Co dělá nerezovou ocel a slitinu titanu ideálními pro výrobu šperků? Přehled výroby a trendy na trhu
Stainless Steel & Titanium Jewelry Making: Techniques for Modern Designers
Stainless steel was initially used in watches and luxury pens from Rolex and other well-known fashion brands. This material is tough, has excellent corrosion resistance, and maintains its color at room temperature, unlike silver jewelry, which easily tarnishes, or alloy jewelry, which can be toxic due to lead content. As a result, it is increasingly applied in the jewelry industry, becoming a common material for trendy fashion accessories. Stainless steel jewelry features a rugged, minimalist, stable, and understated style with a cool metallic appearance, earning recognition and affection from many fashion enthusiasts.
Titanium is highly corrosion-resistant and stable, and its unique silver-gray tone performs well in high polish, silk finish, and matte finishes. It is one of the most suitable decorative metals besides precious metals and is often used in modern jewelry design abroad. Titanium has futuristic qualities, showcasing elegance while remaining timeless. It is lightweight yet exceptionally strong, making it a popular material for jewelry internationally, especially favored by young professionals.

Kroužek z nerezové oceli
Obsah
Section I Stainless Steel Products and Production Processes
1. Introduction to Stainless Steel
1.1 Definition of Stainless Steel
Stainless steel is a general term for steel with a certain chemical stability in solutions such as atmosphere, water, acids, alkalis, salts, or other corrosive media. Generally speaking, steel resistant to corrosion from weak media such as atmosphere, steam, and water is called stainless steel. Steel resistant to corrosion from acidic, alkaline, and saline corrosive media is called corrosion-resistant or acid-resistant steel. Stainless steel has rust resistance but is not necessarily corrosion-resistant, while corrosion-resistant steel generally has better rust resistance.
The corrosion resistance of stainless steel is generally believed to result from the formation of a “passivation film” on its surface under the action of corrosive media. The ability to resist corrosion depends on the stability of the “passivation film.” This is related not only to the chemical composition of the stainless steel but also to the type, concentration, temperature, pressure, flow rate of the corrosive media, and other factors.
Stainless steel has good corrosion resistance due to the addition of chromium to the iron-carbon alloy. Although other elements, such as copper, aluminum, silicon, nickel, and tantalum, can also improve the corrosion resistance of steel, their effectiveness is limited in the absence of chromium. Therefore, chromium is the most important element in stainless steel. The minimum chromium content required for stainless steel with good corrosion resistance depends on the corrosive medium. American Iron and Steel Institute (The AISI) defines the boundary between non-stainless steel and other steels with 4% chromium. The Japanese Industrial Standard JIS G 0203 stipulates that stainless steel is an alloy containing chromium or chromium-nickel to improve corrosion resistance, generally with a chromium content greater than 11%. The German DIN standard and the European standard EN10020 specify that the chromium content of stainless steel is not less than 10.5%. The carbon content is not more than 1.2%. In our country, the chromium content of stainless steel is generally defined as not less than 12%.
1.2 Common Alloying Elements of Stainless Steel
Various elements mainly determine the performance and structure of stainless steel. Currently, there are more than 100 known chemical elements, among which the elements that have the greatest impact on the performance and structure of stainless steel are carbon, chromium, nickel, manganese, nitrogen, titanium, niobium, molybdenum, copper, aluminum, silicon, zirconium, yttrium, boron, and more than a dozen others. Adding these elements leads to changes in the internal structure of the steel, giving it special properties. To deepen our understanding of stainless steel, it is necessary to first understand the impact of various elements on the performance and structure of stainless steel.
(1) Chromium
Chromium is the most fundamental element determining the corrosion resistance of stainless steel. In oxidizing media, chromium can quickly form a layer on the surface of the steel that is impermeable and insoluble to the corrosive medium, which is a chromium-rich oxide film. This oxide film is very dense and firmly bonded to the metal, protecting the steel from further oxidation and corrosion by external media; chromium can also effectively increase the electrode potential of the steel. When the chromium content is not less than 12.5% atoms, it can cause a sudden change in the electrode potential of the steel, rising from a negative potential to a positive electrode potential. Therefore, it can significantly improve the corrosion resistance of the steel. The higher the chromium content, the better the corrosion resistance of the steel. When the chromium content reaches 25%, 37.5% atoms, second and third sudden changes occur, giving the steel even higher corrosion resistance.
(2) Nickel
The effect of nickel on the corrosion resistance of stainless steel can only be fully demonstrated when combined with chromium. This is because low-carbon nickel steel requires a nickel content of 24% to achieve a pure austenitic structure (austenite is a non-magnetic solid solution containing a small amount of carbon in γ-Fe, with a face-centered cubic crystal structure); to significantly alter the corrosion resistance of steel in certain media, the nickel content must be above 27%. Therefore, nickel cannot constitute stainless steel on its own. However, by adding 9% nickel to 18% chromium-containing steel, the steel can achieve a single austenitic structure at room temperature, which can enhance the steel’s corrosion resistance to non-oxidizing media (such as dilute sulfuric acid, hydrochloric acid, phosphoric acid, etc.) and improve the process performance of the steel in welding and cold bending.
(3) Manganese and nitrogen – can replace nickel in chromium-nickel stainless steel
Manganese and nitrogen play a role in stainless steel, similar to nickel. The stabilizing effect of manganese on austenite is comparable to that of 1/2 nickel, while the effect of nitrogen is much greater, about 40 times that of nickel. Therefore, manganese and nitrogen can replace nickel to achieve a single austenitic structure. However, adding manganese will reduce low-chromium stainless steel’s corrosion resistance. Additionally, high-manganese austenitic steel is not easy to process. Therefore, manganese is not used alone in stainless steel; it is only used partially to replace nickel.
(4) Carbon
The content and distribution of carbon in stainless steel largely influence its performance and structure. On one hand, carbon is a stabilizing element for austenite, with an effect approximately 30 times greater than that of nickel. High carbon martensitic stainless steel (martensite is a supersaturated solid solution of carbon dissolving in α-Fe, which is a metastable phase transformed from austenite through a diffusionless phase change) can fully accept quenching strengthening, significantly improving its strength in terms of mechanical properties; on the other hand, due to the strong affinity between carbon and chromium, chromium, which occupies 17 times the amount of carbon in stainless steel, combines with it to form chromium carbide. As the carbon content in steel increases, more chromium forms carbides with carbon, significantly reducing the corrosion resistance of the steel. Therefore, from the perspectives of strength and corrosion resistance, the role of carbon in stainless steel is contradictory. In practical applications, to achieve corrosion resistance, the carbon content in stainless steel is generally low, mostly around 0.1%. To further enhance the corrosion resistance of the steel, especially its resistance to intergranular corrosion, ultra-low carbon stainless steel is often used, with carbon content at 0.03% or even lower; however, for manufacturing rolling bearings, springs, tools, and other stainless steels, higher carbon content is required due to the need for high hardness and wear resistance, generally between 0.85%~1.00%, such as 9Cr18 steel, etc.
(5) Titanium and Niobium
When stainless steel is heated to 450~800℃, the chromium content near the grain boundaries often decreases due to the precipitation of chromium carbides at the grain boundaries, forming a chromium-depleted zone, which leads to a decrease in the electrode potential near the grain boundaries, thereby causing electrochemical corrosion, known as intergranular corrosion. A common occurrence is intergranular corrosion in the heat-affected zone near welds. Sodium and niobium are strong carbide-forming elements, and their affinity for carbon is much greater than that of chromium. By adding titanium or niobium to the steel, the carbon in the steel can first form carbides with titanium or niobium instead of chromium, thus ensuring that intergranular corrosion does not occur due to chromium depletion near the grain boundaries. Therefore, sodium and niobium are often used to fix carbon in steel, enhance the resistance of stainless steel to intergranular corrosion, and improve the welding performance of the steel.
The amount of titanium or niobium to be added should be determined based on the carbon content. Generally, titanium is added five times the carbon content, and niobium is eight times the carbon content.
(6) Molybdenum and Copper
Molybdenum and copper can enhance stainless steel’s corrosion resistance against corrosive media such as sulfuric and acetic acids. Molybdenum can also significantly improve resistance to media containing chloride ions (such as hydrochloric acid) and organic acids. However, molybdenum-containing stainless steel is not suitable for use in nitric acid, as the corrosion rate of molybdenum-containing stainless steel in boiling 65% nitric acid is doubled compared to that without molybdenum; the addition of copper to chromium-manganese-nitrogen stainless steel can accelerate intergranular corrosion of the stainless steel.
Molybdenum hinders the obtaining of a single austenitic structure in steel; therefore, in molybdenum-containing steel, the content of elements such as nickel and manganese should be correspondingly increased to ensure that the steel has a single austenitic structure after heat treatment.
(7) Silicon and Aluminum
The role of silicon in improving the oxidation resistance of chromium steel is significant. Steel containing 5% chromium and 1% silicon can have oxidation resistance comparable to that of 12% chromium steel. If the steel in 1000 ℃ can resist the chemical, containing 0.5% silicon needs 22% of the chromium, such as the addition of 2.5% to 3% of the silicon later, only 12% of the chromium can be. Information indicates that adding 2.5% silicon to Cr15Ni20 chromium-nickel steel can achieve oxidation resistance comparable to that of Cr15Ni60 chromium-nickel alloy.
Adding aluminum to high chromium steel can also significantly improve its oxidation resistance, and its function is similar to adding silicon.
Adding silicon and aluminum to high-chromium steel is intended to further improve the steel’s oxidation resistance and save chromium.
Although silicon and aluminum significantly improve the oxidation resistance of chrome steel, they also have many drawbacks. The main issue is that they cause the steel’s grain to coarsen and increase its tendency to become brittle.
(8) Tungsten and Vanadium
The main role of tungsten and vanadium in steel is to improve its thermal strength.
(9) Boron
0.005% Boron (ferrite, which is a solid solution of carbon in α-Fe, with a body-centered cubic lattice) added to high chromium ferritic stainless steel (Cr17Mo2Ti) can improve the corrosion resistance of the steel in boiling 65% acetic acid; adding trace amounts (0.006‰~0.007‰) of boron to austenitic stainless steel can improve the hot plasticity of the steel; boron has a good effect on improving the thermal strength of steel, significantly enhancing the thermal strength of stainless steel; boron-containing chromium-nickel austenitic stainless steel has special applications in the atomic energy industry. However, the presence of boron in stainless steel can reduce the plasticity and impact toughness of the steel.
In addition to the above elements, some stainless steels contain rare metals and rare earth elements to improve their performance. In stainless steels used in industrial applications, many steels contain several to dozens of alloying elements at the same time. When several elements coexist in this unified body of stainless steel, the structure of the stainless steel is determined by the sum of the influences of various elements.
The influence of various elements on the microstructure of stainless steel can be broadly categorized into two main types based on their commonality: one type consists of elements that form or stabilize austenite, which include carbon, nickel, manganese, nitrogen, and copper, with carbon and nitrogen having the greatest effect; the other type consists of elements that form ferrite, which include chromium, tungsten, tantalum, niobium, silicon, titanium, vanadium, and aluminum. When compared to chromium as a reference, the effect of this type of element in forming ferrite is greater for all other elements than for chromium.
When these two types of elements coexist in stainless steel, the structure of the stainless steel depends on the results of their mutual influence. If the role of the elements that stabilize austenite is predominant, the structure of the stainless steel will be primarily austenitic, with little to no ferrite; if their influence is not sufficient to maintain the austenite in the steel at room temperature, this unstable austenite will undergo a martensitic transformation upon cooling, resulting in a martensitic structure; if the role of the elements that form ferrite becomes predominant, the structure of the steel will be primarily ferritic.
Apart from process factors, stainless steel’s performance mainly depends on the composition of its internal structure, which is determined by the sum of various alloying elements in the steel. Therefore, the alloying elements ultimately determine the performance of stainless steel.
1.3 Classification of Stainless Steel
