Unveiling single-crystal Optimize gemstones like Sapphire, Beryl, and Diamond
Optimization and identification for Sapphire & Ruby Corundum Gemstone, Beryl family gemstones, and Diamond
Gem crystals arranged in a periodic pattern according to certain rules by atoms or molecules are called single-crystal gems. Many single-crystal gems exist, such as rubies, sapphires, diamonds, emeralds, tourmaline, crystals, and zircon. Single crystal gems generally have high transparency and strong luster. The optimization treatment of single-crystal gems is mainly used to improve the color and transparency of allochromatic-colored gems. Most gems colored by trace elements can improve their color and increase transparency through optimization treatment. Different optimization treatment methods are selected based on single-crystal gems’ chemical composition, structure, and color mechanism. For example, natural emeralds and rubies with many fissures often use colorless or colored oil injection for filling. There are many optimization treatment methods for corundum gems, and almost all can be applied to corundum gems. The optimization treatment methods for other types of single-crystal gems should be chosen according to the color principle of the gems.
In addition, some single crystal gems colored by their components, such as garnet, malachite, and peridot, cannot use optimization treatment methods to change the color of the gems.

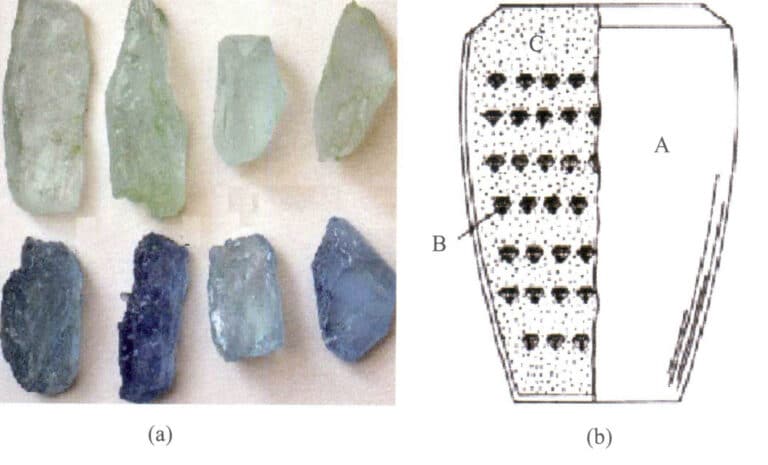
Various colors of corundum gemstones
Sisällysluettelo
Section I Sapphire & Ruby Corundum Gemstone
1. Gemological Characteristics of Corundum Gems
Corundum gemstones are a general term for single crystal gemstones of α— Al2O3. Pure crystals are colorless, but they often exhibit different colors due to the presence of trace amounts of transition metal ions (Table 5-1). Chromium ions color the most precious pigeon blood red rubies, blue sapphires are usually colored by iron and titanium ions, and key ions, etc color-changing sapphires. Rubies, sapphires, diamonds, emeralds, and cat’s eye stones are the five major precious gemstones. Color centers, such as yellow sapphires, color some corundum gemstones.
Table 5-1 Colors of corundum gemstones produced by different coloring ions
| Types of impurities | Jalokiven väri |
|---|---|
| Cr2O3 | Light red, pink, deep red |
| TiO2 + Fe2O3 | Sininen |
| NiO + Cr2O3 | Golden yellow |
| NiO | Keltainen |
| Cr2O3 + V2O5 + NiO | Vihreä |
| V2O5 | Color change (blue-purple under fluorescent light, red-purple under tungsten light) |
Corundum gemstones come in various colors, including red, purple, green, blue, yellow, and black (Figure 5-1). Rubies are limited to the medium to deep red varieties containing chromium, while light pink to orange-yellow ones are generally called Padma gemstones. The remaining colored gem-quality corundum is collectively known as sapphires. When naming corundum gemstones, the color of the gemstone is prefixed before sapphire, such as yellow sapphire. If no specific color is written, it can be assumed to be blue, and sometimes it also refers to the general term.

2. Optimization treatment and identification methods of corundum gemstones
A long time ago, people began to use heat treatment methods to improve the color of sapphire gemstones. According to relevant records, around 1045, a low-temperature heat treatment method for sapphire gemstones appeared, which involved heating with molten gold, most of which can be heated to above 1100℃. Although this method has been used for a long time, it is still used today, albeit with slight variations. The purpose is to weaken or remove the purple tones in rubies and pink sapphires.
In the 1970s, Sri Lankan milky Geuda sapphires changed color to blue after high-temperature heating at 1500℃, transforming from inexpensive paving stones to gem-quality sapphires. Starting in 2001, sapphires treated with beryllium diffusion appeared in large quantities on the market, and it wasn’t until early 2002 that gemologists identified these stones as beryllium-diffused sapphires.
There is also a high-temperature and high-pressure method for treating lighter-colored sapphires, which significantly increases the color concentration and saturation after treatment.
2.1 Classification of Optimization Treatment Methods for Sapphire Gemstones
The sapphires discussed in this section include rubies, padparadscha sapphires, various colored sapphires, and various star sapphires. Corundum gemstones are a common type of gemstone, and many optimization treatment methods are available. Almost all optimization treatment methods can be applied to corundum gemstones, which can currently be divided into three main categories (heat treatment, irradiation, and additive colour matching) and twelve methods, as shown in Table 5-2.
Table 5-2 Classification of Optimization Treatments for Corundum Gemstones
| First Type of Heat Treatment Method | (1) The transformation of color in corundum gemstones containing iron ions from colorless, light yellow to yellow, orange |
| (2) The deepening of color in colorless or light blue corundum gemstones containing iron and titanium ions and the lightening of color in deep blue corundum gemstones | |
| (3) The elimination of purple and blue tones in rubies | |
| (4) The precipitation, elimination, and reformation of star light and fibrous inclusions | |
| (5) The introduction of synthetic gemstone growth patterns and stress relief, as well as fingerprint-like inclusions | |
| (6) Diffusing colorless corundum into various colors or star light | |
| The second type of irradiation method | (7) Colorless turning yellow, pink turning orange, blue turning green, and the elimination of color centers through radioactive irradiation |
| Third type of color enhancement method | (8) Coloring and dyeing, precipitating color materials in the fissures of gemstones |
| (9) Colorless or colored filling, commonly using wax, oil, or plastic | |
| (10) Overgrowth, growing a layer of synthetic corundum on the surface of synthetic or natural corundum gemstones | |
| (11) Composite stones, using corundum-type gemstones or other types of gemstones to splice, increasing weight or improving color | |
| (12) Coating, substrate, surface coating or lamination, sticking or engraving starlight |
Among the 12 optimization treatment methods mentioned above, the most commonly used are six methods in heat treatment. Below, we will analyze each method and principle of optimization treatment one by one.
2.2 Heat Treatment Method
(1) Interchange of corundum gemstones containing iron ions from colorless and light yellowish green to yellow and orange
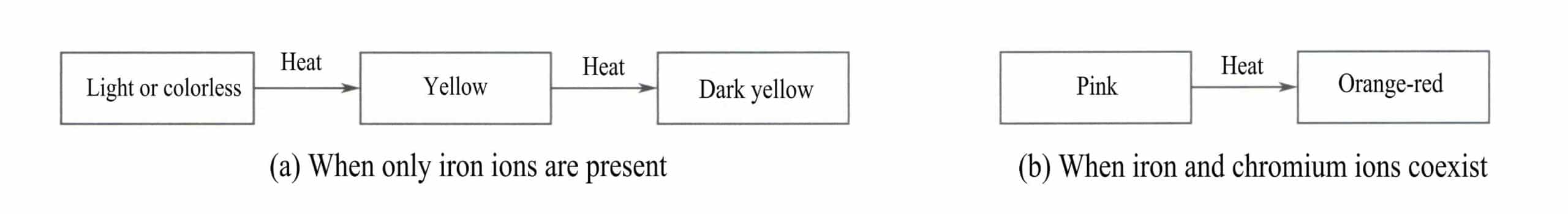
When iron ions exist as divalent in corundum, the gemstone is colorless or slightly greenish. Under high-temperature oxidation conditions, divalent iron can be oxidized to trivalent iron through gas diffusion. With varying content of trivalent iron, the gemstone can exhibit different degrees of yellow [Figure 5-2 (a)].
When the iron content in gemstones far exceeds that of titanium, the charge transfer between iron ions dominates, and the gemstone can still appear yellow. Still, the yellow formed with titanium is much darker than that without titanium.
When iron ions coexist with chromium ions, and iron is divalent, the gemstone is pink; upon oxidation and heating, iron becomes trivalent, and the gemstone appears orange-red [Figure 5-2 (b)].
The temperature required for the heat treatment of corundum gemstones is relatively high, generally required to be above 1500℃, close to but below the melting point of corundum (2050℃). A good temperature control system must be in place during heating; otherwise, the gemstone may partially or completely melt. The atmosphere during heat treatment is oxidizing, often using an open crucible to oxidize Fe2+ to Fe3+, conducted under weak oxidizing conditions in the air, which can yield more vibrant colored corundum gemstones. Due to the high temperature during heating, to prevent the gemstone from cracking, attention must be paid to the heating and cooling rate, requiring slow temperature changes, and chemical agents can also be added to alleviate temperature changes.
(2) The color of colorless or light blue corundum gemstones containing iron and titanium ions deepens, while the color of deep blue corundum gemstones lightens.
Chromophore ions of iron and titanium produce the blue and green colors in sapphires. The different valence states and concentrations of iron and titanium ions in sapphires lead to different colors. The charge transfer of iron and titanium is the main reason for the color change in blue corundum gemstones.
Fe2+ + Ti4+ —> Fe3+ + Ti3+ (5-1)
(Low energy) (High energy)
When light hits the gemstone, single electrons absorb light energy and transfer it from iron to titanium, causing the equation to proceed to the right. The absorption of single electron energy forms a broad absorption band from yellow to red, thus producing blue. This charge transfer characteristic that generates color has a high probability of strong light absorption, resulting in vibrant colors.
In the first process, the color deepens. Iron in light-colored or colorless corundum containing iron and titanium generally exists in the divalent form, while titanium exists in the form of compound TiO2. To drive the equation to the right, titanium TiO2 must exist in ionic form in the corundum, which requires high-temperature heat treatment.
A typical example is the thermal treatment of the “Geuda” corundum in Sri Lanka. This corundum, which ranges in color from cream to yellowish-brown or milky with a blue tint, can be treated at high temperatures to produce varying degrees of blue, some of which can even reach the finest color of sapphire (Figure 5-3).
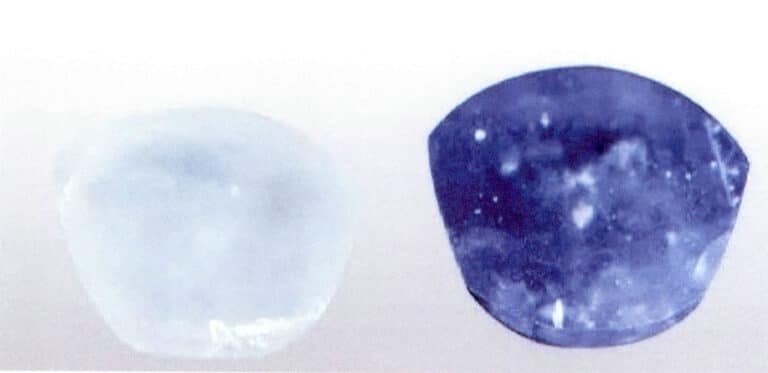
Due to the numerous fissures in natural corundum gemstones, it is important to prevent the gemstones from bursting during the heat treatment process. Before heat treatment, the raw gemstone material should be adjusted to remove some surface fissures and larger inclusions; during heat treatment, some chemicals are often added to prevent bursting during heating and to accelerate the speed of color change. When the heating temperature is lower, it is necessary to extend the holding time; when using a higher temperature, only a short holding time is required.
