Cos'è la placcatura in argento, come si esegue e perché viene utilizzata?
Silver Plating Guide for Jewelry: Processes, Alloys & Troubleshooting
Introduzione:
This article explains what silver plating is – a process of depositing a layer of silver onto a substrate. It details how it is performed using various methods, from traditional cyanide plating solutions to modern cyanide-free alternatives, covering decorative, industrial, and high-speed plating for components like connectors. The text also explores why it’s used, highlighting its excellent conductivity, reflectivity, and application in silver alloys for enhanced properties. Finally, it provides essential troubleshooting guides for common plating faults, making it a comprehensive resource for understanding both the theory and practice of silver electroplating.

Indice dei contenuti
Sezione I Panoramica
Silver (Ag) has an atomic number of 47 in the periodic table, with the element symbol Ag. The symbol originates from the Latin word Argentum (meaning shining thing). Its electrical conductivity, conductance, and visible light reflectivity are the highest among metals. Due to its high light reflectivity, it has traditionally been called white silver. The standard electrode potential of Ag is 0.799 V.
Silver ions have a strong bactericidal effect and are widely used as disinfectants (usually, utensils labeled as treated for sterilization have been processed using silver compounds). Silver has also been applied as a sterilization device in water purifiers in recent years. Some main parameters of silver are shown in Table 2-1.
Table 2-1 Some Main Parameters of Silver
| Characteristic Parameters | Characteristic Values |
|---|---|
|
Nome dell'elemento, simbolo dell'elemento, numero atomico Classificazione Gruppo, periodo Densità, durezza Metal monomer color Massa atomica relativa Raggio atomico Covalent bond radius Chemical valency Struttura cristallina punto di fusione boiling point Calore di vaporizzazione Calore di dissoluzione Capacità termica specifica Conducibilità Conducibilità termica
|
Silver、Ag、47 Metallo di transizione 11.5 10490kg/m3, 2. 5 Bianco argento 107.8682 160pm 153pm 1 Doughnut cube 1234. 93K(961. 78℃) 2435K(2162 ℃) 250. 58kJ/mol 11. 3 kJ/mol 232J/(kg • K) 63X106m • Ω 429W/(m ・ K) |
Silver is a precious metal that easily undergoes chemical changes. When sulfur compounds are present in the air (such as automobile exhaust, hydrogen sulfide in hot springs, etc.), forming Ag2S on the surface of the silver turns it black. Since ancient times, silverware has been used as tableware for the ruling class and wealthy families. There is a saying that when silver comes into contact with food containing arsenic, the tableware changes color to warn the user.
The history of silver plating is long, dating back to 1838 when G. R. Elkington and H. Elkington in the UK proposed a silver plating solution containing silver oxide, potassium cyanide, and sodium cyanide in 1838.
In 1913, F. O. Frary published a paper on using silver nitrate as a plating bath. E. B. Saniger conducted comparative studies on silver electroplating from sulfonates, nitrates, borofluorides, and fluorides, reporting that smooth plating deposits could be obtained from borofluoride solutions. In 1933, H. Hickman reported that a rotating electrode could obtain silver deposits from acidic solutions.
Silver plating has been widely used both in decorative fields and in industry. Especially in recent years, the development of silver plating on connectors for electronic and communication devices and substrates for semiconductors and integrated circuits has been rapid. Moreover, silver plating in these applications differs from conventional plating methods, typically using high-speed plating. The plating solution is generally neutral, with silver salts being potassium silver cyanide and organic acids as the main components. The development of plating for functional parts is also advancing rapidly. However, research on silver plating is still less extensive than gold plating. In particular, silver alloy plating solutions have not yet reached a practical usage level. Since the introduction of silver plating solutions, cyanide-based solutions have been predominantly used. Although there have been several improvements, the mainstream has not moved away from cyanides. Representative cyanide plating solution compositions are shown in Table 2-2. Using cyanide silver plating allows good silver coatings to be obtained over a wide range of temperatures and concentrations, and the operation control is relatively easy. Table 2-2 lists two types of plating solutions: potassium cyanide and sodium cyanide. The potassium salt type is mostly used when bright silver plating is required. The reasons are as follows:
① Fast electroplating deposition rate;
②High conductivity of the plating solution, which can ensure better dispersion and coverage capabilities;
③ Wide tolerance range for carbonates;
④ Has a smoothing effect, etc.
However, due to the high content and toxicity of cyanide, a large number of experimental studies on non-cyanide silver plating have been carried out at home and abroad. Although no plating solution comparable to cyanide has been found, some products have already been launched.
Table 2-2 Basic Composition and Process Conditions of Silver Cyanide Plating Solution
| Composizione e condizioni di processo | N. 1 | No. 2 | N. 3 |
|---|---|---|---|
| Silver cyanide (as silver)/(g/L) | 25 〜 33 | 25 〜 33 | 36 〜 114 |
| Free potassium cyanide/(g/L) | 30 〜 45 | 45 〜 160 | |
| Free sodium cyanide/(g/L) | 30 〜 38 | ||
| Potassium carbonate/(g/L) | 30 〜 90 | 15 〜 75 | |
| Sodium carbonate/(g/L) | 38 〜 45 | ||
| Potassium hydroxide/(g/L) | 4 〜 30 | ||
| Densità di corrente/(A/dm2) | 0. 5 〜 1. 5 | 0. 5 〜 1. 5 | 0. 5 〜 1. 0 |
| Temperatura/°C | 20 〜 25 | 20 〜 25 | 38 〜 50 |
Section II Decorative Silver Plating
Decorative silver plating for ornaments and Western tableware must use bright silver plating. Before the development and use of brighteners, silver decorative pieces were plated with a certain thickness of silver layer, then surface polished to achieve brightness. In 1902, Frary obtained experimental results of bright silver layers by adding a small amount of carbon disulfide ( CS2 ) to the plating solution. This marked the beginning of rapid research on silver plating brighteners.
