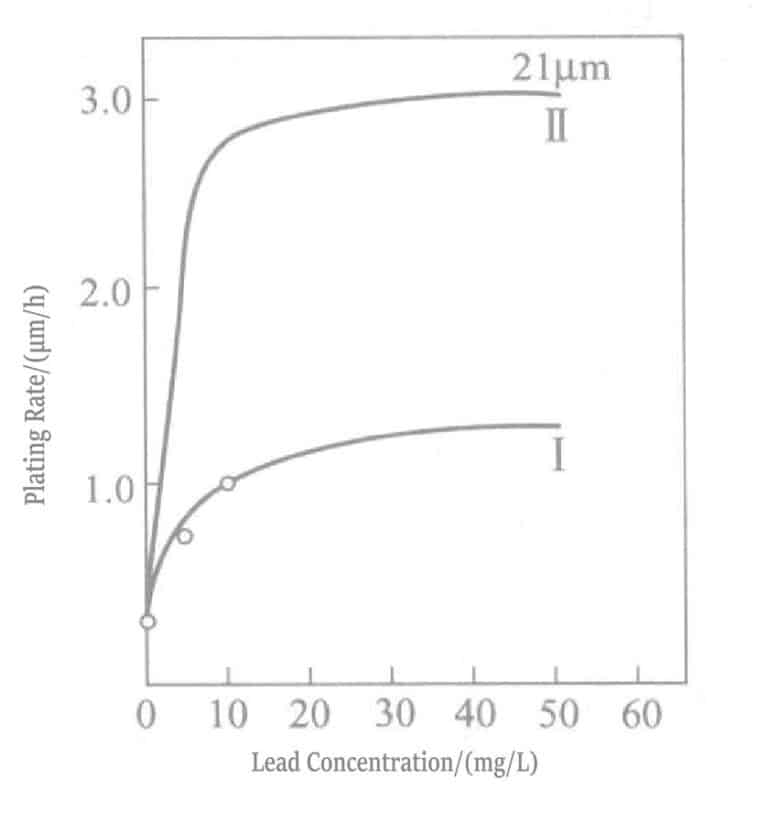
Quadro 1-31 Composição e condições de funcionamento da solução de revestimento de ouro com borohidreto de potássio
| Composição e condições de funcionamento | I | II | Composição e condições de funcionamento | I | II |
|---|---|---|---|---|---|
| Cianeto de potássio e ouro/(g/L) | 2 | 2 | Cianeto de potássio /(g/L) | 10 | 10 |
| Borohidreto de potássio/(g/L) | 2 | 10 | EDTA-2Na/(g/L) | 5 | 5 |
| Cianeto de potássio/(mg/L) | 5 | 5 | Temperatura da solução de galvanização/°C | 75 | 75 |
Tabela 1-32 Composição da solução de revestimento de ouro BMAB
| Composição e condições de funcionamento | Concentração | Composição e condições de funcionamento | Concentração |
|---|---|---|---|
| Cianeto de potássio e ouro/(g/L) | 2 | Cianeto de potássio /(g/L) | 30 |
| BMAB/(g/L) | 10 | EDTA-2Na/(g/L) | 5 |
| Cianeto de potássio/(mg/L) | 5 | рH | 13.6 |
Quadro 1-33 Composição da solução química de douradura com cianeto de ouro (III) de potássio
| Composição | Parâmetros | Composição | Parâmetros |
|---|---|---|---|
| KAu(CN)4/(g/L) | 4 | KOH/(mg/L) | 11.2 |
| KBH4/(g/L) | 3 | Temperatura/℃ | 70 |
| PbCl2/(mg/L) | 0.5 |
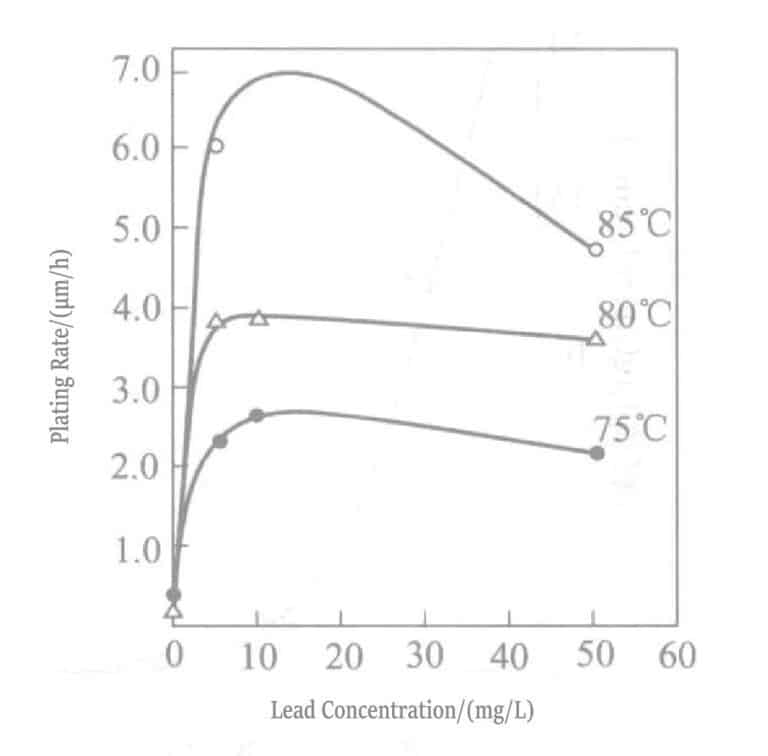
Figura 1-30 Efeito da temperatura e da concentração de chumbo na taxa de precipitação do ouro
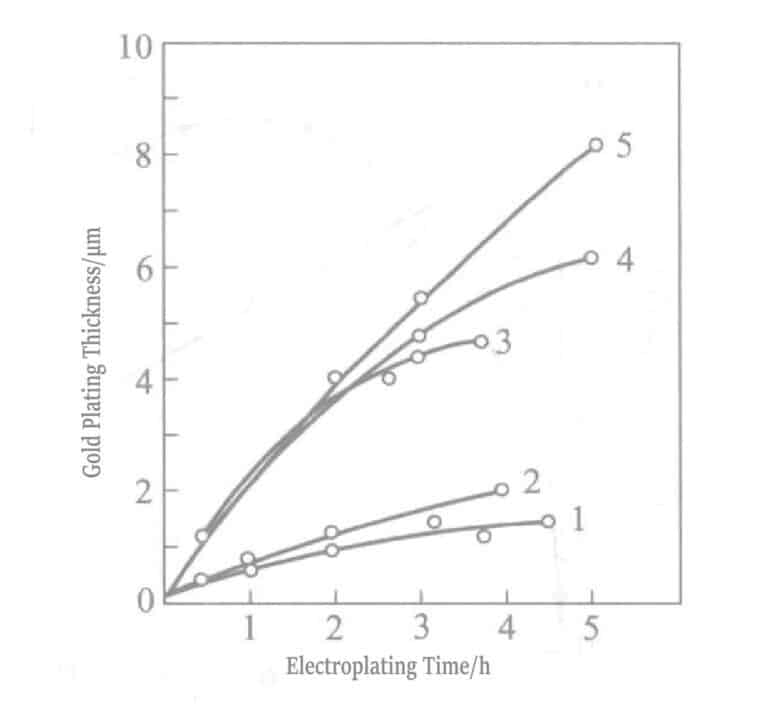
Figura 1-31 Relação entre o tempo de revestimento e a espessura da camada revestida a ouro
1 - Sem agitação Au+ líquido; 2 - Sem agitação Au3+ líquido; 3 - Agitação do Au3+ líquido; 4 - Reabastecimento de Au3+ líquido; e 5 - Renovação de Au3+ líquido
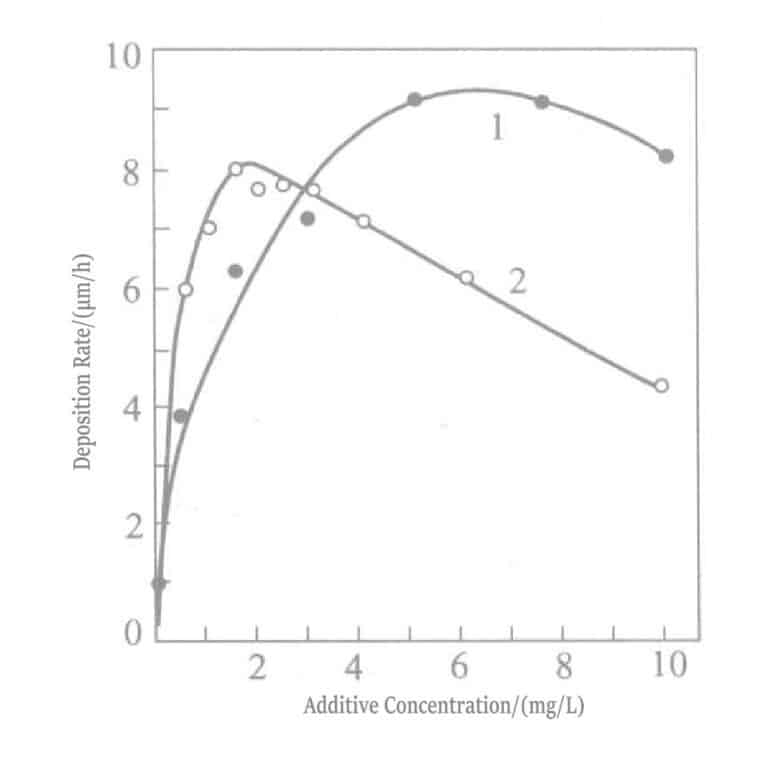
Figura 1-32 Efeito das concentrações adicionadas de chumbo e tálio na taxa de revestimento de ouro
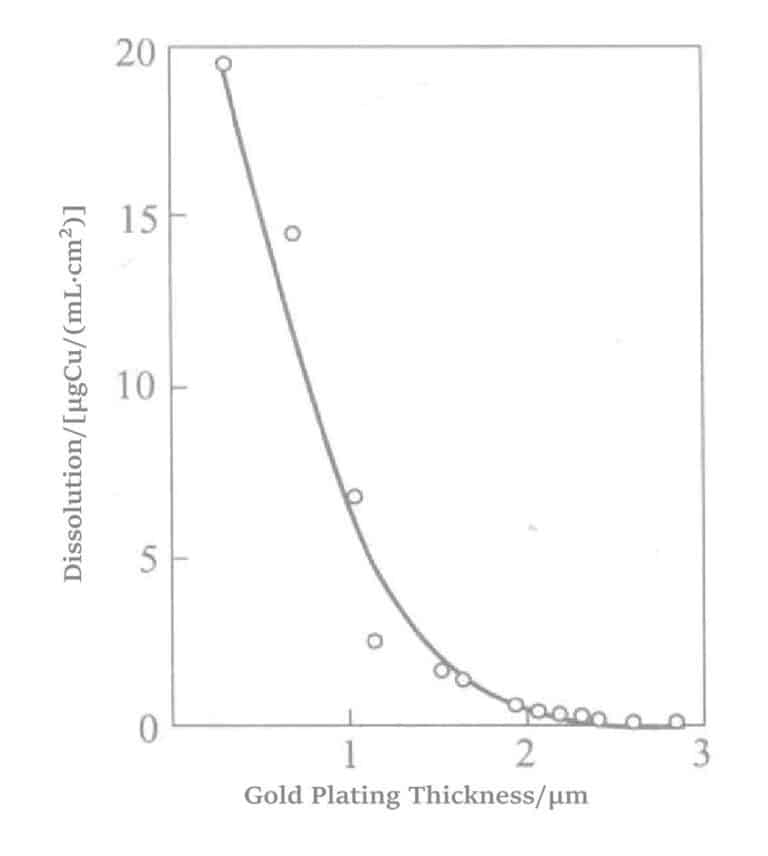
Figura 1-33 Porosidade da camada de revestimento de ouro eletrolítico
O processo químico de revestimento a ouro do vidro cristalizado (SiO2 46% + Al2O3 16% + MgO 17% + K2O 10% + F 4% + B2O3 7% ) é apresentado na Figura 1-34.
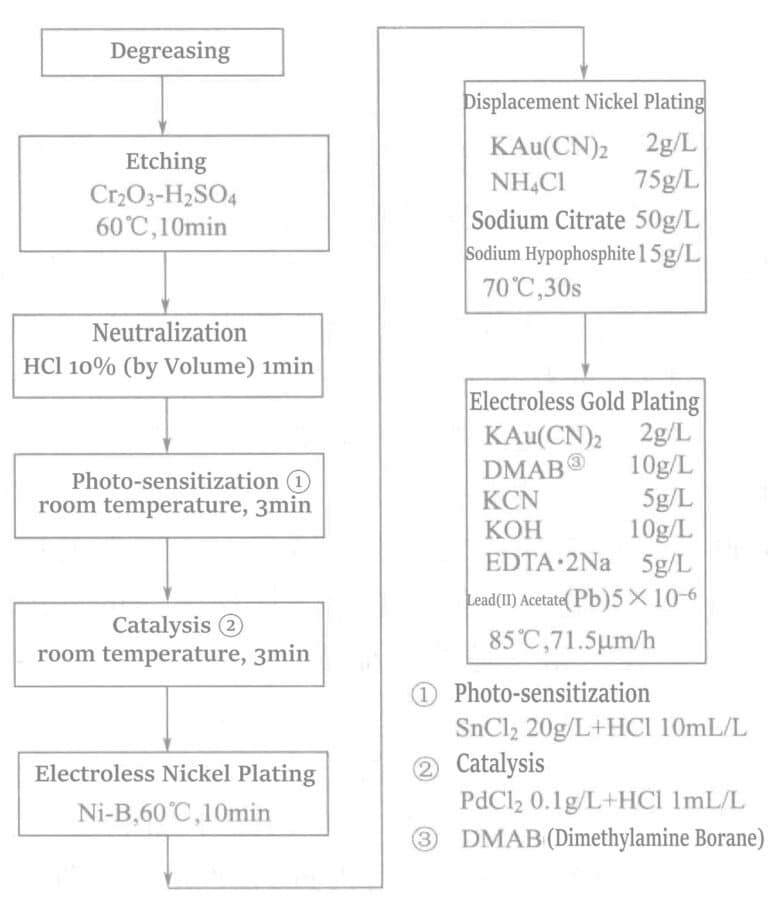
4. Mecanismo de reação da solução química de revestimento de ouro com sal de borohidreto
