En omfattende guide til 10 typer forbedrede edelstener
Kjennetegn ved ulike forbedrede edelstener
Innledning:
Edelstensforbedring er en fascinerende prosess der vitenskap og kunst kombineres for å avdekke edelsteners indre skjønnhet og forvandle dem til fantastiske smykker og dekorativ kunst. Denne oversikten tar for seg de ulike teknikkene som brukes til å forbedre edelstener, for eksempel varmebehandling, kjemiske reaksjoner og fysiske modifikasjoner, som kan forbedre fargen, klarheten og holdbarheten til rubiner, safirer, smaragder og andre edelstener. Den tar også for seg de tradisjonelle og moderne metodene som har blitt brukt for å få frem den indre glansen i disse edelstenene, noe som har gjort dem til stjerner i smykkeverdenen. Enten du er smykkeentusiast, designer, forhandler eller en som ønsker å tilføre glitter til samlingen sin, gir denne guiden deg innsikt i en verden av edelstenforbedring. Den tar for seg metoder som varmebehandling, kjemiske reaksjoner og fysiske modifikasjoner som får frem den indre glansen i rubiner, safirer, smaragder og andre edelstener, og hvordan disse metodene kan brukes til å forbedre kvaliteten og verdien på edelstenene. For dem som jobber i smykkeindustrien, gir denne guiden et omfattende innblikk i teknikker og prosesser som brukes for å øke edelsteners skjønnhet og verdi, slik at de blir mer attraktive for smykker og dekorative formål.
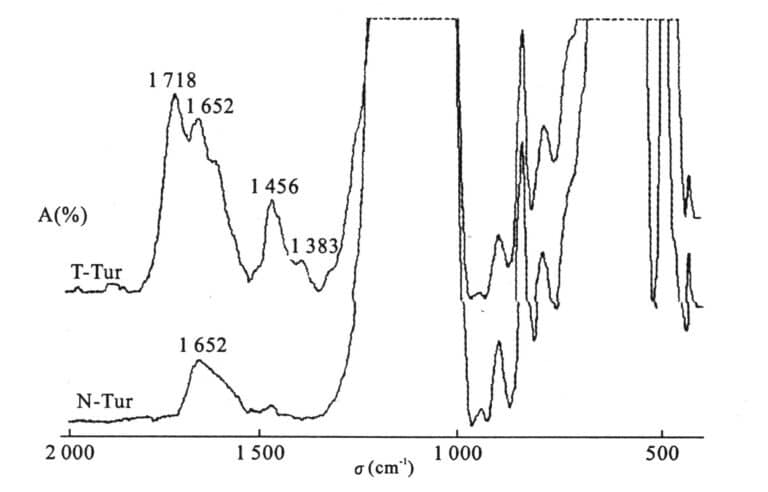
Infrarøde spektre av naturlig og fylt turkis N-Tur: Naturlig turkis; T-Tur: Fylt turkis
Innholdsfortegnelse
Del I Forbedring av diamantenes egenskaper
Diamanter som er dyre og lønnsomme, har ofte ulike feil, for eksempel lav klarhet, dårlig farge eller liten størrelse. For å øke salgsprisen søker folk ulike metoder for å forbedre diamanter.
1. Fylling av diamanter
Ved å fylle diamantsprekker med fargeløst, gjennomsiktig blyglass med høy brytningsindeks, hardt blyglass med lavt smeltepunkt og andre amorfe materialer kan man skjule sprekkene, forbedre klarheten og dermed oppnå høyere fortjeneste.
Injeksjon av fremmedlegemer er et kjennetegn ved fylte diamanter, og manifestasjonen er:
(1) Flash-effekt
Etter at fyllstoffet er injisert langs sprekkene, kan man i mikroskopet se et regnbuelignende, sterkt blinkende fenomen i sprekkenes retning. Når scenen roteres, eller diamanten beveges sakte frem og tilbake, endres også sprekkene, og fargene og områdene på blinkene endres tilsvarende.
(2) Strømningsstruktur
I noen fylte sprekker eller hulrom kan man se et glasslignende stoff som flyter inni, og noen ganger kan man observere svært fine, gjennomsiktige, buede linjer av flytende materiale i fyllstoffet. Siden flytemønstrene i fyllstoffet ikke er lett oppløselige, kan de bare observeres i visse områder av sprekkene. Denne følelsen av flytstruktur oppstår når fyllstoffet sprøytes inn i diamantsprekkene under høy temperatur og høyt trykk, og retningen er i samsvar med sprekkenes retning.
(3) Inklusjoner av gassbobler
På grunn av ufullstendig fylling i sprekker eller hulrom i diamanter, opptar gass ofte disse mellomrommene, noe som resulterer i et fenomen med høy kontrast. Boblene kan være fordelt enten på veggene i sprekkene eller i fyllmaterialet, og kan opptre enkeltvis eller i klynger, noen synlige for det blotte øye, mens andre er svært små.
Når fyllingsdiamanter slipes og poleres til løse diamanter, er fyllingsmaterialets hardhet mye lavere enn diamantens, noe som fører til parabolske fordypninger og sprekker på fasettene. Samtidig er brytningsindeksen i fyllmaterialet lavere enn i diamanten, noe som fører til at det ofte oppstår Becke-linjer langs sprekkene i den fylte diamanten. Hvis diamanten senkes ned i en olje med høy brytningsindeks, blir Becke-linjene mer utpreget. Hvis diamanten senkes ned i bensin og belyses med sterkt lys, kan man se flytende regnbuer i bensinen inne i sprekkene.
Når man utfører en flammeforbrenningstest på fylte diamanter, vaskes fyllmaterialet ut ved høye temperaturer, og man kan se smeltede stoffer i kantene av sprekkene, mens det indre av sprekkene eller hulrommene ser tåkete ut.
(4) Deteksjonsmetoder
① Observasjonsvinkel: Vinkelen for å oppdage fenomenet blinking i fylte diamanter bør være parallell med sprekkene, mens den optimale observasjonsvinkelen for ufylte diamanter bør være vinkelrett på sprekkoverflaten.
② Spotlight-belysning: Ved bruk av fiberoptisk belysning blir blitseffekten spesielt tydelig, noe som avslører fyllingsområdet og eksponerer eventuelle hårfine sprekker i fyllingen. Hvis et polarisasjonsfilter plasseres mellom mikroskopet og edelstenen i forbindelse med en gjennomskinnelig lyskilde, kan det vise fyllingsområdet og bidra til å skille blitseffekten fra naturlig irisering.
Skyggemetoden: Ved å bruke en ugjennomsiktig, svart, ikke-reflekterende lysskjerm mellom diamanten og mikroskopets lyskilde kan det være lettere å observere strømningsstrukturen.
④ Forstørret observasjon: Fylte diamanter er vanligvis over 0,3 ct. For å vurdere om en diamant er fylt, bør den observeres nøye under et mikroskop på 6 x 10 eller 8 x 10, mens et forstørrelsesglass på 10 ganger bare kan avsløre noen grove ledetråder og tegn.
2. Termisk bestrålte diamanter
Fargen på diamanter er hovedsakelig forårsaket av ulike fargesentre som absorberer forskjellige områder av synlig lys, og dannelsen av fargesentre er nært knyttet til ulike defekter i diamantkrystallstrukturen. Eliminering og dannelse av strukturelle defekter har spesielle funksjoner i den termiske bestrålingsprosessen.
