Ποιες είναι οι βασικές μέθοδοι και εφαρμογές της επιπλατινωσης με πλατίνα στη σύγχρονη βιομηχανία;
Platinum Plating Techniques: Solutions, Alloys, and Applications for Jewelry
Εισαγωγή:
Wondering about platinum plating? This guide covers everything from the basics to advanced techniques. Learn about different plating solutions, including chloride and sulfate-based options, and discover how to improve them. Explore thin and thick plating solutions for various applications. Intrigued by platinum alloys? We cover popular ones like Pt-Au, Pt-Co, and Pt-Ir. Plus, dive into chemical plating for unique applications. Whether you’re a jewelry designer, retailer, or custom maker, this comprehensive overview will help you enhance your products with platinum plating.

Πίνακας περιεχομένων
Section I Overview
Platinum has an atomic number of 78 in the periodic table, with the element symbol Pt, a relative atomic mass of 195.7, a density of 21.09g/cm3 (20℃), and a melting point of 1768℃.
Some main parameters of platinum are shown in Table 3-1.
Table 3-1 Some Main Parameters of Platinum
| Χαρακτηριστικές παράμετροι | Χαρακτηριστική τιμή |
|---|---|
|
Όνομα στοιχείου, σύμβολο στοιχείου, ατομικός αριθμός Ταξινόμηση Ομάδα, Περίοδος Πυκνότητα, σκληρότητα Χρώμα Σχετική ατομική μάζα Ατομική ακτίνα Ακτίνα ομοιοπολικού δεσμού Χημική σθένους Κρυσταλλική δομή σημείο τήξης σημείο βρασμού Θερμότητα εξάτμισης Θερμότητα διάλυσης Ειδική θερμοχωρητικότητα Αγωγιμότητα Θερμική αγωγιμότητα |
Platinum、Pt、78 Transition Metal 10(Ⅷ),6 21090kg/m3, 3.5 Grayish white 195.084 135μμ 128pm 2、4 Κυβικός με κέντρο το πρόσωπο 2041. 4K( 1768.3℃) 4098K (3825℃) 510kJ/mol 19:6kJ/mol 130J/(kg • K) 9. 66X 106m ・Ω 71. 6W/(m ・ K) |
Section II Electroplating of Platinum
Table 3-2 Industrial Applications of Pt Plating Coatings
| Product | Υλικά | Πάχος επιμετάλλωσης/μm | Product | Υλικά | Πάχος επιμετάλλωσης/μm |
|---|---|---|---|---|---|
|
Aerospace Components Aviation Components Safety Bulkhead Trays Electrodes |
Niobium-containing superalloys SUS347 Τιτάνιο SUS316 |
10 10 5 10 |
Electrodes Electrodes Electrodes - |
Τιτάνιο Titanium mesh Tungsten Wire - |
2〜7 2〜7 10 - |
Table 3-3 Typical Platinum Salts
| 2, 4-valent salts | Typical platinum salts |
|---|---|
| Pt(II) salts |
Chloroplatinic acid:H2PtCl6 • 6H2O Diammine platinum nitrite:Pt(NH3)2(ΟΧΙ2)2 Platinum nitrite sulfate:H2Pt(NO2)2SO4 |
| Pt(Ⅳ) salts | Sodium hydroxyplatinate:Na2Pt(OH)6 · 2H2O |
1. Various Platinum Plating Solutions
Table 3-4 Various Pt Plating Solution Compositions and Process Conditions
| Σύνθεση και συνθήκες διεργασίας | Chloride | Diammonium sulphite | DNS | Hydroxybasic salts | Φωσφορικό οξύ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Νο. 1 | Αρ. 2 | Αρ. 3 | Αρ. 4 | Αρ. 5 | Αρ. 6 | Αρ. 7 | Αρ. 8 | No. 9 | No. 10 | No. 11 | No. 12 | No. 13 | No. 14 | |
| Chloroplatinic acid H2PtCl6/(g/L) | 10 〜50 | |||||||||||||
| Ammonium chloroplatinate (NH4)2PtCl6/ (g/L) | 15 | |||||||||||||
| Diammine platinum nitrite Pt(NH3)2(ΟΧΙ2)2/(g/L) | 8~16. 5 | 20 | 6~20 | 8 | 6~20 | 16.5 | ||||||||
| Platinum nitrite sulfate H2Pt(NO2)2SO4/ (g/L) | 10 | |||||||||||||
| Sodium hydroxyplatinate Na2Pt(OH)6 ・ 2H2O/(g/L) | 20 | 18.5 | ||||||||||||
| Hydroxyplatinic acid H2Pt (OH)6/ (g/L) | 20 | |||||||||||||
| Potassium hydroxyplatinate K2Pt(OH)6/ (g/L) | 20 | |||||||||||||
| Platinum chloride PtCl4· 5H2O/(g/L) | 7.5 | |||||||||||||
| Ammonia(28%)/(g/L) | ||||||||||||||
| Hydrochloric acid/(g/L) | 180~300 | |||||||||||||
| Κιτρικό νάτριο/(g/L) | 100 | 20~25 | ||||||||||||
| Ammonium chloride/(g/L) | 4~5 | |||||||||||||
| Ammonium nitrate/(g/L) | 100 | |||||||||||||
