¿Qué es el baño de rodio y cómo puede beneficiar a sus joyas?
Rhodium Plating Guide for Jewelry: Anti-Tarnish, Hardness, Silver-White Finish
Introducción:
Rhodium plating is an electroplating process that deposits a thin layer of rhodium, a precious metal from the platinum family, onto jewelry. But what makes it so special? This hard, silver-white coating provides exceptional tarnish and corrosion resistance, preventing jewelry from darkening over time. It also significantly increases surface hardness, making pieces more scratch-resistant and durable. Commonly used as a protective final layer for silver and platinum, it enhances brightness and gives a high-gloss, reflective mirror finish. This article delves into the process, from sulfate-based plating solutions to chemical deposition, explaining how this technique creates long-lasting, beautiful jewelry.

Índice
Sección I
Rhodium has an atomic number of 45 in the periodic table, with the element symbol Rh. It was discovered by W. H. Wollaston in 1803. Its name comes from the Greek word “Rodeos,” meaning rose-colored because rhodium salt solutions are rose-colored.
Rhodium was the first white metal to be industrially applied in electroplating factories. Generally, rhodium is resistant to corrosion by acids and bases (including aqua regia), but it can react with hot, concentrated sulfuric acid, sodium hypochlorite, and others under 300℃. The rhodium plating film has a high mirror reflectivity, exceptionally high hardness reaching Hv 800–1000, excellent corrosion resistance, and low electrical resistance. Unlike Ag, it does not change over time, so it can be used as a contact material. It is also widely used in electronics, electrical, and optical components industries. Rhodium can also be used as an anti-wear coating for advanced scientific instruments. Additionally, rhodium is commonly used to manufacture hydrogenation catalysts, and rhodium-platinum alloys make thermocouples. Rhodium plating is a color and protective layer for silver-white precious metal jewelry such as silver and platinum. Some main parameters of rhodium are shown in Table 5-1.
Table 5-1 Some Main Parameters of Rhodium
| Characteristic parameters | Characteristic value |
|---|---|
|
Element name, element symbol, atomic number Clasificación Group, Period Density, hardness Color Masa atómica relativa Radio atómico Covalent bond radius Chemical valency Crystalline structure melting point boiling point Calor de vaporización Heat of dissolution Capacidad calorífica específica Conductivity Thermal conductivity |
Rhodium、Rh、45 Transition metal 9(Ⅷ), 5 12450kg/m3、6 Silver White 102.90550 135pm 135pm 2、3、4 Cúbico centrado en la cara 2237K (1964℃) 3968K (3695℃) 493kJ/mol 21. 5 kJ/mol 0. 242J/(kg ・ K) 21. 1 X 106m •Ω 150W/(m • K) |
Section II Rhodium Plating and Its Alloys
1. Rhodium Plating
Rhodium is the most widely used platinum group metal in electroplating. Due to rhodium’s excellent corrosion resistance, its plating is harder and more wear-resistant than other precious metals, and its white tone is widely used in the jewelry industry. It is especially indispensable as an anti-tarnish protective coating for silver ( usually plated with 0.05μm flash rhodium ). Moreover, its high mirror reflectivity makes it commonly used as the final flash plating on mirrors. Black rhodium plating is typically used on eyeglass frames and watch cases. It can be used as an electrode in seawater electrolysis and household water treatment electrodes. Additionally, in the electronics industry, it is applied on switch contacts.
The application of rhodium in electroplating began in the 1930s, primarily for decorative purposes. In 1934, Shield applied for the first patent for rhodium electroplating.
The plating solutions for rhodium electroplating include:
① Rhodium sulfate – sulfuric acid plating solution series;
② Rhodium phosphate-sulfuric acid plating solution series;
③ Also, phosphate-based fluoroboric acid plating solution, sulfonic acid plating solution, etc., have not been commercialized.
Rhodium has mainly been studied for its application on spring contacts.
In sulfuric acid plating solutions, there are thin plating solutions for decorative purposes (focusing on reflectivity and gloss), thick plating solutions (focusing on film thickness and contact resistance), and high-speed plating solutions.
1.1 Thin Plating Solution
Table 5-2 Representative Components and Operating Conditions of Rhodium Plating Solutions
| Sulfate-Sulfuric Acid Series | Phosphate-Sulfuric Acid Series | Phosphate-Phosphoric acid series |
|---|---|---|
|
Rhodium (as rhodium sulfate) 1. 5〜2. 0g/L Sulfuric acid (95%~96%) 25〜50mL/L Solution Temperature 40〜50℃ Current density 1〜10A/dm2 Voltage 3〜6V Anode Pt |
Rhodium (as rhodium phosphate) 2. 0g/L Sulfuric acid (95%~96%) 25〜50mL/L Solution Temperature 40〜50℃ Current density 1〜10A/dm2 Voltage 3〜6V Anode Pt
|
Rhodium (as rhodium phosphate) 2. 0g/L Phosphoric acid (85%) 40〜80mL/L Solution Temperature 40〜50℃ Current density 1〜15A/dm2 Voltage 4〜8V Anode Pt |
(1) Corrosion resistance performance:
Rhodium is an extremely stable metal, but the plating film is somewhat inferior. Generally, other metals are first plated on the substrate when plating rhodium and rhodium is plated last. In this case, the corrosion resistance of the underlying plated metal becomes a very important factor. There are two reasons: first, because rhodium is a precious metal, there is a potential difference between it and non-precious metals; second because it is expensive, it cannot be plated too thickly. When plating rhodium on an underlying Ni layer, electrochemical corrosion can easily occur, so a high-potential plating layer can be introduced between the two, such as gold plating, which is better. However, since gold plating increases costs, later 2μmPd or Pd-Ni alloys were introduced to improve corrosion resistance.