The second process is the lightening of deep colors. This is the reaction to the first process, mainly changing and adjusting the content and ratio of impurity elements such as iron and titanium that form sapphire’s deep blue or even black-blue color.
Examples include corundum produced in Shandong, China, Hainan Island, China, and Australia. The improvement of this gemstone is theoretically feasible, but an ideal method has yet to be found in practice.
(3) Elimination of the purple and blue tones in rubies
The purpose of heat treatment for rubies is to change the content and mode of occurrence of impurities (usually iron and titanium) that cause the color variations in rubies so that the impurities do not present color, thereby making the red color presented by chromium ions in the gemstone more vivid.
For example, rubies often have blue or purple tones due to iron ion impurities. The heat treatment of rubies is relatively low in temperature, generally below 1000℃, and in an oxidizing atmosphere, it can remove the blue-purple tones in rubies, making the red color in rubies more vibrant (Figure 5-4). This heat-treated corundum gemstone has good stability, does not fade under light and heat, and does not contain added components, allowing it to be sold as a natural gemstone without needing to be noted in the certificate, directly named as a natural gemstone.
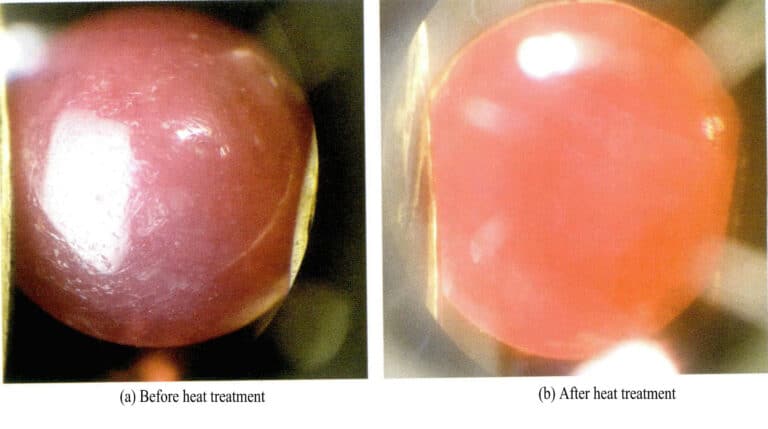
The temperature for this heat treatment is much lower than that for sapphire heat treatment, but if the goal is to eliminate the fibrous inclusions in the ruby, a higher temperature is required.
(4) Elimination, precipitation, and reformation of star-like and fibrous inclusions
Crystals can form solid solutions with impurities at certain temperatures. When the temperature drops to a certain level, the impurities become supersaturated in the crystal and precipitate as push crystals or microcrystals, causing the crystal to produce a milky substance or fibrous inclusions.
Adding a rutile of 0.2% in A12O3 and, synthesizing corundum at high temperatures and cooling at a relatively fast rate, the crystallized crystals remain blue and transparent. However, small fibrous or needle-like inclusions will appear if the crystals are reheated at a temperature of 1100-1500℃ or kept at the same temperature for about a week.
Many extremely small rutile inclusions, oriented needle-like, form three groups of oriented inclusions at the base of the parallel corundum crystals, which are mutually at 120° angles. Clear asterism can appear [Figure 5-5 (a)].
Phase diagram studies indicate a mutual solubility limit between titanium oxides and A12O3 around 1600℃. Above this limit temperature, titanium oxides can dissolve in A12O3 in a certain proportion to form solid solutions. Below this limit temperature, titanium mostly precipitates TiO2 [Figure 5-5 (b)].
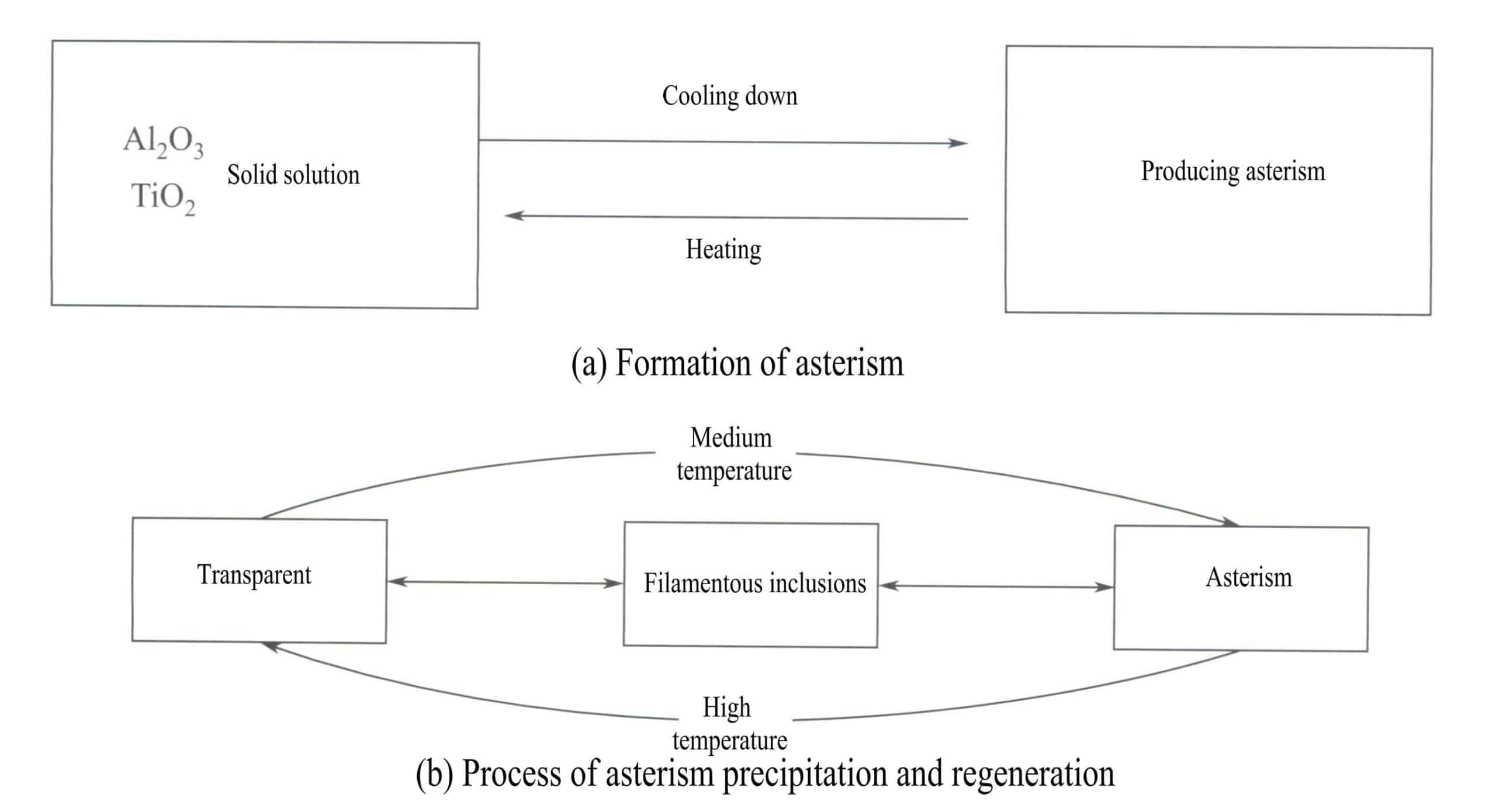
Below the mutual solubility limit, titanium residues in the form of Ti4+(TiO2) :
2Ti2O3 + O2 →4TiO2 (5-2)
Therefor, under the same impurity concentration of (TiO2), different temperature and pressure conditions can cause or eliminate asterism and silk-like inclusions in corundum gemstones.
① To eliminate asterism and silk-like inclusions
Choose natural ruby or sapphire raw materials with poor asterism and unclear star lines.
Treatment method: By rapidly cooling after high-temperature heating, heat the gemstone to a high temperature of 1600℃, where TiO2 and A12O3 form a solid solution, TiO2 dissolves in the gemstone while A12O3 does not, thereby eliminating the silk-like inclusions in the gemstone.
② Starlight extraction:
Raw materials: natural or artificially synthesized rubies and sapphires with a high titanium content.
Treatment method: The sample is heated under high-temperature conditions, maintained at 1100-1500℃ for some time. It should be maintained for about a week at lower temperatures, while at high temperatures, it needs to be maintained for several hours. During this time, the rutile needle-like crystals inside the corundum can form a regular arrangement, resulting in the starlight phenomenon.
③ Starlight recreation:
Choose natural titanium-containing inclusions in gemstone raw materials, mostly sapphires. This is because some naturally produced gemstones have poor starlight, or the fibrous inclusions are coarse and unevenly grown.
Treatment method: These inclusions can be melted into the gemstone through artificial high-temperature melting, and then the temperature is controlled to extract the ideal inclusions, recreating high-quality starlight.
The recreation process combines eliminating and extracting the previous two processes.
Operating steps: At high temperatures (above (1600℃ ), maintain a constant temperature for some time to allow the filamentous and coarse inclusions to melt without melting the gem. It is essential to control the appropriate temperature and time. Then, slowly cool down to a selected temperature between 1500-1100℃, maintaining a constant temperature for some time to give the TiO2 needle-like inclusions enough time to nucleate and grow, and finally, slowly cool down to room temperature.
After processing and polishing into a smooth gem, the raw materials for starlight will show six-ray starlight on the top facet.
The process of precipitation and reformation of starlight is shown in Figure 5-5 (b).
(5) The introduction of synthetic gem growth patterns, stress reduction, and fingerprint-like inclusions.
This method is commonly used to grow rubies and blue sapphires by flame fusion. During the crystallization and cooling process of synthetic gemstones, some obvious defects, such as curved growth lines, internal stresses, curved color bands, etc. , appear due to the uniformity of the ingredients, the stability of the temperature control of the equipment, the orientation of the growth and the rate of crystallization.
To eliminate these defects, a conventional annealing treatment is generally performed after synthesis (around 1300℃ ) to eliminate the brittleness of the gem and enhance the stability of the synthetic gem.
Curved color bands and growth stripes are important criteria for distinguishing synthetic gems from natural gems. To make the synthetic product closer to the natural one, high-temperature treatment is conducted in a thermal field near the gem melting point, with temperatures above 1800℃ for an extended period. High-temperature treatment can eliminate stress, reduce brittleness, and reduce the gem’s curved color bands and growth stripes through high-temperature diffusion or make them less noticeable. However, this method cannot remove small bubbles in the synthesis.
Additionally, uneven heating of synthetic sapphires can cause local fissures to form first, and then heating in certain additives can heal the fissures, resulting in fingerprint-like inclusions that are very close to natural gems.
2.3 Irradiation method
Initially, colorless sapphires were irradiated with X-rays or γ rays to produce light yellow to orange-yellow sapphires. Still, the colors generated by this irradiation are unstable and will fade under the light. Therefore, light fading experiments are the only reliable method for identifying irradiated yellow sapphires (K. Nassau, 1991). In recent years, a new type of irradiation—neutron irradiation—has produced yellow sapphires with color centers similar to those of natural yellow sapphires, which do not fade under light but begin to fade when heated above 250℃. In addition, neutron-irradiated yellow sapphires have the following identification characteristics:
① Orange-yellow ultraviolet fluorescence:
Irradiated yellow sapphires all exhibit strong orange-yellow ultraviolet fluorescence. Natural color center-induced yellow sapphires also have orange-yellow fluorescence, but sapphires with Fe3+ as the main coloring ion do not exhibit ultraviolet fluorescence.
② The composition contains little or no chromium ions.
③ Infrared absorption spectrum:
Neutron-irradiated yellow sapphires show absorption at 3180cm-1 and 3278cm-1.
④ Ultraviolet-visible absorption spectrum characteristics:
The absorption curve of neutron-irradiated yellow sapphires shows a weak Fe3+ absorption peak at 450nm. It decreases starting from 405 nm, indicating increased transparency to violet and ultraviolet light, while other irradiated treatments and natural color center-induced yellow sapphires are opaque to ultraviolet light.