| Sodium nitrite/(g/L) | 10 | |||||||||||||
| Fluoroboric acid/(g/L) | 50~100 | |||||||||||||
| Sodium fluoborate/(g/L) | 80~120 | |||||||||||||
| Sulfonic acid/(g/L) | 20~100 | |||||||||||||
| Phosphoric acid/(g/L) | 80 | 10~100 | ||||||||||||
| Sulfuric acid/(g/L) | 10~100 | pH2 | ||||||||||||
| Sodium acetate/(g/L) | 70 | |||||||||||||
| Sodium carbonate/(g/L) | 100 | |||||||||||||
| Sodium hydroxide/(g/L) | 10 | 5.1 | ||||||||||||
| Sodium oxalate/(g/L) | 5.1 | |||||||||||||
| Sodium sulfate/(g/L) | 30.8 | |||||||||||||
| Potassium hydroxide/(g/L) | 15 | |||||||||||||
| Ammonium Hydrogen Phosphate)(g/L) | 20 | |||||||||||||
| Sodium hydrogen phosphate/(g/L) | 100 | |||||||||||||
| Potassium sulfate/(g/L) | 40 | |||||||||||||
| Θερμοκρασία διαλύματος επιμετάλλωσης/°C | 45~90 | 80~90 | 90~95 | 70~90 | 65~100 | 75~100 | 75~100 | 80~90 | 30~70 | 75 | 65~80 | 75 | 70~90 | 70~90 |
| Πυκνότητα ρεύματος/(A/dm2) | 3.0 | 0.5~1.0 | 0.3~2.0 | 2~5 | 0.2~2 | 0.5~0.3 | 0.5~0.3 | 0.5 | 2.5 | 0.8 | 0.8 | 0.75 | 0.3~1 | 0.3~1 |
| Τρέχουσα απόδοση/% | 15~20 | 70~10 | 10 | 14~18 | 15 | 15 | 15 | 35~40 | 10~15 | 100 | 80 | 100 | 10~50 | 15~50 |
(1) Chloride plating solution
The first technically successful Pt plating solution used chloroplatinic acid (H2PtCl6・6H2O) as the base salt. A soluble Pt electrode was used, and its conditions were 10~15g/L chloroplatinic acid, 180~300g/L hydrochloric acid, plating solution temperature of 45~90℃, current density of 2.5~3.5A/dm2, and cathode current efficiency of 15%~20%. The plating film obtained from this solution can reach 20μm with no cracks and good ductility. However, the pH must be controlled within a narrow range to prevent hydrolysis of the plating solution. When the pH of the plating solution begins hydrolysis it reaches 2.2.
(2) Diamminonitrite Plating Solution
To ensure the concentration of divalent Pt and prevent it from oxidizing to Pt(Ⅳ), an appropriate amount of amine compounds needs to be added to form a complex with Pt (II). The basic component of this plating solution is diamminonitritoplatinum Pt(NH3)2(ΟΧΙ2)2, often referred to as Pt-P salt (II). The plating solution using this salt was discovered by W. Keitel in 1931 (plating solution No. 3 in Table 3-4). When the nitrite concentration in the solution increases, it affects the dissociation of the Pt complex, thereby influencing the behavior of the plating solution. After boiling, NH4OH is added to react with NaNO3 to generate NH4ΟΧΙ2 to restore the initial current efficiency, producing, which then decomposes into nitrogen and hydrogen gases. In this way, almost all the non-metallic components of the Pt-P salt in the plating solution become gases and disappear, making the plating solution’s lifespan longer than that of chloride plating solutions. The advantage of this plating solution is that its component adjustment is relatively easy.
A. B. Triper and others used PR as the power source, achieving an electroplating speed of 5μm/h. The conditions were: 5~6A/dm2, cathode electrolysis time of 5 s and anode electrolysis time of 2 s. The plating solution No. 4 in Table 4-3 was proposed in Lacroix’s 1967 patent in France. This plating solution can produce a coating thickness of up to 7.5μm. Plating solution No. 5 is from a US patent (US PAT. 2984603, 2984604), proposed in 1961, which involves adding sulfonic acid to the Pt-P salt plating solution. No. 6 contains phosphoric acid, while No. 7 uses phosphoric-sulfuric acid as the basic solution, proposed in a 1960 French patent (Fr PAT. 1299226). They used insoluble anodes and flexibly applied crucial methods such as stirring and shaking.