Det er forskjellige fargesentre som gjør at diamanter blir farget, for eksempel den "kanarigule" karakteristikken til N-senteret; N3 senteret er det vanligste blant produsenter av gule diamanter, med en absorpsjonslinje ved 415 nm; N2 senteret er representert ved 478 nm, og viser lysende gul fluorescens under langbølget ultrafiolett lys, og denne diamanten fremstår ofte som en fortryllende ravgul farge i sollys; H3 senteret (med en absorpsjonslinje ved 503 nm) sammen med N3 og N2 sentre er de viktigste fargeskapende faktorene for brune diamanter, mens H3 og H4 sentre er de viktigste årsakene til at type I a fargeløse eller lysegule diamanter viser en lysere gul etter varmebestråling. I tillegg viser diamanter generelt at GRI-hjertet som produseres ved bestråling (som partikler, nøytroner, høyenergielektroner, protoner osv.) manifesterer seg som et svært bredt absorpsjonsbånd (fra 741 nm - i det gulgrønne synlige lysområdet), noe som gjør at diamanter kan vise forskjellige farger som grønt, blått, blågrønt, dypgrønt, svart, gult og mer. De ledige plassene som oppstår når karbonatomer erstattes med boratomer i diamanter av type Ⅱ b, kalles B-hjerter, og får diamanter til å se blå ut. B-hjerter er imidlertid sjeldne i naturlige diamanter. Derfor er fargeforandringen i diamanter hovedsakelig rettet mot gule diamanter.
3. Belagte diamanter
Diamantbelegg er en metode der man ved hjelp av kjemisk dampavsetning (DF) legger et lag med polykrystallinsk diamantfilm på overflaten av en diamant, som har en tydelig granulær struktur som er relativt lett å se under forstørrelse. Raman-spektroskopi viser at diamantfilmens karakteristiske topp ligger nær 1332 cm-1med full bredde ved halv maksimum (FWHM); diamantfilmer av dårlig kvalitet har en betydelig toppforskyvning og redusert intensitet, og kan til og med vise en bred topp i nærheten av 1500 cm-1.
4. GE-prosessering av diamanter
Denne metoden retter seg hovedsakelig mot brune diamanter av typen Ⅱ a -type med en viss klarhet, og flytter eksterne fargesentre under høy temperatur og høyt trykk for å gi den beste indre fargen. Brune diamanter antas å være forårsaket av gitterdefekter som følge av plastisk deformasjon av diamantkrystallgitteret, noe som oppstår under diamantdannelsen fra mantelen til overflaten på grunn av trykkendringer. Derfor burde det være mulig å reparere denne deformasjonen gjennom trykksetting eller trykkavlastning. Det er imidlertid bare ca. 1% av diamantene som faktisk kan behandles, som beskrevet nedenfor.
① De aller fleste viser svak til tydelig hvit eller sjelden brun morfologi. Halvparten har en litt uskarp morfologi, noe som kan skyldes vekstlinjenes spredningseffekt på annet lys.
② Spalting eller fjærlignende sprekker nær overflaten.
③ Mange spaltninger nær overflaten viser "delvis heling", i likhet med de "fingeravtrykkslignende inneslutningene" som ofte finnes i safirsteiner. Andre spaltninger har et frostet eller kornet utseende nær overflaten, men blir glassaktige på større dyp. Et svart område (fjærformet grafittinneslutning) kan sees i noen spaltninger.
Inklusjoner er ofte omgitt av spenningsbrudd, for eksempel grafittinneslutninger omgitt av gjennomskinnelige glorier som stråler utover fra små brudd, og noen grafittinneslutninger er omkranset av et nettverk av fine brudd. Dette strålende bruddmønsteret kan skyldes ulik termisk ekspansjon av inneslutningen og diamanten etter høy temperaturoppvarming. Et sett med sirkulære brudd er fordelt langs den oktaedriske formen, noe som skyldes frigjøring av indre spenning rundt inneslutningene i diamanten. Noen faste, ugjennomsiktige inneslutninger viser ikke de nevnte strålende eller sirkulære bruddene, men har en flytende og smeltende struktur, og noen ganger observeres sky- eller tåkelignende stoffer.
Under et polarisert lysmikroskop kan man observere middels til sterke spenningsmønstre og en kryssformet "Tammie", ordnet i bånd og flekker. Stressinterferensfargene er for det meste første- og andreordens grå, blå eller oransje, mens naturlige Ⅱ a-diamanter vanligvis viser grå og brune interferensfarger med lavere intensitet.
5. Belagte, fargede og laserbehandlede diamanter
(1) Belagte diamanter
Ved å belegge og spraye et svært tynt lag med farget organisk materiale på overflaten av diamanter kan man både forbedre diamantens farge og forsterke dens "ild".
(2) Fargede diamanter
Ved å bruke røde, blå, rosa og andre farger på diamantens omkrets, som kan være vanskelig å oppdage etter metallinnlegg, kan diamanten få en rød eller blå fargetone. For å redusere den gule tonen i diamanten kan gule komplementærfarger (blå eller lilla) brukes til å farge overflaten av diamanten, slik at den fremstår som hvitere.
(3) Laserrengjøringsdiamanter
Ved hjelp av laserboringsteknologi kan "feil" i diamanten fordampes eller korroderes bort med sterk syre, og deretter kan hulrommene fylles med glass for å forbedre diamantens klarhet.
Del II Forbedring av beryll-edelstener
Beryll-edelstener inkluderer blant annet smaragd, akvamarin, gylden beryll, cesisk beryll og Max-ixe (Max-ixe-type) beryll. Smaragder er 0,15%-0,5%beryll erstattet av aluminium og er grønne; Akvamarin er laget av En liten mengde beryll Al3+ og være2+ erstattet av Fe3+ og Fe2+henholdsvis i en sjarmerende himmelblå og blågrønn; fargen på gyllen beryll er gul til brungul, noe som skyldes Fe3+erstatter Al3+ inn i oktaederet i form av isomorfisme. Rosa og lilla rød cesium beryl, fargeionene er hovedsakelig Mn2+ og Mn3+i tillegg til Cs1+og Fe3+ så videre; Maxisi-beryll er en mørkeblå beryll som er farget av et lysere fargesenter.
Vanlige metoder for å forbedre beryl edelstener inkluderer varmebehandling ved lav til middels temperatur, bestrålingsbehandling og infusjonsmetoder. For eksempel kan visse grønne og blågrønne beryller gjennomgå varmebehandling (400-450 °C), noe som kan eliminere gule toner fra lyseblå til himmelblå akvamarin, gjøre noen gylne beryller til fargeløse steiner og forvandle oransjerød beryll til rosa cesian beryll, samt endre cesian beryll til rød eller purpurrød. Bestråling kan forvandle fargeløs, lysegrønn og lyseblå beryll til gul, grønn eller blå, mens noen fargeløse beryller kan bli rosa eller oransjerøde. Noen fargeløse eller rosa beryller kan bli dypblå etter bestråling, men vil raskt falme i sollys. Infusjonsmetoden er den viktigste teknikken for å forbedre naturlige smaragder, som innebærer bløtlegging i sterk syre, etterfulgt av gjentatt vask med rent vann og fortynnet alkalisk løsning, tørking og deretter infusjon med kanadisk balsam ved hjelp av varm infusjon eller høytrykksinfusjon (vakuuminfusjon), forsegling med voks og polering. Noen bruker også fargede fargestoffer eller pigmenter til infusjon.
Forbedrede beryll-edelstener har forskjellige egenskaper på grunn av de ulike forbedringsprosessene. Det er imidlertid vanskelig å skille mellom beryll-edelstener som er behandlet med lav til middels høy temperatur og bestråling, og deres naturlige motstykker.
1. Forbedring av smaragder
(1) Smaragd behandlet med injeksjonsmetoden
Det finnes tre typer injeksjonsmidler: fargeløs olje, farget olje og harpiksfylling, hver med sine egne egenskaper.
① Injisering av fargeløs olje: Hovedformålet er å dekke eksisterende sprekker og hull uten å endre fargen på edelstenen. Det er anerkjent av smykkeindustrien og forbrukere som en optimalisering av edelstenen. Under identifisering kan smaragden plasseres i vann eller en annen fargeløs løsning og observeres under reflektert lys. Ved å rotere edelstenen kan man se interferensfarger forårsaket av den fargeløse oljen eller væskeinneslutninger i én retning. Oppvarmingseksperimenter kan vise oljestrømning, ofte omtalt som "svetting".
② Injeksjon av farget olje: Under forstørrelse kan man se grønn olje fordelt på en trådlignende måte i sprekkene, og noen oljer viser fluorescens. Etter at oljen har tørket, etterlater den et grønt fargestoff i sprekkene.