Afterward, Wilson dissolved 28 g of carbon disulfide in 56 g of ether and added it to 1 L of silver plating, shaking the solution daily. Then, after 7~14d, 75 mL was taken from it and added to 100 L of silver plating solution, resulting in a highly bright plating layer.
Parson dissolved 6 g of carbon disulfide and 30 g of potassium cyanide in 1 L of water and, after shaking for 30 hours, took 7 mL and added it to 100 mL of silver plating solution, obtaining a good bright plating layer. The N, S, and O atoms bonded to the carbon atoms in the brightening agent cause the plating layer to become bright. Commonly used brightening agents include carbon disulfide, ketones, and a mixture of Turkish red oil, all stable brightening agents. Glycerol and potassium antimony tartrate can increase the hardness of the silver plating layer, and sodium selenite mixed with other sulfur-containing compounds helps to smooth the plating layer. All brightening agents act as depolarizers, and sulfides act in colloidal form to achieve their effect. Table 2-3 shows the composition of some silver plating brightening agents.
Table 2-3 Various Silver Plating Brighteners
| Brightener Name | Main Inventors |
|---|---|
| Carbon disulfide and ketone-based polymer |
O. Kardos; US PAT. 2807576(1957) O. H. A. Lammert;US PAT. 2666738(1954) Hanson-Von Winkle-Munning;Swiss PAT. 298147(1954) J. Wernle,Berne;France PAT. 1048094(1953) |
| Xanthates | Sieman, Halskie;German PAT. 731962(1943) |
| ASK compounds (Acrolein Sulfur Disulfide Yellow Polymer) | R. Erdman;Metalloberflache 1,2(1950) |
| Thiocarbazide | H. Schlotter;German PAT. 959775(1957) |
| Thiocarbazide | SEL-REX ( America ) |
| Selenium and antimony compounds |
R. Weiner;US PAT. 2777810(1957) Schering;US PAT. 3215610(1966) |
| Sb-Bi compounds | E. Rank;US PAT. 3219558(1965) |
Section III Pre-Plating Silver
Table 2-4 Composition and Operating Conditions of Pre-plating Silver Solutions
| Substrate Materials | Composizione e condizioni di processo | |
|---|---|---|
| Ag plating solution | Ag-Cu plating solution | |
| Iron base |
Potassium silver cyanide:1.4~2.8g/L Potassium cyanide:60~150g/L Temperature:20~25℃ Current density:1.5~2.5A/dm2 Voltage:4~6V Time:1~2min Anode: SUS plate |
Silver cyanide(in silver):0.8~1.5g/L Copper cyanide (as copper):6.0~7.5g/L Potassium cyanide:50~60g/L Temperature:15~25℃ Current density:0.1~0.2A/dm2 Time:5~10min Anode:SUS plate |
|
Silver cyanide:1.9g/L Copper cyanide(in copper):11.3g/L Potassium cyanide:75g/L Temperature:15~25°C Current density:1.5~2.5A/dm2 Anode:4~6V Time:2~3min |
||
| Copper base |
Silver cyanide:5.6~8.3g/L Potassium cyanide:60~90g/L Temperature:20~35℃ Current density:15A/dm2 Voltage:4~6V Time:1~2min Anode:Ni plate |
|
Table 2-5 Composition and Operating Conditions of Pre-Plated Silver Plating Solution
| Composizione e condizioni di processo | Parametri | Composizione e condizioni di processo | Parametri |
|---|---|---|---|
| Nickel chloride | 240g/L | Densità di corrente | 15A/dm2 |
| Hydrochloric acid (37% by volume) | 120mL/L | Tempo | 1〜2min |
| Temperatura | 20〜35℃ | Anodo | Ni plate |
Table 2-6 Pretreatment Plating Solution Composition for Pre-Silver Plating of Brass Castings, Nickel Silver, etc.