(2) The effect of impurities on plating performance:
Rhodium plating solution is strongly acidic, and during printed circuit board plating, it may cause the dissolution of the mask. When metal impurities are present, the rhodium plating layer will appear blackened, reducing the commercial value of the rhodium plating layer. When organic impurities are present, the internal stress of the rhodium plating layer increases, which, in turn, reduces the adhesion of the plating layer. W. Safranek studied the case of increased plating stress when organic impurities are present in the plating solution; the results are shown in Table 5-3.
Table 5-3 Effects of Organics on the Stress of Rhodium Plating Layers
| Plating solution temperature /℃ | Cleaning fluid/ ( kgf/ mm2) | Masking agent (A) (low sulfur content)/(kgf/mm2) | Masking agent /(kgf/mm2) |
|---|---|---|---|
|
30 40 50 60 70 |
70 87 80 69 59 |
72 89 82 71 61 |
91 114 92 91 100 |
Note: Plating solution composition and conditions:
Rhodium metal 8g/L
H2SO4 30g/L
Current Density 0.5A/dm2
Plating time 30min
Amount of plating solution 200mL
1.2 Thick Plating Solution
(1) The types of sulfonates and the relationship between their concentration in solution and current efficiency.
Aotani et al. studied benzaldehyde-2,4-disulfonate sodium or 1,5-naphthalene disulfonate disodium and amino sulfonic acid in a rhodium-plating solution. When the rhodium concentration was 5g/L, and the current density was 1.5A/dm2, after plating for 60 min, the relationship between sulfonate concentration and current density was examined. The results are shown in Figures 5-1 to 5-3. The results indicate that as the sulfonate concentration increases, the current efficiency almost linearly decreases, and the plating film quality also deteriorates accordingly.
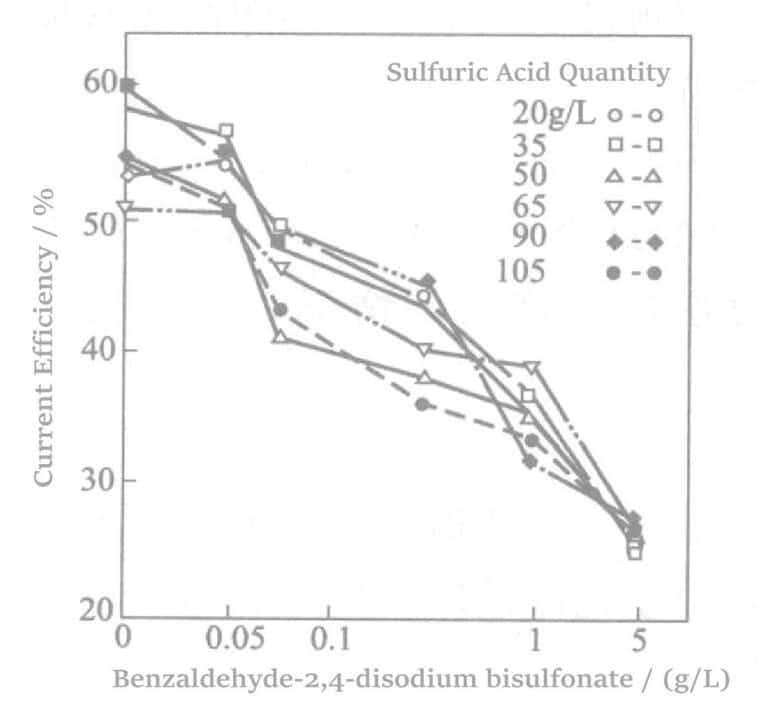
Figure 5-1 Effect of adding sodium 2,4-disulfonate benzaldehyde on current efficiency
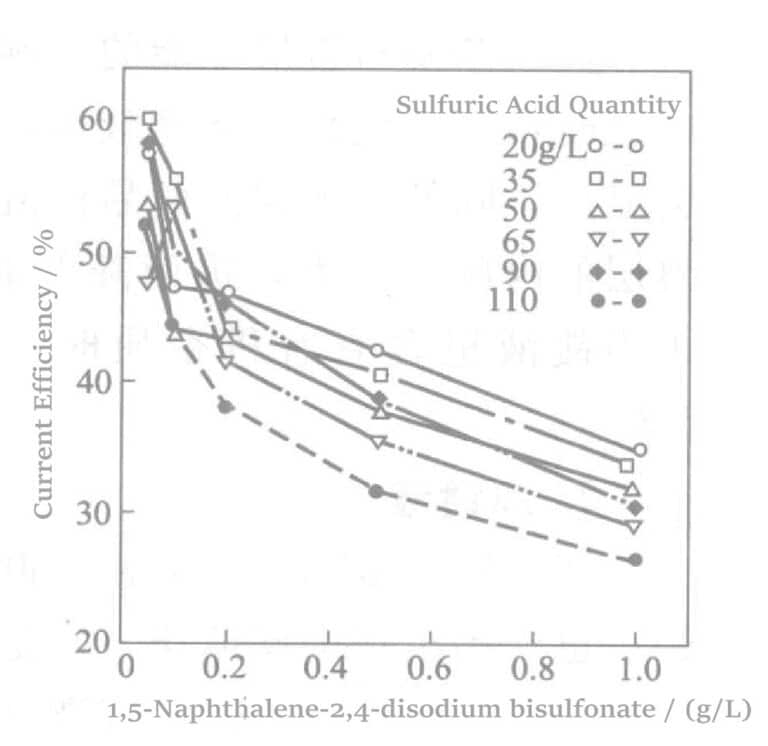
Figure 5-2 Effect of adding disodium 1,5-naphthalene disulfonate on current efficiency
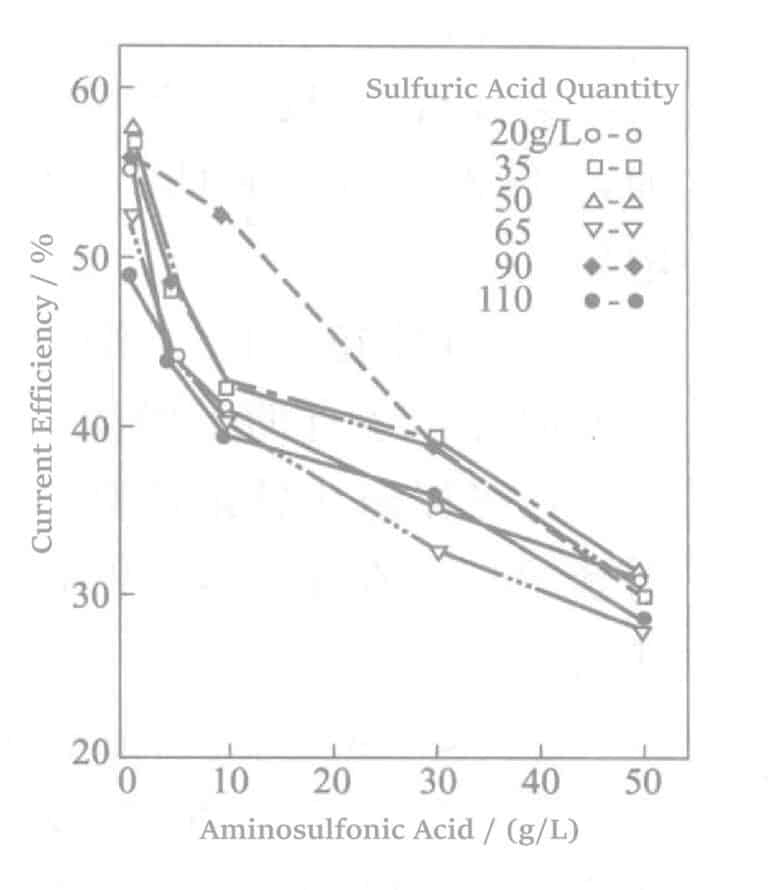
Figure 5-3 Effect of adding amino sulfonic acid on current efficiency
(2) The relationship between thallium nitrate, magnesium sulfate, and aluminum sulfate as stress relief agents and current efficiency.
Additives include 1,5-naphthalene disulfonate disodium and amino sulfonic acid. The relationship between their additive concentration and current efficiency is shown in Figure 5-4. Meanwhile, the changes in current efficiency when various stress relief agents are combined as additives are shown in Figure 5-5.
