¿Qué hace que el acero inoxidable y las aleaciones de titanio sean ideales para la joyería? Perspectivas de producción y tendencias del mercado
Joyería de acero inoxidable y titanio: Técnicas para diseñadores modernos
El acero inoxidable se utilizó inicialmente en relojes y plumas de lujo de Rolex y otras conocidas marcas de moda. Este material es duro, tiene una excelente resistencia a la corrosión y mantiene su color a temperatura ambiente, a diferencia de las joyas de plata, que se empañan con facilidad, o las de aleación, que pueden ser tóxicas por su contenido en plomo. Por eso se utiliza cada vez más en la industria de la joyería y se ha convertido en un material habitual en los accesorios de moda. Las joyas de acero inoxidable presentan un estilo robusto, minimalista, estable y sobrio, con un aspecto metálico fresco, lo que le ha valido el reconocimiento y el afecto de muchos entusiastas de la moda.
El titanio es muy resistente a la corrosión y estable, y su exclusivo tono gris plateado da buenos resultados en los acabados pulido alto, acabado seda y mate. Es uno de los metales decorativos más adecuados, además de los metales preciosos, y se utiliza a menudo en el diseño de joyería moderna en el extranjero. El titanio posee cualidades futuristas, mostrando elegancia sin dejar de ser atemporal. Es ligero pero excepcionalmente fuerte, lo que lo convierte en un material muy popular en joyería a nivel internacional, especialmente entre los jóvenes profesionales.

