Ο απόλυτος οδηγός για την κατασκευή κραμάτων χρυσού Κ που χρησιμοποιούνται για κοσμήματα
A Comprehensive Guide to K Gold Alloys' Properties and features
Introduction:
Master the craft of K gold jewelry with our guide, packed with 70 key terms defining the gold alloy artistry. Discover the perfect balance of Au-Ag, Au-Cu, Au-Ni, and Au-Pd to achieve desired colors and strength. Embrace the technicalities of alloy composition, crucial for casting, corrosion resistance, and skin safety. Navigating the intricacies of K gold filler selection, our guide illuminates the path to creating durable, visually stunning pieces. From preventing brittle fractures in K red gold to refining the whiteness of K white gold, we provide solutions for common production challenges. Enhance your jewelry-making prowess with our expert insights, merging tradition with innovation for timeless elegance.

Πίνακας περιεχομένων
Section Ⅰ Alloying of Gold and K Gold filling Materials
1. Alloying of Gold
Since ancient times, gold has become essential due to its beautiful color, excellent chemical stability, and forming process performance. Jewelry and accessory materials. Jewelry made of pure gold has advantages such as small volume, high value, and portability, and it has good value retention and decorative functions, making it beloved by various ethnic groups in our country throughout history. However, pure gold is too soft in texture, making it unsuitable for shaping and setting, resulting in traditional pure gold jewelry being relatively monotonous and easily deformed or worn.
With the change in consumer attitudes, people’s preference for gold jewelry is no longer just about the quality of the material but rather more focused on its decorative shape and color diversity, which has promoted the development of K gold alloys. Developing K gold alloys aims to improve the mechanical properties, such as strength and hardness of gold, meet users’ sensory requirements, and reduce material costs. By adding a certain proportion of alloying elements to pure gold to create K gold of corresponding quality, K gold jewelry made with gold alloy as the base material, or K gold inlaid jewelry set with various gemstones, excels in color, quality, and style compared to pure gold jewelry. With the continuous improvement of design and processing technology, K gold jewelry is gaining a larger market share with its personalized and artistic creativity.
K gold has different qualities that vary in physical properties, chemical properties, mechanical properties, and process performance due to the various types and proportions of alloying elements added. Standard base alloy systems for jewelry gold include Au-Ag alloy, Au-Cu alloy, Au-Ni alloy, and other binary alloy systems, as well as Au-Ag-Cu, Ag-Ni-Cu, and other ternary alloy systems.
1.1 Au-Ag Alloy
The Au-Ag binary alloy phase diagram is shown in Figure 3-9. Both can dissolve infinitely in both liquid and solid states. Adding silver to gold lowers its melting point. The melting point continuously decreases as the silver content increases, with a tiny temperature gap between the liquidus and solidus lines. Therefore, this alloy has good casting performance, which helps ensure the quality of jewelry castings.
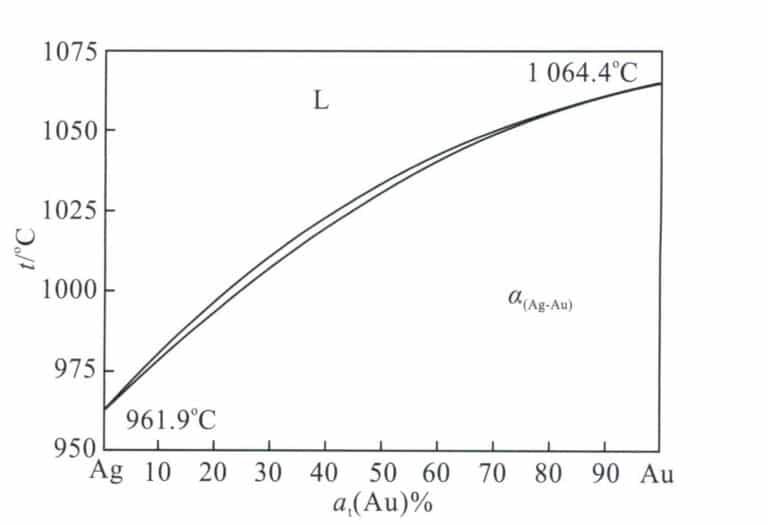
Adding silver to gold can lighten its color and change it to a greenish-yellow direction. Since silver and gold have a face-centered cubic crystal structure and their atomic radii are almost the same, the strengthening effect of silver on gold is not prominent. Taking 18K gold with a composition of 75%Au-25%Ag as an example, its annealed hardness is only HV32, and its tensile strength is only 185 MPa, indicating relatively low strength and hardness. However, the elongation can still reach 36%, showing good flexibility and cold working performance. Therefore, Au-Ag alloy is often used to develop K yellow gold for jewelry.
1.2 Au-Cu Alloy
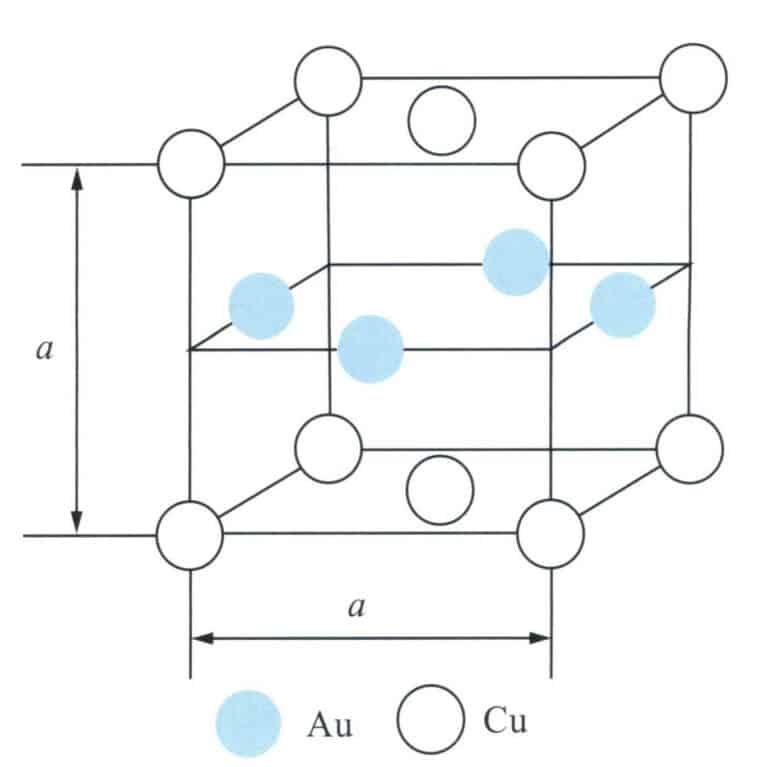
The binary alloy phase diagram is shown in Figure 3-10. The two can be infinitely soluble in the liquid state. As the copper content increases, the alloy’s melting point rapidly decreases, and when the copper content exceeds 20%, the alloy’s melting point gradually increases again. The solidification crystallization interval of the Au-Cu alloy is small, especially in the range of copper content of 15% to 25%, where the crystallization interval of the alloy is almost none, which gives it a good casting performance and a low tendency for shrinkage. After solidification, the alloy is a single solid solution in the high-temperature region. During the continued cooling process, an ordering transformation occurs in the medium temperature environment, forming the AuCu [wt (Au) = 75.6%] intermediate phase and the AuCu3[wt(Au) = 50.8%] intermediate phase.
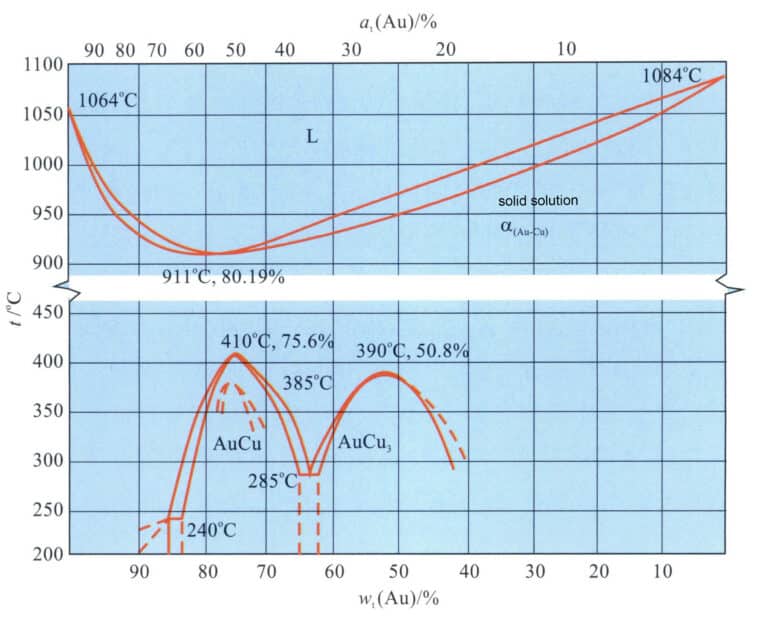
The chemical composition of the Au-Cu alloy significantly impacts its mechanical properties. As the copper content increases, the strength of the solid solution (quenched) alloy rapidly increases, peaking around 25%Cu, and further increasing the copper content causes the strength to decrease rapidly (Figure 3-11). Cu is an effective strengthening element for commonly used K gold. The heat treatment process also dramatically affects the mechanical properties of the Au-Cu alloy. Taking 18K gold with composition 75%Au-25%Cu as an example, its solid solution hardness is HV165, and its tensile strength is 514 MPa. After aging treatment, the ordered phase formed in the alloy can increase its tensile strength to around 910 MPa and hardness to around HV200. Still, flexibility decreases, and the alloy becomes brittle, unfavorable for cold deformation processing.
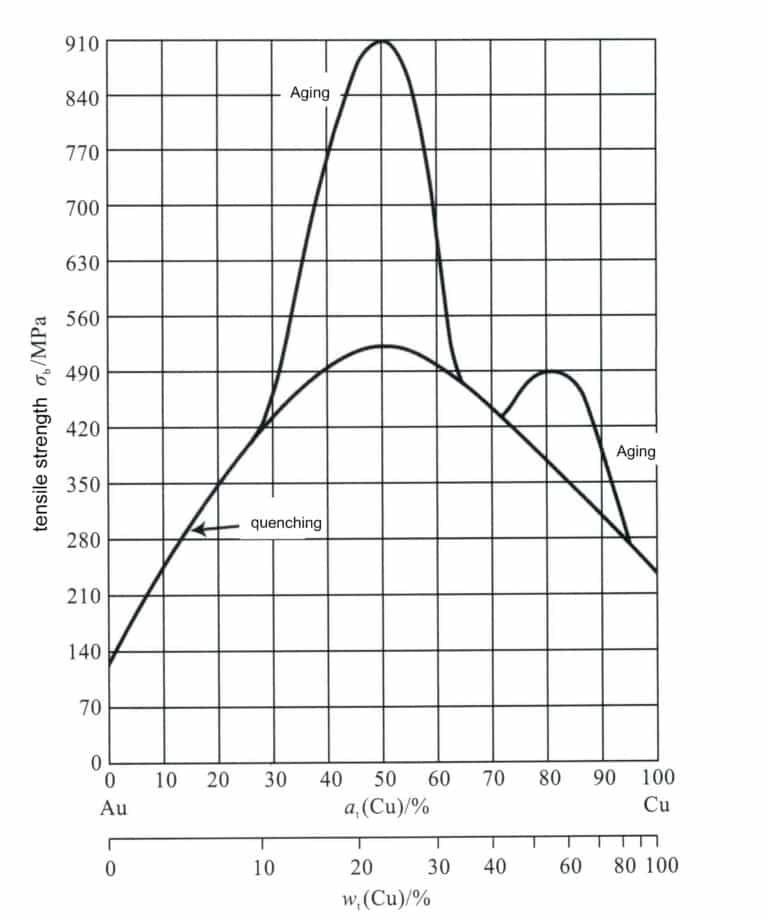
Adding copper to gold changes its color towards red, making it the primary alloying element of K red gold. Copper is also often used in K gold and K white gold to improve the mechanical and processing properties of the alloy.
1.3 Au-Ni Alloy
The binary alloy phase diagram of Au-Ni is shown in Figure 3-12. A certain amount of nickel added to gold lowers the alloy’s melting point, with the melting point reaching its lowest at a nickel content of 18%, approximately 955℃, and the alloy has a minimal crystallization interval, which is beneficial for improving the casting performance of the alloy.
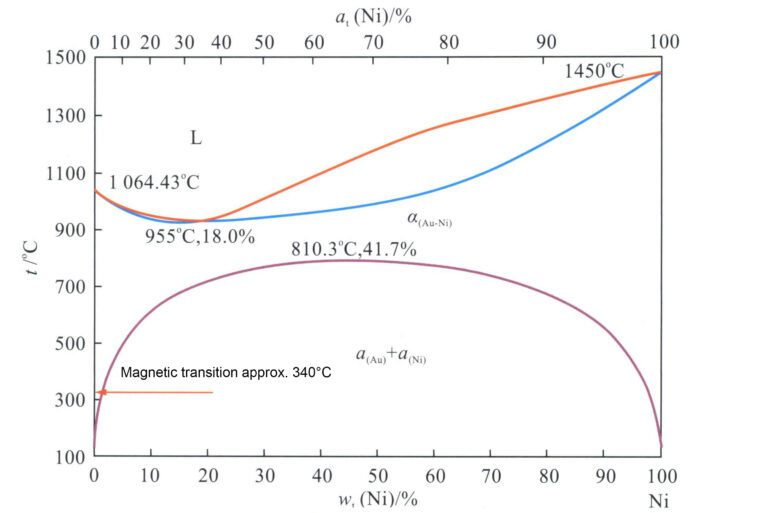
Figure 3-12 Au-Ni Binary Alloy Phase Diagram
The Au-Ni alloy is a single-phase solid solution at high temperatures. The solid solution decomposes into a two-phase structure when the temperature drops below a certain point. Utilizing this characteristic, aging treatment of the Au-Ni alloy can significantly enhance the strength and hardness of the material (Figure 3-13).
Adding nickel to gold lightens its color; when the nickel content reaches a certain level, the alloy presents a grayish-white color close to platinum, making it one of the most effective bleaching elements in K white gold. However, Ni is a sensitizing element, and when its release rate exceeds a certain threshold, there is a risk of causing skin allergies.
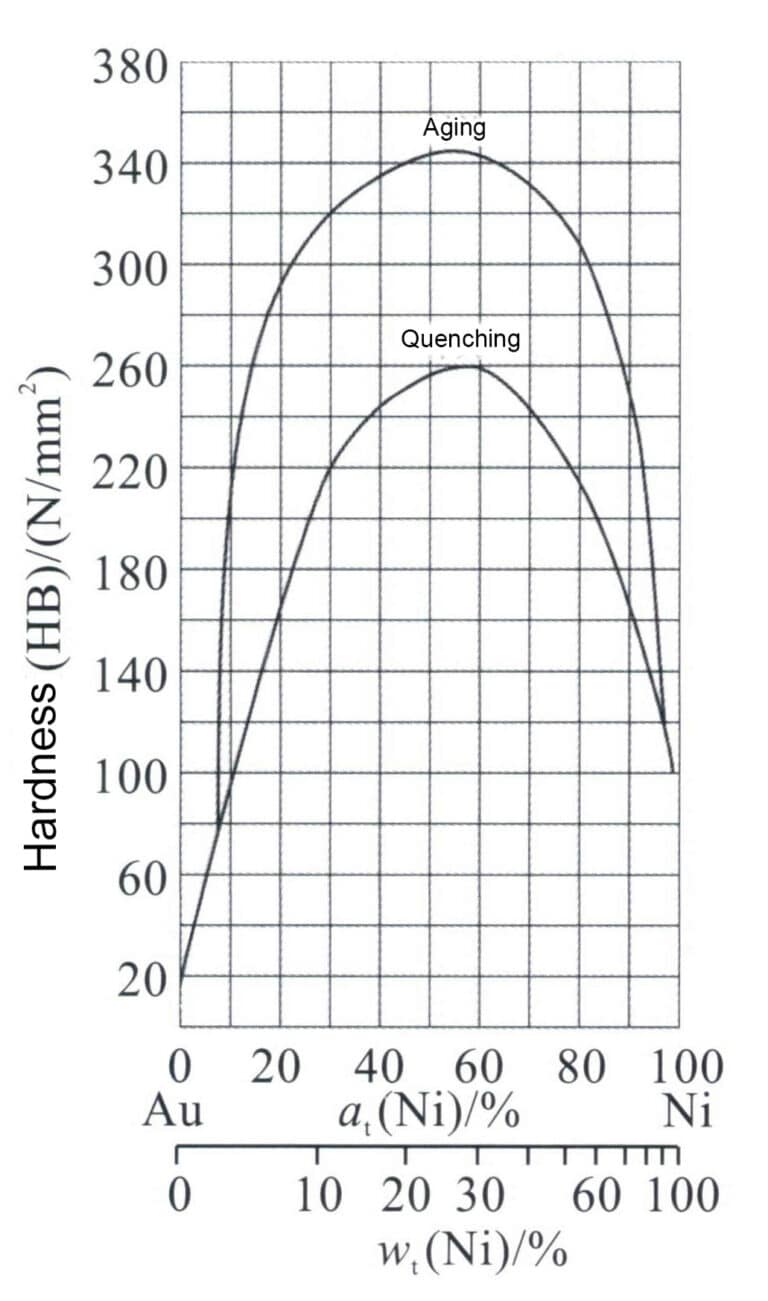
Figure 3-13 The effect of the heat treatment process on Au-Ni alloy hardness
1.4 Au-Pd Alloy
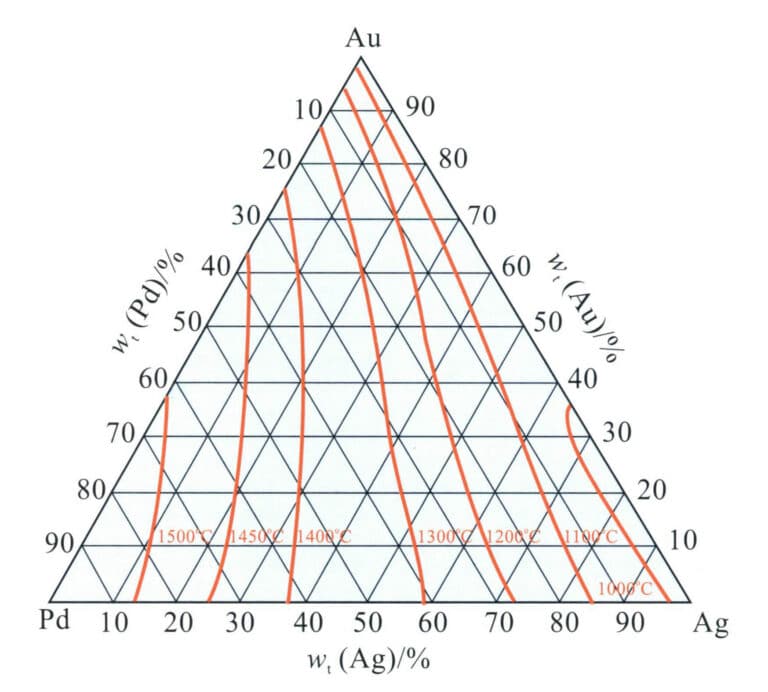
The binary alloy phase diagram is shown in Figure 3-14. The addition of palladium to gold increases the alloy’s melting point, and as the palladium content increases, the alloy’s liquidus and solidus temperatures continue to rise. At the gold-rich end, the crystallization interval is relatively large, reaching about 51℃ when the palladium content is around 17% (at), gradually decreasing towards the palladium-rich end. The alloy has a single solid solution structure at high temperatures, and during the cooling process, alloys with a specific composition range will undergo an ordering transformation and formation order Au3Pd phase and AuPd3 ordered phase, which improves the strength and hardness of the alloy but reduces its ductility and flexibility.
