Τι είναι η επιμετάλλωση με ασήμι, πώς γίνεται και γιατί χρησιμοποιείται;
Silver Plating Guide for Jewelry: Processes, Alloys & Troubleshooting
Εισαγωγή:
This article explains what silver plating is – a process of depositing a layer of silver onto a substrate. It details how it is performed using various methods, from traditional cyanide plating solutions to modern cyanide-free alternatives, covering decorative, industrial, and high-speed plating for components like connectors. The text also explores why it’s used, highlighting its excellent conductivity, reflectivity, and application in silver alloys for enhanced properties. Finally, it provides essential troubleshooting guides for common plating faults, making it a comprehensive resource for understanding both the theory and practice of silver electroplating.

Πίνακας περιεχομένων
Ενότητα Ι Επισκόπηση
Silver (Ag) has an atomic number of 47 in the periodic table, with the element symbol Ag. The symbol originates from the Latin word Argentum (meaning shining thing). Its electrical conductivity, conductance, and visible light reflectivity are the highest among metals. Due to its high light reflectivity, it has traditionally been called white silver. The standard electrode potential of Ag is 0.799 V.
Silver ions have a strong bactericidal effect and are widely used as disinfectants (usually, utensils labeled as treated for sterilization have been processed using silver compounds). Silver has also been applied as a sterilization device in water purifiers in recent years. Some main parameters of silver are shown in Table 2-1.
Table 2-1 Some Main Parameters of Silver
| Characteristic Parameters | Characteristic Values |
|---|---|
|
Όνομα στοιχείου, σύμβολο στοιχείου, ατομικός αριθμός Ταξινόμηση Ομάδα, Περίοδος Πυκνότητα, σκληρότητα Metal monomer color Σχετική ατομική μάζα Ατομική ακτίνα Ακτίνα ομοιοπολικού δεσμού Χημική σθένους Κρυσταλλική δομή σημείο τήξης σημείο βρασμού Θερμότητα εξάτμισης Θερμότητα διάλυσης Ειδική θερμοχωρητικότητα Αγωγιμότητα Θερμική αγωγιμότητα
|
Silver、Ag、47 Μέταλλο μετάπτωσης 11.5 10490kg/m3, 2. 5 Silver white 107.8682 160pm 153pm 1 Doughnut cube 1234. 93K(961. 78℃) 2435K(2162 ℃) 250. 58kJ/mol 11. 3 kJ/mol 232J/(kg • K) 63X106m • Ω 429W/(m ・ K) |
Silver is a precious metal that easily undergoes chemical changes. When sulfur compounds are present in the air (such as automobile exhaust, hydrogen sulfide in hot springs, etc.), forming Ag2S on the surface of the silver turns it black. Since ancient times, silverware has been used as tableware for the ruling class and wealthy families. There is a saying that when silver comes into contact with food containing arsenic, the tableware changes color to warn the user.
The history of silver plating is long, dating back to 1838 when G. R. Elkington and H. Elkington in the UK proposed a silver plating solution containing silver oxide, potassium cyanide, and sodium cyanide in 1838.
In 1913, F. O. Frary published a paper on using silver nitrate as a plating bath. E. B. Saniger conducted comparative studies on silver electroplating from sulfonates, nitrates, borofluorides, and fluorides, reporting that smooth plating deposits could be obtained from borofluoride solutions. In 1933, H. Hickman reported that a rotating electrode could obtain silver deposits from acidic solutions.
Silver plating has been widely used both in decorative fields and in industry. Especially in recent years, the development of silver plating on connectors for electronic and communication devices and substrates for semiconductors and integrated circuits has been rapid. Moreover, silver plating in these applications differs from conventional plating methods, typically using high-speed plating. The plating solution is generally neutral, with silver salts being potassium silver cyanide and organic acids as the main components. The development of plating for functional parts is also advancing rapidly. However, research on silver plating is still less extensive than gold plating. In particular, silver alloy plating solutions have not yet reached a practical usage level. Since the introduction of silver plating solutions, cyanide-based solutions have been predominantly used. Although there have been several improvements, the mainstream has not moved away from cyanides. Representative cyanide plating solution compositions are shown in Table 2-2. Using cyanide silver plating allows good silver coatings to be obtained over a wide range of temperatures and concentrations, and the operation control is relatively easy. Table 2-2 lists two types of plating solutions: potassium cyanide and sodium cyanide. The potassium salt type is mostly used when bright silver plating is required. The reasons are as follows:
① Fast electroplating deposition rate;
②High conductivity of the plating solution, which can ensure better dispersion and coverage capabilities;
③ Wide tolerance range for carbonates;
④ Has a smoothing effect, etc.
However, due to the high content and toxicity of cyanide, a large number of experimental studies on non-cyanide silver plating have been carried out at home and abroad. Although no plating solution comparable to cyanide has been found, some products have already been launched.
