Τι είναι η χημική επιχρύσωση και πώς λειτουργεί;
Χημική επιχρύσωση: Χρυσόχρυση: Τεχνικές, λύσεις και εφαρμογές για κοσμήματα
Εισαγωγή:
Τι είναι η χημική επιχρύσωση; Είναι μια διαδικασία που χρησιμοποιείται για την εναπόθεση ενός λεπτού στρώματος χρυσού σε διάφορα υλικά, ενισχύοντας την εμφάνιση και την αντοχή τους. Πώς λειτουργεί; Η χημική επιχρύσωση περιλαμβάνει τη χρήση ειδικών διαλυμάτων με ιόντα χρυσού και αναγωγικούς παράγοντες. Οι παραδοσιακές μέθοδοι χρησιμοποιούν συχνά κυάνιο, αλλά υπάρχουν και επιλογές χωρίς κυάνιο. Γιατί να επιλέξετε τη χημική επιχρύσωση; Προσφέρει ένα ομοιόμορφο, υψηλής ποιότητας φινίρισμα που είναι ιδανικό για κοσμήματα και άλλα διακοσμητικά αντικείμενα. Είτε είστε κατασκευαστής κοσμημάτων, είτε σχεδιαστής, είτε έμπορος λιανικής πώλησης, η κατανόηση αυτών των τεχνικών μπορεί να αναβαθμίσει τα προϊόντα σας.

Πίνακας περιεχομένων
Τμήμα I Χημική επιχρύσωση με κυάνιο
1. Επισκόπηση
Με την υψηλή πυκνότητα των ηλεκτρονικών εξαρτημάτων, την πολυπλοκότητα του σχεδιασμού γραμμών, τη μικροκατασκευή των κυκλωμάτων και την ανεξαρτησία των ηλεκτρικών ιδιοτήτων, η χημική επιχρύσωση, η οποία λύνει την πολυπλοκότητα της διαδικασίας επιμετάλλωσης, έχει γίνει ένας αναπόφευκτος τρόπος επιχρύσωσης. Ωστόσο, η χημική επιχρύσωση έχει τα ακόλουθα μειονεκτήματα:
① Αργή ταχύτητα,
② Στενές συνθήκες χρήσης και εύρος λειτουργίας, αυξάνοντας τη δυσκολία διαχείρισης,
③ Η επιφάνεια του υλικού πρέπει να είναι απολύτως καθαρή,
④ Η διάρκεια ζωής του διαλύματος επιμετάλλωσης είναι μικρή (επιρρεπής σε αντιδράσεις αυτοοξειδοαναγωγής),
⑤ Το πάχος επιμετάλλωσης είναι πολύ ευαίσθητο στις συνθήκες ανάδευσης.
Ως εκ τούτου, η χημική επιχρύσωση πρέπει να πραγματοποιείται με τη χρήση ειδικού εξοπλισμού. Η συνήθως χρησιμοποιούμενη σύνθεση του διαλύματος χημικής επιχρύσωσης παρουσιάζεται στον πίνακα 1-24, που παρασκευάζεται συνδυάζοντας διάφορα άλατα συντονισμού χρυσού και αναγωγικά μέσα.
Πίνακας 1-24 Τύποι αλάτων συντονισμού χρυσού και αναγωγικών παραγόντων σε χημικό διάλυμα επιχρύσωσης
| Αναγωγικό μέσο | KAu(CN)2 | KAu(CN)4 | Na3Au(SO2)2 | HAuCl4 • 3Η2O | HAuCl4 ・ 3H2O | AuCN | KAu(CN)4 | KAu ・ O2 | KAu(OH)4 | AuI | Δεν είναι συγκεκριμένο |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Υποφωσφορικό νάτριο | 57,58 | 71 | 77 | ||||||||
| Φορμαλδεΰδη | 71 | 78 | |||||||||
| Biazide | 59 | 71 | 76 | 78 | |||||||
| Βοροϋδρίτης | 60,61 | 72 | 78 | 79 | |||||||
| Μεθυλομποράνιο | 60 | 69 | 74 | 75 | 79 | 80 | |||||
| Υδροξυλαμίνη | 62 | ||||||||||
| Αδημοσίευτο | 63 | 73 | 81 | ||||||||
| Θειουρία | 64 | ||||||||||
| Υδροξείδιο του αμμωνίου | 65 | ||||||||||
| Τρυγικό τετραϋδρικό νάτριο (συνδυασμένο) | 66 | 70 | |||||||||
| Ν,Ν-Διμεθυλαμίδιο | 82 | ||||||||||
| Ν,Ν-Διμεθυλογλυκίνη | 67 | ||||||||||
| Κανένα | 68 |
Πίνακας 1-25 Λειτουργίες των συστατικών στο διάλυμα χημικής επιχρύσωσης
| Στοιχείο | Σκοπός | Όνομα ένωσης |
|---|---|---|
| Χρυσό ιόν | Προμήθεια μεταλλικού χρυσού | KAu(CN)2、KAu(CN)4、AUCN、 Na3Au(SO2)2. HAuCl4 • 3Η2O、KAu ・ O2 |
| Αναγωγικός παράγοντας | Αναγωγικοί παράγοντες για την εναπόθεση χρυσού | Τετραϋδρικό τρυγικό νάτριο, βορειοϋδρίτης, αμινοβοράνιο, φορμαλδεΰδη, υποφωσφίτης, Ν,Ν-διμεθυλογλυκίνη, ασκορβικό οξύ |
| Οργανικοί χηλικοί παράγοντες | Ρυθμιστική δράση για την αποφυγή ταχείας εναπόθεσης χρυσού | EDTA, κιτρικό κάλιο, τρυγικό οξύ |
| Σταθεροποιητής | Μάσκα ενεργών πυρήνων για την αποφυγή αποσύνθεσης του διαλύματος επιμετάλλωσης | Θειουρία, κυανιούχο μέταλλο, ακετυλακετόνη |
| Ενεργοποιητής | Η επιβραδυντική επίδραση του μετριοπαθούς χηλικού παράγοντα | Σουκκινικό οξύ |
| Ρυθμιστικός παράγοντας | Διατήρηση ενός συγκεκριμένου pH | Φωσφορικά άλατα, κιτρικά άλατα, τρυγικά άλατα |
| Επιφανειοδραστική ουσία | Τα επιμεταλλωμένα μέρη να είναι εύκολο να βραχούν | Αλειφατικά σουλφονικά άλατα, θειικά άλατα αλκοόλης |
2. Διάλυμα επιμετάλλωσης χρυσού με υποφωσφίτη
Όταν το βασικό μέταλλο και ο χρυσός στο διάλυμα επιμετάλλωσης υφίστανται αντίδραση εκτόπισης, το μέταλλο με χαμηλότερο ηλεκτροχημικό δυναμικό ιονίζεται ευκολότερα από τον χρυσό. Κατά τη διάρκεια της αντίδρασης, μόλις η επιφάνεια του βασικού μετάλλου καλυφθεί πλήρως από χρυσό, η αντίδραση σταματά αμέσως, οπότε μπορεί να ληφθεί μόνο ένα λεπτό στρώμα επιμετάλλωσης χρυσού 0,1~0,3μm. Αυτό το διάλυμα επιμετάλλωσης, ή διάλυμα Brookshire, είναι ένα παλιό διάλυμα επιμετάλλωσης και αποτελεί τη βασική σύνθεση των χημικών διαλυμάτων επιμετάλλωσης χρυσού που κυκλοφορούν σήμερα στο εμπόριο. Χαρακτηριστικά του διαλύματος επιμετάλλωσης: το βασικό στρώμα για την επιμετάλλωση χρυσού πρέπει να είναι νικέλιο για την αντίδραση εκτόπισης- ο χρυσός δεν μπορεί να επιμεταλλωθεί με εκτόπιση σε υλικό χαλκού.
(1) Σύνθεση και συνθήκες του διαλύματος χημικής επιχρύσωσης με υποφωσφίτη
Στον πίνακα 1-26 παρουσιάζεται η τυπική σύνθεση του διαλύματος χημικής επιχρύσωσης με υποφωσφίτη.
Πίνακας 1-26 Σύνθεση και συνθήκες του διαλύματος χημικής επιχρύσωσης με υποφωσφίτη
| Σύνθεση και συνθήκες λειτουργίας | Σειριακός αριθμός | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Κυανιούχο κάλιο χρυσού/(g/L) | 2 | 2 | 20 | 2 | 2 |
| Υποφωσφορικό νάτριο/(g/L) | 10 | 10 | 10 | 10 | 10 |
| Κυανιούχο κάλιο /(g/L) | 0.2 | 0.2 | 80 | 0.4 ① | 0.2 |
| Θερμοκρασία/°C | 96 | 96 | 96 | 96 | 96 |
| pH | 13.5 | 7.5 | 13.5 | 13.5 | 7.1 |
| Ρυθμός φορτίου/(cm2/cm3) | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Ρυθμός επιμετάλλωσης/[mg/(cm2- h)] | 9.8 | 9.85 | 12.3 | 8.2 | 3.86 |
(2) Η επίδραση του νικελίου στον ρυθμό εναπόθεσης του διαλύματος επιμετάλλωσης με υποφωσφίτη
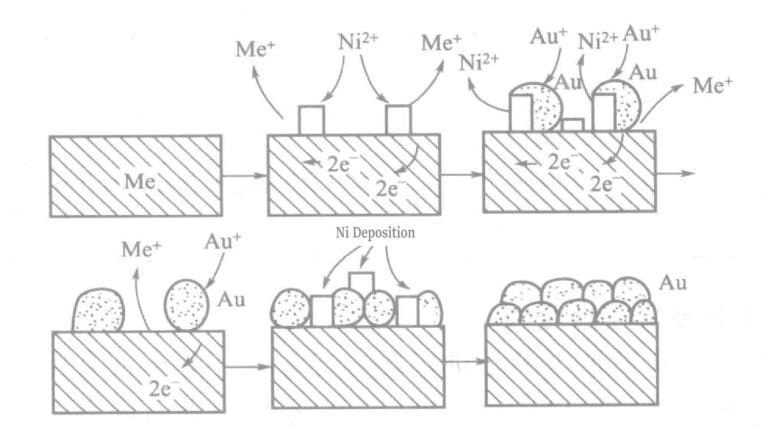
Στο διάλυμα χημικής επιχρύσωσης με υποφωσφίτη ως αναγωγικό μέσο, τα ενεργά ιόντα μετάλλων (Ni2+) προστίθενται. Το μέταλλο (νικέλιο) και ο χρυσός συν-αποτίθενται σε ίχνη στην επιφάνεια των επιχρυσωμένων εξαρτημάτων, δημιουργώντας μια μεγάλη χημική κινητήρια δύναμη και αυξάνοντας την ταχύτητα επιχρύσωσης. Η αρχή παρουσιάζεται στο πρότυπο διάγραμμα του σχήματος 1-25.