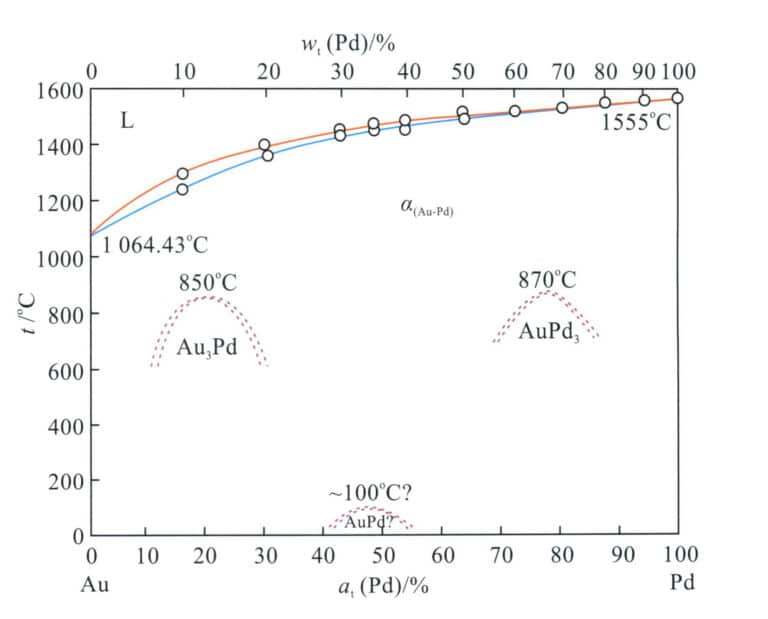
Overall, the melting point of the Au-Pd alloy is relatively high, increasing the difficulty of casting. The hardness of the solid solution Au-Pd alloy is not high, but it has good ductility, which is beneficial for cold deformation processing. Palladium has a good bleaching effect on gold and is one of the base alloy systems for K white gold; however, the high price of palladium leads to increased material costs.
2. K Gold filling Materials
K gold is an alloy composed of a certain proportion of intermediate alloys added to pure gold, commonly referred to as filling materials in the jewelry industry. In set jewelry, common K gold grades include 8K -10K, 14K, and 18K; by color, there are K yellow gold, K white gold, and K red gold, among others. Therefore, the use of filling materials in K gold jewelry is prevalent, and the quality of filling materials directly affects the quality of the jewelry.
When jewelry companies produce K gold jewelry, they mix pure gold with purchased filling materials. The performance of filling materials provided by different suppliers can sometimes vary significantly; even the same grade of filling material from the same supplier may experience performance fluctuations, affecting the production of jewelry companies. When selecting K gold filling materials, the following factors should be considered.
2.1 Physical properties
The surface decorative effect of K gold jewelry is significant. For K gold jewelry, when selecting filling materials, attention should be paid to the impact of the density, color, magnetism, melting point, and other aspects of the filling materials on K gold jewelry.
(1) Density.
The range of alloy elements selected for filling materials is quite broad, and each alloy element has its atomic mass and corresponding density. The density of K gold formulated with different compositions of filling materials varies. For a piece of jewelry with a fixed volume and grade, low-density materials can reduce the jewelry’s weight and lower the product’s cost.
(2) Color.
For K gold jewelry, color is a fundamental physical property. Decorative gold alloys are generally divided into two main categories based on color: colored gold alloys and white gold alloys. By changing the alloy composition ratio of the filler, different colored gold alloy materials can be obtained. The most commonly used colored K gold includes three series: K gold, K white gold, and K red gold, with typical fillers shown in Figure 3-15. In addition, in recent years, a few unique colored K gold filler materials have also been developed abroad, which can form unique colored, complex, and brittle intermetallic compounds with gold.

(a) K gold filler

(b) K white gold filler

(c) K red gold filler
Figure 3-15 Various colored K gold fillers for jewelry
(3) Magnetism.
K gold jewelry, as precious metal jewelry, generally wants the alloy to exhibit something other than magnetism to avoid consumer doubts about the authenticity of the material. Gold is not magnetic; K gold jewelry contains many other metallic elements. When the filler material contains magnetic components such as Fe, Co, Ni, and Ga, it may cause K gold material to exhibit magnetism. For example, K white gold commonly uses nickel as a bleaching element. Figure 3-13 shows that the alloy is a single-phase solid solution below the solidus line and above a certain temperature. When slowly cooled to a specific temperature, phase separation begins, forming a two-phase region. When the temperature drops to about 340℃, a magnetic transition occurs, and the alloy shows a certain degree of magnetism.
(4) Melting Point.
Most K gold jewelry is produced using gypsum mold casting technology. Due to gypsum’s poor high-temperature thermal stability, thermal decomposition occurs when the temperature reaches 1200℃, releasing SO2 gas, which causes the casting to develop pores. If the gypsum mold is not wholly roasted, leaving residual carbon inside the mold, or if the metal liquid is severely oxidized, forming a large amount of cupric oxide, this decomposition temperature will be significantly lowered. Therefore, to ensure the safety of gypsum mold casting, controlling the alloy’s melting point is necessary. Generally, the melting points of K gold and K red gold are around 900℃, and using gypsum mold casting will be fine. However, for K white gold, due to the use of high melting point Ni, Pd as a bleaching element, the melting point of the alloy is higher than that of K gold and K red gold, which poses a risk of thermal decomposition of the gypsum mold. When the content of Ni, Pd is very high, the gypsum mold can no longer guarantee production quality, necessitating costly phosphoric acid bonded casting powder, which undoubtedly increases production costs and difficulties.
2.2 Chemical Properties
For jewelry, chemical stability is essential. The chemical stability of K gold jewelry is mainly reflected in its resistance to tarnishing and corrosion, which is closely related to the filler materials used in K gold. The corrosion resistance of K gold alloys varies with composition; in general, high-purity K gold is beneficial for improving its corrosion resistance. For example, 18K -22K gold has good corrosion resistance in ordinary single inorganic acids, and 14K gold also has good corrosion resistance. Still, it will leach copper and silver from the surface under strong acid conditions. Gold alloys below 9K are not resistant to strong acid corrosion and will tarnish and discolor in poor environments. However, the content of precious metals in K gold materials is not the only factor affecting tarnishing; tarnishing and discoloration are the combined results of the alloy materials’ chemical composition, chemical processes, environmental factors, and microstructure. In low-purity K gold, when the composition of the filler material is favorable for improving the potential of K gold, forming a dense protective film, and improving the microstructure of the alloy, it is still possible to obtain alloys with excellent chemical properties and good resistance to discoloration. Among the three main K gold series, K red gold is prone to surface tarnishing due to its high copper content, and beneficial alloying elements must be utilized in its filler materials for improvement.
2.3 Mechanical Properties
To maintain a high luster for a long time, K gold jewelry needs to improve the hardness of the alloy to meet wear resistance requirements; some structural components of jewelry, such as ear pins, ear hooks, brooches, springs, etc., require good elasticity and also need to improve the hardness of the alloy. However, the hardness strength of gold itself could be higher, making it challenging to meet the requirements for inlaying. One of the purposes of K gold is to enhance the material’s strength, hardness, toughness, and other mechanical properties. Among the three typical K golds, nickel-bleached K white gold has higher strength and hardness, with more excellent elasticity, requiring a balance between strength, hardness, and flexibility; K red gold may undergo an ordering transition and lose flexibility, necessitating adjustments and improvements in the composition of the filler material and the manufacturing process.
2.4 Process Performance
The composition design of the filler material should fully consider the performance requirements of different processing techniques. For example, the melting method can affect the oxidation resistance of the alloy; the same alloy can yield inconsistent results when melted using a torch, induction heating in the atmosphere, or a protective atmosphere or vacuum. Additionally, jewelry production can utilize different processing methods such as casting, stamping, and welding, each with varying performance requirements for K gold, determining the selection and quantity of alloy elements in the filler material. When designing the filler material’s composition, the alloy’s process operability should be fully considered to avoid operational issues caused by a too-narrow processing range. Processing performance mainly finds aspects such as casting performance, Plastic processing performance, polishing performance, welding performance, and recycling performance.
(1) Casting Performance.
The casting performance of the alloy significantly impacts the surface quality of the cast jewelry. The quality of the alloy’s casting performance can be assessed from several aspects, including the fluidity of the molten metal, the tendency for shrinkage and porosity, and the tendency for thermal cracking during deformation. The K gold used for casting should have a small crystallization interval, a low tendency for oxidation, good fluidity, and filling performance. It should not quickly form dispersed shrinkage and deformation cracks, which is conducive to obtaining cast jewelry with complete shape, precise contours, dense crystallization, and sound structure.
(2) Plasticity processing performance.
The Plasticity processing technology has many applications in producing K gold jewelry. In addition to using drawing and rolling machinery to make sheets, wires, and pipes, it is also frequently used to form jewelry, such as turning on machine tools, stamping with stamping machines, and hydraulic pressing. To ensure the quality of Plasticity processing products, it is essential to formulate correctly and strictly adhere to operational process specifications, as well as the inherent Plasticity processing performance of the material, which has a decisive impact. K gold materials are required to have good Plasticity processing performance, especially during operations like drawing, rolling, stamping, and hydraulic pressing, where the hardness of the alloy should not be too high, and the work hardening rate of the alloy should slow down to facilitate operation; the material should also have good flexibility. Otherwise, it is prone to cracking.
(3) Polishing performance.
Jewelry has explicit requirements for surface quality, and most jewelry must be polished to achieve a mirror-like shine. This requires not only the correct execution of polishing operations but also attention to the properties of the alloy itself. For example, the workpiece should have a dense structure with refined and uniform grains, free of defects such as pores and inclusions. If the grains of the workpiece are coarse and there are defects like shrinkage or pores, phenomena such as orange peel, polishing depressions, and comet tails may quickly occur. Scratches and comet tail defects may also quickly appear if there are rigid inclusions.
(4) Reusability performance.
The yield of the casting jewelry process is generally only around 50% or even lower. Each casting brings a large amount of sprue system and waste materials for reuse. Jewelry companies, based on production costs and efficiency, always hope to use as much recycled material as possible. Due to the inevitable issues of volatilization, oxidation, and gas absorption during the alloy’s melting process, the alloy, the composition of the alloy will change to some extent with each casting, affecting the alloy’s metallurgical quality and casting performance. The performance degradation of the alloy during the reuse process is closely related not only to the operational process but also to the inherent reusability performance of the alloy, which mainly depends on the alloy’s tendency for gas absorption and oxidation, as well as its reactivity with crucibles and casting materials. The smaller the tendency for gas absorption and oxidation, the lower the reactivity with crucibles and casting materials, and the better the reusability performance.
(5) Welding performance.
During jewelry-making, it is often necessary to divide the workpiece into several simple small parts for separate production and then weld these small parts together. To achieve good welding quality, in addition to correctly using solder, it is also necessary to assess the welding performance of K gold. If the welded piece has good thermal conductivity, heat will not quickly accumulate at the welding site during the heating process. Still, it will soon be conducted throughout the entire workpiece, which is not conducive to the melting of the solder. Suppose K gold is prone to oxidation during heating. In that case, the formed oxide layer will reduce the wettability of the solder, preventing it from penetrating the weld seam and leading to issues such as weak welding, false welding, and poor welding.
2.5 Safety
Jewelry that comes into direct contact with the human body for a long time must consider safety as one of the critical factors in choosing jewelry materials. Harmful elements to the human body, such as Cd, Pb, and radioactive elements, should be avoided in filler materials. Additionally, allergic reactions caused by jewelry coming into contact with the skin should also be minimized; for example, K gold jewelry that uses Ni as a bleaching element poses a risk of causing skin allergies. Therefore, the European Commission and some other countries have established strict limits on the release rate of Ni in jewelry, meaning that jewelry containing Ni must meet relevant standards regarding Ni release rates.
2.6 Economic Factors
K gold is an alloy made of gold and its filler materials. The price of filler materials is one of the critical factors affecting production costs, especially for low-karat K gold, which requires many filler materials for alloying. Therefore, when selecting alloying elements for filler materials, widely sourced and inexpensive materials should be followed, and expensive precious metals should be avoided or minimized to reduce the cost of K gold.
Section II K Yellow Gold
K yellow gold refers to yellow gold alloy, known in English as karat yellow gold, commonly represented as KY in the jewelry industry, such as 18KY and 14KY. K gold is a traditional color gold alloy that has held an important position in K gold jewelry materials for a long time. However, since the 1990s, with the popularity of white jewelry, the proportion of K gold jewelry has gradually declined.
Nevertheless, due to K gold’s relatively excellent processing and manufacturing properties, it is still widely used in the jewelry industry, and some manufacturers even use K gold to make jewelry blanks and then plate them with rhodium (Rh) to replace K white gold jewelry.
1. The organization and performance of K yellow gold in the Au-Ag-Cu system
The Au-Ag-Cu alloy is the base alloy system of K gold, which largely determines its performance. Ag and Cu are the main alloying elements of K gold, and during production, a certain amount of Zn and a small amount of other components are often added to improve the performance of the alloy. The different ratios of alloying elements impact the physical properties, chemical properties, mechanical properties, and process performance of K yellow gold materials.
1.1 The physical properties of K yellow gold in the Au-Ag-Cu system
(1) Color.
In the Au-Ag-Cu system of K gold, the color of K gold alloys is closely related to their composition. Adjusting the ratio of Ag, Cu, and other alloying elements in the alloys can obtain different colors of K gold alloys.
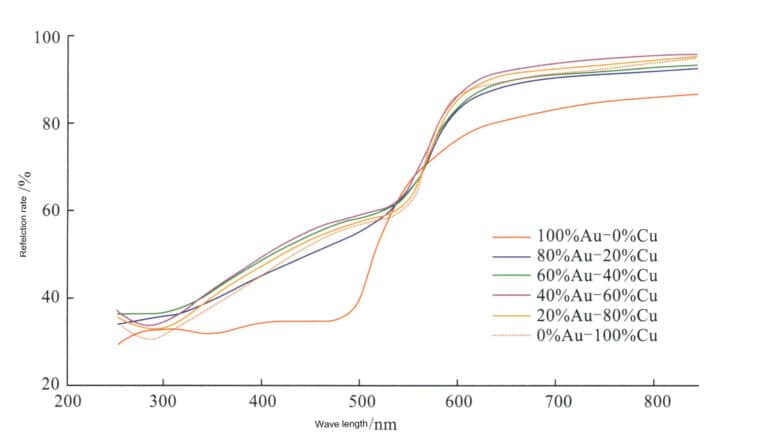
With the increase in Cu content, the electronic transition energy of the alloy decreases, and the reflectance curve shifts towards lower energy, significantly increasing the reflectance in the red light band ( 640 -750 nm ) (Figure 3-16), resulting in a gradual increase in the red index of K gold alloys.