Table 2-2 Basic Composition and Process Conditions of Silver Cyanide Plating Solution
| Σύνθεση και οι συνθήκες επεξεργασίας της | Νο. 1 | Αρ. 2 | Αρ. 3 |
|---|---|---|---|
| Silver cyanide (as silver)/(g/L) | 25 〜 33 | 25 〜 33 | 36 〜 114 |
| Free potassium cyanide/(g/L) | 30 〜 45 | 45 〜 160 | |
| Free sodium cyanide/(g/L) | 30 〜 38 | ||
| Potassium carbonate/(g/L) | 30 〜 90 | 15 〜 75 | |
| Sodium carbonate/(g/L) | 38 〜 45 | ||
| Potassium hydroxide/(g/L) | 4 〜 30 | ||
| Πυκνότητα ρεύματος/(A/dm2) | 0. 5 〜 1. 5 | 0. 5 〜 1. 5 | 0. 5 〜 1. 0 |
| Θερμοκρασία/°C | 20 〜 25 | 20 〜 25 | 38 〜 50 |
Section II Decorative Silver Plating
Decorative silver plating for ornaments and Western tableware must use bright silver plating. Before the development and use of brighteners, silver decorative pieces were plated with a certain thickness of silver layer, then surface polished to achieve brightness. In 1902, Frary obtained experimental results of bright silver layers by adding a small amount of carbon disulfide ( CS2 ) to the plating solution. This marked the beginning of rapid research on silver plating brighteners.
Afterward, Wilson dissolved 28 g of carbon disulfide in 56 g of ether and added it to 1 L of silver plating, shaking the solution daily. Then, after 7~14d, 75 mL was taken from it and added to 100 L of silver plating solution, resulting in a highly bright plating layer.
Parson dissolved 6 g of carbon disulfide and 30 g of potassium cyanide in 1 L of water and, after shaking for 30 hours, took 7 mL and added it to 100 mL of silver plating solution, obtaining a good bright plating layer. The N, S, and O atoms bonded to the carbon atoms in the brightening agent cause the plating layer to become bright. Commonly used brightening agents include carbon disulfide, ketones, and a mixture of Turkish red oil, all stable brightening agents. Glycerol and potassium antimony tartrate can increase the hardness of the silver plating layer, and sodium selenite mixed with other sulfur-containing compounds helps to smooth the plating layer. All brightening agents act as depolarizers, and sulfides act in colloidal form to achieve their effect. Table 2-3 shows the composition of some silver plating brightening agents.
Table 2-3 Various Silver Plating Brighteners
| Brightener Name | Main Inventors |
|---|---|
| Carbon disulfide and ketone-based polymer |
O. Kardos; US PAT. 2807576(1957) O. H. A. Lammert;US PAT. 2666738(1954) Hanson-Von Winkle-Munning;Swiss PAT. 298147(1954) J. Wernle,Berne;France PAT. 1048094(1953) |
| Xanthates | Sieman, Halskie;German PAT. 731962(1943) |
| ASK compounds (Acrolein Sulfur Disulfide Yellow Polymer) | R. Erdman;Metalloberflache 1,2(1950) |
| Thiocarbazide | H. Schlotter;German PAT. 959775(1957) |
| Thiocarbazide | SEL-REX ( America ) |
| Selenium and antimony compounds |
R. Weiner;US PAT. 2777810(1957) Schering;US PAT. 3215610(1966) |
| Sb-Bi compounds | E. Rank;US PAT. 3219558(1965) |
Section III Pre-Plating Silver
Table 2-4 Composition and Operating Conditions of Pre-plating Silver Solutions
| Substrate Materials | Σύνθεση και οι συνθήκες επεξεργασίας της | |
|---|---|---|
| Ag plating solution | Ag-Cu plating solution | |
| Iron base |
Potassium silver cyanide:1.4~2.8g/L Potassium cyanide:60~150g/L Temperature:20~25℃ Current density:1.5~2.5A/dm2 Voltage:4~6V Time:1~2min Anode: SUS plate |
Silver cyanide(in silver):0.8~1.5g/L Copper cyanide (as copper):6.0~7.5g/L Potassium cyanide:50~60g/L Temperature:15~25℃ Current density:0.1~0.2A/dm2 Time:5~10min Anode:SUS plate |
|
Silver cyanide:1.9g/L Copper cyanide(in copper):11.3g/L Potassium cyanide:75g/L Temperature:15~25°C Current density:1.5~2.5A/dm2 Anode:4~6V Time:2~3min |
||
| Copper base |
Silver cyanide:5.6~8.3g/L Potassium cyanide:60~90g/L Temperature:20~35℃ Current density:15A/dm2 Voltage:4~6V Time:1~2min Anode:Ni plate |
|
Table 2-5 Composition and Operating Conditions of Pre-Plated Silver Plating Solution
| Σύνθεση και οι συνθήκες επεξεργασίας της | Parameters | Σύνθεση και οι συνθήκες επεξεργασίας της | Parameters |
|---|---|---|---|
| Nickel chloride | 240g/L | Πυκνότητα ρεύματος | 15A/dm2 |
| Hydrochloric acid (37% by volume) | 120mL/L | Φορά | 1〜2min |
| Θερμοκρασία | 20〜35℃ | Άνοδος | Ni plate |
Table 2-6 Pretreatment Plating Solution Composition for Pre-Silver Plating of Brass Castings, Nickel Silver, etc.