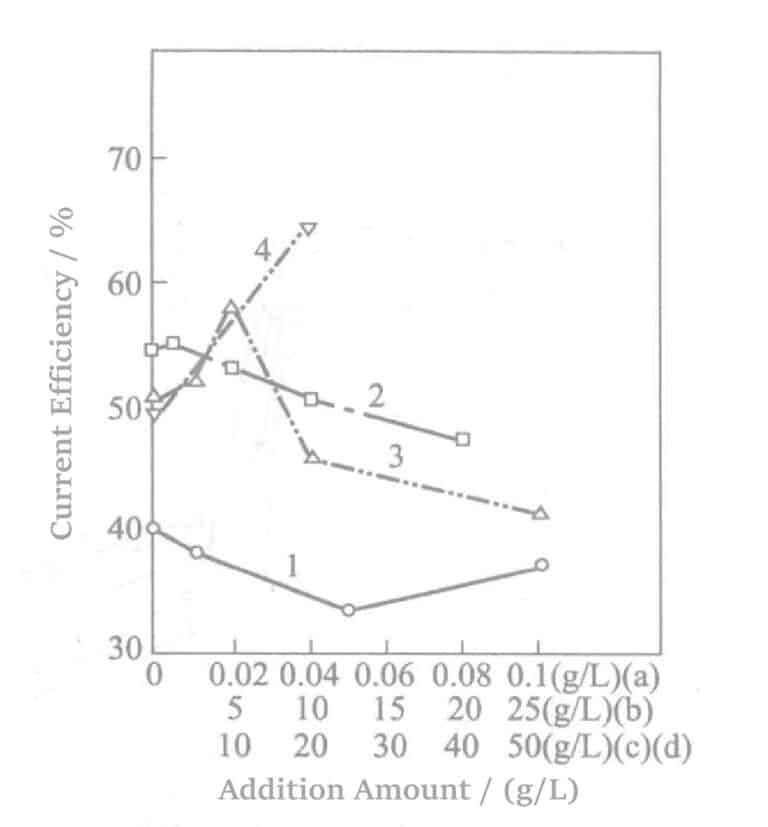
Figure 5-4 Effect of additives on current efficiency
1-sulfuric acid 90g/L, sodium benzaldehyde-2,4-disulfonic acid 0.5g/L, wetting agent for nickel plating;
2-sulfuric acid 20g/L, thallium nitrate 0.05g/L, sulfamic acid;
3-sulfuric acid 35g/L, sulfamic acid 20g/L, magnesium sulfate;
4-sulfuric acid 50g/L, sulfamic acid 5g/L, aluminum sulfate
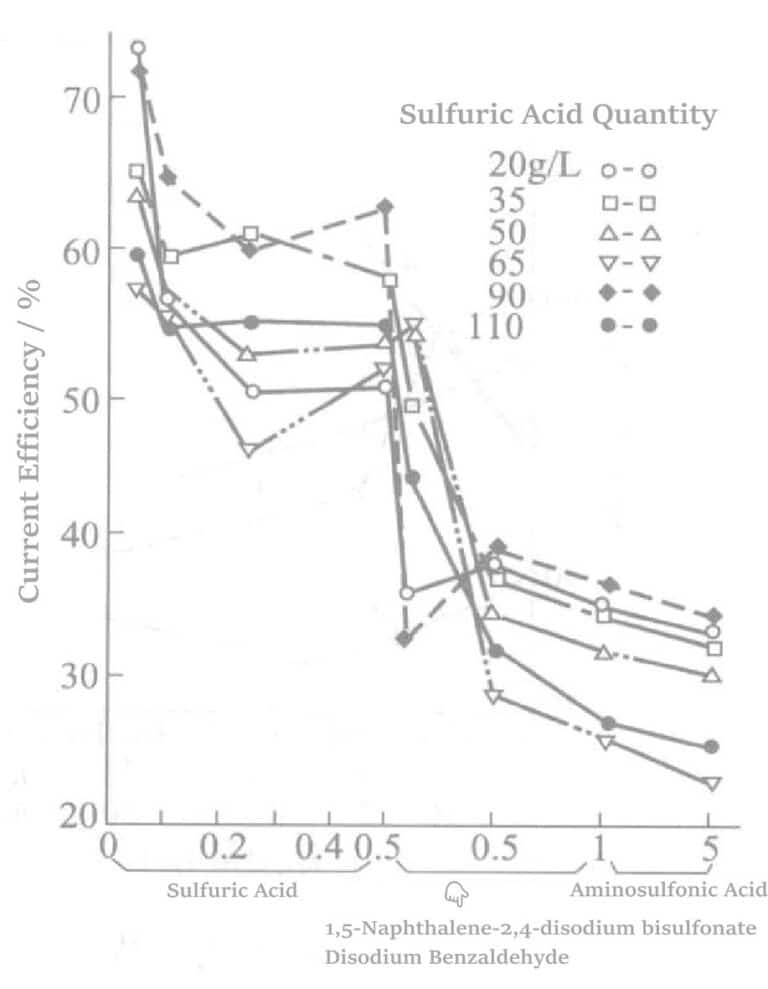
Figure 5-5 The effect of adding thallium nitrate, 1,5-naphthalene disulfonate disodium, benzaldehyde, and amino sulfonic acid on current efficiency
It can be seen that the combined use of sulfonic acid, thallium nitrate, benzaldehyde-2,4-disulfonate sodium or 1,5-naphthalene disulfonate disodium, 2,4-disulfonate sodium, and 1,5-naphthalene disulfonate sodium can produce a semi-bright or higher non-peeling plating layer. The roles of each component are as follows:
① Rhodium: 5g/L is used as the standard, and the current efficiency increases for every 1g/L increase.
② Sulfuric acid: When the concentration of sulfuric acid increases, the brightness slightly increases, but the current efficiency decreases.
③ Sulfonic acid: As a brightening leveling agent, Sulfonic acid can increase leveling (brightness increases, roughness decreases).
