金の電鋳と特殊素材への応用とは?
金めっきを理解する:技術、利点、応用
はじめに
電鋳は、主に金とその合金を使用して、自立した金属部品を作成する高度な電気めっきベースの製造プロセスです。従来のメッキとは異なり、厚く正確な層を形成し、それを基材から分離することに重点を置いている。本書では、装飾品や歯冠のような複雑な金合金部品を作るために、同時蒸着や逐次蒸着のような特定のめっき液や方法がどのように使用されるかを説明しています。また、ステンレス鋼やチタンのような難易度の高い素材にめっきを施す際に、特別な表面処理が重要である理由や、電子機器から航空宇宙用途まで、密着性と機能性能を確保するための不動態化層について詳しく解説しています。
金の電気鋳造とは何か、そしてそれが特殊材料にどのように適用されるか
目次
セクション I 電鋳金と金合金
電鋳とは、金属電気めっきをベースとした製造プロセスのことで、素材へのコーティングを目的とした電気めっきとは異なる。電鋳の違いは、電着後に様々な方法で素材を分離し、金属電着層のみを製品とすることである。一見電気メッキと似ているように見えるが、電鋳の方が高度であり、より高い電気メッキ技術が要求される。特にメッキ層の電流分布や内部応力に関しては、電気メッキよりも専門性が高い。
電鋳はその誕生以来、100年以上の歴史を持っている。1840年、F.V.W.ネットー博士が初めて電鋳に関する論文を発表しました。銅メッキ液を使って、平面や立体の物体に緻密な銅のレプリカを作ったり、造形したり、同様の模型を作ったり、印刷したり、鋳造したりするものです。
現在、電鋳に使用される材料は主に銅とニッケルであり、電鋳用の高濃度めっき液には少量の添加剤が加えられている。電鋳金の台頭は最近のことだが、真に理想的な電鋳金めっき液はまだ存在しない。
1.電鋳用金めっき
電鋳用のめっき液には多くの種類がある。一般的に使用されるめっき液はシアンめっき液で、その他に亜硫酸塩めっき液、塩化物めっき液、これらの混合溶液などがある。一般に、電気メッキに使用されるクエン酸メッキ液は、電鋳には使用できない。これまで電鋳には、リード&ゴルディーが発明した金めっき液が使用されてきた(表1-118)。これらのめっき技術には歴史的な限界があり、実用上も一定の問題がある。Rogersは、14.1g/Lのシアン化金カリウム、18.3g/Lのシアン化カリウム、14.1g/Lの炭酸カリウム、11.4g/Lのホウ酸を含むめっき液から、温度65℃、3.2A/dmの条件で100~125μm/hの金めっき層を得た。2 (ただし、電気化学的等価計算によると、電流効率が100%に達しても、約60μmのめっき層しか得られない)。1967年、日本と英国は、28~36g/Lの金を含むシアン化金カリウムをリン酸で中和した中性めっき液(pH6.5)から7kgの電鋳金を得た。
ナトリウム、カリウム、アンモニウムイオンタイプの亜硫酸塩電鋳金溶液に、電鋳用ヒ素化合物を含む金析出粒精製剤(DOS 2249658, 1972)を添加すると、600μmの金層を生成することができる。
表1-118 電鋳液の組成と使用条件
| シアンめっき液 | 動作条件 |
|---|---|
|
1.シアン化金カリウム 遊離シアン化カリウム リン酸水素二カリウム 温度 電流密度 攪拌 |
6.8 〜 10g/L 31g/L 31g/L 50 〜 60℃ 2.5 A/dm2 カソード攪拌 |
|
2.シアン化金カリウム フェリシアン化カリウム シアン化カリウム 温度 電流密度 |
30g/L 200g/L 7.5g/L 85 ℃ 3 〜 5
|
|
3.シアン化金(II)カリウム シアン化カリウム トルコレッドオイル 温度 電流密度 |
30g/L 70g/L 0.5mL/L 60 〜 65℃ 0.4 〜 1 A/dm2 |
|
4.シアン化金カリウム シアン化カリウム 水酸化カリウム スルファミン酸カリウム
4-ヒドロキシ-3-メトキシベンズアルデヒド 温度 電流密度 |
18g/L 120g/L 4g/L 4g/L 4g/L 80℃ 0.5 〜 1. 8A/dm2 |
| 塩化メッキ液 | 動作条件 |
|---|---|
|
金(塩化物として) 塩酸 塩化ナトリウム 硫酸 温度 電流密度
