Mitä on rodinointi ja miten se voi hyödyttää korujasi?
Rodium Plating Guide for Jewelry: Hopeanvalkoinen viimeistely: Anti-Tarnish, Hardness, Silver-White Finish
Johdanto:
Rodiumpinnoitus on sähköpinnoitusprosessi, jossa koruihin pinnoitetaan ohut kerros rodiumia, platinaan kuuluvaa jalometallia. Mutta mikä tekee siitä niin erityisen? Tämä kova, hopeanvalkoinen pinnoite on poikkeuksellisen tahran- ja korroosionkestävä ja estää koruja tummumasta ajan myötä. Se myös lisää merkittävästi pinnan kovuutta, mikä tekee koruista naarmuuntumattomampia ja kestävämpiä. Sitä käytetään yleisesti hopean ja platinan viimeisenä suojakerroksena, ja se lisää kirkkautta ja antaa kiiltävän, heijastavan peilipinnan. Tässä artikkelissa perehdytään prosessiin sulfaattipohjaisista pinnoitusliuoksista kemialliseen pinnoitukseen ja selitetään, miten tällä tekniikalla saadaan aikaan pitkäikäisiä, kauniita koruja.

Sisällysluettelo
I jakso Yleiskatsaus
Rodiumin järjestysluku jaksollisessa järjestelmässä on 45, ja sen alkuaineen symboli on Rh. Sen löysi W. H. Wollaston vuonna 1803. Sen nimi tulee kreikan kielen sanasta "Rodeos", joka tarkoittaa ruusunväristä, koska rodiumsuolaliuokset ovat ruusunvärisiä.
Rodium oli ensimmäinen valkoinen metalli, jota käytettiin teollisesti galvanointitehtaissa. Yleensä rodium kestää hapon ja emästen (myös kuningasveden) aiheuttamaa korroosiota, mutta se voi reagoida kuuman, väkevän rikkihapon, natriumhypokloriitin ja muiden aineiden kanssa alle 300 ℃:n lämpötilassa. Rodiumpinnoitekalvolla on korkea peiliheijastavuus, poikkeuksellisen suuri kovuus, joka saavuttaa Hv 800-1000, erinomainen korroosionkestävyys ja alhainen sähköinen vastus. Toisin kuin Ag, se ei muutu ajan myötä, joten sitä voidaan käyttää kontaktimateriaalina. Sitä käytetään laajalti myös elektroniikka-, sähkö- ja optisten komponenttien teollisuudessa. Rodiumia voidaan käyttää myös kehittyneiden tieteellisten instrumenttien kulumisenestopinnoitteena. Lisäksi rodiumia käytetään yleisesti hydratuskatalyyttien valmistukseen, ja rodium-platinaseoksista valmistetaan lämpöpareja. Rodiumpinnoitus on väri- ja suojakerros hopeanvalkoisille jalometallikoruille, kuten hopealle ja platinalle. Taulukossa 5-1 esitetään joitakin rodiumin tärkeimpiä parametreja.
Taulukko 5-1 Rodiumin tärkeimmät parametrit
| Ominaisuusparametrit | Ominaisarvo |
|---|---|
|
Alkuaineen nimi, alkuaineen symboli, atomiluku Luokitus Ryhmä, ajanjakso Tiheys, kovuus Väri Suhteellinen atomimassa Atomisäde Kovalenttisen sidoksen säde Kemiallinen valenssi Kiderakenne sulamispiste kiehumispiste Höyrystymislämpö Liukenemislämpö Ominaislämpökapasiteetti Johtavuus Lämmönjohtavuus |
Rodium、Rh、45 Siirtymämetalli 9(Ⅷ), 5 12450kg/m3、6 Hopea Valkoinen 102.90550 135pm 135pm 2、3、4 Kasvokeskeinen kuutiomainen 2237K (1964℃) 3968K (3695℃) 493kJ/mol 21. 5 kJ/mol 0. 242J/(kg ・ K) 21. 1 X 106m -Ω 150W/(m - K) |
II jakso Rodiumpinnoitus ja sen seokset
1. Rodium pinnoitus
Rodium on yleisimmin käytetty platinaryhmän metalli galvanoinnissa. Rodiumin erinomaisen korroosionkestävyyden ansiosta sen pinnoitus on kovempaa ja kulutusta kestävämpää kuin muiden jalometallien, ja sen valkoista sävyä käytetään laajalti koruteollisuudessa. Se on erityisen välttämätön hopean ( yleensä pinnoitettu 0,05μm flash-rodiumilla ) kiiltosuojapinnoitteena. Lisäksi sen korkea peiliheijastavuus tekee siitä yleisesti käytetyn peilien viimeisenä pinnoitteena. Mustaa rodiumpinnoitusta käytetään yleensä silmälasikehyksissä ja kellojen koteloissa. Sitä voidaan käyttää elektrodina meriveden elektrolyysissä ja kotitalouksien vedenkäsittelyelektrodeissa. Lisäksi elektroniikkateollisuudessa sitä käytetään kytkinten koskettimissa.
Rodiumia alettiin käyttää galvanoinnissa 1930-luvulla pääasiassa koristetarkoituksiin. Vuonna 1934 Shield haki ensimmäistä patenttia rodiumin galvanointiin.
Rodiumin galvanoinnissa käytettäviä pinnoitusratkaisuja ovat:
① Rodiumsulfaatti - rikkihappo pinnoitusliuossarja;
② Rodiumfosfaatti-rikkihappo pinnoitusliuossarja;
③ Myöskään fosfaattipohjaista fluoroboorihappopinnoitusliuosta, sulfonihappopinnoitusliuosta jne. ei ole kaupallistettu.
Rodiumia on tutkittu lähinnä sen soveltamiseksi jousikontakteihin.
Rikkihappopinnoitusliuoksissa on olemassa ohuita pinnoitusliuoksia koristetarkoituksiin (joissa keskitytään heijastuskykyyn ja kiiltoon), paksuja pinnoitusliuoksia (joissa keskitytään kalvon paksuuteen ja kosketusresistenssiin) ja suurnopeuspinnoitusliuoksia.