③ Injisering av resin: Bobler kan bli liggende igjen i sprekkene, og noen ganger kan de se tåkete ut eller ha en flytende struktur. I reflektert lys kan man se nettlignende fyllmaterialer på overflaten av edelstenen.
(2) Overflatebeleggmetoder inkluderer to typer: baksidemetoden og beleggmetoden.
① Baksidemetode: Et lag med grønn film eller grønn folie er plassert i bunnen av smaragdringen, noe som ofte ikke er lett å legge merke til etter at den er satt i en spørsmålsstillingsstil. Ved identifisering kan man se sammenføyningssømmen, og det kan være bobler igjen i sømmen. Noen ganger kan filmen rynke seg, sprekke eller falle av, og baksiden viser ingen dikroisme.
② Beleggingsmetode: Det er lett å se et nettverk av sammenvevde sprekker, og når den er nedsenket i vann, kan fargen sees konsentrert i kantene. Fra siden kan et lagdelt distribusjonsfenomen observeres.
2. Forbedring av Maxixe blå beryll
Hovedmetoden for å forbedre Maxixe blå beryl er bestråling. Etter γ-stråle eller kortbølget ultrafiolett bestråling er den koboltblå, og dens synlige lysabsorpsjonsspektrum er 695nm, 655nm sterke absorpsjonsbånd, 628nm, 615nm, 581nm, 550nm svake absorpsjonsbånd.
Del III Forbedring av korund-edelstener
1. Forbedring av Ruby
Korund-edelstenen med en rød farge kalles rubin. Rubinens farger inkluderer lys rød, middels rød, dyp rød og rød med andre fargetoner. I Bibelen er rubinen oppført som en av de mest dyrebare edelstenene. I dag utgjør foredlede rubiner det store flertallet av rubinmarkedet, og de har egenskaper som skiller seg fra naturlige rubiner på grunn av ulike typer foredlingsprosesser.
(1) Termisk behandling
Rubiner som er behandlet ved høye temperaturer, har ofte ujevne farger, og klarheten i de opprinnelige fargebåndene kan endre seg i varierende grad.
(For eksempel kan faste inneslutninger med lavt smeltepunkt delvis smelte, kantene kan bli avrundede, og fibrøse inneslutninger kan bli intermitterende; flytende inneslutninger kan sprekke på grunn av volumutvidelse, og til og med gå inn i nydannede bruddlinjer).
③ Overflaten på edelstener har ofte noen "pockmarks" eller groper.
(2) Injeksjonsbehandling
① Lyse rubiner dyppes i organisk fargestoff (immersion) og varmes opp for å størkne fargestoffet og farge dem.
② Farget olje fylles inn i sprekkene i edelstenen, noe som noen ganger gir fargerike interferensfarger.
Boraks, vannglass, parafin, plast, silisiumdioksyd, blyholdig glass osv. fylles i sprekkene på rubiner, eller kromoksidfargestoffer tilsettes for å forbedre den røde fargen på rubinen.
Hovedformålet med injeksjon langs sprekken er å forbedre fargen og gjennomsiktigheten til rubinen. Dens karakteristiske er at alle injektorene er plassert i edelstenens splittede gud, brytningsindeksen til injektorene er forskjellig fra rubinens, og absorpsjonsspekteret til rubinen kan være forskjellig fra det for den infrarøde spektralanalysen. Raman-spektroskopisk analyse viser at elementer som ikke vises i naturlige rubiner, som bly, bor, silisium, fosfor, kalsium og så videre.
(3) Termisk diffusjonsbehandling
① Kromdiffusjon
Høye temperaturer brukes til å la eksterne kromelementer komme inn i overflatelaget på lyse rubiner ved isomorf substitusjon, okkupere aluminiumsgitteret og danne et rødt diffusjonslag.
Rubiner som er behandlet med termisk diffusjon, har ofte varierende nyanser av rødt, er ujevne eller virker flekkete. Hvis slike rubiner senkes ned i dibrommetan og observeres under diffust, reflektert lys, kan man se en konsentrasjon av rødt på omkretsen, fasettkantene og spalteoverflatene. I tillegg kan termisk diffuse rubiner ha en avvikende brytningsindeks på opptil 1,80.
② Berylliumdiffusjon
Berylliumdiffusjon kan gi korundsteiner en gul, oransje eller brun fargetone, og berylliumelementer kan trenge inn fra rubinens overflate til innsiden av edelstenen, eller til og med hele steinen. Det ytre laget er oransjerødt, mens midten er rosa og rød.
Berylliumdiffusjon rubin, kan også vises krystallvekst, men forskjellen er at den nye krystallen i form av en liten plate eksisterer i perleoverflatens hulrom, men dekker ikke hele perleoverflaten. Den tilfeldige veksten av de festede krystallene kan gradvis vokse til en flat og sekskantet form, og et stort antall aggregater kan danne et solid lag sammensatt av uregelmessige blokker. Fenomenet med festede krystaller på overflaten av perlen er vanligvis lett å observere i det mørke området belysning, og det er lett å se med overført lys, og utseendet er grumsete.
Et annet kjennetegn ved berylliumdiffusjon er at hulrommene inne i edelstenen er fylt med et glassaktig stoff og inneholder sfæriske bobler.
Naturlige rubiner (kromindusert farge) viser sterk fluorescens under ultrafiolett lys og til og med i naturlig lys. Fluorescensen til behandlede rubiner er ikke åpenbar, og det ser ut til å være en veldig svak lysegrønn. For det blotte øye har de generelt en oransjerød fargetone, med merkbar pleokroisme, som viser tydelige oransjegule og oransjerøde toner.
2. Forbedring av safirer
Safirer, som er anerkjent som en av de fire største edelstenene i verden, har høy økonomisk og estetisk verdi. Fargene deres er utrolig varierte og uforutsigbare. To safirer varierer ofte i pris på grunn av ørsmå fargeforskjeller. I dag er ca. 95% av safirene på markedet behandlet, og de vanligste metodene er oppvarming og diffusjon av overflatevarme. Fyll med olje, harpiks, glass eller høymolekylære polymerer for å fjerne hull eller flekker, eller overflatebelegg og farging er for tiden mindre brukte behandlingsmetoder.
(1) Termisk energiprosess
Oppvarming av safirer til mellom 450-900 ℃ og opprettholde den temperaturen i 7 timer til 14 dager, etterfulgt av gradvis avkjøling til romtemperatur, vil gi forskjellige resultater: økt blå, lysning av mørke farger, reduksjon av grønt, fylling av sprekker, forsvinning av mørk silke, etc., og forbedrer dermed edelstenens farge, klarhet og gjennomsiktighet, og til og med produserer en stjerneeffekt. For eksempel ser Geuda melkestein fargeløs eller brunaktig te ut på grunn av at den inneholder Ti, Fe, og oppvarming til 1600 ℃ kan endre Ti-, Fe-tilstanden, noe som forbedrer fargen og gjør den til en verdifull blå safir, samtidig som den forbedrer gjennomsiktigheten og glansen.
(2) Varmediffusjonsbehandling
① Overflatediffusjonsbehandling
Safiren legges i en smeltedigel som inneholder aluminiumoksid og natriumoksid og varmes opp til nær smeltepunktet, slik at forbindelsen kan trenge inn i de grunne lagene i edelstenen og danne et tynt blått lag (0,5 mm) for å oppnå fargeforbedring, karakterisert på samme måte som diffusjonsrubin.
② Dyp berylliumdiffusjon
Dette refererer ofte til safirer fra Madagaskar og Tanzania som Red song, som bruker varmediffusjon for å tilføre beryllium i safiren, til og med i hele perlen, noe som resulterer i en levende oransjegul til rødoransje farge, solgt som eksklusive sjeldne naturlige oransje safirer fra Sri Lanka.
To determine the beryllium diffusion in sapphire, the beryllium content can be measured using SIMS (Secondary Ion Mass Spectrometry). Natural sapphire contains beryllium at 1.5-5PPM±, while the beryllium content after diffusion can be between 10-35PPM.