| Componenti | Concentration | Componenti | Concentration |
|---|---|---|---|
| Mercury Chloride(HgCl2) | 7. 5g/L | Mercury oxide (HgO) | 7. 5g/L |
| Ammonium chloride (NH4Cl) | 4g/L | Sodium cyanide | 60g/L |
| Oppure |
Section IV Cyanide-Free Silver Plating
Table 2-14 Stability Constants of Silver Complexes
| Complexes | Stabilization constant | Complexes | Stabilization constant |
|---|---|---|---|
| Ag(CN)2 | 21.1 | Ag(SO3)2 | 8.4 |
| Ag(CH4N4S)3 | 13.5 | AgBr43- | 8.3 |
| AgI43- | 13.4 | Ag(en)2① | 7.4 |
| Ag(S2O3)2 | 12.5 | Ag(NH3)2+ | 6.5 |
| Ag(SCN)4 | 11.2 | Agcl4 3- | 5.7 |
Table 2-15 Partial Results of Cyanide-Free Silver Plating Published So Far
| Composizione e condizioni di processo | Concentration | Nota |
|---|---|---|
|
1. Silver sulfate Ammoniaca(25%) Ioduro di potassio Pirofosfato di sodio Temperatura della soluzione di placcatura Densità di corrente
|
30g/L 7. 5mL/L 600g/L 60g/L Temperatura ambiente 2A/dm2
|
|
|
2. Nitrato d'argento Ioduro di potassio Polietilene Poliammina Temperatura della soluzione di placcatura Densità di corrente |
30〜40g/L 300〜400g/L 5〜20g/L 10〜100g/L Sopra i 40℃ 0. 5〜3. 0A/dm2 |
|
|
3.Ioduro di argento Alcool polivinilico Tiosolfato di sodio Temperatura della soluzione di placcatura Densità di corrente |
40〜80g/L 400〜600g/L 0. 5〜2. 0g/L Temperatura ambiente 0. 5〜3. 0A/dm2 |
A. Taleat et al. hanno concluso che i rivestimenti ottenuti da questa soluzione hanno una struttura dendritica e una buona resistenza alla decolorazione da H2S |
|
4. Solfato d'argento Solfato di ammonio Acido citrico Solfato di ferro Ammoniaca Temperatura della soluzione di placcatura рH |
40〜80g/L 150g/L 4g/L 0. 4〜3. 0g/L 2〜50mL/L 30℃ 10〜10. 6 |
Sia AgNO3 e (NH4)2SO4 sono stati sciolti in metà della quantità di acqua, quindi diluiti 3 volte e mescolati, poi Ag2SO4 è stato sciolto con NH4OH. Inoltre, l'acido citrico viene sciolto utilizzando metà della quantità d'acqua, quindi vengono aggiunti metalli e sali. |
|
5. Nitrato d'argento Pirofosfato di sodio Ammoniaca Nitrato di sodio Solfato di ammonio Temperatura della soluzione di placcatura Densità di corrente |
20〜30g/L 20〜25g/L 60〜100mL/L 40〜70g/L 40〜70g/L Temperatura ambiente 0. 8〜1. 1 A/dm2 |
S.R. Natarajan e altri hanno precipitato l'argento come cloruro d'argento, lo hanno sciolto in un eccesso di tiosolfato di sodio e hanno aggiunto metabisolfito di potassio. Questa soluzione di placcatura può essere mantenuta per diversi mesi a temperatura ambiente, con una densità di corrente di 0,5~1,25A/cm.2Si può ottenere un'efficienza di corrente catodica di 100%. La durezza del film di placcatura risultante è di 60~63kgf/mm.2. Sebbene sia leggermente più morbida della placcatura ottenuta da soluzioni contenenti cianuro, raggiunge comunque un livello utilizzabile come soluzione di argentatura senza cianuro.
Inoltre, la placcatura senza cianuro utilizza anche la dimetilgliossima come agente complessante. Questa soluzione di placcatura utilizza la dimetilgliossima come agente complessante e il solfito come sale conduttore; la soluzione di placcatura è alcalina. La composizione della soluzione di placcatura e le relative condizioni di processo sono riportate nella Tabella 2-16.
Tabella 2-16 Condizioni di processo che utilizzano la dimetilgliossima come agente complessante
| Ingredients and their process conditions | Parametri | Ingredients and their process conditions | Parametri |
|---|---|---|---|
|
Silver ion concentration Dimethylglycolide Sulfite |
1〜75g/L 50〜250g/L 1〜10g/L |
pH Temperatura della soluzione di placcatura Densità di corrente |
7〜13 30〜90℃ 0. 1〜10A/dm2 |
It is recommended that this be used in the field of silver plating on semiconductor bump pads. This method can produce a fine and smooth plating surface. As a non-cyanide plating solution, it does not require oxygen or air to be bubbled into the plating solution to control silver precipitation. Moreover, the plating solution can be used continuously for a long time.
Suppose the sulfite concentration in this plating solution is too low (below 1g/L). In that case, the grain refinement effect of the plating layer deteriorates, and the inhibition effect on plating nodules also worsens. However, if the sulfite concentration is too high (above 75g/L), the plating solution tends to crystallize and precipitate. This may be related to the weak ability of sulfite to reduce.
This plating solution is suitable for alkaline threshold work, for example, when pH<7, the plating solution tends to become turbid but when pH>13, the plating layer is not bright. Some test results are shown in Table 2-17.