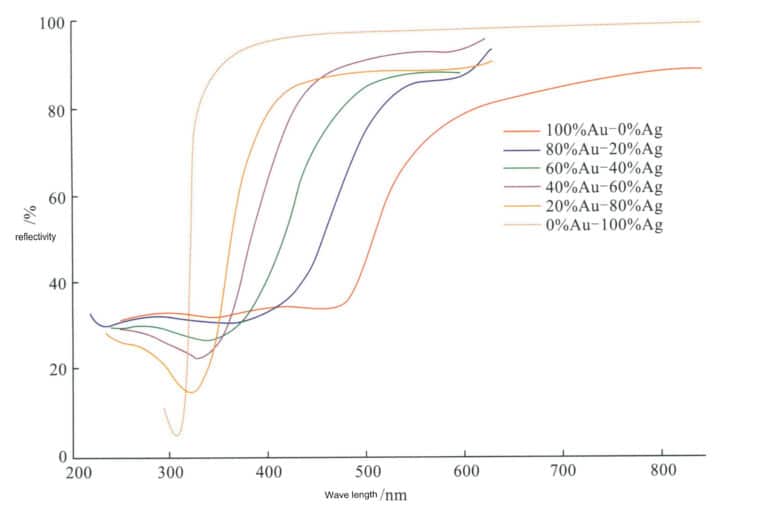
With the increase in Ag content, the Au-Ag alloy’s electronic transition energy increases, and Au’s reflectivity curve nearly migrates parallelly to higher power. As a result, not only are the red and yellow light bands in the visible spectrum strongly reflected, but even the green, blue, and violet bands are strongly reflected, ultimately leading to solid reflection across the entire visible spectrum (Figure 3-17). This causes the bandgap to widen, and the green index of K gold alloy gradually increases. Improving the reflectivity is beneficial when the alloy’s Ag content is high.
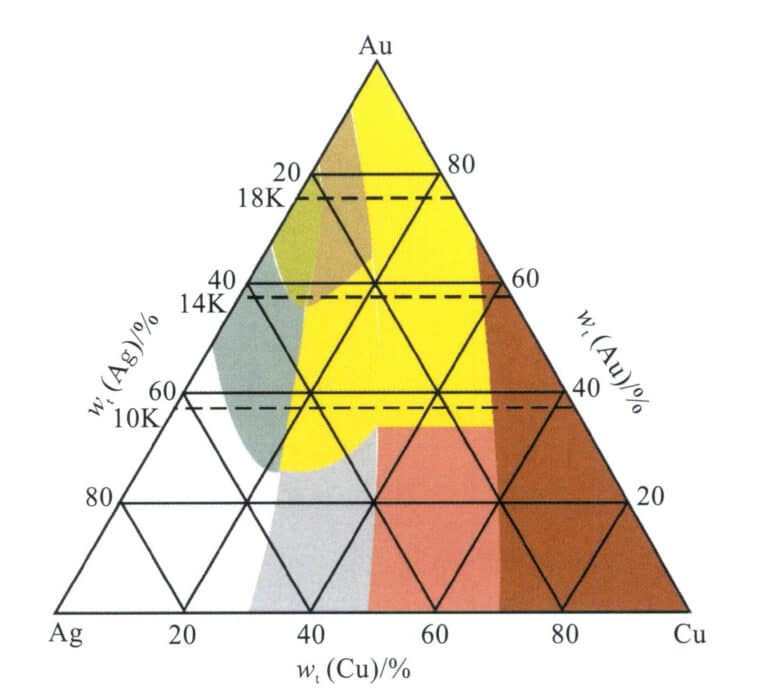
Affected by the comprehensive influence of Ag and Cu on the color of gold alloys, the Au-Ag-Cu alloy displays rich colors and tones (Figure 3-18). The alloy rich in Au appears golden yellow, the alloy rich in Ag seems white, and the alloy rich in Cu appears red. When Ag is added to Au, as the Ag content increases, the color of the alloy gradually changes from yellow to greenish yellow, light greenish-yellow, light white, and finally to white. When Cu is added to Au, as the Cu content increases, the color of the alloy gradually changes from yellow to reddish yellow, pink, and finally to red.
For a specific grade of K yellow gold, adding Zn causes the color of the K gold alloy to lean towards light reddish-yellow or deep yellow.
(2) Density.
Its theoretical density is also constant for K gold with a fixed composition. Since cast jewelry blanks can’t be dense during the production process, it is not appropriate to use casting hardness to explain the impact of alloy element ratios quantitatively. However, the difference between casting density and theoretical density can still indirectly reflect the density of the cast piece, and the required amount of materials can be calculated based on the ratio of alloy density to wax mold density.
Different ratios of alloy elements will have a particular impact on the density of K gold materials. The correspondence between the density of ternary alloys and their chemical composition (Figure 3-19) shows that the solid lines represent the contour lines of alloy density, which tilt towards the Au-Ag axis, indicating that Cu has a more significant impact on alloy density than Ag. As the grade of the alloy increases, the density of the alloy also correspondingly increases; for alloys with a high Au content, the contour lines are parallel. For K gold of the same grade, as the Ag content increases, the density value increases, and the density contour lines gradually shift to higher values.
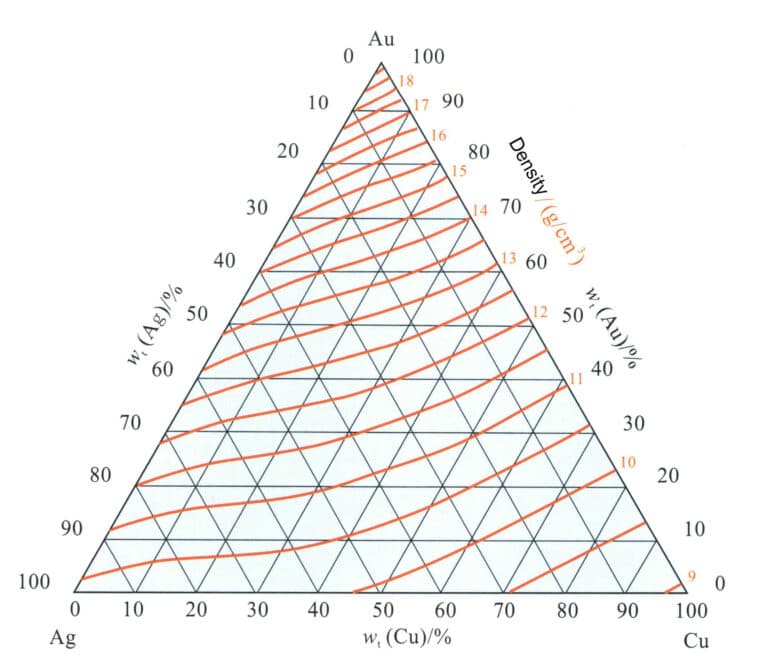
K gold is often alloyed with Zn as an alloying element, and as the Zn content increases, the density of the alloy decreases to some extent.
(3) Melting point.
Figure 3-20 shows the projection of the liquidus line temperature contour of the Au-Ag-Cu alloy on the plane. As the alloy grade increases, its liquidus temperature continuously rises; the combined addition of Ag and Cu causes the melting point of the alloy to decrease, forming a dome-shaped region of melting point contours opening towards the Ag-Cu coordinate line, with the lowest melting point dropping to around 750℃ when the alloy grade is relatively low.
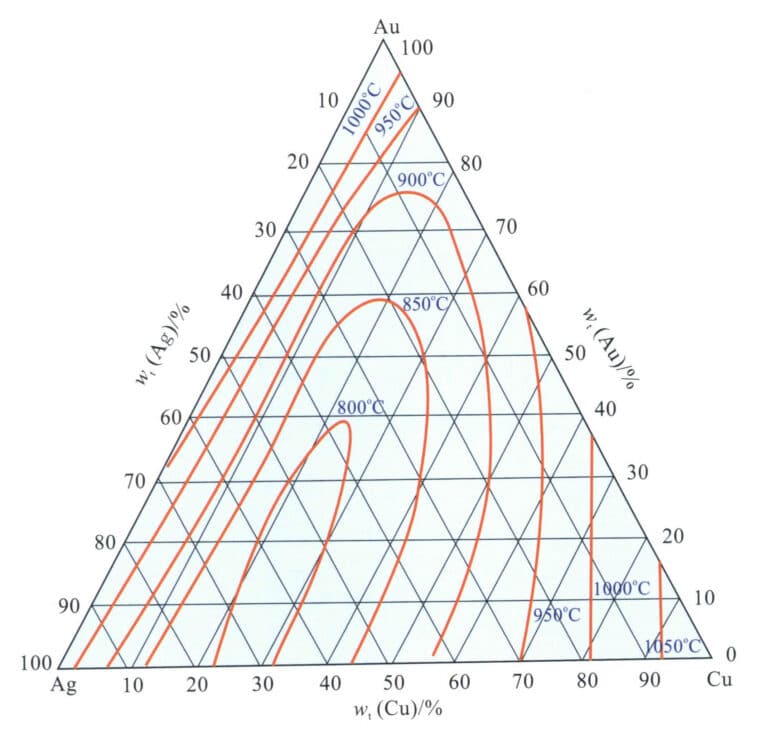
(4) Microstructure.
The phase diagram of the Au-Ag-Cu alloy (Figure 3-21) shows that its three components, Au, Ag, and Cu, can form three types of binary alloys. One is the Au-Ag binary alloy, which is completely miscible in both liquid and solid states; another is the Ag-Cu binary alloy, a typical eutectic alloy with the solubility of Ag and Cu at room temperature very small; another type is Au-Cu binary alloy, which completely dissolves to form a continuous solid solution in the high-temperature region. An ordering transformation occurs upon slow cooling below 410℃, forming AuCu3 and AuCu ordered phases. Therefore, in the Au-Ag-Cu ternary alloy system, Ag-rich and Cu-rich phases exist derived from the Ag-Cu eutectic system, and an immiscible two-phase region develops deeper as the Au content increases. This region appears as an arch towards the Ag-rich corner in the projection plane (Figure 3-22), indicating that the structure of the Au-Ag-Cu ternary alloy is related to the ratio of alloying elements Ag and Cu.
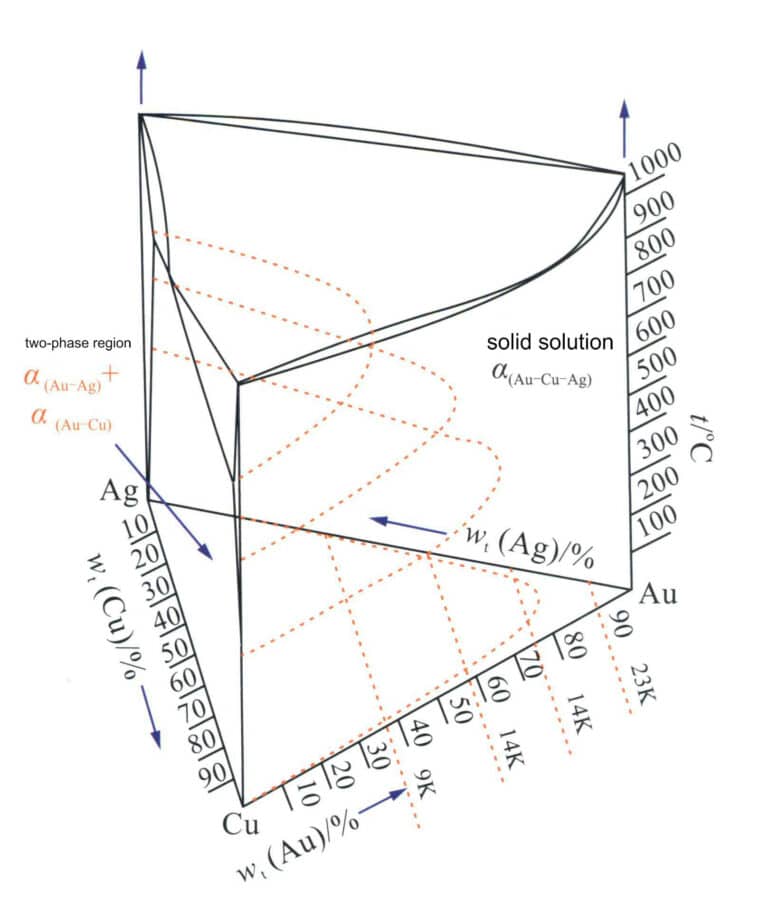
Figure 3-21 The phase diagram of the Au-Ag-Cu alloy
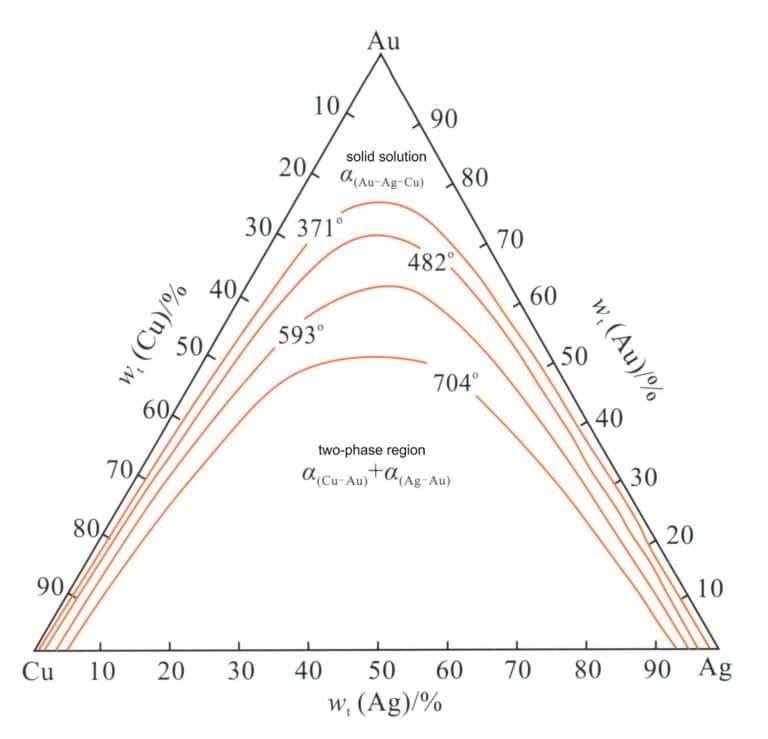
Figure 3-22 Au-Ag-Cu Isothermal two-phase region of the alloy projection of the solid phase boundary at room temperature (According to William S. Rapson, 1990)
For ease of analysis, the content of Ag and Cu is expressed in terms of the conversion ratio Ag, that is:
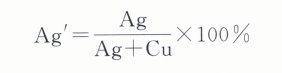
In the formula, Ag and Cu represent the mass fractions of Ag and Cu in the Au-Ag-Cu alloy, respectively.
Using Ag’ as the compositional coordinate, the longitudinal sections corresponding to 18K 14K and 10K three colors in Figure 3-19 are made into quasi-binary section diagrams (Figure 3-23).
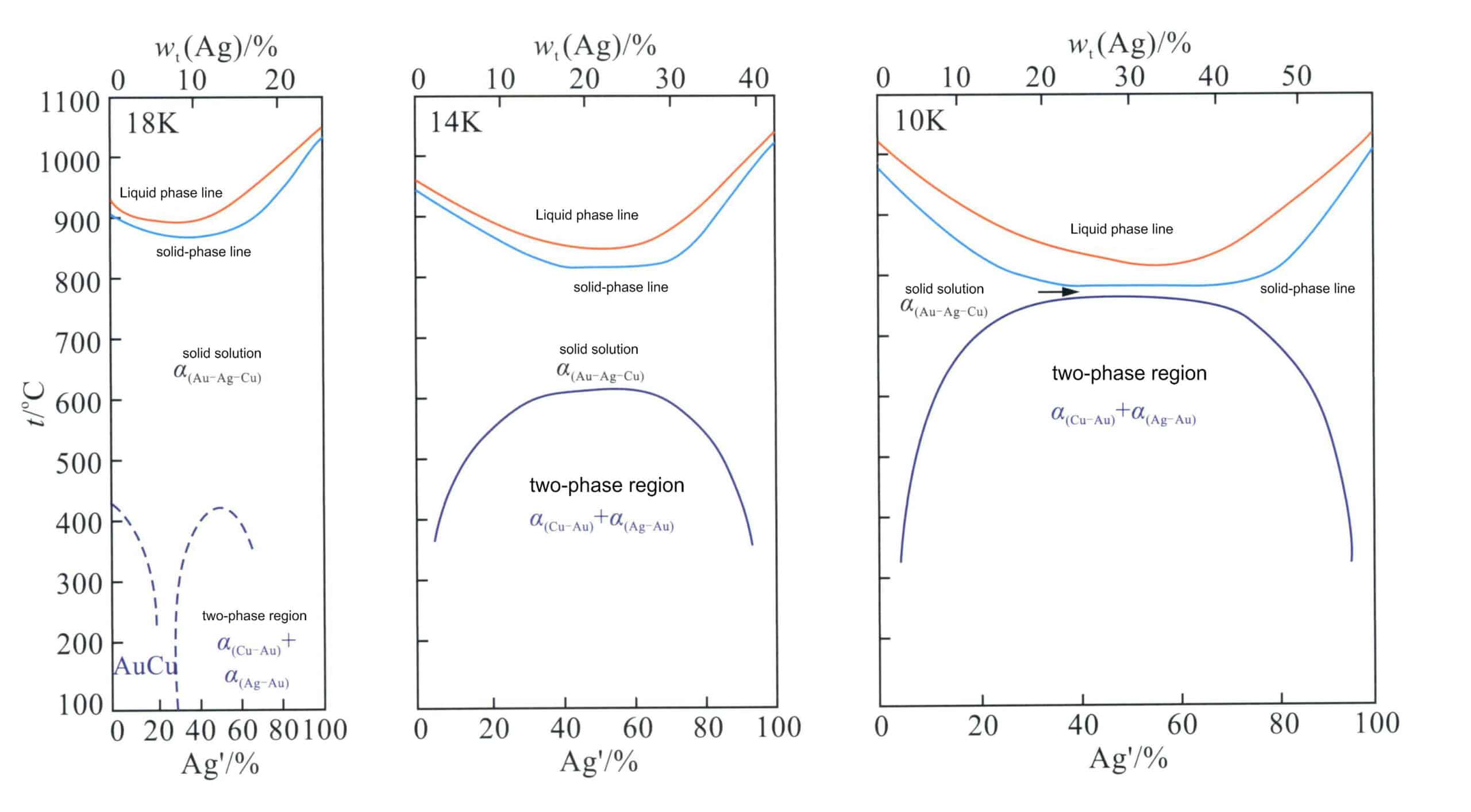
Figure 3-23 Au-Ag-Cu Quasi-binary longitudinal cross-section of the alloy (According to William S. Rapson, 1990)
According to Ag’ and the regions where phase separation occurs, alloys can be divided into different types; for example, 18K Au-Ag-Cu alloys have three typical types.