1.1 Ohut pinnoitusliuos
Taulukko 5-2 Rodiumpinnoitusliuosten edustavat komponentit ja käyttöolosuhteet
| Sulfaatti-rikkihappo-sarja | Fosfaatti-rikkihapposarja | Fosfaatti-fosforihapposarja |
|---|---|---|
|
Rodium (rodiumsulfaattina) 1. 5〜2. 0g/L Rikkihappo (95%~96%) 25〜50mL/L Liuoksen lämpötila 40〜50℃ Virrantiheys 1〜10A/dm2 Jännite 3〜6V Anodi Pt |
Rodium (rodiumfosfaattina) 2. 0g/L Rikkihappo (95%~96%) 25〜50mL/L Liuoksen lämpötila 40〜50℃ Virrantiheys 1〜10A/dm2 Jännite 3〜6V Anodi Pt
|
Rodium (rodiumfosfaattina) 2. 0g/L fosforihappo (85%) 40〜80mL/L Liuoksen lämpötila 40〜50℃ Virrantiheys 1〜15A/dm2 Jännite 4〜8V Anodi Pt |
(1) Korroosionkestävyys:
Rodium on erittäin vakaa metalli, mutta pinnoituskalvo on hieman huonompi. Yleensä rodiumia pinnoitettaessa alustalle pinnoitetaan ensin muita metalleja ja rodium pinnoitetaan viimeisenä. Tällöin alla olevan pinnoitetun metallin korroosionkestävyys on erittäin tärkeä tekijä. Tähän on kaksi syytä: ensinnäkin koska rodium on jalometalli, sen ja muiden metallien välillä on potentiaaliero; toiseksi koska rodium on kallis, sitä ei voida pinnoittaa liian paksusti. Kun rodium pinnoitetaan alla olevan Ni-kerroksen päälle, sähkökemiallinen korroosio voi helposti tapahtua, joten näiden kahden väliin voidaan lisätä korkeapotentiaalinen pinnoituskerros, kuten kullapinnoitus, joka on parempi. Koska kultaus kuitenkin lisää kustannuksia, myöhemmin otettiin käyttöön 2μmPd- tai Pd-Ni-seoksia korroosionkestävyyden parantamiseksi.
(2) Epäpuhtauksien vaikutus pinnoitustehoon:
Rodiumpinnoitusliuos on voimakkaasti hapan, ja painetun piirilevyn pinnoituksen aikana se voi aiheuttaa maskin liukenemisen. Kun metalliepäpuhtauksia on läsnä, rodiumpinnoituskerros näyttää mustalta, mikä vähentää rodiumpinnoituskerroksen kaupallista arvoa. Kun orgaanisia epäpuhtauksia esiintyy, rodiumpinnoituskerroksen sisäinen jännitys kasvaa, mikä puolestaan heikentää pinnoituskerroksen tarttuvuutta. W. Safranek tutki pinnoitusjännityksen lisääntymistä, kun pinnoitusliuoksessa on orgaanisia epäpuhtauksia; tulokset on esitetty taulukossa 5-3.
Taulukko 5-3 Orgaanisten aineiden vaikutukset rodiumpinnoituskerrosten jännitykseen
| pinnoitusliuoksen lämpötila / ℃ | Puhdistusneste/ ( kgf/ mm2) | Peiteaine (A) (vähärikkinen)/(kgf/mm2) | Peiteaine /(kgf/mm)2) |
|---|---|---|---|
|
30 40 50 60 70 |
70 87 80 69 59 |
72 89 82 71 61 |
91 114 92 91 100 |
Huomautus: pinnoitusliuoksen koostumus ja olosuhteet:
Rodiummetalli 8g/L
H2SO4 30g/L
Virrantiheys 0,5A/dm2
pinnoitusaika 30min
Levitysliuoksen määrä 200mL
1.2 Paksu pinnoitusliuos
(1) Sulfonaattityypit ja niiden liuospitoisuuden ja virran tehokkuuden välinen suhde.
Aotani et al. tutkivat bentsaldehydi-2,4-disulfonaattinatriumia tai 1,5-naftaleenidisulfonaattidinatriumia ja aminosulfonihappoa rodiumpinnoitusliuoksessa. Kun rodiumin pitoisuus oli 5 g/l ja virrantiheys 1,5 A/dm2, 60 minuutin pinnoituksen jälkeen tutkittiin sulfonaattipitoisuuden ja virrantiheyden välistä suhdetta. Tulokset esitetään kuvissa 5-1-5-3. Tulokset osoittavat, että sulfonaattikonsentraation kasvaessa virran tehokkuus vähenee lähes lineaarisesti ja pinnoituskalvon laatu heikkenee vastaavasti.
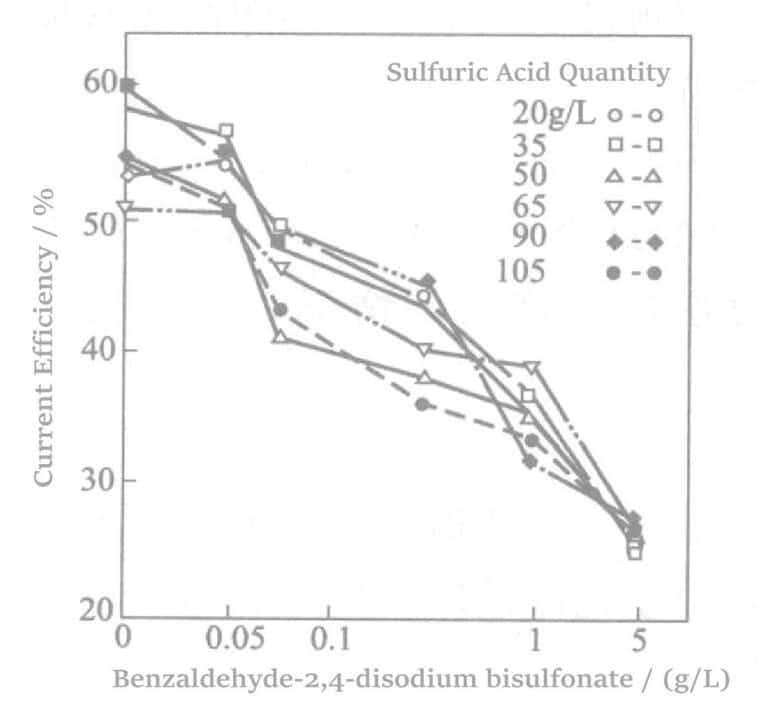
Kuva 5-1 Natrium-2,4-disulfonaatti-bentsaldehydin lisäämisen vaikutus virran hyötysuhteeseen.