Anillo de acero inoxidable
Índice
Sección I Productos y procesos de producción del acero inoxidable
1. Introducción al acero inoxidable
1.1 Definición de acero inoxidable
Acero inoxidable es un término general para designar el acero con cierta estabilidad química en soluciones como la atmósfera, el agua, los ácidos, los álcalis, las sales u otros medios corrosivos. En general, se denomina acero inoxidable al acero resistente a la corrosión de medios débiles como la atmósfera, el vapor y el agua. El acero resistente a la corrosión por medios corrosivos ácidos, alcalinos y salinos se denomina acero resistente a la corrosión o resistente a los ácidos. El acero inoxidable es resistente a la oxidación, pero no necesariamente a la corrosión, mientras que el acero resistente a la corrosión suele ser más resistente a la oxidación.
En general, se cree que la resistencia a la corrosión del acero inoxidable es el resultado de la formación de una "película de pasivación" en su superficie bajo la acción de medios corrosivos. La capacidad de resistir a la corrosión depende de la estabilidad de la "película de pasivación". Esto está relacionado no sólo con la composición química del acero inoxidable, sino también con el tipo, la concentración, la temperatura, la presión, el caudal del medio corrosivo y otros factores.
El acero inoxidable presenta una buena resistencia a la corrosión gracias a la adición de cromo a la aleación de hierro y carbono. Aunque otros elementos, como el cobre, el aluminio, el silicio, el níquel y el tántalo, también pueden mejorar la resistencia a la corrosión del acero, su eficacia es limitada en ausencia de cromo. Por ello, el cromo es el elemento más importante del acero inoxidable. El contenido mínimo de cromo necesario para obtener un acero inoxidable con buena resistencia a la corrosión depende del medio corrosivo. El Instituto Americano del Hierro y el Acero (AISI) define el límite entre el acero no inoxidable y otros aceros con cromo 4%. La norma industrial japonesa JIS G 0203 estipula que el acero inoxidable es una aleación que contiene cromo o cromo-níquel para mejorar la resistencia a la corrosión, generalmente con un contenido de cromo superior a 11%. La norma DIN alemana y la norma europea EN10020 especifican que el contenido de cromo del acero inoxidable no es inferior a 10,5%. El contenido de carbono no es superior a 1,2%. En nuestro país, el contenido de cromo del acero inoxidable se define generalmente como no inferior a 12%.
1.2 Elementos de aleación comunes del acero inoxidable
Varios elementos determinan principalmente el rendimiento y la estructura del acero inoxidable. En la actualidad, se conocen más de 100 elementos químicos, entre los cuales los que más influyen en el rendimiento y la estructura del acero inoxidable son el carbono, el cromo, el níquel, el manganeso, el nitrógeno, el titanio, el niobio, el molibdeno, el cobre, el aluminio, el silicio, el circonio, el itrio, el boro y más de una docena más. La adición de estos elementos provoca cambios en la estructura interna del acero, lo que le confiere propiedades especiales. Para profundizar en el conocimiento del acero inoxidable, es necesario comprender primero el impacto de los distintos elementos en el rendimiento y la estructura del acero inoxidable.
(1) Cromo
El cromo es el elemento fundamental que determina la resistencia a la corrosión del acero inoxidable. En medios oxidantes, el cromo puede formar rápidamente en la superficie del acero una capa impermeable e insoluble al medio corrosivo, que es una película de óxido rica en cromo. Esta película de óxido es muy densa y está firmemente adherida al metal, protegiendo al acero de una mayor oxidación y corrosión por medios externos; el cromo también puede aumentar eficazmente el potencial de electrodo del acero. Cuando el contenido de cromo no es inferior a 12,5% átomos, puede provocar un cambio repentino en el potencial de electrodo del acero, pasando de un potencial negativo a un potencial de electrodo positivo. Por lo tanto, puede mejorar significativamente la resistencia a la corrosión del acero. Cuanto mayor sea el contenido de cromo, mayor será la resistencia a la corrosión del acero. Cuando el contenido de cromo alcanza los 25%, 37,5% átomos, se producen el segundo y tercer cambios bruscos, lo que confiere al acero una resistencia a la corrosión aún mayor.
(2) Níquel
El efecto del níquel en la resistencia a la corrosión del acero inoxidable sólo puede demostrarse plenamente cuando se combina con el cromo. Esto se debe a que el acero al níquel bajo en carbono requiere un contenido de níquel de 24% para lograr una estructura austenítica pura (la austenita es una solución sólida no magnética que contiene una pequeña cantidad de carbono en γ-Fe, con una estructura cristalina cúbica centrada en la cara); para alterar significativamente la resistencia a la corrosión del acero en determinados medios, el contenido de níquel debe ser superior a 27%. Por tanto, el níquel no puede constituir por sí solo un acero inoxidable. Sin embargo, añadiendo níquel 9% al acero con cromo 18%, el acero puede alcanzar una estructura austenítica simple a temperatura ambiente, lo que puede mejorar la resistencia a la corrosión del acero a medios no oxidantes (como ácido sulfúrico diluido, ácido clorhídrico, ácido fosfórico, etc.) y mejorar el rendimiento del proceso del acero en la soldadura y el doblado en frío.
(3) Manganeso y nitrógeno: pueden sustituir al níquel en el acero inoxidable al cromo-níquel.
El manganeso y el nitrógeno desempeñan un papel en el acero inoxidable similar al del níquel. El efecto estabilizador del manganeso en la austenita es comparable al de 1/2 níquel, mientras que el efecto del nitrógeno es mucho mayor, unas 40 veces superior al del níquel. Por lo tanto, el manganeso y el nitrógeno pueden sustituir al níquel para conseguir una estructura austenítica única. Sin embargo, la adición de manganeso reducirá la resistencia a la corrosión del acero inoxidable bajo en cromo. Además, el acero austenítico con alto contenido en manganeso no es fácil de procesar. Por tanto, el manganeso no se utiliza solo en el acero inoxidable; sólo se emplea parcialmente para sustituir al níquel.
(4) Carbono
El contenido y la distribución del carbono en el acero inoxidable influyen en gran medida en su rendimiento y estructura. Por un lado, el carbono es un elemento estabilizador de la austenita, con un efecto aproximadamente 30 veces superior al del níquel. Los aceros inoxidables martensíticos con alto contenido en carbono (la martensita es una solución sólida sobresaturada de carbono que se disuelve en α-Fe, que es una fase metaestable transformada a partir de la austenita mediante un cambio de fase sin difusión) pueden aceptar plenamente el refuerzo por temple, mejorando significativamente su resistencia en términos de propiedades mecánicas; por otra parte, debido a la fuerte afinidad entre el carbono y el cromo, éste, que ocupa 17 veces la cantidad de carbono en el acero inoxidable, se combina con aquél para formar carburo de cromo. A medida que aumenta el contenido de carbono en el acero, más cromo forma carburos con el carbono, lo que reduce considerablemente la resistencia a la corrosión del acero. Por lo tanto, desde el punto de vista de la resistencia y la resistencia a la corrosión, el papel del carbono en el acero inoxidable es contradictorio. En las aplicaciones prácticas, para lograr la resistencia a la corrosión, el contenido de carbono en el acero inoxidable suele ser bajo, en la mayoría de los casos en torno a 0,1%. Para mejorar aún más la resistencia a la corrosión del acero, especialmente su resistencia a la corrosión intergranular, a menudo se utiliza el acero inoxidable ultra bajo en carbono, con un contenido de carbono de 0,03% o incluso inferior; sin embargo, para la fabricación de rodamientos, muelles, herramientas y otros aceros inoxidables, se requiere un mayor contenido de carbono debido a la necesidad de una alta dureza y resistencia al desgaste, generalmente entre 0,85%~1,00%, como el acero 9Cr18, etc.
(5) Titanio y niobio
Cuando el acero inoxidable se calienta a 450~800℃, el contenido de cromo cerca de los límites de grano disminuye a menudo debido a la precipitación de carburos de cromo en los límites de grano, formando una zona pobre en cromo, lo que provoca una disminución del potencial del electrodo cerca de los límites de grano, causando así corrosión electroquímica, conocida como corrosión intergranular. Es frecuente la corrosión intergranular en la zona afectada por el calor cerca de las soldaduras. El sodio y el niobio son fuertes formadores de carburo y su afinidad por el carbono es mucho mayor que la del cromo. Al añadir titanio o niobio al acero, el carbono del acero puede formar primero carburos con titanio o niobio en lugar de cromo, garantizando así que no se produzca corrosión intergranular debido al agotamiento del cromo cerca de los límites de grano. Por lo tanto, el sodio y el niobio se utilizan a menudo para fijar el carbono en el acero, aumentar la resistencia del acero inoxidable a la corrosión intergranular y mejorar el rendimiento de la soldadura del acero.
La cantidad de titanio o niobio que debe añadirse debe determinarse en función del contenido de carbono. Por lo general, el titanio se añade cinco veces el contenido de carbono, y el niobio ocho veces el contenido de carbono.
(6) Molibdeno y cobre
El molibdeno y el cobre pueden mejorar la resistencia a la corrosión del acero inoxidable frente a medios corrosivos como los ácidos sulfúrico y acético. El molibdeno también puede mejorar significativamente la resistencia a medios que contienen iones cloruro (como el ácido clorhídrico) y ácidos orgánicos. Sin embargo, el acero inoxidable que contiene molibdeno no es adecuado para su uso en ácido nítrico, ya que la velocidad de corrosión del acero inoxidable que contiene molibdeno en ácido nítrico 65% en ebullición es el doble que sin molibdeno; la adición de cobre al acero inoxidable al cromo-manganeso-nitrógeno puede acelerar la corrosión intergranular del acero inoxidable.
El molibdeno dificulta la obtención de una estructura austenítica única en el acero; por lo tanto, en los aceros que contienen molibdeno, el contenido de elementos como el níquel y el manganeso debe aumentarse proporcionalmente para garantizar que el acero tenga una estructura austenítica única tras el tratamiento térmico.
(7) Silicio y aluminio
El papel del silicio en la mejora de la resistencia a la oxidación del acero al cromo es significativo. El acero que contiene 5% de cromo y 1% de silicio puede tener una resistencia a la oxidación comparable a la del acero al cromo 12%. Si el acero en 1000 ℃ puede resistir el producto químico, que contiene 0,5% silicio necesita 22% del cromo, como la adición de 2,5% a 3% del silicio más tarde, sólo 12% del cromo puede ser. La información indica que la adición de 2,5% de silicio al acero al cromo-níquel Cr15Ni20 puede lograr una resistencia a la oxidación comparable a la de la aleación de cromo-níquel Cr15Ni60.
Añadir aluminio al acero con alto contenido en cromo también puede mejorar significativamente su resistencia a la oxidación, y su función es similar a la de añadir silicio.
La adición de silicio y aluminio al acero con alto contenido de cromo tiene por objeto mejorar aún más la resistencia a la oxidación del acero y ahorrar cromo.
Aunque el silicio y el aluminio mejoran notablemente la resistencia a la oxidación del acero al cromo, también tienen muchos inconvenientes. El principal es que provocan el engrosamiento del grano del acero y aumentan su tendencia a la fragilidad.
(8) Volframio y vanadio
La función principal del wolframio y el vanadio en el acero es mejorar su resistencia térmica.
(9) Boro
0,005% El boro (ferrita, que es una solución sólida de carbono en α-Fe, con una red cúbica centrada en el cuerpo) añadido al acero inoxidable ferrítico con alto contenido de cromo (Cr17Mo2Ti) puede mejorar la resistencia a la corrosión del acero en ácido acético 65% hirviendo; la adición de trazas (0,006‰~0.007‰) de boro al acero inoxidable austenítico puede mejorar la plasticidad en caliente del acero; el boro tiene un buen efecto en la mejora de la resistencia térmica del acero, mejorando significativamente la resistencia térmica del acero inoxidable; el acero inoxidable austenítico con cromo-níquel que contiene boro tiene aplicaciones especiales en la industria de la energía atómica. Sin embargo, la presencia de boro en el acero inoxidable puede reducir la plasticidad y la tenacidad al impacto del acero.
Además de los elementos mencionados, algunos aceros inoxidables contienen metales raros y elementos de tierras raras para mejorar sus prestaciones. En los aceros inoxidables utilizados en aplicaciones industriales, muchos aceros contienen de varios a decenas de elementos de aleación al mismo tiempo. Cuando varios elementos coexisten en este cuerpo unificado de acero inoxidable, la estructura del acero inoxidable viene determinada por la suma de las influencias de varios elementos.
La influencia de los distintos elementos en la microestructura del acero inoxidable puede clasificarse en dos tipos principales en función de sus características comunes: un tipo consiste en los elementos que forman o estabilizan la austenita, entre los que se incluyen el carbono, el níquel, el manganeso, el nitrógeno y el cobre, siendo el carbono y el nitrógeno los que tienen un mayor efecto; el otro tipo consiste en los elementos que forman la ferrita, entre los que se incluyen el cromo, el wolframio, el tántalo, el niobio, el silicio, el titanio, el vanadio y el aluminio. Si se compara con el cromo como referencia, el efecto de este tipo de elementos en la formación de ferrita es mayor para todos los demás elementos que para el cromo.
Cuando estos dos tipos de elementos coexisten en el acero inoxidable, la estructura de éste depende de los resultados de su influencia mutua. Si el papel de los elementos que estabilizan la austenita es predominante, la estructura del acero inoxidable será principalmente austenítica, con poca o ninguna ferrita; si su influencia no es suficiente para mantener la austenita en el acero a temperatura ambiente, esta austenita inestable sufrirá una transformación martensítica al enfriarse, dando lugar a una estructura martensítica; si el papel de los elementos que forman la ferrita llega a ser predominante, la estructura del acero será principalmente ferrítica.
Aparte de los factores del proceso, el rendimiento del acero inoxidable depende principalmente de la composición de su estructura interna, que viene determinada por la suma de diversos elementos de aleación en el acero. Por tanto, los elementos de aleación determinan en última instancia el rendimiento del acero inoxidable.
1.3 Clasificación del acero inoxidable
El acero inoxidable es una serie de aceros especiales con una amplia gama. En nuestro país se producen más de 100 tipos de calidades de acero inoxidable. En función de sus principales componentes de aleación, estructura metalográfica y aplicaciones industriales primarias, el acero inoxidable puede clasificarse a grandes rasgos como sigue.
(1) Clasificación basada en la composición de aleación del acero inoxidable
Según los principales componentes de aleación del acero inoxidable, puede dividirse en las tres categorías siguientes.
① Acero inoxidable al cromo. Aparte de la base de hierro, este tipo de acero inoxidable contiene principalmente cromo como elemento de aleación. Algunos también contienen uno o más de los elementos Silicio, aluminio, tungsteno, molibdeno, níquel, titanio, vanadio y otros, siendo el contenido de estos elementos en el acero de 1%~3%.
② Acero inoxidable al cromo-níquel. Además de la base de hierro, este tipo de acero inoxidable contiene principalmente cromo y níquel como elementos de aleación. Algunos también contienen uno o más elementos, como titanio, silicio, molibdeno, aluminio, vanadio y boro, estando estos elementos presentes en cantidades inferiores a 4% niveles traza.
③ Acero inoxidable al cromo-manganeso-nitrógeno. Este tipo de acero inoxidable, además de su base de hierro, contiene principalmente cromo y manganeso como elementos de aleación. La mayoría de los aceros contienen también nitrógeno por debajo de 0,5%, y algunos contienen además uno o varios elementos como níquel, silicio y cobre. El contenido de estos elementos en el acero es, respectivamente, inferior a 5%.
(2) Clasificación basada en la estructura del acero inoxidable
El acero inoxidable suele dividirse en tres categorías en función de su estructura (organización metalográfica).
① Tipo ferrítico. Es decir, acero inoxidable que contiene cromo pero no níquel. Este tipo de acero puede endurecerse hasta cierto punto mediante trabajo en frío, pero no mediante tratamiento térmico. Este tipo de acero es siempre magnético.
② Tipo martensítico. Este tipo de acero inoxidable, salvo algunas calidades que contienen una pequeña cantidad de níquel, sólo contiene cromo. Su ventaja es que el tratamiento térmico puede endurecerlo. Este tipo de acero es siempre magnético.
③ Tipo austenítico. Es decir, acero inoxidable que contiene elementos como cromo, níquel, o cromo, níquel, manganeso, o cromo, manganeso, nitrógeno, etc. Este tipo de acero sólo puede endurecerse mediante trabajo en frío; el tratamiento térmico sólo puede ablandarlo. En estado recocido, no es magnético. Después del trabajo en frío, algunos pueden volverse magnéticos.
Las tres clasificaciones anteriores se basan únicamente en la estructura matricial del acero. Debido a la incapacidad de los elementos que estabilizan la austenita y forman la ferrita en el acero para equilibrarse entre sí, las microestructuras reales de los aceros inoxidables utilizados en la industria también incluyen martensita-ferrita, austenita-ferrita, austenita-martensita y otros aceros inoxidables dúplex de transición, así como aceros inoxidables con estructura de martensita-carburo.
2. Accesorios de acero inoxidable
2.1 Requisitos de los materiales de las joyas de acero inoxidable
(1) Propiedades mecánicas
La tecnología de transformación del plástico se ha utilizado ampliamente en la producción de joyas de acero inoxidable. Además de utilizar maquinaria de estirado y laminado para producir chapas, alambres, tubos y otros perfiles, también se utiliza a menudo para el procesamiento de conformado de joyas, como el uso de máquinas herramienta para el acabado, el uso de una máquina de estampación y una prensa hidráulica para operaciones hidráulicas. Para garantizar la calidad de los productos de transformación de plásticos, además de formular correctamente y cumplir estrictamente las especificaciones del proceso operativo, existen requisitos claros para las propiedades mecánicas de los materiales. Las propiedades mecánicas de los materiales se reflejan principalmente en indicadores como la resistencia a la tracción, el límite elástico, la dureza, el alargamiento y la tenacidad. Los materiales de acero inoxidable son necesarios para un buen rendimiento en la transformación de plásticos, especialmente durante operaciones como el estirado, el laminado, el estampado y el prensado hidráulico. La dureza de los materiales no debe ser demasiado alta, y la velocidad de endurecimiento por deformación de los materiales debe ser más lenta para facilitar la operación; los materiales deben tener buena ductilidad; de lo contrario, es probable que se produzcan grietas.
(2) Rendimiento de pulido
La joyería tiene unos requisitos claros en cuanto a la calidad de la superficie, y la mayoría de las joyas deben pulirse para conseguir un brillo similar al de un espejo. Esto requiere no sólo la correcta ejecución del proceso de pulido, sino también que las propiedades inherentes del material tengan un impacto significativo. Por ejemplo, la pieza debe tener una estructura densa con granos finos y uniformes, sin defectos como poros e inclusiones. Si los granos de la pieza son gruesos o hay defectos de contracción o poros, es fácil que se produzcan fenómenos de piel de naranja, depresiones de pulido y cola de cometa. Del mismo modo, si hay inclusiones duras, es fácil que se produzcan arañazos y defectos de cola de cometa.
Entre los factores que afectan al rendimiento del pulido de las joyas de acero inoxidable se incluyen principalmente los siguientes puntos:
- Defectos superficiales de las materias primas, como arañazos, picaduras y decapado excesivo.
- Problemas de calidad de la materia prima. Si la dureza es demasiado baja, es difícil conseguir un pulido brillante, y la superficie es propensa a la piel de naranja durante el estiramiento profundo, lo que afecta a la capacidad de pulido. Una mayor dureza suele mejorar la capacidad de pulido.
- Los productos sometidos a un estiramiento profundo pueden presentar pequeños puntos negros en las zonas con una deformación importante, lo que puede afectar a la calidad del pulido.
(3) Resistencia a la corrosión
La resistencia a la corrosión es muy importante para la joyería. La resistencia a la corrosión de los materiales varía con la composición; el 316 tiene mejor resistencia a la corrosión que el 304, pero la composición no es el único factor que afecta al deslustre. El deslustre y la decoloración son el resultado de una combinación de la composición química, los factores ambientales, la microestructura y el estado de la superficie.
Para determinar la resistencia a la corrosión de las joyas, suelen ser necesarias pruebas de corrosión acelerada, que suelen incluir pruebas de niebla salina y pruebas de inmersión.
(4) Rendimiento del casting
El rendimiento de fundición de las aleaciones influye significativamente en la calidad de la superficie de las joyas fundidas. La calidad del rendimiento de fundición de las aleaciones puede evaluarse desde varios aspectos, como la fluidez del metal fundido, la tendencia a la contracción y la porosidad, y la tendencia al agrietamiento térmico durante la deformación. El acero inoxidable utilizado para la fundición debe tener un intervalo de cristalización menor y una baja tendencia a la oxidación debido a la absorción de gases, buena fluidez y rendimiento de llenado. No debe formar fácilmente porosidad dispersa ni producir grietas de deformación, lo que favorece la obtención de joyas fundidas de forma completa, perfil claro, cristalización densa y estructura sólida.
(5) Rendimiento de la reutilización
En el proceso de fundición de joyas, la tasa de rendimiento suele ser sólo de unos 50% o incluso inferior, y cada fundición genera una gran cantidad de sistema de inyección, materiales de desecho, etc. Las empresas de joyería, basándose en los costes de producción y la eficiencia, siempre esperan utilizar la mayor cantidad posible de material reciclado. Debido a los inevitables problemas de volatilización, oxidación y absorción de gases durante el proceso de fusión de la aleación, la composición de ésta cambiará en cierta medida con cada colada, lo que afectará a su calidad metalúrgica y al rendimiento de la fundición.
La degradación del rendimiento de las aleaciones durante el proceso de reciclado no sólo está relacionada con el proceso operativo, sino también con el rendimiento de reciclado de la propia aleación. Depende principalmente de la tendencia de la aleación a la oxidación por absorción de gas y de su reactividad con los crisoles y los materiales de fundición. Cuanto menor sea la tendencia a la oxidación por absorción de gas y cuanto menor sea la reactividad con los crisoles y los materiales de fundición, mejor será el rendimiento del reciclado.
(6) Seguridad