For the identification of beryllium diffusion-treated sapphires, the immersion method using dibromo methane allows for the observation of color zones around the gemstone. Additionally, if a beryllium diffusion method is used to “lighten” the body color of dark sapphires (basalt host), after immersion in dibromo methane, a faint layer of colorless to yellow color zone can be seen surrounding the blue body color at the periphery, enveloping the entire gemstone.
Due to the different temperatures during the beryllium diffusion treatment process, the results also vary. If the diffusion treatment temperature is at 400-600℃, the color of the sapphire improves, appearing significantly more yellow or brown compared to iron-colored Citrine. If the beryllium diffusion occurs in a high-temperature oxidation environment, beryllium can diffuse into the deeper layers of the gemstone; if the heating time is long, it can diffuse throughout the entire gemstone.
For sapphires subjected to extremely high-temperature beryllium diffusion, the peripheral color zones are no longer visible when identified with dibromo methane. At this point, internal inclusions can be observed to make judgments, such as whether there are healed feather-like cracks, whether the gemstone surface has burn pits, and whether there are new growths of crystals (synthetic corundum). Yellow and red corundum have been treated with beryllium diffusion, and sometimes the internal diffusion of blue color gamut formed by TiO2 due to the release of Ti element at high temperature can be seen.
In summary, the identification of beryllium diffusion sapphires can be comprehensively analyzed and judged based on the above characteristics.
3. Diffusion Star Light
Corundum gemstones treated by heat diffusion can produce star sapphires and star rubies. There are two causes for the star lines: one is that during heat treatment, the originally disordered inclusions in the gemstone become ordered due to heat; the other is formed by surface diffusion. The former is located inside the gemstone, while the latter is on the surface of the gemstone (surface layer).
(1) Heat-treated starlight
Heating Ti-rich sapphires or rubies to 1600-1900℃ causes the disordered Ti-rich inclusions (cloudy) to melt, allowing Ti to enter the corundum lattice. After maintaining the heat for a period of time and then gradually cooling down, TiO2 will dissolve again, forming directionally arranged rutile needle-like inclusions, thus producing the starlight effect. Alternatively, maintaining heat at medium-high temperatures (1100-1300℃) and slowly cooling can also reveal potential starlight effects.
(2) Surface diffusion starlight-
Star rubies and star sapphires formed by surface diffusion methods have already been marketed in our country. After surface diffusion treatment, the refractive index, density, and other physical parameters, as well as the characteristics of inclusions, are the same as those of natural corundum gemstones. The difference between diffusion starlight and natural starlight gemstones is:
① Color: Surface diffusion starry blue sapphire, with a deep blue tone of black and gray, the surface of the gemstone, especially at the bottom of the curved gemstone or on the fracture surface, has red patchy substances.
② Starry light: The surface diffusion of starry light is perfect, with uniform star lines resembling synthetic starry light. Upon magnified inspection, it can be seen that the starry light is limited to the surface of the gemstone. Under a microscope, the surface of the curved gemstone has a very thin layer of fluff formed by tiny white dots, while inside the gemstone, no three groups of directionally arranged golden-red needle-like rutile are visible.
③ Fluorescence: Under SW and LW ultraviolet light, there is no fluorescence, and occasionally, red fluorescent spots can be seen on the surface of the gemstone.
④ Red circle phenomenon: Due to the gemstone surface Cr2O3 content can be as high as 4%; when observed in oil, the surface of the gemstone appears red and has a clearly defined, highly raised red color circle.
Section IV Improving jadeite
1. Heat-treated jadeite
Jade heat treatment, commonly known as color treatment. It involves heating jadeite samples to remove grayish-yellow, brownish-yellow, and other colors, changing them from orange to reddish-brown. Experiments show that yellow and brown jadeite is caused by the dehydration of brown iron ore under natural conditions, resulting in the mineralization of hematite coloring. Hematite dissolves in dilute acid and can be removed. Therefore, after acid washing the sample, it is placed on an iron plate covered with fine sand and evenly heated in a furnace to around 200℃. When the jadeite turns liver-colored, it is cooled, resulting in red, and finally soaked in bleaching water for several hours to ensure full oxidation and color fixation. The identification characteristics are as follows:
① Heated red jadeite: The red has a dry feel and is not easy to distinguish.
② Infrared spectral characteristics: Natural jadeite has a strong absorption band near 1500-1700cm-1, 3500-3700cm-1, while heat-treated products do not.
2. Wax-immersed jadeite
The wax immersion process involves washing the sample with dilute acid. The structural damage is not severe, but it can increase the porosity of jadeite, leading to more paraffin filling into the stone. If the wax-immersed jadeite is left for a long time, it will age and produce white spots, causing a decrease in the transparency of the stone.
Identification features:
① Exposure to high temperatures will cause the wax to ooze out (commonly known as “sweating”), indicating poor durability.
② A blue-white fluorescence can be seen under ultraviolet light.
③ Infrared spectral characteristics: The organic peaks are prominent, exhibiting 2854cm-1, 2920cm-1 characteristic spectrum.
3. Bleached and filled jade
(1) Luster
Often has a resinous luster, waxy luster, or a mixture of glassy luster with resinous and waxy luster.
(2) Color
Lacks depth, with a very white base, green floating on the surface, and color lacking directionality, making it look very uncomfortable.
(3) Structure
Under transmitted light, internal interwoven cracks are visible; under reflected light, surface etching pits or spider web-like patterns can be seen.
(4) Surface Features
Sometimes, more pronounced grooves can form at the native cracks, and even cementing materials or residual bubbles may be visible within them.
(5) Density and Refractive Index
The density of the majority decreases to 3.00-3.43g/cm3, with a refractive index of around 1.65.
(6) Fluorescence
No or weak to strong ultraviolet fluorescence, with a mottled distribution. Under shortwave, weak, appears yellow-green or blue-green (blue-white); under longwave, medium to strong, appears yellow-green or blue-white.
(7) Carbonization
After heating to 200-300℃, the gel undergoes carbonization.
(8) Identification of Large Instruments
Under the Cathode luminescence microscope, its fluorescence colors are mainly yellow, yellow-green, and bluish-green. The color distribution is relatively uniform, and the edge rings appear uneven or incomplete due to erosion. Greenish and deep blue colloidal substances are present in the erosion patterns and cracks (Figure 6-7).
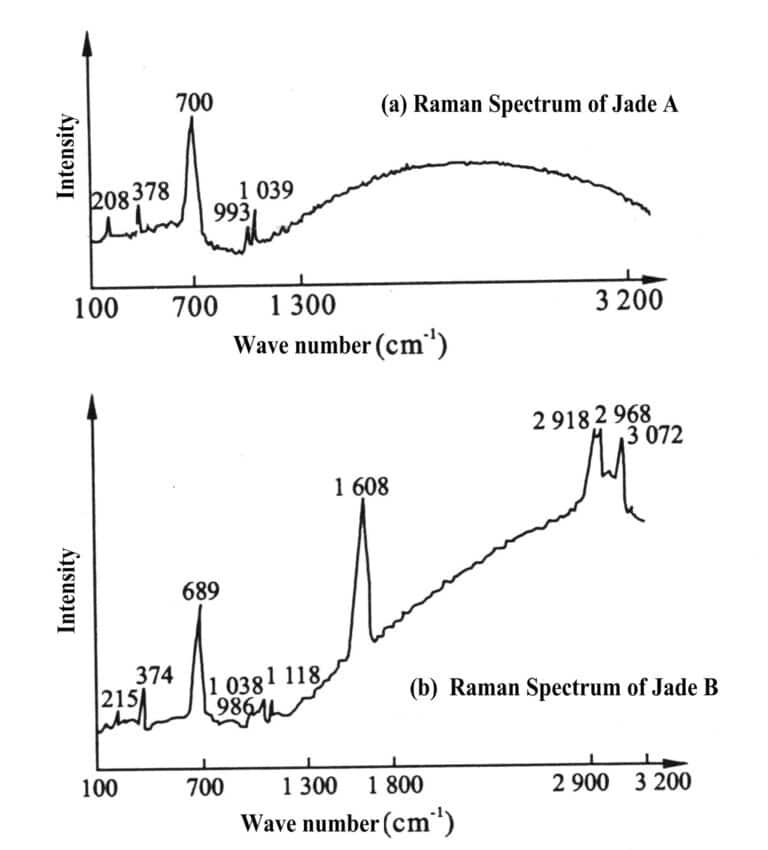
4. Dyed Jadeite
The dyeing process is mostly confidential, usually selecting rough grains of jade with a certain porosity, which are then treated with dilute acid to remove impurities, dried, heated, and subsequently soaked in a dye solution, boiled for several days, allowing the dye to penetrate and fix in the pores (green, purple, etc.). Identification features:
(1) Farge
It is distributed in a silk network, and the precipitation or aggregation of dyes can be seen in the larger lock cracks into color spots and spots to imitate natural jadeite.