| Components | Συγκέντρωση | Components | Συγκέντρωση |
|---|---|---|---|
| Mercury Chloride(HgCl2) | 7. 5g/L | Mercury oxide (HgO) | 7. 5g/L |
| Ammonium chloride (NH4Cl) | 4g/L | Sodium cyanide | 60g/L |
| Or |
Section IV Cyanide-Free Silver Plating
Table 2-14 Stability Constants of Silver Complexes
| Complexes | Stabilization constant | Complexes | Stabilization constant |
|---|---|---|---|
| Ag(CN)2 | 21.1 | Ag(SO3)2 | 8.4 |
| Ag(CH4N4S)3 | 13.5 | AgBr43- | 8.3 |
| AgI43- | 13.4 | Ag(en)2① | 7.4 |
| Ag(S2O3)2 | 12.5 | Ag(NH3)2+ | 6.5 |
| Ag(SCN)4 | 11.2 | Agcl4 3- | 5.7 |
Table 2-15 Partial Results of Cyanide-Free Silver Plating Published So Far
| Σύνθεση και οι συνθήκες επεξεργασίας της | Συγκέντρωση | Σημείωμα |
|---|---|---|
|
1. Silver sulfate Ammonia(25%) Ιωδιούχο κάλιο Sodium pyrophosphate Θερμοκρασία διαλύματος επιμετάλλωσης Πυκνότητα ρεύματος
|
30g/L 7. 5mL/L 600g/L 60g/L Θερμοκρασία δωματίου 2A/dm2
|
|
|
2. Silver nitrate Ιωδιούχο κάλιο Polyethylene Polyamine Θερμοκρασία διαλύματος επιμετάλλωσης Πυκνότητα ρεύματος |
30〜40g/L 300〜400g/L 5〜20g/L 10〜100g/L Above 40℃ 0. 5〜3. 0A/dm2 |
|
|
3.Silver iodide Polyvinyl alcohol Θειοθειικό νάτριο Θερμοκρασία διαλύματος επιμετάλλωσης Πυκνότητα ρεύματος |
40〜80g/L 400〜600g/L 0. 5〜2. 0g/L Θερμοκρασία δωματίου 0. 5〜3. 0A/dm2 |
A. Taleat et al. concluded that the coatings obtained from this solution are dendritic in structure and have good resistance to discoloration by H2S |
|
4. Silver Sulfate Ammonium sulfate Κιτρικό οξύ Iron sulfate Αμμωνία Temperature of plating solution pH |
40〜80g/L 150g/L 4g/L 0. 4〜3. 0g/L 2〜50mL/L 30℃ 10〜10. 6 |
Both AgNO3 and (NH4)2SO4 were dissolved in half the amount of water, then diluted 3 times and mixed, then Ag2SO4 was dissolved with NH4OH. In addition, citric acid is dissolved using half the amount of water, and then metals and salts are added. |
|
5. Silver nitrate Sodium pyrophosphate Αμμωνία Sodium nitrate Ammonium sulfate Temperature of plating solution Πυκνότητα ρεύματος |
20〜30g/L 20〜25g/L 60〜100mL/L 40〜70g/L 40〜70g/L Θερμοκρασία δωματίου 0. 8〜1. 1 A/dm2 |
S.R. Natarajan and others precipitated silver as silver chloride, dissolved it in excess sodium thiosulfate, and added potassium metabisulfite. This plating solution can be maintained for several months and under room temperature, and a current density of 0.5~1.25A/cm2, 100% cathodic current efficiency can be obtained. The hardness of the resulting plating film is 60~63kgf/mm2. Although it is slightly softer than the plating obtained from cyanide-containing solutions, it still reaches a usable level as a cyanide-free silver plating solution.
In addition, cyanide-free plating also uses dimethylglyoxime as a complexing agent . This plating solution uses dimethylglyoxime as the complexing agent and sulfite as the conductive salt, with the plating solution being alkaline. The composition of the plating solution and its process conditions are shown in Table 2-16.
Table 2-16 Process Conditions Using Dimethylglyoxime as the Complexing Agent
| Συστατικά και οι συνθήκες επεξεργασίας τους | Parameters | Συστατικά και οι συνθήκες επεξεργασίας τους | Parameters |
|---|---|---|---|
|
Silver ion concentration Dimethylglycolide Sulfite |
1〜75g/L 50〜250g/L 1〜10g/L |
pH Θερμοκρασία διαλύματος επιμετάλλωσης Πυκνότητα ρεύματος |
7〜13 30〜90℃ 0. 1〜10A/dm2 |
It is recommended that this be used in the field of silver plating on semiconductor bump pads. This method can produce a fine and smooth plating surface. As a non-cyanide plating solution, it does not require oxygen or air to be bubbled into the plating solution to control silver precipitation. Moreover, the plating solution can be used continuously for a long time.
Suppose the sulfite concentration in this plating solution is too low (below 1g/L). In that case, the grain refinement effect of the plating layer deteriorates, and the inhibition effect on plating nodules also worsens. However, if the sulfite concentration is too high (above 75g/L), the plating solution tends to crystallize and precipitate. This may be related to the weak ability of sulfite to reduce.
This plating solution is suitable for alkaline threshold work, for example, when pH<7, the plating solution tends to become turbid but when pH>13, the plating layer is not bright. Some test results are shown in Table 2-17.