No. 8 uses sodium acetate and sodium carbonate to replace ammonium salts, thereby achieving maximum current efficiency and improving the stability of the plating solution. The coating obtained from this solution is smooth and flat, with a plating thickness of up to 10μm without pinholes or cracks.
In Japan, this plating solution is widely used industrially. Below is one example:
|
Platinum(Diammineplatinum Nitrite) Νιτρικό αμμώνιο Sodium nitrite Υδροξείδιο του αμμωνίου |
10g/L 100g/L 10g/L 35g/L |
Solution Temperature Πυκνότητα ρεύματος Τρέχουσα απόδοση - |
90~92℃ 1A/dm2 10%~20% - |
(3) Platinum Nitrosulfuric Acid Plating Solution
This plating solution does not contain ammonia or amine components but uses platinum nitrosulfuric acid[H2Pt (OH)6 · 2H2O]as the basic ingredient. The preparation of the plating solution involves using nitro salts, potassium salts of platinum chloride, or platinum sulfuric acid ([K2Pt(NO2)3Cl, K2Pt (NO2)2Cl2 or K2Pt (NO2)2SO4]). A low current density is used for bright plating, and sulfuric acid is added to adjust the pH below 2.0. Representative compositions are shown in Table 3-4, No. 9. This plating solution can produce relatively thick plating layers.
(4) Alkaline Hydroxyplatinic Acid Metal Salt Plating Solution
In a typical alkaline plating solution, a sodium or potassium salt of hydroxyplatinic acid such as Na2Pt(OH)6 or K2Pt(OH)6 is used. Representative plating solution compositions are shown in Table 3-4, No. 11. The plating solution temperature of 75℃, current density of 0.8A/dm2, and current efficiency can reach 100%, and the anode uses Ni or stainless steel materials.
No. 10 was proposed by A.R. Powell in 1913, and a British patent was obtained (Brit PAT. 363569). A bright coating comparable to the Rh plating solution can
be obtained from this plating solution. When the Pt concentration is below 3g/L, the current efficiency drops sharply. A current density can be up to 2.5A/dm2 when the concentration is high (12g/L ). At a solution temperature of 65 ~ 70°C, the current efficiency can reach about 80%. However, further increasing the temperature does not significantly improve the effect.
(5) Phosphate Plating Solution
As early as 1855, Roseleuer proposed the phosphate scheme. This plating solution uses a tetravalent Pt chloride coordination salt, alkali metal phosphate salts, and ammonium salts as conductive salts. In 1949, W. Pfanhauser proposed the No. 14 plating solution, which, under these conditions, can produce a coating of 0.5μm.
Druve reported experimental results using the same plating solution. The biggest drawback of this plating solution is the difficulty in adjustment. Precipitates formed when newly prepared plating solution must dissolve over a long time. Ammonium phosphate must be used to avoid porous and spongy coatings. Ammonium phosphate helps dissolve the platinum complex. Under certain conditions, an insoluble yellow salt forms on the anode surface in the plating solution, becoming an insulating layer estimated to be ammonium hydroxyplatinate salt.
(6) Sulfate-Based Platinum Plating
Plating platinum on titanium or tantalum is not problematic even if it is not bright, but when plating platinum on decorative items, brightness becomes an important issue, and cracks are also a problem that cannot be ignored. Masashi and others proposed using a sulfate plating solution to address this issue. The characteristics of this plating solution are dissolving platinum salt into sulfate, adding sulfite to the solution, and adjusting the pH to less than 2 with sulfuric acid. Because the addition of sulfite can make the platinum potential more negative than hydrogen ions, it ensures a low hydrogen content in the platinum plating layer, resulting in low internal stress and brightness in the plating layer. However, if the sulfite concentration is too high, platinum may be reduced. If pH>2, the sulfite is easily hydrolyzed. Also pH<2 will help stabilize the platinum complex.
The pretreatment for plating is alkaline→electrolytic degreasing→acid dipping, and 2min cathodic electrolysis.
The plating process is shown in Table 3-5.
Table 3-5 Platinum Plating Process Conditions in Sulfuric Acid Series
| Σύνθεση και συνθήκες διεργασίας | Νο. 1 | Αρ. 2 |
|---|---|---|
|
HAuCl4 (counted as Au) K2SO4 K2SO3 pH (adjusted with sulfuric acid) temperature Πυκνότητα ρεύματος Plating time Πάχος επιμετάλλωσης Στρώμα επιμετάλλωσης |
10g/L 50g/L 1.0g/L 1.0 75℃ 2A/dm2 60min 7 μm Brightness |
10g/L 100g/L 2. 0g/L 2.0 65℃ 1 A/dm2 100min 5/μm Beautiful appearance, good bonding |
In Table 3-5 No.1, a dichroic coating of Pt-Au can be obtained by pre-plating flash gold on the substrate, thickly plating 7μm of platinum, and plating 2μm of gold on the platinum.