Stainless steel is a special steel series with a wide range. More than 100 types of stainless steel grades are produced in our country. Based on their main alloy components, metallographic structure, and primary industrial applications, stainless steel can be roughly classified as follows.
(1) Classification based on the Alloy Composition of Stainless Steel
According to the main alloy components of stainless steel, it can be divided into the following three categories.
① Chromium stainless steel. Apart from the iron base, this type of stainless steel mainly contains chromium as the alloying element. Some also contain one or more of the elements Silicon, aluminum, tungsten, molybdenum, nickel, titanium, vanadium, and others, with the content of these elements in the steel being 1%~3%.
② Chromium-nickel stainless steel. Besides the iron base, this type of stainless steel mainly contains chromium and nickel as alloying elements. Some also contain one or more elements, such as titanium, silicon, molybdenum, aluminum, vanadium, and boron, with these elements present in amounts below 4% to trace levels.
③ Chromium-manganese-nitrogen stainless steel. This type of stainless steel, in addition to its iron base, mainly contains chromium and manganese as alloying elements. Most steels also contain nitrogen below 0.5%, with some also containing one or several elements such as nickel, silicon, and copper. The content of these elements in the steel is respectively only below 5%.
(2) Classification based on the Structure of Stainless Steel
Stainless steel is usually divided into three categories based on its structure (metallographic organization).
① Ferritic type. That is, stainless steel containing chromium but not nickel. This type of steel can be hardened to a certain extent by cold working but not by heat treatment. This type of steel is always magnetic.
② Martensitic type. This type of stainless steel, except for a few grades that contain a small amount of nickel, mostly contains only chromium. Its advantage is that heat treatment can harden it. This type of steel is always magnetic.
③ Austenitic type. That is, stainless steel containing elements such as chromium, nickel, or chromium, nickel, manganese, or chromium, manganese, nitrogen, etc. This type of steel can only be hardened by cold working; heat treatment can only soften it. In the annealed state, it is non-magnetic. After cold working, some may become magnetic.
The above three classifications are based solely on steel’s matrix structure. Due to the inability of the elements that stabilize austenite and form ferrite in steel to balance each other, the actual microstructures of stainless steel used in the industry also include martensite-ferrite, austenite-ferrite, austenite-martensite, and other transitional duplex stainless steels, as well as martensite-carbide-structured stainless steel.
2. Accessories made of Stainless Steel
2.1 Requirements for Materials of Stainless Steel Jewelry
(1) Mechanical Properties
Plastic processing technology has been widely used in producing stainless steel jewelry. In addition to using drawing and rolling machinery to produce sheets, wires, pipes, and other profiles, it is also often used for the forming processing of jewelry, such as using machine tools for finishing, using a stamping machine and a hydraulic press for hydraulic operations. To ensure the quality of plastic processing products, in addition to correctly formulating and strictly adhering to operational process specifications, there are clear requirements for the mechanical properties of the materials. The mechanical properties of the materials are mainly reflected in indicators such as tensile strength, yield strength, hardness, elongation, and toughness. Stainless steel materials are required for good plastic processing performance, especially during operations such as drawing, rolling, stamping, and hydraulic pressing. The hardness of the materials should not be too high, and the work hardening rate of the materials should be slower to facilitate operation; the materials should have good ductility; otherwise, cracks are likely to occur.
(2) Polishing Performance
Jewelry has clear requirements for surface quality, and most jewelry must be polished to achieve a mirror-like shine. This requires not only the correct execution of the polishing process but also the inherent properties of the material to have a significant impact. For example, the workpiece must have a dense structure with fine and uniform grains, free of defects such as pores and inclusions. If the workpiece’s grains are coarse or there are shrinkage or pore defects, it is easy to develop orange peel, polishing depressions, and comet tail phenomena. Similarly, if there are hard inclusions, scratches, and comet tail defects can easily occur.
Factors affecting the polishing performance of stainless steel jewelry mainly include the following points:
- Surface defects of raw materials, such as scratches, pitting, and excessive pickling.
- Raw material quality issues. If the hardness is too low, it is difficult to achieve a bright polish, and the surface is prone to orange peel during deep stretching, which affects the polishability. Higher hardness generally results in better polishability.
- Products undergoing deep stretching may have small black dots on areas with significant deformation, which can affect the polishing quality.
(3) Corrosion Resistance
Corrosion resistance is very important for jewelry. The corrosion resistance of materials varies with composition; 316 has better corrosion resistance than 304, but composition is not the only factor affecting tarnishing. Tarnishing and discoloration result from a combination of chemical composition, environmental factors, microstructure, and surface condition.
Accelerated corrosion tests, which commonly include salt spray tests and immersion tests, are generally required to determine the jewelry’s corrosion resistance.
(4) Casting Performance
The casting performance of alloys significantly impacts the surface quality of cast jewelry. The quality of alloy casting performance can be assessed from several aspects, including the fluidity of the molten metal, the tendency for shrinkage and porosity, and the tendency for thermal cracking during deformation. Stainless steel used for casting must have a smaller crystallization interval and a low tendency for oxidation due to gas absorption, good fluidity, and filling performance. It should not easily form dispersed porosity or produce deformation cracks, which is conducive to obtaining cast jewelry that are complete in shape, clear in profile, dense in crystallization, and sound in structure.
(5) Reusability Performance
For the casting jewelry process, the yield rate is generally only around 50% or even lower, and each casting generates a large amount of gating system, scrap materials, etc. The jewelry companies, based on production costs and efficiency, always hope to use as much recycled material as possible. Due to the inevitable issues of volatilization, oxidation, and gas absorption during the alloy’s melting process, the composition of the alloy will change to some extent with each casting, affecting its metallurgical quality and casting performance.
The performance degradation of alloys during the recycling process is not only related to the operating process but also closely related to the recycling performance of the alloy itself. It mainly depends on the alloy’s tendency for gas absorption oxidation and its reactivity with crucibles and casting materials. The smaller the tendency for gas absorption oxidation and the lower the reactivity with crucibles and casting materials, the better the recycling performance.
(6) Safety
The safety of jewelry materials is an important factor that must be considered, as jewelry is in direct contact with the human body for extended periods. Materials should avoid using harmful elements such as cadmium, lead, and radioactive elements. Additionally, attention should be paid to avoiding allergic reactions caused by contact with the skin and related bacteria-related issues.
Nickel is a typical sensitizing element that poses potential allergic reactions and harm to human skin. Nickel-containing jewelry releases sensitizing nickel ions during wear, causing allergic contact dermatitis. Depending on the severity of the reaction, different symptoms may present. Patients with milder symptoms may only show reactions at the points of contact between the jewelry and skin, such as the ears, neck, wrists, and fingers, with skin itching, erythema, rashes, blisters, erosion, exudation, scabbing, and scaling, with clear boundaries of skin lesions often resembling the shape of the jewelry. In contrast, patients with more severe symptoms may experience systemic allergic reactions, starting with skin redness and swelling, followed by small papules and blisters. There is also a risk of carcinogenesis and teratogenic effects. In response to the commonality and harm of nickel allergies, the European Union established the Nickel. Directive 94/27/EC in the 1990s and the nickel release testing standard EN1811:1998. Subsequently, due to the still high levels of nickel sensitization, the standards were tightened and revised, leading to the issuance of Nickel Directive 2004/96/EC and the nickel release testing standard EN1811:1998+A1:2008. In 2011, an even stricter nickel release testing standard EN1811:2011 was introduced, eliminating the adjustment value for nickel release rates. Given that traditional chromium-nickel stainless steel uses a large amount of nickel as an alloying element, it is essential to assess whether a material meets the nickel release standard requirements before selecting it for use as jewelry.
Research shows that jewelry is prone to harboring bacteria, especially during the summer when sweating is more common. The skin covered by jewelry does not breathe easily, allowing bacteria to proliferate, possibly leading to skin diseases and infections. This is particularly serious with piercings, where the risk of bacterial infection is much higher than surface jewelry, as the piercing is a surgical wound. The piercing creates a tunnel within the tissue without an epithelial covering, supported by the subsequently implanted jewelry. The surrounding tissue cannot come into contact to heal, and the entire healing process involves the epithelial tissue on both surfaces gradually adhering along the inner surface of the tunnel to form a fistula, ultimately resulting in an epithelial canal. During the healing process, if external bacteria are encountered, it can easily lead to infection. For example, when piercing the earlobe, the skin in that area is thin, with little subcutaneous tissue, and the blood vessels are fine and superficial, leading to slow blood flow. After the piercing, the dermal tissue is somewhat damaged. Due to the constant friction and contact between the damaged local tissue and the jewelry, it is easily contaminated by dust, mold, bacteria, etc., leading to infection, which can cause itching around the earlobe hole and, in severe cases, redness, swelling, papules, blisters, suppuration, and erosion, even leading to infectious endocarditis. Given the serious consequences of jewelry carrying bacteria, the World Health Organization recommends that healthcare workers not wear rings or other accessories while providing hospital care. As for the jewelry itself, if its material has good antibacterial properties, it undoubtedly has significant importance in reducing or eliminating bacteria in jewelry. Since stainless steel is widely used as a jewelry material, especially during the healing process of piercings, stainless steel rods are mostly used to expand the piercing hole and prevent the piercing walls from sticking together. Traditional stainless steel does not have antibacterial properties, so antibacterial modification treatment is of great significance for the safety of jewelry use.
(7) Economy
The price of stainless steel jewelry materials is one-factor affecting production costs. The principle of selecting materials should be to choose those with a wide supply and low price and minimize or avoid the use of expensive precious metals to reduce material costs.
2.2 The Main Material of Stainless Steel for Jewelry
(1) Traditional Chromium-Nickel Austenitic Stainless Steel
Traditionally, jewelry uses mainly chromium-nickel austenitic stainless steel, including several typical grades such as 303, 304, 304L, 316, and 316L, with chemical composition ranges shown in Table 5-1.
Table 5-1 Chemical Composition Ranges of Several Decorative Austenitic Stainless Steels
| Steels | Uhlík (C) | Silicon(Si) | Manganese (Mn) | Phosphorus (P) | Sulfur (S) | Nikl (Ni) | Chrom (Cr) | Molybdenum(Mo) |
|---|---|---|---|---|---|---|---|---|
| 303 | ≤0. 15 | ≤1. 00 | ≤2.00 | ≤0. 20 | ≥0. 15 | 8.00~10.00 | 17.00 ~19.00 | ≤0. 6 |
| 304 | ≤0.08 | ≤1. 00 | ≤2.00 | ≤0.045 | ≤0.030 | 8.00~10.50 | 18.00 ~20.00 | - |
| 304L | ≤0.03 | ≤1.00 | ≤2.00 | ≤0.045 | ≤0.030 | 9.00 ~13.50 | 18.00~20.00 | - |
| 316 | ≤0.08 | ≤1.00 | ≤2.00 | ≤0.045 | ≤0.030 | 10.00 ~14.50 | 10.00 ~18.00 | 2.00 ~3.00 |
| 316L | ≤0.03 | ≤1.00 | ≤2.00 | ≤0.045 | ≤0.030 | 12.00~15.00 | 16.00 ~18.00 | 2.00 ~3.00 |
| (Zhu Zhongping, 2004; Gu Jiqing, 2008) | ||||||||
① 303 austenitic stainless steel. 303-type austenitic stainless steel has very good cutting performance, and the surface finish of the machined workpiece is high, which is beneficial for the decorative performance of jewelry. Therefore, this material is sometimes chosen as a material for jewelry. However, 303 stainless steel contains a large amount of sulfides, which can become sources of pitting in corrosive environments, leading to preferential corrosion and the formation of pits, accelerating the anodic dissolution of the surrounding metal and increasing the nickel release rate. However, the measured values greatly exceed this threshold. According to the EN1811:2011 standard, 303 stainless steel is non-compliant in nickel release, whether used for jewelry in direct contact with the skin for long periods or piercing jewelry, posing a risk of nickel sensitization. It is advisable to avoid choosing this material for making jewelry that are in direct contact with the skin for long periods, especially piercing jewelry. 303 stainless steel is usually used in a solid solution state, with the solid solution treatment specification being 1010℃~1150℃ holding for the corresponding time and then quenching. The mechanical properties of 303 type and other types of stainless steel are shown in Table 5-2.
Table 5-2 Mechanical Properties of Decorative Stainless Steel in the Solid Solution State
| Steels | Tensile strength σb /MPa | Mez kluzu σ0.2/MPa | Elongation rate δ/% | Cross-sectional shrinkage rate ψ/% | Hardness/HB | |||