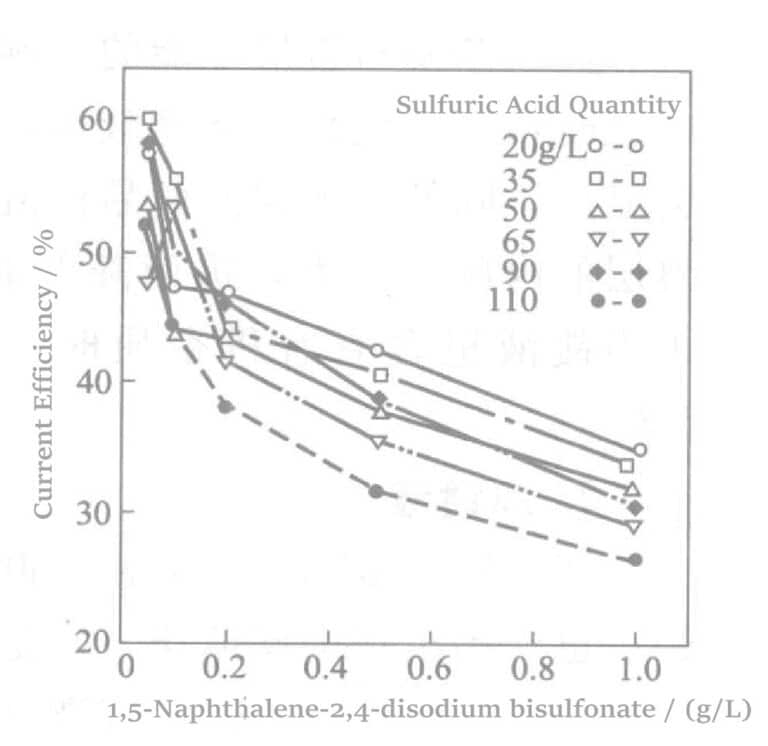
Kuva 5-2 Dinatrium-1,5-naftaleenidisulfonaatin lisäämisen vaikutus virran hyötysuhteeseen.
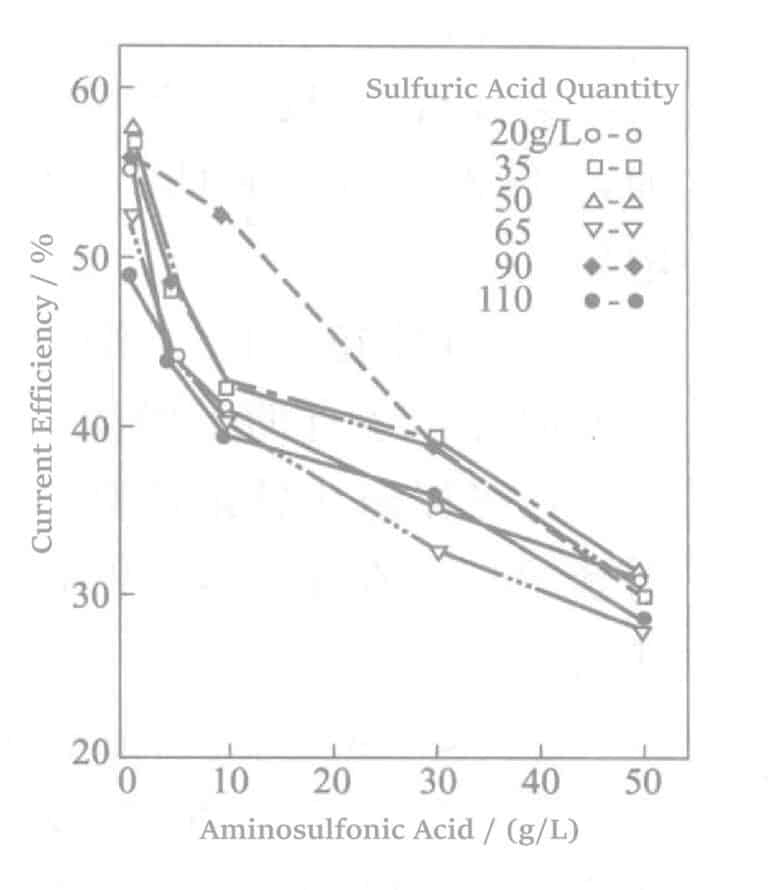
Kuva 5-3 Aminosulfonihapon lisäämisen vaikutus virran hyötysuhteeseen.
(2) Talliumnitraatin, magnesiumsulfaatin ja alumiinisulfaatin suhde stressinpoistoaineina ja nykyinen tehokkuus.
Lisäaineita ovat 1,5-naftaleenidisulfonaattidinatrium ja aminosulfonihappo. Niiden lisäainepitoisuuden ja virran tehon välinen suhde on esitetty kuvassa 5-4. Virran tehokkuuden muutokset, kun erilaisia jännityksenpoistoaineita yhdistetään lisäaineiksi, on esitetty kuvassa 5-5.