2. Thin Plating Solution
3. Thick Plating Solution
(1) Decorative Plating
As mentioned earlier, platinum-plated products such as eyeglass frames and watch cases have emerged due to the emphasis on the platinum brand itself. The plating thickness of platinum-plated products is generally below 5μm.
Recently, another new technology has emerged in the field of decorative items, which is electroforming.
The thickness of electroformed products is generally 100~150μm, and making them hollow can reduce weight and lower costs. When plating with ordinary plating solutions using conventional electroplating methods, cracks will appear once the plating thickness exceeds 10μm, making it technically challenging.
(2) Industrial Applications
The Pt plating of stainless steel parts for aviation has been put into practical use. The process is as follows:
Table 3-6 Performance of Pt Anode Materials
| Σκηνικά θέατρου | Pt | Ti | Nb | Ta |
|---|---|---|---|---|
|
Density(20℃)/(g/cm3) Melting point/°C Hardness (after heat treatment) Thermal conductivity/[W/(m·K)] Resistivity/μΩ·cm Coefficient of linear expansion (x105)/[mm/(mm·K)]
|
21. 45 1769 37〜42 (Vickers) 71. 6 10. 6 9. 1 |
4. 54 1668 120 (Brennel) 16.8 48 8. 5 |
8. 57 2468 84 (Vickers) 67. 4 13. 1 7. 1 |
16. 6 2996 E-60 (Rockwell) 54. 8 12.4 6. 5 |
Generally, the thickness of the Pt plating layer is about 2μm, so the current density is high. Under conditions such as short circuits when the cathode is contacted, and operations involving ammonium bifluoride, fluoroboric acid, strong alkalis, and high-cyanide solutions, the consumption of Pt accelerates. Therefore, extending its lifespan as much as possible is necessary, which can be achieved by increasing the anode-to-cathode area ratio. When plating Pt on Ti electrodes, the Ti can first be roughened by sandblasting, then acid-activated to remove the surface oxide film, followed by Pt electroplating.
The typical aging process of Pt-plated Ti anodes is: ① Ti oxide film at the pinhole of Pt plating is destroyed; ② Ti begins to dissolve; ③ the Pt-Ti interface experiences pitting corrosion as dissolution progresses, and the Pt film peels off. At this time, if it occurs during gold plating, it will cause a sudden increase in the deviation of the gold plating thickness. When encountering such problems in practice, inspecting the anode is best.
4. Other Improvements to the Plating Solution
(1) Improvements to the Pretreatment
There are also methods to improve the adhesion between sodium and its alloys and the platinum plating layer by enhancing the pretreatment process. Kamata proposed in a patent that an acid strike plating is performed in an pH=1 acid strike plating solution, followed by plating the required thickness of the platinum layer in an alkaline plating solution. The main components of the acid strike plating solution are 0.3~3g/L chloroplatinic acid (calculated as platinum) and 5%~15% halide ions (mass fraction). The pH must be controlled below 1; otherwise, the activity of titanium decreases, leading to poor adhesion. Suppose the halide ion concentration is too low. In that case, the removal of the passive film on the titanium surface may be incomplete, which in turn affects the adhesion of the plating layer. The conditions for strike plating are plating solution temperature of 40~80℃ and current density of 5~25A/dm2. The plating conditions and results for platinum plating are shown in Table 3-7.
Table 3-7 Platinum Plating Conditions and Their Results (concentration values are in parentheses)
| Σειριακός αριθμός | Impact plating solution | Platinum plating solution | Πάχος επιμετάλλωσης/μm | Stripping test | ||
|---|---|---|---|---|---|---|
| Platinum ion/(g/L) | Halogen ion (mass fraction)/% | Platinum ion/(g/L) | pH | |||
|
1 2 3 4 5 6 7 8 9 |
H2PtCl6 (0. 1) H2PtCl6 (0. 1) H2PtCl6 (0. 1) H2PtCl6 (1. 0) H2PtCl6 (1. 0) H2PtCl6 (1.0) H2PtCl6 (5.0) H2PtCl6 (5.0) H2PtCl6( 5. 0) |
HCl (5) HCl (5) HCl (5) HCl (10) HCl (10) HCl (10) HCl (20) HCl (20) HCl (20) |
K2Pt(OH)6 (5) K2Pt(OH)6 (10) K2Pt(OH)6 ⑸ Platinum dinitrate (5) Platinum dinitrate (10) Platinum dinitramide (20) K2Pt(OH)6 ⑸ K2Pt(OH)6 (10) K2Pt(OH)6 (20) |
12. 0 13. 0 13. 5 12. 0 13. 0 13. 5 12.0 13. 0 13. 5 |
10 15 20 10 15 20 10 15 20 |
Καλός Καλός Καλός Καλός Καλός Καλός Καλός Καλός Καλός |
(2) Plating Platinum Using a Neutral Plating Solution
Using a nearly neutral plating solution is beneficial for pattern plating, as it avoids using alkali metals such as Na, preventing the adverse effects caused by the accumulation of alkali metals. The platinum plating solution proposed by Otani meets this condition. Table 3-8 shows the composition of the plating solution and its process condition tests.