Table 2-17 Test Results of Non-Cyanide Silver Plating Using Dimethyl Ethylene Urea as a Complexing Agent
| Numero di serie | Silver dimethylglycolide (as silver) /(g/L) | Dimethylglycolideurea/(g/L) | Potassium sulfite/(g/L) | pH | Surface roughness Ra /μm | Aspetto | Height difference /μm | Brightness |
|---|---|---|---|---|---|---|---|---|
|
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 |
1 30 75 1 30 75 1 30 75 1 30 75 1 30 75 1 30 75 1 30 75 1 30 75 1 30 75 0. 8 30 0. 8 80 30 80 |
50 50 50 50 50 50 50 50 200 200 200 200 200 200 200 200 200 200 250 250 250 250 250 250 250 250 250 200 200 200 200 200 200 |
0. 1 3 10 0. 1 3 10 0. 1 3 10 0. 1 3 10 0. 1 3 10 0. 1 3 10 0. 1 3 10 0. 1 3 10 0. 1 3 10 0. 07 0. 07 3 12 12 3 |
7. 0 7.0 7. 0 11. 0 11. 0 11. 0 13. 0 13. 0 13. 0 7.0 7.0 7.0 11. 0 11. 0 11. 0 13. 0 13. 0 13. 0 7. 0 7.0 7.0 11. 0 11. 0 11. 0 13. 0 13. 0 13. 0 5. 0 11. 0 11. 0 13. 5 11.0 11. 0 |
0. 45 0. 33 0. 38 0. 26 0. 16 0. 20 0. 22 0. 20 0. 32 0. 40 0. 35 0. 42 0. 20 0. 13 0. 15 0. 12 0. 20 0. 30 0. 38 0. 36 0. 32 0. 30 0. 18 0. 15 0. 22 0. 18 0. 31 一 — 0. 15 — — 1.0 |
Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Favorable Tumor plating Tumor plating No luster Ag salt precipitation With plating tumor Ag salt precipitation |
0.41 0. 37 0. 39 0. 29 0. 26 0. 19 0. 28 0. 32 0. 34 0. 45 0. 30 0. 35 0. 30 0. 13 0. 15 0. 20 0. 30 0. 35 0. 33 0. 33 0. 38 0. 28 0. 19 0. 25 0. 40 0. 32 0. 40 10 8 3 — 5 一 |
0. 8 0. 5 0. 8 1. 0 1. 1 1. 1 1. 3 1. 2 0. 9 0. 7 0. 8 0. 7 1. 1 1.3 1. 2 1. 3 1. 1 0. 9 0. 8 0. 6 0. 7 1. 0 1. 1 1. 0 1. 2 1. 1 0. 8 <0. 2 <0. 2 0. 2 一 0. 3 —— |
In the table, the plating solution temperature is 60℃, the current density is 1A/dm2, and the plating thickness are 50μm. Surface roughness Ra was measured using a KLA Profiler P-11, appearance was observed with a metallurgical microscope, and brightness was measured with a GAM brightness meter (digital densitometer Model-144).
Adding 2,2’-bipyridine can achieve a mirror-bright coating for cyanide-free silver plating solutions using hydantoin and its derivatives as complexing agents. The composition of the plating solution and its process conditions are shown in Table 2-18.
Table 2-18 Composition and Process Conditions of Cyanide-Free Bright Silver Plating Solution
| Composizione e condizioni di processo | N. 1 | No. 2 | N. 3 | |
|---|---|---|---|---|
|
KOH/(g/L) Acido solfammico/(g/L) Complex of 5,5-dimethylhydantoin/(g/L) Ag(complex of 5,5-dimethylhydantoin)/(g/L) 2,2'-Dipyridine/(g/L) Nicotinamide/(g/L) 2-Aminopyridine/(g/L) 3-Aminopyridine/(g/L) Bright current density range/(A/dm2) |
60 52.5 60 25 0. 8 - - - 5〜20 |
60 52. 5 60 25 0. 4 4. 0 - - 0〜12. 5 |
60 52. 5 60 25 0. 4 - 1.3 - 0〜20 |
60 52. 5 60 25 0. 4 - - 0. 8 0〜20 |
Nella formula, n è un intero di 2~4; R1 e R2 possono essere uguali o diversi e sono gruppi alchilici di C1 ~ C3 o gruppi alchilenici di C2 ~ C6M può essere idrogeno, metalli alcalini, metalli alcalino-terrosi o gruppi amminici.
Può essere utilizzato non solo per l'argentatura, ma anche per la placcatura di leghe d'argento.
Inoltre, è possibile aggiungere tensioattivi per migliorare lo strato di placcatura.
Sezione V Leghe placcate in argento
Anche la storia delle leghe argentate è relativamente lunga, soprattutto perché le leghe argentate possono ottenere proprietà chimiche e meccaniche che l'argentatura pura non può ottenere. Anche se ne esistono molti tipi, tra cui argento-antimonio, argento-piombo, argento-cadmio, argento-rame, argento-nichel, argento-zinco, argento-cobalto, argento-palladio, argento-platino, ecc.
Tra queste, le leghe argento-rame variano di colore a seconda del contenuto di rame, dal bianco al rosso rosato. Inoltre, la placcatura non è fragile e presenta una maggiore resistenza all'usura rispetto alla placcatura in Ag puro. Le leghe argento-piombo possono essere utilizzate come rivestimenti per la riduzione dell'attrito in caso di carichi elevati, come la rotazione ad alta velocità. La lega argento-cadmio ha una forte resistenza alla corrosione, che la rende adatta a resistere alla corrosione dell'acqua di mare. Allo stesso tempo, la sua resistenza allo zolfo e alla decolorazione ad alta temperatura è superiore a quella dell'argentatura pura.
Anche la soluzione di placcatura per le leghe d'argento è per lo più a base di cianuro, con la lega argento-antimonio che è la più comunemente usata tra le leghe. La Tabella 2-19 mostra alcuni processi rappresentativi di placcatura delle leghe d'argento.