|---|---|---|---|---|---|---|---|---|
| 303 | ≥520 | ≥205 | ≥40 | ≥50 | ≤187 | |||
| 304 | ≥520 | ≥205 | ≥40 | ≥60 | ≤187 | |||
| 304L | ≥480 | ≥175 | ≥40 | ≥60 | ≤187 | |||
| 316 | ≥520 | ≥205 | ≥40 | ≥55 | ≤187 | |||
| 316L | ≥480 | ≥175 | ≥40 | ≥60 | ≤187 | |||
| (Zhu Zhongping, 2004; Gu Jiqing, 2008) | ||||||||
② 304 and 304L austenitic stainless steel. 304 is a versatile stainless steel, commonly marked in the market in three ways: 06Cr19Ni10 generally indicates production according to national standards, S30408 generally indicates production according to ASTM standards, and SUS 304 indicates production according to Japanese standards. To maintain the inherent corrosion resistance of stainless steel, the steel must contain more than 17% chromium and 8% nickel content.
304 stainless steel has excellent corrosion resistance, good intergranular corrosion resistance, and excellent hot and cold processing and forming properties. It can be processed into various products such as plates, pipes, wires, strips, and shapes and is suitable for manufacturing cold heading, deep drawing, and deep stretching parts. It has good low-temperature performance, strength, elongation, and reduced area, all good under -180℃ conditions. It has good welding performance and can be welded using conventional welding methods. However, 304 stainless steel also has some shortcomings, such as being sensitive to intergranular corrosion after welding, very sensitive to stress corrosion in water containing chloride ions (including humid atmospheres), relatively low mechanical strength, and poor cutting performance.
304 L stainless steel is a variant of 304 stainless steel with a lower carbon content, used in welding applications. The lower carbon content minimizes the precipitation of carbides in the heat-affected zone near the weld, as carbide precipitation can lead to intergranular corrosion (weld decay) of stainless steel in certain environments.
③ 316 and 316L stainless steel. 316 stainless steel contains a certain amount of molybdenum, and its nickel content is higher than 304 stainless steel. Thus, its corrosion resistance, atmospheric corrosion resistance, and high-temperature strength are superior, allowing it to be used under more stringent conditions, especially since its pitting corrosion resistance is significantly better than that of 304 stainless steel, with its critical pitting temperature higher than that of 304 stainless steel, exhibiting better pitting temperature resistance. Research shows that the critical pitting temperature of 316 stainless steel is significantly sensitive to the concentration of NaCl solution from 0.1% to 0.5%; within this range, the material’s critical pitting temperature sharply drops from close 90℃ to 50℃ . In contrast, the critical pitting temperature of 304 stainless steel shows significant sensitivity to the concentration of NaCl solution from 0.01% to 0.05%, within this range, the critical pitting temperature of the material sharply drops from close 90℃ to around 55℃. From the perspective of sensitivity to chloride ions, 316 stainless steel is also relatively superior to 304 stainless steel in terms of pitting corrosion resistance.
316 L stainless steel is a variant of 316 stainless steel with a carbon content not exceeding 0.03%. It has better resistance to carbide precipitation than 316 stainless steel, making it suitable for applications that cannot be annealed after welding and require maximum corrosion resistance.
316L stainless steel is preferred as an accessory material because it ensures good corrosion resistance. The high-end watch chains and cases in the watch industry also mainly use this type of steel.
(2) New Type of Nickel-Free/Low-Nickel Austenitic Stainless Steel
① Alternative elements for nickel-free/low-nickel austenitic stainless steel. Traditional chromium-nickel austenitic stainless steel expands the austenitic phase region through nickel, delaying its transformation to obtain a single-phase structure. Since nickel is a sensitizer, nickel-containing stainless steel may pose an allergy risk when in prolonged contact with human skin or tissues. Therefore, the research and development of nickel-free austenitic stainless steel that is friendly to the human body has become a current hotspot in metal biomaterials, watch materials, and jewelry materials.
To obtain a single-phase austenitic structure in nickel-free stainless steel, seeking austenite-stabilizing elements that can replace nickel is necessary. The influence of alloying elements on the structure of stainless steel can be converted into the corresponding chromium Creq and nickel equivalent Nieq. To achieve single-phase austenite and avoid the presence of ferrite δ, the composition ratio of each alloying element must be reasonably selected to ensure that the nickel equivalent falls within the single-phase austenite region above the inclined shadow area. To achieve this, the following must be satisfied:
Nieq≥Creq – 8
The Creq, Nieq calculation formula is:
Creq=Cr+1.5Mo+1.5W+0.48Si+2.3V+1.75Nb+2.5Al
Nieq=Ni+Co+0.1Mn-0.01Mn2+18N+30C
Carbon, cobalt, manganese, and nitrogen are the more economical alternative elements for stabilizing austenite. Carbon has the strongest effect in expanding the austenite phase region, but it can sensitize stainless steel; cobalt’s ability to stabilize austenite is similar to that of nickel, but it also carries a risk of allergy, so neither is suitable as a primary substitute for nickel. Manganese stabilizes austenite within a certain range, but when the chromium content exceeds 13%, adding manganese alone cannot achieve a single austenite phase. When the manganese content exceeds 10%, manganese becomes a ferrite stabilizer. Nitrogen is a strong austenite stabilizing element; adding nitrogen to stainless steel suppresses the formation of ferrite phases in the steel, significantly reducing the ferrite content, making the austenite phase more stable, and even preventing stress-induced martensitic transformation under severe cold working conditions. Thus nitrogen is a very suitable substitute for a nickel. However, the Fe-Cr-N thermodynamics of the system indicate that when the chromium content is 12%, nitrogen can achieve austenite within a narrow range; beyond this range, Cr2N and CrN will form, and at high chromium content, ferrite, austenite, and Cr2N will form, and the alloy is also prone to Cr2N during low-temperature aging, which cannot suppress martensitic transformation. Therefore, manganese must be added to Fe-Cr-N, utilizing the synergistic effect of nitrogen and manganese, which is beneficial for obtaining a stable austenitic structure.
② High nitrogen nickel-free / low nickel austenitic stainless steel materials. Countries such as Germany, Bulgaria, Switzerland, Austria, and Japan place great importance on the research and development of high-nitrogen stainless steel and have successively developed some new types of high-nitrogen nickel-free stainless steel materials, such as the BioDur 108 alloy developed by Carpenter Technology Corp in the United States, P2000 by VSG in Germany, P548 developed by Bolher in Austria, and NFS developed by Daido Steel in Japan (Table 5-3). Some of these have already been commercialized and are used in products such as biomedical applications, watches, and jewelry. However, it is difficult to achieve a precise processing degree when producing small precision components, and the costs are high.
Table 5-3 Chemical Compositions of Several High-Nitrogen Nickel-Free Stainless Steels
| Země | Třídy | Composition /wt% | ||||
|---|---|---|---|---|---|---|
| C | Cr | Mn | Mo | N | ||
| Switzerland | PANACEA | ≤0. 15 | 16. 5~17. 5 | 10~12 | 3.0~3. 5 | 0.8~1.0 |
| Austria | P548 | 0.15 | 16.0 | 10.0 | 2.0 | 0.5 |
| Bulgaria | CrMnN18- 11 | ≤0.08 | 17~19 | 10~12 | - | 0. 4~1. 2 |
| Německo | P900 | 0.05 | 18.0 | 18.0 | - | 0. 6~0. 8 |
| Německo | P2000 | ≤0.05 | 16.0 | 14.0 | 3.0 | 0.75~1.0 |
| Japan | NFS | 0.02 | 16.0 | 18.0 | - | 0.43 |
| Spojené státy americké | BioDur 108 alloy | 19~23 | 21~24 | 0. 5~1. 5 | 0.9 | |
| (Yuan Junping, 2012) | ||||||
③ Mechanical properties of high-nitrogen nickel-free/low-nickel austenitic stainless steel. Traditional nickel-containing austenitic stainless steel is classified as a low-strength material under solution treatment conditions and is often strengthened through cold working. Some steels undergo deformation-induced martensitic transformation during significant deformation, giving the material magnetic properties. The strength, plasticity, and other mechanical properties of high-nitrogen stainless steel are closely related to grain size and nitrogen content, with both tensile strength and yield strength significantly increasing with higher nitrogen content. Table 5-4 lists the mechanical properties of some new high-nitrogen austenitic stainless steels in both room-temperature solution-treated and processed states, showing that the strength in the processed state is significantly higher than in the solution-treated state. At the same time, ductility and plasticity remain high, making it difficult to form ferrites and undergo deformation-induced martensitic transformation.
The main ways nitrogen improves the strength of stainless steel are solid solution strengthening, grain size strengthening, and strain hardening. Like carbon, nitrogen occupies the octahedral interstitial sites of the face-centered cubic lattice of austenite. Due to its smaller atomic radius than carbon, it has a stronger lattice expansion effect. Nitrogen atoms interact with dislocations, providing a greater dislocation pinning effect, and can also have a maximum strengthening effect on austenite grain boundaries. In addition, fine-grain strengthening is also an important strengthening mechanism. The transformation pathway shows that compared to 304 stainless steel, high-nitrogen austenitic stainless steel has a significantly more pronounced fine-grain strengthening effect. The effect of nitrogen on the deformation hardening of austenitic stainless steel is also very significant; the increase in nitrogen leads to an increase in slip planes and deformation twins, while the active slip planes and twin layers effectively hinder dislocation movement and twin expansion, thereby greatly increasing the deformation hardening rate of austenitic steel.
Table 5-4 Mechanical Properties of Typical High-Nitrogen Austenitic Stainless Steel at Room Temperature
| Třída slitiny | Status | Tensile strength / MPa | Yield strength/ MPa | Extension rate /% | Cross-sectional shrinkage rate /% | Tvrdost |
|---|---|---|---|---|---|---|
| 15-15HS-® | Solid solution | 828 | 490 | 56 | 79 | HRB95 |
| Cromanite | Solid solution | 850 | 550 | 50 | HB250 | |
| URANUS-® B46 | Solid solution | 650 | 420 | 40 | ||
| URANUS-® B66 | Solid solution | 750 | 420 | 50 | ||
| AL4565TM | Solid solution | 903 | 469 | 47 | HRB90 | |
| Datalloy 2TM | Solid solution | 827 | 760 | 18 | 45 | HRC33 |
| P2000 | Solid solution | 930 | 615 | 56.2 | 77.5 | |
| NMS 140 | Processing | 1010~1117 | 876~1020 | 30~22 | 68~60 | HB311 - 341 |
| P550 | Processing | 1034 | 965 | 20 | 50 | HB300 - 400 |
| P580 | Processing | 1034 | 965 | 20 | 50 | HB350 - 450 |
| Amagnit 600 | Processing | 1034 | 965 | 20 | 50 | HB300 |
| (Yuan Junping, 2012) | ||||||
④ Corrosion resistance. Nitrogen can significantly improve the pitting corrosion and crevice corrosion resistance of austenitic stainless steel in environments containing chloride ions. To describe the relationship between the quantity of alloying elements and corrosion performance, the pitting equivalent is commonly used to represent it:
PRE= %Cr + 3.3%Mo + x%N
The most commonly used value x is 16~30. Therefore, nitrogen has a good effect on stainless steel’s pitting corrosion resistance. However, the mechanism of nitrogen’s action is not yet very clear, and it is generally speculated that there are mainly the following mechanisms.
- Acid consumption theory. Nitrogen forms NH4+ during dissolution and consumes H+ in the process, thereby inhibiting the decrease of pH, slowing down local acidification of the solution and anode dissolution, and suppressing the self-catalytic process of pitting, which is more conducive to the pinning reaction.
- Nitrogen enrichment at the interface. Due to nitrogen’s high reactivity, it accumulates near the metal side of the passivation film-metal interface, affecting the repassivation kinetics and allowing for rapid passivation, thereby inhibiting the stable growth of pitting corrosion.
- The synergistic effect of nitrogen with other elements. Nitrogen further enriches the chromium in the sublayer of the nitrided film, enhancing its stability and density. Nitrogen strengthens the corrosion resistance of chromium, molybdenum, and other elements in austenitic stainless steel, suppressing the over-passivation dissolution of chromium and molybdenum. It can also form a more resistant surface layer during localized corrosion processes.
- Biocompatibility. High-nitrogen nickel-free austenitic stainless steel has good corrosion resistance, especially against pitting and intergranular corrosion, and has high wear resistance. The absence of nickel in the steel avoids the sensitization and other tissue reactions caused by the precipitation of nickel in the human body and on the body surface, demonstrating good biocompatibility.
3. Characteristics of stainless steel jewelry
Stainless steel jewelry has many advantages:
① The metallic luster of stainless steel is very similar to that of platinum. It is both noble and elegant yet modern.
② Stainless steel is resistant to corrosion and heat, can resist dust corrosion, and is easy to clean, requiring only a dry cloth. It does not require polishing cloths or cleaning agents.
③ Stainless steel is harder than silver, not easily deformed, and does not oxidize as easily as silver or other metals. It can maintain a shiny, smooth, and attractive appearance even with long-term wear, making it suitable for processing more minimalist styles without worrying about deformation.
④ Stainless steel can be presented in different styles, usually with a very smooth or matte surface.
⑤ The price of stainless steel jewelry is easily acceptable to the public. Although the price of silver has risen significantly in the past few years, stainless steel remains at an acceptable level.
⑥ Stainless steel has excellent coloring properties and can be colored through various processes, such as chemical oxidation, electrochemical oxidation, ion deposition oxide, high-temperature oxidation, and gas phase cracking, greatly enriching the surface decoration effects of jewelry.
4. Categories of Stainless Steel Jewelry