Table 3-8 Composition and Process Conditions of Neutral Platinum Plating Solution Test
| Συστατικά και οι συνθήκες επεξεργασίας τους | Νο. 1 | Αρ. 2 | Αρ. 3 |
|---|---|---|---|
|
Dinitrodiammine platinum(Pt concentration)/(g/L) Γλυκίνη/(mol/L) Iminodiacetic acid/(mol/L) Diaminotriacetic acid/(mol/L) pH Θερμοκρασία/°C Πυκνότητα ρεύματος/(A/dm2) Precipitation speed/(μm/min) Τρέχουσα απόδοση/%
|
12 0. 57 - - 5.0 70 1. 0 0. 3 80 |
12 - 0. 3 - 5. 0 70 1. 0 0. 2 65 |
12 - 0. 1 0. 1 5. 0 70 1. 0 0. 1 65 |
Since this plating solution is close to neutral, it is favorable for pattern plating and will not adversely affect the counter-plating film.
Kamata from Japan also studied the effect of alkaline earth metals as brighteners. It was found that alkaline earth metals, such as Ca, Ba, Mg, etc., have a brightening effect on alkaline plating solutions. The suitable concentration of alkaline earth ions is (2×100)×10-6. The degree of brightness is also controlled by varying the concentration of alkaline earth metal ions added.
The main components and operating conditions of the plating solution are as follows:
| Main components of plating solution |
KOH 40g/L Pt [added in the form of K2Pt(OH)6] 20g/L Ca [added in the form of CaCl2 aqueous solution] Adequate amount |
| Συνθήκες λειτουργίας |
рH 13.5 Temperature 80℃ Current density 3A/dm2 Base metal Calendered copper plate Plating thickness 20μm |
Table 3-9 Effect of Ca Ion Concentration on the Brightness of the Pt Plating Layer
| Ca ion concentration/x10-6 | Εμφάνιση | Ca ion concentration/x10-6 | Εμφάνιση |
|---|---|---|---|
|
0 0. 1 0. 3 0. 5 0. 7 1. 0 |
Non-glossy Non-glossy Non-glossy Non-glossy Non-glossy Semi-glossy |
1. 5 2. 0 2. 5 3. 0 5. 0 - |
Semi-glossy Semi-glossy Semi-glossy Semi-glossy Mirror Bright - |
Copywrite @ Sobling.Jewelry - Κατασκευαστής προσαρμοσμένων κοσμημάτων, εργοστάσιο κοσμημάτων OEM και ODM
Section III Platinum Alloy Plating
(1) Platinum-Iridium Alloy
Electroplated Pt-Ir alloy can be used on electrodes for soda ash production and electroplating.
The plating process conditions for the alloy proposed by Kamada et al. are shown in Table 3-10.
Table 3-10 Electroplating Pt-Ir Alloy Process Conditions
| Σύνθεση και συνθήκες διεργασίας | Νο. 1 | Αρ. 2 |
|---|---|---|
|
Sodium iridium hexachloride Βορικό οξύ Disodium malonate Sodium tetrachloroplatinate Potassium oxalate Sodium tetrabromoplatinate pH Θερμοκρασία Πυκνότητα ρεύματος |
10g/L 40g/L 0,02mol/L 0. 5~3g/L - - 5 85℃ 0,5 A/dm2 |
10g/L 40g/L - - 0,02mol/L 0. 5〜3g/L 2 85℃ 0,5 A/dm2 |
The electroplating steps are to first flash plate 1μm gold on the brass sheet, then plate gold off, and finally plate a Pt-Ir alloy on top. The coating obtained by this method has good hardness, adhesion, heat resistance, and metal wire bonding connectivity, with a current efficiency reaching 100%.
Regarding this plating solution, if the pH is too low, the current density is too small to be practical; if the pH is too high, hydroxide precipitates are easily formed. If the temperature is too low, the alloy is difficult to deposit; if the temperature is too high, the plating solution evaporates quickly, which is unfavorable for maintaining the plating solution. If the current density is too low, the deposition rate is too slow; if the current density is too high, the cathodic reaction is mainly hydrogen evolution.