|
25 〜 40g/L 23.8 〜 55g/L 10~30g/L 10 〜 20g/L 23 ℃ 8.6 〜 11.0A/dm2 |
| 塩化シアンめっき液 | 動作条件 |
|---|---|
|
金(塩化物として) フェロシアン化カリウム 炭酸カリウム 温度 電流密度 |
10g/L 40g/L 40g/L 30 〜 50℃ 0.1A/dm2 |
| 酸性めっき液 | 動作条件 |
|---|---|
|
シアン化金カリウム エチルグアニジン ギ酸 (85%) H 温度 電流密度 |
30g/L 10g/L 250g/L 4. 0 50℃ 0.2A/dm2 |
| シアンフリーめっき液 | 動作条件 |
|---|---|
|
亜硫酸金ナトリウム リン酸カリウム 亜硫酸ナトリウム 三酸化ヒ素 H 温度 電流密度 |
10g/L 30g/L 50g/L 30mg/L 9 〜 10 90℃ 0.1 〜 0. 6A/dm2 |
| 合金めっき液 | 動作条件 | |
|---|---|---|
|
1.Au-Cu合金めっき液 Au (シアン化金カリウムの形で) 銅(Naの形で2銅EDTA) 銅(Naの形で2銅EDTA) プライベートオファーリング4-3 (85%のHPOの形で)3) 亜硫酸ナトリウム H 温度 電流密度 降水量 陽極 Auの合金比
|
(1) 6 〜 6.5g/L 16~18g/L - 25mL/L - 7.0 〜 7.5 65℃ 0.6 〜 0.6 A/dm2 10 〜 12.7μm/h プラチナ 55% 〜 95%
|
(2) 6 〜 6.5g/L - 16 〜 18g/L 25mL/L 6 〜 8mL/L 7.0 〜 9.0 65℃ 0.6 〜 0. 6A/dm2 10 〜 12.7/μm/h プラチナ 55% 〜 95% |
|
2.金-銅-カドミウム合金めっき液 Au (シアン化金カリウムの形で) 銅(シアン化銅カリウムの形で) カドミウム(シアン化カドミウムカリウムの形で) Ag(シアン化銀カリウムとして) 遊離シアン化カリウム H 温度 現在 めっき電流密度条件:カソード電流密度 めっき時間 陽極電流密度 めっき時間 |
- 1 〜 3g/L 6 〜 13g/L 0.1 〜 0.8g/L 0.01 〜 0.1g/L 3 〜 8g/L 9 〜 11 60 〜8 0℃ PR法(陰極60秒、陽極4秒) 0.5 〜 1.5 A/dm2 4 〜 20s 1.0 〜 3. 0A/dm2 0.5 〜 2s 18K 金-銅-カドミウム合金めっき
|
|
2.電鋳方法
金合金電鋳の一般的な方法としては同時蒸着法と逐次蒸着法がある。
(1) 同時蒸着法
この方法では、金と他の金属2~3種を同時に析出させる合金電鋳が行われる。析出する合金の組成は、めっき液組成だけでなく、電流密度と温度にも依存する。一定の合金析出率を維持するためには、めっき厚は100~300μmに達する必要がある。18K金-銅-カドミウム三元金合金の電鋳の析出の一貫性を確保するためなど、合金を電鋳する場合、電鋳のコンピュータ管理の全過程は、センサーによってめっき液の温度と金属イオンの濃度が自動的に制御され、コンピュータが全電流と製品の表面積を監視する方法で行われる。
電気めっき後の金-銅-カドミウム合金電鋳層は、不活性ガス雰囲気中で熱処理する必要がある。処理設備への投資が非常に大きくなる(めっき液の組成は表1-118の2種類の金合金めっき液と同じ)。
近年、カドミウムに対する環境問題や熱処理の煩雑さから、金以外の合金部品は銀のみを使用するのが一般的である。表1-119の条件を用いて、8Kから18Kまでの金銀合金を電鋳する。日本特許昭和58-130293号では、組成のばらつきが少なく、厚さ150μmの金銀合金電鋳層が得られている。
表 1-119 8K 金銀合金同時析出電鋳用めっき液の組成と条件
| 組成と使用条件 | パラメータ |
|---|---|
|
シアン化金カリウム シアン化銀カリウム 湿潤剤 シアン化カリウム テルル酸 pH 温度 電流密度 |