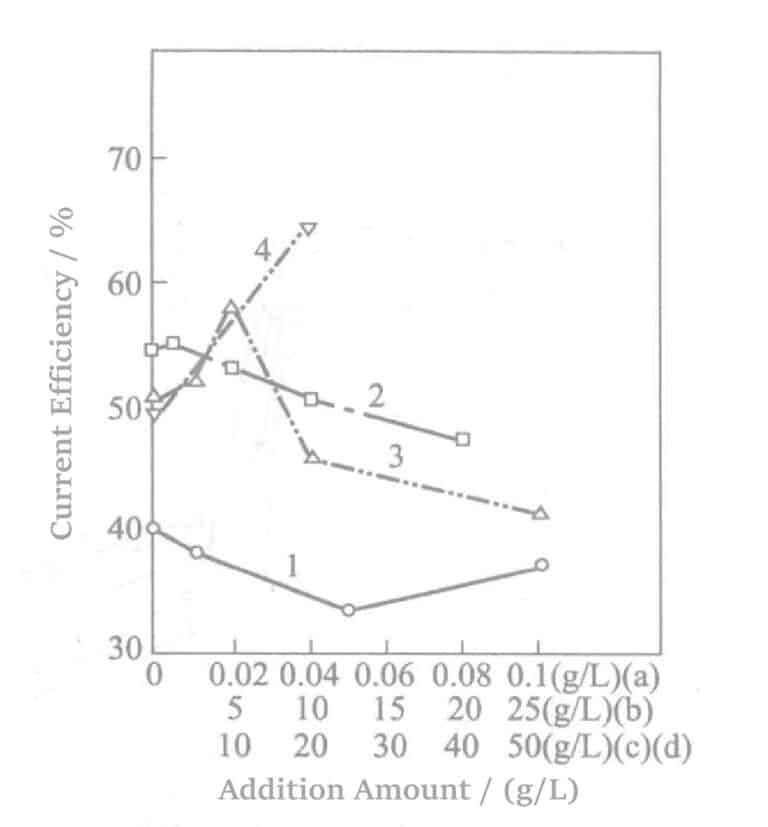
Kuva 5-4 Lisäaineiden vaikutus virran hyötysuhteeseen
1-rikkihappo 90 g/l, natriumbentsaldehydi-2,4-disulfonihappo 0,5 g/l, kostutusaine nikkelöintiin;
2-rikkihappo 20 g/l, talliuminitraatti 0,05 g/l, sulfamiinihappo;
3-rikkihappo 35 g/l, sulfamiinihappo 20 g/l, magnesiumsulfaatti;
4-rikkihappo 50 g/l, sulfamiinihappo 5 g/l, alumiinisulfaatti
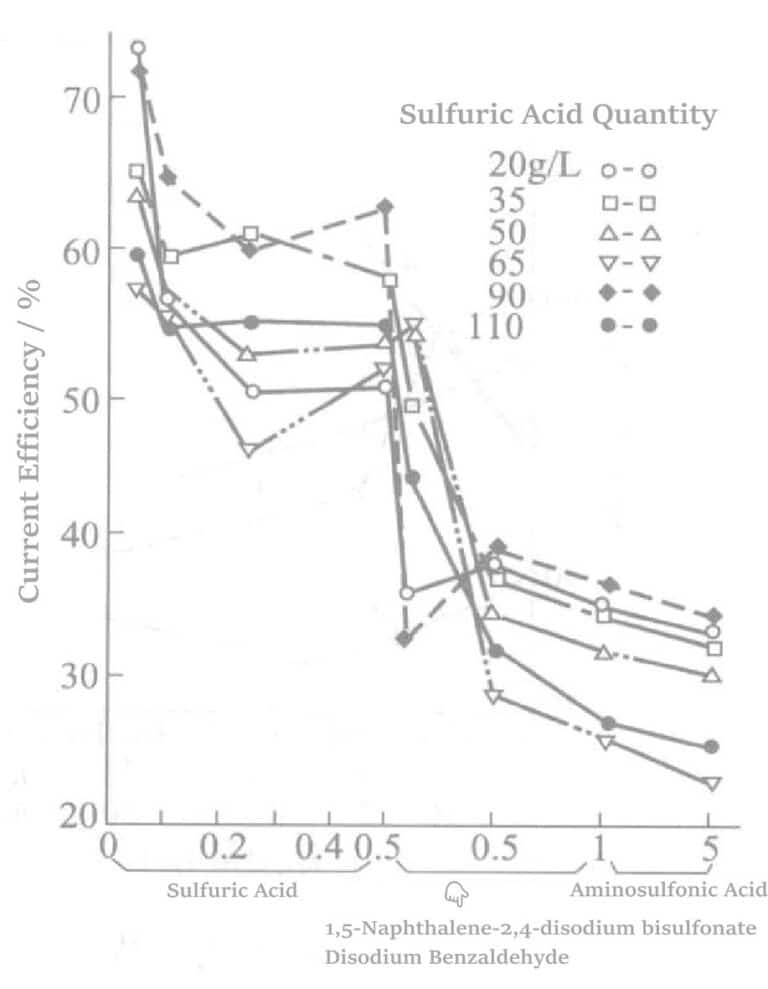
Kuva 5-5 Talliumnitraatin, 1,5-naftaleenidisulfonaattidinatrium-, bentsaldehydi- ja aminosulfonihapon lisäämisen vaikutus virran tehokkuuteen.
Voidaan nähdä, että sulfonihapon, talliumnitraatin, bentsaldehydi-2,4-disulfonaattinatriumin tai 1,5-naftaleenidisulfonaattidinatrium-, 2,4-disulfonaattinatrium- ja 1,5-naftaleenidisulfonaattinatriumin yhdistetyllä käytöllä voidaan saada aikaan puolikirkas tai sitä kirkkaampi pinnoituskerros, joka ei kuoriudu. Kunkin komponentin tehtävät ovat seuraavat:
① Rodium: standardina käytetään 5 g/l, ja nykyinen hyötysuhde kasvaa jokaista 1 g/l:n lisäystä kohden.
② Rikkihappo: Kun rikkihapon pitoisuus kasvaa, kirkkaus kasvaa hieman, mutta virran tehokkuus laskee.