Kroužek z nerezové oceli

Stainless steel bracelet

Stainless steel bangles

Stainless steel earrings
Stainless steel pendents

Stainless steel cufflinks

Stainless steel navel ring
Section II Titanium Alloy Products
1. Introduction to Titanium Alloys
(1) The Discovery of Titanium
Titanium was discovered by the British chemist Gregor R W (1762–1817) in 1791 while studying ilmenite and rutile. Four years later, in 1795, the German chemist Klaproth M H (1743–1817) also discovered this element while analyzing red rutile from Hungary. He proposed naming it after the Greek mythological race of gods, the “Titans,” following the method used for uranium (discovered by Klaproth in 1789). In Chinese, it is named “Tài” based on its phonetic pronunciation.
The titanium discovered by Gregor and Klaproth at that time was powdered titanium dioxide, not metallic titanium. Because titanium oxides are extremely stable, and metallic titanium can react violently with oxygen, nitrogen, hydrogen, carbon, and others, it isn’t easy to obtain elemental titanium. It was not until 1910 that pure metallic titanium with a purity of 99.9% was first produced by American chemist Hunter (Hunter M A).
(2) Properties of Titanium
Pure titanium has a silvery metallic luster and is ductile. Density is 4.51g/cm3, melting point is 1668℃ and boiling point is 3287℃. Valence are +2, +3 and +4. The main characteristics of titanium are its low density and high mechanical strength. The plasticity of titanium mainly depends on its purity. The purer the titanium, the greater the plasticity. It has good corrosion resistance and is not affected by the atmosphere and seawater. At room temperature, titanium is stable in air and is not corroded by dilute hydrochloric acid, dilute sulfuric acid, nitric acid, or dilute alkaline solutions; only hydrofluoric acid, hot concentrated hydrochloric acid, and concentrated sulfuric acid can act on it. Due to its low density, high specific strength, high temperature, and corrosion resistance, titanium alloys are good for making rocket engine casings, artificial satellites, and spacecraft. Titanium is known as “space metal.” Because of these advantages, titanium has become a prominent rare metal since the 1950s.
Due to sodium’s corrosion resistance and high stability, it does not affect its essence after long-term contact with humans and does not cause allergies; it is the only metal that does not affect human autonomic nerves and taste. Titanium has unique medical applications and is known as a “bio-friendly metal.”
Due to titanium’s high melting point, sodium smelting needs to be carried out at high temperatures, and at high temperatures, titanium’s chemical properties become very reactive. Therefore, smelting must be conducted under the protection of inert gases, and using oxygen-containing materials must be avoided, which places high demands on smelting equipment and processes.
(3) Main Categories of Titanium Alloys
According to the composition of the alloy, titanium is divided into two categories: industrial pure titanium and titanium alloys. Industrial pure titanium includes three types: TA1, TA2, and TA3. Titanium alloys are alloys composed of titanium as the base with other elements added, including TA4~TA8, TB1 ~ TB2, TC1 ~ TC10, and other categories, among which the industry’s most widely used titanium alloys are TC4, TA7, and industrial pure titanium (TA1, TA2, and TA3). The main chemical compositions of various titanium alloys are shown in Table 5-5, the allowable impurity element content is shown in Table 5-6, and the mechanical properties of various titanium alloy materials are shown in Table 5-7.
Table 5-5 Main Chemical Composition of Titanium Alloys
| Třídy | Main components (mass fraction) (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti | A1 | Cr | Mo | Sn | Mn | V | Fe | Cu | Si | Zr | B | |
| TA0 | Base | |||||||||||
| TA1 | Base | |||||||||||
| TA2 | Base | |||||||||||
| TA3 | Base | |||||||||||
| TA4 | Base | 2.0~3. 3 | ||||||||||
| TA5 | Base | 3. 3~4.3 | 0.005 | |||||||||
| TA6 | Base | 4.0~5.5 | ||||||||||
| TA7 | Base | 4.0~5.5 | 2. 0~3.0 | 2. 5~3.2 | 1. 0~1.5 | |||||||
| TA8 | Base | 4. 5~5.5 | 2.0~3.0 | |||||||||
| TB1 | Base | 3.0~4.0 | 10.0~11.5 | 7. 0~8. 0 | ||||||||
| TB2 | Base | 2. 5~3.5 | 7.5~8.5 | 4. 7~ 5.7 | 4. 7~ | |||||||
| TC1 | Base | 1. 0~2.5 | 0.8~2.0 | |||||||||
| TC2 | Base | 2.0~3. 5 | 0. 8~2.0 | |||||||||
| TC3 | Base | 4. 5~6.0 | 3. 5~4.5 | |||||||||
| TC4 | Base | 5. 5~6.8 | 3. 5~4.5 | |||||||||
| TC5 | Base | 4. 0~6.2 | 2.0~3.0 | |||||||||
| TC6 | Base | 4.5~6.2 | 1.0~2.5 | 1.0~2.8 | 0. 5~1.5 | |||||||
| TC7 | Base | 5.0~6.5 | 0. 4~0.9 | 0. 25~0. 60 | 0. 25~0. 60 | 0.01 | ||||||
| TC8 | Base | 5. 8~6.8 | 2. 8~3.8 | 0. 20~0. 35 | ||||||||
| TC9 | Base | 5. 8~6.8 | 2. 8~3.8 | 0. 20~0. 40 | ||||||||
| TC10 | Base | 5. 5~6.5 | 5. 5~6.5 | 0. 35~1.0 | 0. 35~1.0 | |||||||
| (Xie Chengmu, 2005; Zhang Xiyan et al., 2005) | ||||||||||||
Table 5-6 Allowable Impurity Element Content in Titanium Alloys
| Třídy | Impurities not greater than (mass fraction) (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Si | C | N | H | O | |||||||
| TA0 | 0.03 | 0.3 | 0.03 | 0.01 | 0.015 | 0.05 | ||||||
| TA1 | 0. 15 | 0.1 | 0.05 | 0.03 | 0.015 | 0.1 | ||||||
| TA2 | 0. 3 | 0.15 | 0.1 | 0.05 | 0. 015 | 0.15 | ||||||
| TA3 | 0.3 | 0.15 | 0.1 | 0.05 | 0.015 | 0.15 | ||||||
| TA4 | 0. 3 | 0.05 | 0.1 | 0.05 | 0. 015 | 0.15 | ||||||
| TA5 | 0. 3 | 0.15 | 0.1 | 0.04 | 0.015 | 0.15 | ||||||
| TA6 | 0. 3 | 0.15 | 0.1 | 0.05 | 0.015 | 0.15 | ||||||
| TA7 | 0. 3 | 0.15 | 0.1 | 0.05 | 0.015 | 0.2 | ||||||
| TA8 | 0.3 | 0.15 | 0.1 | 0.05 | 0.015 | 0.15 | ||||||
| TB1 | 0.3 | 0.15 | 0.1 | 0.04 | 0.015 | 0.15 | ||||||
| TB2 | 0.3 | 0.05 | 0.05 | 0.04 | 0. 015 | 0.15 | ||||||
| TC1 | 0.4 | 0.15 | 0.1 | 0.05 | 0.015 | 0.15 | ||||||
| TC2 | 0.4 | 0.15 | 0.1 | 0.05 | 0. 015 | 0.15 | ||||||
| TC3 | 0.3 | 0.15 | 0.1 | 0.05 | 0.015 | 0.15 | ||||||
| TC4 | 0. 3 | 0.15 | 0.1 | 0.05 | 0.015 | 0.15 | ||||||
| TC5 | 0. 5 | 0.4 | 0.1 | 0.05 | 0.015 | 0.2 | ||||||
| TC6 | 0.4 | 0.1 | 0.05 | 0.015 | 0.2 | |||||||
| TC7 | 0.1 | 0.05 | 0.025 | 0.3 | ||||||||
| TC8 | 0.1 | 0.05 | 0.015 | 0.15 | ||||||||
| TC9 | 0.1 | 0.05 | 0. 015 | 0.15 | ||||||||
| TC10 | 0.15 | 0.1 | 0.04 | 0.015 | 0.2 | |||||||
| (Xie Chengmu, 2005; Zhang Xiyan et al., 2005) | ||||||||||||
Table 5-7 Mechanical Properties of Titanium Alloys
| Třídy | Status | Room temperature performance | High-temperature performance | Poznámky | |||||
|---|---|---|---|---|---|---|---|---|---|
| σ b | δ | ψ | ɑ k | T | σ b | σ 100 | |||
| MPa | % | % | MJ/m2 | ℃ | MPa | MPa | |||
| TA0 | Annealing | ||||||||
| TA1 | Annealing | 350 | 25 | 50 | 0.8 | bar stock | |||
| TA2 | Annealing | 450 | 20 | 45 | 0.7 | bar stock | |||
| TA3 | Annealing | 550 | 15 | 40 | 0.5 | bar stock | |||
| TA4 | Annealing | bar stock | |||||||
| TA5 | Annealing | 700 | 15 | 40 | 0.6 | bar stock | |||
| TA6 | Annealing | 700 | 10 | 27 | 0.3 | 350 | 430 | 400 | bar stock |
| TA7 | Annealing | 800 | 10 | 27 | 0.3 | 350 | 500 | 450 | bar stock |
| TA8 | Quenching Timeliness | 1000 | 10 | 25 | 0. 2 ~ 0. 3 | 500 | 700 | 500 | bar stock |
| TB1 | Quenching Timeliness | ≤1 000 | 18 | 30 | 0.3 | bar stock | |||
| 1 300 | 5 | 10 | 0.15 | ||||||
| TB2 | Quenching Timeliness | ≤1 000 | 18 | 40 | 0.3 | bar stock | |||
| 1 400 | 7 | 10 | 0.15 | ||||||
| TC1 | Annealing | 600 | 15 | 30 | 0.45 | 350 | 350 | 300 | bar stock |
| TC2 | Annealing | 700 | 12 | 30 | 0.4 | 350 | 430 | 400 | bar stock |
| TC3 | Annealing | 900 | 10 | 400 | 600 | 550 | Sheet (1. 0~2. 0) | ||
| TC4 | Annealing | 950 | 10 | 30 | 0.4 | 400 | 630 | 580 | bar stock |
| TC5 | Annealing | 950 | 10 | 23 | 0. 3 | 400 | 600 | 560 | bar stock |
| TC6 | Annealing | 950 | 10 | 23 | 0.3 | 450 | 600 | 550 | bar stock |
| TC7 | Annealing | 1000 | 10 | 23 | 0. 35 | 550 | 600 | bar stock | |
| TC8 | Annealing | 1050 | 10 | 30 | 0.3 | 450 | 720 | 700 | bar stock |
| TC9 | Annealing | 1140 | 10 | 25 | 0. 3 | 500 | 650 | 620 | bar stock |
| TC10 | Annealing | 1 050 | 12 | 25 | |||||
| 1 050 | 12 | 30 | |||||||
| (Xie Chengmu, 2005; Zhang Xiyan et al., 2005) | |||||||||
(4) The Effect of Alloying Elements on the Properties of Titanium Alloys
There are two types of homogeneous and heterogeneous crystals in titanium: below 882℃ is the close-packed hexagonal structure α titanium, and above 882℃ is the body-centered cubic β titanium. Alloying elements can be divided into three categories based on their influence on phase transition temperature.
① Stability α phase: The elements that increase the phase transition temperature are α stable elements, including aluminum, carbon, oxygen, and nitrogen. Aluminum is the main alloying element in titanium alloys, and it significantly improves the alloy’s strength at room and high temperatures, reduces specific gravity, and increases elastic modulus.
② Stable β phase: The elements that lower the phase transition temperature are β stable elements, which can be divided into two types: isomorphic and eutectoid. The former include molybdenum, niobium, and tungsten; the latter include chromium, manganese, copper, iron, and silicon.
③ Neutral elements, such as cobalt and tin, affect the phase transition temperature little.
④ Oxygen, nitrogen, carbon, and hydrogen are the main impurities in titanium alloys. Oxygen and nitrogen have a relatively high solubility in the α phase, significantly strengthening the titanium alloy, but they reduce plasticity. It is usually stipulated that sodium’s oxygen and nitrogen content should be below 0.15%~0.2% and 0.04%~0.05%, respectively. The solubility of hydrogen in the α phase is very low, and excessive hydrogen dissolved in titanium alloys can form hydrides, making the alloy brittle. Typically, the hydrogen content in titanium alloys is controlled to be below 0.015%. The dissolution of hydrogen in titanium is reversible and can be removed by vacuum annealing.
(5) Characteristics of Titanium Alloys
- With high specific strength, the tensile strength can reach 1000~1400MPa, while the density is only 60% that of steel.