Tabella 2-19 Alcuni processi rappresentativi di placcatura dell'argento in lega
| Nome della lega (contenuto)/% | Durezza (Nuc) | Resistenza specifica/(mΩ/cm) | Composizione della soluzione di placcatura |
|---|---|---|---|
|
Sb 0. 7 - 9. 6 -
|
- 100 - 164 - |
- 1.9 - 11.6 - |
Ag: 24g/L Sb: g/L Na2CO325g/L Tartrato: 60g/L NaOH:3〜5g/L |
|
Bi 1〜2. 6 - - - |
- 90〜180 - - - |
- 8〜10.4 - - - |
Ag: 25〜50g/L Bs: 25g/L K2C4O4H235g/L KOH: 25g/L KCN:20-〜50g/L |
|
Cu 20 60 85 - |
- 240 240 340 - |
- 7.5 12 22 - |
K7Ag(P2O7)2 (contato come Ag) 20g/L K6CU(P2O7)2 richiesto K4P2O7 100g/L 20℃、0. 5A/dm2 Nel caso di questa lega, la durezza di Nucor scende a circa 185 dopo circa 26 mesi a temperatura ambiente. |
|
Pb 4 10. 2 - - |
- 180 - - - |
- 10.5 11.5 - - |
AgCN 0. 33mol/L NaCN 0. 3mol/L Acetato di piombo 0,015mol/L NaOH 0,018mol/L Tartrato 0. 21mol/L
|
|
Pd 12 60 90 - |
- 180 250 320 - |
- — 10 — - |
Cianuro di potassio e argento 12g/L, pH 4,5 Cloruro di palladio 22g/L 0. 5A/dm2 Pirofosfato acido di potassio 56g/L, Ag955, Pd 5% Tiocianato di potassio 156g/L (rapporto di lega) Brevetto giapponese: Licenza n. 57-55699
|
|
Tl 9. 5 - - |
- 90 - -
|
- - - -
|
AgCN 32g/L KCN 25g/L K2CO3 30g/L Tl2SO4 6g/L |
Copywrite @ Sobling.Jewelry - Produttore di gioielli personalizzati, fabbrica di gioielli OEM e ODM
Table 2-20 Ag-Pd Alloy Composition and Lattice Constant
| Alloy composition/% | Lattice constant/Å | Alloy composition/% | Lattice constant/Å | ||||
|---|---|---|---|---|---|---|---|
| Ag | Pd | Molten alloy | Alloy Plating | Ag | Pd | Molten alloy | Alloy Plating |
|
100 99 97 95 93 90 |
- 1 3 5 7 10 |
4. 077 4. 077 4. 072 4. 070 4. 061 4. 056 |
4. 077 4. 077 4. 077 4. 071 4. 059 4. 051 |
88 86 85 80 — - |
12 14 15 20 100 - |
4. 054 4. 053 4. 053 4. 031 3. 882 - |
4.054 4. 053 4. 051 4. 020 3. 900 - |
One of the authors of this book researched obtaining Pd-Ag alloys from alkaline ammonia plating solutions to achieve Pd80% (atomic ratio) alloy compositions. The basic composition of this plating solution is:
Pd(NH3)4 (NO3)2 0.1mol/L
Ag(NH3)2NO3 0.01mol/L
NH4NO3 0.4mol/L
Use ammonia water as a pH adjuster.
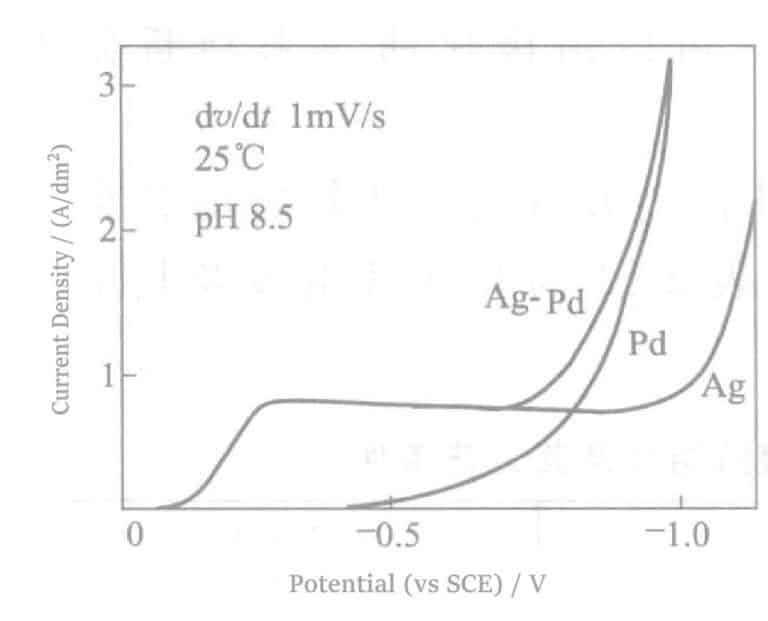
The polarization curves of the Pd, Ag and Pd-Ag alloys are shown in Figure 2-5.