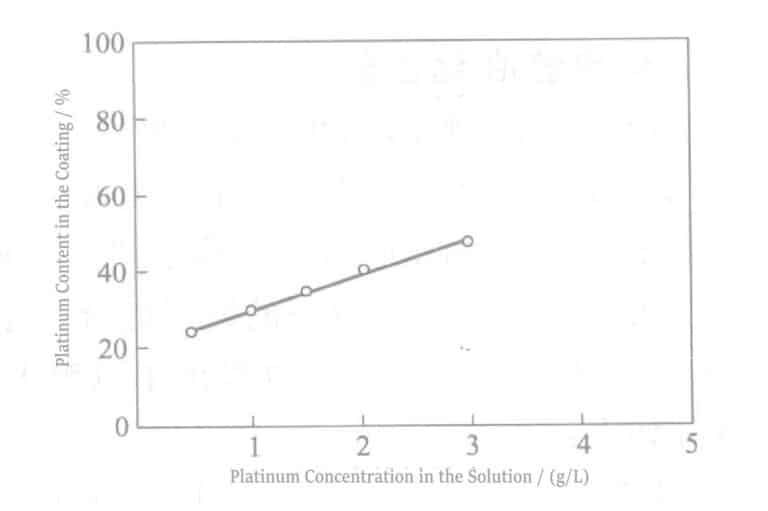
At the same time, the alloy composition in the plating film can also be controlled by adjusting the metal concentration ratio in the plating solution. Figure 3-1 shows the variation of alloy coating composition with the metal concentration ratio in the plating solution.
As can be seen from the figure, within the experimental concentration range, the Pt-Ir composition ratio in the plating layer has a linear relationship with the metal ion concentration ratio in the plating solution.
(2) Electroplating of Platinum-Iron Alloy
Alloys containing Fe are generally used as magnetic materials. The higher the recording density, the better. Platinum-iron alloys have high magnetic anisotropy, good corrosion resistance, and wear resistance and are expected to improve the performance of magnetic films.
Katsutsugu Koda proposed a plating solution formula with good stability that allows continuous electroplating. Since trivalent iron ions in the plating solution tend to form gels, this is detrimental to the appearance of the plating layer and reduces the concentration of divalent iron, negatively affecting the plating solution’s stability. Trivalent iron is generated based on the following reaction:
Pt4+ + 2e–→ Pt2+
2Fe2+ → 2Fe3+ + 2e–
From the above formula, from the perspective of considering the stability of iron ions, tetravalent platinum ions play a negative role, which led to the invention of divalent platinum to replace tetravalent platinum. The practice has proven that divalent platinum can be used for electroplating.
Table 3-11 shows the process conditions and results of binary Pt-Fe alloy electroplating. From the table, it can be seen that the metal atomic ratio of the Pt-Fe alloy coating obtained in No. 1~No. 3. It is close to. When the atomic ratio of the alloy is 50%, it is optimal as a magnetic film for recording.
Table 3-11 Process Conditions for Pt-Fe Binary Alloy Plating and Their Results
| Σύνθεση και συνθήκες διεργασίας | Νο. 1 | Αρ. 2 | Αρ. 3 | Αρ. 4 | Αρ. 5 | |
|---|---|---|---|---|---|---|
| Platinum salt | Τύπος | Pt(NH3)2(ΟΧΙ2)2 | [Pt(NH3)4]Cl2 | Pt(NH3)2(ΟΧΙ2)2 | Pt(NH3)2(ΟΧΙ2)2 | Na[Pt(C2O4)2 |
| Περιεχόμενο | 5g/L | 5g/L | 5g/L | 5g/L | 10g/L | |
| Iron Salt | Τύπος | FeSO4 - 7H2O | FeSO4 - 7H2O | FeSO4 - 7H2O | FeSO4 - 7H2O | FeSO4 - 7H2O |
| Περιεχόμενο | 2g/L | 30g/L | 30g/L | 10g/L | 20g/L | |
| Antioxidants | Τύπος | Sodium sulfite | Hydroxyammonia chloride | L-Ascorbic acid | Citric acid hydrate | Hydroxyammonia sulfate |
| Περιεχόμενο | 5g/L | 3g/L | 3g/L | 40g/L | 50g/L | |
| Συμπλοκοποιητικός παράγοντας | Τύπος | Triammonium citrate | EDTA-2Na | Triammonium citrate | EDTA-2Na | Sodium oxalate |
| Περιεχόμενο | 50g/L | 10g/L | 15g/L | 2g/L | 30g/L | |
| Πρόσθετα | Τύπος | - | Potassium dihydrogen phosphate | Potassium dihydrogen phosphate | Potassium ascorbyl phosphate | - |
| Περιεχόμενο | - | 15g/L | 15g/L | 5g/L | - | |
| Temperature of plating solution | 40℃ | 30℃ | 60℃ | 50℃ | 70℃ | |
| pH | 8 | 2 | 3 | 4 | 8 | |
| Πυκνότητα ρεύματος | 1A/dm2 | 2A/dm2 | 1A/dm2 | 1A/dm2 | 1.5A/dm2 | |
| Plating composition (atomization) | Pt | 51% | 49% | 55% | 72% | 37% |
| Fe | 49% | 51% | 45% | 28% | 63% | |
| Appearance of plated layer | O | O | O | O | O | |
(3) Electroplating of Platinum-Cobalt Alloy
The Pt-Co alloy film has a very high magnetic recording density, which is very attractive for the large capacity of magnetic recording media. Especially when its atomic ratio is 1:1, the performance is optimal.