- The medium-temperature strength is good, and the operating temperature is several hundred degrees higher than that of aluminum alloys. It can still maintain the required strength at medium temperatures and can work for a long time at that temperature of 450~500℃.
- Good corrosion resistance: The surface of titanium immediately forms a uniform and dense oxide film in the atmosphere, which can resist erosion from various media. Generally, titanium has good corrosion resistance in oxidizing and neutral media, and its corrosion resistance is even more excellent in seawater, humid chlorine gas, and chloride solutions.
- Good low-temperature performance, maintaining a certain level of plasticity even at very low temperatures.
- Low elastic modulus, low thermal conductivity, non-ferromagnetic.
2. Decorative Titanium Alloy
Titanium alloys used for jewelry making are generally industrial pure titanium. The difference between industrial pure titanium and chemically pure titanium is that it contains more oxygen, nitrogen, carbon, and other impurity elements (such as iron, silicon, etc.). It is a titanium alloy with a low alloy content. Compared to chemically pure titanium, the presence of more impurity elements significantly increases its strength, and its mechanical properties and chemical characteristics are similar to those of stainless steel (but still lower in strength compared to titanium alloys).
The characteristics of industrial pure titanium are: it has low strength but good plasticity, with certain processing and forming capabilities, and can be processed using techniques such as stamping, welding, and cutting; it has good corrosion resistance in the atmosphere, seawater, humid chlorine gas, and oxidative, neutral, and weakly reducing media, and its oxidation resistance is better than that of most austenitic stainless steels, but its heat resistance is relatively poor, with a not very high operating temperature.
Based on the different impurity content, industrial pure titanium is divided into three grades: TA1, TA2, and TA3. The interstitial impurity elements in these three grades of industrial pure titanium increase gradually, resulting in a corresponding increase in mechanical strength and hardness, while plasticity and toughness decrease accordingly.
The industrial pure sodium in the jewelry industry is TA2 due to its moderate corrosion resistance and comprehensive mechanical properties. When higher corrosion resistance and strength are required, TA3 can be used, and when better-forming performance is needed, TA1 can be used.
Currently, there are many accessories in the country referred to as titanium steel, but the material used is not titanium; it is stainless steel. To attract attention, it is called titanium steel; some even refer to it as titanium alloy accessories, which are stainless steel accessories that do not contain titanium. Titanium steel and stainless steel are two different materials that can be easily distinguished:
- In terms of weight, titanium is lighter than steel; for the same volume, titanium is only about half the weight of steel. The density of titanium is 4.5g/cm3, and that of steel is 7.845g/cm3.
- In terms of color, titanium is a bit darker than steel, while steel is whiter; the difference between the two colors is quite obvious.
3. Characteristics of Titanium Alloy Jewelry
(1) Essential Characteristics
① Light. Titanium’s specific gravity is 4.5, about half that of alloys such as stainless steel, cobalt, and chromium. It is also much lighter than gold and silver, making it advantageous for making earrings, necklaces, and other jewelry.
② Titanium has good corrosion resistance. Titanium is a highly reactive element that easily reacts with oxygen to form TiO2. Still, the oxide film that forms on the surface of titanium is extremely complete and dense, with the ability to self-repair instantly after localized damage, and it is stable in most environments. This is the theoretical basis for titanium’s corrosion resistance. The advantages it demonstrates in jewelry are that it does not corrode or change color, can maintain a good luster for a long time, and is not afraid of water.
③ Titanium can be colored. Titanium metal has a very interesting characteristic: when titanium is placed in an electrolyte and a certain current is applied, its surface will be electrolyzed to form a layer of oxide film, and the thickness of the oxide film can determine the color change without the need for additional elements. The colors that can now be produced include gold, black, blue, brown, and various other colors. This characteristic allows for more colorful and fashionable designs in jewelry.
④ Sodium is not easily deformed and does not need to be reshaped. Titanium has high hardness, is not easily deformed, and unlike ordinary gold and silver jewelry, it does not need to be reshaped after being worn for some time.
(2) Characteristics of Fashion Forward
① New material symbol. The emergence of titanium jewelry marks the breaking of tradition with new materials, challenging the dominance of ancient gold and silver jewelry in the industry. Beyond decoration, jewelry has long become a symbol of status and identity. As a third type of metal—titanium—enters the jewelry industry, it adds health, elegance, and fashion appeal to the pieces.
② Female spirit symbol. Titanium is very lightweight yet extremely tough, representing urban women who are light, beautiful, and resilient.
③ Male spirit symbol. In 1795, German scientist Klaproth discovered titanium while studying rutile. He named it after the Titan (titan) from ancient Greek mythology, which embodies the same meaning of spirit and courage. Its natural strength and texture reflect the heroic spirit of the Titan, showcasing the “Titan” spirit of urban men as sons of the earth.
④ Love symbol. Titanium is highly corrosion-resistant; it does not tarnish like silver and maintains its color for a lifetime at room temperature. Couple jewelry represents the fidelity of love, never betraying, and always maintaining supreme quality.
(3) Health Characteristics
Titanium metal has no harm to the human body. Medical practice has proven that titanium organs can be implanted in the human body for a long time, demonstrating its harmlessness to the body. Titanium jewelry, after long-term contact with the body, will not cause allergies or adverse effects on the skin, nerves, or taste, exhibiting good biocompatibility and stability. Therefore, titanium metal is also known as a biocompatible metal. It is harmless to the human body and can be the preferred jewelry for modern people with skin allergies.
(4) Aviation Characteristics
Sodium is also known as space metal. In our country’s rapidly developing aerospace industry, the public will surely pay more attention to aviation, and titanium, as the preferred material for spacecraft, will inevitably enter the lives of modern people driven by enthusiasm for aerospace. In the repeated journeys of the “Shenzhou” spacecraft into space, titanium can serve as a symbol for ordinary people to commemorate our country’s aerospace achievements.
Kopírování @ Sobling.Jewelry - Výrobce šperků na zakázku, továrna na šperky OEM a ODM
4. Categories of Titanium Alloy Jewelry
Due to the unique silver-gray tone of titanium, whether polished, satin, or matte, performs well and is the most suitable jewelry metal after precious metals like platinum and gold. It is often used in modern jewelry design abroad and is a popular material internationally, highly regarded by young professionals. In addition, titanium crafts are a new generation of high-end gifts on the market. They are a vivid combination of traditional craftsmanship and modern science and technology. They possess practical, storage, aesthetic, and artistic value, making them essential high-end gifts for friends and visiting abroad.
The main product series of titanium jewelry includes the following nine types.
- Titanium rings its products, including ude-saving, stone setting, plating, hollowing, carving, simplicity, and decorative engraving series.
- Titanium pendant.
- Titanium chain. Includes bracelets and necklaces, with a focus on bracelets.
- Titanium cufflinks, tie clips, etc.
- Earrings and body piercing jewelry. Body piercing jewelry is quite popular abroad and is just starting in the domestic market; titanium metal poses no harm to the human body and first caters to the pursuit of health and longevity. Medical practice has proven that titanium organs can be implanted in the human body for a long time, demonstrating their harmlessness to the human body.
- Titanium watch.
- Health products combined with metals such as titanium and germanium. Mainly sodium series health products, which are currently mostly imported and quite expensive; titanium jewelry can promote blood circulation and enhance natural healing ability, while germanium may also replace the functional performance of oxygen. After contact with the skin, starting from a temperature increase of about 0.5℃, it may improve blood circulation and assist in the smooth discharge of waste (cations, protons) from the blood. Germanium can restore the body’s electrical potential to a normal balanced state. One explanation for this phenomenon is that germanium may begin to move electrons to the outermost orbit based on body temperature energy, allowing free electrons to enter and exit freely, thus restoring the chaotic electrical potential balance of nerve circuits to normal operation. This electronic effect of semiconductors may stimulate the activation of nerve cells and alleviate discomfort symptoms in the body.
- The Daily Necessities series offers a wide range of products. For example, there are titanium glasses frames, titanium stationery, titanium canes, titanium swords, titanium ashtrays, titanium prints, titanium wine utensils, and titanium tableware.
- Sports equipment series. Such as golf clubs, tennis rackets, badminton rackets, etc.