Type I: Ag’ is 0%-20%, a rich Cu alloy phase region, a single solid solution at high temperatures, and undergoes an ordering transformation at low temperatures.
Type II: Ag’ is 20%-75%, a single solid solution at high temperatures, and decomposes into two immiscible phases at low temperatures.
Type III: Ag’>75%, a single solid solution at high and low temperatures.
Au-Ag-Cu When Zn and other alloying elements are added to the K gold system, the range of the immiscible two-phase region can be reduced when the Zn content reaches a certain level, making the two-phase region narrower and shorter.
1.2 Au-Ag-Cu K gold's corrosion resistance
The corrosion resistance of Au-Ag-Cu alloys can be divided into four regions (Figure 3-24). Alloys in Region I have a higher grade and good corrosion resistance, able to withstand the corrosion of single inorganic acids; the corrosion resistance of alloys in Region II is inferior to that of Region I but still has relatively good corrosion resistance, with only slight corrosion in solid acids; Region III alloys are further reduced, suffering from strong acid corrosion. Region IV alloys have relatively poor resistance and are prone to darkening and discoloration. Adding a certain amount of {{1}} alloying elements to low-grade Au-Ag-Cu K gold helps improve its corrosion resistance.
However, it still has relatively good corrosion resistance, with only slight corrosion in solid acids; Region III alloys are further reduced, suffering from strong acid corrosion. Region IV alloys have relatively poor resistance and are prone to darkening and discoloration. Adding a certain amount of Zn, Si, and Pd alloying elements to low-grade Au-Ag-Cu K gold helps improve its corrosion resistance.
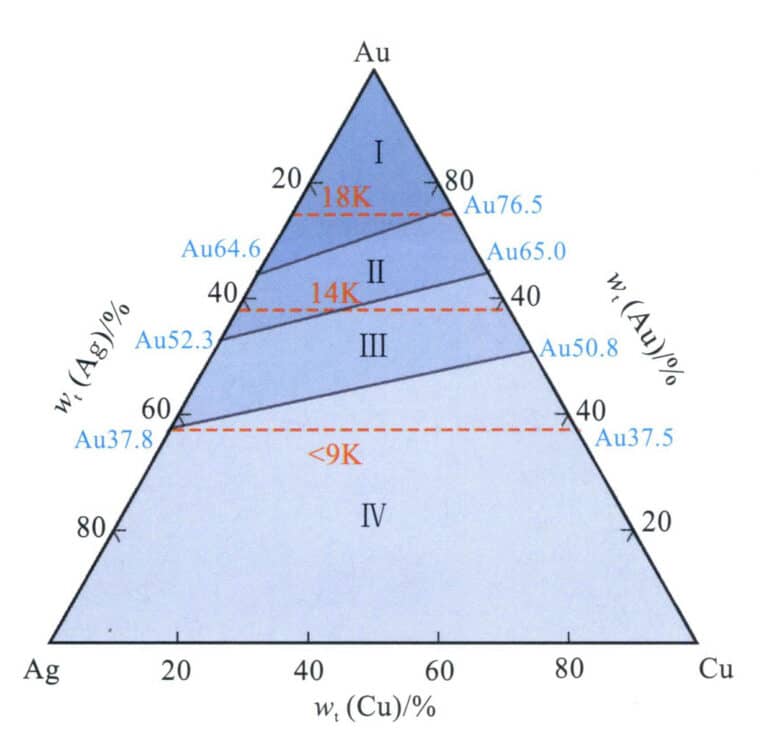
Figure 3-24 Au-Ag-Cu Alloy's corrosion resistance (According to Ning Yuantao et al., 2013)
1.3 Au-Ag-Cu K gold's mechanical properties
In the Au-Ag-Cu alloy, the proportion of Ag and Cu significantly impacts the alloy’s mechanical properties. Alloys with different compositions were quenched after being held at 740℃, and the hardness and elongation in the solid solution state were tested. The hardness of the 50%Au-30%Ag-20%Cu alloy is the highest, reaching HB150, while the elongation is the lowest, only 25%, whereas the alloys near the three corners have lower hardness and higher elongation (Figure 3-25, Figure 3-26).
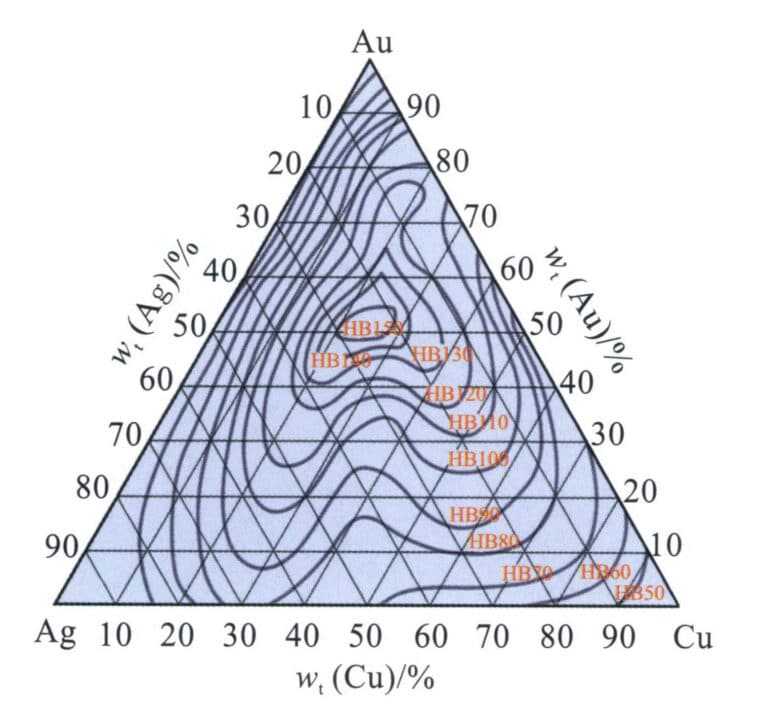
Figure 3-25 The Brinell hardness of Au-Ag-Cu alloy in the solid solution state
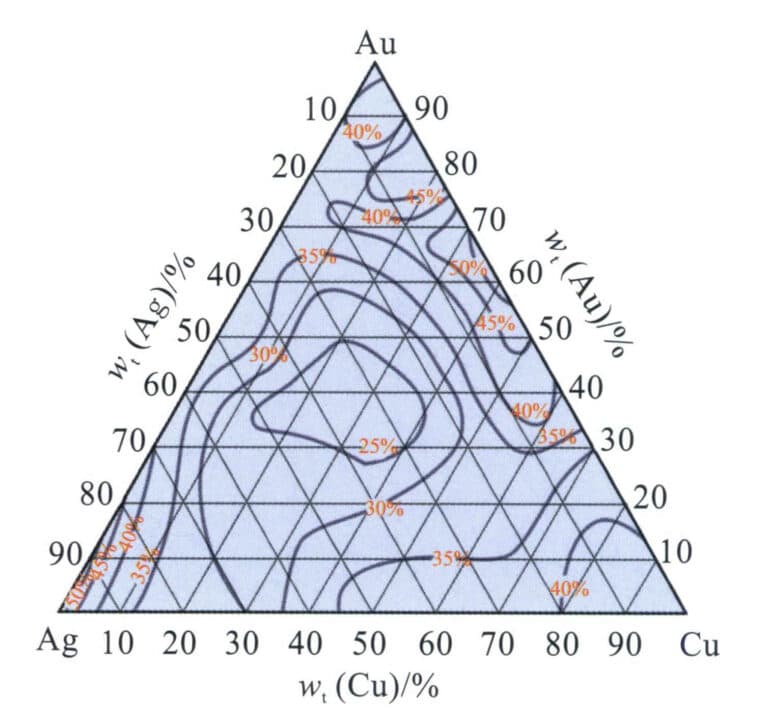
Figure 3-26 The elongation of Au-Ag-Cu alloy in the solid solution state
The differences in the mechanical properties of alloys with different components are also reflected in the impact of heat treatment on them. Taking Au-Ag-Cu 18KY as an example, when the alloy composition is within the range of Type I alloys, aging the solid solution alloy at low temperatures leads to an ordering transformation that enhances strength, increasing the hardness of the alloy but reducing its elasticity and flexibility. When within the range of Type II alloys, aging treatment can cause phase separation in the alloy, improving its strengthening and hardness, resulting in moderate hardness. However, when within the range of Type III alloys, aging treatment cannot be performed, and the hardness of the alloy could be higher.
For Au-Ag-Cu-Zn quaternary alloys, the role of Zn can slightly reduce the alloy’s hardness and the volume of the immiscible solid phase region in the Au-Ag-Cu ternary phase diagram.
1.4 The process performance of Au-Ag-Cu K gold
Au-Ag-Cu K gold has a relatively low melting point, making it suitable for precision casting using gypsum molds. When alloying elements such as Zn Si are added to the alloy, it can further improve the fluidity of the molten metal and reduce the tendency for oxidation, thereby enhancing casting performance.
Au-Ag-Cu K gold has good flexibility in the solid solution state, relatively low hardness, and good cold working performance, which can be processed using cold working techniques such as rolling, drawing, and forging. For alloys that undergo ordering transformations and phase separation, controlling the cooling method during intermediate annealing is essential to avoid reducing ductility and flexibility.
2. Typical Grades and Properties of K Gold for Decoration
The use of K gold has a long history and is a relatively mature gold alloy. A series of colors have been developed to meet the requirements of different processing techniques for decorative K gold, many of which have been commercialized, allowing companies to choose according to their market needs. Table 3-10 lists some typical grades of decorative K gold and their properties.
Table 3-10 Typical Grades and Properties of K Gold for Decoration
| Καθαρότητα | Component content/% | Χρώμα | Melting temperature | Density/ (g/cm3) | Soft hardness HV / (N/mm2) | Soft elongation Rate /% | |||
|---|---|---|---|---|---|---|---|---|---|
| Καθαρότητα | Wt(Au) | Wt (Ag) | Wt (Cu) | Wt (Zn) | Χρώμα | Melting temperature | Density/ (g/cm3) | Soft hardness HV / (N/mm2) | Soft elongation Rate /% |
| 22K | 917 | 55 | 28 | - | Κίτρινο | 995 ~ 1020 | 17.9 | 52 | |
| 22K | 917 | 32 | 51 | - | Deep Yellow | 964 ~ 982 | 17.8 | 70 | 30 |
| 18K | 750 | 160 | 90 | - | Ανοιχτό κίτρινο | 895 ~ 920 | 15.6 | 135 | 35 |
| 18K | 750 | 125 | 125 | - | Κίτρινο | 885 ~ 895 | 15.45 | 150 | 40 |
| 18K | 750 | 14.1 | 10 | 0.9 | Κίτρινο | 887 ~ 920 | 14.99 | 130 | - |
| 14K | 585 | 300 | 115 | - | Κίτρινο | 820 ~ 885 | 14.05 | 150 | 17 |
| 14K | 585 | 265 | 150 | - | Ανοιχτό κίτρινο | 835 ~ 850 | 13.85 | 175 | 30 |
| 14K | 585 | 205 | 210 | - | Ανοιχτό κίτρινο | 830 ~ 835 | 13.65 | 190 | 25 |
| 10K | 417 | 120 | 375 | 88 | Κίτρινο | 778 ~ 860 | 11.42 | 120 (Cast State) | - |
| 9K | 375 | 65 | 450 | 110 | Red and Yellow | 835 ~ 908 | 10.91 | 105 (Casting State) | - |
3. Common Issues in K Gold Jewelry Making
Compared to the other two colors of K gold materials, K gold materials have relatively more mature craftsmanship in jewelry making. However, K gold still often encounters issues in the production process, which are mainly reflected in the following aspects.
3.1 Color Issues of K Gold
In most cases, K gold directly uses its inherent color without further electroplating on the surface, which requires the alloy’s color to meet customer requirements and maintain stability and surface brightness over time. Currently, dozens of K gold alloy models are on the market, all classified as yellow. Still, the actual color perception varies greatly, such as deep yellow, light yellow, greenish-yellow, reddish-yellow, and bluish-yellow. 14K yellow jewelry displays three colors: bluish-yellow, light yellow, and reddish yellow (Figure 3-27). It is not uncommon for companies to receive customer complaints or even returns due to color deviations during production. The color of the alloy depends on its composition and is also related to inspection conditions.

3.2 Issues with the dendritic surface of K gold
The melting point of K gold is lower than that of 24K gold, but dendritic surfaces are rarely seen in 24K gold jewelry during investment casting. In contrast, K gold (especially low-grade K gold) jewelry castings sometimes exhibit dendritic surfaces. The reason is that K gold alloys have a specific solidification range, and their crystallization process often grows in a dendritic shape, forming a dendritic framework that interlocks, leaving residual molten metal between the dendrites. Suppose the molten metal does not wet the mold. In that case, the decomposition of gypsum can produce sulfur dioxide gas, pushing the residual molten metal away from the surface and leaving behind the dendritic framework. This results in a typical dendritic surface structure. Production practice shows that for low-grade K gold, the formation of a large amount of copper oxide or zinc oxide and high casting or mold temperatures increase the likelihood of gypsum decomposition, making it easier to form a dendritic surface.
3.3 The issue of inclusions in K gold
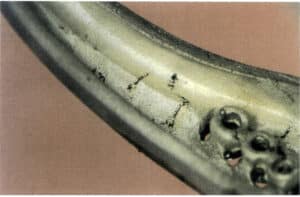
Cu and Zn are the main alloying elements in K gold and are prone to oxidation during smelting, forming oxide inclusions. The inclusion problem caused by Zn is particularly prominent; it forms oxides more easily than Cu in the presence of oxygen, and its oxides do not easily aggregate into liquid slag but instead appear as powder, both floating to the surface of the molten metal and remaining within the molten metal. As a result, once zinc oxide is formed, it will stay in the material, causing voids and surface defects, macroscopically manifested as cat’s paw-shaped inclusions (Figure 3-28).
Section III K White gold
K white gold, or white gold, is a white gold alloy commonly referred to as karat white gold in English and is often represented as KW in the jewelry industry, such as 18KW and 14KW. K white gold was once used as a substitute for platinum, with advantages such as higher strength and better casting performance, and has been widely used in set jewelry, becoming a significant material among decorative gold alloys, occupying a critical position among colored K gold materials.
1. Bleaching of gold and the white grading of K white gold
Gold appears golden yellow, and alloying elements with bleaching effects must be added to make it appear white. Among all metallic elements in nature, except for a few elements like Au and Cu, most metallic elements appear white or gray. Therefore, adding other metals will have a whitening effect on the gold alloy to some extent. Table 3-11 lists some alloying elements’ bleaching capabilities for gold and their main drawbacks when used as bleaching elements. From Table 3-11, it can be seen that there are not many metals that can effectively serve as bleaching elements for K white gold and meet the conventional production process requirements for jewelry. Elements like Ni, Pd, Fe, and Mn have strong bleaching capabilities for gold and are the main bleaching agents to date.
Table 3-11 Bleaching capabilities and main drawbacks of alloying elements for gold (according to Bagnoud et al., 1996)
| Στοιχείο | Bleaching capability | Main Disadvantages |
|---|---|---|
| Ag | General | Causes alloy discoloration when the content is high |
| Pd | Very Good | High cost, increases alloy melting point |
| Pt | Pd Similar to Pd | Higher cost than Pd |
| Ni | Καλός | Skin sensitizer |
| Cr | Αδύναμο | Skin allergen |
| Co | Αδύναμο | Skin allergen |
| In | Αδύναμο | Deteriorates processing performance when the content is high |
| Sn | Αδύναμο | Deteriorates processing performance when the content is high |
| Zn | Αδύναμο | When the content is high, the alloy volatilizes seriously, making recycling difficult. |
| Al | Αδύναμο | Deterioration of processing performance |
| Ti | Αδύναμο | Deterioration of processing performance |
| V | Αδύναμο | Toxic, deteriorates processing performance, highly reactive, difficult to reuse |
| Ta, Nb | Αδύναμο | Highly reactive, difficult to reuse |
| Fe | Καλός | Low solubility in Au, the alloy exhibits ferromagnetism when precipitate phases are present, damaging corrosion resistance. Content exceeds 10% When the alloy is too hard, it deteriorates processing performance and is prone to oxidation during casting |
| Mn | Καλός | Reactivity is strong and processing is difficult when the content exceeds 10% |
Different filling methods may result in color variations for gold alloys of the same fineness. To ensure good communication between supply and demand, the Manufacturing Jewelers and Suppliers of America (MJSA) collaborated with the World Gold Council to define the color grade of K white gold after testing the colors of 10KW, 14KW, and 18KW samples using the CIELab color coordinate system. It defined that the yellowness index value of “K white gold” should be less than 32; exceeding this value means it cannot be called K white gold. The yellowness index value is divided into three levels: Level 1, Level 2, and Level 3, as shown in Table 3-12.