Colorless, light yellow, or light blue corundum gemstones can turn yellow through irradiation, forming yellow sapphires. At least two types of yellow color centers are produced during the irradiation process. One is an unstable color center (YFCC color center) that fades quickly in light, while the other is a more stable color center (YSCC color center) that does not fade in light and at temperatures below 500℃. Deep yellow or orange-yellow sapphires are generally unstable and can fade after low-temperature heating, around 200℃, or exposure to sunlight for a few hours. Chromium-containing light pink sapphires can produce pink-orange sapphires through irradiation.
If a yellow color center exists in chromium-containing pink corundum, it becomes an orange-yellow to pink Padparadscha sapphire. If a yellow color center exists in blue sapphires, it can turn the blue sapphires green. Natural yellow color centers are mostly stable YSCC color centers.
During the irradiation process, optimizing the treatment of gemstones is particularly meaningful for stable color centers. Heating can accelerate the elimination of color centers, requiring around 500℃ to eliminate stable color centers, while eliminating unstable color centers only takes 200℃, comparable to sunlight exposure for a few hours. After heating, yellow turns light yellow or colorless, and green turns blue. If irradiated again, most can revert to their previous colors.
Irradiated sapphires are difficult to detect, but their color usually differs from untreated natural materials. Generally, irradiated sapphires have very bright colors and high saturation.
2.4 Ruby Filling
(1) Filling with Traditional Materials
In addition to using colorants, sometimes colored or colorless wax, colorless oil, colored oil, or plastic is used for filling. The injection of colored oil can be very deceptive. For example, “ruby oil” is a stable mineral oil mixed with red dye and a small amount of a bactericide-type fragrance, which can enhance the red tone of light pink or colorless corundum gemstones, especially those with natural fissures, allowing them to be sold as “rubies.”
The filling of rubies is generally done under vacuum conditions through heating, and it involves the following steps:
① Pre-process the ruby by rough grinding it into the desired shape without needing fine grinding and polishing. Clean with acid to remove impurities from the fissures and dry it.
② Place the filling material and the ruby to be processed into the device, heat it to melt the filling material into a liquid state, and allow it to penetrate the fissures of the ruby under vacuum conditions, maintaining a constant temperature for a period to fully complete the filling process.
③ After the filling, slowly cool down and perform fine grinding, polishing, and other surface treatments on the processed ruby.
After the resin filling, the fissures in the ruby have a resin-like luster, which is distinctly different from the bright glass luster of the ruby. The resin can be moved with a needle, or when touched with a hot needle, there may be an oil phenomenon. Infrared spectroscopy can show absorption peaks of the resin or oil. Rubies treated with oil or resin filling can be observed under a magnifying glass for the iridescent interference colors of the oil or resin and bubbles (Figures 5-6).
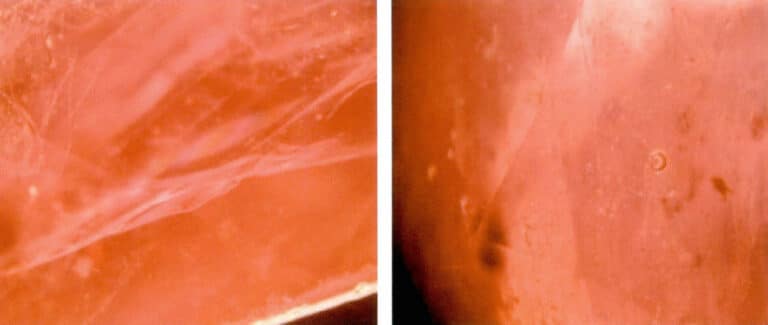
(2) Filling of high-lead glass
Due to the high refractive index and luster of lead glass, the higher the lead content, the greater the refractive index and the stronger the luster. Compared to traditional glass materials, lead glass’s optical properties are closer to ruby’s. Therefore, high-lead glass is a commonly used material for filling rubies in the market. It is worth noting that, as jewelry, too high a lead content is harmful to the body, so the lead content in high-lead glass filling for rubies should be controlled within a reasonable range.
① Filling method:
The glass components generally used for filling rubies are mainly borosilicate aluminum glass, aluminosilicate glass, and phosphate aluminum glass, which can form a molten body at 1500℃ to penetrate the fissures of the ruby, playing a role in repair and purification. The latest application of leaded glass has a strong fluidity of the material, low melting point (about 600°C), refractive index, and luster similar to ruby (strong glass luster), so it is easy to treat it as a natural product without careful observation.
② Detection method:
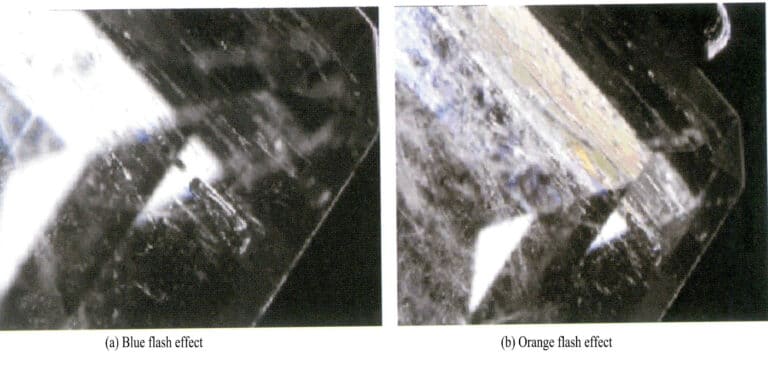
Lead glass fillings appear as white fibrous substances in the fissures of rubies [Figure 5-7 (a)], and over time, they will form yellow fibrous substances. Using a gem microscope for magnified inspection, the filled fissures often show blue or blue-green flashing effects [Figure 5-7 (b)]. In the filled fissures, a white cloudy substance different from the main body of the ruby is displayed.
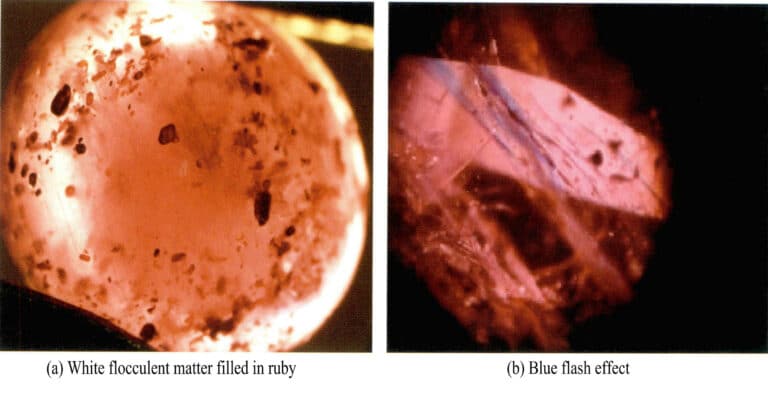
③ Glass filling repair:
Generally uses borosilicate sodium aluminum glass to fill in the ruby with notches or damage at the girdle or pavilion, achieving aesthetic and weight increase effects. This filling is usually localized micro-filling, with a small amount of filling, making it difficult to identify. During identification, carefully observe whether the ruby has damaged parts; if so, magnify to check for filling phenomena inside, and if necessary, use large instruments such as infrared spectrometers or Raman spectrometers for component analysis.
2.5 Composite stones and coatings
Corundum gemstone composite stones have various combinations; commonly seen types include combinations of rubies and synthetic rubies, a synthetic ruby base under a blue sapphire with green; the upper layer is natural blue sapphire, and the lower layer is synthetic blue sapphire, or the upper layer is light blue sapphire, and the lower layer is dark blue sapphire (Figure 5-8), etc.
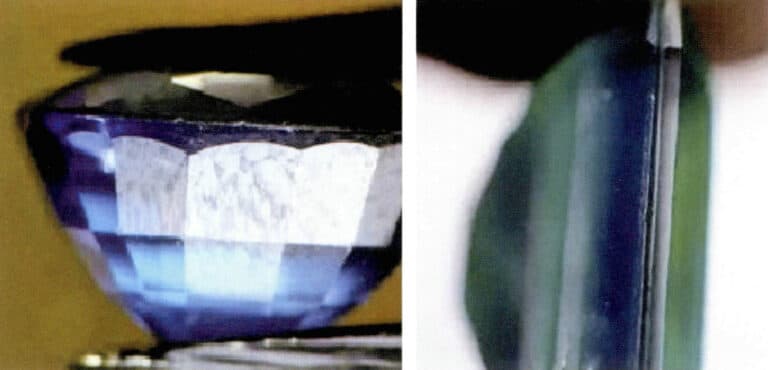
When identifying composite rubies or composite sapphires, it is important to carefully observe the color, luster, and inclusions between the assembled and upper and lower layers. With careful observation, one can find the differences between the two.
What is distinctive is the application of starlight through stickers or engravings. Stripes are applied to the bottom surface of natural or synthetic corundum gemstones using colored or metal pieces, or stripes are carved using relief methods. Chemical etching methods also result in three sets of engraved line patterns at 120° angles on the bottom surface of the gemstone, which look very much like starlight from the table view.
There are many optimization treatment methods for corundum gemstones. For example, overgrowth, involves growing a layer of synthetic corundum on top of synthetic or natural gemstones or coating the surface of corundum gemstones with a diamond film, etc.
2.6 Common methods of additive colour matching
Due to the numerous fissures in natural rubies, colorless or colored oils are generally used to dye rubies. After dyeing, the color of the ruby increases, the structure becomes more solid, and stability improves. It is relatively difficult to identify colorless oil-dyed rubies, and sometimes there may be abnormal fluorescence phenomena; identifying colored oil-dyed rubies is relatively easier, and magnified inspection can reveal color accumulation in the fissures, with lighter colors in areas without fissures. The color distribution is related to its structure (Figure 59). Sometimes, colored oil-dyed rubies may also exhibit fluorescence phenomena.
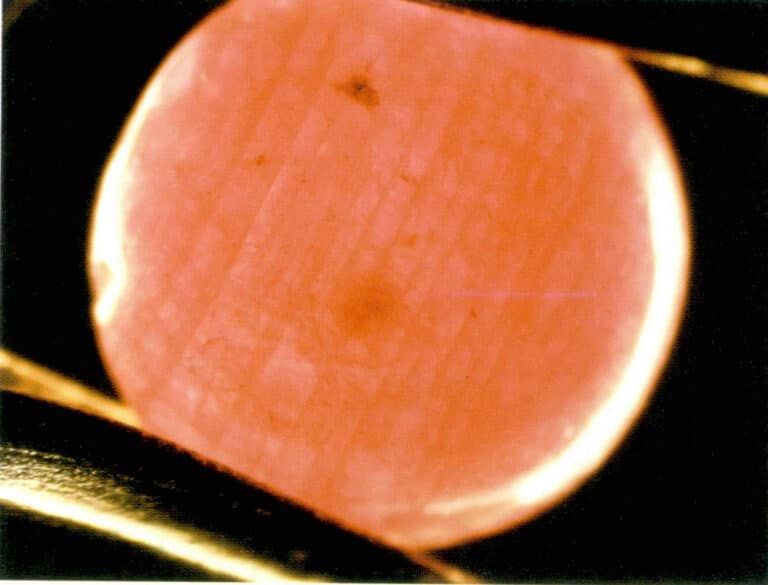
2.7 Identification of improved products
The type of gemstone is determined using conventional testing methods. First, determine whether the sample is a corundum gemstone or natural or synthetic. Then, carefully observe whether the growth lines and fingerprint-like inclusions in the gemstone are artificially implanted; artificially implanted inclusions are generally limited to the surface, and sometimes small bubbles from the synthesis can still be found.
It is easy to identify various color enhancement methods if one observes them. The key to this identification is knowing and considering the possible optimization treatments that may occur during the appraisal.