9g/L 4.5g/L 1mL/L(一部リン酸エステル化) 80g/L 2g/L(TeCl 4g/L、KTeOの場合) 11. 0 40℃ 1.0A/dm2 (100μmめっき可能 100/μm 12K Au-Ag合金めっき)。
|
US PAT.Lechtzinによる米国特許3427231には、PR帯電法(陰極60秒-陽極4秒)を含む実験結果が記録されている。スイス特許CH 529843は、サイクル比5対10対1のPR法を用いている。
US PAT.3427231は、電鋳に超音波を使用する効果について述べており、電流密度を100A/dm以上まで高めることができる。2 また、超音波攪拌と濾過を使用することで、添加物を避けることができる。
(2) 順次沈殿法
この方法では、電鋳合金中の様々な成分が順次析出し、そのサイクルは1回から数十回、数百回に及ぶ。析出物は異なる金属の多層膜を形成する。析出物を熱処理すると、金属成分が互いに拡散し、均一な合金が形成される。合金化のための熱処理は、表1-120のめっき液と条件で一定の膜厚の電気めっきを行った後に行う。
表 1-120 順次析出法におけるめっき液の組成と条件
| 組成と使用条件 | パラメータ |
|---|---|
|
金(シアン化金カリウムとして) Ag(シアン化銀カリウムとして) 銅(シアン化銅カリウムとして) ケーシーエヌ 炭酸水素カリウム pH 温度 |
6g/L 0.5g/L 35g/L 5g/L 100g/L 9.0 60℃ |
| 上記のめっき液を基本条件として、電流密度を変化させて2種類の合金を繰り返しめっきする。300μmの2層複合皮膜をめっきした後、800℃、30分の熱拡散処理を行うと、Au75%-Agl2%-Cul3%の18K金合金を得ることができる。 | |
|
(1)金銀合金の電気めっき条件 電流密度 0.2 電着層の厚さ 0.8μm(4分) (2)金銅合金の電気めっき条件 電流密度 1.2 電解メッキ層の厚さ 0. |
析出物の組成: Au 82% Ag 16% 銅 2% 析出物の組成: Au 65% アグ 5% 銅 30%
|
この方法で得られる金合金めっきには、次のような利点がある:
金銀銅合金の組成は任意に調整できる。
熱拡散後の金銀銅合金の硬度は、通常の電気めっき層よりもはるかに高い。
耐食性が良い。金銀銅合金電気メッキ層は合金ではなく共晶メッキ層である。熱拡散後、完全に合金化され、冶金的に製造された合金と同等の耐食性を持ち、金-銀、金-銅電気メッキ層よりも著しく高い。
金-銅-カドミウム合金メッキ液を使用しない。18K層のメッキ液にはカドミウムを使用していないため、環境に優しく安全です。
3.電鋳金の用途
(1) ペンダントやその他の装飾品の用途
US PAT.446421 ボール状の射出成形品を電鋳して作る小型中空球体。電鋳後、球体に小さな穴を開け、加熱により球体内部のプラスチックを除去し、中空の金属球体を得る。銅、銀、金などの金属を順次電鋳し、熱処理で合金化する特許製法。
GB PAT.2031024 本物の花を電鋳した後、電気炉に入れて24時間熱処理し、花の茎の上部から高圧水を吹き付けて残留物を除去し、電鋳の花飾りを得る。
日本特許(昭和 59-80788)のように、電鋳を用いた金合金時計の外装部品の製造方法が詳細に記載されている特許もある。金ペンダントの製造方法を表1-121に示す。
表1-121 金合金装飾品の製造方法
| ステップ | 方法 |
|---|---|
| モデル |
(1)デザインに合わせてパラフィンで装飾を作り、銀で鋳造して仕上げる。銀模型からゴム模型を作り、射出成形する。 (2)必要なモデル数を鋳造する。現在は主にPb-Zn-Bi合金とZn合金(Zn96%-A14%)を使用しています。 |
| 前処理 |
(1)パラフィンワックス、金属基板表面のバリやでこぼこを除去する必要があり、それ以外の場合は、製品の最終的な品質に影響を与える。 (2)材料がパラフィンである場合、表面半導体を実施する必要があり、次の2つの方法があります。 プラスチックめっき、化学銅めっき メタライゼーション 導電性ニトロラッカーを塗布し、表面に導電性を付与。 |
| 基本めっき |