(2) Spectral characteristics
Appearance of 650 nm broad absorption band. Green colour changes to red under colour filter. Yellow-green or orange-red fluorescence under UV fluorescent lamp. Absorption peaks at 2854cm-1 and 2920cm-1 appear in the infrared spectrum. Appears blue-green and yellow-green fluorescence under cathode rays.
5. Coated Jadeite
The process of applying a colored film is rarely reported. The commonly used material is a green gel-like, highly volatile polymer.
Identification Characteristics:
(1) Farge
Evenly distributed, consistent tone, fully colored. The front and back are the same, with no natural product’s mottled, striped, fine vein, or silk-like color distribution characteristics.
(2) Refractive Index
About 1.65 (Refractive index of film).
(3) Luster
The surface gloss is weak, mostly resinous, with no grainy feel.
(4) Package
Bubbles are visible in some areas.
(5) Surface Features
Visible film peeling mostly occurring at the edges; feels soft to the touch; has a sticky feel when touched by hand. On closer inspection, there are small hair-like scratches on the surface. The orange peel effect and granular structural features (intergranular boundaries) of natural products are not visible.
Copywrite @ Sobling.jewelry - Tilpasset smykkeprodusent, OEM og ODM smykkefabrikk
Section V Improving Agate
Natural agate is beautiful, but improved agate is even more beautiful, not only in color but also in the permanence of its color after improvement. This is due to agate’s characteristics of being micro-transparent and having good permeability, making it easy to improve. We know that agate is a collection composed of microcrystalline quartz, forming various structures (fibrous, radial, filamentous, granular) and textures (banded, fine-threaded, moss-like, striped, lichen-like, branching, and shape-like), creating countless beautiful and captivating patterns. However, there are also many agates with unclear shapes and dull, monotonous colors that require manual improvement. Common methods of improvement include:
(1) Varmebehandling
The uneven light brown agate semi-finished product is heated in an air electric furnace to 700-1000℃ for a period of time. After finishing the dehydration of the limonite, it is slowly cooled to prevent cracking, ultimately achieving a bright red color. The heat treatment does not change the composition of the agate; it only oxidizes the iron content.
The heat-treated red agate is called fire agate or burnt agate, and its transparency and hardness are slightly reduced compared to natural agate, with increased brittleness.
Tiger’s eye, which is similar to agate, can change from brownish-yellow to brown-red when heated under oxidizing conditions and to gray-yellow or gray-white under reducing conditions. It can be used to imitate the cat’s eye effect of chrysoberyl.
(2) Dyeing
Most agate products on the current market have undergone dyeing treatment, especially natural white, gray, and gray-white agate, which have all been dyed. There are two methods of dyeing.
① Chemical precipitation reaction for coloring
When natural agate (chalcedony) is rich in iron, heat treatment can improve its color. However, most agate contains little or no iron oxides, so only chemical reaction methods can be used to infiltrate colored inorganic substances into the pores of the agate, changing the body color of the agate. There are two specific treatment methods.
- Soak the agate in a soluble metal salt dye for a certain period, then take it out, dry it, and place it in a heating furnace to heat, allowing the metal salt to infiltrate into the agate and decompose into colored insoluble oxides, coloring the agate.
- Soak the agate in a dye, take it out after a period, and then place it in a second solvent for soaking, allowing the two solvents to undergo a chemical reaction, precipitating insoluble colored compounds, thus dyeing the agate red, green, blue, yellow, or black.
To dye the agate red, the white agate can be soaked in iron nitrate solution, taken out and dehydrated, then heated in a furnace to about 300℃, at which point the iron nitrate infiltrating the agate pores turns into hematite, or the agate can be soaked in iron chloride solution and then placed in ammonia water for soaking, and after the two undergo a chemical reaction, it is taken out and heated, producing the precipitation of limonite, can yield red agate.
To obtain green agate, one can soak the agate in chromic acid (H2CrO4) or potassium chromate (K2CrO4) solution for a period of time, then take it out and heat it or soak the agate (white) in a solution made from potassium dichromate, an appropriate amount of ferrous sulfite, and dilute sulfuric acid, take it out after a while, and heating can also yield green.
Using two coloring elements, Fe and Co, can turn agate blue. If using Fe ions for coloring, one can first soak the white agate in a solution of potassium ferrocyanide (Ⅱ)K4[Fe(CN)6] at a concentration of 20% for 10-15 days, then take it out and soak it in ferrous sulfate solution for several weeks to generate Prussian blue or Turnbull’s blue K4[Fe(CN)6]3; or using cobalt salts or copper salts with ammonium salts can also yield blue agate.
There are many methods to dye white agate black; a common method is to soak the agate in a sugar solution for several weeks, then take it out and soak it in concentrated sulfuric acid, appropriately heating it for 30 minutes to 2 hours, then take it out, rinse, and dry to complete.
Yellow agate is dyed with potassium dichromate (K2Cr2O7) and can also be soaked in mercury chloride solution and potassium iodide solution to form it. The reaction between the two solvents can result in the formation of an iodine spring (Hg2I) yellow precipitate.
② Dyeing with dyes
The process of dyeing agate with dyes has a history of hundreds of years. Due to the relatively simple process, dyed agate can often be seen in the market. Currently, the dyes used include amines, azo compounds, or sulfide organic dyes. Before dying, the agate undergoes certain chemical pretreatments for bleaching and impurity removal, and then it is soaked in the dye solution. After a period of time, it is taken out and dried, allowing the water-soluble dye to precipitate on the pore walls of the agate, coloring it.
(3) Water injection treatment
When water chalcedony has many cracks or develops cracks during processing, the water inside will slowly flow out until it dries up. If water chalcedony loses moisture, it loses its craft value and economic value. At this point, water injection treatment can be performed. There are two methods for water injection treatment.
① Water-filled agate: Soak the water-filled agate that has lost moisture in water, using capillary action to refill the water or use injection methods to refill the water, and then seal the small gaps with glue or other materials.
② Agate water injection: Agate originally does not contain water (water-filled). To turn it into a water-filled agate product, a small incision can be made in an inconspicuous part of the agate product, hollowing out the inside, injecting water, and then covering the incision with agate pieces, it can be tightly sealed.
(4) Improving agate inspection
① Heat treatment of agate is considered optimization and does not require testing.
② Dyed agate detection is relatively simple. The colors of most blue, green, yellow, and black agate do not appear in natural agate. Currently, there is no simple and reliable detection method for agate treated by chemical precipitation, and it is often unnecessary. Sometimes, a spectroscope can reveal fine Cr absorption lines appearing at the end of the red region in Cr-colored agate; under a color filter, green agate appears red.
③ Water-injected agate can be examined for signs of artificial treatment on the water chamber wall. At suspicious points, scratching with a needle tip can reveal holes or cracks filled with gelatinous or waxy substances.
Section VI Improving Opal
1. Mechanism of Opal Improvement
Colorful and beautifully patterned opal, known as the “palette” of gemstones, is famous worldwide for its unique color-changing effect.
(1) Opal Composition
Natural opal is a sub-microscopic aggregate composed of AG- opal (SiO2 spherical particles are amorphous) and/or CT-opal (a mixture of quartz and feldspar layers) and contains varying amounts of water (generally 4%-9%, up to a maximum of 20%). Its chemical formula is SiO2 - nH2O.