Πίνακας 1-27 Σύνθεση και συνθήκες λειτουργίας του διαλύματος επιμετάλλωσης χρυσού με υποφωσφίτη
| Σύνθεση και συνθήκες λειτουργίας | I | II | Σύνθεση και συνθήκες λειτουργίας | I | II |
|---|---|---|---|---|---|
| Κυανιούχο κάλιο χρυσού/(g/L) | 2, 5, 7 | 2, 5, 7 | Κυανιούχο κάλιο /(g/L) | - | 2 |
| Κιτρικό νάτριο/(g/L) | 75 | 75 | H2NNH2 - H2O /(g/L) | - | 2 |
| Υποφωσφορώδες νάτριο/(g/L) | 15 | 15 | pH | 8.5 | 4.3 |
| EDTA-2Na/(g/L) | - | - | Θερμοκρασία/℃ | 70, 90 | 70, 90 |
| Χλωριούχο νάτριο/(g/L) | - | 5 |
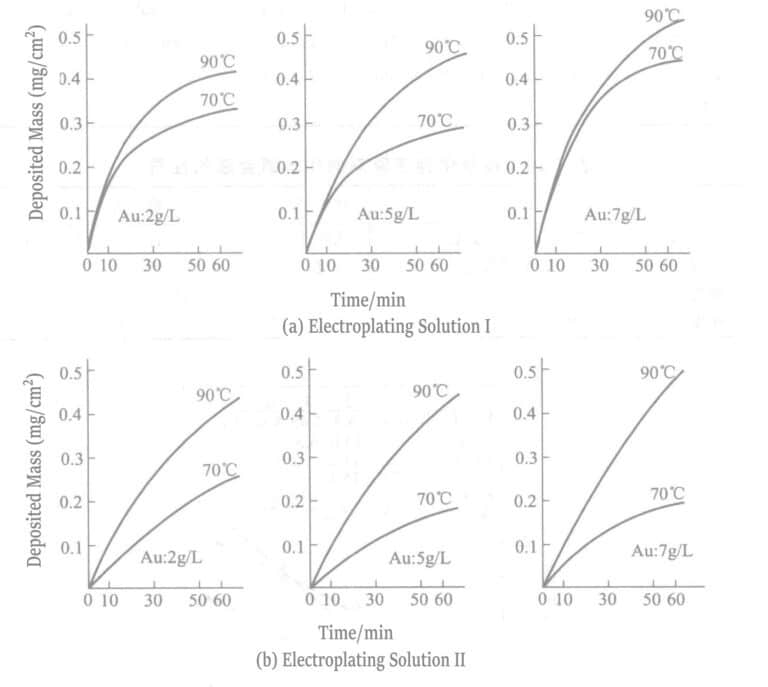
3. Διάλυμα επιμετάλλωσης αλάτων βοροϋδρίτη
(1) Σύνθεση και ιδιότητες του βορειοϋδριδικού καλίου Χημικό διάλυμα επιμετάλλωσης χρυσού
Το 1969, οι Okinaka et al. πρότειναν τη χρήση διαλύματος επιμετάλλωσης με ενώσεις βοροϋδριδίου ως αναγωγικό μέσο. Η σύνθεση και οι συνθήκες του διαλύματος χημικής επιχρύσωσης με βορειοϋδρίτη παρουσιάζονται στους Πίνακες 1-28 και οι ιδιότητες των στρώσεων χρυσού που επιχρύσωσαν με αυτά τα διαλύματα παρουσιάζονται στους Πίνακες 1-29. Το πάχος των επιμεταλλωμένων στρωμάτων που επιμεταλλώθηκαν σε διαφορετικούς χρόνους με διάφορα διαλύματα επιμετάλλωσης υποδεικνύεται στο Σχήμα 1-27. Τα συνοπτικά αποτελέσματα των αναγωγικών μέσων βοροϋδρίτη καλίου και διμεθυλαμινοβοράνιο παρουσιάζονται στον Πίνακα 1-30.
Πίνακας 1-28 Σύνθεση και συνθήκες λειτουργίας του διαλύματος χημικής επιχρύσωσης με βορειοϋδρίτη καλίου
| Σύνθεση και λειτουργία | Σειριακός αριθμός | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Κυανιούχο κάλιο χρυσού/(g/L) | 0. 02mol/L (5.8g/L) | 0. 03mol/L (8.6g/L) | 0. 005mol/L (1. 45g/L) |
| Κυανιούχο κάλιο | 0. 2mol/L (13.0g/L) | 0. 2mol/L (13.0g/L) | 0. lmol/L (6.5g/L) |
| Υδροξείδιο του καλίου | 0. 2mol/L(11.2g/L) | 0. 2mol/L(22.4g/L) | 0. 2mol/L(11.2g/L) |
| Potassium borohydride | 0. 4mol/L(21.6g/L) | 0. 8mol/L(43.1g/L) | 0. 2mol/L(10.8g/L) |
| Θερμοκρασία/°C | 75 | 75 | 75 |
Table 1-29 Properties of Chemical Gold Plating Solution with Potassium Borohydride Reducing Agent
| Nature | Data | Nature | Data |
|---|---|---|---|
| Bonding strength | Excellent bonding strength of the metal layer | Porosity | No pores larger than 1μm on the uniform surface |
| Εμφάνιση | Dull yellow | Καθαρότητα | 99. 9% |
| Πυκνότητα | Gold content 19. 3g/cm3 | Resistance value | 0. 03Ω/cm2 ( Au: 1 μm) |
| Σκληρότητα | Nup hardness 60〜80 | Συγκολλησιμότητα | Excellent |
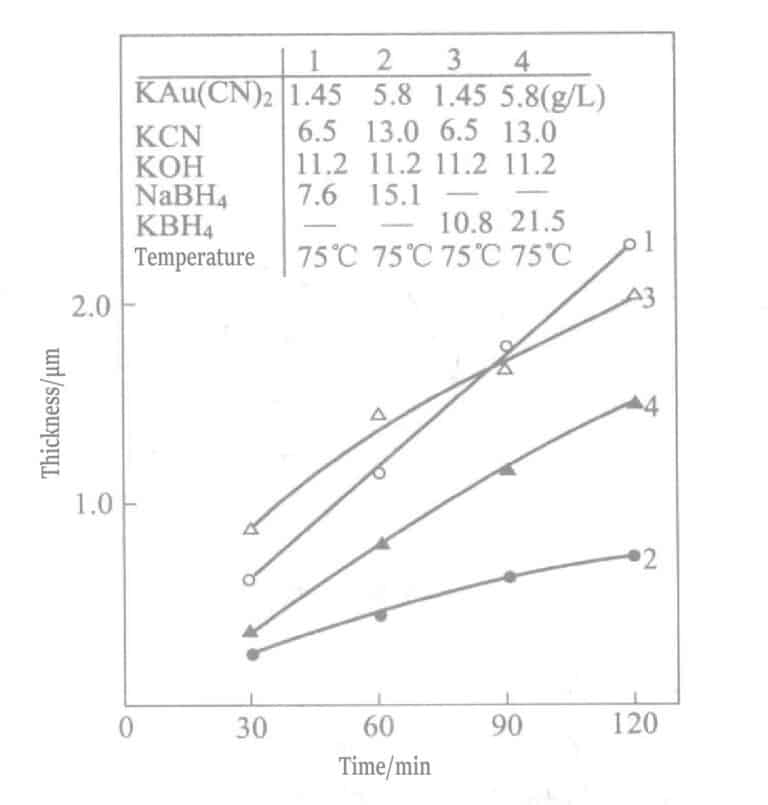
Table 1-30 Functions of Components in Potassium Borohydride Chemical Gold Plating Solution
| Influencing Factors | Potassium Borohydride Plating Solution | DMAB (Dimethylaminoborane) plating solution |
|---|---|---|
| Effect of Gold Concentration | When the gold concentration reaches 0.002mol/L, the precipitation rate rises sharply and decreases above that. | The rate of precipitation increases linearly at 0.01 mol/l gold concentration, but has no effect above that. |
| Effects of Potassium Cyanide |
Unstable plating solution when below 0.005 mol/l. Gold does not precipitate above 0.2 mol/l. |
Same as left |
| Effect of potassium borohydride | BH is not stable at room temperature. Addition of potassium hydroxide improves the stability of the bath, and the rate of precipitation decreases with increasing concentration. | DMAB is stable at room temperature, the precipitation rate increases with the increase of potassium hydroxide. |
| Concentration of reducing agent | Increase in precipitation rate with increase in concentration | |
| Effect of plating bath temperature | Increase the precipitation rate by 10℃, and decompose at 85℃. |
(2) Influence of plating speed and heavy metal ions
Adding trace amounts of heavy metal ions (lead, thallium, etc.) to the chemical gold plating solution significantly increases the deposition rate. Mcintyre explained this effect as follows. Due to low potential deposition, the catalytic effect of adsorbed lead atoms, as shown in mechanisms (1-4) and (1-5), promotes nucleation.
Pb2+ + 2e– → Pbad (1-4)
2Au(CN)2– + Pbad → 2Au + Pb2+ + 4CN– (1-5)
Due to the large difference in work functions between copper, gold materials, and lead ions, lead is reduced and deposited at a potential more positive than the thermodynamic redox potential, forming a monolayer. Due to the catalytic reaction of adsorbed lead atoms as in (1-4) and (1-5), ) the reduction reaction of Au(CN) occurs rapidly, effectively promoting the deposition of gold.
The relationship between lead concentration and deposition rate in potassium borohydride-reducing gold plating solution is shown in Figure 1-28, and the plating solution composition is shown in Table 1-31. Lead is added in the form of lead nitrate. Figure 1-28 shows that the lead addition concentration in the plating solution can only be about 20ml/L, and adding more has no effect.