Identifying colorless oil dye is relatively difficult; generally, it is identified by the fluorescence properties of the oil. However, for oil without fluorescence, it is necessary to observe the blurred outlines of the fissures under a magnifying glass and then touch the suspicious areas with a hot needle to identify them by the emitted odor.
Gems improved by heat treatment can be sold as natural products. The key to identification is looking for evidence of high temperatures. Typical evidence of high temperatures includes unpolished inclusions that may remain after re-polishing, abnormal facets and girdles; there may also be stress fractures left by thermal expansion around included materials, as well as phenomena such as color band diffusion and knots; the absence of an iron absorption line at 450nm can also be observed in the absorption spectrum.
The process of eliminating purple or brown in rubies does not commonly show evidence of high temperatures due to the relatively low temperature.
Yellow stable color centers produced by irradiation can also be sold as natural products, but they are difficult to obtain; unstable color centers have no commercial value due to rapid fading.
The main identification features of high-temperature heat-treated rubies and sapphires are as follows.
(1) Fractures in gas-liquid inclusions
After the fingerprint-like inclusions are heated, the original isolated gas-liquid inclusions rupture to form connected, curved, concentric inclusions that resemble very long, curly, scattered water pipes on the ground, called plumbing healing fissures.
(2) Erosion of solid inclusions
Solid inclusions are eroded, forming circular or elliptical two-phase inclusions composed of glass and bubbles for low-melting-point inclusions; high-melting-point crystal inclusions take on a rounded frosted glass appearance or a surface pitted texture.
(3) Thermal treatment stress fractures
When crystal inclusions melt or decompose due to heating, they may induce or alter pre-existing stress fractures. Common phenomena include:
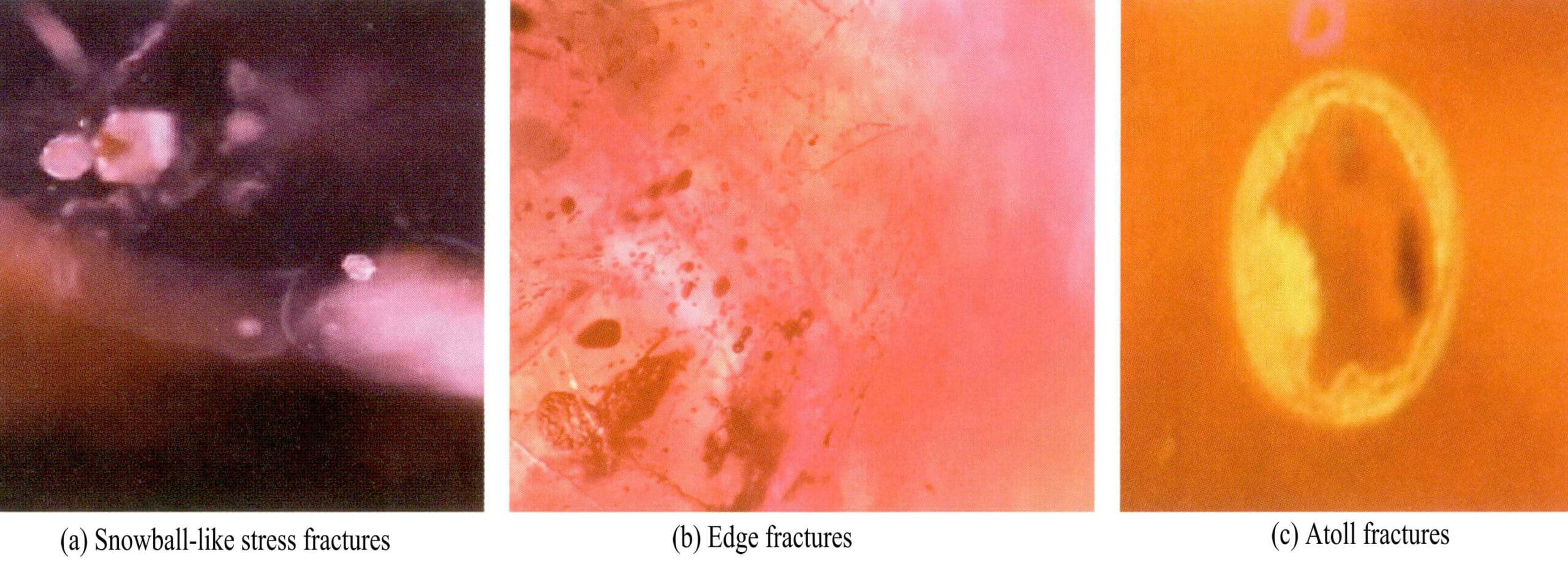
① Snowball:
The crystal inclusion completely melts to form a white sphere or disc, creating stress fractures around it [Figure 5-10 (a)].
② Fringe fractures:
If the crystal inclusion completely or partially melts, the melt may overflow into the fractures, forming a ring of droplets distributed around the crystal or filling other locations in the fractures. The overflow of the melt may also create high-contrast voids around the melted crystal [Figure 5-10 (b)].
③ Atoll fractures:
The crystal inclusion does not melt but forms stress fractures with atoll-like edges. This phenomenon is also visible in heat-treated rubies and blue sapphires, referred to as atoll fractures [Figure 5-10 (c)].
2.8 Diffusion Method Sapphire
(1) Diffusion Treatment of Corundum Gemstones
① Principle of Diffusion Treatment:
Iron, titanium, and chromium ions are introduced into the corundum crystal to replace aluminum ions. Under high-temperature conditions, the coloring ions enter the surface layer of the corundum, causing the gemstone to appear blue or red. The temperature for heat treatment should be just below the melting point of the gemstone, allowing the crystal lattice to expand and facilitating the migration of larger-radius coloring ions. Introducing different coloring ions will produce different colors in the gemstones, with titanium and chromium ions causing blue, chromium ions causing red, an appropriate amount of titanium ions producing a starlight effect, and beryllium ions causing yellow.
② Process of Diffusion Treatment
- Selection of Raw Materials: Colorless or lightly colored transparent natural corundum[Figure 5-11 (a)]. First, these corundumraw materials are polished into various shapes and sizes of rough stones, generally not polished after fine grinding, and then buried in a chemical agent primarily composed of aluminum oxide, containing some coloring ion components [Figure 5-11 (b)].
- Heating: After placing the sample in the crucible as shown in Figure 5-11, continue heating in a high-temperature furnace. The heating time can range from 2 to 200 hours, and the temperature rise ranges from approximately 1600 to 1850°C. Generally, the best temperature range is 0°C to 1800°C.
- Precautions: The corundumdoes not change below 1600℃, but the gem will melt at higher temperatures. Therefore, the heating temperature must be below the phase transition temperature of the corundum( 2050℃) ). During heating, generally at a higher temperature for a longer time, the depth of color penetration is also greater.
There is now a “deep” diffusion method, which differs from this long-term diffusion at high temperatures, using a method of multiple heating of the gem, that is, reheating after the gem cools. Repeated multiple times, with multiple diffusion, the treatment time must be over two months, and the gem’s color is deeper after treatment.
③ The results of diffusion treatment:
The color of the sapphire after diffusion treatment only exists on the surface of the gemstone (Figure 5-12). Robert and others in the United States have measured the thickness of the color layer from diffusion; their method involved cutting three faceted diffusion-treated gemstones perpendicular to the top facet, polishing the cut surface, and then measuring and observing it. Different thicknesses of the color layer introduced by surface diffusion can be seen on the cross-section, with variations in depth believed to be traces of multiple diffusions.
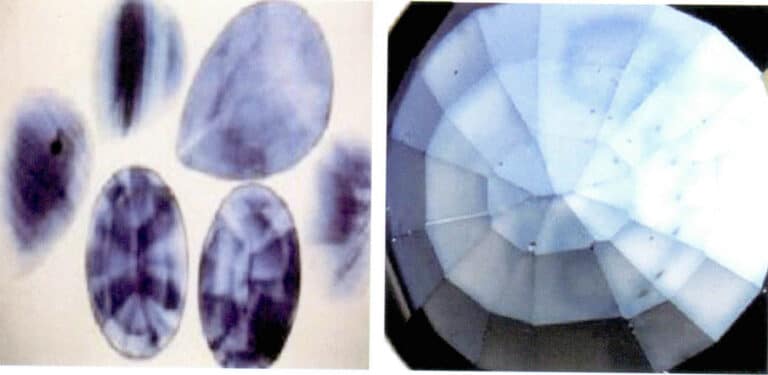
④ Evaluation of diffusion-treated gemstones
- Color origin: The color obtained by diffusion methods is due to the artificial addition of chemical substances other than natural components, and the color only exists on the surface, making the overall color of the gemstone uneven and inconsistent between the interior and exterior. It must be marked as a diffusion gemstone when sold. The letter ” u ” must be marked on the gemstone identification certificate, representing surface diffusion products.
- Pricing principles: The colors obtained by the diffusion method are the same as those formed by natural coloring ions, which have partially entered the lattice. Their physicochemical properties are stable, the preparation cost is not low, and the price should not be set too low. The general pricing principle is below natural sapphires and above synthetic sapphires.
(2) Identification of sapphires treated by diffusion
① Single magnification
- The treated sample’s surface shows partially reflected light and surface-sintered material, which can be partially or completely removed after polishing.
- Diffusion-treated gemstones, when polished lightly, often produce a double-layered band on the polished surface, and a diffusion layer can be seen under magnification.
- In the diffusion treatment of sapphire, deep concentrated colors and diffusion dyes are often deposited in the surface fissures or surrounding pores.
- There are often high-pressure fragments around the inclusions in the gemstone, with some inclusions melted or the “silk” of rutile partially melted into spots or absorbed.
② Oil immersion observation:
The most effective identification method for gemstones treated with diffusion heat is oil immersion observation. Immerse the sample in dibromo methane or other immersion liquids, and observe its appearance with the naked eye or under magnification, which has the typical characteristics of diffusion-treated gemstones.
- High protrusions: Due to the concentration of color, deeper color lines or high protrusions are noticeably present along the junctions of the facets and the girdle area.
- Spotty facets: Finished sapphires treated with diffusion heat often exhibit inconsistencies in color depth across some facets.
- The waist edgeeffect: For diffusion-treated gemstones, the waist is often completely colorless, and the entire waist is visible.
- Blue outline: Regardless of the medium they are immersed in, the edges of diffusion-treated gemstones are very clear, often showing a deep blue outline.
The color of diffusion gemstones observed with the naked eye varies in different solvents. Some other features, such as mottled facets, are more pronounced in glycerin or dichloromethane. The clearest is still dichloromethane, but this solvent is highly toxic.
The refractive index of chromium ion-diffused rubies is relatively high, reaching 1.788-1.790. Some diffusion-treated sapphires exhibit blue-white or blue-green fluorescence under shortwave ultraviolet light. There is also a type of blue diffusion sapphire obtained by diffusing Co2+ into corundum, which can be identified using a Chelsea filter. Under the Chelsea filter, cobalt ion-diffused sapphires appear red.
(3) The coloring mechanism and identification features of beryllium-diffused corundum gemstones.
① The process of beryllium diffusion in corundum gemstones:
In the high-temperature beryllium diffusion process for corundum gemstones, the introduction of beryllium ions is achieved through emerald (BeAl2O4)) powder, and there are two methods for this process.
- Flux method: Add chrysoberylpowder with a mass fraction of 2%-4% to a flux containing boron and phosphorus, and heat the gemstones coated with the flux in an oxidizing atmosphere at 1800℃ for 25 hours.
- Powder method: Mix chrysoberylpowder containing 2%-4% with high-purity alumina powder, or add 0.8% beryllium oxide to the alumina powder, then bury the gemstones in the mixture and heat at 1780℃ in an oxidizing atmosphere for 60-100 h.