③ Sulfonihappo: Sulfonihappo voi kirkastavana tasoitusaineena lisätä tasoitusta (kirkkaus kasvaa, karheus vähenee).
④ Talliumnitraatti: Se voi estää virran tehon heikkenemisen, kun rodiumin pitoisuus laskee, ja vähentää kuoppaantumista.
⑤ Bentsaldehydi-2,4-disulfonaattinatrium tai 1,5-naftaleenidisulfonaattinatrium: voi lisätä pinnoituskerroksen kirkkautta, vähentää pinnoituskyhmyjä ja aiheuttaa samalla virran hyötysuhteen heikkenemistä.
Edellä esitetyn perusteella voidaan olettaa, että seuraavilla koostumuksilla ja käyttöolosuhteilla voidaan saada aikaan pinnoite, jonka paksuus on vähintään 30 μm.
|
Rodiumionien pitoisuus Rikkihapon pitoisuus Talliumnitraatti Sulfonihappo Natriumbentsaldehydi-2,4-disulfonaatti tai dinatrium-1,5-naftaleenisulfonaatti pinnoitusliuoksen lämpötila Nykyinen hyötysuhde |
5g/L 50g/L 0,05g/L 40g/L 0,4 g/l 50℃ Yli 60% |
|
Luontainen vastarinta Kestävyys Korroosionkestävyys Lämmönkestävyys Kovuus Taivutustesti Pinnan kunto |
23×10-6Ω-cm Hyvä Ni-alustaan tunkeutuu vain vähän pisteitä. Ei hilseilyä 450 ℃:ssa, mutta halkeamia on. Keskimääräinen Hv 900 Pohja on ohut näyte, kun kuoriutuu vähemmän, huono leviäminen. Muutamia pinnoituskasvaimia, puolikirkas ja kirkas, mutta kuoppia on läsnä. |
|
Rodium (rodiumsulfaattina) Rikkihappo Selenihappo (HSeO) pinnoitusliuoksen lämpötila Virrantiheys |
10g/L 10〜200mL/L 0. 1〜1. 0g/L 50 〜75℃ 1,2A/dm2 |

Rodiumsuolat voidaan valmistaa seostus-, klooraus- tai sulatusmenetelmillä.
Lisäksi orgaanisia karboksyylihappoja pidetään myös jännityksen lievittäjinä rodiumpinnoituksessa.
1.3 Rodiumpinnoitusprosessin parantaminen
Rodiumpinnoituskerroksissa luontainen vetojännitys on merkittävä vika. Kuten aiemmin mainittiin, jännitystä vähentävän aineen lisääminen voi vähentää jännitystä ja siten lisätä halkeamattoman rodiumpinnoitteen paksuutta. Jännitystä vähentävien aineiden lisääminen aiheuttaa kuitenkin yleensä pinnoituksen kovuuden ja kulutuskestävyyden heikkenemisen.
Armstrong Michael sai aikaan halkeamattoman rodiumpinnoituksen lisäämällä pinnoitusliuokseen kloridi-ioneista peräisin olevia halogeeniyhdisteitä siten, että kovuus ja kulutuskestävyys säilyivät ennallaan. Peruskomponentit ovat seuraavat:
Rodiumsuola (rodiumissa) 5〜15g/L Antaa metalli-ioneja
H2SO4 30〜90mL/L Lisää sähkönjohtavuutta.
HCI (10~300)×10-6 Stressinpoistoaine
Virrantiheys 1~8A/ft2 (0. 1〜0. 8A/dm2 )
HCl voi vähentää pinnoituskerroksen jännitystä vähentämättä kovuutta ja kulutuskestävyyttä. Yleisesti ottaen mitä suurempi kloridi-ionikonsentraatio on, sitä paksumpi halkeamattoman pinnoituskerroksen paksuus voi olla.
Tämä keksintö soveltuu myös painettujen piirilevyjen kuviopinnoitukseen.
On myös muita raportteja, joissa käytetään sulfonihapporyhmiä lisäaineina. Lisäaineen rakennekaava on R-SO3-H. R on suoraketjuinen, haarautunut tai syklinen ryhmä, jossa on alle 20 hiiliatomia. Lisäaineen vaikutus lisää sileyttä ja valkoisuutta, mikä lisää halkeamattoman pinnoituksen paksuutta. Galvanointiliuoksen koostumus on seuraava:
|
Rodium (lisätty sulfaattina tai fosfaattina) Rikki- tai fosforihappo Pyridiini-3-sulfonihappo Pinta-aktiivinen aine Lisäaineet (lisätty R-SO3-H rakenne) |
0. 1〜20g/L 100〜200g/L 0〜5g/L 0. 01〜2g/L 0. 1〜10g/L |
Kokeellisella todentamisella vahvistettiin, että vaikka oktyylisulfonaatin (2 g/l) lisääminen vähentää hieman virran tehoa, se voi tehokkaasti lisätä pinnoitettujen osien valkoisuutta. Lisäämällä oktyylisulfonaattia pinnoituspaksuus voi olla noin 0,3 ~ 0,7 μm.
Joseph ja muut paransivat rodiumsulfaatin valmistusprosessia saadakseen rodiumsulfaattia, joka soveltuu paremmin rodiumpinnoitukseen (ks. kuva 5-7).