Lin Zhongfu et al. propuseram que as soluções de revestimento de ouro com borohidreto de potássio e DMAB (dimetilamina borano) do quadro 1-34 podem tornar-se soluções práticas de revestimento de ouro após a adição de estabilizadores.
(1) Composição de duas soluções de galvanização: A Tabela 1-34 mostra as duas composições.
Quadro 1-34 Soluções de revestimento químico de borohidreto de potássio e DMAB
| Composição da solução de galvanização | Solução de revestimento de borohidreto de potássio | Solução de revestimento DMAB |
|---|---|---|
| Cianeto de ouro e potássio/(mol/L) | 0.02 | 0.02 |
| Cianeto de potássio/(mol/L) | 0.2 | 0.02 |
| Hidróxido de potássio/(mol/L) | 0.2 | 0.8 |
| Borohidreto de potássio/(mol/L) | 0.4 | - |
| DMAB/(mol/L) | - | 0.4 |
| Temperatura/°C | 75 | 85 |
| Velocidade de revestimento/(μm/h) | 0.7 | 0.4 |
(2) Mecanismo de reação: Na solução de revestimento de ouro com borohidreto de potássio, BH4– por si só não produz um efeito redutor, mas o intermediário BH3OH– do seu produto de hidrólise BO2– actua como agente redutor.
BH4– + H2O → BH3OH–+ H2 (1-6)
BH3OH– + H2O → BO2– + 3H2 (1-7)
BH3OH– + 3OH– → BO2– + 3/2 H2 + 2H2O + 3e– (1-8)
Au(CN)2– + e– → Au + 2CN– (1-9)
BH3OH– + 3Au(CN)2– + 3OH– → BO2– + 3/2 H2 + 2H2O + 3Au + 6CN – (1-10)
As reacções de hidrólise das equações (1-6) e (1-7) foram analisadas por polarografia. O resultado mostra que a reação (1-7) é 500 vezes mais rápida do que a reação (1-6). Por conseguinte, a taxa de utilização do borohidreto de potássio na reação de revestimento de ouro é muito baixa. Em condições típicas de revestimento, a taxa de utilização efectiva não excede 2%~3%, permanecendo a maior parte do agente redutor ineficaz devido à hidrólise.
A reação de hidrólise acima referida processa-se rapidamente a pH baixo (reação catalisada por ácido) e a taxa de deposição de ouro é rápida quando a concentração de hidróxido de potássio é baixa. Para evitar a decomposição natural da solução de revestimento de ouro, a concentração de hidróxido de potássio deve ser mantida pelo menos acima de 0,1mol/L. A relação entre a taxa de deposição de ouro e a concentração de hidróxido de potássio é mostrada na Figura 1-35.