② Characteristics of beryllium diffusion corundum gemstones
- During the high-temperature beryllium diffusion process, the element can diffuse throughout the gemstone. The colors of various colored sapphires and rubies can be significantly improved through beryllium diffusion.
- Gemstones treated with flux methods show excellent surface color consistency, while the color of gemstones treated with powder methods almost diffuses throughout the entire gemstone.
③ Coloration Mechanism
- The Role of Beryllium: Ions Beryllium ions act as stabilizers for iron oxide vacancy defect color centers generated at high temperatures, allowing them to remain stable when cooled to room temperature. Beryllium ions are not the direct cause of yellow coloration; rather, they improve the sapphire primarily by strongly absorbing in the blue region of the spectrum, resulting in a strong yellow tint (Figure 5-13).
- The Role of Iron Ions: The content of iron ions plays an important role in the beryllium enhancement process. Iron ions are the main ions responsible for forming orange-yellow coloration, and their coloration mechanism involves the formation of iron oxide vacancy defect color centers. Samples with low iron content appear brown after treatment, while samples with medium to high iron content exhibit yellow.

(4) Beryllium improves the characteristics and identification of gemstones
① Color:
Different colored gemstones will exhibit different colors after beryllium treatment, with varying degrees of yellow-orange tones. The colors produced by different colored sapphires after beryllium ion diffusion are shown in Table 5-3.
Table 5-3 Colors produced by different colored sapphires after beryllium ion diffusion
| Before improvement | Improved |
|---|---|
| Väritön | Yellow to Orange Yellow |
| Vaaleanpunainen | Orange-yellow to pink-orange |
| Dark Red | Bright red to orange-yellow-red |
| Yellow, green | Keltainen |
| Sininen | Yellow or no significant effect |
| Violetti | Orange-yellow to red |
② Instrument test for beryllium ion concentration
- Large instrument testing mainly tests beryllium content in diffusion corundum
- Secondary ion mass spectrometer, beryllium concentration on the surface of natural corundum(1.5-5)×10-6, and the surface beryllium concentration after beryllium diffusion is (1〜5)×10-7. If the Be content is above 1×10-5, further testing is required to confirm whether the corundum has undergone beryllium diffusion treatment.
- Plasma mass spectrometry and X-ray fluorescence spectrometry were used for chemical composition analysis, which revealed that the concentration of beryllium ions in the boron-diffused corundum was distributed in a regular pattern, with lower concentrations in the interior and higher concentrations on the surface.
- Color space: Place the gemstone in a dichloromethane immersion solution; the color space varies in thickness, with irregular secondary color bands.
- Other evidence: Under the microscope, it has the characteristics of high-temperature heat treatment inclusions: molten crystal pseudomorph inclusions, secondary inclusions distributed along the disc-shaped fracture surface (glassy or recrystallized), attached crystals, blue halos, etc.
Section II Beryl family gemstones
The beryl family includes various gemstones, generally named according to their color, such as colorless beryl, yellow beryl, red beryl, etc. The most precious variety is the green emerald, known as the king of green gemstones, which people have always loved. Only when the color reaches a certain concentration can it be classified as emerald. There are also common aquamarine, heliodor, etc. (Figure 5-14).

1. Gemological characteristics of beryl family gemstones
The chemical composition of beryl gemstones is Be3Al2Si60i8 • xH2O, and aluminum can be partially replaced by ions of chromium, iron, magnesium, manganese, and others. Pure beryl is colorless, and different coloring ions can produce different colors. If beryl contains a small amount of chromium and vanadium ions, it will form an emerald; if it contains a small amount of iron ions, it will form blue or blue-green aquamarine.
The crystal structure of beryl is mainly composed of hexagonal rings of silicon-oxygen tetrahedra. Beryl crystals are hexagonal columnar, and the column faces often have distinct parallel longitudinal stripes along the C-axis, sometimes developing into hexagonal bipyramids. Often, small amounts of chromium, iron, and manganese ions replace aluminum ions.
Pure beryl is a colorless transparent crystal, and beryl that only contains potassium ions, sodium ions, and other non-coloring ions is also a colorless transparent crystal; the green color of emerald is due to chromium or vanadium ions, and the color does not need improvement; beryl colored by iron and manganese ions is mostly green, yellow, yellow-green, or aquamarine, and most can undergo color enhancement through methods such as heat treatment and irradiation. The relationship between the color of beryl gemstones and the coloring ions they contain is shown in Table 5-4.
Table 5-4 The relationship between the color of beryl gemstones and the coloring ions they contain
| Jalokivilajikkeet | Väri | Color ion |
|---|---|---|
| Emerald | Bright green | Chromium ion or vanadium ion |
| Aquamarine | Sky blue | Fe2+ , or Fe2+/Fe3+ |
| Goshenite | Väritön | Ei ole |
| Pink beryl | Vaaleanpunainen | Contains Mn2+ , or Cs+ |
| Red beryl | Punainen | Mn3+ |
| Heliodor | Yellow-Golden Yellow | Fe3+ |
| Maxixe-type beryl | Sininen | Color center causes color, unstable |
Copywrite @ Sobling.Jewelry - Custom korujen valmistaja, OEM ja ODM korut tehdas
2. Optimization treatment and identification methods of Beryl family gemstones
Emerald has a slightly lower hardness and is relatively fragile. Natural emeralds contain certain fissures and inclusions, and many types of inclusions have indicative significance for the origin of emeralds. The inclusions and fissures inside emeralds can affect the value and stability of the gemstone, so most emeralds on the market have undergone optimization treatment.
The most common enhancement treatment for emeralds is fracture filling. Oil immersion can conceal the fractures in emeralds and improve transparency. Since oil’s refractive index is similar to emerald’s, it has a minimal impact on the gem’s luster.
Artificial resin filling is also a commonly used method. This method is more durable than oil immersion and can conceal inclusions more easily. However, artificial resin filling can cause irreversible damage to emeralds. After aging, the resin may turn brown or white, making the flaws more apparent.
Slight enhancement treatments have almost no impact on value. Since 2000, GIA certification has provided clarity treatment classification services for emeralds. The certification agency examines unset gems, and the emerald certificates will describe the clarity grades as slight, moderate, or significant. GIA certification emphasizes that the purpose of using the classification system is solely to evaluate the level of treatment, not to provide an overall clarity grade for the gem.
Common enhancement methods for beryl family gemstones include heat treatment, colorless oil (colored oil) filling, irradiation, substrate, coating, and overgrowth.
2.1 Heat Treatment Method
Heat treatment is commonly used for yellow-green beryl or green beryl-containing iron, and it is also suitable for orange beryl colored by both manganese and iron ions. Natural emeralds are rarely treated to change color.
(1) The forms of iron ions present in beryl
Due to various forms of iron ions in beryl, heat treatment can produce different effects. The specific forms of iron ions in the beryl structure mainly include three types:
① If Fe3+ replaces Al3+ , the gemstone appears yellow. As the content of Fe3+ decreases, it can change from golden yellow to colorless, and when containing a very small amount of Fe3+, it is colorless.
② If Fe2+ replaces Al3+, the gemstone does not show color and is colorless.
③ Iron ions exist in the channels of the beryl structure. According to previous studies, the presence of iron ions in the structural channels is believed to be related to the blue color of beryl. Generally, heat treatment has little effect on the color exhibited by these ions, and the coloring mechanism still needs further research.
When Fe2+, Fe3+exists simultaneously within the beryl, the gem often appears green or yellow-green. This type of gem can often be transformed into high-quality aquamarine through heat treatment, with the ideal color being a beautiful sea blue, and its physical and chemical properties are also relatively stable.
Heat treatment can transform orange beryl containing iron and manganese ions into beautiful pink beryl. There is also a type of deep red manganese beryl that can fade when heated to 500℃.
(2) Heat treatment conditions
① Heat treatment temperature: Due to the presence of water in the beryl structure, the heat treatment temperature is relatively low, generally between 250-500℃ and 400℃, and one must be very careful above 400℃. Usually, a few minutes is sufficient. If there is a lot of water, a milky state will appear below 550℃, indicating that the crystal structure has been damaged.
Some beryls can also be heated to high temperatures, such as some beryls from India and Brazil, heated to 700℃ without any change in gem color. This method is often used to eliminate some extremely fine inclusions and fissures.
② Precautions: Due to the many fissures in beryl during the heat treatment process, to prevent the gem from exploding, the heating and cooling must be done slowly, the time at the highest temperature should not be too long, and some protection for the gem is required. For example, these protective measures are quite effective when placing the gem in a closed crucible, filling the coal crucible with fine sand, or wrapping the gem in a clay lump.
2.2 Radioactive Irradiation Method
Radioactive irradiation has a significant impact on the color of beryl. After beryl is irradiated with rays of different energies, it can produce different color changes. Radio irradiation sources commonly include X-rays, high and low-energy electrons, etc. Due to concerns about radioactive residue, neutron irradiation from reactors is rarely used.
(1) Irradiation Methods and Gem Color Changes
Due to the presence of different impurity ions in beryl, different colors can be produced after irradiation. When a small amount of Fe2+ replaces A13+, irradiation can change colorless to yellow, blue to green, and pink to orange-yellow; these colors are stable under light. Maxixe-type colorless, green, yellow, and blue beryl can produce deep cobalt blue beryl after exposure to 7 radiation. The irradiated gemstones have no radioactive residue, but the produced cobalt blue beryl is unstable; the color obtained through irradiation can be transformed or faded back to its original color through heat treatment, and the color obtained through heat treatment can also be restored by irradiation. Most of the cobalt blue beryl currently on the market is beryl that has been irradiated.
Some beryl can produce different colors through different heat treatment atmospheres. For example, iron-containing yellow beryl can become colorless when heated in a reducing atmosphere; green beryl can turn into aquamarine. These colors are stable in light, but the original colors can be restored if irradiated with X-rays or γ radiation.
(2) Identification characteristics of irradiated beryl
Irradiated beryl is generally not easy to detect, but Maxixe-type irradiated blue beryl has the following distinguishing features: the color is cobalt blue, which is significantly different from the sky blue of aquamarine; its visible light absorption spectrum has two absorption bands in the red region (695nm, 655nm), and there are weaker absorption bands in the orange, yellow, and yellow-green regions at 628nm, 615nm, 581nm, and 550nm (some sources also report absorption bands at 688nm, 624nm, 587nm, and 560nm), which are not found in aquamarine. When observing pleochroism, the blue color of Maxixe-type blue beryl appears in the direction of normal light. In contrast, it is mostly colorless in the direction of extraordinary light, whereas in aquamarine, the deep color appears in the direction of extraordinary light. Maxixe-type blue beryl is rich in the metal Cs, with a density of 2.80 g/cm3 and a refractive index of 1.548-1.592, both of which are higher than those of other varieties of beryl.
2.3 Some methods of addictive color matching
Emeralds often have many internal fissures, so they need to be filled to conceal the fissures and improve the stability of the gemstone. After filling treatment, emeralds can also improve the color and clarity of the gemstone.
(1) Injection filling method
The injected oils include various vegetable oils, lubricating oils, liquid paraffin, turpentine, and resins, which can be mixed and injected using one, two, or several materials. The injection methods for emeralds are divided into colorless oil injection, colored oil injection, and resin injection treatment. The injection method is a commonly used optimization treatment for emeralds.
① Colorless oil injection:
After the gemstone undergoes colorless oil injection treatment, the fissures are filled and concealed, making them difficult to detect with the naked eye, thus improving the transparency and brightness of the gemstone. This treatment is recognized by the international jewelry industry and consumers and is very common in the market. The equipment required for colorless oil injection is simple and easy to operate, and the injection steps are as follows:
- Clean the gemstone in ethanol or ultrasonic cleaning, then dry it.
- Soak the gemstone in oil with a refractive index close to emerald under vacuum, pressure, or heating conditions for some time.