La seguridad de los materiales de joyería es un factor importante que debe tenerse en cuenta, ya que las joyas están en contacto directo con el cuerpo humano durante largos periodos. Los materiales deben evitar el uso de elementos nocivos como el cadmio, el plomo y los elementos radiactivos. Además, debe prestarse atención a evitar las reacciones alérgicas causadas por el contacto con la piel y los problemas relacionados con las bacterias.
El níquel es un elemento sensibilizante típico que plantea posibles reacciones alérgicas y daños en la piel humana. Las joyas que contienen níquel liberan iones de níquel sensibilizantes durante su uso, lo que provoca dermatitis alérgica de contacto. Dependiendo de la gravedad de la reacción, pueden presentarse diferentes síntomas. Los pacientes con síntomas más leves pueden mostrar reacciones únicamente en los puntos de contacto entre la joya y la piel, como las orejas, el cuello, las muñecas y los dedos, con prurito cutáneo, eritema, erupciones, ampollas, erosión, exudación, costras y descamación, con límites claros de las lesiones cutáneas que a menudo se asemejan a la forma de la joya. En cambio, los pacientes con síntomas más graves pueden experimentar reacciones alérgicas sistémicas, que comienzan con enrojecimiento e hinchazón de la piel, seguidos de pequeñas pápulas y ampollas. También existe riesgo de carcinogénesis y efectos teratogénicos. En respuesta a lo común y perjudicial de las alergias al níquel, la Unión Europea creó la campaña Níquel. Directiva 94/27/CE en la década de 1990 y la norma de ensayo de liberación de níquel EN1811:1998. Posteriormente, debido a los todavía elevados niveles de sensibilización al níquel, se endurecieron y revisaron las normas, lo que dio lugar a la publicación de la Directiva 2004/96/CE sobre el níquel y la norma de ensayo de liberación de níquel EN1811:1998+A1:2008. En 2011, se introdujo una norma de ensayo de liberación de níquel EN1811:2011 aún más estricta, que eliminaba el valor de ajuste para los índices de liberación de níquel. Dado que el acero inoxidable al cromo-níquel tradicional utiliza una gran cantidad de níquel como elemento de aleación, es esencial evaluar si un material cumple los requisitos de la norma de liberación de níquel antes de seleccionarlo para su uso como joya.
Las investigaciones demuestran que las joyas son propensas a albergar bacterias, especialmente durante el verano, cuando la sudoración es más frecuente. La piel cubierta por joyas no respira fácilmente, lo que permite la proliferación de bacterias, que pueden provocar enfermedades e infecciones cutáneas. Esto es especialmente grave en el caso de los piercings, donde el riesgo de infección bacteriana es mucho mayor que en las joyas superficiales, ya que el piercing es una herida quirúrgica. El piercing crea un túnel dentro del tejido sin revestimiento epitelial, sostenido por la joya implantada posteriormente. El tejido circundante no puede entrar en contacto para cicatrizar, y todo el proceso de cicatrización implica que el tejido epitelial de ambas superficies se adhiere gradualmente a lo largo de la superficie interna del túnel para formar una fístula, dando lugar finalmente a un canal epitelial. Durante el proceso de cicatrización, si se encuentran bacterias externas, puede producirse fácilmente una infección. Por ejemplo, al perforar el lóbulo de la oreja, la piel de esa zona es fina, con poco tejido subcutáneo, y los vasos sanguíneos son finos y superficiales, lo que provoca un flujo sanguíneo lento. Tras la perforación, el tejido dérmico queda algo dañado. Debido a la fricción y el contacto constantes entre el tejido local dañado y la joya, se contamina fácilmente con polvo, moho, bacterias, etc., lo que provoca una infección, que puede causar picor alrededor del agujero del lóbulo de la oreja y, en casos graves, enrojecimiento, hinchazón, pápulas, ampollas, supuración y erosión, llegando incluso a la endocarditis infecciosa. Dadas las graves consecuencias de que las joyas sean portadoras de bacterias, la Organización Mundial de la Salud recomienda que el personal sanitario no lleve anillos ni otros accesorios mientras preste asistencia hospitalaria. En cuanto a la joya en sí, si su material tiene buenas propiedades antibacterianas, sin duda tiene una importancia significativa en la reducción o eliminación de bacterias en las joyas. Dado que el acero inoxidable se utiliza ampliamente como material de joyería, especialmente durante el proceso de cicatrización de los piercings, las varillas de acero inoxidable se utilizan sobre todo para expandir el orificio del piercing y evitar que las paredes del piercing se peguen entre sí. El acero inoxidable tradicional no tiene propiedades antibacterianas, por lo que el tratamiento de modificación antibacteriana es de gran importancia para la seguridad del uso de las joyas.
(7) Economía
El precio de los materiales de joyería de acero inoxidable es uno de los factores que afectan a los costes de producción. El principio de selección de materiales debe ser elegir aquellos con una amplia oferta y bajo precio y minimizar o evitar el uso de metales preciosos caros para reducir los costes de material.
2.2 El principal material del acero inoxidable para joyería
(1) Acero inoxidable austenítico tradicional al cromo-níquel
Tradicionalmente, la joyería utiliza principalmente acero inoxidable austenítico al cromo-níquel, incluyendo varios grados típicos como 303, 304, 304L, 316 y 316L, con rangos de composición química que se muestran en la Tabla 5-1.
Tabla 5-1 Rangos de composición química de varios aceros inoxidables austeníticos decorativos
| Aceros | Carbono (C) | Silicio(Si) | Manganeso (Mn) | Fósforo (P) | Azufre (S) | Níquel (Ni) | Cromo (Cr) | Molibdeno(Mo) |
|---|---|---|---|---|---|---|---|---|
| 303 | ≤0. 15 | ≤1. 00 | ≤2.00 | ≤0. 20 | ≥0. 15 | 8.00~10.00 | 17.00 ~19.00 | ≤0. 6 |
| 304 | ≤0.08 | ≤1. 00 | ≤2.00 | ≤0.045 | ≤0.030 | 8.00~10.50 | 18.00 ~20.00 | - |
| 304L | ≤0.03 | ≤1.00 | ≤2.00 | ≤0.045 | ≤0.030 | 9.00 ~13.50 | 18.00~20.00 | - |
| 316 | ≤0.08 | ≤1.00 | ≤2.00 | ≤0.045 | ≤0.030 | 10.00 ~14.50 | 10.00 ~18.00 | 2.00 ~3.00 |
| 316L | ≤0.03 | ≤1.00 | ≤2.00 | ≤0.045 | ≤0.030 | 12.00~15.00 | 16.00 ~18.00 | 2.00 ~3.00 |
| (Zhu Zhongping, 2004; Gu Jiqing, 2008) | ||||||||
① Acero inoxidable austenítico 303. El acero inoxidable austenítico tipo 303 tiene muy buen rendimiento de corte, y el acabado superficial de la pieza mecanizada es alto, lo que es beneficioso para el rendimiento decorativo de la joyería. Por lo tanto, este material se elige a veces como material para joyería. Sin embargo, el acero inoxidable 303 contiene una gran cantidad de sulfuros, que pueden convertirse en fuentes de picaduras en ambientes corrosivos, dando lugar a una corrosión preferente y a la formación de picaduras, acelerando la disolución anódica del metal circundante y aumentando la tasa de liberación de níquel. Sin embargo, los valores medidos superan ampliamente este umbral. Según la norma EN1811:2011, el acero inoxidable 303 no es conforme en cuanto a la liberación de níquel, tanto si se utiliza para joyas en contacto directo con la piel durante largos periodos como para joyas de piercing, lo que supone un riesgo de sensibilización al níquel. Es aconsejable evitar elegir este material para fabricar joyas que estén en contacto directo con la piel durante largos periodos, especialmente joyas para piercing. El acero inoxidable 303 se utiliza normalmente en estado de solución sólida, siendo la especificación del tratamiento de solución sólida 1010℃~1150℃ mantenerlo durante el tiempo correspondiente y después enfriarlo. Las propiedades mecánicas del tipo 303 y otros tipos de acero inoxidable se muestran en la Tabla 5-2.
Tabla 5-2 Propiedades mecánicas del acero inoxidable decorativo en estado de solución sólida
| Aceros | Resistencia a la tracción σb /MPa | Límite elástico σ0.2/MPa | Velocidad de elongación δ/% | Índice de contracción transversal ψ/% | Dureza/HB | |||
|---|---|---|---|---|---|---|---|---|
| 303 | ≥520 | ≥205 | ≥40 | ≥50 | ≤187 | |||
| 304 | ≥520 | ≥205 | ≥40 | ≥60 | ≤187 | |||
| 304L | ≥480 | ≥175 | ≥40 | ≥60 | ≤187 | |||
| 316 | ≥520 | ≥205 | ≥40 | ≥55 | ≤187 | |||
| 316L | ≥480 | ≥175 | ≥40 | ≥60 | ≤187 | |||
| (Zhu Zhongping, 2004; Gu Jiqing, 2008) | ||||||||
② Acero inoxidable austenítico 304 y 304L. El 304 es un acero inoxidable versátil, comúnmente marcado en el mercado de tres formas: 06Cr19Ni10 indica generalmente la producción según las normas nacionales, S30408 indica generalmente la producción según las normas ASTM, y SUS 304 indica la producción según las normas japonesas. Para mantener la resistencia a la corrosión inherente al acero inoxidable, éste debe contener más de 17% de cromo y 8% de níquel.
El acero inoxidable 304 tiene una excelente resistencia a la corrosión, buena resistencia a la corrosión intergranular y excelentes propiedades de transformación y conformado en frío y en caliente. Puede transformarse en diversos productos, como chapas, tubos, alambres, tiras y formas, y es adecuado para la fabricación de piezas de estampación en frío, embutición profunda y estirado profundo. Tiene un buen comportamiento a bajas temperaturas, resistencia, alargamiento y área reducida, todo ello bueno en condiciones de -180℃. Tiene un buen rendimiento de soldadura y se puede soldar utilizando métodos de soldadura convencionales. Sin embargo, el acero inoxidable 304 también tiene algunas deficiencias, como ser sensible a la corrosión intergranular después de la soldadura, muy sensible a la corrosión bajo tensión en agua que contiene iones de cloruro (incluyendo atmósferas húmedas), resistencia mecánica relativamente baja, y pobre rendimiento de corte.
El acero inoxidable 304 L es una variante del acero inoxidable 304 con un menor contenido de carbono, que se utiliza en aplicaciones de soldadura. El menor contenido de carbono minimiza la precipitación de carburos en la zona afectada por el calor cerca de la soldadura, ya que la precipitación de carburos puede provocar corrosión intergranular (deterioro de la soldadura) del acero inoxidable en determinados entornos.
③ Acero inoxidable 316 y 316L. El acero inoxidable 316 contiene cierta cantidad de molibdeno, y su contenido en níquel es superior al del acero inoxidable 304. Por tanto, su resistencia a la corrosión atmosférica y su resistencia a altas temperaturas son superiores. Por lo tanto, su resistencia a la corrosión, a la corrosión atmosférica y a las altas temperaturas es superior, lo que permite utilizarlo en condiciones más estrictas, especialmente porque su resistencia a la corrosión por picadura es significativamente mejor que la del acero inoxidable 304, con una temperatura crítica de picadura superior a la del acero inoxidable 304, presentando una mejor resistencia a la temperatura de picadura. La investigación muestra que la temperatura crítica de picadura del acero inoxidable 316 es significativamente sensible a la concentración de solución de NaCl de 0,1% a 0,5%; dentro de este rango, la temperatura crítica de picadura del material cae bruscamente de cerca de 90℃ a 50℃ . Por el contrario, la temperatura crítica de picadura del acero inoxidable 304 muestra una sensibilidad significativa a la concentración de solución de NaCl de 0,01% a 0,05%; dentro de este intervalo, la temperatura crítica de picadura del material desciende bruscamente de cerca de 90℃ a alrededor de 55℃. Desde la perspectiva de la sensibilidad a los iones cloruro, el acero inoxidable 316 también es relativamente superior al acero inoxidable 304 en términos de resistencia a la corrosión por picaduras.
El acero inoxidable 316 L es una variante del acero inoxidable 316 con un contenido de carbono no superior a 0,03%. Tiene mejor resistencia a la precipitación de carburos que el acero inoxidable 316, por lo que es adecuado para aplicaciones que no se pueden recocido después de la soldadura y requieren la máxima resistencia a la corrosión.
Se prefiere el acero inoxidable 316L como material para accesorios porque garantiza una buena resistencia a la corrosión. Las cadenas y cajas de alta gama de la industria relojera también utilizan principalmente este tipo de acero.
(2) Nuevo tipo de acero inoxidable austenítico sin níquel/con bajo contenido de níquel
① Elementos alternativos para el acero inoxidable austenítico sin níquel/con bajo contenido de níquel. El acero inoxidable austenítico al cromo-níquel tradicional amplía la región de la fase austenítica a través del níquel, retrasando su transformación para obtener una estructura monofásica. Dado que el níquel es un sensibilizador, el acero inoxidable que contiene níquel puede suponer un riesgo de alergia cuando está en contacto prolongado con la piel o los tejidos humanos. Por lo tanto, la investigación y el desarrollo de acero inoxidable austenítico sin níquel que sea respetuoso con el cuerpo humano se ha convertido en un tema candente en los biomateriales metálicos, los materiales de relojería y los materiales de joyería.
Para obtener una estructura austenítica monofásica en el acero inoxidable sin níquel, es necesario buscar elementos estabilizadores de la austenita que puedan sustituir al níquel. La influencia de los elementos de aleación en la estructura del acero inoxidable puede convertirse en el correspondiente cromo Creq y el equivalente de níquel Nieq. Para conseguir austenita monofásica y evitar la presencia de ferrita δ, la relación de composición de cada elemento de aleación debe seleccionarse razonablemente para garantizar que el equivalente de níquel cae dentro de la región de austenita monofásica por encima de la zona de sombra inclinada. Para conseguirlo, debe satisfacerse lo siguiente:
Nieq≥Creq – 8
El CreqNieq fórmula de cálculo es:
Creq=Cr+1.5Mo+1.5W+0.48Si+2.3V+1.75Nb+2.5Al
Nieq=Ni+Co+0,1Mn-0,01Mn2+18N+30C
El carbono, el cobalto, el manganeso y el nitrógeno son los elementos alternativos más económicos para estabilizar la austenita. El carbono tiene el mayor efecto en la expansión de la región de la fase austenita, pero puede sensibilizar el acero inoxidable; la capacidad del cobalto para estabilizar la austenita es similar a la del níquel, pero también conlleva un riesgo de alergia, por lo que ninguno de los dos es adecuado como sustituto primario del níquel. El manganeso estabiliza la austenita dentro de un cierto rango, pero cuando el contenido de cromo supera los 13%, la adición de manganeso por sí sola no puede conseguir una única fase de austenita. Cuando el contenido de manganeso supera los 10%, el manganeso se convierte en un estabilizador de la ferrita. El nitrógeno es un fuerte elemento estabilizador de la austenita; la adición de nitrógeno al acero inoxidable suprime la formación de fases de ferrita en el acero, reduciendo significativamente el contenido de ferrita, haciendo que la fase de austenita sea más estable, e incluso impidiendo la transformación martensítica inducida por tensiones en condiciones severas de trabajo en frío. Así pues, el nitrógeno es un sustituto muy adecuado del níquel. Sin embargo, la termodinámica Fe-Cr-N del sistema indica que cuando el contenido de cromo es 12%, el nitrógeno puede alcanzar la austenita dentro de un estrecho margen; más allá de este margen, el Cr2N y CrN, y con un alto contenido en cromo se formarán ferrita, austenita y Cr2N, y la aleación también es propensa al Cr2N durante el envejecimiento a baja temperatura, que no puede suprimir la transformación martensítica. Por lo tanto, debe añadirse manganeso al Fe-Cr-N, utilizando el efecto sinérgico del nitrógeno y el manganeso, que es beneficioso para obtener una estructura austenítica estable.
② Alto contenido en nitrógeno sin níquel / bajo contenido en níquelel materiales de acero inoxidable austenítico. Países como Alemania, Bulgaria, Suiza, Austria y Japón conceden gran importancia a la investigación y desarrollo de aceros inoxidables con alto contenido en nitrógeno y han desarrollado sucesivamente algunos nuevos tipos de materiales de acero inoxidable sin níquel con alto contenido en nitrógeno, como la aleación BioDur 108 desarrollada por Carpenter Technology Corp en Estados Unidos, P2000 por VSG en Alemania, P548 desarrollada por Bolher en Austria y NFS desarrollada por Daido Steel en Japón (Tabla 5-3). Algunos de ellos ya se han comercializado y se utilizan en productos como aplicaciones biomédicas, relojes y joyas. Sin embargo, es difícil alcanzar un grado de procesamiento preciso cuando se producen pequeños componentes de precisión, y los costes son elevados.
Tabla 5-3 Composiciones químicas de varios aceros inoxidables sin níquel de alto contenido en nitrógeno
| Country | Grados | Composition /wt% | ||||
|---|---|---|---|---|---|---|
| C | Cr | Mn | Mo | N | ||
| Suiza | PANACEA | ≤0. 15 | 16. 5~17. 5 | 10~12 | 3.0~3. 5 | 0.8~1.0 |
| Austria | P548 | 0.15 | 16.0 | 10.0 | 2.0 | 0.5 |
| Bulgaria | CrMnN18- 11 | ≤0.08 | 17~19 | 10~12 | - | 0. 4~1. 2 |
| Alemania | P900 | 0.05 | 18.0 | 18.0 | - | 0. 6~0. 8 |
| Alemania | P2000 | ≤0.05 | 16.0 | 14.0 | 3.0 | 0.75~1.0 |
| Japón | NFS | 0.02 | 16.0 | 18.0 | - | 0.43 |
| Estados Unidos | BioDur 108 alloy | 19~23 | 21~24 | 0. 5~1. 5 | 0.9 | |
| (Yuan Junping, 2012) | ||||||
③ Mechanical properties of high-nitrogen nickel-free/low-nickel austenitic stainless steel. Traditional nickel-containing austenitic stainless steel is classified as a low-strength material under solution treatment conditions and is often strengthened through cold working. Some steels undergo deformation-induced martensitic transformation during significant deformation, giving the material magnetic properties. The strength, plasticity, and other mechanical properties of high-nitrogen stainless steel are closely related to grain size and nitrogen content, with both tensile strength and yield strength significantly increasing with higher nitrogen content. Table 5-4 lists the mechanical properties of some new high-nitrogen austenitic stainless steels in both room-temperature solution-treated and processed states, showing that the strength in the processed state is significantly higher than in the solution-treated state. At the same time, ductility and plasticity remain high, making it difficult to form ferrites and undergo deformation-induced martensitic transformation.
The main ways nitrogen improves the strength of stainless steel are solid solution strengthening, grain size strengthening, and strain hardening. Like carbon, nitrogen occupies the octahedral interstitial sites of the face-centered cubic lattice of austenite. Due to its smaller atomic radius than carbon, it has a stronger lattice expansion effect. Nitrogen atoms interact with dislocations, providing a greater dislocation pinning effect, and can also have a maximum strengthening effect on austenite grain boundaries. In addition, fine-grain strengthening is also an important strengthening mechanism. The transformation pathway shows that compared to 304 stainless steel, high-nitrogen austenitic stainless steel has a significantly more pronounced fine-grain strengthening effect. The effect of nitrogen on the deformation hardening of austenitic stainless steel is also very significant; the increase in nitrogen leads to an increase in slip planes and deformation twins, while the active slip planes and twin layers effectively hinder dislocation movement and twin expansion, thereby greatly increasing the deformation hardening rate of austenitic steel.
Table 5-4 Mechanical Properties of Typical High-Nitrogen Austenitic Stainless Steel at Room Temperature
| Grado de aleación | Status | Tensile strength / MPa | Yield strength/ MPa | Extension rate /% | Cross-sectional shrinkage rate /% | Dureza |
|---|---|---|---|---|---|---|
| 15-15HS-® | Solución sólida | 828 | 490 | 56 | 79 | HRB95 |
| Cromanite | Solución sólida | 850 | 550 | 50 | HB250 | |
| URANUS-® B46 | Solución sólida | 650 | 420 | 40 | ||
| URANUS-® B66 | Solución sólida | 750 | 420 | 50 | ||
| AL4565TM | Solución sólida | 903 | 469 | 47 | HRB90 | |
| Datalloy 2TM | Solución sólida | 827 | 760 | 18 | 45 | HRC33 |
| P2000 | Solución sólida | 930 | 615 | 56.2 | 77.5 | |
| NMS 140 | Processing | 1010~1117 | 876~1020 | 30~22 | 68~60 | HB311 - 341 |
| P550 | Processing | 1034 | 965 | 20 | 50 | HB300 - 400 |
| P580 | Processing | 1034 | 965 | 20 | 50 | HB350 - 450 |
| Amagnit 600 | Processing | 1034 | 965 | 20 | 50 | HB300 |
| (Yuan Junping, 2012) | ||||||
④ Corrosion resistance. Nitrogen can significantly improve the pitting corrosion and crevice corrosion resistance of austenitic stainless steel in environments containing chloride ions. To describe the relationship between the quantity of alloying elements and corrosion performance, the pitting equivalent is commonly used to represent it:
PRE= %Cr + 3.3%Mo + x%N
The most commonly used value x is 16~30. Therefore, nitrogen has a good effect on stainless steel’s pitting corrosion resistance. However, the mechanism of nitrogen’s action is not yet very clear, and it is generally speculated that there are mainly the following mechanisms.
- Acid consumption theory. Nitrogen forms NH4+ during dissolution and consumes H+ in the process, thereby inhibiting the decrease of pH, slowing down local acidification of the solution and anode dissolution, and suppressing the self-catalytic process of pitting, which is more conducive to the pinning reaction.
- Nitrogen enrichment at the interface. Due to nitrogen’s high reactivity, it accumulates near the metal side of the passivation film-metal interface, affecting the repassivation kinetics and allowing for rapid passivation, thereby inhibiting the stable growth of pitting corrosion.
- The synergistic effect of nitrogen with other elements. Nitrogen further enriches the chromium in the sublayer of the nitrided film, enhancing its stability and density. Nitrogen strengthens the corrosion resistance of chromium, molybdenum, and other elements in austenitic stainless steel, suppressing the over-passivation dissolution of chromium and molybdenum. It can also form a more resistant surface layer during localized corrosion processes.
- Biocompatibility. High-nitrogen nickel-free austenitic stainless steel has good corrosion resistance, especially against pitting and intergranular corrosion, and has high wear resistance. The absence of nickel in the steel avoids the sensitization and other tissue reactions caused by the precipitation of nickel in the human body and on the body surface, demonstrating good biocompatibility.
3. Characteristics of stainless steel jewelry
Stainless steel jewelry has many advantages:
① The metallic luster of stainless steel is very similar to that of platinum. It is both noble and elegant yet modern.
② Stainless steel is resistant to corrosion and heat, can resist dust corrosion, and is easy to clean, requiring only a dry cloth. It does not require polishing cloths or cleaning agents.
③ Stainless steel is harder than silver, not easily deformed, and does not oxidize as easily as silver or other metals. It can maintain a shiny, smooth, and attractive appearance even with long-term wear, making it suitable for processing more minimalist styles without worrying about deformation.
④ Stainless steel can be presented in different styles, usually with a very smooth or matte surface.
⑤ The price of stainless steel jewelry is easily acceptable to the public. Although the price of silver has risen significantly in the past few years, stainless steel remains at an acceptable level.
⑥ Stainless steel has excellent coloring properties and can be colored through various processes, such as chemical oxidation, electrochemical oxidation, ion deposition oxide, high-temperature oxidation, and gas phase cracking, greatly enriching the surface decoration effects of joyería.
4. Categories of Stainless Steel Jewelry