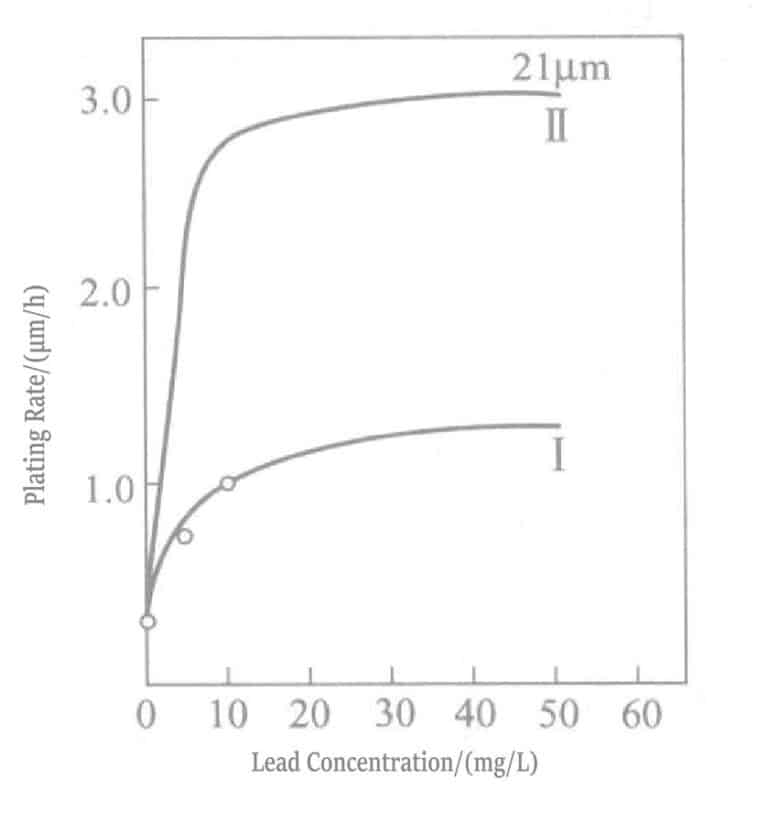
Table 1-31 Composition and Operating Conditions of Potassium Borohydride Gold Plating Solution
| Σύνθεση και συνθήκες λειτουργίας | I | II | Σύνθεση και συνθήκες λειτουργίας | I | II |
|---|---|---|---|---|---|
| Κυανιούχο κάλιο χρυσού/(g/L) | 2 | 2 | Κυανιούχο κάλιο /(g/L) | 10 | 10 |
| Potassium borohydride/(g/L) | 2 | 10 | EDTA-2Na/(g/L) | 5 | 5 |
| Potassium cyanide/(mg/L) | 5 | 5 | Θερμοκρασία διαλύματος επιμετάλλωσης/°C | 75 | 75 |
Table 1-32 Composition of BMAB Gold Plating Solution
| Σύνθεση και συνθήκες λειτουργίας | Συγκέντρωση | Σύνθεση και συνθήκες λειτουργίας | Συγκέντρωση |
|---|---|---|---|
| Κυανιούχο κάλιο χρυσού/(g/L) | 2 | Κυανιούχο κάλιο /(g/L) | 30 |
| BMAB/(g/L) | 10 | EDTA-2Na/(g/L) | 5 |
| Potassium cyanide/(mg/L) | 5 | pH | 13.6 |
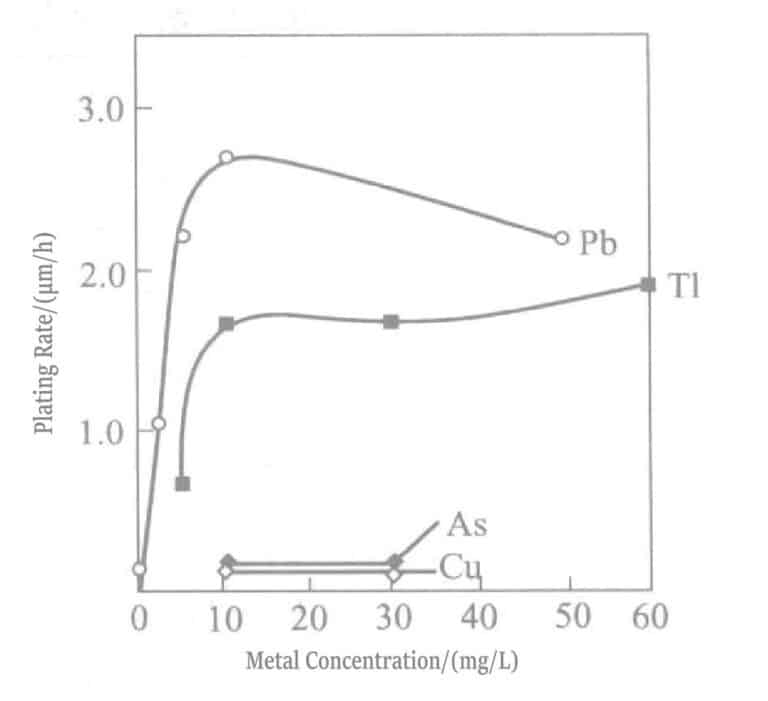
Table 1-33 Composition of Potassium Gold(III) Cyanide Chemical Gold Plating Solution
| Σύνθεση | Parameters | Σύνθεση | Parameters |
|---|---|---|---|
| KAu(CN)4/(g/L) | 4 | KOH/(mg/L) | 11.2 |
| KBH4/(g/L) | 3 | Θερμοκρασία/℃ | 70 |
| PbCl2/(mg/L) | 0.5 |
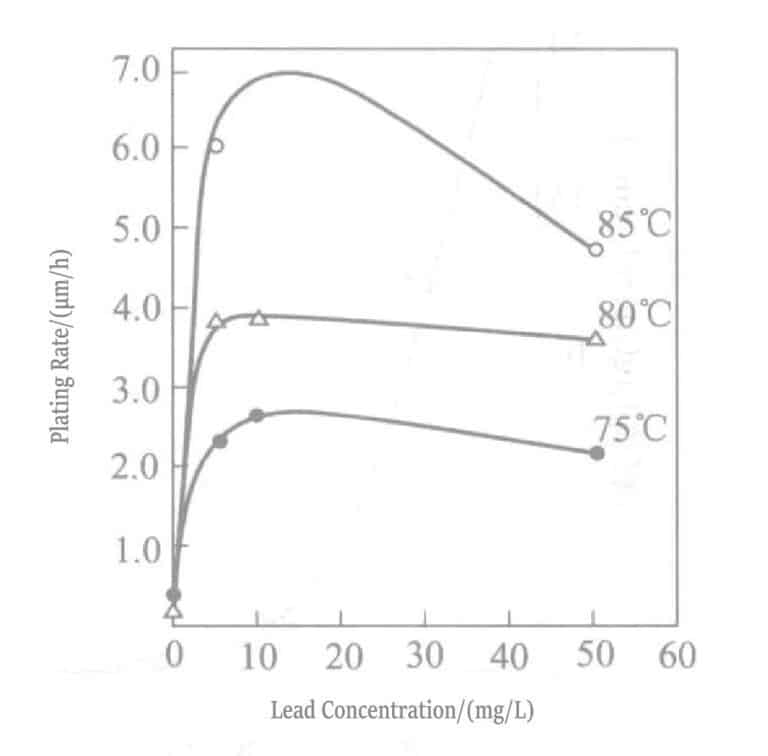
Figure 1-30 Effect of temperature and lead concentration on the rate of gold precipitation
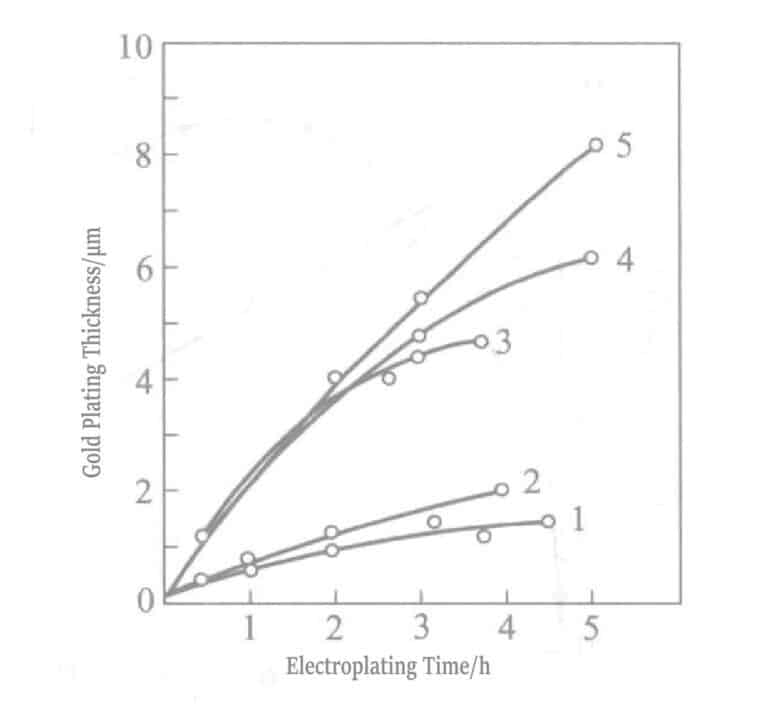
Figure 1-31 Relationship between plating time and thickness of gold plated layer
1 - No stirring Au+ liquid; 2 - No stirring Au3+ liquid; 3 - Stirring Au3+ liquid; 4 - Replenishing Au3+ liquid; and 5 - Renewing Au3+ liquid
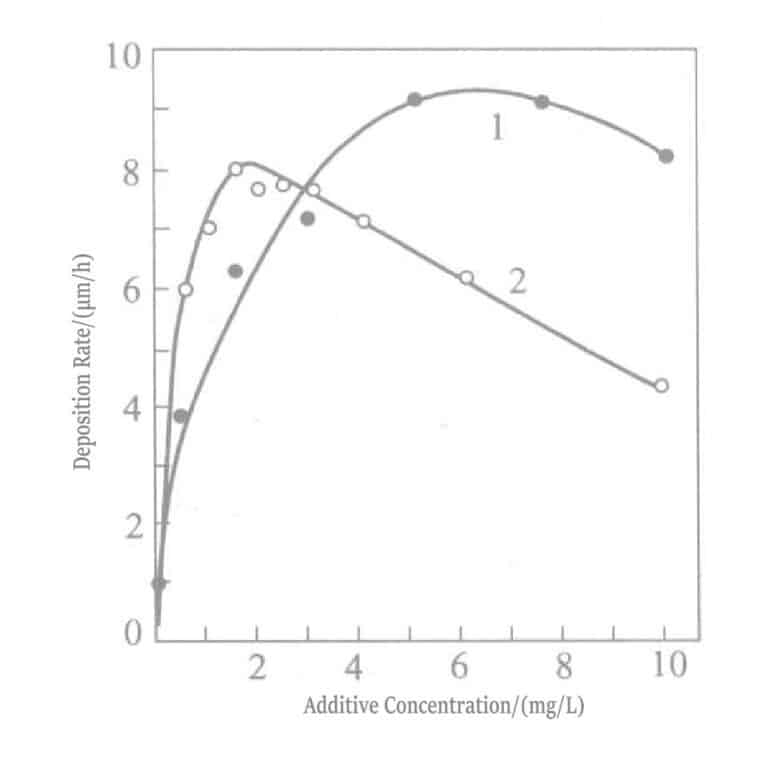
Figure 1-32 Effect of added concentrations of lead and thallium on gold plating rate
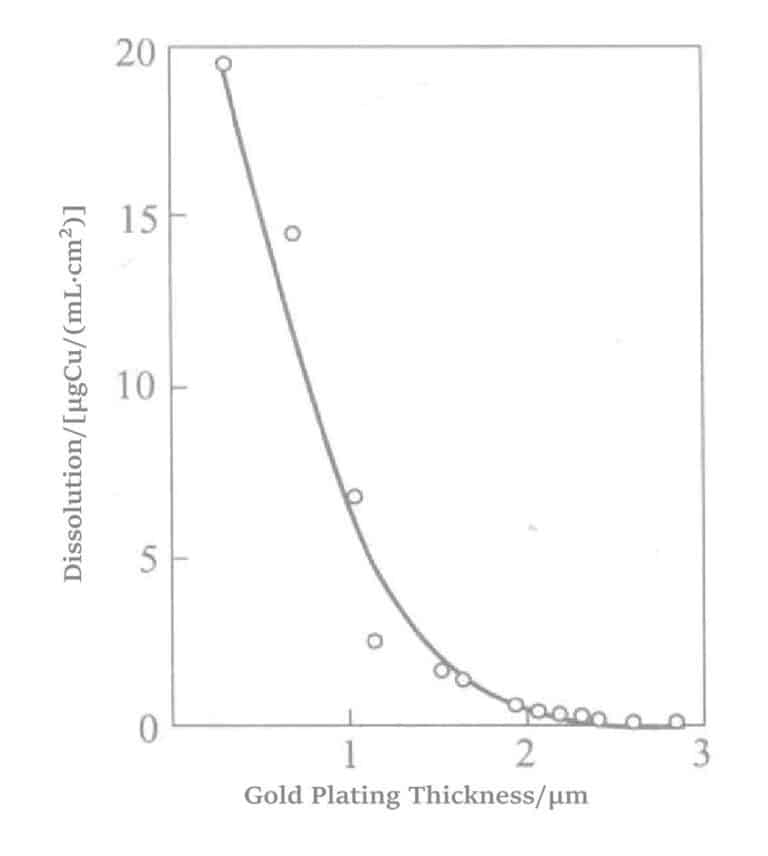
Figure 1-33 Porosity of Electroless Gold Plating Layer
The chemical gold plating process of crystallized glass (SiO2 46% + Al2O3 16% + MgO 17% + K2O 10% + F 4% + B2O3 7% ) is shown in Figure 1-34.