④ Thallium nitrate: Besides serving as a stress-relief agent, Thallium nitrate also helps increase current efficiency, can prevent the decline of current efficiency when rhodium concentration decreases, and reduces pitting.
⑤ Benzaldehyde-2,4-disulfonate sodium or 1,5-naphthalene disulfonate sodium: As brightening leveling agents, can increase the brightness of the plating layer, reduce plating nodules while causing a decrease in current efficiency.
Based on the above, it can be assumed that the following composition and operating conditions can be used to obtain a coating with a thickness of 30μm or more.
|
Rhodium ion concentration Sulfuric acid concentration Thallium nitrate Sulfonic acid Sodium benzaldehyde-2,4-disulfonate or disodium 1,5-naphthalenesulfonate Plating solution temperature Current efficiency |
5 g/L 50 g/L 0.05g/L 40g/L 0.4g/L 50℃ Above 60% |
|
Inherent Resistance Durability Corrosion resistance Heat Resistance Dureza Bending test Estado de la superficie |
23×10-6Ω·cm Bien Few penetration spots to Ni substrate. No flaking at 450℃, but cracks are present. Average Hv 900 The base is thin sample when peeling off less, poor spreading Few plating tumors, semi-bright and bright, but there are pits present |
|
Rhodium (as rhodium sulfate) Ácido sulfúrico Selenic acid(HSeO) Plating solution temperature Current density |
10g/L 10〜200mL/L 0. 1〜1. 0g/L 50 〜75℃ 1.2A/dm2 |

The rhodium salts can be prepared using the alloy, chlorination, or fusion methods.
In addition, organic carboxylic acids are also considered stress relievers in rhodium plating.
1.3 Improvement of the Rhodium Plating Process
In rhodium plating layers, the inherent tensile stress is a major defect. As mentioned earlier, adding a stress-relieving agent can reduce the stress, thereby increasing the thickness of crack-free rhodium plating. However, adding stress-relieving agents usually causes a decrease in the hardness and wear resistance of the plating.
Armstrong Michael obtained crack-free rhodium plating by adding halogen compounds from chloride ions to the plating solution while maintaining the hardness and wear resistance unaffected. The basic components are as follows:
Rhodium salt (in rhodium) 5〜15g/L Provides metal ions
H2SO4 30〜90mL/L Increases electrical conductivity
HCI (10~300)×10-6 Stress Relief Agent
Current density 1~8A/ft2 (0. 1〜0. 8A/dm2 )
HCl can reduce the stress of the plating layer without reducing hardness and wear resistance. Generally speaking, the higher the chloride ion concentration, the thicker the crack-free plating layer can be.
This invention is also suitable for pattern plating on printed circuit boards.
There are also other reports using sulfonic acid groups as additives. The structural formula of the additive is R—SO3—H. Where R is a straight-chain, branched, or cyclic group with less than 20 carbon atoms. The additive effect increases smoothness and whiteness, thereby increasing crack-free plating thickness. The plating solution composition is as follows:
|
Rhodium (added as sulfate or phosphate) Sulfuric or phosphoric acid Pyridine-3-sulfonic acid Tensioactivo Additives (added as R-SO3-H structure) |
0. 1〜20g/L 100〜200g/L 0〜5g/L 0. 01〜2g/L 0. 1〜10g/L |
Through experimental verification, it was confirmed that although adding octyl sulfonate (2g/L) slightly reduces current efficiency, it can effectively increase the whiteness of the plated parts. By adding octyl sulfonate, the plating thickness can reach about 0.3~0.7μm.