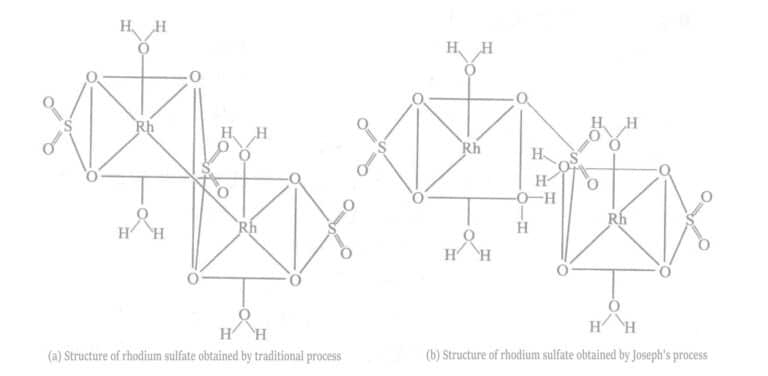
Copywrite @ Sobling.Jewelry - Custom korujen valmistaja, OEM ja ODM korut tehdas
Perinteisessä valmistusmenetelmässä neutralointireaktio suoritetaan huoneenlämmössä. Reaktiolämmöstä johtuen todellinen reaktiolämpötila on paljon korkeampi kuin huoneenlämpötila. Joseph ja muut kontrolloivat reaktiolämpötilaa alle 25 ℃ jäähdyttämällä, mikä voidaan saavuttaa vesijäähdytyksellä. Saatua rodiumsulfaattia käytettiin pinnoituskokeisiin, jolloin saatiin pinnoituskerros, jolla oli alhainen sisäinen jännitys, kirkkaus ja pinnoituspaksuus jopa 1μm.
Lisäksi Japanin vaihekenttä ehdotti menetelmää nopeaa rodiumpinnoitusta varten. Menetelmässä käytetään suihkuvirtausta laitteistoon (kuten kuvassa 5-9 on esitetty), jossa käytetään olemassa olevaa rodiumpinnoitusliuosta nopean pinnoituksen aikaansaamiseksi ja samalla varmistetaan olemassa olevat edut.

Kuva 5-9 Kaaviokuva nopeasta rodiumpinnoituslaitteistosta.
1-pinnoitettu osa (katodi); 2-anodi; 3-suihkujärjestelmä (sisäsäiliö); 4-sulkusäiliö; 5-suutin; 6-johtotanko.
|
Rodiumionien pitoisuus Rikkihapon pitoisuus Lämpötila Virrantiheys Suihkunopeus |
8〜12g/L 70〜90g/L 50〜70℃ 8A/dm2 0. 3〜1. 0m/s |
Kokeissa havaittiin, että virrantiheyden kasvaessa pinnoitusnopeus paranee; mitä korkeampi lämpötila, sitä suurempi pinnoitusnopeus; samalla suihkunopeuden lisääminen voi myös parantaa pinnoitusnopeutta. Taulukossa 5-4 esitetään pinnoitustulokset eri suihkunopeuksilla.
Tällä menetelmällä saadaan yli 5 μm paksuinen pinnoite, joka on kiiltävä, kovarakenteinen ja jonka sisäinen jännitys on alhainen.
Taulukko 5-4 Suihkunopeuden vaikutus pinnoitusnopeuteen
| Galvanointiliuoksen koostumus | pinnoitusolosuhteet | pinnoitusnopeus | Pinnoitustila | ||||
|---|---|---|---|---|---|---|---|
| Rodiumionien pitoisuus | Rikkihappo | Lämpötila | Virrantiheys | Suihkunopeus | Ulkonäkö | Halkeamat | |
|
10g/L 10g/L 10g/L 10g/L 10g/L 10g/L |
80g/L 80g/L 80g/L 80g/L 80g/L 80g/L |
60℃ 60℃ 60℃ 60℃ 60℃ 60℃ |
30A/dm2 30A/dm2 30A/dm2 30A/dm2 30A/dm2 30A/dm2 |
0. 0m/s 0. 2m/s 0. 4m/s 0. 6m/s 0. 8m/s 1. 0m/ s |
1. 70μm/min 1. 73μm/min 1. 84μm/min 1. 90μm/min 2. 10μm/min 2. 22μm/min |
Kiilto Kiilto Kiilto Kiilto Kiilto Kiilto |
Ei Ei Ei Ei Ei Ei |
1.4 Mustan rodiumin elektrolyyttinen pinnoittaminen
Taulukko 5-5 Mustan rodiumpinnoituksen prosessiolosuhteet ja sen anodikäsittelyolosuhteet
| Prosessi | Kohde | Edellytykset | |
|---|---|---|---|
| Galvanointi | Galvanointiliuoksen koostumus |
Rodiumin pitoisuus Rikkihapon pitoisuus Lisäaineet |
2. 5〜3. 5g/L 25〜30g/L Sopiva määrä |
| pinnoitusolosuhteet |
Lämpötila Katodivirran tiheys Sekoittaen Enimmäispaksuus |
20〜25℃ 2〜4 A/dm2 Katodin värähtely 0. 5μm |
|
| Anodisointi | Hoitoratkaisu | Anodikäsittelyneste | 100g/L |
| Hoito-olosuhteet |
Lämpötila Säiliön jännite Käsittelyaika |
20〜30℃ 3V 2〜3min |
|
1.5 Rodium Plating laitteet
(1) Virtalähde:
Koristelutarkoituksessa tehtävä pinnoitus ei ole ongelmallista, mutta paksu pinnoitus on tehtävä ottaen huomioon ampeerimittarin asteikko. On myös suotavaa, että käytössä on kolmivaiheinen kokoaaltomuotoinen aaltomuoto.
(2) pinnoitussäiliö:
Polyvinyylikloridilla päällystettyjä ruostumattomasta teräksestä valmistettuja säiliöitä voidaan käyttää. Rodiumin pinnoitusliuoksen lämpötila on useimmiten 40 ~ 50 ℃, eikä nykyinen hyötysuhde ole kovin korkea. Rikkihapposumun käsittelyyn tarvitaan hyvä ilmanvaihtolaitteisto.
(3) Suodatus:
Tämä riippuu myös säiliön koosta. Jatkuvaa suodatusta ei yleensä käytetä, koska se on voimakkaasti hapanta ja pinnoitusliuos on kallista. Kun orgaanisia epäpuhtauksia sekoittuu, käytetään yleensä säiliön ulkopuolista suodatusta.