Table 2-17 Test Results of Non-Cyanide Silver Plating Using Dimethyl Ethylene Urea as a Complexing Agent
| Σειριακός αριθμός | Silver dimethylglycolide (as silver) /(g/L) | Dimethylglycolideurea/(g/L) | Potassium sulfite/(g/L) | pH | Surface roughness Ra /μm | Εμφάνιση | Height difference /μm | Brightness |
|---|---|---|---|---|---|---|---|---|
|
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 |
1 30 75 1 30 75 1 30 75 1 30 75 1 30 75 1 30 75 1 30 75 1 30 75 1 30 75 0. 8 30 0. 8 80 30 80 |
50 50 50 50 50 50 50 50 200 200 200 200 200 200 200 200 200 200 250 250 250 250 250 250 250 250 250 200 200 200 200 200 200 |
0. 1 3 10 0. 1 3 10 0. 1 3 10 0. 1 3 10 0. 1 3 10 0. 1 3 10 0. 1 3 10 0. 1 3 10 0. 1 3 10 0. 07 0. 07 3 12 12 3 |
7. 0 7.0 7. 0 11. 0 11. 0 11. 0 13. 0 13. 0 13. 0 7.0 7.0 7.0 11. 0 11. 0 11. 0 13. 0 13. 0 13. 0 7. 0 7.0 7.0 11. 0 11. 0 11. 0 13. 0 13. 0 13. 0 5. 0 11. 0 11. 0 13. 5 11.0 11. 0 |
0. 45 0. 33 0. 38 0. 26 0. 16 0. 20 0. 22 0. 20 0. 32 0. 40 0. 35 0. 42 0. 20 0. 13 0. 15 0. 12 0. 20 0. 30 0. 38 0. 36 0. 32 0. 30 0. 18 0. 15 0. 22 0. 18 0. 31 一 — 0. 15 — — 1.0 |
Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Tumor plating Tumor plating No luster Ag salt precipitation With plating tumor Ag salt precipitation |
0.41 0. 37 0. 39 0. 29 0. 26 0. 19 0. 28 0. 32 0. 34 0. 45 0. 30 0. 35 0. 30 0. 13 0. 15 0. 20 0. 30 0. 35 0. 33 0. 33 0. 38 0. 28 0. 19 0. 25 0. 40 0. 32 0. 40 10 8 3 — 5 一 |
0. 8 0. 5 0. 8 1. 0 1. 1 1. 1 1. 3 1. 2 0. 9 0. 7 0. 8 0. 7 1. 1 1.3 1. 2 1. 3 1. 1 0. 9 0. 8 0. 6 0. 7 1. 0 1. 1 1. 0 1. 2 1. 1 0. 8 <0. 2 <0. 2 0. 2 一 0. 3 —— |
In the table, the plating solution temperature is 60℃, the current density is 1A/dm2, and the plating thickness are 50μm. Surface roughness Ra was measured using a KLA Profiler P-11, appearance was observed with a metallurgical microscope, and brightness was measured with a GAM brightness meter (digital densitometer Model-144).
Adding 2,2’-bipyridine can achieve a mirror-bright coating for cyanide-free silver plating solutions using hydantoin and its derivatives as complexing agents. The composition of the plating solution and its process conditions are shown in Table 2-18.
Table 2-18 Composition and Process Conditions of Cyanide-Free Bright Silver Plating Solution
| Σύνθεση και συνθήκες διεργασίας | Νο. 1 | Αρ. 2 | Αρ. 3 | |
|---|---|---|---|---|
|
KOH/(g/L) Σουλφαμικό οξύ/(g/L) Complex of 5,5-dimethylhydantoin/(g/L) Ag(complex of 5,5-dimethylhydantoin)/(g/L) 2,2'-Dipyridine/(g/L) Nicotinamide/(g/L) 2-Aminopyridine/(g/L) 3-Aminopyridine/(g/L) Bright current density range/(A/dm2) |
60 52.5 60 25 0. 8 - - - 5〜20 |
60 52. 5 60 25 0. 4 4. 0 - - 0〜12. 5 |
60 52. 5 60 25 0. 4 - 1.3 - 0〜20 |
60 52. 5 60 25 0. 4 - - 0. 8 0〜20 |
In the formula, n is an integer of 2~4; R1 και Ρ2 can be the same or different, and are alkyl groups of C1 ~ C3 or alkylene groups of C2 ~ C6, M can be hydrogen, alkali metals, alkaline earth metals, or amino groups.
It can be used not only for silver plating but also for plating silver alloy.
Additionally, surfactants can be added to improve the plating layer.
Section V Silver-Plated Alloys
The history of silver-plated alloys is also relatively long, mainly because silver-plated alloys can achieve chemical and mechanical properties that pure silver plating cannot. Although there are many types, including silver-antimony, silver-lead, silver-cadmium, silver-copper, silver-nickel, silver-zinc, silver-cobalt, silver-palladium, silver-platinum, etc.
Among them, silver-copper alloys vary in color depending on the copper content, ranging from white to rose red. Moreover, the plating is non-brittle and has higher wear resistance than pure Ag plating. Silver-lead alloys can be used as friction-reducing coatings for high loads such as high-speed rotation. The silver-cadmium alloy has strong corrosion resistance, making it suitable for resisting seawater corrosion. At the same time, its resistance to sulfur and high-temperature discoloration is higher than that of pure silver plating.
The plating solution for silver alloy plating is also mostly cyanide-based, with silver-antimony alloy being the most commonly used among the alloys. Table 2-19 shows some representative silver alloy plating processes.