Anillo de acero inoxidable

Stainless steel bracelet

Stainless steel bangles

Stainless steel earrings
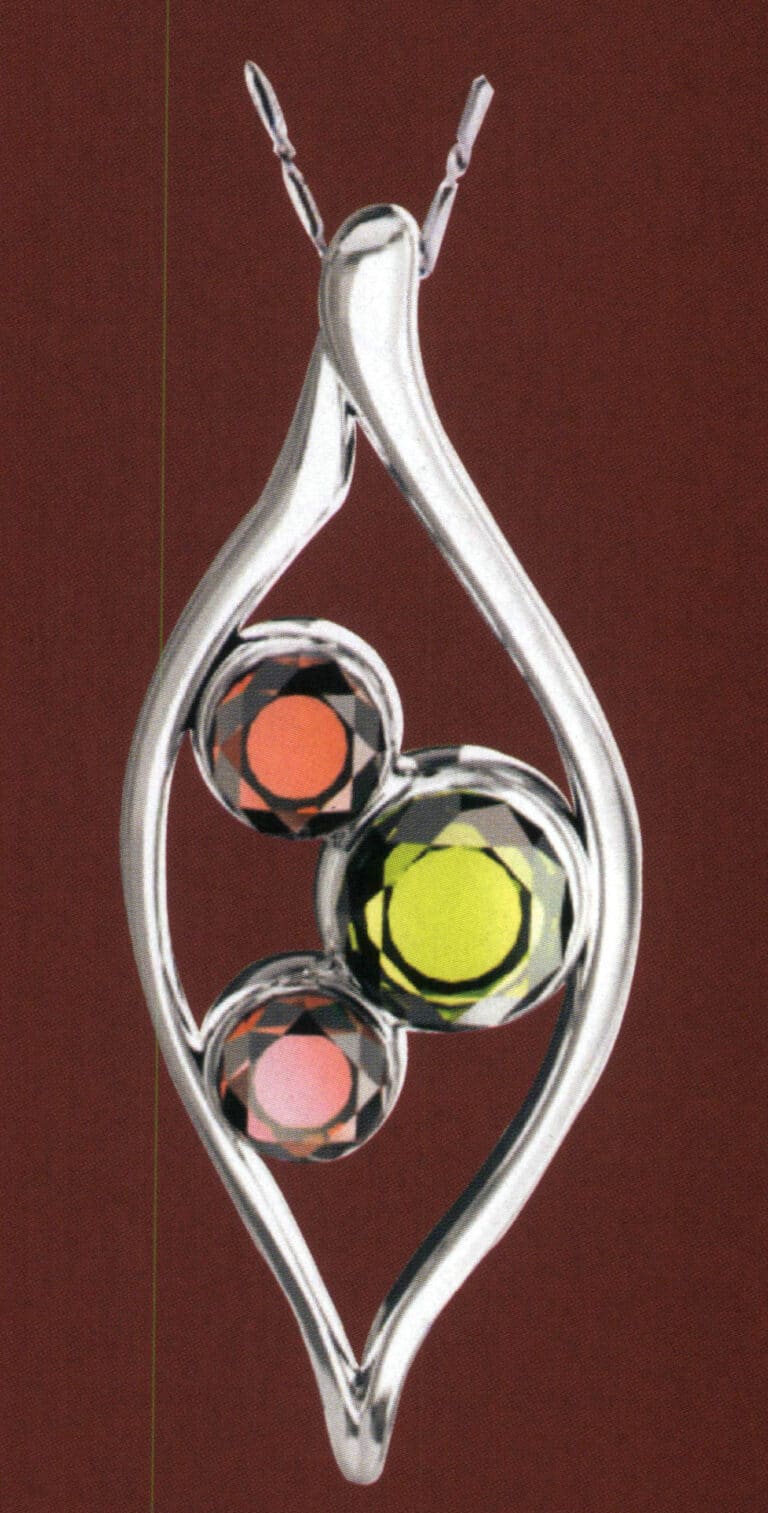
Stainless steel pendents