![Figura 1-35 Curva de variação da taxa de metalização com a concentração de hidróxido de potássio [KAu(CN)2 0,02 mol/L, KCN (linha sólida) 0,2 mol/L, KCN (linha tracejada) 0,1 mol/L, 80 °C]](https://sobling.jewelry/wp-content/uploads/2025/11/Page_58_docsmall.com_副本-768x801.jpg)
Figura 1-35 Curva de variação da velocidade de revestimento com a concentração de hidróxido de potássio
[KAu(CN)2 0,02 mol/L, KCN (linha sólida) 0,2 mol/L, KCN (linha tracejada) 0,1 mol/L, 80 °C]
![Figura 1-36 Efeito da concentração de hidróxido de potássio na taxa de revestimento da solução de revestimento DMAB, DMAB [KAu (CN) 2 0.02mol / L, KCN 0.2mol / L, DMAB 0.4mol / L, 75 ℃]](https://sobling.jewelry/wp-content/uploads/2025/11/Page_58_docsmall.com_副本1-768x560.jpg)
Figura 1-36 Efeito da concentração de hidróxido de potássio na velocidade de revestimento da solução de revestimento DMAB, DMAB
[KAu(CN)2 0,02mol/L, KCN 0,2mol/L, DMAB 0,4mol/L, 75℃]
(CH3)2NH2 - BH3 + OH– → (CH3)2NH2 + BH3OH– (1-11)
(3) Problemas com a solução de galvanoplastia de borohidreto de potássio e com a solução de galvanoplastia de DMAB
A Figura 1-37 mostra que a velocidade de revestimento varia com a concentração de iões de cobre e de níquel na solução de revestimento. Quando a concentração de iões de níquel é de 10-5mol/L, a taxa de revestimento começa a diminuir e quando a concentração de iões de níquel atinge 10-3mol/L, a solução de galvanização decompõe-se. Os iões de cobre não têm praticamente qualquer efeito sobre a velocidade de revestimento. Na fase inicial do revestimento de ouro, os iões de ouro e o níquel sofrem uma reação de deslocamento, dissolvem-se vestígios de iões de níquel e a taxa de revestimento abranda.

Figura 1-37 Efeito dos iões de cobre e dos iões de níquel na taxa de revestimento
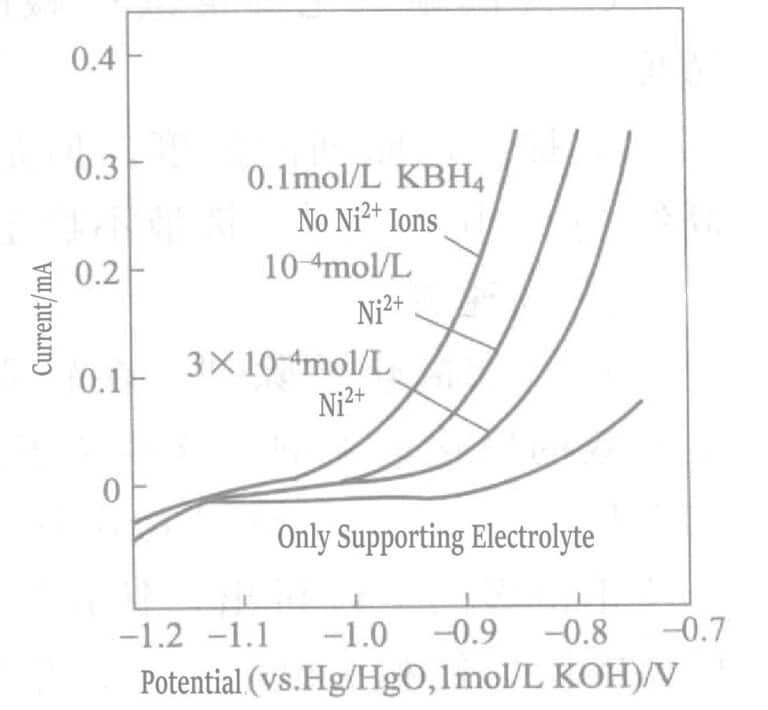
Figura 1-38 Efeito de vestígios de iões de níquel na corrente de oxidação anódica de BH3OH
(Elétrodo rotativo de ouro, 2000r/min, 75℃, KOH 0,2mol/L, KCN 0,1mol/L)
O efeito da adição de quantidades vestigiais de iões de níquel no BH3OH– A corrente de oxidação anódica é mostrada na Figura 1-38. A adição de vestígios de iões de níquel inibe significativamente a reação de oxidação do agente redutor BH3OH– no ânodo do disco rotativo de ouro. Isto deve-se ao facto de os iões de níquel existirem sob a forma de sal ligante de cianeto Ni(CN)42- e este ião complexo adsorve-se preferencialmente na superfície do ouro, dificultando a deposição do ouro.
As camadas de base instáveis (como o nitreto de alumínio) não podem utilizar soluções fortemente alcalinas. O pH da solução de revestimento depende da natureza do agente redutor, o que constitui o maior inconveniente das soluções de revestimento de borohidreto de potássio e DMAB.
Relativamente às questões acima referidas, exceto que o pH da solução de revestimento não pode ser alterado, outros aspectos podem ser melhorados.
(4) Melhorar a solução de revestimento de ouro com borohidreto de potássio e a solução de revestimento de ouro com DMAB
① Aumentar a velocidade de revestimento
- a. Alterar a composição básica da solução de revestimento e as condições de funcionamento para aumentar a velocidade de revestimento.
- b. Aumentar a velocidade de agitação.
- c. Aumentar a temperatura da solução de revestimento.
- d. Reduzir a concentração de cianeto livre.
- e. Diminuir a concentração de álcalis na solução de revestimento de borohidreto de potássio e aumentar a concentração de álcalis na solução de revestimento de DMAB.
- f. Aumentar a concentração do agente redutor. No entanto, quando a taxa de revestimento sem aditivos especiais aumenta em cerca de 2μm/h ou mais, a solução de revestimento torna-se instável e propensa à decomposição.
② Solução de galvanização de alta velocidade
- a. Adicionar eliminadores de polarização. Em soluções de revestimento de ouro macio, o Pb2+ , T1+ são eliminadores eficazes da polarização. Estes iões adsorvem-se fortemente na superfície do ouro, produzindo o chamado fenómeno UPD (under potential deposition), em que o chumbo se deposita a um potencial muito mais positivo do que o Pb2+ / Pb e o tálio deposita-se a um potencial mais positivo do que o potencial de equilíbrio T1+ /T1. Os depósitos de chumbo, tálio e Au(CN)2– sofre reacções de deslocamento, fazendo com que a reação de deposição de ouro se desloque para um potencial mais positivo (enfraquecimento da polarização). Por outro lado, a adição destes iões a soluções químicas de revestimento de ouro, semelhante ao revestimento eletrolítico, desloca o Au(CN)2– na direção positiva, resultando num aumento da taxa de revestimento, que pode atingir cerca de 10μm/h. As soluções de galvanização com adição de Pb2+ , T1+ são apresentados nos quadros 1-35 e 1-36.