(1) 基材が金属の場合、研磨後、酸性銅でメッキする。下地の砂穴や気孔などをふさぐのが目的。 (2)パラフィン素材の場合、導電化後に金を直接電鋳する場合は、下地金属を追加する必要がある。パラフィンワックスは融点が約70℃で水っぽいため、めっき液の温度を40℃程度に確保する必要がある。そうでなければ、パラフィンワックス上に直接金を電鋳することはできない。 |
| 電鋳ゴールド |
最も一般的に使用されるめっき液は、Au-Cu-Cd合金めっき液である。以下は合金めっきプロセスである。 めっき液組成 金 6g/L めっき液温度 70℃。 銅 45g/L 電流密度 0.5~2A/dm2 カドミウム 1g/L 電流効率 1.5A/dm2 午後1時/分 KCN 18g/L 合金組成:Au 5% pH 10 銅 13% カドミウム 7% |
| 三元合金の電気めっきでは、電流密度の変化によって電流効率や金の析出率が大きく変化するため、めっき液中の金属濃度と電気めっき中の電流密度を厳密に管理することが不可欠である。 | |
| 後処理 |
(1)金を電鋳する場合、硝酸、塩酸などの無機酸で合金を溶解しなければならない。不活性ガスに溶解した後、400~500℃、30分加熱処理し、製品の内部応力を除去する。 (2)金合金溶接材によるクローズドホール |
| 仕上げ | 部品の微粉砕、全体のメッキ表面 |
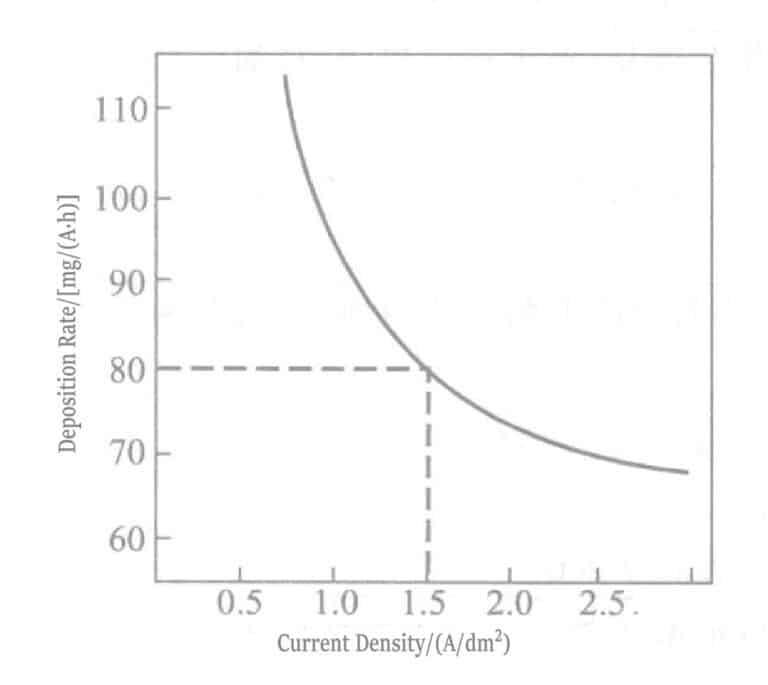
図 1-107 電流密度と蒸着速度の関係
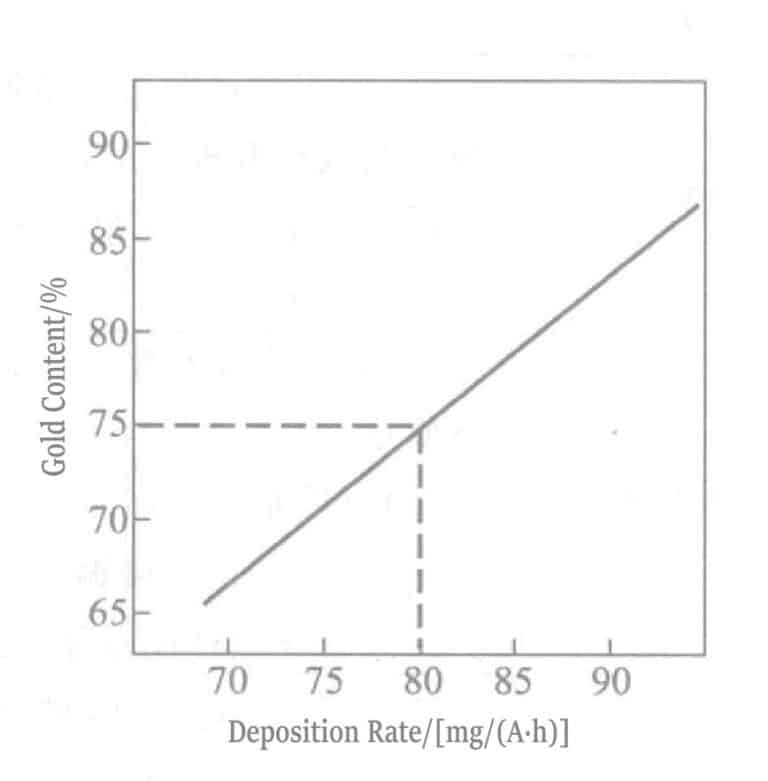
図 1-108 金含有量と沈殿率の関係
(2) 歯科用
歯冠や人工歯は複雑な形状で厚みが薄いため、高い強度と耐食性が要求されます。医療だけでなく、他の多くの分野の技術も関わっています。RogersやVr-i-hoefらは、これらの特殊技術に関する多くの研究報告を提案している。
(3) 機能目的探知機
機能試験には、電子機器、計測器、通信機器、その他の分野の機器が含まれる。
X線フォトマスク 日本特許昭和 58-224427
日本特許 昭和58-200535
赤外線フィルターグレーティング G. チャニン
スパイラル・マイクロメーター ヤング・オグム
突出したパッドライン US PAT.4125441
米国規格協会製のスパイラル・マイクロメーターは、金メッキとニッケルメッキを交互に施している。めっき層の厚さを電流で制御・測定できるため、電子顕微鏡の校正にも使用できる。
セクション II 特殊素材 金メッキ
1.ステンレスめっき
ステンレス鋼は表面に不動態酸化皮膜があるため、耐食性に優れている。しかし、ステンレス鋼の不動態皮膜への電気めっきは非常に難しい。現在、析出接合力に優れた方法として、ステンレス鋼を活性化させながらインパルスニッケルめっき液中で極薄ニッケル層を析出させ、その上に金めっき層を完全かつ強固に接合させる方法がある。しかし、この方法には大きな欠点があり、ステンレス鋼の耐食性を著しく低下させる。以下は、ステンレス鋼に耐食性金層を電気めっきする際の主な問題である。
ニッケルめっきの中間層を使用せず、ステンレス鋼に直接金めっきを行う。
気孔形成を促進するハロゲン化水素酸は使用しない(塩酸による活性化は禁止)。
良好な接着性を確保する。
上記の要求を満たすために、1971年にHAu(CN)4 の製造方法とHAu(CN)4 めっき液の範囲の調整法を開発した。このめっき液は、金リガンド、クエン酸、リン酸塩、リン酸などの弱酸からなり、pH0.1~3.0の範囲で非常によく機能する。
1979年、金(III)シアン化カリウムめっき液を用いて、ステンレス鋼にフラッシュ金めっき(ストライク金めっき)を行う方法が開始された。めっき液には導電性塩の硝酸カリウム、配位子のエチレンジアミン塩酸塩、ニッケル、コバルト、亜鉛、インジウムなどの合金成分を添加し、pHを1.5以下に制御して使用した。
塩素を含まない金(III)シアン化カリウムめっき液が開発され、ステンレス鋼に不利な塩素を含むめっき液として広く使用されるようになった。この金(III)シアン化カリウムめっき液は、特にコバルトと合金化したブラシ金めっきに使用された(表1-122参照)。
表1-122 ステンレス鋼用フラッシュ金めっき液
| 組成と使用条件 | パラメータ | 組成と使用条件 | パラメータ |