Stainless steel cufflinks

Stainless steel navel ring
Section II Titanium Alloy Products
1. Introduction to Titanium Alloys
(1) The Discovery of Titanium
Titanium was discovered by the British chemist Gregor R W (1762–1817) in 1791 while studying ilmenite and rutile. Four years later, in 1795, the German chemist Klaproth M H (1743–1817) also discovered this element while analyzing red rutile from Hungary. He proposed naming it after the Greek mythological race of gods, the “Titans,” following the method used for uranium (discovered by Klaproth in 1789). In Chinese, it is named “Tài” based on its phonetic pronunciation.
The titanium discovered by Gregor and Klaproth at that time was powdered titanium dioxide, not metallic titanium. Because titanium oxides are extremely stable, and metallic titanium can react violently with oxygen, nitrogen, hydrogen, carbon, and others, it isn’t easy to obtain elemental titanium. It was not until 1910 that pure metallic titanium with a purity of 99.9% was first produced by American chemist Hunter (Hunter M A).
(2) Properties of Titanium
Pure titanium has a silvery metallic luster and is ductile. Density is 4.51g/cm3, melting point is 1668℃ and boiling point is 3287℃. Valence are +2, +3 and +4. The main characteristics of titanium are its low density and high mechanical strength. The plasticity of titanium mainly depends on its purity. The purer the titanium, the greater the plasticity. It has good corrosion resistance and is not affected by the atmosphere and seawater. At room temperature, titanium is stable in air and is not corroded by dilute hydrochloric acid, dilute sulfuric acid, nitric acid, or dilute alkaline solutions; only hydrofluoric acid, hot concentrated hydrochloric acid, and concentrated sulfuric acid can act on it. Due to its low density, high specific strength, high temperature, and corrosion resistance, titanium alloys are good for making rocket engine casings, artificial satellites, and spacecraft. Titanium is known as “space metal.” Because of these advantages, titanium has become a prominent rare metal since the 1950s.
Due to sodium’s corrosion resistance and high stability, it does not affect its essence after long-term contact with humans and does not cause allergies; it is the only metal that does not affect human autonomic nerves and taste. Titanium has unique medical applications and is known as a “bio-friendly metal.”
Due to titanium’s high melting point, sodium smelting needs to be carried out at high temperatures, and at high temperatures, titanium’s chemical properties become very reactive. Therefore, smelting must be conducted under the protection of inert gases, and using oxygen-containing materials must be avoided, which places high demands on smelting equipment and processes.
(3) Main Categories of Titanium Alloys
According to the composition of the alloy, titanium is divided into two categories: industrial pure titanium and titanium alloys. Industrial pure titanium includes three types: TA1, TA2, and TA3. Titanium alloys are alloys composed of titanium as the base with other elements added, including TA4~TA8, TB1 ~ TB2, TC1 ~ TC10, and other categories, among which the industry’s most widely used titanium alloys are TC4, TA7, and industrial pure titanium (TA1, TA2, and TA3). The main chemical compositions of various titanium alloys are shown in Table 5-5, the allowable impurity element content is shown in Table 5-6, and the mechanical properties of various titanium alloy materials are shown in Table 5-7.
Table 5-5 Main Chemical Composition of Titanium Alloys
| Grados | Main components (mass fraction) (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti | A1 | Cr | Mo | Sn | Mn | V | Fe | Cu | Si | Zr | B | |
| TA0 | Base | |||||||||||
| TA1 | Base | |||||||||||
| TA2 | Base | |||||||||||
| TA3 | Base | |||||||||||
| TA4 | Base | 2.0~3. 3 | ||||||||||
| TA5 | Base | 3. 3~4.3 | 0.005 | |||||||||
| TA6 | Base | 4.0~5.5 | ||||||||||
| TA7 | Base | 4.0~5.5 | 2. 0~3.0 | 2. 5~3.2 | 1. 0~1.5 | |||||||
| TA8 | Base | 4. 5~5.5 | 2.0~3.0 | |||||||||
| TB1 | Base | 3.0~4.0 | 10.0~11.5 | 7. 0~8. 0 | ||||||||
| TB2 | Base | 2. 5~3.5 | 7.5~8.5 | 4. 7~ 5.7 | 4. 7~ | |||||||
| TC1 | Base | 1. 0~2.5 | 0.8~2.0 | |||||||||
| TC2 | Base | 2.0~3. 5 | 0. 8~2.0 | |||||||||
| TC3 | Base | 4. 5~6.0 | 3. 5~4.5 | |||||||||
| TC4 | Base | 5. 5~6.8 | 3. 5~4.5 | |||||||||
| TC5 | Base | 4. 0~6.2 | 2.0~3.0 | |||||||||
| TC6 | Base | 4.5~6.2 | 1.0~2.5 | 1.0~2.8 | 0. 5~1.5 | |||||||
| TC7 | Base | 5.0~6.5 | 0. 4~0.9 | 0. 25~0. 60 | 0. 25~0. 60 | 0.01 | ||||||
| TC8 | Base | 5. 8~6.8 | 2. 8~3.8 | 0. 20~0. 35 | ||||||||
| TC9 | Base | 5. 8~6.8 | 2. 8~3.8 | 0. 20~0. 40 | ||||||||
| TC10 | Base | 5. 5~6.5 | 5. 5~6.5 | 0. 35~1.0 | 0. 35~1.0 | |||||||
| (Xie Chengmu, 2005; Zhang Xiyan et al., 2005) | ||||||||||||
Table 5-6 Allowable Impurity Element Content in Titanium Alloys
| Grados | Impurities not greater than (mass fraction) (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Si | C | N | H | O | |||||||
| TA0 | 0.03 | 0.3 | 0.03 | 0.01 | 0.015 | 0.05 | ||||||
| TA1 | 0. 15 | 0.1 | 0.05 | 0.03 | 0.015 | 0.1 | ||||||
| TA2 | 0. 3 | 0.15 | 0.1 | 0.05 | 0. 015 | 0.15 | ||||||
| TA3 | 0.3 | 0.15 | 0.1 | 0.05 | 0.015 | 0.15 | ||||||
| TA4 | 0. 3 | 0.05 | 0.1 | 0.05 | 0. 015 | 0.15 | ||||||
| TA5 | 0. 3 | 0.15 | 0.1 | 0.04 | 0.015 | 0.15 | ||||||
| TA6 | 0. 3 | 0.15 | 0.1 | 0.05 | 0.015 | 0.15 | ||||||
| TA7 | 0. 3 | 0.15 | 0.1 | 0.05 | 0.015 | 0.2 | ||||||
| TA8 | 0.3 | 0.15 | 0.1 | 0.05 | 0.015 | 0.15 | ||||||
| TB1 | 0.3 | 0.15 | 0.1 | 0.04 | 0.015 | 0.15 | ||||||
| TB2 | 0.3 | 0.05 | 0.05 | 0.04 | 0. 015 | 0.15 | ||||||
| TC1 | 0.4 | 0.15 | 0.1 | 0.05 | 0.015 | 0.15 | ||||||
| TC2 | 0.4 | 0.15 | 0.1 | 0.05 | 0. 015 | 0.15 | ||||||
| TC3 | 0.3 | 0.15 | 0.1 | 0.05 | 0.015 | 0.15 | ||||||
| TC4 | 0. 3 | 0.15 | 0.1 | 0.05 | 0.015 | 0.15 | ||||||
| TC5 | 0. 5 | 0.4 | 0.1 | 0.05 | 0.015 | 0.2 | ||||||
| TC6 | 0.4 | 0.1 | 0.05 | 0.015 | 0.2 | |||||||
| TC7 | 0.1 | 0.05 | 0.025 | 0.3 | ||||||||
| TC8 | 0.1 | 0.05 | 0.015 | 0.15 | ||||||||
| TC9 | 0.1 | 0.05 | 0. 015 | 0.15 | ||||||||
| TC10 | 0.15 | 0.1 | 0.04 | 0.015 | 0.2 | |||||||
| (Xie Chengmu, 2005; Zhang Xiyan et al., 2005) | ||||||||||||
Table 5-7 Mechanical Properties of Titanium Alloys
| Grados | Status | Room temperature performance | High-temperature performance | Notas | |||||
|---|---|---|---|---|---|---|---|---|---|
| σ b | δ | ψ | ɑ k | T | σ b | σ 100 | |||
| MPa | % | % | MJ/m2 | ℃ | MPa | MPa | |||
| TA0 | Annealing | ||||||||
| TA1 | Annealing | 350 | 25 | 50 | 0.8 | bar stock | |||
| TA2 | Annealing | 450 | 20 | 45 | 0.7 | bar stock | |||
| TA3 | Annealing | 550 | 15 | 40 | 0.5 | bar stock | |||
| TA4 | Annealing | bar stock | |||||||
| TA5 | Annealing | 700 | 15 | 40 | 0.6 | bar stock | |||
| TA6 | Annealing | 700 | 10 | 27 | 0.3 | 350 | 430 | 400 | bar stock |
| TA7 | Annealing | 800 | 10 | 27 | 0.3 | 350 | 500 | 450 | bar stock |
| TA8 | Quenching Timeliness | 1000 | 10 | 25 | 0. 2 ~ 0. 3 | 500 | 700 | 500 | bar stock |
| TB1 | Quenching Timeliness | ≤1 000 | 18 | 30 | 0.3 | bar stock | |||
| 1 300 | 5 | 10 | 0.15 | ||||||
| TB2 | Quenching Timeliness | ≤1 000 | 18 | 40 | 0.3 | bar stock | |||
| 1 400 | 7 | 10 | 0.15 | ||||||
| TC1 | Annealing | 600 | 15 | 30 | 0.45 | 350 | 350 | 300 | bar stock |
| TC2 | Annealing | 700 | 12 | 30 | 0.4 | 350 | 430 | 400 | bar stock |
| TC3 | Annealing | 900 | 10 | 400 | 600 | 550 | Sheet (1. 0~2. 0) | ||
| TC4 | Annealing | 950 | 10 | 30 | 0.4 | 400 | 630 | 580 | bar stock |
| TC5 | Annealing | 950 | 10 | 23 | 0. 3 | 400 | 600 | 560 | bar stock |
| TC6 | Annealing | 950 | 10 | 23 | 0.3 | 450 | 600 | 550 | bar stock |
| TC7 | Annealing | 1000 | 10 | 23 | 0. 35 | 550 | 600 | bar stock | |
| TC8 | Annealing | 1050 | 10 | 30 | 0.3 | 450 | 720 | 700 | bar stock |
| TC9 | Annealing | 1140 | 10 | 25 | 0. 3 | 500 | 650 | 620 | bar stock |
| TC10 | Annealing | 1 050 | 12 | 25 | |||||
| 1 050 | 12 | 30 | |||||||
| (Xie Chengmu, 2005; Zhang Xiyan et al., 2005) | |||||||||
(4) The Effect of Alloying Elements on the Properties of Titanium Alloys
There are two types of homogeneous and heterogeneous crystals in titanium: below 882℃ is the close-packed hexagonal structure α titanium, and above 882℃ is the body-centered cubic β titanium. Alloying elements can be divided into three categories based on their influence on phase transition temperature.
① Stability α phase: The elements that increase the phase transition temperature are α stable elements, including aluminum, carbon, oxygen, and nitrogen. Aluminum is the main alloying element in titanium alloys, and it significantly improves the alloy’s strength at room and high temperatures, reduces specific gravity, and increases elastic modulus.
② Stable β phase: The elements that lower the phase transition temperature are β stable elements, which can be divided into two types: isomorphic and eutectoid. The former include molybdenum, niobium, and tungsten; the latter include chromium, manganese, copper, iron, and silicon.
③ Neutral elements, such as cobalt and tin, affect the phase transition temperature little.
④ Oxygen, nitrogen, carbon, and hydrogen are the main impurities in titanium alloys. Oxygen and nitrogen have a relatively high solubility in the α phase, significantly strengthening the titanium alloy, but they reduce plasticity. It is usually stipulated that sodium’s oxygen and nitrogen content should be below 0.15%~0.2% and 0.04%~0.05%, respectively. The solubility of hydrogen in the α phase is very low, and excessive hydrogen dissolved in titanium alloys can form hydrides, making the alloy brittle. Typically, the hydrogen content in titanium alloys is controlled to be below 0.015%. The dissolution of hydrogen in titanium is reversible and can be removed by vacuum annealing.
(5) Characteristics of Titanium Alloys
- With high specific strength, the tensile strength can reach 1000~1400MPa, while the density is only 60% that of steel.
- The medium-temperature strength is good, and the operating temperature is several hundred degrees higher than that of aluminum alloys. It can still maintain the required strength at medium temperatures and can work for a long time at that temperature of 450~500℃.
- Good corrosion resistance: The surface of titanium immediately forms a uniform and dense oxide film in the atmosphere, which can resist erosion from various media. Generally, titanium has good corrosion resistance in oxidizing and neutral media, and its corrosion resistance is even more excellent in seawater, humid chlorine gas, and chloride solutions.
- Good low-temperature performance, maintaining a certain level of plasticity even at very low temperatures.
- Low elastic modulus, low thermal conductivity, non-ferromagnetic.
2. Decorative Titanium Alloy
Titanium alloys used for jewelry making are generally industrial pure titanium. The difference between industrial pure titanium and chemically pure titanium is that it contains more oxygen, nitrogen, carbon, and other impurity elements (such as iron, silicon, etc.). It is a titanium alloy with a low alloy content. Compared to chemically pure titanium, the presence of more impurity elements significantly increases its strength, and its mechanical properties and chemical characteristics are similar to those of stainless steel (but still lower in strength compared to titanium alloys).
The characteristics of industrial pure titanium are: it has low strength but good plasticity, with certain processing and forming capabilities, and can be processed using techniques such as stamping, welding, and cutting; it has good corrosion resistance in the atmosphere, seawater, humid chlorine gas, and oxidative, neutral, and weakly reducing media, and its oxidation resistance is better than that of most austenitic stainless steels, but its heat resistance is relatively poor, with a not very high operating temperature.
Based on the different impurity content, industrial pure titanium is divided into three grades: TA1, TA2, and TA3. The interstitial impurity elements in these three grades of industrial pure titanium increase gradually, resulting in a corresponding increase in mechanical strength and hardness, while plasticity and toughness decrease accordingly.
The industrial pure sodium in the jewelry industry is TA2 due to its moderate corrosion resistance and comprehensive mechanical properties. When higher corrosion resistance and strength are required, TA3 can be used, and when better-forming performance is needed, TA1 can be used.
Currently, there are many accessories in the country referred to as titanium steel, but the material used is not titanium; it is stainless steel. To attract attention, it is called titanium steel; some even refer to it as titanium alloy accessories, which are stainless steel accessories that do not contain titanium. Titanium steel and stainless steel are two different materials that can be easily distinguished:
- In terms of weight, titanium is lighter than steel; for the same volume, titanium is only about half the weight of steel. The density of titanium is 4.5g/cm3, and that of steel is 7.845g/cm3.
- In terms of color, titanium is a bit darker than steel, while steel is whiter; the difference between the two colors is quite obvious.
3. Characteristics of Titanium Alloy Jewelry
(1) Essential Characteristics
① Light. Titanium’s specific gravity is 4.5, about half that of alloys such as stainless steel, cobalt, and chromium. It is also much lighter than gold and silver, making it advantageous for making earrings, necklaces, and other jewelry.
② Titanium has good corrosion resistance. Titanium is a highly reactive element that easily reacts with oxygen to form TiO2. Still, the oxide film that forms on the surface of titanium is extremely complete and dense, with the ability to self-repair instantly after localized damage, and it is stable in most environments. This is the theoretical basis for titanium’s corrosion resistance. The advantages it demonstrates in jewelry are that it does not corrode or change color, can maintain a good luster for a long time, and is not afraid of water.
③ Titanium can be colored. Titanium metal has a very interesting characteristic: when titanium is placed in an electrolyte and a certain current is applied, its surface will be electrolyzed to form a layer of oxide film, and the thickness of the oxide film can determine the color change without the need for additional elements. The colors that can now be produced include gold, black, blue, brown, and various other colors. This characteristic allows for more colorful and fashionable designs in jewelry.
④ Sodium is not easily deformed and does not need to be reshaped. Titanium has high hardness, is not easily deformed, and unlike ordinary gold and silver jewelry, it does not need to be reshaped after being worn for some time.
(2) Characteristics of Fashion Forward
① New material symbol. The emergence of titanium jewelry marks the breaking of tradition with new materials, challenging the dominance of ancient gold and silver jewelry in the industry. Beyond decoration, jewelry has long become a symbol of status and identity. As a third type of metal—titanium—enters the jewelry industry, it adds health, elegance, and fashion appeal to the pieces.
② Female spirit symbol. Titanium is very lightweight yet extremely tough, representing urban women who are light, beautiful, and resilient.
③ Male spirit symbol. In 1795, German scientist Klaproth discovered titanium while studying rutile. He named it after the Titan (titan) from ancient Greek mythology, which embodies the same meaning of spirit and courage. Its natural strength and texture reflect the heroic spirit of the Titan, showcasing the “Titan” spirit of urban men as sons of the earth.
④ Love symbol. Titanium is highly corrosion-resistant; it does not tarnish like silver and maintains its color for a lifetime at room temperature. Couple jewelry represents the fidelity of love, never betraying, and always maintaining supreme quality.
(3) Health Characteristics
Titanium metal has no harm to the human body. Medical practice has proven that titanium organs can be implanted in the human body for a long time, demonstrating its harmlessness to the body. Titanium jewelry, after long-term contact with the body, will not cause allergies or adverse effects on the skin, nerves, or taste, exhibiting good biocompatibility and stability. Therefore, titanium metal is also known as a biocompatible metal. It is harmless to the human body and can be the preferred jewelry for modern people with skin allergies.
(4) Aviation Characteristics
Sodium is also known as space metal. In our country’s rapidly developing aerospace industry, the public will surely pay more attention to aviation, and titanium, as the preferred material for spacecraft, will inevitably enter the lives of modern people driven by enthusiasm for aerospace. In the repeated journeys of the “Shenzhou” spacecraft into space, titanium can serve as a symbol for ordinary people to commemorate our country’s aerospace achievements.
Copywrite @ Sobling.Jewelry - Fabricante de joyería personalizada, fábrica de joyería OEM y ODM
4. Categories of Titanium Alloy Jewelry
Due to the unique silver-gray tone of titanium, whether polished, satin, or matte, performs well and is the most suitable jewelry metal after precious metals like platinum and gold. It is often used in modern jewelry design abroad and is a popular material internationally, highly regarded by young professionals. In addition, titanium crafts are a new generation of high-end gifts on the market. They are a vivid combination of traditional craftsmanship and modern science and technology. They possess practical, storage, aesthetic, and artistic value, making them essential high-end gifts for friends and visiting abroad.
The main product series of titanium jewelry includes the following nine types.
- Titanium rings its products, including ude-saving, stone setting, plating, hollowing, carving, simplicity, and decorative engraving series.
- Titanium pendant.
- Titanium chain. Includes bracelets and necklaces, with a focus on bracelets.
- Titanium cufflinks, tie clips, etc.
- Earrings and body piercing jewelry. Body piercing jewelry is quite popular abroad and is just starting in the domestic market; titanium metal poses no harm to the human body and first caters to the pursuit of health and longevity. Medical practice has proven that titanium organs can be implanted in the human body for a long time, demonstrating their harmlessness to the human body.
- Titanium watch.
- Health products combined with metals such as titanium and germanium. Mainly sodium series health products, which are currently mostly imported and quite expensive; titanium jewelry can promote blood circulation and enhance natural healing ability, while germanium may also replace the functional performance of oxygen. After contact with the skin, starting from a temperature increase of about 0.5℃, it may improve blood circulation and assist in the smooth discharge of waste (cations, protons) from the blood. Germanium can restore the body’s electrical potential to a normal balanced state. One explanation for this phenomenon is that germanium may begin to move electrons to the outermost orbit based on body temperature energy, allowing free electrons to enter and exit freely, thus restoring the chaotic electrical potential balance of nerve circuits to normal operation. This electronic effect of semiconductors may stimulate the activation of nerve cells and alleviate discomfort symptoms in the body.
- The Daily Necessities series offers a wide range of products. For example, there are titanium glasses frames, titanium stationery, titanium canes, titanium swords, titanium ashtrays, titanium prints, titanium wine utensils, and titanium tableware.
- Sports equipment series. Such as golf clubs, tennis rackets, badminton rackets, etc.