3-12 The white color grade of K white gold
| Color grade | YI Yellow Index YI | Degree of Whiteness | Rhodium Plating |
|---|---|---|---|
| Level 1 | YI< 19 | Very White | No need |
| Level 2 | 19≤YI ≤24.5 | White is better | Can be plated or not |
| Level 3 | 24.5 < YI ≤ 32 | Poor | Must be plated |
Using this white grading indicator allows suppliers, manufacturers, and sellers to quantitatively determine the color requirements of K white gold.
2. Categories and Characteristics of K white gold
Based on the bleaching elements used, K white gold can be roughly divided into four categories: nickel K white gold, palladium K white gold, nickel + palladium K white gold, and low or no nickel (low) palladium K white gold. According to statistics from foreign research institutions, the first two categories account for 76% and 15% of the K white gold jewelry market, while the latter account for 7% and 2%.
2.1 Nickel K white gold
Due to its low price and good bleaching effect, Ni has traditionally been used as a bleaching agent for Au. Among all commercial K white gold, nickel K white gold dominates the market.
The Ni content directly affects the bleaching effect of K white gold. Au alloys with 9% -12%Ni content are nearly white, and as the Ni content gradually decreases, the yellowness of the alloy increases. When the Ni content is below 5%, the whiteness of the alloy significantly decreases, and the color turns yellowish.
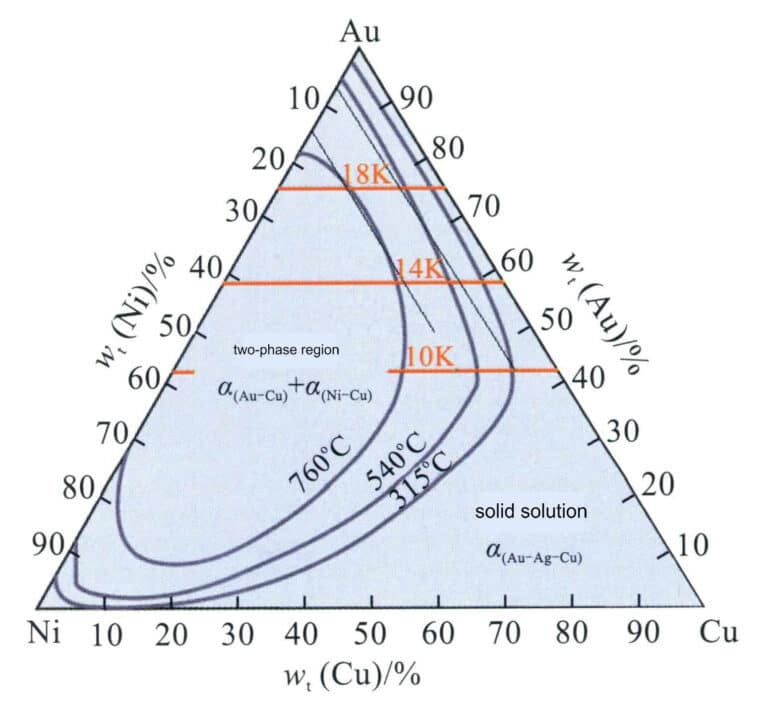
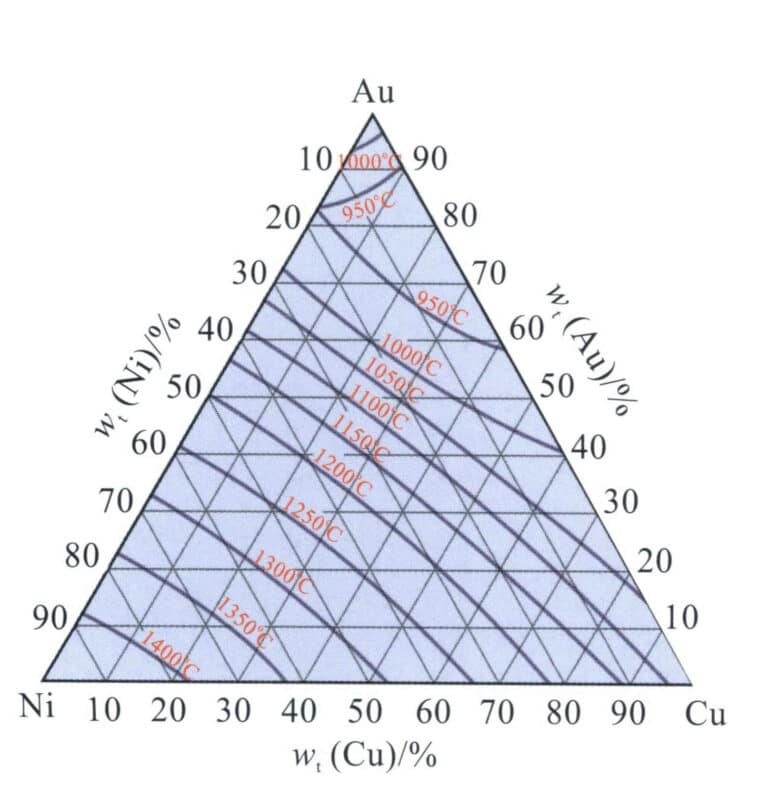
From the Au-Ni binary alloy phase diagram in Figure 3-12, it can be seen that the Au-Ni alloy is a continuous solid solution at high temperatures and can decompose into Au-rich and Ni-rich phases at low temperatures, increasing the hardness of the alloy. Nickel K white gold with high Ni content has poor machinability and is generally formed using the investment casting process. Adding Cu can improve the machinability of the alloy. Thus, the Au-Ni-Cu alloy is the most commonly used base alloy system for jewelry K white gold. The projection of the phase decomposition boundary line of the Au-Ni-Cu ternary alloy on the plane (Figure 3-29) shows that as the Cu content increases, the two-phase decomposition region of the Au-Ni binary alloy system extends into the ternary system. As the temperature decreases, the range of the phase decomposition region expands.
The organization of Au-Ni-Cu ternary alloys is related to the ratio of Ni and Cu, and in order to facilitate the analysis, the converted ratios of Cu and Ni were used to reflect their relative quantities, i.e.
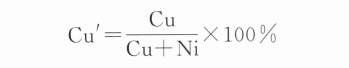
In the formula, Cu Ni represents the mass fraction, respectively. The smaller the Cu value, the higher the Ni content; the more significant the Cu value, the lower the Ni content.
Figure 3-30 shows the quasi-binary longitudinal cross-section of the alloy with Au-Ni-Cu’ as the component coordinates and colors of 18K, 14K, and 10K. It can be seen that the alloy structure is a single-phase solid solution only when the Cu’ value exceeds 80%; below this value, a two-phase immiscible region appears. As the alloy color decreases, the alloy’s melting point continuously increases, the solidification crystallization interval widens, and the range of the solid-state two-phase region also expands.
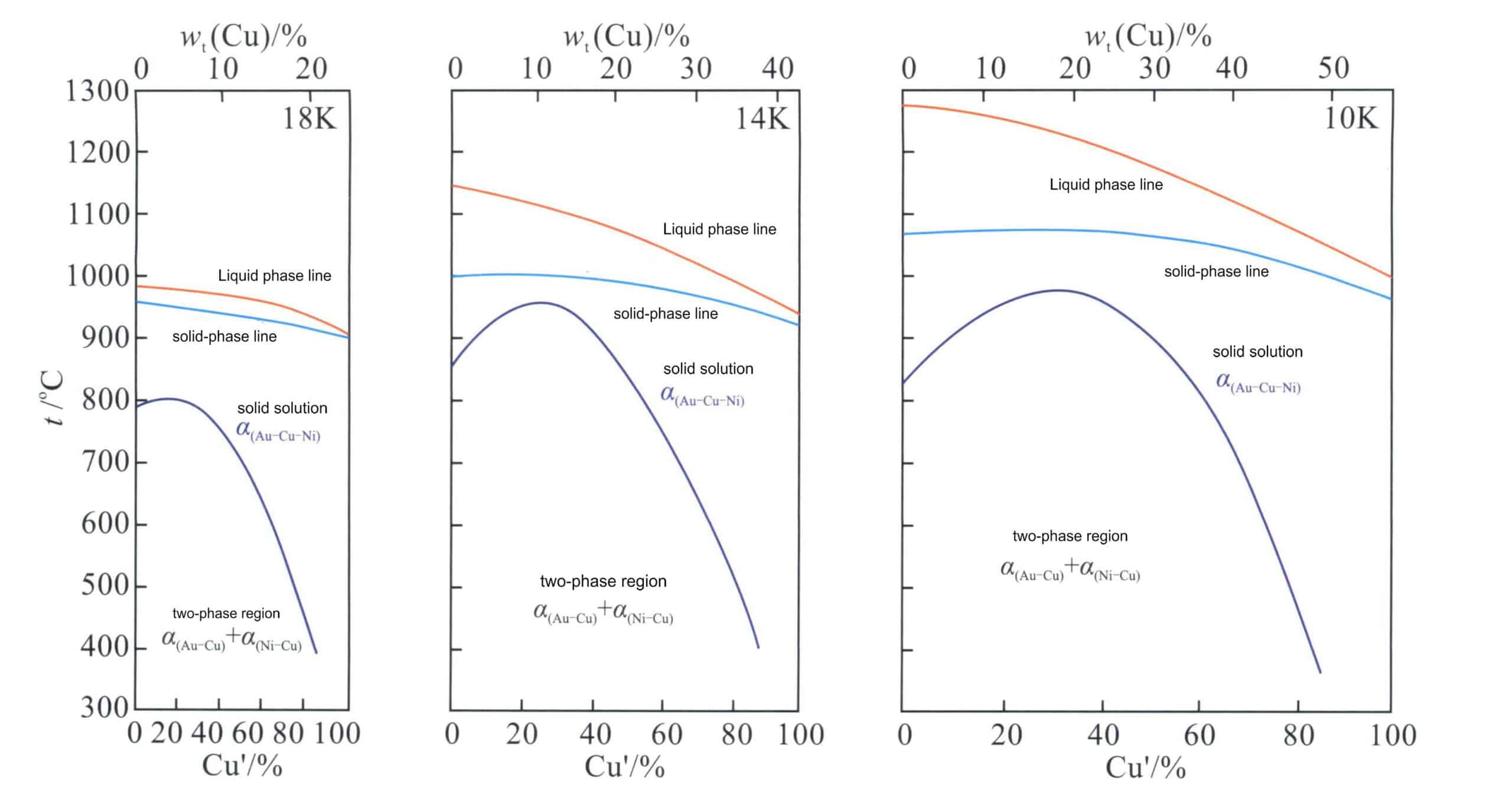
The relationship between the liquidus temperature of the Au-Ni-Cu alloy and composition is shown in Figure 3-31; as the Ni content increases, the alloy’s melting point also rises, indicating that the casting performance of the alloy deteriorates.
The relationship between the color of the Au-Ni-Cu alloy and composition is shown in Figure 3-32. The dashed line indicates the boundary between white and yellow or red. As the Ni content increases, the whiteness of the alloy increases. Its Ni content should not be lower than a specific value to achieve a certain whiteness for the alloy. For 18K, 14K, and three colors of 10K, the alloys corresponding to the bold black line segment in the figure can be used for jewelry making.
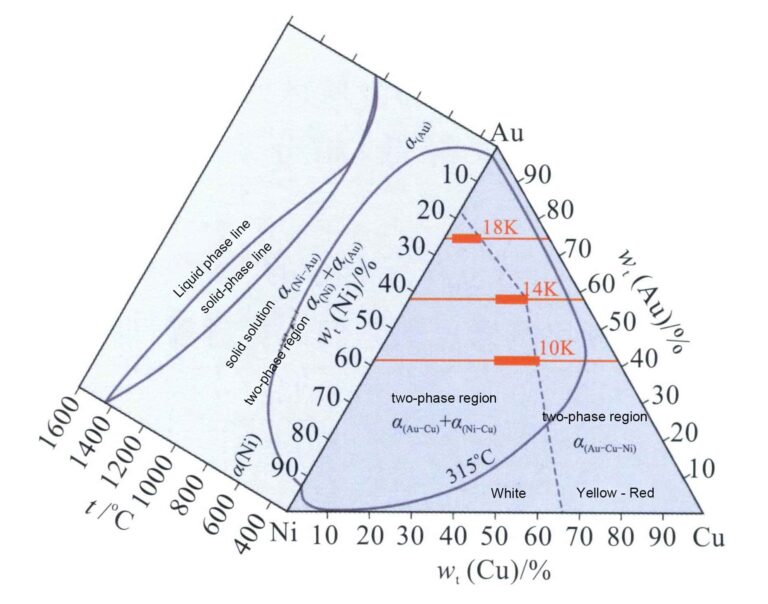
The strength and hardness of the Au-Ni-Cu series alloy are high, but its machinability could be better than that of the Au-Ag-Cu series alloy. In the phase separation of gold at low temperatures, the hardness of the Ni-rich phase is much higher than that of the Au-rich phase. The two phases deform at different rates when the material is rolled or drawn. The soft metal of the Au-rich phase is more straightforward to deform than the Ni-rich phase’s hard metal. When processed to a certain extent, stress appears between the two phases, affecting the alloy’s flexibility and reducing its cold working performance.
To improve the performance of the Au-Ni-Cu alloy, Zn is often chosen as an auxiliary whitening element to compensate for the chromatic effect caused by adding Cu and enhance Ni’s whitening effect. It can also serve as a deoxidizer for investment casting, improving processing performance. However, the volatilization of Zn during the smelting process reduces the alloy’s ductility and poses particular difficulties for the recycling of the alloy.
2.2 Palladium K white gold
Ni poses a risk of causing skin allergies; therefore, palladium K white gold, with Pd as the leading whitening element, is a vital category widely used in Europe.
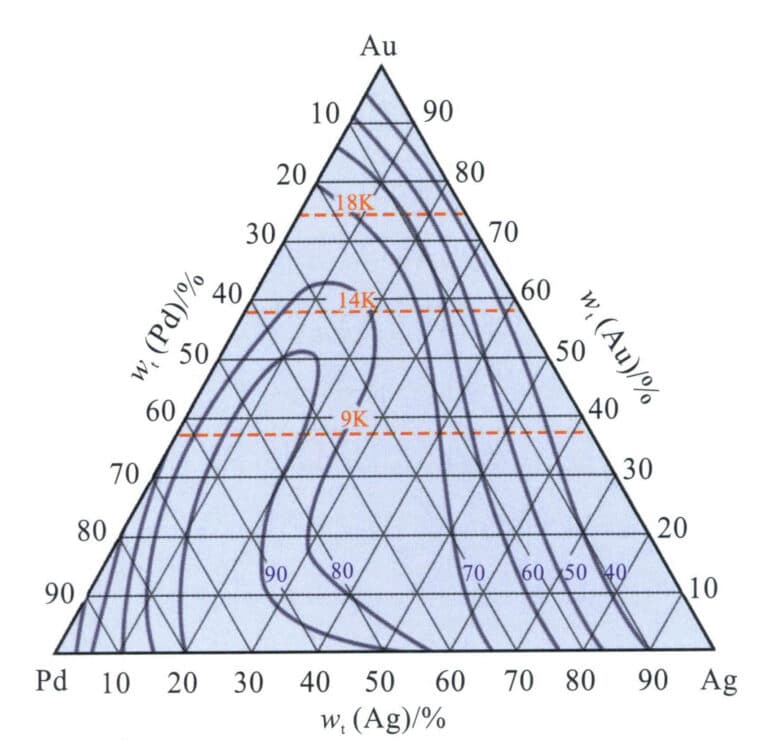
Pd is a platinum group element with a good bleaching ability for gold, allowing alloys to present a warm gray-white color with a comfortable feel. Due to the high price of palladium, Ag is often used as a secondary bleaching element. Au-Pd-Ag The ternary alloy is the base alloy system of palladium K white gold, and the relationship between its color and composition is shown in Figure 3-33. The Pd content must reach a specific value to present a better white color. For standard 18K, 14K, and 9K, three colors, the content should be chosen within the boundary line area. Taking 18K white gold as an example, when the Pd content is 10%-13%, it has excellent white color and does not require rhodium plating.