1.6 Vianmääritys rodiumpinnoituksessa
Taulukko 5-6 Rodiumpinnoituksen yleiset viat ja vastatoimet
| Viat | Vastatoimet |
|---|---|
| Halkeamat |
Rodiumpitoisuuden varmistaminen tapahtuu yleensä silloin, kun pitoisuus on alhainen. Happopitoisuuden varmistaminen tapahtuu yleensä silloin, kun pitoisuus on alhainen. Vahvistus pinnoituskylvyn lämpötilan, yleensä tapahtuu, kun lämpötila on alhainen. |
| Huono sitoutuminen | Edellisen prosessin vahvistaminen on yleensä tarpeen, koska perusmetallin aktiivisuus ei ole riittävä. |
| Rikkihappopitoisuuden nousu | Jos pitoisuus on liian suuri, katodivirran hyötysuhde heikkenee. Se voidaan kierrättää tai pinnoitusliuos voidaan lämmittää ylimääräisen rikkihapon haihduttamiseksi, jäähdyttää ja lisätä puhdasta vettä, ja sitten rodium voidaan muuttaa rodiumhydroksidiksi natriumhydroksidilla ja suodattaa, sitten pestä puhtaalla vedellä ja lopuksi liuottaa rikkihapolla. |
| Tummanharmaa pinnoitus | Rodiumpinnoitussäiliö on yleensä pienikokoinen, ja käytetty anodi on liukenematon anodi, joten pinnoitusliuoksen koostumus vaihtelee suuresti. Alhainen happopitoisuus aiheuttaa rodiumin hydrolyysin ja saostumisen, jolloin pinnoituskerroksesta tulee tummanharmaa. Rodiumhydroksidi saostuu hitaasti pH2:ssa, ja saostuminen lisääntyy, kun pH on 3~4, joten rikkihapon konsentraation hallinta on erittäin tärkeää. |
2. Rodium seos pinnoitus
Rodiumin seospinnoitusta ei ole tutkittu paljon. Aikaisempia ovat Rh-Ni-seospinnoitus. Smith haki patenttia Rh-Ni-seospinnoitukselle asetaattisulfaattiliuoksesta. Sen pääkomponentti on Rh 0,4 g/l, Ni 3,5~13,5 g/l sulfaattia, pH 1,7, virrantiheys 4~10 A/dm.2. Saadaan seoksia, jotka sisältävät 25%~100% Rh. Samaa sarjaa käyttäen voidaan saada Rh-Co-seosta, jos Nin sijasta käytetään Co:ta.
Aotani tutki Rh-Zn-seoksia. Edustava prosessi on esitetty taulukossa 5-7.
Taulukko 5-7 Sulfaattipinnoitus Rh-Zn-prosessi
| Ainesosat ja niiden käsittelyolosuhteet | Koostumus ja komponenttien pitoisuus |
|---|---|
|
Rh[muodossa Rh2(SO4)3] Zn (muodossa ZnSO4 - 7H2O Na2SO4 - 10H2O H3BO3 Virrantiheys
|
0. 03 ~ 1. 0g/L 5 ~ 40g/L 23g/L 10g/L 3 ~ 9A/dm2 |
Rh-Ir-seoksella on hyvä korroosionkestävyys, tiheä kiteytyminen ja vahva tarttuvuus, ja sitä voidaan käyttää myös anodina elektrolyysissä koriste- ja toiminnallisessa pinnoituksessa.
Rh-Ir-seoksen pinnoitusliuoksen pääkomponentit ovat metallinen rodiumsuola, metallinen iridiumsuola, fluoroboraatti johtavana suolana, fluoroboorihappo ja amidosulfonihappo (amidosulfonihapolla on myös jännitystä vähentävä vaikutus) pH-puskureina. Lisäksi voidaan lisätä boorihappoa fluoroboorihapon hydrolyysin estämiseksi. Galvanointiliuosta käytetään noin 50-70 ℃:n lämpötilassa ja virrantiheyden ollessa noin 2-10 A/dm.2, joka voi tuottaa tiheän seosmetallipinnoitekerroksen, jolla on vahva tartunta.
Esimerkki galvanoinnista: Rodiumsuola saadaan RuCl:n reaktiosta.3-3H2O ja NH2SO4H. Iridium-suola saadaan (NH4)2IrCl6 ja NH2SO3H. Rh-Ir:n massasuhde pinnoitusliuoksessa säädetään arvoon 1/1. Erilaisia tuloksia voidaan saada muuttamalla kunkin komponentin pitoisuutta pinnoitusliuoksessa (ks. taulukko 5-8).
Taulukko 5-8 Ru-Ir-seoksen pinnoitusliuoksen koostumus ja olosuhteet
| Ainesosat ja niiden käsittelyolosuhteet | Nro 1 | Nro 2 | Nro 3 | Nro 4 |
|---|---|---|---|---|
|
Ru/(g/L) Ir/(g/L) NaBF4/(g/L) NH2SO3H/(g/L) Virrantiheys/(A/dm2) pinnoitusliuoksen lämpötila/°C pH Ir-pitoisuus pinnoituskerroksessa/% |
8〜9 8〜9 100 30 3 70 0. 9 3〜4 |
8〜9 8〜9 100 20 3 70 0. 8 5〜6 |
3〜4 3〜4 75 14 2 60 0. 9 8〜9 |
3〜4 3〜4 75 4 2 60 1. 2 23 〜24 |
Tuloksena syntyvässä pinnoituskerroksessa ei ole halkeamia ja se on kiiltävä.
Koristelussa ruostumattoman teräksen luonnollinen väri tai kromipinnoituksen vaalean sinivalkoinen väri eivät enää vastaa ihmisten tarpeita. Ihmiset pitävät enemmän puhtaasta ja kirkkaasta ulkonäöstä, joka muistuttaa hopeointia. Hopeointikerros kuitenkin hapettuu ja värjäytyy helposti ilmassa. Rodiumseospinnoituksella voidaan säästää kallisarvoista rodiumia ja parantaa merkittävästi pinnoitteen suorituskykyä (ks. taulukko 5-9).