|---|---|---|---|
| KAu(CN)4(Auとして計算) | 2g/L | pH | < 0. 8 |
| コバルト | 0.2g/L | 温度 | 35 ℃ |
| 硫酸 | 10mL/L | 電流密度 | 1.5A/dm2 |
| リン酸 | 100mL/L | めっき時間 | 30 〜 60s |
表1-123 ステンレス鋼への金めっきの腐食原理
| シリアル番号 | 腐食の原理 | 孔食ダイアグラム |
|---|---|---|
| 1 |
ステンレス鋼の表面は、高濃度の塩酸による活性化処理中に凹みが生じやすい。 表面の窪みのような欠陥部分は、金めっき中に孔の形成を促進する原因となる。 |

|
| 2 | 塩化物を含むフラッシュ金めっき液(1)の場合と同様に、塩素化した基板表面に欠陥が発生する。 |
|
| 3 | シアン化金カリウム[KAu(CN)4]リン酸塩溶液を用いた金フラッシュめっきの場合、塩酸や塩化物を使用しないため、ステンレス鋼の表面にへこみは生じない。 |
![シアン化金カリウム[KAu(CN)4]リン酸塩溶液を用いた金フラッシュめっきの場合、塩酸や塩化物を使用しないため、ステンレス鋼の表面にへこみは生じない。](https://sobling.jewelry/wp-content/uploads/2025/11/3.png)
|
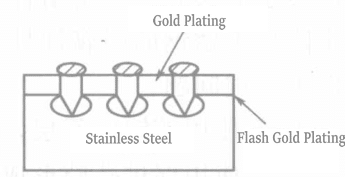
IC用ステンレスリードフレームへの薄金めっきについては、金めっき厚が300Å(30nm)以上の場合、溶接性、ワイヤーボンディング性が450℃1分前後で良好であり、200Å以上の場合、すべての金ワイヤーボンディングが良好であるとの報告がある。
この時、金めっき層の厚さは300Åと非常に薄い。ステンレスの表面粗さが粗いと、金めっき層の均一性に影響し、気孔などの欠陥が発生する。そのため、ステンレス鋼表面の活性化処理では、表面を平滑にし、密着性を向上させるために、処理剤として無機混合酸や有機腐食抑制剤を使用することができる。
耐食性のほか、ICステンレス(SUS430)基板上の金めっき層と銀めっき層を460℃の雰囲気中でそれぞれ0秒、30秒、60秒、240秒加熱した。その後、溶接性、金ワイヤーボンディング性を試験した。ストライクニッケルめっきにはワット浴を使用し、ニッケルめっき層の上に中間めっき層を設ける。最外層が金メッキの場合、中間層として銀メッキ層とニッケルコバルト合金メッキ層が良い効果を示す。最外層が銀メッキの場合は、中間層としてパラジウム-ニッケル合金メッキ層(0.1μm)、無電解ニッケルメッキ層(ニッケル-リン、0.1μm)が良い効果を示す。また、中間層を設けずに、ストライクニッケル層をストライクニッケル-コバルト合金層(0.02μm)に置き換えると、貴金属めっき層の耐熱性を向上させることができる。この効果は、金めっきの中間層として使用した場合に特に顕著である。これは、ニッケル-コバルト合金めっき層が鉄の熱拡散層として機能するためである。
コピーライト @ Sobling.Jewelry - ジュエリー カスタムジュエリーメーカー、OEMおよびODMジュエリー工場
2.チタンおよびチタン合金めっき
金属チタンは軽く、比強度(強度/密度)は鋼鉄の2倍である。大気中や酸性環境下での耐食性、耐熱性に優れ、航空機や航空宇宙産業の製造に広く使用されている。針の標準電極電位はE=-1.75Vで、アルミニウムよりもマイナスだが、酸性環境では酸化皮膜を形成し、不動態化しやすい。不動態化皮膜の厚さは約100Åに達するため、チタンへの電気めっきでは良好な密着性を得ることが難しい。
(1) 腐食方法
ナトリウムの電気めっきに関する研究では、1952年から約33件の事例が報告されている。これらの方法は、いずれも腐食を利用してナトリウム表面の酸化膜を除去し、露出した活性表面にめっき層を析出させることに主眼を置いています。文献からプロセスフローをまとめたものを表1-124に、各種腐食液をシリーズ別にまとめたものを表1-125に示します。
表1-124 チタンへの電気めっき法の概要