The purpose of injecting colorless oil is to “hide fissures,” allowing more gemstone fissures to be filled, making them less noticeable to the naked eye. Upon magnified inspection, the oil appears mostly colorless in surface fissures; over time, it may turn light yellow (Figure 5-15). Under long-wave ultraviolet light and yellow-green fluorescence can be seen, and oil may be exuded upon contact with a heated needle. This practice is commercially accepted, considered optimization, and does not need to be specified; it can be sold as a natural product.
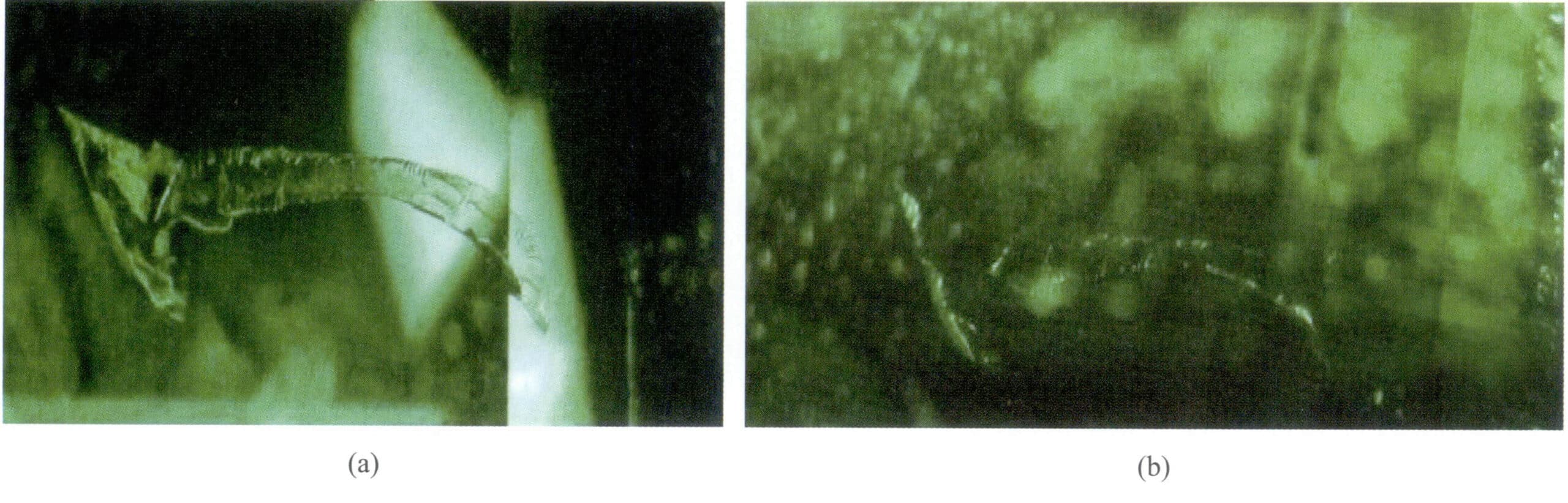
② Colored oil injection:
The method of colored oil injection is the same as that of colorless oil injection. The purpose of this treatment is not only to conceal the gemstone’s micro-fissures but also to change the gemstone’s color. Colored oil injection is divided into two cases: inject colored oil into emeralds to enhance their color and increase their value and inject beryl with many fissures, serving as a substitute for emeralds.
After the emerald is injected with colored oil, it will exhibit the following characteristics, which can be used to determine whether it has been injected with colored oil.
- The dye is distributed filamentous along the fissures and can be seen under magnification with a glass or microscope. A flashing effect can be observed under bright or dark conditions, with abnormal interference colors (Figure 5-16).
- After treatment, the gemstone will release oil and gas from the fissures when heated, and traces of oil can be wiped off with a cotton swab.
- Colored oil can emit strong fluorescence under ultraviolet light.
③ Resin treatment:
After the emerald undergoes resin treatment, the filling area appears misty, with visible flow structures and residual bubbles. Under reflected light, a network of fissure fillings can be seen. Abnormal interference colors are visible. The filling material has low hardness, can be pierced by a steel needle, and has weak luster.
Observing the filling material under a gem microscope, using different lighting and magnification to examine the filling areas of the emerald, can provide important identification information.
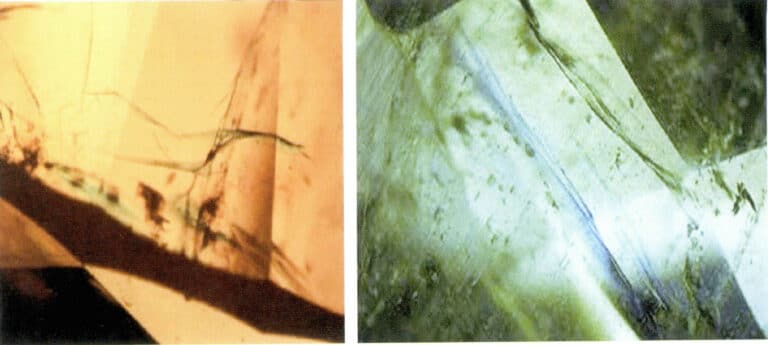
- Flash effect: The flash effect can often be observed in the filled fissures, caused by the different scattering of light by the emerald and the filling material (such as epoxy resin). Under bright conditions, the filling fissures show blue to purple reflected light, while under dark conditions, inclined observation can change it to orange flashes (Figure 5-17).
- Bubbles and Residues: Natural emeralds contain bubbles often found in two-phase or three-phase inclusions. The bubbles are spherical and not distinctly shaped. Bubbles in filled fissures are very obvious and are often flattened. Fissures filled with oil may show a brown flash effect when observed against a bright background due to oxidation, while oxidized residues can form branch-like features.
- Infrared Spectroscopy: Different filling materials have their characteristic absorption peaks, such as the characteristic absorption peaks of olive oil at 2584 cm-1 and 2924 cm-1; the characteristic peaks of palm oil at 2852 cm-1, 2920 cm-1, 3004 cm-1; and the characteristic peaks of epoxy resin at 2925 cm-1, 2964 cm-1, 3034 cm-1, 3053 cm-1. Infrared spectrometers can classify and analyze the components of filling materials, with 2800-3000 cm-1 strong absorption peaks and 3058 cm-1, 3036 cm-1 absorption peaks serving as evidence of resin filling in emeralds.
- Diamond View: The Diamond View can quickly, clearly, and accurately determine whether an emerald has been treated with filling. Observations through the Diamond View allow for a clear view of color bands, color patches, and the distribution of all fissures that are not visible or observable under a microscope. Most importantly, it can distinguish whether there are filling materials within the fissures; under ultraviolet fluorescence, unfilled fissures exhibit blue-white fluorescence, while filled fissures show light yellow-green fluorescence. This allows for determining whether the sample is filled, the area of filling, and the location of the filling. However, the Diamond View also has certain limitations; when color bands are quite pronounced and exhibit strong red fluorescence under ultraviolet light, it may affect the observation of fissure fillings.
- Raman Spectroscopy: The Raman spectrometer can quickly determine the inherent frequency, symmetry, internal forces, and general kinetic properties of molecular vibrations in gemstones, allowing for rapid and effective analysis of the components of inclusions within gemstones. Since different filling materials have different laser Raman spectral characteristics, laser Raman spectrometers can be used to classify and analyze the components of filling materials. The characteristic peak of the gel is 1602 cm-1, 1180 cm-1, 1107 cm-1, 817 cm-1, 633cm-1, and the presence of these absorption peaks can serve as important evidence for whether the emerald has undergone gel filling treatment. However, this method also has certain limitations; when the internal filling material is not near the gemstone’s surface, it is difficult to focus, and the results may not be ideal.
Currently, there are differences in the expression of identification conclusions regarding the filling treatment of emeralds between some domestic and foreign jewelry testing laboratories. Foreign identification certificates usually state “natural emerald” in the conclusion while indicating the degree of filling in the remarks section. Based on the filling material and the degree of filling, it can generally be classified into five levels: none, not obvious, slight, moderate, and obvious. On the other hand, domestic identification certificates directly indicate “emerald (filling treatment)” in the conclusion.
(2) Dyeing and Coloring
Since beryl is a single-crystal gemstone, the dyeing effect is far inferior to that of agate, and generally, gemstones with more fissures are chosen for dyeing. The dyeing and coloring of emeralds are merely remedial measures to enhance color. After dyeing, the color of emeralds is often concentrated in the fissures, resulting in uneven color distribution. When observed with a spectroscope, natural emeralds exhibit a distinct Cr absorption spectrum, while dyed emeralds may show absorption bands formed by the dye at 630-660nm.
(3) Substrate
Substrate is a traditional treatment method, usually involving placing a green film at the bottom of the emerald to enhance its color. Upon magnified inspection, the junction between the green film and the gemstone can be observed at the bottom of the emerald; over time, the film may wrinkle or peel off, and bubbles can be seen at the junction. Treated emeralds show a very vague or even absent Cr absorption spectrum under a spectroscope, with weak or no dichroism.
(4) Overgrowth
A very thin layer of emerald or aquamarine crystals grows on the surface of the light-colored beryl. The identification feature is that the growth layers do not have the inclusion characteristics of natural emeralds but have the inclusion characteristics of synthetic emeralds.
(5) Coating
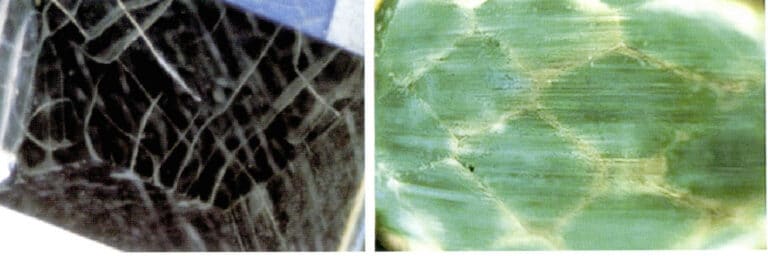
A very thin film is coated on the surface of the emerald, which may be a colorless film or a colored film. The surface of the coated emerald often produces various network-like and radial fissures (Figure 5-18), with color concentrated on the surface; inside, natural beryl’s tubular, raindrop-shaped, and gas-liquid two-phase inclusions can be seen; the outer layer shows synthetic emerald inclusions.
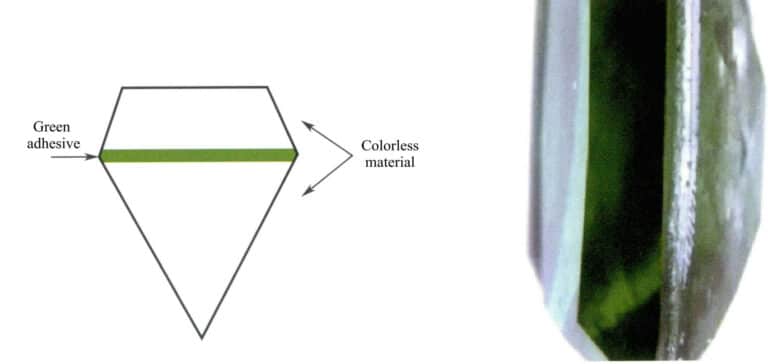
(6) Composite
Emerald-composite stones are often made up of light-colored emeralds and green dye layers, which can be seen under magnification as layers of adhesive and inclusions in the emeralds. The orange region shows a distinct absorption spectrum caused by the dye. There is also a common imitation of emerald composite stone—sudarite (Figure 5-19), with colorless or light-colored glass on the top and bottom layers and green glue in the middle. When observed magnified parallel to the waist ridge, a small amount of deep green adhesive material containing bubbles can be seen at the bonding surface.
Common optimization treatment methods and identification features of emeralds are summarized in Table 5-5.