Quadro 1-35 Solução de revestimento de borohidreto de potássio com adição de Pb2+
| Solução 1 | Solução 1 | ||
|---|---|---|---|
| Cianeto de potássio para ouro | 5g/L | Coloidal | 2g/L |
| Cianeto de potássio | 8g/L | Solução 2 | |
| Citrato de sódio | 50g/L | Borohidreto de potássio | 200g/L |
| EDTA | 5g/L | Hidróxido de sódio | 120g/L |
| Cloreto de chumbo | 0,5g/L | ||
Tabela 1-36 Solução de galvanização de borohidreto de potássio com adição de T1+
| Composição e condições de funcionamento | Parâmetros | Composição e condições de funcionamento | Parâmetros |
|---|---|---|---|
| Cianeto de potássio para ouro | Ajustar conforme necessário | Borohidreto de potássio | 5. 4 ~ 10. 8g/L |
| Cianeto de potássio | 6,5 g/L | Temperatura | 70 〜 80℃ |
| Hidróxido de potássio | 11,2g/L | Taxa de revestimento | <10μm/h |
| Sulfato de tálio | 5 〜 100mg/L |
Au(CN)4– + 2e– → Au(CN)2– + 2CN– (1-12)
![Figura 1-39 Efeito da adição de cloreto de chumbo na curva de polarização da reação de redução do Au(CN)2- do [elétrodo rotativo de ouro, KAu(CN)2 0. 009mol/L, KOH 0. 2mol/L]](https://sobling.jewelry/wp-content/uploads/2025/11/Page_61_docsmall.com_副本-768x578.jpg)
Figura 1-39 Efeito da adição de cloreto de chumbo na formação de Au(CN)2--curva de polarização da reação de redução [Elétrodo de ouro rotativo, KAu(CN)2 0. 009mol/L, KOH 0. 2mol/L]
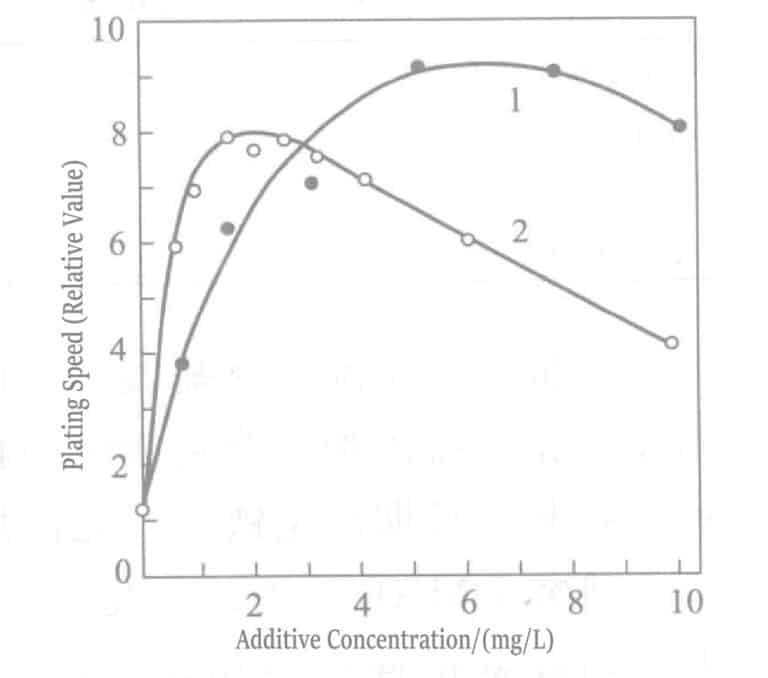
Figura 1-40 Efeito da concentração de cloreto de tálio (curva 1) e de cloreto de chumbo (curva 2) na taxa de douramento da solução de revestimento de borohidreto de potássio
- b. Adição de estabilizadores. A adição de estabilizadores orgânicos que reduzem a atividade catalítica do ouro, o aumento da temperatura da solução de galvanização e a alteração da concentração do agente redutor podem melhorar a velocidade de galvanização da solução. Por exemplo, a velocidade de revestimento de soluções de revestimento contendo ácido aminoacético, ácido hidroxietil etilenodiaminotetracético e ácido diazotriacético ou derivados do ácido succínico pode atingir uma alta taxa de 12 ~ 23μm / h a 90 ℃. No entanto, em altas temperaturas, a reação de hidrólise do agente redutor acelera significativamente, tornando a solução de revestimento difícil de controlar. A adição de quantidades vestigiais de estabilizador de p-dimetilamino azobenzeno não afeta a estabilidade da solução de revestimento e pode aumentar a concentração de uso usual de BH4- em 2 vezes, aumentando a velocidade de revestimento para 5μm / h. Outros estabilizadores eficazes incluem etileno-glicol. Éter etílico, éter etílico de dietilenoglicol e polietilenoimina.
- c. Utilizar sais complexos de cianeto de ouro trivalente. As soluções de galvanização com sais complexos de cianeto de ouro trivalente, tais como as que contêm cloreto de chumbo ou cloreto de titânio, como indicado no Quadro 1-33, são descritas no Quadro 1-37.
Quadro 1-37 Solução de galvanização com cianeto de ouro(III) e borohidreto de potássio
| Composição e condições de funcionamento | Parâmetros | Composição e condições de funcionamento | Parâmetros |
|---|---|---|---|
| Ouro(III) potássico | 3g/L | Cloreto de chumbo | 0. 5mg/L |
| Hidróxido de potássio | 11,2g/L | Temperatura | 70℃ |
| Borohidreto de potássio | 3g/L |
A vantagem da solução de revestimento de sal complexo de cianeto de ouro trivalente é que o KAuO2 e KAu(OH)4 podem ser utilizados como aditivos suplementares de sal de ouro, evitando a acumulação excessiva de iões de cianeto livres na solução de revestimento, reduzindo a taxa de revestimento e favorecendo uma taxa de revestimento estável a longo prazo. A taxa de revestimento da solução de revestimento sem aditivos é de 2~8μm/h.
O estudo do comportamento eletroquímico, da estrutura e das propriedades da solução de revestimento com DMAB mostra que, à semelhança da solução de revestimento com borohidreto de potássio, KAu(CN)2 é utilizado apenas na preparação da solução de revestimento de ouro, e o hidróxido de ouro dissolvido na solução de hidróxido de potássio é adicionado como suplemento para evitar a acumulação de iões de cianeto livres. A composição da solução de revestimento é indicada no Quadro 1-38.
Quadro 1-38 Solução de revestimento com DMAB (hidróxido de ouro dissolvido numa solução de hidróxido de potássio)
| Composição e condições de funcionamento | Parâmetros | Composição e condições de funcionamento | Parâmetros |
|---|---|---|---|
| Cianeto de potássio para ouro | 0,013 ~ 0,018mol/L | Aditivo | Pequena quantidade |
| DMAB | 0,07 〜 0,1 mol/L | pH | 13.1 ~ 13.4 |
| Hidróxido de potássio | 0,09 〜 0,15mol/L | Temperatura | 70 〜 75℃ |
| Cianeto de potássio | 0,007 ~ 0,01mol/L | Taxa de revestimento | 2μm/h |
(5) Melhorar a estabilidade da solução de revestimento.
A utilização de aditivos pode aumentar a taxa de revestimento e a estabilidade da solução de revestimento, mas as impurezas produzidas causarão a decomposição da solução de revestimento.
Substâncias como o EDTA e a etanolamina adicionadas à solução de revestimento formam iões complexos fortes com iões de impurezas metálicas, inibindo a reação entre as impurezas metálicas e o BH4– e BH3OH–melhorando assim a estabilidade da solução de revestimento. A composição da solução de revestimento de borohidreto de potássio com adição de EDTA e etanolamina é mostrada no Quadro 1-39.
Quadro 1-39 Solução de galvanoplastia de borohidreto de potássio com adição de EDTA e etanolamina
| Composição e condições de funcionamento | Parâmetros | Composição e condições de funcionamento | Parâmetros |
|---|---|---|---|
| Cianeto de potássio para ouro | 1,45g/L | EDTA | 5g/L |
| Cianeto de potássio | 11 g/L | Etanolamina | 50mL/L |
| Hidróxido de potássio | 11,2g/L | Temperatura | 72℃ |
| Borohidreto de potássio | 10,8g/L | Taxa de revestimento | 1. 5μm/h |
Quadro 1-40 Solução de galvanização com dois agentes redutores (hidrazina e DMAB)
| Composição e condições de funcionamento | Parâmetros | Composição e condições de funcionamento | Parâmetros |
|---|---|---|---|
| Cianeto de potássio para ouro | 0. 005mol/L | DMAB | 0. 05mol/L |
| Cianeto de potássio | 0. 035mol/L | Hidrazina | 0. 25mol/L |
| Hidróxido de potássio | 0. 8mol/L | Temperatura | 80℃ |
| Carbonato de potássio | 0. 45mol/L | Taxa de revestimento na camada de base de níquel (fase inicial) | 2. 6μm/h |
| Acetato de chumbo | 15X10-6 | Sobre o ouro (valor fixo) | 7. 8μm/h |
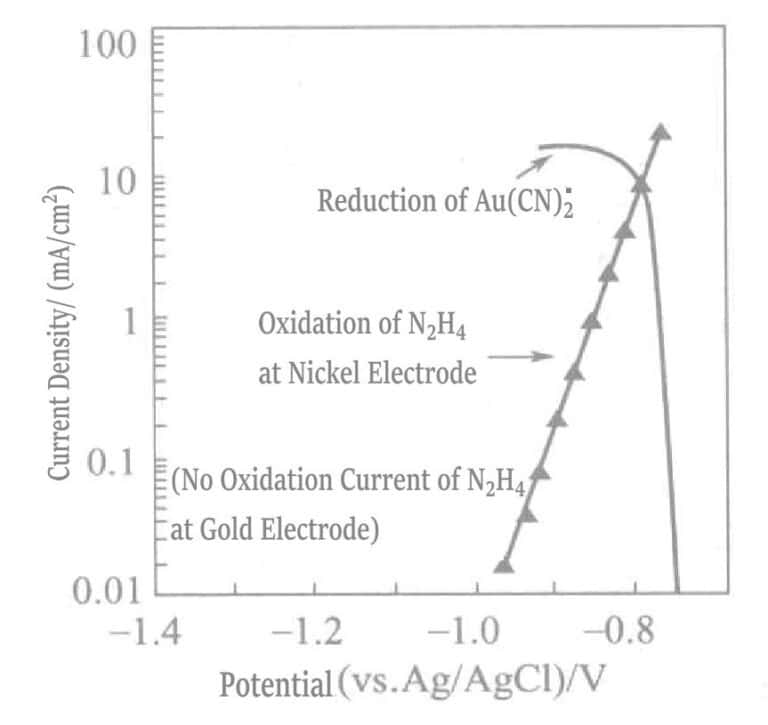
Figura 1-41 Curvas de polarização da reação de oxidação anódica da hidrazina no elétrodo de níquel e da reação de redução catódica do Au(CN)2-.