| シリアル番号 | エッチング液の組成 | 動作条件 | 表面メッキ |
|---|---|---|---|
| 1 |
エチレン・グリコール 高周波 |
陽極エッチング 15 〜 30分 |
シアン化銅衝撃めっき フッ化ホウ酸銅めっき |
| 2 |
(1) エチレングリコール 79% - HF 15% H2O2 6% (2) H3プライベートオファーリング4 54% HF 12.5% NH4高周波2 15. 5% H2O2 18. 1% (3) エチレングリコール 800mL/L - HF 200mL/L フッ化亜鉛 100 g/L
|
陽極エッチング 55 〜 60分 5A/dm2 15~30分 陽極エッチング 3〜5A/dm2 35 〜 45℃ 5 〜 10 分 陽極エッチング 0.6 〜 1. 2A/dm2 25℃ 3 〜 10分
|
シアン化銅衝撃めっき - - - シアン化銅衝撃めっき - - - - シアン化銅衝撃めっき - - |
| 3 |
(1) 氷酢酸 875mL/L HF 125mL/L (2) 氷酢酸 875mL/L HF 125mL/L
|
含浸時間 15分 カソード腐食 40~60Vサイクル電解 - |
シアン化銅めっき 銅、ニッケル クロム - |
| 4 |
濃塩酸 1000mL - |
20 〜 40分 90 〜 100℃, 10 〜 15s
|
無洗浄ダイレクトインパクトニッケルめっき - |
| 5 |
(1) エチレングリコール 800mL/L - HF 200mL/L (2) エチレングリコール 800mL/L - HF 200mL/L 亜鉛華2 100mL/L
|
カソード腐食 5A/dm2 カソード腐食 20 〜25℃ - 6V 1 A/dm2
|
銅、ニッケル - - - 銅、ニッケル - - |
| 6 |
(1) 重クロム酸ナトリウム 390g/L HF 50mL/L (2) 重クロム酸ナトリウム 250g/L HF 25mL/L (3) 重クロム酸ナトリウム 250g/L HF 25mL/L CuSQ4 5g/L (4) CUSO4 225g/L HF 10mL/L
|
82 ℃ 20分 82℃ 20分 82℃ 1分 - 93℃ 30s
|
|
| 7 |
エチレングリコール 800mL/L - HF 200mL/L 亜鉛華2 100mL/L -
|
4A/dm210分間の銅めっき 陽極電解 2A/dm2、10分 カソード電解 50%、HNO3
|
|
| 8 |
フッ化クロム 40g/L HCl 40mL/L
|
80 ℃ 3分
|
|
| 9 |
35% HCI 900mL/L 40% HF 100mL/L FeCl2 50g/L
|
10 〜 15s 2回含浸 -
|
無電解ニッケルめっき - - |
| 10 |
30% H2SO4 - - - - |
93℃含浸 2.7 A/dm2 陽極電解 5 A/dm2 カソード電解
|
衝撃ニッケルめっき - - - - |
| 11 |
- クロロプラチン酸 0.5g/L 濃塩酸 100mL/L -
|
5%四水和物(複合)酒石酸塩で洗浄後、四水和物(複合)酒石酸銅めっき液でめっき。 | |
| 12 |
HF 200 〜 250mL/L 硝酸3 45 〜 50mL/L H2SO4 400mL/L
|
含浸 70 〜 80℃ 0.5 〜 10分
|
|
| 13 |
NaF 100g/L HCl 100g/L シュウ酸 50 〜 100g/L CTAB 0.2 〜 10g/L
|
- 陽極電解 30 〜 80℃ 0.5 〜 10分
|
|
| 14 |
硝酸3 45 〜 50mL/L シュウ酸ナトリウム 200g/L
|
70℃、5分 - |
アルカリニッケルめっき - |
| 15 |
(1) HF 130mL/L 氷酢酸 830mL/L 硝酸3 40mL/L (2) 濃塩酸 82 (3) CrQ3 - 6H2O 210 〜 250g/L - 濃塩酸 1L
|
- - - 82℃、陽極電解 10〜50A/dm2 100℃、陽極電解 30 〜 100A/dm2
|
|
| 16 |