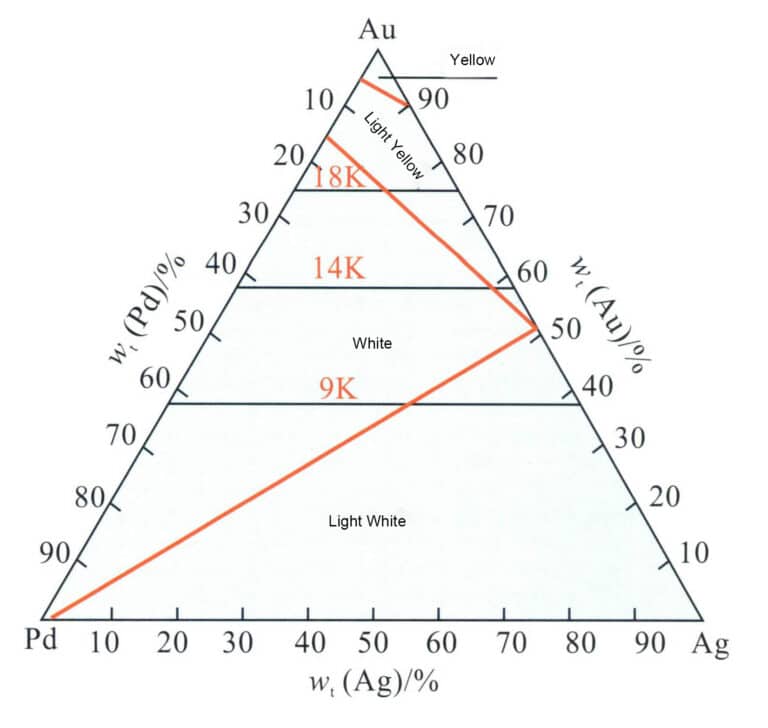
Unlike Au-Ni-Cu alloys, Au-Pd-Ag alloys are single-solid solutions across the entire composition range and do not exhibit phase separation. The liquidus temperature distribution of Au-Pd-Ag alloys is shown in Figure 3-34. Adding palladium to gold increases the alloy’s melting point, and as the Pd content increases, the alloy’s melting point continues to rise. This increases the casting difficulty of the alloy. When the palladium content is very high, conventional gypsum mold casting processes can easily lead to porosity defects in the castings due to the thermal decomposition of the gypsum casting powder.
The distribution of annealed hardness contours for Au-Pd-Ag alloys is shown in Figure 3-35. Its hardness is similar to that of Au-Ag-Cu alloys, significantly lower than that of Au-Ni-Cu alloys. At the same time, this alloy is a single continuous solid solution structure at room temperature, thus exhibiting excellent machinability, making it suitable for rolling, engraving, inlaying, and other operations.
Since Pd Ag belongs to all precious metal elements, Au-Pd-Ag has good corrosion resistance. Adding an appropriate amount of other alloying elements to this alloy can further improve its performance in certain aspects.
2.3 Nickel-Palladium K white gold
This type of K white gold contains both Ni and Pd, using Ni as the base bleaching element and limiting its content to reduce. The risk of low nickel allergy and the improvement of the alloy’s processing performance; to compensate for insufficient bleaching ability, an appropriate amount of Pd is added to the alloy, allowing the alloy to achieve sufficient whiteness while having good processing performance and at the same time avoiding the problem of excessively high material costs when Pd is used solely as the primary bleaching element.
2.4 Nickel-free (low) palladium K white gold
Given the potential toxicity of Ni to human skin, many countries and regions have established regulations on the nickel release rate of jewelry materials, promoting the research and development of nickel-free K white gold materials. In addition to palladium K white gold using Pd as the bleaching element, the development of materials other than Ni Pd is also underway. The preparation of medium to high-quality K white gold with alloying elements has not been satisfactory; in many cases, a considerable amount of Pd still needs to be added to achieve good results. So far, only a few alloy systems have been commercially applied, with added elements including Pt, Fe, Mn, and others. Pt is an excellent whitening agent for Au but is also often used with Pd, which has a long history of application in dental alloys. 18K white gold containing 10%Pt, 10%Pd, 3%Cu, and 2%Zn has been commercialized in the jewelry industry, and due to the high content of Pt and Pd, it is pretty expensive. Fe has been studied as a second whitening agent. Still, a large amount of Pd must also be added to maintain the color and processability of the alloy, especially for low K number alloys (such as 14K). The Au-Fe system is a biphasic structure, which causes hardness and corrosion issues for the alloy. Mn is a promising whitening agent for K gold; when its content is high, better whiteness can be achieved, but the alloy is quite brittle and still requires a certain amount of Pd to improve its performance. Manganese K white gold is prone to oxidation and must be melted in a neutral or reducing atmosphere. When using a torch for melting, hydrogen gas can be chosen as it can consume the oxygen around the metal. The color of manganese K white gold can reach levels two and three, and electroplating is required to achieve satisfactory color. It is prone to discoloration when in contact with chemicals. Therefore, electroplating is essential.
In low K number white gold alloys (such as 8K, 9K, and 10K ), using a high content of Ag as a whitening agent can make the product appear white. These alloys are relatively soft and have good flexibility, and an appropriate amount of Pd, Cu, Zn, or Ni can be added to improve their performance. Still, the amounts of Cu and Zn must be controlled to avoid affecting the color of the alloy. These alloys have poor corrosion resistance and are prone to rusting due to chemical reactions with sulfur in the atmosphere.
3. Performance Requirements of K white gold
For jewelry companies, choosing suitable filler materials ensures product quality and significantly impacts production costs. Multiple aspects must be considered comprehensively to obtain ideal performance from K white gold jewelry materials.
3.1 Color and Corrosion Resistance
As K white gold should have at least a white color, it must meet the basic requirements of YI < 32 and improve the whiteness of the alloy as much as possible without significantly affecting its performance. The alloy should also have a high reflectivity to achieve better brightness during polishing. The alloy has good resistance to dull discoloration and corrosion.
3.2 Melting Point and Volatility
A low melting point is beneficial for smelting and casting. The melting point of K white gold materials is usually higher than that of K gold, especially for materials with good whiteness, a high content of bleaching elements, and, thus, a higher melting point. Alloys with high melting points require higher pouring temperatures, which poses a risk of gypsum thermal decomposition in gypsum precision casting processes. In contrast, using ceramic molds with phosphoric acid-bonded casting powder increases production costs, efficiency, and difficulty. Therefore, for jewelry made using precision casting, it is advisable to choose alloy materials with suitable melting points, ideally within 1050℃. Adding Zn to the alloy helps lower the melting point, but excessive Zn content can increase volatility during the casting process, affecting product quality and reusability.
3.3 Grain Structure
K white gold materials should be conducive to obtaining fine and dense grain structures, which helps improve the polishing effect of the alloy and reduces the likelihood of complex spot defects.
3.4 Hardness and Machinability
K white gold alloy materials should have appropriate as-cast and annealed hardness, good mechanical properties, and cold working performance, not overly strong work hardening, a low tendency for thermal cracking during annealing, and a low tendency for stress corrosion cracking.
3.5 Compliance with Nickel K white gold materials should meet the relevant directive standards.
For nickel-bleached K white gold alloy materials, the requirements of the nickel directive should be met, and the nickel release rate should not exceed the standard.
3.6 Meeting the requirements for environmental protection and cost reduction
In selecting alloying elements, comprehensive material sources, low prices, and environmental friendliness are followed to reduce alloy costs and improve cost-effectiveness.
It should be noted that the relative importance of various performance characteristics changes with the application of materials, and it is often challenging to meet all the above requirements simultaneously. Sometimes, compromises must be made among these requirements to achieve the best optimization effect as much as possible.
4. Composition and Performance of Some K white gold
The types of K white gold supplied in the market are diverse, with specific performance differences. Overall, to improve processing performance or reduce material costs, most commercial K white gold make certain concessions in whiteness, often appearing grayish-white, and usually require rhodium plating. Even alloys with very high whiteness cannot be compared to the color of the rhodium layer, so they often undergo rhodium plating on their surfaces as well. Tables 3-13 and 3-14 list the performance of some nickel K white gold and palladium K white gold, respectively.
Table 3-13 Composition and Properties of Some Nickel K white gold
| Καθαρότητα | Chemical composition (wt)/% | Σκληρότητα HV/(N/mm)2) | Tensile strength ( Fire state) /MPa | Liquidus line Temperature /℃ | Solidus Line Temperature /℃ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Καθαρότητα | Au | Ni | Cu | Zn | Ag | Cast State | Cold working state (70%) | Tensile strength ( Fire state) /MPa | Liquidus line Temperature /℃ | Solidus Line Temperature /℃ |
| 18K | 75 | 11 | 9.5 | 4.5 | - | 307 | 307 | 716 | 950 | 913 |
| 18K | 75 | 7.4 | 14 | 3.6 | - | 291 | 291 | 623 | 943 | 913 |
| 18K | 75 | 6.6 | 15.4 | 3 | - | 187 | 288 | 607 | 946 | 922 |
| 18K | 75 | 5 | 17 | 3 | - | 182 | 276 | 623 | 939 | 915 |
| 18K | 75 | 4 | 17 | 3 | - | 184 | 268 | 612 | 921 | 898 |
| 14K | 58.5 | 11 | 25.5 | 5 | - | 169 | 306 | 747 | 986 | 956 |
| 14K | 58.5 | 8.3 | 28.2 | 5 | - | 145 | 286 | 665 | 987 | 947 |
| 14K | 58.5 | 6.5 | 28.4 | 6.6 | - | 153 | 278 | 706 | 965 | 924 |
| 9K | 37.5 | 10 | 37 | 13.5 | 2 | 127 | 258 | 642 | 923 | 887 |
| 9K | 37.5 | - | 5.5 | 5.5 | 52 | 118 | 189 | 400 | 885 | 874 |
Table 3-14 Composition and performance of part of palladium K white gold
| Καθαρότητα | Chemical composition (wt)/% | Hardness HV/ (N/mm2) | Liquidus line Temperature /℃ | |||||
|---|---|---|---|---|---|---|---|---|
| Καθαρότητα | Au | Pd | Ag | Cu | Zn | Ni | Hardness HV/ (N/mm2) | Liquidus line Temperature /℃ |
| 18K | 75 | 20 | 5 | - | - | - | 100 | 1350 |
| 18K | 75 | 15 | 10 | - | - | - | 100 | 1300 |
| 18K | 75 | 10 | 15 | - | - | - | 80 | 1250 |
| 18K | 75 | 10 | 10.5 | 3.5 | 0.1 | 0.9 | 95 | 1150 |
| 18K | 75 | 6.4 | 9.9 | 5.1 | 3.5 | 1.1 | 140 | 1040 |
| 18K | 75 | 15 | - | 3.0 | - | 7.0 | 180 | 1150 |
| 14K | 58.3 | 20 | 6 | 14.5 | 1 | - | 160 | 1095 |
| 14K | 58.3 | 5 | 32.5 | 3 | 1 | - | 100 | 1100 |
| 10K | 41.7 | 28 | 8.4 | 20.5 | 1.4 | - | 160 | 1095 |
| 9K | 37.5 | - | 52 | 4.9 | 4.2 | 1.4 | 85 | 940 |
5. Common Issues with Nickel K white gold Jewelry Materials
In the manufacturing of K white gold jewelry, Ni is an alloy element that is both inexpensive and can enhance the brightness of the jewelry. Nickel K white gold has good color and physical-mechanical properties, making it the most widely used jewelry material among K white gold. However, nickel K white gold often encounters issues during production and use, which harms consumer health and causes many troubles for jewelry manufacturing companies. The main problems with nickel K white gold include the following aspects.
5.1 Nickel allergy issue
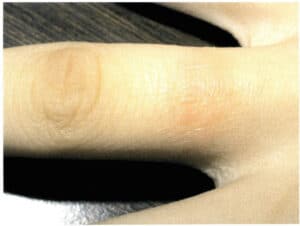
Many facts have proven that Ni has potential allergic and toxic effects on human skin, which can lead to Ni allergy. Ni allergy refers to the allergic reaction that occurs when K white gold jewelry comes into prolonged contact with human skin, causing Ni in the alloy to dissolve under the action of sweat and release Ni ions. These Ni ions can penetrate the skin and combine with specific proteins, leading to allergic reactions. Its particles can cause the skin to develop rashes and localized inflammation, resulting in eczema, itching (Figure 3-36), and even ulceration, severely affecting human health and appearance (Rushforth, 2000). Once a person develops a Ni allergic reaction, they will experience this reaction for the rest of their life.
According to statistics, approximately 10% -15% of women and 2% of men in Europe have allergic reactions to nickel metal, which is higher than in other parts of the world. In response, the European Commission actively addressed this issue by issuing the Nickel Directive 94/27/EC in 1999, which regulates the sale and import of certain products suspected of releasing nickel metal to some extent upon skin contact. For jewelry that comes into prolonged contact with the skin, the maximum release rate of nickel is limited to 0.5g / cm2 / week. Two testing standards, EN1811 and EN12472, were specifically developed to simulate objects with and without coatings, testing the nickel release rate in a specific time, temperature, and artificial sweat conditions. Subsequently, due to the still high sensitization rate of nickel, stricter revisions were made, leading to the issuance of Nickel Directive 2004/96/EC and the nickel release testing standard EN/811:2011, which eliminated the adjustment value for nickel release rates. Based on the effects after the implementation of the directive, the European Commission has tightened the Nickel Directive twice. Countries like the UK, Japan, and China have also established corresponding nickel release requirements for K white gold. The Nickel Directive does not prohibit using nickel materials but restricts the nickel release rates of alloys and materials. When producing K white gold jewelry, jewelry manufacturers first need to determine whether there are restrictions on nickel release in the customer’s country or region and choose appropriate filler materials accordingly. Notably, a considerable portion of the K white gold filler materials available on the market cannot pass the nickel metal release rate test.
5.2 Color issues
K white gold is an alternative material for platinum jewelry, requiring good whiteness. Therefore, most K white gold jewelry is plated with rhodium on the surface. Typically, the rhodium plating time is very short, commonly referred to as “flash plating,” resulting in a skinny layer that wears off after a period of use, exposing the original color of the base metal. In many cases, the color of the K white gold contrasts significantly with the color of the plating, leading to customer complaints or doubts. Furthermore, for a long time, the jewelry industry has mainly used qualitative methods to describe alloy colors, often resulting in disputes between jewelry companies and customers due to inconsistent judgments.
5.3 Magnetic Issues
Gold itself is not magnetic, but nickel K white gold can sometimes exhibit a certain degree of magnetism. This often raises doubts and complaints from consumers, who believe the material purity needs to be improved and that the material is mixed with Fe and others. Therefore, nickel K white gold, as a precious metal material for decoration, generally does not want the alloy to exhibit magnetism in most cases.
In nature, Fe is a well-known magnetic metal element, and besides it, there are a few other elements also exhibit magnetism, such as Co, Ni, and Ga. Whether a substance shows magnetism depends not only on its composition but also on its microstructure. Elements that are the same but have different structures or are in various temperature ranges may sometimes show differences in magnetism. In the case of the Au-Ni-Cu alloy system, phase separation occurs within a specific temperature range, forming a rich Ni phase and a rich Au phase, while the rich Ni phase may exhibit a certain degree of magnetism.
5.4 Poor Processing Performance Issues
The cold working performance of K gold jewelry is a comprehensive representation of various mechanical properties. K white gold jewelry is mainly designed with embedded gemstones, and the cold working performance of the material is an essential factor affecting the ease of the embedding operation. Suppose the material’s stiffness and yield strength are too high. In that case, it becomes difficult to hold the metal claws or edges against the gemstones during embedding, making it hard to secure them, which may even get damaged during the embedding process. The metal claws (nails) can easily break during embedding if the material’s toughness is insufficient. In jewelry production, materials often undergo cold deformation processes such as rolling, drawing, and stamping. If the material’s ductility is poor, cracks are likely to occur. The cold working performance of nickel K white gold is significantly worse than that of K gold, and issues such as processing cracks or breakage often arise during production.
5.5 Stress corrosion cracking issues
There are frequent cases of consumers losing gemstones due to broken claws while wearing nickel K white gold embedded jewelry, mainly caused by stress corrosion cracks in nickel K white gold, which often appear on stamped claws. Various stresses can occur during rolling, stamping, welding, and embedding claws. If measures are not taken to eliminate these internal stresses, residual stresses will form in the jewelry. Table 3-15 lists the possible causes of claw residual stress formation.