Table 2-19 Some Representative Alloy Silver Plating Processes
| Alloy Name(Content)/% | Hardness (Nuc) | Specific resistance/(mΩ/cm) | Σύνθεση διαλύματος επιμετάλλωσης |
|---|---|---|---|
|
Sb 0. 7 - 9. 6 -
|
- 100 - 164 - |
- 1.9 - 11.6 - |
Ag:24g/L Sb:g/L Na2CO3:25g/L Tartrate:60g/L NaOH:3〜5g/L |
|
Bi 1〜2. 6 - - - |
- 90〜180 - - - |
- 8〜10.4 - - - |
Ag:25〜50g/L Bs:25g/L K2C4O4H2:35g/L KOH:25g/L KCN:20-〜50g/L |
|
Cu 20 60 85 - |
- 240 240 340 - |
- 7.5 12 22 - |
K7Ag(P2O7)2 (counted as Ag) 20g/L K6CU(P2O7)2 required K4P2O7 100g/L 20℃、0. 5A/dm2 In the case of this alloy, the hardness of Nucor drops to about 185 after about 26 months at room temperature. |
|
Pb 4 10. 2 - - |
- 180 - - - |
- 10.5 11.5 - - |
AgCN 0. 33mol/L NaCN 0. 3mol/L Lead acetate 0.015mol/L NaOH 0.018mol/L Tartrate 0. 21mol/L
|
|
Pd 12 60 90 - |
- 180 250 320 - |
- — 10 — - |
Potassium silver cyanide 12g/L, pH 4.5 Palladium chloride 22g/L 0. 5A/dm2 Potassium acid pyrophosphate 56g/L, Ag955, Pd 5% Potassium thiocyanate 156g/L(alloy ratio) Japanese Patent: License No. 57-55699
|
|
Tl 9. 5 - - |
- 90 - -
|
- - - -
|
AgCN 32g/L KCN 25g/L K2CO3 30g/L Tl2SO4 6g/L |
Copywrite @ Sobling.Jewelry - Κατασκευαστής προσαρμοσμένων κοσμημάτων, εργοστάσιο κοσμημάτων OEM και ODM
Table 2-20 Ag-Pd Alloy Composition and Lattice Constant
| Alloy composition/% | Lattice constant/Å | Alloy composition/% | Lattice constant/Å | ||||
|---|---|---|---|---|---|---|---|
| Ag | Pd | Molten alloy | Alloy Plating | Ag | Pd | Molten alloy | Alloy Plating |
|
100 99 97 95 93 90 |
- 1 3 5 7 10 |
4. 077 4. 077 4. 072 4. 070 4. 061 4. 056 |
4. 077 4. 077 4. 077 4. 071 4. 059 4. 051 |
88 86 85 80 — - |
12 14 15 20 100 - |
4. 054 4. 053 4. 053 4. 031 3. 882 - |
4.054 4. 053 4. 051 4. 020 3. 900 - |
One of the authors of this book researched obtaining Pd-Ag alloys from alkaline ammonia plating solutions to achieve Pd80% (atomic ratio) alloy compositions. The basic composition of this plating solution is:
Pd(NH3)4 (ΟΧΙ3)2 0.1mol/L
Ag(NH3)2ΟΧΙ3 0.01mol/L
Νιου Χάμσαϊρ4ΟΧΙ3 0.4mol/L
Use ammonia water as a pH adjuster.
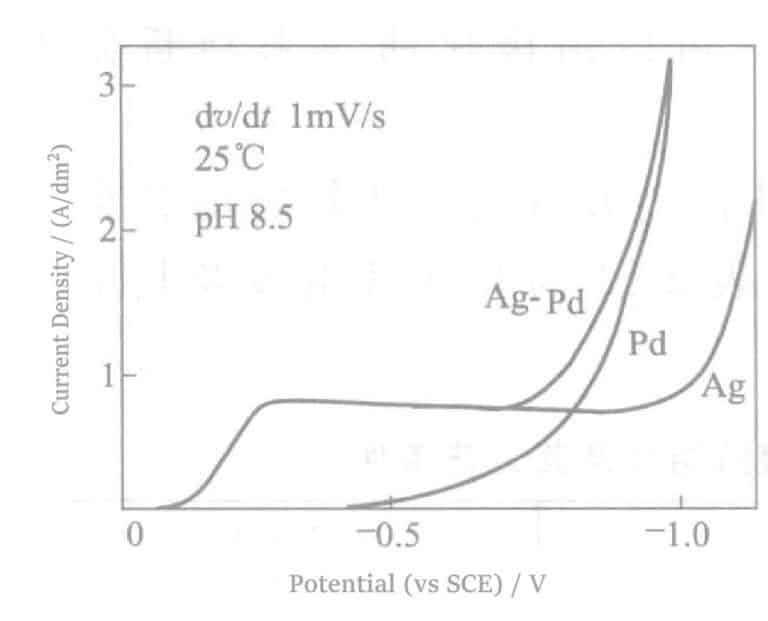
The polarization curves of the Pd, Ag and Pd-Ag alloys are shown in Figure 2-5.
Pd2+ + 4ΝΧ3 → Pd(NH3)42+ β1=6.3×1032
Ag+ + 2ΝΜ3 →Ag(NH3)2+ β2=2.5×107
From the above equation, it can be seen that the stability constants of their complexes differ greatly. Considering also the ammonia water used for pH adjustment, with a total concentration of 1mol/L, according to the Nernst equation, the equilibrium potentials of Pd and Ag in 25℃(relative to NHE) are -0.08 V and +0.24 V yield a more positive potential for Ag. In the polarization curve of the Ag-Pd alloy, it is observed that Ag deposits first, followed by Pd deposition, and finally, the curve moves along the Pd polarization line.