(1) HNO3 300mL/L HF 200mL/L 濃塩酸 100mL/L (2) エチレングリコール 750mL/L - HF 150mL/L - CuSO4-5H2O 225g/L H2SO4 50g/L アル2(SO4)3 50g/L 界面活性剤 1g/L 重クロム酸ナトリウム 100g/L CuSO4 5g/L 塩酸 50mL/L
|
5分含浸 - 煮沸含浸 カソード電解 5A/dm2 50 ~ 60℃ 5 〜 30分 含浸 - - - 90℃ 1分含浸 -
|
鉄メッキ - - - - - - - - - - - - - - |
| 17 |
HF 200mL/L 硝酸3 45 〜 50mL/L - CrO3 高周波 -
|
含浸 25℃ 15分 含浸 50℃ I 30分
|
スルファミン酸ニッケル - 無電解ニッケルめっき - - - |
| 18 |
濃塩酸 - |
含浸 3分
|
酒石酸四水和物で含浸後、酒石酸四水和物を含む銅めっき液でめっき。 |
表1-125 各種エッチング液の組成
|
1.HF-HCl 2.HF-HCl-FeCl3 3.HF-硝酸3 4. HF-CH3 COOH 5.HF-CUSOについて4 6. HF-CrO3 7.HF-ナトリウム2 Cr2 O7 8.HF-ナトリウム2 Cr2 O7 -CuSO4 9.HF-エチレンジアミン 10.HF-エチレンジアミン-ZnF2
|
11.HF-H3 プライベートオファーリング4 -日本4 H F2 -H2 O 12.塩酸 13. HCl-CrO3 14.HCl-CrF3 15. HCl-H2 白金Cl6 16.HCl-NaCr2 O7 -CuSO4 17. HCl-NaF-シュウ酸-CTAB 18. H2 SO4 19. H2 SO4 -CuSO4 -アル2 (SO4 )3 -界面活性剤 20.硝酸3 -クエン酸ナトリウム
|
表 1-126 主な工程手順と試験結果の概要
| そうだ。 | 基本プロセス | 表面メッキ | ボンディング | 総合的な判断 | ||
|---|---|---|---|---|---|---|
| メッキの外観 | 曲げた後 | 480℃ 加熱後2時間 | ||||
| 1 |
エッチング(12%HF + 1%HNO315分 陽極(13%HF + 83%CH3COOH) エッチング40℃、1.6A/dm2 6分
|
スルファミン酸ニッケル 25μm
|
O | X | X | O |
| 2 |
エッチング(5% HF + 40% HNO315分 エッチング(10%HF + 10g/L CrO330分
|
無電解ニッケルめっき 3μm
|
X | X | X | X |
| 3 |
エッチング(10%HF + 70%HNO315分 沸騰後3分でCONCHCIガス発生 シアン化銅衝撃めっき 1分 衝撃ニッケルめっき 3分
|
無電解ニッケルめっき 25μm
|
O | X | X | O |
| 4 |
無電解エッチング(10%HF + 70%HNO315分 陽極腐食(10%HF + 70%HNO3) 5分 シアン化銅衝撃めっき 1分 衝撃ニッケルめっき 3分
|
無電解ニッケルめっき 30μm
|
X | X | X | O |
| 5 |
エッチング(10%HF + 70%HNO315分 沸騰CONCHCl 10分 シアン化銅衝撃めっき 1分 衝撃ニッケルめっき 3分
|
ブライトニッケル 25μm
|
O | X | △ | △ |
| 6 |
エッチング(10%HF + 70%HNO315分 沸騰CONCHCl 10分 シアン化銅による衝撃めっき 1分 衝撃ニッケルめっき 3分
|
ブライトニッケル 25μm
|
X | X | X | X |
| 7 |
エッチング(Na2Cr2O7 + 60% HF) 衝撃ニッケルめっき 3分
|
ブライトニッケル 25μm
|
X | X | X | X |
| 8 |
エッチング (20% HNO3 - 20% クエン酸ナトリウム) - |
アルカリ無電解ニッケルめっき 10μm
|
O | X | X | X |
| 9 |
アノードエッチング 氷酢酸 875mL/L HF 125mL/L エッチング(同上)
|
ブライトニッケル 25μm
|
O | X | X | X |
| 10 |
アノードエッチング エチレングリコール 800mL/L HF 125mL/L