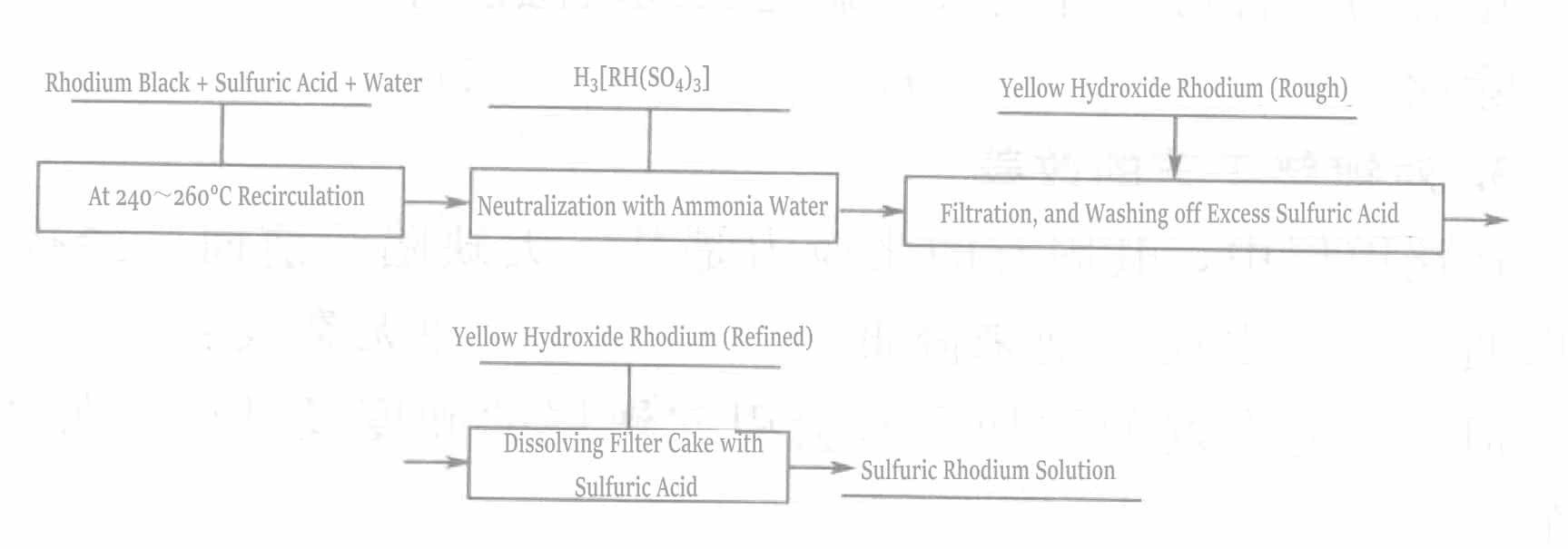
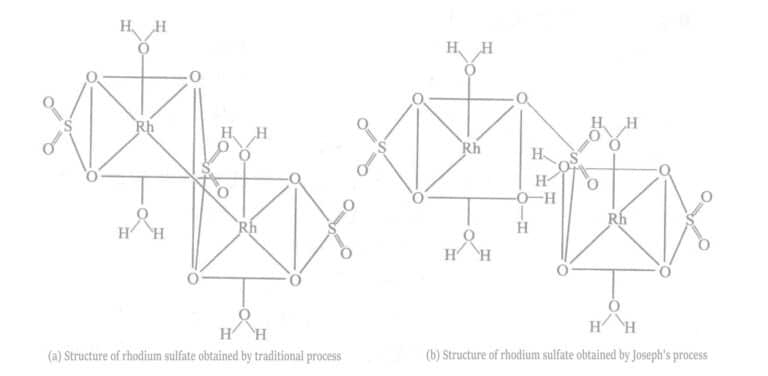
Joseph and others improved the manufacturing process of rhodium sulfate to obtain rhodium sulfate that is more suitable for rhodium plating (see Figure 5-7).
Copywrite @ Sobling.Jewelry - Fabricante de joyería personalizada, fábrica de joyería OEM y ODM
In the traditional preparation method, the neutralization reaction is carried out at room temperature. Due to the heat of the reaction, the actual reaction temperature is much higher than room temperature. Joseph and others controlled the reaction temperature below 25℃ by cooling, which can be achieved by water cooling. The rhodium sulfate obtained was used for plating tests, resulting in a plating layer with low internal stress, brightness, and a plating thickness of up to 1μm.
In addition, Japan’s phase field proposed a method for rapid rhodium plating. The method used is to introduce a jet flow on the equipment (as shown in Figure 5-9), using existing rhodium plating solution, to achieve rapid plating while ensuring the existing advantages.

Figure 5-9 Schematic diagram of rapid rhodium plating equipment
1—plated part (cathode); 2—anode; 3—jet system (inner tank); 4—outer tank; 5—nozzle; 6—conductive rod
|
Rhodium ion concentration Sulfuric acid concentration Temperatura Current density Jet speed |
8〜12g/L 70〜90g/L 50〜70℃ 8A/dm2 0. 3〜1. 0m/s |
Through experiments, it was found that as the current density increases, the plating speed improves; the higher the temperature, the greater the plating speed; at the same time, increasing the jet velocity can also enhance the plating speed. The plating results with varying jet velocities are shown in Table 5-4.
Using this method, a coating with a thickness above 5μm can be obtained, which is glossy, hard in texture, and has low internal stress.
Table 5-4 Effect of Jet Velocity on Plating Speed
| Plating solution composition | Plating conditions | Plating speed | Plating condition | ||||
|---|---|---|---|---|---|---|---|
| Rhodium ion concentration | Ácido sulfúrico | Temperatura | Current density | Jet speed | Apariencia | Grietas | |
|
10g/L 10g/L 10g/L 10g/L 10g/L 10g/L |
80g/L 80g/L 80g/L 80g/L 80g/L 80g/L |
60℃ 60℃ 60℃ 60℃ 60℃ 60℃ |
30A/dm2 30A/dm2 30A/dm2 30A/dm2 30A/dm2 30A/dm2 |
0. 0m/s 0. 2m/s 0. 4m/s 0. 6m/s 0. 8m/s 1. 0m/ s |
1. 70μm/min 1. 73μm/min 1. 84μm/min 1. 90μm/min 2. 10μm/min 2. 22μm/min |
Brillo Brillo Brillo Brillo Brillo Brillo |
No No No No No No |
1.4 Electroplating Black Rhodium
Table 5-5 Process Conditions for Black Rhodium Plating and Its Anode Treatment Conditions
| Proceso | Artículo | Prerequisite | |
|---|---|---|---|
| Electroplating | Plating solution composition |
Rhodium Concentration Sulfuric acid concentration Additives |
2. 5〜3. 5g/L 25〜30g/L Cantidad adecuada |
| Plating conditions |
Temperatura Cathode current density Stirring Maximum thickness |
20〜25℃ 2〜4 A/dm2 Cathode Vibration 0. 5μm |
|
| Anodizing | Treatment solution | Anode treatment fluid | 100g/L |
| Treatment conditions |
Temperatura Tank Voltage Processing time |
20〜30℃ 3V 2〜3min |
|
1.5 Rhodium Plating Equipment
(1) Power Supply:
Flash plating for decorative purposes is not problematic, but the ammeter’s scale must be considered when performing thick plating. It is also preferable to have a three-phase full-wave waveform.