(2) Types of Opal
There are many varieties of opal, which can be broadly classified into four categories: black opal, white opal, fire opal, and “crystalline” opal.
2. Opal Improvement Process
The artificial improvement of natural opal is mainly approached from two angles: first, by attempting to deepen the body color of the opal to highlight the play-of-color effect; second, by injecting foreign substances to fill the voids, thereby producing and enhancing the play-of-color effect.
(1) Dyeing
Natural opal is composed of countless small spheres of diameter 150- 400 nm and SiO2, tightly packed spheres.
There are countless voids between the particles, which provides favorable conditions for the dyeing process. Dyeing can deepen the body color of the opal, making the play-of-color effect more pronounced and making the appearance of the opal more vibrant and enchanting. There are several dyeing methods, as follows:
- Sugar Acid Treatment
The purpose is to enhance the body color to black. This method began in 1960. The process involves first washing, then drying the opal at a low temperature below 100℃, soaking it in a hot sugar solution for several days; after slowly cooling, quickly wiping off the excess sugar juice from the surface of the opal and soaking it in hot concentrated sulfuric acid (100℃±) for one or two days; after cooling, rinsing thoroughly multiple times, then quickly rinsing in carbonate solution, and finally rinsing clean. At this Point, the hydrogen and oxygen in the sugar are removed, leaving carbon in the cracks and voids of the opal, thus creating a dark background.
- Smoke Treatment
The purpose is to make the opal turn black, imitating black opal. The smoke treatment process involves wrapping the opal in paper and then heating it until the paper smokes. After being smoked, the opal’s surface develops a black background.
- Silver Nitrate Exposure Method
The purpose is to imitate black opal. After cleaning the opal and drying it at a low temperature, soak it in a silver nitrate solution, allowing the silver solution to fully penetrate the opal’s pores and cracks, then take it out for exposure; the silver black makes the opal turn black.
- Aniline dyeing method
The purpose is to imitate black opal. Soak the opal in black aniline dye, and once the opal has turned black, take it out and let it dry (or bake it).
(2) Foreign substance injection
The foreign substance injection method is mainly used for porous water protein stones and low-quality protein stones (colorless, black, or red) to create a color-changing effect, conceal flaws, and improve transparency.
- Injection Molding Treatment
The opal is first dried, the water in the pores is removed, and then pumped into a vacuum, and then soaked in a hot (below 100℃) injector, and the injection agent is pressed into the deep hole god by the outside atmospheric pressure to cover the cracks and make the opal (Opal) present a dark background.
- Oil Injection Treatment
This method uses oil injection and waxing to cover the cracks of inferior opals, improving the appearance of the gemstone and making it comparable to high-quality opals.
3. Improving the Characteristics of Opal
(1) Dyed Opal
- Sugar Acid Treated Opal
Upon magnified observation, the color spots appear as fragmented small pieces limited to the surface of the opal, with a granular structure, and small black dot-like carbon dye is visible accumulating in the gaps of the color flakes or granules.
- Smoke Treated Opal
The black color is limited to the surface, with reduced density (1.38-1.39g/cm3)
- Silver nitrate treatment of opal
Upon magnified inspection, a silver-black precipitate can be seen in the pores; acetone or dilute hydrochloric acid can be used to wipe off the discoloration, and chemical analysis can detect silver.
- Aniline-stained opal
The dye precipitates in the pores or cracks, forming speckled pigment clusters as if “pepper powder” has been sprinkled.
(2) Foreign substance injection opal
- Injection-molded opal
Bright colors, stable properties, and high transparency. Upon magnification, bubbles, flow patterns, and flashes can be seen; infrared spectroscopy shows plastic absorption spectral lines; a hot needle test reveals an odor; acetone wipes result in color fading; opal density decreases, and the refractive index diminishes.
- Oiling (or waxing) opal
Greasy or waxy luster may appear, and when tested with a hot needle, oil or wax is extracted.
(3) Heat treatment of opal
Whether it is dyeing treatment or foreign substance injection treatment, opal must be purified and heated to remove impurities, discoloration, and adsorbed water. If the heating temperature is relatively high (300℃), most of the moisture in the opal can be extracted, allowing the dye and injected agents to occupy the moisture position. This indicates that when the opal is heated to 300℃, some isolated water molecules are lost, and all liquid water is lost. Therefore, when improving natural opal, heating should be done at a stable low temperature.
Section VII Improving turquoise
With a unique sky blue turquoise, it is mainly composed of water-containing copper aluminophosphate composed of cryptocrystalline aggregates, which are often Eloite, kaolinite, quartz, mica, limonite, phosphoaluminite and other symbiosis. These symbiotic minerals affect the quality of turquoise.
The pure color of turquoise is determined by the presence of Cu2+ ions, which define its blue base color, while the presence of iron and the loss of copper and water will affect its color changes and structural variations.
In addition, turquoise color, under the action of alcohol, aromatic oil, soapy water and some other organic solvents, can occur fading phenomenon.
Therefore, lower-grade turquoise needs to be artificially improved to enhance its aesthetic and economic value, satisfying the preferences and wear of people from both ancient and modern times, as well as from around the world.
1. Improvement process
Since turquoise has a certain porosity (especially sponge turquoise), various improvement methods can greatly enhance some turquoise that has a poor appearance, loose structure, and undesirable color.
(1) Foreign object injection
- Oil injection
Soaking turquoise in liquids like gasoline to change its color and luster. However, samples soaked in this way are prone to fading. This is a traditional improvement method that is now rarely used.
- Voksing
Boiling turquoise in paraffin (insect wax, Sichuan wax) can deepen the color of the turquoise and seal fine pores.
- Injection molding
Soak turquoise in colorless or colored plastic liquid for infusion, sometimes adding coloring agents. Once the plastic fully penetrates the pores or cracks, remove it and clean off the excess plastic from the surface. This method can enhance the stability of turquoise, increase surface smoothness, reduce surface light scattering, and give turquoise a medium blue tone, improving its appearance.
- Water glass
Soak turquoise in a water glass (sodium silicate) to allow the water glass to penetrate the pores or cracks of the turquoise, condensing and solidifying to enhance the stability of the turquoise and improve its transparency.
(2) Dyeing
Using the porous nature of turquoise, it is immersed in inorganic or organic dyes to dye light-colored or near-white turquoise to the desired color. After the dyeing liquid penetrates into the inside of the gem, the water is heated to make the dyeing liquid undergo a chemical reaction, so that the blue dye (or pigment) is deposited in the pores, making the gem colored.
2. Improving the characteristics of turquoise
Compared to natural turquoise, the improved turquoise has the following characteristics:
(1) Oiled turquoise
Oiled turquoise is very prone to fading and is rarely used now. It smokes when burned, and when probed with a hot needle, it “sweats.”
(2) Wax-impregnated turquoise
A hot needle touches it, and it “sweats”; it fades after exposure to sunlight or heat.
(3) Injection-molded turquoise
Refractive index less than 1.61 density less than 2.76g/cm3(hardness is generally only 3-4, the surface is prone to scratches. Zoom in and see bubbles. Hot needle test, there is a special spicy smell, and there are burn marks. In the infrared spectrum, there is a strong absorption spectrum line caused by plastics (1450-1500cm-1), and in the new injection variety, there is a strong absorption band of 1725 cm-1. X-ray diffraction analysis, there is a block of phosphonamidite phase. (Figure 6-8).
(4) Water glass turquoise
Density decreases, usually 2.40-2.70g/cm3; magnified observation reveals bubbles.
(5) Dyed turquoise
The color is unnatural, deep blue-green or deep green, with an overly uniform distribution; the color darkens at cracks due to dye accumulation; the color layer is very thin, generally around 1 mm; at the peeling areas on the surface of the sample and in the pits behind, an undyed light-colored core may be exposed; wiping with a cotton ball dipped in ammonia can make the cotton ball appear blue-green.