Pd2+ + 4NH3 → Pd(NH3)42+ β1=6.3×1032
Ag+ + 2NH3 →Ag(NH3)2+ β2=2.5×107
From the above equation, it can be seen that the stability constants of their complexes differ greatly. Considering also the ammonia water used for pH adjustment, with a total concentration of 1mol/L, according to the Nernst equation, the equilibrium potentials of Pd and Ag in 25℃(relative to NHE) are -0.08 V and +0.24 V yield a more positive potential for Ag. In the polarization curve of the Ag-Pd alloy, it is observed that Ag deposits first, followed by Pd deposition, and finally, the curve moves along the Pd polarization line.
Effect of plating conditions on alloy deposition: The effect of current density on alloy composition is shown in Figure 2-6. It can be seen from the figure that the Ag content in the coating decreases with increasing current density. When the Pt electrode is rotated, or the plating solution is stirred, the Ag content in the coating increases. This indicates that Ag’s deposition (deposition) is controlled by diffusion of Ag+, consistent with the polarization curves in Figure 2-5.
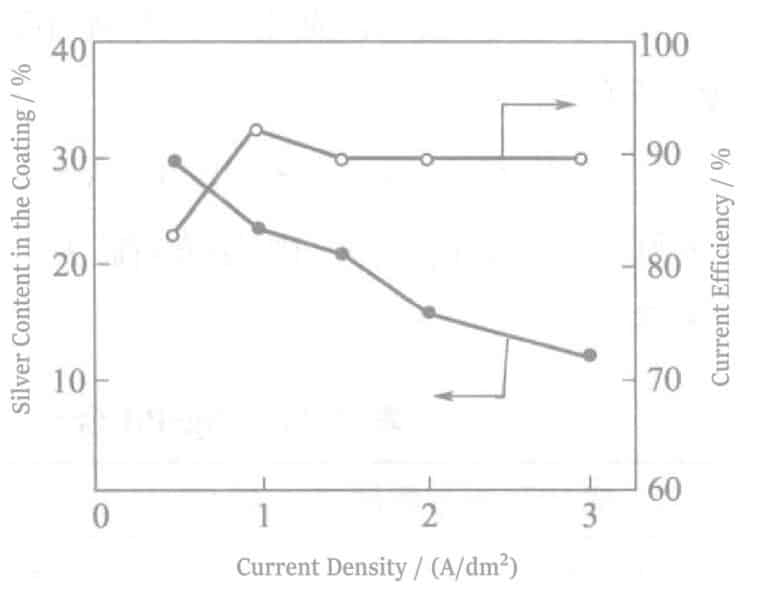
The increase in Ag content is caused by the decrease in current density or the increase in diffusion rate due to the increased concentration of Ag ions in the cathode diffusion layer. From the polarization curve in Figure 2-5, the Ag potential is more positive than the Pd potential, which conforms to the deposition of regular alloys. According to Brenner’s definition of regular deposition, metals with more positive standard electrode potentials increase their content in the alloy as the ion concentration in the diffusion layer increases. In this experiment, the actual potential change is determined by the plating solution composition and can be judged by the polarization curve regarding the positivity or negativity of metal ions.
Koichi Yamakawa et al. proposed alloy plating formulas to achieve good coatings over a relatively wide range of alloy compositions. Table 2-21 shows the composition of their plating solution and its process conditions.
Table 2-21 Composition and Process Conditions of Ag-Pd Alloy Plating Solution
| Composizione e condizioni di processo | N. 1 | No. 2 |
|---|---|---|
|
PdCl2/(g/L) AgNO3/(g/L) KBr/(g/L) KNO2/(g/L) Sodium saccharin/(g/L) Boric acid/(g/L) Sodium naphthalene sulfonate/(g/L) pH (adjusted by NaOH and HNO3) Anodo Temperature of plating solution/°C Densità di corrente/(A/dm2) |
28. 4 15. 3 590. 0 23. 4 0. 5 - - 6. 0 30% Pd-Ag 50 0.5,1,2,5,10 |
33 10. 0 590. 0 15. 0 - 50. 0 1. 0 9 Pt 30 0.5,1,2,5,10
|
Ag+ + 4Br- → AgBr43-
Pd2+ + 4NO22- → Pd(NO2)42-
Table 2-22 Ag-Pd Alloy Coating Results
| Densità di corrente /(A/dm2) | N. 1 | No. 2 | ||||
|---|---|---|---|---|---|---|
| Coating thickness /μm | Aspetto | Pd/(Ag+ Pd)/% | Coating thickness /μm | Aspetto | Pd/(Ag+ Pd)/% | |
|
0. 5 1 2 5 10 |
10 10 3 3 0. 5 |
Gray, Semi-Gloss Gray, Semi-Gloss Silver Gloss Silver Gloss Silver Gloss |
25 20 25 30 40