Titanium ring

Titanium pendents

Titanium bracelets

Titanium cufflinks
Titanium tie clips

Titanium earrings


Titanium watch
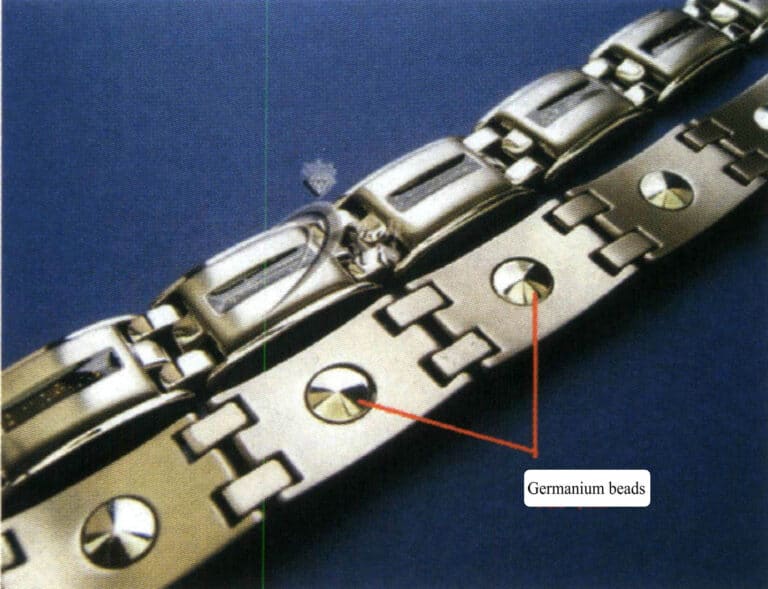
Titanium health bracelet with embedded germanium beads
5. The Market Situation of Titanium Jewelry
Titanium jewelry is an emerging type of jewelry product that is gradually being recognized and accepted by more and more people. Because titanium metal has many excellent properties, it is very suitable for jewelry processing. With improved processing technology, titanium jewelry has gained popularity internationally since 2000. Many people now accept titanium as a metal for producing jewelry, and the demand for titanium jewelry is increasing yearly. Some world-renowned jewelry brands have also begun to launch titanium jewelry, which can attract attention to titanium metal products and stimulate demand.
Due to the high technical requirements for processing sodium, it is difficult to cast and shape it with conventional equipment, and it is also challenging to weld it with ordinary tools, which creates significant difficulties in achieving production scale. Additionally, the technology and knowledge for making titanium jewelry are poorly disseminated in the country. Therefore, although titanium jewelry has been popular in Western countries for a long time, it is still a new concept for Chinese people, and the domestic production capacity is low. Currently, the consumption of titanium products in the country has just begun, and it is not on the same level as traditional gold and silver jewelry. The market is currently expanding, but this presents a rare opportunity. The diversification of jewelry materials will be a major trend in the market, and titanium, as a third metal, will inevitably break the traditional dominance of gold and silver jewelry due to its inherent characteristics.
Section III Forming Process of Stainless Steel and Titanium Alloy Products
1. Mechanical Forming Process
1.1 Machining and Forming
Some structurally simple pieces can be directly processed and formed to produce stainless steel and titanium alloy jewelry. Common methods include machining, electrical discharge machining, and etching.
(1) Cutting and Forming
Using a lathe to directly process stainless steel or titanium alloy profiles into jewelry is most common in ring and bracelet jewelry, accounting for a large proportion. Figures 5-1 and 5-2 show stainless steel and titanium alloy rings shaped using a lathe.

Figure 5-1 Stainless steel ring finished on a lathe

Figure 5-2 Titanium alloy ring shaped by lathe finishing
Finishing is difficult due to the material characteristics of stainless steel and titanium alloy. Based on these properties, it is necessary to select and formulate corresponding processing parameters to ensure the processing accuracy and surface quality of the jewelry.
① Machining of stainless steel rings. In actual production, the processing of stainless steel is relatively difficult. If you cannot master its characteristics, you will not achieve the desired processing quality during cutting and cause significant damage to the tools.
The reasons for the difficulty in machining stainless steel mainly come from the following five aspects.
- The comprehensive mechanical properties of stainless steel are high. Due to the higher content of alloying elements such as chromium and nickel in stainless steel, the material’s mechanical properties have changed significantly. From the perspective of various mechanical performance indicators, the mechanical properties of stainless steel have characteristics that distinguish them from ordinary steel, with both strength performance indicators and plastic toughness indicators being relatively high. This has resulted in the characteristic of stainless steel being difficult to machine.
- The strong chip adhesion makes tool buildup easy. Stainless steel has a higher adhesion, which causes the material to “bond” to the tool during finishing, resulting in “tool buildup.”
- Low thermal conductivity; cutting heat cannot be dissipated in time. The heat transferred to the tool can reach 20%, and the cutting edge of the tool is prone to overheating, losing cutting ability.
- Chips are not easily broken. In metal cutting, the formation of chips from ductile materials (tough materials) goes through four stages: extrusion, slip, fracture, and separation. Due to the generally high elongation, cross-sectional shrinkage, and impact values of stainless steel, especially for 304(L) and 316(L) austenitic chromium-nickel stainless steel used in jewelry, both elongation and toughness are good, making it difficult for chips to curl and break during the cutting process. In boring, drilling, and cutting operations, chip removal is difficult, and chips can easily scratch the processed surface.
- The tendency for work hardening is strong, making the tools prone to wear. Austenitic stainless steel has a strong tendency to work harden, with a high hardness of the work-hardened layer and a certain depth of work hardening, which increases the difficulty of processing and tool wear.
The measures to be taken in stainless steel cutting are as follows.
First, choose a reasonable geometric shape for the cutting tool, making cutting deformation easier, reducing cutting force, and allowing chips to form and discharge smoothly. Different tools should have the following requirements for the geometry of the cutting part:
- Rake angle. A larger rake angle reduces cutting force and heat, decreases vibration during cutting, and weakens the work-hardening effect. The rake angle between 12°~30°can generally be selected depending on the type of tool, tool material, and cutting conditions. At the same time, a positive flank angle enhances the strength of the cutting edge; a negative chamfer is ground on the main cutting edge to strengthen the blade.
- The shape in front. When processing stainless steel, due to the material being relatively tough and soft, the chips experience strong friction with the front of the tool during their formation and curling process, causing a crescent-shaped pit to gradually form on the front of the tool. The center of the crescent-shaped pit is the pressure center of the chips against the front of the tool. Based on the above characteristics, a curved chip groove is pre-ground on the front of the tool to slow down the wear of the cutting edge and enhance the strength of the tip.
- Relief angle. The influence of the relief angle on the cutting process is generally not as sensitive as that of the rake angle. However, due to the significant metal deformation during stainless steel cutting, if the relief angle of the tool is small, it is prone to severe friction with the workpiece surface, resulting in increased surface roughness, work hardening, and exacerbated tool wear. At the same time, this worsens the conditions for subsequent cutting processes. When the relief angle of the finishing tool is α<6°, the workpiece surface exhibits a roughening phenomenon. This phenomenon is particularly severe when the feed rate and the back-cutting amount are relatively small. Therefore, a bit larger relief angle is generally selected when cutting stainless steel. However, if the rear corner is too large, the strength of the cutting edge will be reduced.
Secondly, choose suitable tool materials. Due to the characteristics of stainless steel itself, the cutting part of the tool must have high wear resistance and red hardness during machining, and it is often more important to focus on selecting toughness than durability.
The third point is the selection of cutting parameters. When choosing cutting parameters, the following factors should be considered: the cutting parameters should be selected based on the hardness of stainless steel and various raw materials; the cutting parameters should be selected based on the tool material, welding quality, and the grinding conditions of the lathe tool; the cutting parameters should be selected based on the diameter of the part, the size of the machining allowance, and the precision of the lathe.
Fourth, the requirements for cooling and lubrication. The coolant used for cutting stainless steel must have high cooling performance to remove a large amount of heat. It should also have good lubrication performance to provide effective external lubrication. It should have good permeability to facilitate wedging, diffusion, and internal lubrication. In addition, it should have good washing performance and supply methods to meet the needs of chip removal.
② Machining of titanium alloy rings. The poor machining performance of titanium alloys can be measured in terms of tool durability, the quality of the machined surface, and the difficulty of chip formation and removal. The reasons for the difficulty in machining sodium and titanium alloy materials are mainly reflected in the following aspects.
- The thermal conductivity and thermal diffusivity coefficients are low. The thermal conductivity and thermal diffusivity coefficients of titanium alloy materials are only 1/15 of aluminum and aluminum alloys, of 1/5 of that of steel. They are lower than the thermal conductivity coefficients of stainless steel and high-temperature alloys. The low thermal conductivity and diffusivity result in significant temperature differences and high thermal stress during machining, making it difficult for cutting heat to dissipate and leading to machining adhesion phenomena.
- The contact between the cutting edge and the front cutting surface is small, resulting in high stress at the cutting edge. This stress concentration makes the tool prone to wear and damage.
- High chemical reactivity leads to the formation of an oxide layer during processing, which is very hard and accelerates tool wear.
- A large friction coefficient, a small elastic modulus, and a high yield strength cause significant rebound deformation on the surface of processed products, thereby affecting their processing accuracy.
The measures taken in the cutting processing of titanium alloys are similar to those for stainless steel, but due to the special nature of titanium alloy materials, attention should be paid to the following three points during cutting processing.
The first is the selection of cutting machine tools and fixtures. The cutting machine tools should have high power, good rigidity, and a large range of speed and feed rates. The rigidity of the fixtures should be good, and the clamping force during finishing should not be too large to reduce the deformation of the processed parts and ensure processing accuracy.
The second is the selection of tool materials. In the process of cutting high-strength, high-toughness titanium alloys, the cutting force on the tool is very large, and sometimes the phenomenon of workpiece back-cutting may occur. The hard oxide layer can damage the surface of carbide blades. This requires that the tool material maintains sufficient hardness and good wear resistance at high temperatures and heat resistance. Therefore, when cutting titanium alloys, carbide tools should be prioritized only and high-speed steel tools should only be used when the temperature is relatively low. Never use tool materials that contain titanium, as these materials can easily bond with titanium alloys at high temperatures, leading to rapid tool wear.
The third is to correctly choose the cutting parameters. This includes cutting speed, depth, and feed rate, which can improve processing efficiency and reduce production costs. The cutting temperature of carbide tools should be controlled within 600~800℃, while the cutting temperature of high-speed steel tools should be controlled within 450~560℃.
(2) Electrical Discharge Forming
① Introduction to Electrical Discharge Machining. Electrical discharge machining is conducted in a liquid medium, where the automatic feed adjustment device of the machine tool maintains an appropriate discharge gap between the workpiece and the tool electrode. When a strong pulse voltage is applied between the tool electrode and the workpiece (reaching the breakdown voltage of the medium in the gap), it breaks down at the lowest insulation strength of the medium. Due to the small discharge area and extremely short discharge time, the energy is highly concentrated, causing the temperature in the discharge area to reach 10000-12000℃ instantaneously, resulting in localized melting and even vaporization of the metal on the surfaces of the workpiece and the tool electrode. The locally melted and vaporized metal is ejected into the working fluid under explosive force and is cooled into small metal particles, which are then quickly washed away from the working area by the working fluid, forming a tiny pit on the surface of the workpiece. After each discharge, the insulation strength of the medium recovers, waiting for the next discharge. This process is repeated, continuously eroding the workpiece’s surface and replicating the tool electrode’s shape, thereby achieving the purpose of forming and machining.
Electrical discharge machining includes various forms, such as electrical discharge forming, electrical discharge wire cutting, electrical discharge grinding, electrical discharge drilling, and various specialized electrical discharge machining applications.
Electrical discharge machining is widely used in the production of stainless steel and titanium alloy jewelry, mainly in two aspects: first, electrical discharge wire cutting is used for direct processing of jewelry; second, electrical discharge cutting and forming are used to create molds for subsequent stamping and hydraulic production of jewelry.
② Wire-cut Electrical Discharge Machining (WEDM), sometimes called wire cutting. Its basic working principle is to use a continuously moving fine metal wire (called the electrode wire) as the electrode to perform pulse spark discharge machining on the workpiece, cutting and shaping it. It is mainly used for processing various complex shapes and precision small workpieces, such as punch molds, die molds, convex-concave molds, fixed plates, discharge plates, forming tools, templates, metal electrodes for electrical discharge forming processing, various fine holes, grooves, narrow seams, arbitrary curves, etc. It has outstanding advantages such as small machining allowance, high accuracy, short production cycle, and low manufacturing cost, and has been widely applied in production. Wire-cut electrical discharge machines account for more than 60% of the total number of domestic and international electrical machining machines.
According to the different running speeds of the electrode wire, electric discharge wire cutting machines are usually divided into two categories: one is the high-speed wire feeding electric discharge wire cutting machine, where the electrode wire moves back and forth at high speed, generally with a wire feeding speed of 0.2m/s, the electrode wire can be reused, the processing speed is relatively high, but the fast wire feeding can easily cause the electrode wire to shake and pause during reverse movement, leading to a decline in processing quality; the other is the low-speed wire feeding electric discharge wire cutting machine, where the electrode wire moves in a low-speed unidirectional manner, generally, with a wire feeding speed of less than, the electrode wire is not reused after discharge, the operation is stable and uniform, with little shaking, and the processing quality is better, but the processing speed is lower.
In jewelry production, decorative patterns are often formed through wire cutting, as shown in the example of the stainless steel pendant pattern in Figure 5-3.