Titanium ring

Titanium pendents

Titanium bracelets

Titanium cufflinks
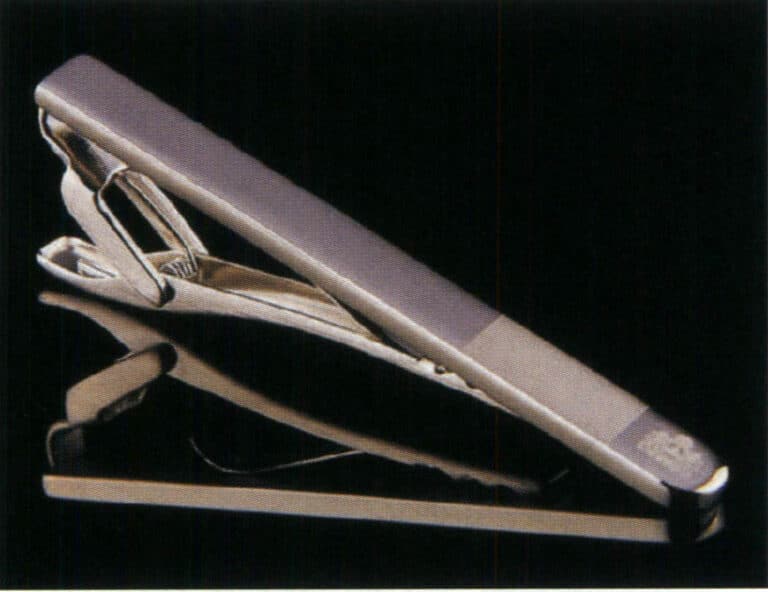
Titanium tie clips

Titanium earrings


Titanium watch
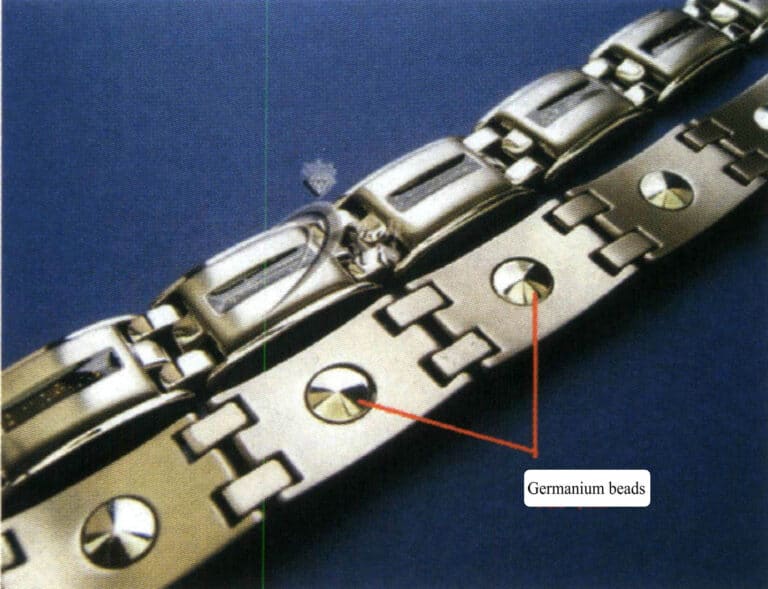
Titanium health bracelet with embedded germanium beads
5. The Market Situation of Titanium Jewelry
Titanium jewelry is an emerging type of jewelry product that is gradually being recognized and accepted by more and more people. Because titanium metal has many excellent properties, it is very suitable for jewelry processing. With improved processing technology, titanium jewelry has gained popularity internationally since 2000. Many people now accept titanium as a metal for producing jewelry, and the demand for titanium jewelry is increasing yearly. Some world-renowned jewelry brands have also begun to launch titanium jewelry, which can attract attention to titanium metal products and stimulate demand.
Due to the high technical requirements for processing sodium, it is difficult to cast and shape it with conventional equipment, and it is also challenging to weld it with ordinary tools, which creates significant difficulties in achieving production scale. Additionally, the technology and knowledge for making titanium jewelry are poorly disseminated in the country. Therefore, although titanium jewelry has been popular in Western countries for a long time, it is still a new concept for Chinese people, and the domestic production capacity is low. Currently, the consumption of titanium products in the country has just begun, and it is not on the same level as traditional gold and silver jewelry. The market is currently expanding, but this presents a rare opportunity. The diversification of jewelry materials will be a major trend in the market, and titanium, as a third metal, will inevitably break the traditional dominance of gold and silver jewelry due to its inherent characteristics.
Section III Forming Process of Stainless Steel and Titanium Alloy Products
1. Mechanical Forming Process
1.1 Machining and Forming
Some structurally simple pieces can be directly processed and formed to produce stainless steel and titanium alloy jewelry. Common methods include machining, electrical discharge machining, and etching.
(1) Cutting and Forming
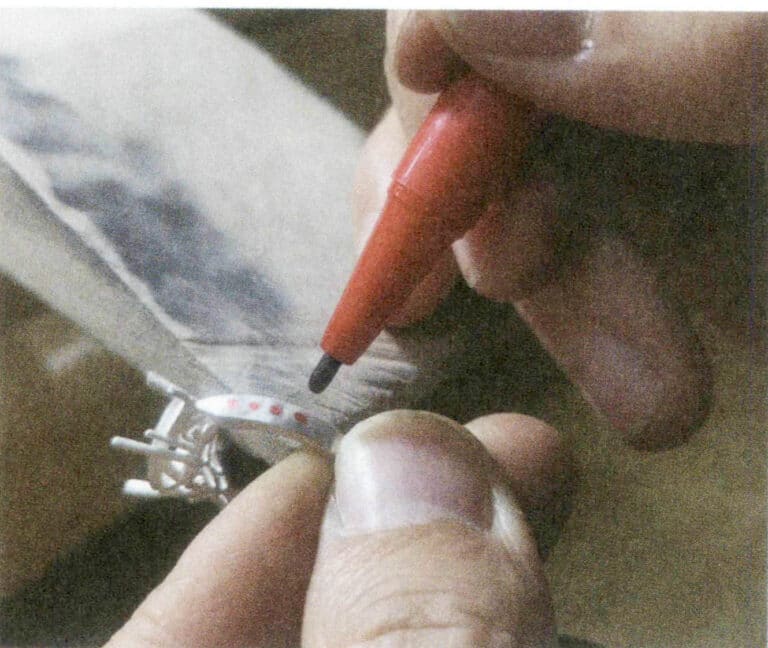
Using a lathe to directly process stainless steel or titanium alloy profiles into jewelry is most common in ring and bracelet jewelry, accounting for a large proportion. Figures 5-1 and 5-2 show stainless steel and titanium alloy rings shaped using a lathe.

Figure 5-1 Stainless steel ring finished on a lathe

Figure 5-2 Titanium alloy ring shaped by lathe finishing
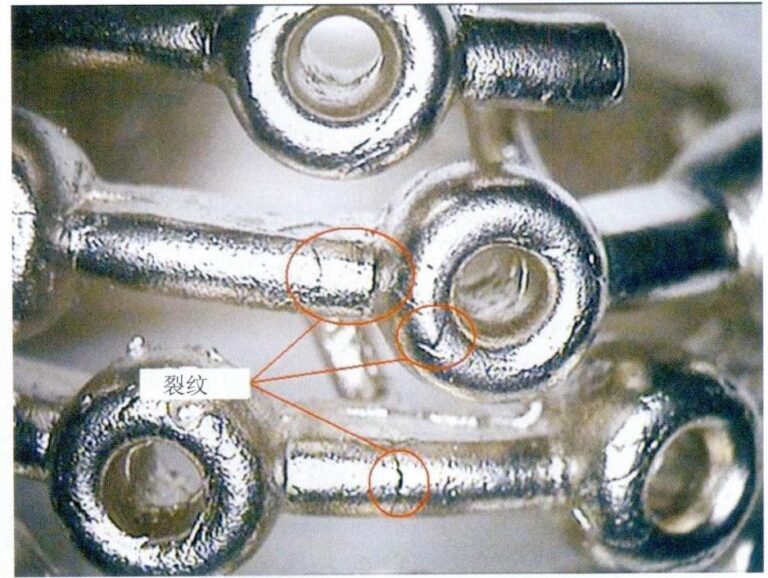
Finishing is difficult due to the material characteristics of stainless steel and titanium alloy. Based on these properties, it is necessary to select and formulate corresponding processing parameters to ensure the processing accuracy and surface quality of the jewelry.
① Machining of stainless steel rings. In actual production, the processing of stainless steel is relatively difficult. If you cannot master its characteristics, you will not achieve the desired processing quality during cutting and cause significant damage to the tools.
The reasons for the difficulty in machining stainless steel mainly come from the following five aspects.
- The comprehensive mechanical properties of stainless steel are high. Due to the higher content of alloying elements such as chromium and nickel in stainless steel, the material’s mechanical properties have changed significantly. From the perspective of various mechanical performance indicators, the mechanical properties of stainless steel have characteristics that distinguish them from ordinary steel, with both strength performance indicators and plastic toughness indicators being relatively high. This has resulted in the characteristic of stainless steel being difficult to machine.
- The strong chip adhesion makes tool buildup easy. Stainless steel has a higher adhesion, which causes the material to “bond” to the tool during finishing, resulting in “tool buildup.”
- Low thermal conductivity; cutting heat cannot be dissipated in time. The heat transferred to the tool can reach 20%, and the cutting edge of the tool is prone to overheating, losing cutting ability.
- Chips are not easily broken. In metal cutting, the formation of chips from ductile materials (tough materials) goes through four stages: extrusion, slip, fracture, and separation. Due to the generally high elongation, cross-sectional shrinkage, and impact values of stainless steel, especially for 304(L) and 316(L) austenitic chromium-nickel stainless steel used in jewelry, both elongation and toughness are good, making it difficult for chips to curl and break during the cutting process. In boring, drilling, and cutting operations, chip removal is difficult, and chips can easily scratch the processed surface.
- The tendency for work hardening is strong, making the tools prone to wear. Austenitic stainless steel has a strong tendency to work harden, with a high hardness of the work-hardened layer and a certain depth of work hardening, which increases the difficulty of processing and tool wear.
The measures to be taken in stainless steel cutting are as follows.
First, choose a reasonable geometric shape for the cutting tool, making cutting deformation easier, reducing cutting force, and allowing chips to form and discharge smoothly. Different tools should have the following requirements for the geometry of the cutting part:
- Rake angle. A larger rake angle reduces cutting force and heat, decreases vibration during cutting, and weakens the work-hardening effect. The rake angle between 12°~30°can generally be selected depending on the type of tool, tool material, and cutting conditions. At the same time, a positive flank angle enhances the strength of the cutting edge; a negative chamfer is ground on the main cutting edge to strengthen the blade.
- The shape in front. When processing stainless steel, due to the material being relatively tough and soft, the chips experience strong friction with the front of the tool during their formation and curling process, causing a crescent-shaped pit to gradually form on the front of the tool. The center of the crescent-shaped pit is the pressure center of the chips against the front of the tool. Based on the above characteristics, a curved chip groove is pre-ground on the front of the tool to slow down the wear of the cutting edge and enhance the strength of the tip.
- Relief angle. The influence of the relief angle on the cutting process is generally not as sensitive as that of the rake angle. However, due to the significant metal deformation during stainless steel cutting, if the relief angle of the tool is small, it is prone to severe friction with the workpiece surface, resulting in increased surface roughness, work hardening, and exacerbated tool wear. At the same time, this worsens the conditions for subsequent cutting processes. When the relief angle of the finishing tool is α<6°, the workpiece surface exhibits a roughening phenomenon. This phenomenon is particularly severe when the feed rate and the back-cutting amount are relatively small. Therefore, a bit larger relief angle is generally selected when cutting stainless steel. However, if the rear corner is too large, the strength of the cutting edge will be reduced.
Secondly, choose suitable tool materials. Due to the characteristics of stainless steel itself, the cutting part of the tool must have high wear resistance and red hardness during machining, and it is often more important to focus on selecting toughness than durability.
The third point is the selection of cutting parameters. When choosing cutting parameters, the following factors should be considered: the cutting parameters should be selected based on the hardness of stainless steel and various raw materials; the cutting parameters should be selected based on the tool material, welding quality, and the grinding conditions of the lathe tool; the cutting parameters should be selected based on the diameter of the part, the size of the machining allowance, and the precision of the lathe.
Fourth, the requirements for cooling and lubrication. The coolant used for cutting stainless steel must have high cooling performance to remove a large amount of heat. It should also have good lubrication performance to provide effective external lubrication. It should have good permeability to facilitate wedging, diffusion, and internal lubrication. In addition, it should have good washing performance and supply methods to meet the needs of chip removal.
② Machining of titanium alloy rings. The poor machining performance of titanium alloys can be measured in terms of tool durability, the quality of the machined surface, and the difficulty of chip formation and removal. The reasons for the difficulty in machining sodium and titanium alloy materials are mainly reflected in the following aspects.
- The thermal conductivity and thermal diffusivity coefficients are low. The thermal conductivity and thermal diffusivity coefficients of titanium alloy materials are only 1/15 of aluminum and aluminum alloys, of 1/5 of that of steel. They are lower than the thermal conductivity coefficients of stainless steel and high-temperature alloys. The low thermal conductivity and diffusivity result in significant temperature differences and high thermal stress during machining, making it difficult for cutting heat to dissipate and leading to machining adhesion phenomena.
- The contact between the cutting edge and the front cutting surface is small, resulting in high stress at the cutting edge. This stress concentration makes the tool prone to wear and damage.
- High chemical reactivity leads to the formation of an oxide layer during processing, which is very hard and accelerates tool wear.
- A large friction coefficient, a small elastic modulus, and a high yield strength cause significant rebound deformation on the surface of processed products, thereby affecting their processing accuracy.
The measures taken in the cutting processing of titanium alloys are similar to those for stainless steel, but due to the special nature of titanium alloy materials, attention should be paid to the following three points during cutting processing.
The first is the selection of cutting machine tools and fixtures. The cutting machine tools should have high power, good rigidity, and a large range of speed and feed rates. The rigidity of the fixtures should be good, and the clamping force during finishing should not be too large to reduce the deformation of the processed parts and ensure processing accuracy.
The second is the selection of tool materials. In the process of cutting high-strength, high-toughness titanium alloys, the cutting force on the tool is very large, and sometimes the phenomenon of workpiece back-cutting may occur. The hard oxide layer can damage the surface of carbide blades. This requires that the tool material maintains sufficient hardness and good wear resistance at high temperatures and heat resistance. Therefore, when cutting titanium alloys, carbide tools should be prioritized only and high-speed steel tools should only be used when the temperature is relatively low. Never use tool materials that contain titanium, as these materials can easily bond with titanium alloys at high temperatures, leading to rapid tool wear.
The third is to correctly choose the cutting parameters. This includes cutting speed, depth, and feed rate, which can improve processing efficiency and reduce production costs. The cutting temperature of carbide tools should be controlled within 600~800℃, while the cutting temperature of high-speed steel tools should be controlled within 450~560℃.
(2) Electrical Discharge Forming
① Introduction to Electrical Discharge Machining. Electrical discharge machining is conducted in a liquid medium, where the automatic feed adjustment device of the machine tool maintains an appropriate discharge gap between the workpiece and the tool electrode. When a strong pulse voltage is applied between the tool electrode and the workpiece (reaching the breakdown voltage of the medium in the gap), it breaks down at the lowest insulation strength of the medium. Due to the small discharge area and extremely short discharge time, the energy is highly concentrated, causing the temperature in the discharge area to reach 10000-12000℃ instantaneously, resulting in localized melting and even vaporization of the metal on the surfaces of the workpiece and the tool electrode. The locally melted and vaporized metal is ejected into the working fluid under explosive force and is cooled into small metal particles, which are then quickly washed away from the working area by the working fluid, forming a tiny pit on the surface of the workpiece. After each discharge, the insulation strength of the medium recovers, waiting for the next discharge. This process is repeated, continuously eroding the workpiece’s surface and replicating the tool electrode’s shape, thereby achieving the purpose of forming and machining.
Electrical discharge machining includes various forms, such as electrical discharge forming, electrical discharge wire cutting, electrical discharge grinding, electrical discharge drilling, and various specialized electrical discharge machining applications.
Electrical discharge machining is widely used in the production of stainless steel and titanium alloy jewelry, mainly in two aspects: first, electrical discharge wire cutting is used for direct processing of jewelry; second, electrical discharge cutting and forming are used to create molds for subsequent stamping and hydraulic production of jewelry.
② Wire-cut Electrical Discharge Machining (WEDM), sometimes called wire cutting. Its basic working principle is to use a continuously moving fine metal wire (called the electrode wire) as the electrode to perform pulse spark discharge machining on the workpiece, cutting and shaping it. It is mainly used for processing various complex shapes and precision small workpieces, such as punch molds, die molds, convex-concave molds, fixed plates, discharge plates, forming tools, templates, metal electrodes for electrical discharge forming processing, various fine holes, grooves, narrow seams, arbitrary curves, etc. It has outstanding advantages such as small machining allowance, high accuracy, short production cycle, and low manufacturing cost, and has been widely applied in production. Wire-cut electrical discharge machines account for more than 60% of the total number of domestic and international electrical machining machines.
According to the different running speeds of the electrode wire, electric discharge wire cutting machines are usually divided into two categories: one is the high-speed wire feeding electric discharge wire cutting machine, where the electrode wire moves back and forth at high speed, generally with a wire feeding speed of 0.2m/s, the electrode wire can be reused, the processing speed is relatively high, but the fast wire feeding can easily cause the electrode wire to shake and pause during reverse movement, leading to a decline in processing quality; the other is the low-speed wire feeding electric discharge wire cutting machine, where the electrode wire moves in a low-speed unidirectional manner, generally, with a wire feeding speed of less than, the electrode wire is not reused after discharge, the operation is stable and uniform, with little shaking, and the processing quality is better, but the processing speed is lower.
In jewelry production, decorative patterns are often formed through wire cutting, as shown in the example of the stainless steel pendant pattern in Figure 5-3.