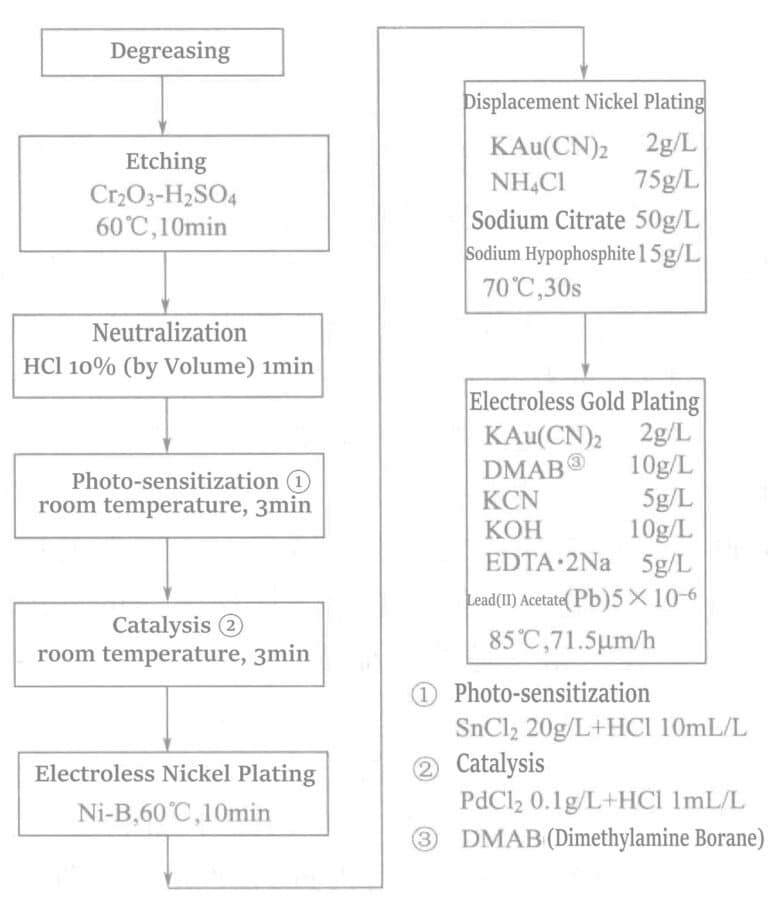
4. Reaction Mechanism of Borohydride Salt Chemical Gold Plating Solution
Lin Zhongfu et al. proposed that the potassium borohydride and DMAB (dimethylamine borane) gold plating solutions in Table 1-34 can become practical gold plating solutions after adding stabilizers.
(1) Composition of two plating solutions: Table 1-34 shows the two compositions.
Table 1-34 Potassium Borohydride and DMAB Chemical Plating Solutions
| Σύνθεση διαλύματος επιμετάλλωσης | Potassium borohydride plating solution | DMAB plating solution |
|---|---|---|
| Potassium gold cyanide/(mol/L) | 0.02 | 0.02 |
| Potassium cyanide/(mol/L) | 0.2 | 0.02 |
| Potassium hydroxide/(mol/L) | 0.2 | 0.8 |
| Potassium borohydride/(mol/L) | 0.4 | - |
| DMAB/(mol/L) | - | 0.4 |
| Θερμοκρασία/°C | 75 | 85 |
| Plating speed/(μm/h) | 0.7 | 0.4 |
(2) Reaction mechanism: In the potassium borohydride gold plating solution, BH4– itself does not produce a reducing effect, but the intermediate BH3Ω– of its hydrolysis product BO2– acts as the reducing agent.
ΒΗ4– + Η2O → BH3Ω–+ Η2 (1-6)
ΒΗ3Ω– + Η2O → BO2– + 3H2 (1-7)
ΒΗ3Ω– + 3OH– → BO2– + 3/2 H2 + 2 ώρες2Ο + 3ε– (1-8)
Au(CN)2– + e– → Au + 2CN– (1-9)
ΒΗ3Ω– + 3Au(CN)2– + 3OH– → BO2– + 3/2 H2 + 2 ώρες2O + 3Au + 6CN – (1-10)
The hydrolysis reactions of equations (1-6) and (1-7) were analyzed using polarography. The result shows that reaction (1-7) is 500 times faster than reaction (1-6). Therefore, the utilization rate of potassium borohydride in the gold plating reaction is very low. Under typical plating conditions, the effective utilization rate does not exceed 2%~3%, with most reducing agent remaining ineffective due to hydrolysis.
The above hydrolysis reaction proceeds rapidly at low pH (acid-catalyzed reaction), and the rate of gold deposition is fast when the potassium hydroxide concentration is low. To prevent the natural decomposition of the gold plating solution, the potassium hydroxide concentration must be maintained at least above 0.1mol/L. The relationship between the gold deposition rate and the potassium hydroxide concentration is shown in Figure 1-35.
![Figure 1-35 Curve of plating rate variation with potassium hydroxide concentration [KAu(CN)2 0.02 mol/L, KCN (solid line) 0.2 mol/L, KCN (dashed line) 0.1 mol/L, 80 °C]](https://sobling.jewelry/wp-content/uploads/2025/11/Page_58_docsmall.com_副本-768x801.jpg)
Figure 1-35 Curve of plating rate variation with potassium hydroxide concentration
[KAu(CN)2 0.02 mol/L, KCN (solid line) 0.2 mol/L, KCN (dashed line) 0.1 mol/L, 80 °C]
![Figure 1-36 Effect of potassium hydroxide concentration on the plating rate of DMAB plating solution, DMAB [KAu(CN)2 0.02mol/L, KCN 0.2mol/L, DMAB 0.4mol/L, 75℃]](https://sobling.jewelry/wp-content/uploads/2025/11/Page_58_docsmall.com_副本1-768x560.jpg)
Figure 1-36 Effect of potassium hydroxide concentration on the plating rate of DMAB plating solution, DMAB
[KAu(CN)2 0.02mol/L, KCN 0.2mol/L, DMAB 0.4mol/L, 75℃]
(Χ.Ε.)3)2Νιου Χάμσαϊρ2 • BH3 + ΩΧ– → (CH3)2Νιου Χάμσαϊρ2 + BH3Ω– (1-11)
(3) Issues with Potassium Borohydride Plating Solution and DMAB Plating Solution
Figure 1-37 shows that the plating rate changes with the concentration of copper and nickel ions in the plating solution. When the nickel ion concentration is at 10-5mol/L, the plating rate begins to decrease, and when the nickel ion concentration reaches 10-3mol/L, the plating solution decomposes. Copper ions have almost no effect on the plating rate. At the initial stage of gold plating, gold ions and nickel undergo displacement reaction, trace amounts of nickel ions dissolve, and the plating rate slows.

Figure 1-37 Effect of Copper Ions and Nickel Ions on Plating Rate
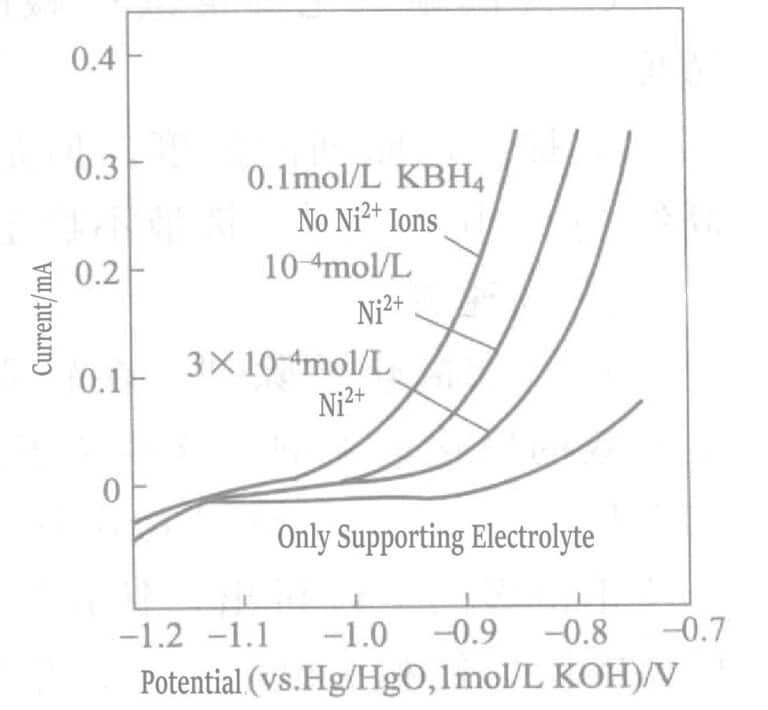
Figure 1-38 Effect of Trace Nickel Ions on the Anodic Oxidation Current of BH3Ω
(Gold Rotating Electrode, 2000r/min, 75℃, KOH 0.2mol/L, KCN 0.1mol/L)
The effect of adding trace amounts of nickel ions on the BH3Ω– anodic oxidation current is shown in Figure 1-38. The addition of trace nickel ions significantly inhibits the oxidation reaction of the reducing agent BH3Ω– on the gold-rotating disk anode. This is because nickel ions exist in the form of cyanide ligand salt Ni(CN)42- , and this complex ion preferentially adsorbs on the gold surface, hindering the deposition of gold.