Taulukko 5-9 Rodium-Rutenium-seoksen pinnoitusliuoksen koostumus ja prosessiolosuhteet rodium-rutenium-seoksen pinnoitusta varten
| Koostumus ja sen käsittelyolosuhteet | Koostumus ja komponenttien pitoisuus |
|---|---|
|
Rodiumsuola [Rh2(SO4)3] Rikkihappo Ruteniumsuola Lisäaine (tyyppi 8701) Lämpötila Katodivirran tiheys Anodi Sekoitusmenetelmä |
1〜2g/L 30mL/L 0. 1〜1g/L 25g/L 40〜50℃ 2〜8A/dm2 Ruteniumilla päällystetty titaaniverkko Katodin liike |
III jakso Kemiallinen rodiumpinnoitus
Kuten muidenkin metallien kemiallisessa pinnoituksessa, kemiallisen pinnoituksen etuna on, että se ei edellytä alustan johtavuutta ja soveltuu erilaisiin muotoihin. Koska kemiallisen pinnoituksen dispergoituvuus on paljon parempi kuin galvanoinnin, samalla galvanoinnin aikana P voi sisältyä pinnoituskerrokseen, ja rodiumin puhtaudella on merkittävä haitallinen vaikutus sen korroosionkestävyyteen ja katalyyttiseen suorituskykyyn. Joidenkin tietojen mukaan, kun jalometallit sisältävät 0,01%~0,001% P:tä, S:ää ja Cl:ää, kaasuturbiinien korroosionkestävyys ja käyttöikä heikkenevät 25%.
Alexander S. Kozlov ehdotti myös patenttia kemialliselle rodiumpinnoitukselle. Sen pääkomponentit ovat liukoiset metallisuolat, kompleksinmuodostajat ja pelkistimet. Tarvittaessa voidaan lisätä myös PH-puskureita ja joitakin lisäaineita, kuten stabilisaattoreita ja pinta-aktiivisia aineita. Tämä koostumus ei sisällä haitallisia aineita tai haihtuvia komponentteja, mikä voi estää sivutuotteiden kertymisen ja siten välttää pinnoitusliuoksen vanhenemisen. Samalla pinnoitusliuos voi myös laskeuttaa metallikomponentit kiehumalla pois ei-toivotut komponentit haihtumalla.
Sen metallisuola on Rh (NH3)3 (NO2)3. Pääkomponentit saadaan reagoimalla K3[Rh(NO2)3Cl3] ammoniakkivedellä seuraavasti: Rh(NH3)3 (NO2)3 (metalli-ionit), ammoniakkivesi (kompleksinmuodostaja) ja hydratsiinihydraatti (pelkistysaine).
Tyypillisen kemiallisen rodiumpinnoituksen pääreaktio on seuraava:
Rh(NH3)3(NO2)3 + 0.75 N2H4-H2O → Rh + 3,75N2 + 6.75H2O
Taulukko 5-10 Kemiallisen rodiumpinnoituksen koetulokset
| Koostumus ja prosessiolosuhteet | Nro 1 | Nro 2 | Nro 3 | Nro 4 | Nro 5 | Nro 6 | Nro 7 | Nro 8 |
|---|---|---|---|---|---|---|---|---|
|
Rh(NH3)3(NO2)3 NH4OH N2H4-H2O pinnoitusmateriaali Esikäsittely Esikäsittely Reaktioaika Pinnoituksen paksuus Pinnoitetun kerroksen pinnan kunto Ominaisuus |
3. 2g/L 50ml/L 1. 5g/L Nikkelifolio Hiekkapaperilla karhennus 70℃ 10min 0. 2μm Tiheä ja kirkas Korroosionkestävä |
1g/L 200ml/L 1g/L Inconel kalvo Hiekkapaperilla karhennus 85℃ 15 min 0. 4μm Tiheä ja kirkas Korroosionkestävä |
0. 5g/L 500ml/L 0. 7g/L Ruostumaton teräs Asetonipuhdistus 75℃ 30min 0. 2μm Tiheä Kirkas Katalyyttinen |
5g/L 100ml/L 2g/L Mg2Al4Si5O18 Herkistetty aktivointi 60℃ 30min 0. 5μm Harmaa univormu Katalysoitu |
1g/L 100ml/L 2. 5g/L SiC-jauhe Herkistetty aktivointi 70℃ 30min 0. 03μm Kirkastava Katalysoitu |
1g/L 200ml/L 0. 2g/L Lasihiutale Herkistymisen aktivointi 60℃ 10min 0. 1μm Peili kirkas Peili |
3g/L 100ml/L 1. 5g/L Alumiinioksidi Herkistetty aktivoitu 75℃ 2h 2. 2μm Ei sileä harmaa Elektroniset komponentit |
7g/L 50ml/L 4. 5g/L Ti-pelti Hiekkapaperilla karhennus 85℃ 3h 3. 5μm Tiukka Puoliksi kirkas Inertti anodi |
Tätä pinnoitusliuoksen koostumusta voidaan soveltaa erilaisiin pinnoitettaviin kappaleisiin suorittamalla pinnoitettaville osille asianmukainen esikäsittely.
Tieteen ja teknologian kehittyessä myös rodiumin kysyntä kasvaa vastaavasti. Niillä on suuri potentiaali, joka perustuu rodiumpinnoituskerrosten ominaisuuksiin, olipa kyse sitten koriste-esineistä tai teollisista sovelluksista. Kun rodiumpinnoitetta käytetään sähkökontakteissa, sen paksuus on alle 0,5 μm, kun se on tarkoitettu ruostumattomuuden estämiseen; kun se on tarkoitettu kulutuskestävyyteen, pinnoitepaksuus on 0,2-2 μm; kun pinnoitepaksuus on tiukasti kulutuskestävyyttä vaativissa osissa, pinnoitepaksuus on 2,5-25 μm. Kun sitä käytetään lyijykehysten kullan aluskatteena, se voi säästää käytetyn kullan määrää.