1.2 Mold Stamping (Hydraulic) Forming
(1) The Introduction to Stamping Process
Stamping is a forming processing method that uses a press and molds to apply external force to metal sheets, strips, pipes, and profiles, causing them to undergo plastic deformation or separation. The surface shape of the mold is clearly replicated, thereby obtaining workpieces (stamped parts) of the desired shape and size. Compared to traditional lost-wax casting, stamping can economically and repeatedly produce large quantities of the same product in a short time, and the surface of the product is smooth, with stable quality, greatly reducing the workload of subsequent processes, improving production efficiency, and lowering production costs. Therefore, stamping has received increasing attention in the jewelry manufacturing industry, and its application is becoming more widespread.
(2) Characteristics and Applicability of Stamped Accessories
Stamping accessories have the following characteristics:
- (Compared with investment casting jewelry, stamped parts have the characteristics of being thin, uniform, light, and strong, and using stamping methods can greatly reduce the wall thickness of the workpiece.
- Stamping produces jewelry with fewer holes, better surface quality, improved quality, and reduced defect rate.
- During mass production, stamping has high production efficiency, good working conditions, and low production costs.
- When the mold’s precision is high, the stamped accessories’ accuracy is high, and the repeatability is good, with consistent specifications, greatly reducing the trimming, grinding, and polishing workload.
- Stamping can achieve a higher degree of mechanization and automation.
However, the following conditions must be considered before the stamping process can produce accessories.
First, the structure of the jewelry should have good stamping processability, and it is best to avoid small holes, narrow grooves, and structures with angles or hollowed-out bottoms that cannot be stamped. A draft angle should be designed. The shape of the stamped parts should be as symmetrical as possible to avoid issues such as stress concentration, eccentric loading, and uneven mold wear. The thickness of the jewelry should not be too large, and the wall thickness difference should not be too big.
Second, the accessories should have a considerable production volume. Due to the stamping process, special molds must be made, which takes a long time and has high mold costs. Therefore, when the production volume is small, the production cost does not have an advantage.
Third, the strength of stainless steel and titanium alloys is relatively high, requiring good flow performance of the material in the cavity during the extrusion process, especially at the edges, corners, and ridges, where it is necessary to fill in completely without causing serious defects such as collapsed corners, edges, or ridges. A larger impact or pressure is needed, so the stamping machinery selected must have sufficient force, and the mold material must have adequate strength, with precise dimensions for the supporting and positioning points and surfaces for stamping.
(3) The Main Process of Stamping Accessories
① Analyze the processability of stamped parts. The product part drawing is an important basis for formulating stamping process plans and mold design. The formulation of stamping process plans should start from the product part drawing. Analyzing the part drawing includes both technical and economic aspects: the economic analysis of stamping processing, which analyzes product costs based on the production program of stamped parts, clarifying the economic benefits that can be achieved by adopting stamping production; the processability analysis of stamped parts refers to the difficulty level of stamping processing for the part. From a technical perspective, it mainly analyzes whether the shape characteristics, size, precision requirements, and material properties of the part meet the requirements of the stamping process. If poor processability is found, modification suggestions for the stamped part product need to be proposed and can only be modified with the consent of the product designer.
② Determine the forming process plan for the stamped parts. After analyzing the processability of the stamped parts, several different stamping process plans are usually developed based on the analysis of the nature of the processes, the number of processes, the sequence of processes, and the combination methods. A comprehensive analysis and comparison are conducted from various aspects, including product quality, production efficiency, equipment occupancy, the difficulty of mold manufacturing and mold life, process costs, and the convenience and safety of operation, to determine the most economical and reasonable process plan suitable for the specific production conditions of the factory.
Then, based on the overall plan for forming the determined parts, determine and design the process plan for each stamping operation. This includes the processing methods for completing the forming of each operation, the main process parameters for each operation, necessary forming process calculations based on the forming limits of each stamping operation, determining the forming force for each operation, calculating the consumption quotas for materials, energy, and labor hours for each operation; calculating and determining the shape and dimensions of each operation piece, and drawing the process diagrams for each operation.
③ Determine the structural form of the stamping die. The stamping die is a special process equipment that processes materials into parts (or semi-finished products) and is an essential piece of equipment for stamping production. The quality of stamped parts, production efficiency, and production costs are directly related to the design and manufacturing of the die. The level of technology in die design and manufacturing is one of the important indicators of a country’s product manufacturing level, and it largely determines the quality, efficiency, and new product development capabilities of the products.
There are many forms of stamping molds, which can generally be classified according to the following two main characteristics.
a. Classified according to the nature of the process as follows.
Punching die: A mold that separates material along closed or open contour lines. Such as blanking die, punching die, cutting die, notching die, trimming die, and splitting die.
Bending die: A mold that causes the blank or other raw materials to undergo bending deformation along a straight line (bending line) to obtain a workpiece with a certain angle and shape.
Deep drawing dies are molds that transform a sheet metal blank into an open hollow part or further change the shape and size of the hollow part.
The molding dies: These are molds that directly copy the shape of the convex and concave molds according to the drawing, using a blank or semi-finished workpiece. The material itself only undergoes local plastic deformation, such as expansion molds, necking molds, flaring molds, undulating forming molds, flanging molds, shaping molds, etc.
b. Classified according to the degree of process combination as follows.
Single-process die: A die completes only one stamping process in a single press stroke.
Composite die: A die with only one station that completes two or more stamping processes simultaneously at the same station during one stroke of the press.
Progressive die (also known as continuous die): A die with two or more stations in the direction of the blank’s feed, completing two or more stamping processes at different stations in one press stroke.
Stamping dies for accessories generally consist of two types of components. The first type is process parts, which directly participate in the completion of the process and have direct contact with the raw materials, including working parts, positioning parts, unloading and pressing parts, etc.; the second type is structural parts, which do not directly participate in the completion of the process and do not have direct contact with the raw materials, but serve to ensure the completion of the process or improve the functionality of the die, including guiding parts, fastening parts, standard parts, and other components. The stamping die manufacturing process is shown in Figure 5-4.
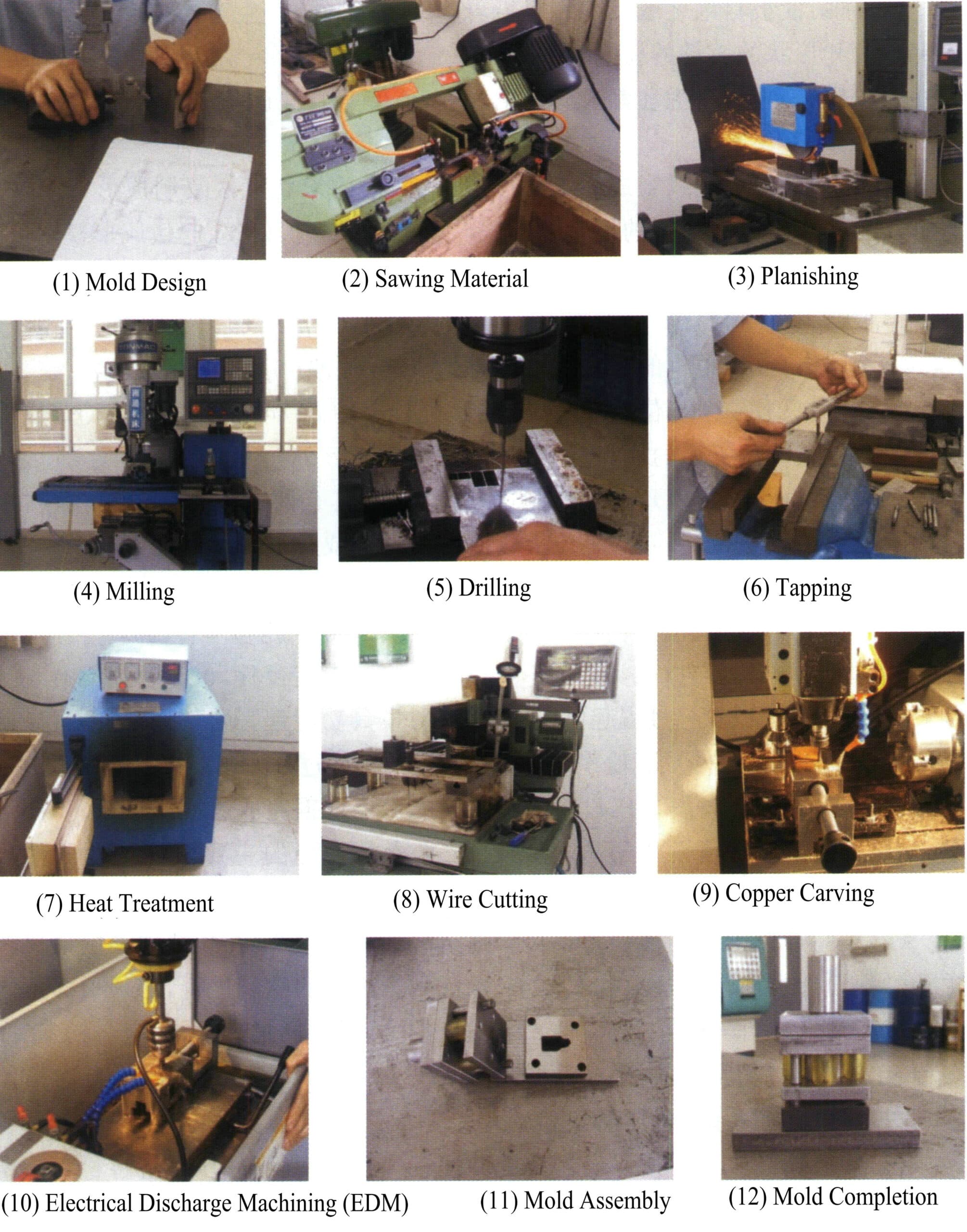
④ Choose stamping equipment. The type of stamping equipment selected is mainly based on the nature of the stamping to be completed, production volume, dimensions of the stamped parts, and precision requirements; the main basis for selecting the technical parameters of the equipment is the dimensions of the stamped parts, the magnitude of the deformation force, and the size of the molds.
⑤ Write stamping process documents. To scientifically organize and implement production, accurately reflect the technical requirements determined in the process design during production, and ensure the smooth progress of the production process, it is necessary to prepare detailed process documents based on different production types, generally presented in the form of a process flow. The content includes process name, number of processes, process sketches (shape and size of semi-finished products), molds used, selected equipment, process inspection requirements, specifications and performance of sheet materials, and the shape and size of rough parts.
⑥ Stamping jewelry production. Shape the material using stamping equipment according to the established stamping process parameters.
(4) Measures to Improve the Cross-Sectional Quality of Stamped Parts
The process of stamping accessories can be classified into two main categories based on the technology: forming processes and separating processes. The purpose of the forming process is to cause the sheet material to undergo plastic deformation without breaking blanks, creating workpieces of the desired shape and size. The separating process, also known as blanking, aims to separate the stamped parts from the sheet material along a specific contour line while ensuring the quality requirements of the separation surface. The quality of the blanking surface depends on the blanking conditions and the properties of the material itself, such as the edge gap and edge shape, the sharpness of the edge, the blanking force, the lubrication conditions, and the quality of the sheet material, and performance, etc. Stamping production requires that the cut parts have a larger bright band and minimize the width of the fracture band area. This is crucially dependent on taking measures to increase plastic deformation and delay the occurrence of shear cracks. For example, reducing the cutting gap, using a pressure plate to compress the strip on the die surface, applying reverse pressure to the strip under the punch with a top plate, reasonably selecting the overlap, paying attention to lubrication, etc. In addition, during cutting, efforts should be made to minimize the collapse angle, burrs, and warping. To achieve this, it is necessary to adopt the lower limit value of reasonable gaps as much as possible. The mold edge can be kept sharp, and the edge value can be reasonably selected. Measures such as pressure plates and ejector plates can be used.
2. Investment Casting Process
2.1 Stainless Steel Casting and Forming
The melting point of 304 stainless steel is 1454℃, and the melting point of 316 stainless steel is 1398℃. This temperature far exceeds the limits that gypsum molds can withstand. Therefore, stainless steel jewelry must be cast using acid-bonded casting powder, which significantly increases production costs.
(1) Casting Powder for Stainless Steel Jewelry
Due to the high casting temperature of stainless steel, casting powder cannot use gypsum as a binder and must use casting powder with higher refractoriness. Like gypsum casting powder, the casting powder used for stainless steel jewelry is also composed of a binder and filler. The filler is usually quartz and feldspar, with a total amount of approximately 80%. Phosphoric acid was initially widely used as a binder, but now methenamine phosphate is preferred. It is a dry powder that can be easily added to the powder mixture, utilizing the chemical reaction of the binder system to solidify the casting powder, as follows:
NH4H2PO4 + MgO +5H2O→ NH4MgPO4-6H2O
The entire phosphoric acid reaction is very complex. When the amount of MgO required for the reaction of methyl phosphonic acid is chemically equal, it often actually requires an excess of MgO, thus forming a NH4MgPO4-6H2O colloid that surrounds the filler and the excess MgO. During roasting, the temperature reaches 1000℃, and the mold undergoes a thermal reaction, resulting Mg2P2O7 in the final products of crystalline filler, excess MgO, and SiO2 filler.
Using phosphate-bonded casting powder, the overall strength of the casting powder is much higher than that of gypsum casting powder, the surface of the mold cavity is smoother and finer, and the surface finish of the castings is higher. However, the residual strength of the mold is also higher, making it somewhat difficult to remove the castings from the mold.
(2) The Casting Process of Stainless Steel Jewelry
Stainless steel jewelry can be produced using centrifugal casting methods, vacuum suction casting, or vacuum pressure casting methods. The casting process involves many procedures (Figure 5-5).