Unstable base layers (such as aluminum nitride) cannot use strongly alkaline solutions. The pH of the plating solution depends on the nature of the reducing agent, which is the biggest drawback of potassium borohydride and DMAB plating solutions.
Regarding the above issues, except that the pH of the plating solution cannot be changed, other aspects can be improved.
(4) Improve potassium borohydride gold plating solution and DMAB gold plating solution
① Increase plating speed
- a. Change the plating solution’s basic composition and operating conditions to increase plating speed.
- b. Increase stirring speed.
- c. Increase the temperature of the plating solution.
- d. Reduce the concentration of free cyanide.
- e. Decrease the alkali concentration in the potassium borohydride plating solution and increase the alkali concentration in the DMAB plating solution.
- f. Increase the concentration of the reducing agent. However, when the plating rate without special additives increases by about 2μm/h or more, the plating solution becomes unstable and prone to decomposition.
② High-speed plating solution
- a. Add polarization eliminators. In soft gold plating solutions, Pb2+ , T1+ ions are effective polarization eliminators. These ions strongly adsorb on the gold surface, producing the so-called UPD (under potential deposition) phenomenon, where lead deposits at a potential much more positive than the Pb2+ / Pb equilibrium potential and thallium deposits at a potential more positive than the T1+ /T1 equilibrium potential. The deposited lead, thallium and Au(CN)2– undergoes displacement reactions, causing the gold deposition reaction to shift to a more positive potential (polarization weakening). On the other hand, adding these ions to chemical gold plating solutions, similar to electrolytic plating, shifts the Au(CN)2– reduction potential in the positive direction, resulting in an increased plating rate, which can reach about 10μm/h. Plating solutions with added Pb2+ , T1+ ions are shown in Tables 1-35 and 1-36.
Table 1-35 Potassium Borohydride Plating Solution with Added Pb2+
| Solution 1 | Solution 1 | ||
|---|---|---|---|
| Potassium gold cyanide | 5g/L | Colloidal | 2g/L |
| Κυανιούχο κάλιο | 8g/L | Solution 2 | |
| Sodium Citrate | 50g/L | Potassium borohydride | 200g/L |
| EDTA | 5g/L | Sodium hydroxide | 120g/L |
| Lead Chloride | 0.5g/L | ||
Table 1-36 Potassium Borohydride Plating Solution with Added T1+
| Σύνθεση και συνθήκες λειτουργίας | Parameters | Σύνθεση και συνθήκες λειτουργίας | Parameters |
|---|---|---|---|
| Potassium gold cyanide | Adjust as needed | Potassium borohydride | 5. 4 ~ 10. 8g/L |
| Κυανιούχο κάλιο | 6.5g/L | Θερμοκρασία | 70 〜 80℃ |
| Υδροξείδιο του καλίου | 11.2g/L | Plating rate | <10μm/h |
| Thallium sulfate | 5 〜 100mg/L |
Au(CN)4– + 2e– → Au(CN)2– + 2CN– (1-12)
![Figure 1-39 Effect of adding lead chloride on the Au(CN)2- reduction reaction polarization curve of the [Rotating gold electrode, KAu(CN)2 0. 009mol/L, KOH 0. 2mol/L]](https://sobling.jewelry/wp-content/uploads/2025/11/Page_61_docsmall.com_副本-768x578.jpg)
Figure 1-39 Effect of adding lead chloride on the Au(CN)2--reduction reaction polarization curve [Rotating gold electrode, KAu(CN)2 0. 009mol/L, KOH 0. 2mol/L]
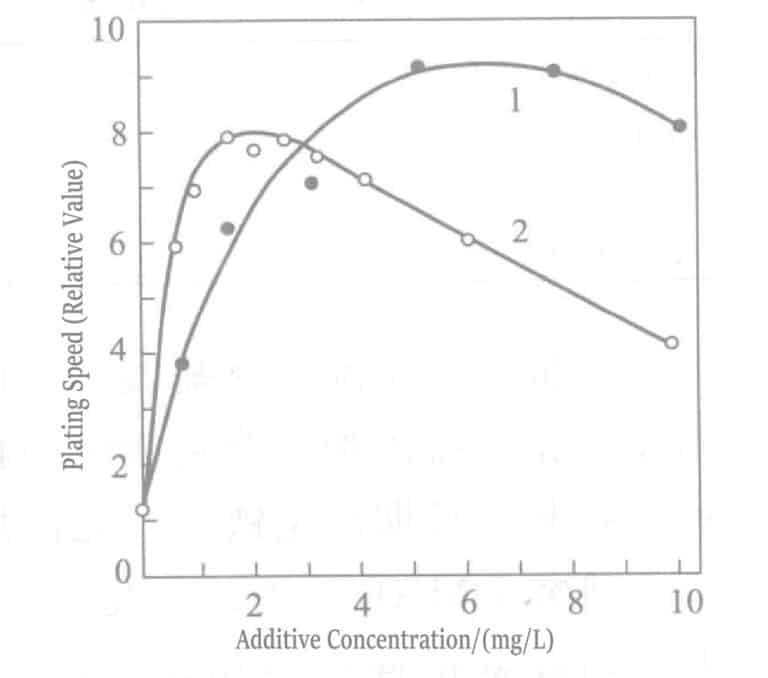
Figure 1-40 Effect of thallium chloride (curve 1) and lead chloride (curve 2) concentration on the gold plating rate of potassium borohydride plating solution
- b. Adding stabilizers. Adding organic stabilizers that reduce the catalytic activity of gold, increasing the plating solution temperature, and changing the concentration of the reducing agent can improve the plating speed of the solution. For example, the plating speed of plating solutions containing amino acetic acid, hydroxyethyl ethylenediaminetetraacetic acid, and diazotriacetic acid or succinic acid derivatives can reach a high rate of 12~23μm/h at 90℃. However, at high temperatures, the hydrolysis reaction of the reducing agent accelerates significantly, making the plating solution difficult to control. Adding trace amounts of p-dimethylamino azobenzene stabilizer does not affect the stability of the plating solution and can increase the usual usage concentration of BH4- by 2 times, raising the plating speed to 5μm/h. Other effective stabilizers include ethylene·glycol. Ethyl·ether, diethylene glycol? ethyl ether, and polyethyleneimine.
- c. Use trivalent gold cyanide complex salts. Plating solutions with trivalent gold cyanide complex salts, such as those with added lead chloride or titanium chloride, as shown in Table 1-33, is detailed in Table 1-37.
Table 1-37 Potassium Gold(III) Cyanide Potassium Borohydride Plating Solution
| Σύνθεση και συνθήκες λειτουργίας | Parameters | Σύνθεση και συνθήκες λειτουργίας | Parameters |
|---|---|---|---|
| Potassium gold(III) | 3g/L | Lead chloride | 0. 5mg/L |
| Υδροξείδιο του καλίου | 11.2g/L | Θερμοκρασία | 70℃ |
| Potassium borohydride | 3g/L |
The advantage of trivalent gold cyanide complex salt plating solution is that KAuO2 and KAu(OH)4 can be used as supplementary gold salt additives, avoiding excessive accumulation of free cyanide ions in the plating solution, reducing the plating rate, and favoring long-term stable plating rate. The plating rate of additive-free the plating solution is 2~8μm/h.
The study of the electrochemical behavior, structure, and properties of the DMAB plating solution shows that similar to the potassium borohydride plating solution, KAu(CN)2 only is used when preparing the gold plating solution, and dissolved gold hydroxide in potassium hydroxide solution is added as a supplement to avoid the accumulation of free cyanide ions. The composition of the plating solution is shown in Table 1-38.
Table 1-38 DMAB Plating Solution (Gold Hydroxide Dissolved in Potassium Hydroxide Solution)
| Σύνθεση και συνθήκες λειτουργίας | Parameters | Σύνθεση και συνθήκες λειτουργίας | Parameters |
|---|---|---|---|
| Potassium gold cyanide | 0.013 ~ 0.018mol/L | Πρόσθετος | Small amount |
| DMAB | 0.07 〜 0.1 mol/L | pH | 13.1 ~ 13.4 |
| Υδροξείδιο του καλίου | 0.09 〜 0.15mol/L | Θερμοκρασία | 70 〜 75℃ |
| Κυανιούχο κάλιο | 0.007 ~ 0.01mol/L | Plating rate | 2μm/h |
(5) Improve the stability of the plating solution.
Using additives can increase the plating rate and the stability of the plating solution, but the impurities produced will cause the plating solution to decompose.
Substances such as EDTA and ethanolamine added to the plating solution form strong, complex ions with metal impurity ions, inhibiting the reaction between metal impurities and BH4– and BH3Ω–, thereby, improving the stability of the plating solution. The composition of the potassium borohydride plating solution with added EDTA and ethanolamine is shown in Table 1-39.