1.2 Mold Stamping (Hydraulic) Forming
(1) The Introduction to Stamping Process
Stamping is a forming processing method that uses a press and molds to apply external force to metal sheets, strips, pipes, and profiles, causing them to undergo plastic deformation or separation. The surface shape of the mold is clearly replicated, thereby obtaining workpieces (stamped parts) of the desired shape and size. Compared to traditional lost-wax casting, stamping can economically and repeatedly produce large quantities of the same product in a short time, and the surface of the product is smooth, with stable quality, greatly reducing the workload of subsequent processes, improving production efficiency, and lowering production costs. Therefore, stamping has received increasing attention in the jewelry manufacturing industry, and its application is becoming more widespread.
(2) Characteristics and Applicability of Stamped Accessories
Stamping accessories have the following characteristics:
- (Compared with investment casting jewelry, stamped parts have the characteristics of being thin, uniform, light, and strong, and using stamping methods can greatly reduce the wall thickness of the workpiece.
- Stamping produces jewelry with fewer holes, better surface quality, improved quality, and reduced defect rate.
- During mass production, stamping has high production efficiency, good working conditions, and low production costs.
- When the mold’s precision is high, the stamped accessories’ accuracy is high, and the repeatability is good, with consistent specifications, greatly reducing the trimming, grinding, and polishing workload.
- Stamping can achieve a higher degree of mechanization and automation.
However, the following conditions must be considered before the stamping process can produce accessories.
First, the structure of the jewelry should have good stamping processability, and it is best to avoid small holes, narrow grooves, and structures with angles or hollowed-out bottoms that cannot be stamped. A draft angle should be designed. The shape of the stamped parts should be as symmetrical as possible to avoid issues such as stress concentration, eccentric loading, and uneven mold wear. The thickness of the jewelry should not be too large, and the wall thickness difference should not be too big.
Second, the accessories should have a considerable production volume. Due to the stamping process, special molds must be made, which takes a long time and has high mold costs. Therefore, when the production volume is small, the production cost does not have an advantage.
Third, the strength of stainless steel and titanium alloys is relatively high, requiring good flow performance of the material in the cavity during the extrusion process, especially at the edges, corners, and ridges, where it is necessary to fill in completely without causing serious defects such as collapsed corners, edges, or ridges. A larger impact or pressure is needed, so the stamping machinery selected must have sufficient force, and the mold material must have adequate strength, with precise dimensions for the supporting and positioning points and surfaces for stamping.
(3) The Main Process of Stamping Accessories
① Analyze the processability of stamped parts. The product part drawing is an important basis for formulating stamping process plans and mold design. The formulation of stamping process plans should start from the product part drawing. Analyzing the part drawing includes both technical and economic aspects: the economic analysis of stamping processing, which analyzes product costs based on the production program of stamped parts, clarifying the economic benefits that can be achieved by adopting stamping production; the processability analysis of stamped parts refers to the difficulty level of stamping processing for the part. From a technical perspective, it mainly analyzes whether the shape characteristics, size, precision requirements, and material properties of the part meet the requirements of the stamping process. If poor processability is found, modification suggestions for the stamped part product need to be proposed and can only be modified with the consent of the product designer.
② Determine the forming process plan for the stamped parts. After analyzing the processability of the stamped parts, several different stamping process plans are usually developed based on the analysis of the nature of the processes, the number of processes, the sequence of processes, and the combination methods. A comprehensive analysis and comparison are conducted from various aspects, including product quality, production efficiency, equipment occupancy, the difficulty of mold manufacturing and mold life, process costs, and the convenience and safety of operation, to determine the most economical and reasonable process plan suitable for the specific production conditions of the factory.
Then, based on the overall plan for forming the determined parts, determine and design the process plan for each stamping operation. This includes the processing methods for completing the forming of each operation, the main process parameters for each operation, necessary forming process calculations based on the forming limits of each stamping operation, determining the forming force for each operation, calculating the consumption quotas for materials, energy, and labor hours for each operation; calculating and determining the shape and dimensions of each operation piece, and drawing the process diagrams for each operation.
③ Determine the structural form of the stamping die. The stamping die is a special process equipment that processes materials into parts (or semi-finished products) and is an essential piece of equipment for stamping production. The quality of stamped parts, production efficiency, and production costs are directly related to the design and manufacturing of the die. The level of technology in die design and manufacturing is one of the important indicators of a country’s product manufacturing level, and it largely determines the quality, efficiency, and new product development capabilities of the products.
There are many forms of stamping molds, which can generally be classified according to the following two main characteristics.
a. Classified according to the nature of the process as follows.
Punching die: A mold that separates material along closed or open contour lines. Such as blanking die, punching die, cutting die, notching die, trimming die, and splitting die.
Bending die: A mold that causes the blank or other raw materials to undergo bending deformation along a straight line (bending line) to obtain a workpiece with a certain angle and shape.
Deep drawing dies are molds that transform a sheet metal blank into an open hollow part or further change the shape and size of the hollow part.
The molding dies: These are molds that directly copy the shape of the convex and concave molds according to the drawing, using a blank or semi-finished workpiece. The material itself only undergoes local plastic deformation, such as expansion molds, necking molds, flaring molds, undulating forming molds, flanging molds, shaping molds, etc.
b. Classified according to the degree of process combination as follows.
Single-process die: A die completes only one stamping process in a single press stroke.
Composite die: A die with only one station that completes two or more stamping processes simultaneously at the same station during one stroke of the press.
Progressive die (also known as continuous die): A die with two or more stations in the direction of the blank’s feed, completing two or more stamping processes at different stations in one press stroke.
Stamping dies for accessories generally consist of two types of components. The first type is process parts, which directly participate in the completion of the process and have direct contact with the raw materials, including working parts, positioning parts, unloading and pressing parts, etc.; the second type is structural parts, which do not directly participate in the completion of the process and do not have direct contact with the raw materials, but serve to ensure the completion of the process or improve the functionality of the die, including guiding parts, fastening parts, standard parts, and other components. The stamping die manufacturing process is shown in Figure 5-4.
④ Choose stamping equipment. The type of stamping equipment selected is mainly based on the nature of the stamping to be completed, production volume, dimensions of the stamped parts, and precision requirements; the main basis for selecting the technical parameters of the equipment is the dimensions of the stamped parts, the magnitude of the deformation force, and the size of the molds.
⑤ Write stamping process documents. To scientifically organize and implement production, accurately reflect the technical requirements determined in the process design during production, and ensure the smooth progress of the production process, it is necessary to prepare detailed process documents based on different production types, generally presented in the form of a process flow. The content includes process name, number of processes, process sketches (shape and size of semi-finished products), molds used, selected equipment, process inspection requirements, specifications and performance of sheet materials, and the shape and size of rough parts.
⑥ Stamping jewelry production. Shape the material using stamping equipment according to the established stamping process parameters.
(4) Measures to Improve the Cross-Sectional Quality of Stamped Parts
The process of stamping accessories can be classified into two main categories based on the technology: forming processes and separating processes. The purpose of the forming process is to cause the sheet material to undergo plastic deformation without breaking blanks, creating workpieces of the desired shape and size. The separating process, also known as blanking, aims to separate the stamped parts from the sheet material along a specific contour line while ensuring the quality requirements of the separation surface. The quality of the blanking surface depends on the blanking conditions and the properties of the material itself, such as the edge gap and edge shape, the sharpness of the edge, the blanking force, the lubrication conditions, and the quality of the sheet material, and performance, etc. Stamping production requires that the cut parts have a larger bright band and minimize the width of the fracture band area. This is crucially dependent on taking measures to increase plastic deformation and delay the occurrence of shear cracks. For example, reducing the cutting gap, using a pressure plate to compress the strip on the die surface, applying reverse pressure to the strip under the punch with a top plate, reasonably selecting the overlap, paying attention to lubrication, etc. In addition, during cutting, efforts should be made to minimize the collapse angle, burrs, and warping. To achieve this, it is necessary to adopt the lower limit value of reasonable gaps as much as possible. The mold edge can be kept sharp, and the edge value can be reasonably selected. Measures such as pressure plates and ejector plates can be used.
2. Investment Casting Process
2.1 Stainless Steel Casting and Forming
The melting point of 304 stainless steel is 1454℃, and the melting point of 316 stainless steel is 1398℃. This temperature far exceeds the limits that gypsum molds can withstand. Therefore, stainless steel jewelry must be cast using acid-bonded casting powder, which significantly increases production costs.
(1) Casting Powder for Stainless Steel Jewelry
Due to the high casting temperature of stainless steel, casting powder cannot use gypsum as a binder and must use casting powder with higher refractoriness. Like gypsum casting powder, the casting powder used for stainless steel jewelry is also composed of a binder and filler. The filler is usually quartz and feldspar, with a total amount of approximately 80%. Phosphoric acid was initially widely used as a binder, but now methenamine phosphate is preferred. It is a dry powder that can be easily added to the powder mixture, utilizing the chemical reaction of the binder system to solidify the casting powder, as follows:
NH4H2PO4 + MgO +5H2O→ NH4MgPO4·6H2O
The entire phosphoric acid reaction is very complex. When the amount of MgO required for the reaction of methyl phosphonic acid is chemically equal, it often actually requires an excess of MgO, thus forming a NH4MgPO4·6H2O colloid that surrounds the filler and the excess MgO. During roasting, the temperature reaches 1000℃, and the mold undergoes a thermal reaction, resulting Mg2P2O7 in the final products of crystalline filler, excess MgO, and SiO2 filler.
Using phosphate-bonded casting powder, the overall strength of the casting powder is much higher than that of gypsum casting powder, the surface of the mold cavity is smoother and finer, and the surface finish of the castings is higher. However, the residual strength of the mold is also higher, making it somewhat difficult to remove the castings from the mold.
(2) The Casting Process of Stainless Steel Jewelry
Stainless steel jewelry can be produced using centrifugal casting methods, vacuum suction casting, or vacuum pressure casting methods. The casting process involves many procedures (Figure 5-5).