Table 3-15 Causes and consequences of residual stress formation in claws
| Operating process | Causes of residual stress | Possible consequences related to stress |
|---|---|---|
| Welding claws onto the ring | Excessive temperature of claws during welding | The stress and cracks on the claws are usually not visible to the naked eye |
| Weld the claw to the ring | The heating speed of the claw during welding is too fast | Thermal stress may cause fractures |
| Weld the claw to the ring (Quenching cracks) | Quenching of the workpiece is too early after welding | External cooling is fast, while central cooling is slow, leading to inconsistent thermal contraction, causing Stress and cracks occur in the insert claw |
| Creating pits on the insert claw | Overheating occurs due to improper operation | Causes brittle fracture and cracks in the prong setting |
| Press the prong clamp onto the surface of the gemstone | Causes changes in the grain structure of the prong setting | Generates residual stress micro-cracks and eventual fracture |
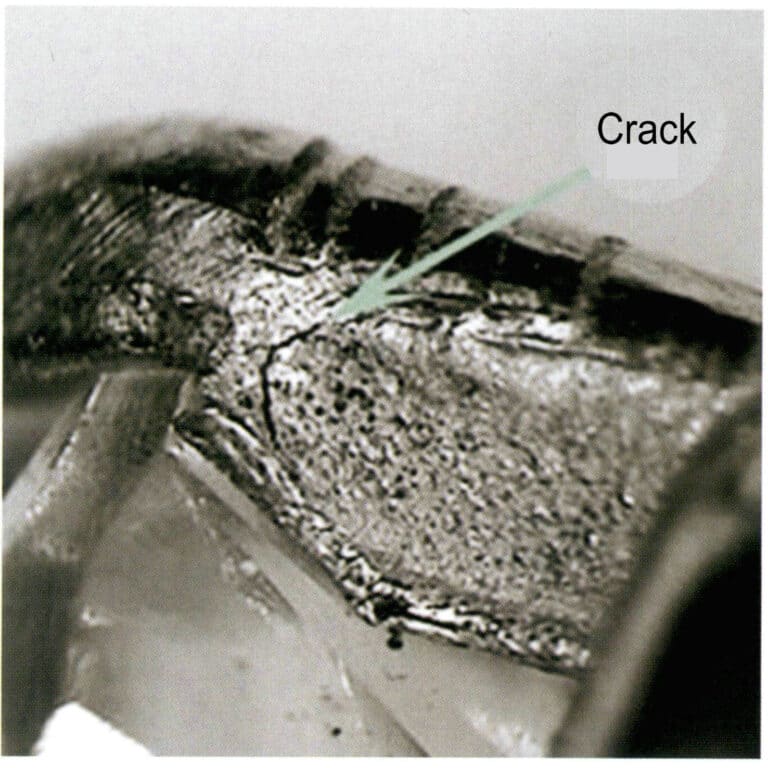
On the one hand, residual stress reduces the electrode potential of the alloy, decreasing the material’s corrosion resistance, while the prong itself is relatively thin, which can even lead to stress corrosion cracks; on the other hand, residual stress can cause micro-cracks (exposed or latent), as shown in Figure 3-37.
These micro-cracks are not easy to detect; they are often places where corrosive media accumulate. During the use of jewelry, dirt, such as sebum, skin flakes, and dust, usually adheres to the inside of the prongs (Figure 3-38). When jewelry comes into contact with various corrosive media, such as human sweat, tap water, chlorine in swimming pools, and different salts, this sebum, Skin flakes can easily absorb corrosive liquids or residual salts. Under these corrosive media, areas with high stress become anode zones, leading to electrochemical corrosion, which weakens the material and can even cause it to break. The higher the concentration of the corrosive media, the longer the contact time, the higher the temperature, and the thinner the prongs, the faster the weakening of the prongs occurs, exacerbating the stress corrosion cracking and leading to failure.
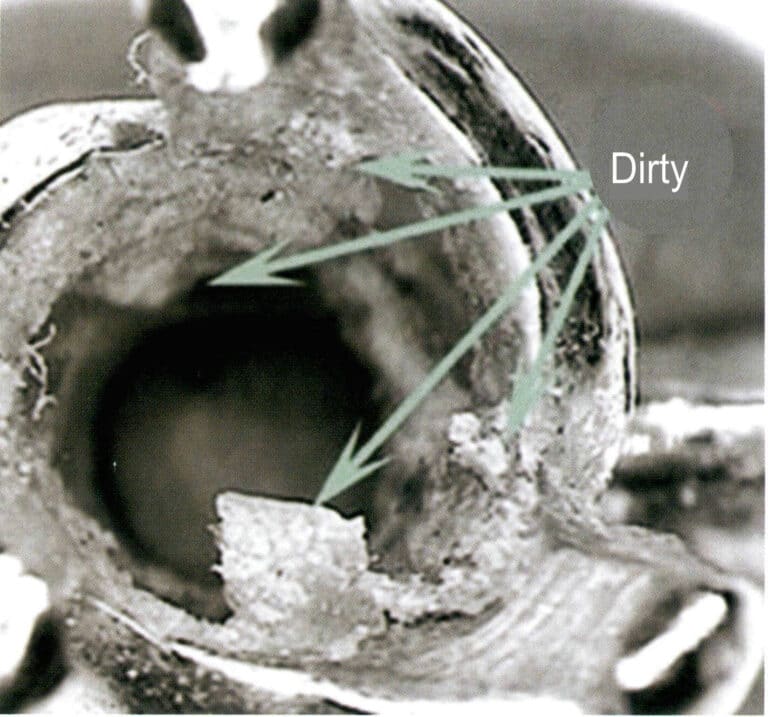
To effectively prevent stress corrosion cracking of nickel K white gold, it is necessary to prioritize materials that are not highly sensitive to stress corrosion. During production, efforts should be made to eliminate residual stress and micro-cracks in the material. During use, it is also essential to regularly clean the jewelry to reduce the accumulation of corrosive media in sensitive areas.
5.6 Casting Defect Issues
Casting nickel K white gold has specific difficulties compared to K gold and silver alloys. Enterprises often encounter casting defects during production, such as sand holes, hard spots, gas holes, shrinkage cavities (porosity), and hot cracks, among which hard spots and gas shrinkage issues are more prominent.
(1) Hard Spot Issues
A hard spot refers to the presence of a foreign object with very high hardness on the surface or inside of nickel K white gold jewelry castings, commonly known as steel sand or gold dross (Figure 3-39). This is a typical complex spot defect found in nickel platinum.

Jewelry castings with hard spots often exhibit severe scratches during polishing, making it very difficult to achieve a bright surface. This issue is usually discovered only in the final polishing stage, forcing jewelry manufacturing companies to spend much labor on repairs, especially for small, dispersed hard spots. Points often take much time; ultimately, the jewelry is scrapped because it is tough to repair satisfactorily.
Hardpoints mainly come from the following aspects:
Ni segregation.
This is primarily caused by incomplete melting and uneven stirring. Due to the high melting point of Ni and its lower density than gold, if the melting time is too short or stirring is not carefully done, Ni segregation is likely to occur, leading to the formation of complex points.
Formation of Ni2Si intermediate compounds.
This occurs when Si in the alloy reacts with Ni, and Ni2Si is a high-hardness dense intermetallic compound. The higher the Si content in the alloy, the greater the likelihood of Ni2Si appearing. When sulfur dioxide gas is present in the molten metal, it exacerbates the reaction between Ni and Si.
Si oxidation forms SiO2.
When nickel-platinum alloys containing Si are smelted, if they are in an oxidizing atmosphere or at excessively high smelting temperatures, Si’s strong reactivity leads to preferential oxidation, making it easy to form SiO2, especially when there is a small amount of metal liquid remaining in the crucible. The subsequent smelting is carried out directly, resulting in a more severe oxidation of Si.
Segregation of grain refinement agents.
Adding Ir, Co, and REE to nickel K white gold can form high melting point heterogeneous nuclei, increasing the number of nuclei and refining the grains. The alloying of these elements is relatively tricky, and improper smelting temperature, time, and operating processes can easily lead to segregation and the formation of tricky spots.
Complex foreign objects mixed in from the outside.
This includes multiple aspects, such as using contaminated materials and smelting tools carrying foreign objects.
Therefore, during production, it is essential to prioritize materials that are not sensitive to complex spot defects. In the casting process, it is necessary to strengthen the management of raw materials and smelting tools and equipment, establish reasonable operating process specifications, and strictly implement them.
(2) Air shrinkage problem
The nucleation and growth of crystals manifest in the solidification of metals. Due to the multi-component nature of alloys and the influence of thermal flow, the initial crystal growth of metals often exhibits dendritic shapes, with residual molten metal between the dendrites. If the molten metal does not wet the mold or there is external Air pressure, the residual molten metal will be pushed away from the surface, leaving behind a dendritic skeleton, forming a typical Air shrinkage porosity defect (Figure 3-40).

The formation of Air shrinkage porosity defects is closely related to the properties of the alloy and the casting process. In the lost wax casting of K gold jewelry, gypsum is generally used as a binder to cast powder material to form the mold. The main gypsum component is CaSO4, a relatively poor thermal stability material. It undergoes thermal decomposition at high temperatures and releases sulfur dioxide Air, leading to defects such as pores and Air shrinkage in the jewelry castings. For nickel K white gold, since Ni raises the alloy’s melting point, the alloy needs to be cast at higher temperatures, increasing the likelihood of gypsum decomposition, mainly when significant oxidation occurs during the alloy melting process, forming substances such as CuO and ZnO. This further lowers the decomposition temperature of gypsum, making it easier for the castings to develop Air shrinkage porosity.
Therefore, when casting nickel K white gold jewelry, it is necessary to establish reasonable melting and casting process specifications.
Section Ⅳ K red gold
K red gold is a red-colored gold alloy, known in English as karat red gold, commonly referred to as KR in the jewelry industry, such as 18KR and 14KR. Among the K gold jewelry material series, K red gold has become a fashionable trend in today’s international jewelry industry due to its elegant and luxurious color, compared to the vibrant K yellow gold and shiny K white gold. Industry insiders have given this material a romantic name based on its unique color, calling it “rose gold,” representing the eternal theme of humanity’s love. Many internationally renowned jewelry and watch brands, such as Cartier, Chanel, Piaget, Titoni, Jaeger-LeCoultre, and Girard-Perregaux, have all launched multiple series of rose gold jewelry and watches, making K red gold one of the popular K gold jewelry theme materials worldwide. Due to traditional customs favoring red in China, rose gold has gained even more market favor and has developed rapidly.
1. Impact of Alloying Elements on the Properties of K red gold jewelry
1.1 The impact of alloy elements on the color of K red gold jewelry
Among all known chemical elements, Cu is the only element that appears red, making it the most basic and primary alloy element in K red gold. According to the alloy color zone diagram in Figure 3-15, Au-Ag-Cu, the higher the Cu content, the redder the K gold color. Taking 18K red gold as an example, when Cu is the only alloy element, the red color of K red gold jewelry is the best, but the brightness value of the alloy is the lowest. Different alloy element ratios will mainly impact the color of K red gold jewelry. After adding white-toned alloy elements such as Ag and Zn, there will be a bleaching effect on the color of K red gold, gradually lightening the red color of the alloy but increasing its brightness. When the total content of Ag and Zn increases to 7%, and the Cu content decreases to around 18%, the color of the alloy appears pink, commonly known as “rose gold.” When the total content of Ag and Zn increases to 10%, and the Cu content decreases to around 15%, the color of the alloy turns yellow. Therefore, for 18K red gold, to achieve a certain degree of redness, the Cu content in the alloy should not be lower than 15%; otherwise, the alloy cannot be classified as K red gold; for 14K red gold, since the Au content is reduced, the Cu content can be lowered somewhat, but it should not be lower than 27%.
1.2 The impact of alloy elements on the structure of K red gold
K red gold is based on the alloy system Au-Ag-Cu, with a very high Cu content. According to the conversion ratio of Ag and Cu content Ag, the Ag of K red gold is very small, belonging to type I in the Au-Ag-Cu alloy. This alloy is a single solid solution at high temperatures. When the temperature drops to a specific value, different intermediate phases will arise based on the various compositions of the alloy. These intermediate phases are characterized by atomic arrangements that exhibit short-range or even long-range order, an ordering transformation in materials metallurgy.
The typical ordered structures include CuAu I type, CuAu II type, and Cu3Au I type, which occur in different composition ranges and temperature intervals. The binary phase diagram Au-Cu in Figure 3-11 shows that the CuAu I type ordered structure and the CuAu II type ordered structure occur within the composition range equivalent to CuAu, with the former forming below 385℃. The Cu atoms and Au atoms are arranged in layers on the 001 crystal plane, with one layer entirely composed of Au atoms, while the adjacent layer is wholly composed of Cu atoms (Figure 3-41)
a. Lattice constant
Due to the smaller size of Cu atoms, the original face-centered cubic lattice is distorted to form a tetragonal lattice of c/a = 0.93; the latter forms between 385℃ and 410℃, representing a long-period structure with an orthorhombic lattice. Its unit cell is equivalent to aligning 10 CuAu I unit cells in parallel along b. After five small unit cells, the type of atoms on the (001) plane changes, meaning the plane initially composed entirely of Au atoms becomes Cu atoms. In contrast, the plane originally composed of Cu atoms becomes entirely Au atoms, thus creating an anti-phase domain boundary at the halfway point of the extended unit cell (Figure 3-42). The third type is an alloy with a composition equivalent to Cu3Au slowly cooled to below 390℃. In the formed structure, Au and Cu atoms are arranged orderly, with Au atoms located at the corners of the face-centered cubic unit cell, while Cu atoms occupy the face-centered positions. The atomic ratio is 3:1, resulting in a Cu3Au I-type ordered structure. Regardless of the ordered structure’s form, it significantly impacts the mechanical properties of Au-Cu alloys. The presence of lattice distortion and ordered domain boundaries increases the resistance to Plasticity deformation of the material, significantly enhancing the strength and hardness of the alloy, but considerably reducing the material’s flexibility, leading the alloy to exhibit obvious brittleness.
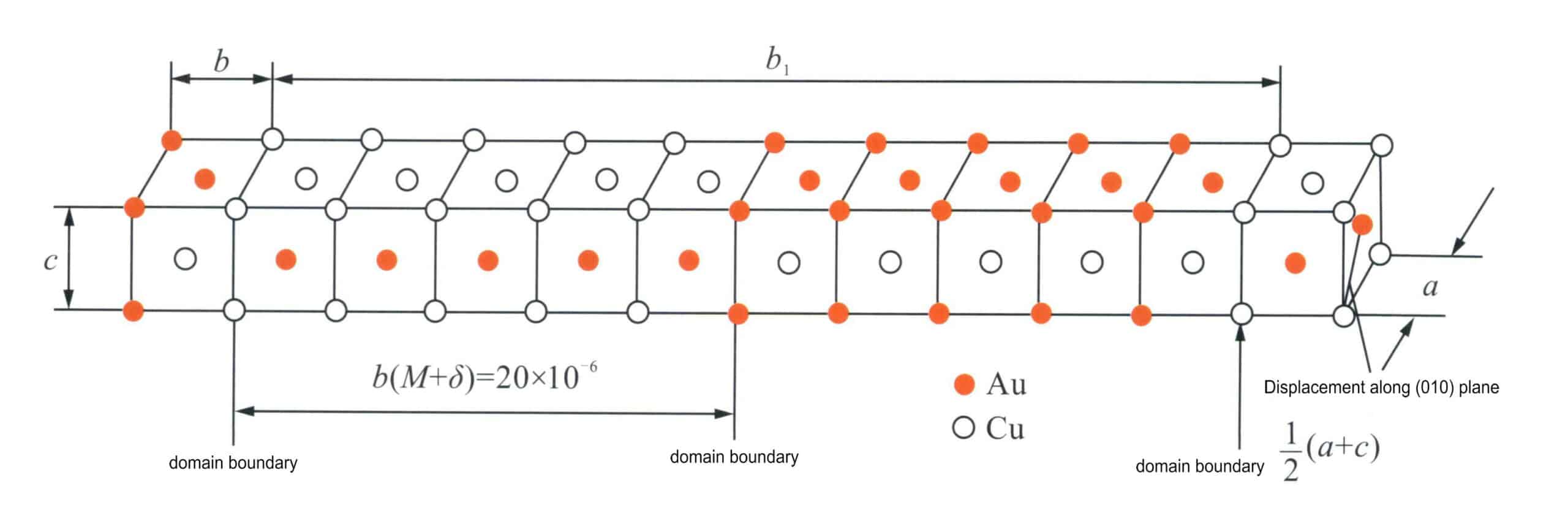
a,b,c, Lattice constant; b1. 10 CuAu I unit cells arranged side by side in the horizontal direction;
M. Half-period of the superlattice; δ. The slight expansion generated in the horizontal direction (from left to right)
The composition of K red gold significantly affects the sensitivity to the ordering transition and the degree of transition. Although ordering transitions can occur over a relatively wide range of compositions, the highest degree of order is only achieved when the corresponding composition ratios meet these ordered structures. Suppose the alloy composition deviates from the ideal composition ratio. In that case, it cannot form a wholly ordered solid solution, only a partially ordered one, thereby improving the performance of the alloy to a certain extent. Therefore, when designing the composition of K red gold, one should not simply use Cu elements for alloying but add a certain amount of other alloying elements to make the Au, Cu atomic ratio deviate from the ideal composition. Although the reduction of Cu components slightly weakens the red color of the alloy, it is beneficial for the processing performance of the alloy and the controllability during the production process.
1.3 The effect of alloying elements on the casting performance of K red gold
The high Cu content in K red gold makes it prone to defects such as oxidation inclusions, pores, and shrinkage during casting. Therefore, adding some alloying elements that help improve casting performance in K red gold is often necessary, such as Zn, Si, rare earths, etc. They can act as deoxidizers for K red gold, purifying the molten metal, improving smelting quality, enhancing filling capacity, reducing the surface roughness of the products, and minimizing the reaction between the molten metal and the mold, which is conducive to obtaining a brighter cast surface.
1.4 The Influence of Alloy Elements on the Processing Performance of K Red Gold
The K red gold with different alloy compositions significantly differs in casting hardness. Generally speaking, alloys with a higher Cu content have a higher casting hardness. Taking 18K red gold as an example, when the Cu content is 18%, the casting hardness is usually below HV170, while when the Cu content exceeds 21%, the initial hardness can exceed HV210. This indicates that the strengthening effect of Cu in K red gold is predominant.
The processing performance of K red gold mainly depends on the alloy composition and the organizational state. Direct rolling under casting conditions is prone to cracking. The work hardening rate is closely related to the Cu content; when the Cu content is low, it shows a relatively linear work hardening rate. As the Cu content increases to a certain level, the work hardening rate appears relatively flat in the early processing stages. In contrast, the alloy hardens rapidly in the later stages, affecting flexibility.