Section VIII Improving Amber
Amber is an organic mixture formed from the resin of coniferous plants from the Mesozoic Era, specifically the Cretaceous to the Cenozoic Era, through geological processes. It is formed from the resin of coniferous trees buried underground, undergoing petrification and diagenesis. It comes in various colors, among which light yellow and honey yellow are called honey wax, red ones are called blood amber, golden yellow ones are called golden amber, those containing biological remains are called insect amber, those that appear blue under ultraviolet light are called blue amber, highly petrified and hard ones are called stone amber, and those with fragrance are called fragrant amber, etc.
Amber is prone to oxidation, which can cause color changes and brittleness, and it often contains impurities such as sand, stones, insects, and grass, so it often needs to be improved and updated. Common types include compressed amber and coated amber.
1. Coated Amber
In recent years, the commonly seen coated amber can be divided into colorless and colored coatings, with the colored coatings further classified into full coating and partial coating.
These coating methods enhance the luster of amber, partially improve its color, and enhance the three-dimensional effect of “sunlight” in light-colored amber, thereby increasing the grade of amber.
(1) Colorless Coated Amber
Due to the low hardness of amber, it is easy to carve and difficult to polish. Now the amber products sold on the market about 99%of its surface are covered with a colorless transparent light film to achieve the purpose of enhancing luster and polishing, and played a certain anti-scratch role. Compared with natural amber, the characteristics of colorless coated amber are as follows:
① Strong luster can reach a bright resin luster.
② There are bubbles in the film; when the coating is thick, a large number of bubbles can be trapped in the depressions of the product, and when pricked with a needle, the film will peel off in sheets.
③ When scratched with a needle, its surface is mostly concave, has a sticky and soft feel, is not easy to crack, and feels similar to scratched plastic products.
④ Infrared spectroscopy detection shows that the composition of the colorless film is complex and varied.
(2) Colored Coated Amber
The commonly seen colored coated amber in the market mainly comes in two types: one has a colored film coated on the bottom of the amber product to enhance the three-dimensional effect of “too much light obstruction” in light-colored amber; the other is a spray of a colored glossy film on the surface of the amber product, making the amber present different shades of red blood amber or brownish-yellow “old beeswax.”
The characteristics of colored coated amber can serve as a basis for identification.
① Characteristics of amber with a colored film on the bottom
- Under magnification, the color layer of the coated amber is shallow, with no transition and uneven coloring.
- The coated surface often retains traces of spraying.
- Using a needle to pry, the film may sometimes peel off in sheets.
- The spectrum in the red area can detect the composition of the film, which is different from amber.
② Characteristics of amber with a colored film on the surface.
- Upon magnified observation, the color layer of the coated amber is shallow, with no transition and uneven coloring.
- Due to the large amount of spraying, there may sometimes be a concentration of color in the recessed areas of the coated amber.
- Due to uneven spraying, there may sometimes be uncolored areas in the recessed parts of the coated amber.
- After pricking with a needle or soaking in acetone, the film may sometimes peel off in sheets.
- Infrared spectroscopy can detect film components in amber that should not be present.
For coated amber, according to the national standard (GB/T16552), the definition of a film is “a film applied to the surface of gemstones using methods such as coating, plating, or lining to improve the luster, color, or produce special effects,” which should be classified as a type of “treatment” for gemstones and must be noted in the identification certificate.
2. Heat Treatment of Amber
To improve the transparency, clarity, color, and size of amber, oil boiling, and reconstruction methods are often used for optimization.
(1) Oil-Boiled Amber
Cloudy amber is heated and boiled in vegetable oil to increase the transparency of amber. This type of heat-treated amber often has leaflike cracks resembling “water lily leaves” and “sunlight rays.”
(2) Reconstructed Amber
The reconstruction of amber has been discussed in the chapter on synthetic gemstones, but during the reconstruction process, thermal energy plays an important role. Therefore, to some extent, reconstructed amber also falls under the category of thermal energy processes.
Reconstructed amber can be divided into three types: fused amber, compressed amber, and molded amber.
Compressed amber is a type of reconstructed amber made from natural amber as raw material, formed into an overall organic gemstone through medium to low-temperature heating and pressure.
Compressed amber has characteristics that are different from natural amber and fused amber, and the obvious indicators for identification are:
① Dark red fibrous bodies
There are dark red filaments, clouds and lattice-like bloodlines visible to the naked eye in the pressed amber. This is a thin red oxide film formed by oxidation of the aged amber raw material, which is more clearly seen under ultraviolet fluorescence. Natural amber is sometimes blasted to form cracks due to temperature, humidity and other effects, and is oxidized to be red, but it is distributed along the cracks in a dendritic shape rather than along the edges of the particles.
② Animal and plant inclusions
In compressed amber, complete and intact animal or plant inclusions are not seen, nor is there any introduction of foreign substances.
③ Bubbles
Compressed amber contains abundant gaseous inclusions; these bubbles not only come from the original natural amber but also form new bubbles between particles and, during stirring, are irregularly distributed throughout the amber and densely small. Although they may burst during heating to form “amber flowers” resembling water lilies, they are particularly small and often arranged in layers of space.
④ Flow structures
Although pressed amber sometimes shows flow structures that are either obvious or not, it is accompanied by indistinct boundaries between particles, appearing very uniform internally; however, this structure can also be found in natural amber.
⑤ Luminescence
Under ultraviolet fluorescent light, pressed amber exhibits the luminescent properties of natural amber, which often reveals the edges and contours of amber particles, allowing clear observation of individual connections and the shapes of the particles. In samples with dark red thread-like bodies, the boundaries of the particles can be seen distributed along the thread-like bodies.
(3) Dyed Amber
The practice of dyeing amber has a long history, with ancient methods using natural plant dyes to color amber in various shades (red, green, purple, etc.) to mimic the characteristics of aged amber. Modern dyeing, some jewelry manufacturers also use organic dyes, because amber is also organic matter, and the two are easy to react, so that the dye chromophore penetrates into the interior of amber, resulting in different colors of amber dyes.
Section IX Improvement of pearls
Pearls are known as the queen of gemstones. They are round, have soft colors, and their luster is captivating. They are pure and beautiful, highly cherished by people. Pearls have a unique body color, accompanying colors, and a combination of iridescence, making them easily distinguishable from any other jewelry or gemstones.
Beautiful pearls undergo optimization treatment, which will enhance their color and increase their commercial value. The methods for improving pearls are divided into two main types: optimization and treatment.
1. Optimized pearls
The optimization process of pearl is generally divided into pretreatment, purification, bleaching, whitening and polishing.
(1) Pretreatment
The quality of pearl pretreatment directly affects the effectiveness of subsequent processes. Pretreatment mainly includes sorting and drilling stages.
① Sorting
Grading of Cultured Pearls,” sorting is done based on the size, shape, luster, color, and thickness of the pearl layer so that they can be treated separately. This not only benefits the economic value utilization but also, due to the different thicknesses of the pearl layers and the varying organic pigment clusters and impurities in different types of pearls, the reagents, dosage, concentration, and time parameters used will differ, making sorting beneficial for optimizing the improvement of effects.
② Drilling
Drilling the sorted pearls, according to processing requirements, can be done as half-drilling or full-drilling. Drilling can also reduce or eliminate surface defects, such as pits on the pearls, and promote purification and whitening effects.
(2) Purification
Purification is the process of using purifying agents to remove dirt and moisture from the surface of the pearls, which involves the following steps:
① Expansion
Soak the pearls in a mixture of benzene (C6H6) and ammonia water (NH4OH) at low temperature (35-50℃) for several hours, then take them out and rinse them several times with deionized water. The purpose of swelling is mainly to enhance the connectivity of the pores in the pearl structure, making it a bit “looser.”
② Dehydration
After swelling and cleaning the pearls, proceed to dehydration. Soak the pearls in a detergent solution for a while, then rinse them several times with clean water and let them dry; use anhydrous ethanol or pure glycerin as a dehydrating agent to remove the adsorbed water in the pores and cracks of the pearl structure.
③ Sunlight
After the pearl is puffed and dehydrated, it is exposed to the sun and dried.