|
2 2 0. 5 0. 3 0. 1 |
Gray, Semi-Gloss Gray, Semi-Gloss Silver Gloss Silver Gloss Silver Gloss |
50 30 50 60 70 |
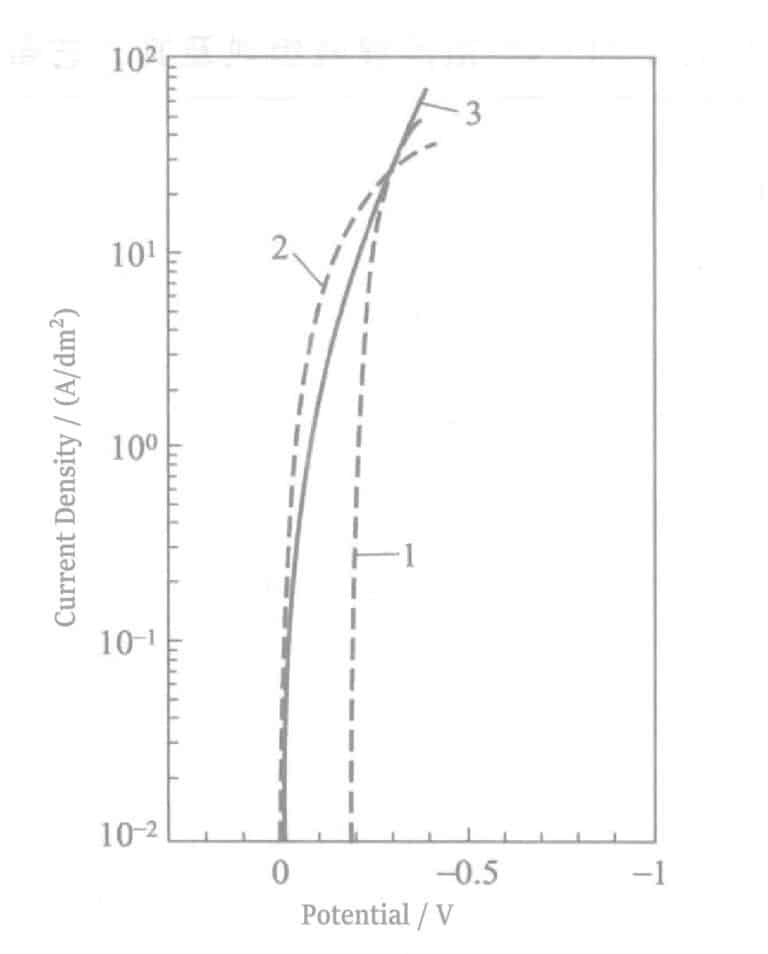
Figure 2-7 Polarization curves of Ag-Pd alloy plating solution
1--Pd deposition current; 2--Ag deposition current; 3--Ag-Pd alloy deposition current
Table 2-23 Sn-Ag and its Sn-Ag-Cu Plating Solution Composition and Process Conditions
| Ingredients and their process conditions | Sn-Ag plating solution | Sn-Ag-Cu plating solution |
|---|---|---|
|
Sulfuric acid/(mL/L) Tin sulfate/(g/L) Silver nitrate/(g/L) Tiourea/(g/L) Polyoxyethylene alkyl ether/(g/L) Copper sulfate pentahydrate/(g/L) Cathode current density/(A/dm2 ) Plating solution temperature/°C Agitazione Deposition speed/(μm/min) |
120 36 1. 5 15 2 - 2 20 Sì 1 |
120 36 1. 5 15 2 4 2 20 Sì 1 |
The coating obtained under the above conditions is dense and smooth.
The composition of the Sn-Ag barrel plating solution and its process conditions are shown in Table 2-24.
Table 2-24 Composition and Process Conditions of Tin-Silver Electroplating Solution
| Composition and its process conditions and properties | N. 1 | No. 2 | N. 3 |
|---|---|---|---|
|
Stannous sulfate (as Sn)/(g/L) Stannous chloride (as Sn)/(g/L) Sodium gluconate/(g/L) Gluconic acid/(g/L) Succinic acid/(g/L) Sodium pyrophosphate/(g/L) EDTA-2Na/(g/L) Silver Acetate(Silver)/(g/L) Silver nitrate (as silver)/(g/L) PEG(#3000)/(g/L) рH Plating solution temperature/°C Anode material Average current density/(A/dm2 ) Plating time/min Spessore del rivestimento/μm Plating Appearance Silver content/% Punto di fusione/°C Brazing wettability (after plating) Brazing wettability (after humidification test) Whisker crystal |
12 - 50 - 20 - - 1. 8 - 1 7. 5 50 Sn plate 0. 1 75 5 White, non-glossy 2. 0 221 Within 1s Within 2s Nessuno |
- 13 60 - - 100 - 0. 5 - 1 8. 1 40 Sn plate 0. 1 75 5 White, non-glossy 3. 8 221 Within 1s Within 2s Nessuno |
- 25 - 96 - 80 50 - 1 1 8. 5 25 Platinized titanium plate 0. 1 75 5 White, non-glossy 3. 3 221 Within 1s Within 2s Nessuno |
The resulting plating layer has good wettability.
Sn-Ag alloy is an additive for alloy plating, which can achieve a plating layer thickness of over 50μm.
When the Sn-Ag alloy is used on raised pads, the requirement for plating thickness increases. However, plating solutions typically used for thin layers tend to have issues such as uneven surfaces and insufficient adhesion when the plating thickness is increased. These problems can be solved by adding certain additives. The main components of the solution proposed by Yachikawa are:
① Add a cationic surfactant containing alkyl amines, whose molecular structure is H(OCH2CH2)nRN(CH2CH2O)nH.
② Water-soluble amines and their derivatives.
③ Glycerol.
④ Urea compounds or reducing agents (where the role of the reducing agent is to prevent the deposition of iodine at the anode when iodide compounds are present).
The implementation process conditions are shown in Table 2-25.