1.5 The Influence of Alloy Elements on the Corrosion Resistance of K red gold
K red gold mainly uses Cu as the alloying element. Cu has poorer chemical stability than the noble metal gold and is prone to react with oxygen, sulfur, etc., forming CuO or CuS. Increasing the Au content helps improve the discoloration performance of K red gold. High-quality K red gold has better resistance to discoloration from sweat than low-quality K red gold. However, the Au content is not the only factor determining resistance to dullness and discoloration. Dullness and discoloration are the combined results of chemical processes, environment, and organizational structure. Adding some oxygen-active elements to K red gold alloy can form a dense transparent oxide film on the alloy surface, which may also lead to better resistance to dullness and discoloration in K red gold.
2. Selection of K red gold Filler
The composition of the alloy plays a decisive role in its performance. When selecting K red gold filler, it is necessary to start from the performance requirements of the alloy and focus on the following aspects:
(1) Color aspect.
It should have an excellent red color and good brightness. In addition, the alloy should have good resistance to dulling, making it less likely to change color during storage and use; the alloy can be left untreated without electroplating.
(2) The alloy should have refined grains and a dense structure, exhibiting good mechanical properties.
To address the brittle fracture often caused by ordering transitions in K red gold, one should avoid the ideal composition ratio that forms the highest degree of order when designing the alloy composition.
(3) The alloy’s adaptability to different processing techniques and the operability of the processes avoid operational issues caused by a too-narrow processing range.
(4) In selecting alloying elements, the principle of broad material sources, low cost, and environmental friendliness is followed to reduce alloy costs.
3. Composition and properties of some K gold
In response to the jewelry market’s demand for K gold in different colors, the industry has developed a series of pink-red K gold, and based on different production process requirements, has developed K gold suitable for both casting and cold processing. The composition and properties of some K gold are shown in Table 3-16.
Table 3-16 Properties of some K gold
| Καθαρότητα | Chemical composition (wt)/% | Melting point /℃ | Density/ (g/cm3) | Soft hardness HV/(N/mm2) | Χρώμα | |||
|---|---|---|---|---|---|---|---|---|
| Καθαρότητα | Au | Ag | Cu | Zn | Melting point /℃ | Density/ (g/cm3) | Soft hardness HV/(N/mm2) | Χρώμα |
| 18K | 750 | 90 | 160 | - | 880 ~ 885 | 15.3 | 160 | Ροζ |
| 18K | 750 | 45 | 205 | - | 855 ~ 890 | 15.15 | 165 | Κόκκινο |
| 14K | 585 | 100 | 277 | 38 | 810 ~ 880 | 13.25 | 148 | Ροζ |
| 14K | 585 | 90 | 325 | - | 850 ~ 885 | 13.30 | 160 | Κόκκινο |
4. Common Issues with K Red Gold Jewelry
K red gold jewelry often encounters various issues during production and use, including the following aspects.
4.1 Brittle Fracture Issues
The brittle fracture issue of K red gold jewelry is a prominent problem that jewelry manufacturing companies frequently encounter when producing K red gold jewelry. A typical form of this crack, made of 18KR material and using conventional K red gold filler materials, shows multiple occurrences of such cracks after casting, with no Plasticity deformation near the fracture, presenting a typical brittle fracture phenomenon Figure 3-43.
Practice shows that the brittleness fracture of red K gold alloy jewelry occurs in both 14KR and 18KR, with the brittleness fracture being particularly prominent in 18KR. Moreover, it does not only appear during the cooling process of casting; it can also make the material brittle during subsequent annealing, welding, and even during the cooling process of the stone-setting fire lacquer procedure. This can cause the jewelry to fracture with slight external force or impact. When the brittleness is severe, the alloy can break easily like dry twigs, unlike the ductile and Plasticity properties of suitable precious metal alloys, causing significant trouble and processing difficulties for jewelry manufacturing enterprises.
The main factors leading to the brittleness fracture of K red gold jewelry are as follows:
(1) The influence of alloy composition.
The binary alloy phase diagram in Figure 3-11 shows that when the Cu content is between 30% and 80%, during the casting process after casting, when the temperature is above 410℃, the Au-Cu binary alloy is completely soluble. When the temperature drops below 410℃, depending on the different compositions of the alloy, it will produce different degrees of ordering transformation that reduces the flexibility of the material, making the alloy brittle. Therefore, choosing materials with a relatively low degree of ordering transformation is preferable when selecting K red gold for repairs.
(2) The influence of cooling rate.
Like other metal materials, K red gold materials experience thermal stress during the cooling process from high temperature to low temperature, especially during rapid cooling, which is more likely to produce significant thermal stress, leading to deformation or even cracks in the jewelry. Therefore, a slow cooling method is generally adopted to reduce thermal stress in processing K gold and K white gold jewelry. However, if this method is used in processing K red gold jewelry, the jewelry is prone to organizational stress due to the ordering transformation. The transition of K red gold from disorder to order does not occur instantaneously; it is a process that relies on atomic migration and rearrangement. Since atomic diffusion and migration require time, it is evident that if K red gold is rapidly cooled from a temperature above the critical transformation temperature to room temperature, it will suppress the occurrence of the ordering process and may even retain the disordered state at high temperatures.
Therefore, in the processing of K red gold, it is not enough to adopt a slow cooling method to reduce thermal stress; the key is to minimize the sum of thermal stress and structural stress. Additionally, during the molding process of jewelry, it is often necessary to perform repair welding on the ornaments or to weld components together; when setting the jewelry, it is expected first to fix the ornaments with fire lacquer, which requires heating the workpiece. Even if no ordering transformation occurs during casting, in subsequent processing, such as slow cooling after heating or maintaining a temperature below the critical temperature for a certain period, ordering transformations will still occur. Therefore, in the subsequent processing of K red gold castings, attention should be paid to the heating temperature range and the cooling rate after heating. Laser welding can repair small sand holes on ornaments or welding connection points if conditions permit to avoid the risk of brittle fracture caused by forming an ordered solid solution after the alloy is heated.
(3) The impact of deformation processes.
In jewelry making, mechanical stamping or hydraulic pressing is commonly used for forming. If the alloy material has already undergone a certain degree of ordering transformation, the material’s flexibility will be significantly affected. It may induce cracks when combined with the effects of work hardening during the deformation process. Therefore, when processing K red gold, the ingot should undergo solution treatment to form a uniformly composed single-phase solid solution. During processing, the material will undergo work hardening, which reduces the material’s flexibility, so intermediate annealing treatment is necessary to eliminate the stress generated during processing.
4.2 Color issues
In K red gold jewelry, the overall expectation is for the alloy to have a good red color. It is well known that among all known chemical elements, only a few metal elements are colored, such as Au, which appears golden yellow; Cu, which appears reddish; Bi, which appears light red; and Ce, which seems light yellow, while the rest of the metal elements are mostly gray-white or silver-white. Cu has a significant impact on the mechanical properties of gold; therefore, Cu is the most basic and primary alloying element for achieving red K gold. The higher the Cu content, the redder the K gold.
For jewelry, the commonly used purities are 18K and 14K. If it is merely an Au-Cu binary alloy, the alloy will present a slightly dull red color and is prone to producing oxidation inclusions during casting. During the cooling process after casting, it is straightforward to undergo ordering transformations, leading to the brittleness of the alloy.
To achieve better processing and casting performance, other alloying elements besides Cu are often added to K gold, which can result in a relatively lighter red color. Some companies sometimes electroplate a layer of K gold on the surface of jewelry, which can achieve a bright and uniform rose-red color, providing an excellent decorative effect. However, once the plating wears off, it can easily create a color contrast that affects the appearance.
4.3 Dullness and discoloration issues
K gold jewelry tends to become dull and discolored after being used or placed for some time, losing its initial brightness and luster.
Taking 18KR as an example, it was soaked in artificial sweat for corrosion testing, measuring the color coordinate values before and after the test and calculating their changes and color difference values, as shown in Table 3-17. It can be seen that as the corrosion time increases, the brightness value L* continuously decreases. In contrast, the a* and b* values rise, and the color difference gradually increases. This indicates that the surface of the alloy gradually becomes dull, and the color gradually turns yellow and red. In the initial 24 hours of corrosion, the rate of change in brightness and chroma values of the alloy is relatively fast, especially the yellow-blue index changes quickly. After 24 hours, the changes in color coordinates stabilize.
Table 3-17 Color coordinate values and changes of 18KR after soaking in artificial sweat for different durations
| Soaking Time | L* | a* | b* | △L* | △a * | △b* | Color Difference △E |
|---|---|---|---|---|---|---|---|
| 0 hours | 85.97 | 9.6 | 18.15 | 0.00 | 0.00 | 0.00 | 0.00 |
| 24 hours | 85.56 | 10.04 | 19.48 | -0.41 | 0.44 | 1.33 | 1.46 |
| 48 hours | 85.31 | 10.29 | 19.75 | -0.66 | 0.69 | 1.6 | 1.86 |
| 72 hours | 85.24 | 10.43 | 19.82 | -0.73 | 0.83 | 1.67 | 2.00 |
The darkening and color change of K gold is closely related to the material properties, and of course, it is also related to the manufacturing process and usage conditions. Some K gold can improve its corrosion resistance for alloy materials by adding a small amount of alloying elements, effectively enhancing its ability to resist darkening and color change. Such K gold should be prioritized during production.
Section V Decorative Gold Solder
1. Performance Requirements for Decorative Gold Solder
Welding is the most commonly used process in jewelry manufacturing. For gold jewelry, the main welding methods are fusion welding and brazing. Fusion welding involves heating the workpiece interface to a molten state without applying pressure to complete the welding. For high-purity gold jewelry welding, such as 24K gold necklaces, fusion welding is generally used directly to ensure the color quality of the welded area. Brazing uses a metal material with a lower melting point than the workpiece as the filler, heating the workpiece and filler to a temperature above the filler melting point and below the workpiece melting point, allowing the liquid filler to wet the workpiece, fill the interface gap, and achieve atomic diffusion with the workpiece, thus completing the welding. Brazing is widely used in most jewelry welding processes, where the filler is the basis for ensuring welding quality. The so-called filler refers to the material used to firmly fill the connection points of jewelry components to bond the workpieces. Decorative gold brazing filler is an alloy material composed of gold as the base and other alloying elements added for welding filling, and it is also an essential component of gold jewelry.
The following requirements generally apply to gold jewelry brazing filler:
(1) The gold content in the solder should be consistent with the jewelry to ensure the color quality requirements.
(2) The solder should have good welding performance.
The melting range of the solder is relatively tiny, and it flows well after melting, wetting the metal body well, making it easy to weld and penetrate small seams. The weld zone has a dense structure, combines well with the metal body, and is not prone to defects such as pores and inclusions.
(3) The solder should have good physical and chemical properties.
It should be comparable to the welded metal body in color, corrosion resistance, and other aspects.
(4) Since jewelry welding often has many dispersed weld points, multiple welding is required to complete the assembly and repair defects of the entire piece. This not only requires the melting point of the solder to be lower than the lowest melting point of the metal body but also requires that the solder consist of a series of solders with different melting points. In the subsequent welding, the melting point of the solder should be lower than that of the previous solder, which requires the formation of what is commonly referred to in the industry as high solder, medium solder, and low solder.
(5) The solder should have good mechanical properties and processing performance.
In the jewelry industry, solder is often rolled into thin sheets or drawn into fine wires for use, requiring the solder to have good cold deformation properties and mechanical properties similar to the metal body at the welding site so as not to cause brittle fracture in the welding area.
(6) The solder is safe and friendly, avoiding toxic elements such as Cd and Pb.
Cd is a traditional alloying element in K gold jewelry solder, which can effectively lower the melting point of Au-Ag-Cu series alloys and improve the fluidity and filling ability of the solder. The mechanical properties of Cd-containing solder are excellent. However, because it quickly generates harmful CdO fumes to the human body when melted, it has contact toxicity and should be restricted in use. According to the national standard “Regulations on the Limit of Harmful Elements in Jewelry” (GB 28480-2012) and the EU RoHS Directive (2005/618/EC) (1) , the total content of harmful elements in gold jewelry solder must not exceed the maximum limit specified by the standard, namely Cr (hexavalent), Hg, Pb content below 1000mg/ kg, Cd content below 100mg/kg, and Ni release below 0.2/ug/ (cm2⸳week).
2. Formulation of K Gold Jewelry Solder
K gold jewelry solder is prepared from Au-Ag-Cu or Au-Ag-Cu-Zn series alloys. Using Au-Ag-Cu series alloy solder ensures that the composition of the solder alloy is the same as that of K gold jewelry alloy, maintaining consistency in composition and color.
However, the melting point of the solder alloy must be lower than the melting point of K gold jewelry alloy, so the ratio of Ag to Cu in the Au-Ag-Cu series alloy must be adjusted to lower the melting point of the solder. Low melting point alloying elements such as Zn, Sn, In, and Ga can be added if further melting point reduction is needed. Among them, Zn has a solubility of up to 33.5%(at) in Au, In, and Ga, around 12%(at), and the limit solubility of Sn is 6.8%(at). Therefore, adding a small amount of In, Ga, Sn to Au can significantly lower the liquid phase temperature of the alloy, but adding too much will reduce the solidus line, expand the melting range, and cause adverse effects. Table 3-18 lists K gold solders’ compositions and melting ranges.
Table 3-18 Composition and Melting Range of Some K Gold Solder Alloys
| Καθαρότητα | Solder Type | Chemical composition (wt)/% | Solidus Line Temperature /℃ | Liquidus line Temperature /℃ | Melting Point /℃ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Καθαρότητα | Solder Type | Au | Ag | Cu | Zn | In | Ga | Solidus Line Temperature /℃ | Liquidus line Temperature /℃ | Melting Point /℃ |
| 9K | Low temperature | 37.5 | 31.88 | 18.13 | 8.12 | 3.12 | 1.25 | 637 | 702 | 65 |
| 9K | High temperature | 37.5 | 29.38 | 19.38 | 10.62 | 2.5 | 0.62 | 658 | 721 | 63 |
| 14K | Low temperature | 58.34 | 13.33 | 15.00 | 8.75 | 4.58 | - | 669 | 741 | 72 |
| 14K | Medium temperature | 58.34 | 14.49 | 14.25 | 9.17 | 3.75 | - | 660 | 745 | 85 |
| 14K | High temperature | 58.34 | 14.16 | 14.58 | 10.00 | 2.92 | - | 668 | 748 | 80 |
| 18K | Low temperature | 75.00 | 6.25 | 8.50 | 5.50 | 4.75 | - | 730 | 765 | 35 |
| 18K | Medium temperature | 75.00 | 5.75 | 9.50 | 6.00 | 3.75 | - | 682 | 767 | 85 |
| 18K | High temperature | 75.00 | 5.25 | 12.25 | 6.50 | 1.00 | - | 792 | 829 | 37 |
| 22K | Low temperature | 91.80 | 2. 40 | 2.00 | 1.00 | 2.80 | - | 850 | 890 | 40 |
| 22K | Medium temperature | 91.80 | 3.00 | 2.60 | 1.00 | 1.60 | - | 895 | 920 | 25 |
| 22K | High temperature | 91.80 | 4.20 | 3.00 | 1.00 | - | - | 940 | 960 | 20 |
3. Preparation of Nickel K white gold Soldering Alloys
The varieties and quantities of Nickel K white gold solder are relatively few, mainly consisting of the following alloy systems:
(1) Au-Cu-Ni-Zn series alloys.
Nickel 18K white gold solder is primarily based on low melting point alloy components from the Au-Ni series. However, for most white K gold jewelry, the melting point of Au-Ni solder alloys
It is still relatively high, making it unsuitable for direct use as solder. It requires adding other components, such as adding Zn to lower the melting point of the solder and adding Cu to improve workability, forming the Au-Cu-Ni-Zn solder alloy.
(2) Au-Ag-Cu-Ni-Zn alloy.
Low gold content white K gold solder can use Au-Ag-Cu-Ni-Zn alloy, with Ni and Zn as bleaching agents to increase the Ag content as solder alloy.
Common Au-Cu-Ni-Zn or Au-Ag-Cu-Ni-Zn series K white gold jewelry solder formulas are shown in Table 3-19. Commercial solders come in forms such as solder sheets, solder wires, solder powders, and solder pastes. Figure 3-44 shows a typical color K gold solder sheet.
Table 3-19 Common nickel K white gold jewelry solder formulas
| Solder Appearance | Chemical composition (wt)/% | Melting Temperature Range /℃ | |||||
|---|---|---|---|---|---|---|---|
| Solder Appearance | Au | Ag | Cu | Ni | Zn | Melting Temperature Range /℃ | |
| 18K | 75.00 | - | 1.00 | 16.50 | 7.50 | 888 ~ 902 | |
| 18K | 75.00 | - | 6.50 | 12.00 | 6.50 | 803 ~ 834 | |
| 14K | 58.33 | 15.75 | 11.00 | 5.00 | 9.92 | 800 ~ 833 | |
| 14K | 58.33 | 15.75 | 5.00 | 5.00 | 15.92 | 707 ~ 729 | |
| 10K | 41.67 | 30.13 | 15.10 | 12.00 | 1.10 | 800 ~ 832 | |
| 10K | 41.67 | 28.10 | 14.10 | 10.00 | 6.13 | 736 ~ 784 | |
| 8K | 33.30 | 42.00 | 10.00 | 5.00 | 9.70 | 721 ~ 788 | |