(KOH 0. 8mol/L, KCN 0. 035mol/L, N2H4 0. 05mol/L, 80℃)
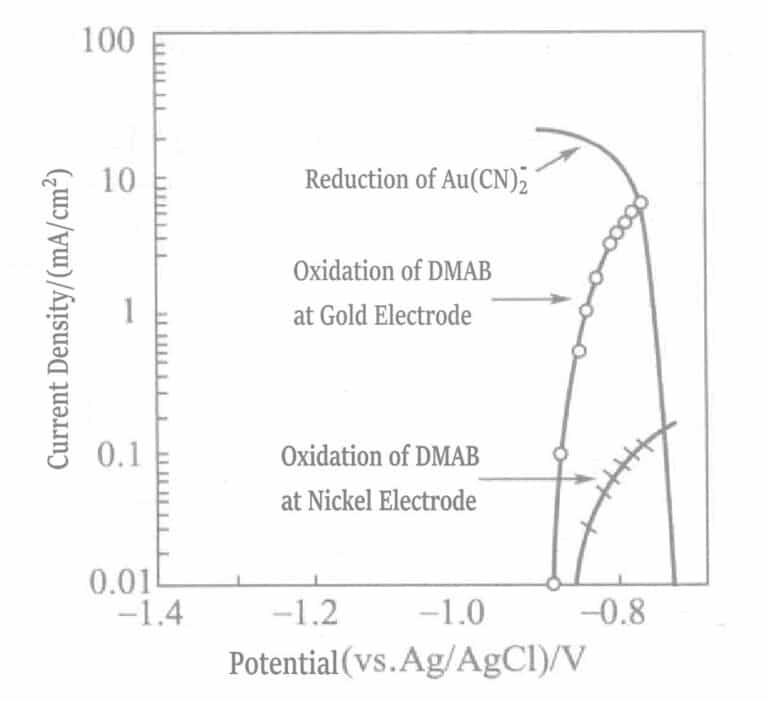
Figura 1-42 Curvas de polarização da reação de oxidação anódica do DMAB em eléctrodos de níquel e ouro e da reação de redução catódica do Au(CN)2-
(KOH 0. 8mol/L, KCN 0. 035mol/L, DMAB 0. 05mol/L, 80℃)
Numa solução de revestimento que contenha apenas o agente redutor hidrazina, o ouro pode ser depositado apenas com base no efeito catalítico da camada de base de níquel. Quando a camada de base está completamente coberta por ouro, a deposição de ouro pára e, neste ponto, a espessura da camada de revestimento de ouro depende da concentração de cianeto livre na solução de revestimento. Embora a espessura máxima de revestimento desta solução de revestimento seja limitada (cerca de 2μm), a camada de revestimento é suficientemente densa para cumprir os requisitos da ligação por pressão chave.
Para além da hidrazina, existem muitos outros agentes redutores disponíveis na solução de revestimento de ouro catalítico de materiais; ver Quadro 1-41.
Tabela 1-41 Outras soluções de galvanização de agentes redutores
| Agente redutor | pH | Temperatura/℃ |
|---|---|---|
| Hipofosfito | 7 〜 7. 5 | 93 |
| 7. 5 〜 13. 5 | 96 | |
| 3 〜 4 | 70 〜 80 | |
| Hidrazina | 7 〜 7. 5 | 92 〜 95 |
| 5. 8 〜 5. 9 | 95 | |
| 11. 7 | 95 | |
| Hidroxilamina | 2. 3 〜 2. 8 | 70 〜 85 |
| Cianoborohidreto de sódio (NaBH3CN) | 3.5 | 90 |
| Hidrazinilborano (N2H4 ・ BH3) | >13 | 60 |
| Tioureia | 6. 5 〜 7. 0 | 83 〜 90 |
| 4.2 | 80 ~ 85 | |
| Ácido ascórbico | 8 | 63 |
| Tricloreto de titânio | 5 | 75 |
Copywrite @ Sobling.Jewelry - Fabricante de jóias personalizadas, fábrica de jóias OEM e ODM
5. Revestimento químico de ligas de ouro
(1) Liga de ouro e prata:
O revestimento químico de ligas de ouro e prata pode ser obtido através da adição contínua de uma solução de cianeto de prata e potássio à solução de revestimento de ouro com borohidreto. Ag(CN)2– é mais facilmente reduzido do que o Au(CN)2–. Ao preparar uma nova solução de revestimento, a prata precipitará primeiro se as duas forem misturadas, e a liga não pode ser revestida.
Adicionando continuamente pequenas quantidades de cianeto de prata e potássio gota a gota à solução de revestimento, mantendo uma condensação relativamente baixa [razão molar de Au:Ag (26:10~(26:0.5)] Pode ser precipitada uma camada de revestimento de liga de ouro-prata com um teor de prata de 80%~20%. Nesta solução de revestimento, a precipitação de prata é um produto de reação de substituição do ouro que precipita primeiro e do Ag(CN)2–, em solução.
(2) Liga de ouro-cobre:
Na solução convencional de revestimento químico de cobre com EDTA baseado em coordenação e agente redutor de formaldeído, apenas o cianeto de ouro e potássio pode revestir uma liga de ouro e cobre. Ao alterar a concentração de cianeto de ouro e potássio, a fração de massa de cobre na camada de revestimento de liga de ouro pode ser ajustada de 1%~94%.
(3) Liga de estanho-ouro:
A solução de revestimento químico de ligas de ouro e estanho utiliza o SnCl2 método do agente redutor. A composição e a gama de concentrações da solução de revestimento são indicadas no Quadro 1-42.
Quadro 1-42 Solução de revestimento de ligas de estanho-ouro
| Composição e condições de funcionamento | Parâmetros | Composição e condições de funcionamento | Parâmetros |
|---|---|---|---|
| Cianeto de potássio de ouro(III) | 4〜10g/L | Triolefina | 0. 05 ~ 0. 5g/L |
| Cianeto de potássio | 5〜15g/L | Temperatura | Temperatura ambiente |
| Hidróxido de potássio | 60〜100g/L | Taxa de revestimento | 1,5μm/h (sem adição de triolefina) |
| Cloreto estanoso | 40〜60g/L | 5μm/h (adição de triolefina 0,5g/L) |
(4) Liga de ouro e índio:
A solução de revestimento da liga de ouro e índio é preparada adicionando Ih2(SO4)3 e EDTA numa solução de borohidreto. O conteúdo de índio na camada de revestimento é 1% ~ 4%. À temperatura ambiente, uma fina camada de revestimento de liga de ouro-índio depositada em n-GaAs ativado por paládio, após tratamento térmico de 350 ℃, tem uma resistência de contato superior ao ouro puro.
(5) Ligas de ouro-níquel e ouro-cobalto:
Uemaki desenvolveu soluções de galvanoplastia de ligas de ouro-níquel e ouro-cobalto de 18~22K. pH 3~5, a camada de galvanoplastia de liga de ouro da solução de galvanoplastia química de cianeto com adição de hipofosfito de sódio como agente redutor tem propriedades físicas, tais como dureza e resistência ao desgaste, inferiores às das camadas de galvanoplastia de liga de ouro contendo níquel e cobalto.
Secção II Douradura química sem cianetos
1. Visão geral
O revestimento de ouro do tipo deslocamento utiliza a diferença de potencial entre o potencial de oxidação do metal da camada de base e o potencial de redução dos iões de ouro como força motriz da reação de revestimento. Em contrapartida, o revestimento químico de ouro do tipo redução utiliza a diferença de potencial entre o potencial de oxidação do agente redutor e o potencial de redução dos iões de ouro como força motriz da reação de revestimento.
Os electrões envolvidos na reação de redução dos iões de ouro são fornecidos quer pela reação de oxidação do metal da camada de base (revestimento de ouro por deslocamento) quer pela reação de oxidação do agente redutor (revestimento de ouro por redução).
Outros métodos incluem: ① Adição de sais de metais pesados à solução de revestimento de ouro do tipo deslocamento, onde a superfície metálica da camada de base adsorve os metais pesados, deslocando o potencial na direção que torna os iões de ouro mais fáceis de reduzir; ② Adição de um agente redutor à solução de revestimento de ouro do tipo deslocamento, permitindo que as reacções redox ocorram simultaneamente para revestir ouro mais espesso; ③ Em soluções de revestimento de ouro contendo agentes redutores, o metal nobre da camada de base actua como um agente redutor e sofre oxidação com um catalisador, realizando o revestimento químico de ouro catalítico da camada de base.