Table 5-5 Common optimization treatment methods and identification features of emeralds
| Processing method | Processing result | Identification features | Optimization or processing |
|---|---|---|---|
| Oil immersion | Soaking in Colorless Oil | The filling position has a flashing effect, oil will come out after heating, and colored oil is distributed in a filamentous manner along the fissures | Optimization |
| Soaked in colored oil | Hoito | ||
| Filling glue | Filling resin | Flash Effect | Hoito |
| Dyeing and coloring | Introducing green dye into fissures | Color concentrated in fissures | Hoito |
| Substrate | Add a layer of green film at the bottom of the emerald | Method to check for visible junction seams, where there may be bubbles, weak dichroism, and the Cr absorption spectrum is not obvious | Hoito |
| Overgrowth | A layer of darker synthetic emerald grows on top of the light-colored emerald | The characteristics of the inner and outer layers are different. | Hoito |
| Coating (regeneration) | A synthetic emerald film grows on the outer layer with natural emerald at the center. | The outer layer of emerald is prone to network and radial fissures | Hoito |
| Composite | Made from two or more types of materials, commonly seen are natural emerald and synthetic emerald, natural emerald and green film, etc. | There are bubbles in the assembly seam, and there are differences in refractive index, luster, etc. of different materials. | Hoito |
Section III Diamond
1. Gemological characteristics of diamonds
Diamonds have high hardness, melting points, insulation properties, and chemical stability. The composition of diamonds is the element C; pure diamonds are colorless and transparent, while diamonds containing different impurities can exhibit different colors. The quality of color plays a decisive role in the evaluation of diamonds. The grading of diamond color is very strict, with flawless and completely transparent being the highest quality; even a slight hint of color can cause prices to plummet. However, colored diamonds are an exception, as the price difference among different colors of colored diamonds can be significant. The common colors of diamonds are colorless and yellow (Figure 5-20).

Diamonds are commonly found in two types of mineral deposits: kimberlite and lamproite. The first kimberlite was discovered in South Africa in 1870, and to date, over 5,000 kimberlite bodies have been discovered worldwide, with over 500 containing diamonds. The production of gem-quality diamonds in lamproite is very low, accounting for only about 10% of the total.
Due to diamonds’ high hardness and strong dispersion, they possess a unique charm and have always been loved by people. Therefore, optimizing the treatment of lower-quality diamond rough has also been a research focus for many gemologists and merchants. There are many methods for optimizing diamonds, such as irradiation, high-temperature and high-pressure treatment, laser drilling, and filling of fissures. Most colored diamonds that have been optimized are due to artificial irradiation, causing internal structural defects in the diamonds, resulting in different color centers that are fundamentally different from the color formation of naturally colored diamonds.
The color formation of diamonds is mainly related to the types of impurities and changes in structural components; different colors have different formation types. The common colors of diamonds and their formation causes are as follows (Table 5-6).
Table 5-6 Types of Causes for Diamond Color
| Diamond Color | Syy |
|---|---|
| Sininen | Contains B Element |
| Keltainen | Contains N Element |
| Pink, Brown | Plastic deformation |
| Vihreä | Color center causes color |
| Black | Inclusion causes color |
2. Optimization treatment and identification methods of diamonds
Due to diamonds’ unique charm, more than diamond production is needed. The methods for optimizing diamond treatment are also constantly improving. The optimization treatment of diamonds mainly includes two aspects: one is to improve the color of diamonds; the other is to treat the inclusions in diamonds to enhance their clarity. Since 1950, irradiation treatment has been used to improve the color of diamonds. With technology to remove dark inclusions in diamonds, laser drilling and filling of fissures gradually developed in 1960. Since 1990, further improvements have been made in crack filling and laser drilling. Synthetic diamond technology has also promoted the optimization treatment of diamonds. Since 2000, high-temperature and high-pressure treatment (HPHT) has improved diamonds with brown and brownish tones.
The multiple treatments of diamonds first appeared in the 1990s to the early 21st century, initially mainly seen in clarity treatments. During the diamond identification process, it was found that diamonds had undergone laser drilling treatment, followed by glass filling along the laser channel; there were also cases where diamonds underwent two filling treatments to improve clarity. With the emergence and maturation of high-temperature and high-pressure treatment methods and irradiation followed by high-temperature quenching techniques, multiple treatments began to change the color of diamonds.
The color of a diamond is an important factor in determining its quality; the higher the color grade, the higher the value. The optimization treatments for diamonds, such as irradiation, traditional coating, substrate, and HPHT, are mostly aimed at improving the color of diamonds. Some optimization methods focus on enhancing the clarity of diamonds, such as laser drilling. The main optimization treatment methods for diamonds include five types: using irradiation treatment to change the color of diamonds; filling and laser drilling methods are used to improve the clarity of the diamonds; surface treatments of diamonds, including surface coatings and filming; high temperature and high-pressure treatment (HPHT); the combination treatment of diamonds.
2.1 Irradiation Treatment
Irradiation can cause diamonds to produce different color centers, thereby changing the color of the diamond. After irradiation treatment, diamonds can almost present any color, and the improved color is stable. This treatment method is suitable for colored diamonds, but irradiation treatment cannot improve the color grade of colorless diamonds above grade K. The residual radiation from diamonds treated with irradiation poses potential hazards to human health, limiting consumer acceptance of irradiated gemstones.
The essence of irradiation is to use a radiation source to generate high-energy ions or rays, causing damage to the diamond structure and creating color centers. Radioactive irradiation can improve the overall color of diamonds. The principle is that irradiation damages part of the diamond lattice, forming disordered areas and point defects. The structural defects affect the gemstone’s absorption of visible light, increasing specific absorption of certain wavelengths of light, thus resulting in color.
The time and dose of irradiation are controlled according to the desired color. The deeper the required color, the longer the irradiation time and the greater the dose. Irradiated diamonds are often yellow-green, green, blue-green, and other colors.
Different types of diamonds can produce different colors, and different radiation sources can also produce different colors. There are four common radiation sources, and the irradiation process and the resulting colors are shown in Table 5-7.
Table 5-7 Radiation Sources and Improved Colors
| Radiation source | Processing process | Final colour |
|---|---|---|
| 60Co | Long irradiation time, unstable color | Green, blue-green, pink-red, golden yellow, etc. |
| Radium salt | Cyclotron irradiation, not commonly used | Green color, black color can be formed after a long time |
| Neutron treatment | Overall color, stable color, most commonly used | Heat treatment at 500 to 900°C produces brown, yellow, orange, or pink-purple colors |
| Electron treatment | Overall color, more commonly used | Light blue-green, heat-treated to produce orange-yellow, pink, brown |
① 60Co Irradiation:
Using 60Co to produce γ radiation diamonds can generate green, blue-green, pink-red, golden yellow, etc. However, it takes a long time, and the color is unstable; this method currently needs to be used.
② Radium salt irradiation:
Diamonds irradiated by a cyclotron can produce green; if the heating time is longer, black can be produced. However, the color is limited to the surface and can produce radioactive residues.
③ Neutron treatment:
Diamonds are placed in a nuclear reactor and bombarded with neutrons, which can directly penetrate the diamond, producing stable green and blue-green colors. After irradiation, heating to 500-900℃, type I a diamonds can produce yellow and orange-yellow; type I b diamonds produce pink and purple-red. This method is relatively commonly used.
④ Electronic treatment:
Treated diamonds can produce light blue or bluish-green colors, are limited to the surface, have no radioactive residue, and have good stability. Heating to 400℃ can produce orange, yellow, blue, brown, etc. This method is relatively common.
Colored diamonds obtained through irradiation treatment can be distinguished by color distribution, absorption spectrum, fluorescence spectrum, or conductivity. Different colors of irradiated colored diamonds have different absorption spectra. The colors after irradiation are relatively stable, but it must be noted at the time of sale that they fall under the category of treated in gemstone optimization treatment. If irradiated diamonds contain radioactive residue, they must be placed until the content is below national standards before being marketed.
(1) Absorption spectrum
In diamonds, there are generally trace amounts of nitrogen atoms. These nitrogen atoms have two modes of occurrence: one replaces carbon atoms in the lattice in a monatomic form, such as nitrogen atoms becoming nitrogen donors, causing the crystal to exhibit characteristic yellow; the other form exists in aggregates within the crystal. Whether it is an aggregate composed of two adjacent nitrogen atoms or one composed of four nitrogen atoms, no absorption occurs in the visible light range, resulting in no color.
Nitrogen-containing colorless diamonds can produce yellow after irradiation and heating treatment. This yellow color is believed to be caused by H3 (503nm) and H4 (496nm) color centers, with H4 color centers being dominant, while natural yellow diamonds do not have H3 or H4 color centers or are not obvious. The absorption lines caused by H4 color centers in the absorption spectrum show that the diamond has been irradiated. However, the absence of H4 color centers does not necessarily indicate that the diamond’s color is natural.
Additionally, irradiated yellow diamonds can also exhibit absorption lines at 595nm. In 1956, researchers from GIA discovered that diamonds treated with irradiation and heat had an absorption peak at 595nm, which natural diamonds do not have. Although later studies found that this absorption peak could disappear under high-temperature treatment (greater than 1000℃ ), two new absorption peaks at 1936nm (HIb) and 2024nm (HIc) would appear. Therefore, any absorption peak at 595nm, 1936nm, and 2024nm can be considered diagnostic spectral lines for artificially irradiated diamonds. Given current technology, it is impossible to have irradiated diamonds without both the 595nm absorption line and the HIb and HIc absorption lines. Thus, any of the three absorption lines appearing at 595nm, 1936nm, and 2024nm can serve as identification features for treated diamonds.
Irradiated blue or green diamonds exhibit an absorption line at 741nm at the end of the red region. However, natural green diamonds can also have this absorption line.
The characteristic absorption line for irradiated pink and purple diamonds is at 637nm, and an additional 595nm, 575nm absorption line may also appear. The 637nm absorption line is the diagnostic line for pink-treated diamonds. Naturally colored pink diamonds primarily show a broad band at 563nm. Blue diamonds coated on type Ia diamonds often display N3 centers and an absorption band at 415nm. In comparison, natural blue diamonds are colored by boron and do not show the 415nm absorption peak. Natural blue diamonds are also conductive, whereas irradiated blue diamonds are not.
(2) Color distribution characteristics
Natural-colored diamonds have color bands that are linear or triangular, with the color bands parallel to the crystal faces; the color of irradiated diamonds is limited to the diamond’s surface; the color of diamonds after irradiation only exists on the surface, often presenting dark marks at the edges of the surface facets. For diamonds treated with a cyclotron, the color is only on the surface, and the distribution pattern of the color is related to the diamond’s cut and the direction of irradiation (Figure 5-21).
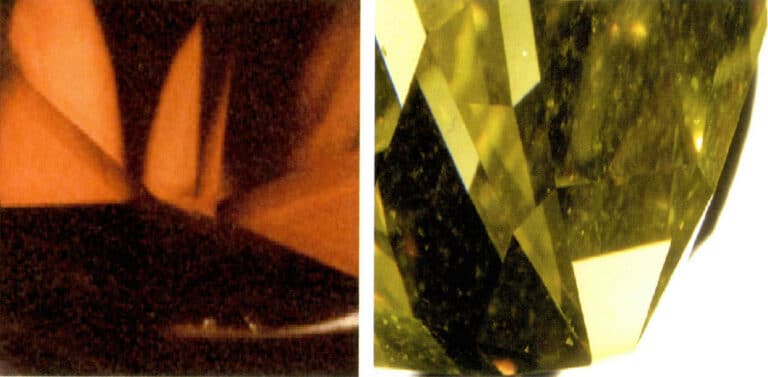
When the irradiation method bombards a brilliant-cut diamond from the pavilion direction, an “umbrella-shaped” color distribution can be observed around the tip of the pavilion when viewed from the table, also known as the umbrella effect; when the radiation starts from the crown direction, a dark ring can be seen around the girdle; if the diamond is bombarded from the side, the side closer to the radiation source will have a deeper color.
(3) Conductivity
Natural type IIb blue diamonds have conductivity, while blue diamonds treated with irradiation do not have conductivity.