Table 1-39 Potassium Borohydride Plating Solution with Added EDTA and Ethanolamine
| Σύνθεση και συνθήκες λειτουργίας | Parameters | Σύνθεση και συνθήκες λειτουργίας | Parameters |
|---|---|---|---|
| Potassium gold cyanide | 1.45g/L | EDTA | 5g/L |
| Κυανιούχο κάλιο | 11 g/L | Αιθανολαμίνη | 50mL/L |
| Υδροξείδιο του καλίου | 11.2g/L | Θερμοκρασία | 72℃ |
| Potassium borohydride | 10.8g/L | Plating rate | 1. 5μm/h |
Table 1-40 Plating Solution with Two Reducing Agents (Hydrazine and DMAB)
| Σύνθεση και συνθήκες λειτουργίας | Parameters | Σύνθεση και συνθήκες λειτουργίας | Parameters |
|---|---|---|---|
| Potassium gold cyanide | 0. 005mol/L | DMAB | 0,05 mol/L |
| Κυανιούχο κάλιο | 0. 035mol/L | Hydrazine | 0,25mol/L |
| Υδροξείδιο του καλίου | 0. 8mol/L | Θερμοκρασία | 80℃ |
| Ανθρακικό κάλιο | 0. 45mol/L | Plating rate On the Nickel Base Layer (Initial Stage) | 2. 6μm/h |
| Lead acetate | 15X10-6 | On the gold (fixed value) | 7. 8μm/h |
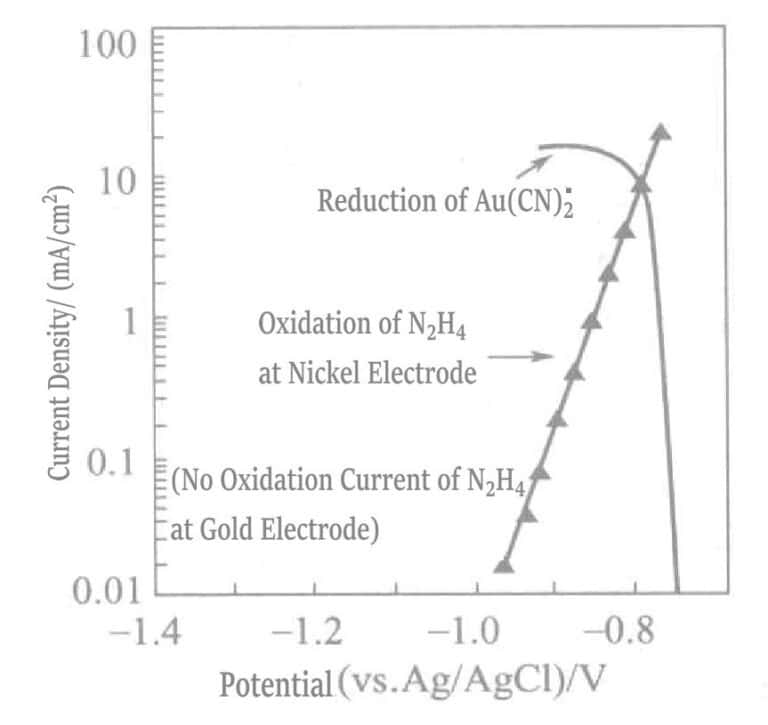
Figure 1-41 Polarization curves of the anodic oxidation reaction of hydrazine on the nickel electrode and the cathodic reduction reaction of Au(CN)2-.
(KOH 0. 8mol/L, KCN 0. 035mol/L, N2H4 0. 05mol/L, 80℃)
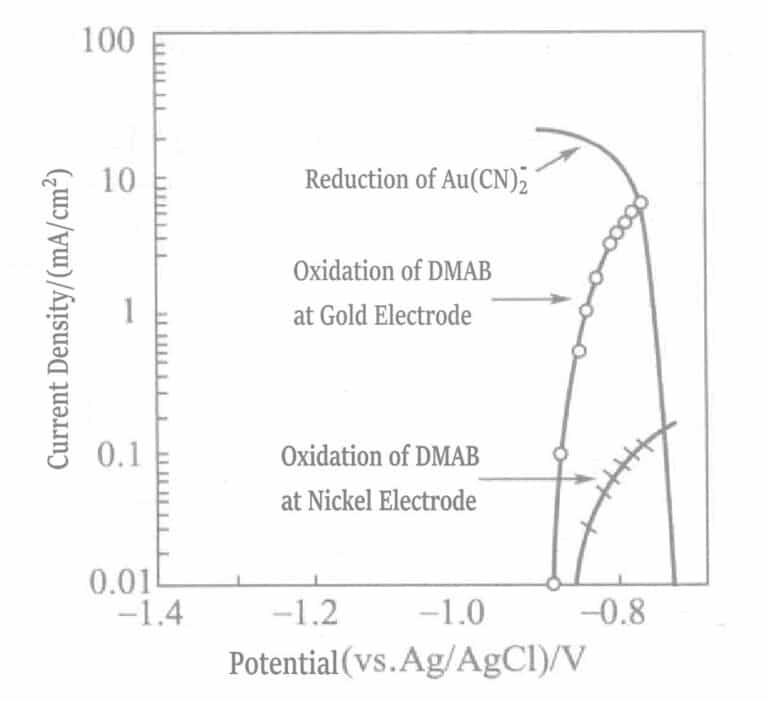
Figure 1-42 Polarization curves of the anodic oxidation reaction of DMAB on nickel and gold electrodes and the cathodic reduction reaction of Au(CN)2-
(KOH 0. 8mol/L, KCN 0. 035mol/L, DMAB 0. 05mol/L, 80℃)
In a plating solution containing only the reducing agent hydrazine, gold can be deposited solely relying on the catalytic effect of the nickel base layer. When the base layer is completely covered by gold, gold deposition stops, and at this point, the thickness of the gold plating layer depends on the concentration of free cyanide in the plating solution. Although the maximum plating thickness of this plating solution is limited (about 2μm), the plating layer is dense enough to meet the requirements of key pressure bonding.
Besides hydrazine, many other available reducing agents are available in the material catalytic gold plating solution; see Table 1-41.
Table 1-41 Other Reducing Agent Plating Solutions
| Αναγωγικό μέσο | pH | Θερμοκρασία/℃ |
|---|---|---|
| Hypophosphite | 7 〜 7. 5 | 93 |
| 7. 5 〜 13. 5 | 96 | |
| 3 〜 4 | 70 〜 80 | |
| Hydrazine | 7 〜 7. 5 | 92 〜 95 |
| 5. 8 〜 5. 9 | 95 | |
| 11. 7 | 95 | |
| Υδροξυλαμίνη | 2. 3 〜 2. 8 | 70 〜 85 |
| Sodium cyanoborohydride (NaBH3CN) | 3.5 | 90 |
| Hydrazinylborane (N2H4 ・ BH3) | >13 | 60 |
| Θειουρία | 6. 5 〜 7. 0 | 83 〜 90 |
| 4.2 | 80 ~ 85 | |
| Ascorbic acid | 8 | 63 |
| Titanium trichloride | 5 | 75 |
Copywrite @ Sobling.Jewelry - Κατασκευαστής προσαρμοσμένων κοσμημάτων, εργοστάσιο κοσμημάτων OEM και ODM
5. Chemical Gold Alloy Plating
(1) Gold-silver alloy:
A chemical gold-silver alloy plating can be achieved by continuously adding potassium silver cyanide solution to the borohydride gold plating solution. Ag(CN)2– is more easily reduced than Au(CN)2–. When preparing a new plating solution, silver will precipitate first if the two are mixed, and the alloy cannot be plated.
By continuously adding small amounts of potassium silver cyanide dropwise into the plating solution, maintaining a relatively low condensation [molar ratio of Au:Ag (26:10~(26:0.5)] A gold-silver alloy plating layer with a silver content of 80%~20% can be precipitated. In this plating solution, the precipitation of silver is a replacement reaction product of the gold that precipitates first and the Ag(CN)2–, in solution.
(2) Gold-Copper Alloy:
In conventional coordination-based EDTA and formaldehyde reducing agent chemical copper plating solution, only potassium gold cyanide can plate a gold-copper alloy. By changing the potassium gold cyanide concentration, the copper mass fraction in the gold alloy plating layer can be adjusted from 1%~94%.
(3) Gold-Tin Alloy:
Gold and tin alloy chemical plating solution use the SnCl2 reducing agent method. The composition and concentration range of the plating solution are shown in Table 1-42.
Table 1-42 Gold-Tin Alloy Plating Solution
| Σύνθεση και συνθήκες λειτουργίας | Parameters | Σύνθεση και συνθήκες λειτουργίας | Parameters |
|---|---|---|---|
| Potassium gold(III) cyanide | 4〜10g/L | Triolefin | 0. 05 ~ 0. 5g/L |
| Κυανιούχο κάλιο | 5〜15g/L | Θερμοκρασία | Θερμοκρασία δωματίου |
| Υδροξείδιο του καλίου | 60〜100g/L | Plating rate | 1.5μm/h (no triolefin addition) |
| Stannous chloride | 40〜60g/L | 5μm/h (triolefin addition 0.5g/L) |
(4) Gold-indium alloy:
The gold-indium alloy plating solution is prepared by adding Ih2(SO4)3 and EDTA to a borohydride solution. The indium content in the plating layer is 1%~4%. At room temperature, a thin gold-indium alloy plating layer deposited on palladium-activated n-GaAs, after 350℃ heat treatment, has a contact resistance superior to pure gold.
(5) Gold-nickel, gold-cobalt alloys:
Uemaki developed 18~22K bright gold-nickel and gold-cobalt alloy plating solutions. pH 3~5, the gold alloy plating layer of cyanide chemical gold plating solution with added reducing agent sodium hypophosphite has physical properties such as hardness and wear resistance inferior to those of gold alloy electroplating layers containing nickel and cobalt.
Section II Cyanide-Free Chemical Gold Plating
1. Επισκόπηση
Displacement-type gold plating uses the potential difference between the oxidation potential of the base layer metal and the reduction potential of gold ions as the plating reaction driving force. In contrast, reduction-type chemical gold plating uses the potential difference between the oxidation potential of the reducing agent and the reduction potential of gold ions as the plating reaction driving force.
The electrons involved in the reduction reaction of gold ions are provided either by the oxidation reaction of the base layer metal (displacement-type gold plating) or by the oxidation reaction of the reducing agent (reductive gold plating).
Other methods include: ① Adding heavy metal salts to the displacement-type gold plating solution, where the base layer metal surface adsorbs the heavy metals, shifting the potential in the direction that makes gold ions easier to reduce; ② Adding a reducing agent to the displacement-type gold plating solution, allowing redox reactions to occur simultaneously to plate thicker gold; ③ In gold plating solutions containing reducing agents, the base layer noble metal acts as a reducing agent and undergoes oxidation with a catalyst, performing base layer catalytic chemical gold plating.
Ligands in chemical gold plating solutions commonly use cyanide, which forms very stable complexes with gold ions (stability constant K=4×1028). Currently, many semiconductor components have switched to using near-neutral, low-cyanide, or cyanide-free gold plating solutions, such as sulfite or thiosulfate gold plating solutions and cyanide-free chemical gold plating solutions with thiol RSH series ligands.
2. Displacement-Type Chemical Gold Plating
Generally, the bonding strength between the reduced-type thick-layer electroless gold plating and the base layer of base metals is relatively poor. Therefore, displacement-type electroless gold plating must be performed before plating a thick gold layer.
In the displacement-type gold plating solution, the base layer’s base metal dissolves (oxidizes) in the electrolyte solution, releasing electrons. In contrast, the gold ions in the solution accept electrons and deposit (reduce) on the non-metal surface. The formation of gold ligands reduces the concentration of gold ions in the plating solution, shifting the reduction potential in the negative direction, as shown in Table 1-43.
Table 1-43 Ligands and Reduction Potentials of Gold Ion Complexes
| Ligands | Ligand | E/V |
|---|---|---|
| H2O |
Au(H2O)2+ Au(H2O)43+ |
1.68 1.50 |
| Cl- |
AuCl2 - AuCl4 - |
1.15 0.92 |
| SCN- |
Au(SCN)2 - Au(SCN)4 - |
0.67 0.64 |
| I- |
AuI2- AuI4- |
0.58 0.57 |
| Νιου Χάμσαϊρ3 | Au(NH)43+ | 0.56 |
| Ω- | Au(OH)4 - | 0.48 |
| Θειουρία | Au(Thu)2+ | 0.38 |
| S2O32- | Au(S2O3)23- | 0.15 |
| SO32- | Au(SO3)23- | 0.06 |
| R-SH | Au(R-S)2- | -0. 3 〜 - 0. 5 |
| ΣΟ- | Au(CN)2 - | -0.65 |
Table 1-43 shows that besides cyanides, other ligands such as thiols, sulfites, and thiosulfates can also form stable complexes with gold ions and exhibit negative potentials.