Os ligandos nas soluções químicas de revestimento de ouro utilizam normalmente o cianeto, que forma complexos muito estáveis com iões de ouro (constante de estabilidade K=4×1028). Atualmente, muitos componentes semicondutores passaram a utilizar soluções de revestimento de ouro quase neutras, com baixo teor de cianetos ou sem cianetos, tais como soluções de revestimento de ouro à base de sulfito ou tiossulfato e soluções químicas de revestimento de ouro sem cianetos com ligandos tiólicos da série RSH.
2. Revestimento químico de ouro por deslocamento
Geralmente, a força de ligação entre o revestimento de ouro electroless de camada espessa do tipo reduzido e a camada de base dos metais de base é relativamente fraca. Por conseguinte, o revestimento de ouro eletrolítico do tipo deslocamento deve ser realizado antes do revestimento de uma camada de ouro espessa.
Na solução de revestimento de ouro do tipo deslocamento, o metal de base da camada de base dissolve-se (oxida) na solução electrolítica, libertando electrões. Em contrapartida, os iões de ouro na solução aceitam os electrões e depositam-se (reduzem) na superfície não metálica. A formação de ligandos de ouro reduz a concentração de iões de ouro na solução de revestimento, deslocando o potencial de redução na direção negativa, como se mostra na Tabela 1-43.
Tabela 1-43 Ligandos e potenciais de redução dos complexos de iões de ouro
| Ligandos | Ligando | E/V |
|---|---|---|
| H2O |
Au(H2O)2+ Au(H2O)43+ |
1.68 1.50 |
| Cl- |
AuCl2 - AuCl4 - |
1.15 0.92 |
| SCN- |
Au(SCN)2 - Au(SCN)4 - |
0.67 0.64 |
| I- |
AuI2- AuI4- |
0.58 0.57 |
| NH3 | Au(NH)43+ | 0.56 |
| OH- | Au(OH)4 - | 0.48 |
| Tioureia | Au(Qui)2+ | 0.38 |
| S2O32- | Au(S2O3)23- | 0.15 |
| SO32- | Au(SO3)23- | 0.06 |
| R-SH | Au(R-S)2- | -0. 3 〜 - 0. 5 |
| CN- | Au(CN)2 - | -0.65 |
A Tabela 1-43 mostra que, para além dos cianetos, outros ligandos, como os tióis, sulfitos e tiossulfatos, também podem formar complexos estáveis com iões de ouro e apresentar potenciais negativos.
Na solução de revestimento de ouro do tipo deslocamento do sistema de sulfito, para além dos iões de sulfito, podem também ser utilizados ligandos como os ácidos policarboxílicos poliamina e respectivos sais, aminas solúveis em água, sais de amina, sais de triacetato de etilenodiamina, estabilizadores como os sais de tetraalquilamónio, tetra (metileno fosfonato) de etilenodiamina, açúcares e compostos de tiol. As concentrações de iões de amónio, de iões cloreto, de iões sulfato, de iões brometo ou de iodeto devem ser mantidas dentro de um intervalo específico e não devem ser demasiado elevadas; caso contrário, podem formar-se ligandos de amónio (complexos)-ouro na solução de revestimento, cujo potencial redox é mais positivo do que o do sulfito de sódio. Quando a solução de revestimento é deixada em repouso ou durante o revestimento de ouro, o sulfito de sódio pode oxidar e reduzir o ouro, causando instabilidade na solução de revestimento.
O succinato de tiol, a acetilcisteína, a cisteína e outros compostos da série tiol e iões de ouro podem formar ligandos estáveis em soluções de revestimento de ouro sem cianetos. Succinato de tiol [HOOCCH(SH)-CH2COOH] e o potencial de redução do ligando iónico de ouro, ou seja, Au(HOOCCHSCH2COOH)2– + e– ⇌Au(s) + 2 ( HOOCCH - SCH2COOH)–O valor correto do potencial do elétrodo padrão para a reação é difícil de obter. O potencial de redução medido da solução de revestimento é de cerca de -0,3~0,5V. O ligando tiol succinato de ouro existe sob a forma de [Au(HOOC - CHSCH2COOH)2]–com o ouro no estado de oxidação +1.
HAuCl4 + 3H2O → Au(OH)3 + 4HCl (1-13)
Au(OH)3 + 4[HOOCCH(SH)CH2 COOH] → [Au(HOOCCH-S-CH2COOH)4 ]– + 3H2O+H+ (1-14)
[Au( HOOCCH-S-CH2COOH)4]– → [Au(HOOCCH-S-CH2COOH)2]– + HOOCH2CHOOCCH-S-S-CHCOOHCH2COOH (1-15)
Tabela 1-44 Solução de revestimento de ouro sem cianeto
| Composição e condições de funcionamento | Sistema de sulfitos | Sistema de ácido mercaptosuccínico | ||
|---|---|---|---|---|
| Clorato de ouro e sódio/(mol/L) | 0.05 | |||
| Tiossulfato de sódio/(mol/L) | 0.028 | 0.015 | ||
| Mercaptosuccinato de ouro/(mol/L) | 0.01 | |||
| Sulfito de sódio/(mol/L) | 0.52 | 0.1 | ||
| Ácido mercaptosuccínico/(mol/L) | 0.25 | 0.25 | ||
| Citrato trissódico/(mol/L) | 0.22 | |||
| Acetilcisteína/(mol/L) | 0.03 | |||
| Cloreto de tetrametilamónio/(mol/L) | 0.8 | |||
| EDTA/( mol/L) | 0.015 | |||
| EDTA - 2Na/(mol/L) | 0.02 | |||
| Amino tris(ácido metilenofosfónico)/(mol/L) | 0.1 | |||
| Carboximetilcelulose de sódio/(g/L) | 10 | |||
| pH | 7.0 | 7.0 | 1,5 (ajustado com ácido clorídrico) | 7.0 |
| Temperatura /℃ | 60 | 85 | 80 ~ 90 | |
3. Camada de revestimento químico de ouro espesso de tipo reduzido
Nas soluções de revestimento de ouro com tiossulfato como ligando, o sulfito de sódio impede a decomposição dos iões tiossulfato. No NaAuCl4 Em soluções de revestimento de ouro que contêm sais de ouro trivalentes, o ouro monovalente é reduzido pelo excesso de tiossulfato. Em soluções de revestimento de ouro fracamente alcalinas, são normalmente adicionados tampões de pH como o cloreto de amónio, o tetraborato de sódio e o ácido bórico.
Os compostos de tiol podem formar ligandos com iões de ouro que têm uma excelente estabilidade e actuam como agentes redutores. Estes compostos de tiol incluem a L-cisteína e a 2-etanamina tiol, entre outros. O quadro 1-45 apresenta a composição e as condições de funcionamento de soluções de revestimento químico de ouro do tipo reduzido, isentas de cianetos, utilizando tiossulfato de sódio e compostos de tiol como ligandos.
Quadro 1-45 Solução para douradura por redução sem cianetos
| Composição e condições de funcionamento | Sistema de tiossulfato | Sistema tiol | |||
|---|---|---|---|---|---|
| Clorato de ouro/(g/L) | 0.01 | ||||
| Clorato de ouro e sódio/(g/L) | 0.0125 | 0.0125 | |||
| Sulfito de ouro de sódio/(g/L) | 0.02 | ||||
| Mercaptosuccinato de ouro/(g/L) | 0.01 | ||||
| Ácido mercaptosuccínico/(g/L) | 0.27 | ||||
| Tiossulfato de sódio/(g/L) | 0.1 | 0.17 | 0.1 | ||
| Sulfito de sódio/(g/L) | 0.1 | 0.4 | |||
| Sulfito de amónio/(g/L) | 0.43 | ||||
| EDTA ・ 2Na/(g/L) | 0.19 | ||||
| Trietanolamina/(g/L) | 0.034 | ||||
| Cloreto de amónio/(g/L) | 0.05 | ||||
| Tetraborato de sódio/(g/L) | 0.13 | ||||
| Di-hidrogenofosfato de potássio/(g/L) | 0.15 | ||||
| Tioureia/(g/L) | 0.0033 | ||||
| Hidroquinona/(g/L) | 0.002 | ||||
| Ácido ascórbico/(g/L) | 0.25 | ||||
| Hidrazina/(g/L) | 0.3 | ||||
| L-cisteína/(g/L) | 0.08 | ||||
| 2-aminoetil mercaptano/(g/L) | 0.2 | ||||
| Benzotriazol de potássio/(g/L) | 0.05 | ||||
| pH | 7.5 | 7.0 | 8.0 | 7.0 | 7.5 |
| Temperatura/℃ | 60 | 80 | 70 | 80 | 80 |
4. Revestimento químico catalítico de ouro da camada de base
O revestimento químico de ouro catalítico da camada de base é um método de revestimento químico de ouro do tipo redução que utiliza um agente redutor. O agente redutor apenas tem atividade catalítica na superfície do substrato, a camada de metal de base (níquel), e não tem atividade catalítica na superfície de ouro depositada. A camada de revestimento químico catalítico de ouro é mais lisa, mais densa e tem menos poros ou poros mais pequenos do que a camada de revestimento de ouro por deslocamento.