(3) Pearl Bleaching
The pearl bleaching process, which began in 1924, is the most important part of pearl optimization, as pearls often exhibit undesirable colors due to the presence of organic pigment clusters and impurity ions, affecting the color grade of the pearls. Pearl bleaching is essentially a chemical reaction the bleaching solution is a mixture of bleaching agents (hydrogen peroxide), solvents (organic solvents, water), surfactants (alcohols, ketones, ethers, etc.), and pH stabilizers (triethanolamine or sodium silicate)]. Currently, the jewelry industry mainly uses two methods: hydrogen peroxide bleaching and chlorine bleaching.
① Hydrogen Peroxide Bleaching Method
The pearl is soaked in a solution of hydrogen peroxide (H2O2) with a concentration of 2%-4%, the temperature is controlled at 20-30℃, the PH value is between 7-8, and it is exposed to sunlight or ultraviolet light, after about 20 days of bleaching, the pearl will become gray or silver white, and it is best to become pure white.
This process mainly includes five steps: soaking, washing, liquid replacement, pearl selection, and decontamination. The required equipment mainly consists of a light and temperature control device, a bleaching container, and a vacuum washing device. The formula for the bleaching solution is confidential; a Japanese research institute proposed a formula in 1930: 3% of H2O2 1000ml, 10 ml of benzene, 10 ml of ether, neutralized with ammonia water, adding an appropriate amount of PH stabilizer, temperature below 30-50℃, with the surfactant being dioxane and the stabilizer being triethanolamine.
② Chlorine Bleaching Method
The bleaching ability of chlorine is stronger than that of hydrogen peroxide. Improper use can make pearls brittle and fragile or leave a chalky, powdery surface on the pearl’s surface. Therefore, this bleaching method is usually not commonly used.
(4) Pearl Whitening
The bleaching method cannot completely eliminate organic pigment clusters, resulting in pearls not turning completely white. After bleaching, the base color of the pearls is primarily white. To enhance the whiteness and luster of the pearls, a fluorescent whitening treatment is still needed. The fluorescent whitening method is an optical whitening method that utilizes the principle of complementary colors in optics to achieve the goal of removing yellow and discoloration from pearls to enhance their whiteness.
Is the whitening agent that makes pearls whiter a special fluorescent coating? It emits blue fluorescence that is complementary to yellow, resulting in a blue-white appearance of the pearls. Commonly used whitening agents include AT, DT, VBL, PBS, WG, RBS, etc., with a typical dosage of around 0.5%-3%.
There are two types of fluorescent whitening agents: direct dye type (water-soluble) and dispersive type.
① Direct dye whitening method
During the bleaching process, the whitening agent can be used simultaneously with the bleaching solution, or it can be used alone.
If used alone, the pearls should be purified in advance and then soaked in the whitening solution. In the whitening solution, in addition to the whitening agent, there are also solvents (water and organic solvents) and surfactants as auxiliaries. This method requires high water quality, free from metal ions such as iron and copper, and generally requires softening treatment.
② Dispersive whitening method
The use of solid powder to whiten the color of pearls is the third-generation whitening process currently adopted in Japan. The specific process is not detailed, but it is likely that some method is used to permeate and fill a certain fluorescent whitening agent into the inner layer of the pearls.
(5) Polishing
Polishing, or buffing. Pearl polishing is also a very important process. Good polishing can enhance the bleaching and whitening effects. The polishing materials currently used include small bamboo pieces, small stones, and paraffin, as well as sawdust, granular salt, and diatomaceous earth.
After polishing the pearl, wash it with detergent and let it dry in the sun.
2. Processing Pearls
(1) Dyed Pearls
Currently, in the market, most colored pearls (black, silver-gray, pink, red, orange-yellow, etc.) are dyed, except for white pearls.
The dyeing process of pearls is similar to the bleaching process. After pre-treatment and purification, the pearls are placed in a vacuum filtration bottle, then immersed in the dye solution (at a temperature below 30 〜40℃ ) for one to two days until the desired color is achieved.
The dye solution consists of dyes (mostly organic dyes), solvents (pure water, organic solvents), and penetrants (potassium iodide or pyridine). Commonly used dyes include peach pink, pink, and magenta.
The dyeing of pearls can be divided into two methods: chemical dyeing and center dyeing.
① Chemical dyeing method
Soak the pearls in certain special chemical solvents to dye them. For example, using dilute silver nitrate and ammonia solution as dye, soaking the pearls turns them black; using cold potassium permanganate as dye can turn them brown.
② Central dyeing method
First, after swelling and removing impurities from the pearls, inject specific dyes into the pores and holes of the pearls to make them show color.
Regardless of the dyeing method, there is a certain level of deception. Dyed pearls have bright colors and uniform luster. Dyes often concentrate in the pores and cracks of the pearls.
(2) Irradiated pearls
The radiation irradiation method is a pearl improvement process that began in the 1960s and is currently widely used. The radiation source used is 60 Co , with an intensity of 3.7 x 1013 Bq , a radiation distance of 1 cm, and an irradiation time of about 30 minutes. Irradiated pearls can produce blue-gray and black colors, with seawater pearls being somewhat darker. Additionally, neutron irradiation of certain freshwater pearls can produce silver-gray colors.
The color of irradiated pearls is stable to light and heat, and it is easy to distinguish from silver nitrate dyeing in tone, but irradiation may cause radioactivity, and not all pearls can utilize irradiation to change color.
(3) Filling Pearls
The surface of pearls often has some small cracks and bumps, affecting the luster and smoothness of the pearls, which must be repaired and healed. There are two treatment methods.
① Peeling and Smoothing
Use very fine tools to carefully peel off the unsightly surface layer of the pearl to achieve a smooth and even surface, hoping a better layer of pearls appears beneath the surface, achieving the goal of transforming it into jade.
② Filling the pores
The small cracks on the surface of the pearl, or the marks left by peeling and polishing, must be repaired and filled. The specific method is to soak the peeled, polished, and cleaned pearls in hot olive oil. The oil’s penetration gradually heals and repairs the cracks and wounds on the pearl’s surface, achieving a smooth, rounded surface with a bright color. If the olive oil is heated to 150℃ , a deep brown color will appear on the surface of the pearl.
3. Improving pearl identification
After the above optimization or treatment, the pearls become bright in color, smooth, and round. The distinguishing features of dyed pearls compared to natural pearls are as follows:
(1) Color characteristics
① Dyed pearls
Dyed black pearls have a uniform color, but in areas with lesions or cracks, the color is deeper, resulting in uneven local color distribution. For drilled dyed pearls, there is often color concentration and small color spots near the hole, on surface cracks, and at peeling areas. On the string of beads, traces of color fading can be seen. If a cotton ball soaked in diluted nitric acid is used to wipe a dyed black pearl, the cotton ball will turn black. Other brightly colored dyed pearls have the same color distribution as dyed black pearls; if they are strung together, their tones and shades are consistent.
② Core
The dyed black nucleated pearls show a strong color difference between the white nucleus and the black nacre when viewed through the drilled hole; nucleated pearls dyed in other colors have both the nucleus and the nacre layer dyed, revealing a black inner core. Nucleated pearls that have been color-changed through irradiation show a black nucleus, while the nacre layer is nearly colorless and transparent.
③ Accompanying colors
Black pearls that have been color-changed through irradiation exhibit vibrant hues in the spectral color, along with a metallic luster, but the color is uniform and lacks the diversity of accompanying colors found in cultured pearls.
(2) Ultraviolet fluorescence
Dyed pearls are often emotional; freshwater pearls often exhibit yellow-green fluorescence, while seawater cultured pearls often show weak blue-white fluorescence.
In addition, generally speaking, dyed black pearls have a diameter greater than 9 mm, while dyed or irradiated pearls are mostly less than 8 mm.
Section X Other improvements for gemstones
In the current jewelry market, almost all natural gemstones can be improved, and even synthetic gemstones have improvement products.
The characteristics of common gemstone improvement products are summarized in Table 6-1, for more details, please visit the website: https://sobling.jewelry/improving-gemstones-the-art-and-science-of-enhancing-jewels/