In the sulfite system displacement-type gold plating solution, besides sulfite ions, ligands such as polyamine polycarboxylic acids and their salts, water-soluble amines, amine salts, ethylenediamine triacetate salts, stabilizers like tetraalkylammonium salts, ethylenediamine tetra (methylene phosphonate), sugars, and thiol compounds can also be used. The concentrations of ammonium ions, chloride ions, sulfate ions, bromide ions, or iodide ions must be maintained within a specific range and not be too high; otherwise, ammonium (complex)–gold ligands may form in the plating solution, whose redox potential is more positive than sodium sulfite. When the plating solution is left standing or during gold plating, sodium sulfite may oxidize and reduce gold, causing instability in the plating solution.
Thiol succinate, acetylcysteine, cysteine, and other thiol series compounds and gold ions can form stable ligands in cyanide-free gold-plating solutions. Thiol succinate [HOOCCH(SH)-CH2COOH] and the reduction potential of the gold ion ligand, that is, Au(HOOCCHSCH2COOH)2– + e– ⇌Au(s) + 2 ( HOOCCH – SCH2COOH)–, the correct value of the standard electrode potential for the reaction is difficult to obtain. The measured reduction potential of the plating solution is about -0.3~0.5V. The thiol succinate gold ligand exists in the form of [Au(HOOC – CHSCH2COOH)2]–, with gold in the +1 oxidation state.
HAuCl4 + 3H2O → Au(OH)3 + 4HCl (1-13)
Au(OH)3 + 4[HOOCCH(SH)CH2 COOH] → [Au(HOOCCH—S—CH2COOH)4 ]– + 3H2O+H+ (1-14)
[Au( HOOCCH—S—CH2COOH)4]– → [Au(HOOCCH—S—CH2COOH)2]– + HOOCH2CHOOCCH—S—S—CHCOOHCH2COOH (1-15)
Table 1-44 Cyanide-Free Displacement Gold Plating Solution
| Σύνθεση και συνθήκες λειτουργίας | Sulfite system | Mercaptosuccinic acid system | ||
|---|---|---|---|---|
| Sodium gold chlorate/(mol/L) | 0.05 | |||
| Sodium thiosulfate/(mol/L) | 0.028 | 0.015 | ||
| Gold mercaptosuccinate/(mol/L) | 0.01 | |||
| Sodium sulfite/(mol/L) | 0.52 | 0.1 | ||
| Mercaptosuccinic acid/(mol/L) | 0.25 | 0.25 | ||
| Trisodium citrate/(mol/L) | 0.22 | |||
| Acetylcysteine/(mol/L) | 0.03 | |||
| Tetramethylammonium chloride/(mol/L) | 0.8 | |||
| EDTA/( mol/L) | 0.015 | |||
| EDTA - 2Na/(mol/L) | 0.02 | |||
| Amino tris(methylenephosphonic acid)/(mol/L) | 0.1 | |||
| Sodium carboxymethyl cellulose/(g/L) | 10 | |||
| pH | 7.0 | 7.0 | 1.5 (Adjusted with hydrochloric ) | 7.0 |
| Θερμοκρασία /℃ | 60 | 85 | 80 ~ 90 | |
3. Reduced-Type Chemical Thick Gold Plating Layer
In gold plating solutions with thiosulfate as the ligand, sodium sulfite prevents the decomposition of thiosulfate ions. In NaAuCl4 plating solutions containing trivalent gold salts, monovalent gold is reduced by excess thiosulfate. In weakly alkaline gold plating solutions, pH buffers such as ammonium chloride, sodium tetraborate, and boric acid are commonly added.
Thiol compounds can form ligands with gold ions that have excellent stability and act as reducing agents. These thiol compounds include L-cysteine and 2-ethanamine thiol, among others. Table 1-45 lists the composition and operating conditions of cyanide-free reduced-type chemical gold plating solutions using sodium thiosulfate and thiol compounds as ligands.
Table 1-45 Cyanide-Free Reduction-Type Gold Plating Solution
| Σύνθεση και συνθήκες λειτουργίας | Thiosulfate system | Thiol system | |||
|---|---|---|---|---|---|
| Gold chlorate/(g/L) | 0.01 | ||||
| Sodium gold chlorate/(g/L) | 0.0125 | 0.0125 | |||
| Sodium gold sulfite/(g/L) | 0.02 | ||||
| Gold mercaptosuccinate/(g/L) | 0.01 | ||||
| Mercaptosuccinic acid/(g/L) | 0.27 | ||||
| Sodium thiosulfate/(g/L) | 0.1 | 0.17 | 0.1 | ||
| Sodium sulfite/(g/L) | 0.1 | 0.4 | |||
| Ammonium sulfite/(g/L) | 0.43 | ||||
| EDTA ・ 2Na/(g/L) | 0.19 | ||||
| Triethanolamine/(g/L) | 0.034 | ||||
| Ammonium chloride/(g/L) | 0.05 | ||||
| Sodium tetraborate/(g/L) | 0.13 | ||||
| Φωσφορικό διυδρογόνο κάλιο/(g/L) | 0.15 | ||||
| Θειουρία/(g/L) | 0.0033 | ||||
| Hydroquinone/(g/L) | 0.002 | ||||
| Ascorbic acid/(g/L) | 0.25 | ||||
| Hydrazine/(g/L) | 0.3 | ||||
| L-cysteine/(g/L) | 0.08 | ||||
| 2-aminoethyl mercaptan/(g/L) | 0.2 | ||||
| Potassium benzotriazole/(g/L) | 0.05 | ||||
| pH | 7.5 | 7.0 | 8.0 | 7.0 | 7.5 |
| Θερμοκρασία/℃ | 60 | 80 | 70 | 80 | 80 |
4. Base Layer Catalytic Chemical Gold Plating
Base layer catalytic chemical gold plating is a reduction-type chemical gold plating method using a reducing agent. The reducing agent only has catalytic activity on the surface of the substrate, the base metal (nickel) layer, and has no catalytic activity on the deposited gold surface. The catalytic chemical gold plating layer is smoother, denser, and has fewer or smaller pores than the displacement-type gold plating layer.
In thiosulfate and sulfite chemical gold plating solutions, gold can be deposited on the nickel surface without adding other reducing agents. On the nickel surface, sulfite only reduces the thiosulfate gold complex and does not act on the gold surface, so it is called a base layer catalytic type of chemical gold plating. A displacement gold plating reaction also occurs simultaneously on the surface of the base nickel plating layer. Therefore, the reducing agent sulfite undergoes different oxidation reactions influenced by the composition of the base nickel plating layer and pretreatment conditions. The concentration of the thiosulfate sodium ligand or the pH of the plating solution affects the different ratios of displacement and reduction reactions, the maximum thickness of the gold plating layer, appearance, porosity, and adhesion, so it is necessary to select an appropriate plating solution composition and gold plating conditions.
5. Stability of Cyanide-Free Chemical Gold Plating Solution
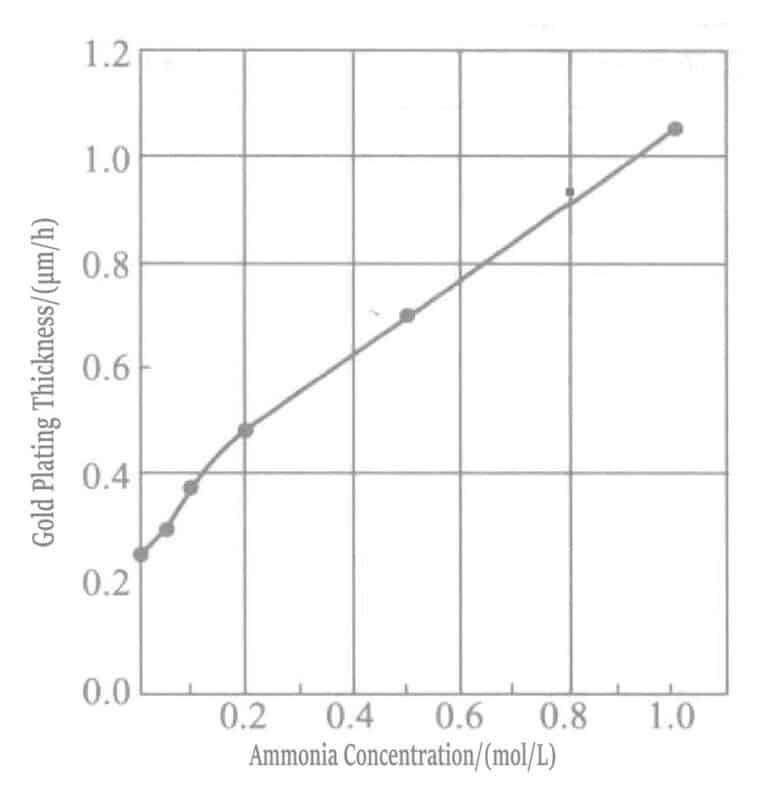
Increasing the concentration of ammonia water accelerates the deposition rate of the gold plating layer. In plating solutions with high ammonia concentration, mercapto succinate acts as a reducing agent, and a reduction reaction occurs simultaneously with the displacement reaction.
The contamination of copper or iron ions is the main cause of instability in cyanide-free chemical gold plating solutions. Copper dissolved from the nickel plating layer’s pores and iron from the substrate easily promote the instability of the chemical gold plating solution.
Reasons: ① Gold ions accept electrons released when these metals oxidize, increasing the reduction rate. ② Copper ions or iron ions catalyze the oxidation reaction of sulfite or thiol, accelerating the reaction rate, which causes defects on the surface of the chemical gold plating layer and reduces welding performance and bonding strength. In chemical gold plating solutions, the dissolution and contamination of these metals should be suppressed by adding ligands that form stable complexes with these dissolved and contaminated metal ions.
6. Cyanide-Free Chemical Gold Plating Solution
(1) Tetrachloroaurate (III) salts and weakly reducing amine borane gold plating solutions
Tetrachloroaurate (III) salts (NaAuCl4) are easily reduced to deposit gold. Ether-substituted tertiary amine borane reducing agents can be used with NaAuCl4 to form an autocatalytic plating solution, or reducing agents such as trimethylamine borane, methylmorpholine borane, and diisopropylamine borane, along with stabilizers like thiols and iodide compounds.