|
ブライトニッケル 25μm
|
O | X | X | X |
| 11 |
エッチング i 硫酸銅 200g/L 硫酸 48g/L 硫酸アルミニウム 24g/L エッチング ii 重クロム酸ナトリウム 100g/L 硫酸銅 5g/L 塩酸 5mL/L
|
ブライトニッケル 25μm
|
O | X | X | X |
| 注:○は好ましい、△は一般的、×は良くない | ||||||
(2) 塩酸の活性化
塩酸をエッチャントとして処理すると、チタンの表面は黒い網目模様となり、その上に直接電気めっきを施しても良好な密着性を得ることができる。各工程におけるニッケルめっき層の密着強度の比較結果を表1-127に示す。このうち、工程No.3による熱処理後のめっき層の密着強度試験結果を表1-128に示す。この結果では、沸騰試料1+1HC1を30分、沸騰試料2+1HC1を15分、沸騰試料2+1HC1を5分処理した後、ニッケル電気めっきを行った。その後、300℃で30分間熱処理し、曲げ試験を行った。その結果、2+1HC1の効果が最も高く、300℃で30分以上の熱処理が必要であることがわかった。
表1-127 チタン電気めっきプロセスステップ
| プロセス | 1 | 2 | 3 |
|---|---|---|---|
| 1.有機溶剤洗浄 | O | O | O |
| 2.アルカリ脱脂 | O | O | O |
| 3.水洗い | O | O | O |
| 4.濃塩酸エッチング | O | O | O |
| 5.水洗い | O | O | O |
| 6.HF(46%)処理 | O | O | X |
| 7.水洗い | O | O | O |
| 8.衝撃ニッケル陽極電解、2.2A/dm22分 | O | O | O |
| 9.衝撃ニッケル陽極電解、2.2A/dm22分 | O | O | O |
| 10.水洗い | O | O | O |
| 11.光沢ニッケルめっき | O | O | O |
表 1-128 電着層の熱処理温度と時間と接着強度の関係
| 熱処理温度 | 加熱時間/分 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 60 | |||||||||||
| 400 | O | O | ||||||||||
| 300 | O | O | ||||||||||
| 250 | X | X | ||||||||||
| 200 | X | X | ||||||||||
(3) 金メッキ
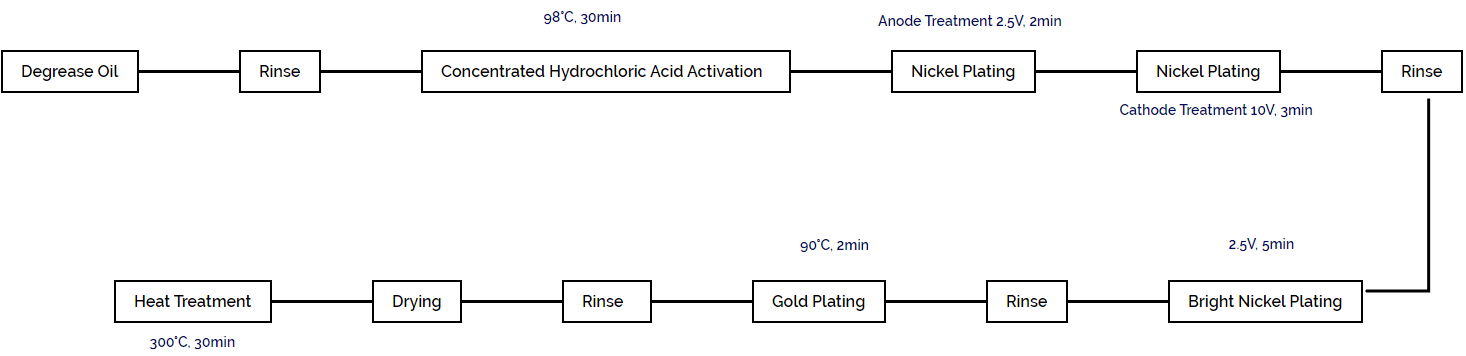
針材への金メッキ工程を図1-109に示す。
(4) その他の方法
チタン材を大気中で熱処理すると表面に安定した酸化皮膜が生成されるので、水溶性還元剤とチタンを溶解する処理液で酸化皮膜を除去し、直ちにメッキする。その工程を図1-110に示す。

第一段階の処理:100~600℃、50~60分の熱処理。
第二段階の処理:水溶性還元剤(次亜リン酸ナトリウム、ヒドラジンなど)とチタンを溶解する塩(酸性フッ化アンモニウム、フッ化ナトリウム)の水溶液を用いた活性化処理。
Liuらは、チタン表面に微細孔を生成する方法を用いたが、そこでは微細孔の数、大きさ、深さを制御することが非常に重要である。そのプロセスを図1-111に示す。微細孔の大きさ、数、皮膜の接着強度の関係を図1-112と1-113に示す。
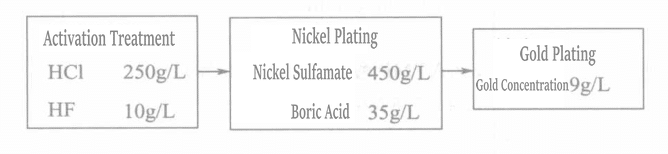
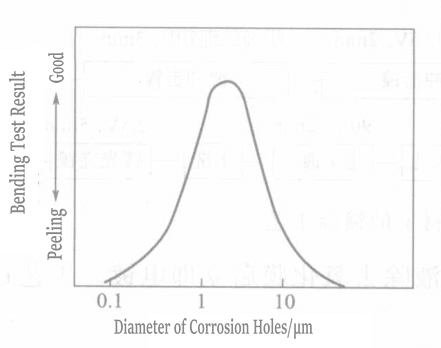
図 1-112 孔食径と接着強度の関係
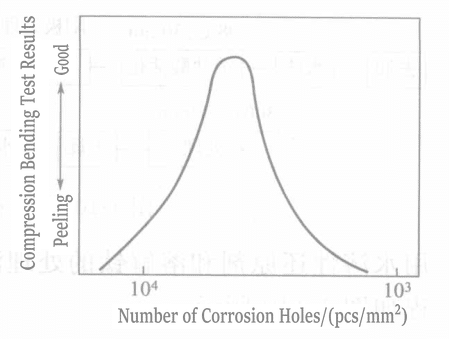
図 1-113 ピット数と接着強度の関係
(5) まとめ
チタン素材への金めっき層の良好な密着性を得るための鍵は以下の通りである:
チタン表面の酸化物を素早く除去し、酸化が起こる前に直ちに電気めっきを行う。
チタン表面に形成された微細孔の固定効果により、接着強度が向上する。
熱処理により、表面からナトリウムを除去し、皮膜中のガスを除去する。