Effect of plating conditions on alloy deposition: The effect of current density on alloy composition is shown in Figure 2-6. It can be seen from the figure that the Ag content in the coating decreases with increasing current density. When the Pt electrode is rotated, or the plating solution is stirred, the Ag content in the coating increases. This indicates that Ag’s deposition (deposition) is controlled by diffusion of Ag+, consistent with the polarization curves in Figure 2-5.
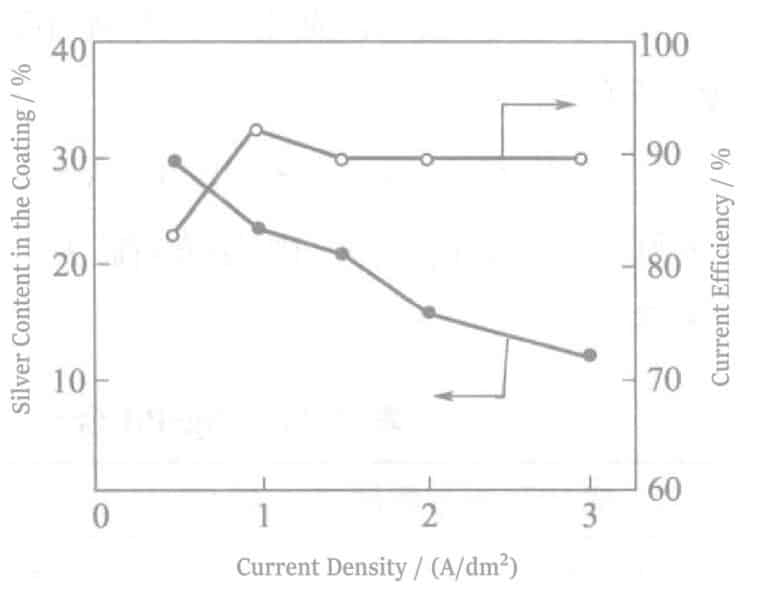
The increase in Ag content is caused by the decrease in current density or the increase in diffusion rate due to the increased concentration of Ag ions in the cathode diffusion layer. From the polarization curve in Figure 2-5, the Ag potential is more positive than the Pd potential, which conforms to the deposition of regular alloys. According to Brenner’s definition of regular deposition, metals with more positive standard electrode potentials increase their content in the alloy as the ion concentration in the diffusion layer increases. In this experiment, the actual potential change is determined by the plating solution composition and can be judged by the polarization curve regarding the positivity or negativity of metal ions.
Koichi Yamakawa et al. proposed alloy plating formulas to achieve good coatings over a relatively wide range of alloy compositions. Table 2-21 shows the composition of their plating solution and its process conditions.
Table 2-21 Composition and Process Conditions of Ag-Pd Alloy Plating Solution
| Σύνθεση και συνθήκες διεργασίας | Νο. 1 | Αρ. 2 |
|---|---|---|
|
PdCl2/(g/L) AgNO3/(g/L) KBr/(g/L) KNO2/(g/L) Sodium saccharin/(g/L) Boric acid/(g/L) Sodium naphthalene sulfonate/(g/L) pH (adjusted by NaOH and HNO3) Άνοδος Temperature of plating solution/°C Πυκνότητα ρεύματος/(A/dm2) |
28. 4 15. 3 590. 0 23. 4 0. 5 - - 6. 0 30% Pd-Ag 50 0.5,1,2,5,10 |
33 10. 0 590. 0 15. 0 - 50. 0 1. 0 9 Pt 30 0.5,1,2,5,10
|
Ag+ + 4Br– → AgBr43-
Pd2+ + 4NO22- → Pd(NO2)42-
Table 2-22 Ag-Pd Alloy Coating Results
| Current density /(A/dm2) | Νο. 1 | Αρ. 2 | ||||
|---|---|---|---|---|---|---|
| Coating thickness /μm | Εμφάνιση | Pd/(Ag+ Pd)/% | Coating thickness /μm | Εμφάνιση | Pd/(Ag+ Pd)/% | |
|
0. 5 1 2 5 10 |
10 10 3 3 0. 5 |
Gray, Semi-Gloss Gray, Semi-Gloss Silver Gloss Silver Gloss Silver Gloss |
25 20 25 30 40
|
2 2 0. 5 0. 3 0. 1 |
Gray, Semi-Gloss Gray, Semi-Gloss Silver Gloss Silver Gloss Silver Gloss |
50 30 50 60 70 |
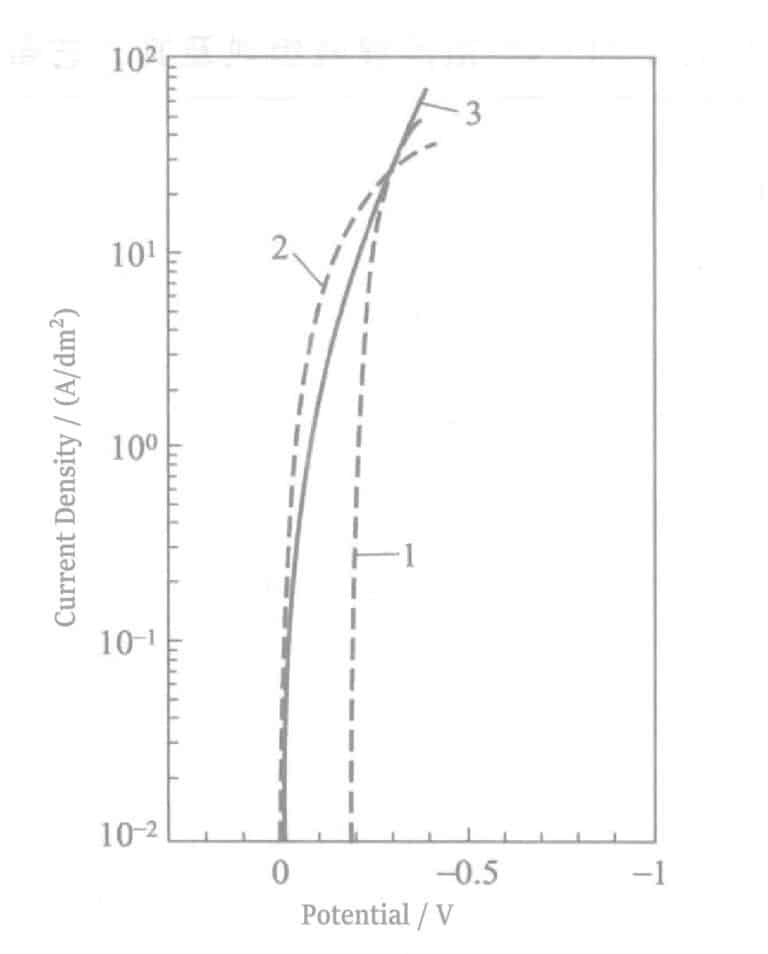
Figure 2-7 Polarization curves of Ag-Pd alloy plating solution
1--Pd deposition current; 2--Ag deposition current; 3--Ag-Pd alloy deposition current