Nas soluções de revestimento químico de ouro com tiossulfato e sulfito, o ouro pode ser depositado na superfície do níquel sem adição de outros agentes redutores. Na superfície do níquel, o sulfito apenas reduz o complexo tiossulfato-ouro e não actua na superfície do ouro, pelo que é designado por revestimento químico de ouro do tipo catalítico da camada de base. A reação de deslocação do banho de ouro também ocorre simultaneamente na superfície da camada de base do banho de níquel. Por conseguinte, o agente redutor sulfito sofre diferentes reacções de oxidação influenciadas pela composição da camada de niquelagem de base e pelas condições de pré-tratamento. A concentração do ligando tiossulfato de sódio ou o pH da solução de revestimento afecta as diferentes proporções das reacções de deslocamento e de redução, a espessura máxima da camada de revestimento de ouro, o aspeto, a porosidade e a adesão, pelo que é necessário selecionar uma composição adequada da solução de revestimento e as condições de revestimento de ouro.
5. Estabilidade da solução química de douradura sem cianetos
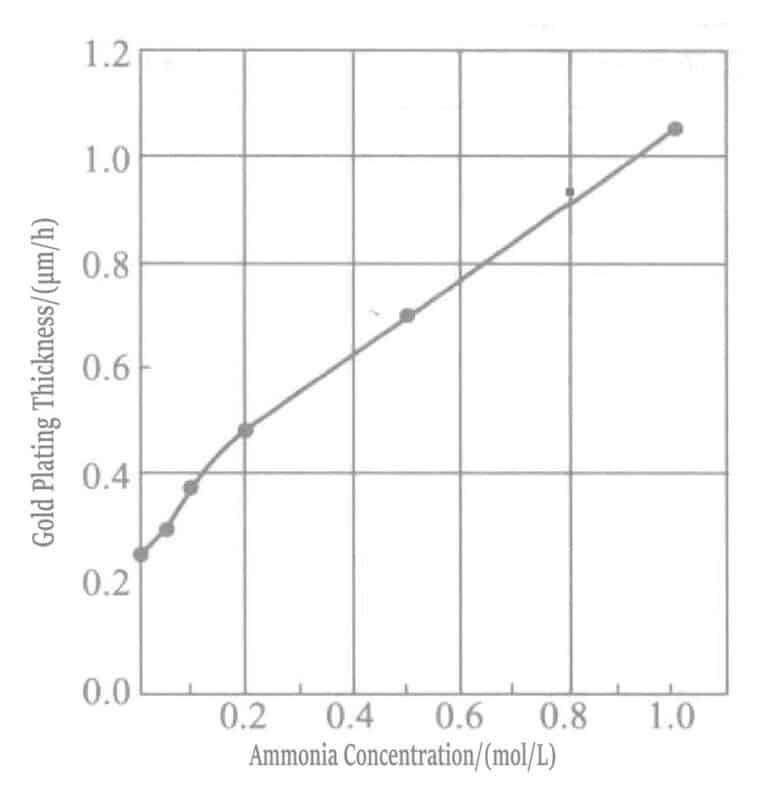
O aumento da concentração de água de amoníaco acelera a taxa de deposição da camada de revestimento de ouro. Em soluções de revestimento com elevada concentração de amoníaco, o mercapto-succinato actua como agente redutor, ocorrendo uma reação de redução em simultâneo com a reação de deslocamento.
A contaminação por iões de cobre ou de ferro é a principal causa de instabilidade das soluções de revestimento químico de ouro sem cianetos. O cobre dissolvido nos poros da camada de revestimento de níquel e o ferro do substrato promovem facilmente a instabilidade da solução química de revestimento de ouro.
Razões: ① Os iões de ouro aceitam os electrões libertados quando estes metais oxidam, aumentando a taxa de redução. ② Os íons de cobre ou íons de ferro catalisam a reação de oxidação do sulfito ou tiol, acelerando a taxa de reação, o que causa defeitos na superfície da camada química de revestimento de ouro e reduz o desempenho da soldagem e a força de ligação. Nas soluções químicas de revestimento de ouro, a dissolução e a contaminação destes metais devem ser suprimidas pela adição de ligandos que formam complexos estáveis com estes iões metálicos dissolvidos e contaminados.
6. Solução química de revestimento a ouro sem cianeto
(1) Sais de tetracloroaurato (III) e soluções fracamente redutoras de amina borano para revestimento de ouro
Sais de tetracloroaurato (III) (NaAuCl4) são facilmente reduzidos a ouro de depósito. Os agentes redutores borano de amina terciária substituída por éter podem ser utilizados com NaAuCl4 para formar uma solução de revestimento autocatalítico, ou agentes redutores como o borano trimetilamina, o borano metilmorfolina e o borano diisopropilamina, juntamente com estabilizadores como os tióis e os compostos de iodeto.
(2) Solução de revestimento de ouro com sal de sulfito de ouro:
Atualmente, é utilizado um grande número de soluções monovalentes de sulfito de ouro, com hipofosfitos, formaldeído, hidrazina, tetrahidroborato e DMAB como agentes redutores. Sal de sulfito de ouro [Na3Au(SO3)2é instável em água e requer a adição de estabilizadores como o 1,2-diaminoetano e o brometo de potássio.
(3) Solução de tiossulfato para revestimento de ouro
① Solução de revestimento de ouro com tioureia e seus derivados como agentes redutores:
A combinação de tiossulfato de ouro monovalente e solução de revestimento de tioureia tem boa estabilidade, não produz gás hidrogénio em pH neutro e não tem porosidade. A composição da solução de revestimento é mostrada na Tabela 1-46 (Solução de revestimento A).
Quadro 1-46 Solução de tiossulfato para douradura
| Composição e condições de funcionamento | Solução de galvanização A | Solução de galvanização B |
|---|---|---|
| NaAuCl4 / (mol/L) | 0.1 | 0.0125 |
| Na2S2O3/(mol/L) | 0.08 | 0.1 |
| Na2SO3 /(mol/L) | 0.4 | 0.1 |
| Na2B4O7/( mol/L) | 0.1 | - |
| NH4Cl/(mol/L) | - | 0.05 |
| Tioureia/(mol/L) | 0.1 | - |
| L-ascorbato de sódio/(mol/L) | - | 0.25 |
| pH | 9.0 | 6.0 |
| Temperatura/℃ | 80 | 60 |
| Taxa de revestimento/(μm/h) | 1. 9 〜 2. 3 | 1. 5 〜 2. 0 |
② Solução de revestimento de ouro com ácido ascórbico como agente redutor:
Na solução de revestimento de tiossulfato com ácido L-ascórbico de sódio como agente redutor, está presente sulfito de sódio, que pode revestir o ouro de forma estável. A composição da solução de revestimento é mostrada na Tabela 1-46 (Solução de revestimento B).
Os agentes redutores eficazes nas soluções de revestimento de tiossulfato de sódio, para além da tioureia e do ascorbato de sódio, incluem o tartarato de sódio, o ácido glicólico e o ácido hipofosforoso.
③ Mecanismo de reação da solução de revestimento de tiossulfato:
O sal de ouro reage com o tiossulfato para produzir Au(S2O3)23-. Quando não há tiossulfato na solução, apenas o sulfito está presente e forma-se Au(SO3)23-. A equação da reação é a seguinte
Au3++ 2S2O3 2- + H2O ⇌ Au(S2O3)23- + SO4 2- + 2H+ (1-16)
Au3+ + 3SO32- + H2O ⇌ Au(SO3 )23- + SO42- + 2H+ (1-17)
Au+ + 2SO32- ⇌ Au(SO3)23- K=1010 (1-18)
Au+ + 2S2O32- ⇌ Au(S2O3)23- K = 1026 (1-19)

Figura 1-44 Comparação das curvas de polarização catódica das reacções de deposição de ouro em soluções de sulfito e de tiossulfato
(NH4Cl 0. 1mol/L, Na2SO3 0. 2mol/L)
O tiossulfato tem uma capacidade de complexação mais forte com o ouro monovalente do que o sulfito, pelo que o ouro monovalente é mais difícil de depositar a partir de soluções de revestimento que contenham tiossulfato de sódio do que as que contenham sulfito.
Na solução de revestimento de ouro de sulfito com o agente redutor ascorbato, devido à diferença nas curvas de polarização catódica entre os iões do complexo de sulfito de ouro e os iões do complexo de tiossulfato de ouro, a taxa de deposição de ouro é apenas 1/10 da da solução de revestimento de tiossulfato de sódio. A figura 1-45 mostra as curvas de polarização catódica e anódica do ascorbato sulfito e do tiossulfato de ouro.