(4) Others
Diamonds treated with radium often show strong residual radioactivity. When this treated diamond is placed on photographic film for some time, a blurred image of the diamond may appear on the film after exposure, which is caused by the radioactivity in the diamond.
2.2 Laser Impurity Removal and Fracture Filling
Laser treatment removes dark mineral inclusions from diamonds, and materials such as resin or glass fill the fractures.
(1) Treatment Methods and Processes
Focus the laser on the diamond to vaporize it, targeting the location where mineral inclusions need to be removed while using the laser to vaporize the mineral inclusions, and then fill the small holes left with a substance that has optical properties similar to diamond by melting it with the laser.
KM laser treatment is a new method that has emerged recently. Laser heating on inclusions connects internal natural fissures with surface fissures, and acid treatment is used to remove dark inclusions. This method suits diamonds containing dark inclusions very close to the surface. After treatment, it generally contains “zigzag” channels extending from the inside to the surface.
(2) Identification of diamonds treated with laser drilling
Under magnifying glasses and gem microscopes, it can be observed that diamonds treated with lasers and filled with fissures have the following characteristics:
① Due to the permanent laser holes on the diamond’s surface and the hardness of the filling material being much lower than that of the diamond, it will form relatively hard-to-detect pits on the surface of the diamond.
② Rotate the diamond and observe the linear laser channels. The laser channels are more pronounced due to the differences in refractive index, transparency, and color of the filling material compared to the diamond (Figure 5-22).
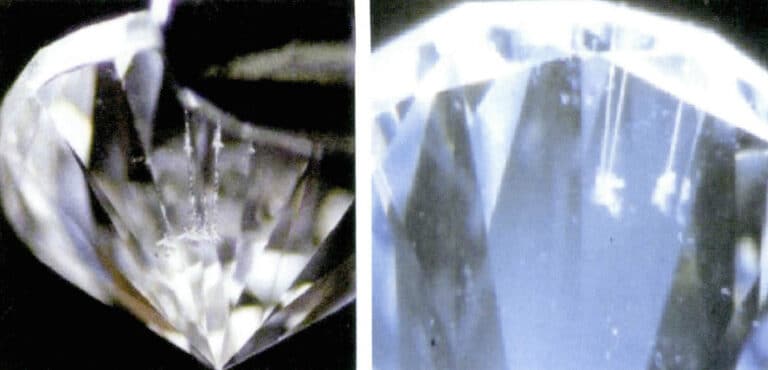
③ There is a difference in color and luster between the laser-filling material and the surrounding diamond (Figure 5-23).

(3) Identification of diamonds with fissure filling treatment
The vast majority of filled diamonds currently on the market can be identified using conventional instruments, exhibiting the following significant characteristics:
① Flashing Effect: When observing the filled fissure surface under magnification, it exhibits an orange-yellow, yellow-green, or purplish-red flashing effect. This flashing phenomenon can show different colors at different positions on the fissure surface, and the flashing color can change with the rotation of the sample (see Figure 5-24).

② Observing Fissure Surface: Characteristics Filled diamonds will exhibit some obvious features when the fissures are filled, including irregular bubbles, flow marks, and fibrous structures of the filling material within the fissures. The filling material may appear light brown or brown-yellow when thick. Sometimes, some filling material may remain on the diamond’s surface, and the luster and color of the filling material at the fissure surface still show subtle differences compared to the diamond.
③ Observing Diamond Color: After fissure filling, the color of the diamond may also change. Under a tenfold magnifying glass, a hazy bluish-purple tone often appears.
In addition to identification using conventional instruments, large detection instruments such as Raman spectrometers, energy spectrometers, and X-X-ray imaging technology can also be used to analyze the filler’s composition, phase, and filling characteristics.
2.3 Surface Treatment
(1) Surface Coating
The oldest method to change the yellowish body color of diamonds is to color the surface of the diamond to mask the true body color. This is a traditional surface treatment method aimed at improving the yellowish body color of diamonds. There are two common methods: the first is to apply a blue substance to the girdle of the diamond, which can significantly improve the yellowish body color, raising the diamond by 1 to 2 color grades; the second is to coat the surface of the diamond with a layer of colored oxide film, which also results in a noticeable improvement in color after coating, and this coating is relatively durable.
Identification method: Observing under a high-power microscope reveals a rainbow-like surface luster, and boiling in strong acid for a few minutes can also cause the surface color to fade. The coated diamond appears orange overall. Since the hardness of the diamond coating material is lower than the diamond’s; scratches are commonly seen on the coating surface (Figure 5-25).

(2) Diamond Coating
Diamond coating is gradually improved from the diamond coating process and is an application of modern technology in gemstone surface treatment.
① Process Method:
Under low pressure and medium temperature conditions, a layer of synthetic diamond or diamond-like carbon film is formed on the surface of diamonds or other materials using a chemical deposition method. The initial process was relatively simple, and the synthetic diamond film was polycrystalline, making it easy to identify. This diamond film is a polycrystalline material composed of carbon atoms with diamond structure and physical-chemical properties, with a thickness generally ranging from tens to hundreds of micrometers. It can be as thick as several millimeters.
According to reports, the American Sumitomo Electric Industries has developed a method to coat nearly colorless natural diamond octahedrons with a synthetic diamond film in sky blue that is up to 20 mm thick. A small amount of blue diamond film is coated on faceted diamonds to cover slight yellow tones and enhance the color of the diamond.
② Identification characteristics of coated diamonds:
Diamonds that have undergone coating treatment generally have a transparent film with the desired color, which can fill in the pits on the gem’s surface, making it smooth and increasing its luster, as well as enhancing the color concentration of the gem. There are often spots or granular areas at the edges where the gem contacts the mounting metal, and the film can also be removed with acid.
Since the film is a polycrystalline aggregate, it has a granular structure that can be easily distinguished from the single crystal of diamond when observed under high magnification microscopy.
Diamond films deposited using chemical vapor deposition or ion beam deposition methods can be checked for color by immersion in oil, specifically by immersing the diamond in dibromo methane, which will produce interference colors on the diamond’s surface. Most of the successfully synthesized diamond films or diamond-like carbon films studied so far are polycrystalline thin films, which have poor transparency and are easier to identify than single-crystal diamonds.
Large instruments such as scanning electron microscopes and Raman spectroscopy can also test and analyze diamond films.
2.4 High-Temperature High-Pressure (HPHT) Treatment
High-temperature, high-pressure treatment involves placing brown diamonds, which have color defects due to plastic deformation, in a high-temperature, high-pressure furnace to restructure their crystal structure and create color centers, thereby improving the color of the diamonds. This is a new optimization treatment method for diamonds, with a very small yield, which is insufficient to meet global diamond 1%.
There are mainly two types of high-temperature, high-pressure treated diamonds, type I a and type II a. Type I a brown diamonds contain color-causing impurities such as nitrogen atoms and vacancies within their crystal structure, which cannot be eliminated under the current high-temperature, high-pressure treatment conditions to improve their color grade. Only based on the existence of lattice defects in the diamond crystal can high-temperature high-pressure treatment enhance its plastic deformation strength and promote the generation of lattice defects to achieve color modification. Generally, through high-temperature, high-pressure technology, brownish-yellow can be transformed into yellow-green, golden-yellow, and a small amount of pink and blue, among others.
High temperature and high-pressure treatment can help type IIa brown diamonds overcome the barriers they face, prompting their structure to reorganize under high temperature and high-pressure conditions, restoring to the initial stable state before plastic deformation, thus changing their color to colorless (Figure 5-26).

(1) The process of high-temperature and high-pressure treatment of diamonds
High temperature and high-pressure laboratory simulations mimic the natural environment for diamond crystal growth, artificially controlling temperature, pressure, and medium conditions, providing sufficient activation potential for defects and impurity atoms within the diamond crystal, intensifying the strength of plastic deformation, thereby improving or altering the lattice defects in the diamond to achieve color change.
Diamonds treated by HPHT mainly fall into two types: brown type IIa and type Ia diamonds. The main treatment methods are as follows:
① Select diamond rough stones or raw stones, choosing samples with fewer fissures and inclusions.
② Determine the heating and pressurization rates to avoid rapid heating that may cause brittle fracture.
③ Reach the maximum temperature and pressure, maintaining for some time; the temperature and pressure conditions vary for different treatment objects. The treatment temperature for type Ia diamonds is about 2100℃. The pressure is (6-7)x109Pa, with a stabilization time of 30 minutes; type IIa diamonds require a slightly lower temperature, around 1900℃, with pressure similar to type Ia diamonds, and a longer stabilization time, requiring several hours.
④ After treatment, first reduce pressure and then slowly lower the temperature, allowing sufficient time for the vacancies in the crystal structure to reorganize and stabilize.
⑤ Remove the sample and re-polish the rough diamond.
Two main types of diamonds are treated with high temperature and high pressure: the GE-POL diamond from GE Company in the United States and the Nova diamond.
(2) GE-POL diamond
The GE-POL diamond uses a new method for color optimization treatment, the high-temperature and high-pressure repair method. This technology, developed by General Electric (GE) in the United States, improves the color of diamonds under high temperatures and high-pressure conditions. It is called the GE-POL diamond because it is a new product exclusively sold by the Israeli subsidiary POL in 1999. The technology involves treating natural diamonds with high temperature and pressure to enhance their color grade, typically improving them by 4〜6 levels. The rough diamond must have a color grade of J or above and be free of impurities, qualifying as a high-clarity type IIa diamonds. Brown and gray type IIa diamonds can be treated to become colorless diamonds. At the same time, diamonds treated with HPHT may also deepen or change color, occasionally resulting in light pink or light blue, reaching the level of fancy diamonds.
Identification features of GE-POL diamonds: The color grades of treated diamonds mostly range from D to G, with slightly cloudy and brown or gray tones. Under high magnification, internal textures of GE-POL diamonds can be seen, commonly featuring feather-like fissures accompanied by reflections. fissures often extend to the diamond’s surface, with some healed fissures, cleavage, and abnormally shaped inclusions. Some treated diamonds exhibit unusually pronounced strain under orthogonally polarized light, resulting in abnormal extinction phenomena. This method treats diamonds like natural diamonds, making identification relatively difficult. General Electric has promised that all diamonds they treat will be laser-engraved with the words “GEPOL” on the girdle surface.
(3) Nova diamond
The high-temperature and high-pressure treatment method transforms type Ia natural brown diamonds into colored diamonds. Previous research suggests that the coloration of brown diamonds is due to dislocations and associated point defects generated by plastic deformation after the diamond’s formation. In 1999, Nova Diamond in the United States used high-temperature and high-pressure technology to treat common type Ia brown diamonds into vibrant yellow-green diamonds, also known as high-temperature and high-pressure enhanced or Nova diamonds.
Nova diamond identification characteristics: This type of diamond exhibits a yellow-green color, with some crystals containing graphite inclusions and surface etch pits. After high-temperature and high-pressure treatment, the diamond structure undergoes significant plastic deformation, showing pronounced abnormal extinction, displaying strong yellow-green fluorescence accompanied by chalky fluorescence, and featuring a characteristic 529nm spectral line and 986nm absorption spectral line.
2.5 Combining treatment
Diamond combining treatment includes two situations: one is to combine two small diamonds into a larger diamond; the other is to use a diamond as the crown (or upper part) and a colorless transparent sapphire or glass as the pavilion (or lower part), combining the two together. When setting, the pavé method is often used to conceal the bonding layer. Composite diamonds have the following identification characteristics:
(1) Observe the characteristics of the combining surface and any possible bubbles;
(2) The gloss of the upper and lower parts of the composite layer, the refractive index of the encapsulation, and the difference in light transmission;
(3) Place the sample in water for testing, observe its layering phenomenon, and use organic immersion oil cautiously for observation, as organic matter may dissolve the combining layer and separate the two parts;
(4) Observe the bright round-cut composite diamonds; the cutting proportions and internal total reflection phenomena are inferior to those of natural diamonds.