Table 2-25 Sn-Ag Process Conditions for Raised Pad Plating
| Composition, process conditions and properties | N. 1 | No. 2 | N. 3 | No. 4 | No. 5 | No. 6 | No. 7 | No. 8 | No. 9 |
|---|---|---|---|---|---|---|---|---|---|
|
Tin pyrophosphate/(g/L) Silver pyrophosphate/(g/L) Pirofosfato di potassio/(g/L) Polyoxyethylene cetylamine/(g/L) Dimethylamine/(g/L) Potassium glycerate/(g/L) Silver iodide/(g/L) Potassium iodide/(g/L) Hypoethyl Urea/(g/L) Hypoethylenediamine/(g/L) Sodium hypophosphite/(g/L) Ammonium polyoxyethylene octadecanoate/(g/L) Triethanolamine/(g/L) Tiourea/(g/L) Hydrazine hydrochloride/(g/L) Trimethylurea/(g/L) Dimethylaminoboron/(g/L) Glycerol/(g/L) Ammonium dipolyoxyethylene dodecanoate/(g/L) Hydroxylamine hydrochloride/(g/L) Ethylenediamine/(g/L) Glycerol acetate/(g/L) Calcium glycerate/(g/L) рH Spessore del rivestimento/μm Tin content of plating/% |
33 2. 5 100 10 20 - - - - - - - - - - - - - - - - - - 11. 0 57 89. 7 |
33 2. 5 100 10 20 0. 5 - - - - - - - - - - - - - - - - - 11. 0 63 91. 6 |
33 - 96 10 20 - 1. 3 83 1. 0 - - - - - - - - - - - - - - 6. 0 63 89. 2 |
33 - 96 - - - 1. 3 83 - 8 2 4 - - - - - - - - - - - 6. 0 67 91. 4 |
33 - 96 - - - 1. 3 83 - - - 10 10 0. 5 2 - - - - - - - - 6. 0 60 91. 6 |
33 - 96 6 - - 1. 3 83 - - - - - - - 0.8 2. 5 0. 8 - - - - - 6. 0 64 91. 0 |
33 - 96 - - - 1. 3 83 - - - - - - - 0. 8 2. 5 0. 8 8 4 - - - 6. 0 61 90. 7 |
33 - 96 - - - 1. 3 83 - - - - - 0. 8 1. 5 - - - 6 - 4 1. 0 - 6. 0 61 88. 7
|
33 - 96 7 10 - 1. 3 - - - 2. 5 - - 0. 3 - - - - - - - - 0. 5 6. 0 59 89. 3
|
Section VI Troubleshooting of Silver Plating
1. Cyanide Plating Solution (usually for Rack Silver Plating) Bright Silver Plating Defects
Table 2-26 Common Silver Plating Defects and Countermeasures
| Fault content | Cause | Countermeasures |
|---|---|---|
| Poor adhesion of the plated layer | Pre-plated silver is not completely covered. Passivation of the base or bottom plating layer | Check the concentration of silver, potassium cyanide and sodium cyanide in the silver pre-plating solution and the surface activity of the plated parts before plating. |
| Ag plating is black or has spots on the surface | Insufficient concentration of free potassium cyanide or free sodium cyanide in the plating solution. | Adjust the concentration of free potassium cyanide and sodium cyanide to standard values. |
| Ag anode is covered by black skin film | Insufficient concentration of free potassium cyanide or free sodium cyanide in the bath. | Adjust the concentration of free potassium cyanide and sodium cyanide to standard values. |
| Hydrogen gas precipitation on the surface of plated parts | The concentration of free potassium cyanide or free sodium cyanide is high compared to the concentration of silver ions in the bath. | Increase the concentration of silver ions or remove part of the plating solution to reduce the amount of plating solution. |
| Roughness of the plated layer | High current density | Reduce the current density to an appropriate value |
| Spots, protrusions, pockmarks on plated surface | Hydrogen adsorption due to impurities in the plating solution. | Filtration with activated carbon |
| Plated layer is not smooth | Contamination of the plating solution, high current density, dirty anode bag (anode sludge floating) | Filter the bath, clean the anode bag, and clean the bath. |
| Thickness of plated layer not, anode passivation | Excessive surface area of product | Increase the anode area by maintaining the proper amount of plated parts. |
2. Issues, Causes, and Countermeasures of High-Speed Silver Plating
Table 2-27 Common Problems and Countermeasures of High-Speed Silver Plating
| Fault content | Cause | Countermeasures |
|---|---|---|
| Dark and coarse plating | Current density is too high, KCN is too low, Ag ion concentration is too low, CO32- concentration is too high, brightener concentration is too low. | Confirm and adjust, analyze and adjust free cyanide ion, remove (cool) CO32- , analyze and add |
| Stepped plating | The concentration ratio of brightener and replacement inhibitor is not coordinated, usually due to its high ratio. | Analyze and dilute the plating solution. |
| Blistering | Replacement of degreasing agent is required, the pre-plated layer is not satisfactory, the bottom layer is passivated. | Confirm and replace the plating solution, replace the pre-plating solution if it is dirty, and confirm the final rinse and plating room. |
| Spots and uneven luster | Insufficient brightener, clogged nozzle, silver in the anode solution, or solid ions in the Pt/Ti anode solution. | Analyze and adjust, remove and replace, remove, wash, replace if greenish black, and perform activated carbon filtration. |