Koda also researched Pt-Co alloys (see Table 3-12).
Table 3-12 Process Conditions and Results of Pt-Co Binary Alloy Plating
| Σύνθεση και συνθήκες διεργασίας | Νο. 1 | Αρ. 2 | Αρ. 3 | Αρ. 4 | Αρ. 5 | |
|---|---|---|---|---|---|---|
| Platinum salt | Τύπος | Pt(NH3)2(ΟΧΙ2)2 | [Pt(NH3)4]Cl2 | Pt(NH3)2(ΟΧΙ2)2 | Pt(NH3)2(ΟΧΙ2)2 | Na[Pt(C2O4)2 |
| Περιεχόμενο | 2g/L | 5g/L | 5g/L | 2g/L | 10g/L | |
| Iron Salt | Τύπος | CoSO4 - 7H2O | CoSO4 - 7H2O | CoSO4 - 7H2O | CoSO4 - 7H2O | CoSO4 - 7H2O |
| Περιεχόμενο | 30g/L | 30g/L | 2g/L | 45g/L | 20g/L | |
| Buffer(1) | Τύπος | EDTA-2Na | Triammonium citrate | Triammonium citrate | Βορικό οξύ | Ammonium oxalate |
| Περιεχόμενο | 30g/L | 5g/L | 50g/L | 30g/L | 30g/L | |
| Buffer(2) | Τύπος | Triammonium citrate | - | - | EDTA-2Na | - |
| Περιεχόμενο | 5g/L | - | - | 2g/L | - | |
| Conductive salt | Τύπος | Sulfamic acid | Ammonium sulfate | Ammonium sulfate | Sulfamic acid | Ammonium sulfate |
| Περιεχόμενο | 15g/L | 15g/L | 15g/L | 20ml/L | 15g/L | |
| Antiprecipitant | Τύπος | - | Αμμωνία | - | - | - |
| Περιεχόμενο | - | 3g/L | - | - | - | |
| Temperature of plating solution | 60℃ | 50℃ | 40℃ | 50℃ | 70℃ | |
| pH | 3 | 2 | 4 | 3 | 4 | |
| Πυκνότητα ρεύματος | 1A/dm2 | 2A/dm2 | 4A/dm2 | 3A/dm2 | 4A/dm2 | |
| Plating composition (atomization) | Pt | 65% | 49% | 30% | 40% | 37% |
| Fe | 35% | 51% | 70% | 60% | 63% | |
| Appearance of plated layer | O | O | O | O | O | |
The alloy atomic ratio of the coating obtained from No. 2 in Table 3-11 is about 50%.
Hu Zhongmin et al. also proposed a plating Pt-Co alloy formula. Its main components are as follows:
|
Pt(NH3)2(ΟΧΙ2)2 (as Co) 0.2~15g/L CoSO4 (as cobalt) 5~70g/L (Maintain Co:Pt=30:1) |
|
pH 1.2 (adjusted with NH2SO3H) Temperature 70℃ Current density 2A/dm2
|
(4) Platinum-Rhodium Alloy
Because Pt-W alloy coating has higher oxidation catalytic ability than Pt coating, people’s interest in Pt-W alloy plating has been aroused. Matsunori Sawada et al. proposed a platinum-tungsten alloy formula that can achieve a uniform appearance, good catalytic ability, and good plating solution stability.
A stable plating solution is obtained by adding organic acids or organic acid salts to the main components and then aging the mixture.
The organic acids used can be acetic acid, citric acid, oxalic acid, tartaric acid, etc. Representative components and concentrations are as follows:
H2PtCl4 2g/L(as Pt)
Na2WO4 • 2H2O 25g/L(as W)
Sodium citrate 5g/L
Citric acid 5g/L
Sodium sulfate 15g/L
Aging conditions 60℃×8h
Plating conditions 65℃ ,6mA/cm2 , 10min
Plating material Stainless steel wire mesh with a diameter of 0.3mm
Pre-plating treatments are:
Electrolytic degreasing→Water rinse→Hydrochloric acid soaking→Water rinse→Flash gold plating→Sulfuric acid soaking→Water rinse→Electroplating Pt-W Alloy
Suppose no aging treatment is applied and plating is done immediately using the prepared plating solution. In that case, the co-deposition of tungsten will be unstable, especially since the initial tungsten deposition will be low. The plating solution will gradually stabilize with continued use, and tungsten co-deposition will increase. A stable tungsten-containing plating layer can be obtained if the above aging treatment is used.