2.2 Casting and Forming of Titanium Alloys
(1) The Requirements of Titanium Alloy Casting for Melting
Due to the high reactivity of titanium alloy liquid, its melting must be carried out under a higher vacuum or protected by inert gases (argon or helium). The crucibles used for melting are all water-cooled copper, with three melting processes.
① Vacuum non-consumable electrode arc furnace smelting. Alloy smelting is carried out under vacuum or inert gas protection. This process mainly prepares consumable electrode smelting, characterized by high-temperature and high-speed melting. The vacuum non-consumable electrode arc furnace maintains the stability of the arc after vacuuming and filling with inert gas, preventing the volatilization of difficult-to-melt metals, especially reactive metals, thus stabilizing the metal composition for refining purposes.
② Vacuum self-consumption electrode arc furnace smelting. It uses self-consumption electrodes made of titanium or titanium alloys as the cathode and a water-cooled copper crucible as the anode. The melted electrode enters the crucible as droplets, forming a molten pool. The surface of the molten pool is heated by the arc, remaining in a liquid state at all times. At the same time, the bottom and the surrounding area in contact with the crucible are subjected to forced cooling, resulting in crystallization from the bottom up. The metal liquid in the molten pool solidifies to become titanium ingots.
③ Vacuum self-consuming electrode shell melting furnace. This type of furnace was developed based on the vacuum self-consuming electrode arc furnace, which integrates melting and centrifugal casting for irregular castings. Its biggest feature is a layer of titanium alloy solid shell between the water-cooled copper crucible and the metal melt known as the shell. This shell, made of the same material, serves as the inner lining of the crucible to form a melt pool for storing titanium liquid, avoiding contamination of the titanium alloy liquid by the crucible. After pouring, the layer of shell left in the crucible can be reused as the inner lining of the crucible.
In recent years, with the development of technology and the needs of production, new methods and equipment for smelting titanium alloys and other reactive metals have been successively researched and developed, mainly including electron beam furnaces, plasma furnaces, and vacuum induction furnaces, which have achieved a certain degree of application. However, regarding technical and economic indicators such as power consumption, melting speed, and cost comparison, self-consuming electrode arc furnaces (including shell furnaces) remain the most economical and suitable smelting method.
For jewelry production, the melting amount is generally small, and the surface quality requirements are high. Therefore, dental casting equipment can generally be used. Figure 5-6 shows a dental titanium casting machine that can be used for casting titanium jewelry. This titanium casting machine integrates pressurization, suction, and centrifugation, is compact, easy to operate, does not require a dedicated space, and utilizes flywheel energy storage and the momentary increase in the device’s force and the rapid flow acceleration to inject the titanium molten liquid into the fine details of the mold cavity, resulting in a higher success rate for the castings. The small melting and casting chambers enable quick vacuum extraction, reduce argon gas consumption, minimize residual air, and ensure relatively good-quality titanium castings.
(2) Requirements for Casting Materials in Titanium Alloy Casting
Titanium and its alloys are highly chemically reactive metals that, in a molten state, almost react with all refractory materials to form brittle compounds, greatly increasing the difficulty of melting and casting titanium alloys.
① There are three types of molds for casting titanium alloys.
- Permanent molds mainly include processed graphite and metal molds (iron and titanium molds). The molds are all mechanically processed. The castings produced have relatively simple structures and lower dimensional accuracy; they are generally used to produce rough parts.
- Disposable molds: They can produce castings with relatively complex shapes and high dimensional accuracy. According to their molding methods, there are two types: compacted graphite sand molds and lost foam molds. The latter can manufacture more complex castings (wall thickness 2 mm) with high dimensional accuracy and low surface roughness (Ra3.2). According to the different materials of the mold shell, the lost foam mold shell is divided into three different systems: the pure graphite mold shell system. It uses graphite powder of different particle sizes as refractory fillers and sand-spraying materials, with resin as the binder. The mold shell has high strength, lightweight, low cost, and a wide range of raw material sources, making it suitable for centrifugal or gravity casting. The second is the refractory metal surface layer mold shell system. This is a composite system, where the surface layer requires special processes due to the different molding materials (such as aluminum powder and other refractory metals), while the back layer is the same as the lost foam casting of cast steel in terms of molding materials and shell-making processes. The third is the oxide ceramic mold shell system. The mold shell’s surface and back layers are made of oxides as molding materials, resulting in high mold shell strength and the lowest thermal conductivity among the three types of mold shells, making it suitable for casting complex, thin-walled shapes. The titanium castings poured using the above three types of shell systems differ little in chemical composition and mechanical properties; however, there is a significant difference in surface quality, with the shrinkage rate of the latter two types of shells significantly lower than that of the graphite shell, resulting in high dimensional accuracy of the castings.
- Embedded casting: Embedded casting is primarily used in the casting of titanium alloy jewelry. It is very similar to oxide ceramic shells, except that layered shells are not used, and the investment method is applied directly.
② Requirements for embedding materials in casting titanium jewelry. The linear shrinkage rate of titanium during pure titanium casting is 1.8%~2.0%. To achieve good dimensional accuracy, the embedding material must provide sufficient expansion to compensate for the casting shrinkage of sodium. The conditions for selecting embedding materials for titanium casting must include the following: minimal reaction with titanium, ability to achieve a good surface shape, no contamination of the castings, moderate expansion to compensate for titanium shrinkage and sufficient strength.
Casting titanium embedding materials can be divided into three categories based on different expansion methods: embedding materials that expand due to the hardening and thermal deformation of silicon, embedding materials that expand due to the oxidation of metal powder zirconium (Zr), and embedding materials that expand due to the formation of spinel (MgO, Al2O3), including embedding materials primarily composed of refractory materials such as silicon oxide, aluminum oxide, magnesium oxide, calcium oxide, and zirconium oxide.
The current expansion of the embedding material mainly relies on SiO2 undergoing an allotropic transformation when heated, accompanied by significant volume expansion, which determines the special position of the SiO2 among embedding material. However, molten titanium can chemically react with it, severely affecting the quality of titanium castings. To address this issue, a certain proportion of ZrO2. It is a high-temperature resistant inert material added to the currently ideal casting titanium embedding material, as it does not chemically react with molten titanium at high temperatures. Castings made with ZrO2-based embedding materials have less contamination beneath the surface and do not stick to sand, resulting in castings with a metallic luster but a smaller expansion coefficient, which can affect the dimensional accuracy of the castings.
The scouring effect of high-temperature molten metal is significant during pouring. When the strength of the embedding material is insufficient, some of the embedding material powder will fall off and mix into the titanium liquid under the scouring of the titanium liquid, causing the fluidity of the titanium liquid to deteriorate and preventing it from reaching the end of the casting cavity. Therefore, the ideal embedding material for casting titanium must have good stability and expansion coefficient and possess a certain strength to withstand the impact of the titanium liquid.
(3) Casting Methods for Titanium Alloy Jewelry
Casting titanium alloy jewelry requires special heat sources, dedicated mold materials, and equipment to prevent titanium surface contamination. Dental-specific titanium casting machines have been introduced into jewelry making, with melting atmospheres protected by vacuum or inert gases (argon or helium). The casting methods include pressure casting, vacuum casting, and centrifugal casting. Due to the high pouring temperature of titanium alloys, their low density, and poor fluidity, it is necessary to complete the filling quickly, with the best method being vacuum centrifugal casting. Figures 5-7 and 5-8 exemplify casting titanium alloy jewelry.

Figure 5-7 Titanium jewelry casting

Figure 5-8 Titanium jewelry produced by casting method
(4) Common Issues in Casting Titanium Jewelry
The common problems when casting titanium alloy jewelry are the following five.
① The casting is incomplete. The incompleteness of the casting is related to the following aspects.
- Casting machine. The model of the casting machine is closely related to the casting flow rate, the casting machine’s vacuum level, and the inert gas’s flow rate.
- Setting of the pouring channel. If the pouring channel is too narrow or too long, or if its position and quantity are inappropriate, it can affect the integrity of the casting.
- Setting of ventilation channels. Titanium is protected by inert gas during the melting process, and the inert gas can also enter the mold cavity. When the melted titanium liquid is injected into the mold cavity, the gas in the cavity’s small areas hinders the titanium liquid’s flow, forming gas holes. Therefore, it is necessary to carefully set up the exhaust channels on the wax mold.
- Molding temperature. A high molding temperature results in fewer casting defects, but the surface contamination layer of the castings is thick, and the mechanical properties are poor. Reducing the molding temperature can decrease surface contamination, but it leads to more casting defects. When the molding temperature is between 350~400℃, it can reduce both contamination and casting defects.
- Titanium material usage. When the number of castings in the mold is too large, and the amount of titanium material is insufficient, incomplete castings will inevitably occur.
② Internal porosity of castings. The appearance of internal porosity in titanium castings is due to the inert gases and residual air being entrained into the mold cavity when the molten sodium is poured into the mold cavity. When the titanium liquid is injected into the cavity, a shell forms immediately, causing the entrained gases to be unable to escape, resulting in internal porosity in the casting. The quantity and type of porosity formed are related to the equipment. Porosity formed in pressurized, suction-type, and pressurized (non-suction) types is dispersed. The porosity formed in pressurized (non-suction) types is less than in pressurized suction types. The porosity in centrifugal casting titanium machines are mostly found at the inner end of the rotating body, and the incidence of porosity is significantly lower than that in pressurized suction and pressurized types.
The permeability of the embedding material is also related to the pores. Embedding materials with good permeability are used for pressurization, and suction-type titanium casting machines can produce more pores. The centrifugal titanium casting machine is unrelated to the permeability of the embedding material. In addition, the positioning of the runner and exhaust duct also has a certain relationship.
③ Shrinkage cavities. The formation of shrinkage cavities inside titanium castings is a difficult problem in titanium casting technology. The volume of molten titanium shrinks by 1% during solidification. If the titanium casting process is not properly controlled and sufficient compensation is not provided, shrinkage cavities will inevitably occur in the titanium castings. Shrinkage cavities in cast titanium jewelry are mostly at the sprue and casting junction. The sprue design is the most important way to control shrinkage cavities in titanium castings, as it regulates the rate, flow, and integrity of the molten metal entering the mold cavity. Factors such as size, type, shape, position, and direction can all affect the quality of the casting.
④ The surface of titanium castings is rough. Surface roughness refers to an uneven surface with bumps or flow marks. Causes may include excessively high mold temperatures, sintering reactions between the embedding material and sodium liquid, mold breakage, sand sticking, or poor quality of the embedding material.
⑤ The surface contamination layer of sodium castings is too thick. Many factors determine the thickness of the surface contamination layer of titanium castings, including the type of flux, mold temperature, equipment vacuum level, and purity of inert gases. Among them, the type of flux has the following general influence on the thickness of the contamination layer: zirconia-based flux has the thinnest layer, followed by alumina, magnesia, and phosphate-based fluxes in order of increasing thickness of the contamination layer.