2.2 Casting and Forming of Titanium Alloys
(1) The Requirements of Titanium Alloy Casting for Melting
Due to the high reactivity of titanium alloy liquid, its melting must be carried out under a higher vacuum or protected by inert gases (argon or helium). The crucibles used for melting are all water-cooled copper, with three melting processes.
① Vacuum non-consumable electrode arc furnace smelting. Alloy smelting is carried out under vacuum or inert gas protection. This process mainly prepares consumable electrode smelting, characterized by high-temperature and high-speed melting. The vacuum non-consumable electrode arc furnace maintains the stability of the arc after vacuuming and filling with inert gas, preventing the volatilization of difficult-to-melt metals, especially reactive metals, thus stabilizing the metal composition for refining purposes.
② Vacuum self-consumption electrode arc furnace smelting. It uses self-consumption electrodes made of titanium or titanium alloys as the cathode and a water-cooled copper crucible as the anode. The melted electrode enters the crucible as droplets, forming a molten pool. The surface of the molten pool is heated by the arc, remaining in a liquid state at all times. At the same time, the bottom and the surrounding area in contact with the crucible are subjected to forced cooling, resulting in crystallization from the bottom up. The metal liquid in the molten pool solidifies to become titanium ingots.
③ Vacuum self-consuming electrode shell melting furnace. This type of furnace was developed based on the vacuum self-consuming electrode arc furnace, which integrates melting and centrifugal casting for irregular castings. Its biggest feature is a layer of titanium alloy solid shell between the water-cooled copper crucible and the metal melt known as the shell. This shell, made of the same material, serves as the inner lining of the crucible to form a melt pool for storing titanium liquid, avoiding contamination of the titanium alloy liquid by the crucible. After pouring, the layer of shell left in the crucible can be reused as the inner lining of the crucible.
In recent years, with the development of technology and the needs of production, new methods and equipment for smelting titanium alloys and other reactive metals have been successively researched and developed, mainly including electron beam furnaces, plasma furnaces, and vacuum induction furnaces, which have achieved a certain degree of application. However, regarding technical and economic indicators such as power consumption, melting speed, and cost comparison, self-consuming electrode arc furnaces (including shell furnaces) remain the most economical and suitable smelting method.
For jewelry production, the melting amount is generally small, and the surface quality requirements are high. Therefore, dental casting equipment can generally be used. Figure 5-6 shows a dental titanium casting machine that can be used for casting titanium jewelry. This titanium casting machine integrates pressurization, suction, and centrifugation, is compact, easy to operate, does not require a dedicated space, and utilizes flywheel energy storage and the momentary increase in the device’s force and the rapid flow acceleration to inject the titanium molten liquid into the fine details of the mold cavity, resulting in a higher success rate for the castings. The small melting and casting chambers enable quick vacuum extraction, reduce argon gas consumption, minimize residual air, and ensure relatively good-quality titanium castings.
(2) Requirements for Casting Materials in Titanium Alloy Casting
Titanium and its alloys are highly chemically reactive metals that, in a molten state, almost react with all refractory materials to form brittle compounds, greatly increasing the difficulty of melting and casting titanium alloys.
① There are three types of molds for casting titanium alloys.
- Permanent molds mainly include processed graphite and metal molds (iron and titanium molds). The molds are all mechanically processed. The castings produced have relatively simple structures and lower dimensional accuracy; they are generally used to produce rough parts.
- Disposable molds: They can produce castings with relatively complex shapes and high dimensional accuracy. According to their molding methods, there are two types: compacted graphite sand molds and lost foam molds. The latter can manufacture more complex castings (wall thickness 2 mm) with high dimensional accuracy and low surface roughness (Ra3.2). According to the different materials of the mold shell, the lost foam mold shell is divided into three different systems: the pure graphite mold shell system. It uses graphite powder of different particle sizes as refractory fillers and sand-spraying materials, with resin as the binder. The mold shell has high strength, lightweight, low cost, and a wide range of raw material sources, making it suitable for centrifugal or gravity casting. The second is the refractory metal surface layer mold shell system. This is a composite system, where the surface layer requires special processes due to the different molding materials (such as aluminum powder and other refractory metals), while the back layer is the same as the lost foam casting of cast steel in terms of molding materials and shell-making processes. The third is the oxide ceramic mold shell system. The mold shell’s surface and back layers are made of oxides as molding materials, resulting in high mold shell strength and the lowest thermal conductivity among the three types of mold shells, making it suitable for casting complex, thin-walled shapes. The titanium castings poured using the above three types of shell systems differ little in chemical composition and mechanical properties; however, there is a significant difference in surface quality, with the shrinkage rate of the latter two types of shells significantly lower than that of the graphite shell, resulting in high dimensional accuracy of the castings.
- Embedded casting: Embedded casting is primarily used in the casting of titanium alloy jewelry. It is very similar to oxide ceramic shells, except that layered shells are not used, and the investment method is applied directly.
② Requirements for embedding materials in casting titanium jewelry. The linear shrinkage rate of titanium during pure titanium casting is 1.8%~2.0%. To achieve good dimensional accuracy, the embedding material must provide sufficient expansion to compensate for the casting shrinkage of sodium. The conditions for selecting embedding materials for titanium casting must include the following: minimal reaction with titanium, ability to achieve a good surface shape, no contamination of the castings, moderate expansion to compensate for titanium shrinkage and sufficient strength.
Casting titanium embedding materials can be divided into three categories based on different expansion methods: embedding materials that expand due to the hardening and thermal deformation of silicon, embedding materials that expand due to the oxidation of metal powder zirconium (Zr), and embedding materials that expand due to the formation of spinel (MgO, Al2O3), including embedding materials primarily composed of refractory materials such as silicon oxide, aluminum oxide, magnesium oxide, calcium oxide, and zirconium oxide.
The current expansion of the embedding material mainly relies on SiO2 undergoing an allotropic transformation when heated, accompanied by significant volume expansion, which determines the special position of the SiO2 among embedding material. However, molten titanium can chemically react with it, severely affecting the quality of titanium castings. To address this issue, a certain proportion of ZrO2. It is a high-temperature resistant inert material added to the currently ideal casting titanium embedding material, as it does not chemically react with molten titanium at high temperatures. Castings made with ZrO2-based embedding materials have less contamination beneath the surface and do not stick to sand, resulting in castings with a metallic luster but a smaller expansion coefficient, which can affect the dimensional accuracy of the castings.
The scouring effect of high-temperature molten metal is significant during pouring. When the strength of the embedding material is insufficient, some of the embedding material powder will fall off and mix into the titanium liquid under the scouring of the titanium liquid, causing the fluidity of the titanium liquid to deteriorate and preventing it from reaching the end of the casting cavity. Therefore, the ideal embedding material for casting titanium must have good stability and expansion coefficient and possess a certain strength to withstand the impact of the titanium liquid.
(3) Casting Methods for Titanium Alloy Jewelry
Casting titanium alloy jewelry requires special heat sources, dedicated mold materials, and equipment to prevent titanium surface contamination. Dental-specific titanium casting machines have been introduced into jewelry making, with melting atmospheres protected by vacuum or inert gases (argon or helium). The casting methods include pressure casting, vacuum casting, and centrifugal casting. Due to the high pouring temperature of titanium alloys, their low density, and poor fluidity, it is necessary to complete the filling quickly, with the best method being vacuum centrifugal casting. Figures 5-7 and 5-8 exemplify casting titanium alloy jewelry.

Figure 5-7 Titanium jewelry casting

Figure 5-8 Titanium jewelry produced by casting method
(4) Common Issues in Casting Titanium Jewelry
The common problems when casting titanium alloy jewelry are the following five.
① The casting is incomplete. The incompleteness of the casting is related to the following aspects.
- Casting machine. The model of the casting machine is closely related to the casting flow rate, the casting machine’s vacuum level, and the inert gas’s flow rate.
- Setting of the pouring channel. If the pouring channel is too narrow or too long, or if its position and quantity are inappropriate, it can affect the integrity of the casting.
- Setting of ventilation channels. Titanium is protected by inert gas during the melting process, and the inert gas can also enter the mold cavity. When the melted titanium liquid is injected into the mold cavity, the gas in the cavity’s small areas hinders the titanium liquid’s flow, forming gas holes. Therefore, it is necessary to carefully set up the exhaust channels on the wax mold.
- Molding temperature. A high molding temperature results in fewer casting defects, but the surface contamination layer of the castings is thick, and the mechanical properties are poor. Reducing the molding temperature can decrease surface contamination, but it leads to more casting defects. When the molding temperature is between 350~400℃, it can reduce both contamination and casting defects.
- Titanium material usage. When the number of castings in the mold is too large, and the amount of titanium material is insufficient, incomplete castings will inevitably occur.
② Internal porosity of castings. The appearance of internal porosity in titanium castings is due to the inert gases and residual air being entrained into the mold cavity when the molten sodium is poured into the mold cavity. When the titanium liquid is injected into the cavity, a shell forms immediately, causing the entrained gases to be unable to escape, resulting in internal porosity in the casting. The quantity and type of porosity formed are related to the equipment. Porosity formed in pressurized, suction-type, and pressurized (non-suction) types is dispersed. The porosity formed in pressurized (non-suction) types is less than in pressurized suction types. The porosity in centrifugal casting titanium machines are mostly found at the inner end of the rotating body, and the incidence of porosity is significantly lower than that in pressurized suction and pressurized types.
The permeability of the embedding material is also related to the pores. Embedding materials with good permeability are used for pressurization, and suction-type titanium casting machines can produce more pores. The centrifugal titanium casting machine is unrelated to the permeability of the embedding material. In addition, the positioning of the runner and exhaust duct also has a certain relationship.
③ Shrinkage cavities. The formation of shrinkage cavities inside titanium castings is a difficult problem in titanium casting technology. The volume of molten titanium shrinks by 1% during solidification. If the titanium casting process is not properly controlled and sufficient compensation is not provided, shrinkage cavities will inevitably occur in the titanium castings. Shrinkage cavities in cast titanium jewelry are mostly at the sprue and casting junction. The sprue design is the most important way to control shrinkage cavities in titanium castings, as it regulates the rate, flow, and integrity of the molten metal entering the mold cavity. Factors such as size, type, shape, position, and direction can all affect the quality of the casting.
④ The surface of titanium castings is rough. Surface roughness refers to an uneven surface with bumps or flow marks. Causes may include excessively high mold temperatures, sintering reactions between the embedding material and sodium liquid, mold breakage, sand sticking, or poor quality of the embedding material.
⑤ The surface contamination layer of sodium castings is too thick. Many factors determine the thickness of the surface contamination layer of titanium castings, including the type of flux, mold temperature, equipment vacuum level, and purity of inert gases. Among them, the type of flux has the following general influence on the thickness of the contamination layer: zirconia-based flux has the thinnest layer, followed by alumina, magnesia, and phosphate-based fluxes in order of increasing thickness of the contamination layer.