Table 2-23 Sn-Ag and its Sn-Ag-Cu Plating Solution Composition and Process Conditions
| Συστατικά και οι συνθήκες επεξεργασίας τους | Sn-Ag plating solution | Sn-Ag-Cu plating solution |
|---|---|---|
|
Sulfuric acid/(mL/L) Tin sulfate/(g/L) Νιτρικό άργυρο/(g/L) Θειουρία/(g/L) Polyoxyethylene alkyl ether/(g/L) Copper sulfate pentahydrate/(g/L) Cathode current density/(A/dm2 ) Θερμοκρασία διαλύματος επιμετάλλωσης/°C Ανάδευση Deposition speed/(μm/min) |
120 36 1. 5 15 2 - 2 20 Ναι 1 |
120 36 1. 5 15 2 4 2 20 Ναι 1 |
The coating obtained under the above conditions is dense and smooth.
The composition of the Sn-Ag barrel plating solution and its process conditions are shown in Table 2-24.
Table 2-24 Composition and Process Conditions of Tin-Silver Electroplating Solution
| Composition and its process conditions and properties | Νο. 1 | Αρ. 2 | Αρ. 3 |
|---|---|---|---|
|
Stannous sulfate (as Sn)/(g/L) Stannous chloride (as Sn)/(g/L) Sodium gluconate/(g/L) Gluconic acid/(g/L) Succinic acid/(g/L) Sodium pyrophosphate/(g/L) EDTA-2Na/(g/L) Silver Acetate(Silver)/(g/L) Silver nitrate (as silver)/(g/L) PEG(#3000)/(g/L) pH Θερμοκρασία διαλύματος επιμετάλλωσης/°C Anode material Average current density/(A/dm2 ) Plating time/min Πάχος επιμετάλλωσης/μm Plating Appearance Silver content/% Melting point/°C Brazing wettability (after plating) Brazing wettability (after humidification test) Whisker crystal |
12 - 50 - 20 - - 1. 8 - 1 7. 5 50 Sn plate 0. 1 75 5 White, non-glossy 2. 0 221 Within 1s Within 2s Κανένα |
- 13 60 - - 100 - 0. 5 - 1 8. 1 40 Sn plate 0. 1 75 5 White, non-glossy 3. 8 221 Within 1s Within 2s Κανένα |
- 25 - 96 - 80 50 - 1 1 8. 5 25 Platinized titanium plate 0. 1 75 5 White, non-glossy 3. 3 221 Within 1s Within 2s Κανένα |
The resulting plating layer has good wettability.
Sn-Ag alloy is an additive for alloy plating, which can achieve a plating layer thickness of over 50μm.
When the Sn-Ag alloy is used on raised pads, the requirement for plating thickness increases. However, plating solutions typically used for thin layers tend to have issues such as uneven surfaces and insufficient adhesion when the plating thickness is increased. These problems can be solved by adding certain additives. The main components of the solution proposed by Yachikawa are:
① Add a cationic surfactant containing alkyl amines, whose molecular structure is H(OCH2CH2)nRN(CH2CH2O)nH.
② Water-soluble amines and their derivatives.
③ Glycerol.
④ Urea compounds or reducing agents (where the role of the reducing agent is to prevent the deposition of iodine at the anode when iodide compounds are present).
The implementation process conditions are shown in Table 2-25.