(2) Gold plating solution with gold sulfite salt:
Currently a large number of monovalent gold sulfite plating solutions are used, with hypophosphites, formaldehyde, hydrazine, tetrahydroborate and DMAB as reducing agents. Gold sulfite salt [Na3Au(SO3)2] is unstable in water and requires the addition of stabilizers such as 1,2-diaminoethane and potassium bromide.
(3) Thiosulfate gold plating solution
① Gold plating solution with thiourea and its derivatives as reducing agents:
The combination of monovalent gold thiosulfate and thiourea plating solution has good stability, does not produce hydrogen gas around neutral pH, and has no porosity. The composition of the plating solution is shown in Table 1-46 (Plating Solution A).
Table 1-46 Thiosulfate Gold Plating Solution
| Σύνθεση και συνθήκες λειτουργίας | Plating Solution A | Plating Solution B |
|---|---|---|
| NaAuCl4 / (mol/L) | 0.1 | 0.0125 |
| Na2S2O3/(mol/L) | 0.08 | 0.1 |
| Na2SO3 /(mol/L) | 0.4 | 0.1 |
| Na2B4O7/( mol/L) | 0.1 | - |
| NH4Cl/(mol/L) | - | 0.05 |
| Thiourea/(mol/L) | 0.1 | - |
| Sodium L-ascorbate/(mol/L) | - | 0.25 |
| pH | 9.0 | 6.0 |
| Θερμοκρασία/℃ | 80 | 60 |
| Plating rate/(μm/h) | 1. 9 〜 2. 3 | 1. 5 〜 2. 0 |
② Gold plating solution with ascorbic acid as a reducing agent:
In the thiosulfate plating solution with L-ascorbic acid sodium as the reducing agent, sodium sulfite is present, which can stably plate gold. The composition of the plating solution is shown in Table 1-46 (Plating Solution B).
Effective reducing agents in sodium thiosulfate plating solutions, besides thiourea and sodium ascorbate, include sodium tartrate, glycolic acid, and hypophosphorous acid.
③ Reaction mechanism of thiosulfate plating solution:
Gold salt reacts with thiosulfate to produce Au(S2O3)23-. When there is no thiosulfate in the solution, only sulfite is present and formed Au(SO3)23-. The reaction equation is as follows:
Au3++ 2S2O3 2- + Η2O ⇌ Au(S2O3)23- + SO4 2- + 2 ώρες+ (1-16)
Au3+ + 3SO32- + Η2O ⇌ Au(SO3 )23- + SO42- + 2 ώρες+ (1-17)
Au+ + 2SO32- ⇌ Au(SO3)23- K=1010 (1-18)
Au+ + 2S2O32- ⇌ Au(S2O3)23- K = 1026 (1-19)

Figure 1-44 Comparison of cathodic polarization curves of gold deposition reactions in sulfite and thiosulfate plating solutions
(NH4Cl 0. 1mol/L, Na2SO3 0. 2mol/L)
Thiosulfate has a stronger complexing ability with monovalent gold than sulfite, so monovalent gold is more difficult to deposit from plating solutions containing sodium thiosulfate than those containing sulfite.
In the sulfite gold plating solution with the reducing agent ascorbate, due to the difference in cathodic polarization curves between gold sulfite complex ions and gold thiosulfate complex ions, the gold deposition rate is only 1/10 of that in the sodium thiosulfate plating solution. Figure 1-45 shows ascorbate sulfite and thiosulfate gold’s cathodic and anodic polarization curves.
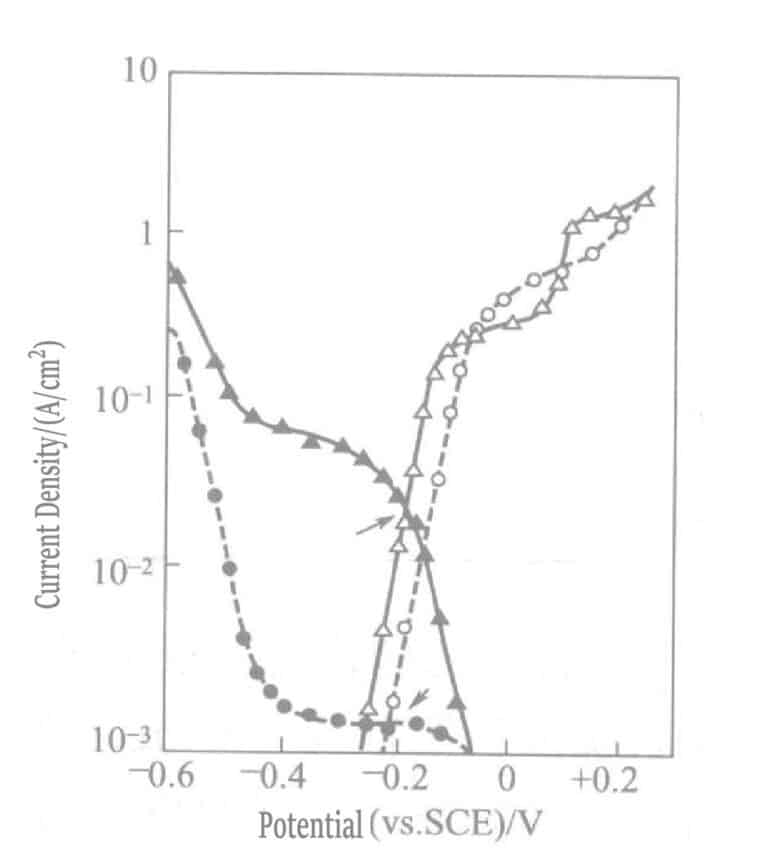
Figure 1-45 Cathodic and anodic polarization curves of ascorbic acid, sulfite plating solution, and thiosulfate gold
(Gold electrode, NH4Cl 0. 1mol/L, pH 6. 0, 60℃. Curve "●" plating solution: Na2SO3 0.2mol/ L and NaAuCl4 0.01mol/L; Curve "▴" same plating solution as before, with the addition of Na2S2O3 0. 1mol/L; Curve "○" plating solution: Na2SO3 0. 2mol/L and sodium ascorbate 0. lmol/L; Curve "△" solution, same as before, with the addition of Na2S2O3 0.1mol/L)
The reaction principle of thiosulfate electroless gold plating solution refers to the oxidation state of the reducing agent, and the local reactions are as follows.
Cathodic reaction:
Au(S2O3)23- + 2e– → Au + 2S2O32- (1-20)
Anodic reaction:
Thiourea plating solution
CS(NH2)2 + 5H2O → CO(NH2)2 + Η2SO4 + 8H+ + 8e– (1-21)
The reaction product CO(NH2)2 is urea.
Ascorbic acid plating solution
C6H8O6 → C6H6O6 + 2 ώρες+ + 2e–
7. Gold Plating Solution of Sugar Additive with Gold Sulfite Salt
After adding saccharide compounds to the gold sulfite plating solution, it can remain stable for a long time, and the gold plating layer is good. The composition of the plating solution is as follows.
Gold sulfite salts: potassium gold sulfite, sodium gold sulfite, ammonium gold sulfite, etc.
Sulfites: potassium sulfite, sodium sulfite, ammonium sulfite, etc.
pH adjuster: Adjust to pH 6~9 with various buffers.
Stabilizers: water-soluble amine compounds, ethylenediamine, diethylenetriamine, triethylenetetramine, etc.; Water-soluble amino acids or salts, ethylenediamine tetraacetic acid, triethylenetetramine hexaacetic acid, trans-1,2-cyclohexane diamine tetraacetic acid or salts, etc.; water-soluble organophosphates or salts, amino tris (methylenephosphonic acid), 1-hydroxyethylidene-1,1-diphosphonic acid, ethylenediaminetetra(methylene phosphonic acid), diethanolamine, pentakis(methylene phosphonic acid) or salts, etc.; water-soluble aromatic nitro compounds can also be added, such as mono-, di-, and tri-nitrobenzol acid, mono- and di-nitrosalicylic acid, nitrobenzene dicarboxylic acid, mono-, di-, and tri-nitrophenol, dinitroaminophenol, mono-, di-, and tri-nitrobenzene, etc.
Sugars other than starch can also include hexoses, glucose, mannose, galactose and other monosaccharides, erythritol, pentitol, hexanol and other sugar alcohols, glucaric acid and other aldaric acids, gluconic acid, hexatonic acid and other aldonic acids, oligosaccharides, etc. These sugar compounds can increase the stability of the plating solution and expand the current density range of the bright plating area.
The plating results are shown in Table 1-47.
Table 1-47 Various Sulfite Gold Plating Baths, Operating Conditions, and Effects of Additives
| Serial Number | Various Sulfite Gold Plating Baths and Operating Conditions | Additive Effect |
|---|---|---|
| Νο. 1 |
Gold sodium sulfite 10g/L Sodium sulfite 65g/L Trisodium citrate 65g/L Ethylenediaminetetramethylenephosphonic acid (EDTMP) 85g/L pH 7 Temperature 60℃ Total current 0.2A Specimen substrate:42Fe-Ni alloy Ideal current density method with strong stirring |
Electrolysis 1080 ℃, electroplating solution decomposition, producing black particles. Appearance of plating layer with different current densities produces spots and scorching. |
| No.1 Add sodium carboxymethyl cellulose (CMC) 10g/L to the plating solution. Other conditions are the same as No.1 | Electrodeposition 1740℃, plating solution decomposition, black particles. The appearance of plating layer is improved, and spots and scorching are obviously reduced. | |
| Αρ. 2 |
Gold sodium sulfite 10g/L Sodium sulfite 130g/L Trisodium citrate 65g/L Triammonium citrate 65g/L p-Nitro(benzene)phenol 1g/L pH 7 Temperature 40℃ Total current 0.2A Specimen substrate: copper Strong stirring |
High current density areas burnt, plating layer appearance is very poor, appear diffuse spots |
| No.2 plating solution with starch 5g/L, other conditions are the same as No.2. | No burning in high current density area, good appearance of plating layer in wide current density range, stable plating solution. | |
| Αρ. 3 |
Sodium gold sulfite 10g/L Sodium sulfite 100g/L Disodium borate 50g/L Boric acid 100g/L p-Nitro(benzene)phenol 1g/L pH 7 Temperature 40℃ Total current 0.2A Specimen substrate: copper Strong stirring |
Poor appearance of plating layer |
| Add 5g/L starch to No.3 plating solution, other conditions are the same as No.3. | Good appearance of plating layer in wide range of current density, stable plating solution. | |
| Αρ. 4 |
Sodium gold sulfite 12g/L Sodium sulfite 100g/L Phosphite 3g/L Ethylenediamine hydrate 30g/L pH 7 Temperature 60°C Total current 0.2A Specimen substrate:42Fe-Ni alloy Strong stirring |
High current density area burnt, plating layer appearance is extremely poor |
| No.4 add starch 5g/L to the plating solution, other conditions are the same as No.4. | Good appearance of plating layer and stability of plating solution in wide range of current density. | |