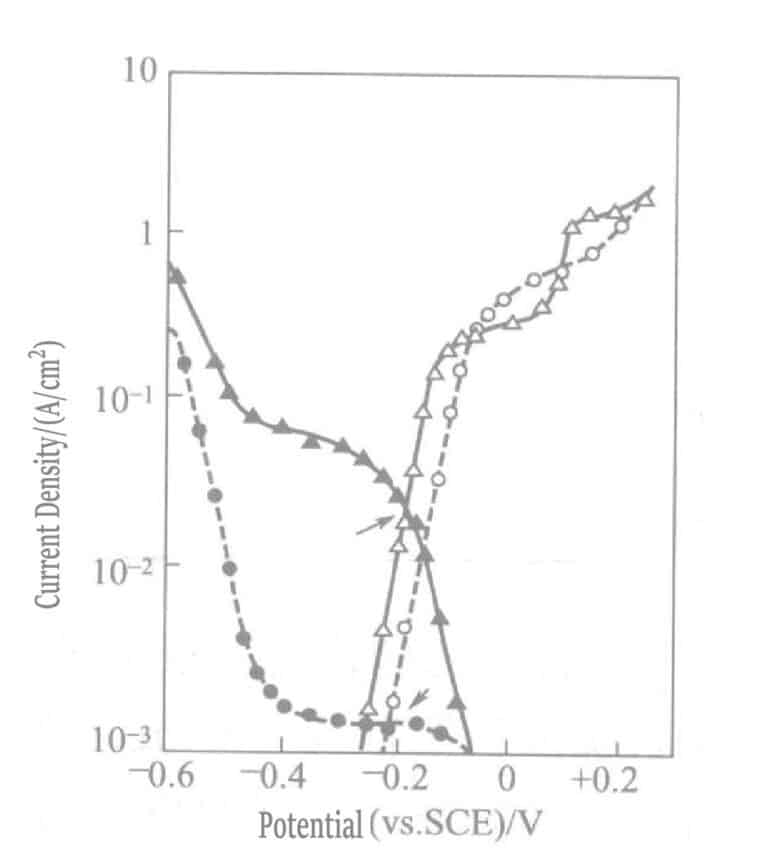
Figura 1-45 Curvas de polarização catódica e anódica de ácido ascórbico, solução de sulfito e tiossulfato de ouro
(elétrodo de ouro, NH4Cl 0. 1mol/L, pH 6. 0, 60℃. Curva "●" solução de revestimento: Na2SO3 0,2mol/ L e NaAuCl4 0,01mol/L; Curva "▴" a mesma solução de plaqueamento anterior, com adição de Na2S2O3 0. 1mol/L; Curva "○" solução de revestimento: Na2SO3 0. 2mol/L e ascorbato de sódio 0. lmol/L; Curva "△" solução, igual à anterior, com adição de Na2S2O3 0,1mol/L)
O princípio de reação da solução de douramento eletrolítico de tiossulfato refere-se ao estado de oxidação do agente redutor, e as reacções locais são as seguintes
Reação catódica:
Au(S2O3)23- + 2e– → Au + 2S2O32- (1-20)
Reação anódica:
Solução de tioureia para revestimento
CS(NH2)2 + 5H2O → CO(NH2)2 + H2SO4 + 8H+ + 8e– (1-21)
O produto da reação CO(NH2)2 é a ureia.
Solução de revestimento com ácido ascórbico
C6H8O6 → C6H6O6 + 2H+ + 2e–
7. Solução de revestimento dourado de aditivo de açúcar com sal de sulfito de ouro
Após a adição de compostos de sacarídeos à solução de revestimento de sulfito de ouro, esta pode manter-se estável durante muito tempo e a camada de revestimento de ouro é boa. A composição da solução de revestimento é a seguinte
Sais de sulfito de ouro: sulfito de ouro de potássio, sulfito de ouro de sódio, sulfito de ouro de amónio, etc.
Sulfitos: sulfito de potássio, sulfito de sódio, sulfito de amónio, etc.
Ajustador de pH: Ajustar o pH 6~9 com vários tampões.
Estabilizadores: compostos de aminas solúveis em água, etilenodiamina, dietilenotriamina, trietilenotetramina, etc.; aminoácidos solúveis em água ou sais, ácido etilenodiamina tetraacético, ácido trietilenotetramina hexaacético, ácido trans-1,2-ciclohexano diamina tetraacético ou sais, etc.; organofosfatos solúveis em água ou sais, amino tris (ácido metilenofosfónico), ácido 1-hidroxietilideno-1,1-difosfónico, ácido etilenodiaminotetra (ácido metilenofosfónico), dietanolamina, ácido pentacis (ácido metilenofosfónico) ou sais, etc.Podem também ser adicionados compostos nitro aromáticos solúveis em água, como o ácido mono, di e tri-nitrobenzol, o ácido mono e di-nitrosalicílico, o ácido nitrobenzeno dicarboxílico, o mono, di e tri-nitrofenol, o dinitroaminofenol, o mono, di e tri-nitrobenzeno, etc.
Os açúcares, para além do amido, podem também incluir hexoses, glucose, manose, galactose e outros monossacáridos, eritritol, pentitol, hexanol e outros álcoois de açúcar, ácido glúcarico e outros ácidos aldáricos, ácido glucónico, ácido hexatónico e outros ácidos aldónicos, oligossacáridos, etc. Estes compostos de açúcar podem aumentar a estabilidade da solução de revestimento e alargar a gama de densidade de corrente da zona de revestimento brilhante.
Os resultados da galvanização são apresentados na Tabela 1-47.
Tabela 1-47 Vários banhos de revestimento de ouro com sulfito, condições de funcionamento e efeitos dos aditivos
| Número de série | Vários banhos de revestimento de ouro com sulfito e condições de funcionamento | Efeito aditivo |
|---|---|---|
| N.º 1 |
Sulfito de sódio dourado 10g/L Sulfito de sódio 65g/L Citrato trissódico 65g/L Ácido etilenodiaminotetrametilenofosfónico (EDTMP) 85g/L pH 7 Temperatura 60℃ Corrente total 0,2A Substrato do provete: liga de 42Fe-Ni Método de densidade de corrente ideal com forte agitação |
Eletrólise 1080 ℃, decomposição da solução de galvanoplastia, produzindo partículas negras. O aparecimento da camada de revestimento com diferentes densidades de corrente produz manchas e queimaduras. |
| N.º 1 Adicionar carboximetilcelulose de sódio (CMC) 10g/L à solução de revestimento. As outras condições são as mesmas que as do N.º 1 | Eletrodeposição 1740 ℃, decomposição da solução de revestimento, partículas pretas. A aparência da camada de revestimento é melhorada, e manchas e queimaduras são obviamente reduzidas. | |
| N.º 2 |
Sulfito de sódio dourado 10g/L Sulfito de sódio 130g/L Citrato trissódico 65g/L Citrato de triamónio 65g/L p-Nitro(benzeno)fenol 1g/L pH 7 Temperatura 40℃ Corrente total 0,2A Substrato do provete: cobre Agitação forte |
Áreas de alta densidade de corrente queimadas, o aspeto da camada de revestimento é muito pobre, aparecem manchas difusas |
| Solução de revestimento n.º 2 com amido 5g/L, as outras condições são as mesmas que as do n.º 2. | Sem queima na área de alta densidade de corrente, boa aparência da camada de revestimento em uma ampla faixa de densidade de corrente, solução de revestimento estável. | |
| N.º 3 |
Sulfito de ouro e sódio 10g/L Sulfito de sódio 100g/L Borato dissódico 50g/L Ácido bórico 100g/L p-Nitro(benzeno)fenol 1g/L pH 7 Temperatura 40℃ Corrente total 0,2A Substrato do provete: cobre Agitação forte |
Mau aspeto da camada de revestimento |
| Adicionar 5g/L de amido à solução de plaqueamento n.º 3, as outras condições são as mesmas que as do n.º 3. | Bom aspeto da camada de revestimento numa vasta gama de densidade de corrente, solução de revestimento estável. | |
| N.º 4 |
Sulfito de ouro e sódio 12g/L Sulfito de sódio 100g/L Fosfito 3g/L Hidrato de etilenodiamina 30g/L pH 7 Temperatura 60°C Corrente total 0,2A Substrato do provete: liga de 42Fe-Ni Agitação forte |
Área de alta densidade de corrente queimada, o aspeto da camada de revestimento é extremamente pobre |
| N.º 4: adicionar amido 5g/L à solução de revestimento, as outras condições são as mesmas que as do n.º 4. | Bom aspeto da camada de revestimento e estabilidade da solução de revestimento numa vasta gama de densidade de corrente. | |