(5) Electroplating Platinum-Nickel Alloy
Hu Zhongmin proposed the main components of the electroplating Pt-Co alloy formula as follows:
(5) Electroplating Platinum-Nickel Alloy
Hu Zhongmin proposed the main components of the electroplating Pt-Co alloy formula as follows:
|
Pt(NH3)2(ΟΧΙ2)2 (as Pt) 0.2~15g/L Nickel sulfamate (as Ni) 5~70g/L (maintain Ni:Pt=30:1) Sulfamic acid Adequate amount |
|
pH 1~1.4 (adjusted with sulfamic acid) Temperature 70°C Current density 2A/dm2 |
Section IV Chemical Plating of Platinum
In addition to being used in jewelry, catalysis, and heat-resistant materials, platinum can also be used as a thin film electrode for semiconductor components. Obtaining platinum thin films through chemical plating is a new approach. The reducing agents are generally hydrazine or hydrazine hydrate; hypophosphite is sometimes used.
Raitian refines platinum salts by passing carbon dioxide into a solution of hexaammineplatinum complex [Pt(NH3)6(OH)4], causing the platinum salt to precipitate and achieving stable and high-speed platinum electroplating.
The specific refining method is to pass carbon dioxide into a solution of hexaammineplatinum complex [Pt(NH3)6(OH)4] for about 3 hours to obtain a platinum salt precipitate. Then, filter, wash, dry the precipitate and dissolve the carbonate with an organic acid to obtain refined platinum salt for electroplating. The purpose of using organic salts is to avoid contamination by inorganic ions. Halide ions tend to adsorb onto the plated parts, reducing the deposition rate and causing the platinum film to darken. The presence of sulfate and nitrate ions can also cause appearance issues with the plating. The organic acids used are sulfonic acids, such as methanesulfonic or ethanesulfonic acid, or low molecular weight organic carboxylic acids, such as acetic or propionic acid.
To facilitate the volatilization and removal of carbon dioxide, the solution can be kept under reduced pressure when dissolving the platinum carbonate precipitate with organic acid.
Plating solution and process conditions:
Pt(NH3)6(Χ.Ε.)3ΕΡΩΤΟΛΟΓΩ)4 (as Pt dissolved in acetic acid) 3g/L
Hydrazine hydrate 3mL/L
Glycerol ester (leveling agent) 20×10-6
pH (Adjusted with ammonia) 11
Temperature 60℃
Plated parts Aluminum oxide plate (activated)
Deposition speed 1.8μm/h
The leveling agent can be polyoxyethylene dodecyl ether, and the reducing agent can be replaced with hypophosphite.
Also using hydrazine hydrate as the reducing agent, Koslov Alexander’s formula is:
Pt(NH3)2(ΟΧΙ2)2 (as Pt) 2g/L
Hydrazine hydrate (reducing agent) 3g/L
Νιου Χάμσαϊρ2OH – HC1(as stabilizer) Adequate amount
pH (adjusted with acetic acid) 3
Temperature 50℃
Deposition speed 0. 1μm/h
Table 3-13 Chemical Plating Pt Test
| Στοιχείο | Test 1 | Test 2 | Test 3 |
|---|---|---|---|
| Test Characteristics | The ion-exchange membrane soaked in 5% (NH4)4PtCl2 solution was placed in a solution of 1g/L sodium hydroboride+1mg/L magnesium carbonate at 50℃ for 1h. | Ion exchange membrane soaked in 5% (NH4)4PtCl2 solution was placed in a solution of 1g/L sodium hydroboride + 10mg/L magnesium sulfate at 30℃ for 1h. |
HPtCl4 1g/L Sodium hydroboride 1g/L Calcium carbonate 10ml/L 80℃,1h Reaction of plate Al in the above solution by immersion |
| Base material | Cation exchange membrane | Cation exchange membrane | Aluminum plate |
| Platinum thickness | 0. 1mm | 0. 1mm | 0. 1mm |
| Platinum particle diameter | Below 10μm | Below 10μm | Below 10μm |
| Surface resistance | 10Ω/cm | 10Ω/cm | 10Ω/cm |
In this reaction, alkaline earth metals are required; they can dissolve with the reducing agent (as in Experiment 1, Experiment 2) or be added to the plating solution (as in Experiment 3). However, the mechanism of action of alkaline earth metals is unclear. The better the compactness of the plating layer, the fewer defects, such as cracks in the plating layer, which can ensure a relatively low resistance and thus guarantee the quality of the electrode.
Kenji Takahashi proposed a chemical plating scheme using tetravalent platinum ammonium salt as the main salt. The general form of the platinum salt is [Pt(NH3)6X]. In the formula X can be a halide ion, OH– group, SO42-, κ.λπ.
Its composition is:
Platinum salt (tetravalent platinum ammonium salt) (in platinum) 0. 5〜5.0g/L
Ammonia (28%) 10〜100g/L
Water and hydrazine (reducing agent) 0. 5〜5g/L
рH 10〜12. 5
Plating solution temperature 50〜70℃