(2) Plating Tank:
Stainless steel tanks coated with polyvinyl chloride can be used. The plating solution temperature for rhodium plating is mostly 40~50℃, and the current efficiency is not very high. Good ventilation equipment is needed to handle sulfuric acid mist.
(3) Filtration:
This also depends on the tank size. Continuous filtration is generally not used since it is strongly acidic, and the plating solution is expensive. When organic impurities are mixed in, external tank filtration is usually employed.
1.6 Troubleshooting Rhodium Plating
Table 5-6 Common Faults and Countermeasures of Rhodium Plating
| Faults | Countermeasures |
|---|---|
| Grietas |
Confirmation of rhodium concentration usually occurs when the concentration is low. Confirmation of acid concentration usually occurs when the concentration is low. Confirmation of plating bath temperature, usually occurs when the temperature is low. |
| Poor bonding | Confirmation of the previous process is usually necessary because the activity of the base metal is not sufficient. |
| Increase in sulfuric acid concentration | If the concentration is too high, the cathode current efficiency will be reduced. It can be recycled, or the plating solution can be heated to evaporate the excess sulfuric acid, cooled down and added with pure water, and then the rhodium can be turned into rhodium hydroxide with sodium hydroxide and filtered, then washed with pure water, and finally dissolved with sulfuric acid. |
| Dark gray plating | The rhodium plating tank is generally of small capacity, and the anode used is insoluble anode, so the composition of the plating solution fluctuates greatly. The low concentration of acid will cause hydrolysis and precipitation of rhodium, which will make the plating layer become dark gray. Rhodium hydroxide precipitates slowly at pH2, and the precipitation increases when the pH is 3~4, so it is very important to manage the concentration of sulfuric acid. |
2. Rhodium Alloy Plating
Alloy plating of rhodium has not been much studied. The earlier ones are Rh-Ni alloy plating.Smith applied for the patent of Rh-Ni alloy plating from acetate sulfate solution. Its main component is Rh 0.4g/L, Ni 3.5~13.5g/L sulfate, pH 1.7, current density 4~10A/dm2. Alloys containing 25%~100% Rh can be obtained. Using the same series, Rh-Co alloy can be obtained if Co is used instead of Ni.
Aotani researched Rh-Zn alloys. The representative process is shown in Table 5-7.
Table 5-7 Sulfate Plating Rh-Zn Process
| Ingredients and their process conditions | Formulation and concentration of components |
|---|---|
|
Rh[in the form of Rh2(SO4)3] Zn (in the form of ZnSO4 • 7H2O Na2SO4 - 10H2O H3BO3 Current density
|
0. 03 ~ 1. 0g/L 5 ~ 40g/L 23g/L 10g/L 3 ~ 9A/dm2 |
Rh-Ir alloy has good corrosion resistance, dense crystallization, and strong adhesion and can also be used as an anode for electrolysis in decorative and functional plating.
The main components of the Rh-Ir alloy plating solution are metallic rhodium salt, metallic iridium salt, fluoroborate as a conductive salt, fluoroboric acid, and amidosulfonic acid (amidosulfonic acid also has a stress-relieving effect) as pH buffers. Additionally, boric acid can be added to prevent the hydrolysis of fluoroboric acid. The plating solution is used at a temperature of about 50~70℃, with a current density of about 2~10A/dm2, which can produce a dense alloy plating layer with strong adhesion.
Electroplating example: Rhodium salt is derived from the reaction of RuCl3·3H2O and NH2SO4H. Iridium salt is derived from the reaction of (NH4)2IrCl6 and NH2SO3H. The mass ratio of Rh-Ir in the plating solution is adjusted to 1/1. Different results can be obtained by changing the content of each component in the plating solution (see Table 5-8).