Table 2-25 Sn-Ag Process Conditions for Raised Pad Plating
| Composition, process conditions and properties | Νο. 1 | Αρ. 2 | Αρ. 3 | Αρ. 4 | Αρ. 5 | Αρ. 6 | Αρ. 7 | Αρ. 8 | No. 9 |
|---|---|---|---|---|---|---|---|---|---|
|
Tin pyrophosphate/(g/L) Silver pyrophosphate/(g/L) Potassium pyrophosphate/(g/L) Polyoxyethylene cetylamine/(g/L) Dimethylamine/(g/L) Potassium glycerate/(g/L) Silver iodide/(g/L) Potassium iodide/(g/L) Hypoethyl Urea/(g/L) Hypoethylenediamine/(g/L) Υποφωσφορώδες νάτριο/(g/L) Ammonium polyoxyethylene octadecanoate/(g/L) Triethanolamine/(g/L) Θειουρία/(g/L) Hydrazine hydrochloride/(g/L) Trimethylurea/(g/L) Dimethylaminoboron/(g/L) Glycerol/(g/L) Ammonium dipolyoxyethylene dodecanoate/(g/L) Hydroxylamine hydrochloride/(g/L) Ethylenediamine/(g/L) Glycerol acetate/(g/L) Calcium glycerate/(g/L) pH Πάχος επιμετάλλωσης/μm Tin content of plating/% |
33 2. 5 100 10 20 - - - - - - - - - - - - - - - - - - 11. 0 57 89. 7 |
33 2. 5 100 10 20 0. 5 - - - - - - - - - - - - - - - - - 11. 0 63 91. 6 |
33 - 96 10 20 - 1. 3 83 1. 0 - - - - - - - - - - - - - - 6. 0 63 89. 2 |
33 - 96 - - - 1. 3 83 - 8 2 4 - - - - - - - - - - - 6. 0 67 91. 4 |
33 - 96 - - - 1. 3 83 - - - 10 10 0. 5 2 - - - - - - - - 6. 0 60 91. 6 |
33 - 96 6 - - 1. 3 83 - - - - - - - 0.8 2. 5 0. 8 - - - - - 6. 0 64 91. 0 |
33 - 96 - - - 1. 3 83 - - - - - - - 0. 8 2. 5 0. 8 8 4 - - - 6. 0 61 90. 7 |
33 - 96 - - - 1. 3 83 - - - - - 0. 8 1. 5 - - - 6 - 4 1. 0 - 6. 0 61 88. 7
|
33 - 96 7 10 - 1. 3 - - - 2. 5 - - 0. 3 - - - - - - - - 0. 5 6. 0 59 89. 3
|
Section VI Troubleshooting of Silver Plating
1. Cyanide Plating Solution (usually for Rack Silver Plating) Bright Silver Plating Defects
Table 2-26 Common Silver Plating Defects and Countermeasures
| Fault content | Αιτίες | Αντίμετρα |
|---|---|---|
| Poor adhesion of the plated layer | Pre-plated silver is not completely covered. Passivation of the base or bottom plating layer | Check the concentration of silver, potassium cyanide and sodium cyanide in the silver pre-plating solution and the surface activity of the plated parts before plating. |
| Ag plating is black or has spots on the surface | Insufficient concentration of free potassium cyanide or free sodium cyanide in the plating solution. | Adjust the concentration of free potassium cyanide and sodium cyanide to standard values. |
| Ag anode is covered by black skin film | Insufficient concentration of free potassium cyanide or free sodium cyanide in the bath. | Adjust the concentration of free potassium cyanide and sodium cyanide to standard values. |
| Hydrogen gas precipitation on the surface of plated parts | The concentration of free potassium cyanide or free sodium cyanide is high compared to the concentration of silver ions in the bath. | Increase the concentration of silver ions or remove part of the plating solution to reduce the amount of plating solution. |
| Roughness of the plated layer | High current density | Reduce the current density to an appropriate value |
| Spots, protrusions, pockmarks on plated surface | Hydrogen adsorption due to impurities in the plating solution. | Filtration with activated carbon |
| Plated layer is not smooth | Contamination of the plating solution, high current density, dirty anode bag (anode sludge floating) | Filter the bath, clean the anode bag, and clean the bath. |
| Thickness of plated layer not, anode passivation | Excessive surface area of product | Increase the anode area by maintaining the proper amount of plated parts. |
2. Issues, Causes, and Countermeasures of High-Speed Silver Plating
Table 2-27 Common Problems and Countermeasures of High-Speed Silver Plating
| Fault content | Αιτίες | Αντίμετρα |
|---|---|---|
| Dark and coarse plating | Current density is too high, KCN is too low, Ag ion concentration is too low, CO32- concentration is too high, brightener concentration is too low. | Confirm and adjust, analyze and adjust free cyanide ion, remove (cool) CO32- , analyze and add |
| Stepped plating | The concentration ratio of brightener and replacement inhibitor is not coordinated, usually due to its high ratio. | Analyze and dilute the plating solution. |
| Blistering | Replacement of degreasing agent is required, the pre-plated layer is not satisfactory, the bottom layer is passivated. | Confirm and replace the plating solution, replace the pre-plating solution if it is dirty, and confirm the final rinse and plating room. |
| Spots and uneven luster | Insufficient brightener, clogged nozzle, silver in the anode solution, or solid ions in the Pt/Ti anode solution. | Analyze and adjust, remove and replace, remove, wash, replace if greenish black, and perform activated carbon filtration. |