Table 5-8 Ru-Ir Alloy Plating Solution Composition and Conditions
| Ingredients and their process conditions | N.° 1 | N.° 2 | N.° 3 | N.° 4 |
|---|---|---|---|---|
|
Ru/(g/L) Ir/(g/L) NaBF4(g/L) NH2SO3H/(g/L) Current density/(A/dm2) Temperatura de la solución de recubrimiento/°C pH Ir content in plating layer/% |
8〜9 8〜9 100 30 3 70 0. 9 3〜4 |
8〜9 8〜9 100 20 3 70 0. 8 5〜6 |
3〜4 3〜4 75 14 2 60 0. 9 8〜9 |
3〜4 3〜4 75 4 2 60 1. 2 23 〜24 |
The resulting plating layer has no cracks and is glossy.
In decoration, the natural color of stainless steel or the pale blue-white of chrome plating can no longer meet people’s needs. People prefer a clean, bright appearance similar to silver plating. However, the silver plating layer easily oxidizes and discolors in the air. Rhodium alloy plating can save precious rhodium and significantly improve the coating’s performance (see Table 5-9).
Table 5-9 Plating Solution Composition and Process Conditions for Rhodium-Ruthenium Alloy Plating
| Composition and its process conditions | Formulation and concentration of components |
|---|---|
|
Rhodium salt [Rh2(SO4)3] Ácido sulfúrico Ruthenium salt Additive (Type 8701) Temperatura Cathode current density Anode Stirring method |
1〜2g/L 30mL/L 0. 1〜1g/L 25g/L 40〜50℃ 2〜8A/dm2 Ruthenium coated titanium mesh Cathode movement |
Section III Chemical Rhodium Plating
Like chemical plating of other metals, the advantage of chemical plating is that it does not require the substrate to be conductive and is suitable for various shapes. Because the dispersibility of chemical plating is much better than that of electroplating, at the same time, during electroplating, P may be incorporated into the plating layer, and the purity of rhodium has a significant adverse effect on its corrosion resistance and catalytic performance. Some data suggest that when precious metals contain 0.01%~0.001% P, S, and Cl, gas turbines’ corrosion resistance and service life will be reduced by 25%.
Alexander S. Kozlov also proposed a patent for chemical rhodium plating. Its main components are soluble metal salts, complexing agents, and reducing agents. If necessary, PH buffers and some additives such as stabilizers and surfactants can also be added. This composition contains no harmful substances or volatile components, which can prevent the accumulation of by-products and thus avoid the aging of the plating solution. At the same time, the plating solution can also deposit the metal components by boiling off unwanted components through evaporation.
Its metal salt is Rh (NH3)3 (NO2)3. The main components can be obtained by reacting K3[Rh(NO2)3Cl3] with ammonia water as follows: Rh(NH3)3 (NO2)3 (metal ions), ammonia water (complexing agent), and hydrazine hydrate (reducing agent).
The main reaction of typical chemical rhodium plating is as follows:
Rh(NH3)3(NO2)3 + 0.75 N2H4·H2O → Rh + 3.75N2 + 6.75H2O
Table 5-10 Experimental Results of Chemical Rhodium Plating
| Composition and process conditions | N.° 1 | N.° 2 | N.° 3 | N.° 4 | No. 5 | No. 6 | No. 7 | No. 8 |
|---|---|---|---|---|---|---|---|---|
|
Rh(NH3)3(NO2)3 NH4OH N2H4·H2O Plating material Pre-treatment Pre-treatment Reaction time Plating thickness Surface condition of plated layer Característica |
3. 2g/L 50ml/L 1. 5g/L Nickel Foil Sandpaper Roughening 70℃ 10min 0. 2μm Dense and bright Corrosion Resistant |
1g/L 200ml/L 1g/L Inconel Foil Sandpaper Roughening 85℃ 15 min 0. 4μm Dense and bright Corrosion Resistant |
0. 5g/L 500ml/L 0. 7g/L Acero inoxidable Acetone cleaning 75℃ 30min 0. 2μm Dense Bright Catalytic |
5 g/L 100ml/L 2 g/L Mg2Al4Si5O18 Sensitized activation 60℃ 30min 0. 5μm Gray uniform Catalyzed |
1g/L 100ml/L 2. 5g/L SiC Powder Sensitized activation 70℃ 30min 0. 03μm Brightening Catalyzed |
1g/L 200ml/L 0. 2g/L Glass Flake Sensitization activation 60℃ 10min 0. 1μm Mirror bright Mirror |
3 g/L 100ml/L 1. 5g/L Aluminum Oxide Sensitized activated 75℃ 2h 2. 2μm Not smooth gray Electronic Components |
7g/L 50ml/L 4. 5g/L Ti plate Sandpaper Roughening 85℃ 3h 3. 5μm Tight Semi-Bright Inert Anode |
This plating solution composition can be applied to various plated items by performing appropriate pretreatment on the plated parts.
With the development of science and technology, the demand for rhodium will also increase accordingly. They hold great potential based on the characteristics of rhodium plating layers, whether for decorative items or industrial applications. When rhodium plating is used on electrical contacts, the thickness for anti-tarnish purposes is below 0.5μm; for wear resistance purposes, the plating thickness is between 0.2~2μm; for parts with strict wear resistance requirements, the plating thickness is between 2.5~25μm. When used as an underlayer plating for gold in lead frames, it can save the amount of gold used.